Significance
Chloroplasts are critical, dynamic organelles in plant cells responsible for photosynthesis and myriad other aspects of metabolism. In recent years, plant cell biologists have increasingly focused on the formation of thin, long extensions from plastids called “stromules.” Although stromules have been observed in all land plant species and cell types investigated, we do not know why these projections form or what they do. Here we demonstrate that stromules form in response to light-related redox signals inside the chloroplast. We then show that chloroplasts extracted from plant cells can make stromules independently. These discoveries suggest that stromules may be involved in transmitting signals from within the chloroplast to other subcellular compartments.
Keywords: chloroplasts, stromules, redox signaling, light signaling, leucoplasts
Abstract
A fundamental mystery of plant cell biology is the occurrence of “stromules,” stroma-filled tubular extensions from plastids (such as chloroplasts) that are universally observed in plants but whose functions are, in effect, completely unknown. One prevalent hypothesis is that stromules exchange signals or metabolites between plastids and other subcellular compartments, and that stromules are induced during stress. Until now, no signaling mechanisms originating within the plastid have been identified that regulate stromule activity, a critical missing link in this hypothesis. Using confocal and superresolution 3D microscopy, we have shown that stromules form in response to light-sensitive redox signals within the chloroplast. Stromule frequency increased during the day or after treatment with chemicals that produce reactive oxygen species specifically in the chloroplast. Silencing expression of the chloroplast NADPH-dependent thioredoxin reductase, a central hub in chloroplast redox signaling pathways, increased chloroplast stromule frequency, whereas silencing expression of nuclear genes related to plastid genome expression and tetrapyrrole biosynthesis had no impact on stromules. Leucoplasts, which are not photosynthetic, also made more stromules in the daytime. Leucoplasts did not respond to the same redox signaling pathway but instead increased stromule formation when exposed to sucrose, a major product of photosynthesis, although sucrose has no impact on chloroplast stromule frequency. Thus, different types of plastids make stromules in response to distinct signals. Finally, isolated chloroplasts could make stromules independently after extraction from the cytoplasm, suggesting that chloroplast-associated factors are sufficient to generate stromules. These discoveries demonstrate that chloroplasts are remarkably autonomous organelles that alter their stromule frequency in reaction to internal signal transduction pathways.
Chloroplasts, the descendants of ancient bacterial endosymbionts, exert impressive influence over processes that are not directly related to their metabolic roles. In recent years, forward genetic screens have led to the discoveries that chloroplasts are critical regulators of leaf shape, cell–cell signaling through plasmodesmata, pathogen defense, and even alternative splicing in the nucleus (1‒8); however, in almost all of these pathways, the signaling route between the chloroplast and the nucleus is unknown. This is a pressing question for plant biology and cell biology in general: how do organelles communicate with the nucleus to coordinate genetic programs and cellular function? One possible route for this communication is through “stromules,” stroma-filled tubular extensions of unknown function from plastids (9‒11).
Stromules were first observed in spinach cells (12), and have since been observed in every cell type and land plant species investigated to date (13). Several studies have identified conditions that can induce or decrease stromule formation (14‒18), concluding that stromule frequency can change in response to abiotic stress, phytohormone signaling, and massive disruption of cellular function (e.g., strong inhibition of cytosolic translation or of actin microfilament dynamics). Almost nothing is known about the genetics of stromules; some mutants with strong morphological defects in plastids, such as mutants with improper plastid division or lacking plastid mechanosensitive channels, cannot form stromules at normal frequencies, but these plastids are so severely misshapen that their stromule frequencies cannot be directly compared with wild-type plastids (19, 20). To date, few experiments have tested whether signals inside plastids can affect stromule frequency, and all of those experiments (e.g., treatment with antibiotics that interfere with plastid genome expression; ref. 17) have suggested that stromule frequency is not regulated by internal plastid biology. Here we test whether light-sensitive redox signaling pathways initiated within chloroplasts regulate stromule activity.
Results and Discussion
Chloroplasts Make More Stromules During the Day.
We began our study by conducting a time course to determine the effects of light on chloroplast stromule formation. For all in planta experiments (except where noted otherwise), we observed stromules in the proximal abaxial epidermis of cotyledons of young N. benthamiana or A. thaliana plants, or in the proximal abaxial epidermis of young leaves at 2 wk after silencing gene expression with virus-induced gene silencing (VIGS). We collected a single z-stack of confocal images of only one leaf of each plant, and considered plants to be independent samples. (The number of plants observed for each treatment for an experiment is designated “n” throughout.) Thus, each experiment considered the stromule frequency determined from hundreds, thousands, or even tens of thousands of plastids.
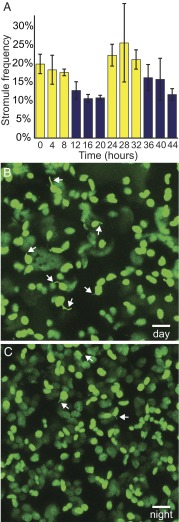
Over the course of 2 d, we measured stromule frequency in cotyledons from young N. benthamiana seedlings every 4 h, and found significantly more stromules during the daytime than at nighttime; 20.8 ± 1.8% of chloroplasts had stromules in the day, compared with only 12.8 ± 0.9% at night (n ≥ 22, P < 0.0005) (Fig. 1 and Fig. S1). (Throughout the paper, stromule frequency is reported as percentage ± SE.) There was no significant difference in stromule frequency between the first and second days or between the first and second nights, indicating that the observed changes were reactions to the changing light environment rather than a progressive developmental change over 48 h.
Fig. 1.
Chloroplast stromule frequency varies with diurnal cycles. (A) Stromule frequency rises in the day (yellow bars) and decreases at night (blue bars) in chloroplasts of N. benthamiana seedlings (n ≥ 22, P < 0.0005). (B and C) Representative images of N. benthamiana epidermal chloroplasts labeled with stromal GFP (green) in the day (B) and at night (C). Some stromules are indicated by white arrows. As a visual aid, here and in other figures; not all stromules are indicated, and the indicated stromules were selected at random. Error bars indicate SE. (Scale bars: 10 μm.)
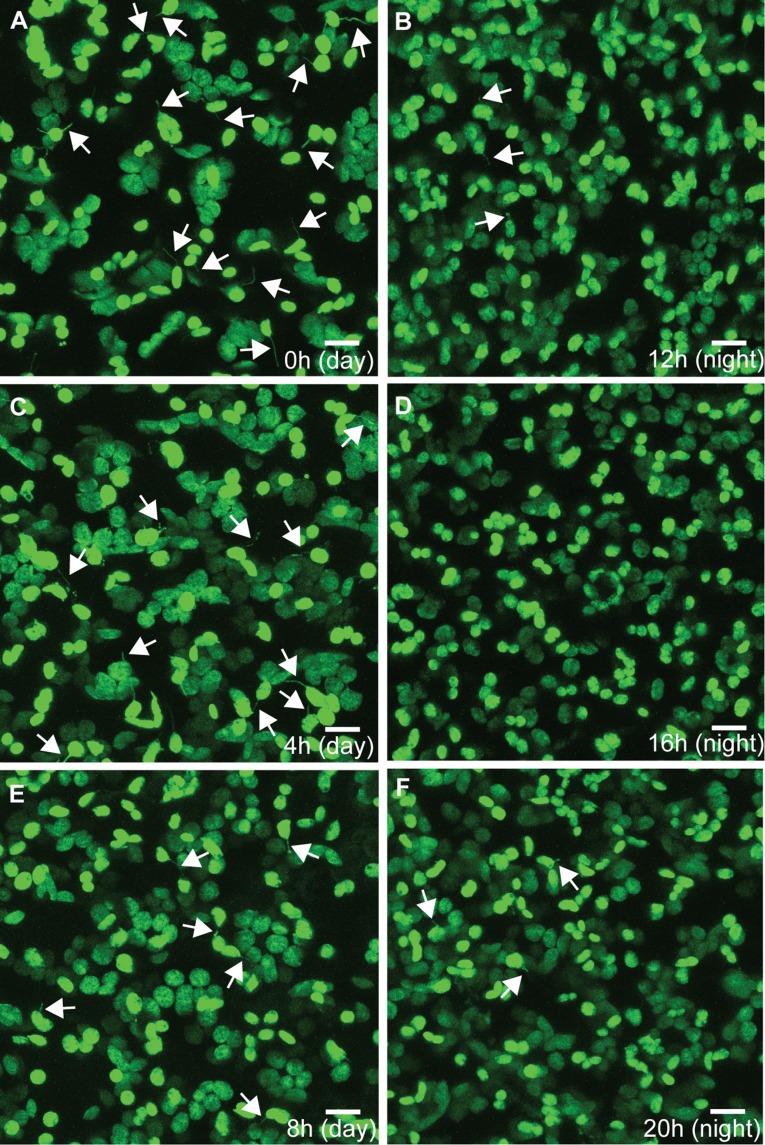
Fig. S1.
Representative images of stromule frequency over the course of 48 h during a 12-h light/12-h dark cycle. N. benthamiana chloroplast stromule frequency is high throughout the day (A, dawn; C, 4 h after dawn; E, 8 h after dawn) and low throughout the night (B, dusk; D, 4 h after dusk; F, 8 h after dusk). Chloroplasts and stromules are labeled with stromal GFP. Some stromules are indicated by white arrows. (Scale bars: 10 μm.)
Previous studies investigating the relationship between chloroplast stromule frequency and light reported that light decreases stromule frequency in seedling hypocotyls during de-etiolation after skotomorphogenesis, and that constant darkness or exposure to only blue light increases stromule frequency after photomorphogenesis (17). The apparent discrepancy between those conclusions and our results showing that light promotes chloroplast stromule formation is explained by the different plastid types used in the de-etiolation experiment (etioplasts transitioning to become chloroplasts) and the dramatic developmental and physiological transitions used in both experiments (constant darkness to constant light, or vice versa), which are not reflective of typical chloroplast stromule behavior in normal, healthy plants. We conclude that light promotes chloroplast stromule formation during the day.
Reactive Oxygen Species Inside Chloroplasts Promote Stromule Formation.
