Abstract
Social cognition is the collection of cognitive processes required to understand and interact with others. The term ‘social brain’ refers to the network of brain regions that underlies these processes. Recent evidence suggests that a number of social cognitive functions continue to develop during adolescence, resulting in age differences in tasks that assess cognitive domains including face processing, mental state inference and responding to peer influence and social evaluation. Concurrently, functional and structural magnetic resonance imaging (MRI) studies show differences between adolescent and adult groups within parts of the social brain. Understanding the relationship between these neural and behavioural observations is a challenge. This review discusses current research findings on adolescent social cognitive development and its functional MRI correlates, then integrates and interprets these findings in the context of hypothesised developmental neurocognitive and neurophysiological mechanisms.
Keywords: Adolescence, fMRI, Social brain, Development, Mentalising, Face processing, Emotion regulation
1. Introduction
Humans are an intensely social species. Humans show a repertoire of social abilities – from rapidly and automatically detecting the presence of another human in our environment, to making inferences about their emotions, beliefs and enduring character traits, and finally using this knowledge to guide interactions (Frith and Frith, 2008, 2010). The last two decades have seen significant progress in understanding the neural underpinnings of human social abilities. The non-invasive in vivo neuroimaging technique functional magnetic resonance imaging (fMRI) has played an important role in this research. Recently, fMRI studies have begun to reveal how the functional neural correlates of social cognition change during development.
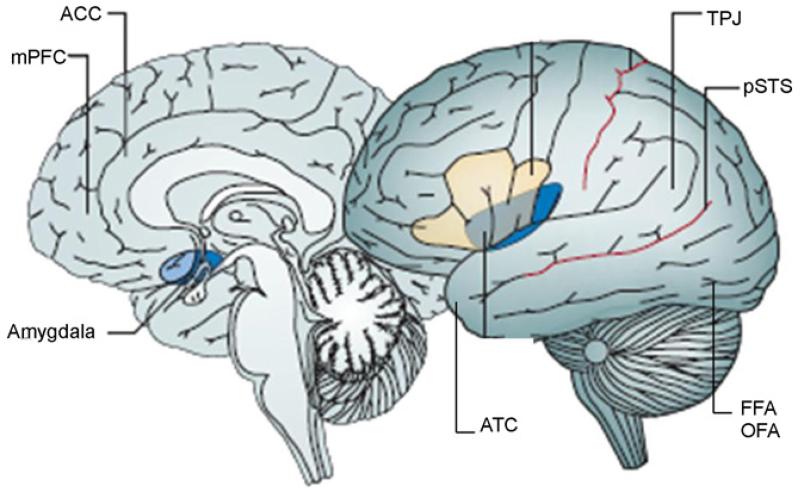
The collection of brain regions subserving social cognition is referred to as the ‘social brain’ (Brothers, 1990; Frith, 2007) (see Fig. 1). The social brain includes the fusiform face area (FFA), posterior superior temporal sulcus (pSTS), amygdala, temporo-parietal junction (TPJ), anterior rostral medial prefrontal cortex (MPFC), the anterior cingulate cortex (ACC) and anterior temporal cortex (ATC). Functional MRI studies show differences between adolescence and adulthood in patterns of activity within these regions and, more recently, in their patterns of functional connectivity. Anatomical MRI studies indicate continuing structural brain development across the period of adolescence, including within certain regions of the social brain.
Fig. 1.
The social brain (adapted from Blakemore, 2008). Regions shown (clockwise from top left) are medial prefrontal cortex (MPFC), anterior cingulate cortex (ACC), temporo-parietal junction (TPJ), posterior superior temporal sulcus (pSTS), fusiform face area (FFA), occipital face area (OFA), anterior temporal cortex (ATC) and amygdala.
Behavioural studies are vital to interpret and qualify developmental neuroimaging findings in terms of preserved vs. changing cognitive abilities. It is reasonable to hypothesise that neuroanatomical reorganisation within social brain regions may alter their functionality, causing changes at a cognitive/behavioural level. At present, the experimental picture is incomplete, although a number of models relating adolescent social cognition to structural and functional development of the social brain have been proposed (see Sections 3 and 4).
In the following section, research on adolescent social cognitive development and its functional neural correlates is summarised, beginning with research on face processing, and proceeding to mentalising, peer influence and then social evaluation. Subsequently, Section 3 summarises current theoretical neurocognitive models accounting for adolescent behavioural and functional neuroimaging changes. Finally, Section 4 evaluates evidence relating to the interpretation of adolescent fMRI findings in the context of structural MRI findings, with consideration of potential neurophysiological mechanisms.
2. Functional neuroimaging and behavioural studies of social cognition in adolescence
2.1. Face processing
2.1.1. Basic face processing
A fundamental requirement for social interaction is the ability to rapidly note the presence of another human being, from visual, auditory and other cues. A particularly salient source of person information is the presence of visual cues indicating a face. Behavioural work with newborn infants has shown a preferential tuning towards face-like objects within hours of being born, with photographs and cartoons of faces being preferred over inverted or non-face objects (Morton and Johnson, 1991; Farroni et al., 2002). It has also been shown that infants prefer looking at faces that engage them in mutual eye gaze, rather than those showing averted gaze (Macchi et al., 2004). In view of these early preferences, it has come as a surprise that specialised face processing mechanisms, at both the behavioural level and the neural level, continue to develop throughout the first and even the second decade of life (Carey et al., 1980; Durand et al., 2007; Mondloch et al., 2003).
An early study by Carey et al. (1980) showed improvement in facial identity recognition across the first decade of life, followed by a brief dip in performance at age 12 (see also Diamond et al., 1983). In a more recent study by Mondloch et al. (2003), a matching task was administered to 6-, 8- and 10-year-old children and adults, which required participants to compare faces on the basis of identity (with facial expression and head orientation varying), facial expression, gaze direction and sound being spoken. Results showed that, in comparison to adults, the 6-year-olds made more errors on every task, and the 8-year-olds made more errors on three of the five tasks, namely when matching the direction of gaze and on the two identity tasks. The 10-year-olds made more errors than did adults on the identity task in which head orientation varied. This suggests that basic face processing abilities, here the ability to recognize identity in a context-invariant manner, continue to develop until at least the end of the first decade of life.
Recent cross-sectional developmental fMRI studies suggest that the prolonged acquisition of face processing abilities is mirrored by protracted functional specialisation within the cortical face processing network (Haxby et al., 2000; Cohen Kadosh and Johnson, 2007; Johnson et al., 2009). In one fMRI study, children (N = 23, 7–11 years, 13 female), adolescents (N = 10, 12–16 years, 5 female) and adults (N = 17, 18–35 years, 8 female) passively viewed photographic images of faces, vs. objects, places or abstract patterns (Golarai et al., 2007) (see Table 1). Results showed an age-related increase in the spatial extent of face-selective suprathreshold activation within right fusiform cortex (the ‘fusiform face area’; FFA): FFA was significantly larger in adult than child groups, and the adolescent group showed an intermediate pattern. The expansion of FFA into surrounding cortex was correlated with a behavioural improvement in recognition memory for facial identity.
Table 1.
