Abstract
Clinical preference for a semisynthetic penicillin (oxacillin or nafcillin) over cefazolin for deep-seated methicillin-susceptible Staphylococcus aureus (MSSA) bloodstream infections (BSI) perseveres despite limited data to support this approach. A retrospective cohort study of patients treated for MSSA BSI with either oxacillin or cefazolin was performed across two medical centers in Chicago, IL. The outcome measures included documented in-hospital treatment failure, all-cause in-hospital mortality, duration of MSSA BSI, and incidence of documented adverse events. Of 161 patients with MSSA BSI, 103 (64%) received cefazolin, and 58 (36%) received oxacillin. The identified sources of BSI were central line (37.9%), osteoarticular (18%), and skin and soft tissue (17.4%). Patients with endocarditis (29/52 [44.2%]) and other deep-seated infections (23/52 [55.8%]) were classified under the subset of deep-seated infections (52/161 [32.3%]). Multivariate models found deep-seated infection (adjusted odds ratio [aOR], 4.52; 95% confidence interval [CI], 1.23 to 16.6; P = 0.023), metastatic disease (aOR, 4.21; 95% CI, 1.13 to 15.7; P = 0.033), and intensive care unit (ICU) onset of infection (aOR, 4.80; 95% CI, 1.26 to 18.4; P = 0.022) to be independent risk factors for in-hospital treatment failure. Treatment group was not an independent predictor of failure (aOR, 3.76; 95% CI, 0.98 to 14.4; P = 0.053). The rates of treatment failure were similar among cefazolin-treated (5/32 [15.6%]) and oxacillin-treated (4/20 [20.0%]) patients (P = 0.72) in the subset of deep-seated infections. Mortality was observed in 1 (1%) and 3 (5.2%) cases of cefazolin- and oxacillin-treated patients, respectively (P = 0.13). Cefazolin was not associated with higher rates of treatment failure and appears to be an effective alternative to oxacillin for treatment of deep-seated MSSA BSI.
INTRODUCTION
Despite the rise and impact of methicillin-resistant Staphylococcus aureus, methicillin-susceptible S. aureus (MSSA) continues to contribute to the overall burden of S. aureus infections, representing ≥50% of clinical S. aureus strains (1–3) with known appreciable mortality outcomes (4, 5). Prevailing evidence supports the use of semisynthetic penicillins, such as oxacillin or nafcillin, or the first-generation cephalosporin cefazolin in preference to vancomycin as optimal therapy for MSSA bloodstream infections (BSI) (6–11). Moreover, an early switch from vancomycin to either nafcillin or cefazolin with definitive MSSA identification was associated with a 69% risk reduction in 30-day in-hospital mortality in a recent retrospective cohort study (11).
Cefazolin offers a convenient dosing scheme with a favorable adverse event profile, allowing many institutions to consider its use in the management of MSSA infections (12). However, deep-seated infections with a high burden of S. aureus have been shown to overproduce certain types of β-lactamases, rendering cefazolin inactive and thus resulting in possible treatment failure (13–15). Furthermore, current practice guidelines, such as those for infective endocarditis, suggest reserving the use of cefazolin for patients with nonanaphylactoid-type penicillin allergies (7). As a result, clinician preference for either nafcillin or oxacillin for more severe or deep-seated MSSA infection has evolved in practice, while the use of cefazolin in this setting remains controversial (12, 16, 17). The purpose of this study was to evaluate and compare treatment outcomes using cefazolin or oxacillin for MSSA BSI, including deep-seated sources of infection.
(Portions of this paper were presented as a poster at the 54th Interscience Conference on Antimicrobial Agents and Chemotherapy, 5 to 9 September 2014, Washington, DC.)
MATERIALS AND METHODS
Study design.
From January 2010 to April 2013, a retrospective cohort study was conducted at Rush University Medical Center (RUMC) and Northwestern Memorial Hospital (NMH), two tertiary care academic medical centers in Chicago, IL. The study methods were reviewed and approved by the institutional review boards at RUMC, NMH, and Midwestern University. Adult patients who received in-hospital definitive treatment with cefazolin or oxacillin within 48 h of finalized blood cultures with MSSA were eligible for inclusion in the study. Patients were included only once, and only the first (index) in-hospital admission during the study period was evaluated. The index blood culture was the first blood culture growing MSSA during the index admission. Patients were excluded if they (i) were <18 years of age, (ii) received antibiotics other than cefazolin or oxacillin for definitive treatment of an MSSA BSI, (iii) received ≥5 days of an empirical antibiotic agent active against MSSA prior to definitive treatment with cefazolin or oxacillin, (iv) had a documented penicillin or cephalosporin allergy, or (v) had polymicrobial bacteremia.
MSSA isolates were identified at each institution according to standard microbiology laboratory procedures. The susceptibility of MSSA isolates at RUMC was determined using MicroScan (Dade Behring, Inc., West Sacramento, CA) and at NMH using Vitek 2 (bioMérieux, La Balmes-les-Grottes, France). All susceptibility profiles were interpreted according to the Clinical and Laboratory Standards Institute (CLSI) interpretive criteria at the time of culture (18–21).
Variables.