Plants sense light with the pigments of the photosynthetic electron transport chain (pETC) in the chloroplast or with photoreceptors elsewhere in the cell (21). We tested whether stromule frequency responds specifically to light sensed by the chloroplast itself by chemically inhibiting pETC activity. We used two pETC inhibitors, 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU) and 2,5-dibromo-6-isopropyl-3-methyl-1,4-benzoquinone (DBMIB). DCMU prevents reduction of plastoquinone at photosystem II and generates singlet oxygen, whereas DBMIB prevents plastoquinols from reducing the cytochrome b6f complex and generates superoxide (8).
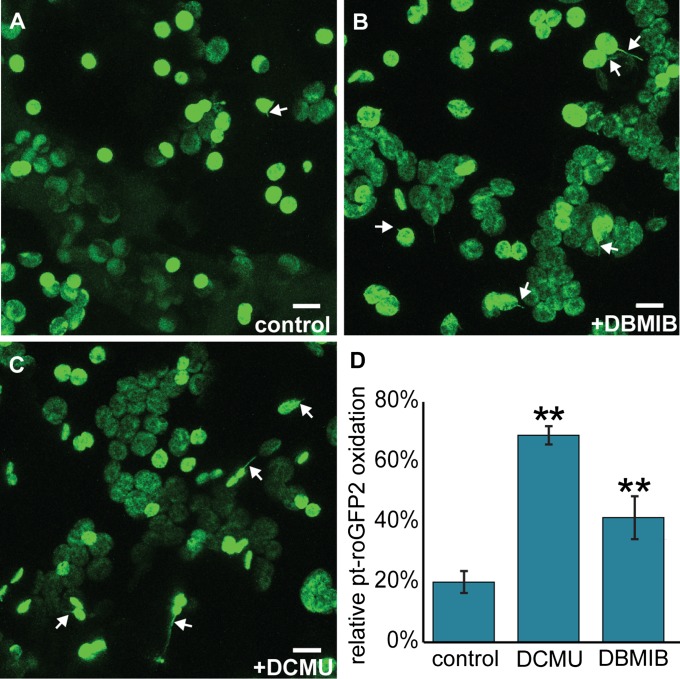
We measured the effects of DCMU and DBMIB on the chloroplast stromal redox status, as monitored by a stromal redox-sensitive transgenic GFP biosensor, pt-roGFP2 (4), to find very low active concentrations of each compound with our treatment technique, and found that 10 μM DCMU or 12 μM DBMIB was sufficient to strongly oxidize redox buffers in the chloroplast stroma. The normalized proportion of oxidized pt-roGFP2 rose from 20.0 ± 3.5% in control conditions to 68.9 ± 3.0% after 10 μM DCMU treatment and to 41.5 ± 7.1% after 12 μM DBMIB treatment (n ≥ 28, P < 0.01) (Fig. S2).
Fig. S2.
Oxidation of stromal redox buffers by ROS from photosynthesis induces stromules in chloroplasts. (A–C) Representative images of stromule formation with control (A), DBMIB (B), and DCMU (C) treatments in N. benthamiana chloroplasts. (D) Both DBMIB and DCMU cause strong oxidation of chloroplast redox buffers, as measured by pt-roGFP2 in A. thaliana cotyledons. n ≥28. **P < 0.01. Chloroplasts and stromules are labeled with stromal GFP. Some stromules are indicated by white arrows. Error bars indicate SE. (Scale bars: 10 μm.)
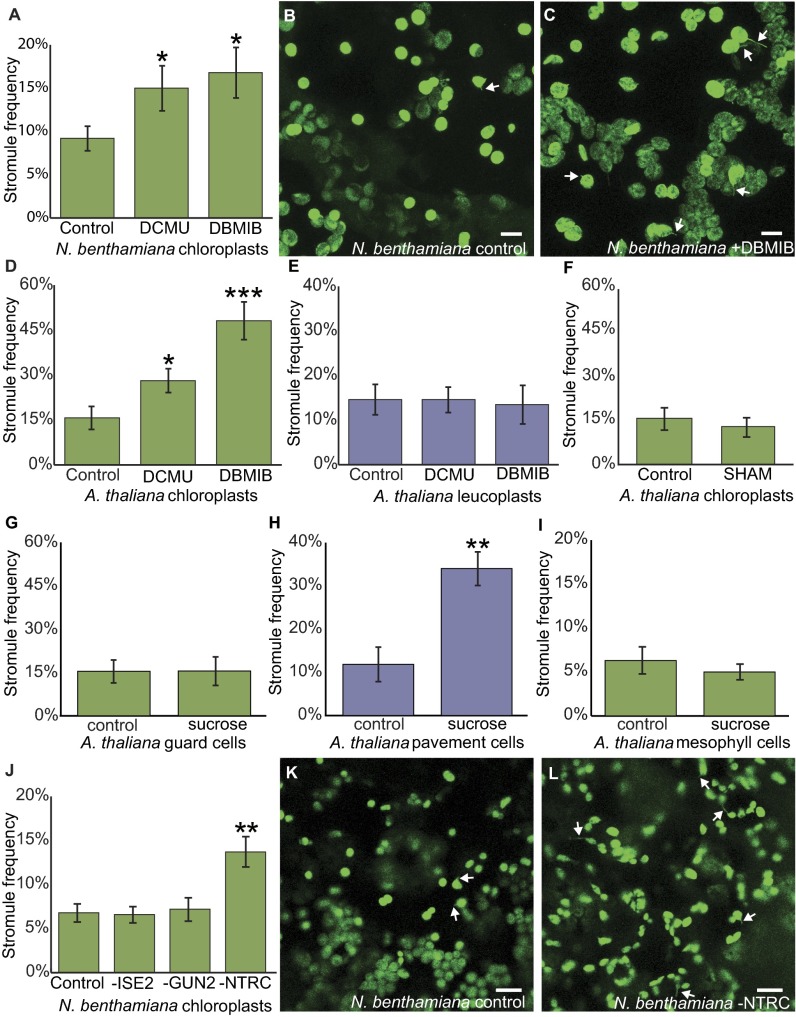
We assessed stromule frequency at 2 h after treating N. benthamiana cotyledons with either of the photosynthesis inhibitors (Fig. 2 A‒C and Fig. S2 A‒C). In the epidermal chloroplasts of mock-treated cotyledons, the average chloroplast stromule frequency was 9.2 ± 1.4%. After treatment with DCMU or DBMIB, stromule frequency increased by more than 50%, to 15.5 ± 2.6% with DCMU and 16.8 ± 2.9% with DBMIB (n ≥ 20, P < 0.05). This finding suggests that stromule formation responds to light-sensitive redox signals inside the chloroplast, to our knowledge the first demonstration that internal chloroplast pathways may regulate stromules.
Fig. 2.
ROS in the chloroplast induce stromules. (A) DCMU and DBMIB treatments both increase stromule frequency in chloroplasts of N. benthamiana seedlings (n ≥ 20, P < 0.05). (B and C) Representative images of N. benthamiana chloroplasts treated with control (B) or with DBMIB (C). (D) A. thaliana epidermal chloroplast stromule frequency increases after DCMU or DBMIB treatment (DCMU, n ≥ 16, P < 0.05; DBMIB, n ≥ 10, P < 0.0005). (E) Stromule frequency in A. thaliana epidermal leucoplasts is unaffected by DCMU or DBMIB treatment (DCMU, n ≥ 16, P = 0.98; DBMIB, n ≥ 10, P = 0.84). (F) A. thaliana epidermal chloroplast stromule frequency is unaffected by SHAM (n ≥ 13, P = 0.57). (G) A. thaliana epidermal chloroplast frequency is similar in chloroplasts with or without sucrose treatment (n ≥ 8, P = 0.96). (H) A. thaliana epidermal leucoplast stromule frequency increases after sucrose treatment (n ≥ 8, P < 0.01). (I) Stromule frequency is not affected by sucrose treatment in A. thaliana mesophyll chloroplasts (n ≥ 8, P = 0.52). (J) Silencing NbNTRC increases stromule frequency in N. benthamiana leaves (n = 8, P < 0.01), but silencing NbISE2 or NbGUN2 does not affect stromule frequency (n = 8, P > 0.82). (K and L) Representative images of N. benthamiana chloroplasts in control (K) or after silencing NbNTRC (L). Chloroplasts and stromules in are labeled with GFP. Some stromules are indicated by white arrows. Error bars indicate SE. *P < 0.05; **P < 0.01; ***P < 0.0005. (Scale bars: 10 μm.)
Unlike N. benthamiana, the epidermis of A. thaliana has two distinct types of plastids: chloroplasts in the guard cells and leucoplasts in the pavement cells (Fig. S3). Leucoplasts are not photosynthetic, but like chloroplasts, they have many other roles in metabolism and storage. As in N. benthamiana epidermal chloroplasts, DCMU and DBMIB promote stromule formation in guard cell chloroplasts of A. thaliana cotyledons, raising stromule frequency from 15.7 ± 3.8% to 28.1 ± 4.0% for DCMU (n ≥ 16, P < 0.05) and to 48.1 ± 6.3% for DBMIB (n ≥ 10, P < 0.0005) (Fig. 2D). This demonstrates that the induction of chloroplast stromules by DCMU and DBMIB is conserved in evolutionarily divergent plants, since N. benthamiana is an asterid and A. thaliana is a rosid, with the last common ancestor of the two species living more than 100 million years ago (22). In contrast, leucoplasts in the epidermis of A. thaliana were unaffected by DCMU and DBMIB treatment [14.6 ± 3.4% in control conditions vs. 14.6 ± 2.8% with DCMU (n ≥ 16, P = 0.98) and 13.5 ± 4.3% with DBMIB (n ≥ 10, P = 0.84)], showing that the effects of DCMU and DBMIB are specific responses to their roles interfering with the pETC (Fig. 2E).
Fig. S3.
