Abstract
We evaluated the in vitro activity of various antimicrobials alone and in combination against 291 extended-spectrum-β-lactamase-producing Escherichia coli (ESBL-EC) isolates causing bacteremia in South Korean hospitals. Ceftazidime, cefepime, and piperacillin-tazobactam in combination with amikacin showed greater activity than found in combination with ciprofloxacin. In settings with a high prevalence of ESBL-producing pathogens, combination aminoglycoside antimicrobial therapy, especially with amikacin, may be considered for empirical therapy against suspected Gram-negative sepsis as a carbapenem-saving strategy.
TEXT
The emergence of extended-spectrum-β-lactamase-producing Escherichia coli (ESBL-EC) in the community, particularly those producing CTX-M β-lactamase enzymes, is one of the most significant epidemiological changes in infectious diseases during recent decades (1). ESBL-EC strains have narrow treatment options that are limited to a small number of antibiotics because coresistance to different classes of antimicrobials is frequent. Because of the multidrug resistance of ESBL-EC strains and the emergence of carbapenem-resistant Enterobacteriaceae, therapeutic options for the treatment of ESBL-EC infections have become limited. For the treatment of severe infections caused by ESBL-EC, carbapenems are generally considered the mainstay for antimicrobial therapy; however, the emergence of resistance is a matter of concern due to the widespread use of carbapenems (2–4). The initial purpose of combination therapy is to broaden the empirical coverage provided by two antimicrobial agents with different activity spectra (5). Advantages include the theoretical possibility of minimizing the emergence of antimicrobial resistance and potential synergistic interactions (6). The purpose of the current study was to analyze nationwide data on the susceptibilities of ESBL-EC isolates causing bacteremia and to improve empirical approaches to therapy for these serious infections.
As a part of the multicenter surveillance study on bacteremia from March 2012 to December 2013, a total of 291 ESBL-EC blood isolates were collected from seven hospitals in various regions of South Korea: Samsung Medical Center (Seoul), Kyunghee University Medical center (Seoul), Keimyung University Dongsan Medical Center (Daegu), Daegu Fatima Hospital (Daegu), Samsung Changwon Hospital (Changwon), Changwon Fatima Hospital (Changwon), and Chungnam National University Hospital (Daejeon). Isolates were maintained in brain heart infusion broth (BD Diagnostics, Sparks, MD) with 50% glycerol and stored at −70°C until use. Isolates were subcultured a minimum of three times prior to experimentation. All antibiotics except fosfomycin were tested by broth microdilution for antimicrobial susceptibility. The MIC of fosfomycin was determined by the agar dilution method using agar medium supplemented with 25 mg/liter of glucose-6-phosphate. MICs were interpreted with category designations according to the criteria of the Clinical and Laboratory Standards Institute (CLSI). E. coli (ATCC 25922) and Pseudomonas aeruginosa (ATCC 27853) were used as the control strains. According to the European Committee on Antimicrobial Susceptibility Testing (EUCAST), susceptible and resistant MIC breakpoints for tigecycline are ≤1 mg/liter and >2 mg/liter, respectively; the CLSI has yet to set values.
The results of the antimicrobial susceptibility testing of each antimicrobial agent were used to evaluate the in vitro activity of antimicrobial combinations. If the isolate was susceptible to either one of two antimicrobial agents taken together, the isolate was considered susceptible to the antimicrobial combination. For instance, if the isolate was resistant to ceftazidime but susceptible to amikacin, the isolate was considered susceptible to the antimicrobial combination. ESBL activity was confirmed via a double-disk synergy test (ceftazidime, cefotaxime, and aztreonam MICs of ≥2 mg/liter) using BD BBL Sensi-Discs (BD Diagnostics, Sparks, MD). ESBL-related genes, including blaTEM, blaSHV, and blaCTX-M, were amplified by PCR as described previously (7, 8). Statistical analysis was performed using SPSS for Windows (version 11.5 software; SPSS, Inc., Chicago, IL).
