Abstract
Fungal infections have increased dramatically in the last 2 decades, and fighting infectious diseases requires innovative approaches such as the combination of two drugs acting on different targets or even targeting a salvage pathway of one of the drugs. The fungal cell wall biosynthesis is inhibited by the clinically used antifungal drug caspofungin. This antifungal activity has been found to be potentiated by humidimycin, a new natural product identified from the screening of a collection of 20,000 microbial extracts, which has no major effect when used alone. An analysis of transcriptomes and selected Aspergillus fumigatus mutants indicated that humidimycin affects the high osmolarity glycerol response pathway. By combining humidimycin and caspofungin, a strong increase in caspofungin efficacy was achieved, demonstrating that targeting different signaling pathways provides an excellent basis to develop novel anti-infective strategies.
INTRODUCTION
Invasive fungal infections are characterized by diagnostic difficulties and high mortality with fatality rates ranging from 30% to 80% in neutropenic patients (1, 2). The yeast Candida albicans and the filamentous fungus Aspergillus fumigatus are by far the most important causes of life-threatening invasive mycoses (3). To combat this life-threatening infection, only a limited number of antifungal agents are available. Among them, the most frequently used are amphotericin B (AMB) and triazole drugs like itraconazole (ITZ), fluconazole, and voriconazole, targeting ergosterol in the fungal cell membrane (4). The echinocandins are the most recent class of antifungal agents. They inhibit the synthesis of β-(1,3)-d-glucan in the fungal cell wall, which is not present in mammalian cells. The first clinically applied echinocandin was caspofungin (CAS) (Cancidas, caspofungin acetate) (5). However, after introduction of CAS in clinical trials, no decrease in the mortality rate of patients with invasive aspergillosis was reported (6). Apparently, there are at least two drawbacks: (i) the increasing resistance against CAS (7) and (ii) the so-called paradoxical effect leading to attenuated activity of echinocandin antifungals at high concentrations (8, 9). However, echinocandins still show promising features as the basis for a combined antifungal therapy because they are poor substrates for cytochrome (CYP) P450 enzymes and P-glycoproteins (10).
The alarming number of cases of life-threatening mycoses and the lack of effective drugs have driven the search for new, broad-spectrum fungicidal agents, including reformulation of existing antifungals as well as the search for synergistic compounds or compounds able to potentiate the effects of known antifungal drugs that can be used for treatment and prophylaxis (11).
In this study, using high-throughput screening (HTS) against A. fumigatus (12), we discovered that extracts of the actinomycete Streptomyces humidus F-100.629 were able to inhibit the growth of A. fumigatus and C. albicans when combined with a sublethal dose of CAS. Bioassay-guided fractionation of these extracts led to the isolation of the novel compound humidimycin, structurally related to the class I lasso peptides siamycins I and II and RP71955/aborycin, all ribosomally synthesized and posttranslationally modified peptides (RiPPs) produced by different strains of Streptomyces (13, 14). Here, we also analyzed the effect of caspofungin and humidimycin on A. fumigatus at the transcriptome level, confirming that they act on different targets. Further analysis of signal transduction mutants, combined with biochemical determination of the phosphorylation status of mitogen-activated protein (MAP) kinases, revealed that the high osmolarity glycerol (HOG) pathway drives the response to humidimycin. This finding led to the discovery of a potential salvage pathway acting during the CAS stress response, which can be used as a novel target to potentiate CAS activity.
MATERIALS AND METHODS
HTS and isolation of humidimycin.
The 20,000 natural product extracts used in the screening against A. fumigatus were obtained from Fundación MEDINA's natural product collection and corresponded to microbial extracts from actinomycetes and fungal strain fermentations prepared as previously described. The HTS was performed in 96- and 384-well plates as described by Monteiro et al. (12).
The test plates contained a volume of 150 μl of RPMI 1640 modified medium/well, including 2.5 × 104 conidia/ml, 0.002% (wt/vol) resazurin, and 7 μl of extract. Each extract was tested with and without a sublethal dose of CAS (0.015 μg/ml). The sublethal dose of CAS was chosen as one-eighth of the minimal effective concentration (MEC) found for this antifungal. Each microtiter plate contained a set of control wells, including growth and no-growth controls and an amphotericin B dose-response curve (from 2 to 0.25 μg/ml). Plates were incubated at 37°C for 30 h, and growth inhibition was quantified by measuring fluorescence (excitation, 570 nm; emission, 615 nm) using a VICTOR2 multilabel counter (PerkinElmer).
The percentages of resazurin reduction and growth inhibition were calculated using the following equations: % reduction= 100 × [(fluorescence intensity of test agent − fluorescence intensity of untreated control)/(fluorescence intensity of reduced resazurin − fluorescence intensity of untreated control)] and % inhibition = 100 − % reduction. Screening data for the resazurin assay were analyzed using the Genedata Screener program (Genedata AG, Switzerland).
The activity of the extracts tested with CAS was subtracted from the activity of the extracts without CAS, and an extract was considered synergistic when this value was ≥60%. The fractional inhibitory concentration index (FICI) was not used because the extracts were not active without the sublethal dose of the antifungal. The RZ′ factor was determined in all experiments.
The activity of the positive extracts was confirmed by analysis of titration curves. The extracts presenting good dose-response curves were analyzed by liquid chromatography-mass spectrometry (LC-MS), and the result was compared with MEDINA's in-house database and with commercial natural product databases (Chapman & Hall Dictionary of Natural Products; http://dnp.chemnetbase.com) in order to detect already known compounds. Those extracts with no match in the databases were selected for scale-up fermentation of the producing strain, extraction and fractionation using SP207ss resin, and preparative high-performance liquid chromatography (HPLC), following a bioassay-guided process in order to isolate the pure compounds responsible for the observed biological activity.
Humidimycin was identified as a secondary metabolite produced by the strain Streptomyces humidus F-100,629 isolated from a soil collected in Almería, Spain. The acetone extract of a culture broth of strain F-100,629 grown in liquid medium for 7 days at 28°C was subjected to bioassay-guided fractionation using SP207ss chromatography (gradient elution with H2O-acetone mixtures) and reversed-phase C-8 preparative HPLC (H2O-CH3CN gradient with 0.1% trifluoroacetic acid [TFA] in both phases; see the supplemental material for further details) to yield humidimycin as the molecule responsible for the observed biological activity.
Biological activity. (i) Strains, media, antifungal compounds, and reagents.
The fungal strains used in this study were Candida albicans MY1055, Aspergillus fumigatus ATCC 46645 (wild-type strain), CEA17 (wild-type strains), and CEA17 mutants. The media used in this work were (i) RPMI 1640 modified medium containing 10.4 g/liter of RPMI 1640 medium (R8755; Sigma), 6.7 g/liter of yeast nitrogen base (YNB) (BD Biosciences), 1.8% (wt/vol) glucose, and 40 mM HEPES (pH 7.1); (ii) yeast extract-peptone-dextrose broth (YPDB)-KCl medium (2% [wt/vol] peptone, 1% [wt/vol] yeast extract, and 4% [wt/vol] KCl), sterilized by autoclaving, and then supplemented with 2% (wt/vol) glucose and 4 μg/ml adenine; (iii) Sabouraud dextrose agar (SDA) (65 g/liter; BD Biosciences); and (iv) potato dextrose agar (PDA) (39 g/liter; BD Biosciences). The chemicals resazurin (R7017; Sigma), Tween 80 (Merck), ITZ (I16657; Sigma), CAS (Merck Sharp & Dohme), and dimethyl sulfoxide (DMSO) (Merck) were used. Aspergillus minimal medium (AMM) was prepared as previously described (15).
