Significance
During transcription, RNA polymerase encounters DNA-encoded signals that interrupt RNA synthesis and cause the transcription elongation complex to pause. An important function of transcription pausing in gene regulation is the halting of transcription to facilitate assembly of regulatory factors into complex with RNA polymerase. An example of this function is found in the bacteriophage λ late-gene transcription, in which the initiation factor σ70 induces a pause that mediates recruitment of the antitermination factor Qλ, thereby allowing the expression of genes downstream of an intrinsic terminator. In this study, we characterize the contributions of two pause signals to σ70-dependent pause kinetics and propose a comprehensive model for σ70-dependent pausing.
Keywords: transcription pausing, σ-dependent pausing, elemental pause, factor-dependent pausing, transcription
Abstract
The movement of RNA polymerase (RNAP) during transcription elongation is modulated by DNA-encoded elements that cause the elongation complex to pause. One of the best-characterized pause sequences is a binding site for the σ70 initiation factor that induces pausing at a site near lambdoid phage late-gene promoters. An essential component of this σ70-dependent pause is the elemental pause site (EPS), a sequence that itself induces transcription pausing throughout the Escherichia coli genome and underlies other complex regulatory pause elements, such as the ops and his operon pauses. Here, we identify and provide a detailed kinetic analysis of a transcription cycle analogous to abortive cycling that underlies the σ70-dependent pause. We show that, in σ70-dependent pausing, the elemental pause acts primarily to modulate the rate at which complexes attempt to disengage the σ70:DNA interaction. Our findings establish the σ70-dependent pause-encoding region as a multipartite element in which several pause-inducing components make distinct mechanistic contributions to the induction and maintenance of a regulatory transcription pause.
Transcription pausing is an important element of gene regulation at the level of RNA synthesis in both prokaryotes and eukaryotes, serving to coordinate the appearance of RNA with its utilization in cellular functions, and to modulate the interaction of regulatory proteins with RNA polymerase (RNAP) (1, 2). The mechanisms by which nucleic acid sequence elements and transcription factors induce transcription pauses are varied (3–6) but are unified in that they must retain the transcription elongation complex (TEC) at a defined position on the DNA template for a period. A notable example of regulatory transcription pausing exists in the Escherichia coli lambdoid phage late-gene operon, where expression of the controlled genes depends upon a σ70-dependent pause that allows engagement of the antiterminator Q to RNAP (7); the terminator-resistant TEC can then “read through” an intrinsic terminator that precedes the coding sequence of the operon.
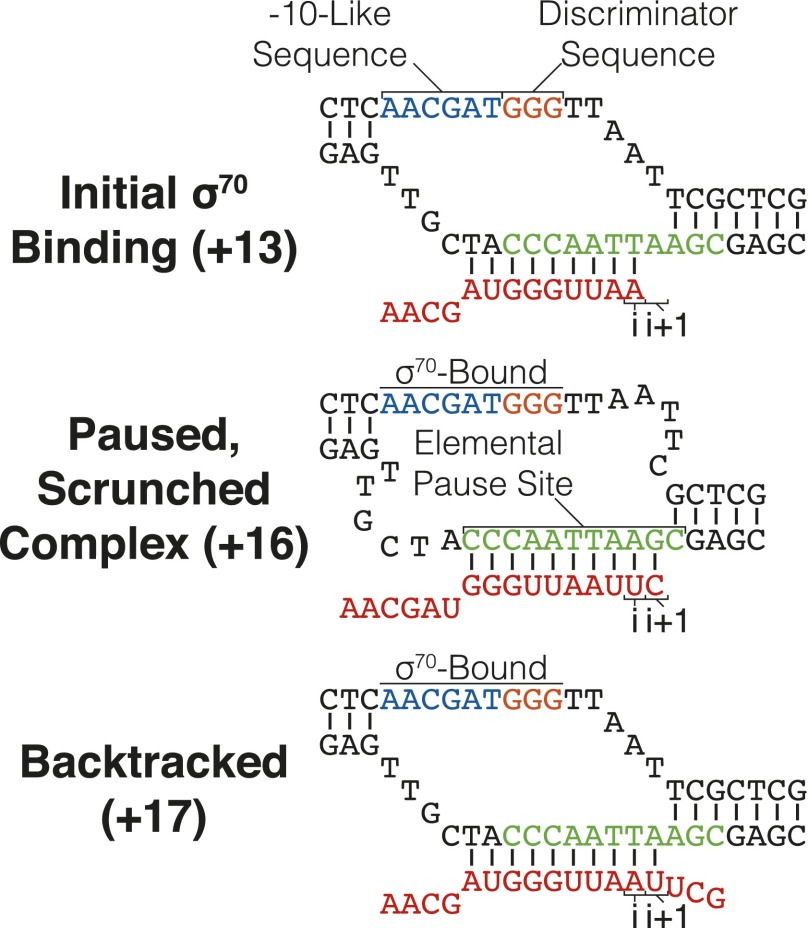
The transcription pause that facilitates assembly of Q into complex with RNAP is dependent on binding of the σ70 initiation factor to a repeat of the −10 promoter element, named the “−10-like sequence” (Fig. 1) (3). The anchoring of RNAP to the DNA site is followed by further RNA synthesis, as downstream DNA is unwound and drawn into RNAP to form a “scrunched” DNA structure that is exactly analogous to the scrunched initial transcribing complex from the promoter (Fig. 1) (8–11). Consistent with its structural similarities to an initial transcribing complex, the σ70-dependent pause comprises a transcription cycle analogous to abortive cycling (10, 12). From the stable pause (at +16 for λpR′), continuation one position further results in either escape downstream and release of σ70, or backtracking that is rescued by Gre-mediated cleavage into a second cycle of synthesis and pausing (Fig. 1) (10, 13). In fact, backtracking is a central component of the pause because it retains RNAP in the pause region.
Fig. 1.
Architecture of σ70-dependent paused TECs. As the TEC reaches +13, σ70-dependent pausing is induced through contacts between the nontemplate strand −10-like and discriminator sequences by σ70 regions 2 and 1.2, respectively. The +16 paused, scrunched complex is formed when the −10-like sequence-bound TEC continues to synthesize RNA by DNA scrunching. The TEC encounters an EPS at +16 and can enter into a stable, paused state. Upon synthesis to +17, if RNAP fails to break the σ70:DNA interaction, the TEC backtracks into an arrested state in which σ70 remains bound to the −10-like sequence and the RNA 3′-OH is displaced downstream from the active center.
A second pause-inducing sequence, located immediately downstream of the −10-like sequence, is essential to the σ70-dependent pause (Fig. 1) (14). Although this segment was first described as a backtrack-inducing sequence, a property it does display, it primarily acts as an elemental pause site (EPS) (15–19). The EPS is a pause-inducing sequence that was identified through global analysis of RNAP distribution within the E. coli genome, and which functions by halting the nucleotide addition cycle so that further elongation cannot occur (17, 18). The EPS may include, or lead to, further conformational rearrangements that contribute to the pause, especially an opening of the clamp that encloses the nucleic acids (20–22).
The most important elements of the EPS are a GC-rich segment at the upstream edge of the RNA:DNA hybrid and a pyrimidine at the pause site followed immediately by a G. These elements interact with various structures of the enzyme, especially a “core recognition element” that is proposed to counteract pausing by favoring translocation (18). The existence of two discrete pause-inducing sequences within the σ70-dependent pause-encoding region raises the question of how each element contributes to σ70-dependent pause kinetics.