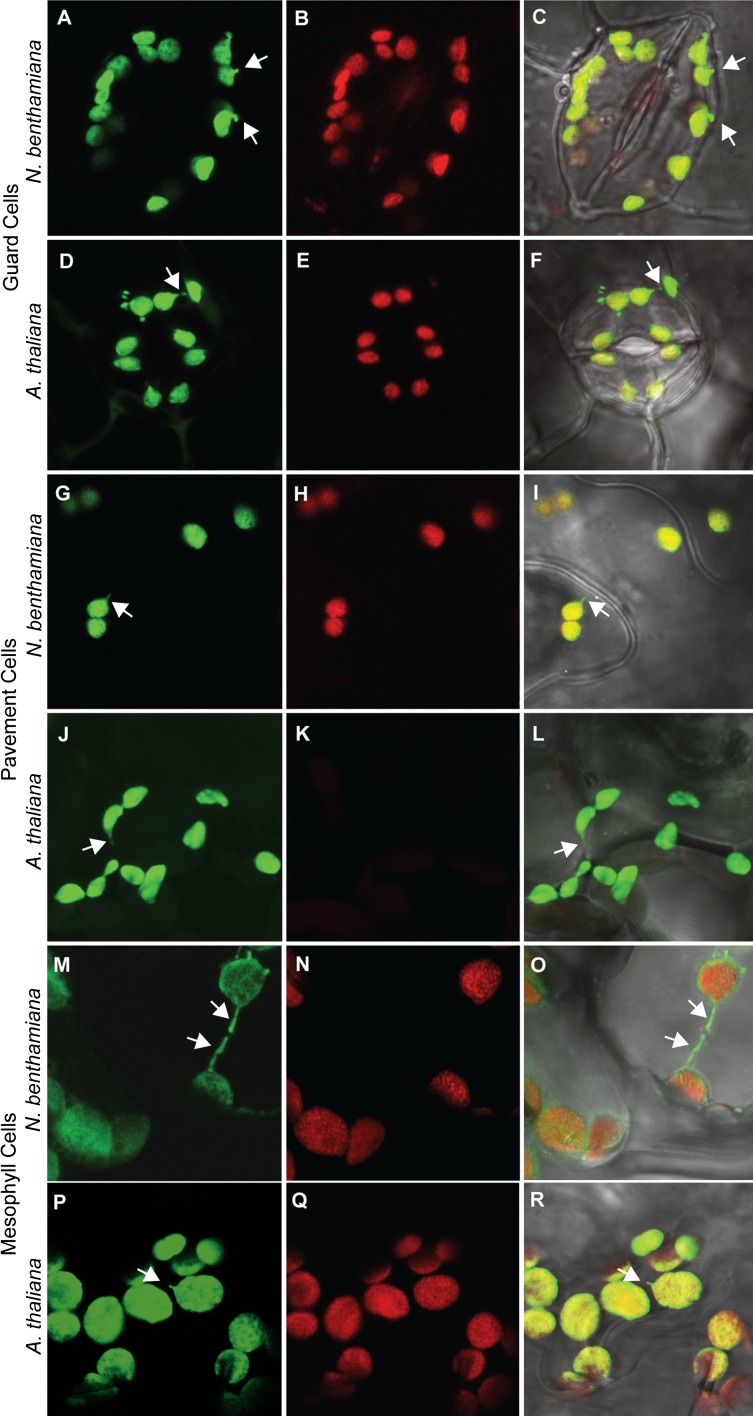
Representative images of chloroplasts and leucoplasts in N. benthamiana and A. thaliana visualized with confocal laser scanning microscopy. Guard cells (A–C and D–F), pavement cells (G–I and J–L), and mesophyll cells (M–O and P–R) all contain chloroplasts in N. benthamiana, as do A. thaliana guard cells (E) and mesophyll cells (Q); however, A. thaliana pavement cells (J–L) have leucoplasts that lack chlorophyll (K). Green, stromal GFP; red, chlorophyll autofluorescence; gray, transmitted light. Some stromules are indicated by white arrows. Error bars indicate SE.
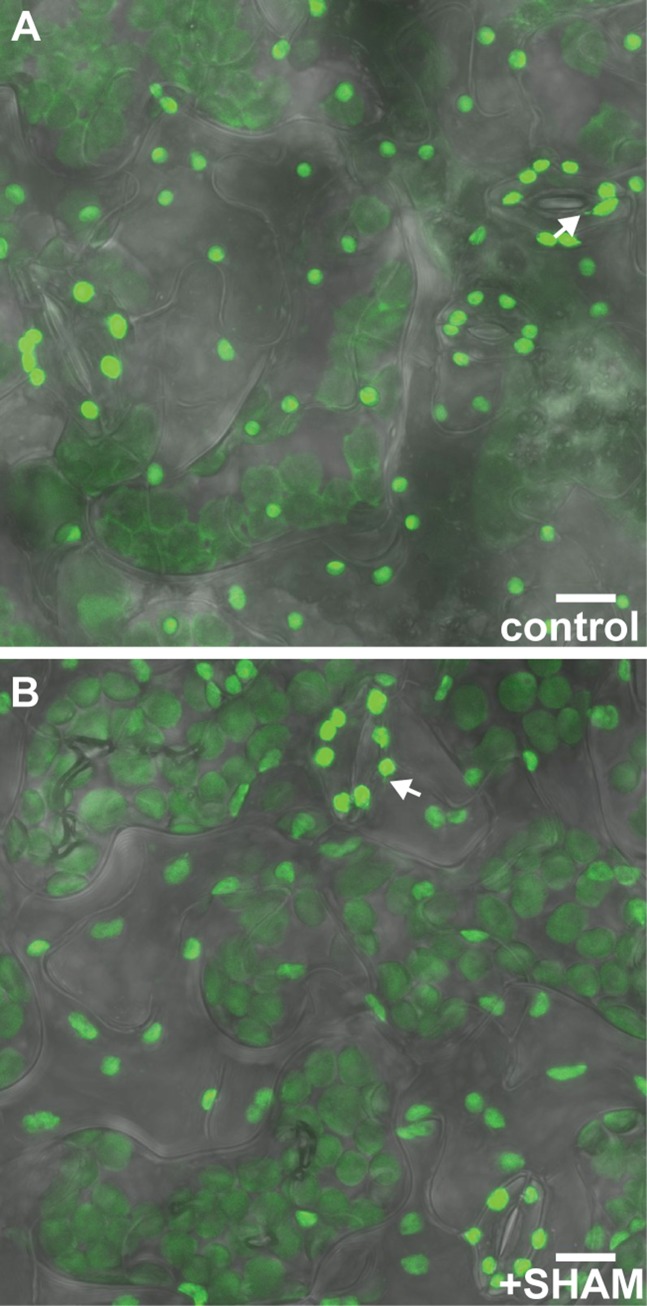
To further test whether stromules form in response to the chloroplast redox status specifically, as opposed to any oxidative stress in the cell, we also treated A. thaliana cotyledons with salicylhydroxamic acid (SHAM). SHAM inhibits the mitochondrial alternative oxidase, which leads to rapid and strong oxidation of mitochondrial redox buffers (4). SHAM did not impact chloroplast stromule formation in A. thaliana (15.7 ± 3.8% in control vs. 12.8 ± 3.3% with SHAM; n ≥ 13, P = 0.57), supporting the hypothesis that chloroplast stromule frequency is specifically regulated by the redox status of the chloroplast (Fig. 2F and Fig. S4).
Fig. S4.
SHAM does not impact stromule frequency in A. thaliana guard cells. Stromule frequency is similar in guard cells with control treatment (A) and those with SHAM treatment (B). Green, stromal GFP; gray, transmitted light. Some stromules are indicated by white arrows. (Scale bars: 10 μm.)
In summary, low concentrations of DCMU or DBMIB are sufficient to induce significant increases in stromule frequency within only 2 h. This is apparently not a secondary effect of broad disruption of cellular metabolism or redox homeostasis, because leucoplasts, which are not photosynthetic, are unaffected by the treatments, and disrupting mitochondrial function and generating reactive oxygen species (ROS) in mitochondria by SHAM treatment does not affect chloroplast stromule frequency. Thus, light-sensitive redox cues inside chloroplasts specifically affect stromule frequency.
NADPH-Dependent Thioredoxin Reductase c Regulates Chloroplast Stromule Frequency.
Signaling from chloroplasts to other organelles within the plant cell is critical for plant survival and development (23, 24). Chloroplast-to-nucleus signaling is transduced through several pathways, some of which are light-sensitive. We used VIGS in N. benthamiana as a reverse genetic approach (25) to determine whether disrupting the light-sensitive chloroplast-to-nucleus signal transduction pathways impacts stromule formation. VIGS strongly reduces gene expression in young leaves within 1–2 wk of infection by generating small RNAs that specifically target a gene for posttranscriptional silencing (25).
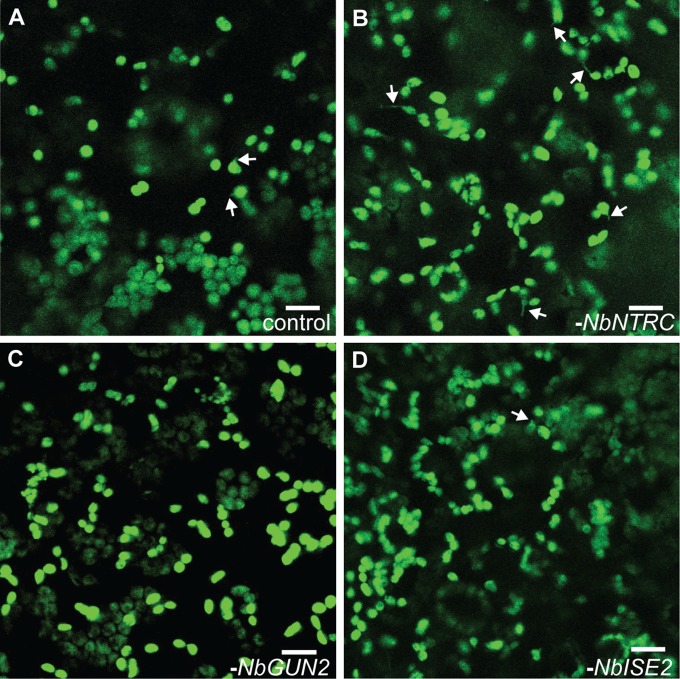
Chloroplasts contain their own genomes encoding approximately 80 proteins (mostly related to photosynthesis or transcription and translation), and light exerts control over plastid genome expression (PGE) at transcriptional and posttranscriptional levels (26). Thus, we first focused on NbISE2, an essential plastid RNA helicase required for healthy chloroplast biogenesis and PGE (3). Without ISE2, hundreds of nuclear genes involved in photosynthesis are strongly down-regulated (3). Silencing NbISE2 gene expression had no impact on stromule frequency, however (6.8 ± 1.0% in controls vs. 6.6 ± 0.9% after silencing NbISE2; n = 8, P = 0.85) (Fig. 2J and Fig. S5), in agreement with previous reports that antibiotics directly interfering with PGE, such as lincomycin, have no effect on stromule frequency (17).
