Abstract
A method for integrating porous polymer membranes such as polycarbonate, polyethersulfone and polyethylene terephthalate to microfluidic devices is described. The use of 3-amino-propyltriethoxysilane as a chemical crosslinking agent was extended to integrate membranes with PDMS and glass microfluidic channels. A strong, irreversible bond between the membranes and microfluidic structure was achieved. The bonding strength in the APTES treated devices was significantly greater than in devices fabricated using either a PDMS “glue” or two-part epoxy bonding method. Evaluation of a filtering microdevice and the pore structure via SEM indicates the APTES conjugation does not significantly alter the membrane transport function and pore morphology.
1. Introduction
In the past decade, the integration of various membranes within microfluidic devices has enabled advanced mass transport control for many different research applications.1 By incorporating membranes with microfluidic channels, compartmentalized systems have been realized to control mass flux between compartments, or exclude components entirely. The integration of membranes into cell handling microdevices has assisted in regulating cellular manipulations and selective introduction of materials to cell cultures via fluid filtration for introducing agonists from a separate flow compartment,2 or membrane transport within a microarray.3 These types of devices can greatly benefit the biomedical and pharmaceutical industries in drug development, conducting clinical diagnostics as well as various biochemical and cell handling applications. One approach toward membrane integration with microfluidic channels has been to construct a laminated device consisting of different layers of aligned microchannels separated by a porous polymer membrane. A critical design consideration for fabricating this type of microfluidic device is how to best achieve reliable bonding between different membrane materials with molded PDMS or glass microfluidic layers.1 Bonding of membrane and device materials is further complicated in devices consisting of multiple channel layers or complex channel designs. Various approaches to bonding PDMS to polymer membranes have been reported, such as direct thermal bonding or bonding following plasma oxidization. In thermal bonding the layers are gently pressed together and cured at a temperature higher than the membrane material’s glass transition temperature. Thermal bonding can create permanently laminated structures, but also tends to cause wrinkling of the membrane due to the different thermal expansion coefficients of the individual layers. It can also cause distortion of the membrane’s pores or complete pore collapse, changing the membrane transport properties. Oxygen plasma activation of both the membrane and PDMS surfaces followed by direct bonding can create laminated structures. However, plasma activation only creates a weak bond between the membrane and the PDMS microdevice for a wide range of membrane materials including polycarbonate (PCTE), polyethersulfone (PES), and polyethylene terephthalate (PETE). Another popular bonding method involves the use of epoxy or PDMS prepolymer as an intermediate “glue” layer between the PDMS and the membrane to improve the bonding strength.1,3–5 This approach presents problems such as partially clogging the membrane’s pores and thus reducing the flux across the membrane, as well as the trapping of air bubbles along the membrane boundaries and PDMS channels.1,5 In one of the reported techniques, the use of PDMS prepolymer liquid as a bonding glue was implemented by adjusting the thickness of the glue layer to avoid gap formation along the edges of the membrane when it is sandwiched between two PDMS layers.3 In this method the thickness of the PDMS glue was adjusted by mixing uncured PDMS to various concentrations in toluene. Thin film deposition was another bonding approach in which the membrane is coated with a thin SiO2 layer by sputtering for direct plasma bonding of the membrane to PDMS structures.6 This method is not only more expensive but also relies upon the amount of SiO2 sputtered on the membrane surface, and this may cause inconsistent bonding results and changes in membrane transport properties. A final approach for incorporating membranes into microfluidic devices is to encapsulate a piece of membrane within a cavity in a bonded PDMS–PDMS device.7 In this type of incorporation, the membrane is not physically bonded with the structure, but is instead constrained at the edges by the PDMS bond. In this case it is possible for fluid to leak around the edges of the membrane at high pressures, and the membrane may become distorted if the PDMS is stretched.
