Abstract
Vulvovaginal candidiasis (VVC) and recurrent VVC (RVVC) remain major health problems for women. VT-1161, a novel fungal CYP51 inhibitor which has potent antifungal activity against fluconazole-sensitive Candida albicans, retained its in vitro potency (MIC50 of ≤0.015 and MIC90 of 0.12 μg/ml) against 10 clinical isolates from VVC or RVVC patients resistant to fluconazole (MIC50 of 8 and MIC90 of 64 μg/ml). VT-1161 pharmacokinetics in mice displayed a high volume of distribution (1.4 liters/kg), high oral absorption (73%), and a long half-life (>48 h) and showed rapid penetration into vaginal tissue. In a murine model of vaginal candidiasis using fluconazole-sensitive yeast, oral doses as low as 4 mg/kg VT-1161 significantly reduced the fungal burden 1 and 4 days posttreatment (P < 0.0001). Similar VT-1161 efficacy was measured when an isolate highly resistant to fluconazole (MIC of 64 μg/ml) but fully sensitive in vitro to VT-1161 was used. When an isolate partially sensitive to VT-1161 (MIC of 0.12 μg/ml) and moderately resistant to fluconazole (MIC of 8 μg/ml) was used, VT-1161 remained efficacious, whereas fluconazole was efficacious on day 1 but did not sustain efficacy 4 days posttreatment. Both agents were inactive in treating an infection with an isolate that demonstrated weaker potency (MICs of 2 and 64 μg/ml for VT-1161 and fluconazole, respectively). Finally, the plasma concentrations of free VT-1161 were predictive of efficacy when in excess of the in vitro MIC values. These data support the clinical development of VT-1161 as a potentially more efficacious treatment for VVC and RVVC.
INTRODUCTION
Vulvovaginal candidiasis (VVC) is a common mucosal fungal infection in women of childbearing age (1). Epidemiological studies where both yeast cultures and symptoms were confirmed (2, 3) suggest a prevalence of several million infections annually in the United States. This prediction is consistent with the estimate of 10 million annual physician visits due to vaginal symptoms and the approximate percentage of about one-third of such infections being caused by yeast (4). Many of these visits represent multiple vaginal yeast infections in any individual in a given year, with four or more infections per year formally defined as recurrent VVC (RVVC). RVVC is regarded as a chronic condition with a serious impact on quality of life (QOL) (5). A recent survey determined its QOL index score being equal to that for asthma or chronic obstructive pulmonary disease (COPD) and worse than that for headache/migraine (6).
Pharmaceutical treatments of fungal infections are classified by the mechanism of action of a given drug (7). For most mucosal yeast infections such as VVC, the class of choice is the azole antifungal drugs (e.g., fluconazole, itraconazole, clotrimazole, etc.). These drugs target fungal CYP51, which is required for the biosynthesis of lanosterol, a key component of the fungal cell membrane (8). For VVC treatment, azole antifungals are administered both topically and systemically, with both routes being largely successful in treating up to 90% of uncomplicated disease (1). A retrospective review showed no efficacy difference between routes but a preference for oral treatment (9).
Treatment of RVVC is less successful. The commonly prescribed oral fluconazole as a maintenance regimen (5) was shown in a randomized longitudinal clinical study to achieve 91% efficacy of maintaining a clinical remission during 6-month prophylaxis, but only 42% efficacy 6 months after the final dose (10). These data suggest that maintenance fluconazole is largely suppressive in nature (i.e., when therapy is stopped, the symptomatic infection returns) and are consistent with relapse most often being due to the same Candida strain (10, 11).
In addition to the difficulty in treatment of recurrent disease, there are indications that drug resistance is increasing, particularly with regard to C. albicans, and the data also reflect increases in the percentages of Candida spp. that are intrinsically less susceptible to antifungal drugs (12). Candida albicans is the predominant yeast species isolated from VVC patients (1) and is inherently sensitive to fluconazole. However, the percentage of non-albicans Candida species that are inherently less sensitive to fluconazole (e.g., C. glabrata) may be increasing (13, 14). Additionally, a higher percentage of non-albicans Candida species (42%) was measured in RVVC patients than in patients with sporadic infrequent VVC (20%) (15), possibly leading to greater difficulty in treating RVVC due to a higher percentage of species with less susceptibility to azole antifungals.
We have recently described a novel fungal CYP51 inhibitor, VT-1161, (16) that was designed for greater selectivity relative to off-target human cytochrome P450 (CYP) enzymes while retaining the same or greater potency for the fungal CYP51 target (17). The potency of VT-1161 against C. albicans CYP51 in a cellular assay was ≤0.5 nM compared to in vitro 50% inhibitory concentration (IC50) values of ∼100 μM or greater against human CYP51 and key xenobiotic-metabolizing CYPs present in human liver microsomes (e.g., CYP2C9, CYP2C19, and CYP3A4), and its in vitro MIC value against wild-type fluconazole-sensitive C. albicans was 0.002 μg/ml (17). We present here the in vitro MIC potency of VT-1161 against several clinical isolates that have reduced susceptibility or were fully resistant to fluconazole and also the in vivo activity of VT-1161 in a murine model of vaginal candidiasis using fluconazole-sensitive wild-type or select fluconazole-resistant C. albicans isolates.
