Abstract
The physiologic roles of voltage-gated KV7 channel subtypes (KV7.1–KV7.5) in detrusor smooth muscle (DSM) are poorly understood. Here, we sought to elucidate the functional roles of KV7.2/KV7.3 channels in guinea pig DSM excitability and contractility using the novel KV7.2/KV7.3 channel activator ICA-069673 [N-(2-chloro-5-pyrimidinyl)-3,4-difluorobenzamide]. We employed a multilevel experimental approach using Western blot analysis, immunocytochemistry, isometric DSM tension recordings, fluorescence Ca2+ imaging, and perforated whole-cell patch-clamp electrophysiology. Western blot experiments revealed the protein expression of KV7.2 and KV7.3 channel subunits in DSM tissue. In isolated DSM cells, immunocytochemistry with confocal microscopy further confirmed protein expression for KV7.2 and KV7.3 channel subunits, where they localize within the vicinity of the cell membrane. ICA-069673 inhibited spontaneous phasic, pharmacologically induced, and nerve-evoked contractions in DSM isolated strips in a concentration-dependent manner. The inhibitory effects of ICA-069673 on DSM spontaneous phasic and tonic contractions were abolished in the presence of the KV7 channel inhibitor XE991 [10,10-bis(4-pyridinylmethyl)-9(10H)-anthracenone dihydrochloride]. Under conditions of elevated extracellular K+ (60 mM), the effects of ICA-069673 on DSM tonic contractions were significantly attenuated. ICA-069673 decreased the global intracellular Ca2+ concentration in DSM cells, an effect blocked by the L-type Ca2+ channel inhibitor nifedipine. ICA-069673 hyperpolarized the membrane potential and inhibited spontaneous action potentials of isolated DSM cells, effects that were blocked in the presence of XE991. In conclusion, using the novel KV7.2/KV7.3 channel activator ICA-069673, this study provides strong evidence for a critical role for the KV7.2- and KV7.3-containing channels in DSM function at both cellular and tissue levels.
Introduction
Voltage-gated K+ (KV7) channels (KV7.1–KV7.5), also known as KCNQ (KCNQ1–KCNQ5) channels, have been suggested as key regulators of detrusor smooth muscle (DSM) excitability and contractility (Argentieri et al., 2002; Sheldon et al., 2002; Afeli et al., 2013; Anderson et al., 2013; Svalø et al., 2015). In DSM, the overall function of KV channels, which includes the KV7 channels, is to maintain and stabilize the resting membrane potential (Petkov, 2012). KV7 channel activation by promoting hyperpolarization limits Ca2+ influx through L-type voltage-gated Ca2+ (CaV) channels, which leads to DSM relaxation. The ability of DSM to generate spontaneous action potentials, which underlies DSM phasic contractility, allows this tissue to facilitate urinary bladder function (Chen et al., 2010; Petkov, 2012, 2014). Under pathophysiological conditions, such as overactive bladder (OAB), the contractile activity of DSM may be abnormally increased (Andersson and Wein, 2004). Thus, the selective pharmacological targeting of bladder-specific KV7 channel subtypes may represent a novel therapeutic strategy to control OAB.
The KV7 channel family consists of five members (KV7.1–KV7.5), which form homomeric or heteromeric tetramers of KV7 pore-forming α-subunits. The diversity of KV7 channel α-subunit combinations, such as KV7.2/KV7.3 or KV7.3/KV7.5, expands their functional and biophysical properties (Robbins, 2001; Soldovieri et al., 2011; Petkov, 2012). For example, heteromeric KV7.2/KV7.3 channels produce whole-cell currents 10-fold higher in amplitude than either homomeric KV7.2 or KV7.3 channels (Soldovieri et al., 2011). KV7 channels can also associate with β-regulatory subunits (KCNE1–KCNE5), which may alter KV7 channel properties and subcellular localization (Abbott and Goldstein, 2001; Wrobel et al., 2012). This diversity of KV7 channel expression and function underlies their attractiveness as potential novel therapeutic targets for OAB.
KV7 channel pharmacological modulators have proven useful for elucidating KV7 channel functional roles in various tissues, including vascular, bronchial, and gastrointestinal smooth muscle (Jepps et al., 2009, 2011, 2014; Ng et al., 2011; Brueggemann et al., 2012, 2014b; Mani et al., 2013; Chadha et al., 2014). Such modulators include KV7 channel activators retigabine (KV7.2–KV7.5) and L-364,373 [5-(2-fuorophenyl)-1,3-dihydro-3-(1H-indol-3-ylmethyl)-1-methyl-2H-1,4-benzodiazepin-2-one] (KV7.1) as well as the KV7 channel inhibitors XE991 [10,10-bis(4-pyridinylmethyl)-9(10H)-anthracenone dihydrochloride] and linopiridine, both of which effectively block all KV7 channels. Potential KV7 channel functional roles in DSM were suggested during the clinical trials of the antiepileptic drug retigabine. Patients undergoing treatment reported an increased occurrence of urinary retention compared with the placebo (Brickel et al., 2012). These findings correspond with in vivo studies in rodents, in which retigabine increased bladder capacity while decreasing voiding frequency (Streng et al., 2004; Svalø et al., 2012).
In guinea pig DSM, mRNA transcripts for all KV7 channel subunits have been identified (Anderson et al., 2013), with quantitative polymerase chain reaction studies indicating that KV7.1, KV7.2, and KV7.5 channel α-subunits are most prevalent in DSM cells (Afeli et al., 2013). KV7 channel inhibition with XE991 was associated with membrane depolarization and an increase in Ca2+ oscillations and phasic contractions in guinea pig DSM (Afeli et al., 2013; Anderson et al., 2013). KV7 channel activation with retigabine caused membrane hyperpolarization and inhibition of DSM phasic contractions (Afeli et al., 2013). KV7 channels also regulate porcine DSM contractility, in which the KV7.2 and KV7.4 channel activator ML-213 [N-(2,4,6-trimethylphenyl)-bicyclo[2.2.1]heptane-2-carboxamide] displayed a comparable effect to retigabine in promoting DSM relaxation (Svalø et al., 2013). Despite emerging developments, KV7 channel subtype-specific physiologic roles remain poorly understood owing to the lack of selective pharmacological modulators capable of distinguishing among KV7 channel subtypes (Xiong et al., 2008).
The novel compound ICA-069673 [N-(2-chloro-5-pyrimidinyl)-3,4-difluorobenzamide] was developed by Icagen, Inc. (Durham, NC) in a screening program to identify subtype-selective KV7 channel activators (Amato et al., 2011). ICA-069673 demonstrated 20-fold greater selectivity for heteromeric KV7.2/KV7.3 channels over KV7.3/KV7.5, with EC50 values of 0.69 and 14.3 µM, respectively (Amato et al., 2011). ICA-069673 did not affect KV7.1 channels, which are predominately expressed in the heart (Amato et al., 2011). A recent study on A7r5 smooth muscle cells demonstrated that ICA-069673 was effective in activating recombinantly expressed KV7.4 channels (Brueggemann et al., 2014a). However, KV7.4 channel protein expression was not observed in guinea pig DSM (Afeli et al., 2013).
Here, we aimed to investigate the functional roles of KV7.2- and KV7.3-containing channels in DSM excitability and contractility by targeting them with the novel KV7.2/KV7.3 channel activator ICA-069673. We applied a multilevel experimental approach including immunocytochemistry with confocal microscopy, Western blot analysis, isometric DSM tension recordings, Ca2+ imaging, and perforated whole-cell patch-clamp electrophysiology to test the hypothesis that KV7.2- and KV7.3-containing channels are among the KV7 channel subtypes critically regulating guinea pig DSM excitation-contraction coupling.
Materials and Methods
Ethical Approval for Animal Use.
Experimental procedures involving the use of animals, as described in this study, were reviewed and approved by the Institutional Animal Care and Use Committee of the University of South Carolina (Columbia, SC; protocol no. 2186).
Animal Housing, Euthanasia, and DSM Tissue Acquisition.
A total of 38 adult male Hartley-Albino guinea pigs, aged 3–12 months (Charles River Laboratories, Raleigh, NC) and weighing 310–575 g (average 463 ± 16 g), were used in these studies. Animals were housed at room temperature (22°C–23°C) within the Animal Resource Facilities at the University of South Carolina. Guinea pigs had free access to food and water and were exposed to 12-hour light/dark cycles. Each animal was euthanized by CO2 inhalation using a SMARTBOX Automated CO2 Delivery System (Euthanex Corp., Palmer, PA), followed by thoracotomy. The urinary bladder was subsequently removed by administering a transverse incision superior to the bladder neck.
DSM Single-Cell Isolation and Collection.
DSM single cells were enzymatically isolated using a combination of papain and collagenase, as previously described (Hristov et al., 2012b). Intact DSM strips with urothelium removed were used for single-cell isolation. Briefly, one to two isolated DSM strips (5–8 mm long, 2–4 mm wide) with urothelium removed were placed in 2 ml dissection solution (see the section below on solutions and drugs) containing 1 mg/ml bovine serum albumin (BSA), 1 mg/ml papain (Worthington Biochemical, Lakewood, NJ), and 1 mg/ml dl-dithiothreitol and incubated for 25–30 minutes at 37°C. Subsequently, isolated DSM strips were transferred to 2 ml dissection solution containing 1 mg/ml BSA, 1 mg/ml collagenase (type II; Sigma-Aldrich, St. Louis, MO), and 100 μM CaCl2 and incubated for 5 to 6 minutes at 37°C. Isolated DSM strips were then washed three times with BSA-containing dissection solution. Enzyme-treated strips were then passed through a fire-polished Pasteur pipette to disperse individual DSM cells, which were used for immunocytochemistry, Ca2+ imaging, and patch-clamp electrophysiology. DSM cells were used for experiments within 12 hours of isolation.
