Abstract
The aim of the study was to identify the plasmid-encoded factors contributing to the emergence and spread of epidemic IncI1-Iγ plasmids obtained from Escherichia coli and Salmonella enterica isolates from animal and human reservoirs. For this, 251 IncI1-Iγ plasmids carrying various extended-spectrum β-lactamase (ESBL) or AmpC β-lactamase genes were compared using plasmid multilocus sequence typing (pMLST). Thirty-two of these plasmids belonging to different pMLST types were sequenced using Roche 454 and Illumina platforms. Epidemic IncI1-Iγ plasmids could be assigned to various dominant clades, whereas rarely detected plasmids clustered together as a distinct clade. Similar phylogenetic trees were obtained using only the plasmid backbone sequences, showing that the differences observed between the plasmids belonging to distinct clades resulted mainly from differences between their backbone sequences. Plasmids belonging to the various clades differed particularly in the presence/absence of genes encoding partitioning and addiction systems, which contribute to stable inheritance during cell division and plasmid maintenance. Despite this, plasmids belonging to the various phylogenetic clades also showed marked resistance gene associations, indicating the circulation of successful plasmid-gene combinations. The variation in traY and excA genes found in IncI1-Iγ plasmids is conserved within pMLST sequence types and plays a role in incompatibility, although functional study is needed to elucidate the role of these genes in plasmid epidemiology.
INTRODUCTION
Escherichia coli strains producing extended-spectrum β-lactamases (ESBLs) have emerged globally in humans as well as in animals during the last decade (1–3). Conjugative plasmids, integrons, and IS elements play a dominant role in the dissemination of the resistance genes in Enterobacteriaceae (3). Many plasmid families have been associated with the carriage of genes encoding specific ESBLs or plasmid-borne AmpC type β-lactamases (pAmpC) and were considered to be epidemic resistance plasmids due to their role in the dissemination of these genes (4). Among them, IncI1-Iγ plasmids have been predominantly associated with ESBL/pAmpC-producing isolates from animals (5–8). Genetically related IncI1-Iγ plasmids carrying blaCTX-M-1 genes were identified in E. coli isolates from poultry sources and in those sustaining infections in humans in The Netherlands, suggesting that transfer of plasmids occurred among strains from food animals and humans (9).
Predominant plasmid-gene combinations have been described in Enterobacteriaceae from both animals and humans and help to determine the epidemiology of ESBL genes. In isolates of animal origin, the blaCTX-M-1, blaTEM-52, and blaSHV-12 genes predominate, each on IncI1-Iγ plasmids, while blaCMY-2 genes can be associated with IncI1-Iγ or IncK plasmids. In contrast, blaCTX-M-15 predominates in human isolates and is typically located on IncFII plasmids and to a much lesser extent on IncI1-Iγ plasmids (7, 8, 10, 11). IncI1-Iγ-associated blaCTX-M-15 genes are also present in isolates from livestock animals but at a very low prevalence (12). The predominant gene-plasmid combinations spread epidemically in bacterial communities and animal or human enterobacterial populations (7). The successful dissemination of epidemic IncI1-Iγ plasmids will most probably be related to plasmid-encoded factors, possibly in association with factors encoded by the host strain.
Plasmid multilocus sequence typing (pMLST) using five conserved genes can discriminate between IncI1-Iγ plasmid subtypes (http://pubmlst.org/plasmid/) (13), and the IncI1-Iγ plasmids deposited in the pMLST database actually belong to 158 different sequence types (STs) and 10 clonal complexes (CCs), indicating their high level of variability. The most frequent IncI1-Iγ ST identified is ST7 (http://pubmlst.org/plasmid/). Recent data showed that dominant STs can be identified among the IncI1-Iγ plasmids and are associated with specific ESBL/pAmpC genes (4, 14). IncI1-Iγ plasmids belonging to ST7 were most frequently associated with blaCTX-M-1, whereas IncI1-Iγ plasmids belonging to ST12 and ST31 were associated with blaCMY-2 and blaCTX-M-15 genes, respectively (http://pubmlst.org/plasmid/). Complete nucleotide sequences of a number of IncI1-Iγ plasmids are available in the GenBank database. All IncI1-Iγ plasmids characterized contained a set of highly conserved core genes involved in essential functions, such as replication, maintenance, stability, and transfer as well as some unique accessory elements (15).
To understand better the plasmid-encoded factors contributing to the emergence and spread of epidemic IncI1-Iγ plasmids, we performed pMLST and comparative sequence analysis of epidemic and rarely detected IncI1-Iγ plasmids obtained from E. coli and Salmonella enterica from animal and human reservoirs.
MATERIALS AND METHODS
Bacterial isolates.
S. enterica and E. coli isolates containing IncI1-Iγ plasmids carrying ESBL/pAmpC genes were selected from existing strain collections in The Netherlands, United Kingdom, and Germany from isolates obtained from 2005 to 2009. These collections were obtained in National Resistance Surveillance programs or dedicated studies for ESBL/pAmpC producers (16) and integrons (17) in which the plasmids were characterized by PCR-based replicon typing (PBRT) (18, 19). This resulted in a collection of 251 E. coli and Salmonella isolates with ESBL, pAmpC, or class 1 integrons on IncI1-Iγ plasmids (see Table S1 in the supplemental material).
β-Lactamase identification.
Whole-cell DNA of the isolates was used for screening for ESBL and/or pAmpC genes by miniaturized microarray (Alere, Jena, Germany). The identity of ESBL/pAmpC genes was confirmed by PCR and sequencing as described previously (20).
Plasmid isolation and characterization.
IncI1-Iγ plasmids were extracted using a crude lysis (miniprep) method (21) or a Qiafilter plasmid midikit (Qiagen). Extracted plasmid DNA was used for electrotransformation of E. coli DH10B cells (Invitrogen), and transformants were selected on Luria-Bertani agar with 1 mg/liter cefotaxime (Sigma).
All 251 ESBL/pAmpC-encoding IncI1-Iγ plasmids were subtyped by pMLST analysis as described previously (13) using DNA extracted from transformants with the DNeasy blood and tissue kit (Qiagen). pMLST amplicons obtained using pubmlst-derived primers (http://pubmlst.org/plasmid/primers/incI1.shtml) were purified and sequenced using an ABI 3730 DNA sequencer (Applied Biosystems, Foster City, CA). Plasmids were assigned to sequence types (STs), and minimum-spanning trees were generated from the allelic profiles using Bionumerics 6.5. A PCR result negative on one of the selected loci was designated an incomplete sequence type. The sizes of the plasmids were estimated using pulsed-field gel electrophoresis (PFGE) of S1 nuclease digests of total DNA (22).
Plasmids selected for sequence analysis.
Based on the variations in pMLST types, 32 transformants were selected for complete plasmid sequence analysis (see Table S1 in the supplemental material). These transformants harbored IncI1-Iγ plasmids that were all obtained from individual E. coli strains, and 28 of the 32 plasmids belonged to the most prevalent pMLST types identified. For each ST (if possible), representative plasmids were chosen that (i) differed in their sizes and (ii) were obtained from E. coli isolates that originated from several animal or human sources and differed in their years of isolation. For comparative reasons, four IncI1-Iγ plasmids belonging to unique or incomplete STs were also selected for sequence analysis.