Patient demographics and data variables were extracted from inpatient electronic medical records, in addition to pharmacy and microbiology databases examined retrospectively by trained reviewers. The variables collected and analyzed included patient demographics (age, gender, and race), past medical history and comorbidities (e.g., hepatic and renal dysfunction, diabetes, osteoarticular manipulation, and malignancies), history of solid organ transplant or receipt of any immunosuppression, hospital disposition (intensive care unit [ICU] versus non-ICU) at the time of index blood culture, length of hospital stay, duration of antibiotics prior to the index blood culture during hospitalization, place of infection acquisition (community versus health care associated), site of infection, serum creatinine level on the day of the index blood culture, modified acute physiology and chronic health evaluation II (APACHE II) components and composite score (22, 23), source control, antimicrobial agent (i.e., cefazolin or oxacillin) and dose utilized, time to first negative blood culture, treatment duration, adverse drug events, change in definitive treatment agent (i.e., switching to an alternative agent), and organ dysfunction.
Definitions.
Renal dysfunction was defined as the presence of documented chronic kidney disease, end-stage renal disease, use of renal replacement therapy, or acute kidney injury defined as an increase in serum creatinine level of ≥0.5 mg/dl or a 50% increase from baseline during the hospital stay (24). Hepatic dysfunction was defined as any liver enzymes at >3 times the upper limit of normal or documented classification of hepatic cirrhosis. Immunocompromised patients included those who received immunosuppressants, corticosteroids, or chemotherapy within the 6 months prior to the hospital stay or those who were diagnosed with HIV/AIDS. Persistent bacteremia was defined as continuous positive cultures growing MSSA for >7 days from the start of treatment (25). All outcomes (including treatment failure) were determined retrospectively by trained reviewers using patient progress notes. Treatment failure was defined by a change in MSSA-directed therapy if documentation was present indicating clinician opinion that either cefazolin or oxacillin was ineffective. Persistent bacteremia without resolution of clinical symptoms, recurrent MSSA infection post-negative culture, and growth of MSSA from alternative site(s) after initiation of therapy were evaluated; however, documentation of ineffective treatment by a clinician was utilized as the definition for treatment failure. In-hospital mortality secondary to complications from infection also defined treatment failure when MSSA infection was the primary factor for worsening clinical status that led to patient death. A transition in definitive therapy from either cefazolin to oxacillin or oxacillin to cefazolin or from either agent to any other Gram-positive agent was defined as a change in therapy to an alternate agent. Transition between study agents was not considered a treatment failure, and patient outcomes were analyzed according to their initial treatment regimen. Modified APACHE II scores were calculated using previously defined methods (22, 23), in which missing values were conservatively assumed to be normal. ICU onset of infection was defined as patient residence in the ICU when the index blood culture became positive for MSSA. A documented source of bacteremia was identified from patient medical records. Deep-seated sources of MSSA BSI were considered infectious endocarditis or other identified deep-seated infectious sources, including bone and joint, deep-seated abscesses, osteomyelitis, pneumonia, or unresolved vascular graft infections. Twenty-four-hour daily doses of cefazolin and oxacillin were defined as the equivalent dose, in grams, per 24 h (e.g., 1 g every 48 h was considered a 0.5-g 24-h dose equivalent) for ease of comparison.
Statistical analysis.
The primary outcome (i.e., dependent variable) was the covariate-adjusted odds of treatment failure among patients with MSSA BSI definitively treated with cefazolin or oxacillin during the in-hospital study period. The initial treatment strategy with either cefazolin or oxacillin was assessed as the primary predictor of interest and was forced into all models. Covariates (i.e., modified APACHE II [m-APACHE II] score on the day of infection, race, history of diabetes, history of prosthetic device implantation, place of acquisition, requirement of ICU admission during admission, history of renal dysfunction, history of therapeutic immunosuppression, receipt of broad-spectrum antibiotics within the last 30 days, presence of a known source of infection, whether source control was achieved, presence of a cerebrospinal fluid source, presence of a central line/dialysis access source, presence of metastatic infection, presence of a deep-seated infection, or receipt of any definitive therapy with a second- or third-generation cephalosporin) were considered for inclusion in the multivariate model of treatment failure. Secondary endpoints included all-cause in-hospital mortality, treatment failure rates among deep-seated infections, duration of MSSA BSI, and the incidence of documented adverse events. Statistical analyses were performed using Intercooled Stata, version 13.0 (StataCorp, College Station, TX).
Continuous variables were evaluated with Student's t test or the Wilcoxon rank sum test. Categorical variables were evaluated with the chi-square or Fisher's exact test where appropriate. Unadjusted odds ratios (ORs) with 95% confidence intervals (CIs) were determined using bivariate logistic regression. Multivariate analyses were performed by constructing models to assess dichotomous outcomes (e.g., treatment failure, mortality, etc.) while controlling for relevant covariates listed above as a forward stepwise procedure. Variables significant at a P value of <0.2 in the univariate analysis having a plausible relationship with the treatment outcome (and described above) were analyzed in logistic regression models (26). Variables were retained in the models if the objective function value changed by >3.84 with each iterative addition (n + 1 model). The final models were secondarily checked for optimal parsimony utilizing the Akaike information criterion (AIC) (26). That is, a model with n variables was compared to a model with n + 1 variables to evaluate the impact of the additional variable on calculated AIC. If model n + 1 was no more explanatory by the likelihood ratio test, model n was selected as the final model, according to the rule of parsimony. Outcome probabilities, adjusting for covariates, were calculated from adjusted odds ratios (aORs). Univariate time-to-event analysis was conducted using Kaplan-Meier curves and log rank tests for significance. Multivariate time-to-event analysis was conducted by assessing the variables identified in multivariate logistic regression models in Cox proportional hazards regression. All tests were two-tailed, with an a priori level of alpha set at 0.05 for statistical significance.
