Significance
Brain-derived neurotrophic factor (BDNF) is a secreted neurotrophin known to mediate activity-dependent synaptic plasticity. Endogenously synthesized BDNF is normally stored and transported in dense core vesicles and secreted at synapses in response to activity. However, secreted BDNF may also be endocytosed by neurons and transported within neuronal cytoplasm in the form of endosomes. By monitoring the endocytosed BDNF bound to fluorescent quantum dots (BDNF-QDs), we found that endocytosed BDNF-QDs could be preferentially localized to postsynaptic sites in cultured hippocampal neurons and became exocytosed in response to synaptic activity. This synaptic release of endocytic BDNF requires a synaptotagmin isoform distinct from that regulates the secretion of dense core vesicles, and may serve as a source for activity-dependent secretion of synaptic BDNF.
Keywords: BDNF, endocytosis, secretion, synaptotagmin, complexin
Abstract
Brain-derived neurotrophic factor (BDNF) is known to modulate synapse development and plasticity, but the source of synaptic BDNF and molecular mechanisms regulating BDNF release remain unclear. Using exogenous BDNF tagged with quantum dots (BDNF-QDs), we found that endocytosed BDNF-QDs were preferentially localized to postsynaptic sites in the dendrite of cultured hippocampal neurons. Repetitive neuronal spiking induced the release of BDNF-QDs at these sites, and this process required activation of glutamate receptors. Down-regulating complexin 1/2 (Cpx1/2) expression eliminated activity-induced BDNF-QD secretion, although the overall activity-independent secretion was elevated. Among eight synaptotagmin (Syt) isoforms examined, down-regulation of only Syt6 impaired activity-induced BDNF-QD secretion. In contrast, activity-induced release of endogenously synthesized BDNF did not depend on Syt6. Thus, neuronal activity could trigger the release of endosomal BDNF from postsynaptic dendrites in a Cpx- and Syt6-dependent manner, and endosomes containing BDNF may serve as a source of BDNF for activity-dependent synaptic modulation.
Brain-derived neurotrophic factor (BDNF), a member of neurotrophin family of secreted factors, is known to play important regulatory roles in neuronal survival and differentiation, synaptic development and plasticity, as well as many cognitive functions (1, 2). The findings that the synthesis and secretion of neurotrophins are regulated by neuronal activity prompted the suggestion that neurotrophins may regulate activity-dependent neural plasticity in the brain (3). Indeed, there is now substantial evidence indicating that activity-induced BDNF secretion at glutamatergic synapses is essential for long-term potentiation (LTP) (4), a cellular substrate for the learning and memory functions of neural circuits.
The BDNF protein is first synthesized in the endoplasmic reticulum as a precursor protein, prepro-BDNF, which is then converted to pro-BDNF by removal of the signal peptide and further cleaved to generate the mature BDNF (5). Immunostaining and electron microscope studies using specific antibodies to the pro- and mature form of BDNF showed that pro-BDNF is colocalized with mature BDNF in secretory granules in presynaptic axon terminals (6), suggesting that the cleavage may occur in the secretory granule. However, under some experimental conditions, the processing of pro-BDNF into mature BDNF may occur extracellularly (7, 8). The secretory granule containing BDNF and pro-BDNF could undergo exocytosis upon neuronal excitation, as readily demonstrated in cell cultures using ELISA or fluorescent protein-tagged BDNF expressed in the neuron (9, 10). Besides secretory granules, neurotrophins within neuronal cytoplasm could also reside in endosomal compartments, resulting from endocytic uptake of extracellular neurotrophins secreted by the neuron itself or other nearby cells. Initially discovered as factors derived by target tissues, neurotrophins exert their actions via binding to neuronal surface receptors, including tropomyosin related kinase B (TrkB) and pan-neurotrophin receptor p75 (11). Neurotrophin binding to its receptor leads to cytoplasmic signaling as well as internalization of the neurotrophin-receptor complexes. These endocytosed neurotrophin-receptor complexes remain active in the form of “signaling endosomes” that could be transported over long distances within neuronal cytoplasm to exert its regulatory functions within the neuron (12–15). In this study, we have examined the possibility that these endosomes may undergo activity-dependent exocytosis at postsynaptic dendrites, thus providing an additional source of synaptic BDNF.
To mark endosomes containing BDNF via the endocytic pathway, it is necessary to monitor BDNF trafficking in neurons. Although YFP-tagged BDNF has been used to study internalization of exogenous BDNF (16), such fluorescent protein-labeled BDNF was not suitable for real-time tracking of BDNF-containing endosomes at a high spatiotemporal resolution. In this study, we used BDNF linked to quantum dots (QDs), which are fluorescent nanoparticles with excellent photostability (17) and could be tracked in live cells with high signal-to-noise ratio and over unprecedented duration. This method has been used to examine endocytic recycling of synaptic vesicles (18) and axonal transport of endosomes containing neurotrophins (19, 20). In this study, we used time-lapse imaging of BDNF-QDs within cultured hippocampal neurons to monitor intracellular transport and localization of these endosomes. Furthermore, the sudden disappearance of cytoplasmic QD fluorescence in a solution containing fluorescence quencher was used to indicate the exocytosis of QD-containing endosomes. Previous studies have shown that extracellular false transmitters, soluble fluorescent markers, and membrane-bound fluorescent lipid dyes could be loaded into endosomes, which undergo exocytosis upon membrane depolarization (21–25). However, whether endosomes formed by receptor-mediated endocytosis is similarly regulated by activity remains unclear. Furthermore, the Ca2+-dependence and the kinetics of exocytosis of different endosomal vesicle populations may be differentially regulated by distinct vesicle-associated proteins.
In the present study, we have explored the role of synaptotagmin (Syt) and complexin (Cpx) in regulating activity-induced exocytosis of BDNF-containing endosomes. As a universal cofactor in all Ca2+-triggered vesicular fusion reactions that have been examined (26), Cpx is known to serve both activating and clamping functions for vesicular exocytosis, by interacting with the Ca2+ sensor Syt and the assembled SNARE complexes at the plasma membrane (27). Various isoforms of Syt play distinct regulatory roles in various types of neurosecretion, presumably via their differential Ca2+ sensitivity. By manipulating the expression of various Syt and Cpx isoforms in cultured hippocampal neurons, we found that Syt6 and Cpx1/2 play essential regulatory roles in activity-dependent exocytosis of BDNF-containing endosomes. These results support the notion that BDNF-containing endosomes may serve as a source of extracellular BDNF for activity-dependent synaptic modulation and that Syt6 specifically regulates the exocytosis of BDNF-containing endosomes.
Results
Intracellular Localization of Endocytosed BDNF-QDs.
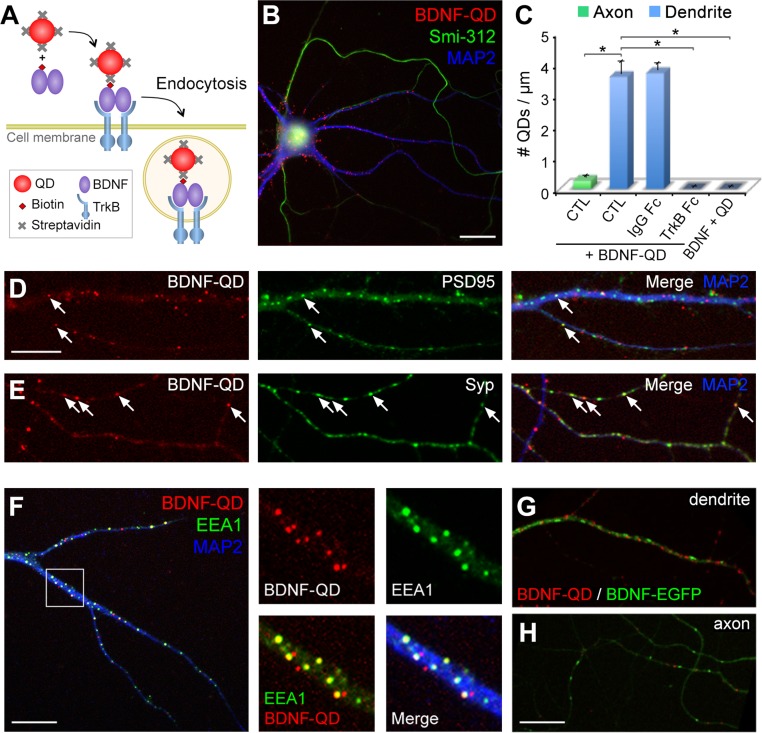
To study intracellular processing and secretion of endocytosed BDNF, we used streptavidin-conjugated QDs (QD655s) (Materials and Methods and Fig. S1) that were linked to biotinylated BDNF. The biological activity of BDNF-QDs has been shown by induced TrkB phosphorylation in TrkB-overexpressing 3T3 cells and Erk1/2 phosphorylation in hippocampal neurons (19). Cultured hippocampal neurons on 13–16 d in vitro (DIV) were treated with 1 nM of BDNF-QD for 10 min (Fig. 1A). Fluorescence imaging of neurons showed many bright and blinking puncta in the soma and neurite processes. Intracellular localization of BDNF-QDs was verified by extracellular addition of a fluorescence quencher, QSY 21 carboxylic acid–succinimidyl ester (QSY21), which effectively quenched the fluorescence of extracellular BDNF-QDs that were bound to the culture substrate and cell surface (28) (Fig. S2A). Endocytosed BDNF-QDs were observed in both dendrites and axons, which were identified as thick processes stained with microtubule-associated protein 2 (MAP2) and thin processes labeled by axon-specific marker Smi-312, respectively (Fig. 1B). In all cases, we found that the density of BDNF-QD puncta in the dendrite was significantly higher than those in the axon. In contrast, very few internalized QDs were observed when a mixture of unmodified BDNF and QDs was used for incubating the cells (Fig. 1C). Moreover, the number of internalized BDNF-QDs was negligible when the neurons were incubated with BDNF-QDs in the presence of TrkB-Fc protein (10 μg/mL), a soluble Fc-tagged TrkB that competes with cell surface TrkB for BDNF binding (29). Thus, internalization of BDNF-QDs depends on BDNF binding to the cell surface.
