Abstract
Background
Harmful practices in the management of childhood diarrhea are associated with negative health outcomes, and conflict with WHO treatment guidelines. These practices include restriction of fluids, breast milk and/or food intake during diarrhea episodes, and incorrect use of modern medicines. We conducted a systematic review of English-language literature published since 1990 to assess the documented prevalence of these four harmful practices, and beliefs, motivations, and contextual factors associated with harmful practices in low- and middle-income countries.
Methods
We electronically searched PubMed, Embase, Ovid Global Health, and the WHO Global Health Library. Publications reporting the prevalence or substantive findings on beliefs, motivations, or context related to at least one of the four harmful practices were included, regardless of study design or representativeness of the sample population.
Results
Of the 114 articles included in the review, 79 reported the prevalence of at least one harmful practice and 35 studies reported on beliefs, motivations, or context for harmful practices. Most studies relied on sub-national population samples and many were limited to small sample sizes. Study design, study population, and definition of harmful practices varied across studies. Reported prevalence of harmful practices varied greatly across study populations, and we were unable to identify clearly defined patterns across regions, countries, or time periods. Caregivers reported that diarrhea management practices were based on the advice of others (health workers, relatives, community members), as well as their own observations or understanding of the efficacy of certain treatments for diarrhea. Others reported following traditionally held beliefs on the causes and cures for specific diarrheal diseases.
Conclusions
Available evidence suggests that harmful practices in diarrhea treatment are common in some countries with a high burden of diarrhea-related mortality. These practices can reduce correct management of diarrheal disease in children and result in treatment failure, sustained nutritional deficits, and increased diarrhea mortality. The lack of consistency in sampling, measurement, and reporting identified in this literature review highlights the need to document harmful practices using standard methods of measurement and reporting for the continued reduction of diarrhea mortality.
Electronic supplementary material
The online version of this article (doi:10.1186/s12889-015-2127-1) contains supplementary material, which is available to authorized users.
Background
Diarrheal disease is a leading cause of mortality in children under five, resulting in around 750,000 deaths each year [1]. The WHO recommends first line management of diarrhea in children under five with continued feeding, increased fluids, and supplemental zinc for 10–14 days to prevent dehydration. In addition, the WHO guidelines state that children exhibiting non-severe dehydration should “receive oral rehydration therapy (ORT) with ORS solution in a health facility”. Antimicrobials are recommended only for the treatment of bloody diarrhea or suspected cholera with severe dehydration [2]. The full guidelines, which have evolved over time, are available at http://www.who.int/entity/maternal_child_adolescent/documents/9241593180/en/index.html.
For decades, health initiatives have targeted the expansion of ORS and ORT, including the UNICEF Growth Monitoring, Oral Rehydration, Breastfeeding and Immunization (GOBI) initiative, the USAID/CDC Africa Child Survival Initiative - Combatting Childhood Communicable Diseases (ACSI-CCCD), and the WHO Integrated Management of Childhood Illness (IMCI) initiative. Despite these efforts, a shift in global attention away from diarrhea management seems likely to have contributed to slowing – and even reversals – in progress toward full coverage for ORT [3, 4].
Many fewer programs have specifically targeted non-adherence to other recommended diarrhea management practices, such as the restriction of fluids, breast milk and/or food intake during diarrhea episodes, and incorrect use of modern medicines. All four of these practices are associated with negative outcomes and conflict with WHO treatment guidelines. Curtailment of fluids and restriction of feeding during diarrhea can increase the risk of dehydration, reduce nutritional intake, and potentially inhibit child growth and development. The use of antibiotics and other medications is appropriate only in the treatment of cholera or dysenteric diarrhea in children. Antidiarrheal drugs and some antiemetics not only have no benefit in diarrhea treatment, but may also cause serious, even life-threatening side effects in children [2]. We have referred to these as “harmful practices” from this point forward, understanding that under some circumstances these practices may not be detrimental.
This review summarizes existing literature on harmful practices in diarrhea case management in children under five years of age, including fluid and breastfeeding curtailment, food restriction, and inappropriate use of medications for diarrhea management in children in low- and middle-income countries. The primary objectives of the review are to:
Determine the documented prevalence of these four harmful practices across low- and middle-income populations, as reported in various studies since 1990;
Describe how these practices have been examined and reported on previously;
Explore beliefs, motivations, and contextual factors associated with harmful practices as reported through both quantitative and qualitative studies; and
Highlight associations between these harmful practices and other characteristics of the episode, child, caregiver, and household.
Findings from this review will identify critical next steps to address harmful practices in diarrhea management and ultimately improve child survival.
Methods
We searched PubMed, Embase, Ovid Global Health, and the WHO Global Health Library in September 2013. Papers were identified that included variations on the combination of the following terms within the publication’s title or abstract or as a keyword: 1) diarrhea; 2) low- and middle-income country; and one or more terms related to 3) a harmful practice or general management of diarrhea. Search terms were developed in PubMed (see Additional file 1) and translated for the three other databases. Publications were restricted to English-language articles published after 1990.
Quantitative articles were included if the paper reported the prevalence of at least one of the four harmful practices associated with caregiver management of diarrhea in children under the age of five, regardless of study design or representativeness of the sample population. Qualitative articles, or quantitative articles not meeting the quantitative inclusion criteria, were included if they presented substantive findings on beliefs, motivations, or context related to at least one of the four practices in caregiver management of childhood diarrhea. Publications were excluded if they exclusively reported data collected prior to 1990, exclusively reported provider practices, reported findings post-intervention only, or did not specifically focus on treatment of children under 5 years of age. Due to the variety of study designs included in the review, study quality was not formally assessed, because multiple quality assessment frameworks would have been required.
Data extraction was completed by the first author (EC). For all studies, information on the study design, study population, and sample size was extracted. For studies reporting prevalence of practices, data were extracted on the definition of the practice measure, the reported prevalence of the practice, and variation in the practice by other factors (reported as stratified prevalence or odds ratio). For non-prevalence studies, data were extracted related to beliefs, motivations, or context directly related to one or more of the harmful practices and then classified by common themes.
We summarize the results for each of the four harmful practices in the results section of the manuscript. For each practice, we: (1) describe how the practice was defined and measured in these studies; (2) summarize reported findings on prevalence, including variations by characteristics of the diarrhea episode, child, caregiver, and household; and (3) report on beliefs, motivations, and contextual factors investigated and relevant results.
Results
The initial search yielded 2,266 articles in Pubmed, 2,512 articles in Embase, 1,512 articles in Ovid Global Health, and 1,890 articles in the WHO Global Health Library. After removing duplicates, 4,270 unique articles remained. Title and abstract review and full article review were conducted by the first author (EC). After reviewing titles and abstracts, 294 articles were identified for full article review. Based on a review of the full article, 157 articles did not meet the inclusion criteria and a full text copy of 23 manuscripts could not be located. In total, 114 publications met the inclusion criteria and were included in the review (Fig. 1). Of the 79 studies reporting the prevalence of at least one harmful practice, 54 studies utilized a population-based cross-sectional sample (3 nationally representative), 12 studies used a non-cross-sectional design but included a representative population sample, and 13 studies employed a non-representative sample. Of the 35 studies reporting on beliefs, motivations, or context for harmful practices, 9 studies used exclusively qualitative methods, 8 studies used mixed-methods, and 18 studies used exclusively quantitative methods (12 with a representative sample, 6 with a non-representative sample). Although there have been summaries of relevant Demographic and Health Survey (DHS) and Multiple Indicator Cluster Survey (MICS) findings [5, 6], we were unable to identify any country-specific secondary analyses on this topic.
Fig. 1.
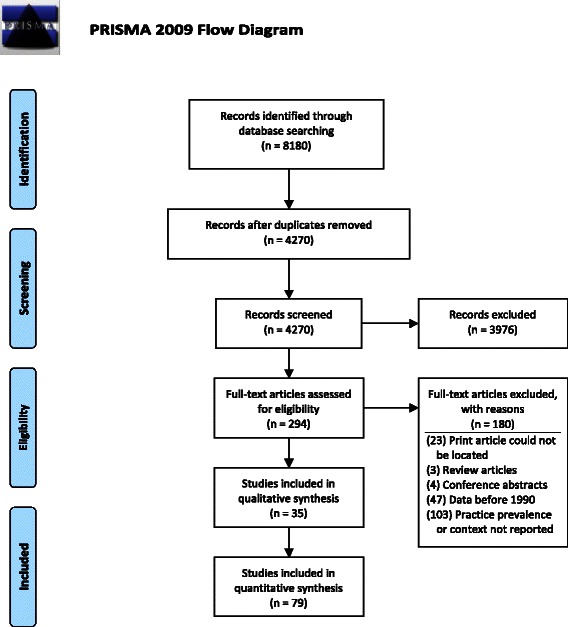
Flow of studies considered in the systematic review
Study characteristics
The publication dates of the 114 studies included in the review were relatively evenly distributed over the period from 1990 to 2013, with publications clustering slightly in the early 1990s and late 2000s/early 2010s. The majority of studies were conducted in South Asia and sub-Saharan Africa (Fig. 2). The number of publications reporting on the prevalence of each of the four practices varied, with the highest proportion reporting on inappropriate medication use (70 %), followed in order of frequency by food restriction (56 %), curtailment of fluids other than breast milk (53 %), and breastfeeding restriction (37 %).
Fig. 2.
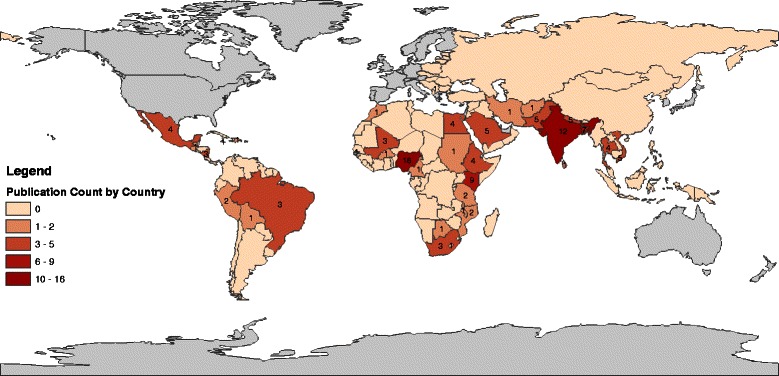
Map with number of studies by country
Respondents in the majority of prevalence studies were caregivers of children under 5 years of age, although some studies interviewed mothers exclusively. The age of children referenced for the practice also varied, with the majority of studies referencing children under 5 years of age. The definition of the diarrhea reference episode also varied, ranging from diarrhea in the past 24 h to the most recent diarrhea event, although the most common reference period was the previous two weeks.
Fluid curtailment
The measurement of fluid intake, and prevalence estimates, varied widely across studies (Table 1, Column 4). Many studies differed in their definition or failed to specify if fluid restriction included or excluded breastfeeding or assessed amount of fluid offered versus consumed. The reported practice of curtailing fluids during a recent episode of diarrhea ranged from as low as 11 % of caregivers in Mirzapur, Bangladesh [7] to over 80 % of caregivers in Kenya’s Nyanza province [8]. Where specified by the study authors, the practice of stopping all fluids was uncommon, generally reported in fewer than 10 % of episodes.
Table 1.
