Abstract
The novel enterovirus protease inhibitor (PI) SG85 effectively inhibits the in vitro replication of 14 rhinoviruses representative of species A and B (median 50% effective concentration, 0.04 μM). A low-level SG85-resistant variant was selected that carried amino acid substitutions S127G and T143A in the 3C protease. Both substitutions are required for low-level resistance to SG85, as demonstrated by reverse genetics. Interestingly, there is no cross-resistance to SG85 and rupintrivir (another PI); a structural explanation is provided for this observation.
TEXT
Rhinoviruses (RV) are nonenveloped, positive-sense, single-stranded RNA viruses that belong to the family of Picornaviridae, genus Enterovirus (1). Currently, more than 150 RV have been identified, which genotypically group into RV-A, RV-B, and RV-C (2). RV infections not only cause common colds but may also trigger exacerbations of asthma and chronic obstructive pulmonary disease (COPD) (3–5). Because of the high (and still rising) number of variants, development of a vaccine will be hard to achieve. Therefore, treatment with antivirals is a more realistic strategy to reduce the burden of these infections. An RV inhibitor, in particular, is needed for the prophylaxis and treatment of RV-induced exacerbations of asthma and COPD (6).
The RV 3C protease (3Cpro) is a promising target for drug development efforts because of the high level of conservation in its substrate-binding site, its role as an indispensable enzyme for virus replication, and its unique cleavage specificity (after Gln), which has not been observed in any other known host cell protease (7–11). Peptidomimetics with Michael acceptor warheads permanently disable the protease by covalent binding to its catalytic site (12, 13). The peptidomimetic rupintrivir (Pfizer AG7088; Fig. 1) effectively inhibits RV and enterovirus replication in vitro but largely failed to fulfill its promise in clinical trials (14–17).
FIG 1.
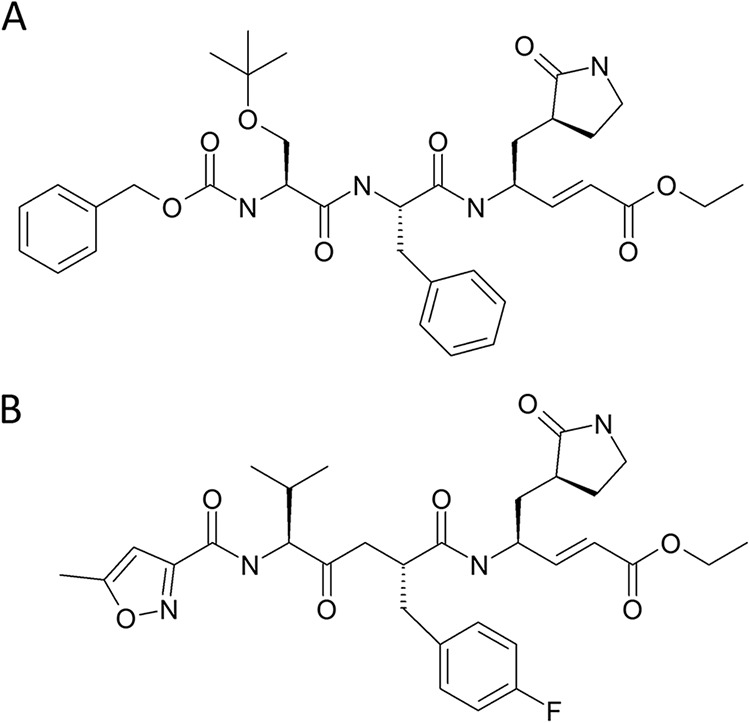
Structural formulae of SG85 (A) and rupintrivir (B).
Comparison of the known crystal structures of enterovirus 3Cpros revealed that the enterovirus 68 (EV68) 3Cpro can be considered an intermediate between the proteases of RV02 and poliovirus (18). Therefore, it was selected for the design of broad-spectrum enterovirus 3Cpro inhibitors, yielding SG85, a peptidic α,β-unsaturated ethyl ester with Michael acceptor properties, as the most promising candidate. SG85 is an efficient inhibitor of EV68 3Cpro and inhibits the replication of enteroviruses in cell-based assays (18, 19).