Plasmid sequencing.
Deep sequencing of the plasmid genomes was performed using Roche 454 XL shotgun sequencing technology or using 150-bp paired-end sequencing libraries (Nextera TAG-mentation sequencing kits [Epicentre]) on an Illumina MiSeq sequencer (23). High-quality filtered reads were subsequently assembled de novo using the Newbler algorithm (v2.5.3) for 454 reads and the AbySS algorithm (abyss version 1.3.3) for Illumina-derived reads. The sequence coverage of the de novo assemblies was on average over 100, with a minimum (for some 454-sequenced plasmids) of 60 sequence reads of coverage per assembled base. High-coverage scaffolds of the genomes were reconstructed by scaffolding the contigs against the closely related IncI1 reference R64 (15). Putative open reading frames (ORFs) were identified by GeneMarkHMMp version 2.6p (24). BLASTP analyses of the putative ORFs against the NCBI nonredundant proteins (NR) database, Pfam (25), and Interpro scan (26) were used to assess their putative functions by identification of structural features and motifs.
Sequence clustering and phylogenetics.
Plasmid sequences were hierarchically clustered and displayed as a phenogram using the BioNJ algorithm (27), where the underlying distance matrix was calculated from the pairwise nonoverlapping maximal unique matches (MUMs) using Nucmer version 3.07 (28). Relative pairwise distances were obtained by dividing the pairwise MUMs' sum by the average genome size of the two paired genomes (MUMi genomic distance) (29). BioNJ trees were generated from the MUMi distance matrix using SplitsTree4 (30).
Nucleotide sequence accession numbers.
The NCBI GenBank accession numbers for the plasmid samples have been submitted under NCBI-BioProject PRJNA263774 and BioSample accession no. SAMN03168474 to SAMN03168504. The accession numbers of the reference plasmids used in this study for comparison are as follows: R64, AP005147; ColIb-P9, AB021078; pEC-Bactec, GU371927; pND11-107, HQ114281; pEK204, EU935740; and R621a, AP011954.
RESULTS AND DISCUSSION
pMLST.
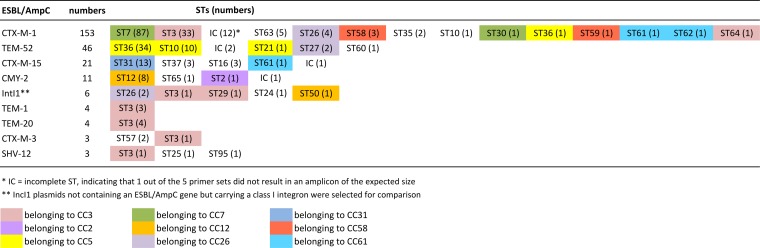
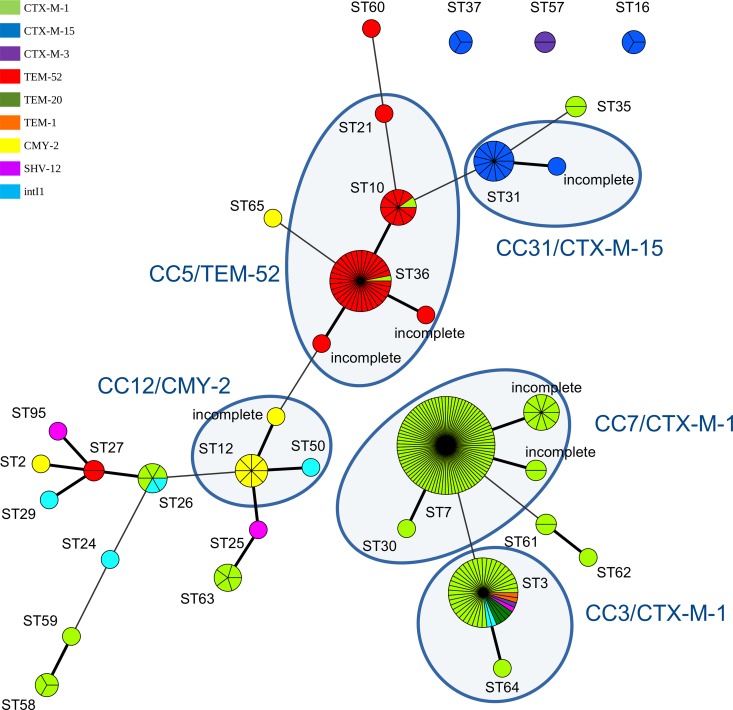
Two hundred fifty-one IncI1-Iγ plasmids carrying ESBL/pAmpC-encoding genes obtained from S. enterica and E. coli isolates from different countries were used in this study. The plasmids were assigned to 28 different pMLST types (Fig. 1 and 2; see Table S1 in the supplemental material). In addition, six incomplete pMLST profiles were observed. The 153 IncI1-Iγ plasmids containing blaCTX-M-1 were assigned to 14 different STs, but the majority belonged to either ST7 (70%) or ST3 (27%) (Fig. 1 and 2). IncI1-Iγ plasmids harboring blaCTX-M-1 were obtained from S. enterica as well as from E. coli isolates, which originated mainly from humans and poultry (9) (see Table S1). IncI1-Iγ plasmids containing the blaCTX-M-15 gene were mainly obtained from E. coli isolates from humans or cattle, and a high percentage (60%) of these plasmids belonged to ST31 (14) (see Table S1). Most (92%) IncI1-Iγ plasmids containing blaTEM-52 were assigned to ST36, ST10, or ST21, all of which belong to CC5. IncI1-Iγ plasmids containing the blaTEM-52 gene were obtained from S. enterica and E. coli isolates and were mainly found in human and poultry reservoirs. IncI1-Iγ plasmids containing the blaCMY-2 gene were mainly (81%) assigned to ST12 (http://pubmlst.org/plasmid/) (31) (Fig. 2). These data confirm the presence of predominant ST-gene combinations among IncI1-Iγ plasmids as previously described (4, 14), suggesting circulation of a number of prevalent IncI1-Iγ plasmids among bacterial species of animal and human reservoirs. However, some pMLST types were associated with a number of different ESBL/AmpC genes: e.g., ST3 plasmids carrying the blaCTX-M-1, blaTEM-20, blaTEM-1, blaSHV-12, and blaCTX-M-3 genes. Previously, ST3 IncI1-Iγ plasmids have been found to be associated with the blaCTX-M-1 gene among E. coli strains of avian and human origins in The Netherlands (9), as well as among E. coli strains isolated from several animal species in other European countries (13, 32, 33).
FIG 1.
Distribution of plasmids to pMLST STs and CCs.
FIG 2.
pMLST analysis of 251 selected IncI1-Iγ plasmids as visualized as a minimum-spanning tree. Plasmids with identical sequence types (STs) were assigned to one circle. Single-locus variants are indicated by a thick line and double-locus variants by a thin line. Sequence types belonging to one clonal complex were grouped by a blue circle. The colors represent the various antibiotic resistance genes found to be associated with the sequence types of the plasmids.
Characteristics of sequenced plasmids.