We have characterized the function of the elemental pause in σ70-dependent pausing by relating the effects of a −10-like sequence consensus mutation and a series of EPS mutations to σ70-dependent pause cycle kinetics. We have analyzed three EPS determinants as follows: (i) the pause site nucleotide; (ii) the GC-rich upstream edge of the RNA:DNA hybrid; and (iii) the internal segment of the RNA:DNA hybrid. We find that the major function of the EPS in the context of σ70-dependent pausing is to slow the pause cycle by limiting the rate at which TECs transition from +16 to +17 and thus attempt to disengage the −10-like sequence. In our model, the −10-like sequence is responsible for inducing the initial capture of RNAP, whereas the EPS determines the frequency at which an escape attempt can be made. Both the strength of σ70 binding to the −10-like sequence and the EPS influence the probability of pause escape. Our study of the λpR′ elemental pause has led to novel mechanistic insights into the nature of σ70-dependent pausing as a transcription pause that uses, first, a cycling effect analogous to that of transcription initiation and, second, a universal pause sequence, to establish a long-lived regulatory pause.
Results
Analysis of the σ70-Dependent Pause Cycle.
To analyze σ70-dependent pausing in vitro, we performed single-round transcription reactions in the presence and absence of the transcript cleavage factor GreB. Inclusion of GreB approximates the in vivo pause cycle because TECs that have backtracked upon failing to disengage the −10-like sequence can reactivate transcription by transcript cleavage and make subsequent escape attempts. Omission of GreB reveals the kinetic properties of individual steps in the pause cycle by showing the change in distribution of paused, backtracked, and escaped complexes in a single cycle. The measurements we make across these two conditions provide a detailed understanding of σ70-dependent pause cycle kinetics and lead to a comprehensive model of σ70-dependent pausing. We note that, as is usual in similar experiments, the kinetic rates we obtain are not purported to be identical to those in vivo, primarily because substrate nucleoside triphosphate concentrations are arbitrary; relative values are, however, meaningful for understanding the pause mechanism.
In the text below, the term “σ70-dependent pause” refers to the σ70-dependent pause cycle as a whole, from the initial binding of σ70 at the −10-like sequence to the release of RNAP into elongation downstream; it is characterized by the terms PrInduction (pause induction, the percentage of complexes that have detectably engaged in the σ70-dependent pause cycle) and t1/2[σP] (overall pause half-life). The term “+16 pause” refers to the transit from +16 to +17 within the λpR′ σ70-dependent pause cycle; it is characterized by the terms CE[16P] (capture efficiency, the percentage of complexes that enter an elemental paused state at +16) and t1/2[16P] (+16 pause half-life). PrEscape is the percentage of complexes that escape the σ70:DNA interaction in a single cycle.
The −10-Like Sequence Modulates σ70-Dependent Pause Kinetics by Influencing the Probability of Pause Escape.
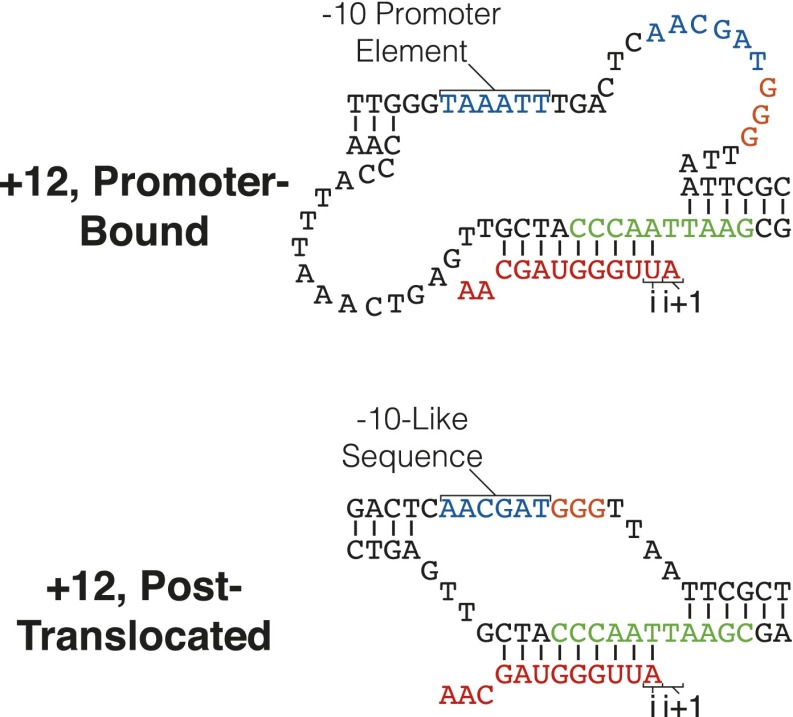
We previously used a λpR′ consensus −10-like sequence mutant (TATAAT substituted for AACGAT) to study the dynamics of σ70-dependent pause escape in the presence of the Qλ antiterminator (Table S1) (11). Below, we use the same consensus variant to examine the kinetic consequences of strengthening the interaction between σ70 and the −10-like sequence. However, the consensus substitution has a second useful property: it lacks a putative elemental pause at +12 that delays transcription from the open complex to +16. Evidence for this pause is a prominent abortive product at +12 from the WT template (Figs. S1, S2A, and S3A) (23). The +12 pause very likely causes asynchrony in further elongation; thus, kinetic analysis with the consensus template benefits from higher synchrony.
Table S1.
Table of mutants used in this study
| Template name | Sequence from +1 to +17 |
| λpR′ | AACGATGGGTTAATTCG |
| ConsλpR′ | TATAATGGGTTAATTCG |
| Cons16G | TATAATGGGTTAATTGG |
| Cons16U | TATAATGGGTTAATTTG |
| Cons16A | TATAATGGGTTAATTAG |
| ConsUE | Nontemplate strand: TATAATGGGTTAATTCG Template strand: ATATTATTCAATTAAGC |
| λpR′IE[CG] | AACGATGGGCCGGCCCG |
| ConsIE[CG] | TATAATGGGCCGGCCCG |
| 3X10 | AACGATGGGTTAGCCCG |
| 3X11 | AACGATGGGCTAACCCG |
| 3X12 | AACGATGGGCCAATCCG |
| 3X13 | AACGATGGGCCGATTCG |
| 2X10 | AACGATGGGTTGGCCCG |
| 2X11 | AACGATGGGCTAGCCCG |
| 2X12 | AACGATGGGCCAACCCG |
| 2X13 | AACGATGGGCCGATCCG |
| 2X14 | AACGATGGGCCGGTTCG |
| 1X10 | AACGATGGGTCGGCCCG |
| 1X11 | AACGATGGGCTGGCCCG |
| 1X12 | AACGATGGGCCAGCCCG |
| 1X13 | AACGATGGGCCGACCCG |
| 1X14 | AACGATGGGCCGGTCCG |
| 1X15 | AACGATGGGCCGGCTCG |
| 12GGC | AACGATGGGTTGGCTCG |
| Cons3x12 | TATAATGGGCCAATCCG |
| Cons2x13 | TATAATGGGCCGATCCG |
| Cons1x14 | TATAATGGGCCGGTCCG |
The sequence of positions +1 to +17 for all λpR′ mutants used in this study is shown. Mutations are underlined.
Fig. S1.
Origin of +12 paused and abortive RNAs. The +12 RNA species from λpR′ is known to be not only abortive (i.e., released), when it is made ab initio from the open complex, but also to be a stable TEC component when it is derived through Gre-mediated (or endogenous) cleavage from longer transcripts (23). The abortive precursor of released +12 RNA is scrunched from the promoter and presumably paused due to an EPS, with its 3′ end in the i + 1 site; the cleavage-derived +12 RNA originates from a σ70-dependent paused complex in which the scrunch is relaxed, and its 3′ end would be in the i site because cleavage was catalyzed by the active site.
Fig. S2.