Western Blot Analysis.
Western blot experiments were performed according to previously described methods (Chen et al., 2010; Hristov et al., 2012a). For these experiments, the primary antibodies used were anti-KCNQ2 (KV7.2) (1:200 dilution; Santa Cruz Biotechnology, Dallas, TX) or anti-KCNQ3 (KV7.3) (1:200 dilution; Santa Cruz Biotechnology) polyclonal antibodies, respectively. The donkey anti-goat IgG-horseradish peroxidase–conjugated secondary antibody (1:500 dilution; Santa Cruz Biotechnology) was used for all Western blot experiments. In control experiments, competing peptides for KV7.2 (Santa Cruz Biotechnology) or KV7.3 (Santa Cruz Biotechnology) were preincubated with the KV7.2 or KV7.3 primary antibodies, respectively.
Immunocytochemistry.
Immunocytochemical experiments were conducted as previously described (Chen et al., 2010; Hristov et al., 2012a). Freshly isolated DSM cells were incubated using either the anti-KCNQ2 (KV7.2) goat primary antibody (1:300 dilution; Santa Cruz Biotechnology) or the anti-KCNQ3 (KV7.3) rabbit primary antibody (1:300 dilution; Santa Cruz Biotechnology) diluted in 1% BSA/phosphate-buffered saline (PBS) overnight at 4°C. DSM cells that were incubated with the anti-KV7.2 goat polyclonal antibody were then incubated with the secondary antibody Cy3-AffiniPure donkey anti-goat IgG (1:500 dilution; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) overnight at 4°C. DSM cells that were incubated with the anti-KV7.3 rabbit polyclonal primary antibody were then incubated with the secondary antibody Cy5-AffiniPure donkey anti-rabbit IgG (1:500 dilution; Jackson ImmunoResearch Laboratories, Inc.) overnight at 4°C. Subsequently, DSM cells were incubated in PBS with the α-smooth muscle actin–fluorescein isothiocyanate antibody (Sigma-Aldrich) for 2 hours in a dark room. Nuclear staining was achieved by incubating DSM cells for 15 minutes in 4′,6-diamidino-2-phenylindole (diluted in PBS 1:10,000; Invitrogen, Grand Island, NY) at room temperature. DSM cells were mounted with 1,4-diazabicyclo[2.2.2]octane. Confocal images were obtained with a ×63 oil immersion objective using a laser scanning LSM 700 confocal microscope (Carl Zeiss, Jena, Germany).
Isometric DSM Tension Recordings.
Isometric DSM tension recordings were performed as previously described using DSM isolated strips without urothelium (Chen et al., 2010; Hristov et al., 2012a; Afeli et al., 2013; Smith et al., 2013). For spontaneous phasic, carbachol-induced, and KCl-induced contractions, the neuronal voltage-gated Na+ channel blocker tetrodotoxin (TTX; 1 µM) was included prior to the application of increasing concentrations of ICA-069673 (100 nM–30 µM). Two separate protocols were conducted involving electrical field stimulation (EFS)–induced DSM contractions, which were performed in the absence of TTX using a PHM-152I programmable stimulator (Med Associates, Inc., Georgia, VT). For the first protocol, continuous stimulation of 10-Hz EFS frequency was delivered at 1-minute intervals and a stable baseline and amplitude were reached prior to applying increasing concentrations of ICA-069673 (100 nM–30 µM). In the second EFS protocol, we applied incremental EFS stimulation frequencies (3.5, 5, 7.5, 10, 12.5, 15, 20, 30, 40, and 50 Hz) at 3-minute intervals for a control period, and then again in the presence of a single concentration of ICA-069673 (either 3 or 10 µM).
Ratiometric Fluorescence Ca2+ Imaging.
We measured the global intracellular Ca2+ levels of freshly isolated DSM cells using fura-2 AM, a ratiometric fluorescent Ca2+ probe, in accordance with previously described procedures (Hristov et al., 2012a). For the experiments with nifedipine, DSM cells were preincubated with 1 µM nifedipine prior to ICA-069673 administration.
Whole-Cell Patch-Clamp Electrophysiology.
Amphotericin-B perforated whole-cell patch-clamp experiments were performed as previously described, using freshly isolated guinea pig DSM cells (Hristov et al., 2012b). Briefly, to record the DSM cell membrane potential, experiments were conducted in current-clamp mode (I = 0) using an Axopatch 200B amplifier, Digidata 1440A, and pCLAMP software (version 10.2; Molecular Devices, Sunnyvale, CA). All patch-clamp electrophysiological experiments were conducted at room temperature (22–23°C).
Solutions and Drugs.
Ca2+-free dissection solution was composed of each of the following: 80 mM monosodium glutamate, 55 mM NaCl, 6 mM KCl, 10 mM glucose, 10 mM HEPES, and 2 mM MgCl2; NaOH was used to attain pH 7.3. Physiologic saline solution containing Ca2+ was freshly prepared each day and contained the following: 119 mM NaCl, 4.7 mM KCl, 24 mM NaHCO3, 1.2 mM KH2PO4, 2.5 mM CaCl2, 1.2 mM MgSO4, and 11 mM glucose, which was aerated with 95% O2/5% CO2 to attain pH 7.4. Physiologic bath solution used for patch-clamp experiments was composed of the following: 134 mM NaCl, 6 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 10 mM glucose, and 10 mM HEPES; NaOH was used to obtain pH 7.4. The patch-pipette solution contained the following: 110 mM potassium aspartate, 30 mM KCl, 10 mM NaCl, 1 mM MgCl2, 10 mM HEPES, and 0.05 mM EGTA; NaOH was used to adjust the pH to 7.2. Amphotericin-B stock solution was freshly prepared daily in dimethylsulfoxide (DMSO) and was included in the pipette solution (200–300 μg/ml) before each experiment and was subsequently replaced every 1 to 2 hours. ICA-069673, XE991, fura-2 AM, and TTX were purchased from Tocris Bioscience (Minneapolis, MN) and were dissolved in DMSO, double-distilled water, and acetate buffer at pH 4.8, respectively. Nifedipine was purchased from Sigma-Aldrich and dissolved in DMSO.
Data Analysis and Statistical Analysis.
Isometric DSM tension recording experiments were conducted using MyoMED software (Catamount Research and Development, St. Albans, VT) and analyzed as previously described (Afeli et al., 2013). Data from isometric DSM tension recordings were normalized and expressed in percentages. For spontaneous phasic, carbachol-induced, KCl-induced, and 10-Hz EFS-induced contractions, the 5-minute period preceding the first application of ICA-069673 (100 nM–30 µM) was taken as the control (100%). Maximal efficacy values were normalized to the control, with 0% of the control indicating full inhibition. The effects of ICA-069673 (100 nM–30 µM) on DSM phasic contraction parameters were determined by analysis of the 5-minute period preceding the subsequent addition of a higher concentration. For 3.5- to 50-Hz EFS-induced contractions, the control period was defined as the contraction amplitude observed at 50-Hz EFS frequency before the addition of ICA-069673 (3 or 10 µM) and taken as 100%. When ICA-069673 was applied in the presence of XE991, the control was defined as the 5-minute period before the addition of ICA-069673 (in the presence of XE991 only). Where indicated in the summarized data, n represents the number of freshly isolated DSM strips or cells, or individual immunocytochemistry/Western blot experiments, and N represents the number of guinea pigs. For spontaneous contraction experiments, statistical analysis for the comparison of ICA-069673 in the absence or presence of XE991, two-way analysis of variance followed by the Bonferroni post hoc test was performed. Remaining statistical analyses were performed using a two-tailed paired t test. Summarized data are reported as means ± S.E.M. P values < 0.05 were considered statistically significant. The CorelDRAW X3 Graphics Suite (Corel Co., Mountain View, CA) and GraphPad Prism 4.03 software (GraphPad Software, La Jolla, CA) were used for data illustration.
Results
KV7.2- and KV7.3-Containing Channels Are Expressed in Freshly Isolated DSM Cells.