We demonstrate here that SG85 effectively inhibits the replication (in multicycle virus-cell-based cytopathic effect [CPE] reduction assays [20]) of 14 RV serotypes that are representative of RV-A and -B (Table 1). Median 50% effective concentrations (EC50s) of 0.04 ± 0.02 μM and 0.02 ± 0.01 μM were obtained against RV-A and RV-B, respectively. The 3Cpro inhibitor rupintrivir (Axon Medchem, The Netherlands) was, on average, 4-fold more active (Table 1), but this varied with the type (e.g., equipotent activity against RV63 and 14-fold more potent against RV02). Akin to rupintrivir, SG85 also strongly inhibited the replication of enterovirus 71 (EV71) and, to a lesser extent, protected cells against coxsackievirus B3 (CVB3), echovirus 11 (ECHO11), and poliovirus 1 (PV1) replication (18) (Table 1).
TABLE 1.
Antiviral activities of SG85 and rupintrivir against 14 RV and 4 enteroviruses in virus-cell-based assays
| Virus strain | Median EC50 (μM) ± MADa |
|
|---|---|---|
| SG85 | Rupintrivir | |
| RV-A | ||
| RV02 | 0.14 ± 0.03a | 0.010 ± 0.006a |
| RV09 | 0.032 ± 0.008a | 0.011 ± 0.001a |
| RV15 | 0.031 ± 0.004a | 0.013 ± 0.001a |
| RV29 | 0.04 ± 0.03a | 0.008 ± 0.002a |
| RV41 | 0.038 ± 0.007a | 0.0036 ± 0.0008a |
| RV59 | 0.04 ± 0.02a | 0.0102 ± 0.0001a |
| RV63 | 0.01 ± 0.01a | 0.0106 ± 0.0009a |
| RV85 | 0.15 ± 0.02a | 0.015 ± 0.008a |
| RV89 | 0.05 ± 0.06a | 0.0040 ± 0.0002a |
| RV-B | ||
| RV14 | 0.055 ± 0.008a | 0.018 ± 0.002a |
| RV42 | 0.02 ± 0.02a | 0.0026 ± 0.0008a |
| RV70 | 0.05 ± 0.05a | 0.0111 ± 0.0001a |
| RV72 | 0.008 ± 0.005a | 0.00356 ± 0.0001a |
| RV86 | 0.02 ± 0.02a | 0.0096 ± 0.0008a |
| EV-A EV71 | 0.10 ± 0.04a | 0.004 ± 0.002a |
| EV-B CVB3 | 0.5 ± 0.1a | 0.25 ± 0.04a |
| EV-B ECHO11 | 33 ± 7a | 2.8 ± 0.7a |
| EV-C PV1 | 39 ± 18 | 8.2 ± 2.7a |
Antiviral activity was determined in a CPE reduction assay with a 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium readout. The results shown are from dose-response curves set up from four or more experiments of which at least two were independent. MAD, median absolute deviation.
One hundred percent inhibition of a virus-induced CPE can be achieved with this compound (as determined by microscopic inspection).
Serial passaging with increasing concentrations of SG85 for extended periods of time did not result in the isolation of SG85-resistant RV14 virus variants (data not shown). Instead, a clonal selection procedure was used. To this end, several hundred infected cultures (in 96-well plates) were treated with a fixed concentration of the compound studied equal to the EC99 or a higher concentration. Supernatants collected from those few cultures where a CPE developed under drug pressure were selected for further expansion of the virus (20). Using this method, we obtained resistant variants in 1 out of 20 cultures in the case of pleconaril (data not shown), whereas for SG85, such variants were observed in only 1 of 273 cultures. This suggests that the virus quasispecies of the inoculum contained very few variants with some natural level of resistance to the compound and/or that it is very difficult for the virus to acquire these substitutions. The variant that was obtained proved to be >4-fold less sensitive than the wild-type virus to the antiviral effect of SG85 (data not shown). Such a low level of drug resistance may not be clinically relevant. However, other factors, such as the pharmacokinetic profile of the compounds in humans, will contribute as well. Genotyping revealed double mutations in the 3Cpro-encoding gene (S127G and T143A). To confirm the causal link between the mutant genotype and the resistant phenotype, single and double mutants were generated by using an infectious clone of RV14 (D. Blaas, Vienna, Austria; referred to as RV14IC here). The two single mutants, i.e., those that carried either S127G or T143A, did not show decreased susceptibility to the antiviral effect of SG85 (Table 2). However, the double mutant (RV14IC S127G T143A) has low-level resistance and proved 3-fold less susceptible than wild-type RV14IC to the antiviral effect of SG85. Of note, the sensitivity of any of the mutants to rupintrivir was not altered (Table 2). The reverse-engineered double mutant had a level of resistance (3-fold) comparable to that of the phenotypically selected variant. It has been reported that in vitro-selected rupintrivir-resistant RV14 (7- to 16-fold resistant) carries four substitutions (i.e., T129A, T131A, Y139H, T143P) in 3C (21). Even though the quadruple mutant also carries a substitution at position 143 (21), we did not observe cross-resistance between SG85 (which selects for the substitutions S127G and T143A) and rupintrivir (Table 2). In the same study, reverse-engineered variants with a single substitution, including one at position T143, showed no reduced susceptibility to the antiviral effect of rupintrivir (21). This is in line with our observations that besides the substitution T143A, the substitution S127G in 3Cpro is also required for resistance to the Michael acceptor-based peptidomimetic SG85.
