Abstract
Chronic stress induces pre-synaptic and post-synaptic modifications in the paraventricular nucleus of the hypothalamus (PVN) that are consistent with enhanced excitatory hypothalamo-pituitary-adrenocortical (HPA) axis drive. The brain regions mediating these molecular modifications are not known. We hypothesized that chronic variable stress (CVS) tonically activates stress-excitatory regions that interact with the PVN, culminating in stress facilitation. In order to identify chronically activated brain regions, ΔFosB, a documented marker of tonic neuronal activation, was assessed in known stress regulatory limbic and brainstem sites. Four experimental groups were included: CVS, repeated restraint (RR) (control for HPA habituation), animals weight-matched (WM) to CVS animals (control for changes in circulating metabolic factors due to reduced weight gain), and non-handled controls. CVS, but not RR or WM, induced adrenal hypertrophy, indicating that sustained HPA axis drive only occurred in the CVS group. CVS (but not RR or WM) selectively increased the number of FosB/ΔFosB nuclei in the nucleus of the solitary tract, posterior hypothalamic nucleus, and both the infralimbic and prelimbic divisions of the medial prefrontal cortex, indicating an involvement of these regions in chronic drive of the HPA axis. Increases in FosB/ΔFosB-immunoreactive cells were observed following both RR and CVS in the other regions (e.g., the dorsomedial hypothalamus), suggesting activation by both habituating and non-habituating stress conditions. The data suggest that unpredictable stress uniquely activates interconnected cortical, hypothalamic, and brainstem nuclei, potentially revealing the existence of a recruited circuitry mediating chronic drive of brain stress effector systems.
Keywords: posterior hypothalamic nucleus, nucleus of the solitary tract, ΔFosB, medial prefrontal cortex, dorsomedial hypothalamic nucleus
Introduction
Appropriate stress regulation is essential for the maintenance of homeostasis, as both elevated and attenuated glucocorticoid levels are associated with physiological and behavioral dysfunction (de Kloet et al., 2008; de Quervain et al., 2009; Handwerger, 2009; Lupien et al., 2007). The ability to adapt to adverse circumstances is a dynamic process that can be perturbed by a number of physiological and behavioral states, including chronic periods of stress. Chronic stress exposure in rats causes exaggerated glucocorticoid responses to novel stress, development of anhedonia, reduced body weight, and adrenal hypertrophy, a constellation of physical and behavioral sequelae consistent with a “depressive-like” state (Akana et al., 1992; Herman et al., 1995; Papp et al., 1991). These physiological and behavioral effects are accompanied by the re-organization of neural structures, characterized by changes in synaptology (Carvalho-Netto et al., 2011), dendritic morphology (Cook and Wellman, 2004), and gene expression (Andrus et al., 2010), all likely mediated by chronically driven stress-sensitive circuits. However, the neural circuits that drive maladaptive responses to chronic stress are unknown.
Previous studies suggest that the paraventricular nucleus of the hypothalamus (PVN) is a site that is essential for the initiation of stress responses. Chronic stress induces changes in PVN peptide expression (Herman et al., 1995; Imaki et al., 1991; Kiss and Aguilera, 1993; Makino et al., 1995), neurotransmitter receptor expression (Cullinan and Wolfe, 2000; Ziegler et al., 2005), and neural excitability (Verkuyl et al., 2004), all of which are consistent with enhanced excitatory drive of PVN neurons. These findings suggest that PVN neurons are hyper-reactive and hyper-responsive to stimuli following repeated stimulation, contributing to the facilitatory effects of chronic stress on acute hormone release. In addition to these largely postsynaptic effects, chronic stress elevates the number of pre-synaptic excitatory neurotransmitter boutons (glutamate and norepinephrine) in apposition to the CRH cell bodies and dendrites (Flak et al., 2009), indicating that the excitatory PVN afferents are also enhanced following chronic stress.
The overall impact of stress on physiological and psychological processes is linked to chronicity, predictability, and severity. Numerous studies suggest that mild repeated stress regimens engender habituation, wherein physiological responses (e.g., HPA axis activation) wane over time (Bhatnagar et al., 2002; Girotti et al., 2006; Rabasa et al., 2011). Animals confronted with homotypic stress regimens, e.g., mild weight restriction, typically have less severe physiological and behavioral phenotypes (e.g., lack of adrenal hypertrophy/thymic atrophy) (Flak et al., 2011), consistent with adaptation. Given that stress pathologies are linked to failures in adaptation, we used an indirect marker of chronic neuronal activation, ΔFosB, to identify neurocircuits that are specifically recruited across the development of an unpredictable stress regimen that consistently generates physical and behavioral pathologies (chronic variable stress (CVS)) (Herman et al., 1995; Jankord et al., 2011; Ulrich-Lai et al., 2006; Willner, 1997). FosB, a member of the fos-related antigen family of immediate early genes, shows peak induction 4–6 hours post-stimulus (Nestler et al., 2001). Following translation, FosB is cleaved and phosphorylated into the more stable ΔFosB (McClung et al., 2004; Ulery et al., 2006), which accumulates in cells and has lasting effects on gene transcription (Nestler et al., 2001). We used FosB/ΔFosB immunohistochemistry to test the hypothesis that chronic unpredictable stress regimen (CVS) selectively recruits circuitry involved in control of stress responses, including afferent inputs to the PVN.
Materials and Methods
Subjects
Male Sprague-Dawley rats from Harlan (Indianapolis, IN), weighing 250–275 g upon arrival, were individually housed in clear polycarbonate cages containing granulated corncob bedding, with food and water available ad libitum. The colony room was temperature- and humidity-controlled on a 12 h light cycle (lights on 6:00 AM; lights off 6:00 PM). Rats were acclimated to the colony facility for one week prior to experimental manipulations. All experimental procedures were conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Animals and approved by the University of Cincinnati Institutional Animal Care and Use Committee. Prior to experimental manipulation, the animals were weighed and placed into groups such that there was no difference in starting body weight between groups (Chronic Variable Stress (CVS) (n=8), Repeated Restraint (RR) (n=8), Weight-Matched (WM) (n=9), and Control (n=8)). All rats were perfused at least 16 hours following the termination of the day 14 stressor, to negate the contribution of acute FosB induction and thereby allow assessment of central ΔFosB expression as a measure of chronic drive.
Weight Matching
Animals were fed a sufficient amount of chow to produce a similar reduction in weight gain as the chronically stressed animals. In order to provide a relatively low-stress means of food restriction, animals were fed 5 grams of chow in the morning at a random time between 7am and 11am and the rest between 5:30 and 6pm. Previous studies indicate that feeding the animals just before lights off does not shift their circadian rhythm (Flak et al., 2011). We added the morning feeding in order to limit the total amount of time without food (since rats typically eat small amounts during the light period of the circadian cycle). WM, RR, and CVS animals did not differ in body weight at any point in the experiment.
Chronic Stress Procedure
Subjects were randomly assigned to either CVS, repeated restraint, weight-matched, or control groups. The chronic stress protocol consisted of twice-daily (morning and afternoon) exposure to randomly assigned stressors for two weeks. Morning stressors were conducted between 8:00 am and 11:30 am and afternoon stressors were administered between 1:30 pm and 5:00 pm. Stressors consisted of rotation stress (1 h at 100 rpm on a platform orbital shaker); warm swim (20 min at 31°C); cold swim (10 min at 18°C), cold room stress (kept in 4°C for one hour); and hypoxia (8% O2 92% N2, 30 min). Repeatedly restrained animals were placed in a plexiglas restraint tube for one hour per day in the AM. The morning following the last afternoon stressor, rats received an overdose of sodium pentobarbital and were perfused with phosphate buffered saline, followed by 4% paraformaldehyde. Brains were post-fixed overnight in 4% paraformaldehyde and transferred to 30% sucrose (4°C).
Immunohistochemistry
Sections were cut at 35 µm on a sliding microtome and sections stored in cryoprotectant (0.1 M phosphate buffer, 30% sucrose, 1% polyvinylpyrrolidone, and 30% ethylene glycol) at −20°C until used for immunohistochemistry. Sections were transferred from cryoprotectant to 50 mM potassium phosphate buffered saline (KPBS, pH 7.2) and 0.9% sodium chloride) at room temperature (RT). Cryoprotectant was rinsed off (5 × 5 min) in KPBS, sections incubated in KPBS + 1.0% H2O2 for 10 minutes at room temperature (RT). Sections were then washed (5 × 5 min) in KPBS at RT and placed in blocking solution (50 mM KPBS, 0.1% bovine serum albumin (BSA), and 0.2% Triton X-100) for 1 hour at RT. Sections were subsequently incubated overnight at 4°C in rabbit anti-FosB/ΔFosB primary (H75, Santa Cruz Biotechnologies; Santa Cruz, CA) diluted 1:300 in blocking solution. This antibody was raised against amino acids 75–100 of the human FosB and detects two bands, one at 35–37 kDa (ΔFosB) and another at 45 kDa (FosB), on Western blot (Marttila et al., 2006). The following morning sections were rinsed in KPBS (5 × 5 min) and incubated in biotinylated anti-rabbit secondary antibody (Vector Laboratories, Inc., Burlingame, CA) (1:500 in KPBS + 0.1% BSA) for 1 hour at RT. Sections were rinsed in KPBS (5 × 5 min) and then treated with avidin-biotin complex (ABC, Vector Laboratories, Inc., Burlingame, CA) (1:1000 in KPBS + 0.1% BSA) for 1 hour at RT. Following this incubation, sections were rinsed again in KPBS (5 × 5 min) and reacted with 0.02% diaminobenzamidine (Sigma Aldrich, St. Louis, MO) with 0.05% hydrogen peroxide. Sections were rinsed a final time in KPBS, mounted, rehydrated through graded ethanols and coverslipped in DPX (Sigma Aldrich, St. Louis, MO). Sections used for quantification of co-labeled for FosB/ΔFosB and dopamine-beta-hydroxylase (DBH) were placed through a similar procedure on the first day of immunohistochemistry, but additionally incubated with mouse anti-DBH (Millipore; Temecula, California) (1:2500). Following overnight incubation with both primary antibodies, the sections were rinsed in KPBS (5 × 5min) and incubated in both Cy3 goat anti-rabbit (Jackson Immuno Research; West Grove, PA) and Alexa 488 donkey anti-mouse (Molecular Probes; Eugene, OR) for thirty minutes. Next, the sections were washed a final time in KPBS and coverslipped with Fluka mounting medium (Sigma Aldrich; St. Louis, MO). The slides were left to dry for a sufficient time before capturing images for quantification.