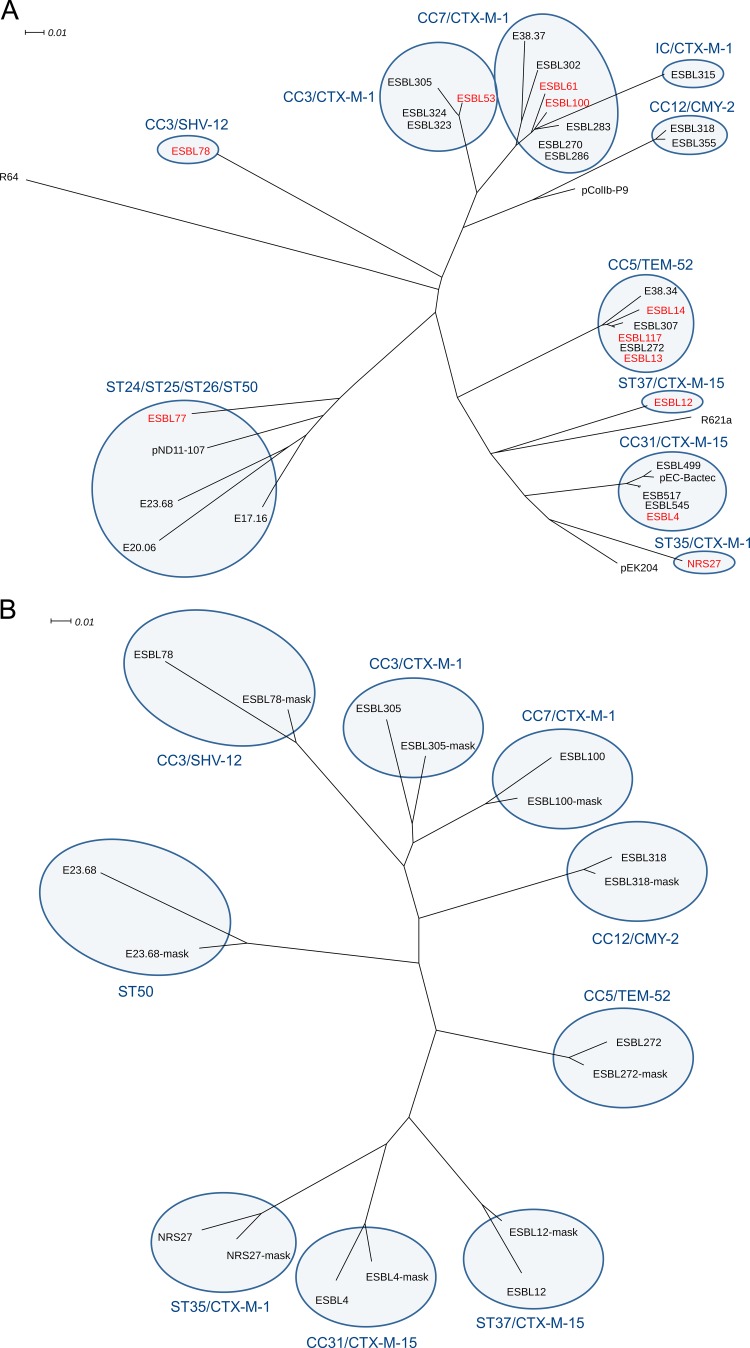
Twenty-eight plasmids belonging to the most prevalent STs and four plasmids belonging to rarely detected STs were selected for complete plasmid sequence analysis (Table 1). All plasmids shared the typical IncI1-Iγ-associated genetic modules, including the replication region, maintenance and stability regions, and the transfer regions. Moreover, the plasmids were supplemented with one to three unique accessory elements, containing the antibiotic resistance genes. Major parts of the plasmid backbone sequences were highly conserved. Based on a phylogenetic analysis of the whole plasmid sequences, including the segments containing the antibiotic resistance genes, the plasmids were classified into a number of distinct clades (Fig. 3A). Similar phylogenetic trees were obtained using the plasmid backbone sequences exclusively (Fig. 3B), which shows that the differences observed between the plasmids belonging to distinct clades were mainly caused by differences in their backbone sequences. Despite this, plasmids belonging to the various phylogenetic clades showed predominant plasmid-gene combinations, indicating the circulation of successful plasmid-gene combinations. Moreover, similar plasmid-gene combinations were obtained from different animal sources as well as from humans, which suggests transmission between animal and human isolates, as was previously assumed (9). Rarely detected plasmids clustered together as a distinct clade.
TABLE 1.
Characteristics of sequenced IncI1 plasmids
| Plasmid | Origin | ESBL | Yr of isolation | Country of origina | Estimated size (kb) | pMLST result |
Presence of colicin | Plasmid with partitionin gene parb | Plasmid(s) with entry exclusion gene: |
Gene(s) for addiction factorsc | Shufflon | Presence of gene: |
Accession no. | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ST | CC | excb | traYb | pilJb | traDb | |||||||||||
| E38.27 | Poultry | CTX-M-1 | 2006 | NL | 88 | ST7 | CC7 | − | R64g | R621a | R621a | pndAC and relBE | BB′C′CA′ | ColIb-P9h | ColIb-P9 | 3168474 |
| ESBL-100 | Human | CTX-M-1 | 2009 | NL | 100 | ST7 | CC7 | + | R64 | R621a | R621a | pndAC and relBE | BB′C′CA′ | ColIb-P9 | ColIb-P9 | 3168475 |
| ESBL-286 | Poultry | CTX-M-1 | 2008 | NL | 110 | ST7 | CC7 | + | R64 | R621a | R621a | pndAC and relBE | BB′C′CA′ | ColIb-P9 | ColIb-P9 | 3168476 |
| ESBL-270 | Poultry | CTX-M-1 | 2007 | NL | 105 | ST7 | CC7 | + | R64 | R621a | R621a | pndAC and relBE | BB′C′CA′ | ColIb-P9 | ColIb-P9 | 3168477 |
| ESBL-283 | Pigs | CTX-M-1 | 2008 | NL | 105 | ST7 | CC7 | + | R64 | R621a | R621a | pndAC and relBE | BB′C′CA′ | ColIb-P9 | ColIb-P9 | CO008736 |
| ESBL-61 | Human | CTX-M-1 | 2009 | NL | 105 | ST7 | CC7 | + | R64 | R621a | R621a | pndAC and relBE | BB′C′CA′ | ColIb-P9 | ColIb-P9 | 3168479 |
| ESBL-302 | Poultry | CTX-M-1 | 2008 | NL | 95 | ICd | NDe | R64 | R621a | R621a | pndAC and relBE | BB′C′CA′ | ColIb-P9 | ColIb-P9 | 3168480 | |
| ESBL-315 | Poultry | CTX-M-1 | 2008 | NL | 90 | IC | + | R64 | R621a | R621a | pndAC and relBE | BB′C′CA′ | ColIb-P9 | ColIb-P9 | CP008738 | |
| ESBL-53 | Human | CTX-M-1 | 2009 | NL | 95 | ST3 | CC3 | − | R64 | pH2291-11 | pH2291-11 | pndAC and relBE | CC′-cas-BB | ColIb-P9 | ColIb-P9 | 3168482 |
| ESBL-305 | Poultry | CTX-M-1 | 2008 | NL | 105 | ST3 | CC3 | − | R64 | pH2291-11 | pH2291-11 | pndAC and relBE | CC′-cas-BB | ColIb-P9 | ColIb-P9 | 3168483 |
| ESBL-323 | Pigs | CTX-M-1 | 2008 | NL | 105 | ST3 | CC3 | + | R64 | pH2291-11 | pH2291-11 | pndAC and relBE | CC′-cas-BB | ColIb-P9 | ColIb-P9 | 3168484 |
| ESBL-324 | Pigs | CTX-M-1 | 2008 | NL | 105 | ST64 | + | R64 | pH2291-11 | pH2291-11 | pndAC and relBE | CC′-cas-BB | ColIb-P9 | ColIb-P9 | 3168485 | |
| ESBL-78 | Human | SHV-12 | 2009 | NL | 105 | ST3 | CC3 | − | R64 | pH2291-11 | pH2291-11 | pndAC and relBE | BB′C′CA′ | ColIb-P9 | ColIb-P9 | 3168486 |
| ESBL-318 | Poultry | CMY-2 | 2008 | NL | 95 | ST12 | CC12 | + | R64 | R64 | R64 (98%) | pndAC, ccdAB, and relBE | BB′A | R64 | R64 | 3168487 |