TABLE 2.
Antiviral activities of SG85, rupintrivir, and pleconaril on the replication of reverse-engineered RV14IC strains that contain mutations in the 3Cpro-encoding gene
| RV14IC strain | EC50 (μM) ± MAD (RR)a |
||
|---|---|---|---|
| SG85 | Rupintrivir | Pleconaril | |
| Wild type | 0.018 ± 0.001 | 0.010 ± 0.001 | 0.327 ± 0.004 |
| S127G mutant | 0.019 ± 0.001 (1) | 0.0094 ± 0.0002 (1) | 0.31 ± 0.01 (1) |
| T143A mutant | 0.018 ± 0.001 (1) | 0.008 ± 0.001 (1) | 0.108 ± 0.004b (0.3) |
| S127G T143A mutant | 0.047 ± 0.001b (3) | 0.012 ± 0.001 (1) | 0.13 ± 0.02c (0.4) |
Antiviral activity was determined in a CPE reduction assay with a 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium readout. Data are in duplicate from three independent assays. The early-stage RV inhibitor pleconaril (kindly provided by V. Makarov, RAS Institute of Biochemistry, Russia) was included in this assay as a reference. MAD, median absolute deviation. RR, relative resistance (EC50 of mutated strain/EC50 of wild type).
P < 0.0001 (unpaired t test).
P < 0.001 (unpaired t test).
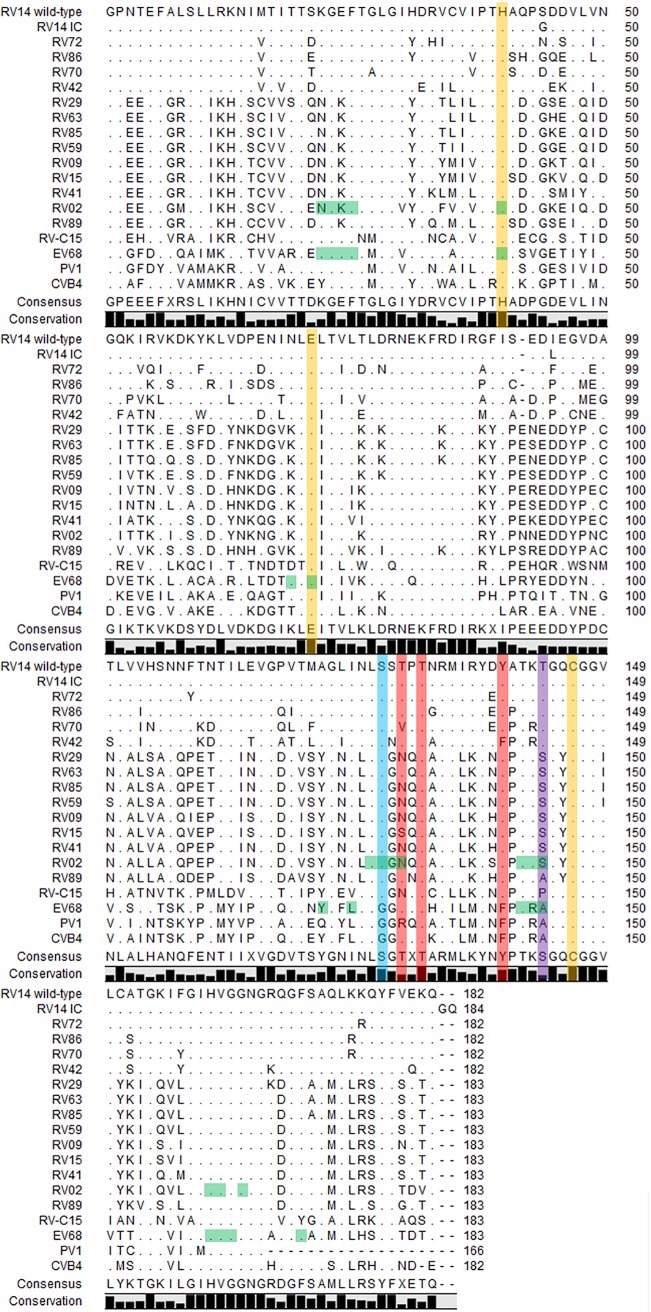
SG85 and rupintrivir are less active against enterovirus species B and C (Table 1). For a better understanding, a sequence alignment was made of 3Cpro of the RV strains that were used in this study, along with the 3C sequences of an RV-C15 isolate (W1, GU 219984.1), CVB4, PV1, and EV68 (Fig. 2). The serine residue at position 127 was conserved throughout the RV strains. At the corresponding position (residue 128), EV68 and PV1 carry a glycine, which corresponds to the substitution that was detected in the low-level SG85-resistant RV14 variant. A crystal structure of RV02 3Cpro in complex with a peptidic Michael acceptor (compound III) revealed the presence of a hydrogen bond between the backbone amide of the P2 residue of the inhibitor and the side chain oxygen of S128 (22). It may be assumed that the same interaction will exist in the SG85 complex of the RV14 protease and will thus be lost with the S127G substitution, presumably resulting in a loss of free energy of binding that decreases the efficiency of the inhibitor. Rupintrivir, however, lacks a P2 backbone amide and such a hydrogen-bonding interaction, and therefore, the S127G substitution does not accumulate as part of a drug resistance mechanism. It should be noted that we made multiple attempts to express both the wild-type and mutant proteases. However, because of the limited solubility of these proteins, we were not able to biochemically characterize these enzymes.
