Abstract
Cytochrome P450 monooxygenases (P450s), which are well-known drug-metabolizing enzymes, are thought to play a signal transduction role in µ opioid analgesia and may serve as high-affinity 3H-cimetidine (3HCIM) binding sites in the brain. 3HCIM binding sites may also be related to opioid or nonopioid analgesia. However, of the more than 100 murine P450 enzymes, the specific isoform(s) responsible for either function have not been identified. Presently, three lines of constitutive P450 gene cluster knockout (KO) mice with full-length deletions of 14 Cyp2c, 9 Cyp2d, and 7 Cyp3a genes were studied for deficiencies in 3HCIM binding and for opioid analgesia. Liver and brain homogenates from all three genotypes showed normal 3HCIM binding values, indicating that gene products of Cyp2d, Cyp3a, and Cyp2c are not 3HCIM-binding proteins. Cyp2d KO and Cyp3a KO mice showed normal antinociceptive responses to a moderate systemic dose of morphine (20 mg/kg, s.c.), thereby excluding 16 P450 isoforms as mediators of opioid analgesia. In contrast, Cyp2c KO mice showed a 41% reduction in analgesic responses following systemically (s.c.) administered morphine. However, the significance of brain Cyp2c gene products in opioid analgesia is uncertain because little or no analgesic deficits were noted in Cyp2c KO mice following intracerebroventricular or intrathecalmorphine administration, respectively. These results show that the gene products of Cyp2d and Cyp3a do not contribute to µ opioid analgesia in the central nervous system. A possible role for Cyp2c gene products in opioid analgesia requires further consideration.
Introduction
Opioid analgesics such as morphine act on µ opioid receptors in the brain and spinal dorsal horn to relieve pain. In the periaqueductal gray, µ receptor stimulation reduces presynaptic GABAergic activity, leading to activation of descending, pain-dampening circuits (Heinricher and Ingram, 2008); however, the relevant cellular mechanisms for this effect remain uncertain. We recently described the development of brain cytochrome P450 monooxygenasis (P450) reductase null (BCPRN) mice (which lack brain neuronal P450 activity) and showed that these mice exhibit defective analgesic responses to morphine (Conroy et al., 2010). P450s are best known as mediators of drug metabolism, but morphine is not metabolized by P450s (Kuo et al., 1991), and brain levels of morphine were identical in BCPRN and control mice (Conroy et al., 2010). Since P450s also catalyze the oxidation of endogenous lipids (Spector, 2009), a pharmacodynamic (versus pharmacokinetic) mechanism best explains the brain P450 requirement for opioid analgesia. Conroy et al. (2010) proposed that µ opioids act in the central nervous system (CNS) by stimulating the release and P450-mediated epoxidation of arachidonic acid. The epoxide products may have pain-relieving properties (Terashvili et al., 2008; Wagner et al., 2013). Subsequent results support this P450/epoxygenase hypothesis for µ agonist action (Zhang and Pan, 2012; Conroy et al., 2013). However, it is not known which of the more than 100 P450 isoforms in the mouse (Nelson et al., 2004) might be important for opioid analgesia.
Earlier work from one of our laboratories focused on the analgesic properties of the histamine H2 receptor antagonist cimetidine and its congener improgan (Hough, 2004). 3H-Cimetidine (3HCIM) exhibits high-affinity, specific binding to unknown protein(s) in brain and liver which are distinct from the H2 receptor. Characterization of 3HCIM-binding proteins in brain suggested a P450 profile, but these were never identified (Stadel et al., 2008). Identification of these proteins is of interest because they could be new analgesic drug targets (Hough et al., 2007). Although this idea has not been confirmed (Stadel et al., 2010), 3HCIM-binding studies led to the discovery that the pain-relieving effects of both nonopioid (Hough et al., 2011) and opioid (Conroy et al., 2010) analgesic drugs require brain P450 activity.
The abundance of murine P450 genes makes it difficult to assign specific roles to particular isoforms; however, the recent development of several P450 gene cluster knockout (KO) mice is important progress toward solving this problem. Constitutive KO genotypes were recently described for the Cyp2c, Cyp2d, and Cyp3a gene clusters (van Herwaarden et al., 2007; Hasegawa et al., 2011; Scheer et al., 2012a,b). Experiments with these new models (Cyp2c, Cyp2d, and Cyp3a KO mice) are clarifying the roles for enzyme families in the metabolism of xenobiotics. Presently, we used these three lines of KO mice to study two P450-related problems: (1) assessing possible mechanistic roles for CYP2C, CYP2D, and/or CYP3A subfamilies in the pain-relieving actions of morphine, and (2) searching for 3HCIM-binding proteins in liver and brain homogenates from these mouse lines.
