Significance
Deregulation of components of the ubiquitin–proteasome system contributes to the development of various diseases. A prominent example is the ubiquitin ligase E6AP/UBE3A, which is associated with three disorders: in complex with the E6 oncoprotein of human papillomaviruses, it contributes to cervical carcinogenesis; loss of E6AP expression results in the development of Angelman syndrome; and increased E6AP expression has been associated with autism spectrum disorders. This indicates that E6AP has to be tightly controlled; however, only little is known about how this is achieved. By analyzing the role of ubiquitin and the E6 oncoprotein in E6AP-mediated ubiquitination, we provide evidence that E6AP exists in an active state and a latent state and that its activity is controlled by allosteric effectors.
Keywords: E6AP/UBE3A, ubiquitin, human papillomavirus, E6 oncoprotein, Angelman syndrome
Abstract
Deregulation of the ubiquitin ligase E6 associated protein (E6AP) encoded by the UBE3A gene has been associated with three different clinical pictures. Hijacking of E6AP by the E6 oncoprotein of distinct human papillomaviruses (HPV) contributes to the development of cervical cancer, whereas loss of E6AP expression or function is the cause of Angelman syndrome, a neurodevelopmental disorder, and increased expression of E6AP has been involved in autism spectrum disorders. Although these observations indicate that the activity of E6AP has to be tightly controlled, only little is known about how E6AP is regulated at the posttranslational level. Here, we provide evidence that the hydrophobic patch of ubiquitin comprising Leu-8 and Ile-44 is important for E6AP-mediated ubiquitination, whereas it does not affect the catalytic properties of the isolated catalytic HECT domain of E6AP. Furthermore, we show that the HPV E6 oncoprotein rescues the disability of full-length E6AP to use a respective hydrophobic patch mutant of ubiquitin for ubiquitination and that it stimulates E6AP-mediated ubiquitination of Ring1B, a known substrate of E6AP, in vitro and in cells. Based on these data, we propose that E6AP exists in at least two different states, an active and a less active or latent one, and that the activity of E6AP is controlled by noncovalent interactions with ubiquitin and allosteric activators such as the HPV E6 oncoprotein.
In eukaryotes, posttranslational modification of proteins by ubiquitin plays a pivotal role in the regulation of many cellular processes, including cell cycle, DNA metabolism (e.g., DNA repair, transcription), and various signal transduction pathways (1–4). The specificity of the ubiquitin-conjugation system is mainly ensured by E3 ubiquitin ligases, which mediate the recognition of target proteins. Based on the presence of distinct domains and their mode of action, E3 proteins can be grouped into three families, RING/RING-like E3s, RING-in-between-RING (RBR) E3s, and HECT E3s (5–7). All E3s have interaction sites for both substrate proteins and E2 ubiquitin-conjugating enzymes. However, whereas in the case of RBR E3s and HECT E3s, ubiquitin is transferred from the E3 to substrates, RING/RING-like E3s function as adaptors between substrates and E2s (i.e., ubiquitin is transferred from the E2 to the substrate).
E6AP, the founding member of the HECT E3 family, was originally identified as an interacting protein of the E6 oncoprotein of cancer-associated human papillomaviruses (HPVs) (8, 9). The E6–E6AP complex targets the tumor suppressor p53 and other proteins—which in the absence of E6 are not targeted by E6AP—for ubiquitination and degradation thereby contributing to HPV-induced cervical carcinogenesis (10, 11). In 1997, it was recognized that alterations in the UBE3A gene, which encodes E6AP, resulting in loss of E6AP expression or in the expression of E6AP variants with compromised E3 activity, are the cause of the Angelman syndrome (AS), a neurodevelopmental disorder (12–14). Recently, it was reported that amplification of the UBE3A gene (i) is found in a certain percentage of patients with autism spectrum disorders (15, 16) and (ii) in mice, results in increased E6AP levels and autistic phenotypes (17).
The notion that alteration of the substrate spectrum, loss of E3 function, and increased E3 function of E6AP contribute to the development of distinct disorders indicates that expression and/or E3 activity of E6AP have to be tightly controlled. Whereas some mechanisms controlling transcription of the UBE3A gene have been identified (e.g., the paternal allele is silenced by a UBE3A antisense transcript) (14, 18), only little is known about how the E3 activity of E6AP is regulated. We recently reported that E6AP binds to HERC2, a member of the HECT E3 family, and that HERC2 acts as an allosteric activator of E6AP (19). The physiological relevance of this interaction is indicated by the finding that a point mutation in the HERC2 gene, resulting in a mutant HERC2 protein with increased turnover rate and hence decreased protein levels, underlies the development of a neurodevelopmental disorder with AS-like features (20).
When analyzing the E6AP–HERC2 interaction, we observed that a ubiquitin variant, in which the so-called canonical hydrophobic patch of ubiquitin is mutated (Ub_hpI), is only poorly used by E6AP for ubiquitination and that HERC2 can partially rescue this disability of E6AP (19). This observation prompted us to take a closer look at the role of ubiquitin in E6AP-mediated ubiquitination. We found that different surface areas of ubiquitin affect the ability of E6AP to catalyze the final transfer of ubiquitin to a substrate protein by different mechanisms, although they are not critically involved in the preceding steps (interaction of E6AP with cognate E2 enzymes, thioester complex formation of E6AP with ubiquitin). Furthermore, we show that the HPV E6 oncoprotein rescues the disability of E6AP to use Ub_hpI, demonstrating that E6 does not only alter the substrate spectrum of E6AP but also acts as a potent allosteric activator of E6AP.
Results
The Hydrophobic Patches of Ubiquitin Are Critical for E6AP-Mediated Ubiquitination.
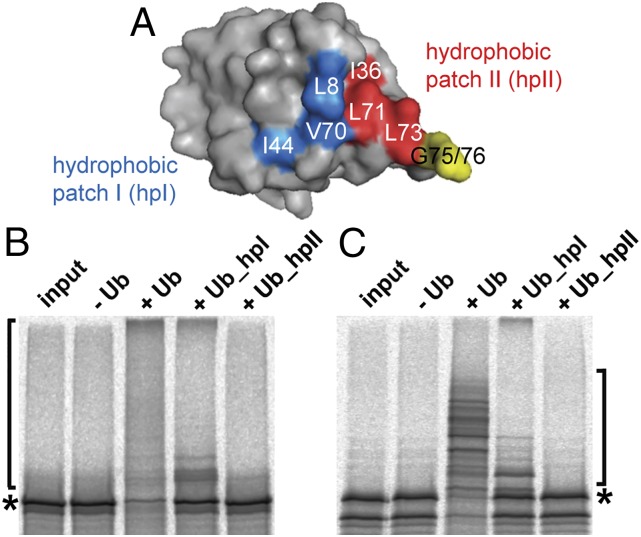
Genetic analyses have shown that three distinct surface areas of ubiquitin are essential for viability of Saccharomyces cerevisiae (21). Two of these “hydrophobic patches”—involving Leu-8 and Ile-44 (termed patch I in the following) and Ile-36, Leu-71, and Leu-73 (termed patch II) (Fig. 1A)—have been more closely examined for their role in ubiquitination mediated by distinct E2 ubiquitin-conjugating enzymes and E3 enzymes (e.g., refs. 22–31). Notably, patch II but not patch I was shown to be required for covalent attachment of ubiquitin to substrate proteins catalyzed by the HECT E3s Rsp5 and NEDD4L (22, 30). In addition, the HECT domains of Rsp5, NEDD4L, NEDD4, and SMURF2, which are all members of the NEDD4 subfamily of HECT E3s, harbor a noncovalent binding site for a second ubiquitin molecule (that is not in thioester complex with the HECT domain) (23, 24, 32, 33). This interaction is mediated by patch I, and although patch I is not essential for isopeptide bond formation (30), it affects the processivity of ubiquitin chain formation (23, 24, 32, 33). In contrast to Rsp5 and NEDD4L, the HECT domain of E6AP does not appear to harbor a noncovalent interaction site for free ubiquitin (24). Nonetheless, both hydrophobic patches are critically involved in E6AP-mediated ubiquitination, as respective ubiquitin mutants (Ub_hpI, Leu-8 and Ile-44 replaced by Ala; Ub_hpII, Ile-36, Leu-71, and Leu-73 replaced by Ala) are not or only poorly used by E6AP for autoubiquitination as well as for ubiquitination of an inactive form of the RING E3 ligase Ring1B (Ring1B-I53S), a known target of E6AP (34) (Fig. 1B).
Fig. 1.
Hydrophobic patches of ubiquitin are critically involved in E6AP-mediated ubiquitination. (A) Surface model of ubiquitin (Protein Data Bank, PDB 1UBQ) with amino acid residues of the canonical hydrophobic patch I and of the noncanonical hydrophobic patch II indicated in blue and red, respectively. (B) For E6AP autoubiquitination, in vitro translated radiolabeled E6AP was incubated with baculovirus-expressed E6AP in the absence or presence of wild-type ubiquitin (Ub) or the ubiquitin mutants Ub_hpI (substitution of Leu-8 and Ile-44 by Ala) and Ub_hpII (substitution of Ile-36, Leu-71, and Leu-73 by Ala) under standard ubiquitination conditions (Materials and Methods) as indicated. Reaction products were analyzed by SDS/PAGE followed by fluorography. (C) As in B but E6AP-mediated ubiquitination of in vitro translated radiolabeled Ring1B-I53S (an inactive form of Ring1B that cannot ubiquitinate itself) (34) was studied. Running positions of the nonmodified form and of the ubiquitinated forms of E6AP and Ring1B-I53S are indicated by asterisks and brackets, respectively.