FIG 2.
Alignment of the 3Cpro amino acid sequences of 15 RV (RV-A and -B) genotypes, EV68 (EV-D), and PV1 (EV-C). A yellow background indicates residues of the catalytic triad, a purple background indicates a residue that is mutated in both the SG85- and rupintrivir-resistant RV14 variants, a blue background indicates residues that are mutated in the SG85-resistant RV14 variant, a red background indicates residues that are mutated only in rupintrivir-resistant RV14 variants, and a green background indicates residues in the structure of the RV02 3Cpro that interact with compounds I and III (22) or residues in EV68 3Cpro that interact with SG85 (18). This sequence alignment was created in CLC sequence viewer 7 (Qiagen).
The threonine at position 143 is conserved in the RV-B strains, whereas the majority of RV-A strains carry a serine (at corresponding position 144) and the RV-C strain included in this analysis carries a proline. It remains to be determined whether RV-C (3Cpro) is sensitive to SG85 (and/or rupintrivir). For RV89, EV68, and PV1, an alanine residue is present at this position, which was also detected in the low-level SG85-resistant RV14 variant. The crystal structures of EV68 3Cpro in complex with SG85 (18) and of RV 3Cpro in complex with compound III or rupintrivir (22) reveal that whereas the main chain of residue 144 is part of the S1 pocket of the protease, its side chain, whether Ala or Ser/Thr, is oriented away from the inhibitor; therefore, it is not immediately clear how the S/T143A substitution would affect the binding of the compound. In agreement with these observations, both the RV14IC S127G T143A double mutant and PV1 show decreased susceptibility to the antiviral effect of SG85, whereas RV89 (which is akin to the RV14IC single mutant, carries only A143) is inhibited as well as wild-type RV14IC.
Protease inhibitors (PI) are being successfully used for the treatment of infections with HIV and hepatitis C virus. The development of novel RV and enterovirus 3C inhibitors should be considered further. Such inhibitors have the potential to exert broad-spectrum antiviral activity and (unlike with most HIV and HCV PI) high-level drug-resistant variants do not, or at least do not readily, develop. Moreover, we demonstrate here that PI of enterovirus and RV 3C with nonoverlapping drug resistance profiles can be developed.
ACKNOWLEDGMENTS
We acknowledge Stijn Delmotte and Caroline Collard for their excellent assistance in the collection of antiviral data.