It is important to note that the FosB antibody used will recognize both cleaved (ΔFosB) and uncleaved forms (FosB). Uncleaved Fos B diminishes to baseline within 6 hours (Nestler et al., 2001), and thus only ΔFosB should be detectable at the time of kill (16h after the last stressor). Nonetheless, to be technically correct, we will refer to the immunohistochemical staining as FosB/ΔFosB.
Cell counts
The number of FosB/ΔFosB immunoreactive neurons within the brain regions was quantified with the Zeiss Axiovision 4.6 software. Image magnifications were chosen in order to assess the number of immunoreactive nuclei in one unilateral image from each section. If possible, four images per animal per region of interest (ROI) were collected. To quantify the number of immunoreactive nuclei, both a threshold gray level and minimum pixel size were determined for each objective using a subset of images for each region with varying signal and background intensities. The program recorded the number of immunoreactive nuclei within a defined ROI. All cell counts were converted to the number of immunoreactive cells per unit area and analyzed. The regions were delineated using characteristics of each nucleus taken from the Paxinos and Watson atlas (Paxinos and Watson, 1997) and quantified at a similar distance from bregma within all experimental animals.
The number of co-labeled FosB/ΔFosB and DBH were counted manually. Images were collected on 2 right and 2 left NTS at approximately −14mm caudal to Bregma (Paxinos and Watson coordinates) (Paxinos and Watson, 1997). Immunoreactive nuclei were first selected in the Cy3 channel. Then, the Alexa channel was added to record the number of cells that also contain cytosolic DBH staining.
Statistics were analyzed using Sigma Stat (Systat Software, San Jose, California). Data are expressed as mean ± standard error. Outliers were determined if the value exceeded both 1.96 times the standard deviation and 1.5 times the interquartile range (McClave, 1994). Outliers were tested for each data set prior to analysis. If data exceeded these limits, the individual piece of data was removed from the specific analysis. The data were analyzed by one-way ANOVA with a Fisher’s LSD post-hoc test using group (CVS, WM, RR, and control) as the between-subjects factor. If an ANOVA failed homogeneity of the variance, the data underwent log transformation prior to analysis.
Results
We used three groups to control for non-CVS-specific changes in FosB induction: non-handled animals were used as a general unstressed control; the weight-matched group controlled for the passive effects of reducing weight gain to CVS levels; and the repeatedly restrained group controlled for neuronal FosB/ΔFosB induction under habituating conditions. At the end of the two week experiment, animals were all killed without acute disturbance, in order to minimize uncleaved FosB production. As previously reported (Herman et al., 1995), CVS caused adrenal hypertrophy {F(3,32)= 15.053, p<.001} and attenuated body weight gain {F(3,32)= 19.094, p<.001}. We used FosB/ΔFosB immunohistochemistry to identify regions containing immunoreactive nuclei. Surprisingly, a number of areas known to be sensitive to behavioral and metabolic disturbance exhibited little or no FosB/ΔFosB immunoreactivity, including the PVN, the ventromedial hypothalamic nucleus, the arcuate nucleus, and the central nucleus of the amygdala (Davis and Shi, 1999; Herman et al., 2008; Karnani and Burdakov, 2011; Lu et al., 2003). Of the sites that contained FosB/ΔFosB immunoreactivity, we selected regions that contain glutamate and/or norepinephrine neurons that project to the PVN for FosB/ΔFosB analysis, given known enhancement of excitatory drive to the PVN seen following CVS (Flak et al., 2009; Miklos and Kovacs, 2012; Verkuyl et al., 2004). Figure 1 contains schematic representations of FosB/ΔFosB immunoreactive cell distributions within a number of key regions of control and stressed conditions. In addition, we also chose to analyze the amygdala, hippocampus, and medial prefrontal cortex (mPFC), as they contained robust FosB/ΔFosB immunoreactivity, exhibit neuroplastic responses to chronic stress, and are up-stream regulators of HPA axis function (Ulrich-Lai and Herman, 2009). To control for widespread effects of our stress paradigm, we analyzed FosB/ΔFosB immunoreactivity within the interstitial nucleus of the posterior limb of the anterior commissure (IPAC) and M1 of the motor cortex, regions that are not currently implicated in acute or chronic activation of neuronal stress responses. The groups did not differ in their FosB/ΔFosB expression (Figure 2) within these two sites, indicating that our reported changes are specific to stress-sensitive neurons and do not reflect global neural activation.
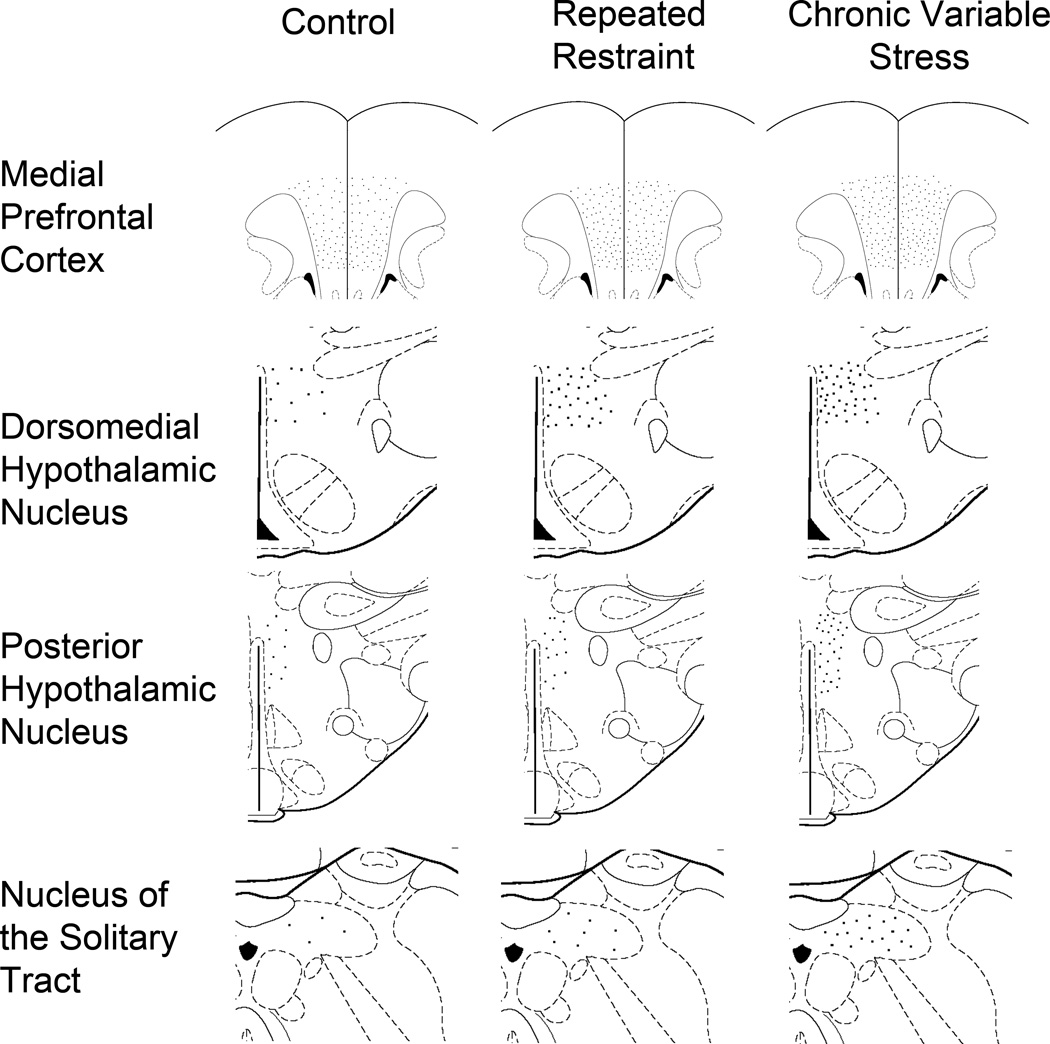
Figure 1.
Schematic Representation of FosB/ΔFosB immunoreactive neurons induced by chronic stress. Using modified images collected from Paxinos and Watson Atlas (Paxinos and Watson, 1997), these schematics illustrate the distribution and relative induction by CVS and RR within the medial prefrontal cotex (mPFC), dorsomedial hypothalamic nucleus (DMH), posterior hypothalamus (PH), and caudal nucleus of the solitary tract (NTS). Each dot represents one cell per unit area and corresponds to a similar density relative to the other regions despite the varying areas presented in the schematic images. Thus, the dots appear smaller in the mPFC because the displayed area is much larger than the hypothalamic regions.