Prevalence of harmful practices by region and country
| Author, Year [reference] | Country | Study design, study population, number of participants | Proportion restricting fluid | Proportion restricting breastfeeding | Proportion restricting food | Proportion using drugs | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Americas | ||||||||||
| Emond et al., 2002 [84] | Brazil | Cross-sectional baseline survey preceding intervention, Northeast Brazil 1997, Caregivers of children with diarrhea in the previous 2 days, n = 922 | Generally give medicines other than ORS | 7 | ||||||
| Strina et al., 2005 [63] | Brazil | Longitudinal survey, Salvador 1997–1999, Caregivers of children ≤36 months with diarrhea in previous 2 weeks, n = 2403 episodes | Gave industrial medicines | 40.9 | ||||||
| Gave industrial medicines & home preparation | 2.7 | |||||||||
| Webb et al., 2010 [85] | Guatemala | Longitudinal survey, Population of Spanish-Mayan Descent 1996–1999, Caregivers of children <36 months with diarrhea in previous 19 days, n = 466 | Stopped or less fluida | 55 | Stopped or less breastfeedingb | 26.6 | Stopped or less food | 15 | ||
| Bachrach et al., 2002 [21] | Jamaica | Case-control hospital based survey, Kingston 2007, Caregivers of children <5 years presenting at hospital, n = 215 total, 117 gastroenteritis cases | Child presenting with gastroenteritis: Gave antidiarrheal/ antimotility drug before coming to hospital | 36 | ||||||
| Martinez et al., 1991 [52] | Mexico | Cross-sectional survey, Rural Highlands of Central Mexico (year not specified), Caregivers of children <5 years, diarrhea episode reference unclear, n = 38 | Give pill as first treatment for diarrhea | 47 | ||||||
| Give over-the-counter drug to child | 53 | |||||||||
| Perez-Cuevas et al., 1996 [40] | Mexico | Cross-sectional survey, Tiaxcala (year not specified), Caregivers of children <5 years with diarrhea in previous 2 weeks, n = 747 | “Withheld” non-breast milk | 27.2 | Stopped breastfeedingb | 12.2 | Stopped or reduced food other than milk | 9.1 | Treated with any drug | 35.2 |
| No liquids given | 3 | Any dietary restriction | 36.6 | |||||||
| Martinez et al., 1998 [86] | Mexico | Cross-section of ethnographic study participants, 3 States (year not specified), Caregivers of children <5 years in reference to most recent diarrhea episode, n = 186 | Gave antimicrobial | 37.1 | ||||||
| Gave antidiarrheal | 28 | |||||||||
| Gave antipyretic | 18 | |||||||||
| Smith et al., 1993 [51] | Nicaragua | Cross-sectional survey, Rural Pacific Coastal Plain (year not specified), Caregivers of infants, diarrhea episode reference unclear, n = 70 | Stopped breastfeeding (among those who reported changing feeding)b | 4 | Did not give solid foods (among those who reported changing feeding)c | 13 | ||||
| Gorter et al., 1995 [79] | Nicaragua | Cross-section of ethnographic study participants, Rural Pacific Coastal Plain 1990, Caregivers of children <5 years with diarrhea in previous 2 weeks, n = 216 | Gave antibiotic | 22 | ||||||
| Gave parasite medicine | 19 | |||||||||
| Gave laxative | 6 | |||||||||
| Vazquez et al., 2002 [33] | Nicaragua | Cross-sectional survey, North of Central Region 1990, Caregivers of children <5 years with diarrhea in previous 2 weeks, n = 187 | Child ate less than usual | 43.5 | Gave any pharmaceutical | 60 | ||||
| Kristiansson et al., 2009 [87] | Peru | Cross-sectional survey, Yurimaguas and Moyobamba Departments 2002, Caregivers of children 6–72 months with illness in previous 2 weeks, n = 780 | Antibiotic use reported by wealth quintile only | |||||||
| Europe | ||||||||||
| Berisha et al., 2009 [16] | Kosovo | Cross-sectional survey, Kosovo 2005, Mothers of children <5 years in reference to most recent diarrhea episode, n = 107 | Less fluid or nonea | 62.6 | Stopped or reduced amount of food or breastfeeding | 43.9 | ||||
| Same fluidsa | 19.6 | Same amount of food or breastfeeding | 48.6 | |||||||
| Eastern Mediterranean | ||||||||||
| Azim et al., 1993 [37] | Afghanistan | Cross-sectional study, Paktika Province 1991, Caregivers of children <5 years with diarrhea in previous 2 weeks, n = 87 | Same or less fluidd | 43.7 | Stopped breastfeedingb | 5.9 | Stopped or less food | 33.5 | Gave any drug | 66 |
| Langsten et al., 1994 [88] | Egypt | Longitudinal survey, Lower Egypt 1990, Caregivers of children <5 years with diarrhea in previous 2 weeks, n = 4900 | Stopped fluids other than BF and milkd | 2.8 | Stopped breastfeedingb | 2.5 | Stopped food | 5.8 | ||
| Reduced other fluidsd | 10.9 | Decreased breastfeedingb | 11.9 | Reduced food | 22.7 | |||||
| Reduced non-breast milkd | 15.3 | |||||||||
| Stopped non-breast milkd | 9.9 | |||||||||
| Langsten et al., 1995 [57] | Egypt | Longitudinal survey, Lower Egypt 1990–1991, Caregivers of children <5 years with diarrhea in previous 2 weeks, n = 4900 | Among acute non-dysenteric cases: Used antibiotics | 46.5 | ||||||
| Among acute non-dysenteric cases: Used antibiotics only | 3.2 | |||||||||
| Among acute non-dysenteric cases: Used other medicine | 63.3 | |||||||||
| Among acute non-dysenteric cases: Used other medicine only | 18.6 | |||||||||
| Among all cases: Used antibiotics | 45.6 | |||||||||
| Among all cases: Used antibiotics only | 3.4 | |||||||||
| Among all cases: Used other medicine | 63.0 | |||||||||
| Among all cases: Used other medicine only | 19.3 | |||||||||
| Jousilahti et al., 1992 [75] | Egypt | Cross-sectional cluster study, Lower Egypt 1992, Caregivers of children <5 years with diarrhea in previous 24 h, n = 766 | Same or less fluidd | 75.6 | Stopped breastfeedingb | 3.7 | Stopped or less solid or semi-solid food | 30.2 | Gave any drug | 54.2 |
| Gave drug and ORS | 17.6 | |||||||||
| Gave drug but no ORS | 36.5 | |||||||||
| El-GIlany et al., 2005 [62] | Egypt | Cross-sectional study, Dakahalia 2002–2003, Caregivers of children <5 years with diarrhea in previous 2 weeks, n = 1052 | Same or less fluide | 29 | Stopped feedinge | 12.7 | Gave any drug | 74.7 | ||
| Among those receiving a drug: | 36.9 | |||||||||
| Antibioticf | 73.9 | |||||||||
| Antidiarrhealf | 73.9 | |||||||||
| Antiemeticf | 16.7 | |||||||||
| Antiprotozoalf | 5.7 | |||||||||
| Antipyreticf | 9.6 | |||||||||
| Antispasmodicf | 1.7 | |||||||||
| Amini-Ranjbar et al., 2007 [53] | Iran | Cross-sectional study, Kerman 2005, Caregivers of children 6–24 months with diarrhea in previous 2 months, n = 330 | Same or less breastfeedingg | 53.8 | Decreased solid foods | 20 | ||||
| WHO, 1991 [89] | Morocco | Cross-sectional study, National 1990, Caregivers of children <5 years with diarrhea in previous 24 h, n = 1066 | Same or less fluide | 70 | Gave any drug | 22.6 | ||||
| Morisky et al., 2002 [90] | Pakistan | Cross-sectional survey, National 1991–1992, Caregivers of children <2 years in reference to most recent episode, n = 5433 | Stop fluidse | 9.2 | Stopped food | 5.9 | Gave antibiotic | 11 | ||
| Reduced food | 6.2 | Gave other medicine | 9.2 | |||||||
| Quadri et al., 2013 [13] | Pakistan | Cross-sectional study (HUAS), Low-Income peri-urban area near Karachi 2007, Caregivers of children <5 years with diarrhea in previous 2 weeks, n = 959 | Did not offer “to drink” (at home before seeking care)e | 22.5 | Did not offer “to eat” (at home before seeking care)c | 44.1 | Gave antibiotic (at home) | 7.7 | ||
| Nasrin et al., 2013 [91] | Pakistan | Cross-sectional study (HUAS), Low-Income periurban area near Karachi 2007, Caregivers of children <5 years with diarrhea in previous 2 weeks, n = 349 | Offered same or less than usual to drink | 33.9 | Offered less than usual to eate | 33.6 | ||||
| Bella et al., 1994 [92] | Saudi Arabia | Case–control study, Eastern Province (year not specified), Caregiver of infant with diarrhea at time of survey versus caregiver of infant without diarrhea, n = 344 total, 68 cases | Stopped bottle feeding (among cases who were bottle feeding) | 35 | ||||||
| al-Mazrou et al., 1995 [93] | Saudi Arabia | Cross-sectional survey, National 1991, Caregivers of children <5 years with diarrhea in the previous 2 weeks, n = 6300 screened | Gave drugs | 40.7 | ||||||
| Gave IV fluids | 4.7 | |||||||||
| Bani et al., 2002 [12] | Saudi Arabia | Cross-sectional hospital based survey, Riyadh City (year not specified), Mothers of children ≤24 months with diarrhea attending primary health clinic, n = 237 | Less fluid givene | 11.3 | Less frequency of breastfeedingb | 24.6 | Less solid/semi-solid food given | 22.7 | ||
| Same fluid givene | 13.2 | Same frequency of breastfeedingb | 37.7 | Same solid/semi-solid food given | 22.6 | |||||
| Moawed et al., 2000 [20] | Saudi Arabia | Cross-sectional hospital based survey, Riyadh City 1998, Mothers of infants with diarrhea attending 2 pediatric hospital diarrhea centers, n = 300 | Stop breastfeeding or milk feeding | 62 | Gave unprescribed medicine | 38 | ||||
| Africa | ||||||||||
| Wilson et al., 2012 [11] | Burkina Faso | Cross-sectional survey, Orodara Health District 2012, Primary caregivers of children <27 months with diarrhea in previous 2 weeks, n = 1067 | Same or less fluide | 64.1 | Stopped breastfeedingb | 1.2 | Stopped or decreased feeding normal diete | 53.2 | Gave any drug other than ORS | 41.2 |
| Gave antibiotic or unidentified drug | 27.6 | |||||||||
| Olango et al., 1990 [17] | Ethiopia | Cross-sectional survey, Rural population in Wolayta district (year not specified), Mothers of children <5 years with diarrhea in previous 2 weeks, n = 619 | Stopped fluids (breastfed children separate category within fluid intake measure) | 8.6 | Stopped food (not weaned are additional category) | 15.2 | Gave injection | 40.8 | ||
| Decreased fluids | 42.3 | Decreased food | 54.4 | Gave tablets | 19.6 | |||||
| Same amount of fluids | 10.3 | Same amount of food | 10.2 | |||||||
| Ketsela et al., 1991 [94] | Ethiopia | Cross-sectional survey, Shewa Administrative Regions 1990, Mothers of children <5 years, diarrhea episode reference unclear, n = 750 | No fluidsa | 26.8 | No breastfeedingg | 3.5 | Gave less fluid thanc | 35.9 | ||
| Less than usual fluida | 31.4 | Gave same fluid as usualc | 38.2 | |||||||
| Same as usual fluida | 23.8 | Gave no foodc | 10.5 | |||||||
| Mash et al., 2003 [95] | Ethiopia | Cross-sectional survey, Oromia Region 1997, Caregivers of children <24 months with diarrhea in the previous fortnight, n = 111 | Stopped or decreased fluidsa | 47.7 | Stopped or decreased breastfeedingb | 67.6 | Stopped or less solid or semi-solid food | 67.6 | ||
| Mediratta et al., 2010 [9] | Ethiopia | Case–control hospital based study, Gondar 2007, Caregivers of children <5 years with diarrhea attending referral hospital, case n = 220 | Less of other fluidsa | 29 | Gave less breast milkb | 24 | “Withheld” food | 46 | ||
| Same amounta | 44 | Same amount of breast milkb | 34 | |||||||
| Saha et al., 2013 [96] | Gambia | Cross-sectional survey, Upper River Region 2009, Caregivers of children <5 years with diarrhea in the previous 2 weeks, n = 258 | Same or less fluide | 36.1 | Less than usual amount of food | 72.5 | Gave antimicrobial (at home) | 9.7 | ||
| Gave antimicrobial (among those seeking care at health facility) | 18.6 | |||||||||
| Gave injectable medicine (among those seeking care at health facility) | 43.7 | |||||||||
| Oyoo et al., 1991 [39] | Kenya | Cross-sectional survey, 6 sites across Kenya 1990, Caregivers of children <5 years with diarrhea in the previous 2 weeks, n = 23884 screened | Same or less fluide | 74 - 96 | Stopped breastfeedingb | 0-3.1 | Stopped feedinga | 19.5 - 53.3 | Gave any drug (range across clusters) | 25.9 - 47.1 |
| Mirza et al., 1997 [97] | Kenya | Longitudinal study with 24 h dietary recall, Kibera Slum 1989–1990, Caregivers of children 3–37 months with diarrhea in the previous 3 days, n = 1496 episodes | Gave less cow’s milk than before diarrhea | 28.7 | ||||||
| Othero et al., 2008 [7] | Kenya | Longitudinal study, Nyanza Province 2004–2006, Caregivers of children <5 years in reference to most recent episode, n = 927 | Offered nothing to drinke | 20.5 | Did not eat anything (among all children) | 39 | Gave anti-diarrheal drugs | 45.3 | ||
| Offered much lesse | 59.9 | |||||||||
| Offered somewhat lesse | 3.3 | |||||||||
| Offered samee | 5.3 | |||||||||
| Burton et al., 2011 [98] | Kenya | Cross-sectional survey, Rural Western Kenya 2005, Caregivers of children <5 years with diarrhea in the previous 2 weeks, n = 188 | Gave antibiotic | 62.4 | ||||||
| Gave antimalarial | 52.4 | |||||||||
| Gave IV fluid | 2.6 | |||||||||
| Olson et al., 2011 [42] | Kenya | Cross-sectional survey, Asembo (n = 371) and Kibera (n = 389) 2007, Caregivers of children <5 years with diarrhea in the previous 2 weeks | Asembo: Stopped fluids other than breast milk and porridge (among those giving fluids in week before illness) | 9 | Asembo: Stopped breastfeedingb | 5 | Asembo: Stopped porridge | 9 | Asembo: Gave oral medication (not ORS or herbs) | 77 |
| Kibera: Stopped fluids other than breast milk and porridge | 18 | Kibera: Stopped breastfeedingb | 16 | Kibera: Stopped porridge | 36 | Kibera: Gave oral medication (not ORS or herbs) | 81 | |||
| Asembo: Decreased fluidsh | 42 | Asembo: Decreased breastfeedingh | 32 | Asembo: Decreased porridgeh | 54 | Asembo: Gave injected medication | 24 | |||
| Kibera: Decreased fluidsh | 47 | Kibera: Decreased breastfeedingh | 47 | Kibera: Decreased porridgeh | 69 | Kibera: Gave injected medication | 28 | |||
| Asembo: Same fluidsh | 47 | Asembo: Same breastfeedingh | 59 | Asembo: Same porridgeh | 41 | Asembo: Gave IV fluids | 8 | |||
| Kibera: Same fluidsh | 22 | Kibera: Same breastfeedingh | 28 | Kibera: Same porridgeh | 18 | Kibera: Gave IV fluids | 7 | |||
| Asembo: Stopped soft or solid food | 10 | |||||||||
| Kibera: Stopped soft or solid food | 37 | |||||||||
| Asembo: Decreased solid foodh | 54 | |||||||||
| Kibera: Decreased solid food< | 70 | |||||||||
| Asembo: Same solid foodh | 41 | |||||||||
| Kibera: Same solid foodh | 23 | |||||||||
| Asembo: Stopped or Decreased feeding (including BF, porridge, solids) | 36 | |||||||||
| Kibera: Stopped or Decreased feeding (including BF, porridge, solids) | 54 | |||||||||
| Omore et al., 2013 [41] | Kenya | Cross-sectional survey (HUAS), Western Kenya 2007, Caregivers of children <5 years with diarrhea in the previous 2 weeks, n = 275 | Offered same amount to drink | 19 | Offered usual amount to eat | 16 | ||||
| Offered less to drink | 67 | Offered less to eat | 83 | |||||||
| Among those offering less: Somewhat less |
52 | Among offering less: Somewhat less |
33 | |||||||
| Much less | 38 | Much less | 30 | |||||||
| Nothing | 10 | Nothing | 37 | |||||||
| Nasrin et al., 2013 [91] | Kenya | Cross-sectional survey (HUAS), Western Kenya 2007, Caregivers of children <5 years with diarrhea in the previous 2 weeks, n = 275 | Gave leftover antibiotics at home | 16 | ||||||
| Zwisler et al., 2013 [68] | Kenya | Cross-sectional survey, 4 Provinces 2012, Caregivers of children <5 years with diarrhea in the previous 2 months, n = 857 | Gave antibiotic | 51.3 | ||||||