Recently, a new method has been proposed for bonding between polymethylmethacrylate (PMMA) and PDMS plates, as well as between two PMMA plates.8 This method is based on the chemical surface modification of the substrates and does not require pressure or high temperatures to initiate the bond. In this method, an organic polymeric substrate is coated with a silane solution of 3-amino-propyltriethoxysilane (APTES), followed by plasma activation of the surfaces to be bonded, resulting in an irreversible bonding of the two substrates. APTES has been used in various applications as a coupling agent or adhesion promoter and pretreatment for coatings. In another study, APTES was used to modify the surfaces of silicon or glass substrates in order to bond these substrates to chemically activated fluorinated ethylene propylene (FEP).9 In this communication, the use of APTES is extended as a method for integration of nanoporous polycarbonate (PCTE), polyethersulfone (PES) and polyester terephthalate (PETE) membranes to PDMS and glass microfluidic channels with an irreversible bond. The three membrane types tested have been selected because they have been specifically developed for low protein binding, and have been widely used in microfluidic systems. Therefore, developing a reliable bonding method can be extremely beneficial to facilitate integration of these types of membranes to microfluidic devices.
2. Experimental
2.1. Bonding of porous polymer membrane to PDMS and glass substrates
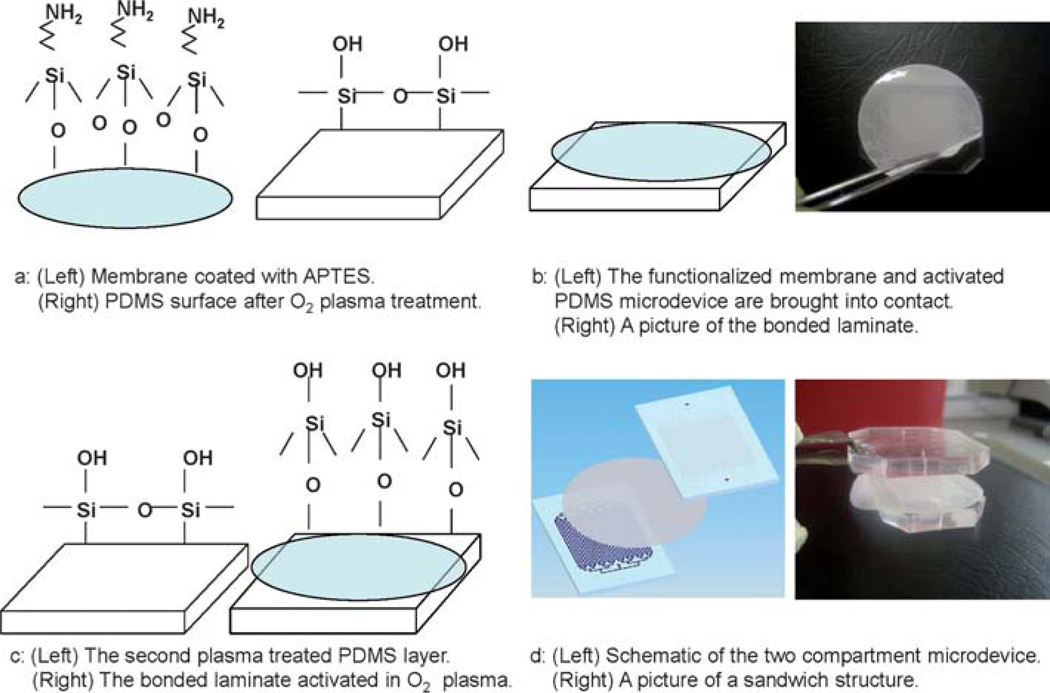
The bonding procedure for direct bonding of a membrane to a PDMS or glass substrate is shown schematically in Fig. 1. A commercial solution of APTES (Sigma-Aldrich, St Louis, USA) was diluted in water to 5% by volume and placed on a ceramic-top hot plate set to 80 °C. PCTE, PES, or PETE (Sterlitech Inc Kent, USA) membranes were activated in an oxygen plasma chamber for 1 min (600 mTorr, 100W) and then immersed in the APTES solution for 20 min. The solution was covered during the heating process to prevent water evaporation. After 20 min, the membrane was removed from the APTES solution with tweezers and was placed on a cleanroom wipe to dry. Then, the surface of a fully cured PDMS microdevice was activated in an oxygen plasma chamber (600 mTorr, 100 W) for 20 s (Fig. 1a). The membrane and the treated microdevice were immediately brought into contact (Fig. 1b). When the membrane comes in contact with the PDMS device, an immediate irreversible bond is formed. In order to bond the membrane to multiple PDMS layers in a sandwich structure, after bonding the membrane to PDMS as described above, the bonded laminate and a second PDMS layer were similarly activated in an oxygen plasma, brought into contact and subsequently pressed together (Fig. 1c and d).
