Abstract
Combination therapies for leishmaniasis, including drugs and immunomodulators, are one approach to shorten treatment courses and to improve the treatment of complex manifestations of the disease. We evaluated a novel T-cell-epitope-enriched DNA vaccine candidate (LEISHDNAVAX) as host-directed immunotherapy in combination with a standard antileishmanial drug in experimental visceral leishmaniasis. Here we show that the DNA vaccine candidate can boost the efficacy of a single suboptimal dose of liposomal amphotericin B in C57BL/6 mice.
TEXT
The leishmaniases are neglected tropical diseases (NTDs) caused by protozoan parasites of the genus Leishmania. Visceral leishmaniasis (VL) is caused by Leishmania donovani and Leishmania infantum and presents as systemic disease with parasitic invasion, primarily of the mononuclear phagocytic system (1). Symptomatic VL is fatal if left untreated. Estimates suggest that there are between 200,000 to 400,000 cases and 20,000 to 40,000 deaths per year worldwide (2). Current drug therapies are unsatisfactory due to toxicity, long treatment courses, challenging routes of administration, and geographical differences in clinical responses to treatment (3, 4). Other disease manifestations include post-kala-azar dermal leishmaniasis (PKDL), which is a complication of VL that presents as a skin condition weeks to months after drug treatment (5), and cutaneous leishmaniasis (CL), which is characterized by skin lesions of variable severity (6). We recently reported on the development of a DNA vaccine candidate for leishmaniasis, based on minimalistic immunogenically defined gene expression vectors modified to foster Th1-type immune responses (MIDGE-Th1 vectors). The vaccine candidate, referred to as LEISHDNAVAX, is an equimass mixture of five independent MIDGE-Th1 vectors encoding different leishmanial antigens (KMP11, TSA, CPA, CPB, and P74) (7). Here we investigated whether LEISHDNAVAX can serve as an adjunct to antileishmanial drug treatment. We selected liposomal amphotericin B as the drug, based on recent developments in the treatment of VL. Single-dose liposomal amphotericin B was shown to be effective and safe in a phase III trial in India (8), is now a recommended first-line treatment for VL in South Asia (4), and also forms part of short-course multidrug therapies, which have recently undergone evaluations in phase III trials (9). Single doses of 10 mg/kg (in monotherapy) or 5 mg/kg (in combined therapy) of liposomal amphotericin B proved to be optimal for patients (8, 9). In this study, a suboptimal dose of liposomal amphotericin B was chosen, to enable demonstration of beneficial treatment effects of combined treatment regimens. From a clinical perspective, delivering reduced doses of this treatment would also result in reduced treatment costs.
The cotherapeutic potential of LEISHDNAVAX was evaluated in female C57BL/6J mice (Charles River, United Kingdom) (7 to 8 weeks of age at the start of experiments and maintained under specific-pathogen-free conditions) that had been infected with 2 × 107 L. donovani amastigotes (strain MHOM/ET/67/HU3), as described previously (10). Parasites were maintained in Rag-1-knockout (B6) mice, and amastigotes were harvested from the spleens of infected animals. Following infection, mice were sorted into groups of 3 or 4 per cage, and 2 cages were assigned per treatment group. Mice were treated with a single intravenous (i.v.) dose (10) of 0.8 mg/kg of liposomal amphotericin B (AmBisome; Gilead) on day 7 postinfection (p.i.). On day 21 (experiment 1), the parasite burden was