Figure 2.
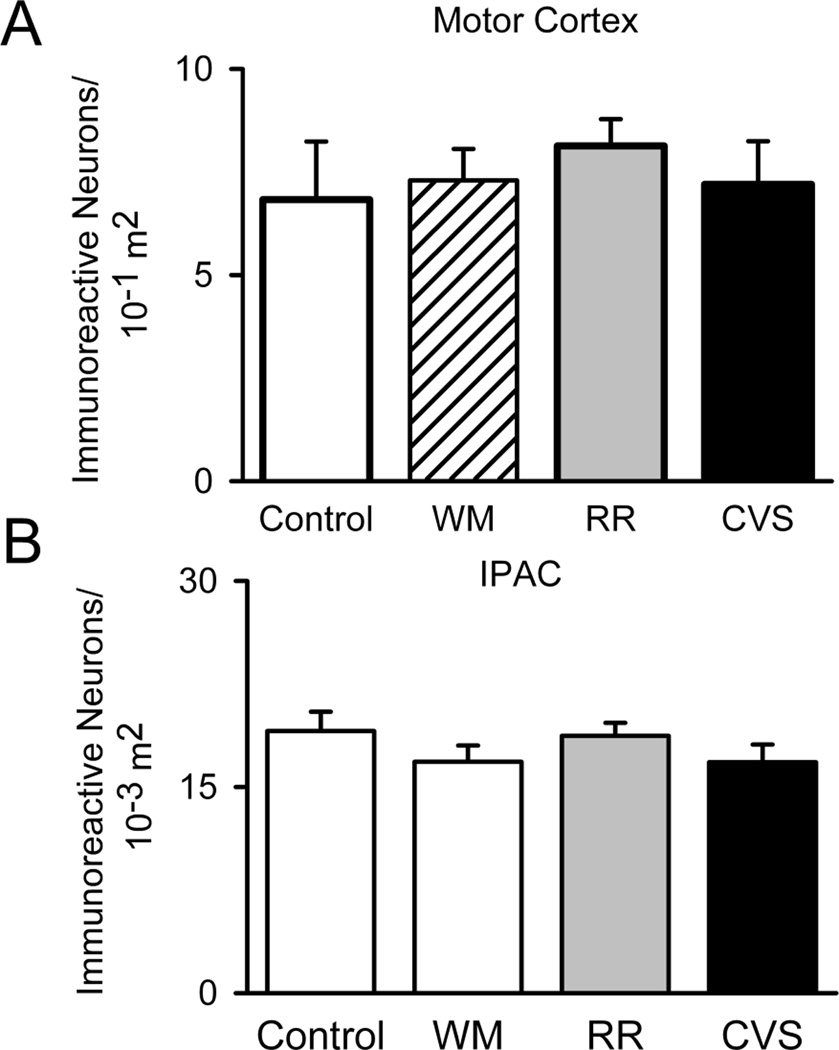
Control Regions. To control for the possibility that the chronic stress regimens globally activate neural structures, we quantified FosB/ΔFosB within the interstitial nucleus of the posterior limb of the anterior commissure (IPAC) (Control n=8, WM n=9, RR n=7, and CVS n=7) and motor cortex (Control n=8, WM n=9, RR n=7, and CVS n=7). The groups did not differ in the number of FosB/ΔFosB immunoreactive nuclei within these two regions. Unit area for IPAC was in 10−3 m2 and 10−1 m2 for motor cortex.
PVN-projecting regions
Our qualitative analyses located several sites that had variable levels of FosB/ΔFosB immunoreactivity across groups, the most notable being the nucleus of the solitary tract (NTS). FosB/ΔFosB in the NTS stood out, since staining was sparse in the brainstem. Quantitative analyses indicated that CVS increased FosB/ΔFosB within the NTS {F(3,30)=47.568, p<.001} (Figure 3A). This area is particularly interesting, as it includes A2/C2 catecholaminergic neurons that project into the PVN (Cunningham and Sawchenko, 1988). Double-label analysis revealed increased co-localization of FosB/ΔFosB with the norepinephrine/epinephrine marker DBH in the NTS of CVS rats {F(3,31)=33.084, p<.001} (Figure 3B), indicating that unpredictable stress tonically activates noradrenergic NTS neurons. Importantly, no increases in NTS FosB/ΔFosB were observed in RR or WM groups. While A1/C1 neurons also project to the PVN (Cunningham and Sawchenko, 1988), FosB/ΔFosB was not observed in this area.
Figure 3.
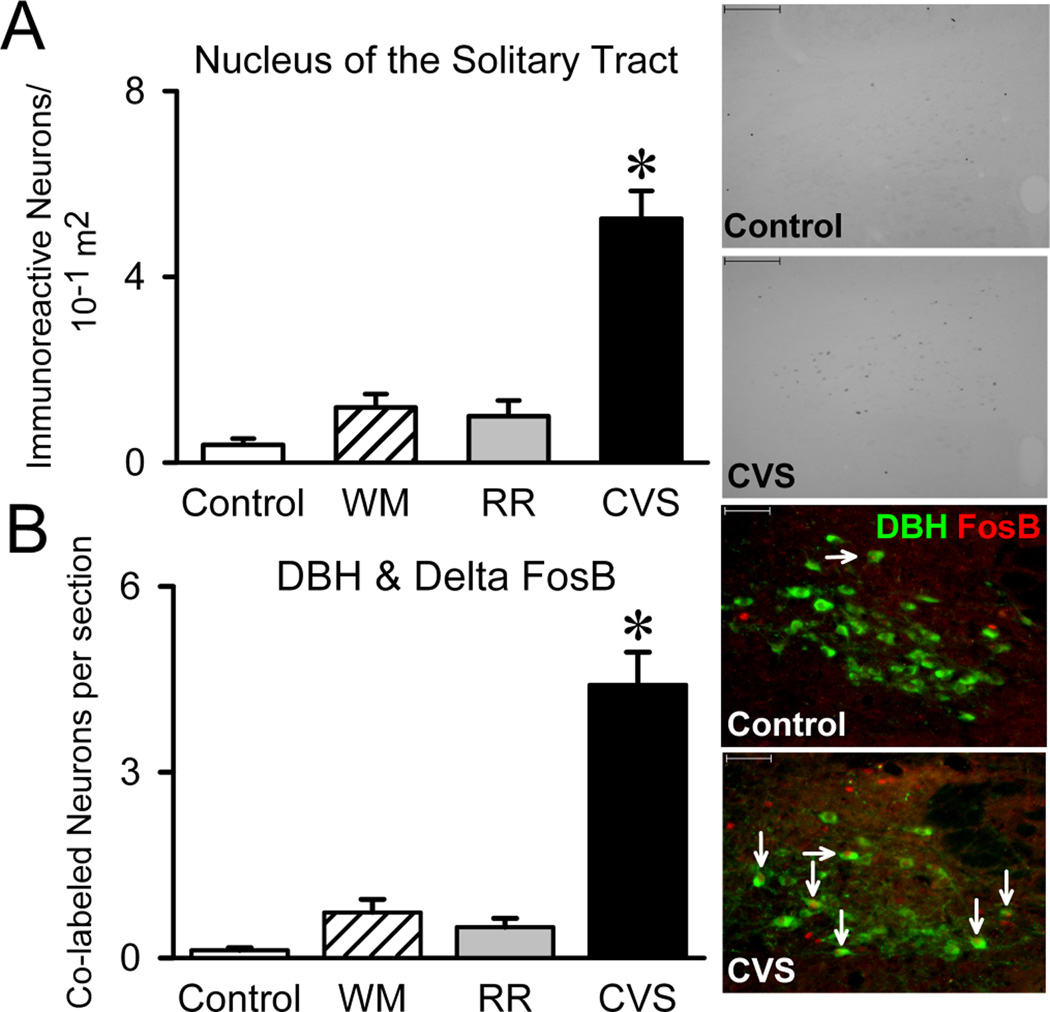
Nucleus of the Solitary Tract. The A2 region of the nucleus of the solitary tract (NTS) supplies the majority of the medial parvocellular PVN with norepinephrine. Thus, we examined FosB/ΔFosB within the NTS (Control n=7, WM n=9, RR n=8, and CVS n=8). CVS increased the number of FosB/ΔFosB immunoreactive neurons within the NTS and especially within DBH-positive neurons (Control n=7, WM n=9, RR n=8, and CVS n=8). Since WM and RR animals did not alter FosB/ΔFosB, the data suggests that unpredictable stress recruits the NTS. * denotes group significantly different from all groups. Unit area was in 10−1 m2.
We also quantified FosB/ΔFosB in select regions known to include glutamate-expressing PVN-projecting neurons. CVS increased FosB/ΔFosB within the dorsomedial hypothalamic nucleus (DMH) {F(3,32)=27.534,p<.001} (Figure 4A) and posterior hypothalamic nucleus (PH) {F(3,23)=12.755,p<.001} (Figure 4B), indicating that chronic unpredictable stress activates these regions. The FosB/ΔFosB cells of each hypothalamic region tended to be in the rostral components of each and, in the case of the posterior hypothalamus, medial to the fornix. Notably, RR also increased DMH FosB/ΔFosB expression (Figure 4B), suggesting that this region is chronically activated during both unpredictable and predictable stress.
Figure 4.