Development differences in fMRI studies of social cognition
| Study | Participants | Paradigm | Results |
|---|---|---|---|
| Basic face processing | |||
| Golarai et al. (2007) | 23 (13F) children aged 7–11; 10 (5F) adolescents aged 12–16; 17 (8F) adults aged 18–35 | Passive viewing of photographs of faces vs. places, objects and abstract patterns | Age-related increase in size of FFA: significant difference between child and adult groups; adolescents showed an intermediate size. |
| Scherf et al. (2007) | 10 (4F) children aged 5–8; 10 (4F) adolescents aged 11–14; 10 (4F) adults aged 20–23 | Passive viewing of dynamic displays of faces vs. places and objects | Age-related increase in size of FFA and face-selective STS between childhood and adolescence. Increased bilaterality between adolescence and adulthood. |
| Cohen Kadosh et al. (2010) | 16 (8F) children aged 7–8; 8 (4F) pre-adolescents aged 10–11; 13 (7F) adults aged 19–37 | Match to sample task with face photographs: matching based on identity, expression or gaze | Connectivity analysis: Basic network comprising FFA, STS and inferior occipital face area present in all age groups. Age-associated increase top-down modulation of intra-network connections depending on task context. |
| Peelen et al. (2009) | 22 (12F) children/adolescents aged 7–17; 22 (13F) adults aged 20–32 | 1-Back task with photographs of faces, headless bodies, tools, and scenes | Age-related increase in FFA selectivity for faces; no age-related increase in fusiform body area for bodies. |
| Facial emotion processing | |||
| Monk et al. (2003) | 17 (8F) children/adolescents aged 9–17; 17 (8F) adults aged 25–36 | Photographs of angry and neutral faces: passive viewing vs. emotional response rating vs. nose width rating | Adolescents vs. adults showed greater activity in right amygdala, ACC and OFC bilaterally during passive viewing of fear vs. neutral faces. |
| Guyer et al. (2008) | 31 (15F) children/adolescents aged 9–17; 30 (13F) adults aged 21–40 | As above | Adolescents vs. adults showed greater activity in amygdala bilaterally and right FFA during passive viewing of fear vs. neutral faces. |
| Mentalising | |||
| Wang et al. (2006) | 12 (6F) children and adolescents aged 9–14; 12 (6F) adults aged 23–33 | Judging sincerity vs. irony (sarcasm) of social exchanges depicted in cartoons | Children/adolescents vs. adults showed greater activity in MPFC, left IFG and right pSTS during irony vs. no irony. |
| Blakemore et al. (2007) | 19 (19F) adolescents aged 12–18; 11 (11F) adults aged 22–38 | Judging likelihood of physical causality vs. intentional causality scenarios | Adolescents vs. adults showed greater activity in MPFC during intentional vs. physical causality. Adults vs. adolescents showed greater activity in right STS for this contrast. |
| Pfeifer et al. (2007) | 12 (6F) children aged 9–11; 12(6F) adults aged 23–32 | Judging whether phrases about academic and social skills accurately described self vs. Harry Potter | Children vs. adults showed greater activity in MPFC and ACC during self- vs. other-knowledge retrieval. |
| Pfeifer et al. (2009) | 12 (7F) adolescents aged 11–14; 12 (6F) adults aged 23–30 | Judging whether phrases about academic and social skills described perceptions of self from different perspectives: self, mother, best friend, classmates | Adolescents vs. adults showed greater activity in MPFC and ACC during self- vs. other-appraisal. |
| Burnett et al. (2009) | 19 (19F) adolescents aged 10–18; 10 (10F) adults aged 22–32 | Judging emotional response during social (embarrassment, guilt) vs. basic (disgust, fear) emotion scenarios | Adolescents vs. adults showed greater activity in MPFC for social vs. basic emotions. Adults vs. adolescents showed greater activity in left ATC during this contrast. |
| Burnett and Blakemore (2009) | 18 (18F) adolescents aged 11–18; 10 (10F) adults aged 22–32 | As above | Functional connectivity analysis: Adolescents vs. adults showed stronger task-dependent connectivity between MPFC and pSTS/TPJ. |
| Social evaluation | |||
| Sebastian et al. (2010b) | 19 (19F) adolescents aged 14–16; 16 (16F) adults aged 23–28 | Rejection-themed emotional Stroop task | Adults showed greater right ventrolateral PFC response to rejection vs. neutral/acceptance words; adolescents showed a greater response in this region to acceptance vs. rejection and no difference to rejection vs. neutral. |
| Sebastian et al. (in press) | 19 (19F) adolescents aged 14–16; 16 (16F) adults aged 23–28 | Modified ‘Cyberball’ online ball-throwing game | Greater response to rejection than acceptance condition in right ventrolateral PFC in adults, but the reverse pattern in adolescence. |
| Gunther Moor et al. (2010) | 12 (7F) children aged 8–10; 14 (8F) young adolescents aged 12–14; 15 (7F) mid adolescents aged 16–17 years; 16 (8F) adults aged 19–25 years | Acceptance or rejection feedback from fictitious peers based on participant’s photo | Age-related increase during expected social feedback in activity within ventral MPFC, ACC and striatum; age-related increase during social feedback in OFC and lateral PFC. |
Gender composition indicated in brackets. Studies which include both child/adolescent and adult comparison groups only are included.
In another fMRI study in which children (N = 10, 5–8 years, 4 females), adolescents (N = 10, 11–14 years, 4 females) and adults (N = 10, 20–23 years, 4 females) freely viewed dynamic displays of faces, places and objects, an age-related increase in size of face-selective FFA was observed between childhood and adolescence, as well as an increase in face-selective superior temporal sulcus (STS) (Scherf et al., 2007). In this study, no further changes in functional specificity for face processing were noted between adolescence and adulthood, but activity became more bilateral with age.
A recent developmental fMRI study implemented dynamic causal modelling analysis (Friston et al., 2003) to examine task-dependent causal interactions among cortical face processing regions, during a match-to-sample task. This connectivity analysis enabled investigation of age group differences in the impact of differing task demands (matching based on identity, emotion or gaze) on effective connectivity between regions, in children (N = 16, 7–8 years, 8 female), pre-adolescents (N = 8, 10–11 years, 4 female) and adults (N = 13, 19–37 years, 7 female) (Cohen Kadosh et al., in press). The same basic cortical network, comprising FFA, STS and inferior occipital gyrus (‘occipital face area’, OFA), was present in all age groups. However, there was an age-related increase in the extent of differential top-down modulation of specific intra-network connections depending on task context. This finding was interpreted as a cumulative effect of exposure and training, such that the cortical network for face processing becomes increasingly fine-tuned with age.
Interestingly, developmental trajectories for the functional neural correlates of face processing seem to differ qualitatively from another social stimulus category, the perception of human bodies. A recent fMRI study compared face and body processing in participants aged 7–32 years (N = 44, 25 female) (Peelen et al., 2009). The previously reported increase in functional specialisation in FFA for face stimuli was replicated, but there was no age-related increase in functional specialisation for body stimuli in the adjacent fusiform ‘body area’ (FBA). This finding suggests a double dissociation between age and the level of proficiency at the brain level for processing distinct classes of social stimuli.
The functional neuroimaging and behavioural data summarised above can be interpreted within the ‘Interactive Specialisation’ theoretical framework (Johnson, 2001; Johnson et al., 2009). According to this account, discrete cognitive functions (e.g. facial identity recognition) are an emergent product of interactions between different brain regions, and between the whole brain and its external environment. Age-associated changes in functional brain activity, as described in this section for faces, are hypothesised to reflect emerging task selectivity or ‘fine-tuning’ within localised neural components, as a consequence of interactions between brain regions and with the environment, leading to an improvement in behavioural performance.
2.1.2. Facial emotion processing
A secondary aspect of face processing, which is particularly important for social interaction, is the ability to interpret facial displays of emotion. This cognitive function recruits a number of brain regions in addition to the basic face-processing regions described above. For example, fMRI studies in which participants view emotional face stimuli often report activity in the amygdala, which is involved in automatic emotion processing (e.g. fear and avoidance;Young et al., 1995; Haxby et al., 2000) and in parts of the prefrontal cortex including those implicated in action and emotion regulation (e.g. ACC and orbitofrontal cortex (OFC)), and in higher-level social processing (e.g. MPFC and ACC; e.g. Blair et al., 1999).
There is some behavioural evidence showing continuing development during adolescence in the ability to recognise facial emotions (for a review, see Blakemore, 2008). An early behavioural study in which male and female participants aged 10–17 matched emotional face pictures to emotion words showed evidence for a brief developmental regression at around the start of adolescence (McGivern et al., 2002). One recent study tested facial emotion recognition accuracy using morphed faces that varied along continua from neutral to fear, neutral to anger, and fear to anger (Thomas et al., 2007). Participants were children (N = 31, 7–13 years, 18 female), adolescents (N = 23, 14–18 years, 9 female) and adults (N = 48, 25–57 years, 41 female). Across all expression morphs, adults were more accurate at identifying the emotion shown than were children and adolescents. However, whereas recognition accuracy for fear showed a linear improvement across cross-sectional age anger showed a quadratic trend, with sharp improvement between adolescence and adulthood. This suggests that adolescence is characterised by continuing improvement in facial emotion recognition, but that the specific developmental trajectory may differ between emotions. This is consistent with other studies, for example Wade et al. (2006). Thomas et al. suggest the finding might reflect discrete neural underpinnings (each with a distinct developmental trajectory) for the detection of anger relative to fear. This interpretation is consistent with evidence from fMRI studies in adults, which show distinct neural components for facial expressions of anger and fear (Fusar-Poli et al., 2009). It is conceivable that these mature functionally at different rates during adolescence.
At the neural level, there is some evidence for continuing development during adolescence of functional activity within brain regions subserving facial emotion processing. It remains a challenge to dissociate (both theoretically and empirically) the fMRI substrates of facial emotion identification from those which subserve downstream processing, e.g. social inference and emotional self-regulation. Perhaps for this reason, inconsistencies are reported in the adult fMRI literature, which poses a challenge to interpretation of developmental findings (see Guyer et al., 2008 for a discussion). Bearing in mind these challenges, of note are two developmental fMRI studies of emotional face processing. In the first (Monk et al., 2003), functional brain activity was compared between fearful and neutral face viewing in adolescent (N = 17, 9–17 years, 8 female) and adult (N = 17, 25–36 years, 8 female) participants. On some trials, participants passively viewed the faces, whereas on other trials, participants were instructed to rate their emotional response to the faces, or to pay attention to a non-emotional feature (nose width). Adolescents showed greater activity than did adults within the right amygdala, ACC and OFC bilaterally during passive viewing of fearful relative to neutral faces, a result which may correspond to the trajectory of emerging behavioural competence in fearful face recognition (Thomas et al., 2007). Relative to adults, adolescents showed greater modulation by task context (attention to nose width vs. passive viewing) of ACC activity during fear vs. neutral face processing, although this result was not shown in a follow-up study.