MATERIALS AND METHODS
For the murine vaginal candidiasis model, female CBA/J mice, 8 to 10 weeks old and weighing ∼20 g, were obtained from the National Institutes of Health (National Cancer Institute [NCI], Frederick, MD). All animals were housed and handled according to institutionally recommended guidelines. All animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the LSU Health Sciences Center. The Candida albicans ATCC 3153A strain was obtained from the ATCC (Rockville, MD). All other C. albicans isolates with various susceptibilities to fluconazole were obtained from the Wayne State Vaginitis Clinic microbiology laboratory organism bank; these isolates were from patients with VVC or RVVC. Cultures were maintained on Sabouraud dextrose agar (Difco Laboratories, Detroit, MI) plates at 4°C and grown to stationary phase in Phytone-peptone broth (overnight culture at 25 C). VT-1161 was supplied by Viamet Pharmaceuticals, Inc. (Durham, NC); estradiol valerate, fluconazole, and Cremaphor EL were purchased from Sigma-Aldrich (St. Louis, MO). Single-use rapid-equilibrium dialysis (RED) plates were from Thermo Fisher Scientific (Waltham, MA). Mouse plasma was from Biochemed (Winchester, VA), and microsomes were from Life Technologies (Grand Island, NY).
In vitro susceptibility test.
Antifungal susceptibility tests were performed using a broth microdilution method, according to CLSI document M27-A3 (18). The range of fluconazole was from 0.125 to 64 μg/ml, and the range of VT-1161 was from 0.015 to 8 μg/ml. A 0.1-ml yeast inoculum of 1.5 (±1.0) × 103 cells/ml in RPMI 1640 medium was added to each microdilution well. The trays were then incubated at 35°C for 48 h. The MICs were read as the lowest antifungal concentration for both VT-1161 and fluconazole with substantially lower turbidity (∼80% growth reduction) than that for growth in the antifungal-free growth well. MICs were determined in duplicate, with either no difference in the values or at most a 1-dilution difference; in the case of 1-dilution differences, the lower value was reported.
Pharmacokinetic studies.
The single-dose oral and intravenous (i.v.) pharmacokinetics (PK) of VT-1161 was determined in mice at TCG Lifesciences (Kolkata, India). A dose of 5 mg/kg VT-1161 in 20% Cremophor EL was administered by oral gavage and a dose of 2 mg/kg VT-1161 in 20% Cremophor EL was administered to BALB/c female mice (age, 6 to 8 weeks; weight, 19 to 21 g) (n = 3/time point), and the plasma concentrations (for oral and i.v. doses) and vaginal tissue concentrations (for oral dose) were determined at 0.5, 1, 2, 4, 8, 24, and 48 h. The pharmacokinetic parameters were determined for individual animals from plasma concentration-time data using noncompartmental modeling (NCA model 201 for intravenous administration or NCA model 200) in WinNonlin Professional (version 5.3; Pharsight Corp., Mountain View, CA). Plasma protein binding was determined at 2.5 μg/ml VT-1161 in triplicate using RED plates for binding dialysis and liquid chromatography/tandem mass spectrometry (LC-MS/MS) for quantification. Microsomal stability was determined in incubations of 1 μM VT-1161 in 1 mg/ml mouse liver microsomes at 37°C at 5, 20, 35, and 65 min, using LC-MS/MS for quantification, and the percentage remaining was referenced to the zero time point.
Murine vaginitis model.
The estrogen-dependent model has been described previously (19). Briefly, animals were injected subcutaneously with estradiol valerate (0.1 mg dissolved in 100 μl sesame oil) 3 days prior to and 4 days after vaginal inoculation. One day prior to inoculation, a blastospore culture of the C. albicans isolate(s) to be used in the study was prepared. On the day of inoculation, blastospores were collected and washed once with phosphate-buffered saline (PBS) and resuspended at 2.5 × 106/ml in PBS for an inoculum of 5 × 104 cells/20 μl PBS. For inoculation, animals were anesthetized “to effect” by isoflurane inhalation. To anesthetized animals, 5 × 104 blastospores in 20 ml PBS were introduced into the vagina, using a Pipetman. Oral drug treatments via once-daily gavages of VT-1161, fluconazole, or vehicle (20% Cremaphor EL) began on day 3 postinoculation and continued through day 6 (i.e., 4 days of treatment). There were 10 animals in each dose group. On day 7, animals were anesthetized, and the vaginal cavity was lavaged with 100 μl of PBS. The lavage fluid was examined microscopically for yeast and cultured for enumeration of organisms (expressed as CFU/100 μl lavage fluid). On day 10, the animals were bled retro-orbitally, humanely sacrificed, and similarly lavaged with subsequent processing for evaluation of the vaginal fungal burden. Blood was processed for plasma and stored for drug-level analyses. In addition, for the first study with wild-type C. albicans, vaginal tissue was collected and quick-frozen for drug-level analyses.
Drug-level analyses.
Each sample was analyzed using LC-MS/MS with electrospray ionization, with quantification against an external calibration curve generated in the same matrix and using the signal response ratio between the sample and the internal standard. Vaginal tissue was homogenized in 50 mM PBS with a Brinkmann Polytron PT10-35 homogenizer fitted with a 12-mm sawtooth generator, and then the compound was extracted with methyl tert-butyl ether (MTBE). The compound was extracted from the plasma samples with MTBE.
Statistical analyses of fungal burden data.
Median values and ranges for fungal burden data were determined for each dose with 10 mice/group. The statistical differences between dose groups were determined by the nonparametric Mann-Whitney U test. Means and standard deviations for plasma concentrations were also determined for each dose group, with no further statistical analyses performed.
