Abstract
Δ9-Tetrahydrocannabinol (THC), the main psychoactive component of marijuana, produces motor and motivational effects via interactions with the dopaminergic system in the caudate-putamen and nucleus accumbens. However, the molecular events that underlie these interactions after THC treatment are not well understood. Our study shows that pretreatment with dopamine D1 receptor (D1R) antagonists before repeated administration of THC attenuated induction of Δ FBJ murine osteosarcoma viral oncogene homolog B (ΔFosB) in the nucleus accumbens, caudate-putamen, amygdala, and prefrontal cortex. Anatomical studies showed that repeated THC administration induced ΔFosB in D1R-containing striatal neurons. Dopamine signaling in the striatum involves phosphorylation-specific effects of the dopamine- and cAMP-regulated phosphoprotein Mr 32 kDa (DARPP-32), which regulates protein kinase A signaling. Genetic deletion of DARPP-32 attenuated ΔFosB expression measured after acute, but not repeated, THC administration in both the caudate-putamen and nucleus accumbens. THC was then acutely or repeatedly administered to wild-type (WT) and DARPP-32 knockout (KO) mice, and in vivo responses were measured. DARPP-32 KO mice exhibited enhanced acute THC-mediated hypolocomotion and developed greater tolerance to this response relative to the WT mice. Agonist-stimulated guanosine 5′-O-(3-[35S]thio)triphosphate ([35S]GTPγS) binding showed that cannabinoid-stimulated G-protein activity did not differ between DARPP-32 KO and WT mice treated with vehicle or repeated THC. These results indicate that D1Rs play a major role in THC-mediated ΔFosB induction in the forebrain, whereas the role of DARPP-32 in THC-mediated ΔFosB induction and modulation of motor activity appears to be more complex.
Introduction
Cannabis and its primary psychoactive active constituent, Δ9-tetrahydrocannabinol (THC), can produce addiction and neuropsychiatric symptoms with repeated use (Volkow et al., 2014). The major effects of THC in the central nervous system are mediated by cannabinoid CB1 receptors (CB1Rs), which are widely distributed throughout the forebrain (Howlett et al., 2002). Functional and anatomic studies have shown that CB1Rs interact with the dopaminergic system in brain circuits that regulate motivated and motor behaviors (Fitzgerald et al., 2012). For example, CB1Rs enhance dopamine release in the striatum by disinhibiting midbrain GABA neurons that regulate dopaminergic neuronal firing (Lupica et al., 2004). In the striatum, CB1Rs are expressed by GABA medium spiny neurons (MSNs) of the direct and indirect pathways, which predominantly express dopamine D1 receptor (D1R)/dynorphin or dopamine D2 receptor (D2R)/enkephalin, respectively (Hohmann and Herkenham, 2000; Pickel et al., 2004). Activation of the D1R-expressing direct pathway promotes movement and drug reward, whereas the D2R-expressing indirect pathway generally inhibits these responses (Lobo and Nestler, 2011; Lenz and Lobo, 2013).
The stable transcription factor Δ FBJ murine osteosarcoma viral oncogene homolog B (ΔFosB) accumulates in striatal neurons during repeated administration of abused drugs, including THC (Perrotti et al., 2008; Lazenka et al., 2014b). Several drugs of abuse induce ΔFosB in D1R positive MSNs of the nucleus accumbens and dorsal striatum as shown using fluorescent reporter bacterial artificial chromosome transgenic mice (Lobo et al., 2013). Similarly, the D1R antagonist SCH23390 [(R)-(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride] blocked cocaine- (Nye et al., 1995) or morphine- (Muller and Unterwald, 2005) mediated induction of ΔFosB in the nucleus accumbens and caudate-putamen. Transgenic overexpression of ΔFosB in D1R-positive MSNs enhanced the rewarding effects of cocaine (Colby et al., 2003) or morphine (Zachariou et al., 2006a), demonstrating significant functional consequences of the modulation of ΔFosB expression in these neurons.
Dopamine signaling is regulated by the dopamine- and cAMP-regulated phosphoprotein Mr 32 kDa (DARPP-32), which is highly expressed in striatal MSNs and dopaminergic terminal fields (Greengard, 2001b; Nairn et al., 2004). DARPP-32 contains several phosphorylation sites that regulate its activity. D1R-stimulated protein kinase A (PKA) phosphorylates DARPP-32 at threonine 34 (T34), which inhibits protein phosphatase-1, thereby enhancing the effects of PKA (Greengard, 2001b; Nairn et al., 2004). In D2R-positive MSNs, phosphorylation of DARPP-32 at T34 is stimulated by adenosine A2AR and inhibited by D2R and N-methyl-d-aspartate (NMDA) receptors (Greengard, 2001b; Nairn et al., 2004). THC-mediated phosphorylation of T34 DARPP-32 is inhibited by administration of a D1R or A2AR antagonist (Borgkvist et al., 2008), suggesting that both MSN populations contribute to cannabinoid-dopamine interactions.