Materials and Methods
Drugs and Solutions.
Morphine sulfate (Mallinckrodt, St. Louis, MO) was dissolved in saline.
Animals.
Three recently developed lines of constitutive P450 gene cluster KO mice (Cyp2c, Cyp2d, and Cyp3a) along with controls (C57BL/6Ntac, Taconic B6-M) were purchased from Taconic Biosciences (Germantown, NY). Cyp2c KOs (B6-Cyp2ctm1104Arte, Taconic 9177-M) contain homozygous deletions of 14 full-length P450 genes (Cyp2c55, Cyp2c65, Cyp2c66, Cyp2c29, Cyp2c38, Cyp2c39, Cyp2c67, Cyp2c68, Cyp2c40, Cyp2c69, Cyp2c37, Cyp2c54, Cyp2c50, and Cyp2c70), but retain a functional Cyp2c44 (Scheer et al., 2012a). Cyp2d KOs [C57BL/6-Del(15Cyp2d22-Cyp2d26)1Arte, Taconic 9178-M] contain homozygous deletions of nine full-length P450 genes (Cyp2d22, Cyp2d11, Cyp2d10, Cyp2d9, Cyp2d12, Cyp2d34, Cyp2d13, Cyp2d40, and Cyp2d26) as recently described (Scheer et al., 2012b). Cyp3a KOs [C57BL/6NTac-Del(5Cyp3a57-Cyp3a59)1Arte, Taconic 8841-M] contain homozygous deletions of seven full-length P450 genes (Cyp3a11, Cyp3a16, Cyp3a25, Cyp3a41, Cyp3a44, Cyp3a57, and Cyp3a59, but have retained Cyp3a13) as described (Hasegawa et al., 2011). All subjects were adult males (greater than 10 weeks of age, 19–44 g). Animals were maintained on a 12-hour light/dark cycle (lights on from 7:00 AM to 7:00 PM), with food and water freely available. All animal experiments were approved by the Institutional Animal Care and Use Committee of Albany Medical College.
Surgery.
Cannulas were chronically implanted for intracerebroventricular (i.c.v.) microinjections. Following anesthesia (pentobarbital sodium, 50 mg/kg, i.p., supplemented with isoflurane), a stainless steel guide cannula was stereotaxically inserted into the left lateral ventricle [coordinates were anterior-posterior −0.5, medial-lateral −1.0, and dorsal ventral −2.0 mm from bregma; following Paxinos and Franklin (2001)]. The cannula was anchored to the skull with stainless steel screws and dental cement. After surgery, subjects were individually housed and allowed to recover for at least 5 days before testing.
I.C.V. Drug Injections.
Subjects were lightly secured in a laboratory pad, the cannula stylet was removed, and the injection cannula was inserted so as to extend 1 mm beyond below the guide. Injections (2 μl) were made over a 1 minute period. One minute later, the injection cannula was clipped and sealed approximately 2 mm above the juncture with the guide cannula. Successful injections were verified by the movement of an air bubble in the tubing. Following testing, animals received pentobarbital sodium (100 mg/kg, i.p.) and India Ink (2 μl). Brains were removed to verify ventricular distribution of the ink. Data from animals with incorrect placements or unsuccessful injections were excluded.
Nociceptive Testing.
The hot water tail immersion test was used to measure morphine analgesia (Sewell and Spencer, 1976). Subjects were restrained in a conical polypropylene tube, the tip of the tail was immersed (2–3 cm) into a 55°C water bath, and latency to sudden tail movement or withdrawal from the water was recorded. Cutoff latencies were 8 seconds. Subjects were baseline tested, received single s.c. or i.c.v. injections, and were retested as described in the legend of Figure 1. Subjects were only used for a single experiment.