RESULTS
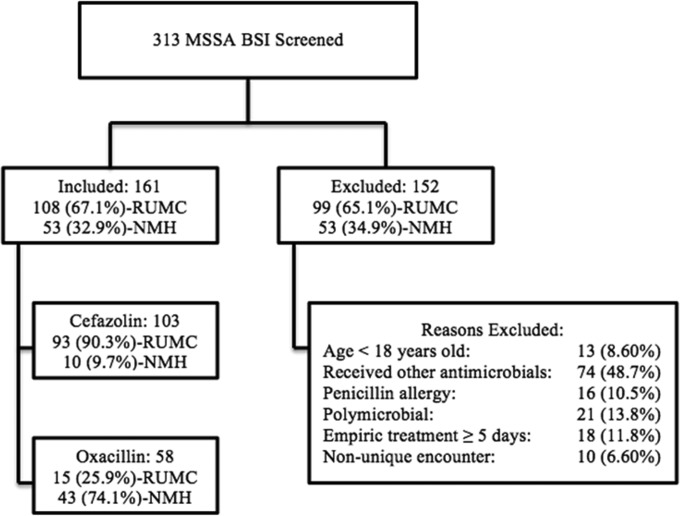
A total of 313 patients with MSSA BSI were screened for inclusion at both study centers, and 161 patients met the inclusion criteria, as shown in Fig. 1. A total of 152 patients were excluded from the analysis. Most of the exclusions (48.7%) were due to the receipt of antibiotics other than cefazolin or oxacillin for definitive treatment of an MSSA BSI.
FIG 1.
Flow chart showing disposition of patients included and excluded from study. BSI, bloodstream infection; MSSA, methicillin-susceptible S. aureus; NMH, Northwestern Memorial Hospital; RUMC, Rush University Medical Center.
Demographics.
The baseline characteristics of the patients treated with cefazolin or oxacillin are displayed in Table 1. Discrepancies in the baseline characteristics between the treatment groups included African-American or Caucasian ethnicity (P = 0.01), m-APACHE score on the day of infection (P = 0.009), presence of renal dysfunction (P = 0.013), history of prosthetic device (P = 0.048), central line source (P = 0.007), and if source control was achieved (P = 0.001), as shown in Table 1. The most common sources for MSSA BSI identified within the cohort were related to central lines (37.9% [61/161]), bone or joints (18% [29/161]), endocardial vegetations (18% [29/161]), skin and soft tissue (17.4% [28/161]), and other sources of infections (10.6% [17/161]).
TABLE 1.
Characteristics of MSSA BSI cohort according to treatment and treatment outcome
| Characteristica | Patients treated with: |
P value | Observed failure | Univariate OR for failure | P value | |
|---|---|---|---|---|---|---|
| Cefazolin | Oxacillin | |||||
| Age (mean [SD]) (yr) | 53.3 (16.7) | 53.6 (18.4) | 0.91 | 52.9 (17.1) | 0.99 | 0.91 |
| Admit wt (mean [SD]) (kg) | 79.5 (22.4) | 81.5 (25.3) | 0.61 | 76.9 (21.1) | 0.99 | 0.60 |
| Male (no. [%]) | 60 (58.3) | 36 (62.1) | 0.64 | 9 (69.2) | 0.63 | 0.56 |
| Race (no. [%]) | 0.019 | 1.32 | 0.31 | |||
| African-American (n = 60) | 46 (44.7) | 14 (24.1) | 0.01 | 7 (53.9) | 2.09 | 0.20 |
| Caucasian (n = 70) | 37 (35.9) | 33 (56.9) | 0.01 | 3 (23.1) | 0.36 | 0.15 |
| Hispanic (n = 24) | 14 (13.6) | 10 (17.2) | 0.53 | 3 (23.1) | 1.81 | 0.41 |
| Other (n = 7) | 6 (5.8) | 1 (1.7) | 0.42 | 0 (0) | >0.99 | |
| m-APACHE score day 0 (mean [SD]) | 13 (6.3) | 10.3 (5.8) | 0.009 | 11.9 (5) | 0.99 | 0.95 |
| Baseline serum creatinine (median [IQR]) | 1.2 (0.8–7.13) | 1.05 (0.81–1.92) | 0.19 | 0.97 (0.74–2.36) | 0.88 | 0.29 |
| ICU admission (no. [%]) | 43 (41.8) | 19 (32.8) | 0.26 | 9 (69.2) | 4.03 | 0.03 |
| Renal dysfunction (no. [%]) | 51 (49.5) | 17 (29.3) | 0.013 | 5 (38.5) | 0.84 | 0.77 |
| History of orthopedic procedure (no. [%]) | 25 (24.3) | 7 (12.1) | 0.06 | 3 (23.1) | 1.23 | 0.72 |
| History of prosthetic device (no. [%]) | 22 (21.4) | 5 (8.6) | 0.048 | 3 (23.1) | 1.55 | 0.46 |
| Source of infection (no. [%]) | ||||||
| Skin/wound | 15 (14.6) | 13 (22.4) | 0.21 | 1 (7.7) | 0.37 | 0.47 |
| Urinary | 2 (1.9) | 1 (1.7) | >0.99 | 0 (0) | >0.99 | |
| Cerebrospinal fluid | 0 (0) | 1 (1.7) | 0.36 | 1 (7.7) | 0.08 | |
| Bone/joint | 21 (20.4) | 8 (13.8) | 0.30 | 4 (30.8) | 2.19 | 0.25 |
| Central line/dialysis access | 47 (45.6) | 14 (24.1) | 0.007 | 5 (38.5) | 1.03 | >0.99 |
| Respiratory | 2 (1.9) | 1 (1.7) | >0.99 | 0 (0) | >0.99 | |
| Source control (no. [%]) | 79 (76.7) | 30 (51.7) | 0.001 | 10 (76.9) | 1.65 | 0.55 |
| Metastatic disease (no. [%]) | 30 (29.1) | 11 (19.0) | 0.16 | 7 (53.9) | 3.91 | 0.014 |