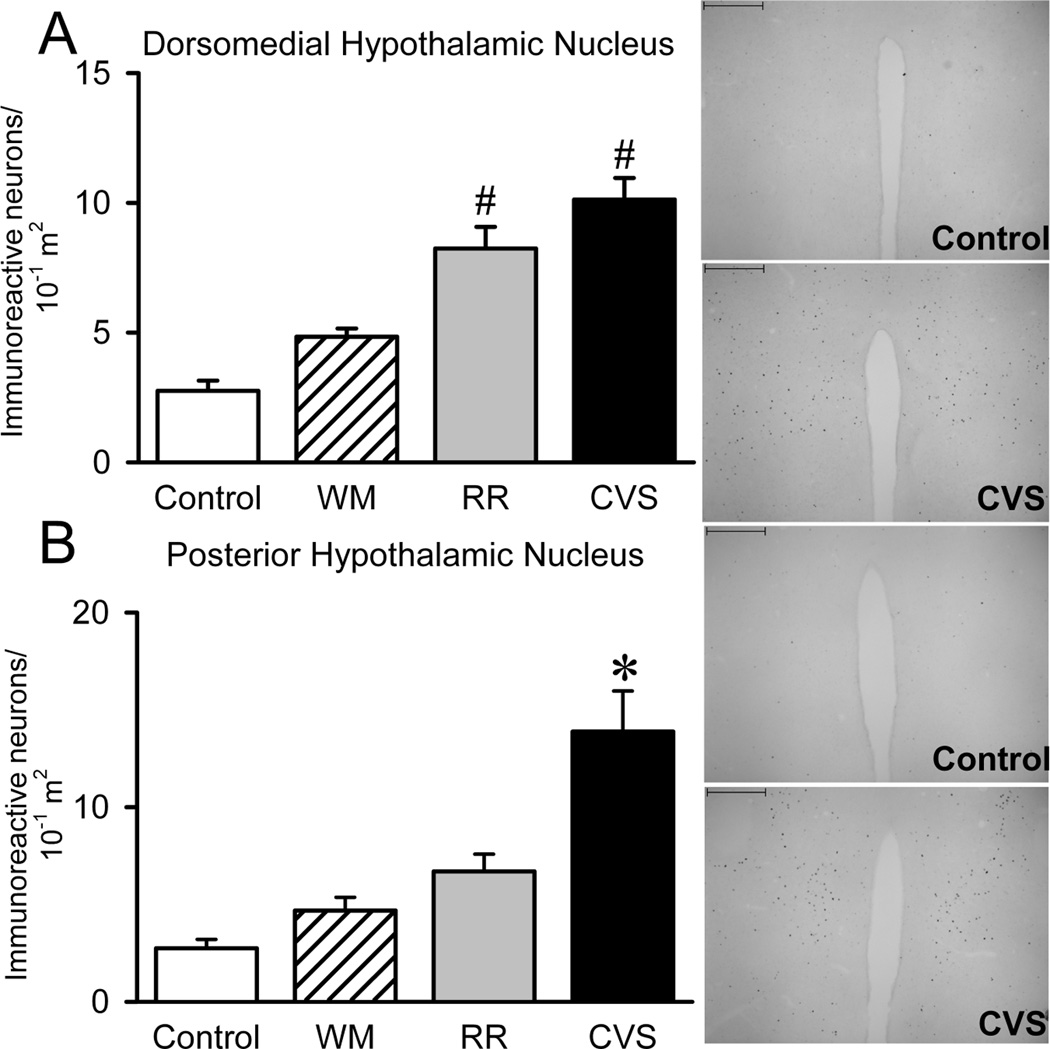
Hypothalamic Nuclei. Since the dorsomedial (DMH) and posterior hypothalamic (PH) nuclei are two regions that supply the PVN with significant amounts of glutamate, we analyzed FosB/ΔFosB within these regions. CVS increased the number of FosB/ΔFosB immunoreactive neurons within the DMH (Control n=8, WM n=9, RR n=8, and CVS n=8) and PH (Control n=4, WM n=7, RR n=6, and CVS n=7), suggesting that unpredictable recruits the DMH and PH. * denotes group significantly different from all groups.# denotes group significantly different from control group. Unit area for DMH and PH were 10−1 m2.
Upstream Limbic Structures
We also quantified FosB/ΔFosB in the amygdala, hippocampus and prefrontal cortex, upstream limbic structures critical for stress regulation. FosB/ΔFosB immunoreactivity was rather abundant in these structures compared to the hypothalamic and brainstem regions previously analyzed. Staining was particularly robust within the mPFC. Because side (Sullivan and Gratton, 1999) and subregion (Radley et al., 2006) effects are reported following lesions of the mPFC, we divided the region of interest into both left and right infralimbic and prelimbic mPFC to assess specific chronic stress-activated areas of the mPFC. CVS elevated the FosB/ΔFosB expression within the left infralimbic {F(3,31)=5.823, p=.03} (Figure 5A), left prelimbic {F(3,32)=7.119, p=.001} (Figure 5B), right infralimbic {F(3,32)=7.808, p<.001} (Figure 5C), and right prelimbic {F(3,31)=9.063, p<.001} (Figure 5D) cortices. In all cases, increased FosB/ΔFosB staining was only observed in the CVS group. We also analyzed FosB/ΔFosB within the basolateral amygdala (BLA), as this nucleus exhibited the most prominent staining in the amygdalar complex (FosB/ΔFosB immunoreactivity in the medial and central nuclei was minimal). BLA FosB/ΔFosB did not differ between treatment groups (Figure 6A). Hippocampal FosB/ΔFosB was restricted to the dentate gyrus. Our analyses found that CVS and RR both increased FosB/ΔFosB in the dentate gyrus {F(3, 31)=4.806, p=.008} (Figure 6B), indicating that this region is responsive to unpredictable as well as predictable stress.
Figure 5.
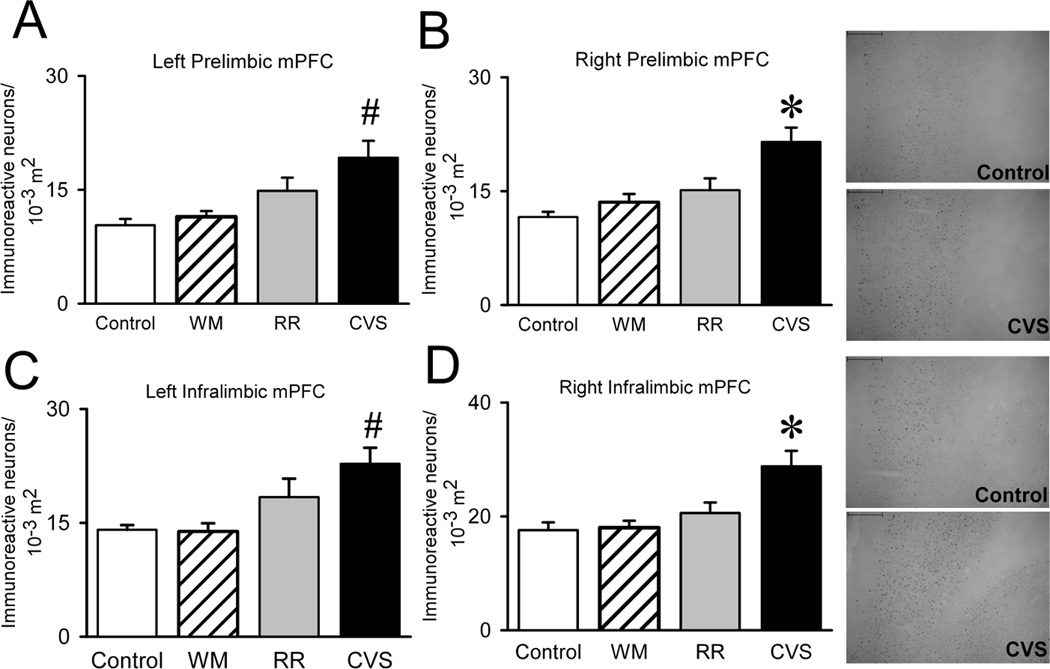
Medial Prefrontal Cortex. Because side and subregion of the mPFC have both been shown to differentially regulate responses to stress, we divided the mPFC into both left and right infralimbic and prelimbic mPFC to locate the specific chronic stress-activated areas of the mPFC. However, CVS elevated the FosB/ΔFosB expression within the right infralimbic (Control n=8, WM n=9, RR n=8, and CVS n=8), right prelimbic (Control n=7, WM n=9, RR n=8, and CVS n=8), left infralimbic (Control n=7, WM n=9, RR n=8, and CVS n=8), and left prelimbic (Control n=8, WM n=9, RR n=8, and CVS n=8), indicating that unpredictable stress recruits all subregions of the mPFC. * denotes group significantly different from all groups. Unit area for mPFC was in 10−3 m2.
Figure 6.