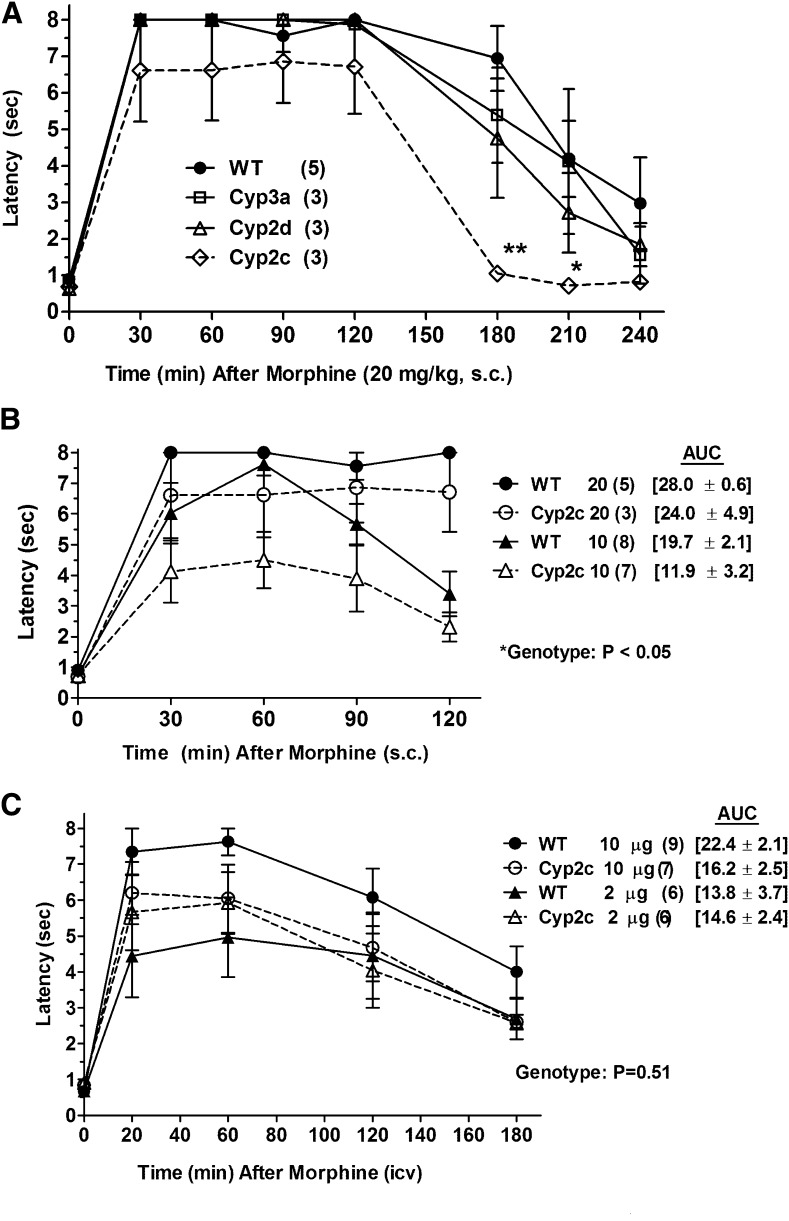
Fig. 1.
Morphine antinociception in P450 KO mice. (A) Control (WT) and three lines of P450 gene cluster KO mice were baseline tested for nociceptive latencies (zero time), received morphine sulfate (20 mg/kg, s.c.), and were retested at the indicated postinjection times [abscissa (minutes)]. Nociceptive latencies (sec, mean ± S.E.M., ordinate) are shown for the number of subjects designated in parentheses (*P < 0.05 and **P < 0.01, respectively) versus WT at the same time. (B) Morphine antinociception in WT and Cyp2c KO mice, shown as in (A), after two doses of morphine (10 and 20 mg/kg, s.c.). Data from the 20 mg/kg group are redrawn from (A). The AUCs (mean ± S.E.M., sec, 30–120 minutes) are given in brackets for the four groups. The *ANOVA of latencies found a significant main effect (P < 0.05) of genotype. (C) WT and Cyp2c KO mice were baseline tested (zero time), received the indicated dose of morphine sulfate (i.c.v.), and were retested at the designated postinjection times [abscissa (minutes)]. Latencies (sec, mean ± S.E.M., ordinate) are shown for the number of subjects in parentheses. The AUCs are given in brackets (mean ± S.E.M., sec, 20–180 minutes) for the four groups. No genotype differences were detected after i.c.v. morphine.
Radioligand Binding.
These experiments were performed following Stadel et al. (2008). Tissues were homogenized in 10 v of Tris-HCl buffer (100 mM, 0.5 mM EDTA, pH 7.4) and centrifuged (26,000g, 15 minutes). Pellets were resuspended in the same volume of buffer, rehomogenized, recentrifuged, and stored at −80° until use. Resuspended pellets (0.3–0.4 mg protein/tube) were incubated in a total volume of 0.1 ml containing 50 nM 3HCIM (80 Ci/mmol; American Radiolabeled Chemicals, Inc., St. Louis, MO) in 100 mM Tris-HCl pH 7.4 for 60 minutes on ice. Burimamide (30 µM) was used to evaluate nonspecific binding. Samples were rapidly filtered through GF/B filters and rinsed three times with 1.5 ml of ice-cold buffer. Filters were placed in 5 ml of Ecoscint (National Diagnostics, Atlanta, GA) and counted in a scintillation counter. Protein content was determined using the bicinchoninic acid method (Pierce Chemical, Rockford, IL).
