Abstract
Methicillin-resistant Staphylococcus aureus (MRSA) isolates have arisen with reduced susceptibility to several anti-MRSA agents. Telavancin (TLV), a novel anti-MRSA agent, retains low MICs against these organisms. Our objective was to determine the MICs for TLV, daptomycin (DAP), vancomycin (VAN), and linezolid (LZD) against daptomycin-nonsusceptible (DNS) S. aureus, vancomycin-intermediate S. aureus (VISA), heteroresistant VISA (hVISA), and linezolid-resistant (LZDr) S. aureus. We also evaluated these agents against each phenotype in pharmacokinetic/pharmacodynamic (PK/PD) models. Seventy DNS, 100 VISA, 180 hVISA, and 25 LZDr MRSA isolates were randomly selected from our library and tested to determine their MICs against TLV, DAP, VAN, and LZD via broth microdilution and a Trek panel. Four isolates were randomly selected for 168-h in vitro models to evaluate treatment with TLV at 10 mg/kg of body weight/day, DAP at 10 mg/kg/day, VAN at 1 g every 12 h (q12h), and LZD at 600 mg q12h. The MIC50/90 for TLV, DAP, VAN, and LZD against 70 DNS S. aureus isolates were 0.06/0.125 μg/ml, 2/4 μg/ml, 1/2 μg/ml, and 2/2 μg/ml, respectively. Against 100 VISA isolates, the MIC50/90 were 0.06/0.125 μg/ml, 1/1 μg/ml, 4/8 μg/ml, and 1/2 μg/ml, respectively. Against 170 hVISA isolates, the MIC50/90 were 0.06/0.125 μg/ml, 0.5/1 μg/ml, 1/2 μg/ml, and 1/2 μg/ml, respectively. Against 25 LZDr isolates, the MIC50/90 were 0.03/0.06 μg/ml, 1/1 μg/ml, 2/2 μg/ml, and 8/8 μg/ml, respectively. The TLV MIC was >0.125 μg/ml for 10/365 (2.7%) isolates. In PK/PD models, TLV was universally bactericidal at 168 h and statistically superior to all antibiotics against DNS S. aureus strain R2334. These data further establish the potency of TLV against resistant MRSA. The model data demonstrate in vitro bactericidal activity of TLV against hVISA, VISA, DNS S. aureus, and LZDr S. aureus strains. Further clinical research is warranted.
INTRODUCTION
Telavancin is a semisynthetic lipoglycopeptide derived from vancomycin, with in vitro activity against Gram-positive bacterial pathogens, including methicillin-resistant Staphylococcus aureus (MRSA). Unlike vancomycin, however, telavancin demonstrates activity against MRSA strains with reduced susceptibility to vancomycin, including heterogeneous vancomycin-intermediate S. aureus (hVISA) and vancomycin-intermediate S. aureus (VISA) (1, 2). Telavancin also demonstrates activity against daptomycin-nonsusceptible (DNS) S. aureus isolates, making it a valuable tool against these periodically reported strains (3, 4). Recent surveillance data indicate 100% susceptibility among >9,500 S. aureus strains from 28 hospitals in the United States, with demonstrated MIC50/90 of 0.03/0.06 μg/ml (5). In this study, telavancin possessed MICs that were 8-fold and 32-fold less than those for daptomycin and vancomycin, respectively. The improved activity and potency of telavancin compared to that of vancomycin is assumed to be related to the dual mechanism of action of telavancin involving both membrane-bound lipid II binding and cell membrane depolarization effects, and it is evidenced by the increased activity of telavancin at lower concentrations (6, 7).
Although extensive global surveillance data exist evaluating telavancin MICs on several thousand S. aureus strains, there are limited data regarding MRSA strains with reduced susceptibility to vancomycin, daptomycin, and linezolid. The purpose of this study was to determine the MICs of telavancin, daptomycin, vancomycin, and linezolid against 365 strains of MRSA that were either DNS, hVISA, VISA, or linezolid resistant (LZDr) to more accurately describe the MIC50/90 of telavancin against isolates that may necessitate its use clinically. The telavancin MICs of each strain were determined via broth microdilution and the Trek Sensititre MIC panel to determine the similarities between these methods (8). We also sought to evaluate telavancin, daptomycin, vancomycin, and linezolid against representative isolates from each of the resistant phenotypes in one-compartment pharmacokinetic/pharmacodynamic (PK/PD) models to determine the optimal regimen for translation to therapeutic efficacy.
MATERIALS AND METHODS
Bacterial strains.
Isolates of MRSA, including 70 DNS, 170 hVISA, 100 VISA, and 25 LZDr strains (total, 365 discrete isolates), were randomly selected from the Anti-Infective Research Laboratory (ARL) (Detroit, MI) strain library. All hVISA strains were proven to be so using the gold standard modified population analysis profile (PAP) (9). One representative strain from each of the four resistant phenotypes (R6669 for hVISA, NJ992 for VISA, R2334 for DNS, and R6246 for LZDr) was randomly chosen for further evaluation in a one-compartment in vitro PK/PD model.