In this follow-up study (Guyer et al., 2008), participants were adolescents (N = 31, 9–17 years, 15 female) and adults (N = 30, 21–40 years, 13 female). The study confirmed the previous finding showing an age-related decrease in amygdala engagement during passive viewing of fearful faces, and also showed greater activity in the FFA in adolescents relative to adults during this contrast. The latter result extends findings in adults that have shown greater activity in FFA during emotional relative to neutral face processing (Vuilleumier et al., 2001). This section has reviewed fMRI and behavioural studies of basic and emotional face processing. Basic face processing abilities show remarkably protracted behavioural development during childhood and adolescence, and functional MRI responses within the cortical network for face processing become more fine-tuned and robust with age. Behavioural studies suggest continuing maturation in facial emotion recognition accuracy during adolescence, although developmental fMRI studies of facial emotion processing are challenging to interpret. There is evidence that the transition from adolescence into adulthood is accompanied by a decrease in FFA and amygdala reactivity to emotional faces: Whether these changes correspond to continuing improvement in recognition accuracy for facial emotions, or to some other cognitive process, remains to be determined experimentally.
2.2. Mentalising
Mentalising, or ‘Theory of Mind’, is the ability to infer mental states such as intentions, beliefs and desires (Premack and Woodruff, 1978; Frith and Frith, 2003). This ability enables one to understand and predict other agents’ behaviours that arise as a direct consequence of their mental states (e.g. in an interpersonal context, an agent’s action towards a coffee pot is more parsimoniously understood in terms of a desire for coffee, than in terms of mechanical forces). A substantial functional neuroimaging literature indicates that mentalising in adults recruits a circumscribed set of brain regions, comprising the ATC, pSTS, TPJ and anterior rostral portion of MPFC (Frith and Frith, 2003). Briefly, the ATC is thought to represent semantic social information (Olson et al., 2007), and the pSTS is important for decoding social gestures and signals to form predictions of action or intent (Haxby et al., 2000; Saxe et al., 2004; Morris et al., 2005). Much evidence implicates both TPJ and anterior rostral MPFC in representing or attending to mental states, although their respective roles are not yet clear (Amodio and Frith, 2006; Abraham et al., 2008; Saxe et al., 2009; Hampton et al., 2008).
2.2.1. Mentalising tasks
Recently, a number of fMRI studies have investigated the neural correlates of mentalising in adolescence (see Blakemore, 2008 for a review). These adolescent studies have used a variety of mentalising tasks – from reflecting on one’s intentions to carry out certain actions (Blakemore et al., 2007), thinking about the preferences and dispositions of oneself or a fictitious story character (Pfeifer et al., 2007, 2009), judging the sincerity or sarcasm of another person’s communicative intentions (Wang et al., 2006) and reflecting on self and other’s emotional response during social situations (Burnett et al., 2009; see Table 1). Despite the variety of tasks used, these studies have consistently shown that activity within anterior rostral MPFC, during mentalising relative to control tasks, correlates negatively with age between adolescence and adulthood (Blakemore, 2008). Some of these studies have shown that activity within posterior and temporal components of the mentalising system, including pSTS, TPJ or ATC, shows the opposite developmental pattern. Recently, it has been shown that the shift in activity within the mentalising system is accompanied by a change in task-dependent interactions (effective connectivity) between anterior rostral MPFC and the pSTS/TPJ (Burnett and Blakemore, 2009).
Given the purported role of anterior rostral MPFC in representing mental states, one hypothesis regarding the age-related decrease in anterior rostral MPFC activity, and changes in its effective connectivity profile, is that it may reflect or underlie a change in mentalising proficiency or strategy. Alternatively, or in addition, the shift in activity may represent increasing regional specialisation, or efficiency within integrated neural circuits (Durston et al., 2006; Brown et al., 2006; Saxe et al., 2009). The development of mentalising proficiency up to the age of five has been studied extensively and is well characterised (e.g. Frith and Frith, 2003), but very little is known about the development of mentalising beyond early childhood. This could be due to a lack of suitable paradigms: in order to create a mentalising task that does not elicit ceiling performance in children aged five and older, the linguistic and executive demands of the task must be increased. This renders any age-associated improvement in performance difficult to attribute to improved mentalising ability per se. However, in view of the increasing complexity of social relationships in adolescence (Brown, 2004) and reports of age-related increases in tolerance for diversity in others’ beliefs (Wainryb et al., 2001), some measure of mentalising proficiency, or its interaction with other cognitive functions, is expected to show continuing development across adolescence.
A recent study by Dumontheil et al. used a novel on-line mentalising task in which children (N = 71, 7–11 years, female) adolescents (N = 70, 11–18 years, female) and adults (N = 36, 19–28 years, female) were instructed to sequentially move objects between a set of shelves as instructed by a ‘Director’ character (Dumontheil et al., 2010). The Director could see the contents of only some of the shelves, and therefore correct interpretation of the Director’s instructions required participants to take into account the Director’s visual perspective and use this mental state information on line in a communicative situation. Results showed continuing development during late adolescence (between age 17 and early adulthood) in performance on visual perspective (mentalising) trials, relative to rule-based control trials relying on executive functions only. Advanced mentalising paradigms are now needed that are less heavily reliant on visual perspective-taking. It will be valuable to evaluate alternative interpretations of the Dumontheil et al. developmental result, for example, that it reflects maturation of allocentric vs. egocentric spatial representation ability, as opposed to mentalising.
2.2.2. Behavioural economic games
Behavioural economic paradigms have recently been used to investigate the use of mental state inferences in strategic social decision-making. Games such as the Ultimatum Game and the Trust Game, which engage participants in structured competitive or co-operative interactions, reveal subtle differences in the degree of mental perspective-taking (among other social variables). This is quantified as the amount of money or tokens exchanged (Berg et al., 1995; Binmore, 2007). A number of fMRI studies in adults have shown task-related activity within the brain’s reward system (e.g. nucleus accumbens) during economic games, consistent with the desire to win monetary rewards; and also within the mentalising system (e.g. pSTS, TPJ, anterior rostral MPFC), consistent with the processing of one’s own and the other player’s actions and intentions (Montague, 2007).
Behavioural studies have shown evidence that the tendency to strategically use mental state information to win money in such games continues to develop during adolescence. A study using a modified Ultimatum Game showed that the tendency to make a generous offer of money was increasingly modulated by the perceived power of one’s co-player to punish a selfish offer between age 9 and 18 (Experiment 2: N = 56, 9–18 years, 26 female; Güroğlu et al., 2009). In contrast, the tendency to act upon basic, inflexible social principles, such as fairness and reciprocity (e.g. acting on the simple rule of reciprocating generosity), is present from a relatively early age (6–9 years) (van den Bos et al., 2009; Sutter and Kocher, 2007). This development could be due to the development of specifically social functions, domain-general executive functions, or indeed the strategic modulation of the latter by social context.
2.3. Peer influence
Peer influence is a nebulous construct in cognitive terms, but it is one that has been studied with particular interest with regards to adolescent social cognition and behaviour. Peer influence is conceptualised as acting on multiple levels – from implicit ‘priming’ effects on bodily gestures and mood, to broader influences on an individual’s social attitudes and activities. In this section, developmental studies will be reviewed that investigate the influence of peers on self-reported attitudes, and the influence of peers on risk-taking in an experimental context. Finally structural and functional MRI correlates will be touched upon.
Much evidence indicates that peer influence in adolescence can promote engagement in beneficial and prosocial behaviours (Eisenberg et al., 2004). However for practical (e.g. public health) reasons, empirical studies tend to focus on peer influence on potentially harmful or ‘risky’ behaviours (see Geier and Luna, 2009). One study investigated age group differences, between adolescence (N = 106, 13–16 years, 54 female), youth (N = 105, 18–22 years, 53 female) and adulthood (N = 95, age 24+, 48 female), in the number of risky decisions made in a driving simulation game, for example speeding through an amber traffic light (Gardner and Steinberg, 2005). The game was played alone or in the presence of two peers, and adolescents took many more risks when driving in the presence of peers, compared to when they were alone. In contrast, adult risk-taking did not differ between the social and solitary conditions, and youths showed an intermediate effect. It would be valuable to identify which aspect of the peers’ present condition was most important in inducing high risk-taking in adolescence: distinct adolescent mechanisms mediating group influence, age differences in norms for risk-seeking or some other factor.
Another study used a self-report questionnaire to chart development during adolescence in the tendency to resist peer influence (Steinberg and Monahan, 2007). More than 3600 male and female participants aged 10–23+ completed a ‘Resistance to Peer Influence’ (RPI) questionnaire consisting of items assessing applicability to self of both morally valenced and neutral (personal preference) statements such as ‘some people go along with their friends just to keep their friends happy’. The results showed a linear increase in RPI between 14 and 18 years.