Fig. 1.
Internalization of BDNF-QDs in cultured hippocampal neurons and their localization in cytoplasmic compartments. (A) Schematic diagram depicting the design of BDNF-QD. Biotinylated BDNF was first linked to streptavidin-conjugated QD655s and then applied extracellularly to cultured neurons for endocytic uptake. (B) Fluorescence images of a hippocampal neuron with internalized BDNF-QDs (red) and immunostained for MAP2 (blue) and Smi-312 (green), which are localized to the somatodendritic region and axon, respectively. (Scale bar, 20 μm.) (C) The number of internalized BDNF-QDs in the axon and dendrites in regular medium (CTL), and medium supplemented with control IgG Fc, BDNF scavenger ligand TrkB-Fc, or uncoupled BDNF and QDs (n = 4 cultures, 8–16 neurons per culture for each condition; *P < 0.001 by one-way ANOVA and Tukey post hoc HSD test). Error bars, SEM. (D and E) Samples of dendrite (D) and axon (E) showing the distribution of BDNF-QDs (red) relative to the immunostained puncta of postsynaptic marker PSD-95 (D, green) or presynaptic marker Syp (E, green) and MAP2 (blue). Arrows: colocalized BDNF-QDs and synaptic markers. (Scale bar, 10 μm.) (F) Coimmunostaining of early endosome marker EEA1 (green) and MAP2 (blue) in neurons containing internalized BDNF-QDs (red). Boxed area is shown at a higher resolution on the right. (Scale bar, 20 μm.) (Magnification, ∼4 × 4.) (G and H) Fluorescence images of sample dendrite (G) and axon (H) containing internalized BDNF-QDs (red) and expressing BDNF-EGFP (green). (Scale bar, 20 μm.)
To characterize the cellular distribution of endocytosed BDNF, we fixed the neurons after 10-min BDNF-QD incubation and immunostained the cells with antibodies against the postsynaptic protein PSD95 and the synaptic vesicle-associated protein synaptophysin (Syp). We found that a subpopulation (15.6 ± 1.6%) of BDNF-QDs in the dendrite colocalized with PSD95 puncta, and 29.8 ± 3.0% of BDNF-QDs in the axon colocalized with Syp puncta (Fig. 1 D and E). Thus, although only a minor population of BDNF-QD–containing vesicles appeared to located at synaptic sites in both dendrites and axons, the percentage is much higher than the average area occupied by PSD95 or Syp puncta along these processes (∼8%) (Fig. S3C), suggesting preferential synaptic location of endocytosed BDNF-QDs. Additional immunostaining studies showed that ∼50% of BDNF-QDs colocalized with the early endosomal protein, EEA1 (Fig. 1F), indicating that BDNF-QDs entered the cells through endocytosis and resided in endosomal compartments.
The intracellular distributions of endocytosed and endogenously synthesized BDNF were also compared. Neurons transfected with a BDNF-EGFP plasmid (Materials and Methods) were incubated with BDNF-QDs for 1 h, and live neurons with EGFP and QD655 fluorescence in the presence of external quencher QSY21 were examined. We found that that BDNF-EGFP distributed in both dendrites and axons in a punctate pattern similar to that of BDNF-QDs, although BDNF-QD puncta were smaller and rounder (Fig. 1 G and H). Moreover, BDNF-QDs and BDNF-EGFP puncta were essentially nonoverlapping, as quantitative fluorescence measurements showed no correlation between QD and EGFP fluorescence [Pearson’s correlation coefficient = −0.096 ± 0.009 (SEM), n = 4 independent cultures], indicating that endocytosed and endogenously synthesized BDNF reside in distinct intracellular compartments.
Cytoplasmic Transport of Internalized BDNF-QDs.
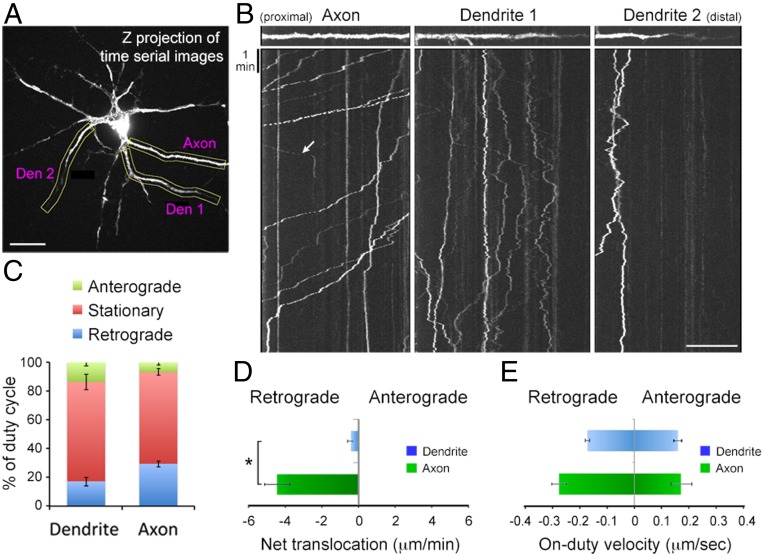
Previous work has demonstrated that BDNF/TrkB endosomes undergo dynein-dependent retrograde transport in the axon toward the cell body after internalization (30). To investigate the transport of internalized BDNF-QDs at both axons and dendrites, hippocampal neurons on 10–16 DIV were perfused with BDNF-QDs for 2 min and then QSY21-containing saline was used to perfuse the culture for 30 min before time-lapse imaging in the presence of QSY21. As shown by representative trajectories and kymographs of axonal transport of BDNF-QDs (Fig. 2 A and B and Movie S1), the majority of transported BDNF-QDs was found to be in the retrograde direction (toward the cell body) with intermittent pauses, consistent with a previous report (19) (Fig. 2 B and C). In contrast, BDNF-QDs in dendrites showed frequent alterations between anterograde and retrograde directions, resulting in very limited net translocation (Fig. 2D). Many BDNF-QDs (69.8 ± 5.4% in dendrites and 64.5 ± 2.1% in axons, n = 7 neurons) were immobilized during the standard period of observation (30–60 min). The mean velocity of translocation (on-duty velocity) in both axons and dendrites was in the range of 0.1–0.3 μm/s (Fig. 2E), consistent with active motor-driven transport.
Fig. 2.
Axonal and dendritic transport of BDNF-QDs. (A) Image of a hippocampal neuron treated with BDNF-QDs for 10 min and immediately examined by time-lapse imaging for transport trajectories of endocytosed BDNF-QDs. (Scale bar, 20 μm.) (B) Kymographs for samples of linearlized axon and dendrite segments (marked by yellow boxes in A). Most BDNF-QDs in the axon (Left) moved retrogradely (toward the cell body), and a few exhibited anterograde movements (arrow). In contrast, BDNF-QDs in the dendrite (Center and Right) moved in both anterograde and retrograde directions. (Scale bar, 20 μm.) (C) Average percentage of time spent in three transport modes for BDNF-QDs in the axon or dendrites, from data of all kymographs (n = 7 neurons from four 10–13 DIV cultures). (D) The average distance of net translocation of BDNF-QDs at axons and dendrites during the standard observation time (30 min) for all neurons examined. Error bars, SEM (*P < 0.01 by Student’s t test). (E) The average velocity of anterograde and retrograde transports in the axon and dendrites observed in all kymographs. Error bars, SEM.
Activity-Induced Endocytosis of BDNF-QDs.
To determine whether the endocytosis of BDNF-QDs could be influenced by neuronal activity, we transfected hippocampal cultures with PSD95-GFP on 6 DIV, and then exposed the cultures on 12–15 DIV to solution containing BDNF-QDs for 10 min with or without the treatment that elevated neuronal activity. Time-lapsed imaging of neurons was performed within 10 min after the incubation with BDNF-QDs. In the absence of treatment that elevates the activity, we found that 17.2 ± 1.9% of BDNF-QDs showed colocalization with PSD95-GFP puncta (Fig. S3A). Because this percentage is much higher than the percentage of dendritic area occupied by PSD95-GFP (7.8 ± 0.5%), internalized BDNF-QDs were not randomly distributed in the dendrite, but preferentially localized to the PSD95-GFP sites. When the neuronal activity was elevated by using BDNF-QD incubating medium containing either 45 mM KCl or 200 μM glycine, which induced depolarization or enhanced activation of NMDA receptors, respectively, the number of BDNF-QDs colocalizing with PSD95-GFP puncta was significantly increased (Fig. S3 A and C). Thus, elevated neuronal activity enhanced either preferential BDNF-QD endocytosis at postsynaptic sites or the trapping of BDNF-QD–containing endosomes at these sites immediately after endocytosis.
