Significance
Epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKI) have demonstrated clinical benefits for patients suffering from non–small cell lung cancer of adenocarcinoma with activating mutation of EGFR. Same mutations observed in lung squamous cell carcinoma do not offer similar benefits. The underlining resistance mechanism revealed in this study turns out to be the concurrent activation of bone morphogenetic proteins (BMPs) signaling pathway in lung squamous cell carcinoma. The combination of EGFR-TKI with inhibitors of BMP receptors signaling pathway overcame the resistance. Such a finding provided a viable clinical strategy to treat those patients.
Keywords: epidermal growth factor receptor tyrosine kinase inhibitor, lung squamous cell carcinoma, bone morphogenetic proteins, drug resistance
Abstract
The empirical criteria for defining a clinical subtype of lung cancer are gradually transiting from histopathology to genetic variations in driver genes. Targeting these driver mutations, such as sensitizing epidermal growth factor receptor (EGFR) mutations, has dramatically improved the prognosis of advanced non–small cell lung cancer (NSCLC). However, the clinical benefit of molecularly targeted therapy on NSCLC appears to be different between lung adenocarcinomas and squamous cell carcinomas (SqCCs). We report here that the resistance of lung SqCC harboring EGFR mutations to EGFR tyrosine kinase inhibitors (EGFR-TKIs) was due to the activation of BMP-BMPR-Smad1/5-p70S6K. The combined treatment of these tumor cells with EGFR-TKI, together with inhibitors specific to BMPR or downstream mTOR, effectively reversed the resistance to EGFR-TKI. Moreover, blocking the whole PI3K-AKT-mTOR pathway with the PI3K/mTOR dual inhibitor BEZ235 also showed efficacy in treating this subtype of lung SqCC. This study details the empirical basis for a feasible clinical solution for squamous cell carcinomas with EGFR mutations.
Traditionally, the classification of lung cancer has been based primarily on histology and morphology (1, 2). With the identification of mutated driver oncogenes, methods from molecular pathology are gradually transforming the definition of the various types of lung cancers (3). Targeting gene aberrances such as epidermal growth factor receptor (EGFR) mutations (4–8) and anaplastic lymphoma kinase (ALK) fusion (9, 10) has significantly improved the prognosis of advanced non–small cell lung cancer (NSCLC). However, the clinical benefits brought by targeted therapies are mainly limited to nonsquamous NSCLC (11), while chemotherapy remains the major therapeutic choice for squamous cell carcinomas (SqCCs).
Recent studies assessing somatic mutations and copy number alteration (CNA) profiles in SqCC performed by the Cancer Genome Atlas project and other investigators have disclosed specific gene mutations, including GRM8, BAI3, ERBB4, RUNX1T1, KEAP1, and FBXW7, and CNAs in 3q26, 24, 27, 32–34, and 8p12.35 in lung SqCC (12, 13). About half of all patients with lung SqCC carry multiple gene aberrances, indicating that complex genomic characterizations are more common in lung SqCC than in adenocarcinoma (ADC). Thus, successful therapeutic strategies that target a single driver gene in lung ADC might not be feasible for lung SqCC patients.
Targeting EGFR mutations by EGFR tyrosine kinase inhibitors (TKIs) is one of the successful strategies in treating lung ADC. EGFR-TKIs obtained median progression-free survival (PFS) of 10–13 mo in EGFR-mutated lung ADC, but only ∼3 mo in lung SqCC with EGFR mutations (11, 14). Moreover, whether EGFR mutations exist in lung SqCC still remains controversial. There is a belief that EGFR mutations might not even occur in pure pulmonary SqCC, and that the occasional detection of these mutations in samples diagnosed as SqCC was due to mixed adenosquamous carcinoma and poorly differentiated ADC (15, 16). In current clinical practice, the utilization of EGFR-TKIs and the assessment of EGFR mutations are still routinely performed in lung SqCC, especially in nonsmokers. Therefore, it is critical to identify the subgroups of lung SqCC patients that are suitable for EGFR-TKI treatment. Exploring the mechanism of resistance to EGFR-TKIs in lung SqCC will deepen our understanding of the differences in tumorigenic profiling between lung SqCC and ADC, and should contribute to guiding clinical therapeutic decisions.
In the present study, we demonstrated that lung SqCC patients with EGFR mutations indeed represent a subset of NSCLC. We further showed that lung SqCC cell lines were resistant to EGFR-TKIs both in vitro and in vivo. We also investigated the mechanisms of resistance to EGFR-TKI in this subset of patients and provided potential strategies for overcoming TKI resistance in these patients.
Results
Lung Squamous Cell Carcinomas with EGFR Mutations Define a Unique Subtype of NSCLC.
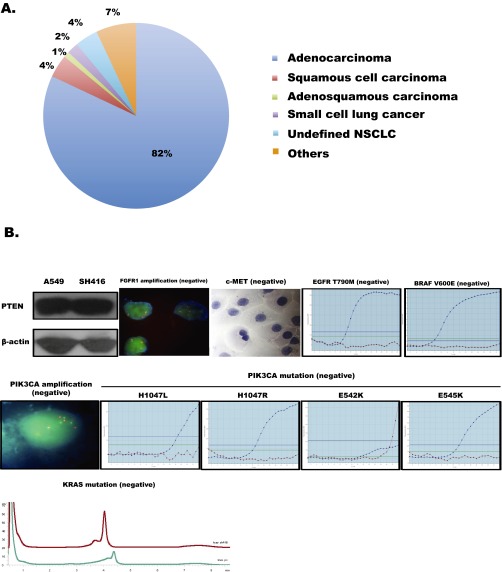
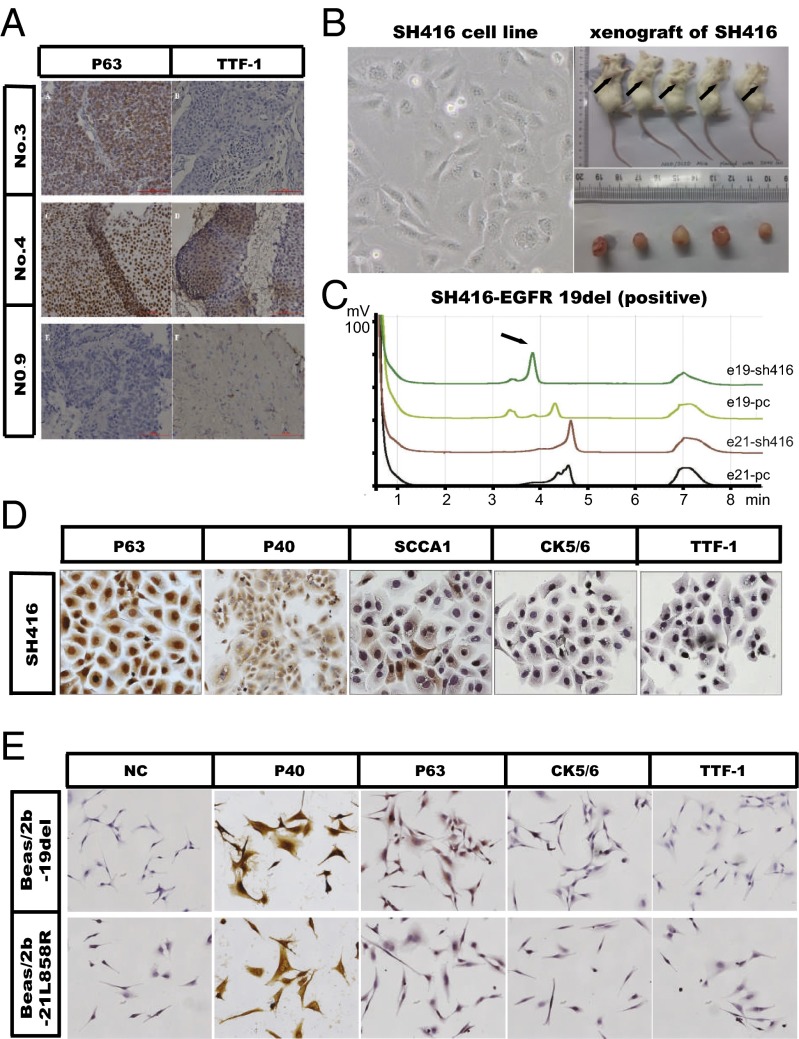
To determine the frequency of EGFR mutations in lung squamous cell carcinomas of Chinese patients, we first examined the percentage of EGFR mutations in Chinese lung cancer patients. We screened tumor tissue specimens from 2525 patients with advanced lung cancer for EGFR mutations (19del and 21L858R). There were 863 patients harboring EGFR mutations, among which lung SqCC contributed about 4.4% (38/863; Fig. S1A). In 396 lung SqCC cases, patients with EGFR mutations accounted for about 9.6% (38/396). In the 38 SqCC patients carrying EGFR mutations, the majority were male (81.6%), smokers (86.8%), and had the EGFR 19del mutation (57.9%) (Table S1). To confirm whether squamous cells were the pure component, we performed immunohistochemistry (IHC) biomarker analyses (P63 and TTF-1) in 18 patients with sufficient biopsied specimens. Most of these specimens (16/18) were positive for P63 and negative for TTF-1 (Table S2 and Fig. 1A).