Antimicrobials and media.
Telavancin analytical powder was provided by Theravance Biopharma Antibiotics, Inc. (George Town, Cayman Islands). Vancomycin and oxacillin were purchased from a commercial source (Sigma Chemical Company, St. Louis, MO). Daptomycin and linezolid were purchased from commercial sources (Cubist Pharmaceuticals, Lexington, MA, and Pfizer, Inc., New York, NY, respectively).
In vitro experiments were performed in Mueller-Hinton broth (MHB) (Difco, Detroit, MI) supplemented with 25 mg/liter calcium and 12.5 mg/liter magnesium. MHB was supplemented with 0.002% polysorbate 80 (Tween 80; Sigma Chemical Company, St. Louis, MO) for all experiments involving telavancin (10). Due to the calcium-dependent nature of daptomycin, MHB supplemented with a total of 50 mg/liter calcium was used for susceptibility testing and in vitro models. Sodium chloride was added to the media at a final concentration of 2% to accurately determine susceptibility to oxacillin, as recommended by the Clinical and Laboratory Standards Institute (CLSI) guidelines (10). Colony counts were determined using tryptic soy agar (TSA) (Difco) plates. Brain heart infusion agar (BHIA) (Difco Laboratories, San Jose, CA) supplemented with vancomycin was used to subculture VISA strains in order to maintain this phenotype. BHIA or Mueller-Hinton agar (MHA) (Difco), supplemented with 3× the MIC of telavancin, was used to screen for the emergence of resistance from the PK/PD models.
Susceptibility testing.
The MICs of the studied antimicrobials were determined in duplicate by broth microdilution at approximately 106 CFU/ml, according to CLSI guidelines (10). Telavancin MICs were determined in duplicate, according to recent CLSI guidelines, incorporating 0.002% polysorbate 80 into dilution broth and via Trek panels revised to align with the 2014 CLSI guidelines (10). MICs determined via the Trek panel were compared to those obtained via broth microdilution. Along with telavancin, MICs for daptomycin, vancomycin, linezolid, oxacillin, quinupristin-dalfopristin, clindamycin, ciprofloxacin, tigecycline, erythromycin, gentamicin, and trimethoprim-sulfamethoxazole were tested via the Trek panel (see Table 3). All samples were incubated at 35°C for 18 to 24 h before being read.
TABLE 3.
MIC50/90 of other antimicrobials tested via Trek panel against all 365 isolates
| Antimicrobiala | MICs (μg/ml) forb: |
|||||||
|---|---|---|---|---|---|---|---|---|
| hVISA |
VISA |
DNS S. aureus |
LZDr
S. aureus |
|||||
| MIC50 | MIC90 | MIC50 | MIC90 | MIC50 | MIC90 | MIC50 | MIC90 | |
| DAP | 0.5 | 1 | 1 | 1 | 2 | 4 | 1 | 1 |
| VAN | 1 | 2 | 4 | 8 | 1 | 2 | 2 | 2 |
| LZD | 1 | 2 | 1 | 2 | 2 | 2 | 8 | 8 |
| OXA | >4 | >4 | >4 | >4 | >4 | >4 | >4 | >4 |
| QD | 0.25 | 0.25 | 0.25 | 0.5 | 0.25 | 0.5 | 0.25 | 0.25 |
| CLI | 2 | >4 | 4 | >4 | >4 | >4 | 4 | >4 |
| CIP | 0.25 | >8 | 1 | >8 | >8 | >8 | 4 | >8 |
| TIG | 0.03 | 0.03 | 0.03 | 0.06 | 0.06 | 0.12 | 0.06 | 0.06 |
| ERY | >16 | >16 | >16 | >16 | >16 | >16 | >16 | >16 |
| GEN | 0.5 | 1 | 0.5 | 1 | 0.5 | 4 | 1 | 1 |
| TS | ≤0.5/9.5 | ≤0.5/9.5 | ≤0.5/9.5 | 1/19 | ≤0.5/9.5 | 1/19 | ≤0.5/9.5 | ≤0.5/9.5 |
DAP, daptomycin; VAN, vancomycin; LZD, linezolid; OXA, oxacillin; QD, quinupristin-dalfopristin; CLI, clindamycin; CIP, ciprofloxacin; TIG, tigecycline; ERY, erythromycin; GEN, gentamicin; TS, trimethoprim-sulfamethoxazole.
VISA, vancomycin-intermediate S. aureus; hVISA, heteroresistant VISA; DNS, daptomycin nonsusceptible; LZDr, linezolid resistant.
In vitro PK/PD model.