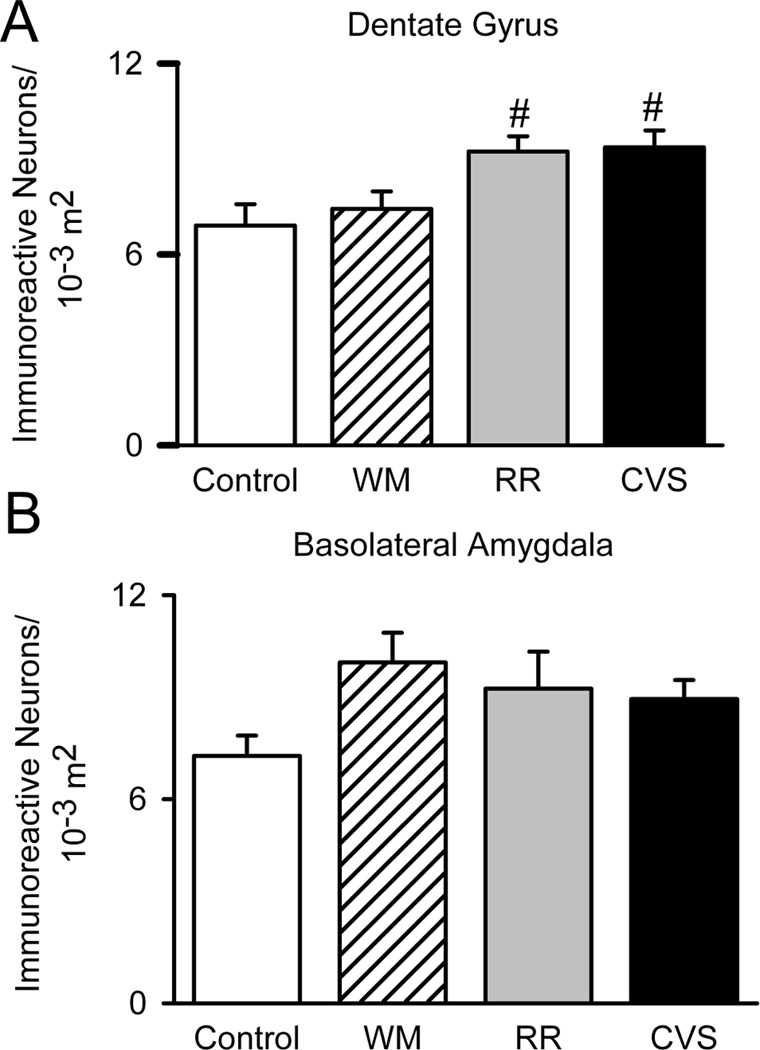
Upstream Limbic Structures. Since chronic stress exposure classically re-wires these stress regulatory regions, we analyzed FosB/ΔFosB within the Basolateral Amygdala (Control n=7, WM n=8, RR n=8, and CVS n=8) and Dentate Gyrus (Control n=8, WM n=9, RR n=8, and CVS n=7). Chronic stress did not alter the number of FosB/ΔFosB immunoreactive neurons within the Basolateral Amygdala. However, both RR and CVS increased the number of FosB/ΔFosB immunoreactive neurons in the dentate gyrus, suggesting that both unpredictable and predictable stress recruits the dentate gyrus.# denotes group significantly different from control group. Unit area for basolateral amygdala and dentate gyrus was in 10−3 m2.
Discussion
The data from the current study suggests that unpredictable stress (i.e., CVS) chronically activates a number of known stress-regulatory regions, including areas that project directly into the PVN (i.e., PH) and upstream limbic structures (i.e., mPFC) that indirectly regulate HPA axis activity. These data support the existence of a ‘recruited chronic stress pathway’ that involves prefrontal cortex, posterior hypothalamus and NTS, putatively responsible for sustaining and amplifying stress responsivity during prolonged stimulation. Other regions, such as the DMH and DG, increase FosB/ΔFosB staining in response to both habituating and non-habituating stress regimens, suggesting that these regions are not required for chronic drive of the HPA axis by unpredictable stress.
Our analyses revealed several sites activated solely by a history of unpredictable stress and known to provide excitatory neurotransmitter input to the PVN. The NTS contained the most pronounced FosB/ΔFosB induction, including the A2/C2 catecholamine cell group. This finding is particularly important, as this cell group provides the majority of norepinephrine afferents to the medial parvocellular PVN (Cunningham and Sawchenko, 1988). Previous evidence suggests that NE/E from medullary regions play an excitatory role in HPA axis drive, as lesions of ascending medullary PVN inputs attenuate CRH immunoreactivity and PVN cFos responses to stress (Li et al., 1996). Furthermore, CVS elevates the NTS TH mRNA and hnRNA (Zhang et al., 2010), suggesting that unpredictable stress exposure enhances the output of A2/C2. In addition, CVS increases the number of PVN DBH-positive boutons in apposition to CRH neurons (Flak et al., 2009), indicating that chronic stress exposure enhances the noradrenergic influence in the initiation of stress responses. Thus, this region appears to be critical for responding to chronic stress. As these neurons are tonically activated over time by chronic stress, the output from this region is continually enhanced, facilitating HPA axis activity and suggesting that these neuroplastic changes within the NTS A2 region may form a final common pathway for mediating chronic stress-related behavioral and physiological dysfunction. However, we should note that NTS neurons project to additional regions important in stress regulation including the rostral ventrolateral medulla, bed nucleus of the stria terminalis, and the amygdala (Ricardo and Koh, 1978), and thus may have indirect as well as or instead of direct effects on PVN activation.
Importantly, some of the FosB/ΔFosB immunoreactive nuclei within the NTS were not DBH-positive. It is likely that non-noradrenergic NTS FosB/ΔFosB neurons (e.g., those that express glucagon-like-peptide-1 (GLP-1) (Larsen et al., 1997)) may also play a role in stress regulation. For example, NTS GLP-1 can contribute to both psychogenic and systemic responses to stress (Kinzig et al., 2003), and GLP-1 neurons innervate numerous stress-regulatory regions, notably including the PVN (Larsen et al., 1997; Llewellyn-Smith et al., 2011; Tauchi et al., 2008). Furthermore, chronic stress reduces GLP-1-positive PVN fiber density and NTS PPG mRNA (Zhang et al., 2010), suggestive of the role of these neurons in chronic stress regulation. It should be noted that GLP-1 and NE are not the sole transmitters expressed in NTS, as the region contains a host of neurotransmitters and peptides (Maley, 1996), including GABA, glutamate, cholecystokinin, calcitonin gene-related peptide, galanin, neuropeptide Y, among others, that could also be modified by chronic stress exposure
In addition to the evidence that PVN NE release is driven by unpredictable but not predictable chronic stress exposure, our analyses found that CVS elevates PH FosB/ΔFosB immunoreactivity. Given evidence for a strong glutamatergic innervations of the PVN by the PH (Ulrich-Lai et al., 2011b), it is possible that unpredictable stress may also drive PVN glutamate release and contribute to enhanced density of glutamatergic appositions onto CRH neurons (Flak et al., 2009). In support of this hypothesis, excitation of the PH can initiate cardiovascular and behavioral stress responses (DiMicco et al., 1986; Shekhar and DiMicco, 1987), and pharmacological blockade can attenuate stress-induced tachycardia (Lisa et al., 1989), suggesting that the PH may also influence other types of stress responses. In addition to glutamatergic neurons, the PH expresses a host of transmitters, including orexin, that are implicated in vigilance and regulating wakefulness (Abrahamson and Moore, 2001). Given its selective recruitment during stress exposure, the PH has the potential to mediation of responses to prolonged stress.
We observed pronounced ΔFosB staining within the medial prefrontal cortex following CVS. Previous studies have indicated that the prelimbic mPFC inhibit HPA axis responses to stress, whereas the infralimbic mPFC elevates glucocorticoid release (Radley et al., 2006) and increases pressor responses following acute stress exposure (Resstel et al., 2006). Our results indicate that CVS activates the prelimbic and infralimbic mPFC to a similar degree, suggesting that the net impact of prefrontal activation may be translated downstream of the cortex. Prior studies indicate that the right mPFC plays a more prominent role than the left side in controlling responses to stress (Sullivan and Gratton, 1999), but we did not find a lateralized effect of CVS on mPFC ΔFosB activation. Overall, CVS clearly activates the mPFC and likely provides lasting changes to gene transcription to this region. In addition to its role in the tuning of the stress responses, the mPFC is critical to short term memory formation (Barsegyan et al., 2010; Floresco et al., 1997), reward processing (Capriles et al., 2003), appraisal (Schmitz and Johnson, 2007), and cognitive control (Maier et al., 2006), all of which can be regulated by chronic stress. The accumulation of FosB/ΔFosB in prefrontal cortical regions may provide a means by which chronic stress promotes long-lasting behavioral as well as hormonal dysfunction.
Our repeated restraint data are consistent with prior work by Perrotti et al (Perrotti et al., 2004), where chronic restraint produced marked FosB/ΔFosB activation in regions such as the prefrontal cortex, BLA, and dentate gyrus. Their work focused on the effects of chronic stress on reward circuits. Our study provides new evidence indicating that BLA and dentate gyrus induction are expressed at similar levels in RR and CVS groups, suggesting that these regions differentially respond to unpredictable stress exposure and are thus unlikely to be involved in stress sensitization (and perhaps pathology). Indeed, activation of these structures during RR may reflect involvement in stress adaptation. In the case of the BLA, this possibility is supported by data indicating a role for the BLA in mediating stress-buffering effects of reward on HPA axis (as well as cardiovascular and behavioral) stress responses (Ulrich-Lai et al., 2011a). In the PFC, the number of FosB/ΔFosB cells is significantly greater following CVS than RR, suggesting that these regions encode predictability (and/or intensity) of the stress regimen. Disproportionate induction by CVS may reflect recruitment of additional output neurons controlling activation of the same subcortical targets, or perhaps addition of neural populations with different subcortical structures. Given our data demonstrating recruitment of down-stream structures (e.g., PH, NTS), both of which are targeted by the mPFC (particularly the infralimbic cortex)(Hurley et al., 1991; Vertes, 2004), the latter is a likely possibility.