| Deep-seated infection (no. [%])b | 32 (31.1) | 20 (34.5) | 0.66 | 9 (69.2) | 5.49 | 0.005 |
| Endocarditis (n = 29) | 17 (53.1) | 12 (60.0) | 0.63 | 6 (66.7) | 1.74 | 0.47 |
| Other deep-seated infections (n = 23) | 15 (46.9) | 8 (40.0) | 3 (33.3) | |||
| Total (no. [%]) | 103 (64) | 58 (36) | 13 (8.1) | |||
m-APACHE, modified APACHE II score; IQR, interquartile range; ICU, intensive care unit.
Deep-seated infection was considered infectious endocarditis or any of the following: bone and joint, deep-seated abscesses, osteomyelitis, pneumonia, or unresolved vascular graft infections.
Outcomes according to treatment group.
The predictors of treatment failure (Table 1) were relatively similar between cefazolin- and oxacillin-treated patients and between those who experienced in-hospital treatment failure or not, with the following exceptions: being in the ICU at the time of culture (P = 0.03), presence of metastatic disease (P = 0.014), and presence of deep-seated infections (P = 0.005). Clinical outcomes by treatment failure and treatment with cefazolin or oxacillin are displayed in Table 2. Treatment failure occurred in 6 cases (5.8%) among cefazolin-treated patients and 7 cases (12.1%) among oxacillin-treated patients (P = 0.16). Treatment failure occurred more often among patients with deep-seated infections than those without (17.3% [9/52] versus 3.7% [4/109]; P = 0.005), patients with metastatic disease than those without (17.1% [7/41] versus 5.0% [6/120]; P = 0.014), and among the subgroup of patients with infective endocarditis than those with other deep-seated infections (20.7% [6/29] versus 13.0% [3/23]; P = 0.71). In the subset of patients with deep-seated infections, the treatment failure rates were similar among cefazolin-treated (5/32 [15.6%]) and oxacillin-treated (4/20 [20.0%]) patients (P = 0.72). The median (interquartile range [IQR]) 24-h daily doses of cefazolin (12 [12 to 12] g versus 12 [12 to 12] g; P = 0.55) and oxacillin (4 [0.66 to 6] g versus 0.66 [0.66 to 0.66] g; P = 0.19) were not different for those who survived and died, respectively.
TABLE 2.
Clinical outcomes of MSSA BSI cohort according to treatment
| Characteristica | Patients treated with: |
P value | |
|---|---|---|---|
| Cefazolin | Oxacillin | ||
| Treatment failure | 6 (5.8) | 7 (12.1) | 0.16 |
| In-hospital mortality | 1 (1.0) | 3 (5.2) | 0.13 |
| Time to treatment failure (median [IQR]) (days) (n = 13) | 6.5 (5–13) | 10 (7–12) | 0.61 |
| Hospital survivor LOS (median [IQR]) (days) | 9 (7–16) | 10 (8–16) | 0.49 |
| Time to source control (median [IQR]) (days) | 2 (1–4) | 1.5 (0–3) | 0.05 |
| Duration of bacteremia (median [IQR]) (days) | 3 (2–4) | 3 (2–4) | 0.57 |
| Duration of treatment (median [IQR]) (days) | 29 (15–42) | 32.5 (15–43) | 0.35 |
| Reinfection after clearance | 5 (4.9) | 3 (5.2) | >0.99 |
| Change in therapy to alternative agent | 21 (20.4) | 25 (43.1) | 0.002 |
| Received infectious disease consult | 74 (71.8) | 45 (77.6) | 0.43 |
| Resolution of deep-seated infection (n = 52)b | 27 (84.4) | 16 (80.0) | 0.72 |
| Resolution of endocarditis (n = 29)c | 13 (76.5) | 10 (83.3) | >0.99 |
| Any ADE | 8 (7.8) | 2 (3.5) | 0.33 |
| Rash | 3 (2.9) | 0 (0) | 0.55 |
| Nephrotoxicity | 1 (1.0) | 0 (0) | >0.99 |
| Hepatotoxicity | 0 (0) | 1 (1.7) | 0.36 |
| Neurotoxicity | 0 (0) | 0 (0) | |
| Total | 103 (64) | 58 (36) | |
All data are reported as no. (%), unless otherwise specified. IQR, interquartile range; LOS, length of stay; ADE, adverse drug event.