determined in untreated and liposomal amphotericin B-treated satellite groups (3 mice/group). Mice were sacrificed, and the livers and spleens were removed. Impression smears were prepared from weighed organs (10), and the numbers of amastigotes per 1,000 nuclei were determined microscopically. Leishman-Donovan units (LDU) were calculated as the number of parasites per host cell nucleus times the organ weight (in milligrams) (11). Different doses of LEISHDNAVAX (20 μg or 40 μg of DNA per antigen, corresponding to 100 μg or 200 μg of total DNA, respectively, in phosphate-buffered saline [PBS], with injection volumes of 1 × 25 μl or 2 × 25 μl, respectively) were administered intradermally (i.d.) at the tail base, using 29-gauge needles (BD Microfine Plus insulin syringes), on day 21 p.i. Control groups included groups treated with the respective monotherapies and an untreated group. DNA vector control groups received a nonexpressing human interleukin 2 (IL-2)-encoding MIDGE-Th1 construct equivalent to 100 μg of total DNA. Mice were sacrificed on day 31 (experiment 1) or day 33 (experiments 2 and 3) p.i., and the parasite burdens in livers and spleens were determined as reported above. Treatment schedules are summarized in Table 1. For histology, organs were fixed in 10% neutral buffered formalin, embedded in paraffin, and routinely stained with hematoxylin and eosin (H&E). Immunohistochemical staining was performed using the avidin-biotin complex (ABC) method (Vector, Peterborough, United Kingdom), with a polyclonal rabbit anti-human CD3 antibody (Dako, Ely, Cambridgeshire, United Kingdom) that cross-reacts with the CD3-equivalent protein in mice (12). The total areas from two longitudinal tissue sections from liver and spleen were examined by light microscopy and digital image analysis (Nikon NIS-Elements). For each slide, the area covered by positive cells was calculated as a percentage of the total area. Statistical significance was evaluated by one-way analysis of variance (ANOVA), assuming Gaussian distribution, and a multiple-comparison test if applicable (GraphPad Prism 6). P values of <0.05 were considered statistically significant.
TABLE 1.
Treatment schedules for L. donovani-infected C57BL/6 mice
| Groupa | Treatment and evaluation scheduleb |
||
|---|---|---|---|
| Day 7 p.i. | Day 21 p.i. | Day 33 (day 31) p.i. | |
| Untreatedc | Sacrifice, sample collection | ||
| A + V(100) | AmBisome (i.v.) | LEISHDNAVAX (i.d.) at 100 μg total DNA | Sacrifice, sample collection |
| A + V(200) | AmBisome (i.v.) | LEISHDNAVAX (i.d.) at 200 μg total DNA | Sacrifice, sample collection |
| Ac | AmBisome (i.v.) | Sacrifice, sample collection | |
| V(100) | LEISHDNAVAX (i.d.) at 100 μg total DNA | Sacrifice, sample collection | |
| V(200) | LEISHDNAVAX (i.d.) at 200 μg total DNA | Sacrifice, sample collection | |
| A + vector | AmBisome (i.v.) | Nonexpressing IL-2-encoding MIDGE-Th1 vectors (i.d.) | Sacrifice, sample collection |
| Vector | Nonexpressing IL-2-encoding MIDGE-Th1 vectors (i.d.) | Sacrifice, sample collection | |
A, liposomal amphotericin B; V(100), LEISHDNAVAX at 100 μg of total DNA; V(200), LEISHDNAVAX at 200 μg of total DNA.
Mice were infected with L. donovani amastigotes on day 0. Treatment and evaluation schedules are given for days postinfection (p.i.).
Satellite groups were sacrificed on day 21 p.i. in one experiment.
Animal experiments were conducted under United Kingdom Home Office license PPL 70/6997, following local ethical approval by the London School of Hygiene and Tropical Medicine and the Royal Veterinary College (London, United Kingdom).