(ii) Viability assays.
Cell viability of A. fumigatus was measured using resazurin as described previously (12). In the case of C. albicans, yeast suspensions from cryovials were streaked on SDA plates and incubated for 18 h at 37°C, and colonies were inoculated in 10 ml Sabouraud dextrose broth (SDB). The overnight culture was adjusted to an optical density (OD) of 0.25 at 660 nm in RPMI 1640 modified medium, diluted 1:10 (∼2 to 5 × 105 cells/ml), and kept on ice until use. After inoculation, 96-well microtiter plates were statically incubated at 37°C for 20 to 24 h. Resazurin was added to the microtiter plates at a final concentration of 0.002% 3 to 4 h before the fluorescence was measured as described above.
(iii) Potentiation assay conditions.
The potentiation assay was performed in 96-well plates, and humidimycin was tested with and without a sublethal dose of clinically used antifungal compounds. The sublethal doses of CAS were 0.015 μg/ml for A. fumigatus and 0.03 μg/ml for C. albicans. A sublethal dose of ITZ (0.06 μg/ml) was used to test A. fumigatus and C. albicans. The sublethal doses were defined as one-eighth of the MEC/MIC obtained for each antifungal compound.
The test plates contained a final volume of 150 μl/well of the inoculum. Culture plates were incubated at 37°C for 30 h. The variation of the color of resazurin was visually observed after the incubation of the plates, and the growth inhibition was quantified by measuring the fluorescence as described above (12). All of the experiments were performed in triplicate. The RZ′ factor obtained in all the experiments was between 0.85 and 0.95.
(iv) Checkerboard tests for antifungal interactions in vitro.
The checkerboard tests were performed in 96-well plates (16, 17). To obtain data for the interactive effects of CAS and humidimycin against A. fumigatus, we determined dose-response curves and measured growth inhibition in 96-well microdilution plate assays with the aid of the redox indicator resazurin. The in vitro drug interaction was evaluated by a two-dimensional, two-agent broth microdilution checkerboard technique, using 96-well flat-bottomed microtitration plates. Interactions were finally determined by checkerboard layouts of doubling dilutions of CAS and humidimycin at 10 final concentrations from 0.24 to 0.0018 μg/ml of CAS (10 columns of the microdilution plate) and 8 final concentrations (8 to 0.015 μg/ml) of humidimycin (8 rows of the same microdilution plate). All of the assays were performed in triplicate.
Flow cytometry experiments.
Flow cytometry (FC) assays were performed with the C. albicans strain treated with the following compounds (concentration ranges): humidimycin (8 to 0.06 μg/ml), CAS (1.0 to 0.004 μg/ml), CAS at a sublethal dose (0.03 μg/ml), ITZ at a sublethal dose (0.06 μg/ml), humidimycin (8 to 0.06 μg/ml) plus CAS at a sublethal dose, and humidimycin (8 to 0.06 μg/ml) plus ITZ at a sublethal dose. Propidium iodide (PI) was used as a probe to determine cell viability and membrane permeability (18). Cells that were not treated, with and without the probe, and AMB curves were used as controls. The C. albicans inoculum was incubated for 6 h with agitation at 37°C until the exponential growth phase was reached in order to obtain a homogeneous population. The assay was performed in 96-well plates containing C. albicans cells (2.5 × 106 cells/ml), and after addition of the different compounds to be tested, plates were incubated overnight at 37°C. After incubation, cells were washed three times with phosphate-buffered saline (PBS) 1× solution and then incubated for 30 min with 100 μl/well of 50 mM PI solution at 37°C in the dark. Plates were then read in a BD Accuri C6 flow cytometer (BD Biosciences) at the wavelengths defined for PI (535 nm/617 nm), with 10,000 events recorded per well. The experiments were performed in triplicate.
Susceptibility tests of A. fumigatus mutants.
Microdilution assays were performed following the EUCAST standard methodology (19). Humidimycin was used to determine the MEC in broth microdilution. The assay was performed in 96-well microtiter plates. The concentrations tested ranged from 8 to 0.015 μg/ml in serial double dilutions. The A. fumigatus strains used in this study are listed in Table 1. Plates were incubated for 24 to 48 h at 37°C, and the endpoint for humidimycin was determined as the concentration that produced a visible change in the morphology of the hyphae compared with the growth in control wells (MEC). For plate susceptibility assays, the experiments were performed as previously reported (20).
TABLE 1.
A. fumigatus strains used in this study
| Strain | Deleted gene | Assigned function | Genetic background | Genotype | Reference |
|---|---|---|---|---|---|
| ATCC 46645 | Wild type | 23 | |||
| CEA17 | Wild type | 40 | |||
| CEA17ΔakuB | AFUA_2G02620 | Ku DNA helicase | CEA17 | akuB::pyrG; PyrG+ | 24 |
| ΔmpkA | AFUA_4G13720 | MAP kinase (MpkA) | CEA17 ΔakuB | akuB; mpkA::ptrA; Ptr+ | 20 |
| ΔsakA | AFUA_1G12940 | MAP kinase (Osm1) | CBS 144.89 | sakA:: hph; HygR | 30 |
| Δskn7 | AFUA_6G12520 | response regulator | ATCC 46645 | skn7::ptrA; Ptr+ | 27 |
| Δyap1 | AFUA_6G09930 | bZIP trans. factor | ATCC 46645 | yap1::hph; HygR | 28 |
Western blot analysis.
Conidia (106/ml) of wild-type and ΔmpkA and ΔsakA mutant strains were cultured for 16 h, and the resulting mycelia were treated with humidimycin (1 μg/ml), CAS (0.1 μg/ml), and humidimycin and CAS together. The mycelia were harvested at different time points and immediately frozen in liquid nitrogen. Protein extraction and Western blot analysis were carried out as previously described (20).
Cytotoxicity and cardiotoxicity assays.
Cell growth and incubation conditions for evaluation of cytotoxicity were similar to those reported previously (21, 22). The cell line Fa2N-4 used in this study was obtained from Xenotech. The healthy Fa2N-4 cells (immortalized hepatocyte cell line) were cultured in MFE essential support medium F with MFE culture medium supplement A. The cell culture was kept at 37°C under a humidified atmosphere of 5% CO2. The methylthiazolyldiphenyl-tetrazolium bromide (MTT) assay test that measures the rate of reduction of MTT as an indicator of the functional integrity of mitochondria was used to determine the cellular viability of the Fa2N-4 cell line. For the in vitro cytotoxicity assay, we used 10,000 cells per well in 96-well plates. Humidimycin was tested at concentrations ranging from 5 to 0.01 μg/ml, with and without the sublethal doses of CAS of 0.015, 0.03, and 0.1 μg/ml. Absorption at 570 nm (OD570) was measured in a Victor2 Wallac spectrofluorometer (see Table S2 in the supplemental material).