| ESBL-355 | Poultry | CMY-2 | 2007 | NL | 100 | ST12 | CC12 | + | R64 | R64 | R64 (98%) | pndAC, ccdAB, and relBE | A | R64 | R64 | 3168488 |
| ESBL-4 | Human | CTX-M-15 | 2009 | NL | 90 | ST31 | CC31 | − | pEC-Bactecj | R621a | R621a | pndAC | BB′D′C′CA | None | None | 3168489 |
| ESBL-499 | Cattle | CTX-M-15 | 2009 | UK | ND | ST31 | CC31 | − | pEC-Bactec | R621a | R621a | pndAC | BB′C′CD′A′ | None | None | 3168490 |
| ESBL-517 | Cattle | CTX-M-15 | 2009 | UK | ND | ST31 | CC31 | − | pEC-Bactec | R621a | R621a | pndAC | BB′C′CD′A′ | None | None | 3168491 |
| ESBL-545 | Cattle | CTX-M-15 | 2009 | UK | ND | ST31 | CC31 | − | pEC-Bactec | R621a | R621a | pndAC | BB′C′CD′A′ | None | None | 3168492 |
| ESBL-12 | Human | CTX-M-15 | 2009 | NL | 100 | ST37 | − | R621ai | R621a | R621a | pndAC | BB′C′CD′A′ | R64 | ColIb-P9 | CP008735 | |
| NRS27 | Human | CTX-M-1 | 2009 | NL | 90 | ST35 | − | R621a | R64 | R64 | pndAC | ND | pH1519-88m | None | 3168494 | |
| ESBL-272 | Poultry | TEM-52 | 2007 | NL | 90 | ST36 | CC5 | − | R64 | pH2291-11 | pH2291-11l | pndCA and vapBC | BB′C′CA′ | ColIb-P9 | ColIb-P9 | 3168495 |
| ESBL-14 | Human | TEM-52 | 2009 | NL | 90 | ST36 | CC5 | − | R64 | pH2291-11 | pH2291-11 | pndCA and vapBC | BB′C′CA′ | ColIb-P9 | ColIb-P9 | 3168496 |
| ESBL-307 | Poultry | TEM-52 | 2008 | NL | 90 | ST36 | CC5 | − | R64 | pH2291-11 | pH2291-11 | pndCA and vapBC | BB′C′CA′ | ColIb-P9 | ColIb-P9 | 3168497 |
| ESBL-117 | Human | TEM-52 | 2009 | NL | 90 | ST36 | CC5 | − | R64 | pH2291-11 | pH2291-11 | pndCA and vapBC | BB′C′CA′ | ColIb-P9 | ColIb-P9 | CP008734 |
| ESBL-13 | Human | TEM-52 | 2009 | NL | 95 | ST36 | CC5 | − | R64 | pH2291-11 | pH2291-11 | pndCA and vapBC | BB′C′CA′ | ColIb-P9 | ColIb-P9 | 3168499 |
| E38.34 | Chicken | TEM-52 | 2009 | NL | 97 | ST36 | CC5 | − | R64 | pH2291-11 | pH2291-11 | pndCA and vapBC | BB′C′CA′ | ColIb-P9 | ColIb-P9 | 3168500 |
| E17.16 | Poultry | IntI1f | 2004 | NL | 100 | ST24 | + | pND11-107k | pH2291-11 | pH2291-11 | pndAC and relBE | BB′C′CA′ | R64 | R64 | CP008733 | |
| E23.68 | Poultry | IntI1 | 2004 | NL | 110 | ST50 | CC12 | + | pND11-107 | R64 | EcH489 | pndAC and relBE | D′BB′C′CA | R64 | R64 | 3168502 |
| E20.06 | Poultry | IntI1 | 2004 | NL | 110 | ST26 | CC26 | + | pND11-107 | R621a | R621a | pndAC and relBE | BB′C′CA′ | R64 | R64 | 3168503 |
| ESBL-77 | Human | SHV-12 | 2009 | NL | 105 | ST25 | + | pND11-107 | R621a | R621a | pndAC and relBE | BB′C′CA′ | R64 | R64 | 3168504 | |
NL, The Netherlands; UK, United Kingdom.
The plasmid/genome with the highest level of identity is indicated.
Identified addiction factors are indicated.
IC, incomplete sequence type.
ND, not detected/not determined.
IncI1 plasmids not containing an ESBL/AmpC gene but carrying a class I integron were selected for comparison.
Accession no. AP005147.
Accession no. AB021078.
Accession no. AP011954.
Accession no. GU371927.
Accession no. HQ114281.
Accession no. KJ484630.
Accession no. KJ484629.
FIG 3.
Relationship of sequenced IncI1-Iγ plasmids. (A) Thirty-two IncI1-Iγ plasmid sequences were hierarchically clustered and are displayed as a phenogram using the BioNJ algorithm, in which the underlying distance matrix was obtained from the pairwise comparison of nonoverlapping maximal unique matches. Clades (tree groups as defined by MLST data) are indicated by colored clouds. Reference plasmids R64, R621a, pEK204, pEC-Bactec, pND11-107, and pColIb-P9 were included for comparison. (B) Randomly selected IncI1-Iγ plasmids from individual clades were hierarchically clustered with their plasmid backbones (lacking the antibiotic resistance cassettes), indicated as the ESBL number followed by “-mask.”
Differences between the sequenced plasmids.
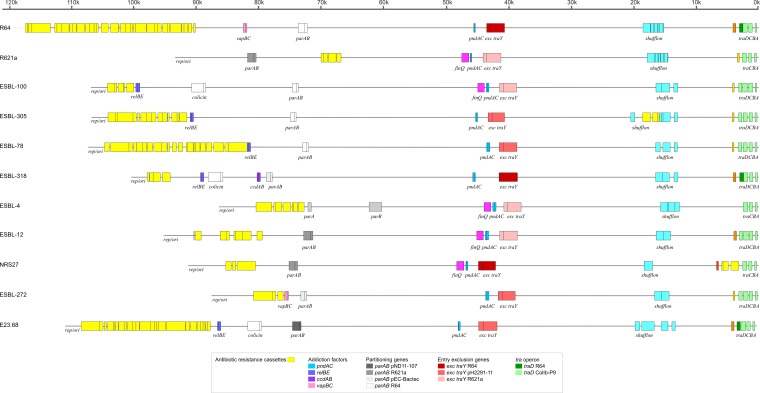
The plasmids belonging to distinct clades mainly differed in the presence/absence of (i) genes encoding colicins ColIb, TraD, PilJ, FinQ, and CcgAII, (ii) genes encoding the proteins involved in partitioning and in surface exclusion, (iii) the number of genes encoding addiction factors, and (iv) the composition of the shufflon (Fig. 4 and Table 1).