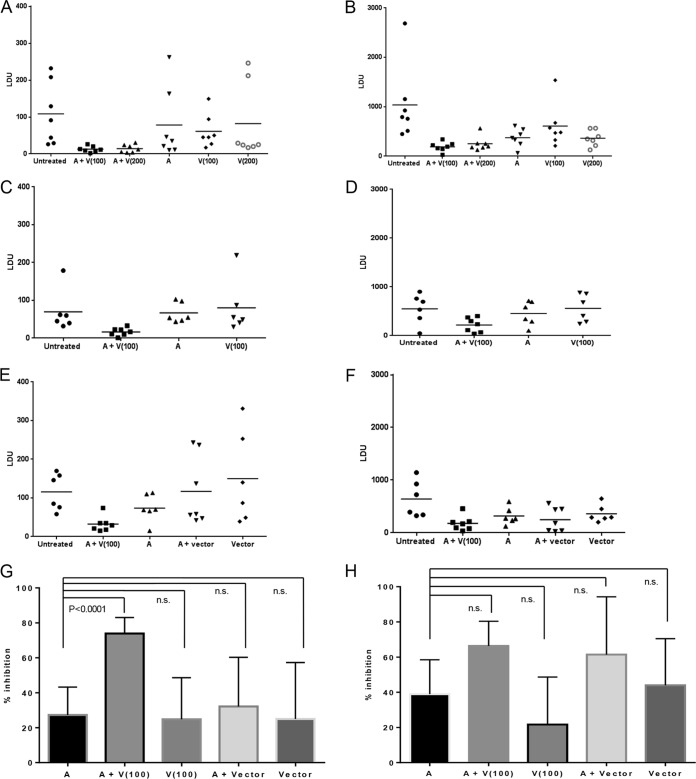
The first experiment, using two different doses of LEISHDNAVAX, showed that treatment groups that received sequential drug therapy and immunotherapy displayed lower parasite burdens in the spleens and livers than did groups that received liposomal amphotericin B treatment alone (Fig. 1A and B). In the spleens, the mean parasite burdens, with corresponding 95% confidence intervals (CIs), were 13 LDU (95% CI, 5 to 20 LDU) with the 100-μg DNA dose and 14 LDU (95% CI, 4 to 24 LDU) with the 200-μg DNA dose, compared to 78 LDU (95% CI, 0 to 168 LDU) with drug treatment alone. Corresponding values in the livers were 190 LDU (95% CI, 101 to 279 LDU) with the 100-μg DNA dose, 251 LDU (95% CI, 116 to 386 LDU) with the 200-μg DNA dose, and 374 LDU (95% CI, 201 to 546 LDU) with drug treatment alone. Levels in livers and spleens at the time of immunotherapy were 1,313 LDU (95% CI, 746 to 1,880 LDU) and 42 LDU (95% CI, 17 to 68 LDU) in the untreated group and 572 LDU (95% CI, 425 to 719 LDU) and 13 LDU (95% CI, 0 to 27 LDU) in the group treated with liposomal amphotericin B, respectively. There was no apparent advantage in administering the higher dose of LEISHDNAVAX; therefore, subsequent experiments were conducted with the 100-μg DNA dose. To exclude the possibility that increased treatment responses in cotherapy groups were caused only by administration of DNA, mice were injected with nonexpressing human IL-2-encoding MIDGE-Th1 vectors following liposomal amphotericin B treatment, in a direct comparison with immunotherapy. These experiments confirmed increased parasite killing by the coadministration regimen (Fig. 1C to F). Additionally, no enhancement of splenic treatment responses was seen in the group treated with drug and DNA vector (117 LDU [95% CI, 34 to 200 LDU]), compared to the group treated with drug only (74 LDU [95% CI, 36 to 111 LDU]). To address the question of whether LEISHDNAVAX can serve as an adjunct to drug treatment, we compared the reductions in parasite burdens between groups that received liposomal amphotericin B alone and groups that received liposomal amphotericin B plus LEISHDNAVAX, or the respective control groups, in an analysis pooling data from experiments 2 and 3. This demonstrated a significant difference in the reductions in splenic parasite burdens for the group treated with coadministered drug and vaccine compared to those of the group treated with drug alone (mean inhibition of 74 ± 4% versus 27 ± 7%; P < 0.0001) (Fig. 1G and H). We further characterized cellular responses in tissue sections with standard H&E staining and staining for the CD3 protein (as a T-cell marker) (Fig. 2). There was no significant difference in the numbers of CD3+ cells between groups. Histological examinations previously revealed degenerative pathological findings in another intracellular infection with tissue involvement in mice, when the mice were treated with DNA vaccines encoding an immunogenic antigen (13). We observed similar histopathological changes in untreated, drug-treated, and drug/immunotherapy-treated mice. These changes were consistent with L. donovani infection and included granulomatous inflammation in the liver (14) and loss of normal tissue architecture in the spleen (15). No weight loss or other signs of adverse reactions were observed in the treated groups. Therefore, the coadministration treatment regimen was well tolerated.
FIG 1.