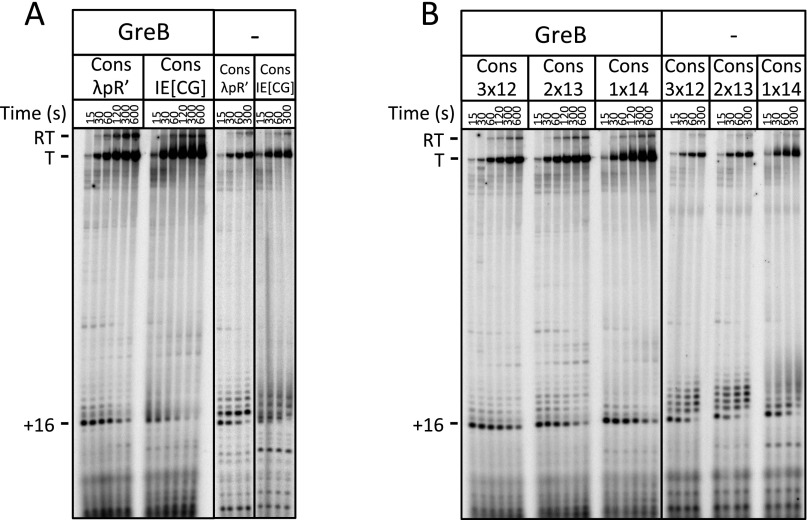
The pause site nucleotide and GC-rich upstream hybrid edge modulate σ70-dependent pause kinetics. (A) Gel image showing single-round in vitro transcription experiments at ConsλpR′, Cons16G, Cons16U, and Cons16A in the presence and absence of GreB. Readthrough (RT), terminated (T), and +16 complexes are shown. Note the absence of abortive transcripts, particularly at +12, in the −GreB reactions (compare with Fig. S3A). The + and − GreB experiments were performed separately. (B) Gel image showing single-round in vitro transcription experiments at ConsλpR′ and ConsUE in the presence and absence of GreB. Readthrough (RT), terminated (T), and +16 complexes are shown. The + and − GreB experiments were performed separately.
Fig. S3.
λpR′IE[CG] mutant scan. (A) Gel image showing single-round in vitro transcription experiments at λpR′ and λpR′IE[CG] in the presence and absence of GreB. Readthrough (RT), terminated (T), +16, and +12 complexes are shown. Note the presence of abortive transcripts, particularly at +12, in the −GreB reactions (compare with Fig. S2A). (B) Graph showing the percent total σ70-dependent pause at 15 s for all restoring scan mutants. (C) Gel images showing single-round in vitro transcription experiments for all restoring scan mutants. Readthrough (RT), terminated (T), and paused complexes (+14, +15, or +16) are shown.
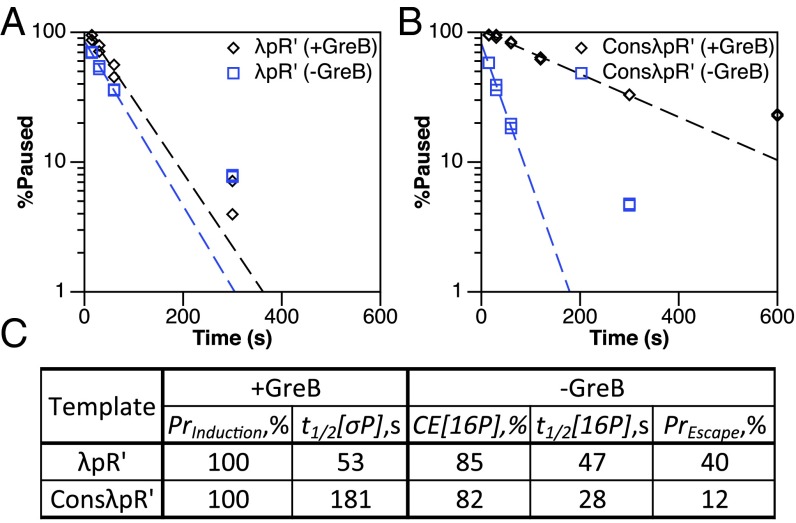
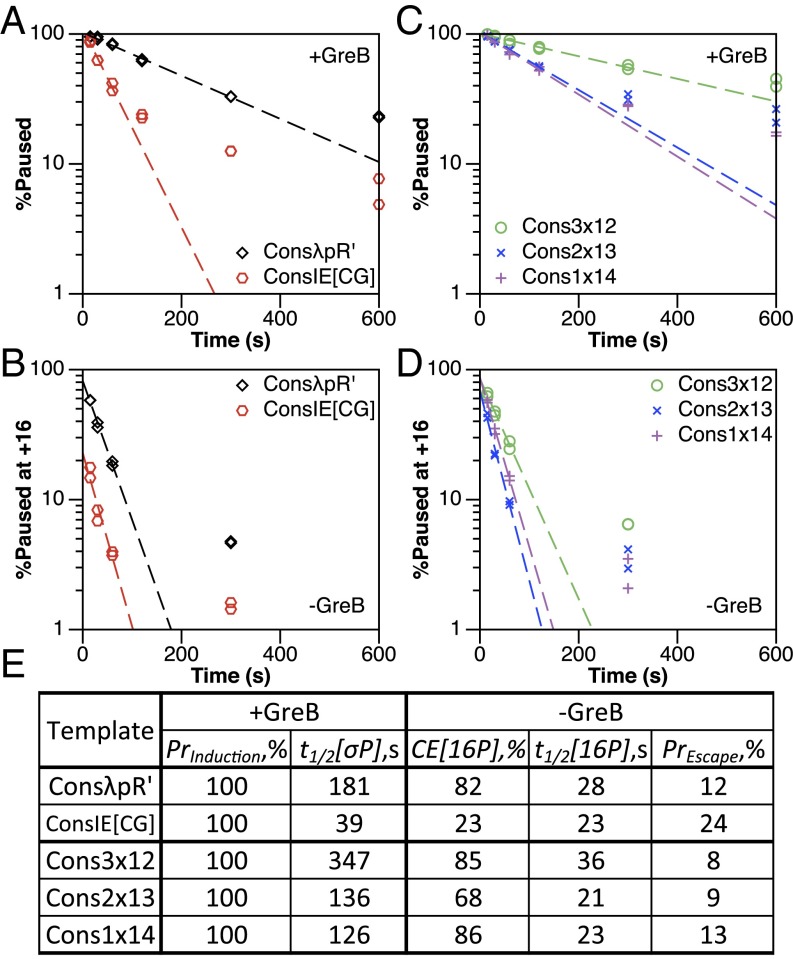
The σ70-dependent pause cycle is evident in the pause kinetics at λpR′ compared with pause kinetics at ConsλpR′. In the presence of GreB, pause induction (PrInduction) is 100% for both templates, whereas overall pause half-life (t1/2[σP]) at λpR′ (53 s) is increased at ConsλpR′ (181 s) (Fig. 2). In the absence of GreB, the probability of escape (PrEscape) is reduced by ∼3.5-fold at ConsλpR′, a change that drives the increase in overall pause half-life by increasing the number of cycles necessary for −10-like sequence-bound TECs to break the σ70:DNA interaction (Fig. 2C). The +16 pause half-life (t1/2[16P]) at λpR′ (47 s) appears reduced at ConsλpR′ (28 s); however, we suggest that this is due to an artificial increase in +16 pause half-life at λpR′ resulting from the asynchrony described above. We argue below that the actual λpR′ +16 pause half-life should be close to that of ConsλpR′; in fact, it is implausible that the −10-like sequence should affect the rate of movement of the TEC from +16 to +17.
Fig. 2.
Strengthening the σ70:DNA interaction increases σ70-dependent pause cycling. (A and B) Single-round in vitro transcription experiments assessing σ70-dependent pause kinetics at λpR′ (A) and ConsλpR′ (B) in the presence and absence of GreB. Scatter plots show the decay of total σ70-dependent paused TECs (+GreB) and +16 paused TECs (−GreB) over time. Dashed trend lines are generated using the average of two replicates, the values of which are shown as points. ConsλpR′ plots are composed of data also shown in Figs. 3A and 4A (+GreB) and Figs. 3B and 4B (−GreB), and are derived from the experiments of Figs. S2A and S4A. (C) Measured values from A and B. PrInduction and t1/2[σP] are total σ70-dependent pause induction and half-life, respectively. CE[16P] and t1/2[16P] are +16 elemental pause capture efficiency and half-life, respectively. PrEscape is the probability of −10-like sequence disengagement following a single escape attempt.