An in vitro one-compartment PK/PD model with a 250-ml capacity and input and outflow ports was used. The apparatus was prefilled with medium, and antimicrobials were administered as boluses over a 168-h period. Prior to each experiment, bacterial lawns from an overnight growth on TSA were suspended and added to each model to obtain a starting inoculum of ∼107 CFU/ml. Fresh medium was continuously supplied and removed from the compartment, along with the drug, via peristaltic pump (Masterflex; Cole-Parmer Instrument Company, Chicago, IL) at an appropriate rate to simulate the average human clearance and half-lives of the antimicrobials. The antimicrobial regimens evaluated were simulations of telavancin at 10 mg/kg of body weight every 24 h (targeted maximum free drug concentration [fCmax], 10.8 μg/ml; average half-life [t1/2], 8.1 h; protein binding, 90%; free drug area under the concentration-time curve from 0 to 24 h [fAUC0–24], 110 μg · h/ml) for 168 h, daptomycin at 10 mg/kg every 24 h (targeted fCmax, 11.3 μg/ml; t1/2, 8 h; protein binding, 92%; fAUC0–24, 115 μg · h/ml) for 168 h, vancomycin at 1,000 mg every 12 h (targeted fCmax, 15.75 μg/ml; t1/2, 6 h; protein binding, 55%; fAUC0–24, 218 μg · h/ml) for 168 h, linezolid at 600 mg every 12 h (targeted fCmax, 10.4 μg/ml; t1/2, 5 h; protein binding, 31%) for 168 h, and a drug-free growth control for 168 h mimicking the pharmacokinetics of telavancin (11–14). The models were performed in duplicate to ensure reproducibility.
Pharmacodynamic analysis.
Samples from each model were collected at 0, 4, 8, 24, 32, 48, 72, 96, 120, 144, and 168 h in duplicate and diluted in 0.9% saline. Colony counts were determined by spiral plating appropriate dilutions using an automatic spiral plater to enumerate the CFU per milliliter and avoid antibiotic carryover. Colonies were counted using a laser colony counter. If the anticipated dilution was near the MIC, vacuum filtration was used to avoid antibiotic carryover. When vacuum filtration was used, samples were washed through a 0.45-μm-pore-size filter with normal saline to remove the antimicrobial agent. For both methods, bacteria were plated on TSA and incubated at 35°C for 24 h before being read. These methods have a lower limit of reliable detection of 2 log10 CFU/ml. The total reduction in log10 CFU/ml over 168 h was determined by plotting model time-kill curves based on the number of remaining organisms over the 168-h period. Bactericidal activity (99.9% kill) was defined as a ≥3-log10 CFU/ml decrease in colony count from that of the initial inoculum. Bacteriostatic activity was defined as a <3-log10 CFU/ml reduction in colony count from that of the initial inoculum.
Pharmacokinetic analysis.
Pharmacokinetic samples were obtained through the injection port of each model at appropriate time points during bacterial model runs for the verification of target antibiotic concentrations. All samples were stored at −70°C until ready for analysis. Telavancin and concentrations were determined using a microbioassay with Kocuria rhizophila (formerly Micrococcus luteus) strain ATCC 9341. Blank 0.25-in.-diameter disks were spotted with 10 μl of standard concentrations or pharmacokinetic samples. Each standard was tested in duplicate by placing the disk on agar plates (antibiotic medium no. 11; Difco, Detroit, MI) and inoculated with a 0.5 McFarland standard suspension of the test organism. Vancomycin concentrations were determined using a fluorescence polarization immunoassay (TDX assay; Abbott Diagnostics). Concentrations of daptomycin and linezolid were determined using a validated high-performance liquid chromatography (HPLC) assay. The half-life, area under the curve from 0 to 24 h (AUC0–24 h), and peak concentrations were determined using the PK Analyst software (version 1.10; MicroMath Scientific Software, Salt Lake City, UT) using the trapezoidal method to calculate the AUC.
Resistance.
The emergence of resistance was evaluated at 168 h by plating 100-μl samples from the model on plates supplemented with telavancin at 3× the MIC. The plates were examined for growth after 24 and 48 h of incubation at 35°C. Resistant colonies grown on screening plates were evaluated by Etest or broth microdilution methods to determine the MIC. If resistance was detected at the end of the model, additional screening was performed to identify the first occurrence of resistance.
Statistical analysis.
Changes in CFU/ml at 168 h were compared by one-way analysis of variance (ANOVA) with Tukey's post hoc test. Descriptive statistics were used to compare differences between broth microdilution and Trek panel MICs for telavancin. A P value of ≤0.05 was considered significant. All statistical analyses were performed using the SPSS Statistical Software (release 21.0; SPSS, Inc., Chicago, IL).
RESULTS
Susceptibility testing.