Fig. 1.
Schematic of the bonding process between a porous membrane and a PDMS substrate. (a) (Left) The surface chemistry of a porous membrane functionalized by APTES and (right) the surface chemistry of PDMS after exposure to O2 plasma. (b) (Left) The membrane is bonded to the first PDMS structure and (right) a picture of bonding a PCTE membrane to a PDMS layer during an experiment. (c) (Left) The second PDMS structure after exposure to O2 plasma and (right) the surface chemistry of the bonded laminate. (d) (Left) Schematic of the two compartment microdevice including the two PDMS microchannels with a thin porous membrane sandwiched in between and (right) a picture of a sandwich structure of a two compartment device with a PCTE membrane and two PDMS layers.
The membrane–PDMS laminate could also be bonded to the second plasma-treated PDMS layer without plasma activation of the bonded laminate. For PCTE and PETE membranes, the bonding was instantaneous and no pressure was applied to initiate the bond. However, for PES membranes, pressure was applied by means of a weight placed on top of the layered structure for 24 h in order to achieve a strong and reliable bond between the PES membrane and PDMS layer.
For glass microdevices the same procedure was carried out substituting glass for the PDMS layers, and a similar irreversible bond was obtained. A longer bonding time (at least 48 h) was required in order to achieve irreversible bonding between PES membranes and glass microdevices.
Also, the surface of the glass or PDMS could be functionalized using a corona discharge instead of an oxygen plasma. However, a corona discharge should not be used to activate the membrane surface since the discharge often damages the membranes creating small tears where the discharge contacts the membrane. It has also been found that while the bonding between oxygen plasma-treated PDMS and a membrane is immediate for PCTE and PETE membranes, it takes about a minute for these membranes to bond to corona-treated PDMS. However, no external pressure is needed to initiate the bonding in either case. The APTES functionalized membranes can be peeled off from corona-treated PDMS and realigned within the first minute. This may be useful for accurate alignment between different layers of multi-layer devices.
2.2. A microfiltration microdevice to evaluate the bonding strength and membrane performance
2.2.1. Microfiltration device design
To evaluate the bonding strength of the APTES coated membrane, a cross-flow filtration microdevice was designed. The device consisted of two PDMS layers with a 200 nm pore size PCTE membrane sandwiched between the layers using the APTES bonding method as described. Each layer consisted of 32 parallel channels, 25 mm long, separated center to center by 800 µm. The sample to be filtered flows through the channels on one side of the membrane, termed reservoir channels, which are 600 µm in width and 135 µm deep, and the filtered analyte flows through the membrane and into the filtrate channels (400 µm wide, 40 µm deep) on the other side. The filtrate channels are slightly thinner than the reservoir channels to allow manual alignment of the two channel structures with enough tolerance to ensure complete channel overlap. A parallel channel design was chosen to allow a high enough flow rate (~tens of µl min−1) to be infused through the network so that sufficient sample could be collected at the device outlet in a reasonable amount of time (<15 min). Each channel has been determined to be fluidically isolated from each other by visual inspection, and no fluid has been observed leaking between adjacent channels under the separating walls during fluid infusion.