Fig. S1.
The distribution of EGFR mutations in lung cancer patients and the genetic characteristics of SH416 cells. (A) Pie chart representation of the distributions of different histologies in tumor tissues carrying EGFR mutations (19del and 21L858R). (B) Possible driver gene aberrances were detected in SH416 cells, including PTEN expression, mutations of KRAS, BRAF V600E, and PIK3CA (E545K, E542K, H1047R, and H1047L), and amplification of FGFR1, PIK3CA, and c-MET. Cell extracts were immunoblotted to detect PTEN protein. FISH was used for identifying amplifications of FGFR1 and PIK3CA. ICC staining was used for identifying c-MET expression. The Amplification-Refractory Mutation System (ARMS) was used for detecting mutations in KRAS as well as in BRAF V600E and PIK3CA.
Table S1.
The clinical characteristics of lung squamous cell carcinoma with sensitizing EGFR mutations (n = 38)
| Characteristics | No. | Present, % |
| Age, y | ||
| Median (range) | 67 (39–84) | |
| Sex | ||
| Male | 31 | 81.6 |
| Female | 7 | 18.4 |
| Smoking status | ||
| Never/light* | 5 | 13.2 |
| Former/current | 33 | 86.8 |
| EGFR mutation status | ||
| 19del | 22 | 57.9 |
| 21L858R | 16 | 42.1 |
Light smokers were defined as patients who had smoked less than 100 cigarettes in their lifetime.
Table S2.
The immunohistochemistry staining status of lung SqCC patients harboring EGFR mutations (n = 18)
| Case number | Sex | Age, y | Smoking status | EGFR mutation status | PFS after EGFR-TKI, mo | P63 | TTF-1 |
| 1 | Female | 84 | Never/light | 19del | 4.2 | + | − |
| 2 | Female | 67 | Never/light | 21L858R | 1.1 | + | − |
| 3 | Male | 53 | Former/current | 19del | 2.3 | + | − |
| 4 | Male | 64 | Former/current | 21L858R | 1.0 | + | + |
| 5 | Male | 57 | Former/current | 21L858R | 2.3 | + | − |
| 6 | Male | 74 | Never/light | 19del | 2.2 | + | − |
| 7 | Male | 42 | Former/current | 19del | 1.9 | + | − |
| 8 | Male | 72 | Former/current | 19 DEL | 1.1 | + | − |
| 9 | Male | 74 | Former/current | 19del | 1.2 | − | − |
| 10 | Male | 55 | Former/current | 21L858R | 1.1 | + | − |
| 11 | Female | 54 | Never/light | 19del | 23.2 | + | — |
| 12 | Female | 32 | Never/light | 19del | 1.0 | + | — |
| 13 | Male | 60 | Former/current | 21L858R | NA | + | — |
| 14 | Female | 39 | Never/light | 21L858R | NA | + | — |
| 15 | Male | 57 | Former/current | 19del | NA | + | — |
| 16 | Male | 53 | Former/current | 21L858R | NA | + | — |
| 17 | Male | 62 | Former/current | 19del | NA | + | — |
| 18 | Male | 72 | Former/current | 21L858R | NA | + | — |
Fig. 1.
SqCCs with EGFR mutations represent a subset of NSCLCs. (A) Representative IHC images of patients with lung SqCC. Formalin-fixed, paraffin-embedded sections (4 μm) were stained for P63 and TTF-1. (B) SH416 (Left), a stable cell line derived from a lung SqCC patient harboring EGFR 19del showed in vivo tumor formation (Right). (C) Denaturing high-performance liquid chromatography (DHPLC) analysis showing that SH416 cells had the EGFR 19del mutation. (D) SH416 cells showed a lung SqCC phenotype, with positivity for P63, P40, and SCCA1, and negativity for TTF-1 and CK5/6. Immunocytochemistry staining was used for these biomarkers. (E) Beas/2b-19del cells and Beas/2b-21L858R cells both showed a lung SqCC phenotype, with positivity for P40, and negativity for TTF-1, P63, and CK5/6. Immunocytochemistry staining was used for these biomarkers.
To further confirm the existence of EGFR mutations in lung SqCC, we also checked IHC biomarkers in a cell line (SH416) derived from a patient diagnosed with advanced lung SqCC, who was initially identified as carrying EGFR 19del (17) (Fig. 1 B and C). immunocytochemistry (ICC) analysis of P63 (positive), P40 (positive), SCCA1 (partially positive), CK5/6 (negative), and TTF-1 (negative) confirmed that this cell line was a pure squamous cell line (Fig. 1D). To test for the existence of possible driver gene aberrances other than EGFR 19del that might affect the efficacy to EGFR-TKI, we detected PTEN loss, mutations in KRAS, mutations of BRAF V600E and PIK3CA (E545K, E542K, H1047R, and H1047L), and amplifications of FGFR1, PIK3CA, and c-MET; none of these aberrances were found (Fig. S1B).
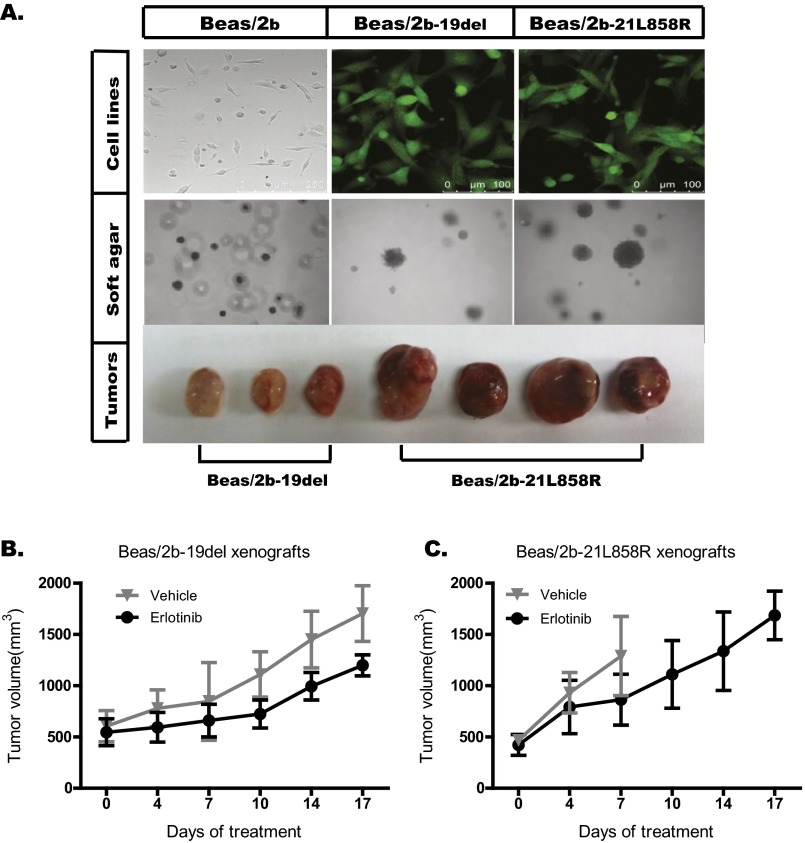
To test whether EGFR mutations are also functional driver mutations in lung SqCC, we engineered cell lines using a human normal epithelial cell line (Beas/2b) in which mutant EGFR genes (EGFR 19 del or EGFR 21L858R) were stably expressed (Fig. S2). Beas/2b cell lines stably expressing mutant EGFR formed colonies at a much higher frequency in soft agar than did the control Beas/2b cell line. More dramatically, these EGFR-expressing mutant Beas/2b cell lines readily formed tumors in BALB/c nude mice, whereas no tumors were observed in mice inoculated with Beas/2b control cell lines (Fig. S2). ICC staining in these two cell lines (Beas/2b-19del cells and Beas/2b-21L858R cells) displayed strong and diffusive P40 positivity and partial P63 positivity, but displayed negative staining of TTF-1 (Fig. 1E), indicating the squamous cell histology of these two cell lines.
Fig. S2.
Construction and confirmation of Beas/2b-19del and Beas/2b-21L858R cells. Beas/2b-19del and Beas/2b-21L858R cell lines were constructed by transfection with the indicated plasmids (pCAG-IN-EGFR-19del -P2A-EGFP and pCAG-IN-EGFR-L858R-P2A-EGFP). (A) Both Beas/2b-19del and Beas/2b-21L858R cells could form foci in soft agar that were significantly larger than those of Beas/2b cells. These two cell lines could also form tumors in nude mice, whereas the Beas/2b cell line could not. (B and C) Balb/C nude mice were s.c. engrafted with Beas/2b-19del and Beas/2b-21L858R cells, and tumors were treated daily with vehicle control or erlotinib at the indicated dose. The tumor volumes (y axis) are plotted over time (x axis). B and C were replotted using data of Fig. 4C to show growth of xenografts following erlotinib treatment.