The Hydrophobic Patches of Ubiquitin Are Not Required for Cognate E2s to Bind to E6AP.
A possible explanation for the requirement of intact patches I and II for E6AP-mediated ubiquitination is that E6AP interacts noncovalently with (one of) these patches, when ubiquitin is in thioester complex with E2 enzymes. If this is the case (i.e., E6AP contacts ubiquitin and E2 simultaneously), it can be predicted that the ubiquitin loading status of cognate E2s affects their affinity for E6AP. Due to both the inherent thermodynamic instability of thioester bonds and the fact that activated ubiquitin is rapidly transferred from E2 to E6AP, it is notoriously difficult to analyze the binding of E2 enzymes in thioester complex with ubiquitin to E6AP. It was recently shown that a UbcH5a variant, in which the catalytic Cys residue was replaced by Lys, can be efficiently loaded with ubiquitin via isopeptide bond formation and that the resulting complex is a reasonable structural mimic of the thioester complex of UbcH5a with ubiquitin (28). Thus, we adopted this strategy to study the interaction of E6AP with UbcH7 and UbcH5b (Fig. S1), which both support E6AP-mediated ubiquitination in vitro (19, 35). As shown by isothermal titration calorimetry and size exclusion chromatography, the ubiquitin loading status of UbcH7 does not have a major influence on the ability of UbcH7 to interact with the isolated HECT domain of E6AP (Fig. S2), confirming previously published data on E6AP–UbcH7 interaction (36). In contrast, whereas the HECT domain of E6AP does not detectably interact with UbcH5b in the absence of ubiquitin, UbcH5b preloaded with ubiquitin (UbcH5b–Ub) binds to the HECT domain with an efficiency similar to UbcH7 (Fig. 2 and Fig. S3). However, neither mutation of patch I nor of patch II had a significant effect on the ability of UbcH5b to bind to the HECT domain (Fig. 2), indicating that they play only a minor role in E6AP–E2 interaction. This notion is further supported by the observation that like UbcH5b–Ub, UbcH5b–Ub_hpI and UbcH5b–Ub_hpII act in a dominant-negative manner in E6AP autoubiquitination [Fig. S4; note that a complex of ubiquitin with UbcH1, an E2 that does not support E6AP-mediated ubiquitination (35), does not interfere with E6AP autoubiquitination, indicating the specificity of the dominant-negative effect of UbcH5b–Ub].
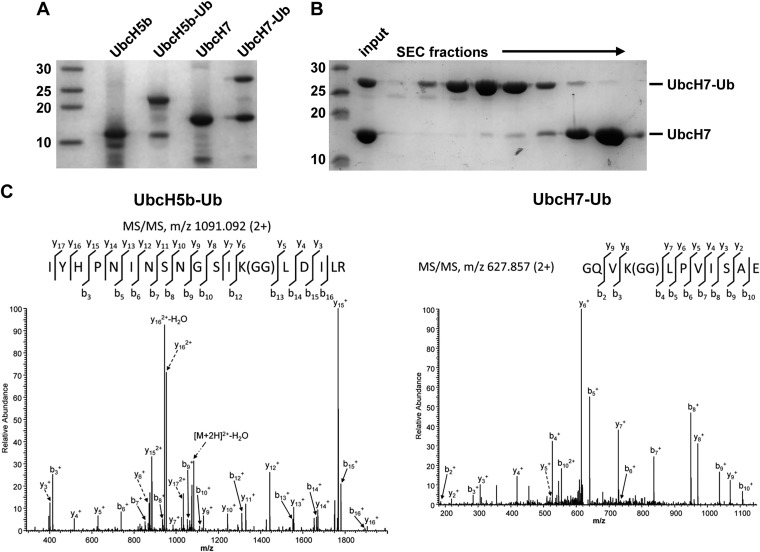
Fig. S1.
Generation of stable conjugates between ubiquitin and UbcH5b and UbcH7. (A) To generate stable conjugates between ubiquitin and UbcH5b or UbcH7 mimicking respective ubiquitin thioester complexes, the catalytic Cys residue of UbcH5b (Cys-85) and of UbcH7 (Cys-86) was substituted by Lys. The respective mutants were incubated with the ubiquitin-activating enzyme and ubiquitin to allow covalent attachment of ubiquitin to Lys-85 of UbcH5b or Lys-86 of UbcH7 via isopeptide bond formation. Whole reaction mixtures were separated by SDS/PAGE with respective input controls (UbcH5b, UbcH7) followed by Coomassie staining. The running positions of molecular mass markers (in kilodaltons) are indicated. (B) For interaction analysis with the HECT domain of E6AP, ubiquitin conjugates of UbcH7 (or UbcH5b) were purified from the nonmodified form by size exclusion chromatography (SEC). Fractions were analyzed by SDS/PAGE followed by Coomassie staining. The running positions of molecular mass markers (in kilodaltons) are indicated. (C) Bands in A representing stable conjugates between ubiquitin and UbcH5b (UbcH5b–Ub) and UbcH7 (UbcH7b–Ub) were cut out, digested by trypsin (5b–Ub) or a trypsin/Glu-C mixture (7-Ub), and desalted. Digested samples were analyzed by reversed phase liquid chromatography-nanospray tandem mass spectrometry (LC-MS/MS) (for details, see SI Materials and Methods). The results obtained showed that UbcH5b and UbcH7 were exclusively modified by ubiquitin at Lys-85 and Lys-86, respectively.
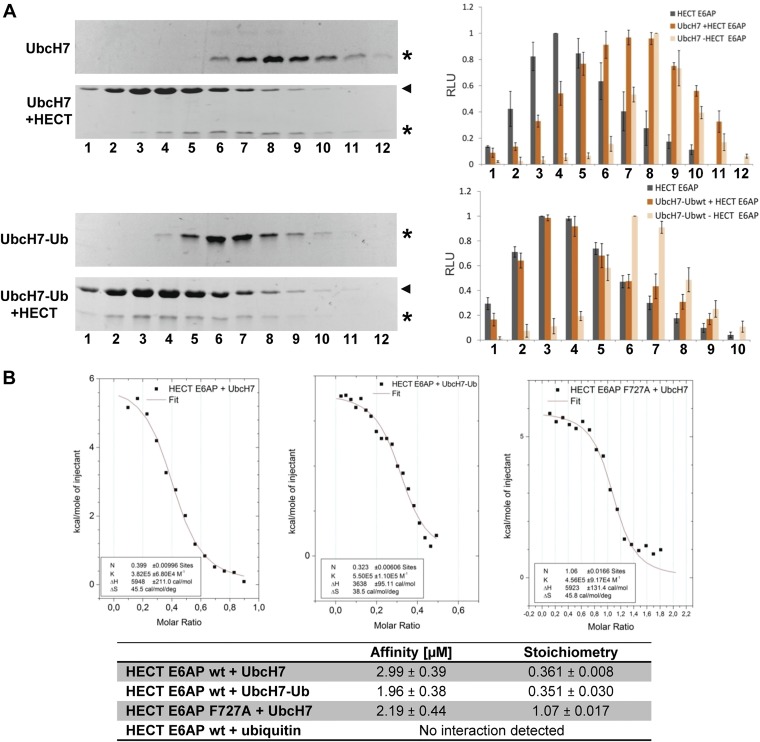
Fig. S2.
The ubiquitin loading status has no major impact on the ability of UbcH7 to interact with E6AP. (A) UbcH7 and the stable UbcH7–ubiquitin conjugate (UbcH7–Ub) were incubated in the absence or in the presence (+HECT) of the HECT domain of E6AP for 5 min. The mixtures were then fractionated by size exclusion chromatography. Fractions were subjected to SDS/PAGE and proteins visualized by Coomassie staining. Intensities of the bands representing the HECT domain, UbcH7, and the different UbcH7–ubiquitin conjugates were quantified by densitometry and are expressed in relative light units (RLU) (Right). Running position of the HECT domain is marked by an arrowhead; running positions of UbcH7 and the different UbcH7–ubiquitin conjugates are marked by an asterisk. Relative fraction numbers are indicated. Error bars represent the SD from at least three independent experiments. (B) The binding affinity of ubiquitin, UbcH7, and the UbcH7–ubiquitin conjugate for the HECT domain of E6AP was determined by isothermal titration calorimetry (for details, see SI Materials and Methods). Origin software was used to fit the data to a single-site binding model and determine the stoichiometry (N), ΔH, ΔS, and the association constant K. Measurements for the interaction of UbcH7 and UbcH7–ubiquitin with the HECT domain of E6AP were done in triplicates. Note that the apparent stoichiometry of UbcH7 to the HECT domain of ∼0.36 is in line with previously published data showing that the HECT domain of E6AP forms trimers at high concentrations and that the trimer is bound to only one UbcH7 molecule (55). Therefore, ITC was repeated with the F727A mutant of the HECT domain (substitution of Phe-727 by Ala), which does not form trimers, revealing that one HECT domain molecule binds to one UbcH7 molecule.