| Gave antimotility agent | 10.4 | |||||||||
| Simpson et al., 2013 [99] | Kenya | Cross-sectional survey, Western Kenya (year not specified), Caregivers of children 6–60 month with diarrhea in the previous 6 months, n = 100 | Gave antibiotic (at any point) | 64 | ||||||
| Gave antimotility (at any point) | 13 | |||||||||
| Gave antibiotic (1st treatment) | 26 | |||||||||
| Gave antibiotic (1st or 2nd treatment) | 46 | |||||||||
| Winch et al., 2008 [71] | Mali | Cross-sectional baseline survey preceding intervention, Southern Mali 2004, Caregivers of children <5 years with diarrhea in the previous 2 weeks, n = 228 | Same or less fluid or breast milk | 82.7 | Gave antibiotics | 57 | ||||
| Stopped feeding or breastfeeding | 46 | Gave metronidazole | 7.5 | |||||||
| Gave antidiarrheal | 2.6 | |||||||||
| Among children with only diarrhea symptoms gave: Antibiotic | 16 | |||||||||
| Antimalarial | 16 | |||||||||
| Paracetamol | 10 | |||||||||
| Perez et al., 2009 [100] | Mali | Cross-sectional survey in intervention comparison area, Mopti Region 2006, Caregivers of children <5 years, reference episode unclear, n = 401 | Gave any drug | 56.1 | ||||||
| Nasrin et al., 2013 [91] | Mozambique | Cross-sectional survey, Rural Southern Mali 2007, Caregivers of children <5 years with diarrhea in the previous 2 weeks, n = 67 | Offered less than usual to eat | 38.3 | Gave leftover antibiotics at home | 3.6 | ||||
| Nhampossa et al., 2013 [15] | Mozambique | Cross-sectional study (HUAS), Rural Southern Mozambique 2007 (Study 1 n = 67) and 2009–2012 (Study 2 n = 246), Caregivers of children <5 years with diarrhea in previous 2 weeks | Study 1: Reduced or stopped breastfeeding/usual fluid intake | 12 | Study 1: Gave antibiotic (Among those seeking treatment) | 14 | ||||
| Study 1: Maintained same fluid or breast milk intake | 73 | |||||||||
| Study 2: Reduced or stopped breastfeeding/usual fluid intake | 79 | |||||||||
| Study 2: Maintained same fluid or breast milk intake | 1 | |||||||||
| Ekanem et al., 1990 [47] | Nigeria | Diarrhea surveillance survey, Periurban Lagos (year not specified), Mothers of children 6–36 months, reference episode is general case, n = 200 | Normal breastfeeding pattern continuedb | 76.9 | ||||||
| Decreased breastfeedingb | 10.4 | |||||||||
| Babaniyi et al., 1994 [10] | Nigeria | Cross-sectional study, Suleja 1991, Caregivers of children <5 years with diarrhea in previous 2 weeks, n = 340 | Normal amount of “other” fluidsai | 55.6 | Stopped breastfeedingb | 7.7 | Stopped or less solid food | 42.4 | Gave any drug (at home) | 53.5 |
| Less “other” fluidsai | 22.6 | |||||||||
| Okoro et al., 1995 [74] | Nigeria | Cross-sectional study, Cross River State 1994, Caregivers of children <5 years with diarrhea in previous 24 h, n = 488 | Gave any drug | 75.6 | ||||||
| Gave drug and ORS/SSS | 51.9 | |||||||||
| Okunribido et al., 1997 [26] | Nigeria | Longitudinal study, Rural Yoruba communities of rural Oyo State (year not specified), Caregivers of children <5 years with diarrhea in previous 2 weeks, n = 98 | Stopped fluids (among those who noticed fluid intake)e | 2 | Child could not suck | 23.4 | Stopped food | 3 | Gave Western medicine: 1sttreatment, among those treating | 37.7 |
| Child refused fluid | 29.5 | Lost appetite | 34.6 | Reduced appetite | 68.8 | Gave Western medicine: 2ndtreatment, among those treating | 30.3 | |||
| Gave Western medicine at any point for watery diarrhea | 50 | |||||||||
| Gave Western medicine at any point for presumed dysentery | 52.7 | |||||||||
| Edet et al., 1996 [101] | Nigeria | Cross-sectional study, Oduknani 1994, Caregivers of children <5 years with diarrhea in previous 24 h, n = 5296 screened | Less fluida | 48.2 | Stopped breastfeedingb | 59.9 | Stopped feeding | 13.8 | ||
| Same fluida | 37.3 | Less food | 32.8 | |||||||
| Same food | 49 | |||||||||
| Omokhodion et al., 1998 [102] | Nigeria | Cross-sectional study, Market women in Ibadan 1996–1997, Market women with children <5 years in reference to any diarrhea episode, Bodia n = 266, Gbagi n = 260 | Bodija Market: Went to chemist to buy drugs | 12 | ||||||
| Gbagi Market: Went to chemist to buy drugs | 19 | |||||||||
| Bodija Market: Used drugs prescribed for previous illness | 7 | |||||||||
| Gbagi Market: Used drugs prescribed for previous illness | 5 | |||||||||
| Ene-Obong et al., 2000 [81] | Nigeria | Surveillance study, Market women in Enugu State 1993–1994, Market women with children <5 years with diarrhea in previous 2 weeks, n = 80 | Gave pharmaceutical | 28.8 | ||||||
| Gave pharmaceutical & sugar-salt solution | 33.8 | |||||||||
| Omotade et al., 2000 [38] | Nigeria | Surveillance study, Oyo State 1993–1994, Caregivers of children <5 years with diarrhea in previous week, n = 158 | Gave antimicrobial | 46.8 | ||||||
| Uchendu et al., 2009 [60] | Nigeria | Cross-sectional hospital based study, Enugu 2006, Caregivers of children <5 years attending health clinic with diarrheal disease and vomiting, n = 156 | Gave antibiotic (at home) | 51.3 | ||||||
| Gave antimotility/antidiarrheal (at home) | 44.9 | |||||||||
| Uchendu et al., 2011 [45] | Nigeria | Cross-sectional hospital based study, Enugu 2006, Caregivers of children <5 years attending health clinic with diarrheal disease and vomiting, n = 156 | Stopped feedse | 5.2 | ||||||
| Ogunrinde et al., 2012 [103] | Nigeria | Cross-sectional hospital based survey, Northwestern Nigeria (year not specified), Caregivers of child 1–59 months attending health clinic with diarrheal disease, n = 186 | As first line treatment gave: | |||||||
| Antibiotic | 23.7 | |||||||||
| Antidiarrheal | 12.7 | |||||||||
| ORS, antibiotic, antidiarrheal | 3 | |||||||||
| Ekwochi et al., 2013 [64] | Nigeria | Cross-sectional hospital based study, Enugu 2012, Caregivers of children ≤5 years attending university teaching hospital, reference any diarrhea episode, n = 210 | Gave unprescribed antibiotic | 46.7 | ||||||
| Cooke et al., 2013 [104] | South Africa | Cross-sectional hospital based study, Capetown 2007–2008, Caregivers of children <65 months with severe diarrhea attending hospital, n = 142 | Same or less fluid among all (but gave some ORS or milk) | 36.6 | Stopped breastfeeding/milk (but gave other fluids)b | 35.2 | ||||
| Haroun et al., 2012 [105] | Sudan | Cross-sectional hospital based study, Gezira (year not specified), Mothers of children <5 years, diarrhea episode reference unclear, n = 110 | Stopped or reduced fluid during episodee | 49 | Stopped feedinge | 30 | ||||
| Same amount of fluid during episodee | 33 | |||||||||
| Stopped or reduced fluid during episode but didn’t change amount of foode | 23 | |||||||||
| Kaatano et al., 1997 [8] | Tanzania | Cross-sectional survey, North-western lake districts (year not specified), Caregivers of children <5 years with diarrhea in previous 2 weeks, n = 89 | Stopped or decreased fluide | 12.6 | Stopped breastfeedingb | 46.7 | Stopped or decreased food | 13.8 | Gave anti-diarrheal | 29.2 |
| Gave antibiotic | 13.5 | |||||||||
| South East Asia | ||||||||||
| Alam et al., 1998 [82] | Bangladesh | Cross-sectional survey, Metropolitan Chittagong 1996–1997, Caregivers of children <5 years with diarrhea in previous 2 weeks, n = 360 | “Inappropriate or non-recommended drug use” among those receiving treatment | 73.5 | ||||||
| Gave metronidazole (denominator all consultations) | 38.6 | |||||||||
| Gave antibiotic (denominator all consultations) | 17.5 | |||||||||
| Gave antiemetic (denominator all consultations) | 12.2 | |||||||||
| Gave antidiarrheal (denominator all consultations) | 8 | |||||||||
| Ali et al., 2000 [27] | Bangladesh | Cross-sectional survey, Brahmanharia district 1993, Caregivers of children <5 years with diarrhea in previous 24 h, n = 186 | Drank less than usual amount of water (not amount offered) | 17 | ||||||
| Taha et al., 2002 [106] | Bangladesh | Cross-sectional survey, Cox’s Bazar district 1994, Mothers of children <5 years, diarrhea episode reference unclear, n = 297 | No fluids for treating diarrheae | 11.7 | Stopped breastfeedingb | 11.7 | Did not give solid or semi-solid foodc | 40.4 | ||
| Baqui et al., 2004 [73] | Bangladesh | Community based controlled trial, Matlab 1998–2000, Caregivers of children 3–59 months with diarrhea in previous week, n = 297 | Gave antibiotic | 34.3 | ||||||
| Gave other medicine | 44.8 | |||||||||
| Gave IV | 0.3 | |||||||||
| Larson et al., 2009 [107] | Bangladesh | Cross-sectional baseline survey preceding intervention, Dhaka 2006, Caregivers of children 6–59 months with diarrhea in previous 2 weeks, n = 640 | Gave antibiotic | 34.7 | ||||||
| Das et al., 2013 [14] | Bangladesh | Cross-sectional survey (HUAS), Rural Mirzapur 2007, Caregivers of children <5 years with diarrhea in previous 2 weeks, n = 1128 | Offered less than usual amount of fluids | 10.8 | Offered less to eat (at home before seeking care) | 28.7 | Gave antibiotics (at home before seeking care) | 2.4 | ||
| Same amount | 61.3 | |||||||||
| Same or less | 72.1 | |||||||||
| Sood et al., 1990 [108] | India | Cross-sectional survey, Rural Haryana State (year not specified), Caregivers of children <5 years, reference any diarrhea episode, n = 108 | Generally stopped breastfeeding | 0 | Some food restricted | 83.33 | ||||
| Rasania et al., 1993 [23] | India | Cross-sectional survey, New Delhi (year not specified), Caregivers of children <5 years, diarrhea episode reference unclear, n = 254 | Restricted breastfeedingb | 12.59 | Gave less food during convalescence | 26.38 | ||||
| Stopped breastfeedingb | 19.29 | Shifted from solid to liquid diet | 45.27 | |||||||
| Stopped all foode | 9.84 | |||||||||
| Restricted “few” foods | 16.53 | |||||||||
| Gupta et al., 2007 [109] | India | Cross-sectional survey, Urban Delhi slum 2004, Caregivers of children <5 years with diarrhea in previous 2 weeks, n = unclear 1307 | Stopped fluide | 20 | Stopped feeding (not clear if food or breastfeeding) | 50 | ||||
| Ahmed et al., 2009 [46] | India | Cross-sectional survey, Kashmir Valley 2006, Caregivers of children <5 years with diarrhea in previous 24 h (n = 1055) and 2 weeks (n = 2836) | Among diarrhea in 15 days: Feeding restrictede | 4 | Diarrhea in last 24 h: Gave antibiotic | 77.9 | ||||
| Diarrhea in last 24 h: Feeding restrictede | 6.9 | |||||||||
| Shah et al., 2012 [31] | India | Cross-sectional survey, Urban slum of Aligarh 2009, Caregivers of children <5 years with diarrhea in previous 2 weeks, n = 101 | Stopped or decreased breastfeeding (among EBF 0-6 m)b | 30.77 | Interrupted, stopped or decreased feeding (among not breastfeeding: 7 m-5 years) | 37.8 | ||||
| Stopped or decreased breastfeeding (among non-EBF 0-6 m)b | 80 | |||||||||
| Zwisler et al., 2013 [68] | India | Cross-sectional survey, 7 States 2012, Caregivers of children <5 years with diarrhea in the previous 2 months, n = 988 | Gave antibiotic | 56.4 | ||||||
| Gave antimotility agent | 3 | |||||||||
| WHO 1991 [110] | Nepal | Cross-sectional survey, Terai (n = 335) and Midhills (n = 526) 1990, Caregivers of children <5 years with diarrhea in previous 24 h | Terai: Same or less fluida | 72 | Terai: Stopped breastfeedingb | 1 | Terai: Stopped or Less Feeding | 25 | Terai: Gave drug, no ORS | 21.5 |
| Midhills: Same or less fluida | 91 | Midhills: Stopped breastfeedingb | 1 | Midhills: Stopped or Less Feeding | 39 | Midhills: Gave drug, no ORS | 14.3 | |||
| Terai: Gave drug and ORS | 4.5 | |||||||||
| Midhills: Gave drug and ORS | 4.9 | |||||||||
| Jha et al., 2006 [111] | Nepal | Cross-sectional hospital based study, Sunsari District (year not specified), Caregivers of children <5 years with diarrhea attending PHC, n = 330 | Not Given Foodec | 2.1 | Gave any drug at any point | 70 | ||||
| Less frequency of food givenec | 12.5 | Gave antibiotic | 19.9 | |||||||
| More liquid mixed food given | 13.1 | Gave antimotility drug | 16.8 | |||||||
| Fed as usual, child refused | 14.6 | Gave anti-vomiting drug | 15.5 | |||||||
| Usual feeding | 57.7 | Gave IV | 17.7 | |||||||
| WHO 1993 [77] | Sri Lanka | Cross-sectional survey, North-western Province 1992, Caregivers of children <5 years with diarrhea in previous 2 weeks, n = 10077 screened | Same or less fluide | 63 | Stopped feedinge | 23 | Gave any medicine | 71 | ||
| Wongsaroj et al., 1991 [65] | Thailand | Cross-sectional survey, 12 Regions 1991, Caregivers of children <5 years with diarrhea in previous 2 weeks, n = 733 | Same or less fluide | 91.8 | Stopped breastfeedingb | 16.6 | Stopped solid foods | 28.7 | Gave any antibiotic or antidiarrheal | 58.6 |
| Gave IV | 6.2 | |||||||||
| Gave antibiotic | 18 | |||||||||
| Gave antidiarrheal | 19.3 | |||||||||
| Gave both antibiotic and antidiarrheal | 21.3 | |||||||||
| Prohmmo et al., 2006 [28] | Thailand | Surveillance survey, Northeast Region 2002, Caregivers of children <5 years with diarrhea in previous 2 weeks, n = 47 episodes | Same or decreased fluid | 42.5 | Stopped breastfeedingb | 0 | Gave antimicrobial | 45 | ||
| Gave antiemetic | 19 | |||||||||
| Gave antidiarrheal | 13 | |||||||||
| Gave cold medicine | 15 | |||||||||
| Gave antipyretic | 25 | |||||||||
| Western Pacific | ||||||||||
| Dearden et al., 2002 [22] | Vietnam | Cross-sectional survey, Rural northern province, Caregivers of children 6–18 months, reference any diarrhea episode, n = 100 | Generally give less or no foods and liquids | 71 | ||||||
| Hoan et al., 2009 [112] | Vietnam | Cross-sectional survey, Rural district (year not specified), Caregivers of children <5 years with diarrhea in previous 2 weeks, n = 133 | Among children with only diarrhea symptoms gave: | 54.1 | ||||||
| Antibiotics | 36.1 | |||||||||
| Anti-diarrheal | 36.1 | |||||||||
| Antihistamine | 3 | |||||||||
| Analgesic/antipyretic | 13.5 | |||||||||
| Cough and cold prep | 0.8 | |||||||||
| Corticosteroid | 2.3 | |||||||||
aExcluding breast milk
bAmong those breastfeeding
cUnclear if only among those receiving solid or semi-solid food before illness
dAmong drinking fluids other than breast milk
eInclusion/exclusion of breastfeeding not specified
fAmong those receiving drug as treatment
gUnclear if only among those breastfeeding at time of illness
hAmong those who continued to receive fluids; breast milk; food
iExplicitly excluding ORS/SSS
Multiple studies explored variations in fluid curtailment by characteristics of the diarrhea episode, child, caregiver, and household (Table 2). Fluid curtailment was associated with diarrhea severity and vomiting in two studies [9, 10], whereas increase in fluid was associated with long illness duration and poor appetite [11]. Studies in Pakistan, Bangladesh, and Saudi Arabia found no clear association between fluid restriction and the age of the child [12–14]. However, a study in Mozambique reported that less fluid was given to infants relative to older children [15]. Younger mothers and mothers who did not work outside the home [12] and less educated mothers [16] were more likely to curtail fluids.
