Abstract
Carriage and noninvasive pneumococcal isolates frequently have a higher prevalence of antimicrobial nonsusceptibility than invasive isolates. From 2009 to 2014, we determined the associated clones in 169 pediatric noninvasive nonsusceptible pneumococci from a total of 506 isolates collected after 7- and 13-valent conjugate vaccine introduction (PCV7/13) to the Irish childhood immunization schedule in 2008 and 2010, respectively. We compared our results to those from 25 noninvasive pediatric pneumococcal isolates collected in 2007, the year before introduction of conjugate vaccines. In 2007, England14-9 and Spain9V-3 accounted for 12% and 32% of nonsusceptible clones, respectively, but in 2009 to 2014, their prevalence fell to 0% and 2.4%. Furthermore, there was a significant decline in Spain6B-2 and its variants from 2009 to 2014 (P = 0.0024). Fluctuations occurred in clonal complex 320 associated with serotype 19A. The prevalence of Sweden15A-25 and its variants and ST558 (a single-locus variant of Utah35B-24) associated with nonvaccine serotypes (NVT) 15A and 35B increased from 0% and 8% in 2007 to 19% and 16% in 2013 to 2014, respectively. Pilus locus 1 (PI-1) is associated with the spread of some nonsusceptible pneumococcal clones. PI-1 was more frequently associated with PCV7/13 serotypes than NVT (P = 0.0020). Our data highlight the value of surveillance of noninvasive pneumococci following conjugate vaccine introduction. Importantly, emerging clones associated with NVT may limit the effectiveness of PCV7/13 in reducing the high rate of nonsusceptibility among pediatric noninvasive pneumococci, with implications for empirical treatment strategies.
INTRODUCTION
Streptococcus pneumoniae causes invasive infections, such as meningitis, or noninvasive infections, including conjunctivitis and otitis media. The organism is frequently carried asymptomatically in the nasopharynx of young children and can be transmitted to adults (1). Antimicrobial-nonsusceptible pneumococci pose a challenge for devising effective empirical antimicrobial treatment strategies.
Pneumococci are encapsulated with 95 serotypes described. Nontypeable (NT) pneumococci lack a capsule. Pneumococci of the same serotype may have genetically distinct backgrounds (i.e., clones), and multilocus sequence typing (MLST) characterizes pneumococci into related clonal groups or sequence types (STs) (2). Antimicrobial nonsusceptibility is common among some pneumococcal clones, such as Spain6B-2, England14-9, Sweden15A-25, and clonal complex 320 (CC320) (3). The presence of a pilus (pilus locus 1 [PI-1]) among some nonsusceptible clones may contribute to their spread (4, 5).
The first pneumococcal conjugate vaccine was the 7-valent pneumococcal conjugate vaccine (PCV7), targeting serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F. These serotypes were previously important in carriage and disease (6). Importantly, PCV7 also targeted several serotypes belonging to antimicrobial-nonsusceptible clones (3). Following PCV7 introduction, non-PCV7 serotypes increased, including serotype 19A (7–11). The frequency of serotype 19A is of concern as many 19A-associated clones, such as CC320, are antimicrobial nonsusceptible (3). The 13-valent pneumococcal conjugate vaccine (PCV13) targeting PCV7 serotypes plus serotypes 1, 3, 5, 6A, 7F, and 19A is now used in several countries and should further impact positively on nonsusceptible pneumococci. Surveillance of pneumococcal serotypes/clones following PCV13 introduction will determine if antimicrobial-nonsusceptible clones expressing nonvaccine serotypes (NVT) emerge. This surveillance should encompass pediatric carriage and noninvasive infections, as greater rates of nonsusceptibility are reported in these isolates (12).
PCV7 was introduced to the Irish childhood immunization schedule in 2008 and replaced by PCV13 in 2010. Previously, we reported on pneumococcal serotypes associated with nonsusceptibility in an Irish pediatric hospital (9). In the present study, we used MLST to further characterize these nonsusceptible noninvasive pneumococci. We also included nonsusceptible pneumococcal isolates from two other Irish pediatric hospitals collected in 2013 to 2014. We determined the prevalence of PI-1 among these isolates, given its association with some nonsusceptible clones (4, 5). Finally, we compared our results to those from a collection of noninvasive antimicrobial-nonsusceptible pneumococci collected before PCV7 introduction.
(Data on serotypes and associated nonsusceptibility patterns from 2007 and 2009 to 2012 were reported in reference 9. Nonsusceptible serotypes from 2013 were presented in part at the 32nd Annual Meeting of the European Society for Pediatric Infectious Diseases [35]. Sequence type data from 2009 to 2011 were presented at the 23rd European Congress for Clinical Microbiology and Infectious Diseases, Berlin, 2013 [36].)
MATERIALS AND METHODS
Isolate collection.
Pneumococci were isolated from carriage and noninvasive infections (i.e., conjunctivitis, nonbacteremic lower respiratory tract infection, and otitis media) at Temple Street Children's University Hospital from January to December 2007 and January 2009 to December 2012 as previously described (9). The serotype data and antimicrobial susceptibilities of isolates from 2009 to 2012 were presented previously (9). From the first quarter of 2013 to the first quarter of 2014, carriage and noninvasive pneumococci isolated at Temple Street Children's University Hospital and two other Irish pediatric hospitals in Dublin, Our Lady's Children's Hospital, Crumlin, and the Adelaide and Meath Hospital, Tallaght, were collected. Just one isolate from each child was included in the study. All hospitals provide pediatric secondary care to catchment localities (population of approximately 1.3 million) and national tertiary referral services. Children from whom pneumococci were isolated ranged in age from 10 days to 16 years. Carriage isolates included nasopharyngeal swabs and aspirates and nasal and throat swabs. Ethical approval was granted by the Ethics Committee at Temple Street Children's University Hospital.
