Abstract
The integrated stress response (ISR) modulates mRNA translation to regulate the mammalian unfolded protein response (UPR), immunity and memory formation. A chemical ISR inhibitor, ISRIB, enhances cognitive function and modulates the UPR in vivo. To explore mechanisms involved in ISRIB action we screened cultured mammalian cells for somatic mutations that reversed its effect on the ISR. Clustered missense mutations were found at the N-terminal portion of the delta subunit of guanine nucleotide exchange factor (GEF) eIF2B. When reintroduced by CRISPR-Cas9 gene editing of wildtype cells, these mutations reversed both ISRIB-mediated inhibition of the ISR and its stimulatory effect on eIF2B GEF activity towards its substrate, eIF2, in vitro. Thus ISRIB targets an interaction between eIF2 and eIF2B that lies at the core of the ISR.
Keywords: Protein synthesis, CRISPR, eIF2α phosphorylation, chemical genetics, Integrated Stress Response
The integrated stress response (ISR) is a widely conserved mechanism for coupling diverse upstream stresses to the phosphorylation of serine 51 in the α subunit of eukaryotic translation initiation factor 2 (eIF2α) (1) (2). Underlying the eIF2α phosphorylation-dependent ISR is a potent attenuation in translation of most mRNAs and selective upregulation of translation of a few special mRNAs that encode transcriptional regulators. The ISR thus activates a broad translational and transcriptional program involved in resistance to unfolded protein stress in the endoplasmic reticulum (ER stress) (3), intermediary metabolism (2), memory (4) and immunity (5).
A small molecule ISR inhibitor (ISRIB) exerts potent effects on the outcome of stress and on memory (6, 7). As expected, ISRIB interfered with the ISR without blocking phosphorylation of eIF2α (Fig. 1A), suggesting that its molecular target(s) lie between eIF2(αP) and its effects on the translational machinery.
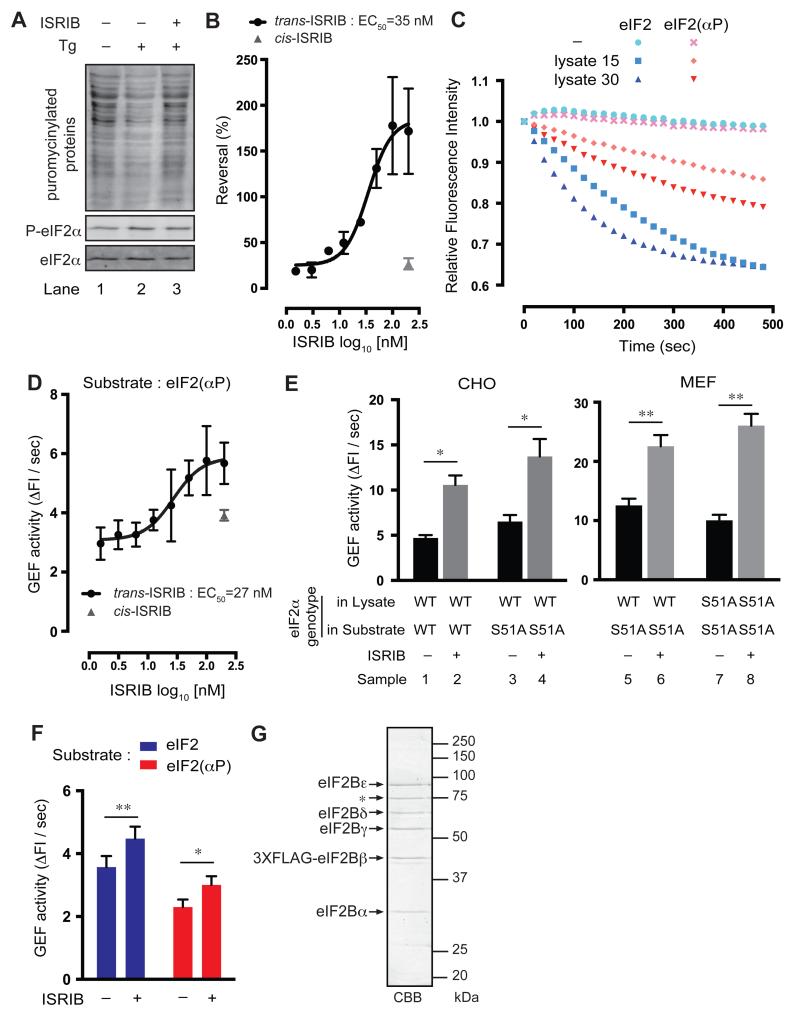
Fig. 1. ISRIB reverses attenuated translation and accelerates eIF2B GEF activity towards eIF2(αP) in vitro.
