SUMMARY
The transcriptional co-activators YAP and TAZ are key regulators of organ size and tissue homeostasis, and their dysregulation contributes to human cancer. Here we discover YAP/TAZ as bona fide downstream effectors of the alternative Wnt signaling pathway. Wnt5a/b and Wnt3a induce YAP/TAZ activation independent of canonical Wnt/β-catenin signaling. Mechanistically, we delineate the ‘alternative Wnt-YAP/TAZ signaling axis’ that consists of Wnt - FZD/ROR - Gα12/13 - Rho GTPases -Lats1/2 to promote YAP/TAZ activation and TEAD-mediated transcription. YAP/TAZ mediate the biological functions of alternative Wnt signaling including gene expression, osteogenic differentiation, cell migration, and antagonism of Wnt/β-catenin signaling. Together, our work establishes YAP/TAZ as critical mediators of alternative Wnt signaling.
INTRODUCTION
Wnt proteins are morphogens that elicit diverse receptor-mediated signaling pathways to control development and tissue homeostasis. Canonical Wnt signaling acts through β-catenin/TCF transcriptional activity (referred to as 'Wnt/β-catenin signaling') (Logan and Nusse, 2004; MacDonald et al., 2009). Wnt3a is a classic canonical Wnt ligand, although it has been shown to elicit both β-catenin-dependent, and independent responses (Angers and Moon, 2009). Noncanonical Wnt signaling mediates biological responses that do not involve β-catenin/TCF activity (referred to as ‘alternative Wnt signaling’), and Wnt5a/b are prototype alternative Wnt ligands (van Amerongen, 2012).
In vertebrate, alternative Wnt signaling is involved in planar cell polarity (PCP), convergent extension movements, dorsoventral patterning, tissue regeneration, and tumorigenesis. During these processes, alternative Wnt signaling induces cytoskeletal and migratory changes and antagonizes canonical Wnt/β-catenin signaling. However, these β-catenin-independent signaling responses remain poorly characterized at the molecular level (van Amerongen, 2012). The Frizzled (FZD) receptors are transducers of both Wnt/β-catenin and alternative Wnt signaling. An interesting, yet controversial aspect of FZD is the requirement of G proteins. Although Gα proteins have been previously shown to modulate Wnt signaling (Katanaev et al., 2005; Liu et al., 2001; Slusarski et al., 1997), recent studies have failed to identify Gα proteins as core components of Wnt/β-catenin signaling (Major et al., 2008; Regard et al., 2011). Thus, identifying G proteins and novel effectors involved in the alternative Wnt signaling is a key unresolved issue in the field.
The Hippo tumor suppressor pathway functions to inhibit the activity of YAP/TAZ transcriptional co-activators. The Hippo-YAP/TAZ pathway has emerged as a hub that integrates diverse stimuli including mechanical and cytoskeletal cues, cell adhesion, apico-basolateral polarity, and mitogens to control cell growth and organ size (Pan, 2010; Yu and Guan, 2013). Recent studies uncovered the critical role of GPCR signaling in YAP/TAZ regulation (Miller et al., 2012; Mo et al., 2012; Yu et al., 2014b; Yu et al., 2012), as well as crosstalk with Wnt or TGFβ signaling (Moroishi et al., 2015; Piccolo et al., 2014). The core Mst1/2-Lats1/2 kinase cascade inhibits YAP/TAZ through direct phosphorylation, which results in cytoplasmic retention via 14–3-3 binding, and further promotes β-TrCP-mediated YAP/TAZ ubiquitination and degradation. Upon inhibition of the Hippo pathway, YAP/TAZ are activated and translocated into the nucleus to bind TEAD family transcription factors to stimulate target gene expression involved in cell proliferation, stem cell self-renewal, and tumorigenesis (Mo et al., 2014).
In the present study, we demonstrate that YAP/TAZ are critical mediators of the alternative Wnt pathway. We identify Wnt5a/b and Wnt3a as potent activators of YAP/TAZ, and further uncover a Wnt signaling pathway termed the 'alternative Wnt-YAP/TAZ signaling axis', which consists of Wnt-FZD/ROR-Gα12/13-Rho-Lats1/2-YAP/TAZ-TEAD. Wnt and FZD-induced YAP/TAZ activation was independent of LRP5/6 co-receptors and β-catenin. Moreover, we show that alternative Wnt ligands WNT5A/B and other secreted Wnt inhibitors, including DKK1, BMP4, and IGFBP4 are major YAP/TAZ-TEAD target genes. Finally, we demonstrate the role of alternative Wnt-YAP/TAZ signaling axis in gene expression, osteogenic differentiation, cell migration, and antagonism of canonical Wnt/β-catenin signaling. Together, our work reveals a critical role of YAP/TAZ in alternative Wnt signaling and its biological responses.
RESULTS
Wnt Ligands Activate YAP/TAZ via Alternative Wnt Pathway
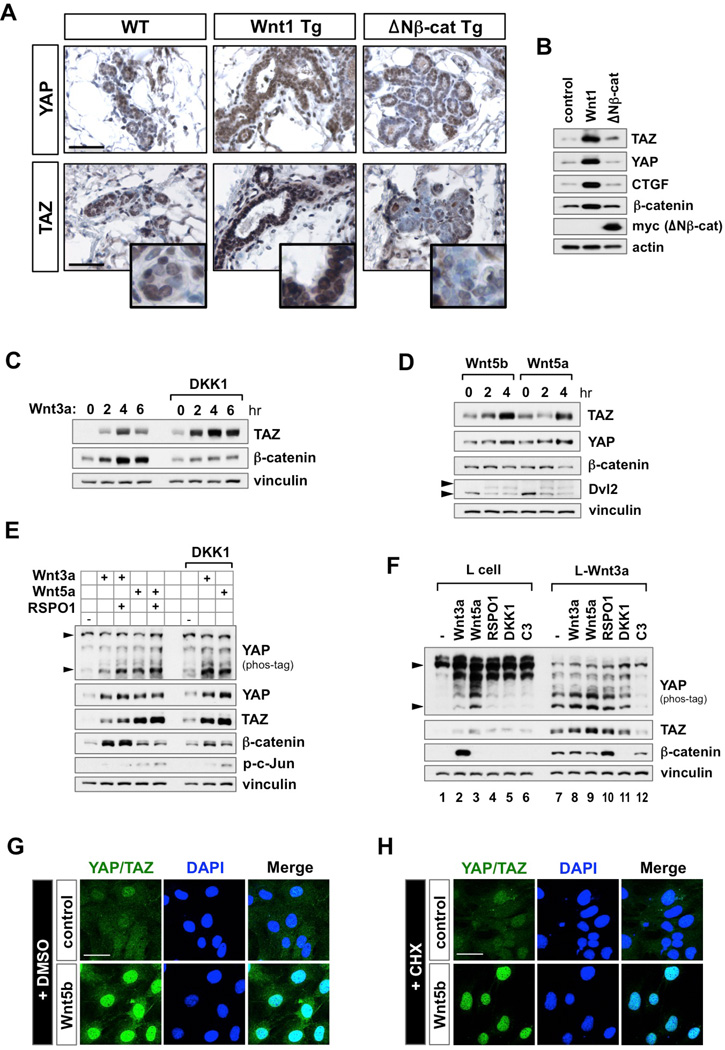
Despite several studies regarding the interaction between Hippo-YAP/TAZ and Wnt signaling, there is no report showing whether Wnt ligands can regulate YAP/TAZ activity in vivo. For this, we examined YAP/TAZ protein expression in mammary gland tissues from 3-month-old MMTV-Wnt1 and myc-tagged constitutively active-β-catenin (MMTV-ΔNβ-cat) transgenic mice (Kim et al., 2008). In the Wnt1-expressing mammary gland, we observed significant accumulation of YAP/TAZ and their target gene CTGF, whereas ΔNβ-catenin-expression had no effect on YAP/TAZ (Figures 1A and 1B).
Figure 1. Wnt Ligands Activate YAP/TAZ Through Alternative Wnt Signaling.
(A) Transgenic expression of Wnt1, but not ΔNβ-catenin, induce YAP/TAZ accumulation in mice. Representative images of YAP/TAZ expression level in Wnt1- and ΔNβ-catenin-expressing hyperplastic mammary glands. Mammary gland tissues from 3 month-old MMTV-Wnt1 and MMTV-ΔNβ-cat transgenic mice were stained with either YAP or TAZ antibodies. Scale bars, 200 µm.
(B) Western blot analysis of YAP/TAZ and CTGF in tissues isolated from Wnt1 and ΔNβ-catenin-expressing hyperplastic mammary glands.
(C) TAZ accumulation by Wnt3a is insensitive to DKK1. MCF10A cells were serum-starved for 16 hr, and then treated with Wnt3a (100 ng/ml) alone, or in the presence of DKK1 (200 ng/ml).
(D) Wnt5a and Wnt5b promote YAP/TAZ accumulation. MCF10A cells were serum-staved and stimulated with either Wnt5a (400 ng/ml) or Wnt5b (400 ng/ml). Upper arrowhead indicates phosphorylated band of Dvl2.
(E) YAP/TAZ activation by Wnt ligands is insensitive to RSPO1 and DKK1. ST2 cells were serum-starved and stimulated for 4 hr with Wnt3a or Wnt5a in the presence of RSPO1 (100 ng/ml) or DKK1 (200 ng/ml). Lower arrowhead corresponds to the dephosphorylated, active form of YAP on a phos-tag gel.
(F) Differential effects of Wnt ligands and inhibitors on YAP/TAZ and β-catenin. Control and Wnt3a stable L cells were serum-starved then stimulated for 4 hr with indicated recombinant proteins or C3 transferase (2 µg/ml), a RhoA inhibitor.
(G and H) Alternative Wnt signaling induces YAP/TAZ nuclear localization. MEFs were serum-starved then pretreated with either DMSO (G) or cycloheximide (CHX, 10 µg/ml) (H) for 1 hr. After Wnt5b stimulation (400 ng/ml) for 4 hr, cells were fixed for immunofluorescence with anti-YAP/TAZ antibody. Scale bar, 20 µm.
See also Figure S1.
Lats1/2 inhibits YAP/TAZ by phosphorylation, which can be readily detected with phospho-YAPS127 antibody or by mobility shift on a phos-tag gel. In addition, the Hippo pathway has notable effect on TAZ protein levels compared to YAP because TAZ has two phosphodegrons (Liu et al., 2010). For the above reasons, we use YAP phosphorylation status and TAZ protein levels as indicators for their regulation by the Hippo pathway. In MCF10A mammary epithelial cells, both TAZ and β-catenin protein levels were increased upon Wnt3a stimulation. Unexpectedly, pretreatment with DKK1, an antagonist of Wnt/β-catenin signaling, suppressed Wnt3a–induced β-catenin accumulation, but did not affect TAZ accumulation (Figure 1C), suggesting that Wnt3a activates YAP/TAZ via the alternative Wnt signaling pathway. To directly test this hypothesis, we utilized alternative Wnt ligands Wnt5a and Wnt5b. Treatment with Wnt5a or Wnt5b induced YAP/TAZ accumulation as well as target gene CTGF and CYR61 expression, while no increase in β-catenin was observed (Figures 1D and S1A). Of note, Wnt5a/b stimulation induced Dvl2 phosphorylation, a hallmark of alternative Wnt pathway activation (Gonzalez-Sancho et al., 2013; Ho et al., 2012). These results suggest that Wnt ligands activate YAP/TAZ via the alternative Wnt pathway.