We recently reported that repeated THC-mediated ΔFosB induction in the striatum was abolished in mice lacking CB1Rs (Lazenka et al., 2014b). Moreover, CB1Rs were colocalized with ∆FosB in striatal neurons and also expressed in puncta surrounding FosB/ΔFosB positive neurons (Lazenka et al., 2014b). These observations are consistent with the idea that THC indirectly induces ΔFosB in striatal neurons. This action could occur via CB1R-mediated dopamine release (Oleson and Cheer, 2012), which would activate D1Rs and promote ΔFosB induction. The primary objective of our current study was to determine the role of D1Rs in repeated THC-mediated effects by assessing whether THC-mediated induction of ΔFosB is D1R-dependent and whether THC-induced ΔFosB is localized to D1R-positive striatal MSNs.
Because DARPP-32 plays an important integrative role in dopamine receptor signaling, we also tested its involvement in THC-mediated ΔFosB induction and motor behaviors, including hypomotility and catalepsy. To assess selectivity of DARPP-32 involvement in THC-induced motor behaviors, other common in vivo responses elicited by THC, including hypothermia and antinociception, were also examined. These four behavioral and physiological measures comprise the cannabinoid tetrad assay (Little et al., 1988).
We report that D1Rs play a necessary role and DARPP-32–mediated signaling plays a modulatory role in THC-mediated ΔFosB induction. Moreover, genetic deletion of DARPP-32 enhances THC-mediated hypolocomotion as well as development of tolerance to this response after repeated THC administration, suggesting involvement of DARPP-32–mediated signaling in the acute and chronic motor effects of THC.
Materials and Methods
THC and CP55,940 [(−)-cis-3-[2-hydroxy-4-(1,1-dimethylheptyl)phenyl]-trans-4-(3-hydroxypropyl)cyclohexanol] were provided by the Drug Supply Program of the National Institute on Drug Abuse (Rockville, MD). Guanosine 5′-O-(3-[35S]thio)triphosphate ([35S]GTPγS; 1250 Ci/mmol) was purchased from PerkinElmer Life Sciences (Boston, MA). Bovine serum albumin and guanosine diphosphate were purchased from Sigma-Aldrich (St. Louis, MO). Rabbit anti-FosB antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Goat anti-preprodynorphin antibody was purchased from Millipore (Billerica, MA). SCH23390 and SCH39166 [(6aS-trans)-11-chloro-6,6a,7,8,9,13-b-hexahydro-7-methyl-5H-benzo[d]naphth[2,1-b]azepi-n-12-ol hydrobromide] were purchased from Tocris Bioscience (Minneapolis, MN). Secondary antibodies were purchased from either LI-COR (Lincoln, NE) or Invitrogen (Grand Island, NY). ProLong Gold anti-fade reagent with DAPI (4ʹ,6-diamidino-2-phenylindole) was purchased from Invitrogen. All other reagent grade chemicals were obtained from Sigma-Aldrich (St. Louis MO) or Fisher Scientific (Pittsburgh PA).
Subjects and Drug Treatments.
Male ICR mice (25–30 g; Harlan Laboratories, Indianapolis, IN) were used to assess the effect of D1R antagonists on THC-mediated ∆FosB induction. The mice were housed four to six per cage and maintained on a 12-hour light/dark cycle in a temperature-controlled environment (20–22°C) with food and water available ad libitum. THC was dissolved in a solution consisting of ethanol, emulphor, and saline in a ratio of 1:1:18. SCH23390 and SCH39166 were dissolved in saline. Each day, mice (n = 7–8 per group) were given 1 mg/kg i.p. SCH23390 or SCH39166, and 30 minutes later they were given a subcutaneous injection of either THC (ramping doses of 10, 20, or 30 mg/kg increased every 2 days) or vehicle at 08:00 and 16:00 hours for 6 days. The injections were given in a volume of 10 ml/kg. On day 7, the mice received morning injections only, and they were sacrificed 24 hours later by decapitation. A separate group of mice (n = 4) was treated using the same protocol with THC or vehicle alone for immunostaining. This treatment paradigm was used because it is known to lead to a high level of ∆FosB in ICR mice over a 1-week period (data not shown).