Data Analysis.
Nociceptive data are expressed as latencies (sec, mean ± S.E.M.) and also as the antinociceptive area under the curve (AUC). AUCs were calculated as the sum of postinjection difference scores for the intervals specified in each experiment. Each difference score was computed as a postinjection latency minus baseline latency. All data were analyzed by analysis of variance (ANOVA), followed by post hoc testing (Statistica, StatSoft, Tulsa, OK). Bonferroni post hoc testing was performed as permitted by ANOVA results. Graphs were produced with Prism 5.04 (Graphpad, San Diego, CA).
Results
Cyp2c KO Mice Show Deficient Analgesic Responses to Systemically Administered Morphine.
A large systemic dose of morphine (20 mg/kg, s.c.) produced robust analgesia in four mouse genotypes lasting up to 2 hours (Fig. 1A). ANOVA [between groups (factor #1): genotype; within groups (factor #2, repeated measures): time] found significant main effects of genotype (F3,10 = 4.07, P < 0.05) and time (F7,21 = 65.9, P < 0.001), with no significant interaction terms. Subsequent analysis showed that the genotype effects were due to the reduced latencies in the Cyp2c KO data. Deficient responses in the Cyp2c KO group were most evident 3 hours after morphine administration, confirmed by post hoc testing (Fig. 1A). Baseline latencies (zero time scores, Fig. 1A) were not different across the four genotypes by one-factor ANOVA.
Because the Cyp2c KO deficit in Fig. 1A was most apparent at the later time points after 20 mg/kg morphine, a lower dose (10 mg/kg, s.c.) was studied in wild-type (WT) control and Cyp2c KO mice (Fig. 1B). Mean latencies in the KO groups after both doses of morphine were smaller than WT responses at all times (Fig. 1B). ANOVA [between groups (factor #1): genotype; (factor #2): dose; within groups (factor #3, repeated measures): time] found significant main effects of genotype (F1,19 = 4.6, P < 0.05), dose (F1,19 = 12.1, P < 0.01), and time (F4,76 = 69.0, P < 0.001), with a significant time by dose (F4,76 = 8.0, P < 0.001) interaction term. Genotype differences at specific times in Fig. 1B were not large enough to be statistically significant by post hoc testing, but the significant genotype term in the ANOVA confirms the diminished morphine responses in the Cyp2c KO mice. AUCs (brackets in 1B) showed 40% and 14% reductions in mean Cyp2c KO responses to morphine at 10 and 20 mg/kg, respectively, compared with WT. However, the genotype-related terms in the ANOVA of AUCs did not reach statistical significance (P = 0.0625 for the main effect of genotype).
Deficient Morphine Analgesia in Cyp2c KO Mice Was Not Clearly Present Following I.C.V. Administration.
To confirm the genotype effects seen after s.c. administration, morphine was administered directly into the CNS. Morphine (2 and 10 µg, i.c.v.) produced prolonged, dose-dependent analgesia in WT mice (Fig. 1C). Compared with respective WT subjects, mean Cyp2c KO responses were reduced after the larger, but not after the smaller, i.c.v. dose of morphine (Fig. 1C). ANOVA [Fig. 1C, between groups (factor #1): genotype; (factor #2)]: dose; within groups (factor #3, repeated measures): time] found significant main effects of time (F4, 96 = 56.9, P < 0.001) but no significant terms related to genotype or dose of morphine. AUC analysis of Fig. 1C data found 28% and 0% reductions in mean KO versus WT responses following 10 and 2 µg of morphine, respectively (brackets in Fig. 1C). However, genotype-related terms in the ANOVA of the i.c.v. AUCs did not reach statistical significance (P = 0.067 for the main effect of genotype).
Normal Analgesic Responses in Cyp2c KO Mice Following I.T. Morphine Administration.
Morphine was also administered into the spinal subarachnoid space of WT and Cyp2c KO mice (Supplemental Fig. 1). Morphine (5 µg, i.t.) produced highly effective analgesia in both genotypes. ANOVA [Supplemental Fig. 1, between groups (factor #1): genotype; within groups (factor #2, repeated measures): time] only found significant main effects of time (F5,50 = 21.7, P < 0.001) with no significant terms related to genotype.