These results demonstrate that lung SqCC with EGFR mutations indeed exist as a significant fraction of the Chinese lung cancer population and that mutated EGFR can have oncogenic properties in the squamous cell background. Thus, these patients present a unique molecular and histological subtype.
Lung SqCCs with EGFR Mutations Are Resistant to EGFR-TKIs Compared with Adenocarcinoma with EGFR Mutations.
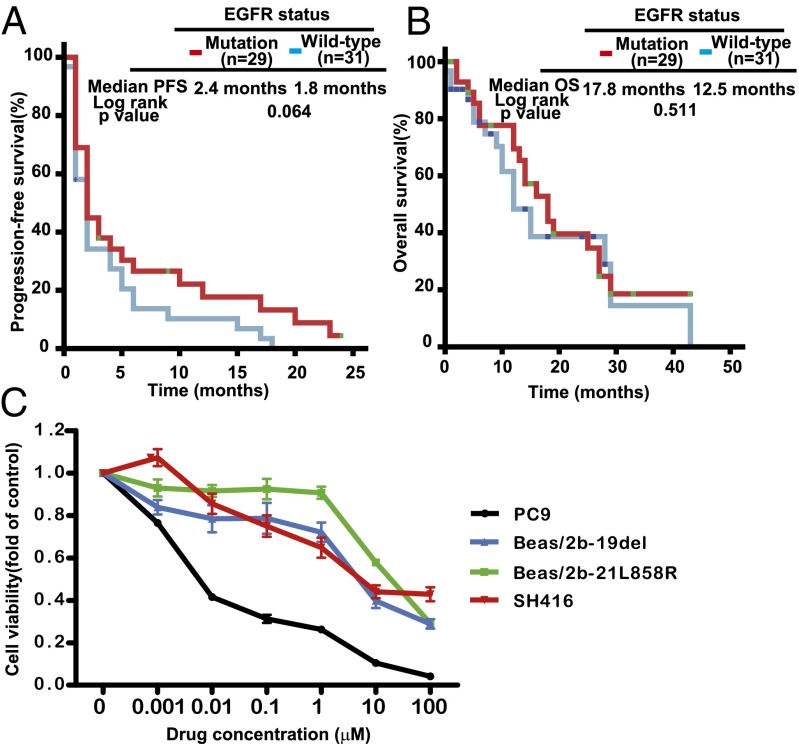
To compare the survival differences after EGFR-TKI treatment between patients with mutant and wild-type EGFRs, we screened 60 patients with advanced lung SqCC who meet following criteria: they received EGFR-TKI treatment and had EGFR mutations diagnosed in the period from June 2005 to June 2012. The median PFS after EGFR-TKI treatment was only 2.4 mo in patients with EGFR mutations, although this was slightly superior to those without EGFR mutations (P = 0.064; Fig. 2A). No significant difference in median overall survival (OS) was observed between patients with mutant and wild-type EGFR (Fig. 2B). The response data and multivariate analysis with COX proportional hazard ratios are presented in Tables S3 and S4. To exclude the possible interference of other common gene aberrances, we also tested for possible concurrent gene variations in PIK3CA, KRAS, DDR2, and FGFR1. Of 29 patients with EGFR mutations, there were nine cases with other key mutations, including two cases with FGFR1 amplification, one case with PIK3CA amplification, five cases with EGFR VIII mutation, and one case with KRAS mutation; there were no cases with PIK3CA mutation or DDR2 mutation. After removing the nine cases with other concurrent mutations, the median PFS and OS after EGFR-TKI treatment remained 2.3 and 17.5 mo, respectively.
Fig. 2.
SqCCs with EGFR mutation were resistant to EGFR-TKIs. (A) Kaplan–Meier curve shows that the median PFS after EGFR-TKI in lung SqCC patients carrying sensitizing EGFR mutation was 2.4 mo, similar to patients with wild-type EGFR (2.4 mo vs. 1.8 mo, P = 0.064). (B) Kaplan–Meier curve shows that the median overall survival after EGFR-TKI in lung SqCC patients carrying sensitizing EGFR mutation was 17.8 mo, similar to those with wild-type EGFR (17.8 mo vs. 12.5 mo, P = 0.511). (C) Cell viability analysis showed that lung SqCC cell lines (Beas/2b-19del, Beas/2b-21L858R, and SH416) presented stronger resistance to erlotinib than did a lung adenocarcinoma cell line with EGFR mutation (PC9). Viability at 72 h was calculated as the ratio of viable erlotinib-exposed cells to viable DMSO-treated cells.
Table S3.
Comparison of the best response to EGFR-TKI between EGFR mutation (n = 29) and wild type (n = 31)
| Best response to EGFR-TKI | EGFR mutation (%) | EGFR wild type (%) | P value |
| PR | 3 (10.4) | 0 (0) | 0.092 |
| SD | 15 (51.7) | 13 (41.9) | |
| PD | 11 (37.9) | 18 (58.1) |
Table S4.
Multivariate analysis by COX proportional hazard model (n = 60)
| Factors | PFS | OS | ||||
| P | HR | 95% CI | P | HR | 95% CI | |
| Sex (male vs. female) | 0.360 | 1.63 | 0.572–4.648 | 0.753 | 0.807 | 0.213–3.062 |
| Age (≤65 vs. >65) | 0.013 | 2.180 | 1.183–4.018 | 0.012 | 2.750 | 1.254–6.031 |
| Smoking status (never/light vs. ever/current) | 0.238 | 1.736 | 0.694–4.346 | 0.769 | 1.180 | 0.390–3.574 |
| Type of EGFR-TKI (gefitinib vs. erlotinib) | 0.877 | 0.948 | 0.483–1.863 | 0.184 | 1.930 | 0.732–5.089 |
| Line of EGFR-TKI (first line vs. second and more than line) | 0.762 | 0.898 | 0.447–1.804 | 0.012 | 0.347 | 0.153–0.790 |
| EGFR mutation status (wild type vs. mutant type)* | 0.023 | 0.500 | 0.275–0.909 | 0.260 | 0.651 | 0.309–1.373 |
EGFR mutation types included the EGFR 19del and 21L858R mutations.
We then tested the responses of lung SqCC cell lines to the EGFR inhibitor erlotinib. Cell viability tests showed that lung SqCC cell lines (SH416, Beas/2b-19del, and Beas/2b-21L858R) were more resistant to erlotinib than a lung ADC cell line with the EGFR 19del mutation (PC9 cell line; Fig. 2C). The IC50 values of the SH416, Beas/2b-19del, and Beas/2b-21L858R cell lines were 9.28, 3.43, and 11.9 μM, respectively. These concentrations were more than 100-fold higher than that of the PC9 cell line (34.36 nM). Further, we tested the response of lung SqCCs to erlotinib with in vivo xenograft experiments. Tumor shrinkage was not observed after erlotinib treatment in either the Beas/2b-19del xenografts or the Beas/2b-21L858R xenografts, although partial suppressions were observed in the two xenograft models compared with the controls (Fig. S2 B and C).
Taken together, these results are consistent with the clinical observation that lung SqCCs with EGFR mutations are indeed resistant to EGFR-TKIs compared with lung ADC cells with the same EGFR mutations.
Activation of the BMP-BMPR-Smad1/5-70S6K Pathway Conferred Resistance to EGFR-TKIs in Lung SqCC Cells and Patients with EGFR Mutations.