Fig. S5.
Representative images of stromule frequency in N. benthamiana after silencing NbNTRC (B), NbGUN2 (C), or NbISE2 (D), versus control (A). Green, stromal GFP. Some stromules are indicated by white arrows. (Scale bars: 10 μm.)
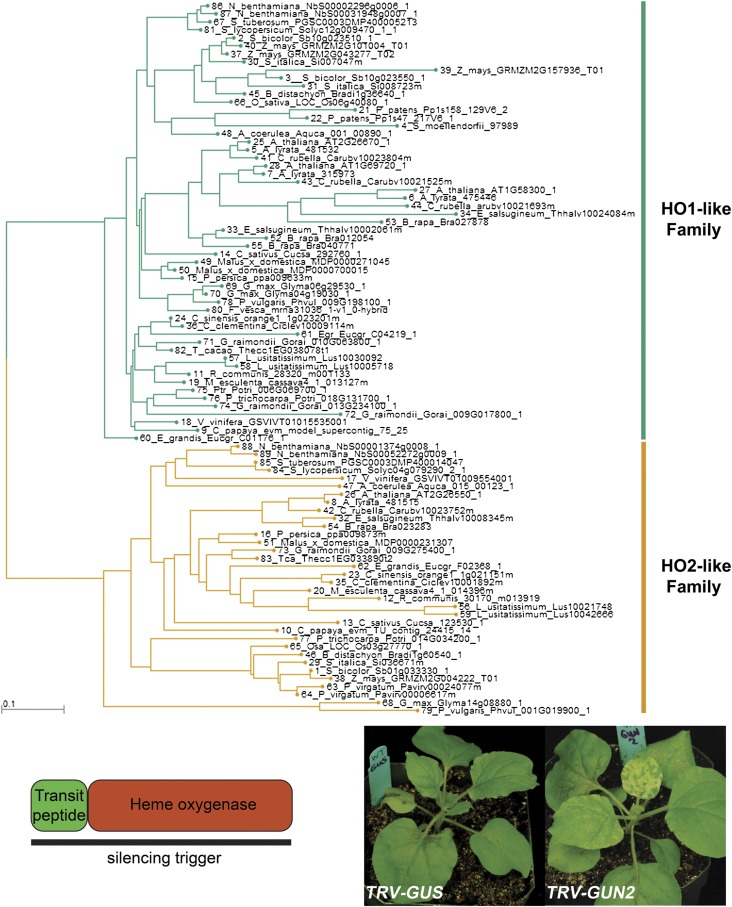
PGE coordinates the expression of photosynthesis-associated nuclear genes through a signal transduction pathway mediated by tetrapyrrole metabolism (23, 24, 27). Genetic disruptions to tetrapyrrole metabolism, specifically defects in the branch point between heme and chlorophyll biosynthesis, interfere with chloroplast biogenesis and photosynthesis (27). We next tested whether loss of NbGUN2, a chloroplast heme oxygenase that participates in chloroplast-to-nucleus communication, impacts stromule formation. As with NbISE2, silencing NbGUN2 gene expression had no impact on chloroplast stromule frequency (7.2 ± 1.3% after silencing NbGUN2; n = 8, P = 0.82) despite causing clear physiological stress and chlorosis (Fig. 2J and Figs. S5, S6, and S7).
Fig. S6.
Identification of GUN2 orthologs in N. benthamiana. A gene tree of heme oxygenases arranged homologs into two clades: HO1 (or GUN2)-like and HO2-like. (Upper) N. benthamiana has two HO1 homeologs. (Lower Left) The silencing trigger against NbGUN2 was designed to cover the entire gene sequence (837 bp). (Lower Right) Silencing NbGUN2 caused expected phenotypes, such as chlorosis.
Fig. S7.
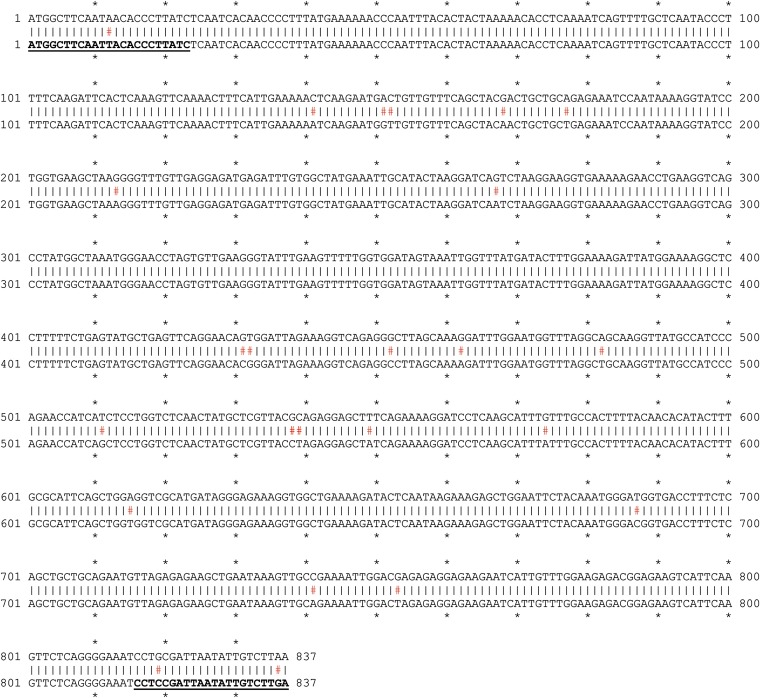
The silencing trigger for NbGUN2 (bottom line) was cloned using primers specific to NbGUN2a (NbS00031948g0007). Here, the silencing trigger is aligned against the homeologous gene, NbGUN2b (NbS00002296g0006). Mismatches are indicated with red hashtags, aligned primer sequences are in bold type and underscored.
We then silenced the expression of the chloroplast NADPH-dependent thioredoxin reductase (NbNTRC) (Figs. S8 and S9), which regulates the redox status and activity of myriad chloroplast proteins and is a critical hub in chloroplast redox signal transduction (28). Silencing NbNTRC more than doubled the stromule frequency (13.7 ± 1.7%; n = 8, P < 0.01), providing genetic evidence that redox signaling within the chloroplast regulates stromule formation (Fig. 2 J‒L and Figs. S5, S8, and S9). Moreover, to our knowledge, NbNTRC is now the first gene identified that regulates stromule frequency without other apparent effects on chloroplast shape.
Fig. S8.
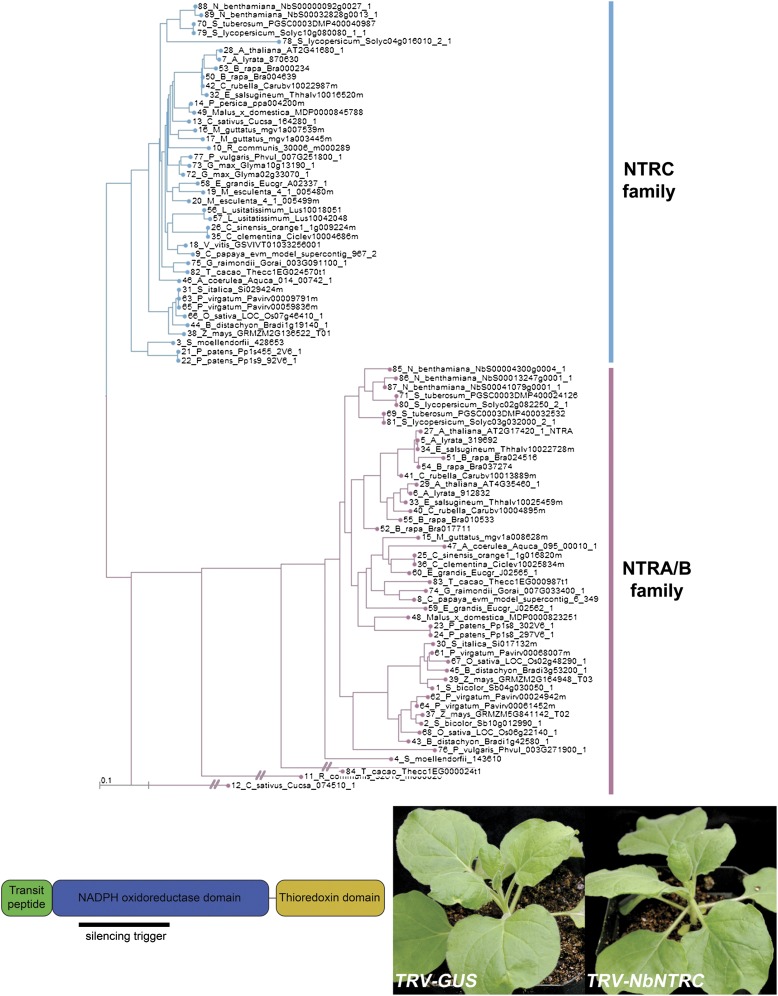
Identification of NTRC orthologs in N. benthamiana. (Upper) A gene tree based on AtNTRC arranged homologs into two clades: NTRA/NTRB-like and NTRC-like. N. benthamiana has two NTRC homeologs. (Lower Left) The silencing trigger against NbNTRC was designed against a sequence that encodes part of the NADPH oxidoreductase domain. (Lower Right) Plants without gene silencing (TRV-GUS) appeared similar to plants silencing NbNTRC expression (TRV-NbNTRC).
Fig. S9.
