Abstract
Diversion of synthetic cannabinoids for abuse began in the early 2000s. Despite legislation banning compounds currently on the drug market, illicit manufacturers continue to release new compounds for recreational use. This study examined new synthetic cannabinoids, AB-CHMINACA (N-[1-amino-3-methyl-oxobutan-2-yl]-1-[cyclohexylmethyl]-1H-indazole-3-carboxamide), AB-PINACA [N-(1-amino-3-methyl-1-oxobutan-2-yl)-1-pentyl-1H-indazole-3-carboxamide], and FUBIMINA [(1-(5-fluoropentyl)-1H-benzo[d]imadazol-2-yl)(naphthalen-1-yl)methanone], with the hypothesis that these compounds, like those before them, would be highly susceptible to abuse. Cannabinoids were examined in vitro for binding and activation of CB1 receptors, and in vivo for pharmacological effects in mice and in Δ9-tetrahydrocannabinol (Δ9-THC) discrimination. AB-CHMINACA, AB-PINACA, and FUBIMINA bound to and activated CB1 and CB2 receptors, and produced locomotor suppression, antinociception, hypothermia, and catalepsy. Furthermore, these compounds, along with JWH-018 [1-pentyl-3-(1-naphthoyl)indole], CP47,497 [rel-5-(1,1-dimethylheptyl)-2-[(1R,3S)-3-hydroxycyclohexyl]-phenol], and WIN55,212-2 ([(3R)-2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenyl-methanone, monomethanesulfonate), substituted for Δ9-THC in Δ9-THC discrimination. Rank order of potency correlated with CB1 receptor-binding affinity, and all three compounds were full agonists in [35S]GTPγS binding, as compared with the partial agonist Δ9-THC. Indeed, AB-CHMINACA and AB-PINACA exhibited higher efficacy than most known full agonists of the CB1 receptor. Preliminary analysis of urinary metabolites of the compounds revealed the expected hydroxylation. AB-PINACA and AB-CHMINACA are of potential interest as research tools due to their unique chemical structures and high CB1 receptor efficacies. Further studies on these chemicals are likely to include research on understanding cannabinoid receptors and other components of the endocannabinoid system that underlie the abuse of synthetic cannabinoids.
Introduction
In the 1960s, Raphael Mechoulam’s isolation and elucidation of the chemical structure of Δ9-tetrahydrocannabinol (Δ9-THC) (Gaoni and Mechoulam, 1964), the primary psychoactive substituent in Cannabis sativa, initiated a concerted effort directed at manipulation of its chemical structure, with one goal being to produce a compound with medicinal effects and no psychoactive properties. Synthesis of phytocannabinoid analogs was followed by development of bicyclic cannabinoids (e.g., CP55,940 [(−)-cis-3-[2-hydroxy-4-(1,1-dimethylheptyl)phenyl]-trans-4-(3-hydroxypropyl)cyclohexanol]) and aminoalkylindoles [e.g., WIN55,212-2 ([(3R)-2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenyl-methanone, monomethanesulfonate)]. The effort was renewed with the discovery and initial characterization of the endocannabinoid system (Devane et al., 1988), which added the structural templates of arachidonic acid derivative agonists (anandamide and 2-arachidonoylglycerol) (Devane et al., 1992; Hanus et al., 2001) and a pyrazole antagonist, rimonabant (Rinaldi-Carmona et al., 1994). Structure-activity relationship studies focused on delineation of the ways in which these diverse chemical structures could bind to the two identified cannabinoid receptors (CB1 and CB2) and differentiation of features that might enhance selectivity for the CB2 cannabinoid receptor. CB1 receptor mediation of the marijuana-like psychoactive effects of cannabinoids was confirmed during this time (Wiley et al., 1995b), and the high correlation between binding affinity and potency for producing these psychoactive effects in mice was noted (Compton et al., 1993). The systematic synthesis of cannabinoids for use as research tools to probe the structure and functioning of the cannabinoid receptors or for use as lead candidates in medication development efforts continued to produce a multitude of novel synthetic cannabinoids (e.g., see Manera et al., 2008). After the initial publication of this medicinal chemistry research, much of it lay dormant, with the exception of occasional retrieval by scientists.
Then, in the 2000s, several of the previously reported compounds were identified in confiscated herbal incense labeled as “Spice” (Vardakou et al., 2010). Largely due to the rapid proliferation of information through the internet, the use of JWH-018 [1-pentyl-3-(1-naphthoyl)indole] and other research chemicals for their intoxicating effects was spreading throughout the world. Legal bans of the cannabinoids contained in early products resulted in the emergence of additional compounds not yet illegal, creating a type of “whack-a-mole” situation between drug control agencies and illicit manufacturers. For example, prevalence of JWH-018 faded as it was subsequently replaced by AM-2201 ([1-(5-fluoropentyl)-1H-indol-3-yl]-1-naphthalenyl-methanone) and then by tetramethylcyclopropyl ketone indoles (XLR-11 [(1-(5-fluoropentyl)-1H-indol-3-yl)(2,2,3,3-tetramethylcyclopropyl)methanone] and UR-144 [(1-pentyl-1H-indol-3-yl)(2,2,3,3-tetramethylcyclopropyl)-methanone]). Most recently, two indazole carboxamide cannabinoids, AB-CHMINACA (N-[1-amino-3-methyl-oxobutan-2-yl]-1-[cyclohexylmethyl]-1H-indazole-3-carboxamide) and AB-PINACA [N-(1-amino-3-methyl-1-oxobutan-2-yl)-1-pentyl-1H-indazole-3-carboxamide], have achieved prominence, resulting in their temporary placement into Schedule I (i.e., compounds with high abuse potential and no accepted medical use) by the US Drug Enforcement Agency (Drug Enforcement Administration, Department of Justice, 2015). An associated problem with the rapid proliferation of synthetic cannabinoids is detection and identification of their metabolites in biologic fluids. This forensic information is often helpful for detection of use for the purposes of medical treatment, employee screening, or legal prosecution.
The purpose of the present study was to examine the in vitro and in vivo pharmacology of AB-CHMINACA and AB-PINACA (Fig. 1). FUBIMINA [(1-(5-fluoropentyl)-1H-benzo[d]imadazol-2-yl)(naphthalen-1-yl)methanone], an analog of a previously identified synthetic cannabinoid of abuse, AM-2201, was also evaluated (Fig. 1). Assessment centered on assays used to predict the abuse liability of cannabinoids (Wiley and Martin, 2009), including binding and activation of CB1 receptors, pharmacological equivalence with Δ9-THC in a battery of four tests in mice, and Δ9-THC discrimination in mice. In the Δ9-THC discrimination procedure, results are also presented for synthetic cannabinoids from different chemical classes, including the prototypic indole-derived synthetic cannabinoid JWH-018, a bicyclic cannabinoid CP47,497 [rel-5-(1,1-dimethylheptyl)-2-[(1R,3S)-3-hydroxycyclohexyl]-phenol], and the aminoalkylindole WIN55,212-2. These representative compounds are from different chemical classes of cannabinoids that have been well characterized previously (Compton et al., 1992a,b; Wiley et al., 1998). They were tested in this work to provide a basis for comparison with the structurally innovative compounds shown in Fig. 1. To assist in the development of forensic markers and to examine metabolic transformations, preliminary analysis of urinary metabolites of the three novel synthetic cannabinoids also was undertaken.
Fig. 1.
Chemical structures of JWH-018, AM-2201, FUBIMINA, AB-CHMINACA, and AB-PINACA.
Materials and Methods
Animals
Adult drug naive male ICR mice (31–34 g; Harlan, Frederick, MD) and C57/BL6J mice (20–25 g; The Jackson Laboratory, Bar Harbor, ME) were used in the tetrad battery and drug discrimination experiments, respectively. All mice were housed singly in polycarbonate mouse cages in a temperature-controlled (20–22°C) environment with a 12-hour light/dark cycle (lights on at 6:00 AM). Water was freely available in the home cage. Whereas separate mice, with unlimited access to food, were used for testing each compound dose in the tetrad battery, mice in the drug discrimination experiments were maintained at 85–90% of free-feeding body weights by restricting daily ration of standard rodent chow and were tested repeatedly. At the start of this project, some of these mice had already been trained to discriminate Δ9-THC from vehicle; however, others were trained to discriminate Δ9-THC de novo. The in vivo studies reported in this manuscript were carried out in accordance with guidelines published in the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011) and were approved by our Institutional Animal Care and Use Committee.
Apparatus
For the tetrad test battery in mice, measurement of spontaneous activity occurred in Plexiglas locomotor activity chambers (47 cm × 25.5 cm × 22 cm). Beam breaks (4 × 8 beam array) were recorded by San Diego Instruments Photobeam Activity System software (San Diego, CA) on a computer located in the experimental room. A standard tail flick device for rodents (Stoelting, Dale, IL) was used to assess antinociception. A digital thermometer (Physitemp Instruments, Clifton, NJ) was used to measure rectal temperature. The ring immobility device consisted of an elevated metal ring (diameter, 5.5 cm; height, 28 cm) attached to a metal stand.