(A) Immunoblot of newly-synthesized puromycinylated proteins in extracts of untreated CHO cells or cells exposed to the ISR-inducing agent thapsigargin (Tg 300 nM, 30 minutes) in the presence or absence of trans ISRIB (100 nM). Phosphorylated (P-eIF2α) and total eIF2α were detected in the immunoblots below. Quantified signal intensities are shown in Fig. S5. (B) Dose-response of ISRIB-stimulation of translation in reticulocyte lysate fitted to a non-linear trace. Shown are mean ± SEM (n = 3) and EC50 (for active trans-ISRIB). Note the inactivity of cis-ISRIB. (C) GEF activity as reflected in time dependent decrease in fluorescence of weakly and heavily phosphorylated eIF2 loaded with Bodipy-FL-GDP and incubated with unlabeled GDP in the presence or absence of cell lysate (μg). Shown is a mean of three independent measurements. (D) Relation between the initial velocities of the release of Bodipy-FL-GDP from heavily phosphorylated eIF2 and ISRIB concentration, fitted to a non-linear trace. Shown are mean ± SEM (n = 3) and EC50 for trans-ISRIB. (E) GEF activity reflected in the initial velocities of GDP release reactions with CHO cell lysate (samples 1-4), wildtype or mutant eIF2αS51A/S51A mouse embryonic fibroblast lysate (MEFs, samples 5-8) and Bodipy-FL-GDP loaded eIF2 of the indicated eIF2α genotype. Shown are mean ± SEM (n = 3 for samples 1-4 and n = 6 for samples 5-8). *P < 0.05, **P < 0.01 (Student’s t test). (F) As in “E”, but with purified eIF2B and Bodipy-FL-GDP loaded non-phosphorylated and phosphorylated eIF2. Shown are mean ± SEM (n = 8). *P = 0.012, **P = 0.0054 (Student’s t test). (G) Coomassie-stained SDS-PAGE of the purified eIF2B used in “F”. The five subunits of eIF2B and PRMT5 (*, a non-specific contaminant) are noted.
We tested ISRIB’s effects on mRNA translation in an in vitro assay - cell-free translation in reticulocyte lysates. Both ISRIB and the eIF2α kinase inhibitor GSK2606414 (8) increased the luminescent signal of reticulocyte lysates programmed with luciferase-encoding mRNA (Fig. S1A). The effect of ISRIB was enhanced further through eIF2α phosphorylation, which was promoted by pre-incubating the lysate at 30°C before adding the luciferase mRNA (Fig. S1B-S1C). ISRIB’s EC50 for stimulating translation, 35 nM, is similar to that in vivo (6) and is restricted to the trans geometric isomer (Fig. 1B). Thus, the ISR imparted by resident eIF2α kinase(s) in the reticulocyte lysate could be reversed by ISRIB.