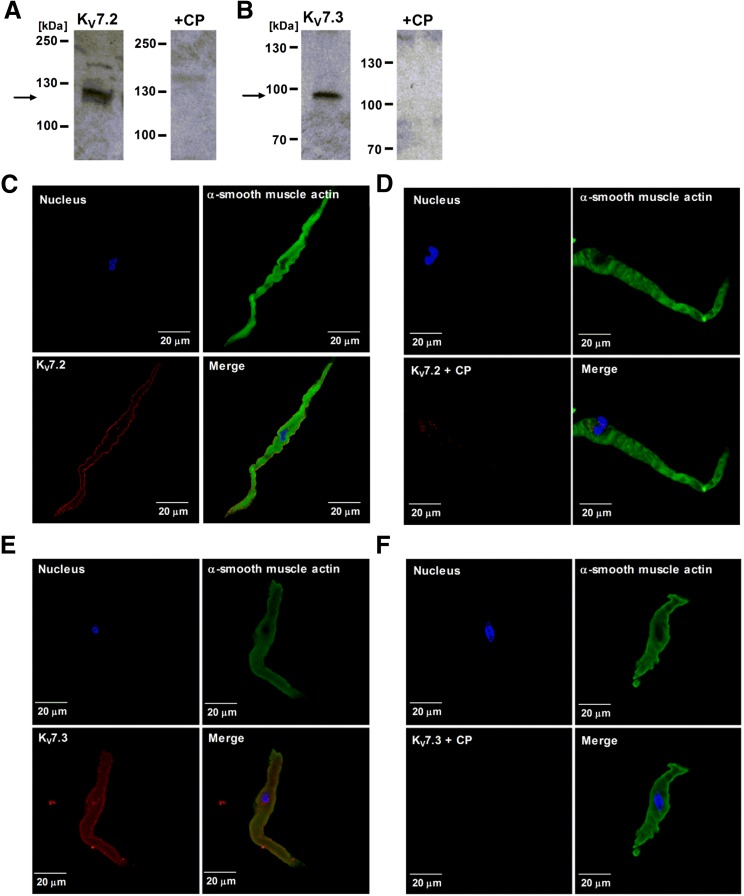
To determine the protein expression of KV7.2 and KV7.3 channel subunits and their subcellular localization in DSM cells, we performed Western blot and immunocytochemical experiments, respectively. Western blot data revealed the protein expression of KV7.2 and KV7.3 channel subunits in whole DSM tissue, which were not observed when the competing peptides for KV7.2 and KV7.3 channel–specific antibodies were present (n = 3, N = 3; Fig. 1, A and B). In freshly isolated DSM cells, immunocytochemical experiments were conducted using antibodies specific for KV7.2 or KV7.3 channel subunits in combination with the α-smooth muscle actin–fluorescein isothiocyanate antibody and 4′,6-diamidino-2-phenylindole nuclear stain (Fig. 1, C–F). The data demonstrated that KV7.2 and KV7.3 channel subunits are expressed in DSM cells and localized within the vicinity of the cell membrane (n = 3, N = 3; Fig. 1, C–F). The detection of KV7.2 and KV7.3 channel protein expression in isolated DSM cells was abolished when the competing peptides for the KV7.2 or KV7.3 channel–specific antibodies were present (Fig. 1, D and F). This confirms the specificity of the KV7.2 and KV7.3 channel–specific antibodies used in this study.
Fig. 1.
KV7.2 and KV7.3 channel subunits are expressed in freshly isolated guinea pig DSM cells. (A and B) Western blot analysis reveals the expression of KV7.2 (A) and KV7.3 (B) channel subunits at the protein level in DSM (n = 3, N = 3). The expected molecular masses of KV7.2 and KV7.3 channel proteins are 120 and 97 kDa, respectively (indicated by the arrows). No protein expression was detected in the presence of the competing peptides for each of the KV7.2 or KV7.3 channel antibodies, respectively. (C and E) Confocal images from immunocytochemical experiments demonstrate the protein expression of KV7.2 (C) and KV7.3 (E) channel subunits in freshly isolated DSM cells, along with membrane localization. Red staining indicates detection of either KV7.2 or KV7.3 channel subunits. Blue staining depicts the nucleus. Cells stained with the α-smooth muscle actin–FITC antibody are shown in green. The merged images for KV7.2 or KV7.3 with α-smooth muscle actin–FITC antibody and 4′,6-diamidino-2-phenylindole nuclear stain is shown at the bottom right. Colocalization of KV7.2 or KV7.3 with the α-smooth muscle actin–fluorescein isothiocyanate antibody is indicated by the yellow color in the merged images, respectively. (D and F) Inclusion of competing peptides for either KV7.2 (D) or KV7.3 (F) channel antibodies resulted in loss of specific staining. Data for all experiments were confirmed using DSM tissues from at least three guinea pigs. Images were acquired with a Zeiss LSM 700 confocal microscope using a 63× oil objective. CP, competing peptide.
The KV7.2/KV7.3 Channel Activator ICA-069673 Inhibits Spontaneous Phasic Contractions in DSM Isolated Strips.
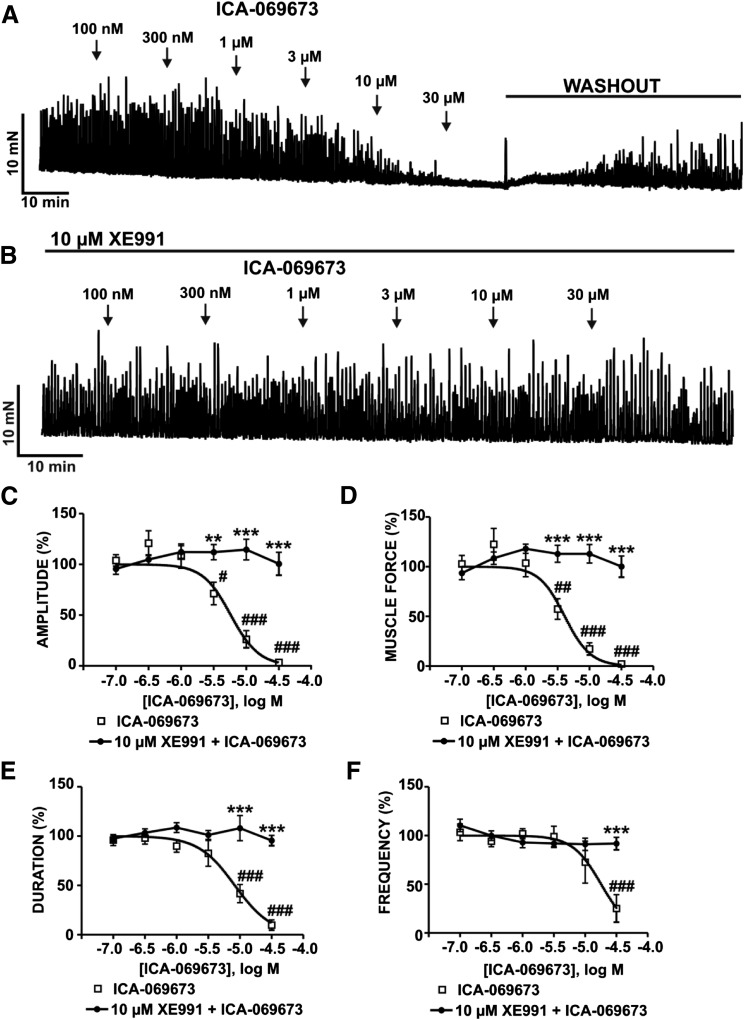
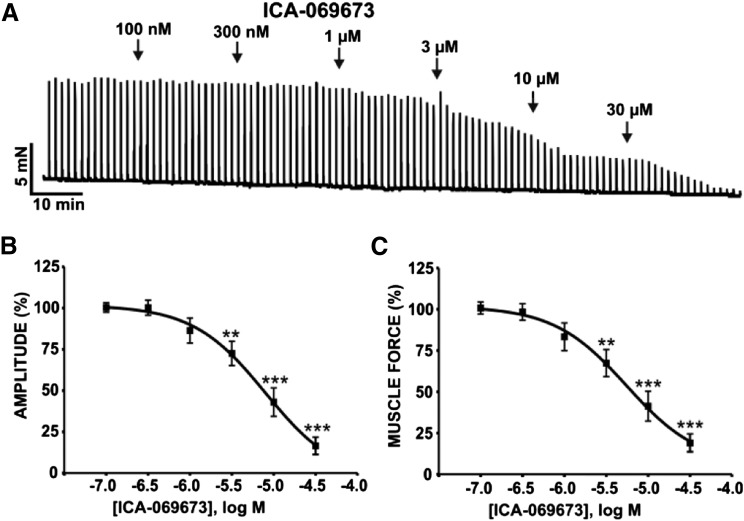
To determine the functional roles of the KV7.2/KV7.3 channels on spontaneous phasic contractions in guinea pig DSM isolated strips, we conducted isometric DSM tension recordings using the novel KV7.2/KV7.3 channel activator ICA-069673 in the presence of 1 µM TTX. As shown in Fig. 2A, ICA-069673 (100 nM–30 µM) caused a concentration-dependent inhibition of spontaneous phasic contractions, an effect that recovered upon washout of ICA-069673 (n = 11, N = 7; P < 0.05). The IC50 and maximal efficacy values for ICA-069673 on DSM spontaneous phasic contraction parameters are summarized in Table 1. To confirm the concentration-dependent inhibition of spontaneous phasic contractions by ICA-069673 was mediated by KV7 channels, we examined its effects in the presence of the KV7 channel inhibitor XE991 (10 µM). In the presence of XE991 (10 µM), there were no measureable inhibitory effects of ICA-069673 on DSM phasic contractions (n = 12, N = 6; P > 0.05; Fig. 2B). These results support the concept that KV7.2- and KV7.3-containing channels are important and physiologically relevant mediators of spontaneous phasic contractions in DSM isolated strips.
Fig. 2.
ICA-069673 inhibits spontaneous phasic contractions in DSM isolated strips. (A) An original isometric DSM tension recording illustrating the inhibition of spontaneous phasic contractions by ICA-069673 (100 nM–30 µM) in a DSM isolated strip. Spontaneous phasic contractions recovered upon washout of ICA-069673. (B) An original isometric DSM tension recording illustrating that blocking KV7 channels with its selective inhibitor XE991 (10 µM) abolished the inhibitory effects of ICA-069673 (100 nM–30 µM) on DSM spontaneous phasic contractions. (C–F) Cumulative concentration-response curves for ICA-069673 on DSM phasic contraction amplitude (C), muscle force (D), duration (E), and frequency (F) in the absence or presence of 10 µM XE991. #P < 0.05; ##P < 0.01; ###P < 0.001, statistically significant effects of ICA-069673 on spontaneous phasic contractions in the absence of XE991 compared with the control level (i.e., 100%) prior to the addition of ICA-069673 (n = 11, N = 7). **P < 0.01; ***P < 0.001, statistically significant differences between the effects of ICA-069673 in the absence versus presence of XE991 (n = 12, N = 6). See Table 1 for potency and maximum efficacy values.
