Significance
Cellular communication along the filaments of heterocyst-forming, nitrogen-fixing cyanobacteria has been discussed for at least 50 y but how this might be accomplished is not fully understood. We recently showed that the septum between heterocysts and vegetative cells is pierced by channels 12 nm in diameter and 20 nm long. Here, we show that three proteins, FraC, FraD, and FraG, participate in the formation of the channels although none of them appears to be a structural component of the channels. Moreover, using gold particle-labeled antibody, FraG was found around the cyanophycin plug as well as associated with the cytoplasmic membrane in the neighborhood of the peptidoglycan that forms the septum.
Keywords: Fra proteins, channels, cell communication, cyanobacteria
Abstract
The filamentous nitrogen-fixing cyanobacterium Anabaena sp. PCC 7120 differentiates specialized cells, heterocysts, that fix atmospheric nitrogen and transfer the fixed nitrogen to adjacent vegetative cells. Reciprocally, vegetative cells transfer fixed carbon to heterocysts. Several routes have been described for metabolite exchange within the filament, one of which involves communicating channels that penetrate the septum between adjacent cells. Several fra gene mutants were isolated 25 y ago on the basis of their phenotypes: inability to fix nitrogen and fragmentation of filaments upon transfer from N+ to N− media. Cryopreservation combined with electron tomography were used to investigate the role of three fra gene products in channel formation. FraC and FraG are clearly involved in channel formation, whereas FraD has a minor part. Additionally, FraG was located close to the cytoplasmic membrane and in the heterocyst neck, using immunogold labeling with antibody raised to the N-terminal domain of the FraG protein.
Cyanobacteria are phototrophic microbes that bear a Gram-negative cell envelope and are capable of oxygenic photosynthesis. Some cyanobacteria, such as the filamentous Anabaena sp. strain PCC 7120 (hereafter called Anabaena), are capable of fixing atmospheric N2 when grown in media lacking combined nitrogen. Nitrogen fixation occurs in heterocysts, specialized cells that differentiate from vegetative cells along the filaments and provide a micro-oxic environment for the process (1). One long-standing attraction of Anabaena is its beautiful pattern of differentiation: new heterocysts differentiate midway between two heterocysts as the distance between them doubles due to division of the vegetative cells. This organism, which belongs to one of the first prokaryotic groups on earth to have evolved multicellularity, had to develop structures for intercellular communication. Intercellular communication between heterocysts and vegetative cells comprises small molecules, such as sucrose moving from vegetative cells to heterocysts (2–5) and a dipeptide, β-aspartyl-arginine, moving from heterocysts to vegetative cells (6, 7). The mechanism of communication between heterocysts and vegetative cells has been debated for the last 50 y. Two pathways have been proposed for such exchanges (1, 8–10). One is through the periplasm, suggested by the continuity of the outer membrane surrounding the entire filament (9, 11, 12). The other proposed means of communication requires structures between adjacent cells in the filament. Several structures connecting vegetative cells and heterocysts and vegetative cells with each other have been observed using freeze-fracture, conventional electron microscopy and cryo fixation with electron tomography (13–17). Different names have been given to these structures: microplasmodesmata, septosomes, septal junctions, or nanopores (12, 13, 18, 19). Using cryopreservation combined with electron tomography, we observed structures we call “channels” traversing the peptidoglycan layer in Anabaena (20). These channels are 12 nm long with a diameter of 12 nm, in the septa between vegetative cells. Longer channels, 21 nm long with a similar diameter of 12 nm, were seen in the septa between vegetative cells and heterocysts (20).
Several Anabaena gene products were proposed to be involved specifically in intercellular communication. Three were characterized initially from a large set of mutants selected on the basis of their inability to fix nitrogen (21). These mutants manifest a fragmentation phenotype, meaning that they fragment into short filaments upon transfer to liquid medium lacking combined nitrogen, after which they die (15, 22, 23). Further characterization of these mutants led to uncovering a role for several fra gene products in intercellular molecular transfer (23–25).
fraC encodes a 179-aa protein with three predicted transmembrane segments; fraD encodes a 343-aa protein with five predicted transmembrane segments and a coiled-coil domain; and fraG (also called sepJ) encodes a 751-aa protein predicted to have an N-terminal coiled-coil domain, an internal linker domain, and a C-terminal permease-like domain with either 10 transmembrane segments (22) or 9 or 11 transmembrane segments (26). fraG deletion prevents heterocyst differentiation and glycolipid layer formation, whereas the deletion of either fraC or fraD allows heterocyst differentiation, but the heterocysts formed show an aberrant neck and do not fix nitrogen (23, 25). Using GFP tags, FraC, FraD, and FraG proteins were shown to be located in the septum between cells (23, 26). FraD was further localized to the septum by immunogold labeling using an antibody raised against the N-terminal coiled-coil part of FraD (25). Fluorescence recovery after photobleaching (FRAP) experiments showed impairment in cell-cell transfer of small molecules such as calcein (622 Da) and 5-carboxyfluorescein (374 Da) in fraC, fraD, and fraG mutants, further indicating a role of these gene products in intercellular communication (23–25).
In the work reported here, cryopreservation combined with electron tomography was used to investigate the role of these three fra gene products in channel formation. We found that FraC and FraG are clearly required for channel formation, whereas FraD plays a minor role. Immunogold labeling with antibody to the N-terminal coiled-coil domain of FraG yielded an improved localization for FraG.
Results
Roles of FraC and FraD in Channel Formation Between Vegetative Cells.
In earlier studies, three deletion mutant strains CSVT1 (∆fraC) CSVT2 (∆fraD), and CSVT22 (double mutant ∆fraC/D) revealed the same fragmentation phenotype: upon transfer to N− medium, the filaments fell apart (15, 23, 25). However, in nitrogen-rich media, these mutants did not show any morphological alteration when examined by light microscopy, although transfer of calcein and 5-CFDA between cells was hampered. In addition, GFP fusions to wild-type FraC and FraD allowed localization of both proteins to the septum connecting vegetative cells. Additionally, immunogold labeling of FraD confirmed its location in the septum (23, 25).
These observations prompted us to investigate the channels in these mutants under nitrogen-replete conditions. We examined 2.2-nm tomographic sections of septa between vegetative cells of the ∆fraC, ∆fraD and ∆fraC/D mutants in three or four tomographic volumes for each strain, including some tomograms covering the whole septum (serial tomograms). We present here only the middle part of the septum between two vegetative cells (Fig. 1). (For the entire septum see supplement Fig. S1). The septum of CSVT1 (∆fraC) shows fewer and wider channels than the WT (Fig. 1 A–D). Note that the septum in each tomogram corresponds to a 200- to 300-nm section of the entire septum. The dimensions and frequency of channels in the septa of CSVT2 (∆fraD) were similar to those observed in the WT (Fig. 1 E and F). The septum between vegetative cells in CSVT22 (∆fraC/D) displays only a single channel, this one appearing to be wider than those observed in WT (Fig. 1 G and H) (other tomograms of CSVT22 show two to three channels in the septum). When the septum is rotated 90° around the y axis, it is clear that CSVT1 and the double mutant contain about 90% fewer channels than the WT. In ∆fraC, as well as in the double mutant, the length of the channels is 12 nm, which is similar to WT, whereas the diameter is 21 nm, noticeably wider than WT (Table 1). Based on these observations, we conclude that FraC plays a role, possibly structural, in assembly of the channels, whereas FraD does not.