Few neuroimaging studies have investigated the neural bases of peer influence. In one fMRI study (Grosbras et al., 2007), pre-adolescent (10-year old) children were divided into two groups on the basis of a median split on RPI score in the Steinberg and Monahan questionnaire, described above. Children then underwent functional imaging while passively viewing clips of angry hand and face gestures. The group who scored high on the resistance to peer influence measure showed stronger functional connectivity between brain regions underlying action perception (e.g. STS) and decision making (e.g. lateral PFC, premotor cortex) than did the group who scored low on the measure. These results are consistent with an account whereby increasing functional integration within task-related networks underpins age-related development in cognitive abilities (e.g. resistance to peer influence), and is associated with development of certain brain structures, although more direct studies are needed. In particular, one outstanding question for theoretical and empirical work is the extent to which these behavioural and neurobiological findings regarding adolescent peer influence should be interpreted within a common conceptual framework.
2.4. Social evaluation: acceptance and rejection
Social psychology studies have shown that adolescents, and by some accounts particularly female adolescents, are more sensitive to being excluded from a social interaction by peers, relative to adults or younger children (O’Brien and Bierman, 1988; Kloep, 1999). In cases where risky behaviour is a group norm, this effect could contribute to the impact of peer influence on risky behaviour. A recent study (Sebastian et al., 2010a) investigated social rejection experimentally, using a computerised ball-passing paradigm known as Cyberball (Williams et al., 2000; Williams and Jarvis, 2006). In Cyberball, participants are told that they are playing a ball-passing game over the Internet with two other players, represented by cartoon drawings. In reality, the players are pre-programmed computer algorithms which systematically include (by passing the ball to) or exclude participants. In this study, adolescents (young adolescents: N = 26, 11–14 years, female; mid-adolescents: N = 25, 14–16 years, female) showed significantly reduced self-reported positive mood following episodes of exclusion (social rejection) than did adults (N = 26, 22–47 years, female). Additionally, levels of anxiety were disproportionately increased following social rejection in younger adolescents (11–13 years) relative to adults, while anxiety was sustainedly high in older adolescents (14–15 years). Thus, female adolescents show heightened sensitivity to social rejection in an experimental context.
Neuroimaging studies are beginning to explore the neural basis of this effect. One study used the Cyberball paradigm, combined with fMRI in a group of adolescents (N = 23, 12–13 years, 14 female) (Masten et al., 2009). The results showed patterns of brain activity that were similar to those in a previous study in adults (Eisenberger et al., 2003): positive correlations were found between self-reported distress and activity within visceral pain and negative affect-related regions (e.g. insula) during social exclusion vs. inclusion; and negative correlations were found between self-reported distress and activity within emotion-regulation regions (e.g. ventrolateral PFC). There were some differences between the adult and adolescent studies; most notably the study in adults found a positive correlation between self-reported distress and activity in dACC, while the study in adolescents found no effect in dACC, but did find a positive relationship with activity in subgenual ACC.
One recent study (Sebastian et al., in press) directly compared adolescents (N = 19, 14–16 years, female) and adults (N = 16, 23–28 years, female) during a modified version of the Cyberball task. Results showed that adults activated ventrolateral PFC (VLPFC) to a greater extent during exclusion than during inclusion conditions, while adolescents exhibited the reverse pattern. Since right VLPFC has previously been associated with the regulation of distress during social exclusion (Eisenberger et al., 2003), it is possible that a reduced engagement of this region in adolescents in response to rejection-related stimuli underlies the increased affective response seen in adolescents in behavioural studies.
A similar result was found in an fMRI study exploring neural responses to the automatic processing of rejection-related information (Sebastian et al., 2010b). This study compared adolescents (N = 19, 14–16 years, female) and adults (N = 16, 23–28 years, female) on a rejection-themed emotional Stroop task in which participants were asked to indicate the ink colour in which rejection, acceptance and neutral words were written. In adults, rejection-themed words activated the right ventrolateral PFC to a greater extent than acceptance or neutral words. However, in the adolescent group, this regulatory region did not discriminate between rejection and neutral words, and responded more to acceptance words than to rejection. These studies are consistent with theories suggesting that prefrontal regulatory mechanisms continue to develop between mid-adolescence and adulthood (e.g. Nelson et al., 2005), and this may be one factor underlying observed adolescent hypersensitivity to rejection.
Rejection by peers is an extreme form of peer evaluation. Peer evaluation which was the subject of a recent fMRI study that used an Internet chat-room paradigm with male and female participants aged 9–17 years (N = 34, 16 female; Guyer et al., 2009). Results showed that in females only, there was an age-related increase in activity during expectation of peer evaluation, within brain regions involved in affective processing (nucleus accumbens, hypothalamus, hippocampus and insula), but no differences within the ACC or other social brain regions. The finding of gender differences in the neural response to social evaluation is in line with reports of greater social anxiety in female adolescents than in males, in response to negative social evaluations in everyday life (La Greca and Lopez, 1998). However, the possibility that female adolescents are more sensitive to social rejection than are males has not been tested empirically. Guyer et al. (2009), for example, did not find gender differences in behaviour on their task.
Recently, an fMRI study investigated peer evaluation and rejection across age, in groups of pre-adolescent (N = 12, 8–10 years, 7 female), young adolescent (N = 14, 12–14 years, 8 females), adolescent (N = 15, 16–17 years, 7 females) and adult (N = 16, 19–25 years, 8 females) participants (Gunther Moor et al., 2010). In this experiment, based on Somerville et al. (2006), participants experienced fictitious evaluation by a panel of peers. Results showed an age-related increase in activity within ventral MPFC, ACC and striatum during evaluation and predicted social feedback, while predicted social rejection resulted in activity within affect-regulation regions, such as OFC and lateral PFC, which increased linearly across age. Activity within parts of OFC during social rejection was positively correlated with self-rated social anxiety in 8–17-year-olds. An interesting point to note in this study is the age-related increase in ventral MPFC activity, while mentalising studies show age-related decreases in the more dorsally situated anterior rostral MPFC (see also Moriguchi et al., 2007). This difference could relate to possible functional subdivisions within MPFC, a large and incompletely functionally characterised brain area (Gilbert et al., 2010).
The above-mentioned fMRI studies of social evaluation show age differences in neural activity. Often, these neural changes correlate with behaviourally assessed changes in the impact of social rejection on mood. For example, fMRI findings correlate with behaviourally measured ability to regulate emotional responses to peer evaluation and rejection. This echoes findings from the studies of peer influence reviewed above. However, new behavioural measures of emotional self-regulation are needed to investigate these relationships. In addition, it is likely that an adolescent’s wider social cognitive skill-set impacts on the response to (and risk of) social rejection. Finally, while the studies reported above hint at possible gender differences in the affective and neural response to peer evaluation, the hypothesis that females are more sensitive to social evaluation should be tested empirically.
This review of the developmental fMRI and behavioural literature on social cognition is not exhaustive. However, the evidence reviewed above suggests continuing development across adolescence in the neural correlates of social cognitive processes including face processing, mentalising, peer influence and the emotional response to social evaluation and rejection. Concurrently, behavioural changes have been demonstrated in many of these domains. In the following section, these behavioural and functional neuroimaging findings are placed in the context of theoretical accounts of neural and cognitive development in adolescence.
3. Theoretical models of adolescent neurocognitive development
Several models have been proposed in which key behavioural and cognitive characteristics of adolescence, as well as the corresponding patterns of fMRI activity, are accounted for as a consequence of neural and hormonal development. These models include the Social Information Processing Network (SIPN) model (Nelson et al., 2005), the Triadic model (Ernst and Fudge, 2009) and other Developmental Mismatch models (e.g. Casey et al., 2008; Steinberg, 2008). The models are broadly compatible, differing in their degree of focus on social cognition and in the level of detailed description at each level of explanation.
The Social Information Processing Network model, which is the most explicitly social model, proposes that a process of ‘social reorienting’ takes place during adolescence, in partial consequence of neuroanatomical remodelling within ‘affective’ and ‘cognitive-regulatory’ brain nodes. This results in key behavioural characteristics of adolescence, such as risk-taking in the presence of peers, and the increasing importance of peer relationships and peer approval. Neuroanatomical remodelling is proposed to result in part from the effects of pubertal gonadal steroids on limbic regions (affective node), which are densely innervated by gonadal steroid receptors, and partly from the gradual maturation of PFC (cognitive-regulatory node), enabling more sophisticated cognitive processing and top-down control (see Dorn, 2006, for a discussion of hormone development). As well as local development within the affective and cognitive nodes, it is hypothesised that greater top-down control results from developing connectivity between these components. This review has discussed some recent data on functional connectivity development, and it is likely that this will continue to be an important direction for research, alongside structural and diffusion measures of connectivity.
In common with the SIPN model, the Triadic model of Ernst and Fudge (2009) distinguishes between affective-motivational and cognitive-regulatory neural systems, which develop anatomically during adolescence. The Triadic model subdivides the affective-motivational system into ‘approach’ and ‘avoidance’ components centred on the striatum and amygdala, respectively. According to this model, an imbalance between the approach and avoidance nodes in adolescence (relative to adulthood) contributes to adolescent risk-taking and social-affiliative characteristics. Specifically, in adolescence, the influence of the striatal approach system is said to be enhanced relative to the influence of the amygdalar avoidance system, due to anatomical development within each node and due to development in their regulation by the regulatory node (MPFC/OFC). This imbalance is hypothesised to result in distinct patterns of activity shown in fMRI studies, and in developmentally heightened risk-taking in adolescence.