TABLE 1.
IC50 and maximal efficacy values for the effects of ICA-069673 on spontaneous phasic, pharmacologically induced, and 10-Hz EFS-induced contractions in guinea pig DSM isolated strips
IC50 values are presented as the mean (95% confidence interval). Maximal efficacy is presented as the mean ± S.E.M and is normalized to the control on a scale from 100% (no relaxation) to 0% (full relaxation). n represents the number of freshly isolated DSM strips or cells, or individual immunocytochemistry/Western blot experiments, and N represents the number of guinea pigs.
| Condition |
Contraction Amplitude |
Muscle Force |
Contraction Duration |
Contraction Frequency |
n/N
|
P Value |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| IC50 |
Maximal Efficacy |
IC50 |
Maximal Efficacy |
IC50 |
Maximal Efficacy |
IC50 |
Maximal Efficacy |
|||
| µM | % | µM | % | µM | % | µM | % | |||
| Spontaneous phasic contractions | 4.5 (2.6–7.9) | 3.3 ± 1.8 | 3.2 (2.0–5.2) | 2.2 ± 1.4 | 8.6 (4.3–17.3) | 9.8 ± 5.2 | 11.9 (1.2–118.3) | 25.0 ± 14.1 | 11/7 | <0.05 |
| Carbachol-induced contractions | 5.5 (3.0–10.2) | 20.8 ± 8.9 | 3.6 (1.9–7.0) | 18.9 ± 8.2 | 13.3 (6.3–28.2) | 36.4 ± 14.9 | n/a | n/a | 10/7 | <0.05 |
| KCl-induced contractions | 5.3 (2.6–11.0) | 10.4 ± 5.6 | 4.2 (2.4–7.6) | 5.3 ± 3.6 | 11.1 (2.8–36.7) | 31.8 ± 13.0 | 12.2 (3.4–44.2) | 22.5 ± 8.9 | 7/7 | <0.05 |
| 10-Hz EFS-induced contractions | 9.8 (1.1–88.0) | 16.6 ± 5.2 | 7.6 (0.6–97.8) | 19.0 ± 5.4 | n/a | n/a | n/a | n/a | 9/9 | <0.05 |
n/a, not applicable.
The KV7.2/KV7.3 Channel Activator ICA-069673 Inhibits Pharmacologically-Induced Contractions in DSM Isolated Strips.
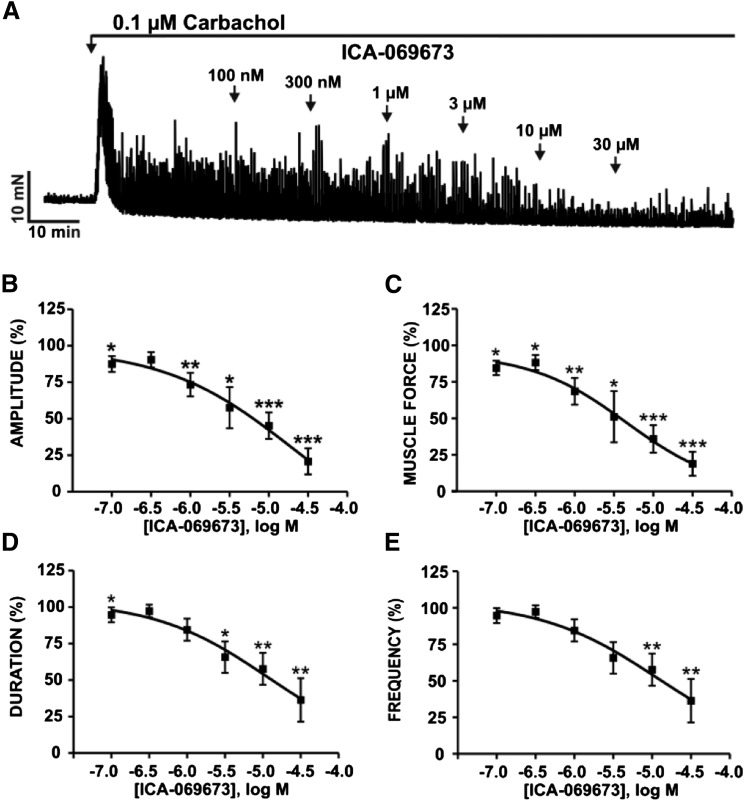
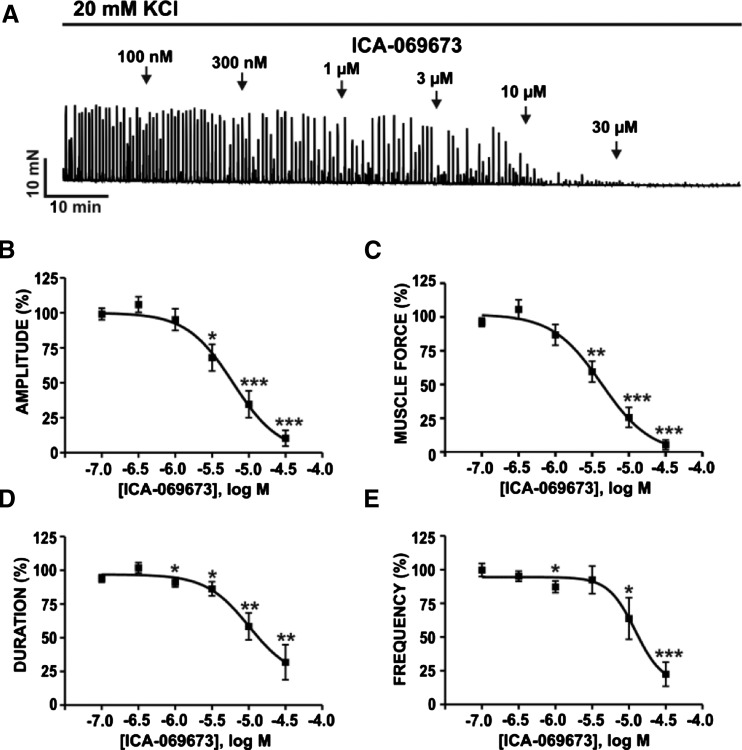
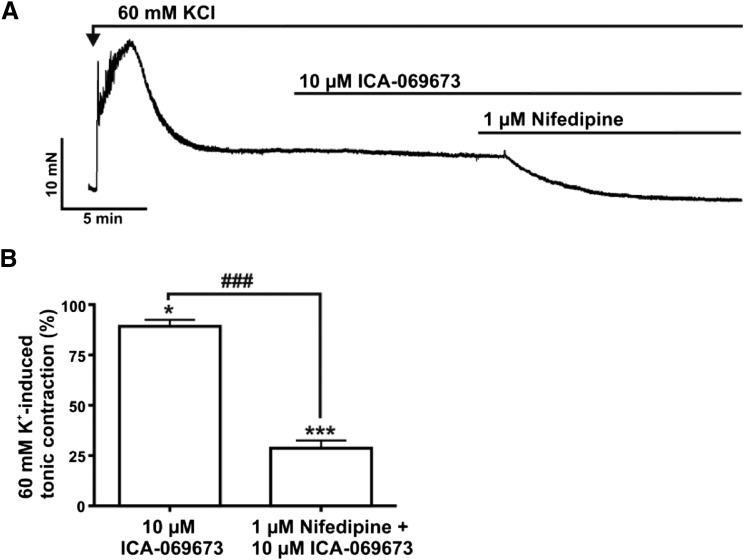
We examined the effects of ICA-069673 on DSM phasic contractions induced by either muscarinic receptor activation or sustained membrane potential depolarization using carbachol or KCl, respectively, in the presence of 1 µM TTX. ICA-069673 (100 nM–30 µM) caused a concentration-dependent inhibition of 0.1 µM carbachol-induced DSM contractions (n = 10, N = 7; P < 0.05; Fig. 3). For 0.1 µM carbachol-induced contractions, the IC50 and maximal efficacy values for ICA-069673 on DSM contraction parameters are summarized in Table 1. DSM phasic contraction frequency was maximally reduced to a lesser extent to 46.5% ± 18.2% of the control (n = 10, N = 7; P < 0.05; Fig. 3E). ICA-069673 (100 nM–30 µM) also concentration-dependently inhibited DSM phasic contractions induced by 20 mM KCl (n = 7, N = 7; P < 0.05; Fig. 4; see Table 1 for IC50 and maximal efficacy values). We then tested the effects of ICA-069673 (10 μM) on DSM tonic contractions induced by 60 mM KCl to determine the role of K+ conductance in mediating the inhibitory effects of ICA-069673 as well to assess the functional interaction with the L-type CaV channels. As illustrated in Fig. 5, under these experimental conditions, ICA-069673 (10 µM) decreased DSM tone to only 89.2% ± 3.2% of the control (n = 8, N = 4; P < 0.05), whereas the subsequent addition of the L-type CaV channel inhibitor nifedipine (1 µM) led to a more significant reduction in DSM tone to 28.7% ± 3.8% of the control (n = 8 to 9, N = 4; P < 0.001; Fig. 5). There was a statistically significant difference between the inhibitory effects of ICA-069673 and nifedipine on DSM tonic contraction (n = 8 to 9, N = 4; P < 0.001). These experiments support the concept that ICA-069673 does not directly inhibit L-type CaV channels and that its effects are consistent with the involvement of K+ conductance.