Serotyping and antimicrobial susceptibility testing.
Serotyping was performed using multiplex PCR and the standard capsular reaction using antisera (Statens Serum Institut, Copenhagen, Denmark) (9, 13). Multiplex PCR detected serotypes 1, 3, 4, 5, 6, 7F/a, 9V/a, 14, 18, 19F, and 23F. The standard capsular reaction confirmed serotypes identified by PCR and identified serotypes not detected by PCR. Susceptibilities to penicillin, cefotaxime, tetracycline, erythromycin, clindamycin, and levofloxacin were determined using Etest (bioMérieux, Marcy l'Etoile, France). Results were interpreted using the 2013 Clinical and Laboratory Standards Institute guidelines (14). Oral and meningitis breakpoints were used to define susceptibilities to penicillin and cefotaxime, respectively (14). Nonsusceptible isolates were defined as those displaying intermediate or complete resistance to one or more of the six antimicrobials tested for. Multidrug-resistant isolates were those with complete resistance to three or more antimicrobials.
DNA extraction.
DNA was purified from overnight cultures grown on 5% sheep blood by either a manual method (GentraPurgene Yeast/Bact kit; Qiagen, N.V., Venlo, The Netherlands) or an automated method (QIAcube HT kit; Qiagen, N.V., Venlo, The Netherlands).
MLST.
All nonsusceptible pneumococci were subjected to MLST, which was performed by standard procedures (2). Allele numbers and sequence types (STs) were assigned using http://pubmlst.org/. eBURST was used to assign clonal complexes (CCs) (15). Sequence types (STs) were compared with the Pneumococcal Molecular Epidemiology Network (PMEN) clones at http://www.sph.emory.edu/PMEN.
Detection of PI-1.
Nonsusceptible pneumococci were screened for PI-1. Primers targeting the rlrA gene were used to detect PI-1 (16). This gene encodes the RlrA protein, necessary for expression of structural genes of PI-1 (17). S. pneumoniae strains TIGR4 and TIGR6 were used as positive and negative controls, respectively.
Statistical analysis.
Proportions were compared using the two-tailed Fisher's exact test on the GraphPadQuickCalcs website http://www.graphpad.com/quickcalcs/contingency1/ (accessed December 2014). A P value of ≤0.05 was considered significant. Simpson's index of diversity (D)—defined as D = Σ n(n − 1)/N(N − 1), where N = total number of isolates and n = total number of isolates of a particular ST—was used to calculate the diversity of STs associated with nonsusceptibility for each year of the study and within selected nonsusceptible serotypes (18). The Shannon index—defined as Σ ƒi log2 ƒi, where ƒi is the frequency of individual STs—was used to calculate the evenness of STs distributed among nonsusceptible pneumococci each year (19).
RESULTS
Prevalence of antimicrobial-nonsusceptible isolates.
The total number of pneumococci collected in 2007 and 2009 to 2014 was n = 611. The distribution of isolates each year was as follows: 2007, n = 105; 2009, n = 46; 2010, n = 121; 2011, n = 89; 2012, n = 83; and 2013 to 2014, n = 167. The numbers of pneumococci isolated from each anatomical site from 2007, 2009, 2010, 2011, 2012, and 2013 to 2014, respectively, were as follows: carriage, n = 44, 18, 48, 39, 30, and 41; conjunctivitis, n = 24, 10, 30, 23, 21, and 75; nonbacteremic lower respiratory tract infections, n = 26, 12, 28, 17, 21, and 30; and otitis media, n = 11, 6, 15, 10, 11, and 21. From 2009 to 2012, most carriage isolates were collected preoperatively from children undergoing upper respiratory surgery. Other carriage isolates collected during this period and 2013 to 2014 were from dermatology clinics, the emergency department, and general practitioners. The numbers of pneumococci nonsusceptible to at least one antimicrobial each year were as follows: 2007, n = 25 (23%); 2009, n = 14 (30%); 2010, n = 38 (31%); 2011, n = 19 (21%); 2012, n = 29 (35%); and 2013 to 2014, n = 69 (41%). The percentage of pneumococci resistant and intermediate to each antimicrobial tested for from 2009 to 2014 is shown in Table 1. The mean age of children overall was 36 months—37 months in children from whom antimicrobial-nonsusceptible pneumococci were isolated. Nonsusceptibility rates among carriage, conjunctivitis, nonbacteremic lower respiratory tract infection, and otitis media from 2009 to 2014 were 29, 28, 43, and 44%, respectively.
TABLE 1.
Percentage of carriage and noninvasive pneumococcal isolates nonsusceptible to penicillin, cefotaxime, tetracycline, erythromycin, clindamycin, and levofloxacin in Irish pediatric hospitals from 2009 to 2014
| Antimicrobial | % of isolates: |
|
|---|---|---|
| Resistant | Intermediate | |
| PEN | 6.5 | 21 |
| CTX | 1 | 8 |
| TET | 20 | 0.6 |
| ERY | 25 | 0 |
| CLI | 19 | 0.6 |
| LVX | 0.2 | 0 |
PEN, penicillin; CTX, cefotaxime; TET, tetracycline; ERY, erythromycin; CLI, clindamycin; LVX, levofloxacin.
