Significance
Channelrhodopsin-2 is a dimeric membrane protein functioning as a light-gated ion channel, which has triggered numerous optogenetic applications. We present the first NMR study, to our knowledge, by which structural details of the retinal cofactor could be resolved. This study was only possible by enhancing the detection sensitivity 60-fold through dynamic nuclear polarization (DNP), a highly promising hybrid method linking EPR with solid-state NMR spectroscopy. Our data show that ground-state channelrhodopsin-2 contains the retinal cofactor in its all-trans configuration with a slightly perturbed polyene chain. Three different photointermediates could be trapped and analyzed. Our study shows that DNP-enhanced solid-state NMR is a key method for bridging the gap between X-ray–based structure analysis and functional studies toward a highly resolved molecular picture.
Keywords: channelrhodopsin, retinal, solid-state NMR, DNP, freeze trapping
Abstract
Channelrhodopsin-2 from Chlamydomonas reinhardtii is a light-gated ion channel. Over recent years, this ion channel has attracted considerable interest because of its unparalleled role in optogenetic applications. However, despite considerable efforts, an understanding of how molecular events during the photocycle, including the retinal trans-cis isomerization and the deprotonation/reprotonation of the Schiff base, are coupled to the channel-opening mechanism remains elusive. To elucidate this question, changes of conformation and configuration of several photocycle and conducting/nonconducting states need to be determined at atomic resolution. Here, we show that such data can be obtained by solid-state NMR enhanced by dynamic nuclear polarization applied to 15N-labeled channelrhodopsin-2 carrying 14,15-13C2 retinal reconstituted into lipid bilayers. In its dark state, a pure all-trans retinal conformation with a stretched C14-C15 bond and a significant out-of-plane twist of the H-C14-C15-H dihedral angle could be observed. Using a combination of illumination, freezing, and thermal relaxation procedures, a number of intermediate states was generated and analyzed by DNP-enhanced solid-state NMR. Three distinct intermediates could be analyzed with high structural resolution: the early K-like state, the slowly decaying late intermediate , and a third intermediate populated only under continuous illumination conditions. Our data provide novel insight into the photoactive site of channelrhodopsin-2 during the photocycle. They further show that DNP-enhanced solid-state NMR fills the gap for challenging membrane proteins between functional studies and X-ray–based structure analysis, which is required for resolving molecular mechanisms.
Since their discovery (1), channelrhodopsins (ChRs) have generated enormous interest because of the rapid development of their applications in optogenetics (2–7). Commonly, ChR2 from Chlamydomonas reinhardtii (8) and its variants are used thanks to their favorable expression levels. They are the only proteins known today functioning as light-gated ion channels (Fig. 1A). Like other microbial retinal proteins, they undergo a periodic photocycle. In ChRs, this photocycle is coupled to channel opening and closing as revealed in electrophysiological recordings (8). A chimera of ChR1 and ChR2 has been crystallized to yield a structure at 2.3-Å resolution (9). However, little is known on how this coupling functions on a molecular level, and a large number of studies based on visible (10–13), IR (11, 14–19), resonance Raman spectroscopy (20, 21), and EPR spectroscopy (22, 23) has been performed to address this question.
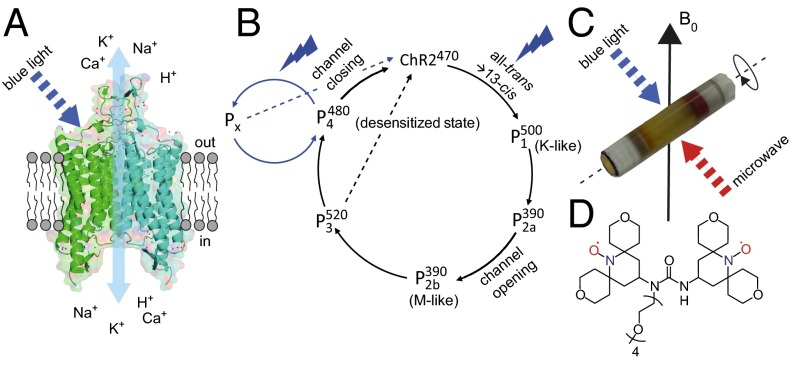
Fig. 1.
(A) Visualization of dimeric ChR2 reconstituted into the lipid bilayer as used in this study [cartoon based on the crystal structure of the ChR1/2 chimera (data from ref. 9)]. Blue light illumination activates ChR2. (B) Single turnover (black arrows) and continuous illumination photocycle (blue arrows) (14, 40). (C) Schematic view of the experimental setup for generating and measuring different photointermediates. (D) The DNP enhancement is generated by magnetization transfer from the biradical AMUPOL to ChR2.
The photocycles of microbial rhodopsins are usually compared with bacteriorhodopsin, the first discovered and most studied light-driven proton pump (24). Without any illumination, microbial retinal proteins thermally equilibrate into a dark state (25). In the case of bacteriorhodopsin, for example, this state contains a mixture of two species termed bacteriorhodopsin568 (all-trans,15-anti retinal Schiff base) and bacteriorhodopsin548 (13-cis,15-syn conformation) (26, 27). On illumination, light adaption occurs from the dark state to the ground state, which contains only the all-trans,15-anti conformer as the photocycle starting point (28). A similar light–dark adaption has been found in halorhodopsin from Halobacterium salinarium (29). However, such a light/dark adaption in conjunction with a conformer mixture does not seem to be a general property of microbial membrane proteins. Other systems have been described where the ground state contains only an all-trans,15-anti retinal Schiff base chromophore [e.g., green proteorhodopsin (30), Anabaena sensory rhodopsin (31), Oxyrrhis marina proteorhodopsin (32), sensory rhodopsin I from H. salinarum (33) and Salinibacter ruber (34), and sensory rhodopsin II from Natronobacterium pharaonis (35, 36) and H. salinarum (37)].
In ChR2, the retinal is covalently bound to the lysine residue 257 conserved in all retinal proteins through a Schiff base linkage (38). The X-ray structure of the ChR chimera shows the retinal in an all-trans configuration (9), although other conformations cannot be excluded at the obtained resolution. Results of retinal extraction in conjunction with resonance Raman studies were interpreted as an isomer mixture containing 30% of a 13-cis retinal in dark- and light-adapted ChR2 (20). In addition, nanosecond IR spectroscopy on the E123T mutant of ChR2 indicated the presence of some 13-cis retinal in the dark state using a similar spectroscopic assignment as in the resonance Raman study (39). In contrast to bacteriorhodopsin, no light adaption was observed using resonance Raman techniques (20) or visual spectroscopy (12). The occurrence of a conformer mixture in the ground state without light adaption would make ChR2 unique among the microbial retinal proteins, but additional data are needed to confirm these observations more directly at improved atomic resolution.
