Abstract
Background
The validity of the 20-item Center for Epidemiological Studies Depression (CES-D) scale for depression screening in Hong Kong Chinese patients with type 2 diabetes remains unknown. We aimed to validate CES-D, compare its psychometric properties with the 9-item Patient Health Questionnaire (PHQ-9), and explore whether one of the two is more suitable for depression screening in Chinese patients with type 2 diabetes.
Methods
Between June 2010 and July 2011, 545 consecutive Chinese patients with type 2 diabetes who underwent structured comprehensive assessments completed the CES-D and PHQ-9. Forty patients were retested within 2–4 weeks by telephone interview and 97 patients were randomly selected to undergo the Mini International Neuropsychiatric Interview (MINI) by psychiatrists for clinical diagnosis of depression.
Results
The internal consistency (Cronbach’s α) of CES-D was 0.85, with a test-retest correlation coefficient of 0.64. The area under the curve for CES-D compared to the clinical diagnosis of major depression was 0.85. A cut-off score of ≥21 for CES-D provided the optimal balance between sensitivity (78.3 %) and specificity (74.3 %) and identified 17.8 % (n = 97) of patients with depression. CES-D and PHQ-9 showed moderate agreement in depression screening (Cohen’s Kappa: 0.45). Compared to non-depressed patients, those who screened positive by PHQ-9 had a higher HbA1c whereas the glycemic differences were not significant when using CES-D.
Conclusion
The CES-D is a valid screening tool for depression in Chinese type 2 diabetic patients although the PHQ-9 was more discriminative in identifying those with suboptimal glycemic control.
Background
Depression and type 2 diabetes are complex diseases with rising prevalence [1, 2]. These two chronic conditions frequently coexist resulting in increased risk of morbidity and mortality with major negative implications on the individuals, families and society [3, 4]. International diabetes guidelines now recommend screening for psychosocial problems including depression, especially when self-management is poor [5, 6]. The 20-item Center for Epidemiological Studies Depression (CES-D) scale and the 9-item Patient Health Questionnaire (PHQ-9) are two most widely used self-administered instruments for depression screening [7, 8]. Originally developed for a general population in a Western setting, both instruments have been validated in other populations including American and Hong Kong Chinese community-dwelling individuals [9–13].
There is emerging evidence suggesting that ethnicity, culture, and acculturation may lead to response bias in these instruments [13–15]. We previously reported the validity of PHQ-9 for depression screening in Hong Kong Chinese patients with type 2 diabetes and reported a lower cutoff point (≥7) for significant depressive symptoms than the conventional one (≥10) which was first validated in primary care settings and obstetrics-gynecology clinics in U.S. [13, 16]. However, there is a paucity of data on the performance of CES-D in Chinese patients with type 2 diabetes. In this study, we aimed to validate CES-D and compare its psychometric properties with PHQ-9 in community-dwelling Chinese patients with type 2 diabetes in Hong Kong.
Methods
Subjects and setting
The study design and patient recruitment have been described previously [16]. In brief, 601 Chinese outpatients with type 2 diabetes aged 25–75 years were recruited consecutively from a hospital-based (Prince of Wales Hospital) and a community-based (Yao Chung Kit Diabetes Assessment Centre) diabetes center between June 2010 and July 2011. All patients underwent a 4-hour diabetes complication assessment using a structured protocol provided by the Joint Asia Diabetes Evaluation Program [17–20]. They were also invited to complete a set of questionnaires to assess their psychological well-being. Significant medical and psychiatric history, social history, family history of diabetes, and medication records were documented. Urine and blood samples were collected after overnight fast for plasma glucose, glycated hemoglobin (HbA1c), total cholesterol, low density-lipoprotein cholesterol (LDL-C), high density-lipoprotein cholesterol (HDL-C), triglycerides, renal function, and urinary albumin-to-creatinine ratio (ACR). This study was approved by the ethics committee of The Chinese University of Hong Kong, and all patients gave informed consent.
Psychological assessment
Symptoms of depression were assessed by the CES-D and PHQ-9 questionnaires. The CES-D scale is a 20-item self-reported instrument developed by Radloff in 1977 [7]. It measures the frequency of common depressive symptoms over the past week. Each item is scored from 0 (rarely or none of the time, less than one day) to 3 (all of the time, 5–7 days). The four positively stated items (item 4, I felt that I was just as good as other people; item 8, I felt hopeful about the future; item 12, I was happy; item 16, I enjoyed life) are reverse-coded for calculating the total score which ranges from 0 to 60. The cut-off value of ≥16 has been widely used to define clinically meaningful depressive symptoms [7, 21]. It was reported to have 96.8 % sensitivity and 67.6 % specificity for clinical depression in Chinese type 2 diabetic patients attending a diabetes centre in Singapore [22]. The PHQ-9 focuses on the frequency of occurrence of 9 depressive symptoms derived from DSM-IV diagnostic criteria over the past two weeks [8]. Each item is scored from 0 (not at all) to 3 (nearly every day), with a total score ranging from 0 to 27. A cutoff value of 10 has been widely used to define probable depression, with 88 % sensitivity and 88 % specificity in the original validation study with majority of participants being Caucasians [8]. In our previous criterion validation in the same group of 99 patients, we identified the optimal value of 7 with 82.6 % sensitivity and 73.7 % specificity [16].
The process of this study has been described previously [16]. Briefly, 40 patients were randomly selected for CES-D and PHQ-9 retest within 2–4 weeks by telephone survey. Another randomly selected subset of patients was referred for assessment by psychiatrists (Dr Marco Lam and Dr Siu-ping Lam) using the Chinese version of Mini International Neuropsychiatric Interview (MINI version 6.0), a short structured diagnostic interview that has been validated and is widely accepted for diagnosing major depression in a research setting [23, 24]. Due to manpower issue, patient was assessed by one psychiatrist only and we were not able to calculate the inter-rater reliability. However the two psychiatrists received training for MINI together. Pilot interview was conducted in three patients and it showed 100 % agreement in the MINI diagnosis for depression.