Fig. 3.
ICA-069673 decreases carbachol-induced phasic contractions in DSM isolated strips. (A) An original isometric DSM tension recording of 0.1 µM carbachol-induced phasic contractions in a DSM isolated strip illustrating the concentration-dependent inhibitory effects of ICA-069673 (100 nM–30 µM). (B–E) Cumulative concentration-response curves for ICA-069673 (100 nM–30 µM) on DSM phasic contraction amplitude (B), muscle force (C), duration (D), and frequency (E) (n = 10, N = 7; *P < 0.05; **P < 0.01; ***P < 0.001). See Table 1 for potency and maximum efficacy values.
Fig. 4.
ICA-069673 decreases 20 mM KCl-induced phasic contractions in DSM isolated strips. (A) An original isometric DSM tension recording illustrating the concentration-dependent inhibitory effects of ICA-069673 (100 nM–30 µM) on DSM phasic contractions induced by 20 mM KCl. (B–E) Cumulative concentration-response curves for the effects of ICA-069673 on DSM phasic contraction amplitude (B), muscle force (C), duration (D), and frequency (E) (n = 7, N = 7; *P < 0.05; **P < 0.01; ***P < 0.001). See Table 1 for potency and maximum efficacy values.
Fig. 5.
Differential effects of ICA-069673 and nifedipine on 60 mM KCl depolarization-induced tonic contraction in DSM isolated strips. (A) An original isometric DSM tension recording illustrating the effects of 10 µM ICA-069673 and 1 µM nifedipine on a depolarization-induced DSM tonic contraction caused by 60 mM KCl in a DSM isolated strip. (B) Summarized data demonstrating the inhibitory effect of ICA-069673 (10 µM) and nifedipine (1 µM) on DSM tonic contraction induced by 60 mM KCl (n = 8 to 9, N = 4; *P < 0.05; n = 6 to 7, N = 3; ***P < 0.0001, respectively). ###P < 0.0001 represents a statistically significant difference for the comparison of ICA-069673 and nifedipine.
The KV7.2/KV7.3 Channel Activator ICA-069673 Inhibits Nerve-Evoked Contractions in DSM Isolated Strips.
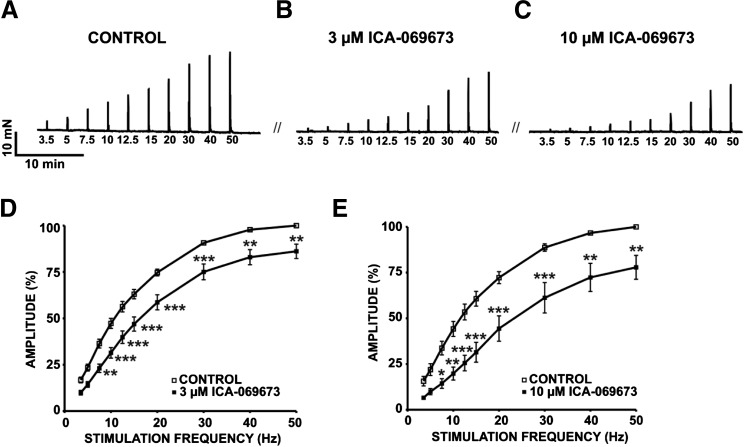
To assess the effects of ICA-069673 on nerve-evoked DSM contractions, we applied increasing concentrations of ICA-069673 to continuous 10-Hz EFS-induced contractions of DSM isolated strips. Under these experimental conditions, ICA-069673 (100 nM–30 µM) displayed a concentration-dependent inhibitory effect on contraction amplitude and muscle force (n = 9, N = 9; P < 0.05; Fig. 6). For 10-Hz EFS-induced contractions, the IC50 and maximal efficacy values for ICA-069673 on nerve-evoked DSM contraction parameters are shown in Table 1. Next, EFS-induced contractions were stimulated over a range of frequencies (3.5–50 Hz) in the absence or presence of either 3 μM or 10 μM ICA-069673, respectively. ICA-069673 (3 μM or 10 μM) significantly decreased the amplitude of EFS-induced contractions stimulated at EFS frequencies from 7.5 to 50 Hz (n = 8–18, N = 4–10; P < 0.05; Fig. 7). Collectively, these data support the concept that KV7.2- and KV7.3-containing channels are critical regulators of nerve-evoked DSM contractions.
Fig. 6.
ICA-069673 decreases nerve-evoked contractions stimulated by 10-Hz EFS in DSM isolated strips. (A) An original isometric DSM tension recording representing the concentration-dependent inhibitory effects of ICA-069673 (100 nM–30 µM) upon nerve-evoked contractions induced by 10-Hz EFS stimulation in a DSM isolated strip. (B) Cumulative concentration-response curves for the effects of ICA-069673 (100 nM–30 µM) on DSM contraction amplitude (B) and muscle force integral (C) (n = 9, N = 9; **P < 0.01; ***P < 0.001). See Table 1 for potency and maximum efficacy values.
Fig. 7.
ICA-069673 decreases 7.5- to 50-Hz EFS-induced contractions in DSM isolated strips. (A–C) The original isometric DSM tension recordings of 3.5- to 50-Hz EFS-induced contractions in DSM isolated strips before (control) (A) and after the addition of 3 µM ICA-069673 (B) or 10 µM ICA-069673 (C). (D and E) Cumulative concentration-response curves demonstrating the effects of 3 µM ICA-069673 (D) and 10 µM ICA-069673 (E) on the amplitude of 3.5- to 50-Hz EFS-induced contractions (n = 8–18, N = 4–10; *P < 0.05; **P < 0.01; ***P < 0.001).
The KV7.2/KV7.3 Channel Activator ICA-069673 Decreases Global Intracellular Ca2+ Levels in Freshly Isolated DSM Cells.
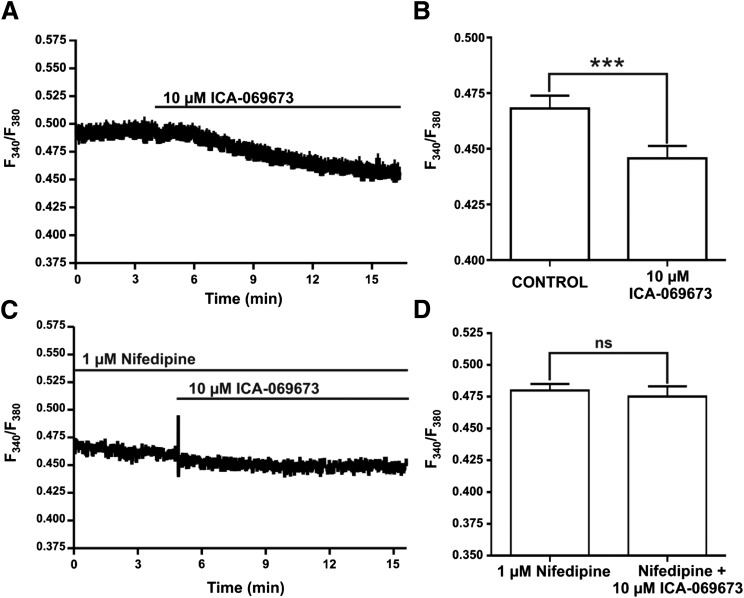
Next, we sought to determine the effects of ICA-069673 on global intracellular Ca2+ levels in DSM single cells by measuring the ratio of fura-2 AM fluorescent emission at 510 nm with excitation at 340 and 380 nm. For the control, the average emitted intensity (F340/F380) prior to ICA-069673 application was 0.470 ± 0.006 (n = 11, N = 5). At 10 μM ICA-069673, there was a significant decrease in the F340/F380 ratio to 0.450 ± 0.006 (n = 11, N = 5; P < 0.001; Fig. 8, A and B). To elucidate the mechanism by which ICA-069673 regulates global intracellular Ca2+ concentrations, we studied the effects of ICA-069673 (10 µM) in the presence or absence of the L-type CaV channel blocker nifedipine (1 µM). In the presence of 1 µM nifedipine, ICA-069673 (10 µM) had no significant effect on global intracellular Ca2+ levels, with the average F340/F380 ratio being 0.470 ± 0.008 compared with the control value of 0.480 ± 0.005 (n = 11, N = 5; P > 0.05; Fig. 8, C and D). These results demonstrate that in guinea pig DSM cells, ICA-069673 decreases global intracellular Ca2+ concentrations by an indirect mechanism involving inhibition of Ca2+ influx through L-type CaV channels, probably as a result of membrane hyperpolarization.
Fig. 8.
ICA-069673 decreases global intracellular Ca2+ levels in freshly isolated DSM cells. (A) Original recordings illustrating that ICA-069673 (10 µM) significantly decreased the global intracellular Ca2+ levels in a freshly isolated DSM cell. (B) Summarized data representing the reduction of global intracellular Ca2+ levels by 10 µM ICA-069673 (n = 11, N = 5; ***P < 0.001). (C) An original recording demonstrating that ICA-069673 has no effect on global Ca2+ levels in the presence of the L-type CaV channel inhibitor nifedipine (1 µM) in a DSM cell. (D) Summarized data representing the effects of ICA-069673 (10 µM) on global intracellular Ca2+ in the presence of nifedipine (1 µM) (n = 10, N = 4; P > 0.05) in DSM cells. Data are reported as the ratio of fura-2 AM fluorescent emission at 510 nm with excitation at 340 and 380 nm. ns, nonsignificant.