Results of the in vitro activity of 21 antimicrobial agents against 291 ESBL-EC isolates are shown in Table 1. More than 80% of these isolates were nonsusceptible to ampicillin, ampicillin-sulbactam, ticarcillin-clavulanic acid, cefotaxime, and aztreonam. The proportion of nonsusceptible isolates to tigecycline, fosfomycin, and amikacin was <12%. Among the carbapenems, only one isolate was nonsusceptible to imipenem, while 5 (1.7%), 6 (2.1%), and 15 (5.2%) were nonsusceptible to doripenem, meropenem, and ertapenem, respectively. Of the CTX-M groups, CTX-M-14 (44.3%) was the most frequent type, followed by CTX-M-15 (34.7%), CTX-M-55 (6.1%), CTX-M-27 (4.4%), and CTX-M-24 (3.0%). A total of 130 isolates (44.6%) produced TEM-1 along with CTX-M-type ESBL, and 3 isolates produced SHV-12 and TEM-1.
TABLE 1.
Results of antimicrobial susceptibility testing of ESBL-EC isolates
| Antimicrobial agent(s) | MIC (mg/liter)a |
No. (%) of nonsusceptible isolates | ||
|---|---|---|---|---|
| 50% | 90% | Range | ||
| Fosfomycin | 2 | 4 | 0.25–256 | 13 (4.5) |
| Ampicillin | >256 | >256 | 0.06–256 | 273 (93.8) |
| Ampicillin-sulbactam | 64/32 | >64/32 | 0.06/0.03–64/32 | 266 (91.4) |
| Amoxicillin-clavulanic acid | 16/8 | >32/16 | 0.03/0.015–32/16 | 230 (79.0) |
| Ticarcillin-clavulanic acid | 128/2 | >256/2 | 0.25/2–256/2 | 246 (84.5) |
| Ceftazidime | 16 | >64 | 0.06–64 | 166 (57.0) |
| Cefotaxime | >128 | >128 | 0.12–128 | 265 (91.1) |
| Cefepimeb | 128 | >128 | 0.12–128 | 207 (71.1) |
| Aztreonam | 32 | >64 | 0.06–64 | 233 (80.1) |
| Piperacillin-tazobactam | 4/4 | 256/4 | 0.25/4–256/4 | 95 (32.6) |
| Imipenem | 0.12 | 0.25 | 0.06–64 | 1 (0.3) |
| Meropenem | 0.06 | 0.12 | 0.06–64 | 6 (2.1) |
| Doripenem | 0.03 | 0.06 | 0.015–16 | 5 (1.7) |
| Ertapenem | 0.03 | 0.25 | 0.03–32 | 15 (5.2) |
| Ciprofloxacin | 32 | >64 | 0.06–64 | 204 (70.1) |
| Amikacin | 8 | 32 | 0.12–128 | 34 (11.7) |
| Gentamicin | 8 | >64 | 0.06–64 | 151 (51.9) |
| Tobramycin | 8 | 64 | 0.06–64 | 151 (51.9) |
| Tigecycline | 0.25 | 1 | 0.06–64 | 10 (3.4) |
| Colistin | 0.25 | 0.5 | 0.06–64 | |
| Trimethoprim-sulfamethoxazole | >32/608 | >32/608 | 0.03/0.59–32/608 | 157 (54.0) |
50% and 90%, MICs at which 50% and 90% of the isolates tested were inhibited.
For cefepime, isolates with MICs between 4 and 8 mg/liter (susceptible-dose dependent) were considered susceptible.
In coresistance analysis, >80% of ESBL-EC isolates resistant to ceftazidime, cefepime, and piperacillin-tazobactam were concurrently resistant to ampicillin, ampicillin-sulbactam, amoxicillin-clavulanic acid, ticarcillin-clavulanic acid, cefotaxime, and aztreonam. In contrast, <25% of isolates resistant to ceftazidime, cefepime, and piperacillin-tazobactam were resistant to fosfomycin, amikacin, tigecycline, and the carbapenems. The proportions of isolates nonsusceptible to ceftazidime, cefepime, and piperacillin-tazobactam were 57.0%, 71.1%, and 32.6%, respectively. The distributions of ceftazidime, cefepime, and piperacillin-tazobactam MICs are shown in Fig. 1. The in vitro efficacy of several antimicrobial combinations was assessed with β-lactam antibiotics (ceftazidime, cefepime, and piperacillin-tazobactam) in combination with amikacin or ciprofloxacin, which are currently available in clinical practice (Fig. 2). For evaluation of the in vitro activity of antimicrobial combinations, if the isolate was susceptible to either of two antimicrobial agents taken together, the isolate was considered susceptible to the antimicrobial combination. Based on the antimicrobial susceptibility data, the three β-lactams (ceftazidime, cefepime, and piperacillin-tazobactam) in combination with amikacin (susceptibility rates, 91.4%, 90.4%, and 92.8%, respectively) showed greater activity than that seen in combination with ciprofloxacin (52.9%, 43.6%, and 71.1%, respectively). The combination of piperacillin-tazobactam with amikacin was the most susceptible regimen, other than the carbapenems; in particular, the rate of susceptibility to cefepime in combination with amikacin was much higher than that of cefepime alone (90.4% versus 28.9%; P < 0.05).