Statistical analyses
All analysis was performed using the Statistical Package for Social Sciences (SPSS version 20.0, IBM). Data were expressed as mean ± SD, median (interquartile range) or number (%), as appropriate. The Student’s t-test, Mann–Whitney U test and Chi-square tests were used for group comparisons. Cronbach’s α was calculated to evaluate the internal consistency. Pearson correlation coefficients were used to measure test-retest correlation and concurrent validity of the PHQ-9 and CES-D as appropriate. Item discrimination was tested by corrected item-total correlation using the Pearson product–moment correlation formula, which has been incorporated into SPSS Reliability analysis. Exploratory factor analysis (EFA) with eigenvalue >1 criteria was performed to establish the construct validity. An oblique (Promax) rotation was used in the EFA based on the assumption that the CES-D would have correlated factors. The response agreement between PHQ-9 and CES-D was evaluated with Cohen’s kappa. Receiver Operator Characteristic (ROC) analysis was used to determine the diagnostic performance and optimal cutoff score for screening major depression against MINI-based clinical diagnosis. AP value < 0.05 (2-tailed) was considered significant.
Results
Characteristics of study participants
A total of 545 participants (mean age 54.6 ± 9.5 years, median[IQR] disease duration 6 [3–11] years, 41.3 % were female) fulfilled the inclusion criteria and had complete CES-D and PHQ-9 data for analysis, among them 97 underwent clinical interview by psychiatrists. Their characteristics were presented in Table 1. Overall, 4.8 % (n = 26) of patients had a history of clinical diagnosed psychiatric disorder, mostly consisting of depression (n = 18), and 8.2 % (n = 44) of patients were concurrently treated with psychotropic medications. 28 (5.1 %) had suicide ideation in the past 2 weeks based on the 9th item of PHQ-9. Of them, 22 (4.0 %), 5 (0.9 %) and 1 (0.2 %) reported suicide ideation for a few days, more than half the days and nearly every day, respectively.
Table 1.
Demographic and clinical characteristics of Chinese type 2 diabetic patients categorised by validated cutoff values of PHQ-9 and CES-D
| Total | CES-D <21 | CES-D ≥21 | p value | PHQ-9 < 7 | PHQ-9 ≥ 7 | P value | |
|---|---|---|---|---|---|---|---|
| Number | 545 | 448 | 97 (17.8) | 449 | 96 (17.6) | ||
| CES-D score | 13.0 ± 8.6 | 9.9 ± 5.3 | 27.4 ± 5.6 | <0.001 | 11.0 ± 6.9 | 22.3 ± 9.5 | <0.001 |
| PHQ-9 score | 3.5 ± 4.4 | 2.3 ± 2.7 | 8.8 ± 6.2 | <0.001 | 1.8± 1.8 | 11.2± 4.6 | <0.001 |
| Age (years) | 54.6 ± 9.5 | 54.7 ± 9.7 | 54.4 ± 8.6 | 0.807 | 54.4 ± 9.4 | 55.4 ± 9.9 | 0.339 |
| Women | 225 (41.3) | 173 (38.6) | 52 (53.6) | 0.007 | 170 (37.9) | 55 (57.3) | <0.001 |
| Disease duration (years) | 6.0 (3.0,11.0) | 7.0 (3.0,10.3) | 6.0 (3.0,13.0) | 0.867 | 7.0 (4.0,10.8) | 6.0 (2.0-13.0) | 0.568 |
| Education | 0.555 | 0.073 | |||||
| Illiterate/primary school | 142 (26.1) | 117 (26.1) | 25 (25.8) | 113 (25.2) | 29 (30.2) | ||
| Middle school | 275 (50.5) | 222 (49.6) | 53 (54.6) | 222 (49.4) | 53 (55.2) | ||
| High school and above | 128 (23.5) | 109 (24.3) | 19 (19.6) | 114 (25.4) | 14 (14.6) | ||
| In employment | 307 (56.3) | 259 (57.8) | 48 (19.5) | 0.134 | 266 (59.2) | 41 (42.7) | 0.003 |
| Current smoker | 56 (10.3) | 49 (11.0) | 7 (7.2) | 0.271 | 43 (9.6) | 13 (13.5) | 0.249 |
| Exercise ≥3 times/week | 262 (48.1) | 216 (48.2) | 46 (47.4) | 0.887 | 217 (48.3) | 45 (46.9) | 0.796 |
| SMBG ≥ weekly | 278 (51.0) | 234 (52.2) | 44 (45.4) | 0.22 | 232 (51.7) | 46 (47.9) | 0.504 |
| Cardio-metabolic risk factors | |||||||
| Body mass index (kg/m2) | 26.1 ± 4.4 | 26.1 ± 4.3 | 26.0 ± 4.9 | 0.802 | 26.1 ± 4.4 | 26.0 ± 4.6 | 0.902 |
| Systolic BP (mmHg) | 130.6 ± 15.7 | 130.3 ± 15.5 | 132.3 ± 16.7 | 0.255 | 130.4 ± 15.6 | 131.7 ± 16.2 | 0.464 |
| Diastolic BP (mmHg) | 79.6 ± 10.0 | 79.3 ± 9.9 | 80.9 ± 10.5 | 0.158 | 79.5 ± 10.0 | 80.2 ± 10.2 | 0.510 |
| Waist circumference, men (cm) | 91.2 ± 10.5 | 91.1 ± 10.2 | 91.8 ± 12.1 | 0.713 | 91.3 ± 10.4 | 90.7 ± 11.3 | 0.742 |