The broth microdilution MICs of telavancin against all organisms are listed in Table 1, along with the MICs from the Trek panel. Based on CLSI interpretive criteria, all hVISA and LZDr isolates were susceptible to telavancin, as 100% of the isolates possessed MICs of ≤0.125 μg/ml (10). Using broth microdilution, 8/100 (8%) VISA isolates possessed a telavancin MIC of 0.25 μg/ml, and 1/100 (1%) possessed a telavancin MIC of 0.5 μg/ml. One (1.5%) of 70 DNS isolates possessed an MIC of 0.25 μg/ml. Using the Trek panel, 5/100 (5%) VISA isolates possessed a telavancin MIC of 0.25 μg/ml. In total, the broth microdilution telavancin MICs were one dilution higher in 93 (25%), the same in 238 (65%), one dilution lower in 32 (9%), and two dilutions higher in 2 (1%) of the S. aureus isolates (Table 2). The MICs for the strains evaluated in PK/PD model experiments are listed in Fig. 1. Trek panel MICs of antibiotics other than telavancin against tested MRSA are listed in Table 3.
TABLE 1.
Broth microdilution and Trek panel telavancin MIC distributions against hVISA, VISA, DNS S. aureus, and LZDr S. aureusa
| Organism by MIC determination method (no. of isolates) | MIC (μg/ml) |
No. (cumulative %) of isolates inhibited at MIC (μg/ml) of: |
||||||
|---|---|---|---|---|---|---|---|---|
| 50% | 90% | ≤0.015 | 0.03 | 0.06 | 0.125 | 0.25 | 0.5 | |
| Broth microdilution | ||||||||
| hVISA (170) | 0.06 | 0.12 | 10 (6) | 94 (61) | 44 (87) | 22 (100) | 0 (100) | 0 (100) |
| VISA (100) | 0.06 | 0.12 | 2 (2) | 18 (20) | 31 (51) | 40 (91) | 8 (99) | 1 (100) |
| DNS S. aureus (70) | 0.06 | 0.12 | 0 (0) | 14 (20) | 28 (60) | 27 (98) | 1 (100) | 0 (100) |
| LZDr S. aureus (25) | 0.03 | 0.06 | 3 (12) | 13 (64) | 8 (96) | 1 (100) | 0 (100) | 0 (100) |
| Trek panel | ||||||||
| hVISA (170) | 0.03 | 0.06 | 20 (12) | 98 (70) | 38 (92) | 14 (100) | 0 (100) | 0 (100) |
| VISA (100) | 0.06 | 0.12 | 4 (4) | 22 (26) | 40 (66) | 29 (95) | 5 (100) | 0 (100) |
| DNS S. aureus (70) | 0.06 | 0.12 | 0 (0) | 13 (19) | 36 (70) | 21 (100) | 0 (100) | 0 (100) |
| LZDr S. aureus (25) | 0.03 | 0.12 | 0 (0) | 18 (72) | 4 (88) | 3 (100) | 0 (100) | 0 (100) |
VISA, vancomycin-resistant S. aureus; hVISA, heteroresistant VISA; DNS, daptomycin nonsusceptible; LZD-R, linezolid resistant.
TABLE 2.
Broth microdilution MICs compared to Trek panel MICs
| Organism (no. of isolates)a | No. (%)_of isolates with given broth microdilution MIC dilution difference compared to Trek |
|||
|---|---|---|---|---|
| +2 | +1 | ±0 | −1 | |
| hVISA (170) | 2 (1) | 39 (23) | 118 (70) | 11 (6) |
| VISA (100) | 0 (0) | 29 (29) | 70 (70) | 1 (1) |
| DNS S. aureus (70) | 0 (0) | 20 (29) | 36 (51) | 14 (20) |
| LZDr S. aureus (25) | 0 (0) | 5 (20) | 14 (56) | 6 (24) |
VISA, vancomycin-resistant S. aureus; hVISA, heteroresistant VISA; DNS, daptomycin nonsusceptible; LZD-R, linezolid resistant.
FIG 1.
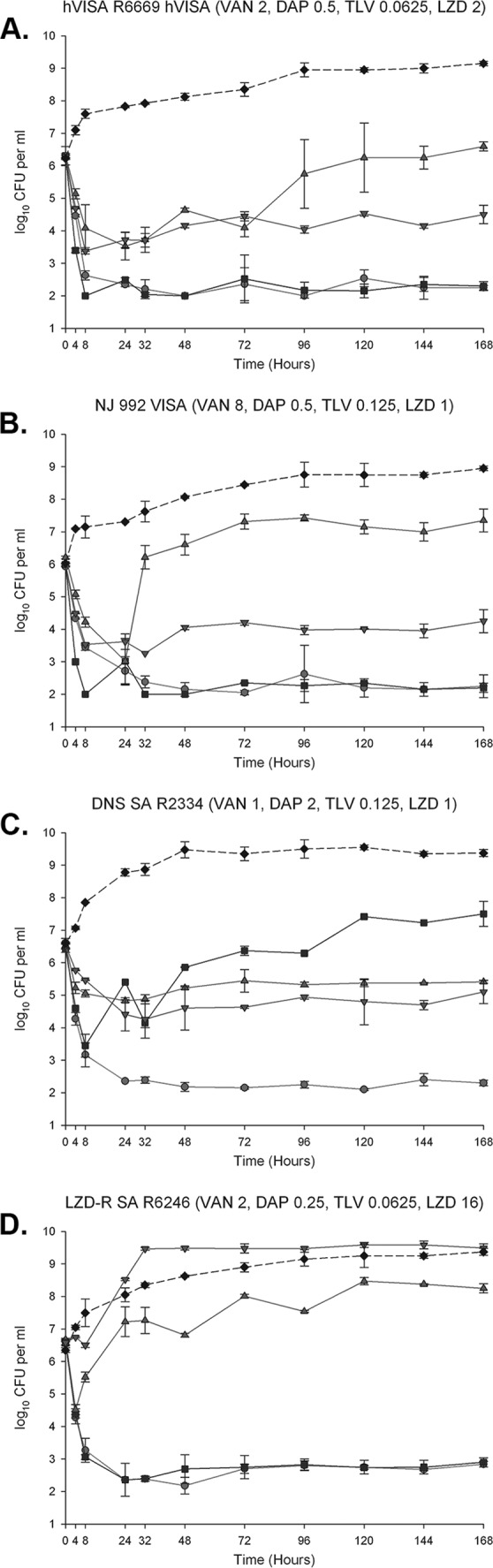