FIG 1.
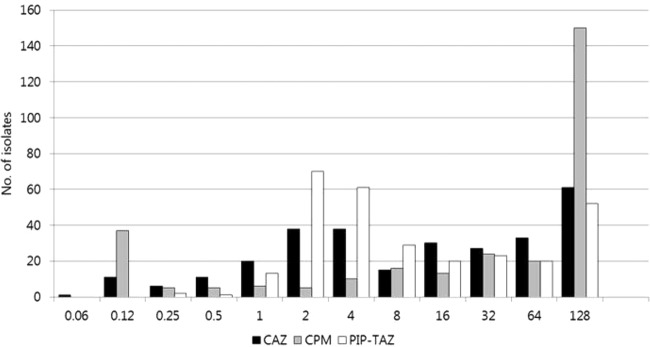
Distribution of MICs (mg/liter) of ceftazidime (CAZ), cefepime (CPM), and piperacillin-tazobactam (PIP-TAZ). The MIC breakpoints for resistance were as follows: ceftazidime, ≥16 mg/liter; cefepime, ≥16 mg/liter; and piperacillin-tazobactam, ≥128/4 mg/liter. For cefepime, isolates with MICs between 4 and 8 mg/liter (susceptible-dose dependent) were susceptible.
FIG 2.
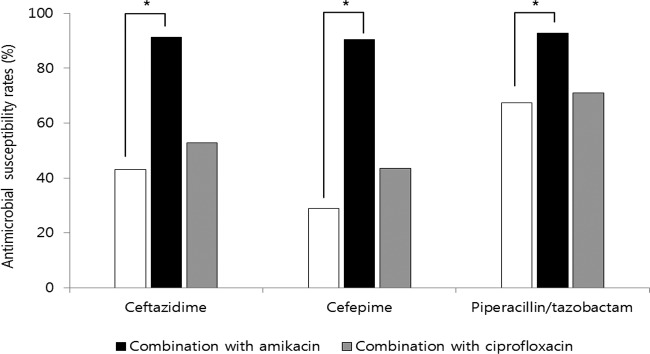
Susceptibility of ESBL-EC to three β-lactam antibiotics alone and in combination with amikacin and ciprofloxacin. *, P < 0.05.
Among the antimicrobial agents other than the carbapenems included in this study, tigecycline and fosfomycin showed good activity in vitro against ESBL-EC. The rates of susceptibility to ceftazidime, cefotaxime, cefepime, and ciprofloxacin were quite low, similar to those in a previous study (9). Carbapenems were the most active agents against ESBL-EC, and only one isolate was nonsusceptible to imipenem. However, increased empirical use of carbapenems in response to an increased prevalence of ESBL-producing isolates may be accompanied by a rapid emergence of carbapenem resistance in other pathogens (10), rendering the genes encoding carbapenem-hydrolyzing enzymes, such as KPCs or metallo-β-lactamases, easier to spread via horizontal gene transfer (11). Therefore, alternatives to the carbapenems should be considered for empirical treatment of suspected Gram-negative sepsis whenever possible.