| Waist circumference, women (cm) | 86.1 ± 11.1 | 85.8 ± 10.9 | 87.2 ± 11.7 | 0.421 | 85.8 ± 11.5 | 87.0 ± 10.0 | 0.475 |
| HbA1c (%) | 7.5 ± 1.4 | 7.5 ± 1.4 | 7.7 ± 1.6 | 0.238 | 7.4 ± 1.3 | 7.9 ± 1.9 | 0.045 |
| HbA1c (mmol/L) | 58.6 ± 15.7 | 58.3 ± 15.2 | 60.4 ± 18.0 | 0.238 | 57.8 ± 14.2 | 62.4 ± 21.2 | 0.045 |
| Total cholesterol (mmol/L) | 4.50 ± 0.88 | 4.48 ± 0.87 | 4.60 ± 0.91 | 0.247 | 4.52 ± 0.89 | 4.40 ± 0.84 | 0.200 |
| HDL-C (mmol/L) | 1.34 ± 0.37 | 1.33 ± 0.37 | 1.35 ± 0.36 | 0.632 | 1.35 ± 0.37 | 1.30 ± 0.37 | 0.223 |
| LDL-C (mmol/L) | 2.49 ± 0.71 | 2.48 ± 0.70 | 2.56 ± 0.75 | 0.284 | 2.51 ± 0.71 | 2.42 ± 0.72 | 0.324 |
| eGFR (ml/min/1.73 m2) | 109.0 ± 23.7 | 109.3 ± 23.4 | 108.1 ± 24.9 | 0.654 | 109.3 ± 23.8 | 108.0 ± 23.2 | 0.653 |
| Spot urinary ACR | 1.10 (0.48,4.02) | 1.09 (0.48,3.90) | 1.11 (0.51,4.56) | 0.442 | 1.05 (0.44,3.90) | 1.25 (0.62,5.03) | 0.096 |
| Hypertension | 430 (78.9) | 356 (79.5) | 74 (76.3) | 0.487 | 349 (77.7) | 81 (84.4) | 0.147 |
| Dyslipidaemia | 463 (85.3) | 383 (85.9) | 80 (82.5) | 0.345 | 380 (85.0) | 83 (86.5) | 0.717 |
| Albuminuria | 166 (30.5) | 136 (30.4) | 30 (30.9) | 0.912 | 135 (30.1) | 31 (32.3) | 0.667 |
| Diabetes-related complications | |||||||
| Cardiovascular disease | 53 (9.7) | 42 (9.4) | 11 (11.3) | 0.554 | 40 (8.9) | 13 (13.5) | 0.164 |
| Retinopathy | 112 (20.6) | 91 (20.3) | 21 (21.6) | 0.786 | 95 (21.2) | 17 (17.7) | 0.448 |
| Chronic kidney disease | 10 (1.8) | 8 (1.8) | 2 (2.1) | 0.695 | 8 (1.8) | 2 (2.1) | 0.689 |
| Sensory neuropathy | 13 (2.4) | 7 (1.6) | 6 (6.2) | 0.016 | 7 (1.6) | 6 (6.2) | 0.015 |
| Medication, target achievement | |||||||
| Use of insulin | 87 (16.0) | 73 (16.3) | 14 (14.4) | 0.65 | 73 (16.3) | 14 (14.6) | 0.684 |
| On OAD | 492 (90.3) | 402 (89.7) | 90 (92.8) | 0.358 | 402 (89.5) | 90 (93.8) | 0.206 |
| Use of antihypertensive drugs | 324 (59.4) | 269 (60.0) | 55 (56.7) | 0.543 | 261 (58.1) | 63 (65.6) | 0.704 |
| Use of lipid lowering drugs | 265 (48.6) | 223 (49.8) | 42 (43.3) | 0.247 | 208 (46.3) | 57 (59.4) | 0.02 |
| Concurrent use of psychotropic drugs | 44 (8.2) | 16 (4.3) | 28 (16.7) | <0.001 | 27 (6.1) | 17 (17.7) | <0.001 |
| HBA1C < 7.0 % (53 mmol/L) | 218 (40.0) | 181 (40.4) | 37 (38.1) | 0.681 | 187 (41.6) | 31 (32.3) | 0.089 |
| LDL-C < 2.6 mmol/L | 312 (57.2) | 255 (56.9) | 57 (58.8) | 0.739 | 252 (56.1) | 60 (62.5) | 0.252 |
| BP < 130/80 mmHg | 206 (37.8) | 173 (38.6) | 33 (34.0) | 0.397 | 175 (39.0) | 31 (32.3) | 0.22 |
PHQ-9 Patients Health Questionnaire-9, CES-D 20-item Center for Epidemiological Studies Depression, SMBG Self-monitoring of blood glucose, BP Blood pressure, eGFR estimated glomerular filtration rate, ACR albumin-to-creatinine ratio, OAD Oral antidiabetic drugs
Data are shown as mean ± SD, number (%) or median (interquartile range)
The definitions of risk factors and complications were as follows: hypertension = known high blood pressure with or without treatment and/or blood pressure ≥ 130/80 mmHg; dyslipidaemia = LDL-C ≥ 2.6mmol/L, HDL-C < 1.0 mmol/L, triglycerides ≥ 2.3 mmol/L, or on any lipid-lowering agents; albuminuria = sport urine albumin/creatinine ratio ≥ 2.5 mg/mmol in men or ≥ 3.5 mg/mmol in women; chronic kidney disease = eGFR <60 ml/min/1.73 m2, cardiovascular disease = coronary heart disease, stroke, and/or peripheral vascular disease
Psychometric properties of the CES-D
Internal reliability and item discrimination
The internal consistency (Cronbach’s α) of CES-D was 0.85, with test-retest correlation coefficient (r) of 0.64 (P < 0.001), similar to PHQ-9 (α = 0.87, r = 0.70, P < 0.001) as shown in our previous study [16]. After removal of the four positive affective items in the CES-D the internal consistency of CES-D questionnaire increased to 0.91. The corrected item-total correlations for individual CES-D items ranged from 0.17 (item 4: feeling good) to 0.66 (item 6: depressed) (Table 2), lower for the four positive items than other items. The corrected item-total correlations for individual PHQ-9 items ranged from 0.48 to 0.68 [16].
Table 2.