The current model of the ChR2 photocycle is shown in Fig. 1B (14, 40). According to this model, blue light excitation leads to a retinal all-trans to 13-cis isomerization, resulting in a red-shifted first intermediate (12) resembling a K-like state, which most likely contains a 13-cis,15-anti retinal Schiff base chromophore similar to Bacteriorhodopsin (27). To our knowledge, such red-shifted K-like intermediates occur in all microbial retinal proteins (38). Schiff base deprotonation leads to the M-like state (10, 11). This state is followed by the red-shifted intermediate , which has previously been correlated with the open state (10). However, later data confirmed that channel opening occurs before formation and might happen during a spectroscopically silent transition between and states (41). The last photocycle intermediate is the long-lived intermediate state (τ = 24 s), which is referred to as the desensitized state with a spectral characteristic similar to the ground state (11, 42). In addition, time-resolved FTIR spectroscopy indicated that could partially convert directly to the ground state (14).
The situation becomes more complicated under continuous light illumination (40, 43). Under these conditions, a high transient current is observed first that is quickly reduced to a much lower steady-state current. After turning off the irradiation, the steady-state current decays biexponentially. This observation can only be explained by a branching of the photocycle. Two open states and two closed states are required to quantitatively describe the observed behavior under continuous light conditions. The two closed states are most likely the ground state and the desensitized state that accumulates under continuous illumination and is identical to the same intermediate from a single turnover (18). One of the open states is probably the open state observed in single-turnover experiments. However, little is known about the identity of the second open state, which only occurs under continuous light conditions. It might be an M-like state, another state, or another unknown state. Light excitation of probably creates this additional state. This state or group of states here is referred to as Px containing at least one open state (Fig. 1B). It is also likely that the open states and Px to some extent can convert directly to the ground state, which is indicated by dashed lines in Fig. 1B.
All of the above-described states were detected by visible and FTIR spectroscopy, and assignments of spectroscopic signatures to conformational and configurational states of the retinal were based on analogous data previously studied. However, detailed information on bond lengths or torsion angles that would also link to quantum chemical calculation is still missing. To fill this gap between static crystallographic data on the one hand and kinetic and functional data based on optical spectroscopy and electrophysiology on the other hand, we applied solid-state magic angle spinning NMR on isotope-labeled ChR2 and retinal to obtain site-resolved structural data directly in a membrane environment under various experimental conditions. In this way, fine details of the chromophore conformation during the photocycle could be resolved, which will be important to understand the link between channel and photocycle activity in ChR2. A limitation using proteoliposomes is the amount of sample that can be studied, because the protein-to-lipid ratio cannot be increased too much without compromising protein integrity. In addition, trapping photointermediates works best using samples with low optical density, which reduces further the usable amount of protein, resulting in a poor NMR signal-to-noise ratio. Therefore, cross-effect dynamic nuclear polarization (DNP) enhanced magic angle spinning (MAS) NMR [review in the work by Maly et al. (44)] was indispensable in overcoming these sensitivity problems (Fig. 1C). This technique requires temperatures around 100 K that are also compatible with trapping of photointermediates as outlined below. DNP-enhanced MAS NMR is not yet a routine method but is applied increasingly to complex, mechanistic studies on retinal proteins (45–48) and other membrane proteins (49–51).
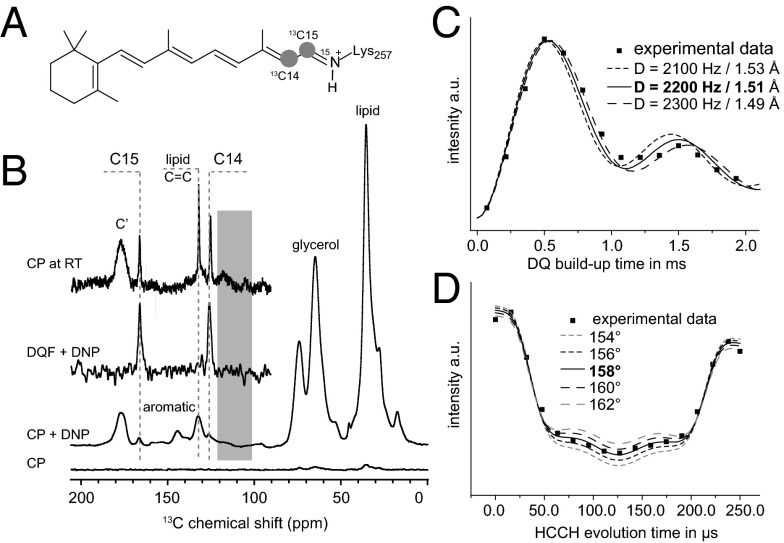
Here, DNP-enhanced solid-state NMR spectroscopy has been applied to 15N-labeled ChR2 carrying 14,15-13C2 retinal reconstituted into lipid bilayers and incubated with the DNP polarizing agent AMUPOL (52) in a glycerol–water mixture. The labeling scheme adopted here is shown in Fig. 2A. The 13C14 chemical shift is sensitive to the configuration of the C13-C14 bond. Together with the neighboring 13C15 atom, the two 13C-labeled spins can be used for double quantum filtering of this spin pair against the natural abundance background and at the same time, offer the possibility to study the length and the dihedral angle of the C14-C15 bond. Furthermore, the chemical shift of the Schiff base nitrogen is also sensitive to the chromophore conformation, reports on the protonation state of the Schiff base, and reflects counterion interactions. Using this approach, we were able to provide a first analysis, to our knowledge, of the retinal–Schiff base chromophore in ChR2 in its ground state as well as three different photointermediate states at atomic resolution.
Fig. 2.
(A) DNP-enhanced MAS NMR has been applied to U-15N-ChR2 containing 14,15-13C all-trans–retinal. (B) A 62-fold signal enhancement is achieved for 13C cross-polarization (CP; CP vs. CP + DNP). The 13C natural abundance background can be efficiently suppressed by a double quantum filter (DQF; DQF + DNP), resulting in a spectrum with only the resonances of C14 and C15. As a control, one additional CP spectrum was acquired at 850 MHz close to room temperature (CP at RT). The gray bar indicates where the 13C14 signal would be expected for a chromophore in the 13-cis,15-syn conformation. (C) 13C14-13C15 double-quantum (DQ) build-up curve. (D) HCCH dephasing curves for the C14-C15 spin system in ChR2 during two rotor periods reporting on the HCCH dihedral angle.
Results and Discussion
Dark State—Ground State.