Mice in the drug discrimination experiment were trained and tested in mouse operant chambers (Coulbourn Instruments, Whitehall, PA), housed within light- and sound-attenuating cubicles. Each chamber contained two retractable response levers or nose poke apertures, with stimulus lights located over each lever/aperture, and a separate house light. A food dispenser delivered 20-mg food pellets (Bioserv, Frenchtown, NJ) into a food cup (with a light) centered between the two levers/apertures. Illumination of lights, delivery of food pellets, and recording of lever presses or nose pokes were controlled by a computer-based system (Graphic State Software, version 3.03; Coulbourn Instruments).
Experimental Procedures
All in vitro and in vivo experimental procedures were similar to those described in our previous publication (Wiley et al., 2013), in which we described results of tests with two tetramethylcyclopropyl ketone indoles, XLR-11 and UR-144, that are also classified as synthetic cannabinoids of abuse.
Receptor Binding.
Transfected cell membrane preparations with human CB1 (hCB1) and human CB2 (hCB2) receptors (PerkinElmer, Waltham, MA) isolated from a HEK-293 expression system were used for cannabinoid-binding assays, as previously described (Zhang et al., 2010). Binding was initiated with the addition of 40 fmol cell membrane proteins to polypropylene assay tubes containing 0.62 nM [3H]CP55,940 (approximately 130 Ci/mmol), a test compound (for displacement studies), and a sufficient quantity of buffer A [50 mM Tris•HCl, 1 mM EDTA, 3 mM MgCl2, 5 mg/ml bovine serum albumin (BSA), pH 7.4] to bring the total incubation volume to 0.5 ml. Nonspecific binding was determined by the inclusion of 10 μM unlabeled CP55,940. All cannabinoid agonists were prepared from a 10 mM ethanol stock by suspension in buffer A. Following incubation at 30°C for 1 hour, binding was terminated by vacuum filtration through GF/C glass fiber filter plates (PerkinElmer), pretreated in 0.1% (w/v) polyethyleneimine for at least 1 hour, in a 96-well sampling manifold (Brandel, Gaithersburg, MD). Reaction vessels were washed three times with ∼2 ml ice-cold buffer B (50 mM Tris•HCl, 1 mg/ml BSA). The filter plates were air-dried and sealed on the bottom. Liquid scintillate was added to the wells, and the top was sealed. Liquid scintillation spectrometry was used to measure radioactivity after incubating the plates in cocktail for at least 30 minutes. Assays were done in duplicate, and results represent combined data from three independent displacement curves.
Agonist-Stimulated [35S]GTPγS Binding.
G protein–coupled signal transduction ([35S]GTPγ) assays of test compounds were conducted in an incubation mixture consisting of a test compound (0.25 nM–20 µM), GDP (20 μM), [35S]GTPγ (100 pM), and the hCB1 and hCB2 membrane preparations described above (40 fmol) in a total volume of 0.45 ml assay buffer [50 mM Tris-HCl, pH 7.4, 1 mM EDTA, 100 mM NaCl, 3 mM MgCl2, 0.5% (w/v) BSA]. Nonspecific binding was determined in the presence of 100 μM unlabeled GTPγS, and basal binding was determined in the absence of drug. Duplicate samples were incubated for 1 hour at 30°C, and the bound complex was filtered from the reaction mixture, as described above, and counted in a liquid scintillation counter.
Mouse Tetrad.
Each mouse was tested in a tetrad of tests, in which cannabinoid agonists produce a profile of in vivo effects (Martin et al., 1991): suppression of locomotor activity, decreased rectal temperature, antinociception, and catalepsy. Prior to injection, baseline values were obtained for rectal temperature and in the tail flick test in each mouse. In the latter procedure, the mouse’s tail was placed under an intense light (radiant heat), and the latency to remove it (in seconds) was recorded. To minimize tail damage, a 10-second maximal latency was employed. After baseline measurements were taken, mice were injected intraperitoneally with vehicle or drug 30 minutes before being placed into individual activity chambers for a 10-minute session. Immediately upon removal from the chambers, tail-flick latency and rectal temperature were measured again, followed by placement on the elevated ring apparatus at 50 minutes postinjection. The amount of time that the animals remained motionless on the ring during a 5-minute period was recorded. If a mouse fell off the ring during the catalepsy test, it was immediately placed back on and timing was continued for up to nine falls. After the tenth fall, the test was terminated for the mouse.
At least 1 week after completion of all agonist tests, combinations of vehicle or 3 mg/kg rimonabant and active doses of each compound (56 mg/kg Δ9-THC, 3 mg/kg AB-CHMINACA, and 30 mg/kg AB-PINACA) were retested in a subset of the same mice. Procedural details were identical to those described above, with the exception that mice received an intraperitoneal injection of vehicle or rimonabant 10 minutes prior to intraperitoneal injection of the agonist test compound.
Because FUBIMINA was not active at doses up to 100 mg/kg i.p., a probe dose of 56 mg/kg (and vehicle) was administered intravenously to separate groups of mice. Evaluation in the tetrad tests proceeded as described above, with the exception that mice were placed into the locomotor chambers 5 minutes after injection and placed on the ring apparatus, 25 minutes postinjection. Subsequently, the effect of 3 mg/kg i.v. rimonabant in combination with the 56 mg/kg i.v. dose of FUBIMINA was assessed. Rimonabant was injected 10 minutes prior to FUBIMINA or vehicle.
Drug Discrimination.
Two groups of adult male mice were trained to discriminate Δ9-THC in standard operant chambers, as described previously (Vann et al., 2009). Mice were trained to press one of two levers following intraperitoneal administration of 5.6 mg/kg Δ9-THC and to press the other lever after intraperitoneal vehicle injection. Ten consecutive responses on the correct (injection-appropriate) lever resulted in delivery of a food pellet [i.e., fixed ratio 10 (FR10)], whereas responses on the incorrect lever reset the ratio requirement on the correct lever. A double alternation sequence of Δ9-THC and vehicle (e.g., drug, drug, vehicle, vehicle) was instituted. Fifteen-minute training sessions were held Monday–Friday until the mice consistently met three criteria, as follows: 1) the first completed FR10 was on the correct lever; 2) ≥80% of the total responding occurred on the correct lever; and 3) response rate was ≥0.17 responses/s.
Substitution tests began after the mice met acquisition criteria. These 15-minute tests usually occurred on Tuesdays and Fridays and were interspersed with training sessions on other weekdays. During test sessions, 10 consecutive responses on either lever delivered reinforcement. To be tested in the experiment, mice must have met the same three criteria as for acquisition on the preceding day and during the previous training session with the alternate training compound (training drug or vehicle). A dose-effect curve was determined with Δ9-THC in both groups of mice. The first group of mice was subsequently tested with several cannabinoid tricyclic dibenzopyran analogs (data not shown) and with two indole-derived synthetic cannabinoids (Wiley et al., 2013). In the present study, the first group of mice was tested with JWH-018, WIN55,212-2, and CP47,497, and a second dose-effect curve was determined Δ9-THC.
The second group of mice was tested with several noncannabinoid compounds (data not shown). Subsequently, their response requirement was changed from lever presses to nose pokes. This change was necessitated by transition of all of the laboratory’s mouse operant equipment to nose poke apertures and was not specifically related to this study. Additional mice were trained de novo on the nose poke response (using the acquisition procedure described above) and were combined with this second group of mice. With the exception of the actual response (nose poke versus lever press), all other procedural details remained the same (e.g., FR10, food reinforcement, testing criteria). After acquisition of the nose poke response, a dose-effect curve with Δ9-THC was determined (i.e., for de novo mice) or redetermined (i.e., for transition mice), followed by dose-effect curve determinations with AB-PINACA, AB-CHMINACA, and FUBIMINA.
Metabolite Analysis.
Twelve mice (n = 4 per drug) were given intraperitoneal injections of 3 mg/kg AB-PINACA, 3 mg/kg AB-CHMINACA, or 100 mg/kg FUBIMINA. Immediately following injections, the mice were placed into metabolism cages and urine was collected over a 24-hour period. Urine from mice dosed with the same compound was pooled for analysis. Samples were extracted using a salting-out liquid-liquid extraction method prior to analysis. Acetonitrile (200 µl) was added to 100 µL urine, and then the samples were vortexed and 50 µl 5 M ammonium acetate was added as a salting out agent. Samples were vortexed and centrifuged at 10,000 rcf for 5 minutes. The top aqueous layer was removed and dried down at 40°C and reconstituted with 50 µl mobile phase A.