One hundred sixty-eight-hour one-compartment PK/PD model. Solid circles, TLV at 10 mg/kg/day; solid squares, DAP at 10 mg/kg/day; triangles, VAN at 1 g q12h; inverted triangles, LZD at 600 mg q12h; solid diamonds, growth control. Shown are R6669 hVISA (A), NJ872 VISA (B), R2334 DNS S. aureus (C), and R6246 LZDr S. aureus (D). TLV, telavancin; DAP, daptomycin; VAN, vancomycin; LZD, linezolid; VISA, vancomycin-intermediate S. aureus; hVISA, heteroresistant VISA; DNS, daptomycin-nonsusceptible; LZDr , linezolid resistant. Values in parentheses represent the MICs in μg/ml. Values are shown as the means and standard deviations.
In vitro PK/PD models.
The average (standard deviation [SD]) observed fCmax for TLV was 11.24 (0.05) μg/ml (target, 10.8 μg/ml), the average (SD) fAUC0–24 was 121.55 (0.04) μg · h/ml (target, 111 μg · h/ml), and the average (SD) t1/2 was 8 (0.05) h (target, 8.1 h). The average (SD) observed fCmax for DAP was 10.96 (0.05) μg/ml (target, 11.3 μg/ml), the average (SD) fAUC0–24 was 115.05 (0.18) μg · h/ml (target, 114 μg · h/ml), and the average (SD) t1/2 was 8.45 (0.04) h (target, 8 h). The average (SD) observed fCmax for VAN was 13.92 (0.19) μg/ml (target, 15.75 μg/ml), the average (SD) fAUC0–24 was 210.68 (0.24) μg · h/ml (target, 218 μg · h/ml), and the average (SD) t1/2 was 5.87 (0.34) h (target, 6 h). The average (SD) observed fCmax for LZD was 10.57 (0.14) μg/ml (target, 10.4 μg/ml), and the average (SD) t1/2 was 4.88 (0.17) h (target, 5 h). No strains developed resistance to telavancin over the course of the 168-h models.
Against hVISA R6669 (Table 4 and Fig. 1A) (telavancin MIC, 0.0625 μg/ml; daptomycin, 0.5 μg/ml; vancomycin, 2 μg/ml; linezolid, 2 μg/ml), telavancin was bactericidal at 8 h and maintained bactericidal activity throughout the 168-h regimen. Daptomycin was also bactericidal at 8 h and maintained bactericidal activity throughout. These two regimens exhibited statistically similar activity and were superior to vancomycin, linezolid, and a drug-free growth control at 168 h (P < 0.001). Linezolid was statistically superior to vancomycin at 168 h (P < 0.001), although bactericidal activity was not achieved. All regimens were superior to the growth control at 168 h (P < 0.05).
TABLE 4.
In vitro activities of regimens against R6669 (hVISA), NJ872 (VISA), R2334 (DNS S. aureus), and R6246 (LZDr S. aureus) at 168 ha
| Regimenb | Log10 CFU/ml at 168 h (change from baseline log10 CFU/ml at 168 h) (mean [SD]) in strain: |
|||
|---|---|---|---|---|
| R6669 | NJ872 | R2334 | R6246 | |
| TLV at 10 mg/kg/day | 2.26 ± 0.07 (−4.02 ± 0.01) | 2.27 ± 0.35 (−3.68 ± 0.34) | 2.30 ± 0.12 (−4.31 ± 0.10) | 2.84 ± 0.08 (−3.79 ± 0.06) |
| DAP at 10 mg/kg/day | 2.30 ± 0.14 (−4.01 ± 0.07) | 2.23 ± 0.17 (−3.80 ± 0.11) | 7.51 ± 0.39 (+1.08 ± 0.40) | 2.90 ± 0.23 (−3.66 ± 0.10) |
| VAN at 1 g q12h | 6.59 ± 0.19 (+0.29 ± 0.42) | 7.35 ± 0.35 (+1.36 ± 0.39) | 5.42 ± 0.05 (−1.12 ± 0.27) | 8.23 ± 0.15 (+1.58 ± 0.18) |
| LZD at 600 mg q12h | 4.47 ± 0.28 (−1.79 ± 0.31) | 4.25 ± 0.29 (−1.77 ± 0.35) | 5.10 ± 0.32 (−1.43 ± 0.42) | 9.49 ± 0.13 (+2.91 ± 0.03) |
| Growth control | 9.15 ± 0.07 (+2.93 ± 0.04) | 8.95 ± 0.07 (+2.92 ± 0.06) | 9.38 ± 0.11 (+2.78 ± 0.13) | 9.38 ± 0.11 (+3.03 ± 0.04) |
VISA, vancomycin-resistant S. aureus; hVISA, heteroresistant VISA; DNS, daptomycin nonsusceptible; LZDr, linezolid resistant.