Next, we investigated YAP/TAZ regulation by Wnt in different cell lines and conditions. In ST2 bone marrow stromal cells, both Wnt3a and Wnt5a induce YAP dephosphorylation, as indicated by the fast migrating bands on the phostag gel (Figure 1E). In parallel, protein levels of both YAP and TAZ were increased. Notably, these effects were not blocked by DKK1, whereas β-catenin accumulation was abolished (Figure 1E). Wnt3a and Wnt5a showed similar kinetics on YAP/TAZ activation (Figure S1B). We also tested control and Wnt3a–stably expressing L cells (L-Wnt3a), which are widely used in the field to generate Wnt3a–conditioned medium. In control L cells, both Wnt3a and Wnt5a induced YAP/TAZ activation, but only Wnt3a increased β-catenin (Figure 1F, lanes 1–3). Importantly, in L-Wnt3a cells, β-catenin and YAP/TAZ were highly activated compared to control cells (Figure 1F, compare lanes 1 and 7). Further treatment with R-spondin1 (RSPO1, an agonist of Wnt/β-catenin signaling) potentiated, whereas DKK1 inhibited β-catenin accumulation. However, neither treatment affected YAP/TAZ activation status, suggesting that Wnt3a–induced YAP/TAZ activation is exclusively through the alternative Wnt pathway (Figure 1F, compare lanes 7, 10, and 11). Consistently, YAP dephosphorylation in the absence of β-catenin accumulation was evident in L-Wnt5a stable cells (Figure S1C). Wnt ligand-induced YAP/TAZ activation was also observed in human stem cells such as HUES9 human embryonic stem cells and primary human bone-marrow stem cells (BMS) (Figures S1D and S1E). In these cells, Wnt3a induced stronger YAP/TAZ activation than β-catenin. Consistent with YAP/TAZ activation, Wnt5b increased nuclear YAP/TAZ (Figure 1G). Moreover, Wnt5b decreased cytoplasmic YAP/TAZ and concomitantly increased nuclear YAP/TAZ in the presence of protein synthesis inhibitor cycloheximide (CHX) (Figures 1H and S1F), indicating that Wnt5b stimulates YAP/TAZ to translocate from the cytoplasm into the nucleus. Together, our results establish a Wnt signaling pathway described as the 'alternative Wnt-YAP/TAZ signaling axis'.
Wnt Activates YAP/TAZ via FZD/ROR-Gα12/13-Rho-Lats1/2 Pathway
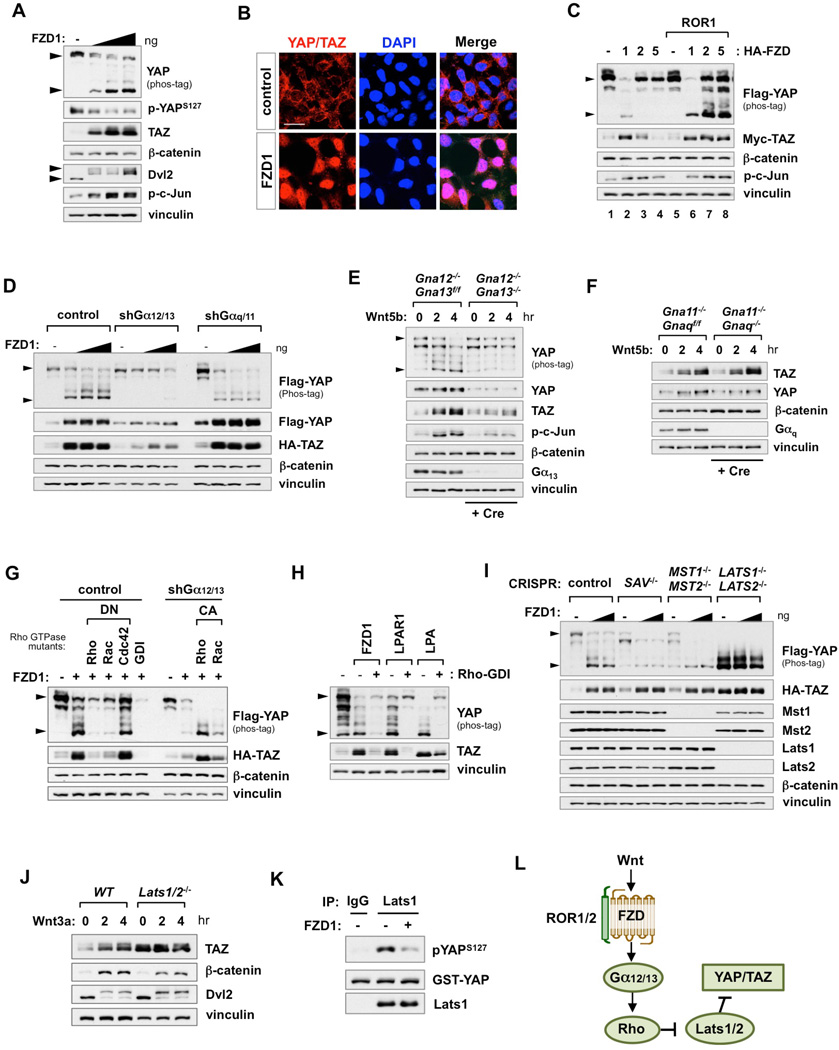
Wnt signals are transduced through FZD family receptors. The LRP5/6 co-receptors are required for activation of Wnt/β-catenin signaling, while FZD alone or together with ROR1/2 co-receptors can trigger alternative Wnt signaling pathways (Niehrs, 2012; Roman-Roman et al., 2004). To elucidate the mechanisms underlying Wnt-induced YAP/TAZ activation, we expressed FZD receptors in HEK293A cells. FZD1 triggered alternative Wnt signaling evident by the induction of Dvl2 and c-Jun phosphorylation, but had no effect on β-catenin accumulation (Figure 2A). Moreover, FZD1 dose-dependently induced endogenous YAP dephosphorylation and TAZ accumulation. Consequently, FZD1 promoted YAP/TAZ nuclear translocation and target gene expression (Figures 2B and S2A). These observations indicate that FZD1 initiates β-catenin-independent alternative Wnt signaling to activate YAP/TAZ. There are multiple members in the FZD family. Unlike FZD1, expression of FZD2 or FZD5 alone did not induce significant YAP/TAZ activation (Figure 2C). The receptor tyrosine kinase ROR1 functions as a co-receptor to potentiate FZD-induced alternative Wnt signaling. ROR1 alone had no effect on YAP/TAZ activation. However, co-expression of ROR1 with either FZD2 or FZD5 induced significant YAP dephosphorylation and TAZ accumulation (Figure 2C). Consistent with previous studies, the tyrosine kinase activity of ROR1 was not required to enhance FZD signaling (Figure S2B) (Grumolato et al., 2010). We also tested the adhesion receptor CELSR (Flamingo), which interacts with FZD to regulate PCP signaling (van Amerongen, 2012). Similar to ROR1, expression of CELSR2 enhanced YAP/TAZ activation by FZD (Figure S2C).
Figure 2. The Wnt-FZD/ROR-Gα12/13-Rho-Lats1/2 Pathway Activates YAP/TAZ.
(A) FZD1 expression activates endogenous YAP/TAZ. HEK293A cells were transfected with FZD1, then serum-starved for 16 hr before harvest. Lower arrowhead in YAP phos-tag blot represents dephosphorylated YAP. Upper arrowhead in Dvl2 blot represents phosphorylated Dvl2.
(B) FZD1 expression promotes YAP/TAZ nuclear localization. HEK293A cells were transfected with FZD1. After starvation for 16 hr, cells were fixed for immunofluorescence with anti-YAP/TAZ antibody. Scale bar, 20 µm.
(C) ROR1 potentiates FZD-induced YAP/TAZ activation.
(D) Knockdown of Gα12/13, but not Gαq/11, suppresses FZD1-induced YAP/TAZ activation.
(E and F) Knockout of Gα12/13, but not Gαq/11, blocks Wnt5b-induced YAP/TAZ activation. Experiments in DKO MEFs were performed within 5 days after Cre infection. DKO cells were serum-starved then stimulated with Wnt5b (400 ng/ml). Cre infection efficiency was confirmed by Gα13 and Gαq blots.
(G) Rho GTPases mediate FZD1-Gα12/13-induced YAP/TAZ activation. FZD1 was co-transfected with dominant negative (DN) or constitutively active (CA) Rho GTPase mutants in control or Gα12/13 depleted HEK293A cells, respectively.
(H) Expression of Rho-GDI inhibits FZD1, LPAR1, and LPA-induced YAP/TAZ activation.
(I) Lats1/2 are required for YAP/TAZ activation by FZD1. HEK293A cells with deletion of Sav1, Mst1/2, or Lats1/2 were transfected with FZD1 then serum-starved for 16 hr before harvest.
(J) Lats1/2 are required for Wnt3a–induced TAZ activation. WT and Lats1/2 DKO MEFs were serum-starved then stimulated with Wnt3a (100 ng/ml).
(K) Inhibition of Lats1 kinase activity by FZD1. In vitro kinase assay was performed with immunoprecipitated Lats1 and recombinant YAP protein.
(L) Components of the alternative Wnt-YAP/TAZ signaling axis.
See also Figure S2.
Recent studies have established GPCR signaling, particularly those coupled to Gα12/13 and Gαq/11, as major upstream activators of YAP/TAZ (Feng et al., 2014; Yu et al., 2014b; Yu et al., 2012). FZDs have the 7-transmembrane topology of a typical GPCR though whether FZDs function as conventional GPCRs has not been unequivocally demonstrated (van Amerongen, 2012). To this end, we examined YAP/TAZ activation by Wnt in Gα-protein knockdown HEK293A cells. Notably, knockdown of Ga12/13 was sufficient to block FZD1-induced YAP/TAZ activation (Figure 2D). However, neither knockdown of Gαq/11 nor inhibition of Gαi/o by pertussis toxin (PTX) affected YAP/TAZ levels (Figures 2D, S2D, and S2E). Consistently, inhibition of PLC or PKC, which are downstream effectors of Gαq/11, had no effect on FZD1-induced YAP activation (Figure S2E). Moreover, knockdown of Gα12/13 also impaired both Wnt3a and Wnt5b–induced YAP/TAZ activation (Figures S2F and S2G). Of note, Gα12/13 knockdown did not affect Wnt3a–induced β-catenin accumulation (Figure S2F), consistent with previous report (Regard et al., 2011).