Efficacy of drug treatment, immunotherapy, and sequential chemotherapy-immunotherapy in spleens and livers of C57BL/6 mice. (A to F) Parasite burdens in spleens (A, C, and E) and livers (B, D, and F) in experiment 1 (A and B), experiment 2 (C and D), and experiment 3 (E and F). Each symbol represents data from an individual mouse; horizontal lines, means (n = 6 to 8 mice/group). A, administration of liposomal amphotericin B; V(100), administration of LEISHDNAVAX at 100 μg of total DNA; V(200), administration of LEISHDNAVAX at 200 μg of total DNA; A + V(100) and A + V(200), coadministration of liposomal amphotericin B and LEISHDNAVAX at 100 μg and 200 μg of total DNA, respectively; vector, administration of nonexpressing human IL-2-encoding MIDGE-Th1 vector. (G and H) Percent inhibition of parasite burdens in the spleen (G) and liver (H), calculated as follows: 100 − [(LDU for individual mouse in treated group)/(mean LDU for untreated group) × 100]. Data were pooled from experiments 2 and 3 (n = 12 to 14 mice/group for groups treated with liposomal amphotericin B or liposomal amphotericin B plus LEISHDNAVAX at 100 μg of total DNA and n = 6 or 7 mice/group for all other groups) and are shown as means with 95% CIs. n.s., not significant.
FIG 2.
Histopathological and immunohistochemical findings for VL-affected organs. Histopathological (H&E staining) (upper) and immunohistochemical (CD3 staining) (lower) evaluations of spleen (A) and liver (B) of C57BL/6 mice sequentially treated with liposomal amphotericin B and LEISHDNAVAX were performed. f, follicles (white pulp); g, granulomas. Arrows, constituent CD3+ cells. Arrowheads, CD3+ cells within granulomas. Images are representative of 5 mice/group from one experiment. Data indicate the percent area of immunohistochemical (IHC) positivity (CD3 staining) for the number of animals indicated (n). Values are group means ± standard deviations. A, administration of liposomal amphotericin B; A + V(100), coadministration of liposomal amphotericin B and LEISHDNAVAX at 100 μg of total DNA. Magnification, ×200.
In summary, we have shown that LEISHDNAVAX can boost the responses to a suboptimal dose of liposomal amphotericin B in experimental VL. The observed differences in the magnitudes of responses between organs are probably due to differences in infection kinetics and immunoregulation. The parasite load in the liver is already in decline 4 weeks after infection, while parasites in the spleen are still multiplying and establish persistence (16, 17). Therefore, the increased efficacy of the combined treatment regimen in the spleen was measured under stringent conditions, as reported previously for an adenovirus-based vaccine (18). LEISHDNAVAX did not show significant antileishmanial efficacy when administered as monotherapy at the highest dose used in prophylactic studies (7); it demonstrated efficacy only when coadministered with liposomal amphotericin B. Lack of efficacy with administration as monotherapy was also observed in repeated-dose studies, which demonstrated good tolerability of LEISHDNAVAX in L. donovani-infected mice (19). Infection inocula and parasite burdens at the end of treatment were similar in this study and the tolerability study (19). Combined treatment approaches are an increasingly attractive treatment option for VL and aim to increase efficacy and to reduce treatment durations and the risk of the emergence of drug resistance (9, 20, 21). An alternative approach to combination treatment or coadministration of small-molecule drugs is coadministration of drugs and immunotherapies targeted to stimulate the immune system. The superiority of immunotherapy plus chemotherapy over chemotherapy alone was shown in the treatment of PKDL patients in Sudan (22) and CL patients in Brazil (23). LEISHDNAVAX is a DNA vaccine that has been designed to induce T-cell-mediated immunity; it is composed of antigens with sequences conserved across different Leishmania species and over time, to facilitate its use in prophylactic and therapeutic regimens for disease manifestations caused by different Leishmania species and strains currently transmitted in areas in which the disease is endemic (7). This brief report is the first demonstration of the therapeutic potential of this novel DNA vaccine. Further studies are needed to investigate the immunological mechanism in a therapeutic setting and to optimize treatment schedules before translation to human studies. Drug treatment started early after the initiation of infection in the current study, unlike human infections, in which patients are more likely to present with established infections. Therefore, future investigations on optimized treatment regimens should consider efficacies in established infections and should include investigations of the pharmacokinetics and biodistribution of liposomal amphotericin B in combined treatment regimens.