Cardiotoxicity was determined by measuring the activity on the potassium ion channel human ether-à-go-go-related gene (hERG) using the FluxOR thallium flux assay. The cell line used in this study was obtained from B'SYS GmbH (Hek293 transfected with the hERG channel). The FluxOR potassium channel assay was performed as outlined in the Invitrogen information sheet in a FLIPR Tetra system (Molecular Devices).
Sequence accession number.
RNA-seq raw data are accessible through the Gene Expression Omnibus (GEO) under accession number GSE55663.
RESULTS
Isolation and structure elucidation of humidimycin (MDN-0010).
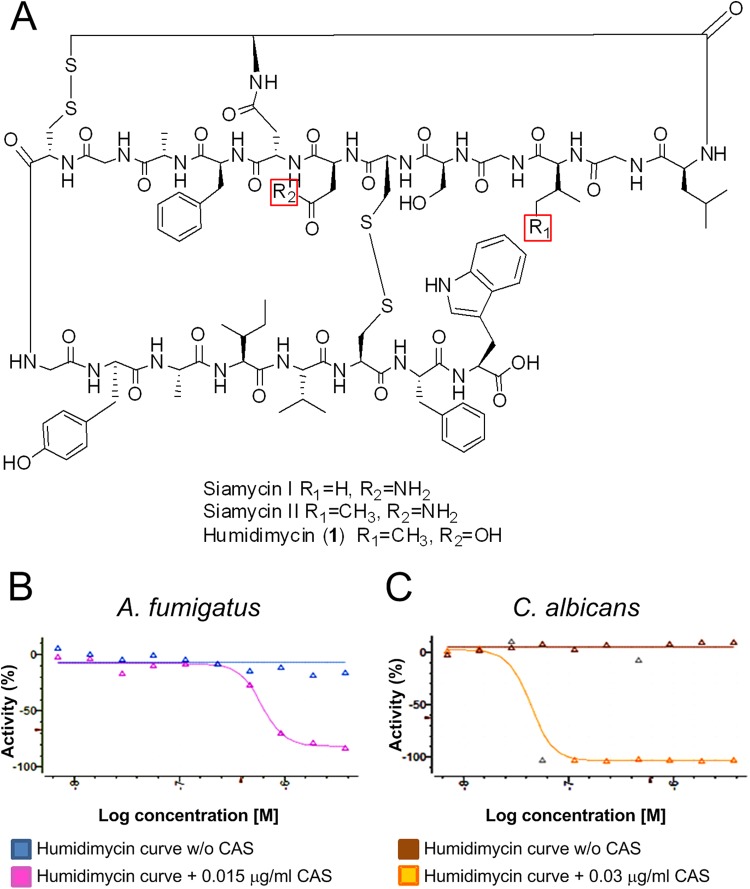
An HTS approach was used to test, in combination with CAS, 20,000 microbial natural product extracts from actinomycetes and fungi (available from the Fundación MEDINA collection) (12). The result was that 0.94% of extracts analyzed displayed a CAS-enhancing activity against the two A. fumigatus wild-type strains tested, ATCC 46645 and CEA17ΔakuB (inhibition cutoff of ≥60%) (23, 24). The extracts showing a good dose-response curve (36.5%) were further characterized, and pure compounds were identified after bioassay-guided fractionation of freshly regrown microorganisms. One of the most promising compounds found in this screen was the new class I lasso peptide humidimycin. By use of different chromatographic methods, the compound was isolated from a fermentation broth of the strain Streptomyces humidus F-100,629. The structure of humidimycin was closely related to those of the known lasso peptides siamycins I and II and RP71955, isolated as well from strains of Streptomyces (13, 14) (Fig. 1A). A molecular formula of C98H132N22O27S4 was proposed for the compound after extensive analysis of the electrospray ionization-time of flight mass spectrometry (ESI-TOF MS) spectra acquired in positive mode (m/z 2194.8839 [M + NH4]+, calculated for C98H136N23O27S4+, 2194.8853; m/z 1097.9453 [M + H + NH4]2+, calculated for C98H137N23O27S42+, 1097.9463) and negative mode (m/z 1087.4124 [M − 2H]2−, calculated for C98H132N23O27S42−, 1087.4174). This molecular formula only differed from that of siamycin II in the replacement of a nitrogen atom by oxygen in humidimycin. Indeed, the 1H and 13C nuclear magnetic resonance (NMR) spectra (see Table S1 in the supplemental material) confirmed the peptidic nature and revealed the complex structure of the molecule. Detailed analysis of the one-dimensional (1D) and two-dimensional (2D) NMR spectra demonstrated nearly identical chemical shifts for all the amino acid residues in humidimycin and siamycin II. Reduction of the two disulfide bridges of the molecule and sequencing by tandem mass spectrometry (MS/MS) allowed us to propose an identical structure for the C terminus of both peptides, from Phe10 to Trp21. The replacement of Asn 8 in siamycin II by Asp in humidimycin was confirmed by a small upfield shift of the 13C signal of the CH2 of the Asp residue in humidymicin compared to that of siamycin II (25) and the absence of primary amide signals in the 1H NMR spectra. The absolute configuration of all the amino acid residues present in the molecule was assumed to be L, the same as in siamycin II, due to the similarity in chemical shifts and the negative value of its optical rotation.
FIG 1.
Humidimycin structure and activity. (A) Humidimycin structure related to those of siamycins I and II. (B and C) Dose-response curve effect of humidimycin (8 to 0.015 μg/ml) in combination with CAS against A. fumigatus (CAS, 0.015 μg/ml) and C. albicans (CAS, 0.03 μg/ml).
Potentiation of the antifungal effect of CAS and itraconazole by humidimycin (MDN-0010).
The potentiation of the antifungal effect was determined by simultaneous testing of humidimycin with and without a sublethal concentration of CAS. Dose-response curves were obtained using the starting concentrations of 8 μg/ml of humidimycin (compound 1) and tested against the two major human pathogens, A. fumigatus and C. albicans. The minimal effective concentration (MEC) measured indicates the concentration that produced a change in the morphology of hyphae compared with that for the control. The sublethal dose of CAS (0.015 μg/ml) was about 1/10 of the MEC for A. fumigatus (0.12 μg/ml) and the MIC for C. albicans (0.25 μg/ml). Doses up to 1 to 2 μg/ml of humidimycin potentiated the CAS antifungal effect against A. fumigatus with a decrease in the CAS MEC from 0.12 to 0.015 μg/ml and against C. albicans with a drop in the CAS MIC from 0.25 to 0.03 μg/ml (Table 2).
TABLE 2.