The Triadic model of Ernst et al. shares some key features with Developmental Mismatch models of Casey et al. and Steinberg et al. (Casey et al., 2008; Steinberg, 2008). A number of researchers have drawn attention to the pattern of heightened risk-taking and emotional sensitivity in adolescence, relative to both childhood and adulthood. This nonlinearity across age suggests heterochronous maturation within neural systems that subserve these processes. It is hypothesised that the limbic system, including the amygdala and striatum, attains functional maturity earlier in development than does the PFC, and that the greatest mismatch in development of these systems occurs during adolescence (Casey et al., 2008). Consequently, during the time lag in functional maturity between PFC and limbic regions, individuals are more greatly affected by emotional context (e.g. reward immediacy, anticipated social rejection) when making decisions. Developmental patterns of activity during fMRI studies that employ emotional and reward-based tasks are also thought to reflect this functional mismatch. In a variant of this model, Steinberg (2008) suggests that remodelling of the dopamine system during adolescence (e.g. reduced limbic and prefrontal dopamine receptor density) increases the salience of social rewards such as peer approval, while gonadal steroid hormone release leads to an increase in sensitivity to social stimuli via effects on oxytocin receptors. The impact of increased levels of circulating gonadal (as well as adrenal) puberty hormones on brain and behaviour in humans is not fully understood (Blakemore et al., 2010).
Implicit in these models is the reasonable assumption that adolescent behavioural and cognitive development is causally related to changes in functional brain activity measured in fMRI, and that the changes in functional brain activity are related to neuroanatomical development. However, there are a number of potentially bidirectional relationships within this scheme. In the following section, evidence will be reviewed for functionally relevant relationships between structural and functional (including fMRI) brain development, and effects on behaviour, as this is of central importance while interpreting the adolescent social brain findings reviewed here.
4. Structure–function relationships in the adolescent social brain
4.1. Structural MRI findings
MRI studies show continuing neuroanatomical development during adolescence (Giedd et al., 1999; Sowell et al., 1999; Gogtay et al., 2004). Two main age-associated changes have been described in volumetric MRI studies. Firstly, cortical grey matter measures (volume, density, thickness; Ashburner and Friston, 2000) decrease across adolescence in a region-specific and commonly non-linear manner (Paus, 2005; Shaw et al., 2008; Tamnes et al., 2009; Ostby et al., 2009). Secondly, white matter volume and density increase across the brain. By many accounts, this increase is linear during the second decade of life, decelerating into adulthood (Giedd et al., 1999; Ostby et al., 2009; Paus et al., 1999). Increases in white matter volume are accompanied by progressive changes in MRI measures of white matter integrity, such as the magnetisation-transfer ratio (MTR) in MRI, and fractional anisotropy (FA) in diffusion-tensor MRI (Macchi et al., 2004; Giorgio et al., 2010; Paus et al., 2008a,b; Fornari et al., 2007).
Research has yet to systematically characterise regional grey and white matter development in specific social brain components (for example within functionally defined FFA). However, the region specificity in patterns of cortical grey matter development can be interpreted as indicating that many social brain regions continue to develop during adolescence. In general, grey matter development is completed prior to adolescence within primary sensory processing regions (e.g. occipital lobe), and during adolescence in association regions (e.g. frontal and temporal lobes). Grey matter density in the frontal and temporal lobes has been reported to follow an inverted-U shaped pattern of development, peaking around puberty onset in the frontal lobe (age 11 in girls and 12 in boys), and at around 16–17 years in the temporal lobe. These peaks in grey matter density are followed by an extended profile of decline throughout the remainder of adolescence and early adulthood (Giedd et al., 1999; Sowell et al., 1999, 2002; Ostby et al., 2009).
There is evidence for distinct trajectories of grey matter development within sub-regions of each cortical lobe (Shaw et al., 2008). In the frontal lobe, precentral (motor) grey matter density peaks prior to adolescence, whereas DLPFC and parts of MPFC attain peak grey matter volume later, at around puberty onset or beyond. Thus, subregions of the frontal lobe implicated in social cognition and executive functions show protracted adolescent grey matter change. In the temporal lobe, regions implicated in social cognition, such as the superior temporal lobe, attain peak grey matter density later (~14 years) than more middle and inferior temporal lobe regions involved in object and perceptual functions (~11–12 years; Shaw et al., 2008). Trajectories of adolescent white matter development in specific tracts related to social brain functions have yet to be delineated.
4.2. Microstructural mechanisms of developmental MRI findings
It has been suggested that adolescent changes in grey matter density, shown in MRI studies, may reflect regional alterations in synaptic density (Giedd et al., 1999). Histological studies have shown evidence for synaptic proliferation in certain brain regions at around the start of adolescence, with protracted synaptic pruning occurring across the remainder of adolescence (e.g. human frontal cortex: Huttenlocher and Dabholkar, 1998; primate: Bourgeois and Rakic, 1993; Rakic et al., 1986). Whether changes in synaptic density would be visible as volumetric changes in MRI scans is debated (see Paus et al., 2008a,b). It is reasonable to hypothesise that changes in synaptic density might be accompanied by yoked changes in glial and other cellular components (Theodosis et al., 2008). Elsewhere, it has been suggested that adolescent decreases in grey matter volume shown in MRI studies are predominantly due to intracortical myelination, resulting in an increase in the volume of tissue that is classified as white matter in MRI volumes (Paus et al., 2008b).
A possible consequence of early-adolescent prefrontal synaptogenesis could be a temporary dip in signal-to-noise ratio within these neural circuits (Blakemore, 2008; see also Rolls and Deco, in press). Subsequent synaptic pruning, perhaps occurring via experience-dependent mechanisms, would then result in more finely tuned, robust and efficient neural circuits. There is little direct evidence in support of this hypothesis at the neurophysiological level, with regards to specifically adolescent development. Results from a recent primate electrophysiology study suggest that the pruning of excitatory synapses in DLPFC could have functional impact, since the synapses which undergo pruning may be functionally mature (Gonzalez-Burgos et al., 2008).
Adolescent changes in white matter shown in structural and diffusion MRI studies, that is increasing volume, density, MTR and FA, are hypothesised to reflect processes including myelination and increasing axonal calibre (Paus et al., 2008a,b; Giorgio et al., 2010; Perrin et al., 2008). As mentioned above, it has been suggested that these cellular processes could also contribute to the volumetric grey matter changes. The probable result of both myelination and increasing axonal calibre would be an increase in axonal conduction speed (Waxman, 1980). This would not only ‘speed up’ neural processing, but allow for greater temporal precision and, e.g. inter-regional synchronisation (Paus et al., 1999; see e.g. Fornari et al., 2007).
4.3. Mechanistic relationships between MRI findings and fMRI signal
A crucial question for adolescent fMRI studies is whether there is a functionally meaningful relationship between structural brain development, reviewed above and the blood oxygenation level-dependent (BOLD) signal in fMRI. This would suggest that age differences in task-elicited BOLD signal index neuronal properties that contribute to age differences in cognition and behaviour. To our knowledge there is no direct developmental evidence focussing on the relationship, across age (particularly during adolescence), between microstructural neuronal properties (e.g. synapse density and myelination) and the BOLD signal (Ernst and Rumsey, 2009), although suggestive relationships have been shown (e.g. Fornari et al., 2007).
It has been observed that there is a ‘diffuse-to-focal’ shift in BOLD signal between childhood and adulthood (Durston et al., 2006; Thomason et al., 2005). The challenge now is to quantify this emerging focality. A good starting point would be to investigate the point-spread function (PSF), which, in the context of fMRI, is the haemodynamic response to a minimal stimulus, for example the BOLD response in striate cortex to a faint spot of light (Shmuel et al., 2007; Sirotin et al., 2009). It would be challenging to establish an equivalent ‘minimal stimulus’ for association cortex such as the TPJ or temporal poles.
The BOLD response is thought to be generated by local excitatory synaptic activity (Logothetis et al., 2001), leading to precisely localised changes in blood flow in response to the release of glutamate (Attwell and Iadecola, 2002). Therefore, changes in the BOLD signal with age could result from a change in local excitatory synaptic drive, either due to progressive myelination (resulting in increased synchronisation of afferent inputs to a region), increased excitatory synaptic density or some other mechanism (see Rolls and Deco, in press, for further discussion). Alternatively, or in addition, increased synapse density, leading to local hypoxia, could trigger an increase in capillary density, with associated changes in neurovascular coupling. This could also give rise to more ‘focal’ BOLD signals.
It is not known whether the relationship between excitatory synaptic activity and local changes in blood flow (neurovascular coupling) remains constant across age. At the low field strengths suitable for scanning children and adolescents (<7 T), the PSF in a region of cortex is thought to be largely determined by proximity to local blood vessels, rather than by local grey matter density, horizontal connections, synapses and so on (Sirotin et al., 2009; Shmuel et al., 2007). There is evidence that local blood supply alters adaptively in response to local excitatory activity, but it is not known whether the nature of this responsiveness changes across development or indeed whether there are periods of ‘neurovascular mismatch’. Further studies are needed to determine the extent to which adolescent BOLD signal changes are attributable to spatial ‘sharpening’ of broad axonal or dendritic arbours, or to increasing density of brain tissue perfusion by capillaries (see e.g. D’Esposito et al., 2003; Attwell et al., 2010).