To further examine the sites of BDNF-QD internalization along the axon, we transfected neurons with Syp-GFP to mark presynaptic sites. We found that 33.8 ± 3.9% of BDNF-QDs were colocalized with Syp-GFP+ sites (Fig. S3B). The percentage of colocalization was increased to 44.0 ± 3.0% after incubation of BDNF-QDs in the presence of 45 mM KCl, suggesting activity also enhanced the localization of endocytosis BDNF-QDs at presynaptic sites. Furthermore, there was no significant increase in the total number of BDNF-QD uptake in the axon (0.032 ± 0.005/μm2 in control vs. 0.025 ± 0.004/μm2 in 45 mM KCl). Taken together, these results indicate that neuronal activity enhances internalization of BDNF-QDs and their localization at both pre- and postsynaptic sites.
Activity-Dependent Exocytosis of BDNF-QDs at Postsynaptic Sites.
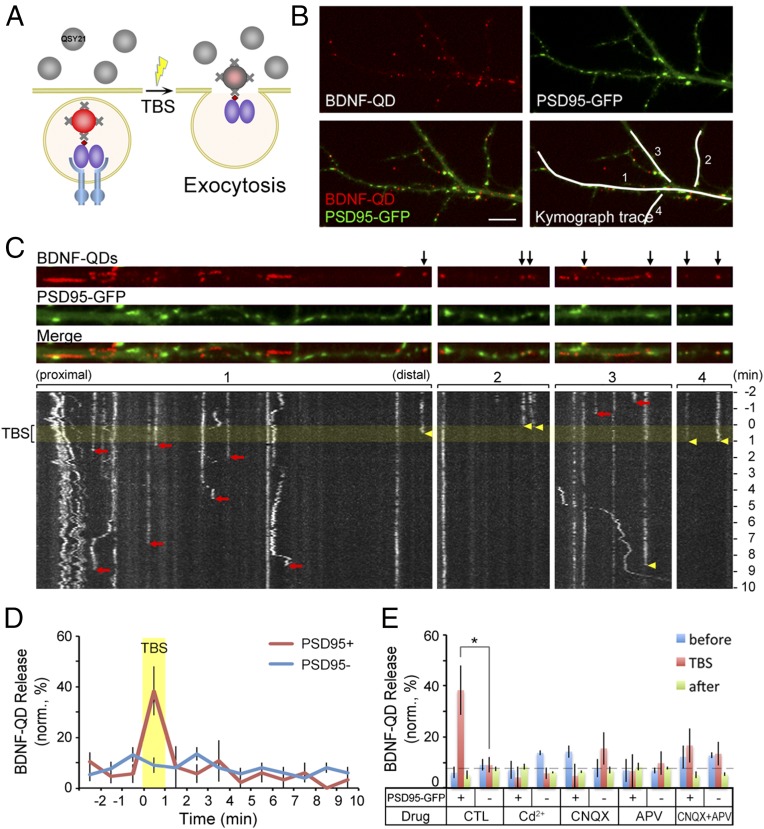
To examine the exocytosis of endosomes containing BDNF-QDs, we monitored the sudden disappearance of the fluorescence of previously loaded BDNF-QDs in the presence of extracellular QSY21, which quenches the QD fluorescence after the fusion of BDNF-QD–containing endosomes with the plasma membrane (Fig. 3A). For this study, we exposed DIV 12–16 hippocampal neurons to a solution containing BDNF-QDs for 60 min, and then perfused the culture with QSY21-containing saline and performed time-lapse imaging of the endocytosed BDNF-QDs (at sampling rate of 0.2 Hz), in the absence or presence of extracellular electrical stimulation. The axon/dendrite identity was determined by the neurite morphology and further confirmed in some cases by immunostaining with somatodendritic marker MAP2 and axonal marker Smi-312 (Fig. S4A). The effectiveness of the electrical stimulation in triggering neuronal spiking was confirmed by fluorescence Ca2+ imaging using the indicator calcium orange. As shown in Fig. S4B, θ-burst stimulation (TBS, 12 trains at 5-s intervals, each train consisting of 10 100-Hz bursts of 5 2-ms pulses spaced by 200 ms) resulted in immediate 30% increase in the calcium orange fluorescence, reflecting neuronal firing (31). We found that a small fraction (12.6 ± 1.7%) of BDNF-QDs vanished after stimulation, suggesting secretion of BDNF-QD–containing endosomes at dendrites.
Fig. 3.
Ca2+-dependence in activity-induced secretion of BDNF-QDs at postsynaptic sites. (A) Schematic diagram depicting the experimental design for detecting secretion of endocytosed BDNF-QDs. Membrane impermeant fluorescence quencher QSY 21 (gray) was added into the culture after the hippocampal neurons had endocytosed BDNF-QDs. Secretion of BDNF-QDs was indicated by the sudden disappearance of QD fluorescence because of fluorescence quenching by extracellular QSY21. (B) Image of an example neuron containing internalized BDNF-QDs and expressing PSD95-GFP. White lines mark four dendritic segments shown in C in linearized manner. (Scale bar, 10 μm.) (C) Images on top show the distribution of BDNF-QDs and PSD95 puncta along four dendritic segments. Black arrows, BDNF-QDs that colocalized with PSD95-GFP puncta. Kymograph traces below show the changes in QD fluorescence with time for the four segments above. Yellow band, the period of TBS. Yellow arrowheads: the time of disappearance of BDNF-QDs that colocalized with PSD95-GFP puncta. Red arrows: time of BDNF-QD disappearance at PSD95-GFP− sites. (D) Quantification of the percentage of BDNF-QDs secreted (in 1-min bins) during the entire imaging session (13 min) for BDNF-QDs located at PSD95-GFP+ and PSD95-GFP− sites (n = 38 neurons from six cultures). Yellow band: the TBS period. (E) Summary of the percentage of BDNF-QDs that were secreted at PSD95-GFP+ and negative sites in the presence of Cd2+ or various blockers of glutamate receptors before (blue), during (red), and after (green) TBS. Error bars indicate SEM in all panels (n = 6 cultures; *P < 0.05 by paired t test).
To further examine whether the BDNF-QD release site is located at the synapse, we transfected cultured hippocampal neurons on 5–6 DIV with vectors expressing PSD95-GFP to mark postsynaptic sites, and exposed the neurons to solution containing BDNF-QDs on 12–16 DIV for 60 min before the time-lapse imaging. We found that most PSD95-GFP puncta (95%) were immobile and colocalized or juxtaposed (within 1 pixel, 1 pixel = 267 nm) to Syp immunostaining, consistent with postsynaptic sites. Furthermore, we found that only about 9.5% of BDNF-QDs colocalized with PSD95-GFP puncta (Fig. 3B), suggesting no apparent concentration of BDNF-QDs at PSD95-GFP sites after 1 h. However, among all PSD95-colocalized BDNF-QDs that were found to disappear during the 12-min period of experiment, 38.3 ± 9.6% of them disappeared during the 1-min period of TBS (yellow arrowheads in Fig. 3 C and D, and yellow open circles in Movie S2). In contrast, those BDNF-QDs not colocalized with PSD95-GFP puncta disappeared during the 12-min period in an apparently random manner uncorrelated with TBS (red arrows in Fig. 3C). These results indicate that endosomal BDNF secretion at postsynaptic dendritic sites is much more sensitive to TBS than that occurred at nonsynaptic sites.
Dependence on Ca2+ Influx and Glutamate Receptor Activation.
To examine whether stimulation-induced release of BDNF-QDs depends on Ca2+ influx, we perfused the culture with extracellular solution containing 20 μM Cd2+, which blocks plasma membrane Ca2+ channels, during the imaging period. For BDNF-QDs colocalizing with PSD95-GFP puncta, we found that the percentage of BDNF-QD release at PSD95+ sites during TBS was reduced to a low level similar to that found at PSD95− sites, and similar to that found before and after TBS (Fig. 3E). Thus, Ca2+ entry in response to neuronal activity is required for the exocytosis of BDNF-QD–containing vesicles at postsynaptic sites.
We also examined the contribution of AMPA and NMDA subtypes of glutamate receptors, which are likely to be activated because of firing of both pre- and postsynaptic neurons in response to TBS. When either AMPA or NMDA receptors were blocked with 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) or d-(−)-2-amino-5-phosphonopentanoate (d-APV), respectively, the percentages of BDNF-QD release at PSD95+ sites during TBS was reduced to a low level similar to that found for the Cd2+ treatment (Fig. 3E). Blocking both AMPA and NMDA receptors had the same effect as blocking either type of glutamate receptors. These results support the notion that Ca2+ influx through NMDA receptors, whose activation is facilitated by membrane depolarization because of AMPA receptor activation, is essential for activity-induced release of BDNF-QDs at postsynaptic dendritic sites.