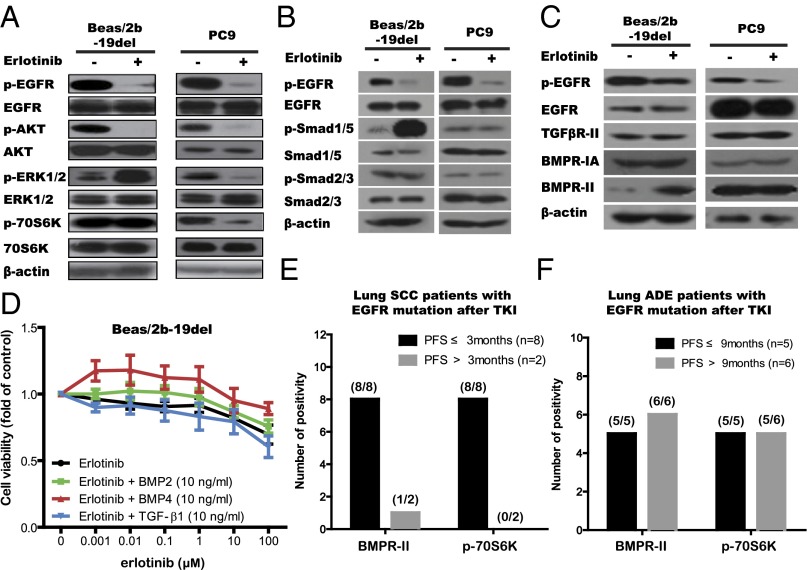
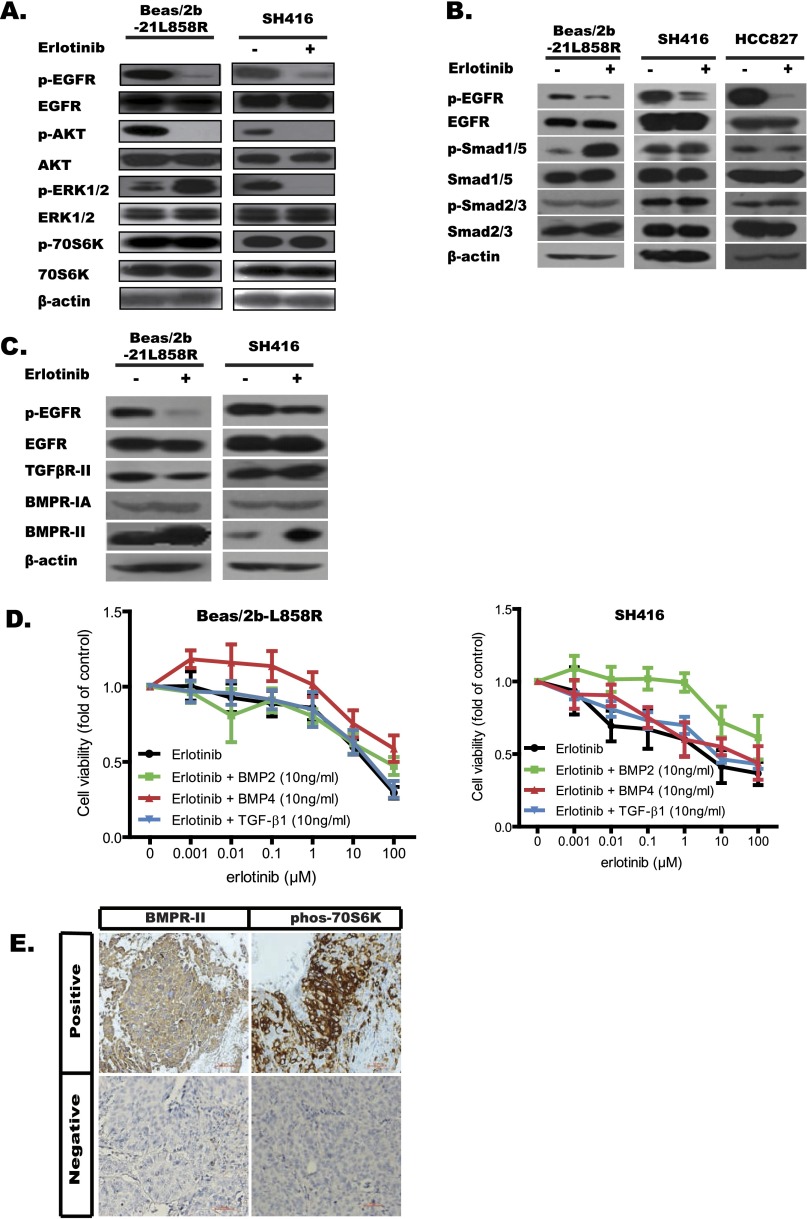
Activation of other signaling cascades has been demonstrated to be a mechanism of drug resistance (18). Therefore, to explore the mechanisms of erlotinib resistance in lung SqCC cells, we first examined the activation of several other intracellular pathways, including PI3K-AKT-mTOR and MAPK. As expected, erlotinib suppressed the activation of EGFR and AKT in all cell lines. Surprisingly, except in the PC9 cell (lung adenocarcinoma cell line), erlotinib did not affect the activation of p70S6 kinase in any of the squamous cell lines (SH416, Beas/2b-19del, and Beas/2b-21L858R cells) as tested by Western blot (Fig. 3A and Fig. S3A). The activation of p70S6K was not observed in the control Beas/2b cells. These results suggest that the activation of p70S6K may lead to the erlotinib resistance observed in these cell lines.
Fig. 3.
Activation of the BMP-BMPR-Smad1/5-70S6K pathway conferred resistance to EGFR-TKIs in lung SqCC cells and patients with EGFR mutations. (A–C) The indicated cell lines were treated with erlotinib (1 μM) for 24 h. Cell extracts were immunoblotted to detect the indicated proteins. (D) Cell viability analysis showed that cotreatment with either BMP2 (10 ng/mL) or BMP4 (10 ng/mL) with erlotinib conferring stronger resistance to erlotinib in Beas/2b-19del cell line. Viability at 72 h was calculated as the ratio of drug-treated cells to viable DMSO-treated cells. (E and F) Formalin-fixed, paraffin-embedded sections (4 μm) were stained for BMPR-II and phos-70S6K. (E) Numbers of cases with positive proteins grouped by PFS after EGFR-TKI in lung SqCC patients (n = 10). (F) Numbers of cases with positive proteins grouped by PFS after EGFR-TKI in lung adenocarcinoma patients (n = 11).
Fig. S3.
Activation of the BMP-BMPR-Smad1/5-70S6K pathway conferred resistance to EGFR-TKIs in lung SqCC cells and patients with EGFR mutations. (A–C) The indicated cell lines were treated with erlotinib (1 μM) for 24 h. Cell extracts were immunoblotted to detect the indicated proteins. (D) Cell viability analysis showed that cotreatment with either BMP2 (10 ng/mL) or BMP4 (10 ng/mL) with erlotinib conferred stronger resistance to erlotinib in Beas/2b-L858R (Left) and SH416 (Right) cell lines. Viability at 72 h was calculated as the ratio of drug-treated cells to viable DMSO-treated cells. (E) Representative IHC images of BMPR-II and p70S6K in stained cells.
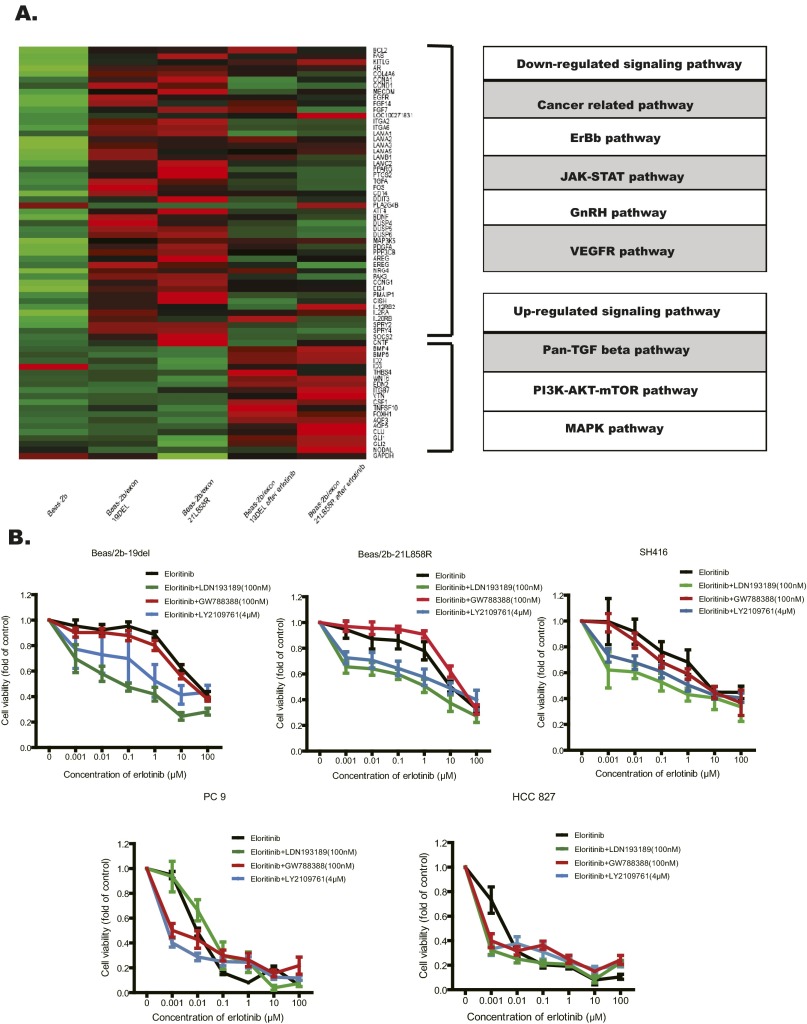
Multiple factors can lead to the activation of p70S6 kinase (19). We hypothesized that these factors were likely increased in the Beas/2b-19del and the Beas/2b-21L858R cells. To identify upstream factors that activate p70S6K, we used RNA sequencing to analyze global gene expression in these two cell lines with or without erlotinib treatment. We found 136 genes (with at least 2.5-fold changes) concurrently up-regulated both in the Beas/2b-19del and in the Beas/2b-21L858R cells after erlotinib treatment. By mapping all of these genes to the KEGG pathway database, we found that the TGF-β, PI3K-AKT-mTOR, and MAPK pathways were the dramatically changed pathways that were activated following erlotinib treatment (Fig. S4A). Because the activation status of MAPK differed between the SH416 and Beas/2b-19del/-21L858R cell lines, we hypothesized that mTOR-p70S6K might be activated by the TGF-β pathway and that this may induce the observed resistance to erlotinib.
Fig. S4.