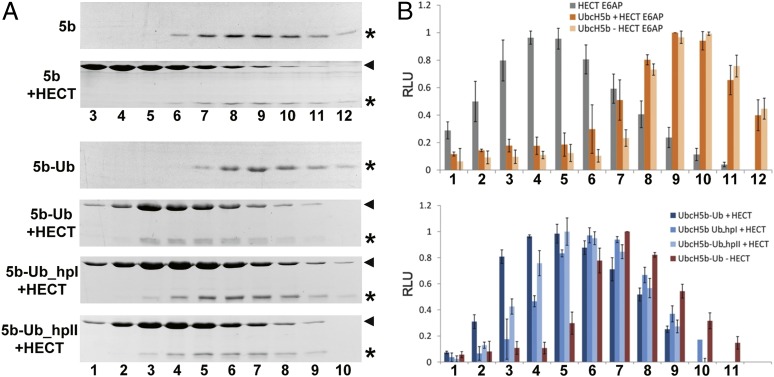
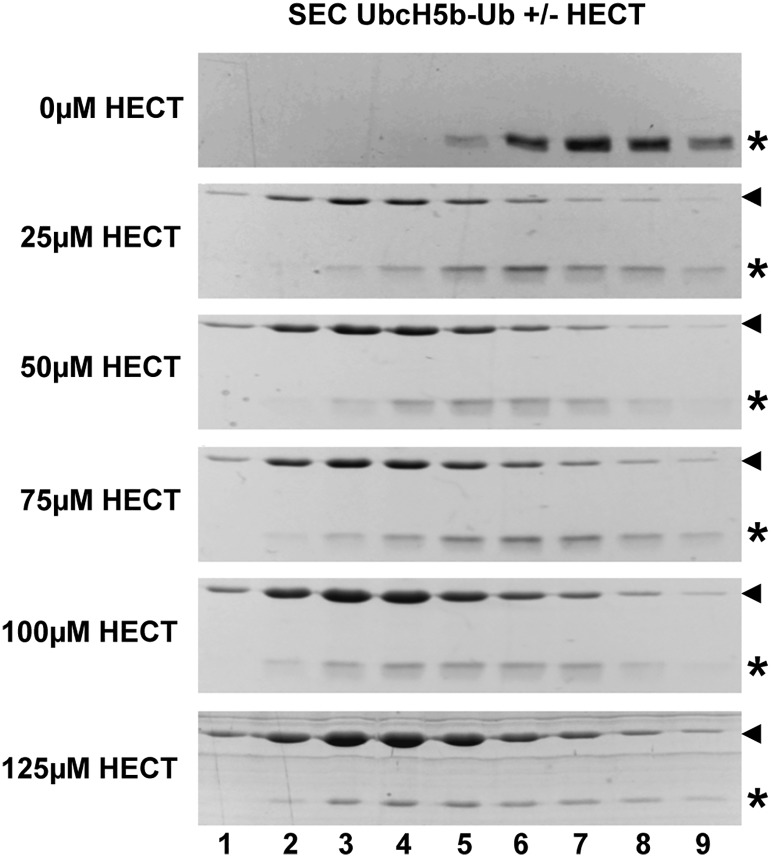
Fig. 2.
The ubiquitin-loading status of UbcH5b affects its ability to interact with E6AP. (A) UbcH5b (5b) and stable conjugates of the catalytically inactive UbcH5b–C85K mutant with wild-type ubiquitin (5b-Ub) or the ubiquitin mutants Ub_hpI and Ub_hpII (5b-Ub_hpI, 5b-Ub_hpII) were incubated in the absence or the presence (+HECT) of the HECT domain of E6AP. After 5 min, the mixtures were fractionated by size exclusion chromatography. Fractions were subjected to SDS/PAGE and proteins visualized by Coomassie staining. Relative fraction numbers are indicated. Running position of the HECT domain is marked by an arrowhead; running positions of UbcH5b and the different UbcH5b–ubiquitin conjugates are marked by an asterisk. (B) Intensities of the bands representing the HECT domain, UbcH5b, and the different UbcH5b–ubiquitin conjugates were quantified by densitometry and are expressed in relative units (RLU). Relative fraction numbers are indicated. Error bars represent the SD from at least three independent experiments. For results obtained for UbcH7–ubiquitin conjugates, see Figs. S1 and S2.
Fig. S3.
The ubiquitin-loading status of UbcH5b affects its ability to interact with E6AP. UbcH5b–ubiquitin conjugates (20 µM) were incubated in the absence or presence of increasing concentrations of the HECT domain of E6AP as indicated for 5 min at room temperature. The mixtures were fractionated by size exclusion chromatography (SEC) and the fractions were analyzed by SDS/PAGE followed by Coomassie staining. Relative fraction numbers are indicated. Running positions of the HECT domain and the UbcH5b–ubiquitin conjugate are marked by an arrowhead and an asterisk, respectively.
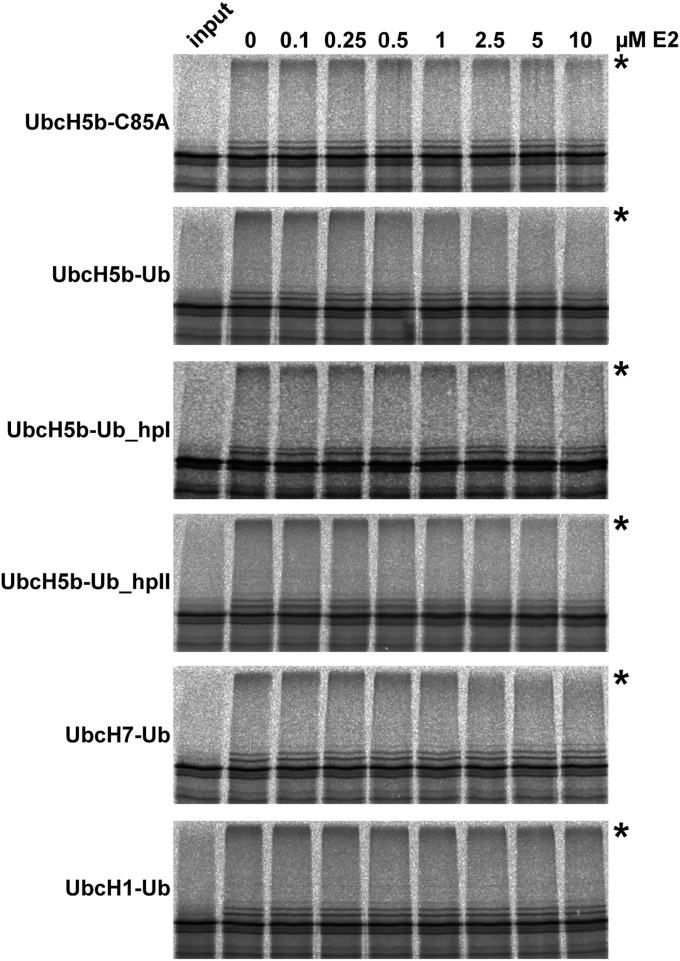
Fig. S4.
Stable UbcH5b–ubiquitin conjugates interfere with E6AP autoubiquitination. In vitro translated radiolabeled E6AP (1 µL) was incubated with 20 µg wild-type ubiquitin in the absence (0) or presence of increasing concentrations (in micromolar) of stable conjugates between the E2 indicated and wild-type ubiquitin (Ub) or the ubiquitin mutants Ub_hpI and Ub_hpII. Reaction mixtures were incubated for 2 h under standard ubiquitination conditions (Materials and Methods), except that recombinant UbcH5b or UbcH7 were not added to limit the efficiency of E6AP autoubiquitination (note that the amount of rabbit UbcH5/UbcH7 orthologs present in 1 µL reticulocyte lysate translate is sufficient to support E6AP-mediated ubiquitination). The stable ubiquitin conjugate of UbcH1–C88K (substitution of catalytic Cys-88 by Lys) was used as control, because this E2 does not support E6AP-mediated ubiquitination. In addition, the catalytically inactive UbcH5b–C85A (substitution of Cys-85 by Ala) was used as control to show that the dominant-negative effect of the UbcH5b–ubiquitin conjugates is dependent on both the presence of UbcH5b and ubiquitin. Running position of ubiquitinated E6AP is indicated by an asterisk.
The Hydrophobic Patches of Ubiquitin Are Involved in E6AP-Catalyzed Isopeptide Bond Formation.
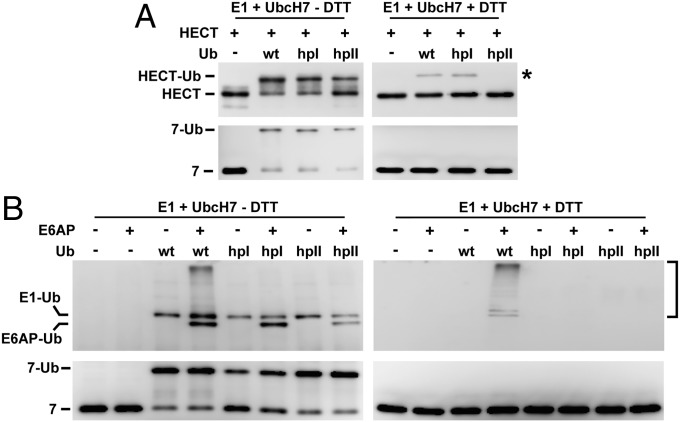
Next, the possibility that patch I and/or patch II are required for efficient transfer of ubiquitin from E2 enzymes to the catalytic Cys residue of E6AP was studied. Hence, the ability of full-length E6AP and of the isolated HECT domain to form thioester complexes with Ub_hpI and Ub_hpII was determined (37). To do so, E1 and UbcH7 were used in concentrations that are not rate limiting for the reaction to ensure that potential differences in the efficiency of the ubiquitin variants to form thioester complexes with E6AP/HECT domain are solely due to the ability of the E6AP/HECT domain to accept these. However, no significant differences were observed for ubiquitin, Ub_hpI, and Ub_hpII in their ability to be transferred from UbcH7 to the catalytic Cys residue of the HECT domain (Fig. 3A) or E6AP (Fig. 3B) (for results obtained with UbcH5b, see Fig. S5A).
Fig. 3.