Samples were analyzed on a Waters Acquity ultraperformance liquid chromatography system coupled to a Waters Synapt G2 HDMS quadrupole time-of-flight mass spectrometer (Waters, Milford, MA). The mass spectrometer was operated under resolution mode, positive electrospray ionization, source temperature of 150°C, desolvation temperature of 500°C, desolvation gas at 1000 l/h, capillary voltage at 2.99 kV, sampling cone at 35 V, and extraction cone at 4.3 V. The mass spectrometer was externally calibrated from 50 to 1000 m/z using a sodium formate solution. Leucine enkephalin was used as a lockmass to correct for mass shifts during acquisition. Full scan data were collected in both low (4 eV) and high (15–40 eV ramp) collision energies nearly simultaneously for every m/z using MSE acquisition mode (Bateman et al., 2002).
Samples were separated on an Acquity BEH C18 column (1.7 μm 2.1 × 50 mm) connected to a Vanguard BEH C18 precolumn (1.7 µm × 2.1 × 5 mm) and held at 30°C. Injection volume was 10 µl. A gradient elution with a flow rate of 500 µl/min was used with mobile phase A consisting of water with 0.1% formic acid and mobile phase B consisting of acetonitrile with 0.1% formic acid. The mobile phase composition for AB-PINACA and AB-CHMINACA was held at 90% A for 1.5 minutes, decreased to 55% A over 15 minutes, decreased to 5% A over 3 minutes, and held at 90% A for 2.9 minutes for column re-equilibration. For FUBIMINA the gradient was held at 90% A for .5 minute, decreased to 85% A over 1 minute to 35% A over 15 minutes, then to 5% A over 3 minutes and held at 90% A for 2.9 minutes for column re-equilibration. For EG-18, the gradient was held at 90% A for 0.5 minute, decreased to 65% A over 1 minute and 35% A over 13 minutes, then decreased to 5% A over 5 minutes and held at 90% A for 2.9 minutes for column re-equilibration. All mobile phase composition changes were done linearly.
Drugs and Chemicals
Δ9-THC, JWH-018, CP47,497, and rimonabant (the prototypic CB1 receptor antagonist/inverse agonist) were obtained from the National Institute on Drug Abuse (Bethesda, MD) through the National Institute on Drug Abuse Drug Supply Program. WIN55,212-2 was purchased commercially (Cayman Chemical, Ann Arbor, MI). AB-PINACA, AB-CHMINACA, and FUBIMINA were provided to RTI by the Drug Enforcement Administration. For the in vivo tests, the vehicle for all compounds was 7.8% Polysorbate 80 N.F. (VWR, Marietta, GA) and 92.2% sterile saline USP (Butler Schein, Dublin, OH). All compounds were injected at a volume of 10 ml/kg.
Guanosine-5′-diphosphate, BSA, ammonium acetate, and formic acid were purchased from Sigma-Aldrich (St. Louis, MO). GTPγS was purchased from Roche Diagnostics (Indianapolis, IN). [35S]GTPγS (1150–1300 Ci/mmol) and scintillation fluid (MicroScint 20) were obtained from PerkinElmer Life Sciences (Boston, MA). High-performance liquid chromatography grade acetonitrile and water were purchased from Fisher Scientific (Fairlawn, NJ). Reference standards and metabolite reference standards for all compounds were obtained from Cayman Chemical (Ann Arbor, MI).
Data Analyses
Binding Data Analysis.
Specific binding was calculated by subtracting nonspecific binding from total binding for each concentration of displacing ligand. For displacement studies, curve-fitting and IC50 calculation were done with GraphPad Prism (version 5; GraphPad Software, San Diego, CA), which fits the data to one- and two-site models and compares the two fits statistically. Ki values were estimated from IC50 values using the Cheng-Prusoff equation.
Data for [35S]GTPγS-binding experiments are reported as mean and S.E. of at least three replicates. Specific binding was calculated by subtracting nonspecific binding from total binding and dividing by the total basal binding minus nonspecific binding. Data were plotted and analyzed with GraphPad Prism. Nonspecific binding was subtracted from each sample. Net-stimulated [35S]GTPγS binding was defined as agonist-stimulated minus basal [35S]GTPγS binding, and percent stimulation was defined as (net-stimulated/basal [35S]GTPγS binding) × 100%. Nonlinear iterative regression analyses of agonist concentration-effect curves were performed with GraphPad Prism. Significance was defined as P ≤ 0.05.
Separate factorial analyses of variance (ANOVAs; compound X receptor) were used to determine differences in ki, EC50, and Bmax. Significant differences were further analyzed with Tukey post hoc tests (α = 0.05), as necessary.
Mouse Tetrad.
Spontaneous activity was measured as total number of photocell beam interruptions during the 10-minute session. For the purpose of potency calculation, it was expressed as percentage of inhibition of activity of the vehicle group. Antinociception was expressed as the percentage of maximum possible effect using a 10-second maximum test latency as follows: [(test − control)/(10 − control)] × 100. Rectal temperature values were expressed as the difference between control temperature (before injection) and temperature following drug administration (Δ°C). For catalepsy, the total amount of time (in seconds) that the mouse remained motionless on the ring apparatus (except for breathing and whisker movement) was used as an indication of catalepsy-like behavior. This value was divided by 300 seconds and multiplied by 100 to obtain a percent immobility. For compounds that produced one or more cannabinoid effects, ED50 was calculated separately using least-squares linear regression on the linear part of the dose-effect curve for each measure in the mouse tetrad, plotted against log10 transformation of the dose. ED50 was defined as the dose at which half-maximal effect occurred. Based on data obtained from numerous previous studies with cannabinoids, maximal cannabinoid effect in each procedure was estimated as follows: 100% inhibition of spontaneous activity, 100% maximum possible effect in the tail flick, −6°C change in rectal temperature, and 100% ring immobility. Separate between-subjects ANOVAs were also used to analyze the four measures for each compound. Significant differences from control (vehicle) were further analyzed with Tukey post hoc tests (α = 0.05), as necessary. Factorial ANOVAs (rimonabant dose × compound dose) were used to analyze results of antagonist tests. Significant main effects and interactions were further analyzed with Tukey post hoc tests (α = 0.05), as necessary.
Drug Discrimination.
For each session, percentage of responses on the drug-associated manipulandum and response rate (responses/s) were calculated. Full substitution was defined as ≥80% responding on the drug-associated manipulandum (Vann et al., 2009). ED50 values were calculated on the linear part of the drug manipulandum selection dose-response curve for each drug using least squares linear regression analysis, followed by calculation of 95% confidence intervals (CI). Because mice that responded less than 10 times during a test session did not respond on either manipulandum a sufficient number of times to earn a reinforcer, their data were excluded from analysis of drug manipulandum selection, but their response rate data were included. Response-rate data were analyzed using repeated-measures ANOVA across dose. Significant ANOVAs were further analyzed with Tukey post hoc tests (α = 0.05) to specify differences between means.
Metabolite Identification.
Liquid chromatography/mass spectrometry data were analyzed using Waters MassLynx 4.1 with the aid of the MetaboLynx application manager. Automated data processing with MetaboLynx was supplemented by manual interrogation of the data using mass defect filtering, precursor ion, and fragment ion searching techniques (Grabenauer et al., 2012). Presence of potential metabolites was determined by exact mass match to predicted elemental compositions in the low energy data function. Further refinement of the site of modification was determined by presence of characteristic fragment ions at the same retention time. Metabolites were provisionally identified by their molecular weight, retention time, and fragment ions. Metabolites were compared with reference standards as available.
Results
Cannabinoid Receptor Binding and Agonist-Stimulated [35S]GTPγS Binding.
All three test compounds displaced [3H]CP55,940 at the CB1 receptor binding site (Fig. 2A), but with varying affinities that ranged from inhibition constants (ki) 0.59 nM for CP55,940 to 296 nM for FUBIMINA (Table 1). In contrast, only two of the three test compounds (AB-CHMINACA and AB-PINACA) stimulated [35S]GTPγ turnover with reasonable potency (Fig. 2B). Rank order of potency was AB-CHMINACA > CP55,940 > AB-PINACA (Table 1). Furthermore, AB-CHMINACA and AB-PINACA exhibited enhanced efficacy compared with CP55,940 (Table 1) [drug X receptor interaction for Bmax: F(3,46) = 10.95, P < 0.05]. Although FUBIMINA also stimulated [35S]GTPγ turnover with efficacy comparable to that obtained with the positive control CP55,940 (Fig. 2B), it did so only at very high concentrations (Table 1), suggesting it serves as a weakly potent agonist at the CB1 receptor.
Fig. 2.
Effects of CP55,940 (filled squares), AB-PINACA (unfilled squares), AB-CHMINICA (filled circles), and FUBIMINA (unfilled circles) on [3H]CP55,940 displacement (A) and [35S]GTPγS turnover (B) in hCB1 receptors expressed in HEK-293 cells. Effects of CP55,940 (filled squares), AB-PINACA (unfilled squares), AB-CHMINICA (filled circles), and FUBIMINA (unfilled circles) on [3H]CP55,940 displacement (C) and [35S]GTPγS turnover (D) in hCB2 receptors expressed in HEK-293 cells. Each concentration-effect curve represents the mean (±S.E.M.) of three to six repetitions.