eIF2α phosphorylation inhibits protein synthesis by inhibiting eIF2B, a guanine nucleotide exchange factor (GEF), which accelerates the exchange of GDP for GTP in the eIF2 complex (9, 10). To measure the effects of ISRIB on eIF2B GEF activity, we established an assay in which the GEF activity in cell lysates (11) promoted the release of BODIPY-FL conjugated GDP (hereafter [b]GDP) from purified eIF2, with an attendant decrease in fluorescent intensity. The eIF2 substrate was purified from Chinese Hamster Ovary (CHO) cells that also expressed a conditionally active eIF2α kinase [Fv2E-PERK (12)] and eIF2 with low or high levels of phosphorylation was generated by treating the cells briefly with the Fv2E-PERK activator, AP20187 (Fig. S2A-S2C). A lysate protein concentration- and time-dependent decrease in fluorescence intensity of eIF2-[b]GDP was observed (Fig. 1C), consistent with lysate-induced release of the bound nucleotide. Importantly, the fluorescent signal declined more slowly in eIF2-[b]GDP with higher levels of phosphorylated eIF2α (Fig. 1C and S2D), consistent with the inhibitory effect of eIF2(αP) on eIF2B GEF activity (13). ISRIB compensated for the inhibitory effect of eIF2(αP) on the GEF activity in cell lysate, with an EC50 of 27 nM; similar to ISRIB’s action in intact cells (Fig. 1D).
ISRIB-mediated acceleration of GEF activity was maintained using an eIF2(αS51A)-[b]GDP substrate that could not be phosphorylated (Fig. 1E, samples 1-4) and was observed in lysates from both wildtype (eIF2α+/+) and mutant (eIF2αS51A/S51A) mouse embryonic fibroblasts (14) (Fig. 1E, samples 5-8). Furthermore, ISRIB stimulated the GEF activity of purified eIF2B on both phosphorylated and non-phosphorylated eIF2 (Fig. 1F-1G), suggesting that the molecular target of ISRIB is present in the pure complex and functions independently of eIF2 phosphorylation.
To isolate ISRIB-resistant cells (ISRIBr) we utilized a CHO-K1 based cell line (CHO-C30) with the ISR-activated promoter of the mouse Ddit3/CHOP gene fused to green fluorescent protein (CHOP::GFP) (15). Activation of CHOP::GFP by unfolded protein stress in the endoplasmic reticulum was only partially inhibited by ISRIB, whilst activation by histidinol, a competitive inhibitor of histidine tRNA synthetase, [that activates the eIF2α kinase GCN2 (16)] was strongly inhibited (Fig. S3). Chemically-induced mutations that reversed the ISRIB-mediated suppression of the histidinol-induced ISR, generated ISRIBr CHO-C30 cells (Fig. S4A-S4B).
We isolated numerous clones with strong or weak ISRIBr phenotypes (Fig. 2A and S4C-S4E). The ISRIBr mutation(s) reversed both the sensitivity of the ISR reporter gene to ISRIB and the ability of ISRIB to promote protein synthesis in stressed cells (Fig. 2B-2C and S5). Furthermore, the eIF2-directed GEF activity in lysates from the mutant clones was not stimulated by ISRIB in vitro (Fig. 2D).
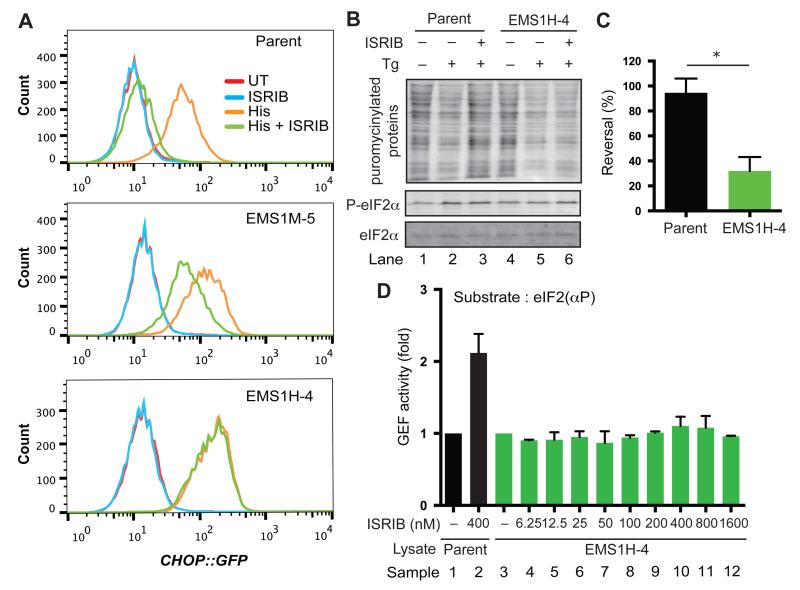
Fig. 2. Selection of ISRIB resistant (ISRIBr) mutations.