For infections caused by Gram-negative bacteria, antimicrobial synergy has traditionally been seen with β-lactam and aminoglycoside combination treatment, as the combination of a β-lactam and an aminoglycoside allows for different mechanisms of bacterial killing (12, 13). Our study evaluated the β-lactams ceftazidime, cefepime, and piperacillin-tazobactam in combination with amikacin or ciprofloxacin. These three β-lactams in combination with amikacin showed greater activity than when combined with ciprofloxacin because the susceptibility rate to amikacin was much higher than that to ciprofloxacin. Similarly, the combination of cefepime with amikacin increased the susceptibility rate from 28.9% to 90.4% against ESBL-EC. The combination of piperacillin-tazobactam with amikacin was the most susceptible regimen (92.8%) among the combinations, comparable to the carbapenems. Ciprofloxacin had low activity against ESBL-EC, with only 29.9% of susceptible isolates. Based on the in vitro susceptibility testing results, amikacin would be the most likely agent to increase the range of antimicrobial coverage against ESBL-EC.
Recent studies suggested that a survival benefit based on initial combination therapy appears to be the greatest in patients at the highest risk of death, such as those with septic shock (14, 15). Although the role of combination therapy in Gram-negative sepsis has been controversial with regard to synergistic effects, it is becoming increasingly important to achieve adequate empirical antimicrobial therapies (16).
In this study, we assessed the in vitro efficacy of 21 antimicrobial agents alone and in combination against ESBL-EC isolates causing bacteremia in South Korean hospitals. Our in vitro results indicate that the combination of β-lactam antibiotics, such as cefepime or piperacillin-tazobactam, with an aminoglycoside or a fluoroquinolone increases isolate susceptibility. In settings with a high prevalence of ESBL-producing pathogens, combination antimicrobial therapy with an aminoglycoside, especially amikacin, can be considered an empirical therapy against suspected Gram-negative sepsis, as one of the carbapenem-saving strategies.
ACKNOWLEDGMENTS
We thank all participating investigators at the KONSID as follows: Jun Seong Son and Soo-Youn Moon, Kyung Hee University Hospital at Gangdong, Seoul, South Korea; Choon Kwan Kim, Seoul Veterans Hospital, Seoul, South Korea; Seung Soon Lee and Jeong-A Lee, Hallym University Sacred Heart Hospital, Seoul, South Korea; Yeon-Sook Kim and Kyung Mok Sohn, Chungnam National University Hospital, Daejeon, South Korea; Ji-Young Rhee, Dankook University Hospital, Cheonan, South Korea; Sook-In Jung, Kyung Hwa Park, and Seung Ju Kang, Chonnam National University Hospital, Gwangju, South Korea; Shin-Woo Kim and Hyun-Ha Chang, Kyungpook National University Hospital, Daegu, South Korea; Seong Yeol Ryu and Hyun Ah Kim, Keimyung University Dongsan Medical Center, Daegu, South Korea; Ki Tae Kwon, Daegu Fatima Hospital, Daegu, South Korea; and Min Hee Lim, Changwon Fatima Hospital, Changwon, South Korea.
This study was supported by the Basic Research Program through the National Research Foundation of Korea (NRF) and funded by the Ministry of Education, Science, and Technology (no. 2010-0021572).
Bacterial isolates were obtained from the Asian Bacterial Bank (ABB) of the Asia Pacific Foundation for Infectious Diseases (APFID).
We declare that we have no conflicts of interest.
REFERENCES
- 1.Rodriguez-Bano J, Pascual A. 2008. Clinical significance of extended-spectrum beta-lactamases. Expert Rev Anti Infect Ther 6:671–683. doi: 10.1586/14787210.6.5.671. [DOI] [PubMed] [Google Scholar]
- 2.Rodriguez-Bano J, Picon E, Gijon P, Hernandez JR, Ruiz M, Pena C, Almela M, Almirante B, Grill F, Colomina J, Gimenez M, Oliver A, Horcajada JP, Navarro G, Coloma A, Pascual A. 2010. Community-onset bacteremia due to extended-spectrum beta-lactamase-producing Escherichia coli: risk factors and prognosis. Clin Infect Dis 50:40–48. doi: 10.1086/649537. [DOI] [PubMed] [Google Scholar]