TLV, telavancin; DAP, daptomycin; VAN, vancomycin; LZD, linezolid.
Telavancin was bactericidal at 24 h and maintained bactericidal activity throughout the 168-h regimen against VISA NJ992 (Table 4 and Fig. 1B) (telavancin MIC, 0.125 μg/ml; daptomycin, 0.5 μg/ml; vancomycin, 8 μg/ml; linezolid, 1 μg/ml). Daptomycin was bactericidal at 8 h and also maintained bactericidal activity throughout the 168-h regimen. These two regimens exhibited statistically similar activity and were superior to vancomycin, linezolid, and a drug-free growth control at 168 h (P < 0.005). Linezolid was statistically superior to vancomycin at 168 h (P = 0.001), although bactericidal activity was not achieved. All regimens were superior to the growth control at 168 h (P < 0.05).
Against DNS S. aureus R2334 (Table 4 and Fig. 1C) (telavancin MIC, 0.125 μg/ml; daptomycin, 2 μg/ml; vancomycin, 1 μg/ml; linezolid, 1 μg/ml), telavancin was statistically superior at 168 h compared to all other regimens (P < 0.001) and was bactericidal at 8 h while maintaining bactericidal activity throughout the 168-h regimen. Vancomycin and linezolid exhibited statistically similar activity at 168 h, and each agent was superior to daptomycin (P < 0.002). All regimens were superior to the growth control at 168 h (P < 0.003).
Both telavancin and daptomycin were bactericidal at 8 h and maintained bactericidal activity throughout the 168-h regimen against LZDr S. aureus R6246 (Table 4 and Fig. 1D) (telavancin MIC, 0.0625 μg/ml; daptomycin, 0.25 μg/ml; vancomycin, 2 μg/ml; linezolid, 16 μg/ml). These two regimens exhibited statistically similar activity and were superior to vancomycin, linezolid, and a drug-free growth control at 168 h (P < 0.001). Vancomycin was statistically superior to linezolid at 168 h (P < 0.001), although net growth was exhibited over the 168-h period. Linezolid exhibited statistically similar activity to the growth control at 168 h, and all other regimens were superior to the growth control and linezolid.
DISCUSSION
Our study evaluated the MICs of telavancin across S. aureus with multiple drug resistances and also evaluated the activity of telavancin against representatives of these phenotypes in 168-h in vitro model experiments. Here, we demonstrated that telavancin maintains activity in the susceptible range against >97% of these resistant S. aureus strains and that it possesses rapid bactericidal activity against hVISA, VISA, DNS S. aureus, and LZDr S. aureus in PK/PD model experiments.
Notably, our data represent a downward shift in telavancin MICs that were published prior to the 2014 CLSI recommendations (10) to include 0.002% polysorbate 80 in experimental broth (15). The previous data reported a telavancin MIC90 of 0.25 μg/ml, with 5% of isolates requiring telavancin concentrations of ≥0.5 μg/ml for inhibition. In our study, even against isolates with decreased susceptibility to vancomycin, daptomycin, or linezolid, only 10 isolates (2.7%) required telavancin at ≥0.25 μg/ml to inhibit growth. Similarly, Mendes and colleagues (5, 16) recently demonstrated that out of 7,264 MRSA isolates, 7,242 (99.7%) were inhibited at 0.0625 μg/ml telavancin. The second of their studies indicated that even among MRSA with vancomycin MICs of 2 to 4 μg/ml and daptomycin MICs of 1 to 2 μg/ml, 100% of isolates were inhibited at 0.125 μg/ml, making every isolate susceptible by the current CLSI breakpoint (5, 10). Our study presents a large evaluation of isolates with elevated resistance to three commonly used agents against MRSA, vancomycin, daptomycin, and linezolid, including several isolates with vancomycin MICs of 8 μg/ml and daptomycin MICs of 4 μg/ml. Even among this resistant subset of MRSA isolates, only 10 of 365 (2.7%) isolates demonstrated telavancin nonsusceptibility, nine of which were VISA strains. Each of these had a vancomycin MIC of ≥4 μg/ml, and the lone non-VISA DNS strain that demonstrated a telavancin MIC of 0.25 μg/ml possessed a vancomycin MIC of 2 μg/ml. The data in our study suggest that telavancin harbors activity in the setting of decreased Gram-positive susceptibility. The data presented in the current study also demonstrate similar efficacy between broth microdilution methods and the Trek panel for telavancin MIC evaluation. Although 93 (25.5%) strains demonstrated +1 dilution in broth microdilution MIC testing compared to that with the Trek panel, and 32 (8.8%) demonstrated −1 dilution in broth microdilution MIC testing compared to that with the Trek panel, these values are within the ±1 dilution allotted by CLSI standards (10). Only 2 (1%) isolates, both hVISA strains, were 2 dilutions higher via broth microdilution testing, attesting to the reliability and reproducibility between these methods.