Deep-seated infection was considered infectious endocarditis or any of the following: bone and joint, deep-seated abscesses, osteomyelitis, pneumonia, or unresolved vascular graft infections. The numbers of patients were 32 for cefazolin treatment and 20 for oxacillin treatment.
The numbers of patients were 17 for cefazolin treatment and 12 for oxacillin treatment.
All-cause in-hospital mortality occurred rarely, and the mortality rates did not differ significantly according to treatment group (3/59 for oxacillin versus 1/103 for cefazolin; P = 0.13). As expected, patients experiencing in-hospital treatment failure were more likely to die (30.8% versus 0%, respectively; P < 0.001). Among those who survived their infection, the subgroup of patients experiencing treatment failure was more likely to have a longer median (IQR) length of hospital stay than those who did not (27 [14 to 33] days versus 9 [7 to 14.5] days, respectively; P < 0.001).
Patients experiencing treatment failure were switched to an alternative treatment regimen from their initial definitive agent (i.e., cefazolin or oxacillin) more often than those who did not experience in-hospital treatment failure (84.6% versus 23.7%, respectively; P < 0.001). Compared to patients receiving treatment with oxacillin, those treated with cefazolin underwent fewer changes in therapy to an alternate agent (20.4% versus 43.1%, respectively; P = 0.002). Patients treated with cefazolin received definitive source control more often than patients treated with oxacillin (76.7% versus 51.7%, respectively) and had a shorter median (IQR) time to achieving source control (1.5 [0 to 3] days versus 2 [1 to 4] days, respectively; P = 0.05).
Univariate and multivariate models of failure.
The multivariate models for in-hospital treatment failure according to treatment are shown in Table 3. The logistic regression models considered all relevant variables for possible inclusion. The final model of failure identified the presence of a deep-seated infection (aOR, 4.52; 95% CI, 1.23 to 16.6; P = 0.023), metastatic disease (aOR, 4.21; 95% CI, 1.13 to 15.7; P = 0.033), and having an ICU onset of infection (aOR, 4.80; 95% CI, 1.26 to 18.4; P = 0.022) as independent risk factors for treatment failure. The model (model n) AIC was 80.0, while a model parameterized with the next most predictive variable (i.e., categorical race, model n + 1) had an AIC of 80.1 and was not significantly different from model n by the likelihood ratio test (P = 0.12). Thus, model n was selected as the final model of failure. Treatment with cefazolin or oxacillin was not independently associated with treatment failure, according to the a priori level of significance after adjusting for relevant confounders (aOR, 3.76; 95% CI, 0.98 to 14.4; P = 0.053).
TABLE 3.
Multivariate model of treatment failurea
| Predictor of treatment failure | Multivariate analysis OR (95% CI) | P value |
|---|---|---|
| Oxacillin treatment | 3.76 (0.98–14.4) | 0.053 |
| Deep-seated infection | 4.52 (1.23–16.6) | 0.023 |
| Metastatic disease | 4.21 (1.13–15.7) | 0.033 |
| ICU onset of infection | 4.80 (1.26–18.4) | 0.022 |
Multivariate regression completed with forward stepwise logistic regression controlling for treatment group, deep-seated infection, metastatic disease, and ICU status.
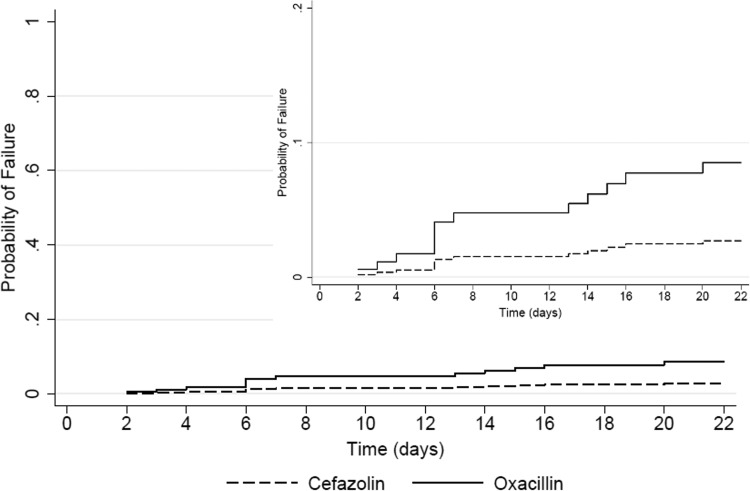
Time to failure was not significantly different by the log rank test among patients treated with cefazolin and those treated with oxacillin (P = 0.17). A Cox proportional hazards regression was conducted using covariates identified in the logistic model building with the results of the time-to-event model stratified by treatment group (Fig. 2). The univariate hazard ratio for failure among patients receiving treatment with oxacillin versus cefazolin was 2.1 (95% CI, 0.7 to 6.2; P = 0.18). The corresponding univariate hazard ratios for treatment failure in the presence of a deep-seated infection, metastatic disease, and ICU onset infection were 5.1 (95% CI, 1.6 to 16.6; P = 0.007), 3.5 (95% CI, 1.2 to 10.5; P = 0.024), and 3.8 (95% CI, 1.2 to 12.3; P = 0.027), respectively. The multivariate adjusted hazard ratio for treatment group adjusting for the previously identified multivariate variables was 3.1 (95% CI, 0.99 to 9.9; P = 0.052), and all previously identified multivariate variables from the univariate analysis remained significant in the multivariate regression model (data not shown).