Normal 3HCIM Binding Values in Liver and Brain from P450 Gene Cluster KO Mice.
3HCIM binding was studied in the P450 gene cluster KO mice in an attempt to identify cimetidine-binding P450 isoforms. Liver homogenates from Cyp2c, Cyp2d, and Cyp3a KO mice yielded specific 3HCIM-binding values that were nearly identical to those from WT subjects (Supplemental Fig. 2). One-factor (genotype) ANOVA of these data found no significant genotype differences (P > 0.05) in 3HCIM binding. Binding values from brain homogenates of the four genotypes were very low (less than half of the magnitude found in rat brain), and were also not significantly different from each other (data not shown).
Discussion
The plethora of murine P450 genes (Nelson et al., 2004) and the extremely low levels of P450s in brain (Iliff et al., 2010; Ferguson and Tyndale, 2011) are formidable obstacles to identifying CNS roles for specific isoforms. However, the clustering of multiple, closely related P450 genes made it possible to delete many genes from a P450 subfamily in single KO models (van Herwaarden et al., 2007; Hasegawa et al., 2011; Scheer et al., 2012a,b). The Cyp2c, Cyp2d, and Cyp3a KO mice tested have full-length deletions of 14, nine, and seven P450 genes, respectively. Thus, the present work screened 30 murine P450 isoforms as potential 3HCIM-binding proteins and as potential transduction elements of opioid analgesic signaling.
Characterization of 3HCIM-binding proteins suggested a P450 profile, but these proteins were never identified (Stadel et al., 2008). Since inhibitors of 3HCIM binding also block the effects of several types of analgesics, 3HCIM-binding proteins were suggested to be targets for analgesic drug development (Hough et al., 2007; Stadel et al., 2010). The absence of deficits in 3HCIM binding in liver homogenates from the genotypes studied (Supplemental Fig. 2) demonstrates that none of 30 P450 isoforms deleted presently contribute to 3HCIM binding.
The hypothesis that P450-catalyzed neuronal epoxygenase activity is required for µ opioid analgesia originated following discovery of attenuated analgesic responses to morphine in BCPRN mice (Conroy et al., 2010); the defective analgesia was not explained by genotype differences in brain morphine levels or by differences in µ opioid receptor properties or signaling (Conroy et al., 2010). Furthermore, P450 inhibitors and epoxygenase inhibitors antagonize morphine analgesia (Conroy et al., 2010, 2013). Consistent with the hypothesis, Zhang and Pan (2012) showed that the in vitro effects of µ (but not δ) opioids in the brain stem are blocked by a P450 inhibitor, but the relevant isoforms are unknown. The present results (Fig. 1A), showing normal responses to morphine in the Cyp2d and Cyp3a KO mice, imply no roles for 16 gene products (Hasegawa et al., 2011; Scheer et al., 2012b) as mediators of µ opioid responses.
Because BCPRN mice (lacking neuronal P450 enzyme function) show defective morphine responses (Conroy et al., 2010), it was presently theorized that the morphine-relevant P450 isoforms might be discovered by demonstrating a deficit in the relevant KO mouse. The blunted maximal effect and shortened duration of action of s.c.-administered morphine in Cyp2c KO mice (Fig. 1, A and B) seemed to support the possible relevance of CYP2C isoforms. Of particular mechanistic relevance might be CYP2C29, CYP2C37, CYP2C38, and CYP2C40, which are absent in the Cyp2c KO mice but are normally expressed in the murine CNS and are thought to have epoxygenase activity (Iliff et al., 2010). However, since systemically administered opioids act in the brain and spinal cord (Heinricher and Ingram, 2008), no CNS role for Cyp2c gene products in opioid analgesia was confirmed when morphine was given by i.c.v. (Fig. 2) or i.t. (Supplemental Fig. 1) dosing. Since synergistic interactions are known to occur between supraspinal and spinal opioid mechanisms (Yeung and Rudy, 1980), a role for the Cyp2c gene products in these interactions remains possible.