Item scores, internal reliability, factor loadings and inter-factor correlations of CES-D
| Score | Internal reliability (α = 0.852) | Factor loadingsb | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Corrected item-total correlation | α if item deleteda | Factor 1 | Factor 2 | Factor 3 | Factor 4 | |
| 1. I was bothered by things that usually don’t bother me. | 0.72 | 0.84 | 0.56 | 0.73 | ||||
| 2. I did not feel like eating; my appetite was poor. | 0.36 | 0.68 | 0.31 | 0.65 | ||||
| 3. I felt that I could not shake off the blues even with help from my family or friends. | 0.40 | 0.71 | 0.62 | 0.69 | ||||
| 4. I felt I was just as good as other people. | 1.65 | 1.19 | 0.17 | 0.86 | 0.77 | |||
| 5. I had trouble keeping my mind on what I was doing | 0.49 | 0.70 | 0.50 | 0.69 | ||||
| 6. I felt depressed. | 0.47 | 0.72 | 0.66 | 0.77 | ||||
| 7. I felt that everything I did was an effort. | 0.48 | 0.72 | 0.48 | 0.65 | ||||
| 8. I felt hopeful about the future | 1.50 | 1.20 | 0.21 | 0.86 | 0.80 | |||
| 9. I thought my life had been a failure. | 0.44 | 0.77 | 0.56 | 0.67 | ||||
| 10. I felt fearful | 0.44 | 0.74 | 0.62 | 0.76 | ||||
| 11. My sleep was restless. | 0.66 | 0.85 | 0.46 | 0.64 | ||||
| 12. I was happy. | 1.39 | 1.16 | 0.33 | 0.85 | 0.87 | |||
| 13. I talked less than usual. | 0.61 | 0.84 | 0.51 | 0.62 | ||||
| 14. I felt lonely. | 0.45 | 0.76 | 0.63 | 0.73 | ||||
| 15. People were unfriendly | 0.39 | 0.66 | 0.51 | 0.88 | ||||
| 16. I enjoyed life. | 1.36 | 1.19 | 0.30 | 0.86 | 0.88 | |||
| 17. I had crying spells. | 0.35 | 0.68 | 0.46 | 0.74 | ||||
| 18. I felt sad | 0.39 | 0.70 | 0.64 | 0.87 | ||||
| 19. I felt that people dislike me | 0.33 | 0.62 | 0.43 | 0.88 | ||||
| 20. I could not get “going.” | 0.14 | 0.48 | 0.51 | 0.65 | ||||
| Factor score | 3.1 ± 4.2 | 3.3 ± 3.2 | 5.9 ± 3.9 | 0.7 ± 1.2 | ||||
| Inter-factor correlation | ||||||||
| Factor 1 | 1.00 | 0.75* | 0.04 | 0.53* | ||||
| Factor 2 | 1.00 | −0.05 | 0.49* | |||||
| Factor 3 | 1.00 | −0.02 | ||||||
aOnly those with increased Cronbach’s α if item deleted were shown in the table
bFactor 1: Depressed affect; factor 2: somatic symptoms; factor 3: positive affect; factor 4: Interpersonal problems
*P for Pearson correlation (r) <0.0001. The correlations between positive affect and other subscales were not significant
Construct validity and item scores of CES-D
The Kaiser-Meyer-Olkin test of sampling adequacy was 0.91 and Bartlett’s test of sphericity was significant (X2 = 5042.6, P < 0.001). EFA using Promax rotation procedure yielded a four-factor structure for the CES-D according to the “eigenvalue >1” rule: 1) depressed affect, 2) somatic symptoms, 3) positive affect, and 4) interpersonal problems. The scree plot was shown as Fig. 1. This four-factor model accounted for 61.1 % of the scale variance, with factor loadings ranging from 0.62 to 0.88 (Table 2).
Fig. 1.
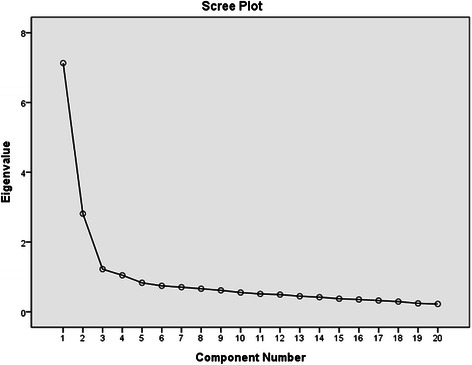
The scree plot for CES-D exploratory factor analysis
The mean CES-D total score was 13.0 ± 8.6 (median12.0, IQR 7.0-17.0), with individual item scores ranging from 0.14 to 1.65. The four positive affect items scored much higher than the other items, with a mean factor score of 5.9 ± 3.9, accounting for 50 % of the total CES-D score. The positive affect factor did not correlate with the somatic symptoms (r = −0.05,P = 0.275), depressed affect (r = 0.04, P = 0.414), or interpersonal problems (r = −0.02, P = 0.703); the latter three factors were significantly inter-correlated, with correlation coefficients ranging from 0.49 to 0.75 (all P < 0.001) (Table 2)
Diagnostic validity
Among the 97 patients who were interviewed by two psychiatrists, 23 patients had a clinical diagnosis of current major depressive episode as measured by the MINI. The area under the curve (AUC) upon ROC analysis was 0.85(95%CI: 0.77-0.92) (Fig. 2). The standard cut-off score of ≥16 for CES-D had an excellent sensitivity (91.3 %) but low specificity (60.8 %) compared to the MINI diagnosis of major depressive episode. As shown in Table 3, a cut-off score of ≥21 on CES-D yielded an optimal balance between sensitivity (78.3 %) and specificity (74.3 %), with positive predictive value (PPV) of 48.6 %, and negative predictive value (NPV) of 91.7 %, similar to the PHQ-9 score ≥ 7. Furthermore, the removal of the four positive items slightly improved the diagnostic performance (Cronbach’s α 0.91, AUC 0.85 [0.77-0.94]), where the optimal cutoff value of 14 yielded a sensitivity of 82.6 % and specificity of 74.3 % (PPV 50.0 %, NPV 93.2 %).
Fig. 2.
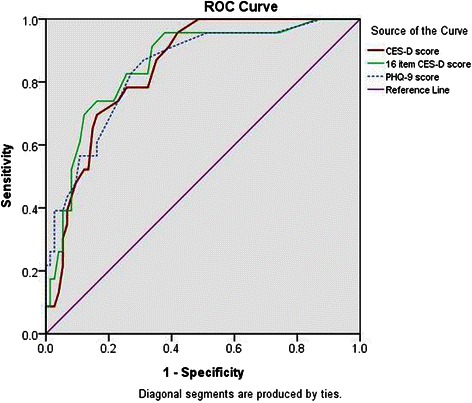
Receiver operating characteristic (ROC) curve analysis of CES-D and PHQ-9 versus clinical interview by MINI in 97 Chinese patients with type 2 diabetes
Table 3.