To unambiguously establish Gα12/13 as critical mediators of the alternative Wnt-YAP/TAZ signaling axis, we generated Gα12/13 and Gαq/11 double knockout (DKO) mouse embryonic fibroblasts (MEFs) by introducing adenovirus expressing Cre recombinase into Gna12−/−Gna13f/f or Gna11−/− Gnaqf/f MEFs. Significantly, Wnt5b–induced YAP/TAZ activation was abolished in Gα12/13 DKO MEFs, but not in Gaq/11 DKO cells (Figures 2E, 2F, and S2H). Moreover, re-expression of Gα12 in the Gα12/13 DKO cells restored YAP/YAZ activation by Wnt5b (Figure S2I). These results provide compelling evidence that Gα12/13 are bone fide mediators of alternative Wnt signaling, leading to YAP/TAZ activation.
The Rho GTPase family is the major effector of Gα12/13 signaling, and also plays a critical role in both alternative Wnt signaling and Hippo-YAP/TAZ regulation (Schlessinger et al., 2009; Yu et al., 2012; Zhao et al., 2012). C3 transferase, a specific RhoA inhibitor, significantly blocked Wnt3a–induced YAP/TAZ activation in L-Wnt3a cells (Figure 1F). To further verify the involvement of Rho GTPases, the effect of Rho family members was tested. The dominant negative (DN) mutants of RhoA and Rac1, but not Cdc42, were able to suppress FZD1-induced YAP/TAZ activation. Moreover, in the Gα12/13-knockdown cells, the constitutively active (CA) forms of RhoA and Rac1 induced YAP/TAZ activation (Figure 2G). Finally, inhibition of Rho family GTPases by Rho-GDI also blocked FZD1-induced YAP/TAZ activation (Figure 2H). LPA and its cognate LPAR1 were included as positive controls (Yu et al., 2012).
Previously, RhoA has been shown to activate YAP/TAZ through inhibiting Lats1/2 activity (Yu et al., 2012). Therefore, we examined FZD1-induced YAP/TAZ activation in SAV1, MST1/2, or LATS1/2 KO HEK293A cells to identify the intersection between FZD1 and the Hippo pathway (Figure 2I). FZD1 expression further activated YAP/TAZ in either SAV1 KO or Mst1/2 DKO cells, but not in Lats1/2 DKO cells. To further examine the role of Lats1/2 in Wnt signaling, we tested Lats1/2 DKO MEFs. In wild type (WT) MEFs, Wnt3a increased TAZ and β-catenin protein levels. However, in Lats1/2 DKO cells, Wnt3a had no effect on TAZ, whereas β-catenin accumulation was retained (Figure 2J). In vitro kinase assay using purified YAP protein as a substrate showed that Lats1 immunoprecipitated from FZD1 expressing cells displayed a significantly lower kinase activity (Figure 2K). Thus, our data suggest that FZD1 activates YAP/TAZ by inhibiting Lats kinase activity via the alternative Wnt pathway. Collectively, we propose that the 'alternative Wnt-YAP/TAZ signaling axis' consists of the following components: Wnt-FZD/ROR-Gα12/13-Rho GTPases-Lats1/2-YAP/TAZ (Figure 2L).
The Alternative Wnt-YAP/TAZ Signaling Axis Is Independent of LRP5/6 and β-Catenin
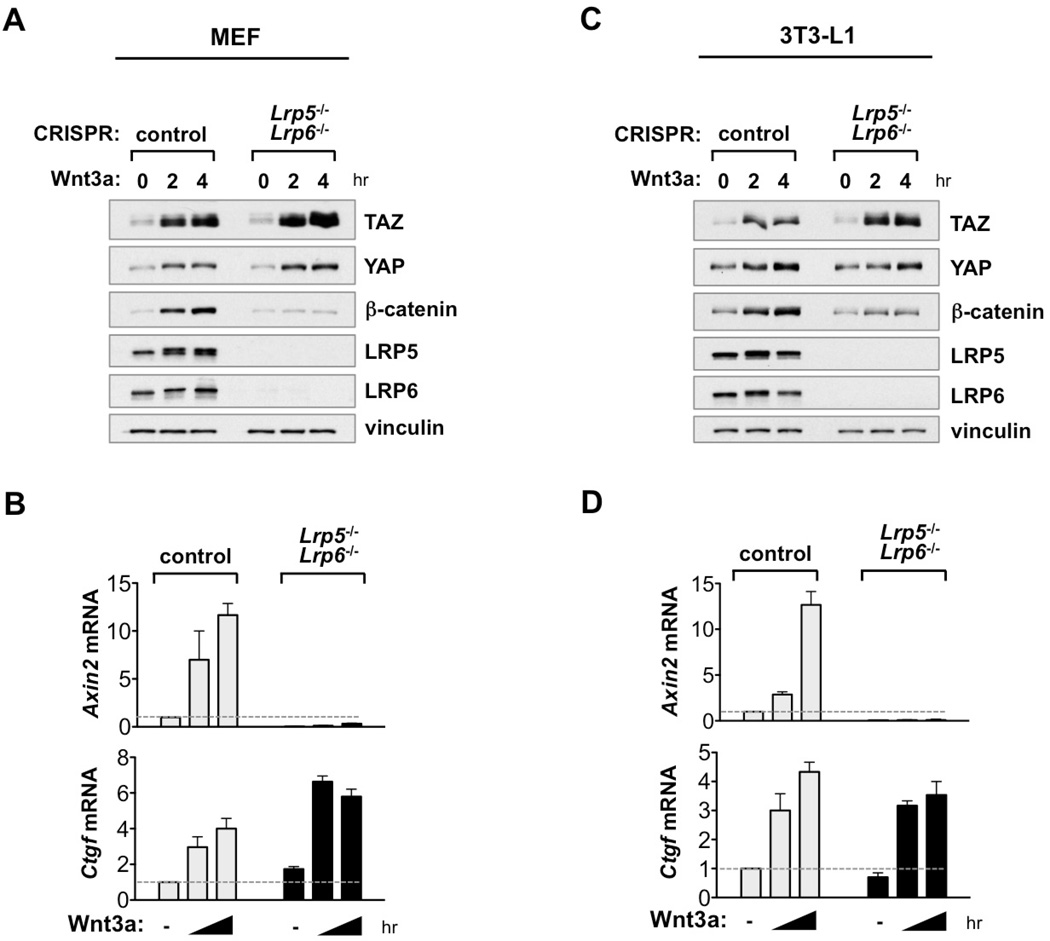
Unlike Wnt5a/b and FZD/ROR, Wnt3a stimulation activates both YAP/TAZ and β-catenin. Previous reports showed that the destruction complex regulates YAP/TAZ degradation via Wnt/β-catenin signaling (Azzolin et al., 2014; Azzolin et al., 2012). To further investigate whether Wnt/β-catenin signaling is involved in Wnt3a–induced YAP/TAZ activation, we generated knockout cell lines of LRP5/6, which are indispensible co-receptors for Wnt/β-catenin signaling. As expected, deletion of LRP5/6 completely abolished β-catenin activation and target gene induction (Axin2) by Wnt3a (Figures 3A-3D, S3A). Strikingly, LRP5/6 DKO did not affect Wnt3a–induced YAP/TAZ activation and target gene expression (Ctgf) (Figures 3A-3D). FZD1-induced YAP/TAZ activation was also retained in LRP5/6 DKO or β-catenin KO cells (Figure S3B). Moreover, in β-catenin KO cells, YAP/TAZ basal levels and regulation were intact (Figure S3C). These results unambiguously show that LRP5/6 and β-catenin, hence canonical Wnt/β-catenin signaling, are dispensable for the alternative Wnt-YAP/TAZ signaling axis.
Figure 3. Wnt3a Activates YAP/TAZ Independent of LRP5/6.
(A) Wnt3a activates YAP/TAZ in LRP5/6 DKO cells. LRP5/6 DKO MEFs were starved for 16 hr then stimulated with Wnt3a (100 ng/ml). LRP5/6 deletion was confirmed by western blot.
(B) LRP5/6 are required for Wnt3a–induced expression of β-catenin target gene Axin2, but not YAP/TAZ target gene Ctgf. Wnt3a–induced expression of Axin2 and Ctgf mRNA was measured by qRT-PCR in control and LRP5/6 DKO MEFs for 0, 2, 4 hr.
(C and D) Experiments similar to panels A and B were performed in 3T3-L1 cells. Data are presented as mean ± SEM.
See also Figure S3.
YAP/TAZ Mediate Alternative Wnt-Induced Biological Responses
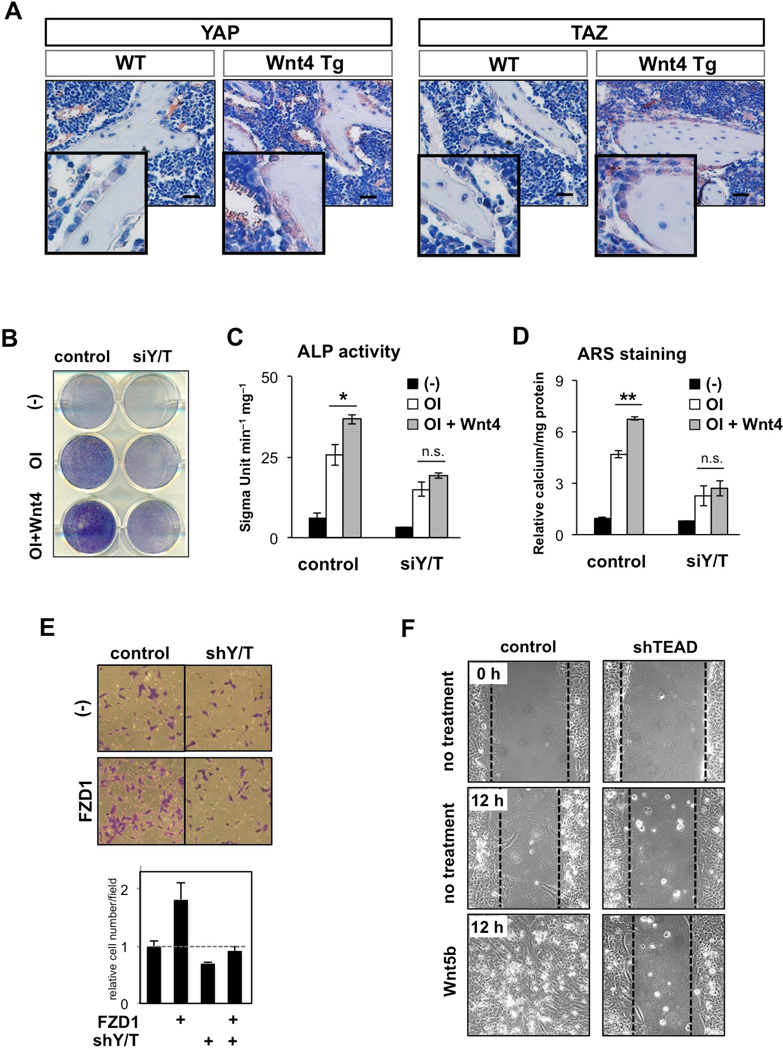
An important function of alternative Wnt signaling is to promote bone formation and cell migration (van Amerongen, 2012; Wang, 2009). The noncanonical Wnt4 ligand promotes bone formation in vitro and in vivo (Chang et al., 2007; Yu et al., 2014a). We found that Wnt4 induced accumulation of YAP/TAZ, but not β-catenin, in murine bone marrow-derived mesenchymal stem cells (MSCs) (Figures S4A and S4B). Furthermore, in osteoblast-specific Wnt4 transgenic mice (OB-Wnt4 Tg), YAP/TAZ were highly accumulated in osteoblasts surrounding the trabecular bones in the femurs, suggesting YAP/TAZ activation by Wnt4 in vivo (Figure 4A). Next, the role of YAP/TAZ in Wnt4-induced osteogenic differentiation was measured in MSCs. In control cells, Wnt4 enhanced osteogenic differentiation, as evidenced by elevated alkaline phosphatase (ALP) activity (Figures 4B and 4C) and alizarin-red staining (ARS) (Figure 4D). Importantly, YAP/TAZ depletion abolished Wnt4-induced enhancement of osteogenic differentiation. In addition, YAP/TAZ depletion significantly suppressed Wnt4-induced gene expression of osteogenic regulators, Runx2 and Sp7, as well as osteogenic markers, Col1a and Bglap (Figures S4C–S 4E).