TABLE 1.
Binding affinity and potency and efficacy for stimulation of [35S]GTPγ turnover at hCB1 and hCB2 receptors
For each measure in all columns, n is shown in italics below the S.E.M.
| Compound | CB1 Kia | [35S]GTPγ Turnover |
CB2 Kia | [35S]GTPγ Turnover |
||
|---|---|---|---|---|---|---|
| CB1 EC50b | CB1 Emaxc | CB2 EC50b | CB2 Emaxc | |||
| CP55,940 | 0.59 | 23.3 | 124 | 0.30 | 2.1 | 63 |
| (0.06) | (4.7) | (9) | (0.04) | (0.8) | (4) | |
| 7 | 12 | 12 | 6 | 10 | 10 | |
| AB-CHMINACA | 0.78 | 7.4 | 205 | 0.45 | 232.4 | 35 |
| (0.11) | (1.5) | (14) | (0.03) | (231.2) | (2) | |
| 3 | 6 | 6 | 3 | 4 | 4 | |
| AB-PINACA | 2.87 | 71 | 192 | 0.88 | 14.9 | 41 |
| (0.69) | (20.9) | (25) | (0.00) | (8.4) | (1) | |
| 3 | 6 | 6 | 3 | 4 | 4 | |
| FUBIMINA | 296.1 | 2466.3 | 122 | 23.45 | 1269.3 | 56 |
| (33.5) | (1037.2) | (7) | (3.21) | (557.1) | (9) | |
| 4 | 6 | 6 | 3 | 6 | 6 | |
Values represent Ki (±S.E.M.) in nM for [3H]CP55,940 displacement at specified (hCB1 or hCB2) receptor.
Values represent EC50 (±S.E.M.) in nM for [35S]GTPγS binding at specified (hCB1 or hCB2) receptor.
Values represent percentage of maximal increase (±S.E.M.) for [35S]GTPγS binding over basal at specified (hCB1 or hCB2) receptor.
Similar to their effects at the CB1 receptor, all three test compounds displaced [3H]CP55,940 at the CB2 receptor binding site (Fig. 2C). Furthermore, affinities of all compounds for the CB2 receptor exceeded those obtained at the CB1 receptor by 1.7- to 12.6-fold (Table 1) [main effect of receptor type for ki: F(1,24) = 60.37, P < 0.05]. In contrast with the greater affinities of the test compounds for the CB2 receptor, they showed reduced efficacy at this receptor compared with the CB1 receptor (Fig. 2D) [main effect of receptor type for Bmax: F(1,46) = 169.91, P < 0.05]. Whereas CP55,940 and FUBIMINA produced similar efficacies at the CB2 receptor, efficacies for AB-PINACA and AB-CHMINACA were somewhat lower (Table 1). Potencies for stimulating [35S]GTPγ turnover at the CB2 receptor varied greatly across the compounds (Table 1). Whereas AB-PINACA was approximately sevenfold less potent than CP55,940, AB-CHMINICA and FUBIMINA were 111- and 604-fold less potent than CP55,940 (Table 1) [main effect of compound for EC50: F(3,46) = 10.58, P < 0.05].
Mouse Tetrad Effects.
For all ANOVAs performed on tetrad test data, significant F values are presented in figure legends of the appropriate figures. Δ9-THC, AB-CHMINACA, and AB-PINACA exhibited the complete profile of cannabinoid effects in the tetrad tests in mice, with each compound producing dose-dependent suppression of spontaneous activity, antinociception, hypothermia, and ring immobility (Fig. 3, A–D, respectively). Across the four tests, AB-CHMINACA was 11- to 58-fold more potent than Δ9-THC, whereas AB-PINACA was 2- to 14-fold more potent than Δ9-THC (Table 2). With the exception of the effect of Δ9-THC on spontaneous activity, one or more doses of Δ9-THC, AB-CHMINACA, and AB-PINACA significantly affected each measure. Although Δ9-THC showed a trend toward decreased spontaneous activity, the effect did not reach statistical significance. In addition to its effects on the tetrad measures, 30 mg/kg AB-PINACA also produced convulsions, flattened body posture (splayed limbs), and labored breathing in most mice within 1 minute after intraperitoneal injection. By the end of tetrad testing, mice had started to recover and were walking around their home cages.
Fig. 3.
Effects of Δ9-THC (filled squares), AB-PINACA (unfilled squares), AB-CHMINACA (filled circles), and FUBIMINA (unfilled circles) on locomotor activity (A), antinociception (B), rectal temperature (C), and catalepsy (D). Values represent the mean (±S.E.M.) of six male ICR mice, with the exception that n = 5 for ring immobility for the 100 mg/kg dose of FUBIMINA due to data excluded for one mouse that fell off the ring 10 times. Asterisks (*) indicate significant differences (P < 0.05) compared with respective vehicle (at left side of each panel). Significant F values are as follows: (A) AB-PINACA [F(6,35) = 6.45, P < 0.05] and AB-CHMINACA [F(5,30) = 9.18, P < 0.05]. (B) Δ9-THC [F(3,20) = 21.69, P < 0.05], AB-PINACA [F(6,35) = 11.97, P < 0.05], AB-CHMINACA [F(5,30) = 23.43, P < 0.05], and FUBIMINA [F(3,20) = 7.27, P < 0.05]. (C) Δ9-THC [F(3,20) = 63.92, P < 0.05], AB-PINACA [F(6,35) = 56.87, P < 0.05], and AB-CHMINACA [F(5,30) = 23.09, P < 0.05]. (D) Δ9-THC [F(3,20) = 27.30, P < 0.05], AB-PINACA [F(6,35) = 11.75, P < 0.05], and AB-CHMINACA [F(5,30) = 17.48, P < 0.05].
TABLE 2.
Potencies in the tetrad tests
Values represent ED50 (±95% CIs) in μmol/kg. All compounds were administered intraperitoneally.
| Compound | Tetrad Tests ED50 |
|||
|---|---|---|---|---|
| SA | MPE | RT | RI | |
| mol. wt. | μmol/kg | |||
| Δ9-THC | 104 | 34 | 30 | 30 |
| (314) | (51–216) | (20–58) | (23–39) | (17–53) |
| AB-CHMINACA | 1.8 | 2.0 | 1.1 | 2.7 |
| (356) | (0.7–4.5) | (1.3–3.0) | (0.7–1.6) | (1.9–3.9) |
| AB-PINACA | 7.6 | 13.7 | 5.3 | 13.9 |
| (330) | (3.3–17.4) | (5.5–34.2) | (3.5–8.0) | (6.8–28.4) |
| FUBIMINA | Not active | Not active | Not active | Not active |
| (360) | (>278) | (>278) | (>278) | (>278) |
MPE, percentage of maximum possible effect in tail flick test; RI, ring immobility; RT, change in rectal temperature in °C; SA, percentage of inhibition of spontaneous activity.
In contrast, FUBIMINA did not affect spontaneous activity or rectal temperature and did not produce ring immobility at intraperitoneal doses up to 100 mg/kg (Fig. 3). Although i.p. doses of 30 and 100 mg/kg FUBIMINA produced statistically significant increases in antinociception, the magnitude of these increases was small and did not approach the maximal effects observed with Δ9-THC, AB-CHMINACA, and AB-PINACA. Despite measurable affinity for the CB1 receptor, FUBIMINA did not exhibit cannabinoid effects in the tetrad tests following intraperitoneal injection. Consequently, a dose of 56 mg/kg i.v. FUBIMINA was tested (Fig. 4). At this dose, FUBIMINA suppressed locomotor activity and produced antinociception, hypothermia, and catalepsy (Fig. 4, A–D, right, respectively), albeit the magnitudes of its antinociceptive and hypothermic effects were somewhat less than a comparable intraperitoneal dose of Δ9-THC.
Fig. 4.
The left side of each panel shows the effects of intraperitoneal injections of vehicle, Δ9-THC (56 mg/kg), AB-PINACA (30 mg/kg), and AB-CHMINACA (3 mg/kg) tested in combination with vehicle (unfilled bars) or 3 mg/kg rimonabant (filled bars). The right side of each panel shows the effects of intravenous injections of vehicle and FUBIMINA (56 mg/kg) tested in combination with vehicle (unfilled bars) or 3 mg/kg rimonabant (filled bars). Dependent measures are spontaneous activity (A), antinociception (B), rectal temperature (C), and catalepsy (D). Values represent the mean (±S.E.M.) of six mice per group. Dollar signs ($) indicate significant main effects (P < 0.05) of compound dose, as compared with vehicle. Asterisks (*) and number symbols (#) indicate significant interactions (with post hoc confirmation of difference) between compound (P < 0.05), relative to the vehicle/vehicle condition or the compound plus vehicle condition, respectively. Significant F values are as follows: (A) Δ9-THC, AB-PINACA, and AB-CHMINACA [main effect: F(3,40) = 7.81, P < 0.05]; FUBIMINA [main effect: F(1,20) = 31.80, P < 0.05]. (B) Δ9-THC, AB-PINACA, and AB-CHMINACA [interaction: F(3,40) = 15.28, P < 0.05]; FUBIMINA [interaction: F(1,20) = 14.44, P < 0.05]. (C) Δ9-THC, AB-PINACA, and AB-CHMINACA [interaction: F(3,40) = 5.66, P < 0.05]; FUBIMINA [interaction: F(1,20) = 23.30, P < 0.05]. (D) Δ9-THC, AB-PINACA, and AB-CHMINACA [interaction: F(3,40) = 28.55, P < 0.05]; FUBIMINA [interaction: F(1,20) = 19.74, P < 0.05].