Hydrophobic patches of ubiquitin do not affect thioester complex formation with E6AP. (A) E1, UbcH7, the HECT domain of E6AP, wild-type (WT) ubiquitin (Ub), and the ubiquitin mutants hpI and hpII (Fig. 1A) were incubated under thioester reaction conditions (Materials and Methods). Reactions were stopped in the presence (+DTT) or the absence (−DTT; to preserve thioester complexes) of a reducing agent. Whole reaction mixtures were subjected to SDS/PAGE followed by Western blot analysis using antibodies directed against E6AP (HECT) or His-tag (UbcH7). Running positions of UbcH7 (marked as 7), the HECT domain of E6AP, and the respective ubiquitin thioester complexes are indicated. An autoubiquitinated form of the HECT domain (which is resistant to DTT treatment) is indicated by an asterisk. (B) As in A but baculovirus-expressed full-length E6AP and His-tagged forms of wild-type (WT) ubiquitin and the ubiquitin mutants hpI and hpII were used. Furthermore, Western blot analysis was performed with an anti-His antibody, because due to the small size difference, the nonmodified form of E6AP is not readily distinguished from the E6AP–ubiquitin thioester complex by using anti-E6AP antibodies. Running positions of the various ubiquitin thioester complexes and the nonmodified form of UbcH7 (marked as 7) are indicated. Autoubiquitinated forms of E6AP (which are resistant to DTT treatment) are indicated by a bracket. For results obtained with UbcH5b, see Fig. S5.
Fig. S5.
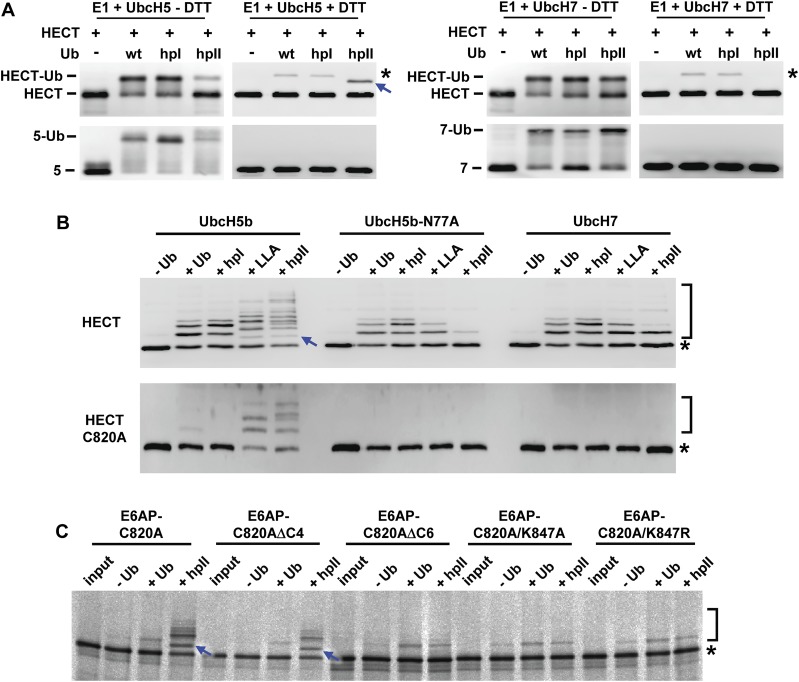
The catalytic Cys residue of E6AP is not required for ubiquitination of the HECT domain by the hpII ubiquitin mutant. (A) E1, UbcH5b or UbcH7, the HECT domain of E6AP (amino acid residues 500–852), wild-type (WT) ubiquitin (Ub), and the ubiquitin mutants hpI and hpII were incubated under thioester reaction conditions (Materials and Methods). Reactions were stopped in the presence (+DTT) or the absence (−DTT; to preserve thioester complexes) of a reducing agent. Whole reaction mixtures were subjected to SDS/PAGE followed by Western blot analysis using antibodies directed against E6AP (HECT) or His-tag (UbcH5b, UbcH7). Running positions of UbcH5b and UbcH7 (marked as 5 and 7, respectively), the HECT domain of E6AP, and the respective ubiquitin thioester complexes are indicated. Monoubiquitinated forms of the HECT domain, which are resistant to DTT treatment (i.e., ubiquitin is linked to the HECT domain via an isopeptide bond), are indicated by an asterisk and a blue arrow. Note that the migration behavior of the monoubiquitinated form observed in presence of Ub_hpII and UbcH5b is different from that observed in the presence of WT ubiquitin or the hpI mutant. This indicates that different lysine residues of the HECT domain serve as attachment sites for the different ubiquitin versions. (B) The HECT domain and its catalytically inactive form (HECT–C820A, substitution of Cys-820 by Ala) were expressed in bacteria. Both forms were incubated in the absence (−Ub) or presence (+Ub) of wild-type (WT) ubiquitin or the ubiquitin mutants hpI, LLA (substitution of Leu-71 and Leu-73 by Ala), and hpII under standard ubiquitination conditions (Materials and Methods). As E2s, UbcH5b, the UbcH5b mutant UbcH5b–N77A (substitution of Asn-77 by Ala), and UbcH7 were used. After 2 h, reactions were stopped and whole reaction mixtures subjected to SDS/PAGE followed by Western blot analysis using an antibody directed against the HECT domain of E6AP. Running positions of nonmodified HECT domain and ubiquitinated forms of HECT are indicated by an asterisk and a bracket, respectively. In addition, a monoubiquitinated form that is only observed in the presence of both Ub_hpII and UbcH5b is marked by a blue arrow. UbcH5b–N77A and UbcH7 can transfer ubiquitin to catalytic Cys residues of HECT and RBR E3s (i.e., they mediate E3–ubiquitin thioester complex formation) but are not or only poorly capable of forming isopeptide bonds between ubiquitin and Lys residues (38, 39). Indeed, the catalytically inactive HECT–C820A mutant was efficiently ubiquitinated in the presence of Ub_hpII and UbcH5b but not in the presence of UbcH5b–N77A or UbcH7. This demonstrates that the ubiquitin mutant Ub_hpII is directly transferred from UbcH5b to a Lys residue of the HECT domain. (C) Full-length E6AP–C820A and mutated forms of it [ΔC4, deletion of the C-terminal 4 amino acids; ΔC6, deletion of the C-terminal 6 amino acids (i.e., deletion of amino acids K847GFGML852); K847A, substitution of Lys-847 by Ala; K847R, substitution of Lys-847 by Arg] were translated in rabbit reticulocyte lysate in the presence of 35S-Met. A total of 1 µL of each translate was incubated with ubiquitin-activating enzyme and UbcH5b in the absence (−Ub) or presence (+Ub) of wild-type ubiquitin or the ubiquitin mutant hpII under standard ubiquitination conditions (Materials and Methods). Whole reaction mixtures were analyzed by SDS/PAGE followed by fluorography. Running positions of nonmodified E6AP–C820A variants and their ubiquitinated forms are indicated by an asterisk and a bracket, respectively. A monoubiquitinated form that is only observed with E6AP–C820A and the ΔC4 variant is indicated by a blue arrow, suggesting that Lys-847 serves as the main attachment site for the hpII mutant. Indeed, mass spectrometric analysis of the HECT domain ubiquitinated by Ub_hpII revealed that Ub_hpII is attached to Lys-847.
In contrast to the reaction with full-length E6AP, a band corresponding to a covalent complex of the HECT domain with Ub_hpI was observed even upon treatment with a reducing agent (DTT) (Fig. 3A), indicating that the isolated HECT domain can use Ub_hpI for autoubiquitination. Indeed, subsequent autoubiquitination experiments showed that the isolated HECT domain uses wild-type ubiquitin and Ub_hpI with similar efficiencies for autoubiquitination (Fig. S5B). In addition, when using UbcH5b instead of UbcH7, the HECT domain was autoubiquitinated not only in the presence of ubiquitin and Ub_hpI but apparently also in the presence of Ub_hpII (Fig. S5 A and B). Closer examination of the latter result revealed that in contrast to ubiquitin and Ub_hpI, covalent attachment of Ub_hpII to E6AP was mainly catalyzed by UbcH5b rather than by the HECT domain itself (i.e., Ub_hpII is directly transferred from the catalytic Cys residue of UbcH5b to a Lys residue of E6AP; for further details, see legend to Fig. S5). This conclusion is based on the findings that the reaction is (i) independent of the catalytic Cys residue of E6AP (Fig. S5 B and C), (ii) only poorly catalyzed by UbcH7 (Fig. S5B), which is known to be weakly active in isopeptide bond formation (38), and (iii) only poorly catalyzed by a UbcH5b mutant (Fig. S5B) that can still catalyze thioester complex formation between ubiquitin and HECT E3s but is impaired in isopeptide bond formation (39).
In conclusion, the data obtained for Ub_hpI and Ub_hpII indicate that both patch I and patch II are involved in E6AP-catalyzed isopeptide bond formation, whereas their integrity is not critical for E6AP–ubiquitin thioester complex formation. Furthermore, patch I and patch II affect E6AP-mediated ubiquitination by different mechanisms: Patch II appears to directly contribute to E6AP-catalyzed isopeptide bond formation, because both the isolated HECT domain and full-length E6AP cannot use Ub_hpII for ubiquitination; in contrast, patch I has an indirect effect, because the isolated HECT domain can use Ub_hpI for ubiquitination, whereas full-length E6AP cannot.
HPV E6 Acts as Allosteric Activator of E6AP.