Fig. 1.
The septum between two vegetative cells of WT Anabaena and three fragmentation mutants. (A) Electron tomographic image of the WT septum. (B) Septum shown in A is rotated 90° around the y axis showing the channel distribution within the septum. Several channels are observed in the middle of the septum (white holes). (C) Electron tomographic image of the septum of CSVT1 mutant (∆fraC). The septum contains fewer channels compared with WT. (D) Septum shown in C is rotated 90° around the y axis. (E) Electron tomographic image of the septum of CSVT2 mutant (∆fraD). The septum contains similar number of channels compared with WT. (F) Septum shown in E is rotated 90° around the y axis. (G) Electron tomographic image of the septum of CSVT22 mutant (∆fraC/D). (H) Septum shown in G is rotated 90° around the y axis showing the channel distribution. Arrowheads indicate the channels observed on their corresponding panel before rotation. “t” indicates thylakoids. All images are composed of 10 superimposed 2.2-nm serial tomographic slices. (Scale bar: 50 nm.)
Fig. S1.
The septum between two vegetative cells of WT Anabaena and three fragmentation mutants. (A) Electron tomographic image of the WT septum. (B) Septum shown in A is rotated 90° around the x axis showing the channel distribution within the septum. Several channels are observed in the middle of the septum (white holes). (C) Electron tomographic image for the septum of ∆FraG. (D) Septum shown in C is rotated 90° around the x axis showing the channel distribution within the septum. Only 2 are observed compared with 15–20 in WT. Arrowheads in D indicates one of channels observed in the septum in C. (E) Electron tomographic image of the septum of the ∆FraC mutant. The septum contains fewer channels compared with WT. (F) Septum shown in E is rotated 90° around the x axis. (G) Electron tomographic image of the septum of the ∆FraD mutant. The septum contains similar number of channels compared with WT. (H) Septum shown in G is rotated 90° around the x axis. (I) Electron tomographic image of the septum of ∆FraC/D. (J) Septum shown in E is rotated 90° around the x axis showing the channel distribution. Arrowheads indicate the channels observed on their corresponding panel before rotation. “t” indicates thylakoids. All images are composed of 10 superimposed 2.2-nm serial tomographic slices. (Scale bar: 50 nm.)
Table 1.
Channel dimensions in WT Anabaena and various fra mutants
| Strain | Diameter of the channels, nm | Length of the channels, nm |
| BG11 (veg-veg) | ||
| PCC 7120 (WT) | 14 ± 4 (7) | 13 ± 4 (7) |
| CSVM34 (∆fraG) | 11 ± 3 (6) | 12 ± 3 (6) |
| CSVT22 (∆fraC/D) | 21 ± 5* (6) | 12 ± 4 (6) |
| BG110 (het-veg) | ||
| PCC 7120 (WT) | 11 ± 2 (10) | 20 ± 6 (10) |
| CSVT22 (∆fraC/D) | 14 ± 2* (8) | 83 ± 26* (8) |
Numbers in parentheses indicate number of channels measured in each case.
Differences from the WT that are statistically significant (P < 0.05).
The Heterocyst-Vegetative Cell Septa in the ∆fraC/D Double Mutant.
Although CSVT22 (∆fraC/D) produces heterocysts in response to nitrogen limitation, it is not able to grow diazotrophically. This phenotype may result from an altered structure of the heterocyst/vegetative cell septa as in the single mutants (25). Four tomograms for the mutant vegetative cell-heterocyst junctions, including one tomogram covering the whole junction (three serial tomograms), were analyzed. The cup-like structure typical of the WT heterocyst neck is missing in the CSVT22 strain, consistent with previously reported results (25). In addition, the septum in the ∆fraC/D mutant appears to be three to four times wider than in the WT (82 ±25 nm in the mutant compared with 21 nm in WT; Fig. 2 and Table 1). The septum also contains fewer channels than WT, one to four channels per septum, compared with ∼20 in WT. These results resemble those for the channels in the septa between vegetative cells of the same mutant, as described in the previous section.
Fig. 2.
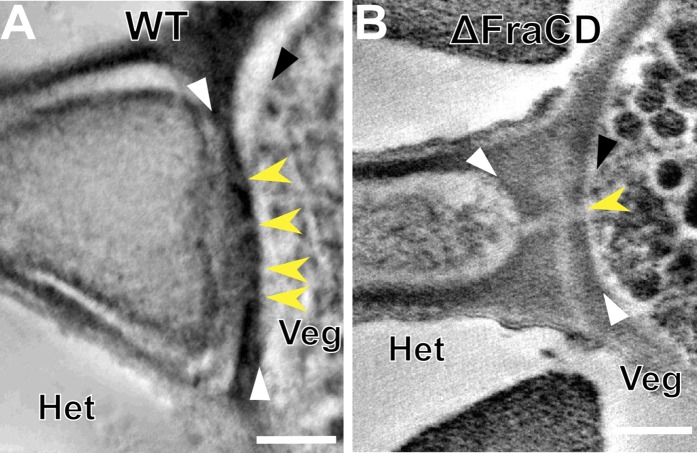
Heterocyst-vegetative cell septa in WT and fragmentation mutants. (A) Electron tomographic image of a WT heterocyst junction. White arrowheads point to the edges of the septum. Yellow arrowheads show the channels that connect the heterocyst and the vegetative cell. (B) Electron tomographic image of the CSVT22 (ΔfraCD) heterocyst junction. The septum in this mutant is thicker and only 1–2 channels are present compared with WT. The yellow arrow points to the only channel observed in this tomogram. Black arrowheads point to the plasma membrane in vegetative cells in each panel. All tomographic images are composed of 10 superimposed 2.2-nm tomographic slices. Het, heterocyst; Veg, vegetative cell. (Scale bar: 200 nm.)
Requirement of FraG for Channel Formation Between Vegetative Cells.
We also studied the septum structure in strain CSVM34 (∆fraG) (27), by electron tomography. We examined 2.2-nm tomographic sections of septa between vegetative cells of the ∆fraG mutant, grown in complete medium, in eight tomographic reconstructions. Only three of the eight reconstructed tomograms showed a few (three or four) channels in the septum (Fig. 3C), compared with 15–20 channels in each of the five WT tomograms. The channels are seen clearly when the tomographic volume is rotated 90° around the y axis, where they appear as white holes in the dark background of the septum. CSVM34 (∆fraG) shows many fewer channels compared with WT (Fig. 3 B and D). The dimensions of the channels observed in ∆fraG are not statistically different from those of WT (Table 1). These results suggest that FraG might provide a dock for initiating channel assembly, to be discussed below.
Fig. 3.