Antimicrobial-nonsusceptible serotypes and clones from 2009 to 2014.
From 2009 to 2014, nonsusceptibility occurred among 15 serotypes, nontypeable pneumococci, and 60 different STs (Table 2). Of the 60 STs, 29 (n = 104 isolates) were Pneumococcal Molecular Epidemiology Network (PMEN) clones and/or single- or double-locus variants (SLV or DLV, respectively). The most common nonsusceptible STs and associated serotypes are outlined in Table 2.
TABLE 2.
Serotypes, STs, CCs or PMEN clones, and associated nonsusceptibilities to six different antimicrobials among carriage and noninvasive pneumococci isolated in Irish pediatric hospitals from 2009 to 2014
| Serotype | ST (no. of isolates) | CC or PMEN clone | Nonsusceptibility pattern (no. of isolates)a |
|||||
|---|---|---|---|---|---|---|---|---|
| PEN | CTX | TET | ERY | CLI | LVX | |||
| 19Ab | 320 (14) | CC320 | R (12), I (2) | R, (4), I (10) | R (14) | R (14) | R (14) | S (14) |
| 63 (11) | Sweden15A-25 | I (11) | S (11) | R (11) | R (11) | R (11) | S (11) | |
| 276 (3) | SLV Denmark14-32 | I (3) | S (3) | R (3) | R (3) | R (3) | S (3) | |
| Other (3) | I (3) | I (1), S (2) | S (2), R (1) | S (2), R (1) | S (2), R (1) | S (3) | ||
| 6Bb | 94 (10) | SLV Spain6B-2 | R (5), I (5) | I (7) S (3) | R (10) | R (10) | R (10) | S (10) |
| 90 (5) | Spain6B-2 | R (1), I (4) | I (4), S (1) | R (4), S (1) | R (5) | R (5) | S (5) | |
| 386 (2) | DLV Poland6B-20 | S (2) | S (2) | R (2) | R (2) | R (2) | S (2) | |
| 9006 (3) | SLV Poland6B-20 | I (3) | S (3) | R (3) | R (3) | R (3) | S (3) | |
| Other (4) | I (2), S (2) | S (4) | R (2), S (2) | R (4) | R (2), S (2) | R (1), S (3) | ||
| 35B | 558 (21) | SLV Utah35B-24 | I (21) | I (2), S (19) | S (21) | R (2), S (19) | S (21) | S (21) |
| 7491 (1) | DLV Utah35B-24 | I (1) | S (1) | S (1) | R (1) | S (1) | S (1) | |
| 15A | 63 (6) | Sweden15A-25 | I (6) | S (6) | R (2), S (4) | R (6) | R (6) | S (6) |
| 374 (5) | SLV Sweden15A-25 | I (5) | S (5) | R (5) | R (5) | R (5) | S (5) | |
| Other (10) | R (3), I (7) | R (1), I (1), S (8) | R (7), S (3) | R (8), S (2) | R (7), S (3) | S (10) | ||
| 19Fb | 2100 (4) | SLV Sweden15A-25 | R (2), I (2) | I (2),S (2) | R (4) | R (4) | R (4) | S (4) |
| 87 (2) | I (2) | S (2) | R (1), S (1) | R (2) | R (2) | S (2) | ||
| Other (9) | R (2), I (4), S (3) | I (3), R (1), S (5) | R (9) | R (9) | R (7), S (2) | S (9) | ||
| NT | 4149 (4) | SLV NorwayNT-42 | R (1), I (3) | I (3), S (1) | R (4) | R (4) | R (3), S (1) | S (4) |
| 344 (3) | NorwayNT-42 | I (2), S (1) | S (3) | R (2), S (1) | R (2), S (1) | R (1), S (2) | S (3) | |
| Other (8) | R (2), I (4), S (2) | I (2) S (6) | R (6), S (2) | R (6), S (2) | R (1), S (7) | S (8) | ||
| 33F | 717 (4) | S (4) | S (4) | R (1), I (3) | R (4) | R (4) | S (4) | |
| 100 (2) | S (2) | S (2) | S (S) | R (2) | S (2) | S (2) | ||
| Other (2) | S (2) | S (2) | S (2) | R (2) | R (2) | S (2) | ||
| 23B | 2372 (3) | I (3) | S (3) | S (3) | S (3) | S (3) | S (3) | |
| 439 (2) | SLV Tennessee23F-4 | S (2) | S (2) | S (2) | R (2) | S (2) | S (2) | |
| Other (2) | I (2) | S (2) | S (2) | S (2) | I (1), S (1) | S (2) | ||
| 6Ab | 473 (6) | I (5), S (1) | S (6) | S (6) | R (6) | S (6) | S (6) | |
| 65 (1) | S (1) | S (1) | S (1) | R (1) | S (1) | S (1) | ||
| 15B/C | 199 (3) | Netherlands15B-37 | S (3) | S (3) | S (3) | R (3) | S (3) | S (3) |
| 7479 (3) | I (3) | S (3) | R (3) | R (3) | I (2), S (1) | S (3) | ||
| 23Fb | 81 (2) | Spain23F-1 | R (2) | I (2) | R (2) | R (1), S (1) | R (1), S (1) | S (2) |
| Other (2) | I (2) | I (1), S (1) | R (1), S (1) | S (2) | S (2) | S (2) | ||
| 11A | 838 (3) | SLV Spain9V-3 | R (3) | I (1), S (2) | S (3) | S (3) | S (3) | S (3) |
| 23A | 42 (1) | DLV Tennessee23F-4 | I (1) | S (1) | S (1) | S (1) | S (1) | S (1) |
| 9752 (1) | S (1) | S (1) | R (1) | R (1) | R (1) | S (1) | ||
| 6C | 386 (2) | DLV Poland6B-20 | S (2) | S (2) | R (2) | R (2) | R (2) | S (2) |
| 9Vb | 156 (1) | Spain9V-3 | I (1) | S (1) | S (1) | S (1) | S (1) | S (1) |
| 13 | 9004 (1) | S (1) | S(1) | R (1) | S (1) | S (1) | S (1) | |
Nonsusceptible isolates were defined as those displaying intermediate or complete resistance to at least one of the six antimicrobials tested for. PEN, penicillin; CTX, cefotaxime; TET, tetracycline; ERY, erythromycin; CLI, clindamycin; LVX, levofloxacin; R, resistant; I, intermediate; S, sensitive.