Telavancin has previously demonstrated excellent in vitro activity against S. aureus in several PK/PD studies. Two models of simulated endocardial vegetations demonstrated the bactericidal activity of telavancin, one study against hVISA and VISA and the other against daptomycin-nonsusceptible S. aureus (4, 17). Our study is the first to evaluate telavancin against representatives of the hVISA, VISA, DNS S. aureus, and LZDr S. aureus phenotypes together in pharmacodynamic models, and telavancin was similarly successful against each of these phenotypes. Of interest, telavancin killed much more rapidly in our study, achieving bactericidal activity against all four resistant strains at 24 h and at 8 h in three of the strains. It is possible that the addition of 0.002% polysorbate 80 to the medium mitigated telavancin binding to the plastic surfaces present in the model and facilitated the availability of free telavancin. This may have resulted in the more rapid bactericidal activity present here than in previous studies, in which the absence of a surfactant may have resulted in lost telavancin due to nonspecific binding to the model apparatus. Another reason for the rapid killing achieved in our study may have been the achieved fAUC0–24 of telavancin. Although our achieved fAUC0–24 of 121.55 μg · h/ml was within 10% of our targeted fAUC0–24, this value is larger than those previously demonstrated in vitro and shown in the package insert when accounting for protein binding (18).
In the study by Leonard and colleagues (17), an AUC0–24 of 968.8 μg · h/ml was achieved. Based on total drug, they would have achieved an estimated free drug AUC0–24 of 96.88 μg · h/ml based on 90% protein binding, which is roughly 25% less than our achieved free AUC0–24 value of 121.55 μg · h/ml. Similarly, the package insert demonstrates an AUC0–24 of 780 μg · h/ml, which when accounting for 90% protein binding is roughly 35% lower than our value. Even with the slightly elevated AUC, our data would suggest that telavancin possesses potent bactericidal activity against MRSA, with decreased susceptibility to vancomycin, daptomycin, or linezolid, even when these strains harbor telavancin MICs at the established telavancin MIC90.
There are some limitations to the current study. We evaluated only four MRSA isolates in the PK/PD model, possibly limiting generalizability of the findings. Also, we did not evaluate any strains that carry both daptomycin nonsusceptibility and vancomycin-intermediate susceptibility, a phenotype that would necessitate creative therapeutic options, such as telavancin. Our models were also run over only 7 days, which would be a much shorter duration of therapy than would be used clinically for deep-seated infections. Further investigation is warranted to confirm the reproducibility of this activity in more MRSA strains.
MRSA has and will continue to pose a therapeutic challenge, especially as isolates arise with reduced susceptibility to vancomycin, daptomycin, and linezolid, three mainstays in the current antimicrobial repertoire. Telavancin presents a novel bactericidal option for treating these resistant infections. The results of our study suggest that telavancin maintains low MICs against a large subset of these resistant strains and is able to provide bactericidal activity against them in PK/PD modeling studies. When these resistant infections arise, it appears that telavancin is a promising therapeutic option that warrants further clinical study.
ACKNOWLEDGMENTS
Theravance Biopharma Antibiotics, Inc. provided funding and materials for this research.
M.J.R. has received grant support, consulted for, or provided lectures for Astellas, Cubist, Forest, Pfizer, Theravance Biopharma Antibiotics, Inc., and Novartis and is partially funded by NIH grant R21 AI109266-01. J.R.S., K.E.B., J.H., and A.R. have nothing to declare.