5. Conclusions
Neuroimaging studies have shown that the social brain – the complex network of brain regions that participate in understanding and interacting with social agents – continues to develop during adolescence. Using a number of social cognition tasks, fMRI studies have shown changes in functional brain activity, which occurrs alongside emerging social cognitive proficiency and neuroanatomical development.
Evidence is awaited that will shed light on the causal links between adolescent social cognitive and social brain development via underlying neurophysiological mechanisms. Neuroanatomical development, leading to enhanced local and interregional neural processing capacity, may be necessary for transitions between each stage of cognitive development. It is also reasonable to predict that experience-dependent behavioural and cognitive progress, leading to differential recruitment of the neural substrates for social cognition, leads to modification of structural brain properties. A conceptual framework that accommodates these potentially bidirectional causal relationships is provided within the Interactive Specialisation account (Johnson et al., 2009). Within this framework, discrete cognitive functions are conceptualised as an emergent product of interactions between brain regions, and between the brain and its external environment, via a process of yoked neural and behavioural fine-tuning.
Understanding developmental fMRI findings on the social brain, and elucidating how these are related to concurrent changes in social cognition and structural brain development, will increase our understanding of the period of adolescence. Delineating typical brain development, and the sequence of emerging social cognitive abilities, may contribute to a better understanding of the rise in vulnerability to certain psychiatric illnesses in adolescence, including social anxiety, schizophrenia and addiction. It is also important to consider the role of individual differences in, for example, genetic variation or early life experience. Development of the social brain may expose an adolescent to certain vulnerabilities presented in an adverse social environment, but at the same time it presents a unique window of opportunity for fostering resilience.
Acknowledgments
The authors are grateful to David Attwell, Geoffrey Bird, Marieke Scholvinck and Hauke Hillebrandt for helpful comments on early versions of this manuscript. SJB has a Royal Society University Research Fellowship. SB was funded by a Wellcome Trust four-year PhD in Neuroscience. CS was funded by the BBSRC. KCK is funded by the ESRC.
Abbreviations
- ACC
anterior cingulate cortex
- ATC
anterior temporal cortex
- BOLD
blood oxygenation level-dependent
- DLPFC
dorsolateral prefrontal cortex
- FA
fractional anisotropy
- FFA
fusiform face area
- fMRI
functional magnetic resonance imaging
- MPFC
medial prefrontal cortex
- MRI
magnetic resonance imaging
- MTR
magnetisation-transfer ratio
- OFA
occipital face area
- OFC
orbitofrontal cortex
- PFC
prefrontal cortex
- pSTS
posterior superior temporal sulcus
- RPI
resistance to peer influence
- STS
superior temporal sulcus
- TPJ
temporo-parietal junction
- VMPFC
ventromedial prefrontal cortex
References
- Abraham A, Werning M, Rakoczy H, von Cramon DY, Schubotz RI. Minds, persons, and space: an fMRI investigation into the relational complexity of higher-order intentionality. Consciousness and Cognition. 2008;17(2):438–450. doi: 10.1016/j.concog.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7(4):268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry—the methods. NeuroImage. 2000;11(6):805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Attwell D, Iadecola C. The neural basis of functional brain imaging signals. Trends in Neurosciences. 2002;25(12):621–625. doi: 10.1016/s0166-2236(02)02264-6. [DOI] [PubMed] [Google Scholar]
- Attwell D, Buchan AM, Charpak S, Lauritzen M, MacVicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468:233–244. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg J, Dickhaut J, McCabe K. Trust, reciprocity, and social history. Games and Economic Behavior. 1995;10(1):122–142. [Google Scholar]
- Binmore KG. Game Theory: A Very Short Introduction. Oxford University Press; USA: 2007. [Google Scholar]
- Blair RJ, Morris JS, Frith CD, Perrett DI, Dolan RJ. Dissociable neural responses to facial expressions of sadness and anger. Brain. 1999;122:883–893. doi: 10.1093/brain/122.5.883. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ. The social brain in adolescence. Nature Reviews Neuroscience. 2008;9(4):267–277. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Den Ouden H, Choudhury S, Frith C. Adolescent development of the neural circuitry for thinking about intentions. Social Cognitive and Affective Neuroscience. 2007;2(2):130. doi: 10.1093/scan/nsm009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ, Burnett S, Dahl RE. The role of puberty in the developing adolescent brain. Human Brain Mapping. 2010;31:926–933. doi: 10.1002/hbm.21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bos W, Westenberg M, van Dijk E, Crone EA. Development of trust and reciprocity in adolescence. Cognitive Development. 2009;25(1):90–102. [Google Scholar]
- Bourgeois JP, Rakic P. Changes of synaptic density in the primary visual cortex of the macaque monkey from fetal to adult stage. Journal of Neuroscience. 1993;13(7):2801. doi: 10.1523/JNEUROSCI.13-07-02801.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brothers L. The neural basis of primate social communication. Motivation and Emotion. 1990;14(2):81–91. [Google Scholar]
- Brown BB. Adolescents’ relationships with peers. In: Lerner RM, Steinberg L, editors. Handbook of Adolescent Psychology. Vol. 2. Wiley; Hoboken, NJ: 2004. pp. 363–394. [Google Scholar]
- Brown TT, Petersen SE, Schlaggar BL. Does human functional brain organization shift from diffuse to focal with development? Developmental Science. 2006;9(1):9. doi: 10.1111/j.1467-7687.2005.00455.x. [DOI] [PubMed] [Google Scholar]
- Burnett S, Bird G, Moll J, Frith C, Blakemore SJ. Development during adolescence of the neural processing of social emotion. Journal of Cognitive Neuroscience. 2009;21(9):1736–1750. doi: 10.1162/jocn.2009.21121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett S, Blakemore SJ. Functional connectivity during a social emotion task in adolescents and in adults. The European Journal of Neuroscience. 2009;29(6):1294. doi: 10.1111/j.1460-9568.2009.06674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey S, Diamond R, Wood B. Development of face recognition – a maturational component. Developmental Psychology. 1980;16(4):257–269. [Google Scholar]
- Casey B, Jones RM, Hare TA. The adolescent brain. Annals of the New York Academy of Sciences. 2008;1124(1):111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Kadosh K, Johnson MH. Developing a cortex specialized for face perception. Trends in Cognitive Sciences. 2007;11(9):267–269. doi: 10.1016/j.tics.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Cohen Kadosh K, Cohen Kadosh R, Dick, Johnson MH. Journal of Cognitive Neuroscience. in press. [Google Scholar]
- D’Esposito M, Deouell LY, Gazzaley A. Alterations in the BOLD fMRI signal with ageing and disease: a challenge for neuroimaging. Nature Reviews Neuroscience. 2003;4:863–872. doi: 10.1038/nrn1246. [DOI] [PubMed] [Google Scholar]
- Diamond R, Carey S, Back K. Genetic influences on the development of spatial skills during early adolescence. Cognition. 1983;13:167–185. doi: 10.1016/0010-0277(83)90021-5. [DOI] [PubMed] [Google Scholar]
- Dorn LD. Measuring puberty. J Adolesc Health. 2006;39:625–626. doi: 10.1016/j.jadohealth.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Dumontheil I, Apperly IA, Blakemore SJ. Online usage of theory of mind continues to develop in late adolescence. Developmental Science. 2010;13(2):331–338. doi: 10.1111/j.1467-7687.2009.00888.x. [DOI] [PubMed] [Google Scholar]
- Durand K, Gallay M, Seigneuric A, Robichon F, Baudouin J-Y. The development of facial emotion recognition: the role of configural information. Journal of Experimental Child Psychology. 2007;97:14–27. doi: 10.1016/j.jecp.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Durston S, Davidson MC, Tottenham N, Galvan A, Spicer J, Fossella JA, Casey BJ. A shift from diffuse to focal cortical activity with development. Developmental Science. 2006;9(1):1–20. doi: 10.1111/j.1467-7687.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Morris AS, McDaniel B, Spinrad TL. Moral cognitions and prosocial responding in adolescence. In: Lerner RM, Steinberg L, editors. Handbook of Adolescent Psychology. 1-12. Wiley; Hoboken, NJ: 2004. pp. 155–188. [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An fMRI study of social exclusion. Science. 2003;302(5643):290. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Ernst M, Fudge JL. A developmental neurobiological model of motivated behavior: anatomy, connectivity and ontogeny of the triadic nodes. Neuroscience and Biobehavioral Reviews. 2009;33(3):367–382. doi: 10.1016/j.neubiorev.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Rumsey J. In: Neuroimaging in Developmental Clinical Neuroscience. 2nd edition. Rumsey J, Ernst M, Cambridge University Press; 2009. [Google Scholar]
- Farroni T, Csibra G, Simion F, Johnson MH. Eye contact detection in humans from birth. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(14):9602. doi: 10.1073/pnas.152159999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornari E, Knyazeva MG, Meuli R, Maeder P. Myelination shapes functional activity in the developing brain. NeuroImage. 2007;38(3):511–518. doi: 10.1016/j.neuroimage.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Harrison L, Penny W. Dynamic causal modelling. NeuroImage. 2003;19:1273–1302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- Frith CD. The social brain? Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences. 2007;362(1480):671. doi: 10.1098/rstb.2006.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CD, Frith U. Implicit and explicit processes in social cognition. Neuron. 2008;60(3):503–510. doi: 10.1016/j.neuron.2008.10.032. [DOI] [PubMed] [Google Scholar]