Figure 4. YAP/TAZ Mediate Alternative Wnt Signaling in Osteogenesis and Cell Migration.
(A) Transgenic expression of Wnt4 induces YAP/TAZ activation. Representative images of YAP/TAZ expression levels in femur sections from 2-month-old WT and OB-Wnt4 mice. Scale bar, 50 µm.
(B and C) ALP staining (B) and activity (C) in MSC after treatment with normal culture media (control), osteoinduction media (OI), and Wnt4 (100ng/ml) for 3 days. Depletion of YAP/TAZ by siRNA impairs Wnt4-induced ALP activity.
(D) ARS staining in MSCs after treatment with OI and Wnt4 (100ng/ml) for 7 days. Depletion of YAP/TAZ by siRNA impairs Wnt4-induced calcium deposition in MSCs.
*P < 0.05, **P < 0.01 (Student’s t-test), n.s.: P > 0.05 by one-way ANOVA with Tukey’s post-hoc test.
(E) YAP/TAZ mediate FZD1-induced cell migration. Migration of HEK293A cells transfected with indicated constructs were assessed by transwell cell migration assays. Data are presented as mean ± SEM.
(F) Depletion of TEAD blocks Wnt5b-induced wound closure. A scratch was made across a confluent monolayer of stable TEAD knockdown MCF10A cells, and Wnt5b-induced wound closure (400 ng/ml) was monitored for 12 hr.
See also Figure S4.
Next, we examined FZD1-induced cell migration by transwell migration assay in control and YAP/TAZ-depleted cells. FZD1 expression enhanced HEK293A cell migration. Notably, YAP/TAZ depletion abolished FZD-induced cell migration, underscoring the importance of YAP/TAZ in this process (Figure 4E). We then tested the role of YAP/TAZ-TEAD in alternative Wnt ligand-induced cell migration. MCF10A cell migration and wound closure were significantly enhanced by Wnt5b stimulation (Figures 4F and S4F, left columns). However, depletion of TEAD transcription factors markedly diminished Wnt5b-induced cell migration (Figures 4F and S4F, right columns). Taken together, these results demonstrate the pivotal role of YAP/TAZ as mediators of the alternative Wnt-induced biological responses including osteogenic differentiation and cell migration.
YAP/TAZ-TEAD Induces Secreted Inhibitors of Wnt/β-Catenin Signaling
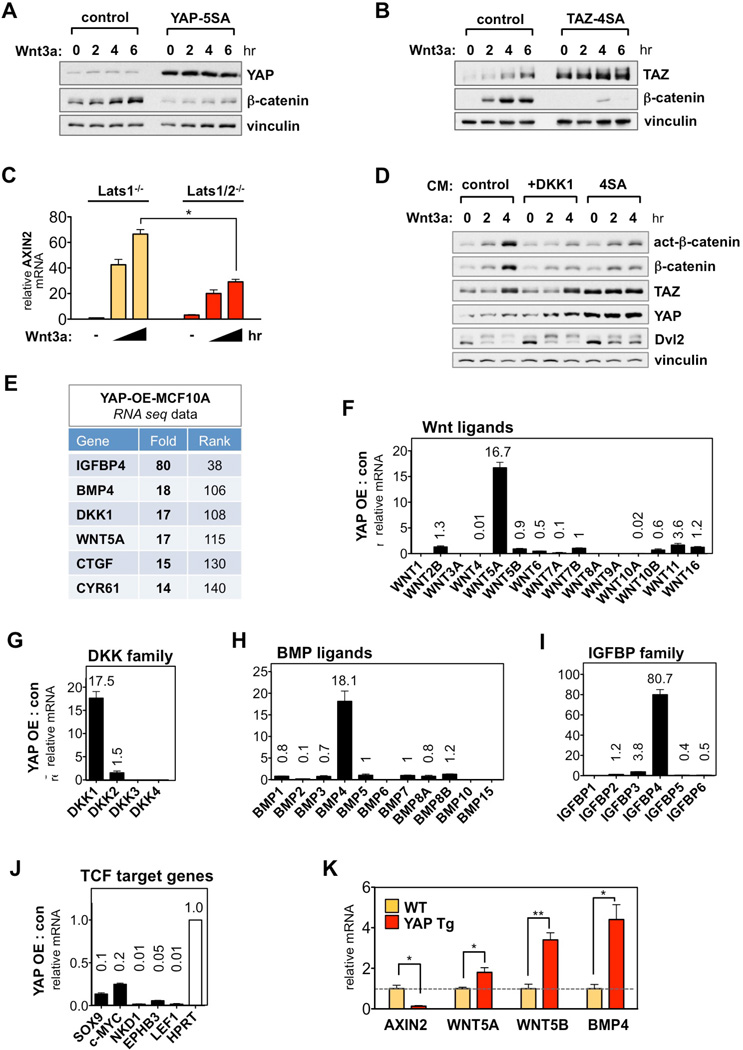
A key function of the alternative Wnt pathway is to antagonize Wnt/β-catenin signaling, which is evident in a broad range of cells during tissue regeneration and development (Angers and Moon, 2009; van Amerongen, 2012). However, the molecular basis for this cross talk is unclear. Therefore, we sought to explore how YAP/TAZ-TEAD regulates Wnt/β-catenin signaling. For this purpose, we utilized active forms of YAP (YAP-5SA) and TAZ (TAZ-4SA), which are constitutively active due to mutations in the inhibitory Lats phosphorylation sites (Zhao et al., 2011). In MCF10A cells stably expressing either YAP-5SA or TAZ-4SA, Wnt3a failed to accumulate β-catenin (Figures 5A and 5B). YAP/TAZ had no effect on β-catenin mRNA level (Figure S5A), suggesting that active YAP/TAZ block Wnt3a–induced β-catenin protein stabilization. We also tested Lats1/2 DKO MEFs, which have higher basal YAP/TAZ activity as indicated by enhanced nuclear localization (Figure S5B). Compared to Lats1 KO, the Lats1/2 DKO MEFs displayed a significantly weaker Wnt3a response, which was restored by YAP/TAZ depletion as indicated by Axin2 levels (Figures 5C and S5C). Together, these results indicate that activation of YAP/TAZ inhibit Wnt/β-catenin signaling.
Figure 5. YAP/TAZ Induce Secreted Inhibitors of Wnt/β-Catenin Signaling.
(A and B) Inhibition of Wnt/β-catenin signaling by nuclear YAP/TAZ. Stable MCF10A–YAP-5SA (A) and TAZ-4SA (B) cells were serum-starved, then stimulated with Wnt3a (100 ng/ml).
(C) Impaired Wnt/β-catenin signaling in Lats1/2 DKO cells. Axin2 mRNA level was measured in Wnt3a (100 ng/ml) stimulated Lats1 KO and Lats1/2 DKO MEFs. Data are presented as mean ± SEM. *P < 0.05 (Student’s t-test).
(D) Inhibition of Wnt/β-catenin signaling by TAZ-4SA conditioned media. HEK293A cells were pretreated for 2 hr with conditioned media (CM) from control and TAZ-4SA-expressing MCF10A cells then stimulated with Wnt3a (100 ng/ml). In lanes 4–6, DKK1 (200 ng/ml) was added in control-CM.
(E) RNA-seq data in YAP-overexpressing (YAP-OE) MCF10A cells. The fold-induction of each gene is ranked among 22,341 genes.
(F-I) Induction of YAP target genes among the Wnt ligands (F), DKK family (G), BMP ligands (H), and IGFBP family (I). Fold-change by YAP is indicated above each bar graph. Ligands without corresponding number were undetectable or below threshold in RNA-seq analysis.
(J) Inhibition of β-catenin/TCF target genes by YAP.
(K) Induction of secreted Wnt inhibitors in YAP transgenic mouse liver. qRT-PCR analysis in the liver of YAP transgenic (YAP Tg) and control (WT) mice fed with 0.2 mg/ml doxycycline for 2 weeks. n = 3 mice per group; Data are represented as mean ± SEM. *P < 0.05, **P < 0.01 (Student’s t-test).
See also Figure S5.
Next, we collected conditioned-media (CM) from active TAZ-4SA-expressing MCF10A cells (4SA-CM), and tested its effect on the Wnt3a response. Pre-treatment with 4SA-CM strongly blocked Wnt3a–induced β-catenin accumulation, comparable to that of purified DKK1 pretreatment (Figure 5D). These data indicate that YAP/TAZ-induced secreted factors are responsible for antagonizing Wnt/β-catenin signaling. Next, we sought to identify YAP/TAZ target genes involved in this process. RNA-seq analysis in YAP overexpressing MCF10A cells revealed secreted inhibitors of Wnt/β-catenin signaling as prominent YAP inducible genes. These include WNT5A, DKK1, BMP4, and IGFBP4 (Figure 5E) (Cruciat and Niehrs, 2013; Haramis et al., 2004; He et al., 2004; Kikuchi et al., 2012; van Amerongen, 2012; Zhu et al., 2008). Moreover, it is worth noting that the classic YAP/TAZ-TEAD target genes CTGF and CYR61 have also been implicated in antagonizing Wnt/β-catenin signaling (Latinkic et al., 2003; Mercurio et al., 2004). On the other hand, there was no considerable mRNA induction among the intracellular Wnt destruction complex such as AXIN, APC, GSK3β, or CK1 (data not shown). These data are consistent with the observation that conditioned medium from TAZ-4SA-expressing cells was sufficient to inhibit Wnt/β-catenin signaling (Figure 5D).