Both CVS and RR induced the expression of ΔFosB within the DMH. The DMH is primarily known to regulate the SNS, in that DMH inactivation attenuates (Stotz-Potter et al., 1996) and stimulation exacerbates cardiovascular responses to psychogenic (Bailey and Dimicco, 2001), but not systemic stress. This region a rich number of intermingled GABAergic and glutamatergic neurons which both project into the PVN and likely regulate responses to stress (Herman et al., 2003). While we do not observe differences in extent of ΔFosB induction in RR vs. CVS groups, it is possible that different DMH populations may be recruited under the two different conditions (e.g., CVS may preferentially activate DMH glutamatergic neurons, while RR may activate GABAergic neurons). Thus, the DMH may be a recruited component for both stress facilitation and stress habituation.
No changes in accumulation of FosB/ΔFosB were observed in any region in the WM group. These data indicate that the effect of CVS is not accounted for by changes in metabolic status induced by lowered body weight. Caloric restriction sensitizes reward circuits (Carr and Wolinsky, 1993; Carroll et al., 1979; Stamp et al., 2008) in order to re-establish previous energy storage levels, which are believed to be partially mediated by FosB/ΔFosB within the nucleus accumbens (Vialou et al., 2011a). Interestingly, RR, CVS, and WM animals all displayed slight, non-significant increases in FosB/ΔFosB within the BLA, another known brain region important for reward processing. Since chronic stress can produce anhedonia, the ΔFosB within reward regions induced by stress may dampen, while caloric restriction may sensitize reward activation.
Recent studies indicate that ΔFosB is required for generation of coping responses to social stress (Vialou et al., 2011b) and the reinforcing effects of sexual experience (Pitchers et al., 2010). Thus, expression of ΔFosB is consistent with a functional role in circuits mediating stress and reward, particularly with regard to experience. In our studies, selective induction in PH and NTS circuits, as well as enhanced induction in IL and PL, suggests that FosB/ΔFosB expression subserves biologically meaningful functions mediating sensitizing effects of chronic stress. However, the use of ΔFosB as a marker for tonic neuronal activation does potentially introduce false negatives, since the necessary mechanisms of FosB transformation into ΔFosB are, to this point, poorly understood. Therefore, it is possible that some nuclei contain neurons that are unable to convert FosB to ΔFosB. For example, the PVN is likely activated in response to each of the stressor of CVS, but this area does not exhibit significant ΔFosB staining, despite displaying numerous other markers of chronic activation (e.g., increased CRH and vasopressin gene transcription, cellular hypertrophy)(Flak et al., 2009). Studies have claimed that the induction of ΔFosB within the PVN following drug administration (Chocyk et al., 2006; Das et al., 2009; Nunez et al., 2010). However, the temporal aspects of these previous studies make it unclear whether it is FosB or ΔFosB is being detected. ‘Acute’ induction of FosB is extinguished by 12 hours, and thus our16 hour interval between the last stressor and study termination is sufficient to parse acute from chronic effects (Perrotti et al., 2004). Previously groups have reported Fos B/ΔFosB staining as late as a week after stress cessation (Perrotti et al., 2004), so there is not a reason to believe that we are ‘missing’ induction at the 16 hour time point. However, our analyses may have missed some areas that are tonically activated by WM, RR, and/or CVS if they are unable to cleave and phosphorylate FosB (e.g., phosphorylation is required for generation of ΔFosB (Hiroi et al., 1999)). In addition, the terminal 21 amino acids of FosB, not present in ΔFosB, are shared by the other Fos-related antigens (McClung et al., 2004), suggesting that the cleavage of this segment of the FosB sequence is required for ΔFosB. Identification of enzymes for phosphorylation and cleavage are required to adequately address heterogeneity of neuronal subtypes in FosB processing. It is also possible that some neuronal subtypes simply do not engage fosB transcription in response to stress. Nonetheless, the lack of a significant FosB/ΔFosB response in known stress-activated regions, such as the PVN, suggests that negative findings using ΔFosB mapping need be interpreted with caution.
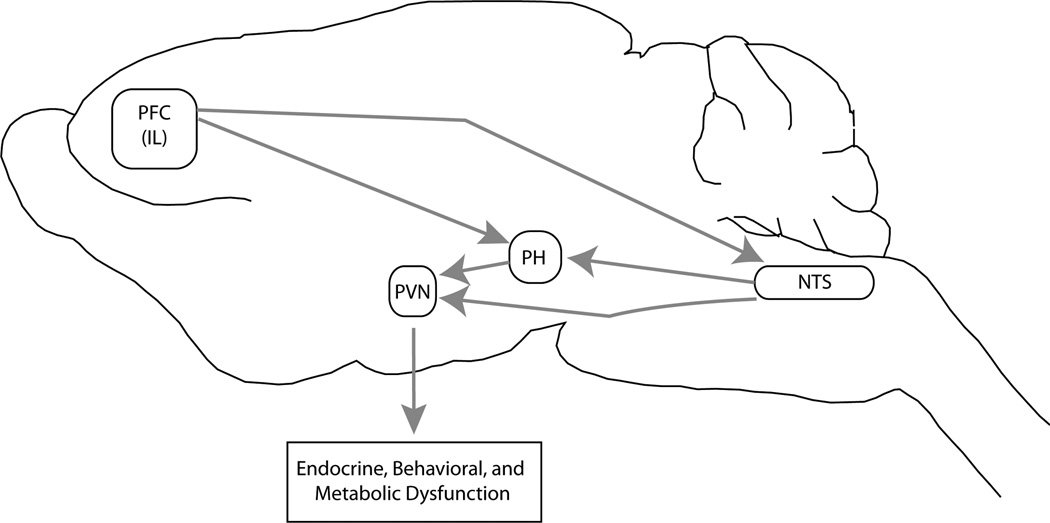
In conclusion, our analyses have located several brain regions specifically recruited by unpredictable stress, including the NTS, PH, and mPFC. Importantly, tract tracing studies indicate significant interconnectivity among these brain regions and the PVN (Figure 7). For example, the infralimbic mPFC has direct projections to the NTS (Vertes, 2004), which sends projections that terminate within the PH (Ciriello et al., 2003), DMH, (Ricardo and Koh, 1978) and the PVN (Cunningham and Sawchenko, 1988). Additionally, the PH (Ulrich-Lai et al., 2011b) projects into the PVN and NTS (Fontes et al., 2001). Finally, the prelimbic mPFC projects to the DMH and attenuates DMH cFos responses to acute stress (Radley et al., 2009), which provides an additional influence on PVN, PH, and NTS activation. Future tract tracing studies accompanied with chronic stress exposure are required to reveal the specific connections among these recruited regions, which may include some of the above proposed circuits and/or additional relays implicated in chronic stress-induced physiological and behavioral dysfunction. These future studies will determine whether these potentially recruited circuit(s) are critical for known consequences of chronic stress exposure (HPA axis facilitation and/or depression-like behavior and/or metabolic disruption and/or hypertension).
Figure 7.
Potential Chronic stress recruited circuitry. Our data suggest the recruitment of a neural circuit underlying chronic drive of the HPA axis, but future studies will have to verify whether these recruited neurons are specifically connected. This circuit begins with the activation of the prefrontal cortex (PFC) projecting to the nucleus of the solitary tract (NTS), which drive neurons within the posterior hypothalamic nucleus (PH). The PH activates the PVN via direct glutamatergic projections to the paraventricular nucleus of the hypothalamus (PVN), known to be a player in endocrine, behavioral, and metabolic homeostasis. Via this pathway, chronic stress may produce endocrine, behavioral, and metabolic dysfunction.
Acknowledgments
Supported by MH049698 and MH069860.