PCV7/13 target serotypes.
Patterns of nonsusceptibility among serotypes and clones from 2009 to 2014.
Sweden15A-25, Spain6B-2, and CC320 and their SLVs or DLVs were the most common sources of penicillin and erythromycin nonsusceptibility (Table 2). Spain6B-2, its variants, and CC320 mostly expressed PCV7/13 serotypes 6B and 19A/19F, respectively. Both PCV13 serotype 19A and NVT 15A were associated with Sweden15A-25 and its variants. Sequence type 558, an SLV of Utah35B-24 expressing NVT 35B, was penicillin nonsusceptible but generally susceptible to other antimicrobials. However, two erythromycin-resistant ST558 isolates occurred in the latter period of the study. Sweden15A-25, Spain6B-2, and CC320 and their variants were the most common multidrug-resistant clones. Multidrug-resistant clone Sweden15A-25 was mostly associated with NVT 15A. The remaining multidrug-resistant clones were mainly PCV7/13 serotypes. According to meningitis breakpoints, 45 isolates were cefotaxime nonsusceptible. If nonmeningeal breakpoints were applied, then just 6 isolates would be considered cefotaxime resistant.
Sweden15A-25 isolates expressing serotype15A were among the most common source of nonsusceptibility in carriage (6/51) and nonbacteremic lower respiratory tract infection (6/46). Spain6B-2 and its variants expressing serotype 6B were also associated with nonsusceptibility among the latter group (10/46; P = 0.0020) but declined in 2013 to 2014 (1/19). Serotype 19A of CC320 was a frequently carried nonsusceptible serotype (5/51) but was more associated with nonsusceptibility in otitis media (6/28; P = 0.0142). Serotype 35B of ST558 was a leading source of nonsusceptibility in carriage (8/51) and conjunctivitis (7/44). Nontypeable pneumococci of NorwayNT-42 and its variants were associated with nonsusceptibility in conjunctivitis (7/44; P = 0.0034) but infrequent in other sites (n = 3). Nonsusceptible NorwayNT-42 and its variants were isolated sporadically throughout the study and were not associated with outbreaks of conjunctivitis.
Changes in circulating nonsusceptible clones from 2007 versus 2009 to 2014.
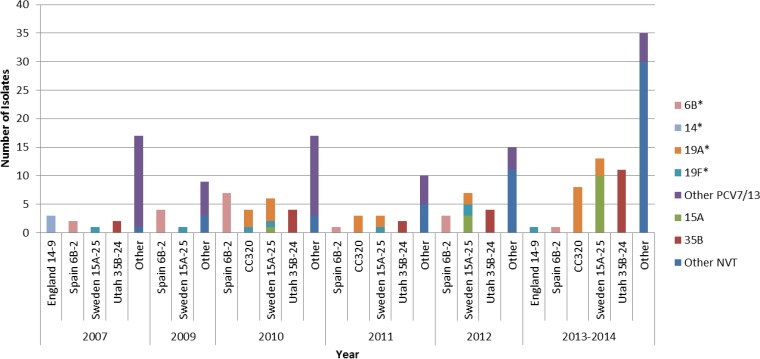
We compared antimicrobial-nonsusceptible clones and associated serotypes after PCV7 and PCV13 introduction to those collected in 2007 before PCV introduction (Fig. 1). Spain 9V-3 and England 14-9, the leading nonsusceptible clones in 2007, were infrequent following conjugate vaccine introduction (i.e., from 2009 to 2014). From 2009 to 2014, only three SLVs of Spain9V-3 expressing serotype 11A and one Spain9V-3 variant expressing serotype 9V occurred (Table 2). PMEN Spain6B-2 and its variants significantly declined in 2014 compared to 2009 (P = 0.0024). CC320 did not occur in 2007 and 2009 but was frequent in 2010 and 2011. In 2012, CC320 was absent, but it did occur in 2013 to 2014 (P = 0.0543). Although not statistically significant, Sweden 15A-25 and Utah 35B-24 and their variants increased (Fig. 1). Interestingly, Sweden 15A-25 was mostly associated with PCV7/13 serotypes 19F and 19A (13/16) in 2009 to 2012 but mostly expressed NVT 15A (7/10) in 2013 to 2014.
FIG 1.