RESULTS
VT-1161 MICs against sensitive and resistant C. albicans.
VT-1161 was tested in microdilution assays against two fluconazole-sensitive C. albicans strains (MICs of 0.25 μg/ml for fluconazole) and 10 isolates taken from VVC patients that varied in their in vitro sensitivity to fluconazole (MICs ranged from 2 to 64 μg/ml, with a MIC50 of 8 μg/ml and a MIC90 of 64 μg/ml) (Table 1). For both sensitive strains and 8 of the 10 resistant clinical isolates, the MIC for VT-1161 was ≤0.015 μg/ml (the lowest concentration tested) (Table 1). The MICs for the other two fluconazole-resistant clinical isolates were 0.12 and 2 μg/ml. Therefore, the MIC50 and MIC90 values were ≤0.015 and 0.12 μg/ml, respectively, which were 500-fold more potent than the corresponding values for fluconazole.
TABLE 1.
VT-1161 and fluconazole MICs against clinical isolates from VVC/RVVC patients
| C. albicans isolate/strain | MIC (μg/ml) |
|
|---|---|---|
| VT-1161 | Fluconazole | |
| 3153A (lab strain) | ≤0.015 | 0.25 |
| MR700-13 | ≤0.015 | 0.25 |
| SM692-08 | ≤0.015 | 2 |
| CC330-10 | ≤0.015 | 4 |
| AJ120-06 | ≤0.015 | 8 |
| BR160-09 | ≤0.015 | 8 |
| JJ330-05 | 0.12 | 8 |
| MG787-08 | ≤0.015 | 8 |
| AC398-07 | ≤0.015 | 32 |
| AF313-10 | ≤0.015 | 32 |
| AR466-06 | ≤0.015 | 64 |
| LP1158-07 | 2 | 64 |
Pharmacokinetics of VT-1161 in mice.
The kinetics of VT-1161 were followed in both plasma and vaginal tissue after a single oral dose and in plasma after a single i.v. dose (Fig. 1 and Table 2). VT-1161 was quickly and efficiently absorbed after an oral dose into plasma (oral bioavailability of 73%) and rapidly and fully equilibrated into tissue; at all time points, the vaginal tissue level was at least 2-fold higher than the plasma level (Fig. 1). VT-1161 had a very long half-life after a single oral dose (>48 h); however, due to insufficient time points, an accurate oral half-life was not determined. From the i.v. study, a large volume of distribution (1.4 kg/liter) was calculated, suggesting that VT-1161 equilibrates into most body compartments, which is consistent with the measured high vaginal tissue levels.
FIG 1.
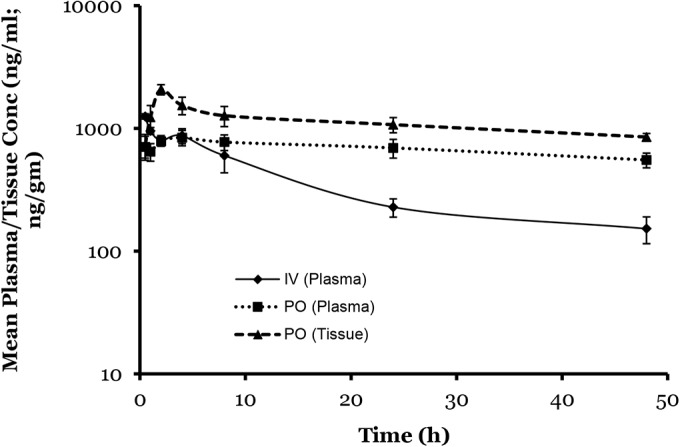
VT-1161 oral and i.v. pharmacokinetics in the mouse. A single dose of VT-1161 was given by oral gavage at 5 (oral) and 2 (i.v.) mg/kg, both in 20% Cremaphor EL, in female BALB/c mice. Plasma and vaginal tissue samples in the oral study and plasma samples in the i.v. study were collected up to 48 h (n = 3 animals per time point). Data points are mean values with error bars representing the standard deviations.
TABLE 2.
In vivo and in vitro pharmacokinetic parameters for VT-1161 in the mouse
| Parametera | Valueb |
|---|---|
| 2 mg/kg i.v. | |
| ∼t1/2 (h) | 11 (2) |
| Vss (liters/kg) | 1.4 (0.2) |
| 5 mg/kg oral | |
| Cmax (μg/ml) | 0.8 (0.2) |
| AUC0−48 h (h · μmol/ml) | 33 (6) |
| ∼t1/2 (h) | >48c |
| F (%) | 73 |
| Plasma protein binding (%) | 97.2 (0.4) |
| Liver microsomal stability (%) | 100d |
t1/2, half-life; Vss, volume of distribution at steady state.
All values are means (SD).
t1/2 values could not be accurately determined due to the lack of data at extended time periods.
All time points showed >92% remaining, with the average value being 98% remaining in comparison to the level seen at the zero time point.
The long half-life can be partly explained by two additional observations. VT-1161 was highly bound to mouse plasma protein (97.2%) and showed virtually no metabolism after 65 min of incubation with mouse liver microsomes (Table 2). Consistent with the long half-life of VT-1161, the plasma concentrations accumulated after repeat dosing. For example, in the rat, which displays an equally long oral half-life for VT-1161, when dosed once daily for 7 days, the maximum concentration of drug in (Cmax) and area under the curve (AUC) exposures increased 6- to 7-fold when parameters derived from data taken on the first day were compared with those from the last day of dosing (E. P. Garvey and R. J. Schotzinger, unpublished data).