References
- Abrahamson EE, Moore RY. The posterior hypothalamic area: chemoarchitecture and afferent connections. Brain Res. 2001;889(1–2):1–22. doi: 10.1016/s0006-8993(00)03015-8. [DOI] [PubMed] [Google Scholar]
- Akana SF, Dallman MF, Bradbury MJ, Scribner KA, Strack AM, Walker CD. Feedback and facilitation in the adrenocortical system: unmasking facilitation by partial inhibition of the glucocorticoid response to prior stress. Endocrinology. 1992;131(1):57–68. doi: 10.1210/endo.131.1.1319329. [DOI] [PubMed] [Google Scholar]
- Andrus BM, Blizinsky K, Vedell PT, Dennis K, Shukla PK, Schaffer DJ, Radulovic J, Churchill GA, Redei EE. Gene expression patterns in the hippocampus and amygdala of endogenous depression and chronic stress models. Mol Psychiatry. 2010 doi: 10.1038/mp.2010.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey TW, Dimicco JA. Chemical stimulation of the dorsomedial hypothalamus elevates plasma ACTH in conscious rats. Am J Physiol Regul Integr Comp Physiol. 2001;280(1):R8–R15. doi: 10.1152/ajpregu.2001.280.1.R8. [DOI] [PubMed] [Google Scholar]
- Barsegyan A, Mackenzie SM, Kurose BD, McGaugh JL, Roozendaal B. Glucocorticoids in the prefrontal cortex enhance memory consolidation and impair working memory by a common neural mechanism. Proc Natl Acad Sci U S A. 2010;107(38):16655–16660. doi: 10.1073/pnas.1011975107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar S, Huber R, Nowak N, Trotter P. Lesions of the posterior paraventricular thalamus block habituation of hypothalamic-pituitary-adrenal responses to repeated restraint. J Neuroendocrinol. 2002;14(5):403–410. doi: 10.1046/j.0007-1331.2002.00792.x. [DOI] [PubMed] [Google Scholar]
- Capriles N, Rodaros D, Sorge RE, Stewart J. A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2003;168(1–2):66–74. doi: 10.1007/s00213-002-1283-z. [DOI] [PubMed] [Google Scholar]
- Carr KD, Wolinsky TD. Chronic food restriction and weight loss produce opioid facilitation of perifornical hypothalamic self-stimulation. Brain Res. 1993;607(1–2):141–148. doi: 10.1016/0006-8993(93)91499-i. [DOI] [PubMed] [Google Scholar]
- Carroll ME, France CP, Meisch RA. Food deprivation increases oral and intravenous drug intake in rats. Science. 1979;205(4403):319–321. doi: 10.1126/science.36665. [DOI] [PubMed] [Google Scholar]
- Carvalho-Netto EF, Myers B, Jones K, Solomon MB, Herman JP. Sex differences in synaptic plasticity in stress-responsive brain regions following chronic variable stress. Physiol Behav. 2011 doi: 10.1016/j.physbeh.2011.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chocyk A, Czyrak A, Wedzony K. Acute and repeated cocaine induces alterations in FosB/DeltaFosB expression in the paraventricular nucleus of the hypothalamus. Brain Res. 2006;1090(1):58–68. doi: 10.1016/j.brainres.2006.03.045. [DOI] [PubMed] [Google Scholar]
- Ciriello J, McMurray JC, Babic T, de Oliveira CV. Collateral axonal projections from hypothalamic hypocretin neurons to cardiovascular sites in nucleus ambiguus and nucleus tractus solitarius. Brain Res. 2003;991(1–2):133–141. doi: 10.1016/j.brainres.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol. 2004;60(2):236–248. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Wolfe TJ. Chronic stress regulates levels of mRNA transcripts encoding beta subunits of the GABA(A) receptor in the rat stress axis. Brain Res. 2000;887(1):118–124. doi: 10.1016/s0006-8993(00)03000-6. [DOI] [PubMed] [Google Scholar]
- Cunningham ET, Jr, Sawchenko PE. Anatomical specificity of noradrenergic inputs to the paraventricular and supraoptic nuclei of the rat hypothalamus. J Comp Neurol. 1988;274(1):60–76. doi: 10.1002/cne.902740107. [DOI] [PubMed] [Google Scholar]
- Das G, Uchida K, Kageyama K, Iwasaki Y, Suda T, Itoi K. Glucocorticoid dependency of surgical stress-induced FosB/DeltaFosB expression in the paraventricular and supraoptic nuclei of the rat hypothalamus. J Neuroendocrinol. 2009;21(10):822–831. doi: 10.1111/j.1365-2826.2009.01902.x. [DOI] [PubMed] [Google Scholar]
- Davis M, Shi C. The extended amygdala: are the central nucleus of the amygdala and the bed nucleus of the stria terminalis differentially involved in fear versus anxiety? Ann N Y Acad Sci. 1999;877:281–291. doi: 10.1111/j.1749-6632.1999.tb09273.x. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, de Jong IE, Oitzl MS. Neuropharmacology of glucocorticoids: focus on emotion, cognition and cocaine. Eur J Pharmacol. 2008;585(2–3):473–482. doi: 10.1016/j.ejphar.2008.03.011. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Aerni A, Schelling G, Roozendaal B. Glucocorticoids and the regulation of memory in health and disease. Front Neuroendocrinol. 2009;30(3):358–370. doi: 10.1016/j.yfrne.2009.03.002. [DOI] [PubMed] [Google Scholar]
- DiMicco JA, Abshire VM, Hankins KD, Sample RH, Wible JH., Jr Microinjection of GABA antagonists into posterior hypothalamus elevates heart rate in anesthetized rats. Neuropharmacology. 1986;25(9):1063–1066. doi: 10.1016/0028-3908(86)90203-0. [DOI] [PubMed] [Google Scholar]
- Flak JN, Jankord RJ, Solomon MB, Krause EG, Herman JP. Opposing effects of chronic stress and weight restriction on cardiovascular, neuroendocrine and metabolic function. Physiol Behav. 2011 doi: 10.1016/j.physbeh.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flak JN, Ostrander MM, Tasker JG, Herman JP. Chronic stress-induced neurotransmitter plasticity in the PVN. J Comp Neurol. 2009;517(2):156–165. doi: 10.1002/cne.22142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Seamans JK, Phillips AG. Selective roles for hippocampal, prefrontal cortical, and ventral striatal circuits in radial-arm maze tasks with or without a delay. J Neurosci. 1997;17(5):1880–1890. doi: 10.1523/JNEUROSCI.17-05-01880.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontes MA, Tagawa T, Polson JW, Cavanagh SJ, Dampney RA. Descending pathways mediating cardiovascular response from dorsomedial hypothalamic nucleus. Am J Physiol Heart Circ Physiol. 2001;280(6):H2891–H2901. doi: 10.1152/ajpheart.2001.280.6.H2891. [DOI] [PubMed] [Google Scholar]
- Girotti M, Pace TW, Gaylord RI, Rubin BA, Herman JP, Spencer RL. Habituation to repeated restraint stress is associated with lack of stress-induced c-fos expression in primary sensory processing areas of the rat brain. Neuroscience. 2006;138(4):1067–1081. doi: 10.1016/j.neuroscience.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Handwerger K. Differential patterns of HPA activity and reactivity in adult posttraumatic stress disorder and major depressive disorder. Harv Rev Psychiatry. 2009;17(3):184–205. doi: 10.1080/10673220902996775. [DOI] [PubMed] [Google Scholar]
- Herman JP, Adams D, Prewitt C. Regulatory changes in neuroendocrine stress-integrative circuitry produced by a variable stress paradigm. Neuroendocrinology. 1995;61(2):180–190. doi: 10.1159/000126839. [DOI] [PubMed] [Google Scholar]
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24(3):151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Herman JP, Flak J, Jankord R. Chronic stress plasticity in the hypothalamic paraventricular nucleus. Prog Brain Res. 2008;170:353–364. doi: 10.1016/S0079-6123(08)00429-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroi N, Fienberg AA, Haile CN, Alburges M, Hanson GR, Greengard P, Nestler EJ. Neuronal and behavioural abnormalities in striatal function in DARPP-32-mutant mice. Eur J Neurosci. 1999;11(3):1114–1118. doi: 10.1046/j.1460-9568.1999.00570.x. [DOI] [PubMed] [Google Scholar]
- Hurley KM, Herbert H, Moga MM, Saper CB. Efferent projections of the infralimbic cortex of the rat. J Comp Neurol. 1991;308(2):249–276. doi: 10.1002/cne.903080210. [DOI] [PubMed] [Google Scholar]
- Imaki T, Nahan JL, Rivier C, Sawchenko PE, Vale W. Differential regulation of corticotropin-releasing factor mRNA in rat brain regions by glucocorticoids and stress. J Neurosci. 1991;11(3):585–599. doi: 10.1523/JNEUROSCI.11-03-00585.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankord R, Solomon MB, Albertz J, Flak JN, Zhang R, Herman JP. Stress vulnerability during adolescent development in rats. Endocrinology. 2011;152(2):629–638. doi: 10.1210/en.2010-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnani M, Burdakov D. Multiple hypothalamic circuits sense and regulate glucose levels. Am J Physiol Regul Integr Comp Physiol. 2011;300(1):R47–R55. doi: 10.1152/ajpregu.00527.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinzig KP, D'Alessio DA, Herman JP, Sakai RR, Vahl TP, Figueiredo HF, Murphy EK, Seeley RJ. CNS glucagon-like peptide-1 receptors mediate endocrine and anxiety responses to interoceptive and psychogenic stressors. J Neurosci. 2003;23(15):6163–6170. doi: 10.1523/JNEUROSCI.23-15-06163.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss A, Aguilera G. Regulation of the hypothalamic pituitary adrenal axis during chronic stress: responses to repeated intraperitoneal hypertonic saline injection. Brain Res. 1993;630(1–2):262–270. doi: 10.1016/0006-8993(93)90665-a. [DOI] [PubMed] [Google Scholar]
- Larsen PJ, Tang-Christensen M, Holst JJ, Orskov C. Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience. 1997;77(1):257–270. doi: 10.1016/s0306-4522(96)00434-4. [DOI] [PubMed] [Google Scholar]
- Li HY, Ericsson A, Sawchenko PE. Distinct mechanisms underlie activation of hypothalamic neurosecretory neurons and their medullary catecholaminergic afferents in categorically different stress paradigms. Proc Natl Acad Sci U S A. 1996;93(6):2359–2364. doi: 10.1073/pnas.93.6.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisa M, Marmo E, Wible JH, Jr, DiMicco JA. Injection of muscimol into posterior hypothalamus blocks stress-induced tachycardia. Am J Physiol. 1989;257(1 Pt 2):R246–R251. doi: 10.1152/ajpregu.1989.257.1.R246. [DOI] [PubMed] [Google Scholar]