The increase in overall pause half-life at ConsλpR′ relative to λpR′ in the presence of GreB shows the importance of escape probability in σ70-dependent pause kinetics and exposes the pause cycle clearly. Furthermore, the increased synchrony of initiation at ConsλpR′ provides us with a system in which to study σ70-dependent pause cycle kinetics without removing the pause from its natural promoter context.
The Pause Site Nucleotide Modulates σ70-Dependent Pause Kinetics.
Consistent with the proposal that downstream portions of the σ70-dependent pause constitute an EPS, both the elemental pause and the σ70-dependent pause are favored by U or C at the pause site and are disfavored by A or G (16, 19). The pause site nucleotide (+16 at λpR′) should not influence the initial σ70-binding event because, at the time, the RNAP active center is positioned several nucleotides upstream of the eventual pause site (Fig. 1). However, the terminal nucleotide could affect the kinetic properties of the +16 elemental pause.
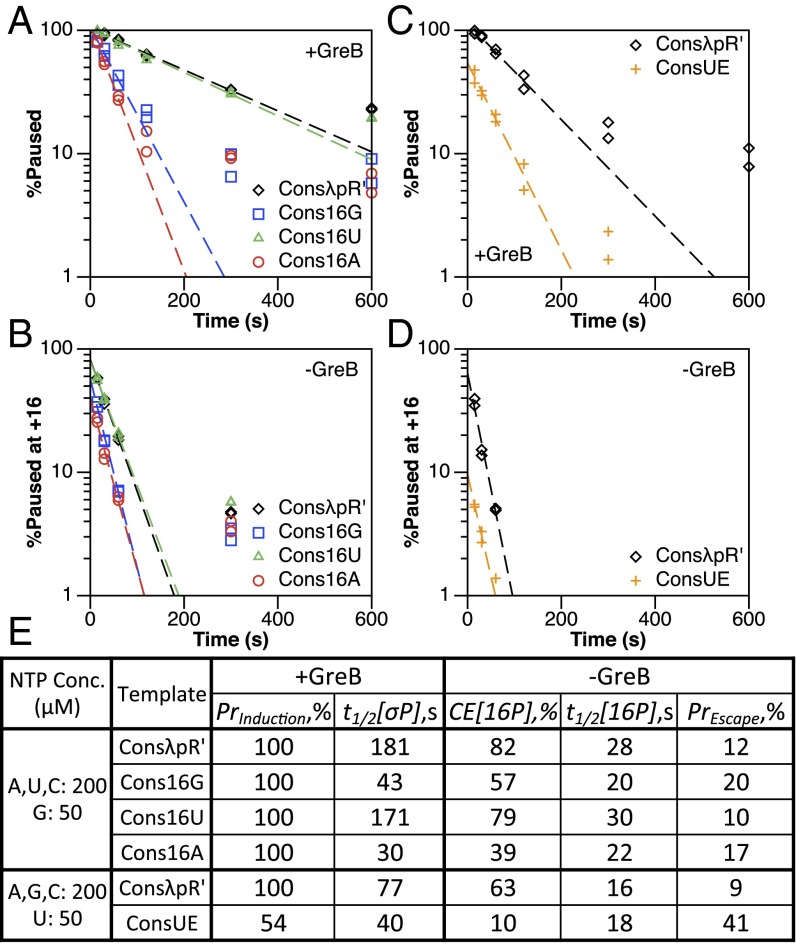
In the presence of GreB, pause induction (PrInduction) is 100% for all +16 nucleotide variants of ConsλpR′ (Table S1), whereas the overall pause half-life (t1/2[σP]) is reduced when the native +16C (181 s) is mutated to G (43 s) or A (30 s), but is almost unchanged when mutated to T (171 s) (Fig. 3 A and E, and Fig. S2A). This indicates that the +16 nucleotide does not contribute to pause induction but does affect the rate of −10-like sequence disengagement. In the absence of GreB, +16 pause capture efficiency (CE[16P]) at ConsλpR′ is reduced when the native +16C (82%) is mutated to G (57%) or A (39%), but is almost unchanged when mutated to T (79%) (Fig. 3 B and E, and Fig. S2A). The +16 pause half-life (t1/2[16P]) at ConsλpR′ is longer for the native +16C (28 s) and the T (30 s) mutant than for the G (20 s) and A (22 s) mutants (Fig. 3 B and E). Furthermore, the probability of escape (PrEscape) is increased for G (20%) and A (17%) mutants relative to the native +16C (12%) and the T (10%) mutant (Fig. 3E). The consistency of mutant effects in the presence and absence of GreB suggests a direct link between the kinetic properties of the +16 elemental pause and the rate at which TECs traverse the σ70-dependent pause cycle. We interpret the short overall pause half-life at Cons16G and Cons16A to result from an increase in the frequency at which −10-like sequence-bound TECs attempt to break the σ70:DNA interaction and an increased probability of success on each attempt.
Fig. 3.
The pause site nucleotide and upstream RNA:DNA hybrid edge modulate σ70-dependent pause kinetics. (A and B) Single-round in vitro transcription experiments assessing the role of the pause site nucleotide (+16) in σ70-dependent pause kinetics (A) and +16 pause kinetics (B). Dashed trend lines are generated using the average of two replicates, the values of which are shown as points. ConsλpR′ plots are composed of data also shown in Figs. 2B (±GreB), 4A (+GreB), and 4B (−GreB), and are derived from the experiments of Figs. S2A and S4A. (C and D) Single-round in vitro transcription experiments assessing the role of the GC-rich upstream RNA:DNA hybrid edge (template strand positions +7 and +8) in σ70-dependent pause kinetics (C) and +16 pause kinetics (D). Dashed trend lines are generated using the average of two replicates, the values of which are shown as points. (E) Measured values from A–D. PrInduction and t1/2[σP] are total σ70-dependent pause induction and half-life, respectively. CE[16P] and t1/2[16P] are +16 elemental pause capture efficiency and half-life, respectively. PrEscape is the probability of −10-like sequence disengagement following a single escape attempt.
The Upstream RNA:DNA Hybrid Edge Modulates σ70-Dependent Pause Kinetics.
Early studies of the λpR′ σ70-dependent pause identified two positions immediately downstream of the −10-like sequence that, when mutated from G to A, reduce the level of pausing through both the nontemplate and template DNA strands (24). The nontemplate strand effect of this sequence is the result of an interaction with σ70 region 1.2 that supplements the binding of σ70 to the −10-like sequence (Fig. 1) (25, 26). The template strand element is part of an EPS at the 82pR′ and λpR′ σ70-dependent pauses that also determines the site of the pause (Fig. 1) (19). Here, we consider the mechanism through which the GC-rich upstream RNA:DNA hybrid edge influences σ70-dependent pause cycle kinetics.
To characterize the contribution of the upstream RNA:DNA hybrid edge to σ70-dependent pausing, we made a ConsλpR′ heteroduplex mutant, named ConsUE, that contains C-to-T mutations at positions +7 and +8 in the template strand, but maintains the WT sequence in the nontemplate strand to preserve σ70 region 1.2 binding (Table S1). To facilitate labeling of the RNA, the experiments of Fig. 3 C and D were performed using 4× lower [UTP] and 4× higher [GTP] than those of Fig. 3 A and B; because G is the next nucleotide incorporated following the +16 pause, the overall pause half-life at ConsλpR′ is reduced from 181 to 77 s, as has been observed for other transcription pauses (27). This does not affect the comparisons made.