This work was supported by a Ph.D. grant from the Agency for Innovation by Science and Technology (IWT, Belgium), by the European Commission through its SILVER project (contract HEALTH-F3-2010-260644), by KU Leuven Geconcerteerde Onderzoeksactie (GOA 10/014), by the BELSPO IUAP consortium BELVIR (Belgium), and by the German Center for Infection Research (DZIF project TTU 01.803).
REFERENCES
- 1.Jacobs SE, Lamson DM, St GK, Walsh TJ. 2013. Human rhinoviruses. Clin Microbiol Rev 26:135–162. doi: 10.1128/CMR.00077-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palmenberg A, Gern J. 2015. Classification and evolution of human rhinoviruses. Methods Mol Biol 1221:1–10. doi: 10.1007/978-1-4939-1571-2_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gern JE, Galagan DM, Jarjour NN, Dick EC, Busse WW. 1997. Detection of rhinovirus RNA in lower airway cells during experimentally induced infection. Am J Respir Crit Care Med 155:1159–1161. doi: 10.1164/ajrccm.155.3.9117003. [DOI] [PubMed] [Google Scholar]
- 4.Gern JE. 2015. How rhinovirus infections cause exacerbations of asthma. Clin Exp Allergy 45:32–42. doi: 10.1111/cea.12428. [DOI] [PubMed] [Google Scholar]
- 5.Mallia P, Message SD, Gielen V, Contoli M, Gray K, Kebadze T, Aniscenko J, Laza-Stanca V, Edwards MR, Slater L, Papi A, Stanciu LA, Kon OM, Johnson M, Johnston SL. 2011. Experimental rhinovirus infection as a human model of chronic obstructive pulmonary disease exacerbation. Am J Respir Crit Care Med 183:734–742. doi: 10.1164/rccm.201006-0833OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thibaut HJ, De Palma AM, Neyts J. 2012. Combating enterovirus replication: state-of-the-art on antiviral research. Biochem Pharmacol 83:185–192. doi: 10.1016/j.bcp.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 7.Binford SL, Maldonado F, Brothers MA, Weady PT, Zalman LS, Meador JW III, Matthews DA, Patick AK. 2005. Conservation of amino acids in human rhinovirus 3C protease correlates with broad-spectrum antiviral activity of rupintrivir, a novel human rhinovirus 3C protease inhibitor. Antimicrob Agents Chemother 49:619–626. doi: 10.1128/AAC.49.2.619-626.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matthews DA, Smith WW, Ferre RA, Condon B, Budahazi G, Sisson W, Villafranca JE, Janson CA, McElroy HE, Gribskov CL, Worland S. 1994. Structure of human rhinovirus 3C protease reveals a trypsin-like polypeptide fold, RNA-binding site, and means for cleaving precursor polyprotein. Cell 77:761–771. doi: 10.1016/0092-8674(94)90059-0. [DOI] [PubMed] [Google Scholar]
- 9.Leong LE, Walker PA, Porter AG. 1993. Human rhinovirus-14 protease 3C (3Cpro) binds specifically to the 5′-noncoding region of the viral RNA. Evidence that 3Cpro has different domains for the RNA binding and proteolytic activities. J Biol Chem 268:25735–25739. [PubMed] [Google Scholar]
- 10.Walker EJ, Younessi P, Fulcher AJ, McCuaig R, Thomas BJ, Bardin PG, Jans DA, Ghildyal R. 2013. Rhinovirus 3C protease facilitates specific nucleoporin cleavage and mislocalisation of nuclear proteins in infected host cells. PLoS One 8:e71316. doi: 10.1371/journal.pone.0071316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malcolm BA. 1995. The picornaviral 3C proteinases: cysteine nucleophiles in serine proteinase folds. Protein Sci 4:1439–1445. doi: 10.1002/pro.5560040801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dragovich PS, Webber SE, Babine RE, Fuhrman SA, Patick AK, Matthews DA, Lee CA, Reich SH, Prins TJ, Marakovits JT, Littlefield ES, Zhou R, Tikhe J, Ford CE, Wallace MB, Meador JW III, Ferre RA, Brown EL, Binford SL, Harr JE, DeLisle DM, Worland ST. 1998. Structure-based design, synthesis, and biological evaluation of irreversible human rhinovirus 3C protease inhibitors. 1. Michael acceptor structure-activity studies. J Med Chem 41:2806–2818. [DOI] [PubMed] [Google Scholar]