The silencing trigger for NbNTRC (top line) was cloned using primers specific to either NbNTRC homeolog. The annotated cDNA sequence of Nb00000092g0027.1 includes an intron (numbered here as nucleotides 610–696; bottom row, top alignment) that was not detected in any of the sequences we cloned, and is thus likely a misannotation or rare isoform. Accounting for this discrepancy, the silencing trigger is ∼99% identical to both NbNTRC homeologs. Mismatches are indicated with red hashtags; aligned primer sequences are in bold type and underscored.
Sucrose Promotes Stromule Formation in Epidermal Leucoplasts, but Not in Chloroplasts.
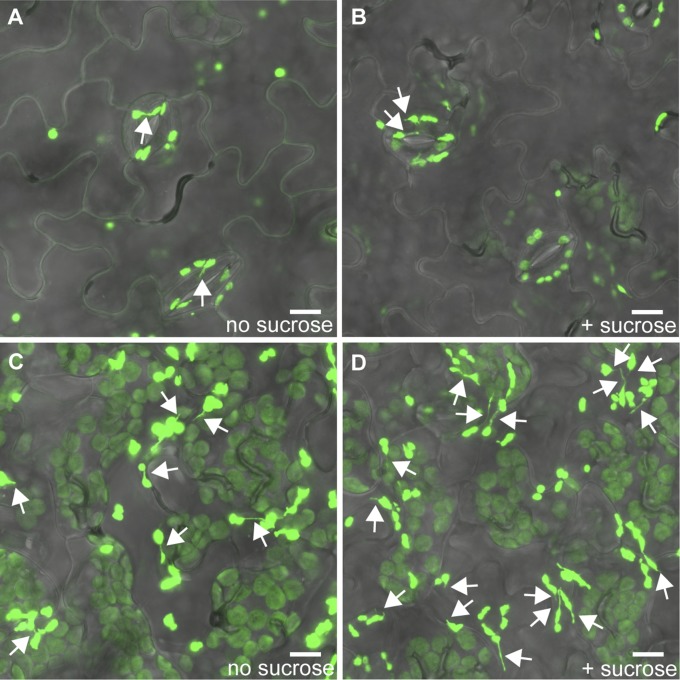
Schattat et al. (29) reported that stromule frequency increases during the day in the epidermal leucoplasts of A. thaliana. Because leucoplasts do not contain pigments and do not respond to DCMU or DBMIB, we sought another hypothesis to explain why leucoplast stromule frequency is light-responsive. Physiologically, one of the major impacts of light on epidermal pavement cells is an increase in sucrose imported from underlying cells that contain photosynthesizing chloroplasts. Previous reports have indicated that stromule frequency is sensitive to sugar levels, but with inconsistent conclusions (16). We found that epidermal leucoplast stromule frequency rises remarkably following sucrose treatments in A. thaliana (33.9 ± 3.8% with sucrose vs. 11.8 ± 3.9% without sucrose; n ≥ 8, P < 0.01) (Fig. 2H and Fig. S10). In contrast, chloroplast stromule frequency did not respond to sucrose treatments in either the epidermal guard cells or mesophyll of A. thaliana [in the epidermis, 15.4 ± 3.9% with sucrose vs. 15.7 ± 3.8% without (n ≥ 8, P = 0.96); in the mesophyll, 5.0 ± 0.9% with sucrose vs. 6.3 ± 1.7% without (n ≥ 8, P = 0.52)] (Fig. 2 G and I and Fig. S10). These results imply that different plastid types use separate signaling pathways to induce stromule formation.
Fig. S10.
Sucrose increases leucoplast but not chloroplast stromule frequency. Representative images of stromule frequency with and without sucrose treatment in A. thaliana epidermal cells. Stromule frequency is similar in chloroplasts (found in guard cells in the epidermis) without sucrose treatment (A) or with sucrose treatment (B). Sucrose treatment increases stromule frequency in leucoplasts (D) compared with control (C). Plastids and stromules are labeled with stromal GFP (green). Some stromules are indicated by white arrows. (Scale bars: 10 μm.)
Isolated Chloroplasts Can Form Stromules.
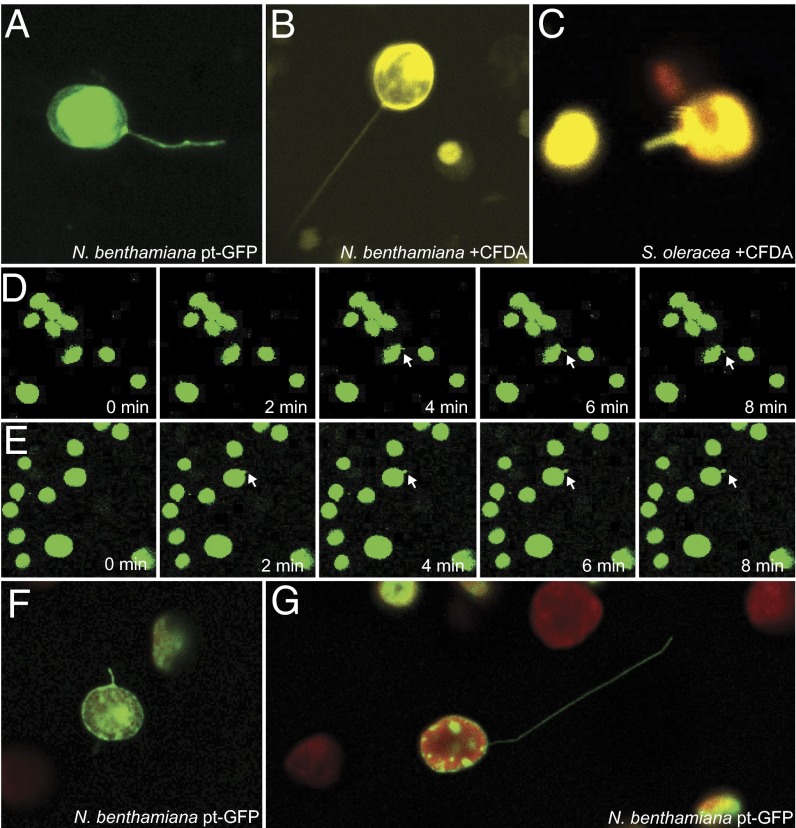
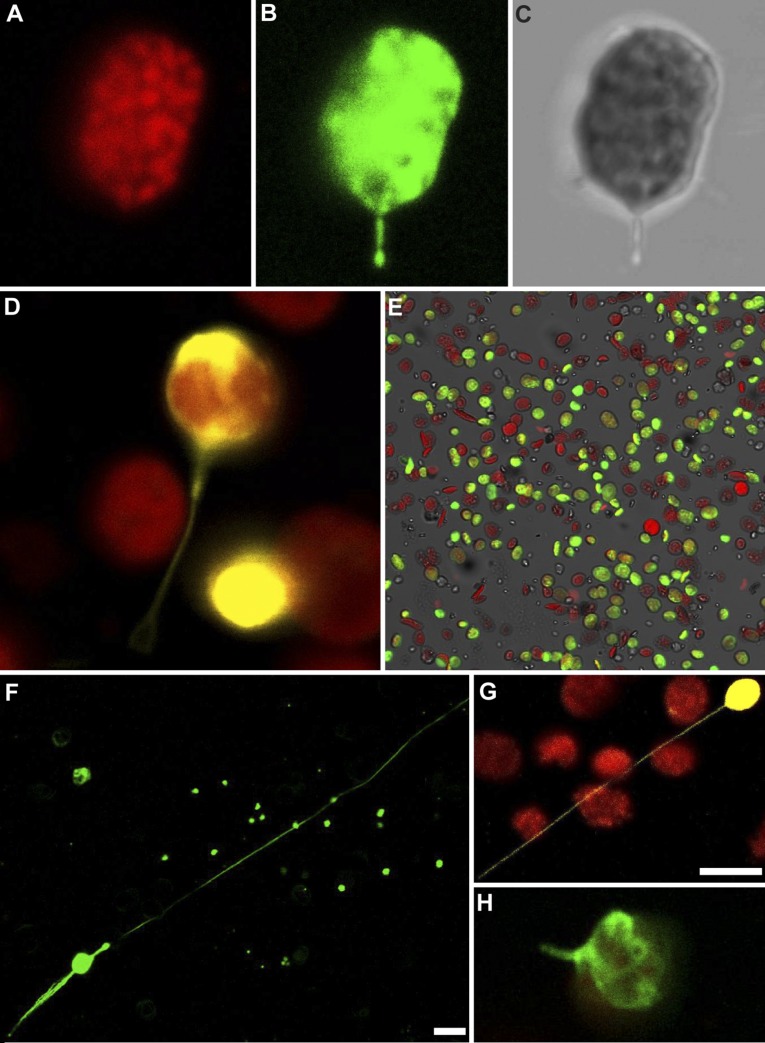
With the finding that signals originating within the chloroplast can trigger stromule formation, we then explored whether stromule formation is dependent on cytosolic structures (e.g., the cytoskeleton), as has been suggested previously (10, 15), or if chloroplasts can make stromules on their own. Previous studies have argued that stromule formation is guided and supported by the cytoskeleton and endoplasmic reticulum, but whether stromule formation requires these external factors is unknown (10, 30). To address this question, we extracted chloroplasts from leaves of N. benthamiana, A. thaliana, and Spinacia oleracea using well-established methods for isolating functional, undamaged chloroplasts (31). We visualized chloroplast stroma either with GFP by extracting chloroplasts from leaves expressing plastid-targeted GFP or with a supravital stain, carboxyfluorescein diacetate (CFDA), which fluoresces only after hydrolysis by carboxylesterases in the chloroplast stroma (32). We readily found isolated chloroplasts with intact stromules in all three species and regardless of staining technique (Fig. 3 and Fig. S11). The stromules were dynamic and could grow very long, sometimes extending more than 150 μm from chloroplasts only 4–6 μm in diameter (Fig. S11). As in plant cells, many stromules were bent and curved along their length, whereas some were very long and straight (Fig. 3 and Fig. S11). Using time-lapse microscopy, we repeatedly observed isolated chloroplasts from apparently new stromules, validating that chloroplasts can generate stromules independently. Stromules are absent in the first frames of chloroplasts shown in Fig. 3 D and E, but they appear and lengthen over the course of 8 min (Movies S1 and S2).
Fig. 3.