Fig. 2B shows the 13C-DNP–enhanced MAS NMR spectrum of ChR2. Using AMUPOL as the polarizing agent, a 62-fold signal enhancement was achieved under our experimental conditions. The observed resonances mainly stem from the 13C natural abundance background of protein, glycerol, and lipid. To suppress these signals and identify the 13C-labeled retinal sites, a double quantum filter has been applied, revealing just two peaks from retinal carbons C14 and C15 at 126.3 and 166.5 ppm, respectively (Fig. 2B). The C14 chemical shift is very sensitive to the conformation of the chromophore (26), and the value of 126.3 ppm is, therefore, a very strong indicator for an all-trans,15-anti chromophore conformation as observed for bacteriorhodopsin and proteorhodopsin (SI Appendix, Table S1). By contrast, the C14 signal of the 13-cis,15-syn chromophore in dark-adapted bacteriorhodopsin appears at 111 ppm. A signal resonating at a similar chemical shift has been observed for a 13-cis,15-syn subpopulation in the A178R mutant of green proteorhodopsin (SI Appendix, Table S1). In ChR2, no signal at or near 111 ppm could be observed (Fig. 2B, gray area). We, therefore, conclude that the chromophore in ChR2 is present in a single all-trans,15-anti conformation. To exclude that this result is an artifact of the sample conditions required for DNP, a 13C-CP spectrum of ChR2 without the addition of radicals or cryoprotectants was recorded at ambient temperature using 832 times the number of scans compared with the DNP experiment (Fig. 2B, CP at RT vs. CP + DNP). In both cases, the retinal resonances compare well. Only the C14 signal is shifted slightly upfield, which can be attributed to temperature effects. The small additional intensities observable in the ambient temperature spectrum result from spinning side bands and can be moved by changing the MAS frequency (SI Appendix, Fig. S1). In addition, keeping the sample in the dark at 4 °C for 24 h did not change the appearance of the DNP NMR spectra (SI Appendix, Fig. S2).
To further study the conformation of the chromophore, the C14-C15 retinal distance has been determined using double-quantum build-up experiments (Fig. 2C). The obtained value of 1.51 ± 0.02 Å is significantly longer than the 1.42 Å observed for green proteorhodopsin (47). This increase in bond length corresponds well with a lower double-bond character of the bond as expected from the blue shift of the absorption maximum compared with green proteorhodopsin. Measurements of the H-C14-C15-H retinal torsional angle revealed a significant out-of-plane twist with an angle of 158° ± 2°. A similar out-of-plane twist has also been observed for bacteriorhodopsin (164°) (53) and green proteorhodopsin (161°) (47), indicating that this out-of-plane twist is a general property of microbial retinal rhodopsins and might help to provide a favorable orientation of the Schiff base during the subsequent photocycle steps.
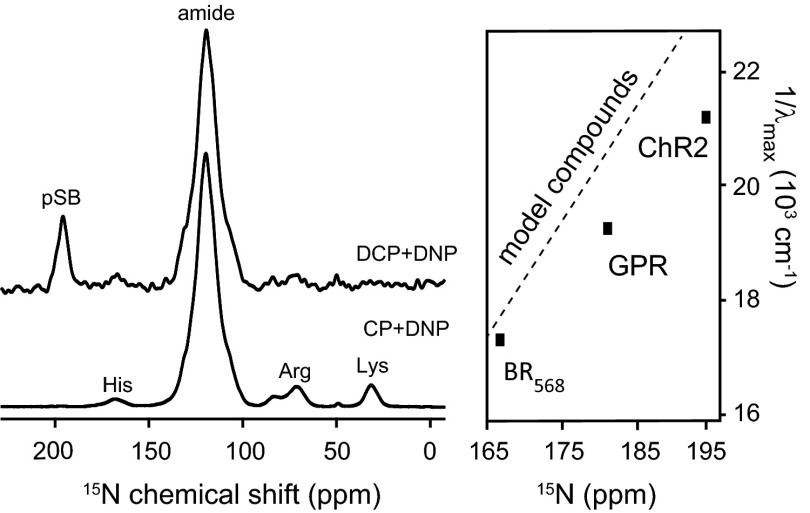
The 15N chemical shift of the protonated Schiff base (pSB) was detected as single resonance at 196.5 ppm using 1H-13C/13C-15N double cross-polarization magnetization transfer from the terminal retinal carbon (C15) to the directly bonded Schiff base nitrogen (Fig. 3). Hu et al. (54) established a relationship between the Schiff base chemical shift and the wavelength of the absorption maximum using Schiff base–counterion model complexes, which is shown in Fig. 3, Inset together with the values for bacteriorhodopsin, green proteorhodopsin, and ChR2. ChR2 (470 nm/196.5 ppm) agrees well with the predicted trend but deviates more strongly from model behavior than bacteriorhodopsin. Based on the model compound geometries, it can be concluded that the distance from the Schiff base to its counterion in ChR2 is shorter than in bacteriorhodopsin. As for C14- or C15-retinal atoms, no peak doubling or splitting is observed, confirming that retinal is present in only one conformation in the ChR2 ground state. The chemical shift difference of the all-trans,15-anti and the 13-cis,15-syn 15N signals observed in dark-adapted bacteriorhodopsin is 8.3 ppm (46), which would be well-resolved under the experimental conditions applied here.
Fig. 3.
DNP-enhanced 15N cross-polarization (CP) spectrum (CP + DNP) and double cross-polarization (DCP) –filtered 15N spectrum (DCP + DNP) of ChR2. (Inset) Comparison of the correlation of the absorption maximum with the 15N Schiff base chemical shift for ChR2, green proteorhodopsin (GPR), bacteriorhodopsin (BR), and model compounds. Modified from ref. 54.
The observation of a 100% all-trans,15-anti conformation of the retinal cofactor in ground-state ChR2 is in line with many other microbial rhodopsins but in contrast to previous reports on ChR2. Previously, a population of 30% 13-cis retinal based on retinal extraction with subsequent HPLC analysis and resonance Raman experiments has been reported (20).
The reason for this discrepancy is the invasive character of retinal extraction in the previously reported work (20). This process required breaking the Schiff base linker by subjecting the protein to EtOH followed by extraction of retinals into hexanes and HPLC purification (55). For bacteriorhodopsin, the results obtained in this way (56) agree well with data from other methods (26, 27). In other cases, however, this protein treatment led to less consistent results. For example, retinal extraction studies of green proteorhodopsin suggested values between 5% and 20% cis-retinal (57, 58). Later, resonance Raman (59) and solid-state MAS NMR (30) studies confirmed that the ground state contains very little (if any) cis-retinal. Similarly, for Anabaena sensory rhodopsin, retinal extraction experiments showed 24% of the 13-cis isomer in the dark-adapted state (60), whereas solid-state MAS NMR experiments showed a purely all-trans configuration (31). Resonance Raman experiments are noninvasive and should give reliable information on the retinal chromophore. However, assignment of the vibrational bands is very challenging and cannot easily be transferred between different systems. In the case of bacteriorhodopsin, assignment was based on differently isotope-labeled retinals (27). Such data are missing for ChR2, resulting in ambiguous interpretation of Raman data (20) (more detailed discussion is in SI Appendix).