ACKNOWLEDGMENTS
This work was supported by European Community Framework Programme 7 (grant 223189).
We thank our colleagues of the LEISHDNAVAX consortium for valuable discussions, the staff of the Biological Services Facility at the London School of Hygiene and Tropical Medicine for expert support, and the staff of the production and quality control departments at Mologen AG for preparation of vaccine material.
C.J. is an employee of Mologen AG, and Mologen AG owns a patent for the MIDGE-Th1 vector (PCT/DE2002/003798). K.S., F.J.S., and S.L.C. have no conflicts of interest to declare.
REFERENCES
- 1.Chappuis F, Sundar S, Hailu A, Ghalib H, Rijal S, Peeling RW, Alvar J, Boelaert M. 2007. Visceral leishmaniasis: what are the needs for diagnosis, treatment and control? Nat Rev Microbiol 5:873–882. doi: 10.1038/nrmicro1748. [DOI] [PubMed] [Google Scholar]
- 2.Alvar J, Velez ID, Bern C, Herrero M, Desjeux P, Cano J, Jannin J, den Boer M. 2012. Leishmaniasis worldwide and global estimates of its incidence. PLoS One 7:e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Croft SL, Olliaro P. 2011. Leishmaniasis chemotherapy: challenges and opportunities. Clin Microbiol Infect 17:1478–1483. doi: 10.1111/j.1469-0691.2011.03630.x. [DOI] [PubMed] [Google Scholar]
- 4.Balasegaram M, Ritmeijer K, Lima MA, Burza S, Ortiz Genovese G, Milani B, Gaspani S, Potet J, Chappuis F. 2012. Liposomal amphotericin B as a treatment for human leishmaniasis. Expert Opin Emerg Drugs 17:493–510. doi: 10.1517/14728214.2012.748036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desjeux P, Ghosh RS, Dhalaria P, Strub-Wourgaft N, Zijlstra EE. 2013. Report of the Post Kala-azar Dermal Leishmaniasis (PKDL) consortium meeting, New Delhi, India, 27–29 June 2012. Parasit Vectors 6:196. doi: 10.1186/1756-3305-6-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reithinger R, Dujardin JC, Louzir H, Pirmez C, Alexander B, Brooker S. 2007. Cutaneous leishmaniasis. Lancet Infect Dis 7:581–596. doi: 10.1016/S1473-3099(07)70209-8. [DOI] [PubMed] [Google Scholar]
- 7.Das S, Freier A, Boussoffara T, Das S, Oswald D, Losch FO, Selka M, Sacerdoti-Sierra N, Schonian G, Wiesmuller KH, Seifert K, Schroff M, Juhls C, Jaffe CL, Roy S, Das P, Louzir H, Croft SL, Modabber F, Walden P. 2014. Modular multiantigen T cell epitope-enriched DNA vaccine against human leishmaniasis. Sci Transl Med 6:234ra56. doi: 10.1126/scitranslmed.3008222. [DOI] [PubMed] [Google Scholar]
- 8.Sundar S, Chakravarty J, Agarwal D, Rai M, Murray HW. 2010. Single-dose liposomal amphotericin B for visceral leishmaniasis in India. N Engl J Med 362:504–512. doi: 10.1056/NEJMoa0903627. [DOI] [PubMed] [Google Scholar]
- 9.Sundar S, Sinha PK, Rai M, Verma DK, Nawin K, Alam S, Chakravarty J, Vaillant M, Verma N, Pandey K, Kumari P, Lal CS, Arora R, Sharma B, Ellis S, Strub-Wourgaft N, Balasegaram M, Olliaro P, Das P, Modabber F. 2011. Comparison of short-course multidrug treatment with standard therapy for visceral leishmaniasis in India: an open-label, non-inferiority, randomised controlled trial. Lancet 377:477–486. doi: 10.1016/S0140-6736(10)62050-8. [DOI] [PubMed] [Google Scholar]
- 10.Nicoletti S, Seifert K, Gilbert IH. 2009. N-(2-Hydroxypropyl)methacrylamide-amphotericin B (HPMA-AmB) copolymer conjugates as antileishmanial agents. Int J Antimicrob Agents 33:441–448. doi: 10.1016/j.ijantimicag.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradley DJ, Kirkley J. 1977. Regulation of Leishmania populations within the host. I. The variable course of Leishmania donovani infections in mice. Clin Exp Immunol 30:119–129. [PMC free article] [PubMed] [Google Scholar]