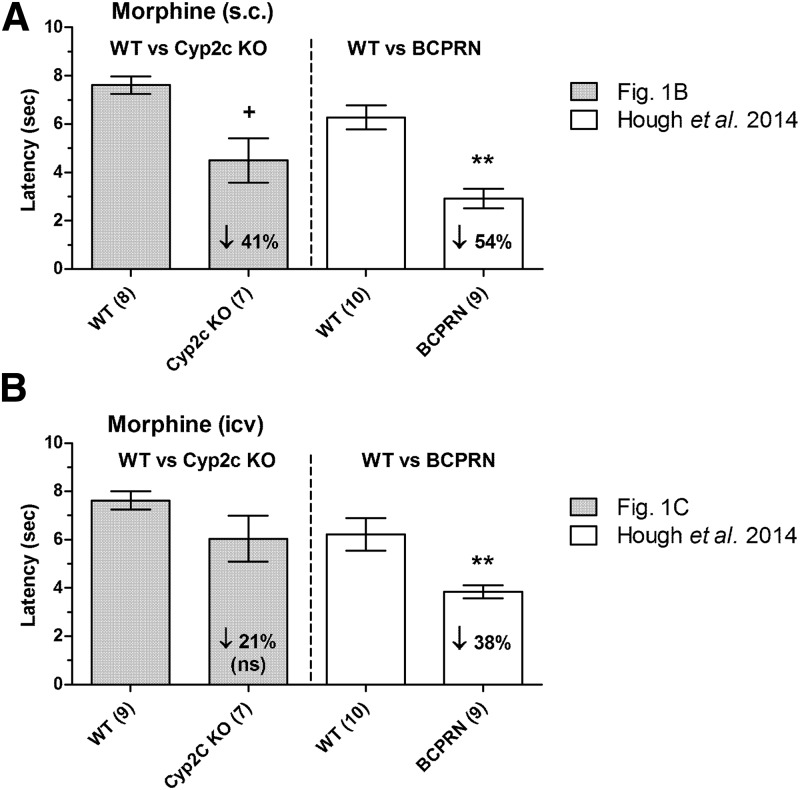
Fig. 2.
Deficits in morphine analgesia in two genotypes of P450-deficient mice. Responses in Cyp2c KO mice are compared with those found in BCPRN mice, a conditional KO lacking microsomal brain neuronal P450 activity. Latencies (ordinates, mean ± S.E.M.) are shown for each treatment of the number of subjects in parentheses. (A) Effects of s.c.-administered morphine in Cyp2c KO and WT control mice (two left-hand-side bars, data are 60 minutes after 10 mg/kg, s.c.; data from Fig. 1B) versus BCPRN and WT mice [two right-hand-side bars, data are 90 minutes after 20 mg/kg, s.c.; data from Hough et al. (2014)]. (B) Effects of i.c.v.-administered morphine (60 minutes after 10 µg) in Cyp2c KO and WT control mice (two left-hand-side bars; data from Fig. 1C) and in BCPRN and WT mice [two right-hand-side bars; data from Hough et al. (2014)]. Downward arrows show mean percent reductions in responses in each mutant genotype versus respective WT controls. **BCPRN, responses significantly different (P < 0.01) versus WT by ANOVA and post hoc comparisons. +Significant (P < 0.05) genotype difference revealed by ANOVA, but not significantly different at this time by post hoc testing.
Figure 2 shows a side-by-side comparison of the deficits in morphine analgesia seen presently in Cyp2c KO mice versus earlier findings with BCPRN mice. Although statistically significant reductions were seen in both mutants following s.c. morphine (41% and 54% reductions, respectively), BCPRN mice also showed a statistically robust (38%) reduction in responses following i.c.v. morphine versus a nonsignificant tendency (28%) toward reduced responding in Cyp2c KO mice. Since BCPRN mice have a neuronal defect in cytochrome P450 reductase (required for all microsomal P450 activity), they are presumed to have reduced activity of many P450 isoforms. Although the nonsignificant tendency toward reduced responding in Cyp2c KO mice prevents strong conclusions, this deficit could imply the importance of multiple P450 subfamilies, including CYP2C. Alternatively, the significant reductions in analgesia seen in Cyp2c KO mice after s.c., but not i.c.v., morphine might be explained by decreases in plasma levels of morphine in the KO mice after s.c. administration. Because morphine is metabolized in mice by glucuronidation, and not by P450 mechanisms (Kuo et al., 1991), Cyp2c KO mice could have adaptive increases in peripheral glucuronidation of morphine, thereby lowering plasma morphine levels. This has not been tested. Unlike the case for humans, mice do not have active morphine glucuronide metabolites (Kuo et al., 1991). Unlike the discrepant findings with i.c.v. morphine (Cyp2c KO versus BCPRN, Fig. 2), no genotype differences in analgesia were seen following i.t. opioids in either Cyp2c KO (Supplemental Fig. 1) or BCPRN mice (Hough et al., 2015). This pattern is consistent with a P450 role for supraspinal, but not spinal opioid, mechanisms.