The observation that full-length E6AP but not the isolated HECT domain is impaired in using Ub_hpI for autoubiquitination indicates that the N-terminal region (with N terminus defined as E6AP without the HECT domain) influences the catalytic properties of the HECT domain. Thus, the N-terminal region may not only represent a binding platform for substrates but also for proteins regulating E6AP activity by driving E6AP into a more active or less active conformation. This hypothesis is supported by the ability of HERC2—which binds to a region (amino acids 150–200) in the N terminus of E6AP but does not represent a substrate for E6AP—to stimulate E6AP activity, and this stimulatory effect is most readily observed when Ub_hpI is used as a source of ubiquitin (19).
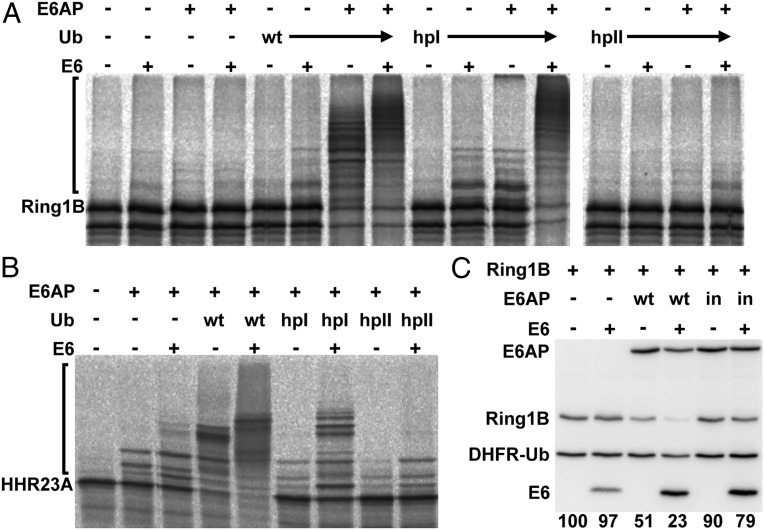
Similar to HERC2, the HPV E6 oncoprotein binds to a distinct region within the N terminus of E6AP (amino acids 378–395) (19, 40) and is not a substrate for E6AP (19, 41). To test if binding of E6 results in activation of the catalytic properties of E6AP, the effect of E6 on the ability of E6AP to use Ub_hpI and Ub_hpII for ubiquitination was determined. Indeed, as shown in Fig. 4, E6AP-mediated ubiquitination of Ring1B-I53S (34) or HHR23A (42) was stimulated in the presence of E6. As for HERC2, the stimulatory effect was particularly prominent, when Ub_hpI was used as a source for ubiquitin. In contrast, E6 could not rescue the disability of E6AP to use Ub_hpII for ubiquitination, supporting the notion that patch I and patch II play different roles in E6AP-mediated ubiquitination.
Fig. 4.
The HPV E6 oncoprotein is an allosteric activator of E6AP. (A) In vitro translated radiolabeled Ring1B-I53S was incubated with baculovirus-expressed E6AP in the absence or presence of the HPV16 E6 oncoprotein and of wild-type (WT) ubiquitin (Ub) or the ubiquitin mutants hpI and hpII (Fig. 1A) under standard ubiquitination conditions (Materials and Methods) as indicated. Reaction products were analyzed by SDS/PAGE followed by fluorography. Running positions of the nonmodified form and of the ubiquitinated forms of Ring1B-I53S are indicated by an asterisk and a bracket, respectively. (B) As in A but in vitro translated radiolabeled HHR23A was used as substrate. (C) H1299–shE6AP cells, in which endogenous E6AP expression is stably down-regulated by RNA interference (50), were transfected with expression constructs for HA-tagged wild-type E6AP (WT) or the catalytically inactive mutant E6AP–C820A (in), HA-tagged HPV16 E6, and a DHFR–HA–ubiquitin fusion protein of HA-tagged Ring1B-I53S (for details on the DHFR–HA–ubiquitin system, see text). Twenty-four hours after transfection, protein extracts were prepared and levels of the various proteins were determined by Western blot analysis using an anti-HA antibody and quantified. The relative ratio of HA-tagged Ring1b-I53S to DHFR–HA–ubiquitin is indicated, with the ratio of HA-tagged Ring1b-I53S to DHFR–HA–ubiquitin in the absence of E6AP set to 100%. Running positions of E6AP, Ring1B, HPV16 E6, and DHFR–HA–ubiquitin are indicated.
Finally, cell culture experiments were performed to determine if the stimulating effect of E6 on E6AP-mediated degradation of Ring1B-I53S can also be observed in cells (Fig. 4C). To do so, the dehydrofolate reductase (DHFR)–ubiquitin fusion protein system was used (43–45). In this system, DHFR–HA–ubiquitin and Ring1B-I53S are expressed as one protein from the same mRNA and cotranslationally cleaved by ubiquitin-specific proteases in a quantitative manner, resulting in two separate proteins. Because upon translation and concomitant cleavage, two separate proteins are generated from a common precursor, comparison of the relative levels of Ring1B-I53S and DHFR–HA–ubiquitin (a mutant form of ubiquitin, in which Lys-48 is replaced by Arg, is used in this system to avoid degradation of DHFR–HA–ubiquitin) provides a direct measure for the effect of E6AP on the turnover rate of Ring1B-I53S (see also ref. 45). As shown in Fig. 4C, coexpression of E6 resulted in significant stimulation of E6AP-mediated degradation of Ring1B-I53S. Furthermore, this effect was not observed in the presence of a catalytically inactive mutant of E6AP, indicating the specificity of the E6 effect (37). Taken together, these data demonstrate that E6 does not only increase the substrate spectrum of E6AP but in addition, acts as a potent allosteric activator of E6AP.
Discussion
Although E6AP has been associated with three different disorders and was the first HECT E3 identified, only little is known about how its activity is regulated at the posttranslational level. By analyzing the functional interaction of defined ubiquitin mutants with E6AP, we provide evidence that the N terminus of E6AP is not only involved in substrate recognition but also has an impact on the ability of the HECT domain to catalyze the final attachment of ubiquitin to substrate proteins. Furthermore, binding of the HPV E6 oncoprotein strongly enhances the catalytic activity of E6AP, indicating that E6AP exists in at least two different states—a fully active form and a catalytically less active or latent form.
It was previously shown that patch II of ubiquitin is involved in both transfer of ubiquitin from E2 to NEDD4 family members and subsequent attachment of ubiquitin to substrate proteins, whereas patch I of ubiquitin affects the processivity of ubiquitin chain formation (22–24, 30, 32, 33). In contrast, both patches contribute to E6AP-mediated isopeptide bond formation, whereas their integrity is not crucial for E6AP–ubiquitin thioester complex formation, supporting the notion that different HECT E3s use different catalytic strategies (46). Nonetheless, it seems likely that similar to NEDD4 family members and, for example, the RNF4–UbcH5a complex, the integrity of patch II is required to create an appropriate environment for nucleophilic attack of the E6AP–ubiquitin thioester bond by the incoming amino group of a Lys residue of a target protein (28, 30, 31). In addition, we propose that in the case of E6AP, patch II serves as selectivity filter. UbcH5b catalyzes the covalent attachment of Ub_hpII to Lys-847 of E6AP, whereas this reaction is not observed with wild-type ubiquitin (Fig. S5C). Thus, it appears that patch II shields the UbcH5b–ubiquitin thioester complex from undesirable attack by the amino group of Lys-847, ensuring selective transthioesterification of ubiquitin from UbcH5b to the catalytic Cys residue of E6AP.
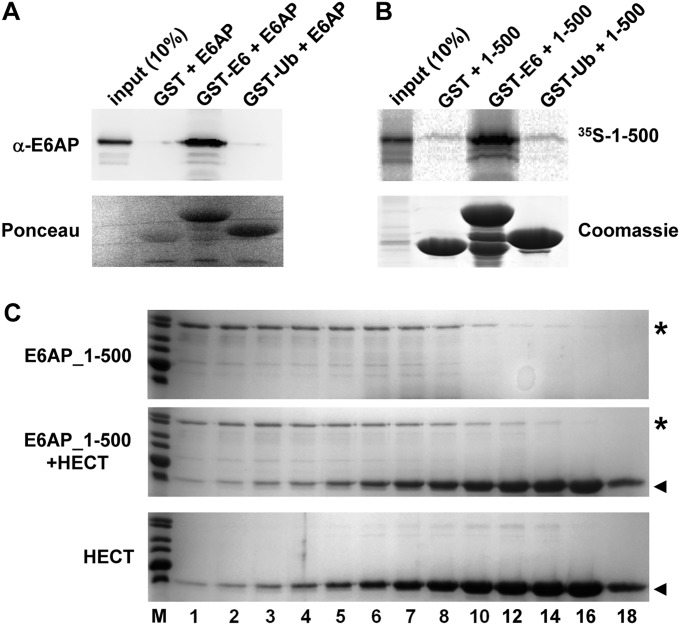
At first glance, the observation that patch I is required for efficient ubiquitination by E6AP is reminiscent of results obtained for ubiquitination mediated by RING E3s or by E2s alone (e.g., refs. 25–28). For example, in the case of the RNF4–UbcH5a complex, Ile-44 and Leu-8 are located at the interface of ubiquitin with UbcH5a and the RNF4 RING domain contributing to the correct positioning of ubiquitin for isopeptide bond formation (28). Such a scenario seems unlikely for E6AP, because the isolated HECT domain uses wild-type ubiquitin and Ub_hpI, in which Ile-44 and Leu-8 are replaced by Ala (Fig. 3 and Fig. S5), with similar efficiencies for autoubiquitination. However, in contrast to full-length E6AP, the isolated HECT domain is not or only weakly active in ubiquitin chain formation (as indicated by the autoubiquitination pattern of the HECT domain, and as reported before) (46). Thus, whereas in the absence of patch I of ubiquitin, the N-terminal region of E6AP interferes with the catalytic activity of the HECT domain, it is crucial for E6AP-mediated ubiquitin chain formation. How can these seemingly contradictory roles of the N terminus be reconciled? An obvious possibility is that (i) as reported for some NEDD4 family members (summarized in ref. 7), the N-terminal region of E6AP physically interacts with the HECT domain, and (ii) either the N-terminal region or the HECT domain of E6AP harbors a noncovalent binding site for ubiquitin. However, we could observe neither a noncovalent interaction of free ubiquitin with E6AP nor a noncovalent interaction of free ubiquitin with E6AP of the isolated N-terminal region with the HECT domain by size exclusion chromatography or coprecipitation experiments (Fig. S6). Thus, we propose that the affinities of the respective interactions are rather low and that the interactions occur in a functionally relevant manner only when the N-terminal region and the HECT domain are present on the same polypeptide chain (i.e., intramolecular interaction) and when ubiquitin is in thioester complex with E6AP (i.e., a single ubiquitin molecule makes the covalent as well as noncovalent contact).