Diagnostic performance for detection of major depressive disorder using MINI clinical interview as reference (n = 97)
| Cutoff value | Sensitivity | Specificity | LR+ | LR- | PPV | NPV | |
|---|---|---|---|---|---|---|---|
| 20-item CES-D | |||||||
| ≥16 | 91.3 % | 60.8 % | 2.33 | 6.99 | 42.0 % | 95.7 % | |
| ≥17 | 87.0 % | 64.9 % | 2.47 | 4.97 | 43.5 % | 94.1 % | |
| ≥18 | 78.3 % | 67.6 % | 2.41 | 3.11 | 42.9 % | 90.9 % | |
| ≥19 | 78.3 % | 71.6 % | 2.76 | 3.29 | 46.2 % | 91.4 % | |
| ≥20 | 78.3 % | 71.6 % | 2.76 | 3.29 | 46.2 % | 91.4 % | |
| ≥21 | 78.3 % | 74.3 % | 3.05 | 3.42 | 48.6 % | 91.7 % | |
| ≥22 | 73.9 % | 77.0 % | 3.22 | 2.95 | 50.0 % | 90.5 % | |
| 16-item CES-D | |||||||
| ≥13 | 82.6 % | 73.0 % | 3.06 | 4.20 | 48.7 % | 93.1 % | |
| ≥14 | 82.6 % | 74.3 % | 3.22 | 4.27 | 50.0 % | 93.2 % | |
| ≥15 | 73.9 % | 78.4 % | 3.42 | 3.01 | 51.5 % | 90.6 % | |
| PHQ-9 | |||||||
| ≥6 | 87.0 % | 68.9 % | 2.80 | 5.28 | 46.5 % | 94.4 % | |
| ≥7 | 82.6 % | 73.0 % | 3.06 | 4.20 | 48.7 % | 93.1 % | |
| ≥8 | 73.9 % | 77.0 % | 3.22 | 2.95 | 50.0 % | 90.5 % | |
| ≥9 | 60.9 % | 83.8 % | 3.75 | 2.14 | 53.8 % | 87.3 % | |
| ≥10 | 56.5 % | 83.8 % | 3.49 | 1.93 | 52.0 % | 86.1 % | |
LR+ Positive likelihood ratio, LR- Negative likelihood ratio, PPV Positive predictive ratio, NPV Negative predictive ratio
Comparison of CES-D and PHQ-9
If the conventional cut-off points (score of ≥16 for CES-D and ≥10 for PHQ-9) were adopted, 31.0 % and 9.0 % of patients were respectively identified to have depression by CES-D and PHQ-9, with fair chance-corrected agreement between the two instruments (Cohen’s kappa: 0.32). However if we used a score of 21 as the cut-off for CES-D, 17.8 % of patients were identified to have possible depression, similar to the depression prevalence reported in the same group of patients using the PHQ-9 ≥ 7 [16], with moderate chance-corrected agreement between the two instruments (Cohen’s kappa: 0.45). The overlap of depressive symptom screen positivity is illustrated in a Venn diagram (Fig. 3), where just over a third of the 545 patients were captured by both PHQ-9 ≥ 7 and CES-D ≥21.
Fig. 3.
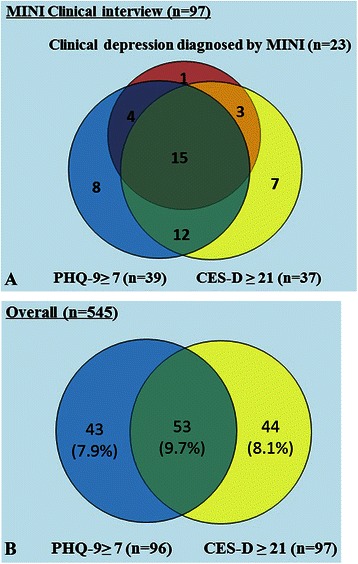
Venn diagram. a for those with MINI interview; b among all the patients
Overall, the demographic and clinical patterns of depression versus non-depression were similar using either PHQ-9 or CES-D as the screening instrument. However, patients with depression by PHQ-9 had significantly higher HbA1c level while there was no such difference between the depressed and non-depressed groups defined by the 20-item CES-D (Table 1), nor did by the 16-item CES-D (7.6 ± 1.7 vs. 7.5 ± 1.4 % [59.2 ± 18.4 vs. 58.6 ± 15.2 mmol/l], P = 0.734). Similarly, PHQ-9 score as a continuous value was positively associated with high HbA1c level (r = 0.12, P = 0.004) but not for CES-D score (r = 0.03, P = 0.423). Patients positive for both CES-D (≥21) and PHQ-9 (≥7) had similar HbA1c to those positive only for PHQ-9 (7.9 ± 1.9 vs. 7.9 ± 2.1 % [62.5 ± 20.6 vs. 62.4 ± 22.5 mmol/l], P = 1.000) but higher HbA1c than those positive only for CES-D although it did not reach statistical significance (7.9 ± 1.9 vs. 7.4 ± 1.3 % [62.5 ± 20.6 vs. 57.8 ± 14.5 mmol/l], P = 0.206). Moreover, using PHQ-9 ≥ 7 as the cutoff value, patients with depression were more likely to use lipid lowering drugs. Such difference was not detected if we used CES-D ≥21 to define depression.
Discussion
To our best knowledge, this is the first study to validate CES-D and systematically compare it with the PHQ-9 for depression screening in Chinese patients with type 2 diabetes. The internal consistency, test-retest reliability, and diagnostic performance of CES-D was comparable with that of the PHQ-9, which had been validated in the same population [16].
Factor structure of CES-D
The four-factor structure of CES-D was similar to the original one proposed by Radloff [7], except that item 20 (“get going”) loaded in the depressed affect factor but not the somatic symptom factor in our Chinese patients with type 2 diabetes. These subtle variations in factor structure might be due to racial/ethnic or culture differences [25, 26]. In a meta-analysis on CES-D factor structure, people from different cultures might conceptualize depressive symptoms in different ways. Besides, the analytic methods used to load various factors, e.g. confirmatory factor analysis (CFA) versus EFA, could also influence the results of CES-D factor structure [25]. In another study involving 138 Hong Kong Chinese married couples (aged 22–70 years) which used CFA to validate the CES-D, the authors reported a 2-factor model (depression and interpersonal problems) [11] compared to the 4-factor model in our study.
In this study, the positive affect factor did not correlate with the other three factors (inter-factor correlation ranged from −0.05 to 0.04), contrary to many other studies in United States (ranged from 0.31 to 0.85) [27–29]. Traditional Chinese and Oriental culture emphasizes modesty, silence, stoicism, and emotional restraint. These beliefs might influence our patients not to endorse positively-stated items in the CES-D (e.g. “I was happy”, the score was reversed during calculation) despite having other negative symptoms, leading to an elevated score on positive affect problems. Our results are consistent with other studies in Chinese subjects [14, 30]. In a study of 168 community-dwelling American Chinese women, native Chinese speakers or Chinese immigrants were 50 % less likely to endorse the four positive items than English speakers or subjects born in United States, albeit having similar mean scores for the other 16 items [14]. This discrepancy has also been reported in other studies probably due to culture influences [30, 31]. American Koreans who were less acculturated to American views were less likely to endorse positive CES-D items than the more acculturated ones [32]. Compared to Americans, Japanese had spuriously lower ratings of positive items whereas the scores for the negative items were comparable between the two groups [30]. Taken together, these cultural or ethnic factors seemed to affect responses to the four positive affect items which might compromise the validity of CES-D. In support of this, the performance of CES-D improved upon exclusion of these 4 positive affect items suggesting that the 16 item CES-D is a better screening tool for depression than the 20 item CES-D, at least in Chinese subjects.