Checkerboard assays of compound humidimycin in combination with CAS and ITZ against A. fumigatus and C. albicansa
| Treatment | A. fumigatus | C. albicans | ||
|---|---|---|---|---|
| CAS | Humidimycin (μg/ml) | CAS in combination (IC50, μg/ml) | Humidimycin (μg/ml) | CAS in combination (IC50, μg/ml) |
| A. fumigatus (IC50, 0.027 μg/ml) | 8 | 0.0059 | 8 | 0.0131 |
| 4 | 0.0063 | 4 | 0.0104 | |
| 2 | 0.0070 | 2 | 0.0085 | |
| 1 | 0.0137 | 1 | 0.0069 | |
| C. albicans (IC50, 0.03 μg/ml) | 0.5 | 0.0177 | 0.5 | 0.0210 |
| 0.25 | 0.0213 | 0.25 | 0.0213 | |
| 0.125 | 0.0237 | 0.125 | 0.0202 | |
| 0.0625 | 0.0220 | 0.0625 | 0.0449 | |
| 0.0313 | 0.0272 | 0.0313 | 0.0254 | |
| 0.0156 | 0.0273 | 0.0156 | 0.0398 | |
| ITZ | Humidimycin (μg/ml) | ITZ in combination (IC50, μg/ml) | Humidimycin (μg/ml) | ITZ in combination (IC50, μg/ml) |
| A. fumigatus (IC50, 0.264 μg/ml) | 8 | 0.0649 | 8 | 0.0124 |
| 4 | 0.0670 | 4 | 0.0109 | |
| 2 | 0.0656 | 2 | 0.0119 | |
| 1 | 0.0649 | 1 | 0.0113 | |
| C. albicans (IC50, 0.031 μg/ml) | 0.5 | 0.0663 | 0.5 | 0.0117 |
| 0.25 | 0.0783 | 0.25 | 0.0119 | |
| 0.125 | 0.1080 | 0.125 | 0.0119 | |
| 0.0625 | 0.1108 | 0.0625 | 0.0119 | |
| 0.0313 | 0.1115 | 0.0313 | 0.0123 | |
| 0.0156 | 0.2646 | 0.0156 | 0.0131 | |
Different concentrations of humidimycin were tested in combination with different concentrations of CAS or ITZ. The most active drug combinations are marked in bold.
Once the potentiating effect of CAS against A. fumigatus and C. albicans was demonstrated, these microorganisms were used to test humidimycin in combination with antifungal agents other than CAS. The strongest synergistic effect was obtained with the combination of humidimycin and itraconazole (ITZ) (0.06 μg/ml) against A. fumigatus. This result characterized humidimycin as an antifungal enhancer (Table 2).
In order to find the optimal combination of the enhancer compound and the clinically used antifungal drug, checkerboard tests were performed. Curves of humidimycin (8 to 0.015 μg/ml) were crossed with curves of CAS (0.24 to 0.002 μg/ml) or ITZ (0.5 to 0.004 μg/ml) against A. fumigatus and C. albicans. The optimal drug concentration of each compound and the maximum potentiation effect were determined. The results presented in Table 2 and illustrated in Fig. 1B and C show that humidimycin enhanced by 4.5-fold the antifungal effect of CAS against A. fumigatus and C. albicans. The potentiation was calculated as the CAS 50% inhibitory concentration (IC50)/humidimycin-CAS IC50 ratio and represents the number of times the CAS IC50 was reduced in the presence of humidimycin. The potentiation for ITZ was comparatively weaker against C. albicans, whereas ITZ exhibited similar values against A. fumigatus.
Flow cytometry (FC) experiments using C. albicans were performed to determine that membrane permeability and cell viability were not affected by the sublethal doses of the antifungal drugs and by humidimycin itself (Fig. 2). These experiments were not performed with A. fumigatus due to the large size of the fungal hyphae, which are not suitable for flow cytometry. The results obtained with this assay permit disregarding of any membrane damage involved in the mode of action of humidimycin and also established that sublethal doses of CAS do not affect the membrane permeability (Fig. 2). Therefore, the potentiation effect observed is not a consequence of the sensitization of the strain in the presence of the compounds but must be due to some other mechanism of interaction that takes place when the two compounds are combined.
FIG 2.
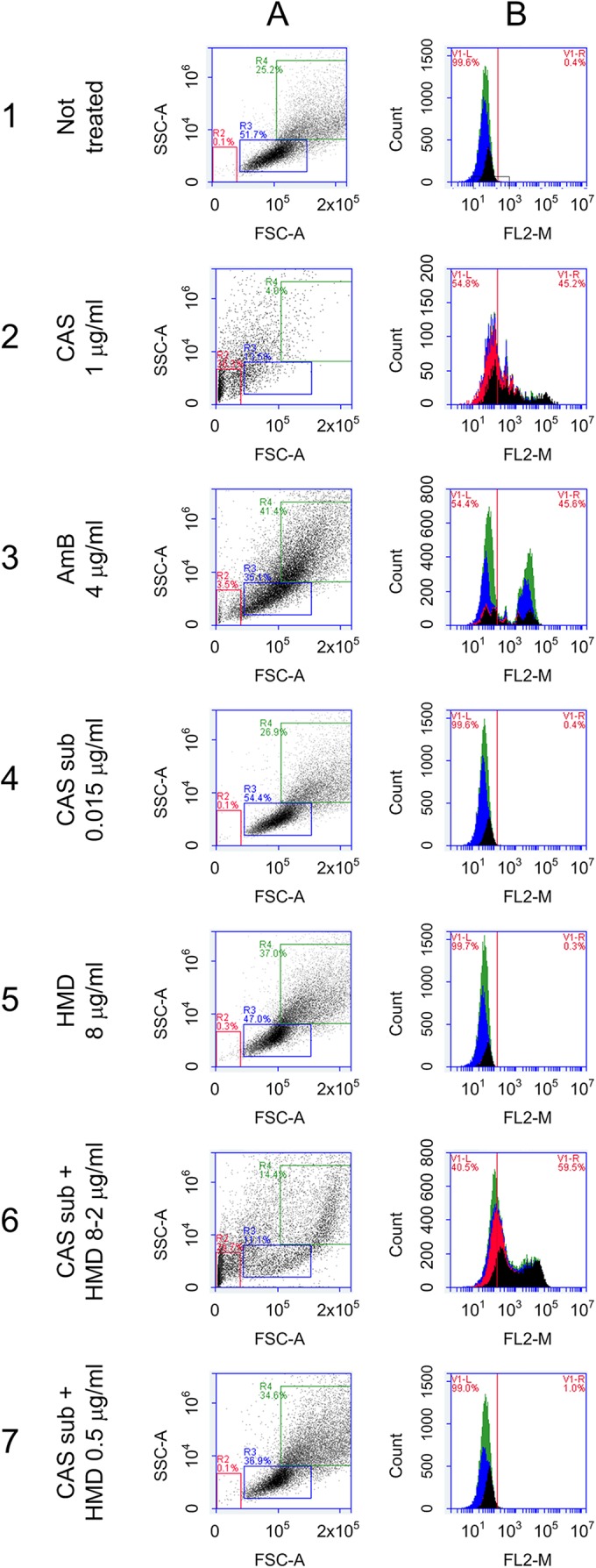
Flow cytometry assays with C. albicans to determine cellular viability by changes in membrane permeability using PI as a probe. The assays were performed with C. albicans and demonstrated that neither the sublethal dose of CAS nor the compound humidimycin itself (used at concentrations below 8 μg/ml) affected the cellular viability. (A) Normal population; (B) background of fluorescence emitted. (1) Nontreated cells. (2) Cells treated with 1 μg/ml of CAS (panel A shows the damaged population and panel B confirmed that PI was able to enter into the cell). (3) Cells treated with amphotericin B (AmB) at 4 μg/ml used as a control. (4) Cells treated with a sublethal dose (sub) of CAS (0.015 μg/ml) (panels A and B) presented the same profile as the nontreated cells (healthy cells). (5) Cells treated with 8 μg/ml of humidimycin (HMD) were also not affected. (6) Cells treated with a sublethal dose of CAS plus humidimycin at concentrations from 8 to 2 μg/ml presented the same damage profile as cells treated with 1 μg/ml of CAS (2). (7) Cells treated with a sublethal dose of CAS + humidimycin at concentrations of <2 μg/ml were not affected.