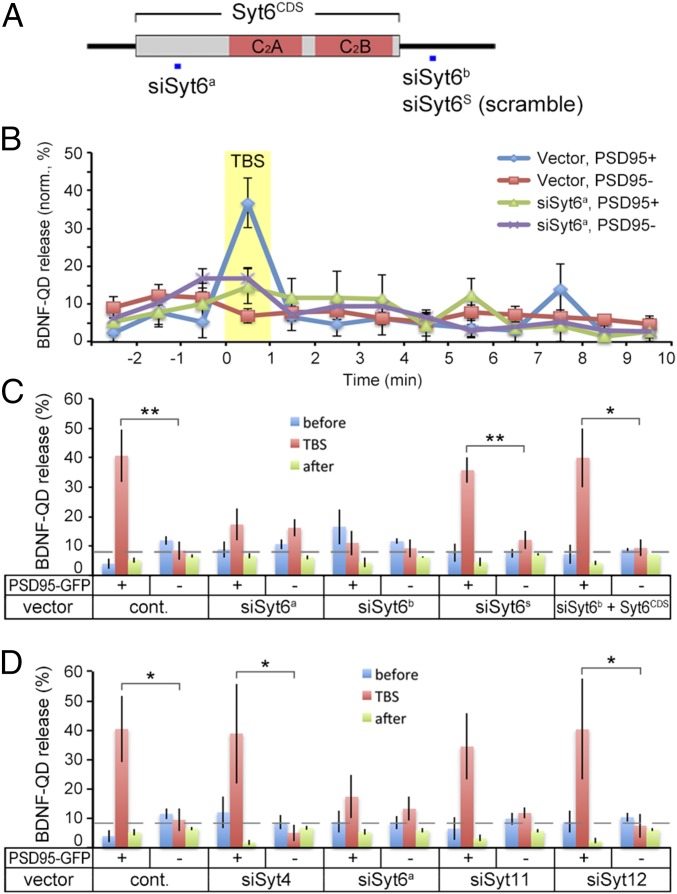
Activity-Induced Secretion Occurred at Syt6+ Sites.
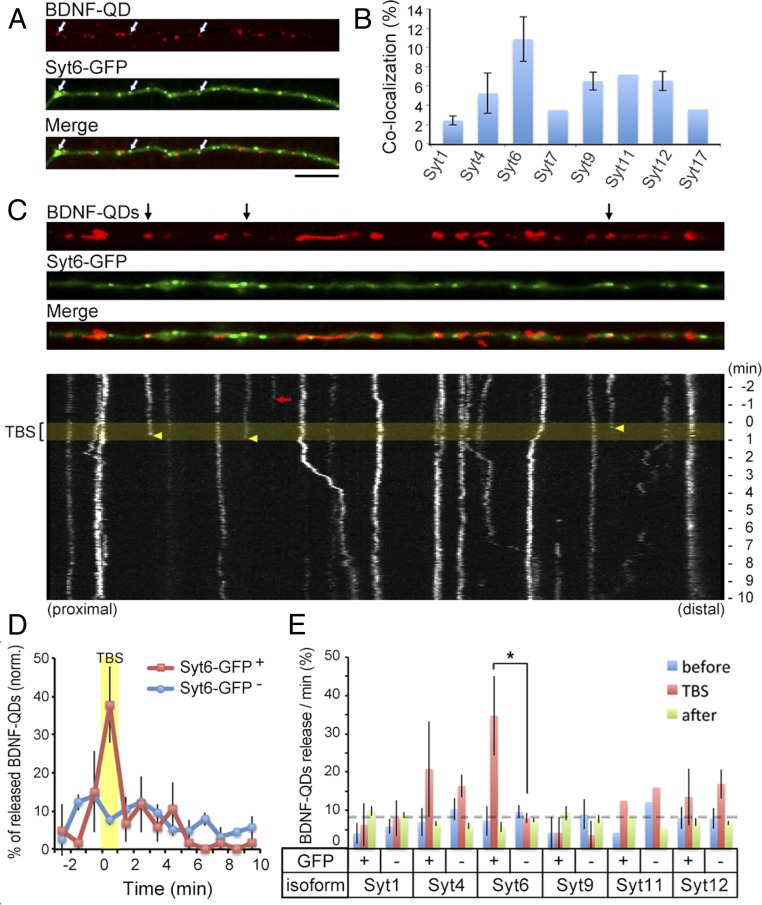
Synaptic vesicle fusion shares a common SNARE-mediated fusion mechanism with other forms of neurosecretion that involves Syt and Cpx (32). To examine whether a specific member of the Syt family is involved in activity-dependent secretion of endocytosed BDNF at postsynaptic sites, we cloned Syt1, Syt4, Syt6, Syt7, Syt9, Syt11, Syt12, and Syt17 from rat embryonic day 19 hippocampi (Table S1) and expressed various GFP-tagged Syt isoforms individually in dissociated hippocampal neurons. Among these isoforms, Syt4, Syt6, Syt9, and Syt12 were expressed in both the axon and dendrites; Syt1, Syt7, and Syt17 were found mainly in the axon; and Syt11 was mainly expressed in dendrites. We found that Syt17-GFP exhibited the highest percentage of juxtaposed colocalization with PSD95 puncta (27.8 ± 4.3%) (Fig. S5), suggesting its presynaptic localization. Moreover, Syt6-, Syt9-, and Syt12-GFP showed an intermediate value (10–12%), and Syt1-, Syt4-, and Syt11-GFP exhibited lowest colocalization (<5%) with PSD95 puncta.
After exposing 12–16 DIV neurons expressing one of the eight Syt-GFPs to BDNF-QDs for 1 h, time-lapse imaging was performed (for 12-min observation with 1-min TBS) to examine BDNF-QD release at sites with and without colocalization of a distinct isoform of Syt-GFP expressed in the neuron. Among eight groups, we found that the percentage of endocytosed BDNF-QDs that colocalized with Syt6-GFP puncta was the highest (Fig. 4 A and B). In addition, there was significantly more overall release of BDNF-QDs from Syt6-GFP–expressing neurons (13.6 ± 2.4%). The percentage of BDNF-QD release at Syt6-GFP sites was also much higher during the TBS period (37.8 ± 9.9%) (Fig. 4 C and D) than that occurred before or after TBS. Furthermore, no significant TBS-related release of BDNF-QDs was observed at sites showing Syt1, Syt4, Syt9, and Syt12 puncta (Fig. 4E). Thus, BDNF-QDs associated with Syt6 at the dendrite were released in an activity-dependent manner, suggesting that Syt6 acts as the specific Ca2+-sensor for promoting the exocytosis of BDNF-containing endosomes.
Fig. 4.
Secretion of BDNF-QDs occurs primarily at Syt6 sites. (A) Representative image of a linearized dendrite segment of a hippocampal neuron containing internalized BDNF-QDs and expressing Syt6-GFP. White arrows, colocalized BDNF-QDs and Syt6-GFP puncta. (Scale bar, 10 μm.) (B) Average percentage of endocytosed BDNF-QDs that colocalized with Syt-GFP puncta in all dendrites examined (n = 3–5 neurons each from three to six 12–15 DIV cultures for Syt1, -4, -6, -9, -12; two cultures for Syt7, -11, -17). Error bars, SEM. (C) An example of linearized dendritic segment and kymographs of BDNF-QD movements. (Upper) Black arrows, BDNF-QDs that colocalized with Syt6-GFP puncta. (Lower) Yellow band, period of TBS. Arrows mark the time of disappearance of BDNF-QDs colocalized with (yellow) or without (red) Syt6-GFP puncta. (D) Average percentage of BDNF-QDs released during 1-min time bins (normalized for each neuron) at Syt6-GFP+ or Syt6-GFP− sites in all DIV 12–16 neurons examined (n = 27 neurons from five cultures). Error bars, SEM. (E) Average percentage of BDNF-QDs released at Syt-GFP+ or Syt-GFP− sites before (blue), during (red), and after (green) TBS, for all neurons expressing one of the six Syt isoforms (n = 3–5 cultures each for all Syt1, -4, -6, -9, -12; 2 cultures for Syt11). Error bars, SEM (*P < 0.01 by paired t test).
Syt6 Knockdown Impaired Activity-Induced Postsynaptic BDNF-QD Release.
To further explore the role of various Syt isoforms in BDNF-QD release, we performed loss-of-function experiments in cultured hippocampal neurons using specific siRNAs against the expression of four Syt isoforms Syt4, Syt6, Syt11, and Syt12 that were found to be localized to dendrites (see above). The knockdown efficiency of siRNA vectors targeting these four Syt isoforms was confirmed in cultured 293T cells by Western blotting and immunostaining (Figs. S6B and S7 B–D and F). Down-regulation of endogenous Syt4 and Syt6 in hippocampal neurons respectively by siRNA against Sy4 (siSyt4) and by either one of the two forms of siRNA against Syt6 (siSyt6a or siSyt6b) was further verified by immunostaining (Figs. S6C and S7 E and G). Next, we transfected DIV 5–6 neurons with siSyt4, siSyt6, siSyt11 or siSyt12 together with PSD95-GFP, and examined TBS-induced release of BDNF-QDs on 12–16 DIV. We found that the percentage of BDNF-QD release at PSD95-GFP+ sites during TBS was reduced to the basal level in neurons transfected with either siSyt6a or siSyt6b (Fig. 5 B and C). In contrast, none of the neurons transfected with siSyt4, siSyt6s (scramble sequence of siSyt6b), siSyt11, siSyt12, or the control vector UI4 had any significant effect on TBS-induced release at PSD95+ sites (Fig. 5 C and D). Furthermore, the knockdown effect of siSyt6b could be prevented by coexpressing siSyt6b-resistant Syt6CDS (Fig. 5C). Thus, specific down-regulation of Syt6 abolished activity-driven release of BDNF-QDs at postsynaptic sites. These findings further support the notion that Syt6 is the isoform specifically involved in regulating activity-dependent secretion of endocytosed BDNF at postsynaptic sites.
Fig. 5.