Higher cut-off point of CES-D in Chinese
Our results suggested an optimal cutoff value of 21, which is higher than the widely used cutoff value of 16 (sensitivity 91.3 %, specificity 60.8 %). Different study designs, settings and populations may contribute to differences in the performance of different cutoff values [33]. Consistently, in studies involving Chinese subjects, the latter tended to have higher CES-D cut-off points in depression screening than Caucasian populations [12, 22, 34]. For example, a CES-D validation study in Hong Kong involving 398 elderly individuals reported an optimal cutoff value of 22 (75 % sensitivity and 51 % specificity), while the conventional cutoff value of 16 had high sensitivity (92 %) but poor specificity (30 %) in detecting depression [12]. In another CES-D validation study in Singaporean Chinese adults using the Schedule for Clinical Assessment in Neuropsychiatry (SCAN) as criterion, the cutoff value of 16 had specificity of 67.6 % only, although the sensitivity was high (96.8 %) [22].
Comparison of CES-D to PHQ-9
In line with other studies [35, 36], our findings showed that both PHQ-9 and CES-D showed similar psychometric performances with respect to the internal consistency, test-retest reliability, and diagnostic validity against MINI-based diagnostic interview. Using the standard cutoff point (≥16), CES-D identified more than 30 % of patients with possible depression, much higher than using the standard cutoff point ≥10 of PHQ-9 (9.0 %, Cohen’s Kappa = 0.32). This finding is consistent with a recent study in systemic sclerosis where Milette and colleagues compared the PHQ-9 to CES-D in 566 patients with systemic sclerosis and found CES-D ≥ 16 identified 34.3 % patients with possible depression, while PHQ-9 ≥ 10 identified 20.7 % (Cohen’s Kappa = 0.49), suggesting that PHQ 9 is more specifically indicative of depression while the CES-D is capturing some of the depression and some of the other emotions present in serious illness.
Compared to conventional cutoff, the CES-D had a higher cutoff value while PHQ-9 had a lower cutoff value. This disparity might be partly explained by the content differences of the two tools. The PHQ-9 is constructed on the basis of the DSM-IV diagnostic criteria for clinical depression; while the CES-D measures depressive symptomatology with emphasis on the affective component and depressed mood [7]. Besides, the PHQ-9 asks about the frequency of depressive symptom in the past two weeks, while the CES-D asks about the frequency of symptoms in the past one week. The shorter duration covered by the CES-D may have captured short-term symptoms including acute hassles and stressors which might not reflect true depression. Besides, since Chinese people tend to give negative response to positive affect items, this may also lead to a higher score of CES-D in our study population.
While the use of PHQ-9 (≥7 or ≥ 10) identified patients with significant depressive symptoms which were associated with poor glycemic control and increased use of lipid-lowering drugs, such association was not found in patients with depressive symptoms detected by CES-D, suggesting that CES-D might identify patients with slightly different profile than those identified by PHQ-9. When evaluating other studies that have examined the association between depression and glycemic control, findings have been mixed where some studies have found an association between depressive symptoms and poor glycemic control whereas others have not [37, 38]. Our results raise the possibility that this inconsistency might be in part due to the different tools used in capturing depressive symptoms. Here, negative emotions can be heterogeneous and complex with different combinations of symptoms which may have common but also distinct biological pathways. This complexity may also explain the inconsistency regarding the associations between depression and glycemic control.
Although theoretically, the CES-D and the PHQ-9 may complement one another to detect depression and negative emotions, this may be not feasible in real-world practice given increased time of testing and redundancy of the items. However, since the two tools cover different time frame (1 week versus 2 weeks) with slightly different attributes (eg. interpersonal problems in CES-D and suicidal tendency in PHQ-9) and complementary aspects as revealed by the Venn diagram, it might be useful to explore the possibility of selecting items from both tools to generate a better depression screening algorithm in future studies.
Both PHQ-9 and CES-D had different cutoff values with PHQ-9 being a better tool than the CES-D in identifying Chinese type 2 diabetic patients with both depression and poor glycemic control. These results highlighted the importance of validating screening tools in local settings. The differences in associations of depression with glycemic control between the two instruments also support the syndromic nature of depression due to possible subphenotypes and aetiologies with variable responses to different screening tools. In this study, we also observed a high suicide risk in these patients that 5 % had suicide ideation using the PHQ-9. Consistently, a study in Italy found more severe suicide ideation in patients with diabetes and its severity was closely associated with older age, polytherapy and lower self-efficacy [39]. Therefore, these results warrant depression screening and subsequent emotional support for patients with diabetes.
Limitations
Although the study population comprised of self-referred patients and those referred by family clinics, the majority of them were attending hospital-based specialist out-patient clinics, who might have more severe disease, longer disease duration, multiple co-morbidities, and more complicated drug regimens than the typical outpatient community-based patients. Thus, our cohort might not fairly represent the general Hong Kong Chinese population with type 2 diabetes. Furthermore, this study was performed in Hong Kong, which has a different health care system and different cultural nuances than that of Mainland China, so caution must be taken when making generalizations to the wider Chinese population. Finally, only a subset of our cohort had a diagnostic interview as the criteria validation measure, so further studies with larger sample sizes are required to confirm these findings.
Conclusions
In summary, the CES-D is a validated tool for detecting major depression in Chinese patients with type 2 diabetes. The improvement in performance after excluding items on positive affect might reflect cultural differences. Between CES-D and PHQ-9, the latter is a preferred screening tool due to its longer coverage period, fewer items associated less administering time, and ability to identify depressed patients with poor metabolic control. The different cutoff values in our population also emphasize the importance of calibrating these tools in different patient groups and settings.
Acknowledgement
We gratefully acknowledge Dr. Larry Cimino of the Dialogue for Diabetes and Depression, Ms. Rebecca Wong, nurse consultant, Ms. Harriet Chung, nurse specialist and all clinical staff concerned at the Prince of Wales Hospital for their contributions.
This study was supported by the European Foundation for Study of Diabetes, the Chinese Diabetes Society, Lilly Foundation, Asia Diabetes Foundation and Liao Wun Yuk Diabetes Memorial Fund, the latter under the Chinese University of Hong Kong.