FIG 4.
Genetic organization of IncI1-Iγ plasmids (aligned at the origin of replication) belonging to various phylogenetic clades. Backbone sequences found to be identical among the various plasmids are indicated by a straight line. Conserved genes are indicated by colors and refer to colors representative for the reference plasmids R64, R621a, pEC-Bactec, ColIb-P9, pND11-107, and pH1519, as indicated below.
ST3 blaCTX-M-1, ST7 blaCTX-M-1, and ST12 blaCMY-2 plasmids as well as the uncommonly isolated plasmid STs contained cib and imm genes similar to the colicin-encoding and immunity-conferring genes of plasmid ColIb-P9 (34). All other plasmids of the present study lacked the cib and imm genes. Expression of colicins can benefit the bacterial host by causing lethality to related enteric bacteria that would otherwise compete for scarce nutrients under conditions of stress (35).
For the correct partitioning of the plasmids among daughter cells, most of the plasmids studied here contained the parAB genes as in the IncI1 reference plasmid R64 (15). Two of the plasmids representing ST37 blaCTX-M-15 and ST35 blaCTX-M-1 contained the parAB genes as in the IncIγ plasmid R621a (36), whereas the plasmids belonging to the uncommon STs contained parAB genes highly homologous to those of pND11_107 (14). The R621a and pND11-107 parAB genes differed significantly both from each other and from those of R64 (15). The ST31 blaCTX-M-15 plasmids lacked the R64/R621a/pND11-107 type of parAB genes. Instead, these plasmids contained the soj-yfhA genes previously suggested to act as a partitioning system in the plasmid pEC-BacTec (37).
Entry exclusion systems inhibit the transfer of closely related conjugative plasmids to prevent plasmid redundancy through recognition of the donor target protein TraY by the recipient protein ExcA or ExcA and ExcB during conjugation (38, 39). Genetic variation between these genes in R64 and R621a allows for cointroduction of these plasmids into a single host cell, resulting in the phenotypic classification of these plasmids into IncI1 and IncIγ groups, respectively (38). Current classification of the plasmids in this study was done based on the PBRT scheme, which detects the presence of RNA interference (RNAi) present in both IncI1 and IncIγ plasmids and cannot discriminate between these (1, 18). Sequence comparison of the plasmids studied here showed that the ST7 blaCTX-M-1, ST31 blaCTX-M-15, ST37 blaCTX-M-15, ST25 blaSHV-12, and ST26 plasmids belonged to the R621a exclusion group, whereas the ST35 blaCTX-M-1 plasmid contained the exclusion system found in R64 (Table 1). The ExcAB proteins encoded by ST12 blaCMY-2 plasmids (40) were exactly like those in R64, while the TraY protein shared 98% identity to the TraY protein of R64. The effect of these amino acid changes in the TraY proteins on the specificity of the entry exclusion is unknown. The ExcAB and TraY proteins encoded by the plasmids belonging to the ST3 blaCTX-M-1, incomplete ST (IC) blaCTX-M-1, ST3 blaSHV-12, ST36 blaTEM-52, and ST24 IntI1 types were identical to ExcAB and TraY proteins of plasmid pH2291-112 (40). The ExcAB proteins encoded by the ST50 blaTEM-1 plasmid were identical to the ExcAB proteins in R64, whereas the TraY protein shared 98% identity to the TraY protein of E. coli EcH489. Based on these results, it is expected that some of the plasmids studied here will phenotypically belong to IncI1 and some to IncIγ, but until functional studies have been carried out, we will adhere to the genotypic classification and refer to these plasmids as IncI1-Iγ.
Addiction systems, such as the toxin-antitoxin modules encoded by plasmids will contribute to the maintenance of the plasmids in their hosts by killing daughter cells that do not inherit the plasmids during cell division (41, 42). One to three different addiction systems were observed in the plasmids included in this study. As expected, all plasmids contained the pndAC system characteristic for IncI1-like plasmids (42). Moreover, except for the blaTEM-52-containing plasmids, including the ST31 blaCTX-M-15, ST37 blaCTX-M-15, and ST35 blaCTX-M-1 plasmids, all plasmids also contained the relBE system (43). The blaTEM-52-containing isolates had, except for the pndAC system, the vapBC system previously described as being a toxin-antitoxin system for maintenance of R64 (44). ST12 blaCMY-2 IncI1-Iγ plasmids contained three addiction systems: the pndAC and relBE systems, as well as the ccdAB system. The ccdAB system has previously been characterized in IncF replicons mainly (42).
Conjugation systems.
All IncI1-Iγ plasmids are characterized by the presence of genes for two types of conjugal pili: a thick pilus required for both liquid and surface matings and a thin pilus required exclusively for liquid matings (45). All plasmids included in this study contained the genes essential for conjugation. Conjugation under liquid conditions was confirmed “in vitro” for a selected number of the plasmids that lacked the traD gene (data not shown).
The shufflon, originally described in plasmid R64, consists of a number of invertible DNA segments, which are separated and flanked by recombination sites (46). The rci gene, located next to the shufflon, mediates site-specific recombination between any of the inverted repeat regions (46). The number of invertible repeats present in the plasmids included in this study varied considerably (Table 1), indicating that the plasmids varied in their ability to conjugate to various host recipients.
Accessory modules and resistance genes.
Accessory modules representative for the various plasmid clades are shown in Table S2 in the supplemental material. All plasmids contained one or two accessory modules encoding in total one to six antimicrobial resistance genes. Although most of the modules were inserted near the replication functions of the plasmids, the exact locations of the accessory modules in the IncI1-Iγ plasmid backbones differed between the various plasmid clades. Some plasmids harbored accessory modules at other locations in the plasmid backbone as well.
Resistance genes of plasmids harboring a blaCTX-M-1 or blaCTX-M-15 gene were all linked to an ISEcp1 element. In the ST7 blaCTX-M-1 and the IC blaCTX-M-1 plasmids, the ISEcp1-blaCTX-M-1 element was inserted near the replication function, whereas in the ST3 blaCTX-M-1 plasmids, the ISEcp1-blaCTX-M-1 element was inserted into the shufflon. The ST7 blaCTX-M-1 and IC blaCTX-M-1 plasmids harbored a second accessory module associated with a class 1 integron, including dfrA1, aadA1, qacEΔ, and sul1 genes.