Down-regulation of Syt6 selectively impaired BDNF-QD release at postsynaptic sites. (A) Schematic structure of Syt6, with sites used for generating siRNA constructs (siSyt6a, siSyt6b, and siSyt6s with scramble sequence) marked by a square in blue. (B) Average percentage of BDNF-QDs released during 1-min time bins at PSD95-GFP+ or PSD95-GFP− site in DIV 12–16 neurons transfected with siSyt6a or control vector. Yellow band, TBS period. Error bars, SEM (n = 3–7 neurons from eight cultures each). (C) Average percentage of BDNF-QDs released per minute at PSD95-GFP+ or PSD95-GFP− sites in neurons transfected with different forms of siRNAs for Syt6 and control vector, before (blue), during (red), and after (green) TBS. Error bars, SEM (n = 3–10 cultures each; *P < 0.05 and **P < 0.01 by Student’s t test). (D) Average percentage of BDNF-QDs released per minute at PSD95-GFP+ or PSD95-GFP− sites in neurons with down-regulation of different Syt isoforms before (blue), during (red), and after (green) TBS. Error bars, SEM (n = 4–8 cultures each; *P < 0.05 by Student’s t test).
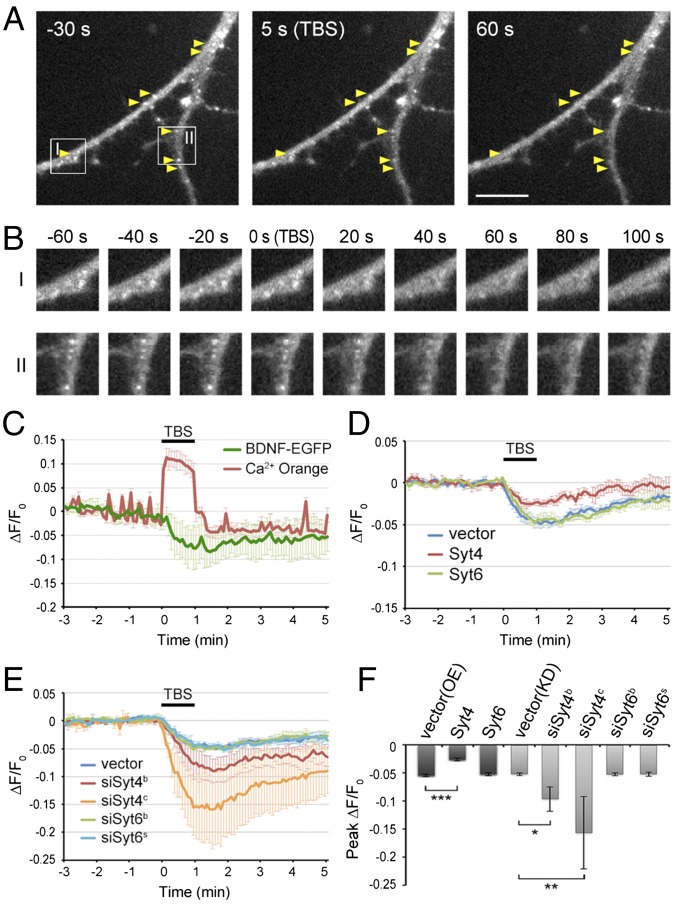
Activity-Induced Secretion of Endogenously Synthesized BDNF.
Recently it was reported that Syt4 is localized to vesicles containing endogenously synthesized BDNF, which is secreted from both the axon and dendrites in response to neuronal depolarization (33). To further investigate whether the mechanism regulating the release of endocytosed BDNF also applies to endogenously synthesized BDNF, we transfected the cultured hippocampal neurons with BDNF-EGFP fusion protein to visualize BDNF release events upon electrical stimulation (34) and examined the effects of overexpression or knockdown of Syt4 or Syt6. We transfected Syt4, Syt6, siSyt4, or siSyt6 together with BDNF-EGFP into DIV 5–6 neurons, and examined TBS-induced release of BDNF-EGFP by time-lapse imaging (at sampling rate of 0.2 Hz) on 13–16 DIV. We found that many BDNF-EGFP puncta in dendrites that were visible before TBS exhibited a reduction of fluorescence upon stimulation, indicating the exocytosis of BDNF-EGFP–containing vesicles (Fig. 6 A and B and Movie S3). Elevation of Ca2+ during TBS was simultaneously monitored together with the reduction of EGFP fluorescence, which reached the maximum (peak) at the end of TBS (0–30 s after TBS) (Fig. 6C). Moreover, knockdown of Syt4 increased, whereas overexpression decreased the secretion of BDNF-EGFP induced by TBS, suggesting Syt4 inhibits BDNF-EGFP secretion in dendrites (Fig. 6 D–F). In contrast, overexpression or knockdown of Syt6 had no significant effect on TBS-induced secretion of BDNF-EGFP. Together, these experiments demonstrate that Syt4 and Syt6 play specific regulatory function for the secretion of endogenously synthesized BDNF and endocytosed BDNF, respectively, and their regulatory actions are opposite: Syt4 inhibits and Syt6 promotes the secretion.
Fig. 6.
Syt4, but not Syt6, regulates activity-induced secretion of BDNF-EGFP in dendrites. (A) Representative images of hippocampal neurons transfected with control vector and BDNF-EGFP, taken at 30 s before, and 3 s and 60 s after TBS application. Arrows, BDNF-EGFP puncta showing fluorescence reduction after TBS. (Scale bar, 10 μm.) (B) Boxed areas I and II in A are shown at a higher resolution. (Magnification, 1.12 × 1.12.) (C) A representative trace of average BDNF-EGFP and Ca2+ Orange fluorescence changes at dendrites in control neurons (shown in A and B), with TBS period marked by the bar. Fluorescence levels were normalized by that before TBS (n = 10). Error bars, SD. (D) Traces of average BDNF-EGFP puncta fluorescence in dendrites of control and Syt4- and Syt6-overexpressing neurons (n = 4 cultures in each group). Error bars, SEM. (E) Traces of average BDNF-EGFP puncta fluorescence in dendrites of control and siSyt4- or siSyt6-transfected neurons (n = 3–5 cultures each). Error bars, SEM. (F) Average peak reduction of BDNF-EGFP puncta fluorescence in dendrites induced by TBS, normalized to the fluorescence intensity before TBS. Error bars, SEM (n = 3–5 cultures each; *P < 0.01, **P < 0.005, ***P < 0.001 by one-way ANOVA and Tukey post hoc test).
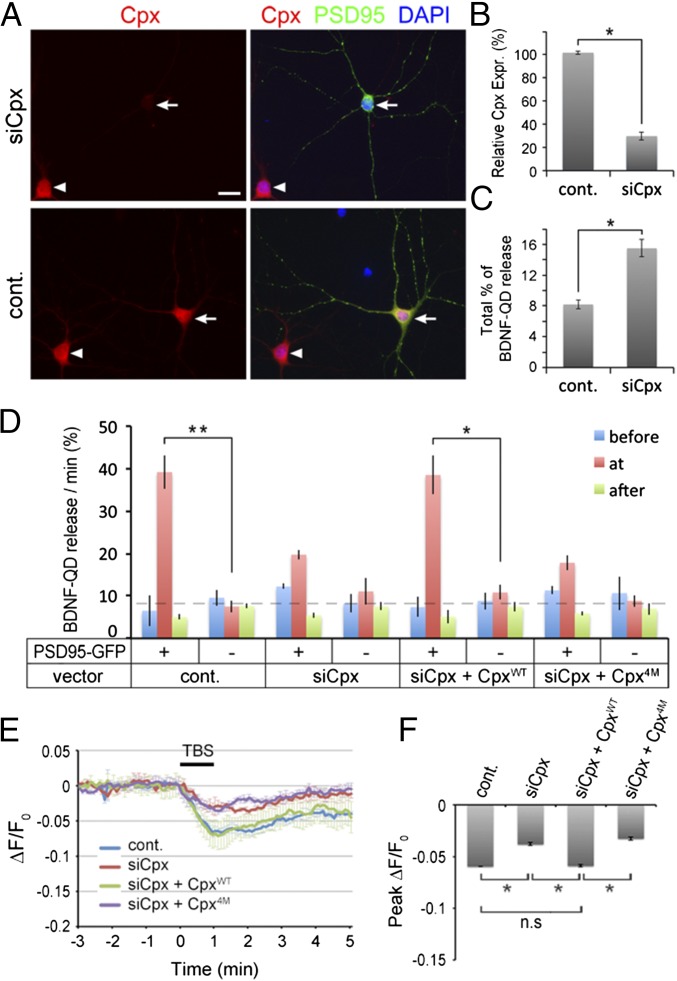
Complexin Down-Regulation Impaired Activity-Dependent Secretion.
To determine whether Cpx is involved in activity-dependent secretion of endocytosed BDNF, we examined the TBS-induced release of BDNF-QDs in Cpx1/2 knockdown neurons. When DIV 5–6 hippocampal neurons were transfected with a microRNA-based siRNA vector (35), siCpx, together with vector expressing PSD95-GFP, we found that immunostaining of endogenous Cpx1/2 was down-regulated efficiently in these neurons on 13–15 DIV, but PSD95-GFP puncta were similar to that in control untransfected cells (Fig. 7 A and B). The overall percentage of BDNF-QD release during the 16-min observation period increased by twofold (Fig. 7C). However, release of BDNF-QDs located at the PSD95-GFP+ site in Cpx knockdown neurons was reduced during the TBS period (Fig. 7D). This siCpx effect was prevented by simultaneous coexpression of a siRNA-resistant full-length Cpx1 (CpxWT), but not by a mutant form of Cpx1 (Cpx4M) that is unable to bind SNARE complexes. These results indicate that Cpx1 is required for activity-dependent exocytosis of BDNF-QDs at postsynaptic sites, but serves as a “clamp” to prevent spontaneous exocytosis of docked endosomes, reminiscent of its role in regulating synaptic vesicle secretion (36).