DARPP-32 knockout (KO) mice on a C57BL/6J background and littermate controls (Hiroi et al., 1999) (n = 8 per group) were given a subcutaneous injection of either 10 mg/kg THC or vehicle at 08:00 and 16:00 hours for 13 days. On day 14, the mice received a single THC injection (08:00), and 24 hours later were assessed for THC-induced antinociception, hypothermia, catalepsy, and locomotor suppression (see Assessment of In Vivo Responses). At 24 hours after in vivo assessment, the mice were sacrificed by decapitation, and their brains were processed for immunoblotting experiments. A separate group of mice was treated with the same paradigm, and their brains were collected 24 hours after the final injection for agonist-stimulated [35S]GTPγS binding. This paradigm was used because it is known to lead to a high level of ∆FosB in the C57BL/6J mice (Lazenka et al., 2014a).
All experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals, 8th edition, as promulgated by U.S. National Institutes of Health and approved by the Institutional Animal Care and Use Committee at Virginia Commonwealth University.
Brain Dissections.
Brain regions were dissected as published elsewhere (Lazenka et al., 2014b) following Franklin and Paxinos (2008). The prefrontal cortex was dissected by collecting tissue rostral to the posterior extent of the anterior olfactory nucleus and removing the olfactory nuclei. A cut was then made anterior to the optic chiasm, and the nucleus accumbens and caudate-putamen were dissected from the resulting coronal section. The amygdala was dissected by cutting caudal to the optic chiasm and then making a second cut caudal to the median eminence. The amygdala sample was isolated using the optic tract as a medial border and the bifurcation of the corpus callosum as dorsal and lateral borders.
Immunoblot.
Each sample was individually homogenized in 20 mM HEPES buffer (pH 7.8) with 0.4 M NaCl, 20.0% glycerol, 5.0 mM MgCl2, 0.5 mM ethylenediaminetetraacetic acid, 0.1 mM ethylene glycol tetraacetic acid, and 1% NP-40 (EMSA buffer) containing 0.5 mM phenylmethanesulfonylfluoride, 10 µg/ml leupepsin, 100 µg/ml benazamide, 2 µg/ml aprotinin, 500 µM dithiothreitol, and Halt protease inhibitor cocktail. Cell lysates were centrifuged at 15,000g for 15 minutes, and the supernatant was transferred to a new centrifuge tube. Protein was quantified according to Bradford (1976), and the samples were diluted to obtain equal protein concentrations.
Samples were then mixed with 4× Laemmli buffer [40% sucrose, 500 mM Tris-HCl (pH 6.8), 0.2 M dithiothreitol, 8% SDS, and 0.04% bromophenol blue] and boiled for 5 minutes before being loaded in 10% Tris-HCl gels with molecular weight markers (Precision Plus Protein Kaleidoscope marker; Bio-Rad Laboratories, Hercules, CA) and separated by electrophoresis. Electrophoresis was conducted at 80 V for 30 minutes through the stacking gel and 120 V for 1.5 hours through the separating gel.
Proteins were transferred electrophoretically to reinforced nitrocellulose at 120 V for 1 hour, blocked in 0.1 M Tris buffer with 0.9% saline (TBS) with 5% Carnation instant nonfat dry milk for 1 hour, and incubated overnight (4°C) in antibodies against FosB (1:1000) and α-tubulin loading control (1:20,000) in 0.1 M TBS containing 0.1% Tween-20 (TBST) with 5% nonfat dry milk. Blots were washed 3 × 10 minutes in TBST and incubated with Alexa 680 goat anti-rabbit IgG (1:12,000) and Alexa 800 goat anti-mouse IgG (1:12,000) in TBST for 45 minutes. Blots were then washed 3 × 10 minutes in TBST and stored in 0.1 M phosphate buffer (pH 7.4) with 0.9% NaCl (phosphate-buffered saline [PBS]). Fluorescent intensity was visualized using the Odyssey LI-COR infrared scanner. LI-COR software version 2.1 was used to measure the integrated intensity between treatments for the band of interest, with subtraction of the background (average of intensities three border widths above and below the band). For each sample, the integrated intensity value for ΔFosB was normalized to α-tubulin in the same sample by calculating the optical density ratio of ΔFosB immunoreactivity/α-tubulin immunoreactivity, and all sample values were averaged by group and expressed as the percentage of vehicle control.
Immunohistochemistry.
Preprodynorphin was used as a marker for D1R/dynorphin MSNs to determine the localization of FosB/ΔFosB after repeated THC administration. Slide-mounted sections (20 µm) containing the caudate-putamen and nucleus accumbens were washed in PBS for 5 minutes and fixed with 4% paraformaldehyde (30 minutes) dissolved in 0.05 M PBS. Slides were washed 3 × 5 minutes in PBS and incubated in PBS containing 1% Triton X-100 for 15 minutes. Slides were then washed 3 × 5 minutes in PBS and incubated in PBS containing 5% normal goat serum for 1 hour. Slides were incubated overnight at 4°C in PBS containing 2.5% normal donkey serum and antibodies against preprodynorphin (1:500; guinea pig) and FosB/ΔFosB (1:500). Images were captured at 40× magnification on a Zeiss 700 laser scanning confocal microscope (Carl Zeiss, Thornwood, NY) using the ZEN 2011 software.