VT-1161 efficacy against sensitive C. albicans in murine vaginitis.
VT-1161 was tested at 4, 10, and 25 mg/kg dosed orally once daily for 4 days in a murine model of vaginal candidiasis using the C. albicans laboratory strain 3153A, which was sensitive in vitro to both VT-1161 and fluconazole (Table 1). Fluconazole served as the positive-control comparator and was dosed at 25 mg/kg orally once daily for 4 days. Vehicle was dosed once daily for 4 days as the negative control. The vaginal fungal burden was measured at both 1 and 4 days after treatment, and compound levels were measured 4 days after the last dose in both plasma and vaginal tissue samples (Table 3). The fungal burden in each treatment group was significantly decreased relative to that in the vehicle control on both days of fungal burden analyses (all P < 0.0001, except for 10 mg/kg VT-1161 at 4 days after treatment, P = 0.0002).
TABLE 3.
VT-1161 efficacy in murine vaginal candidiasis with wild-type C. albicans 3153A
| Treatment | Posttreatment CFUa |
Compound levelb |
||
|---|---|---|---|---|
| Day 1 | Day 4 | Plasma (μg/ml) | Vagina (μg/g) | |
| Vehicle | 1,290 (926–2,893) | 15,166 (12,875–23,292) | ||
| FLUc (25 mg/kg) | 0 (0–70); <0.0001 | 0 (0–70); <0.0001 | 0.040 (0.020) | 0.082 (0.046) |
| VT-1161 (4 mg/kg) | 0 (0–13); <0.0001 | 2790 (0–3739); <0.0001 | 0.83 (0.16) | 1.5 (0.3) |
| VT-1161 (10 mg/kg) | 25 (0–143); <0.0001 | 2790 (18–8500); 0.0002 | 4.0 (1.3) | 5.8 (1.0) |
| VT-1161 (25 mg/kg) | 0 (0–140); <0.0001 | 0 (0–108); <0.0001 | 12 (4) | 16 (3) |
Data are median (interquartile range) CFU/100 μl lavage fluid at 1 day or 4 days posttreatment; P value compared to that of vehicle using the Mann-Whitney U test (bold values indicate significance).
Data are mean (SD) levels 4 days posttreatment.
FLU, fluconazole.
Each treatment group showed essentially the same antifungal activity 1 day after treatment. There were no statistically different fungal burdens between any two treatment comparisons (P values ranged between 0.40 and 0.78). Additionally, each group had approximately the same number of animals showing undetectable vaginal lavage CFU (7/10 for 4 mg/kg VT-1161, 4/10 for 10 mg/kg VT-1161, 6/10 for 25 mg/kg VT-1161, and 5/9 for fluconazole). The fungal burdens 4 days after treatment showed fungal “rebound” in the low- and mid-dose VT-1161 groups (Table 3) (P values of 0.0185 and 0.0288 for the comparison of days 1 and 4 posttreatment data for the 4 and 10 mg/kg groups, respectively). Although fungal regrowth occurred, both doses were still superior to that for the vehicle control (P values of < 0.0001 and 0.0002, respectively). The effects with high-dose VT-1161 or fluconazole were sustained through 4 days posttreatment (P < 0.0001 for each compared to vehicle and 0.7394 or 0.6655, respectively, compared with 1-day posttreatment values). The fungal regrowth in the low- and mid-dose VT-1161 groups was also reflected in the numbers of animals that had undetectable CFU on day 4 after treatment (3/10 for low-dose and 1/10 for mid-dose VT-1161 versus 7/10 for high-dose VT-1161 and 7/9 for fluconazole).
The concentrations of drug in both the plasma and vaginal tissue are also shown in Table 3. Based on the long half-life of VT-1161 in mice (Table 2), the high levels of VT-1161 were expected. Assuming that the accumulation of VT-1161 in mouse plasma was similar to the accumulation previously observed in rat, we expect a Cmax of approximately 25 μg/ml after the fourth and final dose of 10 mg/kg VT-1161. With an estimated half-life of 48 h, plasma levels 4 days after the last dose would then be approximately 6 μg/ml (which is similar to the 4 μg/ml measured). Additionally, based on the short half-life of fluconazole in mice (20), the low levels remaining 4 days after the last dose were also expected. Both drugs fully equilibrated into the vaginal tissue from the blood. The VT-1161 vaginal tissue data were consistent with those for the single-dose PK study, and the fluconazole data were consistent with those in a previous publication (21). Finally, it is noted that the strain of mouse used in the PK study (BALB/c) was different from the strain used in the model (CBA/J), and some PK components may differ. However, it appears that many PK parameters (good absorption, long half-life, and high tissue penetration) were similar between the two strains of mice.
VT-1161 efficacy against resistant C. albicans in murine vaginitis.
Initially, a number of clinical isolates from VVC patients that had been tested in vitro (Table 1) were used in a prestudy to confirm infectibility in vivo. All of the isolates tested established robust infections to approximately the same extent as the wild-type isolates. Subsequently, VT-1161 was tested at 25 mg/kg under the same protocol design as that for the wild-type strain against three of the isolates (AR466-06, JJ330-05, and LP-1158-07) resistant to fluconazole (with MIC values of 64, 8, and 64 μg/ml, respectively). (For clarification, a recent EUCAST recommendation defined a fluconazole MIC of >4 μg/ml as being resistant [22].) Fluconazole at 25 mg/kg and vehicle were again the positive comparator and negative controls, respectively, for each isolate. Because of the large number of animals per study, two studies were conducted with two isolates tested in each study. The isolate AR466-06 was tested in each study to determine the reproducibility between studies. The CFU on days 1 and 4 posttreatment and the plasma concentrations 4 days after treatment are shown in Table 4.