Changes in the most frequent noninvasive pneumococcal clones and their variants nonsusceptible to at least one antimicrobial in 2007 and 2009 to 2014. *, PCV7/13 serotype; PCV7, 7-valent pneumococcal conjugate vaccine serotypes; PCV13, 13-valent pneumococcal conjugate vaccine serotypes; NVT, non-vaccine-type pneumococci; CC, clonal complex. Common clonal complexes that expressed more than one serotype included Sweden15A-25 (serotypes 15A, 19A, and 19F) and CC320 (19A and 19F). Other NVT pneumococci included nontypeable (n = 15), 33F (n = 8), 23B (n = 7), 15B/C (n = 7), 11A (n = 3), 23A (n = 2), 6C (n = 2), and 13 (n = 1).
Genetic diversity among antimicrobial-nonsusceptible isolates.
The Simpson's index of diversity and the Shannon index calculations revealed the pneumococcal STs/CCs associated with antimicrobial nonsusceptibility were quite diverse (Table 3). However, nonsusceptible STs/CCs were less diverse in 2007, before PCV7 introduction. The diversity of STs varied for common nonsusceptible serotypes. Serotype 35B was the least diverse, with a diversity (D) of 0.091 (confidence interval [CI], −0.079 to 0.260), while the diversities of nonsusceptible STs associated with 19A, 6B, and 15A were 0.679 (CI, 0.576 to 0.783), 0.786 (CI, 0.661 to 0.911), and 0.871 (CI, 0.783 to 0.959), respectively.
TABLE 3.
Simpsons index of diversity (D) and Shannon's index (H′) of pneumococcal sequence types associated with noninvasive nonsusceptible pneumococcal isolates in Irish pediatric hospitals in 2007 and 2009 to 2014
| Yr | Simpson's D (CI) | Shannon H′ |
|---|---|---|
| 2007 | 0.893 (0.794–0.993) | 2.366 |
| 2009 | 0.978 (0.944–1.01) | 2.441 |
| 2010 | 0.947 (0.919–0.975) | 2.735 |
| 2011 | 0.965 (0.929–1.000) | 2.552 |
| 2012 | 0.970 (0.945–0.996) | 2.889 |
| 2013–2014 | 0.941 (0.913–0.969) | 3.073 |
Prevalence of the pilus locus from 2009 to 2014.
Of the 169 nonsusceptible pneumococci isolated from 2009 to 2014, 43% (n = 74) were positive for PI-1 and were associated with nonsusceptible PCV7/13 serotypes (n = 46/82) compared to nonsusceptible NVT serotypes (n = 28/87) (P = 0.0020). The most frequent PI-1-positive STs were ST558/SLV Utah35B-24 (n = 21), ST320 (n = 14), ST94/SLV Spain6B-2 (n = 10), and ST90/Spain6B-20 (n = 5). Serotypes 35B (ST558/SLV Utah35B-24), 11A (ST838/SLV Spain9V-3), and 15B/C (ST7479) were the only nonsusceptible NVT pneumococci positive for PI-1.
DISCUSSION
We analyzed STs of nonsusceptible noninvasive pneumococci in Irish pediatric hospitals after PCV7 (2008) and PCV13 (2010) introduction. Important changes occurred in nonsusceptible pneumococcal clones from 2009 to 2014, compared with those from 2007, before PCV7 introduction. Notably, PMEN clones Spain9V-3 and England14-9 and their variants became infrequent or did not occur after conjugate vaccine introduction. The rapid decline of Spain9V-3 and its variants in our population within a short period of PCV7/13 introduction differs from previous studies where Spain9V-3 persisted (20, 21). The high vaccination coverage (92%) of PCV7/13 among Irish children may have contributed to a rapid decline in Spain9V-3 compared to a lower coverage (30.7% to 55%) in regions where this clone persisted (20–22). Furthermore, we mostly included children born after universal conjugate vaccine introduction, whereas in the study by Gherardi et al. where Spain9V-3 persisted, isolates were also obtained from adults likely not to be directly protected by conjugate vaccines (20).
A continuous decline was observed for Spain6B-2, and encouragingly NVT serotypes were not associated with this clone. Conversely, three penicillin-nonsusceptible single-locus variants of Spain9V-3 expressing NVT serotype 11A occurred in the latter period of our study. Expansion of clones expressing NVT may limit the anticipated benefit of conjugate vaccines in reducing nonsusceptible pneumococci.
As previously reported, nonsusceptible pneumococci within CC320 were absent among noninvasive pneumococci before PCV7 introduction (23, 24). However, in 2010 to 2011, nonsusceptible CC320 pneumococci expressing serotype 19A increased in circulation, as reported elsewhere after PCV7 introduction (23, 25).
It has been suggested that pneumococcal clonal diversity may lead to the successful spread of resistance mechanisms (26). In our study, STs among nonsusceptible pneumococci were diverse. However, there was less diversity of antimicrobial-nonsusceptible STs before conjugate vaccine introduction than after conjugate vaccine introduction, suggesting that potential emerging nonsusceptible STs/clones may not yet be fully established in our population. Interestingly, there were low levels of diversity among STs associated with serotype 35B, as described elsewhere (27). Similar to what was reported in Spanish and American studies, penicillin-nonsusceptible serotype 35B of ST558 was common in our study and increased following PCV7 and PCV13 introduction (27–29). In 2013 to 2014, two erythromycin- and penicillin-nonsusceptible 35B/ST558 isolates occurred in our study. If continued clonal expansion of these coresistant pneumococci occurs, it may present a potential problem for empirical treatment of noninvasive infection.