Isolated chloroplasts form stromules. N. benthamiana chloroplasts labeled with stromal GFP (A, green) or CFDA staining (B, yellow) show stromules after isolation from their cellular environment. (C) Chloroplasts isolated from S. oleracea and stained with CFDA (yellow; chlorophyll autofluorescence, red) also have stromules. (D and E) Isolated N. benthamiana chloroplasts labeled with stromal GFP form new stromules over time. Newly forming stromules are indicated by white arrows. (F and G) N. benthamiana chloroplasts isolated with a Percoll purification step and labeled with stromal GFP (green; chlorophyll autofluorescence, red) also have stromules.
Fig. S11.
Stromules of isolated chloroplasts visualized by confocal microscopy and 3D-SIM. (A‒C) After extraction from cells, isolated chloroplasts of N. benthamiana have stromules, as visualized by either stromal GFP fluorescence (B) or transmitted light (C), whereas chlorophyll autofluorescence remains restricted to the thylakoids (A). (D) CFDA also labels stromules in isolated N. benthamiana chloroplasts (CFDA, yellow; chlorophyll, red). (E) Representative example of sample purity after chloroplast isolation protocol. Many chloroplasts are fully intact, with stromal GFP (green); some were damaged during extraction and have lost stromal GFP, but retain the thylakoid membranes (chlorophyll autofluorescence, red); and very small quantitites of nonfluorescent materials remain with the sample (transmitted light, gray). (F and G) After isolation, some N. benthamiana chloroplasts have stromules that are very long, as visualized by GFP (F, in green) or CFDA staining (G, in yellow; chlorophyll autofluorescence, red). (H) Isolated A. thaliana chloroplasts expressing stromal roGFP2 (green) also have stromules after extraction from cells. (Scale bars: 5 μm.)
Superresolution Microscopy Illuminates Stromule Ultrastructure.
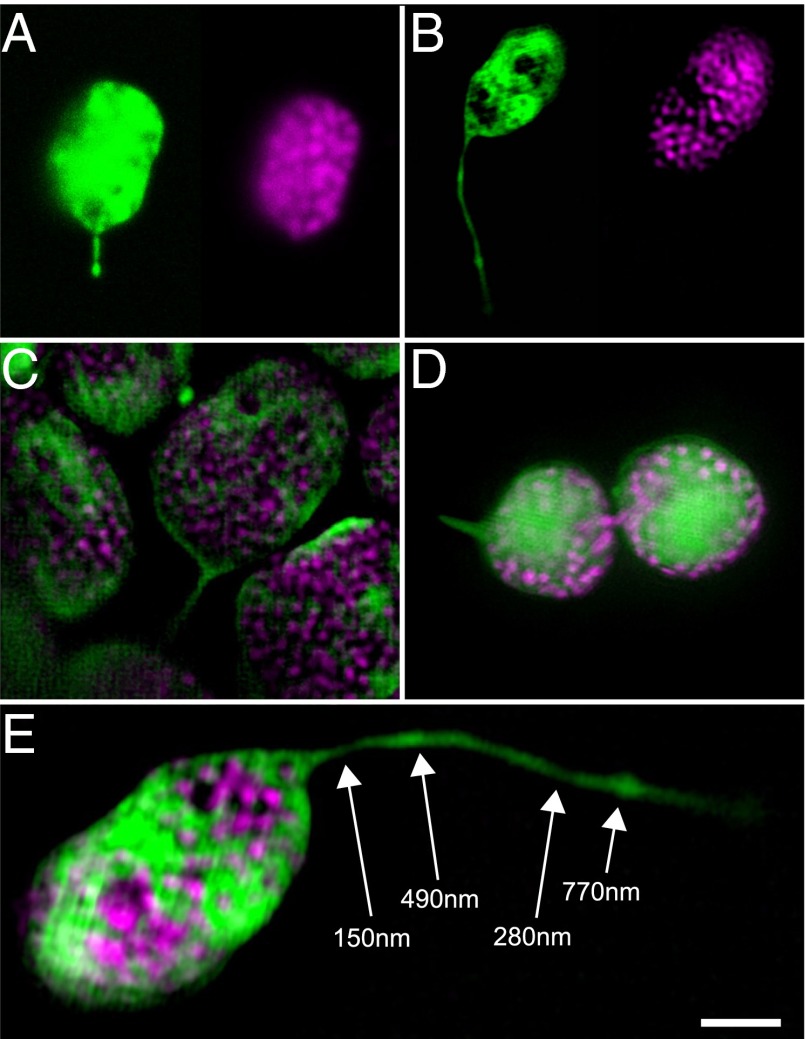
Because stromules can form in isolation after extraction of chloroplasts from their cellular context, we decided to further investigate stromules using superresolution microscopy to gain new insight into their ultrastructure, which will inform future efforts at identifying the chloroplast-associated structural components responsible for stromule formation. The diameter of stromules is postulated to be <200 nm, but this is below the diffraction limit of conventional light microscopy (10, 33), even under optimal conditions. Visualizing stromules by transmission electron microscopy is challenging, because stromule membranes are not easily distinguished from other membranes in thin sections required for conventional electron microscopy. We used 3D structured illumination microscopy (3D-SIM) to obtain the highest-resolution images of wild-type stromules to date (10, 33), and present some representative examples in Fig. 4 and Movie S3. The improved resolution of 3D-SIM is illustrated by the well-defined thylakoid grana in 3D-SIM images (Fig. 4 B‒E) compared with thylakoids visualized by more conventional confocal scanning laser microscopy (Fig. 4A).
Fig. 4.
Examples of fluorescent stromules in N. benthamiana chloroplasts visualized by 3D structured illumination microscopy (3D-SIM). (A and B) Comparison of confocal laser scanning microscopy (A; also shown in Fig. S11) and 3D-SIM (B; also shown in E and in Movie S3) to visualize chloroplast structure (Left: stromal GFP, green; Right: thylakoid chlorophyll, magenta). In particular, note the improved resolution of stromule width and the clarity of the thylakoid grana in the 3D-SIM z-slice (B). (C) 3D-SIM z-slice image of mesophyll chloroplasts with stromules. (D) An epidermal chloroplast connected by a thin bridge that contains both stroma and thylakoids also has a stromule (Left), as shown by SIM. (E) 3D-SIM reveals variability in stromule width. Stromal GFP, green; chlorophyll autofluorescence, magenta. (Scale bar: 2 μm.) One z-slice from a 3D-SIM reconstruction is shown, with measured stromule diameters labeled at indicated positions (white arrows).
At their smallest, we observed stromules <150 nm in diameter (Fig. 4E); given that this is approximately the resolution of 3D-SIM, stromules could be even narrower. 3D-SIM also revealed striking variability in stromule diameter along the length of an individual stromule. Stromules often were narrowest near the chloroplast body, and then typically varied between approximately 200 and 600 nm wide at different positions along their lengths, as shown in Fig. 4E. The variability in stromule width was apparent whether we observed chloroplasts in planta or after isolation, suggesting that structural factors inside the chloroplast could be responsible for the heterogeneous diameter of stromules.
Conclusions
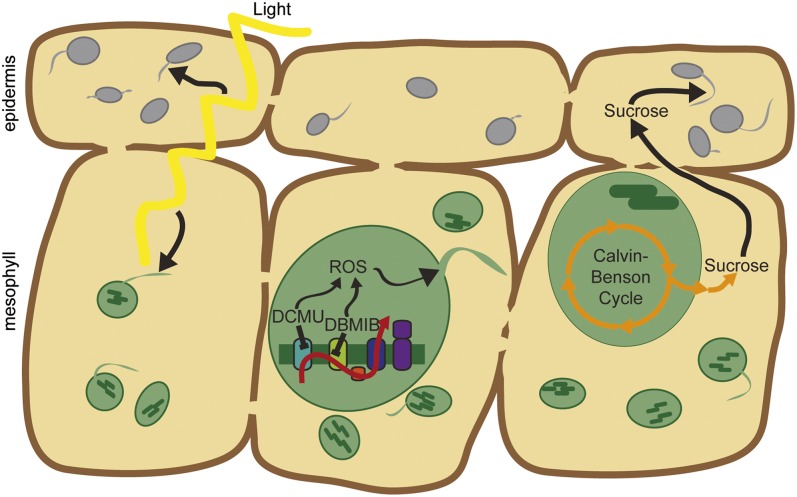
Chloroplasts are extraordinarily independent organelles with their own genomes, as many as 3,000 different proteins, and an array of biochemical activities ranging from photosynthesis and carbon fixation to the synthesis of amino acids, fatty acids, hormones, and pigments. Here we have shown that chloroplasts are even more independent, generating stromules in response to changes in the internal chloroplast redox status in a pathway regulated by the chloroplast NADPH-dependent thioredoxin reductase, NTRC. Leucoplasts, nonphotosynthetic plastids, do not make stromules in response to the same redox cues as chloroplasts, but instead are responsive to sucrose concentration, demonstrating that different types of plastids form stromules in response to different signals. We propose a model consistent with these findings that light promotes stromule formation in leaves by increasing ROS in chloroplasts (34) and by increasing sucrose levels in cells with leucoplasts (Fig. 5).
Fig. 5.
Stromules are initiated by signals within the chloroplast. (Left) Stromule frequency increases in the light (daytime) in both chloroplasts and leucoplasts. (Center) ROS generated from the pETC trigger stromule formation in chloroplasts. (Right) Sucrose promotes stromule formation in leucoplasts, but not chloroplasts. Sucrose is synthesized in the cytosol from products of the Calvin–Benson cycle in chloroplasts and then moves into neighboring heterotrophic pavement cells via plasmodesmata. For simplicity of presentation, only photosynthetic mesophyll cells are shown (and not photosynthetic guard cells), because there is no evidence suggesting that stromules in these cell types behave differently.
Previous reports have investigated stromules using a variety of plastid types in a broad range of species and tissues, generally assuming that stromules act similarly in all cells (9‒19). In light of the clear differences in signals that influence leucoplast and chloroplast stromule formation (Figs. 2 and 5), future work will need to carefully consider the biological context of stromule activity. With the discovery that stromules extend from chloroplasts independently of external structures, analogies to cytonemes could help reveal the roles of stromules, because cytonemes are comparable thin, tubular projections that extend from animal cells to facilitate intercellular communication during development (35, 36). Although the function of stromules remains unknown, it may be speculated that they similarly facilitate signal transduction between organelles, given that stromules have been observed associating with the nucleus, plasma membrane, endoplasmic reticulum, and other plastids (10, 11, 30).