(A) Histograms of the distribution of GFP fluorescence arising from an ISR-inducible CHOP::GFP reporter gene in parental CHO-C30 cells and clones bearing the indicated mutations. The cells were left untreated or treated with histidinol (His; 0.5 mM), ISRIB (100 nM) or both. EMS1M-5 exemplifies a class of clones with a weak and EMS1H-4 a class with a strong ISRIBr phenotype.
(B) Immunoblot of puromycinylated proteins in extracts of parental CHO-C30 cells or a representative strong ISRIBr clone (EMS1H-4) following exposure to thapsigargin (Tg) in the presence or absence of ISRIB (as in Fig. 1A). The images are representative of all three independent experiments that yielded similar results. Quantified signal intensities are shown in (Fig. S5).
(C) Bar diagram, displaying the reversal of translation attenuation by ISRIB in “B” above: Reversal = [(PTg+ISRIB-PTg) ÷ (PUT-PTg)] × 100, (PTg+ISRIB, PTg and PUT are the puro signal from the sample treated with Tg and ISRIB (Lanes 3 or 6), Tg alone (Lanes 2 or 5) and the untreated sample (Lanes 1 or 3), respectively. Shown are mean ± SEM (n = 3). * P < 0.05 (Student’s t test).
(D) Bar diagram of the GEF activity of lysates from parental and strong ISRIBr mutant cells with Bodipy-FL-GDP-loaded eIF2(αP) as a substrate in the absence or presence of ISRIB, as indicated. Shown are mean ± SEM of the initial velocity of the decline in Bodipy-FL-GDP fluorescence upon adding lysate, normalized to the rate in the untreated sample (n = 4).
ISRIB targets the interaction of eIF2B with eIF2. Therefore we examined the coding region of the genes encoding the subunits of eIF2B and eIF2. But for one exception, the coding regions of eIF2B subunits α, β, γ & ε and eIF2α had no mutations (Table S1). This bland mutational landscape contrasted dramatically with that of Eif2b4, encoding eIF2Bδ. The majority of ISRIBr clones isolated had one or more non-synonymous mutations affecting three closely-spaced codons, R171, V178 and L180 (Table S1 and Fig. S6). These mutations cluster in a unique N-terminal region of eIF2Bδ that is not conserved in the other two regulatory subunits of the GEF, but is well conserved among vertebrate eIF2Bδ (Fig. 3A and S7).