2.2.2. Bond strength testing
To measure the maximum pressure that the integrated microdevice was able to withhold without the membrane delaminating from the PDMS layers, water was infused into the inlet of the reservoir channels at a flow rate of 120 µl min−1 via a syringe pump, and the outlet of the reservoir was blocked and connected to a pressure sensor with a maximum pressure reading of 227.8 kPa (Honeywell Sensing and Control, ASDX030G24R). The inlet and outlet of the filtrate channels were blocked to allow pressure to build within the device. The pressure within the device was recorded to determine the burst pressure of the device. In this study, two other commonly used bonding methods were evaluated on identical test devices to compare each technique. In one method, the PCTE membrane was bonded to the PDMS layers by using the previously reported PDMS prepolymer glue technique.3 In another method a low viscosity two-part epoxy (EPO-TEK 301, Epotek, Billerica, MA) was used to bond the PCTE membrane to PDMS. The results obtained from these two techniques were compared with that of using APTES surface modification for bonding. Each device was tested under three conditions to simulate common device storage conditions: evaluating (1) the devices right after infusing water into dry devices; (2) after filling the device with water for 72 h; (3) after submerging the devices in water for 72 h.
2.2.3. Evaluation of APTES coated porous PCTE membrane
The influence of APTES conjugation on the physical properties of the membrane pores and on biofilm formation on the membrane surface was explored in both a flowing and static system. In the flowing system with the described microfiltration device, the channels were first primed using lactated Ringer’s solution (Baxter Healthcare, Deerfield, IL) to purge air bubbles from the main flow, and to remove any unconjugated APTES from the membrane surface. Unwashed unconjugated APTES can cause protein aggregation on the membrane surface. However, this protein aggregate is not adsorbed to the membrane surface and methods such as increasing cross-flow rate on the membrane surface or prewashing the channels can be used to reduce fouling. In some applications the APTES treated surfaces are rinsed with deionized water and ethanol10,11 which may be used to clean the membrane surfaces following device bonding and prior to experiment fluid infusion. These washing/purging steps are similar to preconditioning steps followed in other membrane integration methods. After priming, heparinized sheep’s blood plasma (Hemostat Labs, Dixon, CA) was infused through the reservoir channels for 1 h at a flow rate of 80 µl min−1 and the filtered plasma was collected from the filtrate channel outlets. The filtration flow rate was measured over the experimental period to evaluate the effect of APTES coating to any significant decrease in the permeate flux through the membrane due to buildup of a biofouling layer. After the experiment, nonadherent proteins were washed by infusing Ringer’s solution through the channels of the device. Finally, the device was cut using a razor blade and a small piece of membrane was removed and fixed in 2% glutaraldehyde, dehydrated and evaluated using scanning electron microscopy (AMRAY-1830I, AMRAY, Bedford, MA). A similar experiment was carried out under the same flow rate condition using whole blood instead of blood plasma. Whole blood was infused through the reservoir channels and the filtration flow rate was measured over a 4 h infusion period. In another experiment, both an APTES treated and untreated 200 nm pore size PCTE membrane were soaked in blood plasma for 1 h under static conditions. The membranes were then washed in Ringer’s solution, fixed in 2% glutaraldehyde, dehydrated, and evaluated with SEM to examine any changes in protein biofouling due to the APTES conjugation.