Activation of the BMP-BMPR-Smad1/5-70S6K pathway conferred resistance to EGFR-TKIs in lung SqCC cells and patients with EGFR mutations. (A) Total RNA was extracted from the indicated cells (Beas/2b, Beas/2b-19del, Beas/2b-21L858R cells, and also the treated Beas/2b-19del and Beas/2b-21L858R after 24 h of erlotinib) and analyzed with RNA-seq. Genes with expression up-regulated by more than 2.5-fold were screened out and for further signaling pathway match. (B) Cell viability analyses showed that combining erlotinib treatment with BMPR inhibitor (LDN193189), TGF-β inhibitor (GW788388), and pan-TGF-β inhibitor (LY2109761) overcame the EGFR-TKI resistance in lung SqCC cell lines (Beas/2b-19del, Beas/2b-21L858R, and SH416), but this was not effective in the lung adenocarcinoma cell lines (PC9 and HCC827). The drug concentrations were of the indicated doses. Viability at 72 h was calculated as the ratio of viable drug-exposed cells to viable DMSO-treated cells. (C) Cell viability analyses showed that knockdown via shRNA specific to BMPR-IA (shRNA-636) or BMPR-II (shRNA-1049), but not TGF-βR-II (shRNA-1515), improved sensitivity to erlotinib in both Beas/2b-19del cells and Beas/2b-21L858R cells. Viability at 72 h was calculated as the ratio of viable erlotinib-exposed cells to viable DMSO-treated cells. Moreover, Beas/2b-19del cells were treated with indicated shRNA and/or erlotinib for 24 h. Cell extracts were immunoblotted to detect the indicated proteins. (D) Indicated cells were treated with indicated drug combinations for 24 h. Cell extracts were immunoblotted to detect the indicated proteins. (E) Diagrams showing the speculated mechanisms of erlotinib resistance in EGFR mutant lung SqCCs. The BMP2/4-BMPRII-Smad1/5-p70S6K pathway was bypass-activated following erlotinib treatment.
The TGF-β superfamily includes two classes of members, classic TGF-β proteins and bone morphogenetic proteins (BMPs); these protein classes activate Smad2/3 and Smad1/5, respectively (20–22). In the present study, accumulation of pSmad1/5, but not pSmas2/3, was significantly increased in squamous cells (SH416, Beas/2b-19del, and Beas/2b-21L858R cells); this was not the case in ADC cells (PC9 and HCC827 cells; Fig. 3B and Fig. S3B). Consistently, the amount of BMPR-II protein, but not the amount of TGF-βR-II protein, was increased in squamous cells (SH416, Beas/2b-19del, and Beas2b/21L858R cells) following erlotinib treatment (Fig. 3C and Fig. S3C). To characterize the functional significance of the increase in BMPR-II levels in TKI resistance, we tested multiple TGF-β inhibitors, including LY2109761 (pan-inhibitor to TGF-βR and BMPR), GW788388 (inhibitor to TGF-βR), and LDN193189 (inhibitor to BMPR; Fig. S4B), for their ability to reverse TKI resistance. Both LDN193189 and LY2109761 improved the sensitivity of lung SqCC cells to erlotinib. LDN193189 in particular reversed erlotinib resistance even at 100-nM concentration relative to LY2109761 at 4 μM (Fig. S4B). Consistently, elimination of BMPR-II by shRNA knockdown also improved the sensitivity of lung SqCC cells to erlotinib, a phenomenon not observed in TGF-βR-I knockdown cells (Fig. S4C). In subsequent immunoblotting analyses, the combination of erlotinib and LDN193189 significantly suppressed pEGFR, pAKT, and p70S6K signals (Fig. S4D). We also observed the up-regulated transcription of BMP-related genes such as BMP4, BMP6, and ID2 in the RNA-seq results. These findings suggest that activation of the BMPR pathway resulted in phosphorylation of 70S6K and induced the observed resistance to erlotinib in lung SqCC cells.
To figure out which ligand activated the BMP pathway, we tested the effects of several TGF-β factors and BMPs on erlotinib resistance in lung SqCC cell lines. Cotreatment of BMP2 (10 ng/mL) or BMP4 (10 ng/mL) with erlotinib conferred more resistance to erlotinib compared with erlotinib only treatment in SH416, Beas/2b-19del, and Beas/2b-21L858R cells. This effect was not observed when cells were cotreated with TGF-β1 and erlotinib (Fig. 3D and Fig. S3D). We also detected significantly increased accumulation of BMP2, BMP4, and BMP7 in the conditional medium after erlotinib treatment, whereas the levels of TGF-β1, TGF-β2, and TGF-β3 remained unchanged (data not shown). These results suggest that BMPs such as BMP2 and BMP4 promoted the BMPR signaling pathway, including downstream mTOR-p70S6K signals.
To further confirm the results obtained in the in vitro experiments, we collected tumor tissue specimens from 15 patients with advanced lung SqCC and checked BMPR-II expression. Twelve such specimens were positive for BMPR-II in IHC staining experiments. Of the 15 total patients, 10 cases carried EGFR mutations and received EGFR-TKI treatment; 8 of these patients had PFS of less than 3 mo after EGFR-TKI treatment (group 1), and the other 2 patients had PFS more than 3 mo after EGFR-TKI treatment (group 2). Interestingly, all 8 patients in group 1 presented consistent positivity for staining for both BMPR-II and p70S6K, whereas the 2 patients of group 2 were negative for these signals (Fig. 3E and Fig. S3E). As a control, we performed IHC staining assays of BMPR-II and p70S6K expression in the 11 lung ADC patients with EGFR mutation and did not observe any differences between the changes in the levels of these two proteins with PFS following EGFR-TKI treatment (Fig. 3F). We retrospectively analyzed the expression status of BMPR-II and p70S6K protein in our lung SqCC cell lines and found that all of the cell lines (SH416 and Beas/2b-19del/-21L858R cells) harbored BMPR-II and p70S6K expression. These results indicated that the subset of EGFR mutation-carrying lung SqCC patients with BMPR-II expression and p70S6K activation were resistant to EGFR-TKI treatment (Fig. S4E).
Strategies to Overcome Resistance to EGFR-TKI in EGFR Mutant Lung SqCC Cells.
Based on the aforementioned possible mechanisms of EGFR-TKI resistance in SqCC, we designed three strategies to counter such resistance; these included inhibition of mTOR (rapamycin), simultaneous inhibition of the EGFR and BMP pathways (erlotinib plus LDN193189), and blocking of the entire PI3K-AKT-mTOR pathway (erlotinib plus rapamycin or BEZ235, a dual inhibitor of PI3K and mTOR). Although we demonstrated that simultaneously inhibiting the EGFR and BMP pathways could reverse the sensitivity of SqCC cell lines to erlotinib, this regimen is not suitable for clinical use, owing to the lack of an FDA-approved BMPR-II inhibitor. We thus explored the antitumor activity of several other regimens that might be used in clinical practice that could yield similar activity.
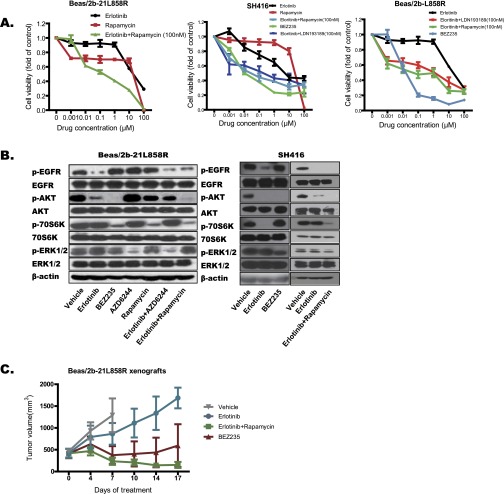
In contrast to erlotinib, the use of rapamycin as single agent failed to exhibit effective antitumor activity in Beas/2b-19del and Beas/2b-21L858R cells. Surprisingly, BEZ235 displayed superior anti-tumor activity in SH416, Beas/2b-19del, and Beas/2b-21L858R cells, compared with erlotinib plus rapamycin or erlotinib plus LDN193189, although both such combination treatments also moderately suppressed the growth of cancer cells (Fig. 4 A and B and Fig. S5A). In the immunoblot, both BEZ235 and erlotinib plus rapamycin significantly suppressed the activation of pAKT and p70S6K in SH416, Beas/2b-19del, and Beas/2b-21L858R cells (Fig. 4C and Fig. S5B). Subsequently, we assessed the antitumor activity of BEZ235 and erlotinib plus rapamycin with in vivo models and observed effective control of tumor growth (Fig. 4D and Fig. S5C). These results suggest that combined treatment with inhibitors specific to BMPR or downstream p70S6K can effectively reverse erlotinib resistance. Moreover, blocking the whole PI3K-AKT-mTOR pathway with an agent such as BEZ235 is also a feasible therapy for this subtype of lung SqCC patients carrying EGFR mutations.
Fig. 4.