Our data, therefore, show unambiguously that ground-state ChR2 contains an all-trans,15-anti chromophore, which also explains the previously reported monoexponential decay of the photoexcited state (12) and the absence of a light adaption step as observed in bacteriorhodopsin, because the amount of this conformer is already close to 100% in the dark.
Observing ChR2 Photointermediates by DNP-Enhanced MAS NMR.
For the analysis of photointermediates, sample illumination has been used in the past [e.g., combined with solution-state NMR to access, for example, the photokinetics of rhodopsin (61, 62) and solid-state NMR for thermal trapping of bacteriorhodopsin or rhodopsin intermediates (46)]. Special consideration has to be given to an efficient illumination setup, because light penetration of the sample significantly decreases with sample thickness (62). The amount of ChR2 proteoliposomes within the optically transparent sapphire rotor used for DNP NMR was, therefore, reduced to about 20% and evenly distributed across the inner rotor surface by short sample rotation at room temperature. In principle, sample illumination can be done in two ways. One possibility is to illuminate the sample outside of the NMR magnet and then, trap the generated state by quickly freezing it and inserting it in the cold probe. However, temperature control using this method can be difficult. Another option is to equip the DNP solid-state probe with a light guide and illuminate the sample directly while it is spinning in the stator. Such an approach has been shown by a number of laboratories using different designs with DNP (46) or standard MAS probes (62–64). The latter approach offers better temperature control, but quick freezing of the spinning samples is not possible. In this study, both methods have been used and yielded similar results as discussed below.
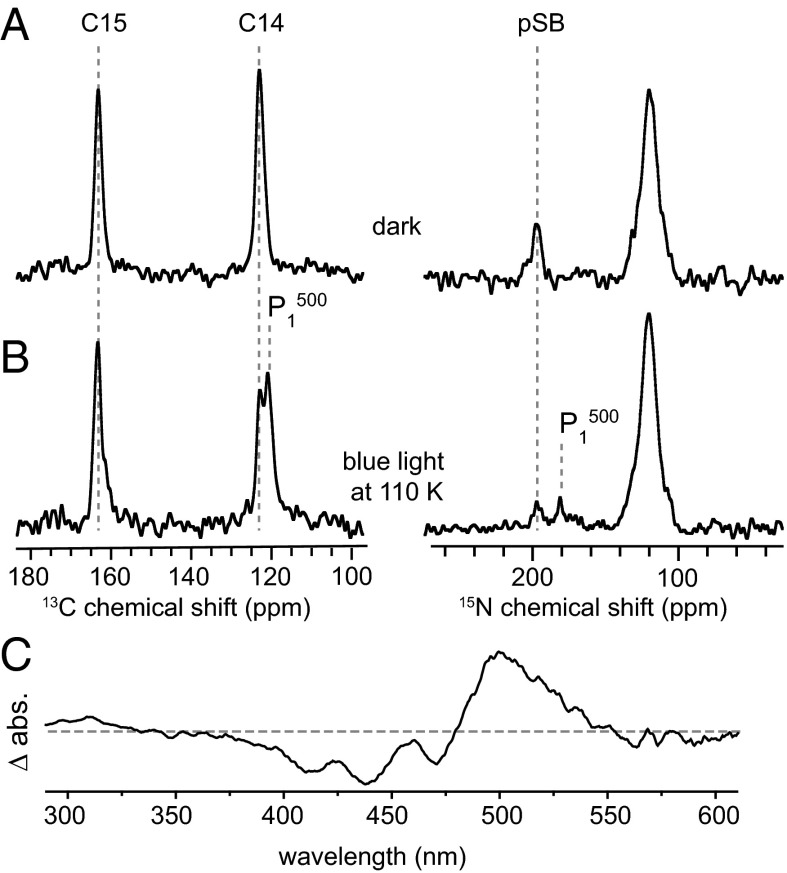
Significant spectral changes are observed on illumination with blue light at 110 K (Fig. 4). For the retinal 13C14 signal, one additional peak at 124.2 ppm is observed in addition to the ground-state signal, whereas the 13C15 resonance broadens slightly. Similarly, a new 15N signal upfield of the pSB ground-state resonance is detected at 181 ppm. It can be concluded that illumination at low temperatures leads to a mixture of two states, one of which corresponds to the ground state.
Fig. 4.
13C double quantum filter- and 15N double cross-polarization–filtered, DNP-enhanced spectra of ChR2 in a sapphire rotor (A) in the dark and (B) after illumination with blue light at 110 K. (C) Optical difference spectrum of ChR2 at 150 K of dark- and blue light-illuminated ChR2 (Δ abs refers to the absorption difference).
For assigning the second state to one of the photointermediates (Fig. 1B), optical spectra on thermally trapped samples prepared under conditions very close to those used for DNP have been recorded. The difference between spectra of a dark sample and those of an illuminated sample shows negative intensities below 480 nm (ground-state bleaching) and positive absorption with a maximum at 500 nm (Fig. 4C). These values are characteristic for the K-like intermediate , which strongly suggests that this state has been trapped and observed by DNP-enhanced MAS NMR in agreement with earlier UV-visible and FTIR spectroscopic studies (11).
The NMR data are also in good agreement with the thermally trapped K state of bacteriorhodopsin, which was accumulated by illumination at 532 nm at 90 K (45, 46). The authors in refs. 45 and 46 reported upfield shifts for both 13C14-retinal and 15N pSB signals with respect to the ground state by 4.9 and 8.7 ppm, respectively. The same trend is observed here, and the newly generated state is, therefore, assigned to the K-like intermediate containing a 13-cis,15-anti chromophore. Because is also photoactive and can be converted to the ground state by light, only a mixture of states can be obtained as reported for bacteriorhodospin (65).