Table 2.
Factors associated with harmful practice
| Level | Factor | Positive association (harmful practice more likely) | Negative association (harmful practice less likely) | No association | No test of significance |
|---|---|---|---|---|---|
| Association with fluid curtailment | |||||
| Episode | Dehydrated (vs not dehydrated) | [57] | |||
| Severe disease | [10] | [57] | |||
| Child vomited (vs did not vomit) | [9] | ||||
| Child was anorexic | [11] | ||||
| Longer duration of episode | [11] | ||||
| Child | Older child age | [15] | [12] | [13, 14] | |
| Caregiver | Older maternal age | [12] | [16] | ||
| Higher maternal education | [16] | [12] | |||
| Older maternal age at marriage | [12] | ||||
| Caregiver employed | [12] | ||||
| Household | Live in urban area (vs rural) | [16] | [95] | ||
| Association with breastfeeding restriction | |||||
| Episode | Dehydrated (vs not dehydrated) | [57] | |||
| Severe disease | [57] | ||||
| Child | Older child age | [12] | |||
| Caregiver | Older maternal age | [12] | |||
| Higher maternal education | [12] | ||||
| Older maternal age at marriage | [12] | ||||
| Caregiver employed | [12] | ||||
| Household | Live in urban area (vs rural) | [33] | [95] | ||
| Association with Food Restriction | |||||
| Episode | Dehydrated (vs not dehydrated) | [40] | [57] | ||
| Severe disease | [40] | [57] | |||
| Child had fever | [11] | ||||
| Child was anorexic | [11] | ||||
| ORS use | [41] | ||||
| Sought care outside home | [41] | ||||
| Child | Older child age | [42] | [12] | [13, 14] | |
| Caregiver | Older maternal age | [12, 16] | [90] | ||
| Higher maternal education | [12, 16] | [90] | |||
| Older maternal age at marriage | [12] | ||||
| Caregiver employed | [12] | ||||
| Household | Greater household income | [90] | |||
| Live in urban area (vs rural) | [16] | [90, 95] | |||
| Association with inappropriate drug use | |||||
| Episode | Dehydrated (vs not dehydrated) | [60] | [40] | [57] | |
| Severe disease | [10, 40] | [57] | |||
| Longer disease duration | [63] | ||||
| Classification of diarrhea | [81] | ||||
| ORS use | [60, 63] | [68] | |||
| Sought care outside home | [11, 41] | ||||
| Child | Older child age | [13, 14] | |||
| Caregiver | Higher maternal education | [64] | [60] | ||
| Household | Greater household income | [60, 87] | |||
| Live in urban area (vs rural) | [93] | ||||
Multiple studies have attributed the practice of fluid curtailment to caregiver beliefs about the impact of fluid intake on a child’s diarrhea episode (Table 3). Multiple studies reported that caregivers often stated that more or specific fluids would increase the severity of the illness [17–19] or could not be digested [20–22]. Two studies suggested these beliefs were informed by caregivers’ observations that reduced fluids decreased stool output and diarrhea intensity [7, 23]. One study reported that certain types of diarrhea are perceived to be manageable by adjusting fluid intake, while others require traditional or spiritual methods, or no treatment at all [24]. The beliefs of family and community members, particularly elderly relatives, have also been reported as influential in determining caregiver practices related to fluids and feeding during childhood diarrhea episodes [22, 24, 25]. In three studies caregivers reported reduced fluid intake due to child refusal, child crying, or decreased thirst [22, 26, 27]. In one study, mothers reported they did not encourage increased fluids because they were inexperienced in how to do this [27].
Table 3.
Beliefs, motivations, and context related to harmful practices by region and country
| Author, Year [reference] | Country | Study design: methods (number conducted), study population | Source of information on diarrhea treatment | Expected effect of treatment | Restriction of specific food or fluid | Treatment specific to type or cause of diarrhea | Drug specific: strength/effectiveness | Drug specific: and source/availability | Other |
|---|---|---|---|---|---|---|---|---|---|
| Americas | |||||||||
| Hudelson et al., 1994 [44] | Bolivia | Qualitative study: Indepth interviews IDIs (65), hypothetical case scenarios (10), and observation (5) of mother and health workers, El Alto 1993, Mothers of children <5 years and health workers | Food: Mothers worry increasing food intake could worsen episode | General: Type of treatment sought is dependent on perceived cause of the illness | Feeding: Diet is already poor so doesn’t vary much during episode | ||||
| Food: Some may offer less food to reduce stool output | Drugs: Drugs are used to treat “diarrea por infeccion” | Food: Reduction in intake due to loss of appetite. Caregivers unaccustomed to encouraging feeding. | |||||||
| Larrea-Killinger et al., 2013 [113] | Brazil | Qualitative study: IDIs (29) and observations, Salvador 1997–2004, Mothers and grandmothers of children <5 years | Combination of ORS and antibiotics believed to reduce severity of episode | ||||||
| McLennan et al., 2002 [49] | Brazil | Qualitative study: IDIs (29) and observations, Salvador 1997–2004, Mothers and grandmothers of children <5 years | Feeding: 1/3 mothers reported restricting some foods | Drugs: 73 % mothers believe child should be given antibiotic for episode | |||||
| Feeding: 95 % believe at least one food item should be restricted | |||||||||
| Food: 38 % believe all solid foods should be restricted | |||||||||
| BF: Few (3 %) believe BF should be suspended | |||||||||
| Granich et al., 1999 [114] | Dominican Republic | Quantitative study: Structured interviews (582), Periurban Santo Domingo 1996, Mothers of children <5 years | Drugs: 71 % of caregivers would give pill or injection for hypothetical episode of diarrhea | ||||||
| Ecker et al., 2013 [115] | Peru | Quantitative study: Structured interviews (1200), Periurban Lima (year not specified), Caregivers of children <5 years | Drugs: 65 % of caregivers believe antibiotic is necessary to treat hypothetical case of non-dysenteric diarrhea | ||||||
| Europe | |||||||||
| Eastern Mediterranean | |||||||||
| Ali et al., 2003 [50] | Pakistan | Quantitative study: Self-administered questionnaire (400), Karachi 2000, Adult females attending clinic | Food: Most caregivers reported receiving information on food restriction from mother or grandmother | Food: Heavy foods, bread, meat commonly restricted | |||||
| Food: 2 % of women believe all food items should be restricted | |||||||||
| Agha et al., 2007 [116] | Pakistan | Quantitative study: Structured interview (647), Gambat, Singh Province (year not specified), Caregivers of children 6–59 months | Fluid: 12 % of caregivers believe less fluid is required during episode | ||||||
| Food: 44 % believe less food is required | |||||||||
| Rasheed et al., 1993 [117] | Saudi Arabia | Quantitative study: Structured interview (240) and self-administered questionnaire (589), Eastern Province 1990, Mothers of children attending government health center and girls attending government high school | Feeding: Fewer mothers than female students believe fluid and foods should be restricted during episode | ||||||
| Drugs: Compared to students, more mothers preferred drugs as treatment | |||||||||
| Africa | |||||||||
| Kaltenthaler et al., 1996 [30] | Botswana | Qualitative study: Focus group discussions FGDs (4) and IDIs (12), KIIs (7) and observations, North-east Botswana 1991–1992, Caregivers of young children, health providers and traditional healers | BF: Pogwana (severe diarrhea with sunken fontanel) is an “African illness” and should be treated with breast feeding cessation and should go to health facility or traditional healer | General: Mothers report using multiple sources of treatment if episode doesn’t improve | |||||
| Nkwi et al., 1994 [34] | Cameroon | Mixed-method study: Structured interviews (256) and hospital observations, 3 provinces in Cameroon, Caregivers of children <5 years | BF: Some diarrhea thought to be caused by “bad breastmilk” - mothers are given herbs to improve quality of milk | ||||||
| Almroth et al., 1997 [36] | Lesotho | Qualitative study: FGDs (19) and IDIs (43), 3 geographically different locations 1991–1992, Mothers and grandmothers of children and nurses | General: Mothers received conflicting advice from grandmothers and nurses | Food: Believe food should be given because it “strengthens the bowels” | Food: Believe you should adjust diet for individual child, if a specific food makes diarrhea worse | Food: Mothers coax children to eat during and after diarrhea | |||
| Feeding: Caregivers report providers still advise caregivers to restrict feeding | General: Mothers report using any treatment that works, sometimes multiple treatments | ||||||||
| Munthali et al., 2005 [35] | Malawi | Qualitative study: IDIs and KIIs (sample size not specified), Rumphi 2000–2002, Old and young men and women and health providers | BF: Perceived causes of diarrhea include contaminated breast milk requires weaning | Drugs perceived to useful in treatment of all illnesses | |||||
| General: Diarrhea due to teething is perceived as requiring no treatment | |||||||||
| Ellis et al., 2007 [78] | Mali | Mixed methods study: Structured interviews (352), illness narratives (14), and IDIs (42), Bougouni District 2003, Caregivers of children <5 years with illness in past 2 weeks or seeking care and health providers | General: Mothers-in-law play important role initiating traditional treatment | Combining several different medicines/therapies is viewed as most efficacious | Treatment of diarrhea typically begins in the home with traditional medicines and/or antibiotics from nearby vendors | ||||
| Ikpatt et al., 1992 [19] | Nigeria | Quantitative study: Self-administered questionnaire (561), Cross River and Akwa Iborn State (year not specified), Household representative | BF: 19 % mothers believe BF should be discontinued | Drugs: 53 % of mothers reported antibiotic and 15 % reported antidiarrheal as treatment for diarrhea | |||||
| Fluid: 15 % believe fluid should not be offered during episode | |||||||||
| Food: 17 % believe solid foods should be withdrawn | |||||||||
| Jinadu et al., 1996 [48] | Nigeria | Mixed method study: Structured interview (335) and FGD (4), Rural Yoruba communities of Osuo State (year not specified), Mothers of children <5 years | Fluid: More mothers believe fluids should not be given for watery diarrhea (65 %) compared to bloody diarrhea (55 %) | ||||||
| Ogunbiyi et al., 2010 [29] | Nigeria | Mixed method study: Structured interviews (250) and FGDs (2), Ibadan 2003–2004, Mothers of child <1 year attending sick baby/immunization clinic of 2 health facilities | BF: “Cultural” reasons for BF restriction - passed from generations | Food: Foods withdrawn because thought to prolong the duration of diarrhea in the child (86 %) and induce vomiting/loss of appetite (14 %) | Food: Indigenous foods rich in protein withdrawn because believed to aggravate diarrhea | BF: Overconsumption of BM thought to cause some diarrhea – therefor reduce BF frequency during episode | |||
| Feeding: 71 % believe some food, fluid, or breast milk should be withdrawn during episode | Food: Withdrawal of other foods also linked to mother’s perception of cause of diarrhea | ||||||||
| Olakunle et al., 2012 [56] | Nigeria | Quantitative study: Structured interview (186), Ilorin West Local Government Area (year not specified), Mothers of children <5 years | Feeding: Majority said food restriction was based on personal view, but some said received information on food restriction from nurses | Feeding: 46 % of mothers believe “some food” should be restricted during episode | Drug: 17 % of mothers believe child should be treated with antibiotic during episode | ||||
| Kauchali et al., 2004 [32] | South Africa | Qualitative study: IDIs (16), FGD (1), Case histories (13) and card sorting, Rural Kwazulu-Natal 2001, Caregivers of young children, grandmothers, CHWs | BF: Perceived causes of diarrhea include “dirty” breast milk requires temporary stop in breastfeeding | ||||||
| Friend du Preeze et al., 2013 [72] | South Africa | Mixed method study: IDIs (17), FGDs (5) and structured interviews (206), Johannesburg and Soweto 2004, Caregivers of children <6 years in longitudinal study and health providers | Drugs: Health care workers reported that mothers commonly use non-prescribed antibiotics | ||||||
| Drugs: Demand for modern medicines is high | |||||||||
| Mwambete et al., 2010 [118] | Tanzania | Qualitative study: Semi-structured interviews (88), Dar es Salaam 2007, Mothers of children <5 years | 35 % of mothers reported metronidazole as most effective chemotherapeutic agent for treating diarrhea | Drugs: Metronidazole (43 %) and Erythromycin + Metronidazole (12 %) were cited as commonly used “therapeutic agents” for diarrhea treatment | |||||
| South East Asia | |||||||||
| Mushtaque et al., 1991 [55] | Bangladesh | Qualitative study: “Socioanthopologic methods,” Central Bangladesh (year not specified), villagers | Food: Certain types of diarrhea require withholding foods that are normally part of the diet | General: Treatments considered appropriate depend on the local classification of the diarrhea | |||||