Fig. 7.
Complexin down-regulation reduced stimulation-induced secretion of BDNF-QD and BDNF-EGFP at postsynaptic sites. (A) Images of hippocampal neurons in a representative culture cotransfected with Cpx1/2 siRNA (siCpx) and PSD95-GFP. Efficient Cpx1/2 down-regulation was observed in the siCpx-transfected neuron (arrowhead, Upper), but not in untransfected cells in the same field (arrow). Arrows: transfected neurons with PSD95-GFP; arrowheads: untransfected neurons. (Scale bar, 20 µm.) (B) Quantitation of Cpx expression levels by measurements of immunostaining intensities of neurons, as illustrated in A. Relative Cpx protein levels were normalized to that of nontransfected neurons in the same image field. Error bars, SEM (n = 3 independent cultures; *P = 0.003 by paired t test). (C) The percentage of total BDNF-QDs released during the standard imaging period in control or Cpx down-regulated neurons (n = 3 independent experiments; *P = 0.003 by paired t test). (D) The average percentage of BDNF-QDs released per minute at PSD95-GFP+ or PSD95-GFP− sites in neurons transfected with indicated plasmids, before (blue), during (red), and after (green) TBS. Error bars, SEM (n = 3–4 independent cultures for each condition; *P < 0.01 and **P < 0.001 by Student’s t test). (E) Traces of average BDNF-EGFP puncta fluorescence in dendrites induced by TBS in control, siCpx, siCpx+CpxWT or siCpx+Cpx4M transfected neurons (n = 3 cultures each). Error bars, SEM. (F) Average peak reduction of BDNF-EGFP puncta fluorescence in dendrites induced by TBS, normalized to the fluorescence intensity before TBS. Error bars, SEM (n = 3 cultures each; *P < 0.001 by one-way ANOVA and Tukey post hoc test).
To test whether Cpx is involved in activity-induced secretion of endogenously synthesized BDNF, we transfected the cultured hippocampal neurons with both the BDNF-EGFP fusion protein and siCpx, and examined TBS-induced release of BDNF-EGFP on 12–16 DIV. The Cpx knockdown decreased the reduction of BDNF-EGFP fluorescence induced by TBS (Fig. 7 E and F), suggesting that Cpx inhibits BDNF-EGFP secretion in dendrites. This effect was rescued by simultaneous expression of a siRNA-resistant Cpx1 (siCpx + CpxWT), but not by the mutant Cpx4M. Thus, consistent with the influence of Cpx on the BDNF-QD exocytosis, these results demonstrate that Cpx is required for the secretion of endogenously synthesized BDNF.
Discussion
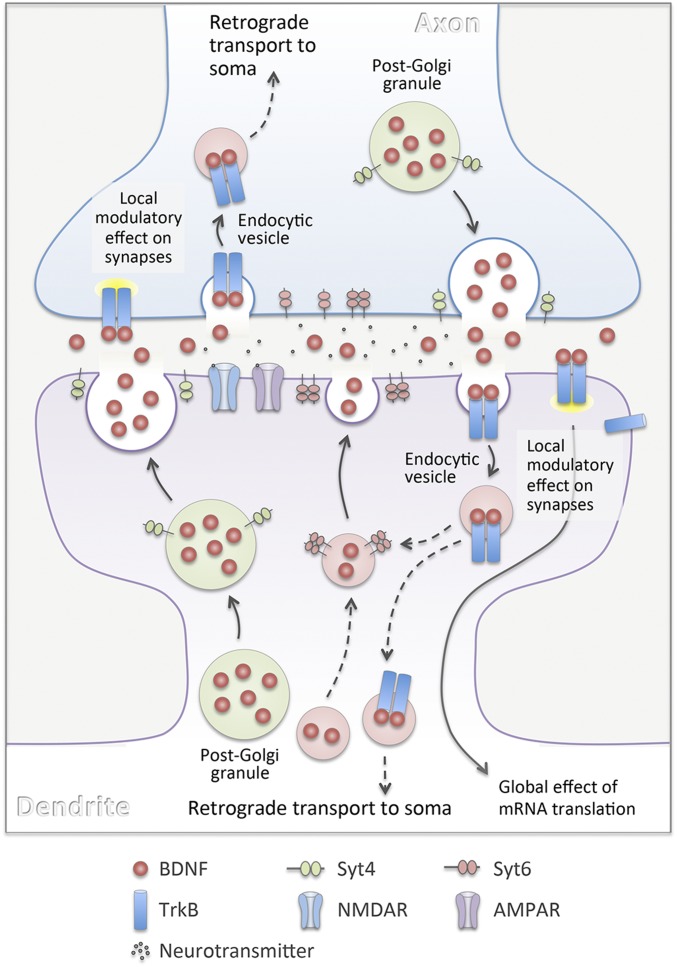
It is generally assumed that exocytosis of BDNF-containing post-Golgi granules represents the main pathway for providing synapse-modulating BDNF. In this study, we found that extracellular BDNF-QDs could be endocytosed by cultured hippocampal neurons in a TrkB-dependent manner (Fig. 1). The endocytosis of BDNF-QDs was preferentially localized to postsynaptic sites on the dendrite, and depolarization facilitated the endocytic process (Fig. S3). Repetitive extracellular stimulation of the culture in the form of TBS greatly increased the release of endocytosed BDNF-QDs only at PSD95+ postsynaptic sites (Fig. 3D), and this release requires the activation of synaptic AMPA and NMDA receptors (Fig. 3E). Among various isoforms of Syt examined, we found that TBS-induced release of BDNF-QDs was significantly facilitated by overexpression of only Syt6 (Fig. 4 C–E) and inhibited by knockdown of only Syt6 (Fig. 5 B–D). Interestingly, Syt6 does not regulate activity-induced secretion of endogenously synthesized BDNF (Fig. 6 D–F). Further studies showed that the Cpx1/2 facilitated activity-induced release of BDNF-QDs (Fig. 7D), but inhibited overall activity-independent BDNF-QD release (Fig. 7C), consistent with that found for synaptic vesicle exocytosis. Taken together, these results delineate a novel endocytic pathway for providing activity-dependent BDNF secretion at the synapse and its distinct regulatory mechanism (Fig. 8).
Fig. 8.
A diagram illustrating the secretion, uptake, and trafficking of BDNF at excitatory synapses. Post-Golgi granules containing BDNF are known to be the major source of BDNF secreted at the synapse. Binding of secreted BDNF to TrkB receptor leads to endocytic uptake of BDNF-TrkB complexes into “signaling endosomes” by either pre- or postsynaptic neuron. Synaptic activation could result in the exocytosis of not only BDNF-containing granules, but also endosomes containing endocytosed BDNF, in a manner requiring the activation of synaptic AMPA and NMDA receptors. Distinct from the Syt4 that is required for exocytosis of BDNF-containing post-Golgi granules, Syt6 is essential for activity-induced exocytosis of BDNF-containing endosomes. Endocytosed BDNF at postsynaptic dendrites may undergo local exocytosis for reuse near the uptake sites, whereas BDNF-containing endosomes formed by axonal terminals may undergo long-range retrograde transport to somatic and dendritic sites of the presynaptic neuron.
Uptake, Transport, and Localization of BDNF-QDs.
We have used brief incubation of cultured neurons with BDNF-QDs to induce endocytic uptake of BDNF-QDs, and fluorescence imaging was performed within 16 min after clearance of extracellular BDNF-QDs. Based on the relatively random distribution of cytoplasmic BDNF-QDs in all dendritic branches and the slow rate of axon-to-dendrite transport of BDNF-QDs, because of prolonged interruption after their arrival at the soma, we inferred that BDNF-QD uptake occurs at both the axon and dendrites. Furthermore, because most BDNF-QDs that colocalized with the postsynaptic marker PSD95 were immobile, BDNF-QD–containing endosomes may form either directly at postsynaptic sites or at extrasynaptic sites, but rapidly became trapped at the synapse.
Postsynaptic BDNF uptake and accumulation of BDNF-containing endosomes are particularly relevant for synaptic modulation. Because BDNF-containing endosomes are likely to be biochemically active (12–15), they could exert prolonged localized action of BDNF-TrkB signaling in the postsynaptic cytoplasm. Mobile endosomes could also spread BDNF-TrkB signals in the extrasynaptic region. Given the small net translocation of mobile BDNF-QDs observed along the dendrite, dendrite-to-axon transport is likely to be very limited. On the other hand, studies using compartmentalized multiwell culture systems have shown that BDNF-QDs endocytosed at axonal terminals were found in the dendrite at a later time (37), indicating the existence of axon-to-dendrite transport. This finding is consistent with the in vivo findings that BDNF applied in the developing Xenopus optic tectum results in retrograde potentiation of bipolar cell inputs to the retinal ganglion cell in an axonal transport-dependent manner (38), and LTP induced by TBS at retinotectal synapses exhibited BDNF-dependent retrograde spread to bipolar cell–retinal ganglion cell synapses (39). Retrograde transport of BDNF-containing endosomes and secretion of endosomal BDNF at the dendrite thus provides a mechanism by which activity-dependent modulation of output synapses of a neuron could exert a coordinated modulation of the input synapses at its dendrites both pre- and postsynaptically.