Figure 4 also shows the results of antagonism tests. Alone, intraperitoneal doses of 56 mg/kg Δ9-THC, 30 mg/kg AB-PINACA, and 3 mg/kg AB-CHMINACA significantly suppressed locomotor activity (Fig. 4A) and produced antinociception (Fig. 4B), hypothermia (Fig. 4C), and catalepsy (Fig. 4D). In each instance, these effects were attenuated by coadministration of 3 mg/kg i.p. rimonabant. Rimonabant (3 mg/kg i.v.) also attenuated the cannabinoid effects of 56 mg/kg FUBIMINA (Fig. 4).
Drug Discrimination in Mice.
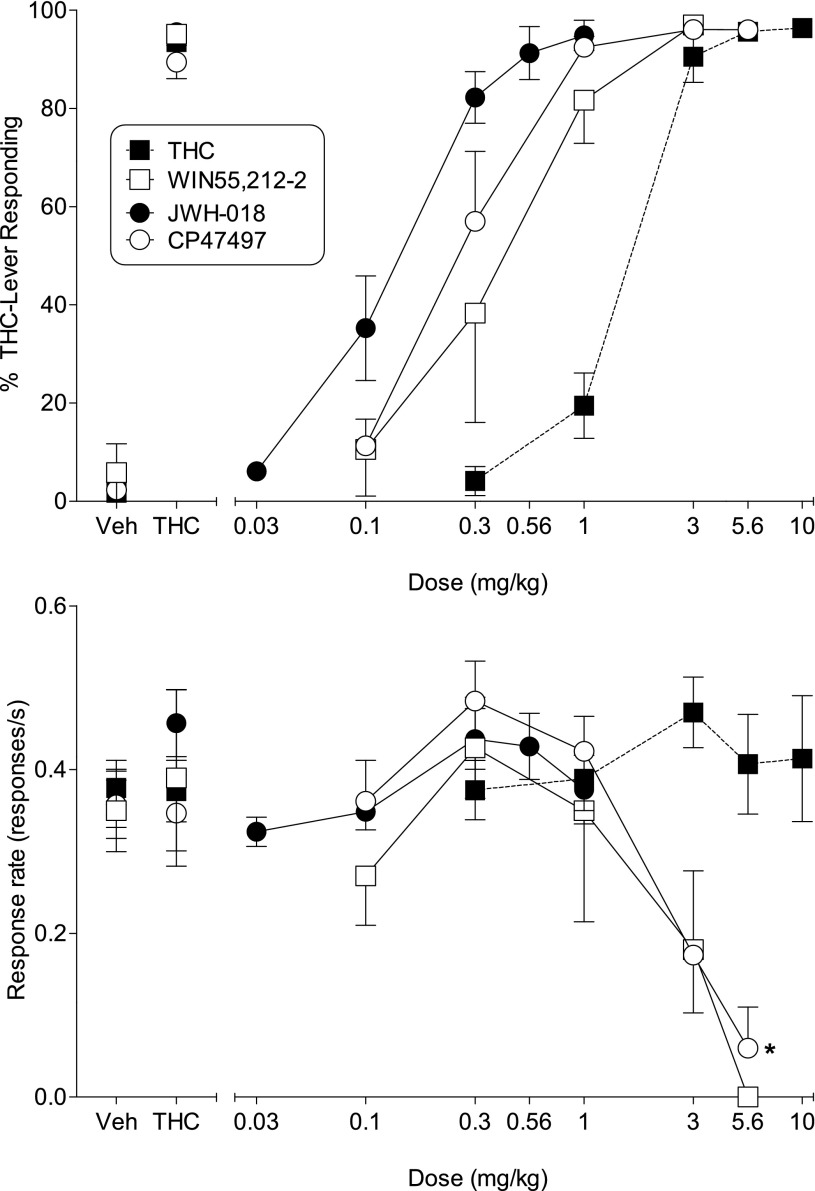
Mice trained to lever press for food reward in a Δ9-THC discrimination procedure showed full dose-dependent substitution for the 5.6 mg/kg Δ9-THC training dose (Fig. 5, top), with a potency similar to that obtained with mice trained in the nose poke procedure (Table 3). The aminoalkylindole WIN55,212-2, a bicyclic cannabinoid CP47,497, and the prototypic indole-derived synthetic cannabinoid JWH-018 also fully and dose-dependently substituted for Δ9-THC (Fig. 5, top). Rank order of potency for substitution was JWH-018 > CP47,497 > WIN55,212-2 > Δ9-THC (Table 3). Whereas CP47,497 produced response rate decreases at higher doses [F(4,28) = 9.18, P < 0.05], Δ9-THC and JWH-018 did not (Fig. 5, bottom), although the highest dose of JWH-018 tested (1 mg/kg) was relatively low compared with those of the other compounds. WIN55,212-2 substantially decreased response rates; however, the small number of mice tested prevented attainment of significance.
Fig. 5.
Effects of Δ9-THC (filled squares), WIN55,212-2 (unfilled squares), JWH-018 (filled circles), and CP47,497 (unfilled circles) on percentage of responses that occurred on the Δ9-THC–associated lever (Top) and response rate (Bottom). Each point represents the mean (±S.E.M.) of data for male C57/BL6J mice: all doses of Δ9-THC (n = 6–7); all doses of JWH-018 (n = 11); at doses of 0.1, 0.3, and 1 mg/kg CP47,497 (n = 8) and WIN55,212-2 (n = 3); at 3 mg/kg CP47,497 (n = 5 for % Δ9-THC-lever responding and n = 6 for response rate); at 5.6 mg/kg CP47,497 (n = 1 for % Δ9-THC-lever responding and n = 2 for response rate); at 3 mg/kg WIN212-2 (n = 2 for % Δ9-THC-lever responding and n = 3 for response rate); at 5.6 mg/kg WIN55,212-2 (n = 1) for response rate. Asterisks (*) indicate significant differences (P < 0.05) compared with vehicle.
TABLE 3.
Potencies for substitution in Δ9-THC discrimination
ED50 (±95% CIs) are expressed in μmol/kg. Molecular weights are provided in parentheses. All compounds were administered intraperitoneally.
| Compound | CB1 Ki | ED50 |
|---|---|---|
| nM | μmol/kg | |
| THC DD (lever) | ||
| Δ9-THC (n = 7) | 41a | 4.5 |
| (314) | (2) | (3.5–5.7) |
| JWH-018 (n = 11) | 9.5a | 0.35 |
| (341) | (4.5) | (0.32–0.50) |
| CP47,947 (n = 8) | 9.5b | 0.85 |
| (318) | (0.35) | (0.47–1.45) |
| WIN 55,212-2 (n = 3) | 1.9a | 0.80 |
| (522) | (0.1) | (0.46–1.38) |
| THC DD (nose poke) | ||
| Δ9-THC (n = 20) | 41a | 5.7 |
| (314) | (2) | (4.8–6.7) |
| AB-CHMINACA (n = 8) | 0.78 | 0.34 |
| (356) | (0.11) | (0.22–0.50) |
| AB-PINACA (n = 9) | 2.87 | 3.78 |
| (330) | (0.69) | (2.42–5.87) |
| FUBIMINA (n = 6) | 296.1 | 131 |
| (360) | (33.5) | (72–233) |
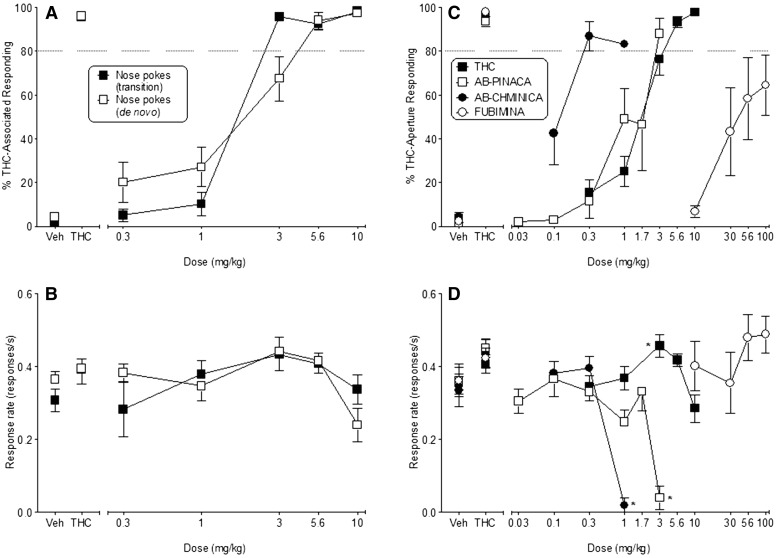
Δ9-THC also produced full dose-dependent substitution for the 5.6 mg/kg Δ9-THC training dose in all mice responding in the nose poke procedure (Fig. 6A), regardless of whether they had received initial training in Δ9-THC discrimination with a different response topography (i.e., transition from lever presses to nose pokes; ED50, 5.4 μmol/kg; 95% CI, 4.8–5.7 μmol/kg) or had been trained de novo with the nose poke response (ED50, 5.7 μmol/kg; 95% CI, 4.5–7.6 μmol/kg). Furthermore, Δ9-THC did not alter response rates (compared with vehicle) in any of the groups across the dose range tested (Fig. 6B). For the purpose of comparison with the test compounds, data from all mice trained to discriminate Δ9-THC using the nose poke response were combined, with a resulting ED50, 5.7 μmol/kg (95% CI, 4.8–6.7 μmol/kg) (Fig. 6C; Table 3). Response rates were significantly increased by 3 mg/kg Δ9-THC [F(5,95) = 5.97, P < 0.05], but significant decreases (compared with vehicle) were not observed at any dose (Fig. 6D). During all control tests with vehicle and 5.6 mg/kg Δ9-THC across the course of the study, mice responded predominantly on the vehicle- and Δ9-THC–associated apertures, respectively (Fig. 6, A and C, left).