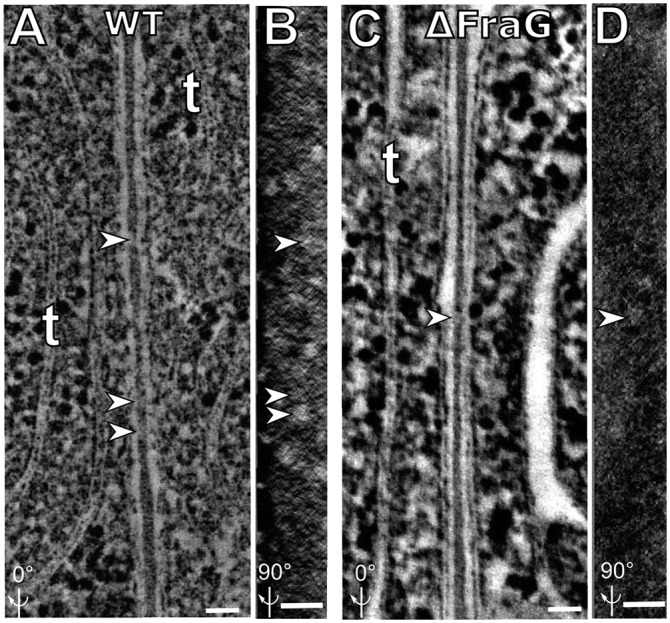
The septum between two vegetative cells of WT Anabaena and of fragmentation mutants. (A) Electron tomographic image of the WT septum. (B) Septum shown in A is rotated 90° around the y axis showing the channel distribution within the septum. Several channels are observed in the middle of the septum (white holes). (C) Electron tomographic image for the septum of mutant CSVM34 (∆fraG). (D) Septum shown in C is rotated 90° around the y axis showing the channel distribution within the septum; only two channels are observed compared with 15–20 in WT. Arrowheads in B and D indicate channels observed in the septum in A and C, respectively. “t” indicates thylakoids. All images are composed of 10 superimposed 2.2-nm serial tomographic slices. (Scale bar: 50 nm.)
FraG Is Localized Around the Cyanophycin Mass in the Heterocyst.
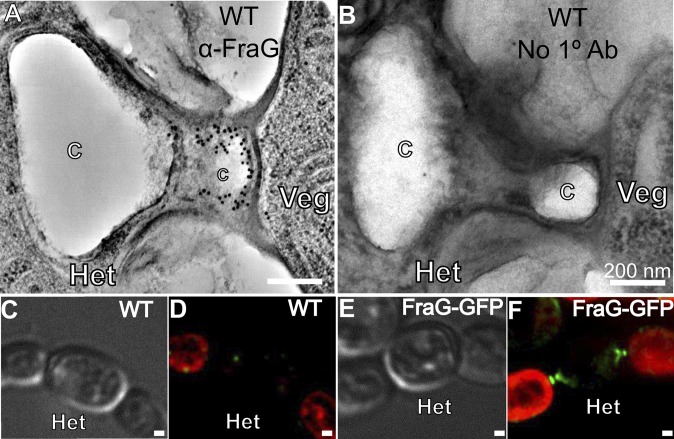
Previous studies, using GFP-tagged FraG, localized FraG to the intercellular septa, but optical microscopy lacks the resolution needed to define this location precisely. To locate FraG better, we immunolabeled samples of the WT strain (grown with or without combined nitrogen) using antibody raised against the FraG N-terminal coiled-coil domain (anti-FraG_CC) and a 10-nm gold-labeled secondary antibody (27). Very few gold particles were observed in cells grown under N+ conditions (Fig. S2), even in septa where FraG-GFP had been localized by confocal microscopy (26). The specificity of the gold particles in the septum could not be confirmed in comparison with gold particles detected in the background, probably due to the low expression level of FraG. This point will be elaborated in the next paragraph. However, with samples grown under nitrogen-fixing conditions, labeling was observed in the heterocyst neck, around the cyanophycin mass, that is, close to the heterocyst-vegetative cell junction. Only a few gold particles were seen on the larger part of the cyanophycin that is close to the polar thylakoid mass. As was the case with cells grown under N+ conditions, very few gold particles were identified in the septum between vegetative cells and between heterocysts and vegetative cells. Fig. 4B shows a control experiment in which WT samples were incubated with the secondary gold-labeled antibody alone, without the FraG primary antibody. No gold particles were detected in this control experiment, which indicates that the signal we detect is due to the FraG antibody. The cells shown in Fig. 4 A and B correspond to samples in which the cyanophycin has dropped out during the preparation of the sample. The absence of cyanophycin must have made the FraG-antigenic domain accessible to the antibody in the heterocyst neck. This hypothesis was confirmed by immunogold labeling of cells with intact cyanophycin, which showed very few bound gold particles (Fig. S3).
Fig. S2.
Immunogold labeling of FraG on WT cells grown in complete media. A and B correspond to two different cells. Very few gold particles can be detected in the septum between vegetative cells (White arrowheads).
Fig. 4.
Subcellular localization of FraG in Anabaena heterocysts. (A) Immunogold labeling of WT Anabaena using antibodies (black dots) raised against the N-terminal coiled-coil domain of FraG. (B) Control immunogold labeling of WT using only secondary antibody; no dots. (C) Light transmission micrograph of WT Anabaena grown under N− conditions. (D) Autofluorescence of the same cells shown in C. Heterocysts do not show autofluorescence due to loss of PS II chlorophyll. (E) Light transmission micrograph of the CSAM137 mutant (FraG-GFP) grown under N− conditions. (F) Autofluorescence (red) and GFP fluorescence (green) of the same cells shown in E. GFP fluorescence locates FraG at the poles of the heterocysts. C, Cyanophycin; Het, heterocyst; Veg, vegetative cell. (Scale bar: 200 nm.)
Fig. S3.
Immunogold labeling of FraG on cells with intact cyanophycin. A and B correspond to two different cells. Arrowheads point to the few gold particles that were observed on the cyanophycin.
In light of these results, we reinvestigated the C-terminal GFP-tagged FraG localization in heterocysts, using 3D deconvolution fluorescence microscopy in the strain CSAM137 (26). The fluorescence is spread through the outermost part of the heterocyst neck. These results agree with the immunolocalization of FraG in the heterocyst neck around the cyanophycin mass (Fig. 4A). Fig. 4F shows two distinguishable spots at each pole of the cell, suggesting the presence of FraG-GFP toward the vegetative cell, as well as around the cyanophycin and more specifically in the part that is present in the heterocyst neck. To further investigate FraG localization around the cyanophycin mass, we collected tomograms for the immunogold-labeled samples (Fig. 5 A–C). The immunotomogram with anti-FraG_CC shows the gold particles around the cyanophycin. Fig. 5 A–C show gold particles at different depths around the cyanophycin mass, between the heterocyst membrane and the cyanophycin. Fig. 5D shows the distribution of gold particles around the cyanophycin mass, confirming the localization of FraG in the heterocyst neck. Note that, in the EM images, the cyanophycin always appears split into two parts. The gap is probably due to the cyanophycin breakage that was observed in all heterocysts in this study and in previous studies (20).
Fig. 5.