Lack of toxicity of the compounds.
The compounds humidimycin (8 to 0.015 μg/ml) and CAS (8 to 0.015 μg/ml) alone or in combination (humidimycin [8 to 0.015 μg/ml] plus CAS at 0.015 μg/ml, 0.03 μg/ml, or 0.09 μg/ml) were tested on the human hepatocyte cell line Fa2N-4 using the MTT assay (Table 3). The lack of cytotoxicity of humidimycin alone and in combination with CAS adds value to the molecule and to its possible use as a synergistic compound with CAS. In addition, it is well established that CAS is a poor substrate for the CYP P450 enzyme system and does not appear to inhibit major CYP 450 isozymes. Thus, drug interactions based on alterations in CYP-mediated metabolism are unlikely to occur with caspofungin (10).
TABLE 3.
Cytotoxicity assay with humidimycin, CAS, and the combination of both on human hepatocyte cell line Fa2N-4 measured by an MTT test
| Treatment | IC50 (mM) for Fa2N-4 cell line |
|---|---|
| Humidimycin (from 8 to 0.015 μg/ml) | >5 |
| CAS (from 8 to 0.015 μg/ml) | >5 |
| Humidimycin (from 8 to 0.015 μg/ml) + CAS (0.015 μg/ml) | >5 |
| Humidimycin (from 8 to 0.015 μg/ml) + CAS (0.03 μg/ml) | >5 |
| Humidimycin (from 8 to 0.015 μg/ml) + CAS (0.09 μg/ml) | >5 |
To assess any potential cardiac liability, we tested the interaction of humidimycin with the cardiac hERG ion channel and in the presence of three different sublethal doses of CAS (0.015, 0.03, and 0.1 μg/ml). The results show the lack of activity of humidimycin as well as its combinations with CAS on the cardiac ion channel tested. The absence of hERG channel blocker activity is particularly relevant for drug candidates and potential drug development.
Transcriptome analysis using RNA-seq revealed drug-induced genes.
In order to get insights into the mode of action underlying the synergistic effect of CAS and humidimycin on A. fumigatus, mycelia were grown in liquid medium and stressed by addition of these compounds alone or in combination. RNA sequencing (RNA-seq) analysis indicated that 668 genes were differentially regulated in response to CAS, 140 in response to humidimycin, and 571 in response to the combination of both compounds (see Fig. S1 in the supplemental material). The differentially regulated genes appeared overall quite scattered. It was not possible to group them in defined categories, and the majority of the known cell wall biosynthetic genes were not affected by the treatments. Additionally, almost half of the detected genes were classified as hypothetical proteins. However, the analysis highlighted at least that CAS stress (0.1 μg/ml) led to differential regulation of genes involved in carbohydrate metabolism, which play a possible role in cell wall biosynthesis, indicating that CAS triggered cell wall stress. Another interesting aspect revealed by this experiment was that CAS affects expression of many (about 50) major facilitator superfamily (MFS) transporters, which were globally downregulated.
To further investigate the effects of humidimycin, A. fumigatus was incubated with 1 μg/ml of drug in liquid culture, which was determined in preliminary experiments to be optimal (Table 2). The number of differentially expressed genes induced at this concentration was rather low (see Fig. S1 in the supplemental material), and also in this case more than half were classified as hypothetical proteins.
When both drugs were combined, the same scenario was observed. What was clear from this experiment was that the total number of differentially expressed genes was lower than that upon CAS treatment alone (see Fig. S1 in the supplemental material), implying that humidimycin reduced the A. fumigatus stress response against CAS. This assumption was confirmed by gene-clustering analysis (see Fig. S2). However, even if this analysis revealed the presence of a group of genes putatively involved in cell wall biosynthesis that were more significantly regulated during CAS stress and less when both drugs were used together, a common denominator among them was not found.
Sensitivity of A. fumigatus signaling mutants to CAS and humidimycin.
To further analyze the mode of action of humidimycin, four A. fumigatus mutants (ΔmpkA, Δskn7, ΔsakA, and Δyap1) displaying deletions in the central regulators of the cellular stress response were tested for sensitivity against CAS and humidimycin (Table 1). Among other functions, the MAP kinase MpkA represents the main element in the regulation of the cell wall integrity (CWI) pathway and of the oxidative stress response (26). Skn7 and Yap1 are transcription factors contributing to the oxidative stress response. Skn7 can also act as a nuclear response regulator in the two-component-like phosphorelay system, contributing to the control of cell wall biosynthesis and osmoregulation (27–29). The MAP kinase SakA plays a role in osmotic and oxidative stress responses and is the major regulator of the HOG pathway (30). The wild-type strains ATCC 46645 and CEA17ΔakuB were used as controls (23, 24). The MECs were measured after 24 to 48 h of static incubation at 37°C. The ΔmpkA mutant was highly sensitive to CAS, while the Δskn7 and ΔsakA mutants showed moderate growth defects (Table 4). Concerning humidimycin, the MECs of the ΔmpkA and Δyap1 mutants were identical to the value for the wild-type control strains (8 μg/ml) (Table 4). In contrast, the A. fumigatus Δskn7 and ΔsakA mutants exhibited MECs considerably lower than those of the wild type and the other mutant strains tested.
TABLE 4.
Minimal effective concentrations of humidimycin and CAS for the A. fumigatus mutant strains testeda
| A. fumigatus strains | MEC (μg/ml) |
|
|---|---|---|
| CAS (24–48 h) | Humidimycin (24–48 h) | |
| ATCC 46645 | 0.038–0.550 | >8, >8 |
| CEA17ΔakuB | 0.034–0.435 | >8, >8 |
| ΔmpkA | 0.008–0.010 | >8, >8 |
| Δskn7 | 0.014–0.315 | 0.015–0.180 |
| ΔsakA | 0.013–0.235 | 0.015–0.020 |
| Δyap1 | 0.012–0.440 | >8, >8 |
Data are geometric mean values of at least two independent determinations.