As discussed, the present results exclude 16 P450 isoforms from the 3a and 2d subfamilies as mediators of opioid analgesia, and four 2c isoforms may be important but require further study. Among these subfamilies, Cyp3a13 (Hasegawa et al., 2011) and Cyp2c44 (Scheer et al., 2012a) gene products were not studied because these genes were not deleted in the present KO mice. The latter is a well-known epoxygenase that deserves further study (Capdevila et al., 2007). It is clear that many additional P450 isoforms remain to be studied in the context of opioid analgesia. Among these, members of the CYP4X (Iliff et al., 2010) and CYP2J (Graves et al., 2013) subfamilies have epoxygenase activity and are found in the CNS. Although the P450 epoxygenase hypothesis has not been confirmed, new classes of analgesic or antihyperalgesic medications are already being developed to mimic the actions of lipid epoxides (Brostram and Falck, 2013) or to inhibit their metabolism (Wagner et al., 2013). The identification of the specific opioid-relevant P450 isoforms in the brain and their analgesic products will catalyze the development of novel pain relievers.
Supplementary Material
Abbreviations
- ANOVA
analysis of variance
- AUC
area under the curve
- BCPRN
brain cytochrome P450 reductase null
- CNS
central nervous system
- 3HCIM
3H-cimetidine
- i.c.v.
intracerebroventricular
- i.t.
intrathecal
- KO
knockout
- P450
cytochrome P450 monooxygenasis
- WT
wild-type
Authorship Contributions
Participated in research design: Hough.
Conducted experiments: Nalwalk.
Contributed new reagents or analytic tools: Scheer.
Performed data analysis: Nalwalk, Hough.
Wrote or contributed to the writing of the manuscript: Hough, Nalwalk, Ding, Scheer.
Footnotes
This work was supported by a grant from the National Institutes of Health National Institute on Drug Abuse [Grant DA027835].
 This article has supplemental material available at dmd.aspetjournals.org.
This article has supplemental material available at dmd.aspetjournals.org.
References
- Brostram L and Falck JR (2013) inventors, Cytometix, Inc., assignee. Arachidonic acid analogs and methods for analgesic treatment using same. U.S. patent 20130023510 A1. 2013 Jan 24.
- Capdevila JH, Falck JR, Imig JD. (2007) Roles of the cytochrome P450 arachidonic acid monooxygenases in the control of systemic blood pressure and experimental hypertension. Kidney Int 72:683–689. [DOI] [PubMed] [Google Scholar]
- Conroy JL, Fang C, Gu J, Zeitlin SO, Yang W, Yang J, VanAlstine MA, Nalwalk JW, Albrecht PJ, Mazurkiewicz JE, et al. (2010) Opioids activate brain analgesic circuits through cytochrome P450/epoxygenase signaling. Nat Neurosci 13:284–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy JL, Nalwalk JW, Phillips JG, Hough LB. (2013) CC12, a P450/epoxygenase inhibitor, acts in the rat rostral, ventromedial medulla to attenuate morphine antinociception. Brain Res 1499:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson CS, Tyndale RF. (2011) Cytochrome P450 enzymes in the brain: emerging evidence of biological significance. Trends Pharmacol Sci 32:708–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves JP, Edin ML, Bradbury JA, Gruzdev A, Cheng J, Lih FB, Masinde TA, Qu W, Clayton NP, Morrison JP, et al. (2013) Characterization of four new mouse cytochrome P450 enzymes of the CYP2J subfamily. Drug Metab Dispos 41:763–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa M, Kapelyukh Y, Tahara H, Seibler J, Rode A, Krueger S, Lee DN, Wolf CR, Scheer N. (2011) Quantitative prediction of human pregnane X receptor and cytochrome P450 3A4 mediated drug-drug interaction in a novel multiple humanized mouse line. Mol Pharmacol 80:518–528. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Ingram SL. (2008) The brainstem and nociceptive modulation, in The Senses: A Comprehensive Reference (Basbaum AI, Bushnell MC, Julius D. eds) vol 5, pp 593–626, Elsevier, New York. [Google Scholar]
- Hough LB. (2004) Improgan-like analgesics: a family of compounds derived from histamine antagonists. Med Chem Res 13:78–87. [Google Scholar]