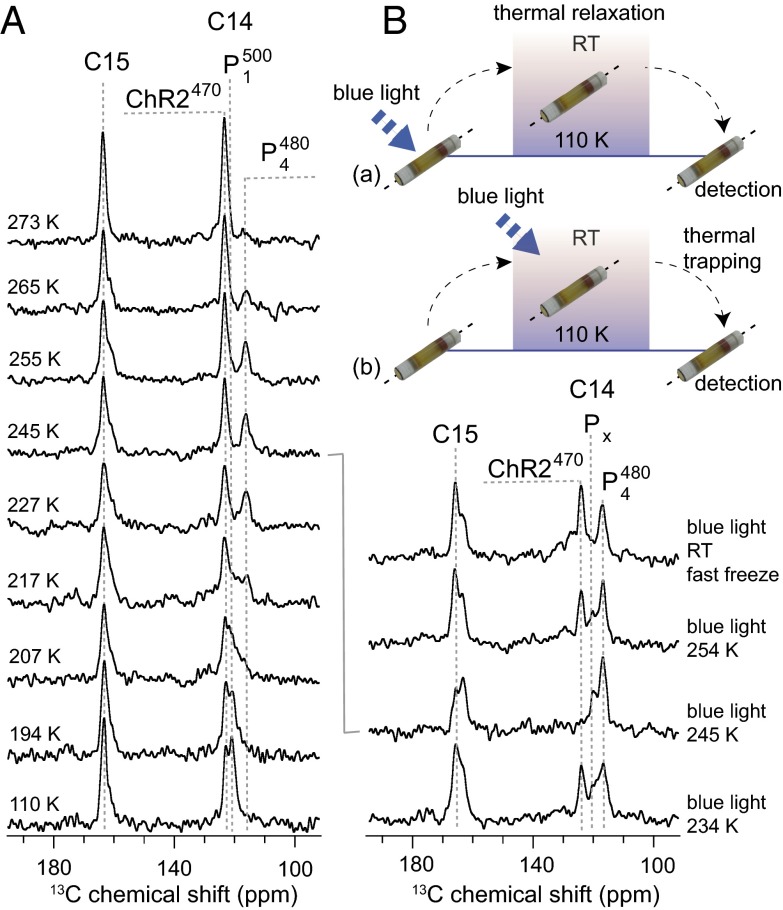
To reach other intermediates during the photocycle, ChR2 was now subjected to a thermal relaxation and a thermal trapping protocol (Fig. 5A). In the first case, the state was created as described above followed by thermal relaxation and equilibration at temperatures between 194 and 273 K, after which the sample was again cooled to 110 K for DNP detection (Fig. 5A). In this way, the thermal energy should allow ChR2 to overcome the energy barrier between its state and subsequent photocycle intermediates. The second protocol involved continuous illumination at temperatures between 234 and 254 K followed by freezing (Fig. 5B). In addition, a sample was illuminated at room temperature and then quickly frozen in liquid nitrogen. The second procedure should make especially those photointermediates accessible, which only occur under continuous illumination (Fig. 1B).
Fig. 5.
(A) 13C double quantum filter (DQF) DNP-enhanced spectra of ChR2 obtained by thermal relaxation. (B) 13C DQF DNP-enhanced spectra of ChR2 obtained by thermal trapping. Dashed lines indicate the observed peak position. RT, room temperature.
The thermal relaxation approach results in altered line shapes of the 13C14- and 13C15-retinal resonances (Fig. 5A). With increasing relaxation temperatures, the 13C14- signal broadens initially, converts into a new signal at 119.3 ppm between 217 and 255 K, and disappears above 265 K. In principle, the 13C15 resonance follows this trend, but changes are less pronounced, because only some line broadening can be detected. At higher temperatures, all additional signals vanish, and the sample returns into the ground-state ChR2470.
The thermal trapping protocol results in a different spectral characteristic of the chromophore as shown in Fig. 5B. Continuous illumination at room temperature is known to accumulate the long-lived intermediate (10), which can be further stabilized for DNP NMR detection by rapid freezing. Therefore, the two signals observed under these conditions for the 13C14 site can be assigned to the ground state (126.0 ppm) and (119.3 ppm). The latter is identical to the 13C14 chemical shift that was observed during the thermal relaxation experiment. We, therefore, conclude that this peak belongs to a population, which can also be generated when lowering the illumination temperature. The ground-state subpopulation is almost completely depopulated at 245 K, which proves that the whole sample can be illuminated sufficiently with the applied setup. At this temperature, an additional signal at 122.7 ppm can be detected, which is also seen in the 234- and 254-K spectra. Because this species is only generated under continuous light, it is tentatively assigned to one of the Px states (Fig. 1B).
The most pronounced photointermediates have been prepared at 245 K in both thermal relaxation as well as trapping approaches. We have, therefore, recorded 15N double cross-polarization–filtered spectra at this temperature using both protocols (SI Appendix, Fig. S3). Under thermal relaxation, only one signal for the pSB is observed at a chemical shift identical to the ground state but with slightly reduced intensity. Furthermore, no evidence for a deprotonated Schiff base species (i.e., an M-like state) has been found. A possible explanation could be that the Schiff base signal of this photo state is similar to the ground state or broadened beyond detection. The experiment was repeated under thermal trapping conditions. Here, the ground state is depleted at 245 K, which results in a loss of the pSB signal at 196.5 ppm and shows that the was not hidden underneath. A new signal occurs at 185 ppm, which is not visible under thermal relaxation conditions and therefore, is assigned to the Px state.
Thermal relaxation and the thermal trapping protocols at 245 K were also compared using optical spectroscopy under nearly the same experimental condition (SI Appendix, Fig. S4). These data confirm that the thermal relaxation and the thermal trapping protocols at 245 K result in a different population of photointermediates.
In summary, three photointermediates (, , and Px) could be generated using different illumination/relaxation protocols. This assignment is also in agreement with UV-visible spectra, which were obtained under cryogenic conditions similar to those used here for DNP on ChR2 samples and subjected to the same illumination and freeze-trapping protocols. Similar to the ground state, we have recorded double-quantum filter build-up and HCCH torsion angle data for all of them (SI Appendix, Fig. S5). The results are given in Table 1 together with the ground-state data. Our data show that the twisting and stretching of the C14-C15 bond observed in the ground state are conserved in all three trapped states.
Table 1.
Parameters obtained for the retinal C14-C15 bond in ChR2 ground state and its photointermediates
| State | δ(C14)/ppm | δ(pSB)/ppm | R/Å | Φ/° |
| ChR2470 | 126.0 ± 0.5 | 196.5 ± 0.5 | 1.51 ± 0.02 | 158 ± 2 |
| P1500 | 124.2 ± 0.5 | 181.0 ± 1.0 | 1.56 ± 0.04 | 156 ± 4 |
| P4480 | 119.3 ± 0.5 | — | 1.53 ± 0.04 | 152 ± 4 |
| Px | 122.7 ± 0.5 | 185.0 ± 1.0 | 1.52 ± 0.02 | 156 ± 2 |
PHI = C14-C15 HCCH torsion angle; R = C14-C15 bond length.
At first glance, it seems surprising that the retinal isomerization in the K-like state does not have a strong effect on the C14-C15 bond, because a hydrogen-out-of-plane band indicative of bond twisting has been reported to occur from the K to the L state of bacteriorhodopsin (66, 67). However, direct solid-state NMR experiments on bacteriorhodopsin, as discussed above, have shown that this bond is already twisted in the ground state, which was observed here for ChR2, and its out-of-plane orientation increases in the M state (53). The latter effect could also be expected for ChR2, for which the deprotonation of the chromophore in the M-like state is accompanied by channel opening (53). However, such a state could not be trapped in this study. The stable conformation of the C14-C15 bond during the ChR2 photocycle shows a strong coupling between retinal and channelopsin-2 and indicates that, most likely, other parts of the retinal cofactor respond more strongly.