Among our multidrug-resistant isolates, Sweden15A-25 and its variants increased following PCV introduction. Similar to ST558, Sweden15A-25 and its clonal variants have undergone successful clonal expansion (27). In the initial years following PCV7 introduction, this clone was associated mostly with PCV13 serotype 19A. However, by the end of the study Sweden15A-25 and its variants mostly expressed NVT serotype 15A. The occurrence of Sweden15A-25 expressing different capsular serotypes is well documented (21, 30). Importantly, NVT expressed by Sweden15A-25 variants, including those not traditionally associated with antimicrobial nonsusceptibility, could lead to antimicrobial nonsusceptibility rates similar to those of the pre-PCV7 era (21, 30).
The PI-1 locus has been associated with the spread of antimicrobial-nonsusceptible clones, including Spain9V-3 and England14-9 and CC320 (5, 21, 31). Moreover, nonsusceptible piliated clones have spread in countries with low antibiotic use (5). In our study, PI-1 was mostly associated with clones expressing PCV7/13 serotypes. The low frequency of PI-1 in nonsusceptible NVT may help limit the spread of these clones.
There were some limitations to our study. Vaccination and antibiotic consumption history for patients was unavailable, and data from 2009 to 2012 was limited to one hospital. Furthermore, pneumococcal strains from carriage and noninvasive infection from children presenting to hospitals may select for more resistant clones, rather than clones more representative of healthy children in the community. Nonetheless, our study illustrates important findings. Introduction of conjugate vaccines to the childhood immunization schedule has resulted in the reduction of clones Spain9V-3, England14-9, and Spain6B-2 associated with PCV7/13 serotypes 14, 9V, and 6B, respectively. This suggests that conjugate vaccines are positively impacting these clones. The increase of Sweden15A-25 and Utah35B-24 and their variants expressing NVT serotypes may lead to continued high antimicrobial resistance rates among noninvasive pneumococci. It is concerning that these nonsusceptible clones associated with NVT pneumococci have occurred in several countries after conjugate vaccine introduction (32, 33). However, the absence of the PI-1 in some nonsusceptible NVT clones is encouraging as this may limit their dissemination. Overall, continued surveillance will determine if nonsusceptible clones, such as Sweden15A-25 and Utah 35B-24 and their variants, or others expressing NVT, increase in pediatric noninvasive infection, with possible implications for dissemination to the adult population and potential issues in effective antimicrobial treatment.
ACKNOWLEDGMENTS
This work was supported by Pfizer (Ireland). The funders had no role in the collection, analysis, or interpretation of data or the writing of the article and the decision to submit it for publication.
We thank the staff in the Microbiology Laboratory, Temple Street Children's University Hospital, Our Lady's Children's Hospital, Crumlin, and the Adelaide and Meath Hospital, Tallaght (all in Dublin, Ireland). We would particularly like to thank Rebecca Rush, Sandra Bennett (Our Lady's Children's Hospital, Crumlin) and Eddie McCullagh (Adelaide and Meath Hospital, Tallaght) for coordinating sample collection and delivery. We thank all of the staff in the Epidemiology and Molecular Biology Unit, Temple Street Children's University Hospital, particularly Desiree Bennett and Robert Mulhall for useful discussion.
This publication made use of the PubMLST website (http://pubmlst.org/) developed by Keith Jolley (34) and sited at the University of Oxford. The development of that website was funded by the Wellcome Trust. We also wish to acknowledge use of the MLST database previously located at Imperial College London and funded by the Wellcome Trust.
M.M.E. performed experiments, analyzed the data, and drafted the manuscript. H.H. was involved in the design of the study, its supervision, and the drafting of the manuscript. R.C. was involved in the design and supervision of the study. M.C. was involved in the design of the study. I.V. was involved in study concept and design and was a technical supervisor for the project. M.M. was a technical supervisor for the project and was involved in drafting the manuscript. All authors have read and approved the final manuscript.
As potential conflicts of interest, H.H. has recent research collaborations with Pfizer and has also recently received lecture and other fees from Novartis, Pfizer, AstraZeneca, and Astellas, and I.V. has received funding support from Pfizer.