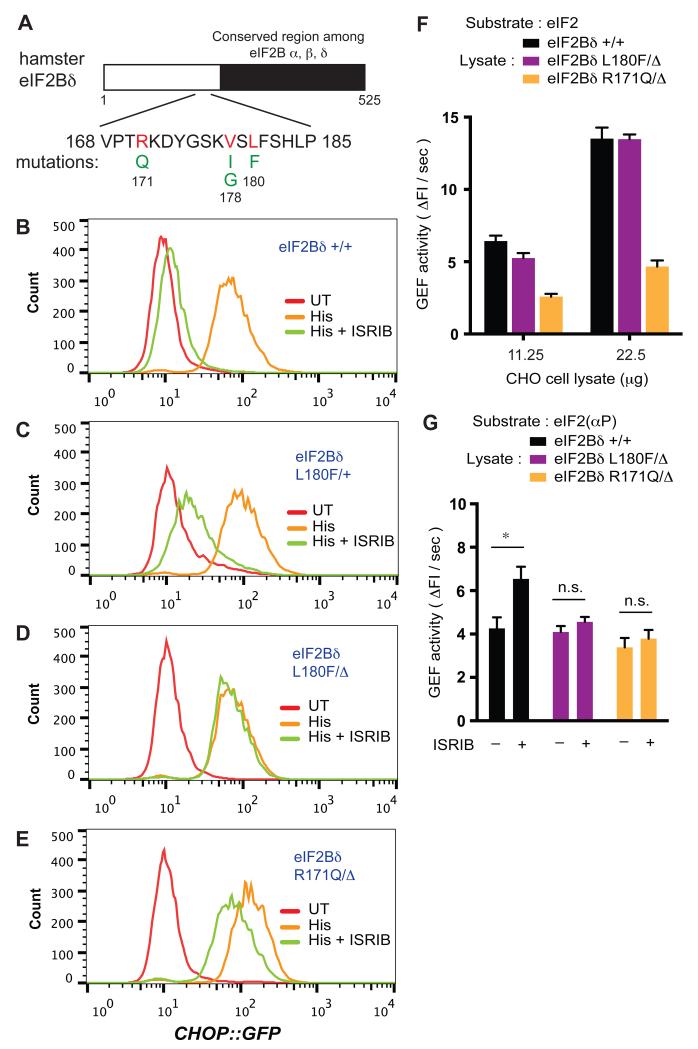
Fig. 3. Clustered mutations in Eif2b4 impart ISRIB resistance.
(A) Schema of eIF2Bδ with the position of the mutations associated with an ISRIBr phenotype showing. These are clustered at the unique N-terminal region that is not conserved in the other regulatory subunits (α, β) of eIF2B.
(B-E) Distribution of CHOP::GFP reporter gene activity in parental CHO-C30 cells and derivative sub-clones bearing the indicated mutations (induced by CRISPR-Cas9 targeted homologous recombination at the Eif2b4 locus). The cells were left untreated or treated for 24 hours with histidinol (His; 0.5 mM) alone or with ISRIB (100 nM).
(F-G) Bar diagram of the GEF activity of lysates from parental or CRISPR-Cas9-induced ISRIBr mutant cells with Bodipy-FL-GDP-loaded eIF2 or eIF2(αP) as substrates in the absence or presence of ISRIB, as indicated. Shown are mean ± SEM (n = 6, for “F”; n=5 for “G”). * P < 0.05, n.s.; not significant (Student’s t test).
To determine if the mutations in these clustered residues of eIF2Bδ were sufficient to impart an ISRIBr phenotype, we promoted homologous recombination at the Eif2b4 locus of parental CHO-C30 cells by CRISPR Cas9-directed editing, offering a homologous directed repair template with either the eIF2BδR171Q or eIF2BδL180F mutation (Fig. S8A). With either repair template, a population of ISRIBr cells emerged after co-transfection of the CRISPR guide and Cas9 nuclease. A single round of enrichment by sorting, delivered clones with weak and strong ISRIBr phenotypes (Fig. S8B-S8D and Table S2). Clones with the weak ISRIBr phenotype retained a wildtype copy of the gene encoding eIF2Bδ, whereas clones with the strong ISRIBr phenotype had gained the mutation and lost both wildtype alleles (Fig. 3B-3E).
In vitro, the baseline GEF activity in lysates from eIF2BδR171Q mutant cells was two-fold lower, whilst that of the eIF2BδL180F was indistinguishable from wildtype (Fig. 3F). Yet both mutations similarly attenuated the effect of ISRIB on lysate GEF activity (Fig. 3G and S9). Thus, mutations in eIF2Bδ can selectively compromise ISRIB action without affecting other aspects of eIF2B function.