Fig. S6.
Interaction studies. (A) Baculovirus-expressed E6AP was incubated with bacterially expressed GST or GST fusion proteins of HPV16 E6 (GST–E6) and ubiquitin (GST–Ub) for 2 h at 4 °C. GST proteins were pulled down by glutathione-Sepharose beads and precipitates analyzed by SDS/PAGE followed by Western blot analysis using an anti-E6AP antibody or by Ponceau staining. Whereas E6AP is efficiently bound by GST–E6, an interaction with GST–Ub was not observed. (B) A C-terminally truncated form of E6AP comprising amino acids 1–500 was translated in rabbit reticulocyte lysate in the presence of 35S-Met. The translate was incubated with bacterially expressed GST, GST–E6, or GST–Ub as indicated for 2 h at 4 °C. GST proteins were pulled down by glutathione-Sepharose beads and precipitates analyzed by SDS/PAGE followed by fluorography (to detect 35S-labeled 1–500) or by Coomassie staining (to visualize GST proteins). (C) The N-terminal 500 amino acids of E6AP (E6AP_1–500) were expressed as a GST fusion protein in bacteria. Upon purification, E6AP_1–500 (final concentration 10 µM) alone (Upper), E6AP_1–500 (10 µM) and the HECT domain (100 µM) (Middle), and the HECT domain (100 µM) alone (Lower) were incubated for 5 min at room temperature. The mixtures were fractionated by size exclusion chromatography and the fractions were analyzed by SDS/PAGE followed by Coomassie staining. Relative fraction numbers are indicated. Running positions of E6AP_1–500 and the HECT domain are marked by an asterisk and an arrowhead, respectively. Note that the presence of the HECT domain did not affect the elution behavior of E6AP_1–500 and vice versa, indicating that under the conditions used, the two proteins did not detectably interact.
Although final proof of the proposed model will have to await elucidation of the structure of full-length E6AP and of E6AP in covalent complex with ubiquitin, it is supported by the data obtained previously for the HERC2–E6AP interaction (19) and the data provided here for the effect of HPV E6 on the catalytic activity of E6AP. Both HERC2 and HPV E6 bind to distinct regions in the N terminus of E6AP (19, 41) and stimulate E6AP activity (note that because the structure of the N terminus has not yet been solved, we cannot exclude that the two binding sites are located within the same domain or at a similar position at the surface of E6AP). Moreover, both proteins rescue the disability of E6AP to use Ub_hpI for ubiquitination, suggesting that (i) in the absence of these proteins, patch I is required to partially relieve the inhibitory effect of the N-terminal region, and (ii) at least in the presence of these proteins, patch I does not contribute or only marginally contributes to the correct positioning of ubiquitin for the final transfer to a target protein. Furthermore, it was previously reported that E6AP autoubiquitination occurs mainly via intermolecular transfer of ubiquitin (i.e., E6AP di- or oligomerizes for autoubiquitination) (47), whereas in the presence of HPV E6, E6AP ubiquitinates itself preferentially in an intramolecular manner (48). Hence, all of the available evidence strongly supports the conclusion that binding of HPV E6 induces a conformational change in E6AP bringing it into a fully active state [note that it was recently reported that E6 induces E6AP oligomerization (49); however, we did not obtain any evidence that this is the case under the conditions used].
Thus far, studies concerning the E6–E6AP interaction have focused on the ability of E6 to increase the substrate spectrum of E6AP (i.e., identification of proteins that in the absence of E6 are not targeted by E6AP). Although several proteins have been reported to represent substrates of E6AP in the absence of E6 (summarized in ref. 7), the physiological relevance of many of these interactions remains unclear, in particular with respect to their role in the development of Angelman syndrome. The data presented here strongly indicate that E6 has a dramatic effect on the ability of E6AP to ubiquitinate its (i.e., E6AP’s) regular substrate proteins. Thus, detailed analysis of the effect of E6 on E6AP will not only contribute to further our understanding of the role of the E6–E6AP complex in cervical carcinogenesis but should also provide valuable insights into the physiological relevance of reported and yet to be identified substrate proteins of E6AP.
Materials and Methods
For plasmids, antibodies, and bacterial protein expression, see SI Materials and Methods.
Generation of Isopeptide-Linked E2–Ubiquitin Conjugates.
Synthesis of different E2–ubiquitin conjugates was carried out as described previously (28). Briefly, UbcH5b–C85K, UbcH7–C86K, or UbcH1–C88K (each 200 µM) were incubated with UBA1 (1.5 µM) and either ubiquitin, Ub_hpI, or Ub_hpII (each 200 µM) at 37 °C for 20–22 h in a buffer containing 50 mM Tris⋅HCl pH 10.0, 150 mM NaCl, 5 mM MgCl2, 3 mM ATP, and 0.8 mM TCEP. Subsequently, respective E2–Ub conjugates were purified by size exclusion chromatography. Fractions containing E2–Ub conjugates were pooled, dialyzed against 25 mM Tris⋅HCl, 50 mM NaCl, 1 mM DTT, pH 7.5, concentrated by ultrafiltration, and stored at 4 °C.
Interaction Studies by Size Exclusion Chromatography.
To study complex formation of UbcH5b, UbcH7, and the different ubiquitin conjugates of UbcH5b and UbcH7 (UbcH5b–Ub, UbcH5b–Ub_hpI, UbcH5b–Ub_hpII, and UbcH7–Ub) with the HECT domain of E6AP, 20 µM of the respective E2 or E2–ubiquitin conjugate were mixed with 125 µM HECT E6AP. After a 5-min incubation at room temperature, mixtures were subjected to size exclusion chromatography. As control, 20 µM of UbcH5b, UbcH7, and the various E2–ubiquitin conjugates were subjected to size exclusion chromatography in the absence of the HECT domain. Fractions were analyzed by SDS/PAGE followed by Coomassie staining and the intensities of the bands in each fraction were quantified by densitometry. The ability of UbcH5b, UbcH7, and the various E2–ubiquitin conjugates to bind to the HECT domain of E6AP was indicated by changes in their retention times in presence and absence of the HECT domain. For each E2 variant, size exclusion experiments were done in triplicate.
In Vitro Ubiquitination and Thioester Assays.
For in vitro ubiquitination, 1 µL of rabbit reticulocyte lysate-translated 35S-labeled substrate (E6AP, Ring1B-I53S, and HHR23A) was incubated with 50 ng of baculovirus-expressed E1, 50 ng of E2 enzyme (UbcH5b or UbcH7), 200 ng of baculovirus-expressed E6AP, 20 µg of ubiquitin or ubiquitin mutants (Ub_hpI and Ub_hpII) in the absence or presence of GST-16 E6 (200 ng) in 40-µL volumes. In addition, reactions contained 25 mM Tris⋅HCl pH 7.5, 50 mM NaCl, 1 mM DTT, 2 mM ATP, and 4 mM MgCl2. After incubation at 25 °C for 2 h, total reaction mixtures were electrophoresed in 8–15% (vol/vol) SDS-polyacrylamide gels, and 35S-labeled proteins were detected by fluorography.
For in vitro thioester assays, 50 ng of E1, 50 ng of UbcH7 or UbcH5b, and 250 ng of E6AP or the HECT domain of E6AP were incubated with 20 µg of ubiquitin or the ubiquitin mutants (Ub_hpI and Ub_hpII) for 1 min at 30 °C in 40-µL volumes. In addition, reactions contained 25 mM Tris⋅HCl pH 7.5, 50 mM NaCl, 0.1 mM DTT, 4 mM ATP, and 10 mM MgCl2. Reactions were terminated by incubating the mixtures for 15 min at 30 °C in 50 mM Tris⋅HCl pH 6.8, 2% (wt/vol) SDS, 4 M urea, 10% (vol/vol) glycerol (to preserve ubiquitin thioester complexes) or by boiling the mixtures in the same buffer containing 100 mM DTT instead of urea (37). Whole reaction mixtures were separated on 8–15% (vol/vol) SDS-polyacrylamide gels and subjected to Western blot analysis using anti-E6AP or anti-His antibodies.
Degradation Assay in Cells.
H1299–shE6AP cells (stable knockdown of E6AP expression) (50) were grown in DMEM supplemented with 10% (vol/vol) FBS. For degradation assays, one 6-cm plate of cells was transfected with expression constructs encoding HA-tagged E6AP or the catalytically inactive mutant E6AP–C820A (2.5 µg), HA-tagged HPV16 E6 (1.5 µg), and DHFR–HA–ubiquitin–HA–Ring1B-I53S (1 µg) as indicated (Fig. 4C). Twenty-four hours after transfection, cells were lysed and levels of E6AP, DHFR–HA–ubiquitin, HA–Ring1B-I53S, and HA–E6 were determined by SDS/PAGE followed by Western blot analysis using an anti-HA antibody. Quantification of the intensity of the signals was performed with the Aida 4.08 software package (Raytest).