With respect to synaptic localization of BDNF-QDs, we found that 15.6 ± 1.6% of BDNF-QDs were located at immunostained PSD95+ sites (Fig. 1D) and 17.2 ± 1.9% of BDNF-QDs colocalized with PSD95-GFP puncta (Fig. 3C) along the dendrite after a brief incubation (10 min) of cultured neurons with BDNF-QDs. These percentages were higher than that expected if endocytosed BDNF-QDs were randomly distributed along the dendrite, because the area occupied by the PSD95-GFP puncta along the dendrite was only about 7.8 ± 0.5% (Fig. S3C). This finding suggests that endocytosed BDNF-QDs showed some preferential localization to the postsynaptic sites in the dendrite. In experiments with prolonged incubation (60 min) of cultured neurons with BDNF-QDs, however, we found only about 9.5% of BDNF-QDs colocalized with PSD95-GFP sites (Fig. 3B). This may be caused by lateral redistribution of endocytosed BDNF-QDs over the 1-h period. To avoid photodamage, imaging time in our experiments was kept within 30 min. This limited time window has prevented us from following individual BDNF-QDs from their endocytosis to subsequent activity-induced release. Thus, we were unable to directly determine whether BDNF-QD–containing endosomes formed at postsynaptic sites may be transported to other dendritic locations for their exocytosis.
Regulatory Functions of Syt and Cpx.
Our results showed that TBS promotes the release of BDNF-QDs at PSD95+ postsynaptic sites, but had no effect on the release at extrasynaptic sites, where Ca2+ elevation as a result of synaptic activation will be largely dissipated. In the absence of applied electrical stimulation, spontaneous release of BDNF-QDs may occur through a fusion mechanism that requires a Syt isoform functional to the resting level of cytoplasmic Ca2+. Recent studies have shown that synchronous and asynchronous phases of neurotransmitter release in hippocampal neurons are selectively regulated by Syt1 and Syt7, respectively, with fast Ca2+-dependent release facilitated by Syt1 and slower form of Ca2+-triggered release mediated by Syt7, whose function is normally occluded by Syt1 (40). Similar dual Syt regulation may also operate for the secretion of endosomal BDNF in the present case. However, it remains possible that the lack of siRNA knockdown effect on extrasynaptic release of BDNF-QDs was simply because of the low frequency of extrasynaptic release events that rendered the effect of siRNAs undetectable.
Our present results showed that the overall release of endocytosed BDNF-QDs from the dendrite is elevated after down-regulation of Cpx1/2. The elevation could be attributed to the increase in spontaneous activity-independent release, because TBS-induced release at postsynaptic sites was greatly reduced. This finding agreed with the notion that Cpx binding to SNARE proteins serves as a clamp to prevent spontaneous fusion of docked vesicles (27, 36), and removal of the clamp in response to activity requires the action of the Ca2+-sensing Syt (41). Further support to the idea that Cpx plays a role in postsynaptic exocytosis comes from the finding that postsynaptic Cpx regulates AMPA receptor delivery to synapses during LTP (42). In the present study, Syt6 is the Ca2+ sensor that specifically promotes the fusion of BDNF-containing endosomes at postsynaptic sites.
The role Syt6 plays in the activity-induced release of BDNF-QDs is opposite to that of Syt4 in the secretion of BDNF-containing secretory granules. In hippocampal neurons and Syt4 knockout mice, Syt4 was found to inhibit depolarization-evoked BDNF release in both axons and dendrites (33). This result is in agreement with our finding that knockdown of Syt4 increased and overexpression decreased the activity-induced secretion of BDNF-EGFP (Fig. 6 D–F). Our study also showed that Syt6 promotes activity-induced release of BDNF-QDs, but the effect on spontaneous release of BDNF-QDs was not detected, possibly because of the low frequency of spontaneous release events. Furthermore, overexpression and knockdown of Syt6 elevated and abolished TBS-induced BDNF-QD release, respectively, without affecting TBS-induced secretion of BDNF-EGFP. The distinct actions of Syt6 and Syt4 may be attributed to the structural difference between the two proteins. Both Syt4 and Syt6 are composed of a short intravesicular sequence at the amino terminal, a transmembrane region, a central linker sequence, and two carboxyl-terminal C2-domains known as C2A and C2B (43). In Syt6, the C2-domains were predicted to bind two to three Ca2+ ions via four to five acidic residues in two flexible loops. In contrast to Syt6, the C2A domain of Syt4 lacks the canonical aspartate residues required for Ca2+ binding, and the C2B-domain is unable to bind Ca2+ although it includes all of the requisite Ca2+-binding sequence (44). These structural differences may result in differential Syt binding to endosomes vs. secretary granules and to SNARE proteins, as well as differential Ca2+ sensitivities for activity-induced exocytosis of these two types of vesicles. Further characterization of the Ca2+-dependence of the exocytosis process may also reveal neuronal activity patterns that are required for triggering BDNF secretion via these two different pathways.
Although we have observed endocytosed BDNF-QDs at the axon (Fig. 1 B–E), the total number was much lower than that found at the dendrite. We have thus focused our attention on BDNF-QD secretion at the dendrite. Studies using cultured hippocampal neurons expressing fluorescently tagged-BDNF have shown BDNF secretion at both axons and dendrites (45, 46). However, BDNF and its propeptide have been found in presynaptic dense core vesicles, but not in the postsynaptic dendrites of the adult mouse hippocampus (6), suggesting an anterograde mode of action of BDNF. Previous work has shown that extracellular BDNF released by either pre- or postsynaptic neurons could be recycled back to the synaptic sites via binding to TrkB receptors on the dendrites of postsynaptic neurons (46, 47). In the present work, we further showed that BDNF could be internalized and released at postsynaptic sites via physiologically relevant TBS. Indeed, extracellularly provided recombinant BDNF has been found to promote late-LTP in the absence of postsynaptic synthesis at Schaffer collateral–CA1 synapses (16). Thus, endosomes containing BDNF could serve as secondary storage sites in addition to endogenous BDNF-containing secretory granules, available for future activity-induced secretion and synaptic modulation.
Materials and Methods
For time-lapse imaging of BDNF-QD, 0.5 μL of 1 μM biotinylated BDNF (19) was incubated with 0.5 μL of 1 μM Qdot655 streptavidin conjugate (Invitrogen) at 4 °C overnight to form the BDNF-QD complex. DIV 12–16 hippocampal neurons were incubated in an extracellular solution with 2% BSA containing 1 or 0.2 nM BDNF-QD for 10 or 60 min, respectively. Coverslips with BDNF-QD–loaded cells were transferred to a field stimulation chamber in a solution containing 4 μM QSY 21 carboxylic acid, succinimidyl ester (QSY21; Invitrogen). Time-lapse images were acquired (acquisition time, 200 ms) at 0.2 Hz with a 14-bit EMCCD camera controlled by Micro-Manager (48) or NIS-Elements microscope imaging software (Nikon Instruments) during TBS (12 trains at 5-s intervals, each train consisting of five 5-Hz bursts of five 2-ms pulses at 100 Hz). After acquisition, the images were processed for viewing and analyzed with NIH ImageJ software.
Other detailed experimental procedures of vector construction, cell cultures, immunostaining, preparation of BDNF-QDs, electrical stimulation, time-lapse imaging, and statistical analysis are included in SI Materials and Methods. All animal protocols were approved by the Animal Care and Use Committee of University of California, Berkeley.
Supplementary Material
Acknowledgments
We thank Drs. D. L. Turner for providing siRNA vectors (UI4-puro and UI4-GFP) and T. C. Südhof for providing complexin knockdown and rescue constructs (siCpx + Cpx14M and siCpx + Cpx1WT). This work was supported by National Institutes of Health Grant NS 036999 (to M.-m.P).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1511830112/-/DCSupplemental.