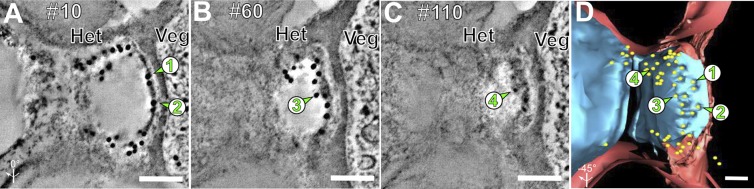
Serial immunoelectron tomography of WT Anabaena grown under N− conditions. (A–C) Serial 2.2-nm tomographic slice images (every 50th slice) through a heterocyst neck labeled with anti-FraG antibody. Note that as one proceeds from the top of the section (A) to the bottom (C), different groups of 10-nm gold labels, indicated by arrows and numbers, are seen at different depths within the cyanophycin interior; 1 and 2 at the top (A), 3 in the middle (B), and 4 at the bottom (C) of the cyanophycin plug. (D) Tomographic model of the immunoelectron tomogram showing the location of FraG around the cyanophycin (blue). Green arrows indicate gold particles in the cyanophycin at different depth with n° 1 and 2 seen in section 10 (A), n° 3 in section 60 (B), and n° 4 in section 110 (C). Note that there is no contact between the gold particle and the channels (or peptidoglycan). Peptidoglycan layer is red. Het, heterocyst; Veg, vegetative cell. (Scale bar: 100 nm.)
FraG Immunogold Localization in the Septum Between Vegetative Cells.
The weak gold signals in the WT vegetative cell septa could be explained by the low expression of FraG. In order better to localize FraG between vegetative cells, we constructed a mutant, W30, overexpressing FraG. The mutant did not show any phenotypic difference compared with WT (same growth rate in N+ and same pattern and morphology for differentiating heterocysts). Western blots of W30 extracts show a sevenfold increase in the amount of FraG compared with WT (Fig. S4). Immunogold labeling of W30 using anti-FraG_CC shows the N-terminal domain mainly on the edge of the septa between vegetative cells (Fig. 6 A and C) distributed close to the cytoplasmic membrane. More than 100 cells were analyzed and all of them show similar gold distribution. (See Fig. S5 for more cells.) The septal localization of the native FraG protein corroborates the data obtained with the FraG-GFP fusion protein (26). The specificity of the signal in W30 was confirmed by immunolabeling the deletion mutant CSVM34, in which no signal was detected (Fig. 6B).
Fig. S4.
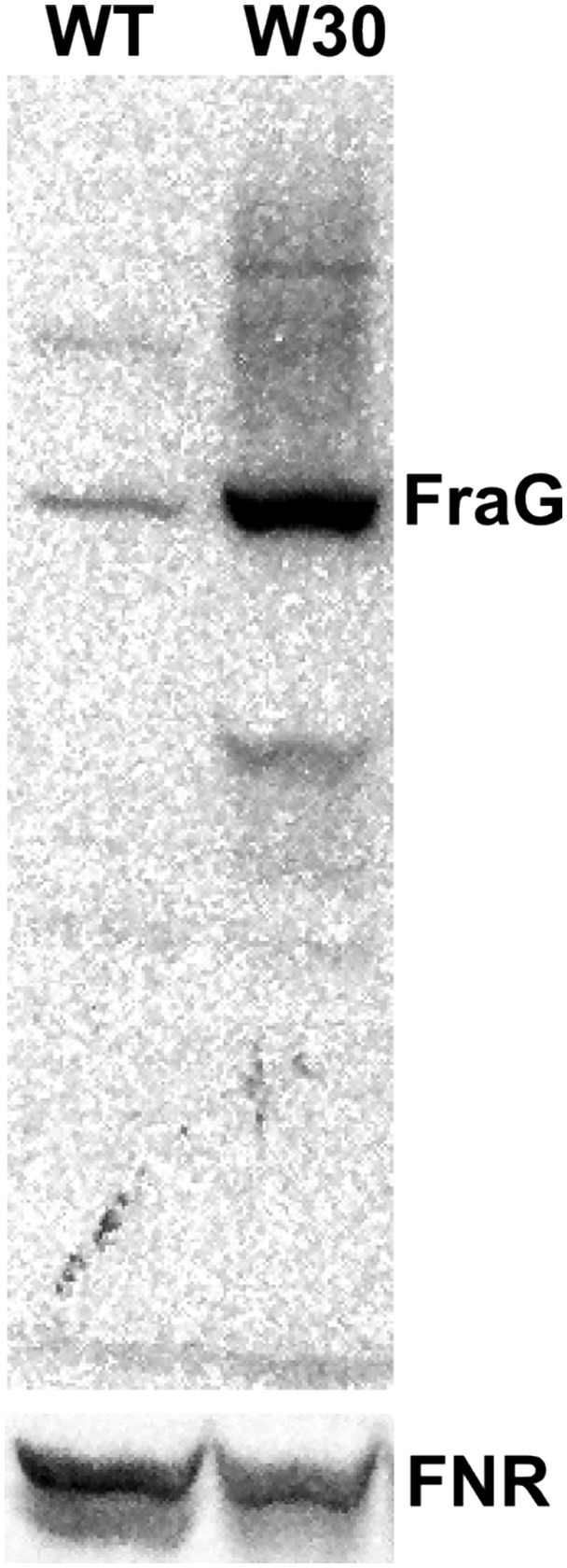
Western blot using the N-terminal FraG antibody on total cell extracts of the WT and W30, overexpressing FraG. The FNR antibody was used as a loading control. The FraG protein is located at 82 kDa and is 7 times more abundant in W30 compared with WT.
Fig. 6.
FraG localization between vegetative cells. (A) Immunogold labeling of W30, overexpressing FraG, using anti-FraG antibody. (B) Immunogold labeling of CSVM34 (ΔfraG) shows no gold particles. (C) Zoom in to the septum in A; 11 gold particles seen on both sides of the septum. (Scale bar: 200 nm.)
Fig. S5.
Immunogold labeling of FraG on W30 cells. Gold particles are localized in the septum between vegetative cells. B, D, F, and H correspond to zoomed-in image of the septa in A, C, E, and G, respectively.
FraG topology.
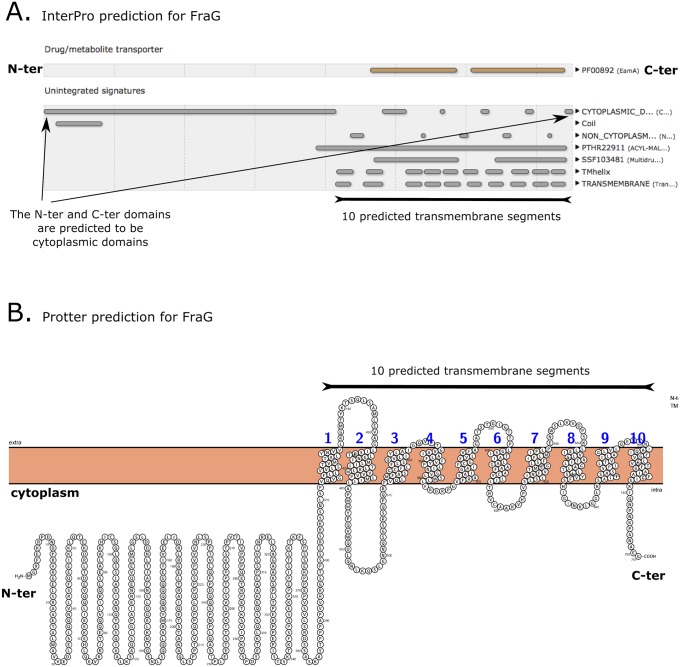
Topology prediction of FraG is not clear, because analysis of homologous sequences from different cyanobacteria predict 9, 10, or 11 transmembrane segments (28). Our analyses for FraG topology in Anabaena using interPro, an integrated database of predictive protein signatures (29), and Protter (30) supported a 10-transmembrane span model (Fig. S6). This fact affects localization of the coiled-coil domain, which has been described as essential for the function of the protein (26).