SI Materials and Methods
Plasmids and Antibodies.
Bacterial expression constructs for the ubiquitin-activating enzyme E1, UbcH1, ubiquitin, the ubiquitin mutant UbLIA, and a GST fusion protein of HPV16 E6 were described previously (9, 19, 35, 51). Codon-optimized cDNAs (GeneArt) for UbcH5b, UbcH7, and the HECT domain of E6AP (amino acids 500–852; numbering according to E6AP isoform 1, with nucleotide 1 referring to A of the start codon) (52) were cloned into pET21a and pET15b, respectively. The UbcH5b mutants C85A, C85K, and N77A, the UbcH7 mutant C86K, the UbcH1 mutant C88K, the HECT domain mutants C820A and F727A, and the ubiquitin mutant ILA (substitution of Ile-36, Leu-71, and Leu-73 by Ala) were generated by PCR-based approaches (further details will be provided upon request).
Expression constructs (in vitro translation, transient transfection experiments) encoding HA-tagged wild-type E6AP (isoform 1), the HA-tagged catalytically inactive mutant E6AP–C820A (substitution of Cys-820 by Ala), the catalytically inactive mutant Ring1b-I53S (substitution of Ile-53 by Ser), a DHFR–HA–ubiquitin fusion protein of HA-tagged Ring1b-I53S, and HHR23A were described previously (34, 45, 50, 53).
Where indicated, HA-tagged proteins were detected by the mouse monoclonal HA.11 (Hiss Diagnostics), His-tagged proteins were detected by the mouse monoclonal anti-6His-POX (Sigma), and full-length E6AP or the HECT domain of E6AP were detected by a mouse monoclonal antibody against E6AP (54).
Bacterial Protein Expression.
His-tagged human UBA1 was expressed in Escherichia coli BL21 and purified as described (51). His-tagged versions of the various E2 enzymes (UbcH1, UbcH5b and mutants, and UbcH7 and mutants) and of the HECT domain of E6AP were expressed in E. coli BL21. Upon lysis, extracts were loaded onto HisTrapFF columns (GE Healthcare) and the respective proteins were eluted by using a linear gradient of imidazole from 5 to 500 mM in 25 mM Tris⋅HCl pH 7.5, 300 mM NaCl. Eluate fractions were analyzed by SDS/PAGE followed by Coomassie staining; fractions containing the respective protein were pooled and dialyzed against 25 mM Tris⋅HCl pH 7.5, 50 mM NaCl, 1 mM DTT. Proteins were stored at 4 °C.
Isothermal Titration Calorimetry.
To determine the binding affinity of ubiquitin, UbcH7, and the UbcH7–ubiquitin conjugate for the HECT domain of E6AP by isothermal titration calorimetry (ITC), an iTC200 apparatus (MicroCal) was used. Experiments were performed at 25 °C in 25 mM Tris⋅HCl pH 7.5, 50 mM NaCl, 1 mM DTT using 1.5 mM to 200 μM of UbcH7 or the respective ubiquitin conjugate in the sample syringe and 140–60 µM of the HECT domain in the sample cell. For ubiquitin, an 800 µM solution was used in the sample syringe with 100 µM HECT domain in the sample cell. A total of 15× or 20× 2-µL injections were performed at 5-min intervals. Origin software (OriginLab) was used to fit the data to a single-site binding model and determine the stoichiometry (N), ΔH, ΔS, and the association constant K.
Mass Spectrometric Analysis of Isopeptide-Linked E2–Ubiquitin Conjugates.
Stable conjugates between ubiquitin and UbcH5b or UbcH7 were subjected to SDS/PAGE and visualized by Coomassie staining. Respective bands were cut out, digested by trypsin (UbcH5b–ubiquitin) or a trypsin/Glu-C mixture (UbcH7–ubiquitin), and desalted. Digested samples were analyzed by reversed phase liquid chromatography-nanospray tandem mass spectrometry (LC-MS/MS) using an LTQ-Orbitrap mass spectrometer (Thermo Fisher) and an Eksigent nano-HPLC. After sample injection, the column was washed for 5 min with 95% mobile phase A (0.1% formic acid) and 5% mobile phase B (0.1% formic acid in acetonitrile), and peptides were eluted using a linear gradient of 5% mobile phase B to 40% mobile phase B in 65 min, then to 80% B in an additional 5 min, at 250 nL/min. The LTQ-Orbitrap mass spectrometer was operated in a data-dependent mode in which each full MS scan (30,000 resolving power) was followed by five MS/MS scans, where the five most abundant molecular ions were dynamically selected and fragmented by collision-induced dissociation (CID) using a normalized collision energy of 35% in the LTQ ion trap. Dynamic exclusion was allowed. Tandem mass spectra were searched against a hand-made database implemented into Mascot Server software (Matrix Science) with Proteome Discoverer 1.3 (Thermo Scientific). “Trypsin/P” or “Trypsin/Glu-C” was selected as enzyme setting. Carbamidomethylation of Cys was used as fixed modification and Met oxidation and Lys-ε-GlyGly were set as variable modifications.
Acknowledgments
We are grateful to Thomas Kapitza, Nicole Richter-Müller, and Ulf Gündisch for excellent technical assistance, and the Proteomics Facility at the University of Konstanz for mass spectrometric validation of ubiquitin conjugates. This work was supported by the Deutsche Forschungsgemeinschaft (SFB 969 B2, B3).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1505923112/-/DCSupplemental.
References
- 1.Kerscher O, Felberbaum R, Hochstrasser M. Modification of proteins by ubiquitin and ubiquitin-like proteins. Annu Rev Cell Dev Biol. 2006;22:159–180. doi: 10.1146/annurev.cellbio.22.010605.093503. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz AL, Ciechanover A. Targeting proteins for destruction by the ubiquitin system: Implications for human pathobiology. Annu Rev Pharmacol Toxicol. 2009;49:73–96. doi: 10.1146/annurev.pharmtox.051208.165340. [DOI] [PubMed] [Google Scholar]
- 3.Varshavsky A. The ubiquitin system, an immense realm. Annu Rev Biochem. 2012;81:167–176. doi: 10.1146/annurev-biochem-051910-094049. [DOI] [PubMed] [Google Scholar]
- 4.Popovic D, Vucic D, Dikic I. Ubiquitination in disease pathogenesis and treatment. Nat Med. 2014;20(11):1242–1253. doi: 10.1038/nm.3739. [DOI] [PubMed] [Google Scholar]
- 5.Metzger MB, Hristova VA, Weissman AM. HECT and RING finger families of E3 ubiquitin ligases at a glance. J Cell Sci. 2012;125(Pt 3):531–537. doi: 10.1242/jcs.091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dove KK, Klevit RE. RING-between-RINGs--keeping the safety on loaded guns. EMBO J. 2012;31(19):3792–3794. doi: 10.1038/emboj.2012.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scheffner M, Kumar S. Mammalian HECT ubiquitin-protein ligases: Biological and pathophysiological aspects. Biochim Biophys Acta. 2014;1843(1):61–74. doi: 10.1016/j.bbamcr.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 8.Huibregtse JM, Scheffner M, Howley PM. A cellular protein mediates association of p53 with the E6 oncoprotein of human papillomavirus types 16 or 18. EMBO J. 1991;10(13):4129–4135. doi: 10.1002/j.1460-2075.1991.tb04990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huibregtse JM, Scheffner M, Howley PM. Cloning and expression of the cDNA for E6-AP, a protein that mediates the interaction of the human papillomavirus E6 oncoprotein with p53. Mol Cell Biol. 1993;13(2):775–784. doi: 10.1128/mcb.13.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scheffner M, Huibregtse JM, Vierstra RD, Howley PM. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell. 1993;75(3):495–505. doi: 10.1016/0092-8674(93)90384-3. [DOI] [PubMed] [Google Scholar]
- 11.Beaudenon S, Huibregtse JM. HPV E6, E6AP and cervical cancer. BMC Biochem. 2008;9(Suppl 1):S4. doi: 10.1186/1471-2091-9-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kishino T, Lalande M, Wagstaff J. UBE3A/E6-AP mutations cause Angelman syndrome. Nat Genet. 1997;15(1):70–73. doi: 10.1038/ng0197-70. [DOI] [PubMed] [Google Scholar]
- 13.Matsuura T, et al. De novo truncating mutations in E6-AP ubiquitin-protein ligase gene (UBE3A) in Angelman syndrome. Nat Genet. 1997;15(1):74–77. doi: 10.1038/ng0197-74. [DOI] [PubMed] [Google Scholar]
- 14.Dagli A, Buiting K, Williams CA. Molecular and clinical aspects of Angelman syndrome. Mol Syndromol. 2012;2(3-5):100–112. doi: 10.1159/000328837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glessner JT, et al. Autism genome-wide copy number variation reveals ubiquitin and neuronal genes. Nature. 2009;459(7246):569–573. doi: 10.1038/nature07953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hogart A, Wu D, LaSalle JM, Schanen NC. The comorbidity of autism with the genomic disorders of chromosome 15q11.2-q13. Neurobiol Dis. 2010;38(2):181–191. doi: 10.1016/j.nbd.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith SE, et al. Increased gene dosage of Ube3a results in autism traits and decreased glutamate synaptic transmission in mice. Sci Transl Med. 2011;3(103):103ra97. doi: 10.1126/scitranslmed.3002627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meng L, et al. Towards a therapy for Angelman syndrome by targeting a long non-coding RNA. Nature. 2015;518(7539):409–412. doi: 10.1038/nature13975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kühnle S, et al. Physical and functional interaction of the HECT ubiquitin-protein ligases E6AP and HERC2. J Biol Chem. 2011;286(22):19410–19416. doi: 10.1074/jbc.M110.205211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harlalka GV, et al. Mutation of HERC2 causes developmental delay with Angelman-like features. J Med Genet. 2013;50(2):65–73. doi: 10.1136/jmedgenet-2012-101367. [DOI] [PubMed] [Google Scholar]