Here we used a chemical genetic approach to identify proteins implicated in ISRIB action. We found that a small segment of eIF2Bδ is involved in the response to ISRIB providing a molecular clue to the how ISRIB might work. Though it is not clear if ISRIB binds eIF2B directly, ISRIB’s ability to promote GEF activity in vitro together with the identification of a clustered set of mutations in the δ subunit that selectively eliminate this response (imparting an ISRIBr phenotype on cells), suggest that direct modulation of the GEF lies at the heart of ISRIB-mediated reversal of the ISR. The active form of eIF2B is a dimer of pentamers (17-19), whereas the active, trans-isomer of ISRIB has perfect two-fold symmetry. Perhaps stabilization of the eIF2B decamer by binding of a symmetric molecule across the interface of its constituent pentamers is important for ISRIB’s action and the ISRIBr mutations, identified here, interfere with this process.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank P. Walter and C. Sidrauski (UCSF) for their gift of ISRIB (used to confirm their observations), R. Schulte from the flow cytometery core and R. Antrobus from the mass spectrometry core at the CIMR for their technical assistance. Supported by grants from the Wellcome Trust (Wellcome 084812/Z/08/Z and a strategic award Wellcome 100140), and by fellowships to YS from the Daiichi Sankyo Foundation of Life Science and the Japan Society for the Promotion of Science for research abroad. DR is a Wellcome Trust Principal Research Fellow.
Footnotes
Publisher's Disclaimer: This manuscript has been accepted for publication in Science. This version has not undergone final editing. Please refer to the complete version of record at http://www.sciencemag.org/. The manuscript may not be reproduced or used in any manner that does not fall within the fair use provisions of the Copyright Act without the prior, written permission of AAAS.
REFERENCES AND NOTES
- 1.Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell. 2003;11:619–633. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 2.Baird TD, Wek RC. Eukaryotic initiation factor 2 phosphorylation and translational control in metabolism. Advances in nutrition. 2012;3:307–321. doi: 10.3945/an.112.002113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harding H, Zhang Y, Bertolotti A, Zeng H, Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol. Cell. 2000;5:897–904. doi: 10.1016/s1097-2765(00)80330-5. [DOI] [PubMed] [Google Scholar]
- 4.Costa-Mattioli M, Gobert D, Harding HP, Herdy B, Azzi M, Bruno M, Ben Mamou C, Marcinkiewicz E, Yoshida M, Imataka H, Cuello AC, Seidah N, Sossin W, Lacaille J-C, Ron D, Nader K, Sonenberg N. Translational control of hippocampal synaptic plasticity and memory by an eIF2 kinase, GCN2. Nature. 2005;436:1166–1173. doi: 10.1038/nature03897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Munn DH, Sharma MD, Baban B, Harding HP, Zhang Y, Ron D, Mellor AL. GCN2 Kinase in T Cells Mediates Proliferative Arrest and Anergy Induction in Response to Indoleamine 2,3-Dioxygenase. Immunity. 2005;22:633–642. doi: 10.1016/j.immuni.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 6.Sidrauski C, Acosta-Alvear D, Khoutorsky A, Vedantham P, Hearn BR, Li H, Gamache K, Gallagher CM, Ang KK, Wilson C, Okreglak V, Ashkenazi A, Hann B, Nader K, Arkin MR, Renslo AR, Sonenberg N, Walter P. Pharmacological brake-release of mRNA translation enhances cognitive memory. Elife. 