Footnotes
Competing interests
J.C. is the Chief Executive Officer of the Asia Diabetes Foundation on a probono basis. She has received honorarium for consultancy or giving lectures from Bayer, Boehringer Ingelheim, Daiichi-Sankyo, Eli-Lilly, GlaxoSmithKline, Merck Sharp & Dohme, Merck Serono, Pfizer, Astra Zeneca, Sanofi, Novo-nordisk and/or Bristol-Myers Squibb. YKW has received sponsorship from Lundbeck Export A/S, Servier Hong Kong Ltd and Celki Medical Company and was a part-time paid consultant for Renascence Therapeutics Limited. A.K. has received honorarium for consultancy or giving lectures from Nestle Nutrition Institute, Merck Serono, Pfizer, Eli Lilly, Roche, Sanofi, Jassen and Astra Zeneca. N.S. has been given honoraria for participating in scientific meetings organized by Lilly, Servier, and Takeda, and his association has received unrestricted grants from Lilly, Lundbeck and Pfizer. No other potential conflicts of interest relevant to this article were reported.
Authors’ contribution
YZ researched data, contributed to the discussion, and wrote, reviewed, and edited the manuscript. RT contributed to the discussion, and wrote, reviewed, and edited the manuscript. ML, SL and HN researched data, contributed to the discussion and reviewed the manuscript. RY, AK, AL, and RO contributed to the discussion, reviewed, and edited the manuscript. YKW, NS and JC conceptualized the study, contributed to the discussion, and reviewed and edited the manuscript. JC finalized the manuscript. All authors read and approved the final manuscript.
Contributor Information
Yuying Zhang, Email: jennyzhang@adf.org.hk.
Rose Z W Ting, Email: pigpig350@gmail.com.
Marco H B Lam, Email: lhb352@ha.org.hk.
Siu-Ping Lam, Email: joycelam@cuhk.edu.hk.
Roseanne O. Yeung, Email: roseanne.yeung@gmail.com
Hairong Nan, Email: nan.fang08@gmail.com.
Risa Ozaki, Email: risaozaki@cuhk.edu.hk.
Andrea O Y Luk, Email: andrealuk@cuhk.edu.hk.
Alice P S Kong, Email: alicekong@cuhk.edu.hk.
Yun-Kwok Wing, Email: ykwing@cuhk.edu.hk.
Norman Sartorius, Email: sartorius@normansartorius.com.
Juliana C N Chan, Phone: (852) 2632 3138, Email: jchan@cuhk.edu.hk.
References
- 1.Chan JC, Cho NH, Tajima N, Shaw J. Diabetes in the Western Pacific Region-Past, present and future. Diabetes Res Clin Pract. 2013;103(2):244–55. doi: 10.1016/j.diabres.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 2.Hidaka BH. Depression as a disease of modernity: explanations for increasing prevalence. J Affect Disord. 2012;140(3):205–214. doi: 10.1016/j.jad.2011.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Molosankwe I, Patel A, Jose Gagliardino J, Knapp M, McDaid D. Economic aspects of the association between diabetes and depression: a systematic review. J Affect Disord. 2012;142(Suppl):S42–55. doi: 10.1016/S0165-0327(12)70008-3. [DOI] [PubMed] [Google Scholar]
- 4.Egede LE, Ellis C. Diabetes and depression: global perspectives. Diabetes Res Clin Pract. 2010;87(3):302–312. doi: 10.1016/j.diabres.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 5.American Diabetes Association. Standards of medical care in diabetes--2014. Diabetes Care. 2014;37 Suppl 1:S14–80. [DOI] [PubMed]
- 6.International Diabetes Federation. Global Guideline for Type 2 Diabetes, 2012.
- 7.Radloff LS: The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement, 1(3):385–481
- 8.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen TM, Huang FY, Chang C, Chung H. Using the PHQ-9 for depression screening and treatment monitoring for Chinese Americans in primary care. Psychiatr Serv. 2006;57(7):976–981. doi: 10.1176/ps.2006.57.7.976. [DOI] [PubMed] [Google Scholar]
- 10.Yu X, Tam WW, Wong PT, Lam TH, Stewart SM. The Patient Health Questionnaire-9 for measuring depressive symptoms among the general population in Hong Kong. Compr Psychiatry. 2012;53(1):95–102. doi: 10.1016/j.comppsych.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Cheung CK, Bagley C. Validating an American scale in Hong Kong: the Center for Epidemiological Studies Depression Scale (CES-D) J Psychol. 1998;132(2):169–186. doi: 10.1080/00223989809599157. [DOI] [PubMed] [Google Scholar]
- 12.Cheng ST, Chan AC. The center for epidemiologic studies depression scale in older chinese: thresholds for long and short forms. Int J Geriatr Psychiatry. 2005;20(5):465–470. doi: 10.1002/gps.1314. [DOI] [PubMed] [Google Scholar]
- 13.Manea L, Gilbody S, McMillan D. Optimal cut-off score for diagnosing depression with the Patient Health Questionnaire (PHQ-9): a meta-analysis. CMAJ. 2012;184(3):E191–196. doi: 10.1503/cmaj.110829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Z, Hicks MH. The CES-D in Chinese American women: construct validity, diagnostic validity for major depression, and cultural response bias. Psychiatry Res. 2010;175(3):227–232. doi: 10.1016/j.psychres.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Demirchyan A, Petrosyan V, Thompson ME. Psychometric value of the Center for Epidemiologic Studies Depression (CES-D) scale for screening of depressive symptoms in Armenian population. J Affect Disord. 2011;133(3):489–498. doi: 10.1016/j.jad.2011.04.042. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Ting R, Lam M, Lam J, Nan H, Yeung R, et al. Measuring depressive symptoms using the Patient Health Questionnaire-9 in Hong Kong Chinese subjects with type 2 diabetes. J Affect Disord. 2013;151(2):660–666. doi: 10.1016/j.jad.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 17.So WY, Raboca J, Sobrepena L, Yoon KH, Deerochanawong C, Ho LT, et al. Comprehensive risk assessments of diabetic patients from seven Asian countries: The Joint Asia Diabetes Evaluation (JADE) program. J Diabetes. 2011;3(2):109–118. doi: 10.1111/j.1753-0407.2011.00115.x. [DOI] [PubMed] [Google Scholar]