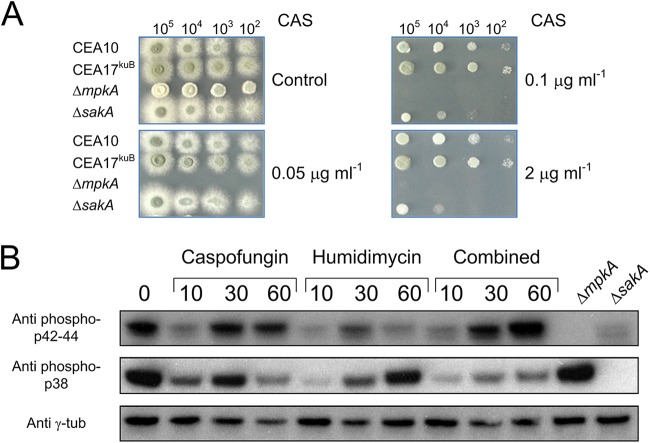
To further determine the role of central MAP kinases, different spore concentrations of the ΔmpkA and ΔsakA mutant strains were inoculated on agar plates supplemented with increasing concentrations of CAS (Fig. 3A). As expected, the ΔmpkA strain did not grow in the presence of CAS even at low concentrations (0.05 μg/ml). The growth of the ΔsakA strain was only inhibited by CAS concentrations of >0.1 μg/ml. However, as observed for the wild-type strain, a further increase in the CAS concentration led to the paradoxical effect exerted by this drug, which was also observed for the ΔsakA mutant. This experiment indicated that the SakA signaling pathway is most likely involved in the CAS response, but even when the sakA gene was deleted, CAS still acted fungistatically.
FIG 3.
Sensitivity of the ΔsakA and ΔmpkA strains to different CAS concentrations and Western blot analysis. (A) The indicated numbers of conidia were spotted on AMM agar plates with or without different concentrations of CAS. (B) The wild-type strain was stressed by humidimycin and CAS alone or in combination, and samples were collected at the indicated time points (minutes after stress). The anti-phospho p42-44 antibody was used to detect the phosphorylated form of MpkA, while the anti-phospho p38 antibody was used for SakA phosphorylated forms. As negative controls, proteins extracted from the ΔsakA and the ΔmpkA strains under nonstressed condition were used. γ-Tubulin was used as a control (Anti γ-tub).
To clarify the role of MpkA and SakA in the response to humidimycin and CAS, the phosphorylation status of both kinases was investigated by Western blot analysis in a wild-type strain stressed by these compounds alone or in combination. Addition of CAS to the medium decreased the phosphorylation levels of MpkA and SakA at the early time point (10 min after stress induction). However, the phosphorylation levels of both kinases increased 30 min after induction, confirming that the activation of both kinases was associated with the stress response against CAS. At 60 min after CAS induction, MpkA was still phosphorylated, while the SakA phosphorylation level was decreased. On the other hand, the addition of humidimycin alone reduced the MpkA and SakA phosphorylation levels, but only the SakA phosphorylation level then increased at a later exposition time (Fig. 3B). When both compounds were simultaneously given, the MpkA activation induced by CAS was not affected by the presence of humidimycin, while the SakA activation was inhibited at all time points (Fig. 3B).
DISCUSSION
The search for compounds to increase the activity of the clinically used CAS against A. fumigatus and C. albicans led to the discovery of humidimycin. Compounds with CAS-enhancing activity are of high interest not only for their potential application in the clinic to improve current therapies but also in basic research to reveal salvage pathways against CAS. It was reasonable to assume that salvage pathways can be identified by a combination of transcriptome analyses and the use of mutants defective in various signal transduction pathways. The analysis of transcriptomic data did not reveal any possible target for humidimycin. Additionally, the resulting data were not clear enough to draw a picture about the modes of action of the compounds used. However, this approach at least showed that the global response of A. fumigatus to CAS stress was reduced by the presence of humidimycin. Consistently, the absolute number of differentially regulated genes induced by CAS decreased in the presence of humidimycin, suggesting that potential salvage pathways were affected.
Inhibition assays demonstrated that the A. fumigatus mutant ΔmpkA is far more susceptible to CAS than the wild type. This result was not surprising because the cell wall is the primary target of echinocandins like CAS, and MpkA is the major regulator of the CWI pathway (20, 26). Consistently, the addition of CAS to culture broth affected the phosphorylation of MpkA. Similarly, the Δskn7 and ΔsakA mutant strains displayed enhanced sensitivity to CAS. Because Δskn7 and ΔsakA mutant strains are also more sensitive against oxidative stress (27, 28, 30), it was conceivable that CAS induced this type of stress. However, this assumption was excluded because the Δyap1 mutant, which lacks one of the major regulators of the fungal response against reactive oxygen intermediates, did not display enhanced susceptibility to CAS (28).
Humidimycin belongs to the group of siamycins, which were shown to have strong antimicrobial activities against Gram-positive bacteria and also to possess anti-HIV activity (31, 32). In a noncompetitive way siamycin I inhibits the autophosphorylation of membrane sensor kinases in Enterococcus faecalis (33). Sensor kinases are members of two-component systems, which were discovered in prokaryotes and later found in some eukaryotes, but not in the animal kingdom. This is one of the reasons why they are considered good candidates for antifungal targets (34). In fungi, sensor kinases belong to a hybrid form consisting of both histidine kinases and response regulator receiver domains. Such hybrid histidine kinases are responsible for the activation of the HOG pathway via a two-component-like phosphorelay system (29). The yeast Saccharomyces cerevisiae encodes a single histidine kinase, Sln1, which regulates the histidine-containing phosphotransfer protein Ypd1. Ypd1 has a dual function: it activates Skn7 in response to oxidative or cell wall stress (35) and inhibits the MAP kinase Ssk2, which is an element of the HOG pathway (29). In S. cerevisiae, deletion of sln1 caused constitutive activation of the HOG pathway, which is lethal for yeast cells (36). The increased phosphorylation of SakA, the major kinase of the HOG pathway, during incubation with humidimycin suggested that this drug also affected histidine kinase two-component systems. In A. fumigatus, the ΔsakA and the Δskn7 mutant strains showed increased sensitivity to humidimycin compared to that for the wild-type strains. Both components are supposed to work downstream of putative histidine kinases. However, this observation needs to be further clarified to better assign a function to these two genes. Additionally, A. fumigatus encodes 14 putative hybrid histidine kinases (29), which makes it difficult to predict whether humidimycin only inhibits a specific histidine kinase or several kinases.
As shown here, the combination of CAS and humidimycin led to the activation of MpkA, but not of SakA. Our data thus support a scenario in which the synergistic effect exerted by the drug combination is caused by the misbalancing of the HOG signaling pathway, which leads to a reduced response against both CAS and humidimycin. The increased sensitivity of the sakA deletion mutants against CAS is in line with this hypothesis.
As a second hypothesis, the possibility that elements of the hybrid histidine kinase family can be involved in the CAS stress response and that the inhibition of their activity can decrease the global stress response must be considered. The active involvement of histidine kinases in the cell wall stress response was already demonstrated in S. cerevisiae (37). This hypothesis is suggested by the decrease in the total number of differentially expressed genes detected by using the two drugs in combination in comparison to the response induced by CAS alone. However, all sensor kinases studied so far in filamentous fungi are mainly involved in morphology and differentiation, but none of them was related to stress sensing, besides some role in osmosensing (38). Therefore, the role of hybrid sensor kinases in stress sensing in filamentous fungi remains to be elucidated.