Our data show that the K-like state is relatively stable and that thermal relaxation only allows accumulating the long-lived intermediate. This finding is in contrast to bacteriorhodopsin, for which K, L, and late M states could be generated in this way (46). The observed differences imply that the energy barriers between these states are significantly lower in ChR2 compared with bacteriorhodopsin. Furthermore, the detection of a new intermediate Px under continuous illumination shows that this branch of the photocycle indeed exists, because it is required to explain the electrophysiological data for the WTs (40, 43) and mutants with slow photocycles (17, 68).
Conclusion and Outlook
Here, we presented the first NMR study, to our knowledge, of ChR2 using DNP-enhanced solid-state MAS NMR. Our data show a pure all-trans,15-anti retinal Schiff base with a stretched C14-C15 bond length and a significant out-of-plane twist of the H-C14-C15-H dihedral angle in the ground state of ChR2. Three different photostates could be generated using thermal relaxation/trapping protocols, including a so-far unknown intermediate that only occurs under continuous light conditions. Additional intermediates will become accessible using ChR2 variants like C128T and D156A with long-lived and states (68). Our data provide novel insight into the photoactive site of ChR2 and show that DNP-enhanced solid-state NMR fills the gap between functional and X-ray–based structure analysis, which is required to resolve its molecular mechanism. Additional studies using extensively labeled retinals incorporated into isotope-labeled opsin for more structural insight during channel-opening and -closing events will be reported in the future.
Methods
15N-ChR2 was expressed in Pichia pastoris, generated with 14–15-13C2–all-trans retinal, and after purification, reconstituted into liposomes. Solid-state MAS NMR was performed under cryogenic conditions (100 K) using cross-effect DNP enhancement provided by doping the proteoliposomes with AMUPOL and applying high-power microwave irradiation to the sample. Trapping of the different photointermediates was achieved using protocols combining illumination, freezing, and thermal relaxation. Optical data were recorded under conditions that resembled the NMR samples as closely as possible. SI Appendix has a detailed description of the applied methods.
Supplementary Material
Acknowledgments
Oliver Ouari and Paul Tordo are acknowledged for providing the polarizing agent AMUPOL. The work was funded by Deutsche Forschungsgemeinschaft/Sonderforschungsbereich 807 Transport and Communications across Membranes. The dynamic nuclear polarization experiments were enabled through DFG Equipment Grant GL 307/4-1 and the Cluster of Excellence Frankfurt: Macromolecular Complexes Frankfurt. Work at the Center for Biomolecular Magnetic Resonance is supported by the State of Hesse.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1507713112/-/DCSupplemental.
References
- 1.Nagel G, et al. Channelrhodopsin-1: A light-gated proton channel in green algae. Science. 2002;296(5577):2395–2398. doi: 10.1126/science.1072068. [DOI] [PubMed] [Google Scholar]
- 2.Fenno L, Yizhar O, Deisseroth K. The development and application of optogenetics. Annu Rev Neurosci. 2011;34(1):389–412. doi: 10.1146/annurev-neuro-061010-113817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagel G, et al. Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid Behavioral responses. Curr Biol. 2005;15(24):2279–2284. doi: 10.1016/j.cub.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 4.Liewald JF, et al. Optogenetic analysis of synaptic function. Nat Methods. 2008;5(10):895–902. doi: 10.1038/nmeth.1252. [DOI] [PubMed] [Google Scholar]
- 5.Zhang F, et al. Multimodal fast optical interrogation of neural circuitry. Nature. 2007;446(7136):633–639. doi: 10.1038/nature05744. [DOI] [PubMed] [Google Scholar]
- 6.Hegemann P, Moglich A. Channelrhodopsin engineering and exploration of new optogenetic tools. Nat Methods. 2011;8(1):39–42. doi: 10.1038/nmeth.f.327. [DOI] [PubMed] [Google Scholar]
- 7.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8(9):1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 8.Nagel G, et al. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc Natl Acad Sci USA. 2003;100(24):13940–13945. doi: 10.1073/pnas.1936192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kato HE, et al. Crystal structure of the channelrhodopsin light-gated cation channel. Nature. 2012;482(7385):369–374. doi: 10.1038/nature10870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bamann C, Kirsch T, Nagel G, Bamberg E. Spectral characteristics of the photocycle of channelrhodopsin-2 and its implication for channel function. J Mol Biol. 2008;375(3):686–694. doi: 10.1016/j.jmb.2007.10.072. [DOI] [PubMed] [Google Scholar]
- 11.Ritter E, Stehfest K, Berndt A, Hegemann P, Bartl FJ. Monitoring light-induced structural changes of Channelrhodopsin-2 by UV-visible and Fourier transform infrared spectroscopy. J Biol Chem. 2008;283(50):35033–35041. doi: 10.1074/jbc.M806353200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verhoefen MK, et al. The photocycle of channelrhodopsin-2: Ultrafast reaction dynamics and subsequent reaction steps. ChemPhysChem. 2010;11(14):3113–3122. doi: 10.1002/cphc.201000181. [DOI] [PubMed] [Google Scholar]
- 13.Stehfest K, Ritter E, Berndt A, Bartl F, Hegemann P. The branched photocycle of the slow-cycling channelrhodopsin-2 mutant C128T. J Mol Biol. 2010;398(5):690–702. doi: 10.1016/j.jmb.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 14.Lórenz-Fonfría VA, et al. Transient protonation changes in channelrhodopsin-2 and their relevance to channel gating. Proc Natl Acad Sci USA. 2013;110(14):E1273–E1281. doi: 10.1073/pnas.1219502110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neumann-Verhoefen MK, et al. Ultrafast infrared spectroscopy on channelrhodopsin-2 reveals efficient energy transfer from the retinal chromophore to the protein. J Am Chem Soc. 2013;135(18):6968–6976. doi: 10.1021/ja400554y. [DOI] [PubMed] [Google Scholar]