- 21.Sloper-Mould KE, Jemc JC, Pickart CM, Hicke L. Distinct functional surface regions on ubiquitin. J Biol Chem. 2001;276(32):30483–30489. doi: 10.1074/jbc.M103248200. [DOI] [PubMed] [Google Scholar]
- 22.Kamadurai HB, et al. Insights into ubiquitin transfer cascades from a structure of a UbcH5B approximately ubiquitin-HECT(NEDD4L) complex. Mol Cell. 2009;36(6):1095–1102. doi: 10.1016/j.molcel.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim HC, Steffen AM, Oldham ML, Chen J, Huibregtse JM. Structure and function of a HECT domain ubiquitin-binding site. EMBO Rep. 2011;12(4):334–341. doi: 10.1038/embor.2011.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maspero E, et al. Structure of the HECT:ubiquitin complex and its role in ubiquitin chain elongation. EMBO Rep. 2011;12(4):342–349. doi: 10.1038/embor.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saha A, Lewis S, Kleiger G, Kuhlman B, Deshaies RJ. Essential role for ubiquitin-ubiquitin-conjugating enzyme interaction in ubiquitin discharge from Cdc34 to substrate. Mol Cell. 2011;42(1):75–83. doi: 10.1016/j.molcel.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wickliffe KE, Lorenz S, Wemmer DE, Kuriyan J, Rape M. The mechanism of linkage-specific ubiquitin chain elongation by a single-subunit E2. Cell. 2011;144(5):769–781. doi: 10.1016/j.cell.2011.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dou H, Buetow L, Sibbet GJ, Cameron K, Huang DT. BIRC7-E2 ubiquitin conjugate structure reveals the mechanism of ubiquitin transfer by a RING dimer. Nat Struct Mol Biol. 2012;19(9):876–883. doi: 10.1038/nsmb.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Plechanovová A, Jaffray EG, Tatham MH, Naismith JH, Hay RT. Structure of a RING E3 ligase and ubiquitin-loaded E2 primed for catalysis. Nature. 2012;489(7414):115–120. doi: 10.1038/nature11376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pruneda JN, et al. Structure of an E3:E2∼Ub complex reveals an allosteric mechanism shared among RING/U-box ligases. Mol Cell. 2012;47(6):933–942. doi: 10.1016/j.molcel.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kamadurai HB, et al. Mechanism of ubiquitin ligation and lysine prioritization by a HECT E3. eLife. 2013;2:e00828. doi: 10.7554/eLife.00828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maspero E, et al. Structure of a ubiquitin-loaded HECT ligase reveals the molecular basis for catalytic priming. Nat Struct Mol Biol. 2013;20(6):696–701. doi: 10.1038/nsmb.2566. [DOI] [PubMed] [Google Scholar]
- 32.French ME, Kretzmann BR, Hicke L. Regulation of the RSP5 ubiquitin ligase by an intrinsic ubiquitin-binding site. J Biol Chem. 2009;284(18):12071–12079. doi: 10.1074/jbc.M901106200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ogunjimi AA, et al. The ubiquitin binding region of the Smurf HECT domain facilitates polyubiquitylation and binding of ubiquitylated substrates. J Biol Chem. 2010;285(9):6308–6315. doi: 10.1074/jbc.M109.044537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zaaroor-Regev D, et al. Regulation of the polycomb protein Ring1B by self-ubiquitination or by E6-AP may have implications to the pathogenesis of Angelman syndrome. Proc Natl Acad Sci USA. 2010;107(15):6788–6793. doi: 10.1073/pnas.1003108107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nuber U, Scheffner M. Identification of determinants in E2 ubiquitin-conjugating enzymes required for hect E3 ubiquitin-protein ligase interaction. J Biol Chem. 1999;274(11):7576–7582. doi: 10.1074/jbc.274.11.7576. [DOI] [PubMed] [Google Scholar]
- 36.Purbeck C, Eletr ZM, Kuhlman B. Kinetics of the transfer of ubiquitin from UbcH7 to E6AP. Biochemistry. 2010;49(7):1361–1363. doi: 10.1021/bi9014693. [DOI] [PubMed] [Google Scholar]
- 37.Scheffner M, Nuber U, Huibregtse JM. Protein ubiquitination involving an E1-E2-E3 enzyme ubiquitin thioester cascade. Nature. 1995;373(6509):81–83. doi: 10.1038/373081a0. [DOI] [PubMed] [Google Scholar]
- 38.Wenzel DM, Lissounov A, Brzovic PS, Klevit RE. UBCH7 reactivity profile reveals parkin and HHARI to be RING/HECT hybrids. Nature. 2011;474(7349):105–108. doi: 10.1038/nature09966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu PY, et al. A conserved catalytic residue in the ubiquitin-conjugating enzyme family. EMBO J. 2003;22(19):5241–5250. doi: 10.1093/emboj/cdg501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huibregtse JM, Scheffner M, Howley PM. Localization of the E6-AP regions that direct human papillomavirus E6 binding, association with p53, and ubiquitination of associated proteins. Mol Cell Biol. 1993;13(8):4918–4927. doi: 10.1128/mcb.13.8.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tomaić V, Pim D, Banks L. The stability of the human papillomavirus E6 oncoprotein is E6AP dependent. Virology. 2009;393(1):7–10. doi: 10.1016/j.virol.2009.07.029. [DOI] [PubMed] [Google Scholar]
- 42.Kumar S, Talis AL, Howley PM. Identification of HHR23A as a substrate for E6-associated protein-mediated ubiquitination. J Biol Chem. 1999;274(26):18785–18792. doi: 10.1074/jbc.274.26.18785. [DOI] [PubMed] [Google Scholar]
- 43.Bachmair A, Finley D, Varshavsky A. In vivo half-life of a protein is a function of its amino-terminal residue. Science. 1986;234(4773):179–186. doi: 10.1126/science.3018930. [DOI] [PubMed] [Google Scholar]
- 44.Varshavsky A. Ubiquitin fusion technique and related methods. Methods Enzymol. 2005;399:777–799. doi: 10.1016/S0076-6879(05)99051-4. [DOI] [PubMed] [Google Scholar]
- 45.Kühnle S, Mothes B, Matentzoglu K, Scheffner M. Role of the ubiquitin ligase E6AP/UBE3A in controlling levels of the synaptic protein Arc. Proc Natl Acad Sci USA. 2013;110(22):8888–8893. doi: 10.1073/pnas.1302792110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang M, Pickart CM. Different HECT domain ubiquitin ligases employ distinct mechanisms of polyubiquitin chain synthesis. EMBO J. 2005;24(24):4324–4333. doi: 10.1038/sj.emboj.7600895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nuber U, Schwarz SE, Scheffner M. The ubiquitin-protein ligase E6-associated protein (E6-AP) serves as its own substrate. Eur J Biochem. 1998;254(3):643–649. doi: 10.1046/j.1432-1327.1998.2540643.x. [DOI] [PubMed] [Google Scholar]
- 48.Kao WH, Beaudenon SL, Talis AL, Huibregtse JM, Howley PM. Human papillomavirus type 16 E6 induces self-ubiquitination of the E6AP ubiquitin-protein ligase. J Virol. 2000;74(14):6408–6417. doi: 10.1128/jvi.74.14.6408-6417.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ronchi VP, Klein JM, Edwards DJ, Haas AL. The active form of E6-associated protein (E6AP)/UBE3A ubiquitin ligase is an oligomer. J Biol Chem. 2014;289(2):1033–1048. doi: 10.1074/jbc.M113.517805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuballa P, Matentzoglu K, Scheffner M. The role of the ubiquitin ligase E6-AP in human papillomavirus E6-mediated degradation of PDZ domain-containing proteins. J Biol Chem. 2007;282(1):65–71. doi: 10.1074/jbc.M605117200. [DOI] [PubMed] [Google Scholar]
- 51.Berndsen CE, Wolberger C. A spectrophotometric assay for conjugation of ubiquitin and ubiquitin-like proteins. Anal Biochem. 2011;418(1):102–110. doi: 10.1016/j.ab.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamamoto Y, Huibregtse JM, Howley PM. The human E6-AP gene (UBE3A) encodes three potential protein isoforms generated by differential splicing. Genomics. 1997;41(2):263–266. doi: 10.1006/geno.1997.4617. [DOI] [PubMed] [Google Scholar]
- 53.Glockzin S, Ogi FX, Hengstermann A, Scheffner M, Blattner C. Involvement of the DNA repair protein hHR23 in p53 degradation. Mol Cell Biol. 2003;23(24):8960–8969. doi: 10.1128/MCB.23.24.8960-8969.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hengstermann A, et al. Growth suppression induced by downregulation of E6-AP expression in human papillomavirus-positive cancer cell lines depends on p53. J Virol. 2005;79(14):9296–9300. doi: 10.1128/JVI.79.14.9296-9300.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang L, et al. Structure of an E6AP-UbcH7 complex: Insights into ubiquitination by the E2-E3 enzyme cascade. Science. 1999;286(5443):1321–1326. doi: 10.1126/science.286.5443.1321. [DOI] [PubMed] [Google Scholar]