We hypothesized that the synergistic effect of combining humidimycin and ITZ is likely due to the involvement of the CWI pathway in the azole stress response. It was previously reported that A. fumigatus mutants having a defective cell wall signaling pathway are more sensitive to azole derivatives (39); thus, it is not surprising that this compound potentiates the activity of ITZ in vitro.
In conclusion, we discovered the novel natural product humidimycin, which enhances the activity of CAS and ITZ. As shown here, it is an appropriate strategy to increase antifungal activity of clinically used drugs by targeting potential compensatory mechanisms. Moreover, since humidimycin did not exhibit its antifungal effects alone, it is unlikely that resistance against such a compound will emerge. The lack of in vitro cytotoxicity of humidimycin against a human hepatocyte cell line suggests promising utilization for the potential development of a combined treatment of invasive fungal infections.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the project ERA-NET Pathogenomics (7th FP) ANTIFUN “The Cell Wall as a Target to Improve Antifungal Therapy against Aspergillosis,” by BFU2008-04709-E/BMC (German Federal Ministry of Education and Research: BMBF FKZ 0315439), by the European Science Fundation (Fuminomics 06-RNP-132), and by the Ministerio de Ciencia e Innovación (grants PCT-010000-2010-4 [NMR] and INP-2011-0016-PCT-010000-ACT6 [polarimeter, HPLC, and IR]).
We thank Silke Steinbach for excellent technical assistance. We also thank Mercedes de la Cruz, Juan Cantizani, and C. Moreno for the help in the early stage of the screening process.
Merck, Sharp & Dohme GmbH (Germany) provided the caspofungin used in the experiments.
We declare no conflicts of interest.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00683-15.
REFERENCES
- 1.Lackner M, Lass-Florl C. 2013. Up-date on diagnostic strategies of invasive aspergillosis. Curr Pharm Des 19:3595–3614. doi: 10.2174/13816128113199990323. [DOI] [PubMed] [Google Scholar]
- 2.Brakhage AA. 2005. Systemic fungal infections caused by Aspergillus species: epidemiology, infection process and virulence determinants. Curr Drug Targets 6:875–886. doi: 10.2174/138945005774912717. [DOI] [PubMed] [Google Scholar]
- 3.Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. 2012. Hidden killers: human fungal infections. Sci Transl Med 4:165rv13. doi: 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- 4.Cowen LE, Steinbach WJ. 2008. Stress, drugs, and evolution: the role of cellular signaling in fungal drug resistance. Eukaryot Cell 7:747–764. doi: 10.1128/EC.00041-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bowman JC, Hicks PS, Kurtz MB, Rosen H, Schmatz DM, Liberator PA, Douglas CM. 2002. The antifungal echinocandin caspofungin acetate kills growing cells of Aspergillus fumigatus in vitro. Antimicrob Agents Chemother 46:3001–3012. doi: 10.1128/AAC.46.9.3001-3012.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karthaus M. 2011. Prophylaxis and treatment of invasive aspergillosis with voriconazole, posaconazole and caspofungin: review of the literature. Eur J Med Res 16:145–152. doi: 10.1186/2047-783X-16-4-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gardiner RE, Souteropoulos P, Park S, Perlin DS. 2005. Characterization of Aspergillus fumigatus mutants with reduced susceptibility to caspofungin. Med Mycol 43(Suppl 1):S299–S305. [DOI] [PubMed] [Google Scholar]
- 8.Wiederhold NP. 2007. Attenuation of echinocandin activity at elevated concentrations: a review of the paradoxical effect. Curr Opin Infect Dis 20:574–578. doi: 10.1097/QCO.0b013e3282f1be7f. [DOI] [PubMed] [Google Scholar]
- 9.Moretti S, Bozza S, D'Angelo C, Casagrande A, Della Fazia MA, Pitzurra L, Romani L, Aversa F. 2012. Role of innate immune receptors in paradoxical caspofungin activity in vivo in preclinical aspergillosis. Antimicrob Agents Chemother 56:4268–4276. doi: 10.1128/AAC.05198-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deresinski SC, Stevens DA. 2003. Caspofungin. Clin Infect Dis 36:1445–1457. doi: 10.1086/375080. [DOI] [PubMed] [Google Scholar]
- 11.Morschhäuser J. 2010. Regulation of multidrug resistance in pathogenic fungi. Fungal Genet Biol 47:94–106. doi: 10.1016/j.fgb.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Monteiro MC, de la Cruz M, Cantizani J, Moreno C, Tormo JR, Mellado E, De Lucas JR, Asensio F, Valiante V, Brakhage AA, Latge JP, Genilloud O, Vicente F. 2012. A new approach to drug discovery: high-throughput screening of microbial natural extracts against Aspergillus fumigatus using resazurin. J Biomol Screen 17:542–549. doi: 10.1177/1087057111433459. [DOI] [PubMed] [Google Scholar]
- 13.Detlefsen DJ, Hill SE, Volk KJ, Klohr SE, Tsunakawa M, Furumai T, Lin PF, Nishio M, Kawano K, Oki T, et al. 1995. Siamycins I and II, new anti-HIV-1 peptides: II. Sequence analysis and structure determination of siamycin I J Antibiot (Tokyo) 48:1515–1517. [DOI] [PubMed] [Google Scholar]
- 14.Tsunakawa M, Hu SL, Hoshino Y, Detlefson DJ, Hill SE, Furumai T, White RJ, Nishio M, Kawano K, Yamamoto S, et al. 1995. Siamycins I and II, new anti-HIV peptides: I. Fermentation, isolation, biological activity and initial characterization. J Antibiot (Tokyo) 48:433–434. [DOI] [PubMed] [Google Scholar]
- 15.Ebel F, Schwienbacher M, Beyer J, Heesemann J, Brakhage AA, Brock M. 2006. Analysis of the regulation, expression, and localisation of the isocitrate lyase from Aspergillus fumigatus, a potential target for antifungal drug development. Fungal Genet Biol 43:476–489. doi: 10.1016/j.fgb.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 16.Marchetti O, Moreillon P, Glauser MP, Bille J, Sanglard D. 2000. Potent synergism of the combination of fluconazole and cyclosporine in Candida albicans. Antimicrob Agents Chemother 44:2373–2381. doi: 10.1128/AAC.44.9.2373-2381.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orhan G, Bayram A, Zer Y, Balci I. 2005. Synergy tests by E test and checkerboard methods of antimicrobial combinations against Brucella melitensis. J Clin Microbiol 43:140–143. doi: 10.1128/JCM.43.1.140-143.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramani R, Gangwar M, Chaturvedi V. 2003. Flow cytometry antifungal susceptibility testing of Aspergillus fumigatus and comparison of mode of action of voriconazole vis-à-vis amphotericin B and itraconazole. Antimicrob Agents Chemother 47:3627–3629. doi: 10.1128/AAC.47.11.3627-3629.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Subcommittee on Antifungal Susceptibility Testing of the ESCMID European Committee for Antimicrobial Susceptibility Testing. 2008. EUCAST technical note on the method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for conidia-forming moulds. Clin Microbiol Infect 14:982–984. doi: 10.1111/j.1469-0691.2008.02086.x. [DOI] [PubMed] [Google Scholar]