REFERENCES
- 1.Mendes RE, Sader HS, Farrell DJ, Jones RN. 2011. Update on the telavancin activity tested against European staphylococcal clinical isolates (2009–2010). Diagn Microbiol Infect Dis 71:93–97. doi: 10.1016/j.diagmicrobio.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 2.Mendes RE, Sader HS, Farrell DJ, Jones RN. 2012. Telavancin activity tested against a contemporary collection of Gram-positive pathogens from USA hospitals (2007–2009). Diagn Microbiol Infect Dis 72:113–117. doi: 10.1016/j.diagmicrobio.2011.09.023. [DOI] [PubMed] [Google Scholar]
- 3.Xiong YQ, Hady WA, Bayer AS, Chen L, Kreiswirth BN, Yang SJ. 2012. Telavancin in therapy of experimental aortic valve endocarditis in rabbits due to daptomycin-nonsusceptible methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 56:5528–5533. doi: 10.1128/AAC.00922-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steed ME, Vidaillac C, Rybak MJ. 2012. Evaluation of telavancin activity versus daptomycin and vancomycin against daptomycin-nonsusceptible Staphylococcus aureus in an in vitro pharmacokinetic/pharmacodynamic model. Antimicrob Agents Chemother 56:955–959. doi: 10.1128/AAC.05849-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mendes RE, Sader HS, Flamm RK, Farrell DJ, Jones RN. 2015. Telavancin in vitro activity against a collection of methicillin-resistant Staphylococcus aureus isolates, including resistant subsets, from the United States. Antimicrob Agents Chemother 59:1811–1814. doi: 10.1128/AAC.04616-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Higgins DL, Chang R, Debabov DV, Leung J, Wu T, Krause KM, Sandvik E, Hubbard JM, Kaniga K, Schmidt DE Jr, Gao Q, Cass RT, Karr DE, Benton BM, Humphrey PP. 2005. Telavancin, a multifunctional lipoglycopeptide, disrupts both cell wall synthesis and cell membrane integrity in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 49:1127–1134. doi: 10.1128/AAC.49.3.1127-1134.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lunde CS, Rexer CH, Hartouni SR, Axt S, Benton BM. 2010. Fluorescence microscopy demonstrates enhanced targeting of telavancin to the division septum of Staphylococcus aureus. Antimicrob Agents Chemother 54:2198–2200. doi: 10.1128/AAC.01609-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones RN, Streit JM, Fritsche TR. 2004. Validation of commercial dry-form broth microdilution panels and test reproducibility for susceptibility testing of dalbavancin, a new very long-acting glycopeptide. Int J Antimicrob Agents 23:197–199. doi: 10.1016/j.ijantimicag.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 9.Wootton M, Howe RA, Hillman R, Walsh TR, Bennett PM, MacGowan AP. 2001. A modified population analysis profile (PAP) method to detect hetero-resistance to vancomycin in Staphylococcus aureus in a UK hospital. J Antimicrob Chemother 47:399–403. doi: 10.1093/jac/47.4.399. [DOI] [PubMed] [Google Scholar]
- 10.CLSI. 2014. Performance standards for antimicrobial susceptibility testing; 24th informational supplement. CLSI document M100-S24 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 11.Benvenuto M, Benziger DP, Yankelev S, Vigliani G. 2006. Pharmacokinetics and tolerability of daptomycin at doses up to 12 milligrams per kilogram of body weight once daily in healthy volunteers. Antimicrob Agents Chemother 50:3245–3249. doi: 10.1128/AAC.00247-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gee T, Ellis R, Marshall G, Andrews J, Ashby J, Wise R. 2001. Pharmacokinetics and tissue penetration of linezolid following multiple oral doses. Antimicrob Agents Chemother 45:1843–1846. doi: 10.1128/AAC.45.6.1843-1846.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaw JP, Seroogy J, Kaniga K, Higgins DL, Kitt M, Barriere S. 2005. Pharmacokinetics, serum inhibitory and bactericidal activity, and safety of telavancin in healthy subjects. Antimicrob Agents Chemother 49:195–201. doi: 10.1128/AAC.49.1.195-201.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Werth BJ, Steed ME, Kaatz GW, Rybak MJ. 2013. Evaluation of ceftaroline activity against heteroresistant vancomycin-intermediate Staphylococcus aureus and vancomycin-intermediate methicillin-resistant S. aureus strains in an in vitro pharmacokinetic/pharmacodynamic model: exploring the “seesaw effect”. Antimicrob Agents Chemother 57:2664–2668. doi: 10.1128/AAC.02308-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mendes RE, Sader HS, Farrell DJ, Jones RN. 2012. Worldwide appraisal and update (2010) of telavancin activity tested against a collection of Gram-positive clinical pathogens from five continents. Antimicrob Agents Chemother 56:3999–4004. doi: 10.1128/AAC.00011-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mendes RE, Farrell DJ, Sader HS, Streit JM, Jones RN. 2014. Update of the telavancin activity in vitro tested against a worldwide collection of Gram-positive clinical isolates (2013), when applying the revised susceptibility testing method. Diagn Microbiol Infect Dis 81:275–279. [DOI] [PubMed] [Google Scholar]
- 17.Leonard SN, Vidaillac C, Rybak MJ. 2009. Activity of telavancin against Staphylococcus aureus strains with various vancomycin susceptibilities in an in vitro pharmacokinetic/pharmacodynamic model with simulated endocardial vegetations. Antimicrob Agents Chemother 53:2928–2933. doi: 10.1128/AAC.01544-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Theravance. 2014. Telavancin package insert. Theravance, South San Francisco, CA: http://www.vibativ.com/docs/VIBATIV_PI_Final.pdf. [Google Scholar]