- Frith U, Frith C. The social brain: allowing humans to boldly go where no other species has been. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences. 2010;365(1537):165. doi: 10.1098/rstb.2009.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith U, Frith CD. Development and neurophysiology of mentalizing. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences. 2003;358(1431):459. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, Benedetti F, Abbamonte M, Gasparotti R, Barale F, Perez J, McGuire P, Politi P. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. Journal of Psychiatry & Neuroscience. 2009;34(6):418–432. [PMC free article] [PubMed] [Google Scholar]
- Gardner M, Steinberg L. Peer influence on risk taking. Risk preference, and risky decision making in adolescence and adulthood: an experimental study. Developmental Psychology. 2005;41(4):625–635. doi: 10.1037/0012-1649.41.4.625. [DOI] [PubMed] [Google Scholar]
- Geier C, Luna B. The maturation of incentive processing and cognitive control. Pharmacology Biochemistry and Behavior. 2009;93(3):212–221. doi: 10.1016/j.pbb.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience. 1999;2:861–862. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Henson RN, Simons JS. The scale of functional specialization within human prefrontal cortex. Journal of Neuroscience. 2010;30(4):1233. doi: 10.1523/JNEUROSCI.3220-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio A, Watkins KE, Chadwick M, James S, Winmill L, Douaud G, De Stefano N, Matthews PM, Smith SM, Johansen-Berg H, James AC. Longitudinal changes in grey and white matter during adolescence. Neuroimage. 2010;49(1):94–103. doi: 10.1016/j.neuroimage.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(21):8174. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golarai G, Ghahremani DG, Whitfield-Gabrieli S, Reiss A, Eberhardt JL, Grosbras MH, Jansen M, Leonard G, McIntosh A, Osswald K, Poulsen C, Steinberg L, et al. Neural mechanisms of resistance to peer influence in early adolescence. Journal of Neuroscience. 2007;27(30):8040. doi: 10.1523/JNEUROSCI.1360-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Kroener S, Zaitsev AV, Povysheva NV, Krimer LS, Barrionuevo G, Lewis DA. Functional maturation of excitatory synapses in layer 3 pyramidal neurons during postnatal development of the primate prefrontal cortex. Cerebral Cortex. 2008;18(3):626–637. doi: 10.1093/cercor/bhm095. [DOI] [PubMed] [Google Scholar]
- Grosbras MH, Jansen M, Leonard G, McIntosh A, Osswald K, Poulsen C, Steinberg L, Toro R, Paus T. Neural mechanisms of resistance to peer influence in early adolescence. Journal of Neuroscience. 2007;27(30):8040–8045. doi: 10.1523/JNEUROSCI.1360-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther Moor B, van Leijenhorst L, Rombouts SARB, Crone EA, van der Molen MW. Do you like me? Neural correlates of social evaluation and developmental trajectories. Social Neuroscience. 2010;23:1–22. doi: 10.1080/17470910903526155. [DOI] [PubMed] [Google Scholar]
- Güroğlu B, van den Bos W, Crone EA. Fairness considerations: increasing understanding of intentionality during adolescence. Journal of Experimental Child Psychology. 2009;104(4):398–409. doi: 10.1016/j.jecp.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Guyer AE, Monk CS, McClure-Tone EB, Nelson EE, Roberson-Nay R, Adler AD, Fromm SJ, Leibenluft E, Pine DS, Ernst M. A developmental examination of amygdala response to facial expressions. Journal of Cognitive Neuroscience. 2008;20(9):1565–1582. doi: 10.1162/jocn.2008.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, McClure-Tone EB, Shiffrin ND, Pine DS, Nelson EE. Probing the neural correlates of anticipated peer evaluation in adolescence. Child Development. 2009;80(4):1000. doi: 10.1111/j.1467-8624.2009.01313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton AN, Bossaerts P, O’Doherty JP. Neural correlates of mentalizing-related computations during strategic interactions in humans. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(18):6741. doi: 10.1073/pnas.0711099105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends in Cognitive Sciences. 2000;4(6):223–232. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. The Journal of Comparative Neurology. 1998;387(2):167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Johnson MH. Functional brain development in humans. Nature Reviews Neuroscience. 2001;2:475–483. doi: 10.1038/35081509. [DOI] [PubMed] [Google Scholar]
- Johnson MH, Grossman T, Cohen Kadosh K. Mapping functional brain development: building a social brain through interactive specialization. Developmental Psychology. 2009;45(1):151–159. doi: 10.1037/a0014548. [DOI] [PubMed] [Google Scholar]
- Kloep M. Love is all you need? Focusing on adolescents’ life concerns from an ecological point of view. Journal of Adolescence. 1999;22(1):49–63. doi: 10.1006/jado.1998.0200. [DOI] [PubMed] [Google Scholar]
- La Greca AM, Lopez N. Social anxiety among adolescents: linkages with peer relations and friendships. Journal of Abnormal Child Psychology. 1998;26(2):83–94. doi: 10.1023/a:1022684520514. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412(6843):150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- Macchi C, Turati C, Simion F. Can a nonspecific bias toward top-heavy patterns explain newborns’ face preference? Psychological Science. 2004;15(6):379–383. doi: 10.1111/j.0956-7976.2004.00688.x. [DOI] [PubMed] [Google Scholar]
- Masten CL, Eisenberger NI, Borofsky LA, Pfeifer JH, McNealy K, Mazziotta JC, Dapretto M. Neural correlates of social exclusion during adolescence: understanding the distress of peer rejection. Social Cognitive and Affective Neuroscience. 2009;4(2):143. doi: 10.1093/scan/nsp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGivern RF, Andersen J, Byrd D, Mutter KL, Reilly J. Cognitive efficiency on a match to sample task decreases at the onset of puberty in children. Brain Cogn. 2002;50:73–89. doi: 10.1016/s0278-2626(02)00012-x. [DOI] [PubMed] [Google Scholar]
- Mondloch CJ, Geldart S, Maurer D, Le Grand R. Developmental changes in face processing skills. Journal of Experimental Child Psychology. 2003;86:67–84. doi: 10.1016/s0022-0965(03)00102-4. [DOI] [PubMed] [Google Scholar]
- Monk CS, McClure EB, Nelson EE, Zarahn E, Bilder RM, Leibenluft E, Charney DS, et al. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. NeuroImage. 2003;20(1):420–428. doi: 10.1016/s1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- Montague PR. Neuroeconomics: a view from neuroscience. Functional Neurology. 2007;22:219–234. [PubMed] [Google Scholar]
- Moriguchi Y, Ohnishi T, Mori T, Matsuda H, Komaki G. Changes of brain activity in the neural substrates for theory of mind during childhood and adolescence. Psychiatry and Clinical Neurosciences. 2007;61(4):355–363. doi: 10.1111/j.1440-1819.2007.01687.x. [DOI] [PubMed] [Google Scholar]
- Morris JP, Pelphrey KA, McCarthy G. Regional brain activation evoked when approaching a virtual human on a virtual walk. Journal of Cognitive Neuroscience. 2005;17(11):1744–1752. doi: 10.1162/089892905774589253. [DOI] [PubMed] [Google Scholar]
- Morton J, Johnson MH. CONSPEC and CONLERN: a two-process theory of infant face recognition. Psychological Review. 1991;98(2):164–181. doi: 10.1037/0033-295x.98.2.164. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, McClure EB, Pine DS. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine. 2005;35(2):163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- O’Brien SF, Bierman KL. Conceptions and perceived influence of peer groups: interviews with preadolescents and adolescents. Child Development. 1988;59(5):1360–1365. doi: 10.1111/j.1467-8624.1988.tb01504.x. [DOI] [PubMed] [Google Scholar]
- Olson IR, Plotzker A, Ezzyat Y. The Enigmatic temporal pole: a review of findings on social and emotional processing. Brain. 2007;130(7):1718–1731. doi: 10.1093/brain/awm052. [DOI] [PubMed] [Google Scholar]
- Ostby Y, Tamnes CK, Fjell AM, Westlye LT, Due-Tonnessen P, Walhovd KB. Heterogeneity in subcortical brain development: a structural magnetic resonance imaging study of brain maturation from 8 to 30 years. Journal of Neuroscience. 2009;29(38):11772. doi: 10.1523/JNEUROSCI.1242-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, et al. Structural maturation of neural pathways in children and adolescents: in vivo study. Science. 1999;283:1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- Paus T. Mapping brain maturation and cognitive development during adolescence. Trends in Cognitive Science. 2005;9(2):60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Paus T, Toro R, Leonard G, Lerner JV, Lerner RM, Perron M, Pike GB, et al. Morphological properties of the action-observation cortical network in adolescents with low and high resistance to peer influence. Social Neuroscience. 2008a;3(3):303–316. doi: 10.1080/17470910701563558. [DOI] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nature Reviews Neuroscience. 2008b;9(12):947–957. doi: 10.1038/nrn2513. doi:10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelen MV, Glaser B, Vuilleumier P, Eliez S. Differential development of selectivity for faces and bodies in the fusiform gyrus. Developmental Science. 2009;12(6):F16–25. doi: 10.1111/j.1467-7687.2009.00916.x. [DOI] [PubMed] [Google Scholar]
- Perrin JS, Hervé P, Leonard G, Perron M, Pike GB, Pitiot A, Richer L, et al. Growth of white matter in the adolescent brain: role of testosterone and androgen receptor. The Journal of Neuroscience. 2008;28(38):9519–9524. doi: 10.1523/JNEUROSCI.1212-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer JH, Lieberman MD, Dapretto M. “I know you are but what am I?!”: neural bases of self- and social knowledge retrieval in children and adults. Journal of Cognitive Neuroscience. 2007;19(8):1323–1337. doi: 10.1162/jocn.2007.19.8.1323. doi:10.1162/jocn.2007.19.8.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer JH, Masten CL, Borofsky LA, Dapretto M, Fuligni AJ, Lieberman MD. Neural correlates of direct and reflected self-appraisals in adolescents and adults: when social perspective-taking informs self-perception. Child Development. 2009;80(4):1016–1038. doi: 10.1111/j.1467-8624.2009.01314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premack D, Woodruff G. Does the chimpanzee have a theory of mind? Behav. Brain. Sci. 1978;1:515–526. [Google Scholar]
- Rakic P, Bourgeois JP, Eckenhoff MF, Zecevic N, Goldman-Rakic PS. Concurrent overproduction of synapses in diverse regions of the primate cerebral cortex. Science. 1986;232(4747):232–235. doi: 10.1126/science.3952506. (New York, N. Y.) [DOI] [PubMed] [Google Scholar]
- Rolls, E.T., Deco, G. (in press).