In the presence of GreB, pause induction (PrInduction) and overall pause half-life (t1/2[σP]) at ConsλpR′ (100% and 77 s) are reduced at ConsUE (54% and 40 s) (Fig. 3 C and E, and Fig. S2B). Because the ConsUE mutations do not interfere with σ70:DNA binding, decreased pause induction likely reflects complexes that bind the −10-like sequence but disengage rapidly so as not to be detectably paused. In the absence of GreB, +16 pause capture efficiency (CE[16P]) at ConsλpR′ (63%) was reduced at ConsUE (10%), whereas +16 pause half-life (t1/2[16P]) was comparable (16 and 18 s, respectively) (Fig. 3 D and E, and Fig. S2B). The probability of escape (PrEscape) was increased at ConsUE (41%) relative to ConsλpR′ (9%) (Fig. 3E). Notably, in contrast to most complexes we describe, ConsUE TECs at +17 appear to chase by 300 s, suggesting that these complexes exist in equilibrium between a GreB-sensitive backtracked state and transcription-competent posttranslocated state (Fig. S2B). Because this effect is specific to +17 complexes (TECs from +18 to +24 appear stably arrested), it is unlikely that the intrinsic cleavage activity of RNAP contributes meaningfully to the decay at +17. Our findings are consistent with the proposal that the elemental pause is stabilized by a GC-rich upstream RNA:DNA hybrid edge (17, 18). An AT-rich upstream hybrid edge increases the rate at which TECs traverse the σ70-dependent pause cycle by promoting unimpeded transit from +16 to +17. Furthermore, the favorability of restoring strong base pairs at the upstream hybrid edge promotes backtracking at +17, as in WT, whereas weak base pairs at these positions increase the probability of escape by reducing the propensity for TECs to backtrack.
The λpR′ +10 to +15 Region Modulates σ70-Dependent Pause Kinetics.
The composition of the RNA:DNA hybrid has been shown to influence the rate of translocation by RNAP and the strength of factor-independent transcription pausing (6). Previous work indicated that the internal sequence of the EPS downstream of the G/C rich element is an important determinant of σ70-dependent pausing at 82pR′ (14), but did not distinguish the separate role of the terminal nucleotide. Here, we replaced the λpR′ native sequence from +10 to +15, 5′-TTAATT-3′, with the sequence 5′-CCGGCC-3′ to create the mutant λpR′IE[CG] (Table S1). Consistent with previous results, σ70-dependent pausing at λpR′IE[CG] was dramatically reduced both in the presence and absence of GreB (Fig. S3A).
To further characterize the RNA:DNA hybrid internal segment, we made a −10-like sequence consensus variant of λpR′IE[CG] (Table S1). In the presence of GreB, pause induction (PrInduction) is 100% for both ConsλpR′ and ConsIE[CG], whereas overall pause half-life (t1/2[σP]) at ConsλpR′ (181 s) is reduced at ConsIE[CG] (39 s) (Fig. 4 A and E, and Fig. S4A). In the absence of GreB, +16 pause capture efficiency (CE[16P]) at ConsλpR′ (82%) is reduced at ConsIE[CG] (23%), whereas +16 pause half-life (t1/2[16P]) is modestly reduced (28 and 23 s, respectively) (Fig. 4 B and E, and Fig. S4A). The probability of escape (PrEscape) is increased at ConsIE[CG] (24%) relative to ConsλpR′ (12%) (Fig. 4E). As for ConsUE, TECs that have moved to +17 at ConsIE[CG] are GreB sensitive while also chasing by 300 s, suggesting that these can isomerize between the backtracked and posttranslocated states (Fig. S4A). This may result from the GC-rich RNA:DNA hybrid stabilizing the forward position of the active center relative to the backtracked complex. Our results indicate that the internal RNA:DNA hybrid is a determinant of elemental pausing and further emphasize the influence of the +16 pause on overall σ70-dependent pause kinetics.
Fig. 4.
The RNA:DNA hybrid internal segment modulates σ70-dependent pause kinetics. (A and B) Single-round in vitro transcription experiments assessing the role of the RNA:DNA hybrid internal segment (+10 to +15) in σ70-dependent pause kinetics (A) and +16 pause kinetics (B). Dashed trend lines are generated using the average of two replicates, the values of which are shown as points. ConsλpR′ plots are composed of data also shown in Figs. 2B (±GreB), 3A (+GreB), and 3B (−GreB), and are derived from the experiments of Figs. S2A and S4A. (C and D) Single-round in vitro transcription experiments assessing σ70-dependent pause kinetics (C) and +16 pause kinetics (D) for restoring scan mutants. Dashed trend lines are generated using the average of two replicates, the values of which are shown as points. (E) Measured values from A–D. PrInduction and t1/2[σP] are total σ70-dependent pause induction and half-life, respectively. CE[16P] and t1/2[16P] are +16 elemental pause capture efficiency and half-life, respectively. PrEscape is the probability of −10-like sequence disengagement following a single escape attempt.
Fig. S4.
The RNA:DNA hybrid internal segment modulates σ70-dependent pause kinetics. (A) Gel image showing single-round in vitro transcription experiments at ConsλpR′ and ConsIE[CG] in the presence and absence of GreB. Readthrough (RT), terminated (T), and +16 complexes are shown. In the absence of GreB, the ConsIE[CG] pause pattern is fuzzy, presumably due to structures formed by high localized GC content, but individual bands can be resolved. The −GreB reactions were performed as a single experiment, however, the gel has been spliced to remove an intervening +GreB reaction. The + and − GreB experiments were performed separately. (B) Gel image showing single-round in vitro transcription experiments at Cons3x12, Cons2x13, and Cons1x14 in the presence and absence of GreB. Readthrough (RT), terminated (T), and +16 complexes are shown. The + and − GreB experiments were performed separately.
A Detailed Mutant Scan of the λpR′ +10 to +15 Region.
To identify elements within the GC-rich segment of λpR′IE[CG] that interfere with σ70-dependent pausing, we performed a scan comprising 3-, 2-, and 1-bp mutations in which λpR′IE[CG] sequence was restored to WT (Table S1 and Fig. S3 B and C). These templates are named in the format nxp, where n refers to the number of bases restored and p refers to the position of the first restored base. The mutants with the greatest increase in pausing for each category (3x12, 2x13, and 1x14) are clustered within a 3-bp stretch of the +10 to +15 segment, from +12 to +14 (Fig. S3B and Table S1). To determine whether the +12 to +14 segment is sufficient to produce the full effect of λpR′IE[CG], we mutated λpR′ such that positions +12 to +14 are replaced with the corresponding λpR′IE[CG] sequence (Table S1; 12GGC). Consistent with the observation that 2- and 3-bp substitutions outside the +12 to +14 segment can also significantly affect pausing at λpR′IE[CG], the 12GGC mutation reduced pausing but failed to reproduce the full effect of λpR′IE[CG] (Fig. S3B).
We then tested consensus variants of 3x12, 2x13, and 1x14 to determine whether changes in the kinetic properties of the +16 pause account for improved overall σ70-dependent pausing (Table S1). In the presence of GreB, pause induction (PrInduction) is 100% for all mutants, and overall pause half-life (t1/2[σP]) at Cons3x12 (347 s), Cons2x13 (136 s), and Cons1x14 (126 s), is increased relative to ConsIE[CG] (39 s) (Fig. 4 C and E, and Fig. S4B). In fact, the Cons3x12 overall pause half-life of 347 s is nearly twice that of ConsλpR′. By measurements in the absence of GreB, we find that the overall pause half-life for Cons3x12, Cons2x13, and Cons1x14 correlates with increased +16 pause capture efficiency (CE[16P]; 85%, 68%, and 86%, respectively), decreased probability of escape (PrEscape; 8%, 9%, and 13%, respectively), and, for Cons3x12, increased +16 pause half-life (t1/2[16P]; 36 s) (Fig. 4 D and E, and Fig. S4B).
The restoring scan yielded a second class of four mutants (1x11, 1x12, 2x11, and 3x10) that appear to backtrack at positions upstream (+14, +15) of the native pause (+16) as shown by the persistence of arrested TECs to at least 300 s in the absence of GreB (Fig. S3C). These mutants are related in that they restore positions +11 and +12 to WT λpR′ sequence and may result from the formation of a sequence that promotes the rapid entry of −10-like sequence-bound complexes into a backtracked state.
Analysis of σ70-Dependent Pause Kinetics.