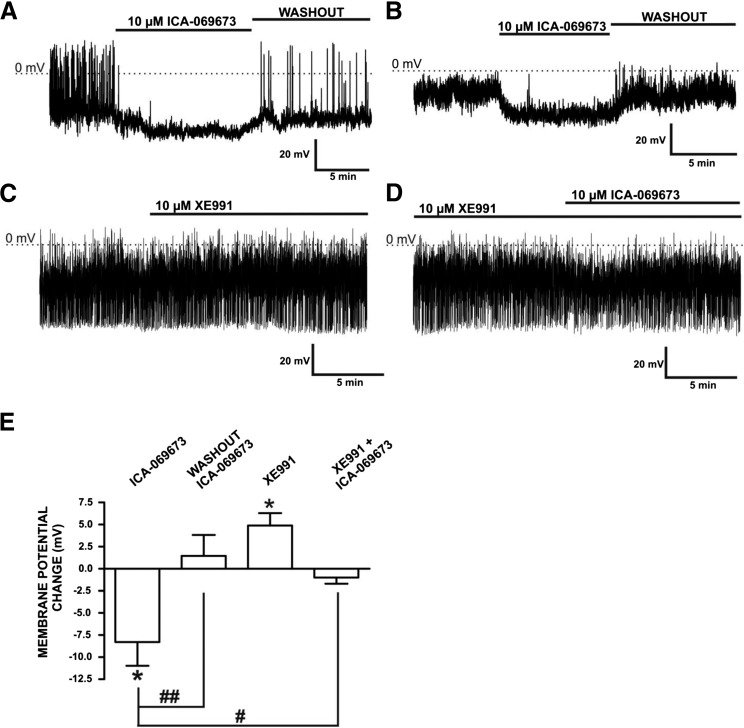
ICA-069673 Hyperpolarizes the Resting Membrane Potential and Inhibits Spontaneous Action Potentials in Freshly Isolated DSM Cells.
To determine the role of the KV7.2/KV7.3 channels in regulating the DSM cell membrane potential, we employed the KV7.2/KV7.3 channel activator ICA-069673 and the perforated whole-cell patch-clamp technique in current-clamp mode. The effects of ICA-069673 were examined in seven DSM cells (N = 6), which had mean cell capacitance of 43.4 ± 4.3 pF and membrane potential of −16.8 ± 2.8 mV. Two of these seven DSM cells displayed distinguishable spontaneous action potentials, which were abolished by ICA-069673 concurrent with membrane hyperpolarization (Fig. 9A). In all DSM cells, ICA-069673 hyperpolarized the DSM cell membrane potential by 8.3 ± 2.7 mV (n = 7, N = 6; P < 0.05; Fig. 9, A, B, and E). ICA-069673–mediated hyperpolarization was fully reversible upon washout (Fig. 9, A and B). We then examined the effects of the KV7 channel inhibitor XE991. As illustrated by the original recording in Fig. 9C and summarized data in Fig. 9E, XE991 (10 µM) depolarized the DSM cell membrane potential by 4.9 ± 1.4 mV (n = 5, N = 5; P < 0.05). To further investigate the mechanism underlying ICA-069673–induced hyperpolarization, specifically its dependence on KV7 channel subtypes, the effects of this channel modulator was examined in DSM cells pretreated with XE991 (10 µM). The subsequent addition of ICA-069673 in the continued presence of XE991 did not have a significant hyperpolarizing effect on the membrane potential of DSM cells (n = 7, N = 5; P > 0.05; Fig. 9, D and E). There was a statistically significant difference in the effects of ICA-069673 in the absence versus presence of XE991 (n = 5–7, N = 5 to 6; P < 0.05; Fig. 9E). Collectively, these data support the novel concept that KV7.2- and KV7.3-containing channels are key regulators of DSM cell excitability.
Fig. 9.
ICA-069673 hyperpolarizes the resting membrane potential and inhibits spontaneous action potentials in freshly isolated DSM cells. (A) An original membrane potential recording in current-clamp mode of a DSM cell exhibiting spontaneous action potentials. ICA-069673 (10 µM) hyperpolarized the DSM cell membrane potential and inhibited spontaneous action potentials in a manner reversible upon washout. (B) An original membrane potential recording of a DSM cell that does not exhibit spontaneous action potentials. ICA-069673 (10 µM) hyperpolarized the membrane potential, an effect that was recovered upon washout. (C) An original membrane potential recording illustrating that the KV7 channel inhibitor XE991 (10 µM) depolarized the DSM cell membrane potential. (D) A continuation of the recording in (C) illustrating that in the presence of XE991 (10 µM), the hyperpolarizing effects of ICA-069673 (10 µM) on the DSM cell membrane potential are attenuated. (E) Summarized data for the effects of ICA-069673 (10 µM), washout of ICA-069673, XE991 (10 µM), and ICA-069673 (10 µM) in the presence of XE991 (10 µM) on the DSM cell membrane potential. The y-axis represents the change in the membrane potential, either depolarization (+) or hyperpolarization (−). With the exception of when ICA-069673 was applied in the presence of XE991, the control period was in the absence of any pharmacological treatment. *P < 0.05, versus the control for the treatments shown. #P < 0.05; ##P < 0.01, statistically significant differences for the comparisons shown. Each data point in (E) is n = 5–7, N = 5 to 6.
Discussion
This study sought to elucidate the physiologic roles of KV7.2- and KV7.3-containing channels in DSM excitability and contractility by targeting their activity with the novel and selective KV7.2/KV7.3 channel activator ICA-069673. This was achieved by applying a multilevel experimental approach at both cellular and tissue levels in DSM. Western blot analysis detected protein expression for KV7.2 and KV7.3 channel subunits in DSM tissue. In isolated DSM cells, immunocytochemical experiments further revealed protein expression for KV7.2 and KV7.3 channel subunits localized within the vicinity of the cell membrane. Our pharmacological studies demonstrated that ICA-069673 significantly hyperpolarized the membrane potential, inhibited spontaneous action potentials, and decreased global intracellular Ca2+ concentrations of DSM cells. Our functional studies on DSM contractility revealed that ICA-069673 inhibited spontaneous phasic, pharmacologically induced, and nerve-evoked contractions in DSM isolated strips. The inhibitory effects of ICA-069673 on DSM excitability and contractility were blocked by the KV7.1–KV7.5 channel inhibitor XE991, supporting the involvement of KV7 channel subtypes in the regulation of DSM excitability and contractility. Our combined results suggest a critical role for KV7.2- and KV7.3-containing channels in DSM excitation-contraction coupling.
From all KV7 channel subunits, mRNA transcripts for KV7.1 and KV7.2 channels are most predominant in guinea pig DSM cells (Afeli et al., 2013). The expression of KV7 channels at the protein level has only been identified in DSM tissue via immunohistochemistry (Afeli et al., 2013; Anderson et al., 2013), which has the inherent limitation of potentially detecting KV7 channel expression from neighboring non-DSM cell types such as neurons or interstitial cells in the absence of cell-specific labeling. Thus, as illustrated in Fig. 1, C–F, novel immunocytochemical experiments with confocal microscopy reveal the expression of KV7.2- and KV7.3-containing channels in isolated DSM cells, where these channels localize within the vicinity of the cell membrane. Furthermore, Western blot experiments detected protein expression for both KV7.2 and KV7.3 channel subunits in DSM (Fig. 1, A and B), consistent with the previous reports by our group and others using immunohistochemistry (Afeli et al., 2013; Anderson et al., 2013).
KV7 channel activators and inhibitors such as retigabine and XE991 have provided a useful pharmacological approach for elucidating KV7 channel roles in urinary bladder function (Argentieri et al., 2002; Sheldon et al., 2002; Streng et al., 2004; Rode et al., 2010; Svalø et al., 2012, 2013, 2015; Petkov, 2012; Afeli et al., 2013; Anderson et al., 2013). However, retigabine and XE991 do not effectively distinguish among KV7.2–KV7.5 and KV7.1–KV7.5 channel subtypes, respectively. The lack of subtype selectivity of these pharmacological modulators has thus been a substantial limitation for gaining an improved understanding of the KV7 channel subtypes most critical to DSM function. A novel KV7 channel activator, ICA-069673, has demonstrated selectivity for heteromeric KV7.2/KV7.3 channels and is a pyrimidine analog of ICA-27243 [N-(6-chloro-pyridin-3-yl)-3,4-difluoro-benzamide] (Roeloffs et al., 2008; Wickenden et al., 2008; Amato et al., 2011). Although it is less potent than its related analog ICA-27243, ICA-069673 displays greater efficacy and improved selectivity for heteromeric KV7.2/KV7.3 over KV7.1 channels (Amato et al., 2011). In contrast with retigabine, which binds to the pore region (S5 to S6) of KV7.2–KV7.5 channels (Gunthorpe et al., 2012), evidence suggests that ICA-069673 is among an emerging novel class of compounds known as “gating modifiers,” named so for their ability to bind to the S1–S4 voltage-sensing domain of KV7 channels (Wickenden et al., 2008; Peretz et al., 2010; Brueggemann et al., 2014a).