FIG 2.
Time to clinical failure stratified by treatment group (cefazolin versus oxacillin). The Cox regression model was parameterized with the same covariates as identified in the logistic regression model build (i.e., treatment group, deep-seated infection, metastatic disease, and ICU admission). The y axis is scaled 0 to 100% in the main panel and 0 to 20% in the smaller panel for ease of viewing.
We evaluated the impact of the study center on multivariate predictions of treatment failure and to control for intersite heterogeneity. The univariate risk of failure according to study site was not significantly different (NMH, 5.7%, versus RUMC, 9.3%; P = 0.55). When study center was evaluated in multivariate models of failure in an exploratory analysis, the predictions of failure were not significantly improved (according to AIC) compared to the n − 1 models without study site included (data not shown).
Comparative safety.
Adverse events were rare among patients treated with both cefazolin and oxacillin, as shown in Table 2. Ten patients in total experienced an adverse drug event. The composite of all recorded adverse drug events was numerically but not significantly (P = 0.33) higher among patients receiving cefazolin (7.8% [8/103]) than among those receiving oxacillin (3.4% [2/58]). Neurotoxicity was not identified in any patient receiving oxacillin or cefazolin.
DISCUSSION
After adjusting for multiple confounders, our findings suggest that cefazolin is no worse than oxacillin for the treatment of MSSA BSI with respect to clinical cure, duration of bacteremia, and time to failure. Our results are consistent with those from other related recently published studies (12, 16, 17, 27). However, in comparison to previous studies, we observed higher rates of endocarditis, in which 16.5% (17/103) of patients carrying the diagnosis of infectious endocarditis were treated with cefazolin, and 20.7% (n = 12/58) were treated with oxacillin. In-hospital treatment failures in this subgroup were similarly common, at 23.5% (n = 4/17) of the cefazolin-treated cases and 16.7% (2/12) of the oxacillin-treated cases. Additionally, our cohort included 32 (31.1%) cefazolin-treated and 20 (34.5%) oxacillin-treated patients with overall deep-seated infections, also resulting in similarly common in-hospital treatment failure rates, at 15.6% (5/32) for cefazolin and 20% (4/20) for oxacillin. Higher failure rates with cefazolin as a result of overproduction of certain types of β-lactamases in deep-seated infections have been reported (14, 15). However, neither cefazolin nor oxacillin in our study was independently associated with treatment failure, even with the presence of deep-seated infections. A further investigation of the prevalence and clinical impact of β-lactamase production and its ability to render cefazolin ineffective in deep-seated infections is warranted.
In our study, agent selection was made at the discretion of the managing physicians, with each institution preferentially utilizing different formulary agents (i.e., oxacillin was preferred at NMH, and cefazolin was preferred at RUMC). Per the institutional guidelines in place during the study period, patients with MSSA infections at RUMC were treated with cefazolin for the entire course of therapy unless a treatment failure occurred, while patients at NMH were initiated on and discharged home with oxacillin unless intolerance to the therapy was observed. The differing patterns of use at each site allowed for an exploration of the efficacy of each agent in general and specifically among infections complicated by deep-seated infections across a broad range of comorbidities and patient acuity. We did not detect differences in efficacy according to study center, and the inclusion of the study center in our multivariate models failed to significantly improve predictions of failure. Recent studies have observed higher discontinuation rates with semisynthetic penicillins than with cefazolin (17, 27), which may partially explain the findings in our composite endpoint of slightly higher failure rates with oxacillin than with cefazolin. However, we did not detect significant differences in the adverse drug events between these agents.
Health care systems seek to provide high-quality patient care while holding at bay the rising costs of care. Based on Red Book average wholesale prices (AWP), the daily cost of treating a patient with normal renal function is approximately $169.68 for oxacillin (2 g every 4 h) compared to $26.28 for cefazolin (2 g every 8 h) (28, 29). In our study, there were no dosing differences seen between those who failed treatment and those who did not, with the majority of patients receiving 6 g and 12 g of cefazolin and oxacillin, respectively. Thus, cefazolin may have similar efficacy for MSSA BSI, with an improved cost profile compared to that of oxacillin.