Strategies for overcoming EGFR mutant lung SqCCs with BMPR-II and p70S6K expression. (A) Combinational treatment with erlotinib plus mTOR inhibitor [100 nM rapamycin (Rap)] overcame the EGFR-TKI resistance in lung Beas/2b-19del cell line. (B) Treatment with BEZ235 or combinational treatment with erlotinib plus BMPR inhibitor (100 nM LDN193189, LDN) overcame the EGFR-TKI resistance in lung Beas/2b-19del cell line. Viability at 72 h was calculated as the ratio of drug-exposed cells to viable DMSO-treated cells. (C) Beas/2b-19del cell lines were treated with the indicated drug combinations for 24 h. Cell extracts were immunoblotted to detect the indicated proteins. (D) Balb/C nude mice were s.c. engrafted with Beas/2b-19del cells, and tumors were treated daily with vehicle control, erlotinib, erlotinib plus rapamycin, or BEZ235 at the indicated dose. The tumor volumes (y axis) are plotted over time (x axis).
Fig. S5.
Strategies for overcoming EGFR mutant lung SqCCs with BMPR-II and p70S6K expression. (A) Combinational treatment with erlotinib plus mTOR inhibitor (100 nM rapamycin) overcame the EGFR-TKI resistance in lung Beas/2b-L858R cell line (Left). Treatment with BEZ235 or combinational treatment with erlotinib plus BMPR inhibitor (100 nM LDN193189) overcame the EGFR-TKI resistance in SH416 (Center) and Beas/2b-L858R (Right) cell lines. Viability at 72 h was calculated as the ratio of drug-exposed cells to viable DMSO-treated cells. (B) Beas/2b-21L858R cell lines were treated with the indicated drug combinations for 24 h. Cell extracts were immunoblotted to detect the indicated proteins. (C) Balb/C nude mice were s.c. engrafted with Beas/2b-21L858R cells, and tumors were treated daily with vehicle control, erlotinib, erlotinib plus rapamycin, or BEZ235 at the indicated dose. The tumor volumes (y axis) are plotted over time (x axis).
Discussion
Increasing knowledge about aberrances in driver genes is now promoting the understanding of tumorigenic profiles and is boosting the development of special molecular target agents that bring significant survival benefits in lung cancer treatment. However, lung cancers with different histology, such as squamous cancer and ADC, harbor distinct frequencies of driver genetic aberrances (10, 13, 23). It has been shown that cancers with the same driver mutation, but different histological subtypes, can have differential sensitivity to targeted therapies. In terms of EGFR mutations, lung SqCC patients harboring these gene variations showed inferior efficacy and survival rates compared with patients with lung ADCs when treated with TKIs (11, 14). The mechanism underlying this phenomenon remains unclear. Here, we systemically analyzed the in vitro and in vivo responses of lung SqCC with sensitizing EGFR mutations to EGFR-TKI, and experimentally evaluated both the mechanisms of resistance and strategies to overcome this resistance.
The discrepancies of morphology and function in histopathology between SqCC and ADC result from the different regions of airway tissue from which these cancer cells originate and may be associated with the variations of somatic mutations. In lung SqCCs, EGFR mutations are considered as a rare molecular event; they are reported in less than 5% of Caucasians and are reported at seemingly higher rates in Asians, ranging from 0% to 20.3% (10, 14, 24–30). In our study, the frequency of EGFR mutation in advanced lung SqCC patients was 9.6%. The divergence of these frequencies might be due to different detection methods, tumor heterogeneity, and/or ethnic differences in genetic background. Rekhtman et al. (15) postulated that EGFR mutation did not exist in pure SqCC and held that so-called “EGFR mutant lung SCC” was mixed in with ADC components (16). In the present study, we detected EGFR mutations in biopsied specimens and confirmed pure squamous components by IHC staining. Moreover, we confirmed that EGFR mutation is a functional driver mutation in lung SqCC using an EGFR mutant SqCC cell line and Beas/2b-based mutant EGFR cell lines. Because the success rate in establishing primary lung cancer cell line, especially SqCC cell line, still remains very low (2.3%), only one EGFR mutant SqCC cell line SH416 was used in this study. Although Beas/2b-based mutant EGFR cell lines formed colonies more rapidly in soft agar than did the parental Beas/2b cell line, they were still not as perfect for the in vitro cell model as patient-derived SqCC cells such as SH-416. Additional EGFR mutant SqCC cell lines were urgently needed for further studying function of mutant EGFR in SqCC cells and their responses to TKIs. Nevertheless, these results suggest that pure lung SqCC with EGFR mutations do exist and represent a unique subtype of NSCLC.
Targeting EGFR mutation by EGFR-TKI improves survival outcomes in lung ADCs, but does little with lung SqCCs. In this study, the median PFS after EGFR-TKI treatment in lung SqCC patients carrying EGFR mutations was less than 3 mo, although this was slightly superior to PFS in lung SqCC patients with wild-type EGFR. The survival time observed in present study was similar to previous reports, all of which show significantly shorter survival for SqCC than for lung ADC with EGFR mutations. Further, in vitro and in vivo experiments confirmed the relatively stronger resistance to erlotinib in lung SqCCs compared with ADCs harboring EGFR mutations. Our clinical and experimental results suggest that histopathology plays an important role in lung “EGFRoma.”
With the exception of some identified mechanisms of resistance to EGFR-TKI such as EGFR T790M, c-MET amplification, KRAS mutation, epithelial–mesenchymal transitions, and transformation to SCLC (3), it is generally held that the activation of the classical TGF-β signaling pathway was related to secondary resistance to EGFR-TKI in lung ADC (31). Here, we observed the continuous phosphorylation of 70S6K due to the activation of another TGF-β superfamily BMP-BMPR protein that was significantly enhanced by erlotinib treatment. Although several studies have demonstrated that high expression of BMP2/4 is associated with poor prognosis and that inhibition of BMPR can decrease the invasiveness and proliferation of lung cancer cell lines (32), the current study provides, to our knowledge, the first clear association between BMPR activation and EGFR-TKI resistance. Importantly, we found that only lung SqCC patients with BMPR-II and p70S6K expression showed resistance to EGRR-TKI. Our results indicate that the mechanism of EGFR-TKI resistance differs between lung squamous and adenocarcinomas. This finding supports the potential importance of selecting EGFR mutant lung SqCC patients without BMPR-II and p70S6K expression as candidates for EGFR-TKI treatment.
Because the enhanced activation of the BMP-BMPR-p70S6K pathway confers EGFR-TKI resistance, erlotinib combined with a BMPR inhibitor (LDN193189) or an mTOR inhibitor (rapamycin) effectively reversed primary resistance. Similarly, BEZ235, a dual inhibitor of PI3K and mTOR could inhibit the proliferation of EGFR mutant lung SqCC cells. Taken together, these results all indicate that simultaneously blocking the activation of upstream and bypassing downstream pathways is an effective strategy for treating lung SqCCs with EGFR mutations. We speculate that lung SqCC would respond more rapidly to EGFR-TKI treatment than would ADC, through an activated bypass pathway in a feedback loop. Our results show different biological behaviors between lung squamous carcinoma and adenocarcinoma. Thus, the effect of histological types on molecular targeted treatment should be considered before therapy with EGFR-TKI.
Materials and Methods
This study was approved by the Institutional Ethics Committee of Peking University Cancer Hospital. Written informed consent was obtained from all patients. All animal studies reported here were approved by the Animal Care Committee of the National Institute of Biological Sciences (Beijing). SI Materials and Methods contains summaries of patient information, cell line details, and reagent information. Details of the methods, including gene aberrance detection, IHC, ICC, cell viability tests, Western blot analysis, soft agar foci formation, RNA sequencing analysis, protein chip analysis, and the s.c. in vivo experiments can be found in SI Materials and Methods.
SI Materials and Methods
Patients.
This study was approved by the Institutional Ethics Committee at Peking University Cancer Hospital. All patients signed informed consent to participate in this study and gave permission for the use of their tissues. First, we determined the histology distribution of EGFR mutations in lung cancer. Lung cancer patients with EGFR mutations were enrolled in the study in the period from June 2005 to June 2012. Clinical factors were collected including sex, age, and smoking status. In the patients with EGFR mutations, 18 patients provided sufficient tumor tissue specimens for further immunohistochemistry analyses. We also investigated the efficacy and survival of EGFR-TKI treatment for lung SqCC. Patients with histologically confirmed SqCC received erlotinib (150 mg) or gefitinib (250 mg) orally daily. The responses were characterized as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD) according to response evaluation criteria in solid tumors (RECIST 1.1). The overall response rate was defined as the sum of CR and PR. The disease control rate was defined as the sum of CR, PR, and SD. Progression-free survival was calculated from the start of EGFR-TKI until the date of the first documented PD or death. Overall survival was calculated from the start of EGFR-TKI to the date of death.
Detection of EGFR Sensitive and Resistant Related Genetic Aberrances.
Genetic aberrances involved in this study included mutations in EGFR (19del, 21L858R, T790M, EGFRVIII), PIK3CA, DDR2, KRAS, or BRAF V600E, and amplification in PIK3CA or FGFR1. We also evaluated the expression of c-MET and PTEN in cell lines. Briefly, EGFR sensitizing mutations were detected by DHPLC according to previously described methods (26). An amplification refractory mutation system (ARMS) was used to reevaluate the EGFR wild-type patients by DHPLC. Other mutations in EGFR T790M, PIK3CA (including H1047L, H1047R, E542K, and E545K), KRAS, and BRAF V600E were also detected by ARMS. EGFRVIII mutation was detected via RT-PCR according to previously described methods.