3. Results and discussion
3.1. Bond strength results
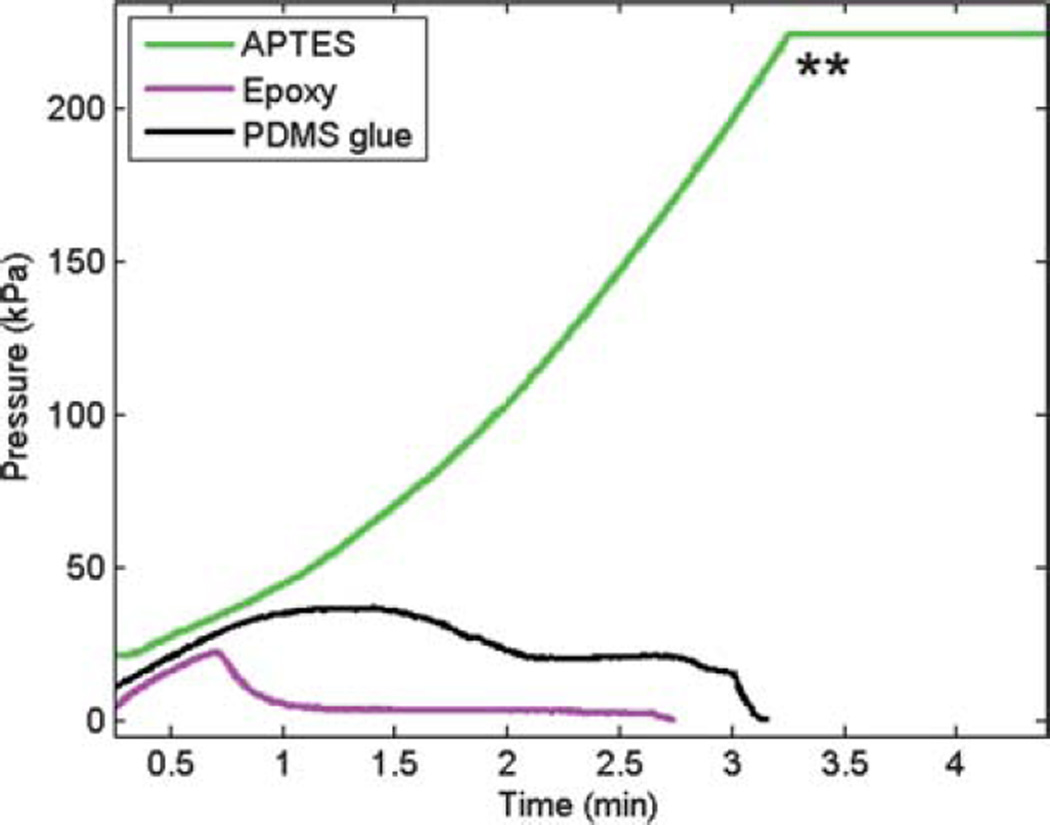
The use of APTES for microchannel–membrane bonding allowed the fabrication of complex channel designs with integrated porous membranes and multi-layer PDMS or glass multichannel structures. Irreversible bonding between the microchannel and membrane layers without deformation of the channel structures or membrane was achieved. The bonding was leakage free along the channels and membrane edges. The bonding strength was preliminarily evaluated after bonding PDMS or glass to both PCTE and PETE membranes by attempting to manually peel the membrane from a glass or PDMS microfluidic device. The membrane could not be peeled from the microchannel surface without breaking the membrane into small pieces, or ripping a piece of PDMS from the structure indicating the strength of the bond was greater than the fracture strength of the materials. The results from testing the APTES bonding strength under applied pressure via a pressure sensor showed that the membrane bond was able to withhold more than 227.8 kPa, which was the maximum limit of the pressure sensor, without any sign of delamination (Fig. 2). The pressure in this test was only relieved by the inlet tubing becoming physically dislodged from the device. To compare the bonding strength of this technique over those using PDMS prepolymer or two-part epoxy glue, identical test devices were used under applied pressure. The results shown in Table 1 demonstrate that for the same experimental conditions there is a significant increase in bonding strength using the APTES surface modification technique. To further test the bonding strength in the presence of water, two additional tests were carried out. In one test, the channels of the microdevices were filled with water and stored at room temperature for 72 h. In another test the microdevices were filled and completely immersed in water for 72 h.
Fig. 2.
Representative plots showing burst pressure for dry devices using three different bonding techniques. The APTES coated membrane was able to withhold much higher pressure (more than 227.8 kPa) compared to PDMS or epoxy bonded membranes. ** Maximum limit of the pressure sensor.
Table 1.