Among the Wnt family, YAP exclusively induced the mRNA level of alternative Wnt ligand WNT5A, and to a lesser extent WNT11, while the other alternative Wnt ligands (WNT4, WNT6, and WNT16), and canonical Wnt ligands (WNT7A, WNT10A, and WNT10B) were either unchanged or suppressed (Figure 5F). WNT3A was not detected in the RNA-seq data, and qRT-PCR experiments showed negligible effect of YAP/TAZ on WNT3A expression (Figures S5D and S5E). YAP also specifically induced DKK1, BMP4, and IGFBP4 within each family (Figures 5G-5I). TAZ also induced the secreted Wnt inhibitors in MCF10A cells. Notably, the TEAD-binding deficient mutants YAP-5SA/S94A and TAZ-4SA/S51A failed to induce the expression of WNT5A, DKK1, BMP4, and IGFBP4 indicating that they are YAP/TAZ-TEAD target genes (Figures S5D and S5E), and consistent with previous reports (Lai and Yang, 2013; Seo et al., 2013). Interestingly, the expression of sFRP1, a secreted Wnt inhibitor that suppresses both Wnt/β-catenin and alternative Wnt signaling (Cruciat and Niehrs, 2013), was significantly abolished (Figure S5F). Consistent with the induction of secreted inhibitors specific to Wnt/β-catenin signaling, YAP significantly decreased a panel of major β-catenin/TCF target genes, including SOX9, MYC, EPHB3, NKD1, and LEF1 (Figure 5J). To extend this observation in vivo, we analyzed gene expression from transgenic mouse livers with a doxycycline (Dox)-inducible YAP. After 2-weeks of Dox treatment, we found significant induction of YAP/TAZ target genes, Wnt5a, Wnt5b, and Bmp4, as well as a marked decrease of β-catenin target genes, Axin2 and COUP-TFII (Figures 5K and S5G). These data indicate that YAP/TAZ induce the expression of secreted Wnt inhibitors and thereby suppress Wnt/β-catenin signaling.
WNT5A/B Are Wnt Target Genes Mediated by YAP/TAZ-TEAD
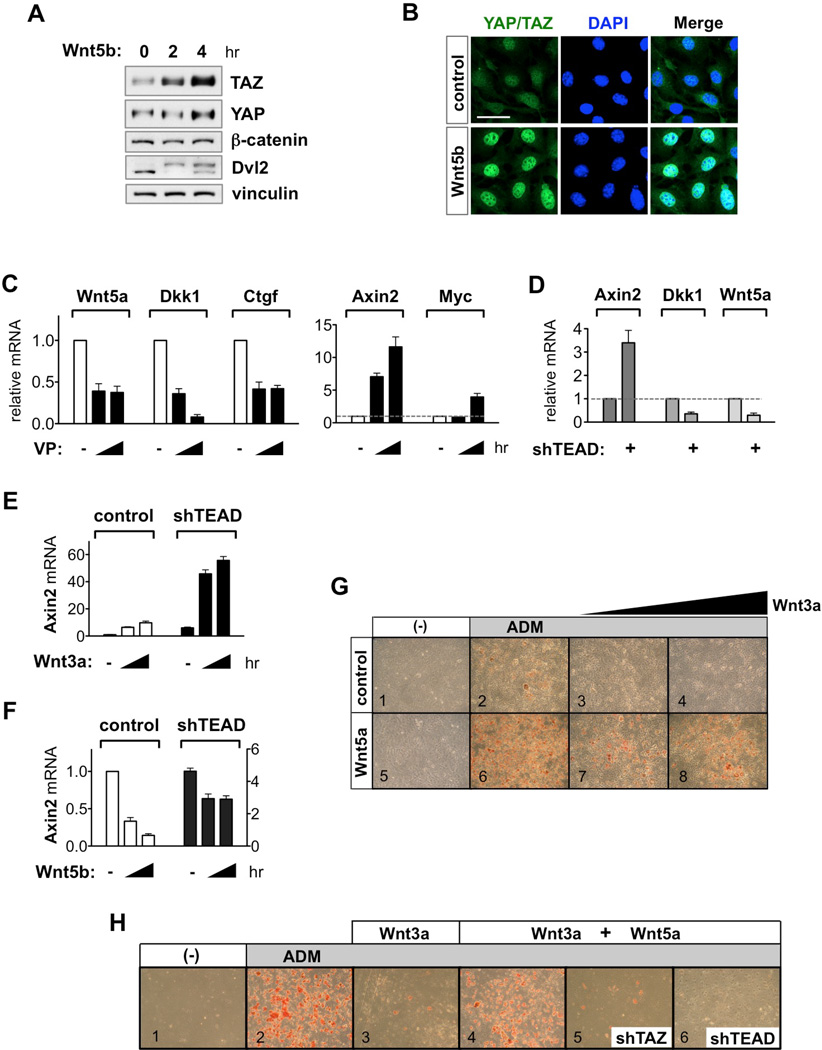
We further investigated WNT5A/B as YAP/TAZ-TEAD target genes because they are prototypes of alternative Wnt ligands and also antagonize Wnt/β-catenin signaling. Wnt5a/b protein levels were dramatically increased in TAZ-4SA expressing cells compared to control MCF10A cells (Figure 6A). In this context, basal levels of Dvl2 phosphorylation, an indicator of alternative Wnt signaling, were evident, whereas β-catenin level was repressed and could not be induced by Wnt3a. Notably, the TEAD-binding deficient TAZ mutant (4SA/S51A) reversed these effects (Figures 6A and S6A). These data suggest that YAP/TAZ induce Wnt5a/b expression and simultaneously inhibit Wnt/β-catenin signaling through TEAD-dependent transcription. We then investigated whether WNT5A is a direct transcriptional target of TEAD. Two TEAD binding sequences (TBS; GGAATG) were identified in the WNT5A promoter region (Figure 6B). Chromatin immunoprecipitation (ChIP) showed that endogenous TEAD1 binds to the TBS of WNT5A (Figure 6C). CTGF and GAPDH promoters were positive and negative controls, respectively. These results indicate that WNT5A is a direct target gene of YAP/TAZ-TEAD.
Figure 6. WNT5A/B Are YAP/TAZ-TEAD-Mediated Wnt Target Genes.
(A) TEAD induce Wnt5a/b and inhibit Wnt/β-catenin signaling. Stable MCF10A-4SA and 4SA/S51A cells were serum-starved for 16 hr then stimulated with Wnt3a (100 ng/ml).
(B) WNT5A promoter region contains two TEAD binding sequences (TBS; GGAATG). Arrows underline primers used to amplify the region with each TBS.
(C) TEAD1 binds WNT5A promoter. ChIP experiment using anti-TEAD1 antibody was performed in TAZ-4SA-MCF10A cells. CTGF, positive control; GAPDH and WNT5A–CR (coding region), negative controls. Data are presented as mean ± SEM. *IgG versus anti-TEAD1 antibody, P < 0.05 (Student’s t-test).
(D) Activation of YAP/TAZ-Wnt5a/b axis by FZD1/ROR1. Stable MCF10A–FZD1/ROR1 cells were stimulated with Wnt3a (100 ng/ml). Note that Wnt3a induces Wnt5a/b accumulation in control cells (lanes 1–3).
(E) VP suppresses FZD1/ROR1-induced WNT5A gene expression. TEAD target genes, WNT5A and CTGF, and TCF target gene MYC mRNA were measured in MCF10A–FZD1/ROR1 (F+R) cells after VP (7 mM) treatment for 0, 2, 4 hr. Data are presented as mean ± SEM. *Compared to no VP treatment, P < 0.05 (Student’s t-test).
(F and G) TEAD mediates Wnt3a–induced WNT5A and WNT5B gene expression. Wnt3a (100 ng/ml) treatment for 0, 2, 4 hr induced mRNA expression of WNT5A (F) and WNT5B (G) in MCF10A and 3T3-L1 cells, respectively. In TEAD knockdown cells, Wnt3a–induced WNT5A/B expression was impaired. Data are presented as mean ± SEM. *Control versus shTEAD at 4 hr, P < 0.05 (Student’s t-test).
(H) Effect of oncogenic PIK3CA mutations on YAP/TAZ activation. PIK3CA-E545K and H1047R knockin MCF10A were starved for 16 hr then harvested.
(I) VP (7 µM) suppresses PIK3CA-H1047R–induced WNT5A and CTGF gene expression. Data are presented as mean ± SEM. *Compared to no VP treatment, P < 0.05 (Student’s t-test).
(J) VP (7 µM) induces β-catenin/TCF target genes in PIK3CA-H1047R knockin cells.
See also Figure S6.
The FZD/ROR receptors are transducers of the alternative Wnt-YAP/TAZ signaling axis (Figure 2). In MCF10A cells, FZD1/ROR1 stable expression induced YAP/TAZ activation, as well as robust induction of Wnt5a/b (Figure 6D). In parallel with the activation of alternative Wnt signaling, FZD1/ROR1 suppressed Wnt3a–induced β-catenin accumulation. To verify the role of the YAP/TAZ-TEAD complex in FZD/ROR-mediated transcriptional regulation, we utilized verteporphin (VP), a drug that disrupts YAP/TAZ-TEAD interaction (Liu-Chittenden et al., 2012). FZD1/ROR1 significantly induced WNT5A and CTGF mRNA levels (Figure 6E). Treatment with VP inhibited expression of both WNT5A and CTGF whereas it potentiated β-catenin/TCF target gene c-MYC expression. We next sought to test the effect of Wnt ligand on WNT5A/B gene induction. Wnt3a treatment induced WNT5A and WNT5B mRNA in MCF10A and 3T3-L1 cells, respectively, which was suppressed by TEAD depletion (Figures 6F, 6G, and S6B). Moreover, the induction of WNT5A/B by Wnt3a stimulation was confirmed at the protein level (Figures 6A and 6D, lanes 1–3). These data establish WNT5A/B as bona fide YAP/TAZ-TEAD-mediated Wnt target genes.
Recent gene expression profiling revealed WNT5A as one of the most significantly upregulated genes in PIK3CA-mutated breast tumors (Cizkova et al., 2010). We sought to determine whether YAP/TAZ-TEAD is required for PIK3CA mutation-induced WNT5A gene expression. We utilized mutant PIK3CA-knockin (KI) MCF10A cells, which express the oncogenic E545K or H1047R mutant PI3K at endogenous genomic locus (Gustin et al., 2009). In both KI cells, we observed accumulation of YAP/TAZ, but not β-catenin (Figure 6H). Importantly, in PIK3CA-H1047R KI cells, WNT5A and CTGF mRNA levels were significantly elevated (Figure 6I). Disruption of YAP/TAZ-TEAD interaction by VP completely diminished the induction of WNT5A and CTGF, whereas VP concomitantly increased β-catenin/TCF target genes, MYC and SOX9 (Figures 6I and 6J).