The plasmid obtained from NRS27 (ST35 blaCTX-M-1) was highly homologous to pEK204 (44). Instead of the blaCTX-M-3 and the blaTEM-1 genes present in pEK204, the ST35 blaCTX-M-1 plasmid contained a blaCTX-M-1 gene and a blaTEM-33 gene. In pEK204, the ISEcp1-blaCTX-M-3 element and the blaTEM-1 gene were both linked to the Tn3 elements and were located close to the replication functions (44). In contrast, in the ST35 blaCTX-M-1 plasmid, the ISEcp1-blaCTX-M-1 element was located in the transfer region between the genes encoding PilJ and TraC.
The ST31 blaCTX-M-15 plasmids were highly homologous to pEC-Bactec, originally obtained from an E. coli isolate from a horse (37). As in pEC-Bactec, the ST31 blaCTX-M-15 plasmids carried an ISEcp1-blaCTX-M-15 element linked to Tn3 elements as well as to a blaTEM-1 gene. The ST37 blaCTX-M-15 plasmid showed a high level of identity to R621a (36), which was originally isolated from Salmonella enterica serovar Typhimurium and classified as belonging to incompatibility group Iγ. The ST37 blaCTX-M-15 plasmid lacked the Tn10-like and IS2 elements present in R621a and had an accessory element containing the blaCTX-M-15 and blaTEM-1 resistance genes instead. CC5 blaTEM-52 plasmids formed a distinct clade and did not show a high level of similarity to any IncI1-Iγ plasmids sequenced previously. The resistance-associated module contained the blaTEM-52 gene linked to Tn3 elements. ST12 blaCMY-2 plasmids carried the blaCMY-2 gene associated with an ISEcp1 element and the blc and sugE genes, as previously described for pCVM29188-101 (47). Highly similar resistance elements were previously identified in Salmonella and other Enterobacteriaceae (48), supporting the concept of a common resistance gene pool available to various bacterial communities (47, 49). The accessory module of the ST3 blaSHV-12 plasmid was very similar to the accessory module of the IncI1-Iγ plasmid pND11-107 (14) but contained an additional blaSHV-12 gene associated with a Tn1722 element (50). The rarely detected IncI1-Iγ plasmids not associated with ESBL/pAmpC genes established a distinct clade with the plasmids pSD107 (51), TY474p2 (CP002489) (52), and pND11-107 (14). The resistance regions of these plasmids differed from those of the plasmids in other clades with respect to the sizes and compositions of the incorporated resistance genes. In addition to the antimicrobial resistance genes, some of the rarely detected IncI1-Iγ plasmids also contained genes putatively providing protection against mercury.
Concluding remarks.
All of the epidemic IncI1-Iγ plasmids studied encoded ESBLs or pAmpC enzymes and differed mainly by the presence/absence of genes contributing to partitioning systems and addiction systems, which contribute to stable inheritance during cell division and plasmid maintenance. The sequence variation identified in traY and excA genes is expected to represent the divide between the IncI1 and IncIγ incompatibility groups, and functional analysis is under way to be able to explain what roles these genes have played in the evolution of the IncI1-Iγ plasmids. In addition to plasmid-encoded factors, undefined host strain-encoded factors are also likely to contribute to the successful dissemination of these epidemic IncI1-Iγ plasmid lineages.
Supplementary Material
ACKNOWLEDGMENTS
Authors G.W., N.W., S.S., B.G., and I.R. represent the EU-SAFEFOODERA-ESBL Consortium. We thank the remaining members of the consortium: Cindy Dierikx, Central Veterinary Institute (CVI), Wageningen University and Research Centre (UR), Lelystad, The Netherlands; Martin J. Woodward, Nick Coldham, Muna Anjum, and Manal AbuOun, Animal and Plant Health Agency, Weybridge, United Kingdom; Anne-Kathrin Schink and Kristina Kadlec, Friedrich-Loeffler-Institut, Neustadt-Mariensee, Germany; Reiner Helmuth and Janine Beutlich, Federal Institute for Risk Assessment, Berlin, Germany; and John Threlfall, John Wain, Michaela Day, and Marie Chattaway, Public Health England, Colindale, London, United Kingdom.
The work was conducted as part of the EU-SAFEFOODERA project 19008176, entitled “The Role of Commensal Microflora of Animals in the Transmission of Extended Spectrum β-Lactamases,” and the KB-12-005.01-018 project “ESBLs, Epidemic Plasmids.”
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.05006-14.
REFERENCES
- 1.Carattoli A. 2008. Animal reservoirs for extended spectrum beta-lactamase producers. Clin Microbiol Infect 14(Suppl 1):S117–S123. [DOI] [PubMed] [Google Scholar]
- 2.Ewers C, Bethe A, Semmler T, Guenther S, Wieler LH. 2012. Extended-spectrum beta-lactamase-producing and AmpC-producing Escherichia coli from livestock and companion animals, and their putative impact on public health: a global perspective. Clin Microbiol Infect 18:646–655. doi: 10.1111/j.1469-0691.2012.03850.x. [DOI] [PubMed] [Google Scholar]
- 3.Liebana E, Carattoli A, Coque TM, Hasman H, Magiorakos AP, Mevius D, Peixe L, Poirel L, Schuepbach-Regula G, Torneke K, Torren-Edo J, Torres C, Threlfall J. 2013. Public health risks of enterobacterial isolates producing extended-spectrum beta-lactamases or AmpC beta-lactamases in food and food-producing animals: an EU perspective of epidemiology, analytical methods, risk factors, and control options. Clin Infect Dis 56:1030–1037. doi: 10.1093/cid/cis1043. [DOI] [PubMed] [Google Scholar]
- 4.Carattoli A. 2011. Plasmids in Gram negatives: molecular typing of resistance plasmids. Int J Med Microbiol 301:654–658. doi: 10.1016/j.ijmm.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Cloeckaert A, Praud K, Lefevre M, Doublet B, Pardos M, Granier SA, Brisabois A, Weill FX. 2010. IncI1 plasmid carrying extended-spectrum-β-lactamase gene blaCTX-M-1 in Salmonella enterica isolates from poultry and humans in France, 2003 to 2008. Antimicrob Agents Chemother 54:4484–4486. doi: 10.1128/AAC.00460-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madec JY, Doublet B, Ponsin C, Cloeckaert A, Haenni M. 2011. Extended-spectrum beta-lactamase blaCTX-M-1 gene carried on an IncI1 plasmid in multidrug-resistant Salmonella enterica serovar Typhimurium DT104 in cattle in France. J Antimicrob Chemother 66:942–944. doi: 10.1093/jac/dkr014. [DOI] [PubMed] [Google Scholar]
- 7.Carattoli A. 2013. Plasmids and the spread of resistance. Int J Med Microbiol 303:298–304. doi: 10.1016/j.ijmm.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Dierikx C, van der Goot J, Fabri T, van Essen-Zandbergen A, Smith H, Mevius D. 2013. Extended-spectrum-beta-lactamase- and AmpC-beta-lactamase-producing Escherichia coli in Dutch broilers and broiler farmers. J Antimicrob Chemother 68:60–67. doi: 10.1093/jac/dks349. [DOI] [PubMed] [Google Scholar]