Fig. S6.
FraG structure prediction. (A) FraG structure prediction using InterPro. The C-terminal domain is predicted to have 10 transmembrane segments. It also predicts a cytoplasmic localization for both the C-terminal and N-terminal domains. (B) FraG structure prediction using Protter. Similar results were obtained using Protter compared with InterPro (A).
To investigate FraG topology experimentally, we fused GFP to the first 391 amino acids of FraG. This sequence includes the N-terminal domain and most of the linker domain of FraG, but excludes all potential transmembrane domains (Fig. 7A). The construct, called CCL-GFP (coiled-coil-Linker-GFP), was expressed in WT strain yielding strain WGF. Additionally, to exclude any localization due to interaction with the WT FraG, the reporter construct was expressed in a ∆fraG background producing strain ∆GF. Vegetative cells of both WGF and ∆GF show a signal in the division plane, forming a ring similar to the FtsZ division ring (Fig. 7 B, D, and E). No signal was detected in the mature septa. Surprisingly, in heterocysts of WGF, the CCL-GFP construct was also localized in the poles even though it lacks the predicted transmembrane domains of FraG (Fig. 7C).
Fig. 7.
FraG N-terminal localization. (A) Cartoon showing the different domains of FraG and the location of the GFP insertion in the linker domain of FraG in the pRGF plasmid. The plasmid was introduced into both WT and ∆fraG yielding WGF and ∆GF. (B and C) Autofluorescence (red) and GFP fluorescence (green) in WGF grown in N+ and N−, respectively. (D) Autofluorescence (red) and GFP fluorescence (green) in W30 grown in N+ (Note that the mutant cannot grow in N− media). (E) Same micrograph shown in D rotated 45° around the y axis and showing only GFP fluorescence. Notched arrowheads indicate the location of the CCL-GFP construct in the divisome plane. Straight arrowheads indicate cells at the end of division, hence the presence of a GFP signal. GFP fluorescence shows the FraG N-terminal-linker domain as rings in the divisome plane of vegetative cells. CC, predicted Coiled Coil; C ter, C-terminal domain; L, predicted Linker domain; M1, FraG first Methionine; N-ter, N-terminal domain; P391, Proline- the 391st amino acid in FraG linker domain where GFP was fused; TM, Transmembrane domain. (Scale bar: 2 μm.)
Discussion
Septa of several fragmentation mutants that are impaired in intercellular communication were examined by electron tomography. None of the fraC, fraD, and fraG mutants showed a total loss of channels in their septa, but rather a decrease in the number of channels and different sizes for these channels were observed, especially for ∆fraC and ∆fraG, indicating that FraC and FraG either affect the assembly of the channels or are involved in the assembly of different channels.
The difference in channel distribution between CSVT1 and CSVT2 suggest different functions for FraC and FraD, respectively. fraC deletion results in the reduction of channel number in the septum, which suggests that FraC is either a part of the channel structure or a regulator of channel assembly. On the other hand, fraD deletion does not seem to affect channel formation between vegetative cells. FraD is probably involved in maintaining a stable cell-cell contact in the septum. We cannot exclude a role for FraD in recruiting components of other types of channels that are not detected using our methods. The ∆fraC/D double mutant (CSVT22) showed a sevenfold increase of the width of the septa between vegetative cells and heterocysts compared with the twofold increase observed in corresponding WT septa. These results suggest that FraC or FraD or both play a role in expansion of the peptidoglycan and the channels that connect heterocysts with vegetative cells. Aberrant heterocyst neck structures were previously shown in fraC and fraD mutants (25), suggesting that FraC and FraD are important for maintaining a tight junction at the septum during the restructuring or remodeling of the peptidoglycan layer throughout the heterocyst differentiation process. The heterocyst neck in these mutants lacks the typical cup-like structure found in the WT. The increase in the septum width and the decrease in the contact area between the heterocyst and the vegetative cell could explain the fragmentation phenotype of the fraC, fraD, and fraC/D mutants (15, 23, 25).
Tomograms for the septa between vegetative cells of CSVM34 (∆fraG), using cells fixed with potassium permanganate, were previously analyzed (12). Structures called “septosomes” were measured to be 18 nm long in CSVM34 compared with 27 nm in WT. The septosome frequency was difficult to measure due to lack of resolution. Using cryo-fixation and staining with osmium tetroxide, which highlights peptidoglycan, and two-axis tomograms, we observed channels with similar length in WT and CSVM34 although there were fewer channels in CSVM34. This difference in observations suggests that different structures connecting cells might be revealed when using cryo-fixation and tomography compared with the structures observed in cells fixed with potassium permanganate (12). However, in agreement with our observations, strain CSVM34 shows a reduced number of septal peptidoglycan nanopores (15% of the wild type) (31).
FraG-GFP has been seen near the septum between vegetative cells (26). In this work we have improved the resolution of the localization of FraG by means of immunogold labeling. The N-terminal coiled-coil domain of FraG was detected close to the septum between vegetative cells and in the heterocyst, in the neck around the cyanophycin mass. The positions of the gold particles around the cyanophycin mass in heterocysts assign the location of the N-terminal domain of FraG to a position facing the cyanophycin cavity. We cannot exclude the possibility that FraG is anchored to a putative hydrophobic layer surrounding the cyanophycin plug. However, due to the fact that the gold particles could be ∼30 nm from their antigenic target (32), the transmembrane domains of FraG could be located in the cytoplasmic membrane with its N-terminal coiled-coil domain facing toward the cyanophycin plug, as modeled in Fig. 8.
Fig. 8.
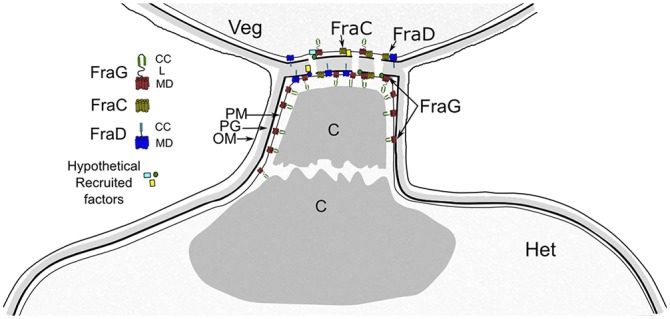
Model for the heterocyst-vegetative cell junction showing putative localization and interactions of FraC, FraD, and FraG. These proteins appear to be located in the plasma membrane and/or septum and involved in channel formation, either directly or by recruiting other factors. In a fully-developed heterocyst, FraG is found in the heterocyst neck around the cyanophycin, implicating FraG in an additional role to channel formation, possibly assembly or maintenance of heterocyst neck formation. C, Cyanophycin; CC, predicted Coiled Coil; L, predicted Linker domain; MD, Transmembrane domain; OM, Outer membrane; PG, Peptidoglycan; PM, Plasma membrane.