| BF: Injection of breast milk into woman used to correct “polluted” breast milk | |||||||||
| Singh et al., 1994 [43] | India | Quantitative study: Structured interviews (208), Jaipur District (year not specified), Mothers of children <5 years | Feeding: Mothers believe intestine becomes weak and child unable to digest heavy foods (roti and milk) during episode | ||||||
| Feeding: Tea water and banana believed to help reduce frequency of diarrhea | |||||||||
| Chandrashekar et al., 1995 [25] | India | Qualitative study: Semi-structured interviews (300), Rural South India 1991, Mothers of children age 3 days - 17 months | Feeding: Elderly relatives are source of information on feeding practices | BF: Some caregivers believe breastfeeding should be restricted when mother is experiencing diarrhea or respiratory infection | |||||
| Buch et al., 1995 [119] | India | Quantitative study: Structured interview (1600), Kashmir 1992, Caregivers of infants with acute diarrhea attending hospital pediatric OPD | Feeding: 19 % of caregivers believe child should have complete dietary restriction | Drugs: 55 % of caregivers believe diarrhea should be treated with antidiarrheal & antispasmodic drugs, while 32 % should be treated with drugs and ORT | |||||
| Fluid: 77 % believe milk should be restricted | |||||||||
| Bhatia et al., 1999 [54] | India | Quantitative study: Structured interview (120), Rural Chandigarh 1996, Mothers of children <5 years | Feeding: 47 % of mothers believe certain foods/fluids should be restricted including chapatti, milk and pulses | ||||||
| Datta et al., 2001 [120] | India | Quantitative study: Structured interview (75), Rural Maharashtra 2000, Caregivers of children <5 years attending hospital pediatric OPD | BF: 16 % of caregivers not aware child has to be given breastfeeding during episode of diarrhea | ||||||
| Vyas et al., 2009 [121] | India | Quantitative study: Structured interview (380), Ganhinagar district (year not specified), Women of reproductive age (15–44) | BF: 52 % of women did not know breastfeeding should be continued during episode | ||||||
| Food: 50 % did not know other foods should be continued | |||||||||
| Bolam et al., 1998 [122] | Nepal | Quantitative study: Structured interview (105), Kathmandu 1994–1996, Women delivering at Kathmandu General Hospital | BF: 3 months postpartum, 53 % of mothers did not know to continue BF during episode | ||||||
| Adhikari et al., 2006 [123] | Nepal | Quantitative study: Structured interview (510), Kathmandu 2005, Married women age 18–38 from 2 village development committees | BF: 7 % of women believe breastfeeding aggravates diarrhea | ||||||
| Ansari et al., 2012 [24] | Nepal | Qualitative study: FGDs (2) and IDIs (8), Morang 2010, Mothers of children <45 months with diarrhea in the previous 6 months | General: Elders recommend traditional treatment practices | Food: Spicy, oily and rotten food commonly believed to be harmful | General: Certain types of diarrhea are perceived to be manageable with ORS/SSW, while others require traditional/spiritual methods. | ||||
| BF: Breast milk sometimes considered harmful | |||||||||
| Baclig et al., 1990 [58] | Thailand | Mixed method study: FGDs (2) and structured interviews (98), Tambon Korat and Koongyang (year not specified), Mothers and grandmothers of children <5 years | Feeding: Mothers believe no changes should be made to the child’s diet to manage poh (a mild self-limiting diarrhea) | ||||||
| Pylypa et al., 2009 [18] | Thailand | Qualitative study: Semi-structured interviews (200) as part of ethnographic study, Rural Northeast Thailand 2000–2001, Caregivers of children <5 years, traditional healers, and health providers | General: Grandmothers and elders are important sources of information for classifying/managing diarrhea | Fluid/BF: Some mothers restricted water or breast milk out of concern that it would make diarrhea worse, belief child could not drink much because he was small, or would vomit | Food: Most mothers didn’t change quantity/type of food given for diarrhea occurring in normal developmental stages (not illness) although expected children would eat less in than normal | Medicines were frequently obtained from health workers – most clinicians consulted gave antibiotics routinely for watery diarrhea, and for diarrhea with fever | Drugs: Some mothers took the medicines themselves to pass to infants through breast milk | ||
| Drugs: Medicines were commonly administered for childhood diarrhea considered illness | |||||||||
| Western Pacific | |||||||||
| Okumura et al., 2002 [70] | Vietnam | Quantitative study: Structured interviews (505), 4 Provinces of Vietnam 1997, Mothers of children <5 years | Antibiotics to be stocked at home (55 % of households) for various anticipated symptoms as if they were panaceas | ||||||
| Le et al., 2011 [69] | Vietnam | Qualitative study: IDIs (5) and FGDs (4), Ha Tay province (year not specified), Mothers of children <5 years and health workers/drug sellers | Drugs: Drugs bought on drug seller recommendation or previous prescriptions | Western medicine considered necessary but more dangerous than traditional therapy | Drugs are available without prescription and small amount can be purchased to give for 2–3 days | ||||
| Rheinlander et al., 2011 [67] | Vietnam | Qualitative study: Semi-structured interviews (43), FGDs (3), and observations, Ethnic minorities in Lao Cai 2008, Caregivers of children <7 years with diarrhea in the past month | General: Elders are in charge of deciding, preparing, and administering treatment for a sick child | Drugs: Medicines chosen based on perceived compatibility with the child and the disease | Antibiotics perceived as very powerful and potentially harmful compared to natural medicines | Drugs: common to receive 2–4 prescribed drugs for diarrhea | |||
| Drugs: To limit intake and harm of western drugs, caregivers gave smaller doses than prescribed, or shifted from one drug to another if recovery was slow |
Beliefs, motivations, and context related to:
BF: Breastfeeding
Fluid: Fluid restriction
Food: Food restriction
Feeding: Fluid, breastfeeding, and food restriction, or non-specific as to type of feeding
Drug: Use of modern medicines
General: Decision making around treatment or perception of diarrhea not specific to one of the harmful practice
Breastfeeding reduction
Many studies reported the practice of breastfeeding reduction or cessation during diarrhea episodes (Table 1, Column 5). Most studies found that among mothers breastfeeding their child prior to the onset of diarrhea, fewer than 10 % of mothers stopped breastfeeding during the episode. The practice of breastfeeding cessation ranged from no mothers reporting breastfeeding cessation in a surveillance study in northeast Thailand to 62 % of mothers reporting stopping breast or milk feeding in a hospital-based study in Saudi Arabia [20, 28]. The practice of breastfeeding cessation was higher in hospital samples compared to samples from the general population. Where breastfeeding reduction was reported, on average one quarter of mothers reported reducing breastfeeding, although there was significant variation in the practice.
Multiple studies assessed variance in breastfeeding restriction by factors including characteristics of the diarrhea episode, child, caregiver, and household (Table 2). One study found younger and less educated mothers were more likely to reduce breastfeeding during episodes of diarrhea [12].
Mothers reported ceasing or reducing breastfeeding when their child had diarrhea for various reasons (Table 3). Mothers reported stopping or reducing breastfeeding because of beliefs that breastmilk was too fatty to be digested [20]. Others reported continued breastfeeding would not reduce the duration of diarrhea [20, 29] or could cause or worsen the diarrhea [18, 19, 29]. Caregivers in two studies believed specific types of diarrhea must be treated with breastfeeding cessation [29, 30]. In multiple cultures, “dirty” breast milk or secretion of ingested food through breast milk was thought to cause certain types of diarrhea. Mothers received treatment or a modified diet to improve the quality of their breast milk [31–34] or children were weaned [35]. Some caregivers stated they were following the advice of healthcare providers by restricting breastfeeding [20, 36]. Older relatives were also important sources of information on feeding practices during diarrhea episodes [25, 31]. In some studies, mothers continued feeding but diluted milk or formula [29], switched to powdered or goat’s milk [37], or only gave water [38].
Food restriction
The measurement of food restriction, and prevalence estimates, varied widely across studies (Table 1, Column 6). Many studies differed in their definition or failed to specify if food restriction was measured only among those eating solid foods prior to illness, whether breastfeeding was included or excluded, and whether amount of food offered versus consumed was measured. Findings on restriction of specific foods have been included for context but not in prevalence estimates of overall food restriction (Table 1). The practice of stopping all food ranged from as low as 3 % of mothers stating they stopped giving solid or semi-solid foods during the episode in Oyo State, Nigeria [26] to as high as 53 % of mothers reporting they stopped feeding in Kenya [39]. As expected, measures that included the reduction of feeding in addition to complete restriction of feeding showed higher rates of food restriction, mostly within the range of 30–60 % of episodes.
Multiple studies addressed the variance of food restriction by other factors, including characteristics of the diarrhea episode, child, caregiver, and household (Table 2). Food curtailment was associated with dehydration and more severe disease [40], seeking care outside of the home, and ORS use [41]. In one study, caregivers were more likely to withhold food if a child had fever or a low appetite [11]. Another study found children less than 2 years of age were more likely to receive continued feeding compared to older children [42]. Two studies found that less educated mothers were more likely to restrict foods [12, 16].
Motivation for food restriction differed (Table 3). Some caregivers reported that a child’s diet should be restricted because of beliefs that a child cannot eat or digest as much during a diarrhea episode [22, 43] and feeding can exacerbate or prolong diarrhea episodes [19, 22, 29, 44–46]. Belief that only certain foods should be restricted because they can aggravate diarrhea was common across countries and included a range of foods such as meat, milk, sweet food, greasy food, high carbohydrate and high protein foods [29, 37, 38, 43, 47–54]. Alternatively, in two studies some caregivers reported that specific foods were customary and should be given during a diarrhea episode to strengthen the bowel or soothe the stomach [36, 52]. Some caregivers reported that restriction of certain foods was based on long held folk tradition [29, 47]. Others reported that diet alteration is based on the type or perceived cause of the diarrhea [18, 29, 55]. Elderly relatives, neighbors, and health care providers were reported to influence mothers’ feeding practices in many contexts [22, 23, 25, 27, 29, 36, 53, 56, 57]. Some caregivers reported that a child’s diet was not restricted during diarrhea because it was already limited [27, 44, 58]. One study reported mothers coaxed their child to eat more [36], but others reported some mothers of children with decreased appetite were unfamiliar with encouraging children to eat [22, 44] or had little time to prepare additional food because they were caring for the child [22]. One study suggested caregivers felt continued feeding was less important if they had been given some treatment at a health facility [31].
Inappropriate medication use
Many studies reported the use of drugs to treat diarrhea in children under five (Table 1, Column 7). The most commonly reported measures were the use of an antibiotic or antimicrobial, followed by use of any medicine, and the use of an antidiarrheal or antimotility agent. While antibiotics are recommended for treatment of dysentery or cholera, most studies did not differentiate between simple and dysenteric diarrhea when reporting on antibiotic use. The Lives Saved Tool (LiST) attributes 7 % of diarrhea cases in children under 5 to dysentery [59], therefor it may be inferred that high antibiotic use rates are inclusive of inappropriate antibiotic use. A hospital-based study in Enugu, Nigeria highlights the difficultly of collecting information on the type of medicine used to treat diarrhea. The study reported that 70 % of mothers misclassified antibiotics and analgesics as antimotility agents when self-reporting drugs used in diarrhea treatment [60]. Multiple studies outside of this review have shown that the accuracy of drug recall varies by questionnaire design and method of assessment [61].