By analyzing gene expression from a panel of 967 cancer cell lines obtained from Cancer Cell Line Encyclopedia (CCLE) (Barretina et al., 2012), we observed a positive correlation between the expression level of YAP/TAZ and the secreted Wnt inhibitors WNT5A, WNT5B, IGFBP4, BMP4, and DKK1 (Figure S6C). In contrast, there was either minor or inverse correlation between YAP/TAZ and β-catenin/TCF target genes, AXIN2, MYC, NKD1, and LEF1. These observations are consistent with a role of YAP/TAZ-TEAD as inhibitors of Wnt/β-catenin signaling.
The Alternative Wnt-YAP/TAZ Axis Antagonizes Wnt/β-Catenin Signaling in Adipocyte Differentiation
Adipocyte differentiation is a biological response in which Wnt/β-catenin and alternative Wnt signaling exert opposite effects (Ross et al., 2000; van Tienen et al., 2009). Wnt/β-catenin signaling by Wnt3a inhibits adipogenesis in 3T3-L1 preadipocytes, whereas alternative Wnt signaling by Wnt5a/b promote this process (Figure S7A). Here, we sought to investigate whether YAP/TAZ mediate the antagonism of Wnt5a/b on Wnt3a in the context of adipogenesis. In 3T3-L1 cells, we confirmed that alternative Wnt signaling by Wnt5a/b promotes YAP/TAZ accumulation and nuclear translocation (Figures 7A, 7B, and S7B). Disruption of YAP/TAZ and TEAD interaction by VP treatment significantly reduced Wnt5a, Dkk1, and Ctgf mRNA. Simultaneously, VP treatment increased β-catenin/TCF target genes Axin2 and Myc expression (Figure 7C). The inhibitory effect of YAP/TAZ-TEAD on Wnt/β-catenin signaling was further confirmed by TEAD depletion. Knockdown of TEAD increased Axin2 levels, and reduced Wnt5a and Dkk1 levels (Figure 7D). These observations indicate that alternative Wnt-YAP/TAZ-TEAD pathway functions to restrict Wnt/β-catenin signaling in preadipocytes. Importantly, depletion of TEAD significantly potentiated Wnt3a–induced Wnt/β-catenin signaling, as indicated by enhanced Axin2 induction (Figure 7E). Furthermore, TEAD depletion attenuated the activity of Wnt5b to inhibit Axin2 induction, demonstrating that TEAD is required for Wnt5b to antagonize Wnt/β-catenin signaling (Figure 7F).
Figure 7. Alternative Wnt-YAP/TAZ-TEAD Axis Antagonizes Wnt/β-Catenin Signaling in Adipogenesis.
(A and B) Wnt5b promotes YAP/TAZ accumulation (A), and nuclear translocation (B) in 3T3-L1 preadipocytes. Scale bar, 20 µm (B).
(C and D) Differential regulation of YAP/TAZ-TEAD and β-catenin/TCF target genes by VP (7 µM) (C) and TEAD depletion (D).
(E) TEAD knockdown potentiates Wnt3a–induced Wnt/β-catenin signaling. Axin2 mRNA was measured after Wnt3a (100 ng/ml) stimulation for 0, 2, 4 hr in serum-starved control and shTEAD 3T3-L1 cells. Data are presented as mean ± SEM.
(F) TEAD knockdown impairs Wnt5b-induced inhibition of Wnt/β-catenin signaling. Note that left y-axis is for control, and right y-axis is for shTEAD 3T3-L1 cells. Data are presented as mean ± SEM.
(G) Wnt5a antagonizes Wnt/β-catenin signaling in adipogenesis. Control and stable 3T3-L1-Wnt5a cells were treated with Wnt3a (0, 50, 100 ng/ml) after induction of adipogenesis. Experiment was terminated within 6 days after ADM treatment to compare panels 2 and 6. Stable expression of Wnt5a derepressed Wnt3a–induced adipogenesis inhibition.
(H) TAZ and TEAD mediate the antagonistic effect of Wnt5a on Wnt3a in adipogenesis. In control cells, pretreatment of Wnt5a (400 ng/ml) impaired the effect of Wnt3a (100 ng/ml) in adipogenesis. In contrast, Wnt5a did not antagonize the effect of Wnt3a in TAZ or TEAD-knockdown 3T3-L1 cells.
See also Figure S7.
Based on the above observations, we hypothesized that YAP/TAZ-TEAD may mediate the inhibitory effect of Wnt5a on Wnt/β-catenin signaling during adipogenesis. In 3T3-L1 cells, Wnt3a treatment blocked adipogenesis differentiation medium (ADM)-induced adipogenesis in a dose-dependent manner (Figure 7G, panels 1–4). Stable expression of Wnt5a alone did not induce adipogenesis but enhanced the effect of ADM treatment. Importantly, Wnt3a failed to inhibit adipogenesis in Wnt5a expressing cells (Figure 7G, panels 5–8). These data support the notion that Wnt5a antagonizes the effect of Wnt3a on adipogenesis. To test if the YAP/TAZ-TEAD complex is important in this process, we depleted these components, then added ADM and Wnt3a on Wnt5a–pretreated cells. Similar with the results observed from Wnt5a–stable cells, addition of purified Wnt5a suppressed the inhibitory effect of Wnt3a on adipogenesis (Figure 7H, compare panels 1–4). However, depletion of YAP/TAZ or TEAD significantly abolished the antagonistic effect of Wnt5a on Wnt3a (Figure 7H, compare panels 4–6, S7C and S7D). Of note, we have previously shown that YAP suppresses the adipogenic activity of cAMP (Yu et al., 2013). While these experiments were performed in the presence of cAMP elevating drugs forskolin or IBMX, the current study utilized Wnt ligands and was designed to test the crosstalk between Wnt5a and Wnt3a. Thus, effects of YAP/TAZ on adipogenesis, though not directly involved, may be context-dependent. Together, these results support our notion that the alternative Wnt-YAP/TAZ-TEAD signaling axis is required for Wnt5a to antagonize the function of Wnt/β-catenin signaling.
DISCUSSION
Differential Regulation of YAP/TAZ and β-Catenin by Wnt
Our study reveals a mechanism of YAP/TAZ regulation via alternative Wnt signaling and the functional significance of YAP/TAZ as downstream effectors. Wnt3a simultaneously activates both YAP/TAZ and β-catenin, although distinct signaling mechanisms are utilized. Wnt3a–induced YAP/TAZ activation was insensitive to DKK1 inhibition and LRP5/6 knockout, whereas β-catenin activation by Wnt3a was sensitive to both. FZD1-induced YAP/TAZ activation was also intact in LRP5/6 DKO and β-catenin KO cells. Moreover, deletion of β-catenin had no effect on either YAP/TAZ protein levels or their degradation. Furthermore, Wnt5a/b activates YAP/TAZ, but not β-catenin. Interestingly, Wnt5a/b ligands are not only upstream activators, but also downstream target genes of YAP/TAZ-TEAD, indicating a potential positive feedback loop.
These results are dissimilar with previous reports that show Wnt3a–induced YAP/TAZ and β-catenin activation share a common mechanism and function via interaction with the destruction complex, which was independent of the Hippo pathway (Azzolin et al., 2014; Azzolin et al., 2012). Notably, recent unbiased proteomic studies have not identified the destruction complex as YAP/TAZ interacting proteins while these studies have validated many known YAP/TAZ binding partners (Couzens et al., 2013; Kohli et al., 2014; Kwon et al., 2013; Wang et al., 2014). Consistent with our results, Byun et al. also showed that Wnt3a activates TAZ by dephosphorylating Lats1/2 target sites, demonstrating the link between Wnt3a and Hippo pathway (Byun et al., 2014).
YAP/TAZ-TEAD Target Genes Inhibits Wnt/β-Catenin Signaling
The depth of research underscores the complex interplay between Hippo-YAP/TAZ and Wnt/β-catenin signaling. However, the field lacks a unified model regarding the inhibitory mechanism of YAP/TAZ in Wnt/β-catenin signaling. Consistent with our observations, other reports show that YAP/TAZ inhibit Wnt/β-catenin signaling in vivo and in vitro (Barry et al., 2013; Imajo et al., 2012; Varelas et al., 2010). Several studies show that this inhibition is achieved by cytosolic YAP/TAZ via binding and suppressing Dvl2 or β-catenin.
Here, we show that nuclear and transcriptionally competent YAP/TAZ exert an inhibitory effect on Wnt/β-catenin signaling based on the following evidence. YAP/TAZ induce the expression of secreted Wnt inhibitors, and conditioned medium from active TAZ expressing cells blocks Wnt3a–induced β-catenin accumulation. The transcriptionally defective YAP/TAZ mutants fail to suppress Wnt/β-catenin signaling. Inhibition of YAP/TAZ-TEAD function, either by pharmacological inhibitor verteporfin or TEAD knockdown, abolishes the antagonistic effect of Wnt5a/b on β-catenin-induced biological responses. Finally, Lats1/2 DKO cells show reduced β-catenin activity due to hyperactive YAP/TAZ. Consistent with this notion, Seo et al. reported that SOX2-induced YAP-TEAD activation inhibits Wnt/β-catenin signaling by inducing DKK1 (Seo et al., 2013). LPA, which is a potent activator of YAP/TAZ, antagonizes Wnt/β-catenin signaling and blocks Wnt3a–mediated differentiation of hESC (Blauwkamp et al., 2012). In addition, Gα12/13 and RhoA, which activate YAP/TAZ, were shown to inhibit Wnt/β-catenin signaling (Regard et al., 2011; Rodrigues et al., 2014). Together, these results support the model that YAP/TAZ antagonize Wnt/β-catenin signaling via TEAD-mediated gene transcription.
Gα12/13 and YAP/TAZ: Implications in Alternative Wnt Signaling
Although the heterotrimeric Gproteins, such as GαO or Gαq, have been implicated in both Wnt/β-catenin and alternative Wnt signaling, the requirement of Gα proteins and the GPCR nature of FZD remain controversial in the field (Dijksterhuis et al., 2014; van Amerongen, 2012). Here, we provide the first genetic and biochemical evidence supporting the involvement of Gα12/13, and its role in alternative Wnt-YAP/TAZ signaling. Both genetic deletion and shRNA-mediated knockdown of Gα12/13 abolish YAP/TAZ activation in response to Wnt3a or Wnt5a/b treatment and FZD1 expression. However, Gα12/13 is not required for Wnt3a–induced β-catenin accumulation. Consistent with our model, Regard et al. showed that Gα12/13 antagonize Wnt/β-catenin signaling (Regard et al., 2011). In addition, we identify Rho GTPases, RhoA and Rac1, as downstream mediators of Gα12/13 in the alternative Wnt-YAP/TAZ axis. Together, we propose FZD as a bona fide Gα 12/13-coupled GPCR, linking alternative Wnt signaling to YAP/TAZ activation.