TABLE 4.
VT-1161 efficacy in murine vaginal candidiasis using clinical isolates of C. albicans with reduced susceptibility to fluconazole
| Treatment | Posttreatment CFUa |
Plasma compound level (μg/ml)b | |
|---|---|---|---|
| Day 1 | Day 4 | ||
| AR466-06 first study | |||
| Vehicle | 1,300 (868–3,758) | 825 (15–4,413) | |
| FLUc (25 mg/kg) | 1,000 (583–1,488); 0.3042 | 1,100 (525–2,813); 0.5659 | 0.082 (0.020) |
| VT-1161 (25 mg/kg) | 142 (38–298); 0.0019 | 52 (0–136); 0.0610 | 16 (5) |
| P(FLU)d | 0.0064 | <0.0001 | |
| AR466-06 second study | |||
| Vehicle | 3,342 (1,563–14,250) | 290 (73–1,475) | |
| FLU (25 mg/kg) | 1,775 (1,198–6,142); 0.4040 | 1,075 (450–2,388); 0.1594 | 0.11 (0.05) |
| VT-1161 (25 mg/kg) | 450 (66–1,788); 0.0089 | 65 (8–130); 0.0713 | 14 (4) |
| P(FLU) | 0.0431 | 0.0003 | |
| JJ330-05 | |||
| Vehicle | 47,916 (35,250–166,250) | 11,250 (4,288–26,500) | |
| FLU (25 mg/kg) | 1,875 (950–4,500); <0.0001 | 10,300 (5,817–40,458); 0.8111 | 0.039 (0.024) |
| VT-1161 (25 mg/kg) | 4,583 (863–10,625); 0.0003 | 52 (164–2,400); 0.0005 | 16 (3) |
| P(FLU) | 0.2395 | 0.0021 | |
| LP1158-07 | |||
| Vehicle | 19,250 (9,300–213,500) | 4,500 (1,850–8,033) | |
| FLU (25 mg/kg) | 12,000 (4,375–28,667); 0.2727 | 7,000 (3,250–19,500); 0.1889 | 0.061 (0.032) |
| VT-1161 (25 mg/kg) | 7,000 (3,500–13,000); 0.0854 | 18,000 (11,625–39,167); 0.0011 (inferior) | 14 (4) |
| P(FLU) | 0.3050 | 0.0647 | |
Data are median (interquartile range) CFU/100 μl lavage fluid at 1 day or 4 days posttreatment; P value compared to that for vehicle.
Data are mean (SD) levels 4 days posttreatment.
FLU, fluconazole.
P(FLU), P value compared to that for FLU. P values were determined using the Mann-Whitney U test. Bold values indicate significance.
Isolate AR466-06 (VT-1161-sensitive; fluconazole-resistant).
The two independent studies with C. albicans AR466-06 gave reproducible results for both test compounds (Table 4). As expected based on its MIC of ≤0.015 μg/ml and the results above with the C. albicans 3153A strain, VT-1161 showed significant efficacy in both studies at 1 day posttreatment compared to that of either the vehicle control or fluconazole (P values ranging from 0.043 to 0.0019), and a trend toward sustained activity at 4 days posttreatment compared to that of the vehicle control (P values of 0.061 and 0.071) with continued efficacy relative to that of fluconazole (P values < 0.0001 and 0.0003). Also, as expected based on its MIC of 64 μg/ml, fluconazole repeatedly had no effect on the vaginal lavage fungal burden measured at either time point in the two studies (P values of 0.30 and 0.40 for day 1, respectively, and P values of 0.56 and 0.16 for 4 days posttreatment, respectively).
Isolate JJ330-05 (VT-1161-intermediate sensitive; fluconazole-intermediate resistant).
Based on C. albicans JJ330-05 being less susceptible to VT-1161 in vitro (MIC of 0.12 μg/ml), the in vivo antifungal activity of VT-1161 was in question prior to the study. VT-1161 retained significant suppression of the vaginal lavage CFU compared to the vehicle control when measured at either time point (P values of 0.0003 and 0.0005 at 1 day and 4 days posttreatment, respectively) (Table 4). Fluconazole's in vivo activity against this isolate was partial. It was active when the fungal burden was measured at 1 day posttreatment (P < 0.0001); however, when the fungal burden was measured at 4 days posttreatment, no antifungal activity was observed (P = 0.81). These data for partial activity were consistent with its in vitro MIC of 8 μg/ml, which is only slightly higher than the guidelines of >4 μg/ml being considered resistant (22). The statistical comparison between VT-1161 and fluconazole with this isolate was as expected based on the P values versus vehicle control; i.e., no difference was observed for day 1 data (P = 0.24), and VT-1161 was superior for day 4 data (P = 0.0021).
Isolate LP1158-07 (VT-1161-intermediate resistant; fluconazole-resistant).