- 9.Leverstein-van Hall MA, Dierikx CM, Cohen Stuart J, Voets GM, van den Munckhof MP, van Essen-Zandbergen A, Platteel T, Fluit AC, van de Sande-Bruinsma N, Scharinga J, Bonten MJ, Mevius DJ, National ESBL Study Group . 2011. Dutch patients, retail chicken meat and poultry share the same ESBL genes, plasmids and strains. Clin Microbiol Infect 17:873–880. doi: 10.1111/j.1469-0691.2011.03497.x. [DOI] [PubMed] [Google Scholar]
- 10.Voets GM, Platteel TN, Fluit AC, Scharringa J, Schapendonk CM, Stuart JC, Bonten MJ, Hall MA, National ESBL Surveillance Working Group . 2012. Population distribution of beta-lactamase conferring resistance to third-generation cephalosporins in human clinical Enterobacteriaceae in the Netherlands. PLoS One 7:e52102. doi: 10.1371/journal.pone.0052102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woodford N, Ward ME, Kaufmann ME, Turton J, Fagan EJ, James D, Johnson AP, Pike R, Warner M, Cheasty T, Pearson A, Harry S, Leach JB, Loughrey A, Lowes JA, Warren RE, Livermore DM. 2004. Community and hospital spread of Escherichia coli producing CTX-M extended-spectrum beta-lactamases in the UK. J Antimicrob Chemother 54:735–743. doi: 10.1093/jac/dkh424. [DOI] [PubMed] [Google Scholar]
- 12.Fischer J, Rodriguez I, Baumann B, Guiral E, Beutin L, Schroeter A, Kaesbohrer A, Pfeifer Y, Helmuth R, Guerra B. 2014. BlaCTX-M-15-carrying Escherichia coli and Salmonella isolates from livestock and food in Germany. J Antimicrob Chemother 69:2951–2958. doi: 10.1093/jac/dku270. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Fernandez A, Chiaretto G, Bertini A, Villa L, Fortini D, Ricci A, Carattoli A. 2008. Multilocus sequence typing of IncI1 plasmids carrying extended-spectrum beta-lactamases in Escherichia coli and Salmonella of human and animal origin. J Antimicrob Chemother 61:1229–1233. doi: 10.1093/jac/dkn131. [DOI] [PubMed] [Google Scholar]
- 14.Johnson TJ, Shepard SM, Rivet B, Danzeisen JL, Carattoli A. 2011. Comparative genomics and phylogeny of the IncI1 plasmids: a common plasmid type among porcine enterotoxigenic Escherichia coli. Plasmid 66:144–151. doi: 10.1016/j.plasmid.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Sampei G, Furuya N, Tachibana K, Saitou Y, Suzuki T, Mizobuchi K, Komano T. 2010. Complete genome sequence of the incompatibility group I1 plasmid R64. Plasmid 64:92–103. doi: 10.1016/j.plasmid.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Wu G, Day MJ, Mafura MT, Nunez-Garcia J, Fenner JJ, Sharma M, van Essen-Zandbergen A, Rodriguez I, Dierikx C, Kadlec K, Schink AK, Wain J, Helmuth R, Guerra B, Schwarz S, Threlfall J, Woodward MJ, Woodford N, Coldham N, Mevius D. 2013. Comparative analysis of ESBL-positive Escherichia coli isolates from animals and humans from the UK, The Netherlands and Germany. PLoS One 8:e75392. doi: 10.1371/journal.pone.0075392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Essen-Zandbergen A, Smith H, Veldman K, Mevius D. 2007. Occurrence and characteristics of class 1, 2 and 3 integrons in Escherichia coli, Salmonella and Campylobacter spp. in the Netherlands J Antimicrob Chemother 59:746–750. [DOI] [PubMed] [Google Scholar]
- 18.Carattoli A, Bertini A, Villa L, Falbo V, Hopkins KL, Threlfall EJ. 2005. Identification of plasmids by PCR-based replicon typing. J Microbiol Methods 63:219–228. doi: 10.1016/j.mimet.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 19.Carattoli A. 2009. Resistance plasmid families in Enterobacteriaceae. Antimicrob Agents Chemother 53:2227–2238. doi: 10.1128/AAC.01707-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dierikx CM, van Duijkeren E, Schoormans AH, van Essen-Zandbergen A, Veldman K, Kant A, Huijsdens XW, van der Zwaluw K, Wagenaar JA, Mevius DJ. 2012. Occurrence and characteristics of extended-spectrum-beta-lactamase- and AmpC-producing clinical isolates derived from companion animals and horses. J Antimicrob Chemother 67:1368–1374. doi: 10.1093/jac/dks049. [DOI] [PubMed] [Google Scholar]
- 21.Sambook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 22.Barton BM, Harding GP, Zuccarelli AJ. 1995. A general method for detecting and sizing large plasmids. Anal Biochem 226:235–240. doi: 10.1006/abio.1995.1220. [DOI] [PubMed] [Google Scholar]
- 23.Brouwer MS, Bossers A, Harders F, van Essen-Zandbergen A, Mevius DJ, Smith HE. 2014. Complete genome sequences of IncI1 plasmids carrying extended-spectrum β-lactamase genes. Genome Announc 2(4):e00859-14. doi: 10.1128/genomeA.00859-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Besemer J, Lomsadze A, Borodovsky M. 2001. GeneMarkS: a self-training method for prediction of gene starts in microbial genomes. Implications for finding sequence motifs in regulatory regions. Nucleic Acids Res 29:2607–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finn RD, Mistry J, Tate J, Coggill P, Heger A, Pollington JE, Gavin OL, Gunasekaran P, Ceric G, Forslund K, Holm L, Sonnhammer EL, Eddy SR, Bateman A. 2010. The Pfam protein families database. Nucleic Acids Res 38:D211–D222. doi: 10.1093/nar/gkp985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hunter S, Apweiler R, Attwood TK, Bairoch A, Bateman A, Binns D, Bork P, Das U, Daugherty L, Duquenne L, Finn RD, Gough J, Haft D, Hulo N, Kahn D, Kelly E, Laugraud A, Letunic I, Lonsdale D, Lopez R, Madera M, Maslen J, McAnulla C, McDowall J, Mistry J, Mitchell A, Mulder N, Natale D, Orengo C, Quinn AF, Selengut JD, Sigrist CJ, Thimma M, Thomas PD, Valentin F, Wilson D, Wu CH, Yeats C. 2009. InterPro: the integrative protein signature database. Nucleic Acids Res 37:D211–D215. doi: 10.1093/nar/gkn785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gascuel O. 1997. BIONJ: an improved version of the NJ algorithm based on a simple model of sequence data. Mol Biol Evol 14:685–695. doi: 10.1093/oxfordjournals.molbev.a025808. [DOI] [PubMed] [Google Scholar]
- 28.Kurtz S, Phillippy A, Delcher AL, Smoot M, Shumway M, Antonescu C, Salzberg SL. 2004. Versatile and open software for comparing large genomes. Genome Biol 5:R12. doi: 10.1186/gb-2004-5-2-r12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deloger M, El Karoui M, Petit MA. 2009. A genomic distance based on MUM indicates discontinuity between most bacterial species and genera. J Bacteriol 191:91–99. doi: 10.1128/JB.01202-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huson DH, Bryant D. 2006. Application of phylogenetic networks in evolutionary studies. Mol Biol Evol 23:254–267. [DOI] [PubMed] [Google Scholar]