Our study has several limitations that must be acknowledged. First, although MicroScan and Vitek 2 are able to capture oxacillin susceptibility and MICs, cefazolin susceptibility was not directly reported at NMH, and oxacillin was the formulary agent of choice, which may have led to a native selection bias across study centers. However, we failed to detect significant differences in outcomes according to study center. Second, this was a retrospective study and is subject to inherent biases; however, no prospective and randomized analyses exist to date. Third, while the Infectious Diseases Society of America (IDSA) guidelines on the management of infectious endocarditis (7) support the use of combination therapy (i.e., addition of aminoglycosides and/or rifampin) for persistent MSSA infections and endocarditis, we did not assess the impact of concurrent aminoglycoside therapy on clinical outcomes. However, previous studies have evaluated the impact of combination therapy on native valve endocarditis and BSI, and an evaluation of these outcomes was not the focus of the current study. Fourth, we were unable to ascertain whether the blaZ β-lactamase was present in the clinical isolates in our study, and our results may be less generalizable to populations in which type A blaZ-producing S. aureus is prevalent. At our centers, genotyping of S. aureus isolates is not routinely performed; future studies evaluating outcomes according to genotype should be performed.
In conclusion, cefazolin produced clinical outcomes similar to those of standard-of-care therapy with oxacillin for the treatment of MSSA BSI. Our study suggests that patients with culture-proven MSSA BSI, including deep-seated infection, can be effectively managed with cefazolin as definitive therapy in combination with source control and close monitoring. Oxacillin and other semisynthetic penicillins will continue to play a major role in the setting of treatment failure and in infections for which cefazolin has poor distribution (i.e., central nervous system infections). Until larger prospective trials can provide additional insight into the comparative effectiveness of cefazolin and semisynthetic penicillins across the spectrum of clinical diseases, we suggest that these two therapeutic approaches may be considered interchangeable for the majority of MSSA BSI.
ACKNOWLEDGMENTS
We acknowledge Ryan Heraty and Brandon Chiu, who supported the development of this article, assisted with data collection, and provided reviews of the data presented here.
No external funding was received for this study.
We declare no conflicts of interest.
REFERENCES
- 1.Miro JM, Anguera I, Cabell CH, Chen AY, Stafford JA, Corey GR, Olaison L, Eykyn S, Hoen B, Abrutyn E, Raoult D, Bayer A, Fowler VG Jr, International Collaboration on Endocarditis Merged Database Study Group. 2005. Staphylococcus aureus native valve infective endocarditis: report of 566 episodes from the International Collaboration on Endocarditis Merged Database. Clin Infect Dis 41:507–514. doi: 10.1086/431979. [DOI] [PubMed] [Google Scholar]
- 2.Mostofsky E, Lipsitch M, Regev-Yochay G. 2011. Is methicillin-resistant Staphylococcus aureus replacing methicillin-susceptible S. aureus? J Antimicrob Chemother 66:2199–2214. doi: 10.1093/jac/dkr278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sievert DM, Ricks P, Edwards JR, Schneider A, Patel J, Srinivasan A, Kallen A, Limbago B, Fridkin S, National Healthcare Safety Network (NHSN) Team and Participating NHSN Facilities. 2013. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect Control Hosp Epidemiol 34:1–14. doi: 10.1086/668770. [DOI] [PubMed] [Google Scholar]
- 4.Cosgrove SE, Sakoulas G, Perencevich EN, Schwaber MJ, Karchmer AW, Carmeli Y. 2003. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin Infect Dis 36:53–59. doi: 10.1086/345476. [DOI] [PubMed] [Google Scholar]
- 5.Klein E, Smith DL, Laxminarayan R. 2007. Hospitalizations and deaths caused by methicillin-resistant Staphylococcus aureus, United States, 1999–2005. Emerg Infect Dis 13:1840–1846. doi: 10.3201/eid1312.070629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang FY, Peacock JE Jr, Musher DM, Triplett P, MacDonald BB, Mylotte JM, O'Donnell A, Wagener MM, Yu VL. 2003. Staphylococcus aureus bacteremia: recurrence and the impact of antibiotic treatment in a prospective multicenter study. Medicine (Baltimore) 82:333–339. doi: 10.1097/01.md.0000091184.93122.09. [DOI] [PubMed] [Google Scholar]
- 7.Baddour LM, Wilson WR, Bayer AS, Fowler VG Jr, Bolger AF, Levison ME, Ferrieri P, Gerber MA, Tani LY, Gewitz MH, Tong DC, Steckelberg JM, Baltimore RS, Shulman ST, Burns JC, Falace DA, Newburger JW, Pallasch TJ, Takahashi M, Taubert KA, Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association, Infectious Diseases Society of America . 2005. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation 111:e394–e434. doi: 10.1161/CIRCULATIONAHA.105.165564. [DOI] [PubMed] [Google Scholar]
- 8.Stryjewski ME, Szczech LA, Benjamin DK Jr, Inrig JK, Kanafani ZA, Engemann JJ, Chu VH, Joyce MJ, Reller LB, Corey GR, Fowler VG Jr. 2007. Use of vancomycin or first-generation cephalosporins for the treatment of hemodialysis-dependent patients with methicillin-susceptible Staphylococcus aureus bacteremia. Clin Infect Dis 44:190–196. doi: 10.1086/510386. [DOI] [PubMed] [Google Scholar]