2013;2:e00498. doi: 10.7554/eLife.00498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Prisco GV, Huang W, Buffington SA, Hsu CC, Bonnen PE, Placzek AN, Sidrauski C, Krnjevic K, Kaufman RJ, Walter P, Costa-Mattioli M. Translational control of mGluR-dependent long-term depression and object-place learning by eIF2alpha. Nat. Neurosci. 2014;17:1073–1082. doi: 10.1038/nn.3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Axten JM, Medina JR, Feng Y, Shu A, Romeril SP, Grant SW, Li WH, Heerding DA, Minthorn E, Mencken T, Atkins C, Liu Q, Rabindran S, Kumar R, Hong X, Goetz A, Stanley T, Taylor JD, Sigethy SD, Tomberlin GH, Hassell AM, Kahler KM, Shewchuk LM, Gampe RT. Discovery of 7-Methyl-5-(1-{[3-(trifluoromethyl)phenyl]acetyl}-2,3-dihydro-1H-indol-5-yl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (GSK2606414), a Potent and Selective First-in-Class Inhibitor of Protein Kinase R (PKR)-like Endoplasmic Reticulum Kinase (PERK) J. Med. Chem. 2012;55:7193–7207. doi: 10.1021/jm300713s. [DOI] [PubMed] [Google Scholar]
- 9.Ranu RS, London IM. Regulation of protein synthesis in rabbit reticulocyte lysates: additional initiation factor required for formation of ternary complex (eIF-2·GTP·Met-tRNAf) and demonstration of inhibitory effect of heme-regulated protein kinase. Proc. Natl. Acad. Sci. U. S. A. 1979;76:1079–1083. doi: 10.1073/pnas.76.3.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Haro C, Ochoa S. Further studies on the mode of action of the heme-controlled translational inhibitor. Proc. Natl. Acad. Sci. U. S. A. 1979;76:1741–1745. doi: 10.1073/pnas.76.4.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kimball SR, Everson WV, Flaim KE, Jefferson LS. Initiation of protein synthesis in a cell-free system prepared from rat hepatocytes. Am. J. Physiol. 1989;256:C28–34. doi: 10.1152/ajpcell.1989.256.1.C28. [DOI] [PubMed] [Google Scholar]
- 12.Lu PD, Jousse C, Marciniak SJ, Zhang Y, Novoa I, Scheuner D, Kaufman RJ, Ron D, Harding HP. Cytoprotection by pre-emptive conditional phosphorylation of translation initiation factor 2. EMBO J. 2004;23:169–179. doi: 10.1038/sj.emboj.7600030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kimball SR, Fabian JR, Pavitt GD, Hinnebusch AG, Jefferson LS. Regulation of guanine nucleotide exchange through phosphorylation of eukaryotic initiation factor eIF2alpha. Role of the alpha- and delta-subunits of eiF2b. J. Biol. Chem. 1998;273:12841–12845. doi: 10.1074/jbc.273.21.12841. [DOI] [PubMed] [Google Scholar]
- 14.Scheuner D, Song B, McEwen E, Gillespie P, Saunders T, Bonner-Weir S, Kaufman RJ. Translational control is required for the unfolded protein response and in-vivo glucose homeostasis. Mol. Cell. 2001;7:1165–1176. doi: 10.1016/s1097-2765(01)00265-9. [DOI] [PubMed] [Google Scholar]
- 15.Novoa I, Zeng H, Harding H, Ron D. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2a. J. Cell Biol. 2001;153:1011–1022. doi: 10.1083/jcb.153.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang P, McGrath BC, Reinert J, Olsen DS, Lei L, Gill S, Wek SA, Vattem KM, Wek RC, Kimball SR, Jefferson LS, Cavener DR. The GCN2 eIF2alpha kinase is required for adaptation to amino acid deprivation in mice. Mol. Cell. Biol. 2002;22:6681–6688. doi: 10.1128/MCB.22.19.6681-6688.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wortham NC, Martinez M, Gordiyenko Y, Robinson CV, Proud CG. Analysis of the subunit organization of the eIF2B complex reveals new insights into its structure and regulation. FASEB J. 2014;28:2225–2237. doi: 10.1096/fj.13-243329. [DOI] [PubMed] [Google Scholar]