Amplifications in FGFR1 and PIK3CA were both determined by FISH. Briefly, BAC RP11-667I6 and RP11-100C1 (Children’s Hospital Oakland Research Institute) were used as break-apart probes for the EML4 and ALK genes, respectively. BAC DNA was labeled with either spectrum red dUTP or spectrum green-11-dUTP by nick translation (Vysis) according to the manufacturer’s recommended conditions. Slides for metaphase FISH from the cell lines were prepared using standard cytogenetic methods. Paraffin-embedded slides were prepared as described previously. The probes were hybridized and washed according to standard FISH procedures.
C-MET amplification and PTEN expression were determined by Western blot and immunocytochemistry staining, respectively (see the following sections).
Cell Lines and Reagents.
The Beas/2b cell line and the PC9 cell line were kindly provided by the National Institute of Biologic Sciences and the Guangdong Provincial Lung Cancer Research Institute, respectively. The SH-416 cell line derived from a squamous cell lung carcinoma with EGFR 19 deletion was kindly provided as a gift from the Shanghai Institutes for Biological Sciences. The HCC827 cell line was purchased from the Peking Union Medical College. The Beas/2b and PC9 cell lines were cultured in RPMI 1640 supplemented with 10% (vol/vol) FBS and 1:100 streptomycin/penicillin. The SH-416 cell line was cultured in BEGM medium (Lonza). Erlotinib-HCl (EGFR tyrosine kinase inhibitor), LDN193189 (inhibitor of BMPR), GW78838 (inhibitor of TGF-βR), LY2109761 (inhibitor of BMPR and TGF-βR), rapamycin (mTOR inhibitor), BEZ235 (PI3K/mTOR dual inhibitor), AZD6244 (MEK1/2 inhibitor), and BMP factors (BMP2 and BMP4) were purchased from Selleck Chemicals, LLC.
Construction of Stable Cell Lines (Beas/2b-19del and Beas/2b-21L858R) by Transfecting pCAG-IN-EGFR-19del/L858R-P2A-EGFP Plasmids into Beas/2b Cells.
The plasmids were kindly provided by Liang Chen (National Institute of Biological Science, Beijing). Cells were seeded onto six-well plates at an initial density of 2 × 105 cells per well at the first day. Transient transfection of indicated plasmids (pCAG-IN-EGFR-19del-P2A-EGFP and pCAG-IN-EGFR-L858R-P2A-EGFP) was performed in a manner mediated by Lipofectamine 2000. Stable clones were selected by adding G418 800 µg/mL for 14 d, and these were then confirmed by Western blotting and sequencing to exclude false positive clones.
Immunohistochemistry and Immunocytochemistry.
Immunohistochemistry biomarkers for determining phenotypes for P63, P40, CK5/6, SCCA1, and TTF-1 were analyzed in lung tissue samples and cell lines using IHC and ICC, respectively. The expression of functional proteins such as BMPR-II and p70S6K were characterized by IHC. c-MET expression in SH416 cells was detected by ICC. The primary antibodies included P63 (1:200; Abcam), P40 (1:500; Abcam), CK5/6 (1:1; Abcam), SCCA1 (1:100; Santa Cruz), TTF-1 (1:100; Santa Cruz), c-MET (1:200; Cell Signaling Technology), BMPR-II (1:1,000; Millipore), and p70S6K (1:500; Cell Signaling Technology). The secondary antibodies included anti-rabbit/mouse secondary antibody (1:1; Gene Tech).
Dried 4-μm slides with formalin-fixed, paraffin-embedded tissue were prepared. Combined treatment with sodium citrate (pH 6.0) and incubation in a pressure cooker (3 min, 125 °C) was used for antigen retrieval. Slides were then incubated overnight at 4 °C with primary monoclonal antibody at the aforementioned dilutions. A two-step polymer-HRP method (Gene Tech) was used for detection. No staining was observed for negative controls, which included incubation of samples with a nonimmune primary antibody. IHC “positive” immunoreactivity was defined as the case where more than 10% of cancer cells had staining. IHC and ICC staining was evaluated independently by different investigators (H.B. and S.F.) and a pathologist.
Cell Viability Analysis.
Cell viability tests were conducted using a Cell Counting Kit-8 (Dojindo). Briefly, cells were seeded in sextuplicate in 96-well plates containing 100 µL media at a density of 3 × 103 cells per well for 24 h, and then cultured with increased concentrations of the indicated drugs for an additional 72 h. Afterward, 10 µL of water-soluble tetrazolium salt was added to each well and incubated for 3 h. The absorbance was measured via scanning with a microplate reader at 450 nm. Relative viability was calculated as follows: (%/control) = [A450 (treated) − A450 (blank)]/[A450 (control) − A450 (blank)].
Western Blot Analysis.
The expression of protein in cells was evaluated with Western blotting. Whole-cell lysates were prepared by extraction in cell lysis buffer (Cell Signaling Technology) followed by protein quantification using a bicinchoninic acid assay (Applygen) and lysis in Laemmli sample buffer. A total of 30 µg of the protein sample was run on a 10% (wt/vol) Tris-glycine gel and transferred to nitrocellulose. Primary antibodies were added and incubated overnight at 4 °C, and secondary antibodies were conjugated to horseradish peroxidase for 2 h at room temperature. Blots were developed by enhanced chemiluminescence and photographed using a Fujifilm Dark Box II and Image Reader LAS-1000 Plus software. Primary antibodies included EGFR, AKT, ERK1/2, RPS6, beta-actin (these antibodies at 1:2,000 dilutions; Cell Signaling Technology), and pEGFR, pAKT, pERK1/2, pRPS6 (these antibodies at 1:1,000 dilutions; Cell Signaling Technology). Peroxidase-labeled anti-rabbit or anti-mouse secondary antibodies (Cell Signaling Technology) were used.
RNA-Seq Analysis.
Total RNA was extracted from Beas/2b, Beas/2b-19del, and Beas/2b-21L858R cells, and also the treated Beas/2b-19del and Beas/2b-21L858R cells after 24 h of erlotinib using TRIzol (Life Technologies) according to the manufacturer’s protocol. The mRNA was purified for RNA-seq library construction and whole transcriptome analysis. The Homo sapiens genome sequences and annotated gene models were downloaded from University of California, Santa Cruz (genome.ucsc.edu/). TopHat v1.4.1 was used to align the raw reads to genome sequences. Cufflinks v2.0.2 was used to assemble transcripts and to calculate transcript abundances. Differences in RNA transcript levels between cell types were identified using the Cuffdiff criterion. The presence of more than three FPKM (fragments per kilobase of exon per million fragments mapped) in total exons of a gene was the criterion for gene detection.
Construction of Special shRNA and Knockdown.
The LV-3 (pGLVH1/GFP + Puro) vector expression plasmid containing shRNA against TGF-βII (5′-GGGACCTCAAGAGCTCCAATA-3′), BMPIA (5′-GACAGAATCTGGATAGTATGC-3′), and BMP II (5′-GCAGCAAGCACAAATCAAACT-3′) were purchased from GenePharma. The lentiviruses were mixed with polybrene (5 mg/mL) and added to BEAS/2b-19del, BEAS/2b-L858R, and SH416 cells. After 24 h, puromycin (5 μg/mL; Sigma-Aldrich) in new culture medium was added to select for transduced cells. Puromycin was present continuously in the media until cells were harvested. After incubation in culture medium for a further 48 h, the cells were observed by fluorescence microscopy (Olympus).
Protein Chip for Cytokines.
Lung cancer cell lines (Beas/2b-19del, Beas/2b-21L858R, and SH416) were treated with erlotinib for 24 h. Matched cell-free media samples (before and after erlotinib treatment) were prepared for cytokines analysis. The detection procedures were performed commercially by BGI Co. Semiquantitative sandwich-based antibody arrays (RayBio Human Cytokine Array G-Series 2000) were used to detect panels of 60 (catalog no. AAH-CYT-G6) and 20 serum markers (catalog no. QAH-CYT-1). Briefly, after blocking the glass chips were incubated with cell culture media overnight at 4 °C. After washing steps chips were incubated with the corresponding biotin-conjugated antibodies for 2 h at room temperature (RT) and then washed four times. Finally, slides were incubated with fluorescent dye-conjugated streptavidin for 2 h at RT in the dark and then washed. After complete drying the slides were scanned with an Axon Confocal Laser Scanner and images were processed with GenePix Pro software.
Soft Agar Foci Formation Analysis.
Anchorage-independent growth of cells was assessed by a colony formation assay in soft agar. For inhibitor treatments, vehicle (DMSO) or erlotinib, BEZ235 was added to the medium at 30 nM and 100 nM concentrations. Approximately 2 × 103 cells were seeded in triplicate in six-well plates and maintained at 37 °C for 14 d. The total number of clones with a diameter more than 50 nm was counted in five representative fields of view within each well. The results of these assays are expressed as means ± SD.