Numerous studies in just the past few years have demonstrated the vital importance of chloroplast-to-nucleus signaling in plant growth and responses to stress (1‒8, 23, 24, 27), with critical agricultural implications, but the structural pathways underlying this signal transduction remain largely uncharacterized. Stromules may contribute to these pathways, because they dynamically respond to physiological signals inside the chloroplast. Continued study of stromules may illuminate how chloroplasts physically interact with their environment to coordinate cellular function.
Methods
Plant and Chemical Materials.
For most N. benthamiana studies, we used a stable transgenic stromal fluorescent marker line, 35SPRO:FNRtp:EGFP, designated pt-GFP herein (30). We also used the N. benthamiana wild-type accession Nb-1 for isolation of wild-type chloroplasts. For A. thaliana studies, we used a stable transgenic stromal fluorescent marker line (4), 35SPRO:RbcStp:roGFP2, designated pt-roGFP2 herein. pt-roGFP2 was also used for measuring stromal redox status.
We obtained DCMU (also known as Diuron; product no. D2425), DBMIB (product no. 271993), and SHAM (product no. S607) from Sigma-Aldrich. Concentrated stock solutions were prepared in DMSO.
Microscopy.
All standard stromule visualization experiments were performed using a Zeiss LSM 710 confocal microscope equipped with an acousto-optical tunable filter to tightly control laser power. GFP was excited with a 488-nm laser with <0.25 mW original power, and emissions from 500 to 530 nm were detected. The 3D-SIM was performed using a Zeiss Elyra PS.1 microscope equipped with standard GFP and Cy5 filter sets.
Diurnal Time Course Experiment.
N. benthamiana stably expressing pt-GFP was grown for 5 d under 100 μmol of photons m−2s−1 (measured with a LI-COR 250A light meter with a LI-190R quantum sensor that detects photosynthetically active radiation, 400–700 nm) with 12-h day length. Epidermal chloroplasts of cotyledons of intact plants were observed every 4 h over the course of 48 h. A green light-emitting diode was used during night time points to prevent exposure to photosynthetically active radiation.
pETC Inhibitor Treatments.
A. thaliana pt-roGFP2 plants were stratified for 3 d at 4 °C and then grown for 14 d under 100 μmol of photons m−2s−1 of light with 16 h day length. The redox status of pt-roGFP2 in A. thaliana cotyledons was measured after treatment with 10 μM DCMU, 12 μM DBMIB, or 0.1% DMSO (control treatment), following the protocol described by Stonebloom et al. (4) but using a Zeiss LSM 710 confocal microscope with 405-nm and 488-nm lasers, collecting emissions ranging from 500 to 530 nm.
N. benthamiana stably expressing pt-GFP was grown for 14 d under 100 μmol of photons m−2s−1 of light with a 16-h day length. Cotyledons were painted with DCMU (10 μM from 10 mM stock solution in DMSO), DBMIB (12 μM from 12 mM stock solution in DMSO), or control 2 h before observation of epidermal chloroplasts.
To measure stromule frequency in A. thaliana (grown as above to measure redox status), pt-roGFP2 cotyledons were removed and placed on 0.5× Murashige and Skoog plates (0.8% agar, pH 5.6) with DCMU (10 μM from 10 mM stock solution in DMSO), DBMIB (12 μM from 12 mM stock solution in DMSO), SHAM (200 μM in DMSO), sucrose (30 mM, or 1% wt/vol), or control for 2 h before imaging of cotyledon epidermal leucoplasts (of pavement cells) and chloroplasts (of guard cells).
VIGS.
pt-GFP plants were grown for 3 wk under 100 μmol of photons m−2s−1 of light with a 16-h day length before agroinfiltration with the appropriate VIGS vectors. Details of VIGS vector construction are provided in SI Methods. Two weeks later, young leaves with silenced gene expression were cut, and the epidermal chloroplasts of the basal region of the leaf were visualized immediately.
Chloroplast Extraction.
Intact chloroplasts were extracted from mature leaves of pt-GFP (N. benthamiana), Nb-1 (wild-type N. benthamiana), pt-roGFP2 (A. thaliana), and spinach by grinding leaves in extraction buffer (50 mM Hepes NaOH, 330 mM sorbitol, 2 mM EDTA, 1 mM MgCl2, 1 mM MnCl2, pH 6.9), filtering the homogenate through several layers of cheesecloth, and then pelleting by centrifugation and resuspending in isolation buffer (50 mM Hepes NaOH, 330 mM sorbitol, 2 mM EDTA, 1 mM MgCl2, 1 mM MnCl2, 10 mM KCl, 1 mM NaCl, pH 7.6) as described previously (31). More complex protocols, such as inclusion of a Percoll gradient for purification (35% vol/vol), had no discernable effect on chloroplast stromule formation. Isolated spinach or wild-type N. benthamiana chloroplasts were incubated with an equal volume of 25 mg/L 5 (6)-carboxyfluorescein diacetate (Sigma-Aldrich, product no. 21879) for 5 min (32), centrifuged at 700 × g for another 60 s, and resuspended in isolation buffer. Chloroplasts were visualized by confocal or structured illumination microscopy immediately after isolation.
Data Analysis.
Stromule frequencies were counted using ImageJ software (imagej.nih.gov/ij/) to scan through z-stacks of confocal images using a focal depth of 1 Airy unit, which allowed us to visualize stromules extended in any axis from the plastid. Stromules were counted regardless of length but only if <1 μm in diameter, as described by Hanson and Sattarzadeh (10). Most previous studies reported stromule frequencies per cell, considering multiple cells from a single leaf as independent samples. Schattat and Klösgen (16), for example, found little variation in stromule frequencies within an individual leaf, but dramatic variation in stromule frequencies between different leaves. This led them to treat separate fields of view within a single leaf as distinct samples, reducing the apparent variation and effectively increasing statistical power. We also found that stromule frequency varied very little among cells within a leaf, but varied notably among leaves of the same age and condition from different plants. Therefore, we considered one leaf per plant as an individual sample (n), and observed many plants for each experiment. Throughout an experiment, all of the analyzed leaves experienced the same growth conditions and were observed at the same age and size.
We conducted power analysis (α = 0.05; β = 0.20) on pilot studies under our growth conditions to determine the sufficient sample size to confidently assert whether or not a treatment caused changes in stromule frequency, and found an approximate minimal n ≥16 for N. benthamiana time course and chemical treatment experiments, and n ≥ 8 for all other experiments. Per treatment, we counted more than 5,000 plastids, at least 100 cells, and ∼20 plants for each chemical treatment or 8 plants for each silencing experiment. Mean stromule frequencies were compared with the Student t test, with significance indicated at P < 0.05.
We also analyzed all data using angular transformations to account for differences in variation in datasets with very high or low stromule frequencies, but the transformation had no impact on the statistical significance of our results, so we present the data as raw frequencies for the purpose of clear presentation. SEs are presented throughout to describe stromule frequencies. R (www.r-project.org) was used for all statistical analyses.
SI Methods
VIGS.
GUN2 (TAIR ID At2g26670) and NTRC (TAIR ID At2g41680) orthologs in N. benthamiana were identified from gene trees generated by BLAST searches using A. thaliana protein sequence queries (Figs. S6–S9). N. benthamiana is an allotetraploid resulting from the hybridization of two Nicotiana species, and thus often has two homeologs of genes that are single copy in A. thaliana. Moreover, both GUN2 and NTRC are members of larger gene families. In A. thaliana, there are three NADPH-dependent thioredoxin reductases (NTRA and NTRB in the cytosol and mitochondria, and NTRC in plastids) and four heme oxygenases (HO1, HO3, and HO4 are closely related and partially redundant, whereas HO2 serves a distinct function) (37, 38).
To identify all true orthologs of NTRC and HO1 and design silencing constructs targeting these genes, but not orthologs of NTRA/NTRB or HO2, we used A. thaliana protein sequences in a BLAST search against the N. benthamiana genome (v0.4.4 predicted cDNAs) from the Sol Genomics Network (solgenomics.net) and also against the land plant genomes available at Phytozome (www.phytozome.net). Amino acid sequences of these putative homologs were aligned with MAFFT (mafft.cbrc.jp) using the highly accurate L-INS-i algorithm. Aligned residues were used to generate a gene tree with the neighbor-joining method using 100 samples for bootstrapping. Silencing triggers were then designed to silence all copies of the target genes but no other genes in the N. benthamiana genome, using BLAST searches to confirm that no genomic sequence between 20 and 24 nucleotides long matched the silencing trigger with fewer than three mismatches.
NbISE2, NbGUN2, and NbNTRC were silenced using VIGS as described by Brunkard et al. (25) alongside a viral negative control, pYC1 [Tobacco rattle virus (TRV) with a mock silencing trigger against β-glucoronidase]. In brief, to prepare the silencing constructs, RNA was extracted from N. benthamiana genotype Nb-1 (Spectrum Plant Total RNA Kit; Sigma-Aldrich) and treated with DNase I (New England Biolabs). This RNA was used to synthesize cDNA with SuperScript III Reverse Transcriptase (Invitrogen) and oligo(dT)20 primers. Silencing triggers were then amplified and inserted into the TRV-derived VIGS vector, pYL156, using standard restriction enzyme-based methods (25).
Oligonucleotides.
Oligonucleotides used to clone silencing triggers are shown with restriction sites underscored and nucleotides matching the genomic sequences in upper case:
NbGUN2_F: 5ʹgattctagaATGGCTTCAATTACACCCTTATC3ʹ
NbGUN2_R: 5ʹgatctcgagTCAAGACAATATTAATCGGAGG3ʹ
NbNTRC_F: 5ʹgtatctAGAACTTGGTAATAATAGGATCAGGTCC3ʹ
NbNTRC_R: 5ʹctgctcgagCAAAATTCATCTTCACG3ʹ
Supplementary Material
Acknowledgments
We thank J. Mathur for the generous gift of pt-GFP N. benthamiana seeds, M. A. Ahern for corroborating stromule counts, and S. E. Ruzin and D. Schichnes of the University of California Berkeley College of Natural Resources Biological Imaging Facility for microscopy support. Research reported in this publication was supported in part by the National Institutes of Health S10 program under award numbers 1S10RR026866-01 and 1S10OD018136-01. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. J.O.B. and A.M.R. were supported by predoctoral fellowships from the National Science Foundation.