- 13.Dragovich PS, Prins TJ, Zhou R, Fuhrman SA, Patick AK, Matthews DA, Ford CE, Meador JW III, Ferre RA, Worland ST. 1999. Structure-based design, synthesis, and biological evaluation of irreversible human rhinovirus 3C protease inhibitors. 3. Structure-activity studies of ketomethylene-containing peptidomimetics. J Med Chem 42:1203–1212. [DOI] [PubMed] [Google Scholar]
- 14.Dragovich PS, Prins TJ, Zhou R, Webber SE, Marakovits JT, Fuhrman SA, Patick AK, Matthews DA, Lee CA, Ford CE, Burke BJ, Rejto PA, Hendrickson TF, Tuntland T, Brown EL, Meador JW III, Ferre RA, Harr JE, Kosa MB, Worland ST. 1999. Structure-based design, synthesis, and biological evaluation of irreversible human rhinovirus 3C protease inhibitors. 4. Incorporation of P1 lactam moieties as l-glutamine replacements. J Med Chem 42:1213–1224. [DOI] [PubMed] [Google Scholar]
- 15.Patick AK, Binford SL, Brothers MA, Jackson RL, Ford CE, Diem MD, Maldonado F, Dragovich PS, Zhou R, Prins TJ, Fuhrman SA, Meador JW, Zalman LS, Matthews DA, Worland ST. 1999. In vitro antiviral activity of AG7088, a potent inhibitor of human rhinovirus 3C protease. Antimicrob Agents Chemother 43:2444–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayden FG, Turner RB, Gwaltney JM, Chi-Burris K, Gersten M, Hsyu P, Patick AK, Smith GJ III, Zalman LS. 2003. Phase II, randomized, double-blind, placebo-controlled studies of ruprintrivir nasal spray 2-percent suspension for prevention and treatment of experimentally induced rhinovirus colds in healthy volunteers. Antimicrob Agents Chemother 47:3907–3916. doi: 10.1128/AAC.47.12.3907-3916.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patick AK, Brothers MA, Maldonado F, Binford S, Maldonado O, Fuhrman S, Petersen A, Smith GJ III, Zalman LS, Burns-Naas LA, Tran JQ. 2005. In vitro antiviral activity and single-dose pharmacokinetics in humans of a novel, orally bioavailable inhibitor of human rhinovirus 3C protease. Antimicrob Agents Chemother 49:2267–2275. doi: 10.1128/AAC.49.6.2267-2275.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan J, George S, Kusov Y, Perbandt M, Anemuller S, Mesters JR, Norder H, Coutard B, Lacroix C, Leyssen P, Neyts J, Hilgenfeld R. 2013. 3C protease of enterovirus 68: structure-based design of Michael acceptor inhibitors and their broad-spectrum antiviral effects against picornaviruses. J Virol 87:4339–4351. doi: 10.1128/JVI.01123-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tijsma A, Franco D, Tucker S, Hilgenfeld R, Froeyen M, Leyssen P, Neyts J. 2014. The capsid binder vapendavir and novel protease inhibitor SG85 inhibit enterovirus 71 replication. Antimicrob Agents Chemother 58:6990–6992. doi: 10.1128/AAC.03328-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lacroix C, Querol-Audi J, Roche M, Franco D, Froeyen M, Guerra P, Terme T, Vanelle P, Verdaguer N, Neyts J, Leyssen P. 2014. A novel benzonitrile analogue inhibits rhinovirus replication. J Antimicrob Chemother 69:2723–2732. doi: 10.1093/jac/dku200. [DOI] [PubMed] [Google Scholar]
- 21.Binford SL, Weady PT, Maldonado F, Brothers MA, Matthews DA, Patick AK. 2007. In vitro resistance study of rupintrivir, a novel inhibitor of human rhinovirus 3C protease. Antimicrob Agents Chemother 51:4366–4373. doi: 10.1128/AAC.00905-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matthews DA, Dragovich PS, Webber SE, Fuhrman SA, Patick AK, Zalman LA, Hendrickson T, Love RA, Prins TJ, Marakovits JT, Zhou R, Tikhe J, Ford CE, Meador JW, Ferre RA, Brown EL, Binford SL, Brothers MA, DeLisle DM, Worland ST. 1999. Structure-assisted design of mechanism-based irreversible inhibitors of human rhinovirus 3C protease with potent antiviral activity against multiple rhinovirus serotypes. Proc Natl Acad Sci U S A 96:11000–11007. doi: 10.1073/pnas.96.20.11000. [DOI] [PMC free article] [PubMed] [Google Scholar]