- 16.Radu I, et al. Conformational changes of channelrhodopsin-2. J Am Chem Soc. 2009;131(21):7313–7319. doi: 10.1021/ja8084274. [DOI] [PubMed] [Google Scholar]
- 17.Ritter E, Piwowarski P, Hegemann P, Bartl FJ. Light-dark adaptation of channelrhodopsin C128T mutant. J Biol Chem. 2013;288(15):10451–10458. doi: 10.1074/jbc.M112.446427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eisenhauer K, et al. In channelrhodopsin-2 Glu-90 is crucial for ion selectivity and is deprotonated during the photocycle. J Biol Chem. 2012;287(9):6904–6911. doi: 10.1074/jbc.M111.327700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuhne J, et al. Early formation of the ion-conducting pore in channelrhodopsin-2. Angew Chem Int Ed Engl. 2015;54(16):4953–4957. doi: 10.1002/anie.201410180. [DOI] [PubMed] [Google Scholar]
- 20.Nack M, Radu I, Bamann C, Bamberg E, Heberle J. The retinal structure of channelrhodopsin-2 assessed by resonance Raman spectroscopy. FEBS Lett. 2009;583(22):3676–3680. doi: 10.1016/j.febslet.2009.10.052. [DOI] [PubMed] [Google Scholar]
- 21.Bruun S, et al. The chromophore structure of the long-lived intermediate of the C128T channelrhodopsin-2 variant. FEBS Lett. 2011;585(24):3998–4001. doi: 10.1016/j.febslet.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 22.Krause N, Engelhard C, Heberle J, Schlesinger R, Bittl R. Structural differences between the closed and open states of channelrhodopsin-2 as observed by EPR spectroscopy. FEBS Lett. 2013;587(20):3309–3313. doi: 10.1016/j.febslet.2013.08.043. [DOI] [PubMed] [Google Scholar]
- 23.Sattig T, Rickert C, Bamberg E, Steinhoff HJ, Bamann C. Light-induced movement of the transmembrane helix B in channelrhodopsin-2. Angew Chem Int Ed Engl. 2013;52(37):9705–9708. doi: 10.1002/anie.201301698. [DOI] [PubMed] [Google Scholar]
- 24.Oesterhelt D, Stoeckenius W. Rhodopsin-like protein from the purple membrane of Halobacterium halobium. Nat New Biol. 1971;233(39):149–152. doi: 10.1038/newbio233149a0. [DOI] [PubMed] [Google Scholar]
- 25.Lozier RH, Bogomolni RA, Stoeckenius W. Bacteriorhodopsin: A light-driven proton pump in Halobacterium Halobium. Biophys J. 1975;15(9):955–962. doi: 10.1016/S0006-3495(75)85875-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harbison GS, et al. Dark-adapted bacteriorhodopsin contains 13-cis, 15-syn and all-trans, 15-anti retinal Schiff bases. Proc Natl Acad Sci USA. 1984;81(6):1706–1709. doi: 10.1073/pnas.81.6.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith SO, Lugtenburg J, Mathies RA. Determination of retinal chromophore structure in bacteriorhodopsin with resonance Raman spectroscopy. J Membr Biol. 1985;85(2):95–109. doi: 10.1007/BF01871263. [DOI] [PubMed] [Google Scholar]
- 28.Stoeckenius W, Bogomolni RA. Bacteriorhodopsin and related pigments of halobacteria. Annu Rev Biochem. 1982;51(1):587–616. doi: 10.1146/annurev.bi.51.070182.003103. [DOI] [PubMed] [Google Scholar]
- 29.Kamo N, Hazemoto N, Kobatake Y, Mukohata Y. Light and dark adaptation of halorhodopsin. Arch Biochem Biophys. 1985;238(1):90–96. doi: 10.1016/0003-9861(85)90144-4. [DOI] [PubMed] [Google Scholar]
- 30.Pfleger N, Lorch M, Woerner AC, Shastri S, Glaubitz C. Characterisation of Schiff base and chromophore in green proteorhodopsin by solid-state NMR. J Biomol NMR. 2008;40(1):15–21. doi: 10.1007/s10858-007-9203-5. [DOI] [PubMed] [Google Scholar]
- 31.Wang S, et al. Solid-state NMR spectroscopy structure determination of a lipid-embedded heptahelical membrane protein. Nat Methods. 2013;10(10):1007–1012. doi: 10.1038/nmeth.2635. [DOI] [PubMed] [Google Scholar]
- 32.Janke C, et al. Photocycle and vectorial proton transfer in a rhodopsin from the eukaryote Oxyrrhis marina. Biochemistry. 2013;52(16):2750–2763. doi: 10.1021/bi301412n. [DOI] [PubMed] [Google Scholar]
- 33.Yan B, Nakanishi K, Spudich JL. Mechanism of activation of sensory rhodopsin I: Evidence for a steric trigger. Proc Natl Acad Sci USA. 1991;88(21):9412–9416. doi: 10.1073/pnas.88.21.9412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kitajima-Ihara T, et al. Salinibacter sensory rhodopsin: Sensory rhodopsin I-like protein from a eubacterium. J Biol Chem. 2008;283(35):23533–23541. doi: 10.1074/jbc.M802990200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirayma J, Kamo N, Imamoto Y, Shichida Y, Yoshizawa T. Reason for the lack of light-dark adaptation in pharaonis phoborhodopsin: Reconstitution with 13-cis-retinal. FEBS Lett. 1995;364(2):168–170. doi: 10.1016/0014-5793(95)00381-i. [DOI] [PubMed] [Google Scholar]
- 36.Imamoto Y, et al. Chromophore configuration of pharaonis phoborhodopsin and its isomerization on photon absorption. Biochemistry. 1992;31(9):2523–2528. doi: 10.1021/bi00124a012. [DOI] [PubMed] [Google Scholar]
- 37.Scharf B, Hess B, Engelhard M. Chromophore of sensory rhodopsin II from Halobacterium halobium. Biochemistry. 1992;31(49):12486–12492. doi: 10.1021/bi00164a027. [DOI] [PubMed] [Google Scholar]
- 38.Ernst OP, et al. Microbial and animal rhodopsins: Structures, functions, and molecular mechanisms. Chem Rev. 2014;114(1):126–163. doi: 10.1021/cr4003769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lórenz-Fonfría VA, et al. Pre-gating conformational changes in the ChETA variant of channelrhodopsin-2 monitored by nanosecond IR spectroscopy. J Am Chem Soc. 2015;137(5):1850–1861. doi: 10.1021/ja5108595. [DOI] [PubMed] [Google Scholar]
- 40.Nikolic K, et al. Photocycles of channelrhodopsin-2. Photochem Photobiol. 2009;85(1):400–411. doi: 10.1111/j.1751-1097.2008.00460.x. [DOI] [PubMed] [Google Scholar]
- 41.Lórenz-Fonfría VA, Heberle J. Channelrhodopsin unchained: Structure and mechanism of a light-gated cation channel. Biochim Biophys Acta. 2014;1837(5):626–642. doi: 10.1016/j.bbabio.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 42.Nack M, et al. Kinetics of proton release and uptake by channelrhodopsin-2. FEBS Lett. 2012;586(9):1344–1348. doi: 10.1016/j.febslet.2012.03.047. [DOI] [PubMed] [Google Scholar]