Subcutaneous in Vivo Experiments.
To generate tumor xenografts, Beas/2b-19del (3 × 106), Beas/2b-21L858R (3.0 × 106), and SH416 tumor cells (2.0 × 106) in 100 μL PBS were injected into the s.c. flanks of 6-wk-old female BALB/c nude mice. Body weight and tumor volume were recorded twice weekly. Tumor volumes were calculated as π/6 × a2 × b, where a was the smaller measurement of the tumor and b was the larger one, and expressed in cubic millimeters. When the tumor volumes reached an average of ∼300 mm3 mice (Beas/2b-19del xenografts and Beas/2b-21L858R xenografts) were randomly assigned to one of the following treatment groups: (a) control oral (p.o.) administration of vehicle daily; (b) erlotinib (25 mg/kg) p.o. daily; (c) BEZ235 (45 mg/kg) p.o. daily; (d) erlotinib (25 mg/kg) p.o. daily plus rapamycin (2 mg/kg) i.p. twice weekly (n = 5 per group of the two cell line xenografts). Animals were killed due to tumor burden. Log-rank tests were performed to compare survival curves between different treatment groups using GraphPad Prism version 6.00 for Windows (GraphPad Software). All animal studies reported were approved by the National Institute of Biologic Sciences’ Animal Care Committee (Beijing).
Statistical Analysis.
The relationships between EGFR mutations and the response to EGFR-TKI were analyzed using Pearson’s χ2 test or Fisher’s exact test, as appropriate. Median PFS and median OS were both calculated using the Kaplan–Meier method, and comparisons between groups were made using log-rank tests. Cox’s proportional hazards regression model was used to identify the independent factors of PFS and OS. For comparison of continuous variables between two groups, two-tailed Student’s t tests and Mann–Whitney Wilcoxon tests were used. All statistical tests were two-tailed, with significance defined as P < 0.05. All analyses were performed using SPSS for Windows, version 17.0.
Acknowledgments
This work was supported by Beijing Nova Program Grants xx2014B051 and xx2012070 from the Beijing Municipal Commission of Science and Technology, National Basic Science 973 Grants 2010CB835400 and 2012CB837400 from the Chinese Ministry of Science and Technology; National Natural Sciences Foundation Key Program 81330062; Education Ministry Innovative Research Team Program IRT13003; Fund for Peking University–Tsinghua University Joint Center for Life Sciences Clinical Investigator; Special Research Foundation of State Key Laboratory of Medical Genomics; National High Technology Research and Development Program 863 (SS2015AA020403); Beijing Technology Project Z141100000214013; Center for Molecular and Translational Medicine; Collaborative Innovation Center for Cancer Medicine; Special Research Foundation for the Doctoral Program of Higher Education; and Beijing Excellent Talents Project 2013D003034000019.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1510837112/-/DCSupplemental.
References
- 1.Brambilla E, Travis WD, Colby TV, Corrin B, Shimosato Y. The new World Health Organization classification of lung tumours. Eur Respir J. 2001;18(6):1059–1068. doi: 10.1183/09031936.01.00275301. [DOI] [PubMed] [Google Scholar]
- 2.Travis WD, Brambilla E, Riely GJ. New pathologic classification of lung cancer: Relevance for clinical practice and clinical trials. J Clin Oncol. 2013;31(8):992–1001. doi: 10.1200/JCO.2012.46.9270. [DOI] [PubMed] [Google Scholar]
- 3.Sequist LV, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3(75):75ra26. doi: 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mok TS, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 5.Maemondo M, et al. North-East Japan Study Group Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 6.Mitsudomi T, et al. West Japan Oncology Group Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol. 2010;11(2):121–128. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 7.Rosell R, et al. Spanish Lung Cancer Group in collaboration with Groupe Français de Pneumo-Cancérologie and Associazione Italiana Oncologia Toracica Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 8.Zhou C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): A multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 9.Kwak EL, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363(18):1693–1703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaw AT, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol. 2009;27(26):4247–4253. doi: 10.1200/JCO.2009.22.6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shukuya T, et al. Efficacy of gefitinib for non-adenocarcinoma non-small-cell lung cancer patients harboring epidermal growth factor receptor mutations: A pooled analysis of published reports. Cancer Sci. 2011;102(5):1032–1037. doi: 10.1111/j.1349-7006.2011.01887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Network TCGAR. Cancer Genome Atlas Research Network Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489(7417):519–525. doi: 10.1038/nature11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim Y, et al. Integrative and comparative genomic analysis of lung squamous cell carcinomas in East Asian patients. J Clin Oncol. 2014;32(2):121–128. doi: 10.1200/JCO.2013.50.8556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hata A, et al. 2013. How sensitive are epidermal growth factor receptor-tyrosine kinase inhibitors for squamous cell carcinoma of the lung harboring EGFR gene-sensitive mutations? J Thorac Oncol 8(1):89–95.
- 15.Rekhtman N, et al. 2012. Clarifying the spectrum of driver oncogene mutations in biomarker-verified squamous carcinoma of lung: Lack of EGFR/KRAS and presence of PIK3CA/AKT1 mutations. Clin Cancer Res 18(4):1167–1176.
- 16.Roggli VL, et al. Lung cancer heterogeneity: A blinded and randomized study of 100 consecutive cases. Hum Pathol. 1985;16(6):569–579. doi: 10.1016/s0046-8177(85)80106-4. [DOI] [PubMed] [Google Scholar]
- 17.Zheng C, Sun YH, Ye XL, Chen HQ, Ji HB. Establishment and characterization of primary lung cancer cell lines from Chinese population. Acta Pharmacol Sin. 2011;32(3):385–392. doi: 10.1038/aps.2010.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bean J, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci USA. 2007;104(52):20932–20937. doi: 10.1073/pnas.0710370104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zoncu R, Efeyan A, Sabatini DM. mTOR: From growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12(1):21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Langenfeld E, Hong CC, Lanke G, Langenfeld J. Bone morphogenetic protein type I receptor antagonists decrease growth and induce cell death of lung cancer cell lines. PLoS One. 2013;8(4):e61256. doi: 10.1371/journal.pone.0061256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Attisano L, Wrana JL. Signal transduction by the TGF-beta superfamily. Science. 2002;296(5573):1646–1647. doi: 10.1126/science.1071809. [DOI] [PubMed] [Google Scholar]
- 22.Kretzschmar M, Liu F, Hata A, Doody J, Massagué J. The TGF-beta family mediator Smad1 is phosphorylated directly and activated functionally by the BMP receptor kinase. Genes Dev. 1997;11(8):984–995. doi: 10.1101/gad.11.8.984. [DOI] [PubMed] [Google Scholar]
- 23.Cancer Genome Atlas Research Network Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511(7511):543–550. doi: 10.1038/nature13385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi YL, et al. EGFR mutation testing in patients with advanced non-small cell lung cancer: A comprehensive evaluation of real-world practice in an East Asian tertiary hospital. PLoS One. 2013;8(2):e56011. doi: 10.1371/journal.pone.0056011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marchetti A, et al. EGFR mutations in non-small-cell lung cancer: Analysis of a large series of cases and development of a rapid and sensitive method for diagnostic screening with potential implications on pharmacologic treatment. J Clin Oncol. 2005;23(4):857–865. doi: 10.1200/JCO.2005.08.043. [DOI] [PubMed] [Google Scholar]
- 26.Bai H, et al. Epidermal growth factor receptor mutations in plasma DNA samples predict tumor response in Chinese patients with stages IIIB to IV non-small-cell lung cancer. J Clin Oncol. 2009;27(16):2653–2659. doi: 10.1200/JCO.2008.17.3930. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka T, et al. 2010. Frequency of and variables associated with the EGFR mutation and its subtypes. Int J Cancer 126(3):651–655.
- 28.Pao W, Girard N. New driver mutations in non-small-cell lung cancer. Lancet Oncol. 2011;12(2):175–180. doi: 10.1016/S1470-2045(10)70087-5. [DOI] [PubMed] [Google Scholar]
- 29.Tseng JS, et al. Retrospective study of erlotinib in patients with advanced squamous lung cancer. Lung Cancer. 2012;77(1):128–133. doi: 10.1016/j.lungcan.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 30.Miyamae Y, et al. Significance of epidermal growth factor receptor gene mutations in squamous cell lung carcinoma. Oncol Rep. 2011;25(4):921–928. doi: 10.3892/or.2011.1182. [DOI] [PubMed] [Google Scholar]
- 31.Serizawa M, Takahashi T, Yamamoto N, Koh Y. 2013. Combined treatment with erlotinib and a transforming growth factor-beta type I receptor inhibitor effectively suppresses the enhanced motility of erlotinib-resistant non-small-cell lung cancer cells. J Thorac Oncol 8(3):259–269.
- 32.Fei ZH, Yao CY, Yang XL, Huang XE, Ma SL. Serum BMP-2 up-regulation as an indicator of poor survival in advanced non-small cell lung cancer patients. Asian Pac J Cancer Prev. 2013;14(9):5293–5299. doi: 10.7314/apjcp.2013.14.9.5293. [DOI] [PubMed] [Google Scholar]