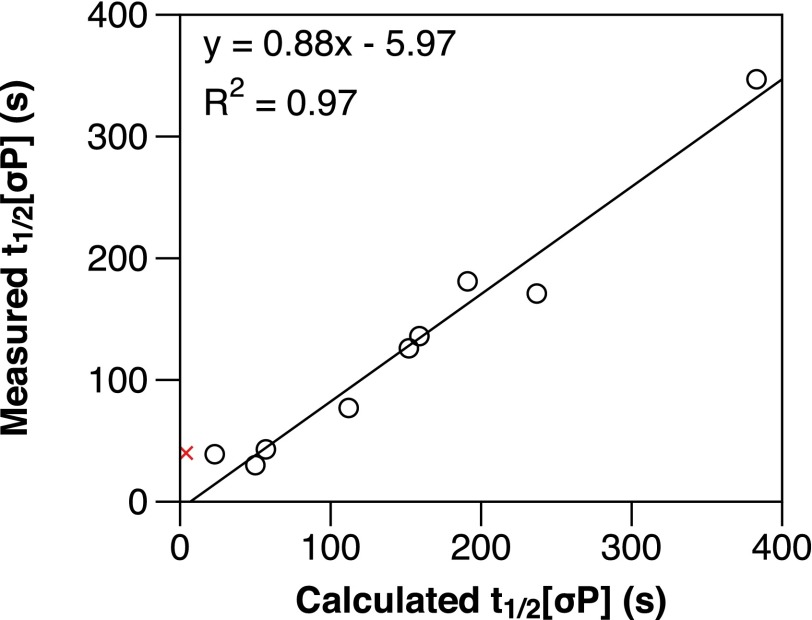
In Fig. 5, we plot measured σ70-dependent pause half-lives against predicted σ70-dependent pause half-lives that were calculated by applying the +16 pause measurements t1/2[16P], CE[16P], and PrEscape to the following equation:
| [1] |
In Eq. 1, we assume that backtracking, transcript cleavage, and resynthesis to +16 are instantaneous relative to the elemental pause half-life. Therefore, kσPause should be dependent on the properties of the +16 elemental pause (k+16Pause and Pr+16Capture) and the probability that a TEC will disengage the −10-like sequence (PrEscape). Eq. 1 is a good predictor of the measured σ70-dependent pause half-life for 9 of the 10 templates tested (Fig. 5; black circles). The one exception, ConsUE (Fig. 5; red X), displayed a decreased tendency to backtrack, indicating that the ConsUE pause cycle may also depend on the rate of backtracking and transcript cleavage. (This might be true as well for ConsIE[CG], although to a lesser extent.) Nonetheless, the strong correlation between measured and calculated overall σ70-dependent pause half-lives suggests that the +16 elemental pause and the probability of −10-like sequence disengagement are the primary determinants of pause cycle kinetics.
Fig. 5.
Plot of measured vs. calculated t1/2[σP] values. Measured t1/2[σP] values taken in the presence of GreB from Figs. 3 A and C, and 4 A and C, are plotted against calculated t1/2[σP] values that were obtained by applying the measurements taken in the absence of GreB from Figs. 3 B, D, and E, and 4 B, D, and E, to Eq. 1. The trend line equation and R2 value are shown. Points plotted as black circles are included in the trend line. The ConsUE value is shown as a red X and is not included in the trend line. For reasons described in the text, WT λpR′ is not plotted.
Consistent with our proposal that asynchrony of initiation obscured the +16 pause half-life at λpR′, the application of λpR′ −GreB measurements to Eq. 1 produces a calculated overall half-life of 100 s, nearly twice the measured value (53 s). Because the strength of the interaction between σ70 and the −10-like sequence should not interfere with +16 pause kinetics, the +16 elemental pause should behave identically at both λpR′ and ConsλpR′ and only the probability of escape should differ. Therefore, we should be able to use the PrEscape measurement from λpR′ and the Pr+16Capture and k+16Pause measurements from ConsλpR′ to approximate t1/2[σP] at λpR′. This calculation produces a predicted λpR′ t1/2[σP] of 57 s, which approximates the measured t1/2[σP] of 53 s and therefore further validates Eq. 1 as a description of σ70-dependent pause kinetics. Furthermore, if the correct t1/2[16P] for WT is actually ∼28 s, whereas t1/2[σP] is 53 s, Gre-mediated cleavage and cycling contribute importantly to the strength of the WT pause.
Discussion
We have characterized the kinetics of a transcription cycle that underlies the σ70-dependent pause of the lambdoid bacteriophage late-gene regulatory region. The pause depends upon σ70:DNA binding and a widespread transcription pause sequence, the EPS, which stabilizes the TEC in an off-pathway paused and scrunched state. The elemental pause prevents the TEC from advancing to the next nucleotide or beyond, which would create an unstable state leading to either disengagement of σ70 and escape from the pause, or backtracking to relieve the scrunched conformation. In effect, the elemental pause limits the rate at which TECs attempt to disengage the σ70:DNA interaction that anchors RNAP to the −10-like sequence; furthermore, the elements of the EPS favor backtracking and arrest over pause escape. We provide a detailed kinetic model of the transit of RNAP through the pause site, in which the overall pause characteristics are explained by the rates and probabilities of individual steps.
In Fig. 6, we show a refined model of the pause at λpR′. Transcription through the σ70-dependent pause can be divided into three stages: induction, cycling, and escape. Induction and escape are defined by the engagement and disengagement, respectively, of σ70 with the −10-like sequence. The cyclic nature of σ70-dependent pausing results from Gre-mediated transcript cleavage allowing TECs that have failed to escape the σ70:DNA bond and consequently have backtracked, to repeat the pause cycle until successful escape. The overall pause time thus depends upon (i) the rate at which TECs traverse the cycle, and (ii) the probability of successful escape.
Fig. 6.
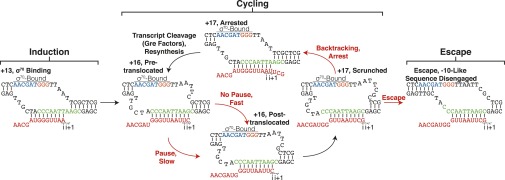
The λpR′ σ70-dependent pause cycle. Pause induction occurs when, at +13, σ70 binds the −10-like sequence and anchors RNAP to the DNA template. The −10-like sequence-bound TEC continues RNA synthesis to +16, at which point RNAP either proceeds rapidly to a posttranslocated conformation or enters an elemental paused state induced by the EPS. Upon entering a posttranslocated conformation at +16, RNA synthesis continues to +17 and RNAP either disengages the −10-like sequence and escapes the pause or fails to disengage the −10-like sequence and enters a backtracked conformation. The backtracked complex is then rescued by Gre-mediated transcript cleavage and so that subsequent escape attempts can be made until σ70 releases the −10-like sequence.
The rate at which TECs traverse the λpR′ σ70-dependent pause cycle is limited by the +16 elemental pause. Correspondingly, EPS mutations that reduce +16 pause capture and/or half-life, including those of Cons16G, Cons16A, ConsUE, and ConsIE[CG], allow TECs to proceed from +16 to +17 rapidly, resulting in a short overall σ70-dependent pause half-life when the presence of GreB permits the entire pause cycle to occur. The probability that σ70 will disengage the −10-like sequence on a given escape attempt determines the number of cycles necessary for all complexes to escape the pause. Because the σ70:DNA interaction is the barrier to escape, a stronger σ70:DNA interaction reduces the probability of pause escape, as shown directly by substitution of the −10 element consensus for the natural −10-like sequence. A second element that should determine the probability of σ70:DNA disengagement is the energy provided by the competing reaction, backtracking, which relieves unwinding energy without loss of σ70. Therefore, an important function of the GC-rich RNA:DNA hybrid upstream edge is to favor backtracking. Mutation of this sequence in ConsUE increases the probability of escape, likely by disfavoring backtracking. Consistent with this model, translocation to +17 breaks two G:C base pairs at the upstream RNA:DNA hybrid edge that stabilize the elemental pause (Fig. 6). We suggest that, as the TEC advances from +16 to +17, it reaches an EPS-induced “tipping point” from which RNAP either escapes the σ70:DNA bond or backtracks and arrests. This model is consistent with the observation that inserting foreign segments between the −10-like sequence and the EPS moves the site of σ70-dependent pausing downstream, implying that the extent of scrunching does not define the tipping point; instead, it is the stabilization provided by the EPS (19).