References
- 1.Leibrock J, et al. Molecular cloning and expression of brain-derived neurotrophic factor. Nature. 1989;341(6238):149–152. doi: 10.1038/341149a0. [DOI] [PubMed] [Google Scholar]
- 2.Park H, Poo MM. Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci. 2013;14(1):7–23. doi: 10.1038/nrn3379. [DOI] [PubMed] [Google Scholar]
- 3.Thoenen H. The changing scene of neurotrophic factors. Trends Neurosci. 1991;14(5):165–170. doi: 10.1016/0166-2236(91)90097-e. [DOI] [PubMed] [Google Scholar]
- 4.Korte M, et al. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc Natl Acad Sci USA. 1995;92(19):8856–8860. doi: 10.1073/pnas.92.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greenberg ME, Xu B, Lu B, Hempstead BL. New insights in the biology of BDNF synthesis and release: implications in CNS function. J Neurosci. 2009;29(41):12764–12767. doi: 10.1523/JNEUROSCI.3566-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dieni S, et al. BDNF and its pro-peptide are stored in presynaptic dense core vesicles in brain neurons. J Cell Biol. 2012;196(6):775–788. doi: 10.1083/jcb.201201038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pang PT, et al. Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science. 2004;306(5695):487–491. doi: 10.1126/science.1100135. [DOI] [PubMed] [Google Scholar]
- 8.Nagappan G, et al. Control of extracellular cleavage of ProBDNF by high frequency neuronal activity. Proc Natl Acad Sci USA. 2009;106(4):1267–1272. doi: 10.1073/pnas.0807322106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haubensak W, Narz F, Heumann R, Lessmann V. BDNF-GFP containing secretory granules are localized in the vicinity of synaptic junctions of cultured cortical neurons. J Cell Sci. 1998;111(Pt 11):1483–1493. doi: 10.1242/jcs.111.11.1483. [DOI] [PubMed] [Google Scholar]
- 10.Balkowiec A, Katz DM. Activity-dependent release of endogenous brain-derived neurotrophic factor from primary sensory neurons detected by ELISA in situ. J Neurosci. 2000;20(19):7417–7423. doi: 10.1523/JNEUROSCI.20-19-07417.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chao MV. Neurotrophins and their receptors: A convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4(4):299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- 12.Cohen MS, Bas Orth C, Kim HJ, Jeon NL, Jaffrey SR. Neurotrophin-mediated dendrite-to-nucleus signaling revealed by microfluidic compartmentalization of dendrites. Proc Natl Acad Sci USA. 2011;108(27):11246–11251. doi: 10.1073/pnas.1012401108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cosker KE, Courchesne SL, Segal RA. Action in the axon: Generation and transport of signaling endosomes. Curr Opin Neurobiol. 2008;18(3):270–275. doi: 10.1016/j.conb.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grimes ML, Beattie E, Mobley WC. A signaling organelle containing the nerve growth factor-activated receptor tyrosine kinase, TrkA. Proc Natl Acad Sci USA. 1997;94(18):9909–9914. doi: 10.1073/pnas.94.18.9909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riccio A, Pierchala BA, Ciarallo CL, Ginty DD. An NGF-TrkA-mediated retrograde signal to transcription factor CREB in sympathetic neurons. Science. 1997;277(5329):1097–1100. doi: 10.1126/science.277.5329.1097. [DOI] [PubMed] [Google Scholar]
- 16.Santi S, et al. Hippocampal neurons recycle BDNF for activity-dependent secretion and LTP maintenance. EMBO J. 2006;25(18):4372–4380. doi: 10.1038/sj.emboj.7601303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pinaud F, et al. Advances in fluorescence imaging with quantum dot bio-probes. Biomaterials. 2006;27(9):1679–1687. doi: 10.1016/j.biomaterials.2005.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Q, Li Y, Tsien RW. The dynamic control of kiss-and-run and vesicular reuse probed with single nanoparticles. Science. 2009;323(5920):1448–1453. doi: 10.1126/science.1167373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie W, Zhang K, Cui B. Functional characterization and axonal transport of quantum dot labeled BDNF. Integr Biol (Camb) 2012;4(8):953–960. doi: 10.1039/c2ib20062g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cui B, et al. One at a time, live tracking of NGF axonal transport using quantum dots. Proc Natl Acad Sci USA. 2007;104(34):13666–13671. doi: 10.1073/pnas.0706192104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Betz WJ, Bewick GS. Optical monitoring of transmitter release and synaptic vesicle recycling at the frog neuromuscular junction. J Physiol. 1993;460:287–309. doi: 10.1113/jphysiol.1993.sp019472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dan Y, Poo MM. Quantal transmitter secretion from myocytes loaded with acetylcholine. Nature. 1992;359(6397):733–736. doi: 10.1038/359733a0. [DOI] [PubMed] [Google Scholar]
- 23.Dan Y, Song HJ, Poo MM. Evoked neuronal secretion of false transmitters. Neuron. 1994;13(4):909–917. doi: 10.1016/0896-6273(94)90256-9. [DOI] [PubMed] [Google Scholar]
- 24.Morimoto T, Popov S, Buckley KM, Poo MM. Calcium-dependent transmitter secretion from fibroblasts: Modulation by synaptotagmin I. Neuron. 1995;15(3):689–696. doi: 10.1016/0896-6273(95)90156-6. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Q, Cao YQ, Tsien RW. Quantum dots provide an optical signal specific to full collapse fusion of synaptic vesicles. Proc Natl Acad Sci USA. 2007;104(45):17843–17848. doi: 10.1073/pnas.0706906104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Südhof TC. Calcium control of neurotransmitter release. Cold Spring Harb Perspect Biol. 2012;4(1):a011353. doi: 10.1101/cshperspect.a011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maximov A, Tang J, Yang X, Pang ZP, Südhof TC. Complexin controls the force transfer from SNARE complexes to membranes in fusion. Science. 2009;323(5913):516–521. doi: 10.1126/science.1166505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jablonski AE, Kawakami T, Ting AY, Payne CK. Pyrenebutyrate leads to cellular binding, not intracellular delivery, of polyarginine-quantum dots. J Phys Chem Lett. 2010;1:1312–1315. doi: 10.1021/jz100248c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McAllister AK, Katz LC, Lo DC. Opposing roles for endogenous BDNF and NT-3 in regulating cortical dendritic growth. Neuron. 1997;18(5):767–778. doi: 10.1016/s0896-6273(00)80316-5. [DOI] [PubMed] [Google Scholar]
- 30.Heerssen HM, Pazyra MF, Segal RA. Dynein motors transport activated Trks to promote survival of target-dependent neurons. Nat Neurosci. 2004;7(6):596–604. doi: 10.1038/nn1242. [DOI] [PubMed] [Google Scholar]
- 31.Smetters D, Majewska A, Yuste R. Detecting action potentials in neuronal populations with calcium imaging. Methods. 1999;18(2):215–221. doi: 10.1006/meth.1999.0774. [DOI] [PubMed] [Google Scholar]
- 32.Südhof TC. Neurotransmitter release: The last millisecond in the life of a synaptic vesicle. Neuron. 2013;80(3):675–690. doi: 10.1016/j.neuron.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dean C, et al. Synaptotagmin-IV modulates synaptic function and long-term potentiation by regulating BDNF release. Nat Neurosci. 2009;12(6):767–776. doi: 10.1038/nn.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu H, Park H, Poo MM. Spike-timing-dependent BDNF secretion and synaptic plasticity. Philos Trans R Soc Lond B Biol Sci. 2014;369(1633):20130132. doi: 10.1098/rstb.2013.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chung KH, et al. Polycistronic RNA polymerase II expression vectors for RNA interference based on BIC/miR-155. Nucleic Acids Res. 2006;34(7):e53. doi: 10.1093/nar/gkl143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang X, Kaeser-Woo YJ, Pang ZP, Xu W, Südhof TC. Complexin clamps asynchronous release by blocking a secondary Ca(2+) sensor via its accessory α helix. Neuron. 2010;68(5):907–920. doi: 10.1016/j.neuron.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xie W. 2014. Trafficking and axodendritic transcytosis of BDNF in hippocampal neurons. Phd dissertation (Stanford University, Stanford, CA)
- 38.Du JL, Poo MM. Rapid BDNF-induced retrograde synaptic modification in a developing retinotectal system. Nature. 2004;429(6994):878–883. doi: 10.1038/nature02618. [DOI] [PubMed] [Google Scholar]
- 39.Du JL, Wei HP, Wang ZR, Wong ST, Poo MM. Long-range retrograde spread of LTP and LTD from optic tectum to retina. Proc Natl Acad Sci USA. 2009;106(45):18890–18896. doi: 10.1073/pnas.0910659106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bacaj T, et al. Synaptotagmin-1 and synaptotagmin-7 trigger synchronous and asynchronous phases of neurotransmitter release. Neuron. 2013;80(4):947–959. doi: 10.1016/j.neuron.2013.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang J, et al. A complexin/synaptotagmin 1 switch controls fast synaptic vesicle exocytosis. Cell. 2006;126(6):1175–1187. doi: 10.1016/j.cell.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 42.Ahmad M, et al. Postsynaptic complexin controls AMPA receptor exocytosis during LTP. Neuron. 2012;73(2):260–267. doi: 10.1016/j.neuron.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perin MS, Fried VA, Mignery GA, Jahn R, Südhof TC. Phospholipid binding by a synaptic vesicle protein homologous to the regulatory region of protein kinase C. Nature. 1990;345(6272):260–263. doi: 10.1038/345260a0. [DOI] [PubMed] [Google Scholar]
- 44.Dai H, et al. Structural basis for the evolutionary inactivation of Ca2+ binding to synaptotagmin 4. Nat Struct Mol Biol. 2004;11(9):844–849. doi: 10.1038/nsmb817. [DOI] [PubMed] [Google Scholar]
- 45.Matsuda N, et al. Differential activity-dependent secretion of brain-derived neurotrophic factor from axon and dendrite. J Neurosci. 2009;29(45):14185–14198. doi: 10.1523/JNEUROSCI.1863-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hartmann M, Heumann R, Lessmann V. Synaptic secretion of BDNF after high-frequency stimulation of glutamatergic synapses. EMBO J. 2001;20(21):5887–5897. doi: 10.1093/emboj/20.21.5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang SH, et al. BDNF-dependent recycling facilitates TrkB translocation to postsynaptic density during LTP via a Rab11-dependent pathway. J Neurosci. 2013;33(21):9214–9230. doi: 10.1523/JNEUROSCI.3256-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Edelstein A, Amodaj N, Hoover K, Vale R, Stuurman N. 2010. Computer control of microscopes using μManager. Curr Protoc Mol Biol, Chapter 14:Unit14.20. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.