- Saxe R, Xiao D, Kovacs G, Perrett DI, Kanwisher N. A region of right posterior superior temporal sulcus responds to observed intentional actions. Neuropsychologia. 2004;42(11):1435–1446. doi: 10.1016/j.neuropsychologia.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Saxe RR, Whitfield-Gabrieli S, Scholz J, Pelphrey KA. Brain regions for perceiving and reasoning about other people in school-aged children. Child Development. 2009;80(4):1197–1209. doi: 10.1111/j.1467-8624.2009.01325.x. [DOI] [PubMed] [Google Scholar]
- Scherf KS, Behrmann M, Humphreys K, Luna B. Visual category-selectivity for faces, places and objects emerges along different developmental trajectories. Developmental Science. 2007;10(4):F15–30. doi: 10.1111/j.1467-7687.2007.00595.x. [DOI] [PubMed] [Google Scholar]
- Sebastian C, Viding E, Williams KD, Blakemore SJ. Social brain development and the affective consequences of ostracism in adolescence. Brain and Cognition. 2010a;72(1):134–145. doi: 10.1016/j.bandc.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Sebastian CL, Roiser JP, Tan GC, Viding E, Wood NW, Blakemore SJ. Effects of age and MAOA genotype on the neural processing of social rejection. Genes Brain & Behavior. 2010b;9:628–637. doi: 10.1111/j.1601-183X.2010.00596.x. [DOI] [PubMed] [Google Scholar]
- Sebastian CL, Tan GCY, Roiser JP, Viding E, Dumontheil I, Blakemore SJ. Developmental influences on the neural bases of responses to social rejection: implications of social neuroscience for education. NeuroImage. doi: 10.1016/j.neuroimage.2010.09.063. in press. [DOI] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, Greenstein D, et al. Neurodevelopmental trajectories of the human cerebral cortex. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2008;28(14):3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmuel A, Yacoub E, Chaimow D, Logothetis NK, Ugurbil K. Spatiotemporal point-spread function of fMRI signal in human gray matter at 7 Tesla. NeuroImage. 2007 Apr;35(2):539–552. doi: 10.1016/j.neuroimage.2006.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirotin YB, Hillman EMC, Bordier C, Das A. Spatiotemporal precision and hemodynamic mechanism of optical point spreads in alert primates. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(43):18390–18395. doi: 10.1073/pnas.0905509106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Heatherton TF, Kelley WM. Anterior cingulate cortex responds differentially to expectancy violation and social rejection. Nature Neuroscience. 2006;9(8):1007–1008. doi: 10.1038/nn1728. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Batth R, Jernigan TL, Toga AW. Localizing age-related changes in brain structure between childhood and adolescence using statistical parametric mapping. NeuroImage. 1999;9(6 Pt 1):587–597. doi: 10.1006/nimg.1999.0436. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Trauner DA, Gamst A, Jernigan TL. Development of cortical and subcortical brain structures in childhood and adolescence: a structural MRI study. Developmental Medicine and Child Neurology. 2002;44(1):4–16. doi: 10.1017/s0012162201001591. [DOI] [PubMed] [Google Scholar]
- Steinberg L. A social neuroscience perspective on adolescent risk-taking. Developmental Review. 2008;28(1):78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L, Monahan KC. Age differences in resistance to peer influence. Developmental Psychology. 2007;43(6):1531–1543. doi: 10.1037/0012-1649.43.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter M, Kocher MG. Trust and trustworthiness across different age groups. Game Econ. Behav. 2007;59:364–382. [Google Scholar]
- Tamnes CK, Ostby Y, Fjell AM, Westlye LT, Due-Tonnessen P, Walhovd KB. Brain maturation in adolescence and young adulthood: regional age-related changes in cortical thickness and white matter volume and microstructure. Cerebral Cortex. 2009;20(3):534–548. doi: 10.1093/cercor/bhp118. [DOI] [PubMed] [Google Scholar]
- Theodosis DT, Poulain DA, Oliet SHR. Activity-dependent structural and functional plasticity of astrocyte-neuron interactions. Physiological Reviews. 2008;88(3):983–1008. doi: 10.1152/physrev.00036.2007. [DOI] [PubMed] [Google Scholar]
- Thomas LA, De Bellis MD, Graham R, LaBar K. Development of emotional face recognition in late childhood and adolescence. Developmental Science. 2007;10(5):547–558. doi: 10.1111/j.1467-7687.2007.00614.x. [DOI] [PubMed] [Google Scholar]
- Thomason ME, Burrows BE, Gabrieli JDE, Glover GH. Breath holding reveals differences in fMRI BOLD signal in children and adults. NeuroImage. 2005;25(3):824–837. doi: 10.1016/j.neuroimage.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Armony JL, Driver J, Dolan RJ. Effects of attention and emotion on face processing in the human brain: an event-related fMRI study. Neuron. 2001;30:829–841. doi: 10.1016/s0896-6273(01)00328-2. [DOI] [PubMed] [Google Scholar]
- Wade AM, Lawrence K, Mandy W, Skuse D. Charting the development of emotion recognition from 6 years of age. Journal of Applied Statistics. 2006;33(3):297–315. [Google Scholar]
- Wainryb C, Shaw LA, Laupa M, Smith KR. Children’s, adolescents’, and young adults’ thinking about different types of disagreements. Developmental Psychology. 2001;37(3):373–386. doi: 10.1037//0012-1649.37.3.373. [DOI] [PubMed] [Google Scholar]
- Wang AT, Lee SS, Sigman M, Dapretto M. Developmental changes in the neural basis of interpreting communicative intent. Soc. Cogn. Affect. Neurosci. 2006;1:107–121. doi: 10.1093/scan/nsl018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman SG. Determinants of conduction velocity in myelinated nerve fibers. Muscle & Nerve. 1980;3(2):141–150. doi: 10.1002/mus.880030207. [DOI] [PubMed] [Google Scholar]
- Williams KD, Cheung CK, Choi W. Cyberostracism: effects of being ignored over the Internet. Journal of Personality and Social Psychology. 2000;79(5):748–762. doi: 10.1037//0022-3514.79.5.748. [DOI] [PubMed] [Google Scholar]
- Williams KD, Jarvis B. Cyberball: a program for use in research on interpersonal ostracism and acceptance. Behavior Research Methods. 2006;38(1):174–180. doi: 10.3758/bf03192765. [DOI] [PubMed] [Google Scholar]
- Young AW, Aggleton JP, Hellawell DJ, Johnson M, Broks P, Hanley JR. Face processing impairments after amygdalotomy. Brain: A Journal of Neurology. 1995;118(Pt 1):15–24. doi: 10.1093/brain/118.1.15. [DOI] [PubMed] [Google Scholar]