Footnotes
The authors declare no conflict of interest.
See Commentary on page 9799.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1511570112/-/DCSupplemental.
References
- 1.Koussevitzky S, et al. Signals from chloroplasts converge to regulate nuclear gene expression. Science. 2007;316(5825):715–719. [PubMed] [Google Scholar]
- 2.Estavillo GM, et al. Evidence for a SAL1-PAP chloroplast retrograde pathway that functions in drought and high light signaling in Arabidopsis. Plant Cell. 2011;23(11):3992–4012. doi: 10.1105/tpc.111.091033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burch-Smith TM, Brunkard JO, Choi YG, Zambryski PC. Organelle-nucleus cross-talk regulates plant intercellular communication via plasmodesmata. Proc Natl Acad Sci USA. 2011;108(51):E1451–E1460. doi: 10.1073/pnas.1117226108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stonebloom S, et al. Redox states of plastids and mitochondria differentially regulate intercellular transport via plasmodesmata. Plant Physiol. 2012;158(1):190–199. doi: 10.1104/pp.111.186130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiao Y, et al. Retrograde signaling by the plastidial metabolite MEcPP regulates expression of nuclear stress-response genes. Cell. 2012;149(7):1525–1535. doi: 10.1016/j.cell.2012.04.038. [DOI] [PubMed] [Google Scholar]
- 6.Nomura H, et al. Chloroplast-mediated activation of plant immune signalling in Arabidopsis. Nat Commun. 2012;3:926. doi: 10.1038/ncomms1926. [DOI] [PubMed] [Google Scholar]
- 7.Avendaño-Vázquez AO, et al. An uncharacterised apocarotenoid-derived signal generated in ζ-carotene desaturase mutants controls leaf development and the expression of chloroplast and nuclear genes in Arabidopsis. Plant Cell. 2014;26(6):2524–2537. doi: 10.1105/tpc.114.123349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petrillo E, et al. A chloroplast retrograde signal regulates nuclear alternative splicing. Science. 2014;344(6182):427–430. doi: 10.1126/science.1250322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Köhler RH, Cao J, Zipfel WR, Webb WW, Hanson MR. Exchange of protein molecules through connections between higher plant plastids. Science. 1997;276(5321):2039–2042. doi: 10.1126/science.276.5321.2039. [DOI] [PubMed] [Google Scholar]
- 10.Hanson MR, Sattarzadeh A. Stromules: Recent insights into a long neglected feature of plastid morphology and function. Plant Physiol. 2011;155(4):1486–1492. doi: 10.1104/pp.110.170852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schattat MH, et al. Differential coloring reveals that plastids do not form networks for exchanging macromolecules. Plant Cell. 2012;24(4):1465–1477. doi: 10.1105/tpc.111.095398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wildman SG, Hongladarom T, Honda SI. Chloroplasts and mitochondria in living plant cells: Cinephotomicrographic studies. Science. 1962;138(3538):434–436. doi: 10.1126/science.138.3538.434. [DOI] [PubMed] [Google Scholar]
- 13.Natesan SK, Sullivan JA, Gray JC. Stromules: A characteristic cell-specific feature of plastid morphology. J Exp Bot. 2005;56(413):787–797. doi: 10.1093/jxb/eri088. [DOI] [PubMed] [Google Scholar]
- 14.Holzinger A, Buchner O, Lütz C, Hanson MR. Temperature-sensitive formation of chloroplast protrusions and stromules in mesophyll cells of Arabidopsis thaliana. Protoplasma. 2007;230(1-2):23–30. doi: 10.1007/s00709-006-0222-y. [DOI] [PubMed] [Google Scholar]
- 15.Natesan SK, Sullivan JA, Gray JC. Myosin XI is required for actin-associated movement of plastid stromules. Mol Plant. 2009;2(6):1262–1272. doi: 10.1093/mp/ssp078. [DOI] [PubMed] [Google Scholar]
- 16.Schattat MH, Klösgen RB. Induction of stromule formation by extracellular sucrose and glucose in epidermal leaf tissue of Arabidopsis thaliana. BMC Plant Biol. 2011;11:115. doi: 10.1186/1471-2229-11-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gray JC, et al. Plastid stromules are induced by stress treatments acting through abscisic acid. Plant J. 2012;69(3):387–398. doi: 10.1111/j.1365-313X.2011.04800.x. [DOI] [PubMed] [Google Scholar]
- 18.Erickson JL, et al. Agrobacterium-derived cytokinin influences plastid morphology and starch accumulation in Nicotiana benthamiana during transient assays. BMC Plant Biol. 2014;14:127. doi: 10.1186/1471-2229-14-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holzinger A, Kwok EY, Hanson MR. Effects of arc3, arc5 and arc6 mutations on plastid morphology and stromule formation in green and nongreen tissues of Arabidopsis thaliana. Photochem Photobiol. 2008;84(6):1324–1335. doi: 10.1111/j.1751-1097.2008.00437.x. [DOI] [PubMed] [Google Scholar]
- 20.Veley KM, Marshburn S, Clure CE, Haswell ES. Mechanosensitive channels protect plastids from hypoosmotic stress during normal plant growth. Curr Biol. 2012;22(5):408–413. doi: 10.1016/j.cub.2012.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hughes J. Phytochrome cytoplasmic signaling. Annu Rev Plant Biol. 2013;64:377–402. doi: 10.1146/annurev-arplant-050312-120045. [DOI] [PubMed] [Google Scholar]
- 22.Bell CD, Soltis DE, Soltis PS. The age and diversification of the angiosperms re-revisited. Am J Bot. 2010;97(8):1296–1303. doi: 10.3732/ajb.0900346. [DOI] [PubMed] [Google Scholar]
- 23.Woodson JD, Chory J. Coordination of gene expression between organellar and nuclear genomes. Nat Rev Genet. 2008;9(5):383–395. doi: 10.1038/nrg2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chi W, Sun X, Zhang L. Intracellular signaling from plastid to nucleus. Annu Rev Plant Biol. 2013;64:559–582. doi: 10.1146/annurev-arplant-050312-120147. [DOI] [PubMed] [Google Scholar]
- 25.Brunkard JO, Burch-Smith TM, Runkel AM, Zambryski P. Investigating plasmodesmata genetics with virus-induced gene silencing and an Agrobacterium-mediated GFP movement assay. In: Heinlein M, editor. Plasmodesmata: Methods and Protocols. Humana; New York: 2015. pp. 185–198. [DOI] [PubMed] [Google Scholar]
- 26.Barkan A. Expression of plastid genes: Organelle-specific elaborations on a prokaryotic scaffold. Plant Physiol. 2011;155(4):1520–1532. doi: 10.1104/pp.110.171231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Terry MJ, Smith AG. A model for tetrapyrrole synthesis as the primary mechanism for plastid-to-nucleus signaling during chloroplast biogenesis. Front Plant Sci. 2013;4:14. doi: 10.3389/fpls.2013.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michalska J, Zauber H, Buchanan BB, Cejudo FJ, Geigenberger P. NTRC links built-in thioredoxin to light and sucrose in regulating starch synthesis in chloroplasts and amyloplasts. Proc Natl Acad Sci USA. 2009;106(24):9908–9913. doi: 10.1073/pnas.0903559106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schattat MH, Klösgen RB, Mathur J. New insights on stromules: Stroma filled tubules extended by independent plastids. Plant Signal Behav. 2012;7(9):1132–1137. doi: 10.4161/psb.21342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schattat M, Barton K, Baudisch B, Klösgen RB, Mathur J. Plastid stromule branching coincides with contiguous endoplasmic reticulum dynamics. Plant Physiol. 2011;155(4):1667–1677. doi: 10.1104/pp.110.170480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joly D, Carpentier R. Rapid isolation of intact chloroplasts from spinach leaves. In: Carpentier R, editor. Photosynthesis Research Protocols. Humana; New York: 2011. pp. 321–325. [DOI] [PubMed] [Google Scholar]
- 32.Schulz A, Knoetzel J, Scheller HV, Mant A. Uptake of a fluorescent dye as a swift and simple indicator of organelle intactness: Import-competent chloroplasts from soil-grown Arabidopsis. J Histochem Cytochem. 2004;52(5):701–704. doi: 10.1177/002215540405200514. [DOI] [PubMed] [Google Scholar]
- 33.Shaw SL, Ehrhardt DW. Smaller, faster, brighter: Advances in optical imaging of living plant cells. Annu Rev Plant Biol. 2013;64:351–375. doi: 10.1146/annurev-arplant-042110-103843. [DOI] [PubMed] [Google Scholar]
- 34.Lai AG, et al. CIRCADIAN CLOCK-ASSOCIATED 1 regulates ROS homeostasis and oxidative stress responses. Proc Natl Acad Sci USA. 2012;109(42):17129–17134. doi: 10.1073/pnas.1209148109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bischoff M, et al. Cytonemes are required for the establishment of a normal Hedgehog morphogen gradient in Drosophila epithelia. Nat Cell Biol. 2013;15(11):1269–1281. doi: 10.1038/ncb2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roy S, Huang H, Liu S, Kornberg TB. Cytoneme-mediated contact-dependent transport of the Drosophila decapentaplegic signaling protein. Science. 2014;343(6173):1244624. doi: 10.1126/science.1244624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reichheld JP, et al. Inactivation of thioredoxin reductases reveals a complex interplay between thioredoxin and glutathione pathways in Arabidopsis development. Plant Cell. 2007;19(6):1851–1865. doi: 10.1105/tpc.107.050849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gisk B, Yasui Y, Kohchi T, Frankenberg-Dinkel N. Characterization of the haem oxygenase protein family in Arabidopsis thaliana reveals a diversity of functions. Biochem J. 2010;425(2):425–434. doi: 10.1042/BJ20090775. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.