The functional studies on DSM contractility revealed that ICA-069673 effectively inhibited spontaneous phasic contractions in DSM isolated strips in a concentration-dependent manner (Fig. 2). Occurring locally in DSM, spontaneous phasic contractions do not initiate urine voiding; however, these contractile events have been associated with involuntary nonvoiding contractions, which are considerably increased in some forms of OAB (Andersson and Wein, 2004; Petkov, 2014). The inhibition of DSM contractility was not observed when ICA-069673 was administered in the presence of the KV7 channel inhibitor XE991, which indicates that the inhibitory effects of ICA-069673 on spontaneous phasic contractions were mediated by KV7 channels (Fig. 2B). Similarly, DSM phasic contractions induced by the muscarinic receptor agonist carbachol (0.1 µM) or through slight membrane depolarization by KCl (20 mM) were significantly inhibited by ICA-069673. Under conditions of high extracellular K+ (60 mM), which significantly changes the driving force for K+ and causes sustained membrane depolarization that in turn activates the L-type CaV channels, ICA-069673 (10 µM) had a rather modest inhibitory effect on DSM tone (approximately 10% of the maximum relaxation; Fig. 5). By contrast, the subsequent inhibition of L-type CaV channels with nifedipine decreased DSM tonic contractions by approximately 7-fold compared with ICA-069673 alone (Fig. 5). These results support the concept that ICA-069673 does not directly inhibit L-type CaV channels in DSM. These data are consistent with a previous report, in which ICA-069673 demonstrated no direct effects on L-type CaV channels as well as voltage-gated Na+ (NaV1.5), hERG, KV7.1, or GABAA channels (Amato et al., 2011). The reduced relaxation of ICA-069673 under high 60 mM K+ compared with the physiologic levels of K+ and the effects of nifedipine also supports that K+ channel conductance underlies the relaxant effects of this novel KV7.2/KV7.3 channel activator.
We next examined how ICA-069673 affects nerve-evoked DSM contractions induced by EFS. The results showed that ICA-069673 significantly decreased 10-Hz EFS-induced contractions (Fig. 6) and contractions induced over a large range of EFS frequencies (7.5–50 Hz) (Fig. 7), indicating that KV7.2- and KV7.3-containing channels sensitive to ICA-069673 are essential regulators of urinary bladder nerve-evoked contractions. Qualitatively, the effects of ICA-069673 on DSM contractility were consistent with those reported for retigabine (Afeli et al., 2013). This similarity suggests the involvement of the same molecular targets in mediating the effects of retigabine and ICA-069673, indicating a prominent role for the KV7.2- and KV7.3-containing channels in DSM function.
The contraction of DSM is ultimately triggered in response to a rise in the intracellular Ca2+ concentration (Andersson and Arner, 2004). Therefore, we sought to determine the effects of ICA-069673 on global intracellular Ca2+ levels and to elucidate the role of the KV7.2/KV7.3 channels in controlling DSM cell Ca2+ signaling by conducting ratiometric fluorescence Ca2+ imaging on isolated DSM cells. ICA-069673 (10 µM) significantly decreased global intracellular Ca2+ levels in DSM cells (Fig. 8, A and B), consistent with the inhibitory effects of ICA-069673 on DSM contractility. The reduction of intracellular Ca2+ concentrations by ICA-069673 is in agreement with the opposite phenomenon, in which the KV7 channel inhibitor XE991 increased the frequency of Ca2+ oscillations in DSM tissue (Anderson et al., 2013). To further assess the interactions between the KV7.2/KV7.3 channels and the L-type CaV channels, we investigated the effects of ICA-069673 in the presence of the L-type CaV channel blocker nifedipine (1 µM). The results showed that when L-type CaV channels were inhibited by nifedipine, ICA-069673 had no effect on global intracellular Ca2+ levels (Fig. 8, C and D). These observations support the concept that in isolated DSM cells, ICA-069673 reduces global intracellular Ca2+ levels by inhibiting L-type CaV channels indirectly through activation of KV7.2- and KV7.3-containing channels and associated membrane hyperpolarization. This is consistent with the well established fact that Ca2+ entry via L-type CaV channels is the predominant mechanism initiating DSM contractions, because blocking L-type CaV channels with nifedipine completely eliminates spontaneous phasic contractions (Chen et al., 2010; Petkov, 2012). Because ICA-069673 had no effects on global Ca2+ levels in the presence of nifedipine, this suggests the L-type CaV channels, as opposed to other Ca2+ entry pathways, mediate the effects of ICA-069673.
To our knowledge, our group was the first to record spontaneous action potentials in freshly isolated DSM cells and to report the effects of retigabine on spontaneous action potentials using the perforated patch-clamp technique in current-clamp mode (Afeli et al., 2013). This study revealed that ICA-069673 (10 µM) hyperpolarized the membrane potential and inhibited spontaneous action potentials in isolated DSM cells (Fig. 9). The hyperpolarizing effects of ICA-069673 on the DSM cell membrane potential were comparable to that of retigabine (Afeli et al., 2013). ICA-069673–mediated hyperpolarization was blocked when the KV7 channels were inhibited by XE991 (Fig. 9, D and E). XE991 alone induced depolarization, as measured with the perforated patch-clamp technique (Fig. 9, C and E). The combined results from our patch-clamp studies indicate a key role for KV7.2- and KV7.3-containing channels in regulating spontaneous action potentials and the resting membrane potential of isolated DSM cells.
In conclusion, using the novel KV7.2/KV7.3 channel activator ICA-069673, this study provides strong evidence to suggest a critical role for the KV7.2- and KV7.3-containing channels in DSM function at both cellular and tissue levels. Pharmacological activation of KV7.2/KV7.3 channels with ICA-069673 hyperpolarized the membrane potential and inhibited spontaneous action potentials in freshly isolated DSM cells, which blocked Ca2+ influx through L-type CaV channels to reduce global intracellular Ca2+ concentrations, causing relaxation of DSM isolated strips. Although our study demonstrates important roles for KV7.2- and KV7.3-containing channels in DSM cells and tissue, evidence suggests a prominent role for KV7.4 and KV7.5 channels in non-DSM human smooth muscle tissues (Greenwood and Ohya, 2009; Ng et al., 2011; Stott et al., 2014). Therefore, it is important for future studies to investigate the expression and function of KV7 channels in human DSM at both cellular and tissue levels, which is critical for further evaluating their therapeutic potential as novel targets for the treatment of OAB.
Acknowledgments
The authors thank Drs. Wenkuan Xin, Kiril L. Hristov, Shankar P. Parajuli, and Vitor S. Fernandes for critical evaluation of the manuscript, as well as Dr. Gary P. Schools for assistance with confocal microscopy.
Abbreviations
- BSA
bovine serum albumin
- CaV
L-type voltage-gated Ca2+ channel
- DMSO
dimethylsulfoxide
- DSM
detrusor smooth muscle
- EFS
electrical field stimulation
- ICA-069673
N-(2-chloro-5-pyrimidinyl)-3,4-difluorobenzamide
- ICA-27243
N-(6-chloro-pyridin-3-yl)-3,4-difluorobenzamide
- KV
voltage-gated K+ channel
- L-364,373
5-(2-fluorophenyl)-1,3-dihydro-3-(1H-indol-3-ylmethyl)-1-methyl-2H-1,4-benzodiazepin-2-one
- ML-213
N-(2,4,6-trimethylphenyl)-bicyclo[2.2.1]heptane-2-carboxamide
- OAB
overactive bladder
- PBS
phosphate-buffered saline
- TTX
tetrodotoxin
- XE991
10,10-bis(4-pyridinylmethyl)-9(10H)-anthracenone dihydrochloride
Authorship Contributions
Participated in research design: Provence, Malysz, Petkov.
Conducted experiments: Provence, Malysz, Petkov.
Performed data analysis: Provence, Malysz, Petkov.
Wrote or contributed to the writing of the manuscript: Provence, Malysz, Petkov.
Footnotes
This work was supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grants R01-DK084284 (to G.V.P.) and F31-DK104528 (to A.P.)]; and the National Institutes of Health National Institute of General Medical Sciences [Grant P20-GM109091 (to G.V.P.)]. A.P. was supported by a Support to Promote Advancement of Research and Creativity (SPARC) Graduate Research Grant from the Office of the Vice President for Research at the University of South Carolina.
References
- Abbott GW, Goldstein SA. (2001) Potassium channel subunits encoded by the KCNE gene family: physiology and pathophysiology of the MinK-related peptides (MiRPs). Mol Interv 1:95–107. [PubMed] [Google Scholar]
- Afeli SA, Malysz J, Petkov GV. (2013) Molecular expression and pharmacological evidence for a functional role of kv7 channel subtypes in Guinea pig urinary bladder smooth muscle. PLoS One 8:e75875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amato G, Roeloffs R, Rigdon GC, Antonio B, Mersch T, McNaughton-Smith G, Wickenden AD, Fritch P, Suto MJ. (2011) N-pyridyl and pyrimidine benzamides as KCNQ2/Q3 potassium channel openers for the treatment of epilepsy. ACS Med Chem Lett 2:481–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson UA, Carson C, Johnston L, Joshi S, Gurney AM, McCloskey KD. (2013) Functional expression of KCNQ (Kv7) channels in guinea pig bladder smooth muscle and their contribution to spontaneous activity. Br J Pharmacol 169:1290–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson KE, Arner A. (2004) Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol Rev 84:935–986. [DOI] [PubMed] [Google Scholar]
- Andersson KE, Wein AJ. (2004) Pharmacology of the lower urinary tract: basis for current and future treatments of urinary incontinence. Pharmacol Rev 56:581–631. [DOI] [PubMed] [Google Scholar]
- Argentieri T, Sheldon J, and Bowlby M (2002), inventors, American Home Products Corporation, assignee. Methods for modulating bladder function. U.S. patent 6348486. 2002 Feb 19.