- 18.Ko GT, So WY, Tong PC, Le Coguiec F, Kerr D, Lyubomirsky G, et al. From design to implementation--the Joint Asia Diabetes Evaluation (JADE) program: a descriptive report of an electronic web-based diabetes management program. BMC medical informatics and decision making. 2010;10:26. doi: 10.1186/1472-6947-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan JC, So W, Ko G, Tong P, Yang X, Ma R, et al. The Joint Asia Diabetes Evaluation (JADE) Program: a web-based program to translate evidence to clinical practice in Type 2 diabetes. Diabet Med. 2009;26(7):693–9. [DOI] [PubMed]
- 20.Chan JC, Sui Y, Oldenburg B, Zhang Y, Chung HH, Goggins W, et al. Effects of telephone-based peer support in patients with type 2 diabetes mellitus receiving integrated care: a randomized clinical trial. JAMA Internal Med. 2014;174(6):972–981. doi: 10.1001/jamainternmed.2014.655. [DOI] [PubMed] [Google Scholar]
- 21.Lewinsohn PM, Seeley JR, Roberts RE, Allen NB. Center for Epidemiologic Studies Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychology and aging. 1997;12(2):277–287. doi: 10.1037/0882-7974.12.2.277. [DOI] [PubMed] [Google Scholar]
- 22.Stahl D, Sum CF, Lum SS, Liow PH, Chan YH, Verma S, et al. Screening for depressive symptoms: validation of the center for epidemiologic studies depression scale (CES-D) in a multiethnic group of patients with diabetes in Singapore. Diabetes Care. 2008;31(6):1118–1119. doi: 10.2337/dc07-2019. [DOI] [PubMed] [Google Scholar]
- 23.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiat. 1998;59 Suppl 20:22–33. [PubMed] [Google Scholar]
- 24.Chan JW, Lam SP, Li SX, Yu MW, Chan NY, Zhang J, et al. Eveningness and insomnia: independent risk factors of nonremission in major depressive disorder. Sleep. 2014;37(5):911–917. doi: 10.5665/sleep.3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim G, Decoster J, Huang CH, Chiriboga DA. Race/ethnicity and the factor structure of the center for epidemiologic studies depression scale: a meta-analysis. Cultur Diver Ethnic Minor Psychol. 2011;17(4):381–396. doi: 10.1037/a0025434. [DOI] [PubMed] [Google Scholar]
- 26.Williams CD, Taylor TR, Makambi K, Harrell J, Palmer JR, Rosenberg L, et al. CES-D four-factor structure is confirmed, but not invariant, in a large cohort of African American women. Psychiat Res. 2007;150(2):173–180. doi: 10.1016/j.psychres.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 27.Makambi KH, Williams CD, Taylor TR, Rosenberg L, Adams-Campbell LL. An assessment of the CES-D scale factor structure in black women: the black women’s health study. Psychiat Res. 2009;168(2):163–170. doi: 10.1016/j.psychres.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong YL. Measurement properties of the center for epidemiologic studies-depression scale in a homeless population. Psychol Assess. 2000;12(1):69–76. doi: 10.1037/1040-3590.12.1.69. [DOI] [PubMed] [Google Scholar]
- 29.Edwards MC, Cheavens JS, Heiy JE, Cukrowicz KC. A reexamination of the factor structure of the Center for Epidemiologic Studies Depression Scale: is a one-factor model plausible? Psychol Assess. 2010;22(3):711–715. doi: 10.1037/a0019917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iwata N, Roberts CR, Kawakami N. Japan-U.S. comparison of responses to depression scale items among adult workers. Psychiatry Res. 1995;58(3):237–245. doi: 10.1016/0165-1781(95)02734-E. [DOI] [PubMed] [Google Scholar]
- 31.Cho MJ, Kim KH. Use of the Center for Epidemiologic Studies Depression (CES-D) scale in Korea. J Nerv Ment Dis. 1998;186(5):304–310. doi: 10.1097/00005053-199805000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Jang Y, Kim G, Chiriboga D. Acculturation and manifestation of depressive symptoms among Korean-American older adults. Aging Ment Health. 2005;9(6):500–507. doi: 10.1080/13607860500193021. [DOI] [PubMed] [Google Scholar]
- 33.Hermanns N, Kulzer B, Krichbaum M, Kubiak T, Haak T. How to screen for depression and emotional problems in patients with diabetes: comparison of screening characteristics of depression questionnaires, measurement of diabetes-specific emotional problems and standard clinical assessment. Diabetologia. 2006;49(3):469–477. doi: 10.1007/s00125-005-0094-2. [DOI] [PubMed] [Google Scholar]
- 34.Yang HJ, Soong WT, Kuo PH, Chang HL, Chen WJ. Using the CES-D in a two-phase survey for depressive disorders among nonreferred adolescents in Taipei: a stratum-specific likelihood ratio analysis. J Affect Disord. 2004;82(3):419–430. doi: 10.1016/j.jad.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 35.Khamseh ME, Baradaran HR, Javanbakht A, Mirghorbani M, Yadollahi Z, Malek M. Comparison of the CES-D and PHQ-9 depression scales in people with type 2 diabetes in Tehran, Iran. BMC Psychiatry. 2011;11:61. doi: 10.1186/1471-244X-11-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Milette K, Hudson M, Baron M, Thombs BD, Canadian Scleroderma Research G Comparison of the PHQ-9 and CES-D depression scales in systemic sclerosis: internal consistency reliability, convergent validity and clinical correlates. Rheumatology. 2010;49(4):789–796. doi: 10.1093/rheumatology/kep443. [DOI] [PubMed] [Google Scholar]
- 37.Lustman PJ, Anderson RJ, Freedland KE, de Groot M, Carney RM, Clouse RE. Depression and poor glycemic control: a meta-analytic review of the literature. Diabetes Care. 2000;23(7):934–942. doi: 10.2337/diacare.23.7.934. [DOI] [PubMed] [Google Scholar]
- 38.Waitzfelder B, Gerzoff RB, Karter AJ, Crystal S, Bair MJ, Ettner SL, et al. Correlates of depression among people with diabetes: the Translating Research Into Action for Diabetes (TRIAD) study. Primary Care Diabetes. 2010;4(4):215–222. doi: 10.1016/j.pcd.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pompili M, Lester D, Innamorati M, De Pisa E, Amore M, Ferrara C, et al. Quality of life and suicide risk in patients with diabetes mellitus. Psychosomatics. 2009;50(1):16–23. doi: 10.1176/appi.psy.50.1.16. [DOI] [PubMed] [Google Scholar]