REFERENCES
- 1.Bogaert D, De Groot R, Hermans PW. 2004. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis 4:144–154. doi: 10.1016/S1473-3099(04)00938-7. [DOI] [PubMed] [Google Scholar]
- 2.Enright MC, Spratt BG. 1998. A multilocus sequence typing scheme for Streptococcus pneumoniae: identification of clones associated with serious invasive disease. Microbiology 144:3049–3060. doi: 10.1099/00221287-144-11-3049. [DOI] [PubMed] [Google Scholar]
- 3.Liñares J, Ardanuy C, Pallares R, Fenoll A. 2010. Changes in antimicrobial resistance, serotypes and genotypes in Streptococcus pneumoniae over a 30-year period. Clin Microbiol Infect 16:402–410. doi: 10.1111/j.1469-0691.2010.03182.x. [DOI] [PubMed] [Google Scholar]
- 4.Sadowy E, Kuch A, Gniadkowski M, Hryniewicz W. 2010. Expansion and evolution of the Streptococcus pneumoniae Spain9V-ST156 clonal complex in Poland. Antimicrob Agents Chemother 54:1720–1727. doi: 10.1128/AAC.01340-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sjöström K, Blomberg C, Fernebro J, Dagerhamn J, Morfeldt E, Barocchi MA, Browall S, Moschioni M, Andersson M, Henriques F, Albiger B, Rappuoli R, Normark S, Henriques-Normark B. 2007. Clonal success of piliated penicillin nonsusceptible pneumococci. Proc Natl Acad Sci U S A 104:12907–12912. doi: 10.1073/pnas.0705589104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shouval DS, Greenberg D, Givon-Lavi N, Porat N, Dagan R. 2006. Site-specific disease potential of individual Streptococcus pneumoniae serotypes in pediatric invasive disease, acute otitis media and acute conjunctivitis. Pediatr Infect Dis 25:602–607. doi: 10.1097/01.inf.0000220231.79968.f6. [DOI] [PubMed] [Google Scholar]
- 7.Chiba N, Morozumi M, Shouji M, Wajima T, Iwata S, Ubukata K. 2014. Changes in capsule and drug resistance of pneumococci after introduction of PCV7, Japan, 2010-2013. Emerg Infect Dis 20:1132–1139. doi: 10.3201/eid2007.131485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grall N, Hurmic O, Al Nakib M, Longo M, Poyart C, Ploy MC, Varon E, Raymond J, ORP Ile de France Ouest. 2011. Epidemiology of Streptococcus pneumoniae in France before introduction of the PCV-13 vaccine. Eur J Clin Microbiol Infect Dis 30:1511–1519. doi: 10.1007/s10096-011-1251-9. [DOI] [PubMed] [Google Scholar]
- 9.McElligott M, Vickers I, Cafferkey M, Cunney R, Humphreys H. 2014. Non-invasive pneumococcal serotypes and antimicrobial susceptibilities in a paediatric hospital in the era of conjugate vaccines. Vaccine 32:3495–3500. doi: 10.1016/j.vaccine.2014.04.047. [DOI] [PubMed] [Google Scholar]
- 10.Parra EL, De La Hoz F, Diaz PL, Sanabria O, Realpe ME, Moreno J. 2013. Changes in Streptococcus pneumoniae serotype distribution in invasive disease and nasopharyngeal carriage after the heptavalent pneumococcal conjugate vaccine introduction in Bogota, Colombia. Vaccine 31:4033–4038. doi: 10.1016/j.vaccine.2013.04.074. [DOI] [PubMed] [Google Scholar]
- 11.Wroe PC, Lee GM, Finkelstein JA, Pelton SI, Hanage WP, Lipsitch M, Stevenson AE, Rifas-Shiman SL, Kleinman K, Dutta-Linn MM, Hinrichsen VL, Lakoma M, Huang SS. 2012. Pneumococcal carriage and antibiotic resistance in young children before 13-valent conjugate vaccine. Pediatr Infect Dis J 31:249–254. doi: 10.1097/INF.0b013e31824214ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lambertsen LM, Harboe ZB, Konradsen HB, Christensen JJ, Hammerum AM. 2010. Non-invasive erythromycin-resistant pneumococcal isolates are more often non-susceptible to more antimicrobial agents than invasive isolates. Int J Antimicrob Agents 35:72–75. doi: 10.1016/j.ijantimicag.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Vickers I, O'Flanagan D, Cafferkey M, Humphreys H. 2011. Multiplex PCR to determine Streptococcus pneumoniae serotypes causing otitis media in the Republic of Ireland with further characterisation of antimicrobial susceptibilities and genotypes. Eur J Clin Microbiol Infect Dis 30:447–453. doi: 10.1007/s10096-010-1108-7. [DOI] [PubMed] [Google Scholar]
- 14.Clinical and Laboratory Standards Institute. 2013. Performance standards for antimicrobial susceptibility testing; twenty-third informational supplement. CLSI document M100-S23 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 15.Feil EJ, Li BC, Aanensen DM, Hanage WP, Spratt BG. 2004. eBURST: inferring patterns of evolutionary descent among clusters of related bacterial genotypes from multilocus sequence typing data. J Bacteriol 186:1518–1530. doi: 10.1128/JB.186.5.1518-1530.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aguiar SI, Serrano I, Pinto FR, Melo-Cristino J, Ramirez M. 2008. The presence of the pilus locus is a clonal property among pneumococcal invasive isolates. BMC Microbiol 8:41. doi: 10.1186/1471-2180-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hava DL, Hemsley CJ, Camilli A. 2003. Transcriptional regulation in the Streptococcus pneumoniae rlrA pathogenicity islet by RlrA. J Bacteriol 185:413–421. doi: 10.1128/JB.185.2.413-421.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simpson EH. 1949. Measurement of diversity. Nature 163:688. doi: 10.1038/163688a0. [DOI] [Google Scholar]
- 19.Shannon CE. 1948. A mathematical theory of communication. Bell Syst Tech J 27:379–423. doi: 10.1002/j.1538-7305.1948.tb01338.x. [DOI] [Google Scholar]