Fig. 6.
(Left) Effects of Δ9-THC on percentage of responses that occurred on the Δ9-THC–associated aperture (A) and response rate (B) in mice trained to discriminate Δ9-THC from vehicle in a nose poke procedure following transition from a lever press procedure (filled squares) or de novo (unfilled squares). Each point represents the mean (±S.E.M.) of data for seven male C57/BL6J mice for the transitional group and n = 11–13 for the de novo group. (Right) Effects of Δ9-THC (filled squares), AB-PINACA (unfilled squares), AB-CHMINACA (filled circles), and FUBIMINA (unfilled circles) on percentage of responses that occurred on the Δ9-THC–associated aperture (C) and response rate (D). Each point represents the mean (±S.E.M.) of data for 18–20 male C57/BL6J mice for Δ9-THC and n = 6–9 for the synthetic compounds, except for % Δ9-THC–associated aperture responding at 3 mg/kg AB-PINACA (n = 2) and 1 mg/kg AB-CHMINACA (n = 1). Asterisks (*) indicate significant differences compared with respective vehicle.
As shown in Fig. 6C, AB-CHMINACA and AB-PINACA produced full, dose-dependent substitution for Δ9-THC. Both compounds were more potent than Δ9-THC by 16- and 1.5-fold for AB-CHMINACA and AB-PINACA, respectively (Table 3). Of the two test compounds, the effects of AB-CHMINACA bore the most resemblance to those of Δ9-THC, in that the lowest dose producing full substitution (0.3 mg/kg) did not affect response rates (Fig. 6D). Although full substitution also occurred at 1 mg/kg AB-CHMINACA, this effect was accompanied by an overall reduction in response rates [F(3,21) = 36.09, P < 0.05]. In fact, of the seven mice tested at this dose, only one responded on either aperture during the entire session.
The profile of AB-PINACA was distinct from that produced by Δ9-THC and AB-CHMINACA, in that full substitution was observed only at a dose (3 mg/kg; Fig. 6C) that also severely reduced response rates [F(4,32) = 16.48, P < 0.05] (Fig. 6D). Only two of nine mice tested with this dose responded on either aperture. Furthermore, the response rate dose-effect function was steep, with no effect at 1.7 mg/kg and nearly complete suppression at a dose (3 mg/kg) only 1/2 log higher (Fig. 6D).
Results of substitution tests with FUBIMINA revealed considerable variability in choice of aperture across the mice. Although FUBIMINA did not fully substitute for Δ9-THC (i.e., >80% Δ9-THC aperture responding), dose-dependent increases in responding on the Δ9-THC aperture (partial substitution) were observed (Fig. 6C; Table 3). FUBIMINA did not significantly affect response rates (Fig. 6D).
Metabolite Identification.
In urine from mice administered AB-PINACA, only monohydroxylations and their corresponding glucuronide conjugates were observed. Three distinct monohydroxylated metabolites were observed, none of which matched the retention times of 4-hydroxy AB-PINACA or 5-hydroxy AB-PINACA reference standards. Fragment ions observed for one of the hydroxylated metabolites were m/z 231, m/z 302, and m/z 330, where m/z 231 is indicative of hydroxylation located on the 1-pentyl-1H-indazole moiety with an attached carbonyl. Other metabolites were identified in in vitro AB-PINACA and confirmed in human urine specimens (Wohlfarth et al., 2015), but not observed in the present study.
Similar to AB-PINACA, a single hydroxylated metabolite and its corresponding glucuronide conjugate were identified in the urine from mice dosed with AB-CHIMINACA. Characteristic fragments observed at both retention times were m/z 328, m/z 356, and m/z 257. The fragment ion at m/z 257 is indicative of hydroxylation on the 1-(cyclohexylmethyl)-1H-indazole moiety with an attached carbonyl. At the time of analysis, no reference standard was available for hydroxylated AB-CHIMINACA; however, Cayman Chemical AB-CHIMINACA metabolites M2, M3A, M4, M5A, and M6 were run as reference standards and were not observed in the in vivo sample.
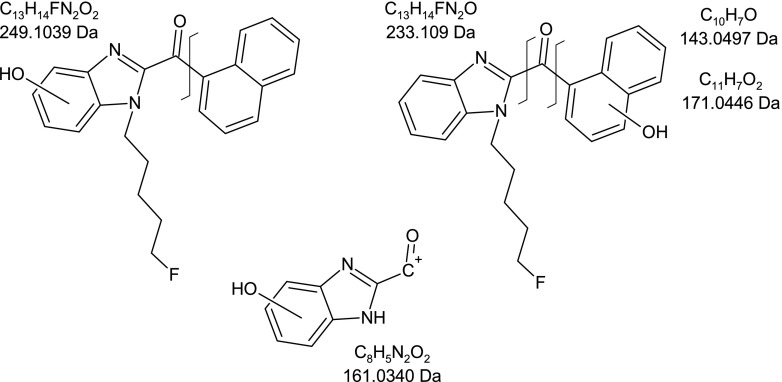
A summary of the metabolites found in urine from mice dosed FUBIMINA is shown in Table 4, in which I represents the phase I metabolites and II represents the phase II glucuronide conjugates. Unlike for AB-PINACA and AB-CHIMINACA, nonmetabolized, intact parent compound was observed in the pooled in vivo urine sample. Major metabolites identified were multiple monohydroxylations and their corresponding glucuronide conjugates (Fig. 7). Hydroxylation for FUBIMINA was confirmed to occur on the 1-(5-fluoropentyl)-1H-benzimidazole moiety by the presence of a fragment ion at m/z 249 (with the carbonyl attached). Several of the hydroxylated metabolites also had a fragment ion at m/z 161, indicating that hydroxylation was on the benzimidazole moiety. Hydroxylation was also observed on the naphthyl moiety, as determined by the presence of fragment ions at m/z 171, m/z 143, and m/z 233.
TABLE 4.
Metabolic transformations observed in pooled urine from mice dosed with FUBIMINA
| Metabolite | FUBIMINA | |
|---|---|---|
| I | II | |
| Parent | X | |
| Monohydroxylation | X | X |
| Dihydrodiol formation | X | X |
| Saturation | X | |
| Hydration | X | X |
| Defluorination + hydroxylation | X | X |
| Defluorination + carboxylation | X | |
| Defluorination + carboxylation + hydroxylation | X | |
Fig. 7.
Structure of FUBIMINA showing characteristic fragment masses for monohydroxylated metabolites. Hydroxylation was confirmed on the 1-(5-fluoropentyl)-1H-benzimidazole moiety by the presence of a fragment ion at m/z 249. Hydroxylation was also confirmed on the benzimidazole moiety by the presence of a fragment ion at m/z 161 and on the naphthyl moiety by the presence of fragment ions at m/z 171, m/z 143, and m/z 233.