- 31.Folster JP, Tolar B, Pecic G, Sheehan D, Rickert R, Hise K, Zhao S, Fedorka-Cray PJ, McDermott P, Whichard JM. 2014. Characterization of blaCMY plasmids and their possible role in source attribution of Salmonella enterica serotype Typhimurium infections. Foodborne Pathog Dis 11:301–306. doi: 10.1089/fpd.2013.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Accogli M, Fortini D, Giufre M, Graziani C, Dolejska M, Carattoli A, Cerquetti M. 2013. IncI1 plasmids associated with the spread of CMY-2, CTX-M-1 and SHV-12 in Escherichia coli of animal and human origin. Clin Microbiol Infect 19:E238–E240. doi: 10.1111/1469-0691.12128. [DOI] [PubMed] [Google Scholar]
- 33.Dahmen S, Haenni M, Madec JY. 2012. IncI1/ST3 plasmids contribute to the dissemination of the blaCTX-M-1 gene in Escherichia coli from several animal species in France. J Antimicrob Chemother 67:3011–3012. doi: 10.1093/jac/dks308. [DOI] [PubMed] [Google Scholar]
- 34.Mankovich JA, Hsu CH, Konisky J. 1986. DNA and amino acid sequence analysis of structural and immunity genes of colicins Ia and Ib. J Bacteriol 168:228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Majeed H, Gillor O, Kerr B, Riley MA. 2011. Competitive interactions in Escherichia coli populations: the role of bacteriocins. ISME J 5:71–81. doi: 10.1038/ismej.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takahashi H, Shao M, Furuya N, Komano T. 2011. The genome sequence of the incompatibility group Igamma plasmid R621a: evolution of IncI plasmids. Plasmid 66:112–121. doi: 10.1016/j.plasmid.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 37.Smet A, Van Nieuwerburgh F, Vandekerckhove TT, Martel A, Deforce D, Butaye P, Haesebrouck F. 2010. Complete nucleotide sequence of CTX-M-15-plasmids from clinical Escherichia coli isolates: insertional events of transposons and insertion sequences. PLoS One 5:e11202. doi: 10.1371/journal.pone.0011202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sakuma T, Tazumi S, Furuya N, Komano T. 2013. ExcA proteins of IncI1 plasmid R64 and IncIgamma plasmid R621a recognize different segments of their cognate TraY proteins in entry exclusion. Plasmid 69:138–145. doi: 10.1016/j.plasmid.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 39.Furuya N, Komano T. 1994. Surface exclusion gene of IncI1 plasmid R64: nucleotide sequence and analysis of deletion mutants. Plasmid 32:80–84. doi: 10.1006/plas.1994.1047. [DOI] [PubMed] [Google Scholar]
- 40.Wang J, Stephan R, Power K, Yan Q, Hachler H, Fanning S. 2014. Nucleotide sequences of 16 transmissible plasmids identified in nine multidrug-resistant Escherichia coli isolates expressing an ESBL phenotype isolated from food-producing animals and healthy humans. J Antimicrob Chemother 69:2658–2668. doi: 10.1093/jac/dku206. [DOI] [PubMed] [Google Scholar]
- 41.Doumith M, Dhanji H, Ellington MJ, Hawkey P, Woodford N. 2012. Characterization of plasmids encoding extended-spectrum beta-lactamases and their addiction systems circulating among Escherichia coli clinical isolates in the UK. J Antimicrob Chemother 67:878–885. doi: 10.1093/jac/dkr553. [DOI] [PubMed] [Google Scholar]
- 42.Mnif B, Vimont S, Boyd A, Bourit E, Picard B, Branger C, Denamur E, Arlet G. 2010. Molecular characterization of addiction systems of plasmids encoding extended-spectrum beta-lactamases in Escherichia coli. J Antimicrob Chemother 65:1599–1603. doi: 10.1093/jac/dkq181. [DOI] [PubMed] [Google Scholar]
- 43.Christensen SK, Gerdes K. 2003. RelE toxins from bacteria and archaea cleave mRNAs on translating ribosomes, which are rescued by tmRNA. Mol Microbiol 48:1389–1400. doi: 10.1046/j.1365-2958.2003.03512.x. [DOI] [PubMed] [Google Scholar]
- 44.Woodford N, Carattoli A, Karisik E, Underwood A, Ellington MJ, Livermore DM. 2009. Complete nucleotide sequences of plasmids pEK204, pEK499, and pEK516, encoding CTX-M enzymes in three major Escherichia coli lineages from the United Kingdom, all belonging to the international O25:H4-ST131 clone. Antimicrob Agents Chemother 53:4472–4482. doi: 10.1128/AAC.00688-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Komano T, Yoshida T, Narahara K, Furuya N. 2000. The transfer region of IncI1 plasmid R64: similarities between R64 tra and Legionella icm/dot genes. Mol Microbiol 35:1348–1359. [DOI] [PubMed] [Google Scholar]
- 46.Gyohda A, Furuya N, Ishiwa A, Zhu S, Komano T. 2004. Structure and function of the shufflon in plasmid r64. Adv Biophys 38:183–213. doi: 10.1016/S0065-227X(04)80166-7. [DOI] [PubMed] [Google Scholar]
- 47.Fricke WF, McDermott PF, Mammel MK, Zhao S, Johnson TJ, Rasko DA, Fedorka-Cray PJ, Pedroso A, Whichard JM, Leclerc JE, White DG, Cebula TA, Ravel J. 2009. Antimicrobial resistance-conferring plasmids with similarity to virulence plasmids from avian pathogenic Escherichia coli strains in Salmonella enterica serovar Kentucky isolates from poultry. Appl Environ Microbiol 75:5963–5971. doi: 10.1128/AEM.00786-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Su LH, Chen HL, Chia JH, Liu SY, Chu C, Wu TL, Chiu CH. 2006. Distribution of a transposon-like element carrying bla(CMY-2) among Salmonella and other Enterobacteriaceae. J Antimicrob Chemother 57:424–429. doi: 10.1093/jac/dki478. [DOI] [PubMed] [Google Scholar]
- 49.Fricke WF, Wright MS, Lindell AH, Harkins DM, Baker-Austin C, Ravel J, Stepanauskas R. 2008. Insights into the environmental resistance gene pool from the genome sequence of the multidrug-resistant environmental isolate Escherichia coli SMS-3-5. J Bacteriol 190:6779–6794. doi: 10.1128/JB.00661-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Partridge SR. 2011. Analysis of antibiotic resistance regions in Gram-negative bacteria. FEMS Microbiol Rev 35:820–855. doi: 10.1111/j.1574-6976.2011.00277.x. [DOI] [PubMed] [Google Scholar]
- 51.Bleicher A, Schofl G, Rodicio MD, Saluz HP. 2013. The plasmidome of a Salmonella enterica serovar Derby isolated from pork meat. Plasmid 69:202–210. doi: 10.1016/j.plasmid.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 52.Richardson EJ, Limaye B, Inamdar H, Datta A, Manjari KS, Pullinger GD, Thomson NR, Joshi RR, Watson M, Stevens MP. 2011. Genome sequences of Salmonella enterica serovar Typhimurium, Choleraesuis, Dublin, and Gallinarum strains of well-defined virulence in food-producing animals. J Bacteriol 193:3162–3163. doi: 10.1128/JB.00394-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.