Reported use of antidiarrheal and antimotility agents was generally lower than reported use of antibiotics. Use of antibiotics at any point in an episode ranged from 10-77 %. Antidiarrheal use ranged from 3–45 % of diarrhea episodes, with the exception of very high reported use (74 %) in Egypt in 2002 [62]. Use of any drug for a diarrhea episode occurring in the previous 2 weeks ranged from 26–76 %. Studies that used a shorter reference period limited to the previous 24 h reported lower rates of drug use at around 20 %.
Multiple studies addressed variance in inappropriate medication use by factors including characteristics of the diarrhea episode, child, caregiver, and household (Table 2). A hospital-based study in Nigeria found children who had received an antibacterial or antidiarrheal at home presented to the hospital with more severe dehydration than those children who did not receive these drugs [60]. Antibiotic and/or antidiarrheal use were associated with seeking care outside of the home [11, 41] and use of ORT [60, 63]. Two studies in Enugu, Nigeria reported conflicting associations between maternal education and antibiotic use [60, 64].
Caregivers reported using antibiotics and other drugs to treat diarrhea because they were accessible and believed to be efficacious (Table 3). Multiple studies reported caregiver beliefs that modern medicines are powerful [64–67], and more effective in treating diarrhea than ORS [65, 68]. Multiple studies reported drugs were widely available and affordable in the public and private sector, typically without prescription [35, 38, 40, 44, 49, 52, 64, 69]. In many contexts, caregivers stocked drugs at home, purchasing them in advance or saving leftover medication from previous illnesses [33, 37, 38, 52, 70]. Caregivers perceived drugs to be cheaper and more accessible than ORS, particularly given the flexibility to purchase a few tablets for little money [64, 65, 71]. Use of antibiotics in the treatment of pediatric diarrhea has become routine for both health care providers and caregivers in some contexts [18, 40, 66]. Caregivers may have also influenced provider behavior as caregivers’ preference for drug therapies creates pressure on providers to give medications in addition or instead of ORS [28, 33, 65, 72]. Drugs were given in sub-clinical doses in multiple studies [67, 69, 73]. It was common in studies for children to receive multiple drugs for a single episode of diarrhea, often from the same source [67, 74–77]. A study in Brazil found drugs were used more commonly to treat episodes of longer duration [63], although initial treatment of diarrhea at home with drugs was common in a study in Mali [78]. Multiple studies suggested treatment with modern medicines may be related to the perceived cause or type of diarrhea [18, 52, 60, 79–81]. Treatment seeking was often related to inappropriate use of medicine for diarrhea management [33, 57, 62, 82].
Discussion
This is the first review, to our knowledge, that addresses harmful practices related to fluids, feeding and medication use during episodes of childhood diarrhea. The findings indicate that there have been many studies – both quantitative and qualitative – that have documented these harmful practices. However, reported prevalence varies greatly across study populations, and we were unable to identify clearly defined patterns across regions, countries, or time periods. A limited number of studies looked at the variation of these harmful practices across potential influencing factors, including characteristics of the diarrhea episode and child, caregiver, or household-level traits. Findings of association differed across studies.
The motivation for harmful practices during diarrhea treatment also appears to vary across populations, although studies consistently report general caregiver concern for their child’s health and caregiver action to treat the illness to the best of their knowledge and abilities. Caregivers reported that their actions were based on the advice of health care providers, community members, or elderly relatives, as well as their own observations or understanding of the efficacy of certain treatments for diarrhea. Others reported following traditionally held beliefs on the causes and cures for specific diarrheal diseases.
Across studies, the measurement of harmful practices was inconsistent and not guided by a conceptual or theoretical framework. Most studies were focused on general practices in diarrhea treatment, and harmful practices were rarely a primary outcome of interest. This has limited the availability and quality of data on the topic. Variations in study design, sample populations, diarrhea episode reference periods, and measurement definitions make drawing comparisons and conclusions across studies challenging. This is further compounded by inconsistent quality in data collection and reporting. Most studies relied on sub-national population samples and many were limited to small sample sizes. The variation in treatment practices by perceived type of diarrhea highlights the importance of using local terminology in order to capture all episodes of diarrhea as perceived by the community [83]. Although the majority of studies included in this review used a recall period of diarrhea in the past two weeks, there was some variation ranging from the past 24 h to past six months or the “most recent” episode of diarrhea. Fischer-Walker and her colleagues highlight the importance of using a shorter recall period for capturing episodes of diarrhea of varying severity [83].
Although this systematic review highlighted limitations of existing research, the available evidence suggests that harmful practices in diarrhea treatment are common in certain populations. A multicountry analysis using MICS data from 28 countries between 2005–2007 reported the majority of mothers did not maintain their child’s nutritional intake during illness [5]. Analysis of DHS data from 14 countries between 1986–2003 suggests a decreasing trend in continued feeding in a majority of countries [6]. These practices can reduce correct management of diarrheal disease in children and result in treatment failure and sustained nutritional deficits. The lack of consistency in sampling, measurement, and reporting identified in this literature review highlights the need to document harmful practices using standard methods of measurement and reporting. Going forward, studies in this area would benefit from the development and use of a broader conceptual framework to ensure that the research is theory-driven and regularly synthesized. Multi-country analyses using MICS and DHS data have been conducted in the past, but they have tended to focus on positive treatment practices rather than harmful practices [5, 6]. Assessing harmful practices with nationally representative data and standardized measurements, through the analysis of the most recently available DHS and MICS data, can contribute to the discussion on improved care of diarrheal disease in children under five.
The strengths of this literature review include applying a systematic process for searching and summarizing the literature, and accessing articles during a time frame in which global efforts focused on improving coverage. This review was limited by the inclusion of only peer-reviewed literature and the exclusion of non-English language publications. Additionally, the quality of individual articles was not assessed, allowing for the potential inclusion of studies with misrepresentative findings.
Conclusions
Harmful practices in the management of childhood diarrhea are prevalent to varying degrees across cultures and include fluid and breastfeeding curtailment, food restriction, and inappropriate medication use. Inappropriate management of diarrhea episodes can result in higher risk of mortality through increased levels of dehydration or lasting health consequences as a result of nutritional restrictions or prolonged diarrhea illness. These practices must therefore be addressed as a matter of urgency in maternal, newborn and child health programs. These programs need to target not only the behaviors of child caregivers, but the broader social network, because our findings show that these practices are often informed by traditional beliefs, popular knowledge, and the instruction of authority figures, including elderly community members and health workers. Broader health systems interventions are also needed to address the alarming findings of high rates of inappropriate use of medications during diarrhea episodes. In addition, the global health community must do a better job or measuring the prevalence of these practices in standard ways, to produce evidence that can be used as the basis for action.
Acknowledgements
The authors would like to thank Christa Fischer-Walker and Cesar Victora for their helpful inputs on earlier drafts of this paper, and Peggy Gross for her technical assistance in developing literature search criteria.
This work was funded through a sub-grant from the U.S. Fund for UNICEF under the Countdown to 2015 for Maternal, Newborn and Child Survival grant from the Bill & Melinda Gates Foundation. The funders had no role in the conceptualization of the paper or in the material presented.
Additional file
PubMed Search Terms. (PDF 68 kb)
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
JB and HN conceptualized the systematic review. EC developed the search criteria, conducted the systematic review, and prepared the first draft of the manuscript. JB, HN, and JP reviewed the search criteria and drafts of the manuscript. All authors approved the final version of the manuscript.
Contributor Information
Emily Carter, Email: ecarter@jhu.edu.
Jennifer Bryce, Email: jbrycedanby@aol.com.
Jamie Perin, Email: jperin@jhu.edu.
Holly Newby, Email: hnewby@unicef.org.
References
- 1.STATISTICS BY AREA/Child Survival and Health: Diarrhoea [http://www.childinfo.org/diarrhoea.html]
- 2.World Health Organization . The treatment of diarrhoea: a manual for physicians and other senior health workers. Geneva: WHO; 2005. pp. 1–50. [Google Scholar]
- 3.Wardlaw T, Salama P, Brocklehurst C, Chopra M, Mason E. Diarrhoea: why children are still dying and what can be done. The Lancet. 2010;375(9718):870–872. doi: 10.1016/S0140-6736(09)61798-0. [DOI] [PubMed] [Google Scholar]
- 4.Wilson SE, Morris SS, Gilbert SS, Mosites E, Hackleman R, Weum KL, et al. Scaling up access to oral rehydration solution for diarrhea: Learning from historical experience in low–and high–performing countries. J Glob Health. 2013;3:1. [DOI] [PMC free article] [PubMed]
- 5.Arabi M, Frongillo EA, Avula R, Mangasaryan N. Infant and young child feeding in developing countries. Child Dev. 2012;83(1):32–45. doi: 10.1111/j.1467-8624.2011.01675.x. [DOI] [PubMed] [Google Scholar]
- 6.Forsberg BC, Petzold MG, Tomson G, Allebeck P. Diarrhoea case management in low- and middle-income countries--an unfinished agenda. Bull World Health Organ. 2007;85(1):42–48. doi: 10.2471/BLT.06.030866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Othero DM, Orago AS, Groenewegen T, Kaseje DO, Otengah PA. Home management of diarrhea among underfives in a rural community in Kenya: household perceptions and practices. East Afr J Public Health. 2008;5(3):142–146. doi: 10.4314/eajph.v5i3.38992. [DOI] [PubMed] [Google Scholar]
- 8.Kaatano GM, Muro AI, Medard M. Caretaker’s perceptions, attitudes and practices regarding childhood febrile illness and diarrhoeal diseases among riparian communities of Lake Victoria, Tanzania. Tanzan Health Res Bull. 2006;8(3):155–161. doi: 10.4314/thrb.v8i3.45113. [DOI] [PubMed] [Google Scholar]
- 9.Mediratta RP, Feleke A, Moulton LH, Yifru S, Sack RB. Risk factors and case management of acute diarrhoea in North Gondar Zone, Ethiopia. J Health Popul Nutr. 2010;28(3):253–263. doi: 10.3329/jhpn.v28i3.5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Babaniyi OA, Maciak BJ, Wambai Z. Management of diarrhoea at the household level: a population-based survey in Suleja, Nigeria. East Afr Med J. 1994;71(8):531–535. [PubMed] [Google Scholar]
- 11.Wilson SE, Ouedraogo CT, Prince L, Ouedraogo A, Hess SY, Rouamba N, et al. Caregiver recognition of childhood diarrhea, care seeking behaviors and home treatment practices in rural Burkina Faso: a cross-sectional survey. PLoS One. 2012;7(3):e33273. [DOI] [PMC free article] [PubMed]
- 12.Bani IA, Saeed AA, Othman AA. Diarrhoea and child feeding practices in Saudi Arabia. Public Health Nutr. 2002;5(6):727–731. doi: 10.1079/PHN2002354. [DOI] [PubMed] [Google Scholar]
- 13.Quadri F, Nasrin D, Khan A, Bokhari T, Tikmani SS, Nisar MI, et al. Health care use patterns for diarrhea in children in low-income periurban communities of karachi, Pakistan. Am J Trop Med Hyg. 2013;89(Suppl1):49–55. [DOI] [PMC free article] [PubMed]