Whereas no antifungal activity was expected for fluconazole with C. albicans LP1158-07 (MIC of 64 μg/ml), it was again uncertain if VT-1161 would retain in vivo activity with this isolate considering its in vitro activity (MIC of 2 μg/ml). Neither compound had activity significantly different from that of either the vehicle control or each other (Table 4), with P values ranging from 0.065 to 0.30 for 5/6 comparisons. The comparison of the day-4 VT-1161 and vehicle control data showed that the vehicle control's CFU was significantly lower than that for VT-1161 (P = 0.0011). However, given the equivalencies between fluconazole and the vehicle control and VT-1161 and fluconazole at this time point, it is uncertain if this finding is reproducible. Regardless, no analysis indicated that either compound had antifungal efficacy against this isolate.
Plasma drug concentrations.
Plasma levels of VT-1161 and fluconazole in the studies of resistant isolates are shown in Table 4. All values are essentially within the experimental error ranges determined for all of the isolates and of the values determined in the first study using C. albicans 3153A (Table 3). This interstudy reproducibility is consistent with the relatively small standard deviations observed within each study, which reflect low interanimal variability (Tables 3 and 4). Together, these data indicate highly reproducible pharmacokinetics for both drugs.
DISCUSSION
Vulvovaginal candidiasis is one of the most prevalent fungal infections for which patients seek medical treatment (1), and recurrent VVC is the most troublesome form of the disease in both discomfort (6) and difficulty of treatment (10). Although the exact prevalence is unclear (23), RVVC has been estimated to occur in as many as 9% of women living in Europe and the United States (24). The current standard of care for RVVC is maintenance fluconazole, which has only a 42% success rate of maintaining a disease-free state for 6 months after treatment (10). New therapies with greater efficacy are needed. In addition, because of the need for treatment over extended periods of time for this chronic condition, new therapies ideally would be dosed infrequently and have few, if any, side effects.
VT-1161 is a novel fungal CYP51 inhibitor that has completed phase 2a clinical studies on both superficial and mucosal fungal infections (registration no. NCT01891305 and NCT01891331, respectively; www.clinicaltrials.gov). It was rationally designed to be highly selective relative to human CYP enzymes while maintaining potent inhibition of the fungal CYP target (16, 17). Its intrinsic in vitro antifungal potency against susceptible C. albicans was reproduced in this study where the MIC was at or below the lowest concentration tested of 0.015 μg/ml. In addition, VT-1161 maintained this in vitro potency against 8 out of 10 clinical isolates that showed various degrees of resistance to fluconazole (2 to 64 μg/ml). A much larger number of sensitive and resistant clinical isolates are required to fully characterize the in vitro antifungal activity of VT-1161. However, these data coupled with MIC data from other laboratories (16, 17) and the biochemical data demonstrating nanomolar potency against the CYP51 target (17) indicate that VT-1161 is one of the most potent CYP51 inhibitors of C. albicans yet described.
Coupled with this in vitro antifungal activity, VT-1161 showed excellent oral pharmacokinetics in the mouse; the oral absorption was 73% with a high volume of distribution consistent with the high levels measured in the vaginal tissue. Consistent with a long oral PK half-life (>48 h), VT-1161 was highly bound to mouse plasma protein and did not show any metabolism in mouse microsome incubations. These mouse PK and in vitro characteristics were consistent with those observed in the guinea pig (25) and in the rat, dog, and human (Garvey and Schotzinger, unpublished data).
Given its intrinsic antifungal potency, safety, and pharmacokinetics, VT-1161 is an ideal candidate for treating a number of diverse candidal infections (e.g., invasive, mucosal, and cutaneous). Specifically, it was a prime candidate to test in a stringent murine model of vaginal candidiasis where infection is allowed to establish itself for 3 days prior to treatment (19). In the initial study using a fluconazole-sensitive strain of C. albicans, oral doses as low as 4 mg/kg VT-1161 were highly statistically efficacious in reducing the fungal burdens in vaginal lavage samples at both 1 and 4 days after the last treatment. The two lower-dose groups of VT-1161 showed some rebound of fungal growth on day 4 of evaluation. However, even with these increases, the values were highly statistically suppressed relative to those for the vehicle controls. The dose of 25 mg/kg VT-1161 showed no regrowth at day 4 relative to day 1, in a comparison of either day 1 and day 4 posttreatment mean values for fungal burden or the numbers of animals that had undetectable fungus in the lavage samples. Additionally, in a direct comparison at the same dose of 25 mg/kg, VT-1161 was equivalent to fluconazole in this model, again both in a comparison of the mean values for fungal burdens and in the numbers of animals that had undetectable fungus.
Plasma concentrations of VT-1161 after 4 days of dosing and another 4 days off drug were similar to expectations based on the approximate half-life of each drug (i.e., relatively high for VT-1161 and low for fluconazole). VT-1161 concentrations were also determined in the vaginal tissue and at each dose showed full equilibration between the plasma and vagina tissue, consistent with the single-dose PK study and high volume of distribution.
VT-1161 largely retained in vitro activity against fluconazole-resistant clinical isolates, and we hypothesized that VT-1161 would suppress fungal growth in vivo against isolates that were fully sensitive to VT-1161. However, it was unclear if VT-1161 could suppress in vivo growth of isolates less susceptible to it. As would be predicted based on MIC values reflecting full sensitivity to VT-1161 (≤0.015 μg/ml) and full resistance to fluconazole (64 μg/ml), VT-1161 was reproducibly efficacious in suppressing in vivo growth of the AR466-06 isolate, whereas fluconazole was inactive. When the JJ330-05 isolate with intermediate susceptibility was used, VT-1161 (MIC of 0.12 μg/ml) remained highly efficacious relative to the vehicle control. Fluconazole (MIC of 8 μg/ml) had “partial” in vivo activity insofar as being able to suppress growth 1 day after treatment but did not show sustained effects 4 days after treatment. Finally, neither inhibitor had any effect on growth measured either 1 or 4 days after treatment with the isolate LP-1158-07 (MICs of 2 and 64 μg/ml for VT-1161 and fluconazole, respectively). The plasma levels of both drugs were very similar in all of these studies with relatively low interanimal variability, demonstrating reproducibility of their oral pharmacokinetics.