Discussion
Psychoactive cannabinoid agonists produce a characteristic profile of in vitro and in vivo pharmacological effects, including binding to and activating CB1 receptors, dose-dependent activity in a tetrad battery of tests in mice, and Δ9-THC–like discriminative stimulus effects (Wiley and Martin, 2009). In the present study, the in vitro positive control CP55,940 showed low nM Ki for both CB1 and CB2 receptors, similar to a number of indole and pyrrole-derived synthetic cannabinoids (Huffman and Padgett, 2005; Wiley et al., 2014a). Furthermore, it stimulated [35S]GTPγS turnover with high potency and efficacy at both receptors, suggesting that CP55,940 would act as a potent CB1 receptor agonist in vivo. Indeed, this prediction has proved true, as CP55,940 produces cannabimimetic effects in the tetrad battery in mice (Compton et al., 1992b) and substitutes and cross-substitutes for Δ9-THC in drug discrimination in rats (Gold et al., 1992; Wiley et al., 1995a). Similarly, the in vivo positive control Δ9-THC suppressed locomotor activity and showed hypothermic, antinociceptive, cataleptic, and Δ9-THC–like discriminative stimulus effects in mice. Δ9-THC–like discriminative stimulus effects were also observed with JWH-018, WIN55,212-2, and CP47,497, as has been shown previously with the former two compounds (Compton et al., 1992a; Wiley et al., 2014b) and with the C-8 homolog of CP47,497 (Gatch and Forster, 2014). Previous research has shown that these three compounds bind to the CB1 receptor with high affinity and produce cannabimimetic effects in the tetrad battery (Compton et al., 1992a,b; Wiley et al., 1998). Together, these data show that representative compounds from the tetrahydrocannabinol, bicyclic, and aminoalkylindole cannabinoid classes produce similar in vivo and in vitro pharmacological profiles in these assays.
In contrast, the three novel synthetic cannabinoids tested in this study produced distinct profiles in the battery of in vitro and in vivo assays, with quantitative differences compared with typical cannabinoid effects. The profiles of AB-CHMINACA and FUBIMINA most closely matched those obtained previously with other synthetic cannabinoids, differing only quantitatively in their respective affinities, potencies, and efficacies. Similar to CP55,940 (present study) and other full dual CB1/CB2 agonists (Huffman and Padgett, 2005), AB-CHMINACA and FUBIMINA displaced [35H]CP55,940 from both cannabinoid receptor types. Although both compounds showed higher CB2 receptor affinity, FUBIMINA exhibited several-fold greater selectivity than AB-CHMINACA or CP55,940. Both compounds also activated CB1 receptors with full efficacy comparable to (FUBIMINA) or greater than (AB-CHMINACA) that produced by CP55,940 (present study) or other full agonists such as WIN55,212-2 (Griffin et al., 1998), albeit FUBIMINA showed limited potency that was consistent with its lower binding affinity. Consistent with the magnitudes of their respective CB1 receptor affinities, AB-CHMINACA produced the full profile of cannabinoid effects in the tetrad battery, whereas FUBIMINA was inactive when administered intraperitoneally and produced a cannabimimetic profile only at a 56 mg/kg i.v. dose. Tetrad effects of AB-CHMINACA and FUBIMINA were attenuated by rimonabant, suggesting CB1 receptor mediation. In Δ9-THC discrimination, 0.3 mg/kg AB-CHMINACA, a dose that did not affect response rates, fully and potently substituted for Δ9-THC. This pattern of results resembles that obtained with the control compounds from different chemical classes of cannabinoids (see Fig. 6). In contrast, FUBIMINA only partially substituted for Δ9-THC and did so with considerable intrasubject variability. Although tested up to the limits of solubility, FUBIMINA failed to decrease response rates. Together, these results suggest that AB-CHMINACA is a potent and efficacious psychoactive CB1 receptor agonist that is likely to possess abuse liability in humans, a finding that has been implicitly supported through its recent placement in Schedule I (Drug Enforcement Administration, Department of Justice, 2015). FUBIMINA also appears to share cannabimimetic effects with Δ9-THC; however, its low potency may limit its abuse, as illicit manufacturers tend to focus on compounds with greater CB1 receptor affinity and high potency. Nevertheless, FUBIMINA has been detected in products in Japan (Uchiyama et al., 2014), and, with sufficient concentrations, cannabimimetic effects are likely. Metabolic transformations observed for these compounds were similar to those described for many similarly structured synthetic cannabinoids and primarily consisted of monohydroxylation and glucuronide conjugation, with FUBIMINA also undergoing defluorination, as is typical for synthetic cannabinoids with a 5-fluoropentyl group (e.g., AM-2201).
Although in vitro results showed that AB-PINACA resembled AB-CHMINACA in its high affinities for CB1 and CB2 receptors and its high efficacy for stimulation of CB1 receptors, differences between their profiles emerged in the in vivo experiments. Both compounds produced rimonabant-reversible effects in the complete tetrad battery; however, administration of 30 mg/kg i.p. AB-PINACA was accompanied by short-lived convulsive behavior, an effect that we do not typically observe with Δ9-THC or other cannabinoids at doses that produce tetrad effects. In the Δ9-THC discrimination procedure, AB-PINACA substituted fully and dose-dependently for Δ9-THC, but full substitution was achieved only at a dose that was accompanied by substantial decreases in response rate, with only a small percentage (22%) of mice responding at this dose. Previously, we have observed response rate decreases only with doses of other synthetic cannabinoids that were suprathreshold for full substitution (Fig. 5; Wiley et al., 1995a, 2013). Hence, AB-PINACA’s lack of separation between doses that were Δ9-THC-like and those that substantially suppressed responding was unusual compared with the profile seen with other synthetic cannabinoids. These results suggest that AB-PINACA is a potent psychoactive CB1 receptor agonist, but they also suggest that the doses that induce intoxication may be very close to (or indistinguishable from) doses associated with behavioral toxicity.
In summary, synthetic cannabinoids that were originally developed as research tools or as candidate medications have been diverted to drugs of abuse in the form of products labeled with such terms as “herbal incense,” “fake weed,” “spice,” and “K2.” AB-CHMINACA, AB-PINACA, and FUBIMINA are among the chemicals that have been identified in recent confiscations. The results of the present study demonstrate that the pharmacological effects of AB-CHMINACA, AB-PINACA, and FUBIMINA overlap with those of psychoactive cannabinoids from different chemical classes, including Δ9-THC, JWH-018, CP47,497, and WIN55,212-2. Each of these three compounds binds to and activates CB1 and CB2 cannabinoid receptors, produces a characteristic tetrad of cannabimimetic effects in mice, and produces dose-dependent increases in responding on the Δ9-THC–associated aperture in Δ9-THC discrimination. A primary difference among the compounds is their potency, with rank order of potency being correlated with their CB1 receptor-binding affinities: FUBIMINA < Δ9-THC < AB-PINACA < AB-CHMINACA. Notably, all three of these compounds are high efficacy agonists in the [35S]GTPγS-binding assay, as compared with the low partial agonism of Δ9-THC. Ironically, AB-PINACA and AB-CHMINACA are of potential interest to the scientific community as research tools due to their unique chemical structures and their high CB1 receptor efficacies (i.e., >CP55,940). Further use of these chemicals is likely to include greater emphasis on the original purpose for which they were developed: research with a primary goal of increased understanding of cannabinoid receptors and other components of the endocannabinoid system that underlie the abuse of plant-derived and synthetic cannabinoids.
Acknowledgments
The authors thank Dr. Jordan Trecki of the Drug Enforcement Agency for helpful discussion on the human aspects of the abuse of these novel synthetic cannabinoids.
Abbreviations
- AB-CHMINACA
N-[1-amino-3-methyl-oxobutan-2-yl]-1-[cyclohexylmethyl]-1H-indazole-3-carboxamide
- AB-PINACA
N-(1-amino-3-methyl-1-oxobutan-2-yl)-1-pentyl-1H-indazole-3-carboxamide
- AM-2201
[1-(5-fluoropentyl)-1H-indol-3-yl]-1-naphthalenyl-methanone
- ANOVA
analysis of variance
- BSA
bovine serum albumin
- CI
confidence interval
- CP47,497
rel-5-(1,1-dimethylheptyl)-2-[(1R,3S)-3-hydroxycyclohexyl]-phenol
- CP55,940
(−)-cis-3-[2-hydroxy-4-(1,1-dimethylheptyl)phenyl]-trans-4-(3-hydroxypropyl)cyclohexanol
- FR10
fixed ratio 10
- FUBIMINA
(1-(5-fluoropentyl)-1H-benzo[d]imadazol-2-yl)(naphthalen-1-yl)methanone
- hCB
human CB
- JWH-018
1-pentyl-3-(1-naphthoyl)indole
- Δ9-THC
Δ9-tetrahydrocannabinol
- UR-144
(1-pentyl-1H-indol-3-yl)(2,2,3,3-tetramethylcyclopropyl)-methanone
- WIN55,212-2
[(3R)-2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenyl-methanone, monomethanesulfonate
- XLR-11
(1-(5-fluoropentyl)-1H-indol-3-yl)(2,2,3,3-tetramethylcyclopropyl)methanone
Authorship Contributions
Participated in research design: Wiley, Thomas, Lefever.