- 14.Das SK, Nasrin D, Ahmed S, Wu Y, Ferdous F, Farzana FD, et al. Health care-seeking behavior for childhood diarrhea in mirzapur, Rural Bangladesh. Am J Trop Med Hyg. 2013;89(Suppl1):62–8. [DOI] [PMC free article] [PubMed]
- 15.Nhampossa T, Mandomando I, Acacio S, Nhalungo D, Sacoor C, Nhacolo A, et al. Health care utilization and attitudes survey in cases of moderate-to-severe diarrhea among children ages 0–59 months in the District of Manhica, southern Mozambique. Am J Trop Med Hyg. 2013;89(1 Suppl):41–8. [DOI] [PMC free article] [PubMed]
- 16.Berisha M, Hoxha-Gashi S, Gashi M, Ramadani N. Maternal practice on management of acute diarrhea among children under five years old in Kosova. Turk Silahl Kuvvetleri Koruyucu Hekimlik Bulteni. 2009;8(5):369–372. [Google Scholar]
- 17.Olango P, Aboud F. Determinants of mothers’ treatment of diarrhea in rural Ethiopia. Soc Sci Med. 1990;31(11):1245–1249. doi: 10.1016/0277-9536(90)90131-B. [DOI] [PubMed] [Google Scholar]
- 18.Pylypa J. Elder authority and the situational diagnosis of diarrheal disease as normal infant development in northeast Thailand. Qual Health Res. 2009;19(7):965–975. doi: 10.1177/1049732309338867. [DOI] [PubMed] [Google Scholar]
- 19.Ikpatt NW, Young MU. Preliminary study on the attitude of people in two states of Nigeria on diarrhoeal disease and its management. East Afr Med J. 1992;69(4):219–222. [PubMed] [Google Scholar]
- 20.Moawed SA, Saeed AA. Knowledge and practices of mothers about infants’ diarrheal episodes. Saudi Med J. 2000;21(12):1147–1151. [PubMed] [Google Scholar]
- 21.Bachrach LR, Gardner JM. Caregiver knowledge, attitudes, and practices regarding childhood diarrhea and dehydration in Kingston. Jamaica. Rev Panam Salud Publica. 2002;12(1):37–44. doi: 10.1590/S1020-49892002000700006. [DOI] [PubMed] [Google Scholar]
- 22.Dearden KA, Quan LN, Do M, Marsh DR, Schroeder DG, Pachon H, et al. What influences health behavior? Learning from caregivers of young children in Viet Nam. Food Nutr Bull. 2002;23(4 SUPP):119–29. [PubMed]
- 23.Rasania SK, Gulati N, Sahgal K. Maternal beliefs regarding diet during acute diarrhea. Indian Pediatr. 1993;30(5):670–672. [PubMed] [Google Scholar]
- 24.Ansari M, Ibrahim MI, Hassali MA, Shankar PR, Koirala A, Thapa NJ. Mothers’ beliefs and barriers about childhood diarrhea and its management in Morang district, Nepal. BMC Res Notes. 2012;5:576. doi: 10.1186/1756-0500-5-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chandrashekar S, Chakladar BK, Rao RS. Infant feeding--knowledge and attitudes in a rural area of Karnataka. Indian J Pediatr. 1995;62(6):707–712. doi: 10.1007/BF02825124. [DOI] [PubMed] [Google Scholar]
- 26.Okunribido OO, Brieger WR, Omotade OO, Adeyemo AA. Cultural perceptions of diarrhea and illness management choices among yoruba mothers in oyo state, Nigeria. Int Q Community Health Educ. 1997;17(3):309–318. doi: 10.2190/W07W-B4FX-TEX7-WC3K. [DOI] [PubMed] [Google Scholar]
- 27.Ali M, Atkinson D, Underwood P. Determinants of use rate of oral rehydration therapy for management of childhood diarrhoea in rural Bangladesh. J Health Popul Nutr. 2000;18(2):103–108. [PubMed] [Google Scholar]
- 28.Prohmmo A, Cook LA, Murdoch DR. Childhood diarrhoea in a district in northeast Thailand: incidence and treatment choices. Asia Pac J Public Health. 2006;18(2):26–32. doi: 10.1177/10105395060180020501. [DOI] [PubMed] [Google Scholar]
- 29.Ogunbiyi BO, Akinyele IO. Knowledge and belief of nursing mothers on nutritional management of acute diarrhoea in infants, Ibadan, Nigeria. (Special Issue: Diversity of research.) Afr J Food Agric Nutr Dev. 2010;10(3):2291–2304. [Google Scholar]
- 30.Kaltenthaler EC, Drasar BS. Understanding of hygiene behaviour and diarrhoea in two villages in Botswana. J Diarrhoeal Dis Res. 1996;14(2):75–80. [PubMed] [Google Scholar]
- 31.Shah MS, Ahmad A, Khalique N, Afzal S, Ansari MA, Khan Z. Home-based management of acute diarrhoeal disease in an urban slum of Aligarh, India. J Infect Dev Ctries. 2012;6(2):137–142. doi: 10.3855/jidc.1374. [DOI] [PubMed] [Google Scholar]
- 32.Kauchali S, Rollins N, Van den Broeck J. Local beliefs about childhood diarrhoea: importance for healthcare and research. J Trop Pediatr. 2004;50(2):82–89. doi: 10.1093/tropej/50.2.82. [DOI] [PubMed] [Google Scholar]
- 33.Vazquez ML, Mosquera M, Kroeger A. People’s concepts on diarrhea and dehydration in Nicaragua: the difficulty of the intercultural dialogue. Revista Brasileira de Saude Materno Infantil. 2002;2(3):223–237. [Google Scholar]
- 34.Nkwi PN. Perceptions and treatment of diarrhoeal diseases in Cameroon. J Diarrhoeal Dis Res. 1994;12(1):35–41. [PubMed] [Google Scholar]
- 35.Munthali AC. Change and continuity in the management of diarrhoeal diseases in under-five children in rural Malawi. Malawi Med J. 2005;16(2):43–46. doi: 10.4314/mmj.v16i2.10859. [DOI] [Google Scholar]
- 36.Almroth S, Mohale M, Latham MC. Grandma ahead of her time: traditional ways of diarrhoea management in Lesotho. J Diarrhoeal Dis Res. 1997;15(3):167–172. [PubMed] [Google Scholar]
- 37.Azim SM, Rahaman MM. Home management of childhood diarrhoea in rural Afghanistan: a study in Urgun, Paktika Province. J Diarrhoeal Dis Res. 1993;11(3):161–164. [PubMed] [Google Scholar]
- 38.Omotade OO, Adeyemo AA, Kayode CM, Oladepo O. Treatment of childhood diarrhoea in Nigeria: need for adaptation of health policy and programmes to cultural norms. J Health Popul Nutr. 2000;18(3):139–144. [PubMed] [Google Scholar]
- 39.Oyoo A, Burstrom B, Forsberg B, Makhulo J. Rapid feedback from household surveys in PHC planning: An example from Kenya. Health Policy Plan. 1991;6(4):380–383. doi: 10.1093/heapol/6.4.380. [DOI] [Google Scholar]
- 40.Perez-Cuevas R, Guiscafre H, Romero G, Rodriguez L, Gutierrez G. Mothers’ health-seeking behaviour in acute diarrhoea in Tlaxcala, Mexico. J Diarrhoeal Dis Res. 1996;14(4):260–268. [PubMed] [Google Scholar]
- 41.Omore R, O'Reilly CE, Williamson J, Moke F, Were V, Farag TH, et al. Health care-seeking behavior during childhood diarrheal illness: results of health care utilization and attitudes surveys of caretakers in western Kenya, 2007–2010. Am J Trop Med Hyg. 2013;89(1 Suppl):29–40. [DOI] [PMC free article] [PubMed]
- 42.Olson CK, Blum LS, Patel KN, Oria PA, Feikin DR, Laserson KF, et al. Community case management of childhood diarrhea in a setting with declining use of oral rehydration therapy: findings from cross-sectional studies among primary household caregivers, Kenya, 2007. Am J Trop Med Hyg. 2011;85(6):1134–40. [DOI] [PMC free article] [PubMed]
- 43.Singh MB. Maternal beliefs and practices regarding the diet and use of herbal medicines during measles and diarrhea in rural areas. Indian Pediatr. 1994;31(3):340–343. [PubMed] [Google Scholar]
- 44.Hudelson P, Aguilar E, Charaly MD, Marca D, Herrera M. Improving the home management of childhood diarrhoea in Bolivia. Int Q Community Health Educ. 1994;15(1):91–104. doi: 10.2190/KEB1-F3V0-9JHK-691T. [DOI] [PubMed] [Google Scholar]
- 45.Uchendu UO, Emodi IJ, Ikefuna AN. Pre-hospital management of diarrhoea among caregivers presenting at a tertiary health institution: implications for practice and health education. Afr Health Sci. 2011;11(1):41–47. [PMC free article] [PubMed] [Google Scholar]
- 46.Ahmed F, Farheen A, Ali I, Thakur M, Muzaffar A, Samina M. Management of diarrhea in under-fives at home and health facilities in Kashmir. Int J Health Sci (Qassim) 2009;3(2):171–175. [PMC free article] [PubMed] [Google Scholar]
- 47.Ekanem EE, Akitoye CO. Child feeding by Nigerian mothers during acute diarrhoeal illness. J R Soc Health. 1990;110(5):164–165. doi: 10.1177/146642409011000506. [DOI] [PubMed] [Google Scholar]
- 48.Jinadu MK, Odebiyi O, Fayewonyom BA. Feeding practices of mothers during childhood diarrhoea in a rural area of Nigeria. Trop Med Int Health. 1996;1(5):684–689. doi: 10.1111/j.1365-3156.1996.tb00096.x. [DOI] [PubMed] [Google Scholar]
- 49.McLennan JD. Home management of childhood diarrhoea in a poor periurban community in Dominican Republic. J Health Popul Nutr. 2002;20(3):245–254. [PubMed] [Google Scholar]
- 50.Ali NS, Azam SI, Noor R. Women’s beliefs regarding food restrictions during common childhood illnesses: a hospital based study. J Ayub Med Coll Abbottabad. 2003;15(1):26–28. [PubMed] [Google Scholar]
- 51.Smith GD, Gorter A, Hoppenbrouwer J, Sweep A, Perez RM, Gonzalez C, et al. The cultural construction of childhood diarrhoea in rural Nicaragua: relevance for epidemiology and health promotion. Soc Sci Med. 1993;36(12):1613–24. [DOI] [PubMed]
- 52.Martinez H, Saucedo G. Mothers’ perceptions about childhood diarrhoea in rural Mexico. J Diarrhoeal Dis Res. 1991;9(3):235–243. [PubMed] [Google Scholar]
- 53.Amini-Ranjbar S, Bavafa B. Iranian mother’s child feeding practices during diarrhea: A study in Kerman. Pakistan J Nutr. 2007;6(3):217–219. doi: 10.3923/pjn.2007.217.219. [DOI] [Google Scholar]
- 54.Bhatia V, Swami HM, Bhatia M, Bhatia SP. Attitude and practices regarding diarrhoea in rural community in Chandigarh. Indian J Pediatr. 1999;66(4):499–503. doi: 10.1007/BF02727156. [DOI] [PubMed] [Google Scholar]
- 55.Mushtaque A, Chowdhury R, Kabir ZN. Folk terminology for diarrhea in rural Bangladesh. Rev Infect Dis. 1991;13(Suppl 4):S252–254. doi: 10.1093/clinids/13.supplement_4.s252. [DOI] [PubMed] [Google Scholar]
- 56.Olakunle JM, Valentine UO, Kamaldeen AS, Buhari ASM. Assessment of mothers’ knowledge of home management of childhood diarrhea in a Nigerian setting. Int J Pharmaceut Res Bio Sci. 2012;1(4):168–184. [Google Scholar]
- 57.Langsten R, Hill K. Treatment of childhood diarrhea in rural Egypt. Soc Sci Med. 1995;40(7):989–1001. doi: 10.1016/0277-9536(94)00163-N. [DOI] [PubMed] [Google Scholar]
- 58.Baclig PV, Patrick WK. The cultural definition of an infantile diarrhea in Tambon Korat and Koongyang, northeast Thailand: community perceptions in diarrhea control. Asia Pac J Public Health. 1990;4(1):59–64. doi: 10.1177/101053959000400110. [DOI] [PubMed] [Google Scholar]
- 59.Walker CLF, Walker N. The Lives Saved Tool (LiST) as a model for diarrhea mortality reduction. BMC Med. 2014;12(1):70. doi: 10.1186/1741-7015-12-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Uchendu UO, Ikefuna AN, Emodi IJ. Medication use and abuse in childhood diarrhoeal diseases by caregivers reporting to a Nigerian tertiary health institution. South Afr J Child Health. 2009;3(3):83–89. [Google Scholar]
- 61.Gama H, Correia S, Lunet N. Questionnaire design and the recall of pharmacological treatments: a systematic review. Pharmacoepidemiol Drug Saf. 2009;18(3):175–187. doi: 10.1002/pds.1703. [DOI] [PubMed] [Google Scholar]
- 62.El-Gilany AH, Hammad S. Epidemiology of diarrhoeal diseases among children under age 5 years in Dakahlia, Egypt. East Mediterr Health J. 2005;11(4):762–775. [PubMed] [Google Scholar]
- 63.Strina A, Cairncross S, Prado MS, Teles CA, Barreto ML. Childhood diarrhoea symptoms, management and duration: observations from a longitudinal community study. Trans R Soc Trop Med Hyg. 2005;99(6):407–416. doi: 10.1016/j.trstmh.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 64.Ekwochi U, Chinawa JM, Obi I, Obu HA, Agwu S. Use and/or misuse of antibiotics in management of diarrhea among children in Enugu, Southeast Nigeria. J Trop Pediatr. 2013;59(4):314–316. doi: 10.1093/tropej/fmt016. [DOI] [PubMed] [Google Scholar]
- 65.Wongsaroj T, Thavornnunth J, Charanasri U. Study on the management of diarrhea in young children at community level in Thailand. J Med Assoc Thai. 1997;80(3):178–182. [PubMed] [Google Scholar]
- 66.Hoa NQ, Ohman A, Lundborg CS, Chuc NTK. Drug use and health-seeking behavior for childhood illness in Vietnam-A qualitative study. Health Policy. 2007;82(3):320–329. doi: 10.1016/j.healthpol.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 67.Rheinlander T, Samuelsen H, Dalsgaard A, Konradsen F. Perspectives on child diarrhoea management and health service use among ethnic minority caregivers in Vietnam. BMC Public Health. 2011;11:690. doi: 10.1186/1471-2458-11-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zwisler G, Simpson E, Moodley M. Treatment of diarrhea in young children: results from surveys on the perception and use of oral rehydration solutions, antibiotics, and other therapies in India and Kenya. J Glob Health. 2013;3(1):10403. doi: 10.7189/jogh.03.010403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Le TH, Ottosson E, Nguyen TK, Kim BG, Allebeck P. Drug use and self-medication among children with respiratory illness or diarrhea in a rural district in Vietnam: a qualitative study. J Multidiscip Healthc. 2011;4:329–336. doi: 10.2147/JMDH.S22769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Okumura J, Wakai S, Umenai T. Drug utilisation and self-medication in rural communities in Vietnam. Soc Sci Med. 2002;54(12):1875–1886. doi: 10.1016/S0277-9536(01)00155-1. [DOI] [PubMed] [Google Scholar]