The above data can be used to predict what drug concentrations should be targeted to achieve VT-1161 in vivo efficacy. For invasive fungal infections, it is widely accepted that the plasma concentration of free drug is the most relevant value and that the area under the curve (AUC) is the most relevant PK parameter (26). If the relevance of free drug is also true for mucosal infections such as VVC, then VT-1161 binding to mouse plasma (97.2%) needs to be considered in analyzing these data. Because only single-point plasma levels were determined in these studies, the AUC values could not be calculated. During the 4 days of dosing, VT-1161 accumulated to reach a Cmax after the last dose and then was slowly eliminated. Using the approximate half-life of VT-1161 in the mouse of 48 h, the Cmax after the last dose is estimated to be ∼4 times higher than the concentration measured 4 days later. This estimated range of the total plasma concentration can be multiplied by 0.029 to obtain an estimate of free drug, and both total and free levels can then be compared to the intrinsic MIC potency for each isolate examined in these studies.
With this approach, both total and free plasma concentrations of VT-1161 were above the in vitro MIC values for the wild-type, AR466-06, and JJ330-05 isolates, consistent with the antifungal efficacy observed in those studies, but not distinguishing between total and free VT-1161 concentrations. Whereas the concentration range of total VT-1161 in the LP-1158-07 study was well above the MIC against the isolate (2 μg/ml), the range of free VT-1161 was below, and thus correlated with the lack of efficacy observed with LP-1158-07. Additionally, the predicted total vaginal tissue levels were well above the MIC of 2 μg/ml for this insensitive isolate. Therefore, taken together, these data indicate that free VT-1161 in the plasma was the best predictor of efficacy in this murine model of vaginal candidiasis.
In summary, the in vivo efficacy of VT-1161 in the murine model of vaginal candidiasis provides strong support toward clinical development of this agent in treating VVC. Likewise, the excellent in vitro and in vivo activity profiles that VT-1161 displayed against resistant C. albicans bode well for its ability to treat infections caused by isolates that are resistant to currently approved CYP51 inhibitor drugs. The strong correlate of efficacy to free drug in plasma based on the MIC allows for possible monitoring for more accurate dosing that may result in even fewer treatments. Furthermore, because of the high selectivity of VT-1161 for its fungal CYP target relative to that for off-target human CYP enzymes (17), few if any side effects are expected. Finally, because of the long half-life of VT-1161, infrequent dosing regimens such as once-weekly maintenance dosing after an initial loading dose are attractive regimens for chronic infections such as RVVC. Given all of the above, a phase 2b study of VT-1161 treatment of RVVC using a loading dose/maintenance dose regimen is now in progress (registration no. NCT02267382; www.clinicaltrials.gov).
ACKNOWLEDGMENTS
All LC-MS/MS drug level measurements were done at OpAns, LLC (Durham, NC).
All work described in the manuscript was supported by Viamet Pharmaceuticals, Inc. (Durham, NC).
REFERENCES
- 1.Sobel JD. 2007. Vulvovaginal candidosis. Lancet 369:1961–1971. doi: 10.1016/S0140-6736(07)60917-9. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad A, Khan AU. 2009. Prevalence of Candida species and potential risk factors for vulvovaginal candidiasis in Aligarh, India. Eur J Obstet Gynecol Reprod Biol 144:68–71. doi: 10.1016/j.ejogrb.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 3.Rathod SD, Klausner JD, Krupp K, Reingold AL, Madhivanan P. 2012. Epidemiologic features of vulvovaginal candidiasis among reproductive-age women in India. Infect Dis Obstet Gynecol 2012:859071. doi: 10.1155/2012/859071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kent HL. 1991. Epidemiology of vaginitis. Am J Obstet Gynecol 165:1168–1176. doi: 10.1016/S0002-9378(12)90722-X. [DOI] [PubMed] [Google Scholar]
- 5.Sobel JD. 2006. Management of recurrent vulvovaginal candidiasis: unresolved issues. Curr Infect Dis Rep 8:481–486. doi: 10.1007/s11908-006-0023-7. [DOI] [PubMed] [Google Scholar]
- 6.Aballéa S, Guelfucci F, Wagner J, Khemiri A, Dietz J-P, Sobel J, Toumi M. 2013. Subjective health status and health-related quality of life among women with recurrent vulvovaginal candidiasis (RVVC) in Europe and the U S A. Health Qual Life Outcomes 11:169. doi: 10.1186/1477-7525-11-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen CA, Sorrell TC. 2007. Antifungal agents. Med J Aust 187:404–409. [DOI] [PubMed] [Google Scholar]