We have identified an internal segment of the λpR′ EPS that contributes importantly to the pause, consisting mostly of the sequence AAU 3–5 nt from the pause end. Original reports of the EPS found a preference for U at −3 (15, 17), and an analysis of bases that oppose translocation (6) also showed preference for U or A at this site. Changing the AAU sequence and surrounding nucleotides to a G/C-rich segment strongly inhibited capture of TECs in an elemental pause. It is noteworthy that a similar sequence similarly located promotes abortive initiation (11).
We can conclude that the σ70-dependent pause depends entirely on both the σ70:DNA interaction and the elongation effects of the EPS. Rendering the −10-like sequence inactive by mutating the essential +2A and +6T nucleotides completely removes pausing at +16 (28). Similarly, our kinetic model for the σ70-dependent pause cycle predicts that removal of the slow elemental pause step would cause even a consensus −10-like sequence σ70-dependent pause to decay on a millisecond timescale and be undetectable. Thus, the functions of the −10-like sequence and the EPS are completely interdependent in σ70-dependent pausing.
The role of the EPS in σ70-dependent pausing offers some insight into the antipausing activity of the antiterminator Q. Incorporation of Q into a σ70-dependent paused TEC effects rapid escape from the σ70-dependent pause (29). In the absence of GreB, Q causes the +16 pause to decay rapidly; however, Q-dependent arrested complexes appear both from +17 to +19 and further downstream in a cluster focused at +25 (11). Notably, these complexes all backtrack to around +13 to +15, indicating that σ70 remains bound to the −10-like sequence. Therefore, because Q does not appear to disrupt the σ70:DNA interaction [and in fact, could supplement it through contacts with the DNA-encoded Q-binding element (23, 30)], the antipausing activity of Q likely functions by modifying the properties of the +16 elemental pause such that the overall rate of pause cycling, and thus the frequency of escape attempts, is increased.
Materials and Methods
Plasmids.
The source of template for transcription and mutagenesis was pM650 (31). Plasmids for protein purification were pET-28a-σ70 (32), pES3 (for GreB) (11), and pVS10 (for RNAP; a gift from I. Artsimovitch, The Ohio State University, Columbus, OH) (33). All mutants were constructed using QuikChange site-directed mutagenesis.
Proteins.
RNAP (33), GreB (34), σ70 (32), and NusA (35) were purified as described.
In Vitro Transcription.
Reaction mixtures containing 5 nM template and 25 nM RNAP (25 nM core reconstituted with 50 nM σ70) were incubated in transcription buffer (20 mM Tris⋅HCl, pH 8.0, 0.1 mM EDTA, 1 mM DTT, and 50 mM KCl), 0.1 mg/mL BSA, a nucleotide mix of either 200 μM ATP, UTP, CTP, and 50 μM GTP containing 0.5 μCi/μL [α-32P]GTP (Figs. 2, 3 A and B, and 4, and Figs. S2A, S3, and S4) or 200 μM ATP, GTP, CTP, and 50 μM UTP containing 0.5 μCi/μL [α-32P]UTP (Fig. 3 C and D, and Fig. S2B), and 150 nM NusA for 10 min at 37 °C to form open complexes. Single-round transcription reactions were initiated by addition of magnesium chloride to 5 mM and rifampicin to 10 μg/mL. Total reaction volume was 25 μL. When present, GreB was added to 200 nM before formation of open complex. Reactions were stopped by adding 125 μL of stop solution (0.6 M Tris, pH 8.0, 12 mM EDTA, 0.16 mg/mL transfer RNA).
Heteroduplex Templates.
Heteroduplex templates were constructed as described previously (24) and sequenced to confirm purity. Transcription was performed as described above.
Purification, Fractionation, and Analysis of Transcription Reactions.
Stopped transcription reactions were phenol extracted by addition of 150 μL of phenol/chloroform/isoamyl alcohol (25:24:1), vortexing, centrifugation, and collection of the aqueous phase. Ethanol precipitation of RNA was performed by adding 450 μL of 100% ethanol to each reaction, followed by storage at −20 °C overnight. Precipitated RNA was resuspended in transcription loading dye [1× transcription buffer, 80% (vol/vol) formamide, 0.05% bromophenol blue, and xylene cyanol]. Reactions were fractionated by electrophoresis using 12% (wt/vol) denaturing polyacrylamide gels containing 7 M urea. Reactive bases were detected by an Amersham Biosciences Typhoon 9400 Variable Mode Imager. Quantitation was performed using ImageQuant.
Data Analysis.
Normalization.
For all experiments, individual bands were normalized for incorporation of [α-32P]GTP (or [α-32P]UTP in the case of Fig. 3 C and D) and nucleotide content to obtain relative numbers of RNA molecules.
Quantification of +GreB experiments.
For +GreB reactions, “%Paused” was calculated for each time by dividing the sum of all paused complexes, as determined by decay over time in the presence of GreB, by the sum of all quantifiable bands (paused complexes, terminated, and readthrough bands) at 120 s, when accumulation of long products is complete. The 120-s time total was used as the divisor for all times to account for the presence of nonquantifiable bands at early times. By 120 s, the complexes represented by these bands have accumulated in the terminated and readthrough bands and are therefore quantifiable.
The %Paused values described above were used to generate an exponential trend line from the average of two replicates. For most templates, the pause half-life remained constant until the %Pause dropped below ∼30%, after which a slower decay occurred. For this reason, the number of data points to which each exponential trend was fit varied from 3 to 5 and was determined by assessing the point at which measured values deviated from the initial decay of the pause. The trend line generated as described above was then used to determine pause half-life and induction (the y intercept of the trend line). For most templates, pause induction was several percentage points above 100%; we report these values as 100%.
Quantification of −GreB experiments.
For −GreB reactions, “%Paused at +16” was calculated for each time by dividing the +16 pause band by the sum of all quantifiable bands (arrested, terminated, and readthrough). In contrast to the calculation described for +GreB samples, the low level of nonquantifiable bands allowed us to perform this calculation within each lane. The exception to this is ConsUE, which, because of the prevalence of nonquantifiable bands at early time points, required use of the 300-s time point as the divisor for all time points.
The %Paused at +16 values were used to determine +16 pause half-life and capture efficiency by generating an exponential trend line from the average of two replicates. A third value, the probability that σ70 will disengage the −10-like sequence on a given escape attempt, was determined for the 60-s time point by dividing the sum of the terminated and readthrough bands by the sum of arrested, terminated, and readthrough bands. The 60-s time point was used for this calculation because at 60 s most elongation complexes have proceeded beyond +16; however, there is not sufficient time for the endogenous transcript cleavage activity of RNAP to rescue arrested TECs, which would allow additional cleavage attempts and could obscure the measurement of escape probability.
Acknowledgments
We thank David Shalloway for helpful discussions regarding kinetic models, and the referees for their insightful suggestions. This work was funded by NIH Grant GM21941.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1512986112/-/DCSupplemental.