- 3.Gudiol C, Calatayud L, Garcia-Vidal C, Lora-Tamayo J, Cisnal M, Duarte R, Arnan M, Marin M, Carratala J, Gudiol F. 2010. Bacteraemia due to extended-spectrum beta-lactamase-producing Escherichia coli (ESBL-EC) in cancer patients: clinical features, risk factors, molecular epidemiology and outcome. J Antimicrob Chemother 65:333–341. doi: 10.1093/jac/dkp411. [DOI] [PubMed] [Google Scholar]
- 4.Oteo J, Perez-Vazquez M, Campos J. 2010. Extended-spectrum [beta]-lactamase producing Escherichia coli: changing epidemiology and clinical impact. Curr Opin Infect Dis 23:320–326. doi: 10.1097/QCO.0b013e3283398dc1. [DOI] [PubMed] [Google Scholar]
- 5.Tamma PD, Cosgrove SE, Maragakis LL. 2012. Combination therapy for treatment of infections with Gram-negative bacteria. Clin Microbiol Rev 25:450–470. doi: 10.1128/CMR.05041-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chow JW, Yu VL. 1999. Combination antibiotic therapy versus monotherapy for Gram-negative bacteraemia: a commentary. Int J Antimicrob Agents 11:7–12. doi: 10.1016/S0924-8579(98)00060-0. [DOI] [PubMed] [Google Scholar]
- 7.Kang CI, Wi YM, Lee MY, Ko KS, Chung DR, Peck KR, Lee NY, Song JH. 2012. Epidemiology and risk factors of community onset infections caused by extended-spectrum beta-lactamase-producing Escherichia coli strains. J Clin Microbiol 50:312–317. doi: 10.1128/JCM.06002-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim J, Lim YM, Jeong YS, Seol SY. 2005. Occurrence of CTX-M-3, CTX-M-15, CTX-M-14, and CTX-M-9 extended-spectrum beta-lactamases in Enterobacteriaceae clinical isolates in Korea. Antimicrob Agents Chemother 49:1572–1575. doi: 10.1128/AAC.49.4.1572-1575.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang CI, Cha MK, Kim SH, Ko KS, Wi YM, Chung DR, Peck KR, Lee NY, Song JH. 2013. Clinical and molecular epidemiology of community-onset bacteremia caused by extended-spectrum beta-lactamase-producing Escherichia coli over a 6-year period. J Korean Med Sci 28:998–1004. doi: 10.3346/jkms.2013.28.7.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang CI, Park SY, Chung DR, Peck KR, Song JH. 2012. Piperacillin-tazobactam as an initial empirical therapy of bacteremia caused by extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae. J Infect 64:533–534. doi: 10.1016/j.jinf.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Thomson KS. 2010. Extended-spectrum-beta-lactamase, AmpC, and carbapenemase issues. J Clin Microbiol 48:1019–1025. doi: 10.1128/JCM.00219-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hancock RE, Raffle VJ, Nicas TI. 1981. Involvement of the outer membrane in gentamicin and streptomycin uptake and killing in Pseudomonas aeruginosa. Antimicrob Agents Chemother 19:777–785. doi: 10.1128/AAC.19.5.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar A, Zarychanski R, Light B, Parrillo J, Maki D, Simon D, Laporta D, Lapinsky S, Ellis P, Mirzanejad Y, Martinka G, Keenan S, Wood G, Arabi Y, Feinstein D, Kumar A, Dodek P, Kravetsky L, Doucette S. 2010. Early combination antibiotic therapy yields improved survival compared with monotherapy in septic shock: a propensity-matched analysis. Crit Care Med 38:1773–1785. doi: 10.1097/CCM.0b013e3181eb3ccd. [DOI] [PubMed] [Google Scholar]
- 14.Leekha S, Standiford HC. 2011. Empiric antimicrobial therapy for Gram-negative sepsis: back to the future. Crit Care Med 39:1995–1996. doi: 10.1097/CCM.0b013e318223b94b. [DOI] [PubMed] [Google Scholar]
- 15.Kumar A, Safdar N, Kethireddy S, Chateau D. 2010. A survival benefit of combination antibiotic therapy for serious infections associated with sepsis and septic shock is contingent only on the risk of death: a meta-analytic/meta-regression study. Crit Care Med 38:1651–1664. doi: 10.1097/CCM.0b013e3181e96b91. [DOI] [PubMed] [Google Scholar]
- 16.Johnson MT, Reichley R, Hoppe-Bauer J, Dunne WM, Micek S, Kollef M. 2011. Impact of previous antibiotic therapy on outcome of Gram-negative severe sepsis. Crit Care Med 39:1859–1865. doi: 10.1097/CCM.0b013e31821b85f4. [DOI] [PubMed] [Google Scholar]