- 20.Valiante V, Jain R, Heinekamp T, Brakhage AA. 2009. The MpkA MAP kinase module regulates cell wall integrity signaling and pyomelanin formation in Aspergillus fumigatus. Fungal Genet Biol 46:909–918. doi: 10.1016/j.fgb.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 21.Tormo JR, DePedro N, Royo I, Barrachina I, Zafra-Polo MC, Cuadrillero C, Hernandez P, Cortes D, Pelaez F. 2005. In vitro antitumor structure-activity relationships of threo/trans/threo/trans/erythro bis-tetrahydrofuranic acetogenins: correlations with their inhibition of mitochondrial complex I. Oncol Res 15:129–138. [DOI] [PubMed] [Google Scholar]
- 22.Royo I, DePedro N, Estornell E, Cortes D, Pelaez F, Tormo JR. 2003. In vitro antitumor SAR of threo/cis/threo/cis/erythro bis-THF acetogenins: correlations with their inhibition of mitochondrial complex I. Oncol Res 13:521–528. [DOI] [PubMed] [Google Scholar]
- 23.Jahn B, Koch A, Schmidt A, Wanner G, Gehringer H, Bhakdi S, Brakhage AA. 1997. Isolation and characterization of a pigmentless-conidium mutant of Aspergillus fumigatus with altered conidial surface and reduced virulence. Infect Immun 65:5110–5117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.da Silva Ferreira ME, Kress MR, Savoldi M, Goldman MH, Hartl A, Heinekamp T, Brakhage AA, Goldman GH. 2006. The akuBKU80 mutant deficient for nonhomologous end joining is a powerful tool for analyzing pathogenicity in Aspergillus fumigatus. Eukaryot Cell 5:207–211. doi: 10.1128/EC.5.1.207-211.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Constantine KL, Friedrichs MS, Detlefsen D, Nishio M, Tsunakawa M, Furumai T, Ohkuma H, Oki T, Hill S, Bruccoleri RE, et al. 1995. High-resolution solution structure of siamycin II: novel amphipathic character of a 21-residue peptide that inhibits HIV fusion. J Biomol NMR 5:271–286. [DOI] [PubMed] [Google Scholar]
- 26.Jain R, Valiante V, Remme N, Docimo T, Heinekamp T, Hertweck C, Gershenzon J, Haas H, Brakhage AA. 2011. The MAP kinase MpkA controls cell wall integrity, oxidative stress response, gliotoxin production and iron adaptation in Aspergillus fumigatus. Mol Microbiol 82:39–53. doi: 10.1111/j.1365-2958.2011.07778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lamarre C, Ibrahim-Granet O, Du C, Calderone R, Latge JP. 2007. Characterization of the SKN7 ortholog of Aspergillus fumigatus. Fungal Genet Biol 44:682–690. doi: 10.1016/j.fgb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 28.Lessing F, Kniemeyer O, Wozniok I, Loeffler J, Kurzai O, Haertl A, Brakhage AA. 2007. The Aspergillus fumigatus transcriptional regulator AfYap1 represents the major regulator for defense against reactive oxygen intermediates but is dispensable for pathogenicity in an intranasal mouse infection model. Eukaryot Cell 6:2290–2302. doi: 10.1128/EC.00267-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bahn YS. 2008. Master and commander in fungal pathogens: the two-component system and the HOG signaling pathway. Eukaryot Cell 7:2017–2036. doi: 10.1128/EC.00323-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Du C, Sarfati J, Latge JP, Calderone R. 2006. The role of the sakA (Hog1) and tcsB (sln1) genes in the oxidant adaptation of Aspergillus fumigatus. Med Mycol 44:211–218. doi: 10.1080/13693780500338886. [DOI] [PubMed] [Google Scholar]
- 31.Nakayama J, Tanaka E, Kariyama R, Nagata K, Nishiguchi K, Mitsuhata R, Uemura Y, Tanokura M, Kumon H, Sonomoto K. 2007. Siamycin attenuates fsr quorum sensing mediated by a gelatinase biosynthesis-activating pheromone in Enterococcus faecalis. J Bacteriol 189:1358–1365. doi: 10.1128/JB.00969-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fréchet D, Guitton JD, Herman F, Faucher D, Helynck G, Monegier du Sorbier B, Ridoux JP, James-Surcouf E, Vuilhorgne M. 1994. Solution structure of RP 71955, a new 21 amino acid tricyclic peptide active against HIV-1 virus. Biochemistry 33:42–50. doi: 10.1021/bi00167a006. [DOI] [PubMed] [Google Scholar]
- 33.Ma P, Nishiguchi K, Yuille HM, Davis LM, Nakayama J, Phillips-Jones MK. 2011. Anti-HIV siamycin I directly inhibits autophosphorylation activity of the bacterial FsrC quorum sensor and other ATP-dependent enzyme activities. FEBS Lett 585:2660–2664. doi: 10.1016/j.febslet.2011.07.026. [DOI] [PubMed] [Google Scholar]
- 34.Chauhan N, Calderone R. 2008. Two-component signal transduction proteins as potential drug targets in medically important fungi. Infect Immun 76:4795–4803. doi: 10.1128/IAI.00834-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown JL, Bussey H, Stewart RC. 1994. Yeast Skn7p functions in a eukaryotic two-component regulatory pathway. EMBO J 13:5186–5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Posas F, Wurgler-Murphy SM, Maeda T, Witten EA, Thai TC, Saito H. 1996. Yeast HOG1 MAP kinase cascade is regulated by a multistep phosphorelay mechanism in the SLN1-YPD1-SSK1 “two-component” osmosensor. Cell 86:865–875. doi: 10.1016/S0092-8674(00)80162-2. [DOI] [PubMed] [Google Scholar]
- 37.Shankarnarayan S, Malone CL, Deschenes RJ, Fassler JS. 2008. Modulation of yeast Sln1 kinase activity by the CCW12 cell wall protein. J Biol Chem 283:1962–1973. doi: 10.1074/jbc.M706877200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grice CM, Bertuzzi M, Bignell EM. 2013. Receptor-mediated signaling in Aspergillus fumigatus. Front Microbiol 4:26. doi: 10.3389/fmicb.2013.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dirr F, Echtenacher B, Heesemann J, Hoffmann P, Ebel F, Wagener J. 2010. AfMkk2 is required for cell wall integrity signaling, adhesion, and full virulence of the human pathogen Aspergillus fumigatus. Int J Med Microbiol 300:496–502. doi: 10.1016/j.ijmm.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 40.d'Enfert C. 1996. Selection of multiple disruption events in Aspergillus fumigatus using the orotidine-5′-decarboxylase gene, pyrG, as a unique transformation marker. Curr Genet 30:76–82. doi: 10.1007/s002940050103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.