- 20.Gherardi G, D'Ambrosio F, Visaggio D, Dicuonzo G, Del Grosso M, Pantosti A. 2012. Serotype and clonal evolution of penicillin-nonsusceptible invasive Streptococcus pneumoniae in the 7-valent pneumococcal conjugate vaccine era in Italy. Antimicrob Agents Chemother 56:4965–4968. doi: 10.1128/AAC.00830-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simões AS, Pereira L, Nunes S, Brito-Avô A, de Lencastre H, Sá-Leão R. 2011. Clonal evolution leading to maintenance of antibiotic resistance rates among colonizing pneumococci in the PCV7 era in Portugal. J Clin Microbiol 49:2810–2817. doi: 10.1128/JCM.00517-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Health Protection Surveillance Centre. 2015. Early childhood immunisation uptake statistics Q1 1999–Q3 2014. Health Protection Surveillance Centre, Dublin, Ireland: http://www.hpsc.ie/A-Z/VaccinePreventable/Vaccination/ImmunisationUptakeStatistics/Immunisationuptakestatisticsat12and24monthsofage/File,954,en.pdf. [Google Scholar]
- 23.Gene A, del Amo E, Iñigo M, Monsonis M, Pallares R, Muñoz-Almagro C. 2013. Pneumococcal serotypes causing acute otitis media among children in Barcelona (1992-2011): emergence of the multiresistant clone ST320 of serotype 19A. Pediatr Infect Dis J 32:e128–e133. doi: 10.1097/INF.0b013e31827c54dc. [DOI] [PubMed] [Google Scholar]
- 24.Gertz RE Jr, McEllistrem MC, Boxrud DJ, Li Z, Sakota V, Thompson TA, Facklam RR, Besser JM, Harrison LH, Whitney CG, Beall B. 2003. Clonal distribution of invasive pneumococcal isolates from children and selected adults in the United States prior to 7-valent conjugate vaccine introduction. J Clin Microbiol 41:4194–4216. doi: 10.1128/JCM.41.9.4194-4216.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beall BW, Gertz RE, Hulkower RL, Whitney CG, Moore MR, Brueggemann AB. 2011. Shifting genetic structure of invasive serotype 19A pneumococci in the United States. J Infect Dis 203:1360–1368. doi: 10.1093/infdis/jir052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de la Pedrosa EG, Baquero F, Loza E, Nadal-Serrano JM, Fenoll A, Del Campo R, Cantón R. 2009. High clonal diversity in erythromycin-resistant Streptococcus pneumoniae invasive isolates in Madrid, Spain (2000-07), J Antimicrob Chemother 64:1165–1169. doi: 10.1093/jac/dkp364. [DOI] [PubMed] [Google Scholar]
- 27.Hanage WP, Huang SS, Lipsitch M, Bishop CJ, Godoy D, Pelton SI, Goldstein R, Huot H, Finkelstein JA. 2007. Diversity and antibiotic resistance among nonvaccine serotypes of Streptococcus pneumoniae carriage isolates in the post-heptavalent conjugate vaccine era. J Infect Dis 195:347–352. doi: 10.1086/510249. [DOI] [PubMed] [Google Scholar]
- 28.Sánchez-Tatay D, Arroyo LA, Tarragó D, Lirola MJ, Porras A, Fenoll A, Hausdorff WP, Brueggemann AB, Obando I. 2008. Antibiotic susceptibility and molecular epidemiology of nasopharyngeal pneumococci from Spanish children. Clin Microbiol Infect 14:797–801. doi: 10.1111/j.1469-0691.2008.02025.x. [DOI] [PubMed] [Google Scholar]
- 29.Richter SS, Diekema DJ, Heilmann KP, Dohrn CL, Riahi F, Doern GV. 2014. Changes in pneumococcal serotypes and antimicrobial resistance after introduction of the 13-valent conjugate vaccine in the United States. Antimicrob Agents Chemother 58:6484–6489. doi: 10.1128/AAC.03344-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ardanuy C, de la Campa AG, García E, Fenoll A, Calatayud L, Cercenado E, Pérez-Trallero E, Bouza E, Liñares J. 2014. Spread of Streptococcus pneumoniae serotype 8-ST63 multidrug-resistant recombinant clone, Spain. Emerg Infect Dis 20:1848–1856. doi: 10.3201/eid2011.131215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Del Grosso M, Camilli R, D'Ambrosio F, Petrucci G, Melchiorre S, Moschioni M, Gherardi G, Pantosti A. 2013. Increase of pneumococcal serotype 19A in Italy is due to expansion of the piliated clone ST416/CC199. J Med Microbiol 62:1220–1225. doi: 10.1099/jmm.0.061242-0. [DOI] [PubMed] [Google Scholar]
- 32.Golden AR, Adam HJ, Gilmour MW, Baxter MR, Martin I, Nichol KA, Demczuk WH, Hoban DJ, Zhanel GG. 2015. Assessment of multidrug resistance, clonality and virulence in non-PCV-13 Streptococcus pneumoniae serotypes in Canada, 2011-13. J Antimicrob Chemother 70:1960–1964. doi: 10.1093/jac/dkv061. [DOI] [PubMed] [Google Scholar]
- 33.Richter SS, Heilmann KP, Dohrn CL, Riahi F, Beekmann SE, Doern GV. 2009. Changing epidemiology of antimicrobial-resistant Streptococcus pneumoniae in the United States, 2004-2005. Clin Infect Dis 48:e23–e33. doi: 10.1086/595857. [DOI] [PubMed] [Google Scholar]
- 34.Jolley KA, Maiden. 2010. BIGSdb: scalable analysis of bacterial genome variation at the population level. BMC Bioinformatics 11:595. doi: 10.1186/1471-2105-11-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McElligott M, Vickers I, Meehan M, Cafferkey M, Cunney R, Humphreys H. The positive impact of pneumococcal conjugate vaccine 13 on serotypes representing carriage and causing non-invasive pneumococcal infection in children, poster 0066. 32nd Annu Meet Eur Soc Paediatr Infect Dis. [Google Scholar]
- 36.McElligott M, Vickers I, Cafferkey M, Cunney R, Humphreys H. 2013. Serotypes and sequence types of antimicrobial-resistant pneumococci among paediatric carriage and non-invasive infections in a Dublin hospital, abstr O401. Abstr 23rd Eur Congr Clin Microbiol Infect Dis. [Google Scholar]