Comparison of bonding methods in different storage conditions
| Test method | Dry device | Channels filled with water (72 h) |
Device submerged in water (72 h) |
|---|---|---|---|
| APTES surface modification | >227.8 kPa | >227.8 kPa | 132.2 kPa |
| PDMS glue | 26.58 kPa | 12.5 kPa | 0 kPa, membrane delaminated |
| Epoxy glue | 22.31 kPa | 13.25 kPa | 0 kPa, membrane delaminated |
The bonding strength for APTES coated membranes remained very strong (over 227.8 kPa) in devices stored at room temperature for 72 h with the device channels filled with water, but decreased dramatically in glued membranes. Complete immersion of the devices in water for an extended period of time weakened the membrane bonding strength for all three tested bonding methods. The maximum pressure the APTES bonded membrane withheld after complete immersion in water was 132.2 kPa, compared to the glued devices which completely delaminated when water infusion began and thus held no pressure. While the APTES bonded membrane started to delaminate from the channel walls at a pressure of 132.2 kPa, it did not separate from the PDMS at the edges of the microdevice. After about 10 min, the PDMS began to bulge which eventually caused the membrane to rupture. The APTES bonding strength was further evaluated in a device stored at room temperature for 30 days with the device channels filled with water, and was able to withstand over 187 kPa, which still indicates a robust bond between the APTES coated membrane and PDMS layers.
3.2. Membrane performance and SEM results
In order to evaluate the ability of the APTES coated membrane to function properly in microfluidic systems, a cross-flow microfiltration device was infused with blood plasma at 80 µl min−1 for 1 h and the reservoir and filtrate outlet flow rate were measured at 74 and 6 µl min−1 respectively with no changes over the infusion time. In a similar experiment whole blood was also infused through the reservoir channels at 80 µl min−1 and the reservoir and filtrate outlet fractions were collected at a flow rate of 72 and 8 µl min−1 respectively and did not alter over a 4 h infusion period. These results showed no significant decrease in reservoir or filtrate output flow rates due to biofilm formation or membrane clogging throughout the experiments. The difference in reservoir and filtrate flow rates between the plasma and whole blood infusion experiments is attributed to the higher viscosity of whole blood increasing the reservoir channel hydraulic flow resistance.
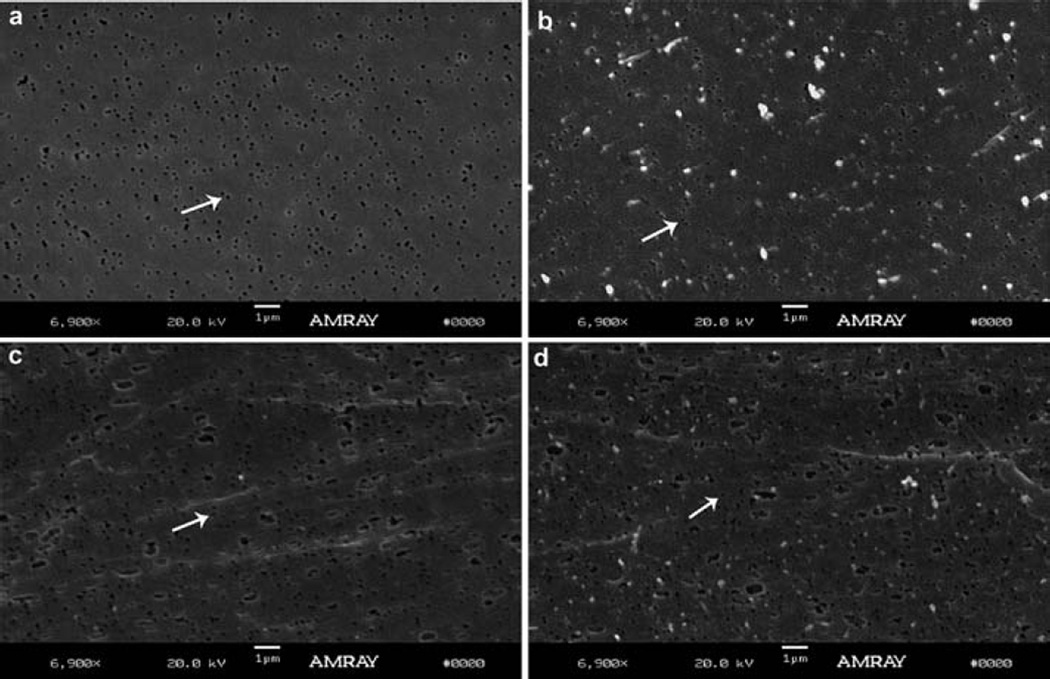
Fig. 3 shows SEM photographs of an untreated membrane (Fig. 3a), an APTES treated membrane after exposure to blood plasma in a flowing system (Fig. 3b), an untreated membrane after exposure to blood plasma in a static system (Fig. 3c), and an APTES treated membrane after exposure to blood plasma in a static system (Fig. 3d). Although some protein aggregation is seen on the membrane surface after exposure to blood plasma, the underlying pores in the membrane can be clearly seen and are not clogged. The extent of protein adsorption under static conditions was greater than under flow conditions, likely due to a lack of fluid shear which prevents protein buildup under flow conditions. However, the protein adsorption between the APTES conjugated and untreated membranes is indistinguishable. Finally, no gross changes were observed in pore morphology of the membranes due to the APTES conjugation or formation of a biofouling layer under either static or flow conditions.