- Brickel N, Gandhi P, VanLandingham K, Hammond J, DeRossett S. (2012) The urinary safety profile and secondary renal effects of retigabine (ezogabine): a first-in-class antiepileptic drug that targets KCNQ (K(v)7) potassium channels. Epilepsia 53:606–612. [DOI] [PubMed] [Google Scholar]
- Brueggemann LI, Haick JM, Cribbs LL, Byron KL. (2014a) Differential activation of vascular smooth muscle Kv7.4, Kv7.5, and Kv7.4/7.5 channels by ML213 and ICA-069673. Mol Pharmacol 86:330–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brueggemann LI, Haick JM, Neuburg S, Tate S, Randhawa D, Cribbs LL, Byron KL. (2014b) KCNQ (Kv7) potassium channel activators as bronchodilators: combination with a β2-adrenergic agonist enhances relaxation of rat airways. Am J Physiol Lung Cell Mol Physiol 306:L476–L486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brueggemann LI, Kakad PP, Love RB, Solway J, Dowell ML, Cribbs LL, Byron KL. (2012) Kv7 potassium channels in airway smooth muscle cells: signal transduction intermediates and pharmacological targets for bronchodilator therapy. Am J Physiol Lung Cell Mol Physiol 302:L120–L132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadha PS, Jepps TA, Carr G, Stott JB, Zhu HL, Cole WC, Greenwood IA. (2014) Contribution of kv7.4/kv7.5 heteromers to intrinsic and calcitonin gene-related peptide-induced cerebral reactivity. Arterioscler Thromb Vasc Biol 34:887–893. [DOI] [PubMed] [Google Scholar]
- Chen M, Kellett WF, Petkov GV. (2010) Voltage-gated K(+) channels sensitive to stromatoxin-1 regulate myogenic and neurogenic contractions of rat urinary bladder smooth muscle. Am J Physiol Regul Integr Comp Physiol 299:R177–R184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood IA, Ohya S. (2009) New tricks for old dogs: KCNQ expression and role in smooth muscle. Br J Pharmacol 156:1196–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunthorpe MJ, Large CH, Sankar R. (2012) The mechanism of action of retigabine (ezogabine), a first-in-class K+ channel opener for the treatment of epilepsy. Epilepsia 53:412–424. [DOI] [PubMed] [Google Scholar]
- Hristov KL, Chen M, Afeli SA, Cheng Q, Rovner ES, Petkov GV. (2012a) Expression and function of K(V)2-containing channels in human urinary bladder smooth muscle. Am J Physiol Cell Physiol 302:C1599–C1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hristov KL, Chen M, Soder RP, Parajuli SP, Cheng Q, Kellett WF, Petkov GV. (2012b) KV2.1 and electrically silent KV channel subunits control excitability and contractility of guinea pig detrusor smooth muscle. Am J Physiol Cell Physiol 302:C360–C372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepps TA, Bentzen BH, Stott JB, Povstyan OV, Sivaloganathan K, Dalby-Brown W, Greenwood IA. (2014) Vasorelaxant effects of novel Kv 7.4 channel enhancers ML213 and NS15370. Br J Pharmacol 171:4413–4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepps TA, Chadha PS, Davis AJ, Harhun MI, Cockerill GW, Olesen SP, Hansen RS, Greenwood IA. (2011) Downregulation of Kv7.4 channel activity in primary and secondary hypertension. Circulation 124:602–611. [DOI] [PubMed] [Google Scholar]
- Jepps TA, Greenwood IA, Moffatt JD, Sanders KM, Ohya S. (2009) Molecular and functional characterization of Kv7 K+ channel in murine gastrointestinal smooth muscles. Am J Physiol Gastrointest Liver Physiol 297:G107–G115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani BK, O’Dowd J, Kumar L, Brueggemann LI, Ross M, Byron KL. (2013) Vascular KCNQ (Kv7) potassium channels as common signaling intermediates and therapeutic targets in cerebral vasospasm. J Cardiovasc Pharmacol 61:51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng FL, Davis AJ, Jepps TA, Harhun MI, Yeung SY, Wan A, Reddy M, Melville D, Nardi A, Khong TK, et al. (2011) Expression and function of the K+ channel KCNQ genes in human arteries. Br J Pharmacol 162:42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretz A, Pell L, Gofman Y, Haitin Y, Shamgar L, Patrich E, Kornilov P, Gourgy-Hacohen O, Ben-Tal N, Attali B. (2010) Targeting the voltage sensor of Kv7.2 voltage-gated K+ channels with a new gating-modifier. Proc Natl Acad Sci USA 107:15637–15642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkov GV. (2012) Role of potassium ion channels in detrusor smooth muscle function and dysfunction. Nat Rev Urol 9:30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkov GV. (2014) Central role of the BK channel in urinary bladder smooth muscle physiology and pathophysiology. Am J Physiol Regul Integr Comp Physiol 307:R571–R584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J. (2001) KCNQ potassium channels: physiology, pathophysiology, and pharmacology. Pharmacol Ther 90:1–19. [DOI] [PubMed] [Google Scholar]
- Rode F, Svalø J, Sheykhzade M, Rønn LC. (2010) Functional effects of the KCNQ modulators retigabine and XE991 in the rat urinary bladder. Eur J Pharmacol 638:121–127. [DOI] [PubMed] [Google Scholar]
- Roeloffs R, Wickenden AD, Crean C, Werness S, McNaughton-Smith G, Stables J, McNamara JO, Ghodadra N, Rigdon GC. (2008) In vivo profile of ICA-27243 [N-(6-chloro-pyridin-3-yl)-3,4-difluoro-benzamide], a potent and selective KCNQ2/Q3 (Kv7.2/Kv7.3) activator in rodent anticonvulsant models. J Pharmacol Exp Ther 326:818–828. [DOI] [PubMed] [Google Scholar]
- Sheldon J, Davis A, Wesley Norton N, Woods M, Carson N, Wang Q, Mitchell R, Littrell J, and Argentieri T (2002) Evidence for KCNQ gene expression and M-current activity in rat and human urinary bladder smooth muscle (Abstract), in ASPET–Ray Fuller Symposium Series: Diseases of Aging-1: Lower Urinary Tract Disorders–Physiology, Pharmacology and Therapeutic Approaches; 2002 Jul 6–7; San Francisco, CA. American Society for Pharmacology and Experimental Therapeutics, Bethesda, MD. [Google Scholar]
- Smith AC, Hristov KL, Cheng Q, Xin W, Parajuli SP, Earley S, Malysz J, Petkov GV. (2013) Novel role for the transient potential receptor melastatin 4 channel in guinea pig detrusor smooth muscle physiology. Am J Physiol Cell Physiol 304:C467–C477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldovieri MV, Miceli F, Taglialatela M. (2011) Driving with no brakes: molecular pathophysiology of Kv7 potassium channels. Physiology (Bethesda) 26:365–376. [DOI] [PubMed] [Google Scholar]
- Stott JB, Jepps TA, Greenwood IA. (2014) K(V)7 potassium channels: a new therapeutic target in smooth muscle disorders. Drug Discov Today 19:413–424. [DOI] [PubMed] [Google Scholar]
- Streng T, Christoph T, Andersson KE. (2004) Urodynamic effects of the K+ channel (KCNQ) opener retigabine in freely moving, conscious rats. J Urol 172:2054–2058. [DOI] [PubMed] [Google Scholar]
- Svalø J, Bille M, Parameswaran Theepakaran N, Sheykhzade M, Nordling J, Bouchelouche P. (2013) Bladder contractility is modulated by Kv7 channels in pig detrusor. Eur J Pharmacol 715:312–320. [DOI] [PubMed] [Google Scholar]
- Svalø J, Hansen HH, Rønn LC, Sheykhzade M, Munro G, Rode F. (2012) Kv 7 positive modulators reduce detrusor overactivity and increase bladder capacity in rats. Basic Clin Pharmacol Toxicol 110:145–153. [DOI] [PubMed] [Google Scholar]
- Svalø J, Sheykhzade M, Nordling J, Matras C, Bouchelouche P. (2015) Functional and molecular evidence for Kv7 channel subtypes in human detrusor from patients with and without bladder outflow obstruction. PLoS One 10:e0117350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickenden AD, Krajewski JL, London B, Wagoner PK, Wilson WA, Clark S, Roeloffs R, McNaughton-Smith G, Rigdon GC. (2008) N-(6-chloro-pyridin-3-yl)-3,4-difluoro-benzamide (ICA-27243): a novel, selective KCNQ2/Q3 potassium channel activator. Mol Pharmacol 73:977–986. [DOI] [PubMed] [Google Scholar]
- Wrobel E, Tapken D, Seebohm G. (2012) The KCNE tango - how KCNE1 interacts with Kv7.1. Front Pharmacol 3:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Q, Gao Z, Wang W, Li M. (2008) Activation of Kv7 (KCNQ) voltage-gated potassium channels by synthetic compounds. Trends Pharmacol Sci 29:99–107. [DOI] [PubMed] [Google Scholar]