In heterocysts, the (artifactual) lost cyanophycin apparently made FraG antigens accessible to gold labeling; FraG antibodies reacted with their respective antigen within the 200-nm section. In the case of vegetative cells and that of heterocysts with intact cyanophycin, immunogold-labeled antibodies react only with their respective antigens that are located on the surface of each section. Some gold particles can be located on the surface near the septum between vegetative cells (Fig. S2) and between heterocysts and vegetative cells (Fig. 4A and Fig. S3). In these areas, the gold-labeled secondary antibody is probably bound to exposed FraG coiled coils. The distribution of the gold particles around the cytoplasmic membrane between vegetative cells (Fig. 6 A and C) suggests a localization of the N-terminal closer to the cytoplasmic membrane of the cells rather than the peptidoglycan.
It has been established in bacteria that GFP fused to the C-terminal domain of cytoplasmic membrane proteins fluoresce when the GFP is located in the cytoplasm (33), or in the periplasm when it is exported by the TAT system (9). The C-terminal GFP-tagged FraG shows its fluorescence signal around the heterocyst neck (Fig. 4), which indicates that the FraG C-terminal domain is located in the cytoplasm. Moreover, the C-terminal GFP-tagged FraG (CCL-GFP) containing the coiled-coil and part of the linker domain of FraG and lacking the transmembrane domain (Fig. 7A), shows its fluorescence signal in a ring in dividing vegetative cells and in the heterocyst poles (Fig. 7). Because a TAT signal peptide is not detected in the N-terminal sequence of FraG, GFP fluorescence indicates that the FraG N-terminal domain might be located in the cytoplasm. The absence of GFP signals in all septa between vegetative cells in WGF and ΔGF is probably due to the absence of the transmembrane domain that anchors the protein to the plasma membrane. The GFP fluorescence signal in the division planes of vegetative cells in the CCL-GFP construct are in agreement with recent results showing that FraG interacts with FtsQ, a protein involved in cell division (34). On the other hand, the presence of the GFP signal in WGF within the cyanophycin plug could be due to interaction of the CCL-GFP construct with either WT FraG or the cyanophycin.
The Anabaena ORF alr2338 was first denoted fraG (22). Later, sepJ was preferred because it better reflected the subcellular localization found in ref. 26. In this work, we show that the alr2338 product is not located exclusively in the septum. Therefore, we prefer fraG for that gene, reflecting its phenotype.
Experimental procedures
Anabaena Strains and Growth Conditions.
Anabaena sp. strain PCC 7120 and derivative strains were grown photoautotrophically at 30 °C under constant white light (35 μE m−2 s−1), in a CO2 enriched atmosphere. The medium composition is similar to BG11 with some modification (35). For heterocyst induction, cells were harvested by centrifugation and resuspended in NO3− deficient medium, for which NaNO3 was replaced by NaCl. Mutant strains CSVT1 (∆fraC), CSVT2 (∆fraD), CSVT22 (∆fraC/D), and CSVM34 (∆fraG) have been described (23, 25, 27). Growth media were supplemented when appropriate with Neomycin (50 μg mL−1). Strain W30 was constructed by transferring pAN130 (22), a replicative plasmid containing the promoter and coding region of fraG, to WT Anabaena by conjugation as described (36).
To express the CC-linker GFP-tagged FraG, a 1,941-bp fragment covering the 5′ noncoding region and part of the fraG orf was amplified using primers FFB (GGATCCTGAAATATGAGTTATGGCTGGGGAC) and FRN (GCTaGcTGGTGCA GGCGGAGGAGTTG), which also creates BamHI and NheI restriction sites, respectively. DNA from Anabaena sp. PCC7120 was used as template. The PCR product, digested with BamHI and NheI, was cloned into pRL25N (37) digested with the same enzymes, yielding pRCG. The plasmid was sequenced to verify the fidelity of the PCR. pRCG was transferred to WT and to CSVM34 by conjugation as described above, yielding WGF and ∆GF, respectively.
Embedding Anabaena for Electron Microscopy.
Anabaena cells were harvested by centrifugation and transferred to an aluminum sample holder and cryoprotected with 0.15 M sucrose. Samples were frozen in a Baltec HPM 010 high-pressure freezer, then freeze-substituted in 2% (wt/vol) osmium tetroxide (EMS), using an Automated Freezing Substitution machine (ASF2, Leica), in anhydrous acetone at −80 °C for 72 h. The temperature was then increased from −80 °C to −20 °C over 12 h. Samples were then washed with acetone three times at −20 °C, then transferred to 4 °C, held overnight, and then warmed to room temperature. Samples were then infiltrated with increasing concentrations of EPON resin [5%, 10%, 25%, 50%, 75%, and 100% (wt/vol)] finally polymerized at 60 °C for 24 h (see ref. 38 for more details).
For immunogold labeling, the high-pressure frozen samples were substituted in 0.1% uranyl acetate in acetone at −80 °C for 3 d and then warmed to −50 °C for 12 h. After three acetone rinses, samples were slowly infiltrated under controlled time and temperature conditions in a Leica AFS system at −50 °C with Lowicryl HM20 resin according to the following schedule: 5%, 10%, 25%, 50%, 75%, and 100% (wt/vol) (24 h incubation for each concentration). After the last incubation with 100% HM20, samples were rinsed with fresh 100% HM20 three times, with 1 h for each wash. Samples were finally polymerized at the same temperature under UV light for 32 h.
Immunocytochemistry.
Rabbit antibodies raised against the 188-aa coiled-coil domain of FraG were used to detect FraG in Anabaena heterocysts and vegetative cells. Samples embedded in Lowicryl HM20 were cut into 150-nm-thick sections and placed on Formvar-coated gold slot grids. Immunocytochemistry was performed essentially as described by Otegui et al. (39). Sections were blocked for 20 min with a 5% (wt/vol) solution of nonfat milk in TBS plus 0.1% Tween 20 (TBST). Primary antibodies were diluted 1:20 in a solution of 2.5% (wt/vol) nonfat milk in TBST at room temperature for 1 h. The sections were rinsed in a stream of TBS plus 0.5% Tween 20 and then transferred to the secondary antibody (goat anti-rabbit IgG 1:20 in TBST) conjugated to 10-nm gold particles, for 1 h. Control procedures omitted the primary antibody. For gold quantification, over 1,000 gold particles were counted in more than 30 pairs of cells. See Fig. S7 for the calculation of gold distribution in the septum vs. the rest of the cell and the background.
Fig. S7.
Quantification of gold particles in W30. Gold particles were counted in 30 different micrographs that include two cells. An average of gold distribution across the cells was calculated. Around 14 gold particles are present in the septum between two vegetative cells, whereas 0–2 gold particles are detected elsewhere, per same surface area, in the cell and in the background.
Sectioning.