References
- 1.Kwak H, Lis JT. Control of transcriptional elongation. Annu Rev Genet. 2013;47:483–508. doi: 10.1146/annurev-genet-110711-155440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santangelo TJ, Artsimovitch I. Termination and antitermination: RNA polymerase runs a stop sign. Nat Rev Microbiol. 2011;9(5):319–329. doi: 10.1038/nrmicro2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ring BZ, Yarnell WS, Roberts JW. Function of E. coli RNA polymerase sigma factor sigma 70 in promoter-proximal pausing. Cell. 1996;86(3):485–493. doi: 10.1016/s0092-8674(00)80121-x. [DOI] [PubMed] [Google Scholar]
- 4.Artsimovitch I, Landick R. Pausing by bacterial RNA polymerase is mediated by mechanistically distinct classes of signals. Proc Natl Acad Sci USA. 2000;97(13):7090–7095. doi: 10.1073/pnas.97.13.7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan CL, Landick R. Dissection of the his leader pause site by base substitution reveals a multipartite signal that includes a pause RNA hairpin. J Mol Biol. 1993;233(1):25–42. doi: 10.1006/jmbi.1993.1482. [DOI] [PubMed] [Google Scholar]
- 6.Bochkareva A, Yuzenkova Y, Tadigotla VR, Zenkin N. Factor-independent transcription pausing caused by recognition of the RNA-DNA hybrid sequence. EMBO J. 2012;31(3):630–639. [Google Scholar]
- 7.Roberts JW, et al. Antitermination by bacteriophage lambda Q protein. Cold Spring Harb Symp Quant Biol. 1998;63:319–325. doi: 10.1101/sqb.1998.63.319. [DOI] [PubMed] [Google Scholar]
- 8.Kapanidis AN, et al. Initial transcription by RNA polymerase proceeds through a DNA-scrunching mechanism. Science. 2006;314(5802):1144–1147. doi: 10.1126/science.1131399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Revyakin A, Liu C, Ebright RH, Strick TR. Abortive initiation and productive initiation by RNA polymerase involve DNA scrunching. Science. 2006;314(5802):1139–1143. doi: 10.1126/science.1131398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marr MT, Roberts JW. Function of transcription cleavage factors GreA and GreB at a regulatory pause site. Mol Cell. 2000;6(6):1275–1285. doi: 10.1016/s1097-2765(00)00126-x. [DOI] [PubMed] [Google Scholar]
- 11.Strobel EJ, Roberts JW. Regulation of promoter-proximal transcription elongation: Enhanced DNA scrunching drives λQ antiterminator-dependent escape from a σ70-dependent pause. Nucleic Acids Res. 2014;42(8):5097–5108. doi: 10.1093/nar/gku147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsu LM. Promoter clearance and escape in prokaryotes. Biochim Biophys Acta. 2002;1577(2):191–207. doi: 10.1016/s0167-4781(02)00452-9. [DOI] [PubMed] [Google Scholar]
- 13.Borukhov S, Sagitov V, Goldfarb A. Transcript cleavage factors from E. coli. Cell. 1993;72(3):459–466. doi: 10.1016/0092-8674(93)90121-6. [DOI] [PubMed] [Google Scholar]
- 14.Perdue SA, Roberts JW. A backtrack-inducing sequence is an essential component of Escherichia coli σ70-dependent promoter-proximal pausing. Mol Microbiol. 2010;78(3):636–650. doi: 10.1111/j.1365-2958.2010.07347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herbert KM, et al. Sequence-resolved detection of pausing by single RNA polymerase molecules. Cell. 2006;125(6):1083–1094. doi: 10.1016/j.cell.2006.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hein PP, Palangat M, Landick R. RNA transcript 3′-proximal sequence affects translocation bias of RNA polymerase. Biochemistry. 2011;50(32):7002–7014. doi: 10.1021/bi200437q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larson MH, et al. A pause sequence enriched at translation start sites drives transcription dynamics in vivo. Science. 2014;344(6187):1042–1047. doi: 10.1126/science.1251871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vvedenskaya IO, et al. Interactions between RNA polymerase and the “core recognition element” counteract pausing. Science. 2014;344(6189):1285–1289. doi: 10.1126/science.1253458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bird J. 2013. Characterization of novel DNA elements necessary for sigma-dependent promoter proximal transcriptional pausing. PhD thesis (Cornell University, Ithaca, NY)
- 20.Toulokhonov I, Zhang J, Palangat M, Landick R. A central role of the RNA polymerase trigger loop in active-site rearrangement during transcriptional pausing. Mol Cell. 2007;27(3):406–419. doi: 10.1016/j.molcel.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 21.Imashimizu M, et al. Intrinsic translocation barrier as an initial step in pausing by RNA polymerase II. J Mol Biol. 2013;425(4):697–712. doi: 10.1016/j.jmb.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weixlbaumer A, Leon K, Landick R, Darst SA. Structural basis of transcriptional pausing in bacteria. Cell. 2013;152(3):431–441. doi: 10.1016/j.cell.2012.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yarnell WS, Roberts JW. The phage lambda gene Q transcription antiterminator binds DNA in the late gene promoter as it modifies RNA polymerase. Cell. 1992;69(7):1181–1189. doi: 10.1016/0092-8674(92)90639-t. [DOI] [PubMed] [Google Scholar]
- 24.Ring BZ, Roberts JW. Function of a nontranscribed DNA strand site in transcription elongation. Cell. 1994;78(2):317–324. doi: 10.1016/0092-8674(94)90300-x. [DOI] [PubMed] [Google Scholar]
- 25.Haugen SP, et al. rRNA promoter regulation by nonoptimal binding of sigma region 1.2: An additional recognition element for RNA polymerase. Cell. 2006;125(6):1069–1082. doi: 10.1016/j.cell.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 26.Feklistov A, et al. A basal promoter element recognized by free RNA polymerase sigma subunit determines promoter recognition by RNA polymerase holoenzyme. Mol Cell. 2006;23(1):97–107. doi: 10.1016/j.molcel.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 27.Landick R, Wang D, Chan CL. Quantitative analysis of transcriptional pausing by Escherichia coli RNA polymerase: His leader pause site as paradigm. Methods Enzymol. 1996;274:334–353. doi: 10.1016/s0076-6879(96)74029-6. [DOI] [PubMed] [Google Scholar]
- 28.Deighan P, Pukhrambam C, Nickels BE, Hochschild A. Initial transcribed region sequences influence the composition and functional properties of the bacterial elongation complex. Genes Dev. 2011;25(1):77–88. doi: 10.1101/gad.1991811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grayhack EJ, Yang XJ, Lau LF, Roberts JW. Phage lambda gene Q antiterminator recognizes RNA polymerase near the promoter and accelerates it through a pause site. Cell. 1985;42(1):259–269. doi: 10.1016/s0092-8674(85)80121-5. [DOI] [PubMed] [Google Scholar]
- 30.Nickels BE, Roberts CW, Sun H, Roberts JW, Hochschild A. The σ70 subunit of RNA polymerase is contacted by the λQ antiterminator during early elongation. Mol Cell. 2002;10(3):611–622. doi: 10.1016/s1097-2765(02)00648-2. [DOI] [PubMed] [Google Scholar]
- 31.Ko DC, Marr MT, Guo J, Roberts JW. A surface of Escherichia coli sigma 70 required for promoter function and antitermination by phage lambda Q protein. Genes Dev. 1998;12(20):3276–3285. doi: 10.1101/gad.12.20.3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marr MT, Roberts JW. Promoter recognition as measured by binding of polymerase to nontemplate strand oligonucleotide. Science. 1997;276(5316):1258–1260. doi: 10.1126/science.276.5316.1258. [DOI] [PubMed] [Google Scholar]
- 33.Belogurov GA, et al. Structural basis for converting a general transcription factor into an operon-specific virulence regulator. Mol Cell. 2007;26(1):117–129. doi: 10.1016/j.molcel.2007.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perederina AA, et al. Cloning, expression, purification, crystallization and initial crystallographic analysis of transcription elongation factors GreB from Escherichia coli and Gfh1 from Thermus thermophilus. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2006;62(Pt 1):44–46. doi: 10.1107/S1744309105040297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmidt MC, Chamberlin MJ. Amplification and isolation of Escherichia coli nusA protein and studies of its effects on in vitro RNA chain elongation. Biochemistry. 1984;23(2):197–203. doi: 10.1021/bi00297a004. [DOI] [PubMed] [Google Scholar]