During development, alternative Wnt ligands, FZD, Gα12/13, RhoA/Rac1, and Yki/YAP share similar functions in PCP (Habas et al., 2001; Lin et al., 2005; van Amerongen, 2012; Zecca and Struhl, 2010), however functional engagement between these components has been enigmatic. Here we demonstrate the link between PCP components and YAP/TAZ, and further show that CELSR2 can also augment FZD-induced YAP/TAZ activation. These data suggest the involvement of alternative Wnt-YAP/TAZ signaling axis in PCP. In addition, Wnt5a and ROR1/2 are potent inducers of tumor metastasis and their expression correlates with decreased patient survival in various human malignancies (Anastas and Moon, 2013). Here, we identified YAP/TAZ as potent effectors of Wnt5a and ROR1, and WNT5A as a direct target gene of YAP/TAZ. We further show positive correlations between YAP/TAZ and WNT5A expression in various cancer cells as well as in PIK3CA mutations. Therefore, elucidation of the alternative Wnt-YAP/TAZ signaling axis will not only expand mechanistic insights, but also provide therapeutic targets in the alternative Wnt pathway.
EXPERIMENTAL PROCEDURES
RNA Extraction, cDNA Synthesis, and Quantitative Real-Time PCR (qRT-PCR) Analysis
Cells or tissues were harvested for RNA extraction using RNeasy Plus mini kit (Qiagen). RNA samples were reverse-transcribed to complementary DNA (cDNA) using iScript reverse transcriptase (Bio-Rad). qRT-PCR was performed using KAPA SYBR FAST qPCR kit (Kapa Biosystems) and the 7300 real-time PCR system (Applied Biosystems). Expression levels are normalized to HPRT. Detailed list of primer sequence information are provided in the Extended Experimental Procedures.
Generation of Knockout Cells Using CRISPR/Cas9 Genome Editing
pSpCas9(BB)-2A–Puro (PX459; Addgene plasmid #48139) and lentiCRISPRv2 (Addgene plasmid #52961) were gifts from Dr. Feng Zhang (Ran et al., 2013; Sanjana et al., 2014). The 20 nucleotide guide sequences were designed using the CRISPR design tool at http://www.genome-engineering.org/crispr or were taken from archived guide sequences from the Genome-scale CRISPR knock-out (GeCKO2) library (Sanjana et al., 2014). Single guide RNAs (sgRNAs) were cloned into PX459 expression vector containing the human codon-optimized Cas9. HEK293A cells were transfected using PolyJet DNA in vitro Transfection Reagent according to the manufacturer’s instructions. In addition, sgRNAs were cloned into the lentiviral vector lentiCRISPRv2. Lentivirus was prepared in 293T cells, and the viral supernatant was collected, and added to MEFs or 3T3-L1 cells with polybrene. 16 hr after infection, the media was changed. 24 hr post transfection, cells were selected with puromycin for 2–3 days. Following removal of puromycin, cells were allowed to recover in regular growth media for 24 hr before being single-cell sorted by FACs (UCSD; Human Embryonic Stem Cell Core, BDInflux) into 96-well plate format. Single clones were expanded and screened by protein immunoblotting, genomic sequencing, and functional assays. Detailed list of guide sequence information are provided in the Extended Experimental Procedures.
Supplementary Material
HIGHLIGHTS.
Wnt ligands promote YAP/TAZ activation via the alternative Wnt signaling pathway.
Alternative Wnt-YAP/TAZ signaling consists of Wnt-FZD/ROR-Ga12/13-Rho-Lats1/2-YAP/TAZ.
YAP/TAZ mediate alternative Wnt signaling-induced osteogenesis and cell migration.
YAP/TAZ antagonize Wnt/β-catenin signaling by inducing secreted Wnt inhibitors.
ACKNOWLEDGMENTS
We thank the following colleagues for generously sharing the following materials: Dr. Duojia Pan for YAP transgenic mice, Dr. Ben Ho Park for PIK3CA mutation knockin MCF10A cells, Dr. Yingzi Yang for Gna11−/− Gnaqf/f and Gna12−/− Gna13f/f MEFs, Dr. Dae-Sik Lim for Lats1−/−Lats2f/f MEFs, Dr. Xi He for FZD1, FZD2, and FZD5 plasmids, and Dr. Liguang Chen for ROR1 plasmids. We also thank Dr. Karl Willert for the helpful discussion. This work was supported by grants from the NIH (EY022611, CA132809, and DE015964) to K.-L.G. and UCSD Cellular and Molecular Pharmacology training grant (T32 GM007752) to S.W.P.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer. 2013;13:11–26. doi: 10.1038/nrc3419. [DOI] [PubMed] [Google Scholar]
- Angers S, Moon RT. Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol. 2009;10:468–477. doi: 10.1038/nrm2717. [DOI] [PubMed] [Google Scholar]
- Azzolin L, Panciera T, Soligo S, Enzo E, Bicciato S, Dupont S, Bresolin S, Frasson C, Basso G, Guzzardo V, et al. YAP/TAZ Incorporation in the beta-Catenin Destruction Complex Orchestrates the Wnt Response. Cell. 2014;158:157–170. doi: 10.1016/j.cell.2014.06.013. [DOI] [PubMed] [Google Scholar]
- Azzolin L, Zanconato F, Bresolin S, Forcato M, Basso G, Bicciato S, Cordenonsi M, Piccolo S. Role of TAZ as mediator of Wnt signaling. Cell. 2012;151:1443–1456. doi: 10.1016/j.cell.2012.11.027. [DOI] [PubMed] [Google Scholar]
- Barretina J, Caponigro G, Stransky N, Venkatesan K, Margolin AA, Kim S, Wilson CJ, Lehar J, Kryukov GV, Sonkin D, et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483:603–607. doi: 10.1038/nature11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry ER, Morikawa T, Butler BL, Shrestha K, de la Rosa R, Yan KS, Fuchs CS, Magness ST, Smits R, Ogino S, et al. Restriction of intestinal stem cell expansion and the regenerative response by YAP. Nature. 2013;493:106–110. doi: 10.1038/nature11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blauwkamp TA, Nigam S, Ardehali R, Weissman IL, Nusse R. Endogenous Wnt signalling in human embryonic stem cells generates an equilibrium of distinct lineage-specified progenitors. Nat Commun. 2012;3:1070. doi: 10.1038/ncomms2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byun MR, Hwang JH, Kim AR, Kim KM, Hwang ES, Yaffe MB, Hong JH. Canonical Wnt signalling activates TAZ through PP1A during osteogenic differentiation. Cell Death Differ. 2014;21:854–863. doi: 10.1038/cdd.2014.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J, Sonoyama W, Wang Z, Jin Q, Zhang C, Krebsbach PH, Giannobile W, Shi S, Wang CY. Noncanonical Wnt-4 signaling enhances bone regeneration of mesenchymal stem cells in craniofacial defects through activation of p38 MAPK. J Biol Chem. 2007;282:30938–30948. doi: 10.1074/jbc.M702391200. [DOI] [PubMed] [Google Scholar]
- Cizkova M, Cizeron-Clairac G, Vacher S, Susini A, Andrieu C, Lidereau R, Bieche I. Gene expression profiling reveals new aspects of PIK3CA mutation in ERalpha-positive breast cancer: major implication of the Wnt signaling pathway. PLoS One. 2010;5:e15647. doi: 10.1371/journal.pone.0015647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couzens AL, Knight JD, Kean MJ, Teo G, Weiss A, Dunham WH, Lin ZY, Bagshaw RD, Sicheri F, Pawson T, et al. Protein interaction network of the mammalian Hippo pathway reveals mechanisms of kinase-phosphatase interactions. Sci Signal. 2013;6:rs15. doi: 10.1126/scisignal.2004712. [DOI] [PubMed] [Google Scholar]
- Cruciat CM, Niehrs C. Secreted and transmembrane wnt inhibitors and activators. Cold Spring Harb Perspect Biol. 2013;5 doi: 10.1101/cshperspect.a015081. a015081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijksterhuis JP, Petersen J, Schulte G. WNT/Frizzled signalling: receptor-ligand selectivity with focus on FZD-G protein signalling and its physiological relevance: IUPHAR Review 3. Br J Pharmacol. 2014;171:1195–1209. doi: 10.1111/bph.12364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X, Degese MS, Iglesias-Bartolome R, Vaque JP, Molinolo AA, Rodrigues M, Zaidi MR, Ksander BR, Merlino G, Sodhi A, et al. Hippo-independent activation of YAP by the GNAQ uveal melanoma oncogene through a trio-regulated rho GTPase signaling circuitry. Cancer Cell. 2014;25:831–845. doi: 10.1016/j.ccr.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Sancho JM, Greer YE, Abrahams CL, Takigawa Y, Baljinnyam B, Lee KH, Lee KS, Rubin JS, Brown AM. Functional consequences of Wnt-induced dishevelled 2 phosphorylation in canonical and noncanonical Wnt signaling. J Biol Chem. 2013;288:9428–9437. doi: 10.1074/jbc.M112.448480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumolato L, Liu G, Mong P, Mudbhary R, Biswas R, Arroyave R, Vijayakumar S, Economides AN, Aaronson SA. Canonical and noncanonical Wnts use a common mechanism to activate completely unrelated coreceptors. Genes Dev. 2010;24:2517–2530. doi: 10.1101/gad.1957710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustin JP, Karakas B, Weiss MB, Abukhdeir AM, Lauring J, Garay JP, Cosgrove D, Tamaki A, Konishi H, Konishi Y, et al. Knockin of mutant PIK3CA activates multiple oncogenic pathways. Proc Natl Acad Sci U S A. 2009;106:2835–2840. doi: 10.1073/pnas.0813351106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas R, Kato Y, He X. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell. 2001;107:843–854. doi: 10.1016/s0092-8674(01)00614-6. [DOI] [PubMed] [Google Scholar]
- Haramis AP, Begthel H, van den Born M, van Es J, Jonkheer S, Offerhaus GJ, Clevers H. De novo crypt formation and juvenile polyposis on BMP inhibition in mouse intestine. Science. 2004;303:1684–1686. doi: 10.1126/science.1093587. [DOI] [PubMed] [Google Scholar]
- He XC, Zhang J, Tong WG, Tawfik O, Ross J, Scoville DH, Tian Q, Zeng X, He X, Wiedemann LM, et al. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-beta-catenin signaling. Nat Genet. 2004;36:1117–1121. doi: 10.1038/ng1430. [DOI] [PubMed] [Google Scholar]
- Ho HY, Susman MW, Bikoff JB, Ryu YK, Jonas AM, Hu L, Kuruvilla R, Greenberg ME. Wnt5a–Ror-Dishevelled signaling constitutes a core developmental pathway that controls tissue morphogenesis. Proc Natl Acad Sci U S A. 2012;109:4044–4051. doi: 10.1073/pnas.1200421109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imajo M, Miyatake K, Iimura A, Miyamoto A, Nishida E. A molecular mechanism that links Hippo signalling to the inhibition of Wnt/beta-catenin signalling. EMBO J. 2012;31:1109–1122. doi: 10.1038/emboj.2011.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katanaev VL, Ponzielli R, Semeriva M, Tomlinson A. Trimeric G protein-dependent frizzled signaling in Drosophila. Cell. 2005;120:111–122. doi: 10.1016/j.cell.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Kikuchi A, Yamamoto H, Sato A, Matsumoto S. Wnt5a: its signalling, functions and implication in diseases. Acta Physiol (Oxf) 2012;204:17–33. doi: 10.1111/j.1748-1716.2011.02294.x. [DOI] [PubMed] [Google Scholar]