EPON sections (100–300 nm) were cut using a Leica EM AFS2 Automatic Freeze-Substitution Processor and collected on 1% Formvar (EMS) copper slot grids. Sections were stained with 2% (wt/vol) uranyl acetate and 0.5% lead citrate for 8 and 5 min, respectively. For tomogram collection, 300-nm sections were used and 10 μL of 15-nm colloidal gold (BBI solutions) were applied for 10 min on each side of the grid as fiduciary markers.
Electron Tomography.
Tomograms were collected using a Tecnai G2 F30 (FEI) electron microscope operating at 300 kV. Images were taken at 15,000× from −60° to +60° with 1° interval. Each tomogram was collected in two perpendicular axes. Etomo was used to build the tomograms and to merge the two single-axis tomograms into one dual-axis tomogram. Tomograms were then displaced, analyzed and modeled using the 3DMOD software (40).
Fluorescence Microscopy.
Anabaena cells were visualized with a Leica DM6000B fluorescence microscope and an ORCA-ER camera (Hamamatsu) using an FITC L5 filter [excitation, band-pass (BP) 480/40 filter; emission, BP 527/30 filter] and the Leica SP5 2-photon confocal microscope. The images, including BlindDeblur deconvolution of 3D images, were produced using the LAS AF Leica software.
Preparation of Cell Extracts and Western Blots.
Total cell extracts were prepared as described in ref. 41. Proteins were separated using Novex 14% Tris-Glycine gels (Novex, Life Technology). Chlorophyll concentration was used to ensure equivalent loading of cell extracts. A total of 1.2 μg of chl was loaded per 1-mm well for blotting and Coomassie staining.
For immunoblots, proteins were transferred to PVDF membranes (immobilon, Millipore) using a Bio-Rad gel transfer system (Bio-Rad). Blots were blocked with Tris-buffered saline supplemented with 0.1% Tween and 5% (wt/vol) dry skimmed milk and incubated with the primary antibody (1:500 dilution for FraG and 1:10,000 dilution for FNR) overnight at 4 °C. After washing, blots were incubated 1 h at room temperature with a 1:15,000 dilution of peroxidase-conjugated anti-rabbit IgG (Promega). The signal was visualized using ECL chemiluminescent substrate (SuperSignal West Pico Chemiluminescent, Thermo Scientific). Images were generated with a CCD camera and analyzed using ImageJ software.
Acknowledgments
We thank Prof. Enrique Flores for support in part from Grant BFU2011-22762 from Plan Nacional de Investigación, Spain, co-financed by the European Regional Development Fund. We also thank Sean Callahan for plasmid pAN130 and Amel Latifi for plasmid pRL25N. William Buikema and Ghada Ajlani provided critical reading of the paper. This work was supported by the Ellison Medical Foundation and The University of Chicago.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1512232112/-/DCSupplemental.
References
- 1.Haselkorn R. Cell-cell communication in filamentous cyanobacteria. Mol Microbiol. 2008;70(4):783–785. doi: 10.1111/j.1365-2958.2008.06475.x. [DOI] [PubMed] [Google Scholar]
- 2.Jüttner F. 14C-labeled metabolites in heterocysts and vegetative cells of Anabaena cylindrica filaments and their presumptive function as transport vehicles of organic carbon and nitrogen. J Bacteriol. 1983;155(2):628–633. doi: 10.1128/jb.155.2.628-633.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cumino AC, Marcozzi C, Barreiro R, Salerno GL. Carbon cycling in Anabaena sp. PCC 7120. Sucrose synthesis in the heterocysts and possible role in nitrogen fixation. Plant Physiol. 2007;143(3):1385–1397. doi: 10.1104/pp.106.091736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.López-Igual R, Flores E, Herrero A. Inactivation of a heterocyst-specific invertase indicates a principal role of sucrose catabolism in heterocysts of Anabaena sp. J Bacteriol. 2010;192(20):5526–5533. doi: 10.1128/JB.00776-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vargas WA, Nishi CN, Giarrocco LE, Salerno GL. Differential roles of alkaline/neutral invertases in Nostoc sp. PCC 7120: Inv-B isoform is essential for diazotrophic growth. Planta. 2011;233(1):153–162. doi: 10.1007/s00425-010-1288-5. [DOI] [PubMed] [Google Scholar]
- 6.Hejazi M, et al. Isoaspartyl dipeptidase activity of plant-type asparaginases. Biochem J. 2002;364(Pt 1):129–136. doi: 10.1042/bj3640129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burnat M, Herrero A, Flores E. Compartmentalized cyanophycin metabolism in the diazotrophic filaments of a heterocyst-forming cyanobacterium. Proc Natl Acad Sci USA. 2014;111(10):3823–3828. doi: 10.1073/pnas.1318564111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flores E, Herrero A. Compartmentalized function through cell differentiation in filamentous cyanobacteria. Nat Rev Microbiol. 2010;8(1):39–50. doi: 10.1038/nrmicro2242. [DOI] [PubMed] [Google Scholar]
- 9.Mariscal V, Herrero A, Flores E. Continuous periplasm in a filamentous, heterocyst-forming cyanobacterium. Mol Microbiol. 2007;65(4):1139–1145. doi: 10.1111/j.1365-2958.2007.05856.x. [DOI] [PubMed] [Google Scholar]
- 10.Mariscal V, Flores E. Multicellularity in a heterocyst-forming cyanobacterium: Pathways for intercellular communication. Adv Exp Med Biol. 2010;675:123–135. doi: 10.1007/978-1-4419-1528-3_8. [DOI] [PubMed] [Google Scholar]
- 11.Flores E, Herrero A, Wolk CP, Maldener I. Is the periplasm continuous in filamentous multicellular cyanobacteria? Trends Microbiol. 2006;14(10):439–443. doi: 10.1016/j.tim.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Wilk L, et al. Outer membrane continuity and septosome formation between vegetative cells in the filaments of Anabaena sp. PCC 7120. Cell Microbiol. 2011;13(11):1744–1754. doi: 10.1111/j.1462-5822.2011.01655.x. [DOI] [PubMed] [Google Scholar]
- 13.Giddings TH, Staehelin LA. Plasma membrane architecture of Anabaena cylindrica: Occurrence of microplasmodesmata and changes associated with heterocyst development and the cell cycle. Eur J Cell Biol. 1978;16:235–249. [Google Scholar]
- 14.Giddings TH, Staehelin LA. Observation of microplasmodesmata in both heterocyst-forming and non-heterocyst forming filamentous cyanobacteria by freeze-fracture electron microscopy. Arch Microbiol. 1981;129(4):295–298. [Google Scholar]
- 15.Bauer CC, Buikema WJ, Black K, Haselkorn R. A short-filament mutant of Anabaena sp. strain PCC 7120 that fragments in nitrogen-deficient medium. J Bacteriol. 1995;177(6):1520–1526. doi: 10.1128/jb.177.6.1520-1526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lang NJ, Fay P. The Heterocysts of Blue-Green Algae. II. Details of Ultrastructure. Proc R Soc Lond B Biol Sci. 1971;178(1051):193–203. [Google Scholar]