- Llewellyn-Smith IJ, Reimann F, Gribble FM, Trapp S. Preproglucagon neurons project widely to autonomic control areas in the mouse brain. Neuroscience. 2011;180:111–121. doi: 10.1016/j.neuroscience.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu XY, Barsh GS, Akil H, Watson SJ. Interaction between alpha-melanocyte-stimulating hormone and corticotropin-releasing hormone in the regulation of feeding and hypothalamo-pituitary-adrenal responses. J Neurosci. 2003;23(21):7863–7872. doi: 10.1523/JNEUROSCI.23-21-07863.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, Maheu F, Tu M, Fiocco A, Schramek TE. The effects of stress and stress hormones on human cognition: Implications for the field of brain and cognition. Brain Cogn. 2007;65(3):209–237. doi: 10.1016/j.bandc.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Maier SF, Amat J, Baratta MV, Paul E, Watkins LR. Behavioral control, the medial prefrontal cortex, and resilience. Dialogues Clin Neurosci. 2006;8(4):397–406. doi: 10.31887/DCNS.2006.8.4/smaier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino S, Smith MA, Gold PW. Increased expression of corticotropin-releasing hormone and vasopressin messenger ribonucleic acid (mRNA) in the hypothalamic paraventricular nucleus during repeated stress: association with reduction in glucocorticoid receptor mRNA levels. Endocrinology. 1995;136(8):3299–3309. doi: 10.1210/endo.136.8.7628364. [DOI] [PubMed] [Google Scholar]
- Maley BE. Immunohistochemical localization of neuropeptides and neurotransmitters in the nucleus solitarius. Chem Senses. 1996;21(3):367–376. doi: 10.1093/chemse/21.3.367. [DOI] [PubMed] [Google Scholar]
- Marttila K, Raattamaa H, Ahtee L. Effects of chronic nicotine administration and its withdrawal on striatal FosB/DeltaFosB and c-Fos expression in rats and mice. Neuropharmacology. 2006;51(1):44–51. doi: 10.1016/j.neuropharm.2006.02.014. [DOI] [PubMed] [Google Scholar]
- McClave JTaD, F H., II Statistics. 1994 [Google Scholar]
- McClung CA, Ulery PG, Perrotti LI, Zachariou V, Berton O, Nestler EJ. DeltaFosB: a molecular switch for long-term adaptation in the brain. Brain Res Mol Brain Res. 2004;132(2):146–154. doi: 10.1016/j.molbrainres.2004.05.014. [DOI] [PubMed] [Google Scholar]
- Miklos IH, Kovacs KJ. Reorganization of synaptic inputs to the hypothalamic paraventricular nucleus during chronic psychogenic stress in rats. Biol Psychiatry. 2012;71(4):301–308. doi: 10.1016/j.biopsych.2011.10.027. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, Self DW. DeltaFosB: a sustained molecular switch for addiction. Proc Natl Acad Sci U S A. 2001;98(20):11042–11046. doi: 10.1073/pnas.191352698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez C, Martin F, Foldes A, Luisa Laorden M, Kovacs KJ, Victoria Milanes M. Induction of FosB/DeltaFosB in the brain stress system-related structures during morphine dependence and withdrawal. J Neurochem. 2010;114(2):475–487. doi: 10.1111/j.1471-4159.2010.06765.x. [DOI] [PubMed] [Google Scholar]
- Papp M, Willner P, Muscat R. An animal model of anhedonia: attenuation of sucrose consumption and place preference conditioning by chronic unpredictable mild stress. Psychopharmacology (Berl) 1991;104(2):255–259. doi: 10.1007/BF02244188. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereo-taxic coordinates. 1997 [Google Scholar]
- Perrotti LI, Hadeishi Y, Ulery PG, Barrot M, Monteggia L, Duman RS, Nestler EJ. Induction of deltaFosB in reward-related brain structures after chronic stress. J Neurosci. 2004;24(47):10594–10602. doi: 10.1523/JNEUROSCI.2542-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitchers KK, Frohmader KS, Vialou V, Mouzon E, Nestler EJ, Lehman MN, Coolen LM. DeltaFosB in the nucleus accumbens is critical for reinforcing effects of sexual reward. Genes Brain Behav. 2010;9(7):831–840. doi: 10.1111/j.1601-183X.2010.00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabasa C, Delgado-Morales R, Munoz-Abellan C, Nadal R, Armario A. Adaptation of the hypothalamic-pituitary-adrenal axis and glucose to repeated immobilization or restraint stress is not influenced by associative signals. Behav Brain Res. 2011;217(1):232–239. doi: 10.1016/j.bbr.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Arias CM, Sawchenko PE. Regional differentiation of the medial prefrontal cortex in regulating adaptive responses to acute emotional stress. J Neurosci. 2006;26(50):12967–12976. doi: 10.1523/JNEUROSCI.4297-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Gosselink KL, Sawchenko PE. A discrete GABAergic relay mediates medial prefrontal cortical inhibition of the neuroendocrine stress response. J Neurosci. 2009;29(22):7330–7340. doi: 10.1523/JNEUROSCI.5924-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resstel LB, Joca SR, Guimaraes FG, Correa FM. Involvement of medial prefrontal cortex neurons in behavioral and cardiovascular responses to contextual fear conditioning. Neuroscience. 2006;143(2):377–385. doi: 10.1016/j.neuroscience.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Ricardo JA, Koh ET. Anatomical evidence of direct projections from the nucleus of the solitary tract to the hypothalamus, amygdala, and other forebrain structures in the rat. Brain Res. 1978;153(1):1–26. doi: 10.1016/0006-8993(78)91125-3. [DOI] [PubMed] [Google Scholar]
- Schmitz TW, Johnson SC. Relevance to self: A brief review and framework of neural systems underlying appraisal. Neurosci Biobehav Rev. 2007;31(4):585–596. doi: 10.1016/j.neubiorev.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhar A, DiMicco JA. Defense reaction elicited by injection of GABA antagonists and synthesis inhibitors into the posterior hypothalamus in rats. Neuropharmacology. 1987;26(5):407–417. doi: 10.1016/0028-3908(87)90020-7. [DOI] [PubMed] [Google Scholar]
- Stamp JA, Mashoodh R, van Kampen JM, Robertson HA. Food restriction enhances peak corticosterone levels, cocaine-induced locomotor activity, and DeltaFosB expression in the nucleus accumbens of the rat. Brain Res. 2008;1204:94–101. doi: 10.1016/j.brainres.2008.02.019. [DOI] [PubMed] [Google Scholar]
- Stotz-Potter EH, Willis LR, DiMicco JA. Muscimol acts in dorsomedial but not paraventricular hypothalamic nucleus to suppress cardiovascular effects of stress. J Neurosci. 1996;16(3):1173–1179. doi: 10.1523/JNEUROSCI.16-03-01173.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Gratton A. Lateralized effects of medial prefrontal cortex lesions on neuroendocrine and autonomic stress responses in rats. J Neurosci. 1999;19(7):2834–2840. doi: 10.1523/JNEUROSCI.19-07-02834.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauchi M, Zhang R, D'Alessio DA, Stern JE, Herman JP. Distribution of glucagon-like peptide-1 immunoreactivity in the hypothalamic paraventricular and supraoptic nuclei. J Chem Neuroanat. 2008;36(3–4):144–149. doi: 10.1016/j.jchemneu.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulery PG, Rudenko G, Nestler EJ. Regulation of DeltaFosB stability by phosphorylation. J Neurosci. 2006;26(19):5131–5142. doi: 10.1523/JNEUROSCI.4970-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Christiansen AM, Ostrander MM, Jones AA, Jones KR, Choi DC, Krause EG, Evanson NK, Furay AR, Davis JF, Solomon MB, de Kloet AD, Tamashiro KL, Sakai RR, Seeley RJ, Woods SC, Herman JP. Pleasurable behaviors reduce stress via brain reward pathways. Proc Natl Acad Sci U S A. 2011a;107(47):20529–20534. doi: 10.1073/pnas.1007740107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Figueiredo HF, Ostrander MM, Choi DC, Engeland WC, Herman JP. Chronic stress induces adrenal hyperplasia and hypertrophy in a subregion-specific manner. Am J Physiol Endocrinol Metab. 2006;291(5):E965–E973. doi: 10.1152/ajpendo.00070.2006. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10(6):397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Jones KR, Ziegler DR, Cullinan WE, Herman JP. Forebrain origins of glutamatergic innervation to the rat paraventricular nucleus of the hypothalamus: Differential inputs to the anterior versus posterior subregions. J Comp Neurol. 2011b;519(7):1301–1319. doi: 10.1002/cne.22571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkuyl JM, Hemby SE, Joels M. Chronic stress attenuates GABAergic inhibition and alters gene expression of parvocellular neurons in rat hypothalamus. Eur J Neurosci. 2004;20(6):1665–1673. doi: 10.1111/j.1460-9568.2004.03568.x. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51(1):32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Vialou V, Cui H, Perello M, Mahgoub M, Yu HG, Rush AJ, Pranav H, Jung S, Yangisawa M, Zigman JM, Elmquist JK, Nestler EJ, Lutter M. A Role for DeltaFosB in Calorie Restriction-Induced Metabolic Changes. Biol Psychiatry. 2011a doi: 10.1016/j.biopsych.2010.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vialou V, Robison AJ, Laplant QC, Covington HE, 3rd, Dietz DM, Ohnishi YN, Mouzon E, Rush AJ, 3rd, Watts EL, Wallace DL, Iniguez SD, Ohnishi YH, Steiner MA, Warren BL, Krishnan V, Bolanos CA, Neve RL, Ghose S, Berton O, Tamminga CA, Nestler EJ. DeltaFosB in brain reward circuits mediates resilience to stress and antidepressant responses. Nat Neurosci. 2011b;13(6):745–752. doi: 10.1038/nn.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology (Berl) 1997;134(4):319–329. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- Zhang R, Jankord R, Flak JN, Solomon MB, D'Alessio DA, Herman JP. Role of glucocorticoids in tuning hindbrain stress integration. J Neurosci. 2010;30(44):14907–14914. doi: 10.1523/JNEUROSCI.0522-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler DR, Cullinan WE, Herman JP. Organization and regulation of paraventricular nucleus glutamate signaling systems: N-methyl-D-aspartate receptors. J Comp Neurol. 2005;484(1):43–56. doi: 10.1002/cne.20445. [DOI] [PubMed] [Google Scholar]