- 43.Hegemann P, Ehlenbeck S, Gradmann D. Multiple photocycles of channelrhodopsin. Biophys J. 2005;89(6):3911–3918. doi: 10.1529/biophysj.105.069716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maly T, et al. Dynamic nuclear polarization at high magnetic fields. J Chem Phys. 2008;128(5):052211. doi: 10.1063/1.2833582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bajaj VS, Mak-Jurkauskas ML, Belenky M, Herzfeld J, Griffin RG. Functional and shunt states of bacteriorhodopsin resolved by 250 GHz dynamic nuclear polarization-enhanced solid-state NMR. Proc Natl Acad Sci USA. 2009;106(23):9244–9249. doi: 10.1073/pnas.0900908106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mak-Jurkauskas ML, et al. Energy transformations early in the bacteriorhodopsin photocycle revealed by DNP-enhanced solid-state NMR. Proc Natl Acad Sci USA. 2008;105(3):883–888. doi: 10.1073/pnas.0706156105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mao J, et al. Structural basis of the green-blue color switching in proteorhodopsin as determined by NMR spectroscopy. J Am Chem Soc. 2014;136(50):17578–17590. doi: 10.1021/ja5097946. [DOI] [PubMed] [Google Scholar]
- 48.Mehler M, et al. The EF loop in green proteorhodopsin affects conformation and photocycle dynamics. Biophys J. 2013;105(2):385–397. doi: 10.1016/j.bpj.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jacso T, et al. Characterization of membrane proteins in isolated native cellular membranes by dynamic nuclear polarization solid-state NMR spectroscopy without purification and reconstitution. Angew Chem Int Ed Engl. 2012;51(2):432–435. doi: 10.1002/anie.201104987. [DOI] [PubMed] [Google Scholar]
- 50.Ong YS, Lakatos A, Becker-Baldus J, Pos KM, Glaubitz C. Detecting substrates bound to the secondary multidrug efflux pump EmrE by DNP-enhanced solid-state NMR. J Am Chem Soc. 2013;135(42):15754–15762. doi: 10.1021/ja402605s. [DOI] [PubMed] [Google Scholar]
- 51.Reggie L, Lopez JJ, Collinson I, Glaubitz C, Lorch M. Dynamic nuclear polarization-enhanced solid-state NMR of a 13C-labeled signal peptide bound to lipid-reconstituted Sec translocon. J Am Chem Soc. 2011;133(47):19084–19086. doi: 10.1021/ja209378h. [DOI] [PubMed] [Google Scholar]
- 52.Sauvée C, et al. Highly efficient, water-soluble polarizing agents for dynamic nuclear polarization at high frequency. Angew Chem Int Ed Engl. 2013;52(41):10858–10861. doi: 10.1002/anie.201304657. [DOI] [PubMed] [Google Scholar]
- 53.Lansing JC, et al. Chromophore distortions in the bacteriorhodopsin photocycle: Evolution of the H-C14-C15-H dihedral angle measured by solid-state NMR. Biochemistry. 2002;41(2):431–438. doi: 10.1021/bi011529r. [DOI] [PubMed] [Google Scholar]
- 54.Hu J, Griffin RG, Herzfeld J. Synergy in the spectral tuning of retinal pigments: Complete accounting of the opsin shift in bacteriorhodopsin. Proc Natl Acad Sci USA. 1994;91(19):8880–8884. doi: 10.1073/pnas.91.19.8880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scherrer P, Mathew MK, Sperling W, Stoeckenius W. Retinal isomer ratio in dark-adapted purple membrane and bacteriorhodopsin monomers. Biochemistry. 1989;28(2):829–834. doi: 10.1021/bi00428a063. [DOI] [PubMed] [Google Scholar]
- 56.Pettei MJ, Yudd AP, Nakanishi K, Henselman R, Stoeckenius W. Identification of retinal isomers isolated from bacteriorhodopsin. Biochemistry. 1977;16(9):1955–1959. doi: 10.1021/bi00628a031. [DOI] [PubMed] [Google Scholar]
- 57.Friedrich T, et al. Proteorhodopsin is a light-driven proton pump with variable vectoriality. J Mol Biol. 2002;321(5):821–838. doi: 10.1016/s0022-2836(02)00696-4. [DOI] [PubMed] [Google Scholar]
- 58.Imasheva ES, et al. Formation of a long-lived photoproduct with a deprotonated Schiff base in proteorhodopsin, and its enhancement by mutation of Asp227. Biochemistry. 2005;44(32):10828–10838. doi: 10.1021/bi050438h. [DOI] [PubMed] [Google Scholar]
- 59.Dioumaev AK, et al. Proton transfers in the photochemical reaction cycle of proteorhodopsin. Biochemistry. 2002;41(17):5348–5358. doi: 10.1021/bi025563x. [DOI] [PubMed] [Google Scholar]
- 60.Vogeley L, et al. Anabaena sensory rhodopsin: A photochromic color sensor at 2.0 A. Science. 2004;306(5700):1390–1393. doi: 10.1126/science.1103943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stehle J, et al. Characterization of the simultaneous decay kinetics of metarhodopsin states II and III in rhodopsin by solution-state NMR spectroscopy. Angew Chem Int Ed Engl. 2014;53(8):2078–2084. doi: 10.1002/anie.201309581. [DOI] [PubMed] [Google Scholar]
- 62.Concistrè M, et al. Light penetration and photoisomerization in rhodopsin studied by numerical simulations and double-quantum solid-state NMR spectroscopy. J Am Chem Soc. 2009;131(17):6133–6140. doi: 10.1021/ja809878c. [DOI] [PubMed] [Google Scholar]
- 63.Daviso E, Diller A, Alia A, Matysik J, Jeschke G. Photo-CIDNP MAS NMR beyond the T1 limit by fast cycles of polarization extinction and polarization generation. J Magn Reson. 2008;190(1):43–51. doi: 10.1016/j.jmr.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 64.Tomonaga Y, et al. An active photoreceptor intermediate revealed by in situ photoirradiated solid-state NMR spectroscopy. Biophys J. 2011;101(10):L50–L52. doi: 10.1016/j.bpj.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xie AH. Quantum efficiencies of bacteriorhodopsin photochemical reactions. Biophys J. 1990;58(5):1127–1132. doi: 10.1016/S0006-3495(90)82455-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ames JB, et al. Bacteriorhodopsin’s M412 intermediate contains a 13-cis, 14-s-trans, 15-anti-retinal Schiff base chromophore. Biochemistry. 1989;28(9):3681–3687. doi: 10.1021/bi00435a009. [DOI] [PubMed] [Google Scholar]
- 67.Rödig C, Chizhov I, Weidlich O, Siebert F. Time-resolved step-scan Fourier transform infrared spectroscopy reveals differences between early and late M intermediates of bacteriorhodopsin. Biophys J. 1999;76(5):2687–2701. doi: 10.1016/S0006-3495(99)77421-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bamann C, Gueta R, Kleinlogel S, Nagel G, Bamberg E. Structural guidance of the photocycle of channelrhodopsin-2 by an interhelical hydrogen bond. Biochemistry. 2010;49(2):267–278. doi: 10.1021/bi901634p. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.