- 17.Wildon D, Mercer F. The Ultrastructure of the Vegetative Cell of Blue-Green Algae. Aust J Biol Sci. 1963;16(3):585–596. [Google Scholar]
- 18.Lehner J, et al. Prokaryotic multicellularity: A nanopore array for bacterial cell communication. FASEB J. 2013;27(6):2293–2300. doi: 10.1096/fj.12-225854. [DOI] [PubMed] [Google Scholar]
- 19.Mariscal V. Cell-Cell joining proteins in heterocyst-forming cyanobacteria. In: Flores E, Herrero A, editors. The Cell Biology of Cyanobacteria. Poole, UK: Caister Academic Press; 2014. pp. 293–304. [Google Scholar]
- 20.Omairi-Nasser A, Haselkorn R, Austin J. Visualization of channels connecting cells in filamentous nitrogen-fixing cyanobacteria. FASEB J. 2014;28(7):3016–3022. doi: 10.1096/fj.14-252007. [DOI] [PubMed] [Google Scholar]
- 21.Buikema WJ, Haselkorn R. Isolation and complementation of nitrogen fixation mutants of the cyanobacterium Anabaena sp. strain PCC 7120. J Bacteriol. 1991;173(6):1879–1885. doi: 10.1128/jb.173.6.1879-1885.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nayar AS, Yamaura H, Rajagopalan R, Risser DD, Callahan SM. FraG is necessary for filament integrity and heterocyst maturation in the cyanobacterium Anabaena sp. strain PCC 7120. Microbiology. 2007;153(Pt 2):601–607. doi: 10.1099/mic.0.2006/002535-0. [DOI] [PubMed] [Google Scholar]
- 23.Merino-Puerto V, Mariscal V, Mullineaux CW, Herrero A, Flores E. Fra proteins influencing filament integrity, diazotrophy and localization of septal protein SepJ in the heterocyst-forming cyanobacterium Anabaena sp. Mol Microbiol. 2010;75(5):1159–1170. doi: 10.1111/j.1365-2958.2009.07031.x. [DOI] [PubMed] [Google Scholar]
- 24.Mullineaux CW, et al. Mechanism of intercellular molecular exchange in heterocyst-forming cyanobacteria. EMBO J. 2008;27(9):1299–1308. doi: 10.1038/emboj.2008.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merino-Puerto V, et al. FraC/FraD-dependent intercellular molecular exchange in the filaments of a heterocyst-forming cyanobacterium, Anabaena sp. Mol Microbiol. 2011;82(1):87–98. doi: 10.1111/j.1365-2958.2011.07797.x. [DOI] [PubMed] [Google Scholar]
- 26.Flores E, et al. Septum-localized protein required for filament integrity and diazotrophy in the heterocyst-forming cyanobacterium Anabaena sp. strain PCC 7120. J Bacteriol. 2007;189(10):3884–3890. doi: 10.1128/JB.00085-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mariscal V, Herrero A, Nenninger A, Mullineaux CW, Flores E. Functional dissection of the three-domain SepJ protein joining the cells in cyanobacterial trichomes. Mol Microbiol. 2011;79(4):1077–1088. doi: 10.1111/j.1365-2958.2010.07508.x. [DOI] [PubMed] [Google Scholar]
- 28.Nürnberg DJ, et al. Branching and intercellular communication in the Section V cyanobacterium Mastigocladus laminosus, a complex multicellular prokaryote. Mol Microbiol. 2014;91(5):935–949. doi: 10.1111/mmi.12506. [DOI] [PubMed] [Google Scholar]
- 29.Mitchell A, et al. The InterPro protein families database: The classification resource after 15 years. Nucleic Acids Res. 2015;43(Database issue):D213–D221. doi: 10.1093/nar/gku1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Omasits U, Ahrens CH, Müller S, Wollscheid B. Protter: Interactive protein feature visualization and integration with experimental proteomic data. Bioinformatics. 2014;30(6):884–886. doi: 10.1093/bioinformatics/btt607. [DOI] [PubMed] [Google Scholar]
- 31.Nürnberg DJ, et al. Intercellular diffusion of a fluorescent sucrose analog via the septal junctions in a filamentous cyanobacterium. MBio. 2015;6(2):e02109–e02114. doi: 10.1128/mBio.02109-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hermann R, Walther P, Müller M. Immunogold labeling in scanning electron microscopy. Histochem Cell Biol. 1996;106(1):31–39. doi: 10.1007/BF02473200. [DOI] [PubMed] [Google Scholar]
- 33.Drew D, et al. Rapid topology mapping of Escherichia coli inner-membrane proteins by prediction and PhoA/GFP fusion analysis. Proc Natl Acad Sci USA. 2002;99(5):2690–2695. doi: 10.1073/pnas.052018199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramos-León F, Mariscal V, Frías JE, Flores E, Herrero A. Divisome-dependent subcellular localization of cell-cell joining protein SepJ in the filamentous cyanobacterium Anabaena. Mol Microbiol. 2015;96(3):566–580. doi: 10.1111/mmi.12956. [DOI] [PubMed] [Google Scholar]
- 35.Ughy B, Ajlani G. Phycobilisome rod mutants in Synechocystis sp. strain PCC6803. Microbiology. 2004;150(Pt 12):4147–4156. doi: 10.1099/mic.0.27498-0. [DOI] [PubMed] [Google Scholar]
- 36.Elhai J, Vepritskiy A, Muro-Pastor AM, Flores E, Wolk CP. Reduction of conjugal transfer efficiency by three restriction activities of Anabaena sp. strain PCC 7120. J Bacteriol. 1997;179(6):1998–2005. doi: 10.1128/jb.179.6.1998-2005.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang L-C, Risoul V, Latifi A, Christie JM, Zhang C-C. Exploring the size limit of protein diffusion through the periplasm in cyanobacterium Anabaena sp. PCC 7120 using the 13 kDa iLOV fluorescent protein. Res Microbiol. 2013;164(7):710–717. doi: 10.1016/j.resmic.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 38.Austin JR., 2nd . In: High-Pressure Freezing and Freeze Substitution of Arabidopsis for Electron Microscopy. Arabidopsis Protocols, Methods in Molecular Biology. Sanchez-Serrano JJ, Salinas J, editors. Humana; Totowa, NJ: 2014. pp. 473–486. [DOI] [PubMed] [Google Scholar]
- 39.Otegui MS, Mastronarde DN, Kang B-H, Bednarek SY, Staehelin LA. Three-dimensional analysis of syncytial-type cell plates during endosperm cellularization visualized by high resolution electron tomography. Plant Cell. 2001;13(9):2033–2051. doi: 10.1105/TPC.010150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kremer JR, Mastronarde DN, McIntosh JR. Computer visualization of three-dimensional image data using IMOD. J Struct Biol. 1996;116(1):71–76. doi: 10.1006/jsbi.1996.0013. [DOI] [PubMed] [Google Scholar]
- 41.Omairi-Nasser A, de Gracia AG, Ajlani G. A larger transcript is required for the synthesis of the smaller isoform of ferredoxin:NADP oxidoreductase. Mol Microbiol. 2011;81(5):1178–1189. doi: 10.1111/j.1365-2958.2011.07739.x. [DOI] [PubMed] [Google Scholar]