- 9.Lodise TP Jr, McKinnon PS, Levine DP, Rybak MJ. 2007. Impact of empirical-therapy selection on outcomes of intravenous drug users with infective endocarditis caused by methicillin-susceptible Staphylococcus aureus. Antimicrob Agents Chemother 51:3731–3733. doi: 10.1128/AAC.00101-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim SH, Kim KH, Kim HB, Kim NJ, Kim EC, Oh MD, Choe KW. 2008. Outcome of vancomycin treatment in patients with methicillin-susceptible Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 52:192–197. doi: 10.1128/AAC.00700-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schweizer ML, Furuno JP, Harris AD, Johnson JK, Shardell MD, McGregor JC, Thom KA, Cosgrove SE, Sakoulas G, Perencevich EN. 2011. Comparative effectiveness of nafcillin or cefazolin versus vancomycin in methicillin-susceptible Staphylococcus aureus bacteremia. BMC Infect Dis 11:279. doi: 10.1186/1471-2334-11-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee S, Choe PG, Song KH, Park SW, Kim HB, Kim NJ, Kim EC, Park WB, Oh MD. 2011. Is cefazolin inferior to nafcillin for treatment of methicillin-susceptible Staphylococcus aureus bacteremia? Antimicrob Agents Chemother 55:5122–5126. doi: 10.1128/AAC.00485-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nannini EC, Singh KV, Murray BE. 2003. Relapse of type A beta-lactamase-producing Staphylococcus aureus native valve endocarditis during cefazolin therapy: revisiting the issue. Clin Infect Dis 37:1194–1198. doi: 10.1086/379021. [DOI] [PubMed] [Google Scholar]
- 14.Nannini EC, Stryjewski ME, Singh KV, Bourgogne A, Rude TH, Corey GR, Fowler VG Jr, Murray BE. 2009. Inoculum effect with cefazolin among clinical isolates of methicillin-susceptible Staphylococcus aureus: frequency and possible cause of cefazolin treatment failure. Antimicrob Agents Chemother 53:3437–3441. doi: 10.1128/AAC.00317-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Livorsi DJ, Crispell E, Satola SW, Burd EM, Jerris R, Wang YF, Farley MM. 2012. Prevalence of blaZ gene types and the inoculum effect with cefazolin among bloodstream isolates of methicillin-susceptible Staphylococcus aureus. Antimicrob Agents Chemother 56:4474–4477. doi: 10.1128/AAC.00052-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paul M, Zemer-Wassercug N, Talker O, Lishtzinsky Y, Lev B, Samra Z, Leibovici L, Bishara J. 2011. Are all beta-lactams similarly effective in the treatment of methicillin-sensitive Staphylococcus aureus bacteraemia? Clin Microbiol Infect 17:1581–1586. doi: 10.1111/j.1469-0691.2010.03425.x. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Echevarria KL, Hughes DW, Cadena JA, Bowling JE, Lewis JS Jr. 2014. Comparison of cefazolin versus oxacillin for treatment of complicated bacteremia caused by methicillin-susceptible Staphylococcus aureus. Antimicrob Agents Chemother 58:5117–5124. doi: 10.1128/AAC.02800-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clinical and Laboratory Standards Institute. 2010. Performance standards for antimicrobial susceptibility testing. CLSI document M100-S20. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 19.Clinical and Laboratory Standards Institute. 2011. Performance standards for antimicrobial susceptibility testing. CLSI document M100-S21. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 20.Clinical and Laboratory Standards Institute. 2012. Performance standards for antimicrobial susceptibility testing. CLSI document M100-S22. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 21.Clinical and Laboratory Standards Institute. 2013. Performance standards for antimicrobial susceptibility testing. CLSI document M100-S23. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 22.Hamilton KW, Bilker WB, Lautenbach E. 2007. Controlling for severity of illness in assessment of the association between antimicrobial-resistant infection and mortality: impact of calculation of Acute Physiology and Chronic Health Evaluation (APACHE) II scores at different time points. Infect Control Hosp Epidemiol 28:832–836. doi: 10.1086/518751. [DOI] [PubMed] [Google Scholar]
- 23.Thom KA, Shardell MD, Osih RB, Schweizer ML, Furuno JP, Perencevich EN, McGregor JC, Harris AD. 2008. Controlling for severity of illness in outcome studies involving infectious diseases: impact of measurement at different time points. Infect Control Hosp Epidemiol 29:1048–1053. doi: 10.1086/591453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, Acute Dialysis Quality Initiative Workgroup. 2004. Acute renal failure–definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 8:R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levine DP, Fromm BS, Reddy BR. 1991. Slow response to vancomycin or vancomycin plus rifampin in methicillin-resistant Staphylococcus aureus endocarditis. Ann Intern Med 115:674–680. doi: 10.7326/0003-4819-115-9-674. [DOI] [PubMed] [Google Scholar]
- 26.Hosmer DW, Lemeshow S. 2000. Applied logistic regression, 2nd ed Wiley, New York, NY. [Google Scholar]
- 27.Youngster I, Shenoy ES, Hooper DC, Nelson SB. 2014. Comparative evaluation of the tolerability of cefazolin and nafcillin for treatment of methicillin-susceptible Staphylococcus aureus infections in the outpatient setting. Clin Infect Dis 59:369–375. doi: 10.1093/cid/ciu301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Truven Health Analytics. 1 July 2013. Cefazolin. Red Book Online. Truven Health Analytics, Ann Arbor, MI. [Google Scholar]
- 29.Truven Health Analytics. 1 July 2013. Oxacillin. Red Book Online. Truven Health Analytics, Ann Arbor, MI. [Google Scholar]