- Kim YC, Clark RJ, Ranheim EA, Alexander CM. Wnt1 expression induces short-range and long-range cell recruitments that modify mammary tumor development and are not induced by a cell-autonomous beta-catenin effector. Cancer Res. 2008;68:10145–10153. doi: 10.1158/0008-5472.CAN-08-2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli P, Bartram MP, Habbig S, Pahmeyer C, Lamkemeyer T, Benzing T, Schermer B, Rinschen MM. Label-free quantitative proteomic analysis of the YAP/TAZ interactome. Am J Physiol Cell Physiol. 2014;306:C805–818. doi: 10.1152/ajpcell.00339.2013. [DOI] [PubMed] [Google Scholar]
- Kwon Y, Vinayagam A, Sun X, Dephoure N, Gygi SP, Hong P, Perrimon N. The Hippo signaling pathway interactome. Science. 2013;342:737–740. doi: 10.1126/science.1243971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai D, Yang X. BMP4 is a novel transcriptional target and mediator of mammary cell migration downstream of the Hippo pathway component TAZ. Cell Signal. 2013;25:1720–1728. doi: 10.1016/j.cellsig.2013.05.002. [DOI] [PubMed] [Google Scholar]
- Latinkic BV, Mercurio S, Bennett B, Hirst EM, Xu Q, Lau LF, Mohun TJ, Smith JC. Xenopus Cyr61 regulates gastrulation movements and modulates Wnt signalling. Development. 2003;130:2429–2441. doi: 10.1242/dev.00449. [DOI] [PubMed] [Google Scholar]
- Lin F, Sepich DS, Chen S, Topczewski J, Yin C, Solnica-Krezel L, Hamm H. Essential roles of G{alpha}12/13 signaling in distinct cell behaviors driving zebrafish convergence and extension gastrulation movements. J Cell Biol. 2005;169:777–787. doi: 10.1083/jcb.200501104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CY, Zha ZY, Zhou X, Zhang H, Huang W, Zhao D, Li T, Chan SW, Lim CJ, Hong W, et al. The hippo tumor pathway promotes TAZ degradation by phosphorylating a phosphodegron and recruiting the SCF{beta}-TrCP E3 ligase. J Biol Chem. 2010;285:37159–37169. doi: 10.1074/jbc.M110.152942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, DeCostanzo AJ, Liu X, Wang H, Hallagan S, Moon RT, Malbon CC. G protein signaling from activated rat frizzled-1 to the beta-catenin-Lef-Tcf pathway. Science. 2001;292:1718–1722. doi: 10.1126/science.1060100. [DOI] [PubMed] [Google Scholar]
- Liu-Chittenden Y, Huang B, Shim JS, Chen Q, Lee SJ, Anders RA, Liu JO, Pan D. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012;26:1300–1305. doi: 10.1101/gad.192856.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major MB, Roberts BS, Berndt JD, Marine S, Anastas J, Chung N, Ferrer M, Yi X, Stoick-Cooper CL, von Haller PD, et al. New regulators of Wnt/beta-catenin signaling revealed by integrative molecular screening. Sci Signal. 2008;1:ra12. doi: 10.1126/scisignal.2000037. [DOI] [PubMed] [Google Scholar]
- Mercurio S, Latinkic B, Itasaki N, Krumlauf R, Smith JC. Connective-tissue growth factor modulates WNT signalling and interacts with the WNT receptor complex. Development. 2004;131:2137–2147. doi: 10.1242/dev.01045. [DOI] [PubMed] [Google Scholar]
- Miller E, Yang J, DeRan M, Wu C, Su AI, Bonamy GM, Liu J, Peters EC, Wu X. Identification of serum-derived sphingosine-1-phosphate as a small molecule regulator of YAP. Chem Biol. 2012;19:955–962. doi: 10.1016/j.chembiol.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Mo JS, Park HW, Guan KL. The Hippo signaling pathway in stem cell biology and cancer. EMBO Rep. 2014;15:642–656. doi: 10.15252/embr.201438638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo JS, Yu FX, Gong R, Brown JH, Guan KL. Regulation of the Hippo-YAP pathway by protease-activated receptors (PARs) Genes Dev. 2012;26:2138–2143. doi: 10.1101/gad.197582.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroishi T, Hansen CG, Guan KL. The emerging roles of YAP and TAZ in cancer. Nat Rev Cancer. 2015;15:73–79. doi: 10.1038/nrc3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehrs C. The complex world of WNT receptor signalling. Nat Rev Mol Cell Biol. 2010;13:767–779. doi: 10.1038/nrm3470. [DOI] [PubMed] [Google Scholar]
- Pan D. The hippo signaling pathway in development and cancer. Dev Cell. 2010;19:491–505. doi: 10.1016/j.devcel.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolo S, Dupont S, Cordenonsi M. The biology of YAP/TAZ: hippo signaling and beyond. Physiol Rev. 2014;94:1287–1312. doi: 10.1152/physrev.00005.2014. [DOI] [PubMed] [Google Scholar]
- Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F. Genome engineering using the CRISPR-Cas9 system. Nat Protoc. 2013;8:2281–2308. doi: 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regard JB, Cherman N, Palmer D, Kuznetsov SA, Celi FS, Guettier JM, Chen M, Bhattacharyya N, Wess J, Coughlin SR, et al. Wnt/beta-catenin signaling is differentially regulated by Galpha proteins and contributes to fibrous dysplasia. Proc Natl Acad Sci U S A. 2011;108:20101–20106. doi: 10.1073/pnas.1114656108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues P, Macaya I, Bazzocco S, Mazzolini R, Andretta E, Dopeso H, Mateo-Lozano S, Bilic J, Carton-Garcia F, Nieto R, et al. RHOA inactivation enhances Wnt signalling and promotes colorectal cancer. Nat Commun. 2014;5:5458. doi: 10.1038/ncomms6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman-Roman S, Shi DL, Stiot V, Hay E, Vayssiere B, Garcia T, Baron R, Rawadi G. Murine Frizzled-1 behaves as an antagonist of the canonical Wnt/beta-catenin signaling. J Biol Chem. 2004;279:5725–5733. doi: 10.1074/jbc.M309233200. [DOI] [PubMed] [Google Scholar]
- Ross SE, Hemati N, Longo KA, Bennett CN, Lucas PC, Erickson RL, MacDougald OA. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289:950–953. doi: 10.1126/science.289.5481.950. [DOI] [PubMed] [Google Scholar]
- Sanjana NE, Shalem O, Zhang F. Improved vectors and genome-wide libraries for CRISPR screening. Nat Methods. 2014;11:783–784. doi: 10.1038/nmeth.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger K, Hall A, Tolwinski N. Wnt signaling pathways meet Rho GTPases. Genes Dev. 2009;23:265–277. doi: 10.1101/gad.1760809. [DOI] [PubMed] [Google Scholar]
- Seo E, Basu-Roy U, Gunaratne PH, Coarfa C, Lim DS, Basilico C, Mansukhani A. SOX2 regulates YAP1 to maintain stemness and determine cell fate in the osteo-adipo lineage. Cell Rep. 2013;3:2075–2087. doi: 10.1016/j.celrep.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slusarski DC, Corces VG, Moon RT. Interaction of Wnt and a Frizzled homologue triggers G-protein-linked phosphatidylinositol signalling. Nature. 1997;390:410–413. doi: 10.1038/37138. [DOI] [PubMed] [Google Scholar]
- van Amerongen R. Alternative Wnt pathways and receptors. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a007914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tienen FH, Laeremans H, van der Kallen CJ, Smeets HJ. Wnt5b stimulates adipogenesis by activating PPARgamma, and inhibiting the beta-catenin dependent Wnt signaling pathway together with Wnt5a. Biochem Biophys Res Commun. 2009;387:207–211. doi: 10.1016/j.bbrc.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Varelas X, Miller BW, Sopko R, Song S, Gregorieff A, Fellouse FA, Sakuma R, Pawson T, Hunziker W, McNeill H, et al. The Hippo pathway regulates Wnt/beta-catenin signaling. Dev Cell. 2010;18:579–591. doi: 10.1016/j.devcel.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Wang W, Li X, Huang J, Feng L, Dolinta KG, Chen J. Defining the protein-protein interaction network of the human hippo pathway. Mol Cell Proteomics. 2014;13:119–131. doi: 10.1074/mcp.M113.030049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. Wnt/Planar cell polarity signaling: a new paradigm for cancer therapy. Mol Cancer Ther. 2009;8:2103–2109. doi: 10.1158/1535-7163.MCT-09-0282. [DOI] [PubMed] [Google Scholar]
- Yu B, Chang J, Liu Y, Li J, Kevork K, Al-Hezaimi K, Graves DT, Park NH, Wang CY. Wnt4 signaling prevents skeletal aging and inflammation by inhibiting nuclear factor-kappaB. Nat Med. 2014a;20:1009–1017. doi: 10.1038/nm.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FX, Guan KL. The Hippo pathway: regulators and regulations. Genes Dev. 2013;27:355–371. doi: 10.1101/gad.210773.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FX, Luo J, Mo JS, Liu G, Kim YC, Meng Z, Zhao L, Peyman G, Ouyang H, Jiang W, et al. Mutant Gq/11 promote uveal melanoma tumorigenesis by activating YAP. Cancer Cell. 2014b;25:822–830. doi: 10.1016/j.ccr.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FX, Zhang Y, Park HW, Jewell JL, Chen Q, Deng Y, Pan D, Taylor SS, Lai ZC, Guan KL. Protein kinase A activates the Hippo pathway to modulate cell proliferation and differentiation. Genes Dev. 2013;27:1223–1232. doi: 10.1101/gad.219402.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH, Zhao J, Yuan H, Tumaneng K, Li H, et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150:780–791. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecca M, Struhl G. A feed-forward circuit linking wingless, fat-dachsous signaling, and the warts-hippo pathway to Drosophila wing growth. PLoS Biol. 2010;8:e1000386. doi: 10.1371/journal.pbio.1000386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Li L, Wang L, Wang CY, Yu J, Guan KL. Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Genes Dev. 2012;26:54–68. doi: 10.1101/gad.173435.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Tumaneng K, Guan KL. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat Cell Biol. 2011;13:877–883. doi: 10.1038/ncb2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Shiojima I, Ito Y, Li Z, Ikeda H, Yoshida M, Naito AT, Nishi J, Ueno H, Umezawa A, et al. IGFBP-4 is an inhibitor of canonical Wnt signalling required for cardiogenesis. Nature. 2008;454:345–349. doi: 10.1038/nature07027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.