Conducted experiments: Antonazzo, Cortes, Moore, Patel, Wallgren.
Performed data analysis: Wiley, Thomas, Grabenauer, Moore.
Wrote or contributed to the writing of the manuscript: Wiley, Thomas, Marusich, Lefever, Grabenauer, Moore.
Footnotes
This work was supported by the Department of Justice, Drug Enforcement Agency [Contract DJD-14-HQ-P-0713]; the National Institutes of Health National Institute on Drug Abuse [Grant R01DA-003672]; and the National Institute of Justice, Office of Justice Programs, US Department of Justice [Contract 2012-R2-CX-K001]. None of the funding agencies had any other role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication. The opinions, findings, and conclusions or recommendations expressed in this publication are those of the authors and do not necessarily reflect those of the Department of Justice, the Drug Enforcement Agency, or the National Institutes of Health.
References
- Bateman RH, Carruthers R, Hoyes JB, Jones C, Langridge JI, Millar A, Vissers JPC. (2002) A novel precursor ion discovery methodon a hybrid quadrupole orthogonal acceleration time-of-flight (Q-TOF) mass spectrometer for studying protein phosphorylation. J Am Soc Mass Spectrom 13:792–803. [DOI] [PubMed] [Google Scholar]
- Compton DR, Gold LH, Ward SJ, Balster RL, Martin BR. (1992a) Aminoalkylindole analogs: cannabimimetic activity of a class of compounds structurally distinct from delta 9-tetrahydrocannabinol. J Pharmacol Exp Ther 263:1118–1126. [PubMed] [Google Scholar]
- Compton DR, Johnson MR, Melvin LS, Martin BR. (1992b) Pharmacological profile of a series of bicyclic cannabinoid analogs: classification as cannabimimetic agents. J Pharmacol Exp Ther 260:201–209. [PubMed] [Google Scholar]
- Compton DR, Rice KC, De Costa BR, Razdan RK, Melvin LS, Johnson MR, Martin BR. (1993) Cannabinoid structure-activity relationships: correlation of receptor binding and in vivo activities. J Pharmacol Exp Ther 265:218–226. [PubMed] [Google Scholar]
- Devane WA, Dysarz FA, 3rd, Johnson MR, Melvin LS, Howlett AC. (1988) Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol 34:605–613. [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. (1992) Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258:1946–1949. [DOI] [PubMed] [Google Scholar]
- Drug Enforcement Administration, Department of Justice (2015) Schedules of controlled substances: temporary placement of three synthetic cannabinoids into schedule I. Final order. Fed Regist 80:5042–5047. [PubMed] [Google Scholar]
- Gaoni Y, Mechoulam R. (1964) Isolation, structure, and partial synthesis of an active constituent of hashish. J Am Chem Soc 86:1646–1647. [Google Scholar]
- Gatch MB, Forster MJ. (2014) Δ9-Tetrahydrocannabinol-like discriminative stimulus effects of compounds commonly found in K2/Spice. Behav Pharmacol 25:750–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold LH, Balster RL, Barrett RL, Britt DT, Martin BR. (1992) A comparison of the discriminative stimulus properties of delta 9-tetrahydrocannabinol and CP 55,940 in rats and rhesus monkeys. J Pharmacol Exp Ther 262:479–486. [PubMed] [Google Scholar]
- Grabenauer M, Krol WL, Wiley JL, Thomas BF. (2012) Analysis of synthetic cannabinoids using high-resolution mass spectrometry and mass defect filtering: implications for nontargeted screening of designer drugs. Anal Chem 84:5574–5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin G, Atkinson PJ, Showalter VM, Martin BR, Abood ME. (1998) Evaluation of cannabinoid receptor agonists and antagonists using the guanosine-5′-O-(3-[35S]thio)-triphosphate binding assay in rat cerebellar membranes. J Pharmacol Exp Ther 285:553–560. [PubMed] [Google Scholar]
- Hanus L, Abu-Lafi S, Fride E, Breuer A, Vogel Z, Shalev DE, Kustanovich I, Mechoulam R. (2001) 2-arachidonyl glyceryl ether, an endogenous agonist of the cannabinoid CB1 receptor. Proc Natl Acad Sci USA 98:3662–3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman JW, Padgett LW. (2005) Recent developments in the medicinal chemistry of cannabimimetic indoles, pyrroles and indenes. Curr Med Chem 12:1395–1411. [DOI] [PubMed] [Google Scholar]
- Manera C, Tuccinardi T, Martinelli A. (2008) Indoles and related compounds as cannabinoid ligands. Mini Rev Med Chem 8:370–387. [DOI] [PubMed] [Google Scholar]
- Martin BR, Compton DR, Thomas BF, Prescott WR, Little PJ, Razdan RK, Johnson MR, Melvin LS, Mechoulam R, Ward SJ. (1991) Behavioral, biochemical, and molecular modeling evaluations of cannabinoid analogs. Pharmacol Biochem Behav 40:471–478. [DOI] [PubMed] [Google Scholar]
- National Research Council (2011) Guide for the Care and Use of Laboratory Animals, National Academies Press, Washington, D.C. [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Héaulme M, Shire D, Calandra B, Congy C, Martinez S, Maruani J, Néliat G, Caput D, et al. (1994) SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett 350:240–244. [DOI] [PubMed] [Google Scholar]
- Showalter VM, Compton DR, Martin BR, Abood ME. (1996) Evaluation of binding in a transfected cell line expressing a peripheral cannabinoid receptor (CB2): identification of cannabinoid receptor subtype selective ligands. J Pharmacol Exp Ther 278:989–999. [PubMed] [Google Scholar]
- Uchiyama N, Shimokawa Y, Matsuda S, Kawamura M, Kikura-Hanajiri R, Goda Y. (2014) Two new synthetic cannabinoids, AM-2201 benzimidazole analog (FUBIMINA) and (4-methylpiperazin-1-yl)(1-pentyl-1H-indol-3-yl)methanone (MEPIRAPIM), and three phenethylamine derivatives, 25H-NBOMe 3,4,5-trimethoxybenzyl analog, 25B-NBOMe, and 2C-N-NBOMe, identified in illegal products. Forensic Toxicol 32:105–115. [Google Scholar]
- Vann RE, Warner JA, Bushell K, Huffman JW, Martin BR, Wiley JL. (2009) Discriminative stimulus properties of delta9-tetrahydrocannabinol (THC) in C57Bl/6J mice. Eur J Pharmacol 615:102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardakou I, Pistos C, Spiliopoulou Ch. (2010) Spice drugs as a new trend: mode of action, identification and legislation. Toxicol Lett 197:157–162. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Barrett RL, Lowe J, Balster RL, Martin BR. (1995a) Discriminative stimulus effects of CP 55,940 and structurally dissimilar cannabinoids in rats. Neuropharmacology 34:669–676. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Compton DR, Dai D, Lainton JA, Phillips M, Huffman JW, Martin BR. (1998) Structure-activity relationships of indole- and pyrrole-derived cannabinoids. J Pharmacol Exp Ther 285:995–1004. [PubMed] [Google Scholar]
- Wiley JL, Lefever TW, Cortes RA, Marusich JA. (2014b) Cross-substitution of Δ9-tetrahydrocannabinol and JWH-018 in drug discrimination in rats. Pharmacol Biochem Behav 124:123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Lowe JA, Balster RL, Martin BR. (1995b) Antagonism of the discriminative stimulus effects of delta 9-tetrahydrocannabinol in rats and rhesus monkeys. J Pharmacol Exp Ther 275:1–6. [PubMed] [Google Scholar]
- Wiley JL, Martin BR. (2009) Preclinical pharmacological and brain bioassay systems for CB1 cannabinoid receptors, in The Cannabinoid Receptors (Reggio PH. ed) pp 329–360, Humana Press, New York. [Google Scholar]
- Wiley JL, Marusich JA, Huffman JW. (2014a) Moving around the molecule: relationship between chemical structure and in vivo activity of synthetic cannabinoids. Life Sci 97:55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Marusich JA, Lefever TW, Grabenauer M, Moore KN, Thomas BF. (2013) Cannabinoids in disguise: Δ9-tetrahydrocannabinol-like effects of tetramethylcyclopropyl ketone indoles. Neuropharmacology 75:145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlfarth A, Castaneto MS, Zhu M, Pang S, Scheidweiler KB, Kronstrand R, Huestis MA. (2015) Pentylindole/pentylindazole synthetic cannabinoids and their 5-fluoro analogs produce different primary metabolites: metabolite profiling for AB-PINACA and 5F-AB-PINACA. AAPS J 17:660–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Gilliam A, Maitra R, Damaj MI, Tajuba JM, Seltzman HH, Thomas BF. (2010) Synthesis and biological evaluation of bivalent ligands for the cannabinoid 1 receptor. J Med Chem 53:7048–7060. [DOI] [PMC free article] [PubMed] [Google Scholar]