- 18.Gordiyenko Y, Schmidt C, Jennings MD, Matak-Vinkovic D, Pavitt GD, Robinson CV. eIF2B is a decameric guanine nucleotide exchange factor with a gamma2epsilon2 tetrameric core. Nature communications. 2014;5:3902. doi: 10.1038/ncomms4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bogorad AM, Xia B, Sandor DG, Mamonov AB, Cafarella TR, Jehle S, Vajda S, Kozakov D, Marintchev A. Insights into the architecture of the eIF2Balpha/beta/delta regulatory subcomplex. Biochemistry. 2014;53:3432–3445. doi: 10.1021/bi500346u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sajid A, Arora G, Gupta M, Singhal A, Chakraborty K, Nandicoori VK, Singh Y. Interaction of Mycobacterium tuberculosis elongation factor Tu with GTP is regulated by phosphorylation. J. Bacteriol. 2011;193:5347–5358. doi: 10.1128/JB.05469-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shang X, Marchioni F, Sipes N, Evelyn CR, Jerabek-Willemsen M, Duhr S, Seibel W, Wortman M, Zheng Y. Rational design of small molecule inhibitors targeting RhoA subfamily Rho GTPases. Chem. Biol. 2012;19:699–710. doi: 10.1016/j.chembiol.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmidt EK, Clavarino G, Ceppi M, Pierre P. SUnSET, a nonradioactive method to monitor protein synthesis. Nat Methods. 2009;6:275–277. doi: 10.1038/nmeth.1314. [DOI] [PubMed] [Google Scholar]
- 23.Scorsone KA, Panniers R, Rowlands AG, Henshaw EC. Phosphorylation of eukaryotic initiation factor 2 during physiological stresses which affect protein synthesis. J. Biol. Chem. 1987;262:14538–14543. [PubMed] [Google Scholar]
- 24.Ronda C, Pedersen LE, Hansen HG, Kallehauge TB, Betenbaugh MJ, Nielsen AT, Kildegaard HF. Accelerating genome editing in CHO cells using CRISPR Cas9 and CRISPy, a web-based target finding tool. Biotechnol. Bioeng. 2014;111:1604–1616. doi: 10.1002/bit.25233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 2013;8:2281–2308. doi: 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harding HP, Zyryanova AF, Ron D. Uncoupling proteostasis and development in vitro with a small molecule inhibitor of the pancreatic endoplasmic reticulum kinase, PERK. J. Biol. Chem. 2012;287:44338–44344. doi: 10.1074/jbc.M112.428987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohammad-Qureshi SS, Haddad R, Palmer KS, Richardson JP, Gomez E, Pavitt GD. Purification of FLAG-tagged eukaryotic initiation factor 2B complexes, subcomplexes, and fragments from Saccharomyces cerevisiae. Methods Enzymol. 2007;431:1–13. doi: 10.1016/S0076-6879(07)31001-X. [DOI] [PubMed] [Google Scholar]
- 28.McEwen DP, Gee KR, Kang HC, Neubig RR. Fluorescent BODIPY-GTP analogs: real-time measurement of nucleotide binding to G proteins. Anal. Biochem. 2001;291:109–117. doi: 10.1006/abio.2001.5011. [DOI] [PubMed] [Google Scholar]
- 29.Yoshida H, Okada T, Haze K, Yanagi H, Yura T, Negishi M, Mori K. ATF6 Activated by Proteolysis Binds in the Presence of NF-Y (CBF) Directly to the cis-Acting Element Responsible for the Mammalian Unfolded Protein Response. Mol. Cell. Biol. 2000;20:6755–6767. doi: 10.1128/mcb.20.18.6755-6767.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Note added in proof: The delta subunit of eIF2B has been independently fingered as a likely target for ISRIB action by Sidrauski C, Tsai JC, Kampmann M, Hearn BR, Vedantham P, Jaishankar P, Sokabe M, Mendez AS, Newton BW, Tang EL, Verschueren E, Johnson JR, Krogan NJ, Fraser CS, Weissman JS, Renslo AR, Walter P. Pharmacological dimerization and activation of the exchange factor eif2b antagonizes the integrated stress response. Elife. 2015;4 doi: 10.7554/eLife.07314. 10.7554/eLife.07314.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.