Fig. 3.
Representative SEM images of porous PCTE membranes. SEM images of (a) untreated PCTE membrane, (b)APTES treated PCTE membrane under a flow condition and exposure to blood plasma, (c) untreated membrane soaked in blood plasma for 1 h in a static system and (d) APTES treated membrane soaked in blood plasma for 1 h in a static system. The white arrows highlight one representative pore on the membrane surface for each condition evaluated.
This method, based on surface modification of porous polymer membranes using APTES, exhibited a significant increase in bonding strength over other bonding techniques such as PDMS glue or biocompatible adhesives for similar test devices. The strong integration of porous polymer membranes to microdevices presented in this work did not alter the membrane function and pore morphology and can greatly expand the reliable use of these types of membrane in more complex microstructures.
Acknowledgements
This work was supported by the National Institutes of Health National Heart Lung and Blood Institute grant No. 1R21HL084367-01A1 and Wallace H. Coulter Foundation Early Career Translational Research Awards in Biomedical Engineering.
Notes and references
- 1.de Jong JH, Lammertink RGH, Wessling L. Lab Chip. 2006;6:1125–1139. doi: 10.1039/b603275c. [DOI] [PubMed] [Google Scholar]
- 2.Neeves KB, Diamond SL. Lab Chip. 2008;8:701–709. doi: 10.1039/b717824g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chueh BH, Huh D, Kyrtsos CR, Houssin T, Futai N, Takayama S. Anal. Chem. 2007;79:3504–3508. doi: 10.1021/ac062118p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu D, Steckl AJ. Lab Chip. 2009;9:1890–1896. doi: 10.1039/b823409d. [DOI] [PubMed] [Google Scholar]
- 5.Wu H, Huang B, Zare RN. Lab Chip. 2005;5:1393–1398. doi: 10.1039/b510494g. [DOI] [PubMed] [Google Scholar]
- 6.Kimura H, Yamamoto T, Sakai H, Sakai Y, Fujii T. Lab Chip. 2008;8:741–746. doi: 10.1039/b717091b. [DOI] [PubMed] [Google Scholar]
- 7.Kuo TC, Cannon DM, Jr, Chen Y, Tulock JJ, Shannon MA, Sweedler JV, Bohn PW. Anal. Chem. 2003;75:1861–1867. doi: 10.1021/ac025958m. [DOI] [PubMed] [Google Scholar]
- 8.Vlachopoulou ME, Tserepi A, Pavli P, Argitis P, Sanopoulou M, Misiakos K. J. Micromech. Microeng. 2009;19:015007. [Google Scholar]
- 9.Bart J, Tiggelaar R, Yang M, Schlautmann S, Zuilhof H, Gardeniers H. Lab Chip. 2009;9:3481. doi: 10.1039/b914270c. [DOI] [PubMed] [Google Scholar]
- 10.Yamada K, Yoshii S, Kumagai S, Miura A, Uraoka Y, Fuyuki T, Yamashita I. Jpn. J. Appl. Phys. 2006;45:8946–8951. [Google Scholar]
- 11.Vashist SK, Raiteri R, Tewari R, Bajpa RP, Bharadwaj LM. J. Phys.: Conf. Ser. 2006;34:806–811. [Google Scholar]