- Hough LB, Nalwalk JW, Cleary RA, Phillips JG, Fang C, Yang W, Ding X. (2014) Deficits in neuronal cytochrome P450 activity attenuate opioid analgesia but not opioid side effects. Eur J Pharmacol 740:255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hough LB, Nalwalk JW, Phillips JG, Kern B, Shan Z, Wentland MP, de Esch IJ, Janssen E, Barr T, Stadel R. (2007) CC12, a high-affinity ligand for 3H-cimetidine binding, is an improgan antagonist. Neuropharmacology 52:1244–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hough LB, Nalwalk JW, Yang J, Conroy JL, VanAlstine MA, Yang W, Gargano J, Shan Z, Zhang SZ, Wentland MP, et al. (2011) Brain P450 epoxygenase activity is required for the antinociceptive effects of improgan, a nonopioid analgesic. Pain 152:878–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hough LB, Nalwalk JW, Yang W, Ding X. (2015) Neuronal cytochrome P450 activity and opioid analgesia: relevant sites and mechanisms. Brain Res 1616:10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff JJ, Jia J, Nelson J, Goyagi T, Klaus J, Alkayed NJ. (2010) Epoxyeicosanoid signaling in CNS function and disease. Prostaglandins Other Lipid Mediat 91:68–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo CK, Hanioka N, Hoshikawa Y, Oguri K, Yoshimura H. (1991) Species difference of site-selective glucuronidation of morphine. J Pharmacobiodyn 14:187–193. [DOI] [PubMed] [Google Scholar]
- Nelson DR, Zeldin DC, Hoffman SM, Maltais LJ, Wain HM, Nebert DW. (2004) Comparison of cytochrome P450 (CYP) genes from the mouse and human genomes, including nomenclature recommendations for genes, pseudogenes and alternative-splice variants. Pharmacogenetics 14:1–18. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. (2001) The Mouse Brain in Stereotaxic Coordinates, Academic Press, San Diego. [Google Scholar]
- Scheer N, Kapelyukh Y, Chatham L, Rode A, Buechel S, Wolf CR. (2012a) Generation and characterization of novel cytochrome P450 Cyp2c gene cluster knockout and CYP2C9 humanized mouse lines. Mol Pharmacol 82:1022–1029. [DOI] [PubMed] [Google Scholar]
- Scheer N, Kapelyukh Y, McEwan J, Beuger V, Stanley LA, Rode A, Wolf CR. (2012b) Modeling human cytochrome P450 2D6 metabolism and drug-drug interaction by a novel panel of knockout and humanized mouse lines. Mol Pharmacol 81:63–72. [DOI] [PubMed] [Google Scholar]
- Sewell RDE, Spencer PSJ. (1976) Antinociceptive activitiy of narcotic agonist and partial agonist analgesics and other agents in the tail-immersion test in mice and rats. Neuropharmacology 15:683–688. [DOI] [PubMed] [Google Scholar]
- Spector AA. (2009) Arachidonic acid cytochrome P450 epoxygenase pathway. J Lipid Res 50 (Suppl):S52–S56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadel R, Carpenter AB, Nalwalk JW, de Esch IJ, Janssen E, Hough LB. (2010) Inhibition of brain [3H]cimetidine binding by improgan-like antinociceptive drugs. Eur J Pharmacol 632:33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadel R, Yang J, Nalwalk JW, Phillips JG, Hough LB. (2008) High-affinity binding of [3H]-cimetidine to a heme-containing protein in rat brain. Drug Metab Dispos 36:614–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terashvili M, Tseng LF, Wu HE, Narayanan J, Hart LM, Falck JR, Pratt PF, Harder DR. (2008) Antinociception produced by 14,15-epoxyeicosatrienoic acid is mediated by the activation of β-endorphin and Met-enkephalin in the rat ventrolateral periaqueductal gray. J Pharmacol Exp Ther 326:614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Herwaarden AE, Wagenaar E, van der Kruijssen CM, van Waterschoot RA, Smit JW, Song JY, van der Valk MA, van Tellingen O, van der Hoorn JW, Rosing H, et al. (2007) Knockout of cytochrome P450 3A yields new mouse models for understanding xenobiotic metabolism. J Clin Invest 117:3583–3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner K, Inceoglu B, Dong H, Yang J, Hwang SH, Jones P, Morisseau C, Hammock BD. (2013) Comparative efficacy of 3 soluble epoxide hydrolase inhibitors in rat neuropathic and inflammatory pain models. Eur J Pharmacol 700:93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung JC, Rudy TA. (1980) Multiplicative interaction between narcotic agonisms expressed at spinal and supraspinal sites of antinociceptive action as revealed by concurrent intrathecal and intracerebroventricular injections of morphine. J Pharmacol Exp Ther 215:633–642. [PubMed] [Google Scholar]
- Zhang Z, Pan ZZ. (2012) Signaling cascades for δ-opioid receptor-mediated inhibition of GABA synaptic transmission and behavioral antinociception. Mol Pharmacol 81:375–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.