- 71.Winch PJ, Gilroy KE, Doumbia S, Patterson AE, Daou Z, Diawara A, et al. Operational issues and trends associated with the pilot introduction of zinc for childhood diarrhoea in Bougouni district, Mali. J Health Popul Nutr. 2008;26(2):151–62. [PMC free article] [PubMed]
- 72.Friend-du Preez N, Cameron N, Griffiths P. “So they believe that if the baby is sick you must give drugs…” The importance of medicines in health-seeking behaviour for childhood illnesses in urban South Africa. Soc Sci Med. 2013;92:43–52. doi: 10.1016/j.socscimed.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 73.Baqui AH, Black RE, El Arifeen S, Yunus M, Zaman K, Begum N, et al. Zinc therapy for diarrhoea increased the use of oral rehydration therapy and reduced the use of antibiotics in Bangladeshi children. J Health Popul Nutr. 2004;22(4):440–2. [PubMed]
- 74.Okoro BA, Jones IO. Pattern of drug therapy in home management of diarrhoea in rural communities of Nigeria. J Diarrhoeal Dis Res. 1995;13(3):151–154. [PubMed] [Google Scholar]
- 75.Jousilahti P, Madkour SM, Lambrechts T, Sherwin E. Diarrhoeal disease morbidity and home treatment practices in Egypt. Public Health. 1997;111(1):5–10. doi: 10.1038/sj.ph.1900318. [DOI] [PubMed] [Google Scholar]
- 76.World Health Organization . Diarrhoeal diseases household case management survey, Nepal, June, 1990 (Extended WER) Geneva: WHO; 1991. p. 22. [Google Scholar]
- 77.Diarrhoeal disease control (CDD) and acute respiratory infections (ARI). Combined CDD/ARI/breast-feeding survey, 1992. Wkly Epidemiol Rec 1993;68(17):120–122. [PubMed]
- 78.Ellis AA, Winch P, Daou Z, Gilroy KE, Swedberg E. Home management of childhood diarrhoea in southern Mali--implications for the introduction of zinc treatment. Soc Sci Med. 2007;64(3):701–712. doi: 10.1016/j.socscimed.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 79.Gorter AC, Sanchez G, Pauw J, Perez RM, Sandiford P, Smith GD. Childhood diarrhoea in rural Nicaragua: beliefs and traditional health practices. Boletin de la Oficina Sanitaria Panamericana. 1995;119(5):377–390. [PubMed] [Google Scholar]
- 80.Hudelson PM. ORS and the treatment of childhood diarrhea in Managua, Nicaragua. Soc Sci Med. 1993;37(1):97–103. doi: 10.1016/0277-9536(93)90322-U. [DOI] [PubMed] [Google Scholar]
- 81.Ene-Obong HN, Iroegbu CU, Uwaegbute AC. Perceived causes and management of diarrhoea in young children by market women in Enugu State, Nigeria. J Health Popul Nutr. 2000;18(2):97–102. [PubMed] [Google Scholar]
- 82.Alam MB, Ahmed FU, Rahman ME. Misuse of drugs in acute diarrhoea in under-five children. Bangladesh Med Res Counc Bull. 1998;24(2):27–31. [PubMed] [Google Scholar]
- 83.Fischer Walker CL, Fontaine O, Black RE. Measuring coverage in MNCH: current indicators for measuring coverage of diarrhea treatment interventions and opportunities for improvement. PLoS Med. 2013;10(5):e1001385. doi: 10.1371/journal.pmed.1001385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Emond A, Pollock J, Da Costa N, Maranhao T, Macedo A. The effectiveness of community-based interventions to improve maternal and infant health in the Northeast of Brazil. Rev Panam Salud Publica. 2002;12(2):101–110. doi: 10.1590/S1020-49892002000800005. [DOI] [PubMed] [Google Scholar]
- 85.Webb AL, Ramakrishnan U, Stein AD, Sellen DW, Merchant M, Martorell R. Greater years of maternal schooling and higher scores on academic achievement tests are independently associated with improved management of child diarrhea by rural Guatemalan mothers. Matern Child Health J. 2010;14(5):799–806. doi: 10.1007/s10995-009-0510-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Martinez H, Ryan GW, Guiscafre H, Gutierrez G. An intercultural comparison of home case management of acute diarrhea in Mexico: implications for program planners. Arch Med Res. 1998;29(4):351–360. [PubMed] [Google Scholar]
- 87.Kristiansson C, Gotuzzo E, Rodriguez H, Bartoloni A, Strohmeyer M, Tomson G, et al. Access to health care in relation to socioeconomic status in the Amazonian area of Peru. Int J Equity Health. 2009;8:11. [DOI] [PMC free article] [PubMed]
- 88.Langsten R, Hill K. Diarrhoeal disease, oral rehydration, and childhood mortality in rural Egypt. J Trop Pediatr. 1994;40(5):272–278. doi: 10.1093/tropej/40.5.272. [DOI] [PubMed] [Google Scholar]
- 89.Diarrhoeal Diseases Control Programme: diarrhoea morbidity and case management survey, Morocco. Weekly Epidemiological Record 1991, 66(13):89–91. [PubMed]
- 90.Morisky DE, Kar SB, Chaudhry AS, Chen KR, Shaheen M, Chickering K. Update on ORS usage in Pakistan: results of a national study. Pakistan J Nutr. 2002;1(3):143–150. doi: 10.3923/pjn.2002.143.150. [DOI] [Google Scholar]
- 91.Nasrin D, Wu Y, Blackwelder WC, Farag TH, Saha D, Sow SO, et al. Health care seeking for childhood diarrhea in developing countries: evidence from seven sites in Africa and Asia. Am J Trop Med Hyg. 2013;89(1 Suppl):3–12. [DOI] [PMC free article] [PubMed]
- 92.Bella H, Ai-Freihi H, El-Mousan M, Danso KT, Sohaibani M, Khazindar MS. Knowledge, Attitudes and Practices related to Diarrhoea in Eastern Province, Saudi Arabia. J Family Community Med. 1994;1(1):40–44. [PMC free article] [PubMed] [Google Scholar]
- 93.Al-Mazrou YY, Aziz KM, Khan MU, Farag MK, Al-Shehri SN. ORS use in diarrhoea in Saudi children: is it adequate? J Trop Pediatr. 1995;41(Suppl 1):53–58. doi: 10.1093/tropej/41.supplement_1.53. [DOI] [PubMed] [Google Scholar]
- 94.Ketsela T, Asfaw M, Belachew C. Knowledge and practice of mothers/care-takers towards diarrhoea and its treatment in rural communities in Ethiopia. Ethiop Med J. 1991;29(4):213–224. [PubMed] [Google Scholar]
- 95.Mash D, Aschenaki K, Kedamo T, Walternsperger K, Gebreyes K, Pasha O, et al. Community and facility surveys illuminate the pathway to child survival in Liben Woreda, Ethiopia. East Afr Med J. 2003;80(9):463–9. [DOI] [PubMed]
- 96.Saha D, Akinsola A, Sharples K, Adeyemi MO, Antonio M, Imran S, et al. Health Care Utilization and Attitudes Survey: understanding diarrheal disease in rural Gambia. Am J Trop Med Hyg. 2013;89(1 Suppl):13–20. [DOI] [PMC free article] [PubMed]
- 97.Mirza NM, Caulfield LE, Black RE, Macharia WM. Risk factors for diarrheal duration. Am J Epidemiol. 1997;146(9):776–785. doi: 10.1093/oxfordjournals.aje.a009354. [DOI] [PubMed] [Google Scholar]
- 98.Burton DC, Flannery B, Onyango B, Larson C, Alaii J, Zhang X, et al. Healthcare-seeking behaviour for common infectious disease-related illnesses in rural Kenya: a community-based house-to-house survey. J Health Popul Nutr. 2011;29(1):61–70. [DOI] [PMC free article] [PubMed]
- 99.Simpson E, Zwisler G, Moodley M. Survey of caregivers in Kenya to assess perceptions of zinc as a treatment for diarrhea in young children and adherence to recommended treatment behaviors. J Glob Health. 2013;3(1):10405. doi: 10.7189/jogh.03.010405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Perez F, Ba H, Dastagire SG, Altmann M. The role of community health workers in improving child health programmes in Mali. BMC Int Health Hum Rights. 2009;9:28. doi: 10.1186/1472-698X-9-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Edet EE. Fluid intake and feeding practices during diarrhoea in Odukpani, Nigeria. East Afr Med J. 1996;73(5):289–291. [PubMed] [Google Scholar]
- 102.Omokhodion FO, Oyemade A, Sridhar MK, Olaseha IO, Olawuyi JF. Diarrhoea in children of Nigerian market women: prevalence, knowledge of causes, and management. J Diarrhoeal Dis Res. 1998;16(3):194–200. [PubMed] [Google Scholar]
- 103.Ogunrinde OG, Raji T, Owolabi OA, Anigo KM. Knowledge, attitude and practice of home management of childhood diarrhoea among caregivers of under-5 children with diarrhoeal disease in Northwestern Nigeria. J Trop Pediatr. 2012;58(2):143–146. doi: 10.1093/tropej/fmr048. [DOI] [PubMed] [Google Scholar]
- 104.Cooke ML, Nel ER, Cotton MF. Pre-hospital management and risk factors in children with acute diarrhoea admitted to a short-stay ward in an urban South African hospital with a high HIV burden. South Afr J Child Health. 2013;7(3):84–87. doi: 10.7196/sajch.472. [DOI] [Google Scholar]
- 105.Haroun HM, Mahfouz MS, El Mukhtar M, Salah A. Assessment of the effect of health education on mothers in Al Maki area, Gezira state, to improve homecare for children under five with diarrhea. J Family Community Med. 2010;17(3):141–146. doi: 10.4103/1319-1683.74332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Taha AZ. Assessment of mother’s knowledge and practice in use of oral rehydration solution for diarrhea in rural Bangladesh. Saudi Med J. 2002;23(8):904–908. [PubMed] [Google Scholar]
- 107.Larson CP, Saha UR, Nazrul H. Impact monitoring of the national scale up of zinc treatment for childhood diarrhea in Bangladesh: repeat ecologic surveys. PLoS Med. 2009;6(11):e1000175. doi: 10.1371/journal.pmed.1000175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sood AK, Kapil U. Knowledge and practices among rural mothers in Haryana about childhood diarrhea. Indian J Pediatr. 1990;57(4):563–566. doi: 10.1007/BF02726770. [DOI] [PubMed] [Google Scholar]
- 109.Gupta N, Jain SK, Chawla U, Hossain S, Venkatesh S. An evaluation of diarrheal diseases and acute respiratory infections control programmes in a Delhi slum. Indian J Pediatr. 2007;74(5):471–476. doi: 10.1007/s12098-007-0080-4. [DOI] [PubMed] [Google Scholar]
- 110.Diarrhoeal disease control programme Household survey of diarrhoea case management, Nepal. Wkly Epidemiol Rec. 1991;66(37):273–276. [PubMed] [Google Scholar]
- 111.Jha N, Singh R, Baral D. Knowledge, attitude and practices of mothers regarding home management of acute diarrhoea in Sunsari, Nepal. Nepal Med Coll J. 2006;8(1):27–30. [PubMed] [Google Scholar]
- 112.Hoan LT, Chuc NTK, Ottosson E, Allebeck P. Drug use among children under 5 with respiratory illness and/or diarrhoea in a rural district of Vietnam. Pharmacoepidemiol Drug Saf. 2009;18(6):448–453. doi: 10.1002/pds.1730. [DOI] [PubMed] [Google Scholar]
- 113.Larrea-Killinger C, Munoz A. The child’s body without fluid: mother’s knowledge and practices about hydration and rehydration in Salvador, Bahia, Brazil. J Epidemiol Community Health. 2013;67(6):498–507. doi: 10.1136/jech-2012-201919. [DOI] [PubMed] [Google Scholar]
- 114.Granich R, Cantwell MF, Long K, Maldonado Y, Parsonnet J. Patterns of health seeking behavior during episodes of childhood diarrhea: a study of Tzotzil-speaking Mayans in the highlands of Chiapas, Mexico. Soc Sci Med. 1999;48(4):489–495. doi: 10.1016/S0277-9536(98)00356-6. [DOI] [PubMed] [Google Scholar]
- 115.Ecker L, Ochoa TJ, Vargas M, Del Valle LJ, Ruiz J. Factors affecting caregivers’ use of antibiotics available without a prescription in Peru. Pediatrics. 2013;131(6):e1771–1779. doi: 10.1542/peds.2012-1970. [DOI] [PubMed] [Google Scholar]
- 116.Agha A, White F, Younus M, Kadir MM, Alir S, Fatmi Z. Eight key household practices of integrated management of childhood illnesses (IMCI) amongst mothers of children aged 6 to 59 months in Gambat, Sindh, Pakistan. J Pak Med Assoc. 2007;57(6):288–293. [PubMed] [Google Scholar]
- 117.Rasheed P. Perception of diarrhoeal diseases among mothers and mothers-to-be: implications for health education in Saudi Arabia. Soc Sci Med. 1993;36(3):373–377. doi: 10.1016/0277-9536(93)90022-V. [DOI] [PubMed] [Google Scholar]
- 118.Mwambete KD, Joseph R. Knowledge and perception of mothers and caregivers on childhood diarrhoea and its management in Temeke municipality, Tanzania. Tanzan J Health Res. 2010;12(1):47–54. doi: 10.4314/thrb.v12i1.56278. [DOI] [PubMed] [Google Scholar]
- 119.Buch NA, Hassan M, Bhat IA. Parental awareness and practices in acute diarrhea. Indian Pediatr. 1995;32(1):76–79. [PubMed] [Google Scholar]
- 120.Datta V, John R, Singh VP, Chaturvedi P. Maternal knowledge, attitude and practices towards diarrhea and oral rehydration therapy in rural Maharashtra. Indian J Pediatr. 2001;68(11):1035–1037. doi: 10.1007/BF02722350. [DOI] [PubMed] [Google Scholar]
- 121.Sheetal V. Impact of education on rural women about preparing ORS and SSS: a study of the primary health centre, Uvarsad, Gandhinagar. Health Popul Perspect Issues. 2009;32(3):124–130. [Google Scholar]
- 122.Bolam A, Manandhar DS, Shrestha P, Ellis M, Costello AM. The effects of postnatal health education for mothers on infant care and family planning practices in Nepal: a randomised controlled trial. BMJ. 1998;316(7134):805–811. doi: 10.1136/bmj.316.7134.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Adhikari P, Dhungel S, Shrestha R, Khanal S. Knowledge attitude and practice (KAP) study regarding facts for life. Nepal Med Coll J. 2006;8(2):93–96. [PubMed] [Google Scholar]