- 8.Fromtling RA. 1988. Overview of medically important antifungal azole derivatives. Clin Microbiol Rev 1:187–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watson MC, Grimshaw JM, Bond CM, Mollison J, Ludbrook A. 2002. Oral versus intra-vaginal imidazole and triazole anti-fungal agents for the treatment of uncomplicated vulvovaginal candidiasis (thrush): a systematic review. BJOG 109:85–95. doi: 10.1111/j.1471-0528.2002.01142.x. [DOI] [PubMed] [Google Scholar]
- 10.Sobel JD, Wisenfeld HC, Martens M, Danna P, Hooton TM, Rompalo A, Sperling M, Livengood C III, Horowitz B, Von Troon J, Edwards L, Panzer H, Chu T-C. 2004. Maintenance fluconazole therapy for recurrent vulvovaginal candidiasis. N Engl J Med 351:876–883. doi: 10.1056/NEJMoa033114. [DOI] [PubMed] [Google Scholar]
- 11.El-Din SS, Reynolds MT, Ashbee HR, Barton RC, Evans EG. 2001. An investigation into the pathogenesis of vulvo-vaginal candidiasis. Sex Transm Infect 77:179–183. doi: 10.1136/sti.77.3.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marchaim D, Lemanek L, Bheemreddy S, Kaye KS, Sobel JD. 2012. Fluconazole-resistant Candida albicans vulvovaginitis. Obstet Gynecol 120:1407–1414. doi: 10.1097/AOG.0b013e31827307b2. [DOI] [PubMed] [Google Scholar]
- 13.Buitrón García-Figueroa R, Araiza-Santibanez J, Basurto-Kuba E, Bonitaz-Trujillo A. 2009. Candida glabrata: an emergent opportunist in vulvovaginitis. Cir Cir 77:423–427. [PubMed] [Google Scholar]
- 14.Kumari V, Banerjee T, Kumar P, Pandey S, Tilak R. 2013. Emergence of non-albicans Candida among candidal vulvovaginitis cases and study of their potential virulence factors, from a tertiary care center, North India. Indian J Pathol Microbiol 56:144–147. doi: 10.4103/0377-4929.118703. [DOI] [PubMed] [Google Scholar]
- 15.Richter SS, Galask RP, Messer SA, Hollis RJ, Diekema DJ, Pfaller MA. 2005. Antifungal susceptibility of Candida species causing vulvovaginitis and epidemiology of recurrent cases. J Clin Microbiol 43:2155–2162. doi: 10.1128/JCM.43.5.2155-2162.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoekstra WJ, Garvey EP, Moore WR, Rafferty SW, Yates CM, Schotzinger RJ. 2014. Design and optimization of highly-selective fungal CYP51 inhibitors. Bioorg Med Chem Lett 24:3455–3458. doi: 10.1016/j.bmcl.2014.05.068. [DOI] [PubMed] [Google Scholar]
- 17.Warrilow AGS, Martel CM, Parker JE, Garvey EP, Hoekstra WJ, Moore WR, Schotzinger RJ, Kelly DE, Kelly SL. 2014. The clinical candidate VT-1161 is a highly potent inhibitor of Candida albicans CYP51 but fails to bind the human enzyme. Antimicrob Agents Chemother 58:7121–7127. doi: 10.1128/AAC.03707-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard, 3rd ed CLSI document M27-A3. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 19.Fidel PL Jr, Cutright JL, Sobel JD. 1997. Efficacy of D0870 treatment of experimental Candida vaginitis. Antimicrob Agents Chemother 41:1455–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Humphrey MJ, Jevons S, Tarbit MH. 1985. Pharmacokinetic evaluation of UK-49,858, a metabolically stable triazole antifungal drug, in animals and humans. Antimicrob Agents Chemother 28:648–653. doi: 10.1128/AAC.28.5.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Houang ET, Chappatte O, Byrne D, Macrae PV, Thorpe JE. 1990. Fluconazole levels in plasma and vaginal secretions of patients after a 150-milligram single oral dose and rate of eradication of infection in vaginal candidiasis. Antimicrob Agents Chemother 34:909–910. doi: 10.1128/AAC.34.5.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cuesta I, Bielza C, Larrangag P, Cuenca-Estrella M, Laguna F, Rodriguez-Pardo D, Almirante B, Pahissa A, Rodriguez-Tudela JL. 2009. Data mining validation of fluconazole breakpoints established by the European Committee on Antimicrobial Susceptibility Testing. Antimicrob Agents Chemother 53:2949–2954. doi: 10.1128/AAC.00081-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rathod SD, Buffler PA. 2014. Highly-cited estimates of the cumulative incidence and recurrence of vulvovaginal candidiasis are inadequately documented. BMC Women's Health 14:43. doi: 10.1186/1472-6874-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foxman B, Muraglia R, Dietz JP, Sobel JD, Wagner J. 2013. Prevalence of recurrent vulvovaginal candidiasis in 5 European countries and the United States: results from an Internet panel survey. J Low Genit Tract Dis 17:340–345. doi: 10.1097/LGT.0b013e318273e8cf. [DOI] [PubMed] [Google Scholar]
- 25.Garvey EP, Hoekstra WJ, Moore WR, Schotzinger RJ, Long L, Ghannoum MA. 2015. VT-1161 dosed once daily or once weekly exhibits potent efficacy in treatment of dermatophytosis in a guinea pig model. Antimicrob Agents Chemother 59:1992–1997. doi: 10.1128/AAC.04902-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andes D. 2006. Pharmacokinetics and pharmacodynamics of antifungals. Infect Dis Clin North Am 20:676–697. doi: 10.1016/j.idc.2006.06.007. [DOI] [PubMed] [Google Scholar]