- 12.Aranday-Cortes E, Bull NC, Villarreal-Ramos B, Gough J, Hicks D, Ortiz-Pelaez A, Vordermeier HM, Salguero FJ. 2013. Upregulation of IL-17A, CXCL9 and CXCL10 in early-stage granulomas induced by Mycobacterium bovis in cattle. Transbound Emerg Dis 60:525–537. doi: 10.1111/j.1865-1682.2012.01370.x. [DOI] [PubMed] [Google Scholar]
- 13.Taylor JL, Turner OC, Basaraba RJ, Belisle JT, Huygen K, Orme IM. 2003. Pulmonary necrosis resulting from DNA vaccination against tuberculosis. Infect Immun 71:2192–2198. doi: 10.1128/IAI.71.4.2192-2198.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murray HW. 2001. Tissue granuloma structure-function in experimental visceral leishmaniasis. Int J Exp Pathol 82:249–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smelt SC, Engwerda CR, McCrossen M, Kaye PM. 1997. Destruction of follicular dendritic cells during chronic visceral leishmaniasis. J Immunol 158:3813–3821. [PubMed] [Google Scholar]
- 16.Bankoti R, Stager S. 2012. Differential regulation of the immune response in the spleen and liver of mice infected with Leishmania donovani. J Trop Med 2012:639304. doi: 10.1155/2012/639304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smelt SC, Cotterell SE, Engwerda CR, Kaye PM. 2000. B cell-deficient mice are highly resistant to Leishmania donovani infection, but develop neutrophil-mediated tissue pathology. J Immunol 164:3681–3688. doi: 10.4049/jimmunol.164.7.3681. [DOI] [PubMed] [Google Scholar]
- 18.Maroof A, Brown N, Smith B, Hodgkinson MR, Maxwell A, Losch FO, Fritz U, Walden P, Lacey CN, Smith DF, Aebischer T, Kaye PM. 2012. Therapeutic vaccination with recombinant adenovirus reduces splenic parasite burden in experimental visceral leishmaniasis. J Infect Dis 205:853–863. doi: 10.1093/infdis/jir842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riede O, Seifert K, Oswald D, Endmann A, Hock C, Winkler A, Salguero FJ, Schroff M, Croft SL, Juhls C. 2015. Preclinical safety and tolerability of a repeatedly administered human leishmaniasis DNA vaccine. Gene Ther doi: 10.1038/gt.2015.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Musa AM, Noazin S, Khalil EA, Modabber F. 2010. Immunological stimulation for the treatment of leishmaniasis: a modality worthy of serious consideration. Trans R Soc Trop Med Hyg 104:1–2. doi: 10.1016/j.trstmh.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 21.Dalton JE, Kaye PM. 2010. Immunomodulators: use in combined therapy against leishmaniasis. Expert Rev Anti Infect Ther 8:739–742. doi: 10.1586/eri.10.64. [DOI] [PubMed] [Google Scholar]
- 22.Musa AM, Khalil EA, Mahgoub FA, Elgawi SH, Modabber F, Elkadaru AE, Aboud MH, Noazin S, Ghalib HW, El-Hassan AM. 2008. Immunochemotherapy of persistent post-kala-azar dermal leishmaniasis: a novel approach to treatment. Trans R Soc Trop Med Hyg 102:58–63. doi: 10.1016/j.trstmh.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 23.Nascimento E, Fernandes DF, Vieira EP, Campos-Neto A, Ashman JA, Alves FP, Coler RN, Bogatzki LY, Kahn SJ, Beckmann AM, Pine SO, Cowgill KD, Reed SG, Piazza FM. 2010. A clinical trial to evaluate the safety and immunogenicity of the LEISH-F1+MPL-SE vaccine when used in combination with meglumine antimoniate for the treatment of cutaneous leishmaniasis. Vaccine 28:6581–6587. doi: 10.1016/j.vaccine.2010.07.063. [DOI] [PubMed] [Google Scholar]