To determine the percentage of cells in which proteins were colocalized, the number of cells that were positive for DAPI was first counted. Then, the number of cells positive for FosB/ΔFosB-ir + dynorphin-ir or FosB/ΔFosB-ir alone was counted by overlaying the two images. We counted 40–50 cells per image and averaged based on three images per animal (n = 4 animals per treatment group) in the same field using consistent stereotaxic coordinates for caudate-putamen and nucleus accumbens.
Assessment of In Vivo Responses.
Mice (n = 8 per group) were assessed in the tetrad assay, which consists of the following four end points: locomotor activity, immobility, tail withdrawal latency, and rectal temperature (Little et al., 1988), as we have published elsewhere (Long et al., 2009). After baseline assessment of each measure, separate groups of mice were given an intraperitoneal injection of 70 mg of THC, which was the ED84 dose determined in pilot studies (unpublished data), or vehicle. Catalepsy was determined in the bar test, antinociception was evaluated in the warm-water tail immersion test at 52.0°C, and body temperature was measured by inserting a thermocouple probe 1.2 cm into the rectum. For locomotor activity, each mouse was placed in a clear Plexiglas box (42.7 × 21.0 × 20.4 cm) for a 5-minute assessment period, and Anymaze software (Stoelting, Wood Dale, IL) was used to determine the duration of time spent immobile. Mice were tested in separate chambers for baseline and THC trials to avoid habituation. Because locomotor activity undergoes habituation with repeated sessions, this measure was assessed only once at 20 minutes after injection. Catalepsy, antinociception, and hypothermia were assessed at 30, 60, 120, and 180 minutes after injection, based on the published time course for these cannabinoid-mediated effects (Andersson et al., 2005).
Agonist-Stimulated [35S]GTPγS Binding.
Agonist-stimulated [35S]GTPγS binding was conducted as published (Sim et al., 1995) with minor modification. Briefly, tissues were placed in 5 ml of cold membrane buffer (50 mM Tris-HCl, 3 mM MgCl2, 1 mM EGTA, pH 7.4) and homogenized. Samples were centrifuged at 50,000g for 10 minutes, the supernatant was removed, and the samples were resuspended in assay buffer (100 mM NaCl, 3 mM MgCl2, 0.2 mM EGTA, 50 mM Tris-HCl, pH 7.4). Membranes (4–8 µg of protein) were incubated with adenosine deaminase (4 mU/ml) in assay buffer at 30°C for 10 minutes. Concentration-effect curves were generated by incubating membranes in assay buffer with varying concentrations of CP55,940 in the presence of 30 µM guanosine diphosphate, 0.1 nM [35S]GTPγS, and 0.1% bovine serum albumin at 30°C for 2 hours. Basal binding was measured in the absence of agonist, and nonspecific binding was measured with 20 µM unlabeled GTPγS. The reaction was terminated by vacuum filtration though grade GF/B glass fiber filters, followed by three washes with cold Tris buffer (50 mM Tris-HCl, pH 7.4). Bound radioactivity was determined by liquid scintillation spectrophotometry at 95% efficiency after overnight extraction in Econo-Safe scintillation fluid (Research Products International, Mount Prospect, IL). Data are reported as mean ± S.E.M. of at least six experiments, each performed in triplicate. Nonspecific binding was subtracted from each sample. Net stimulated [35S]GTPγS binding was defined as agonist-stimulated minus basal [35S]GTPγS binding, and percentage of stimulation was defined as (Net-stimulated/Basal [35S]GTPγS binding) × 100%.
Statistical Analysis.
For immunoblot experiments, data were analyzed with Prism version 6 (GraphPad Software, San Diego, CA) Three-way analysis of variance (ANOVA) for in vivo studies was performed using SPSS statistics version 22 (IBM, Armonk, NY). Two-way ANOVA with Bonferroni post-hoc test was used for comparisons of ΔFosB expression in D1R antagonist studies, ΔFosB expression in DARPP-32 KO mice, colocalization experiments, and comparisons of catalepsy and locomotor activity. For comparisons in the development of tolerance to hypothermia and antinociception, a mixed three-way ANOVA was used followed by a two-way ANOVA comparing any significant interactions followed by the Bonferroni post-hoc test. P < 0.05 was considered statistically significant.
For agonist-stimulated [35S]GTPγS binding, nonlinear iterative regression analyses of agonist concentration-effect curves were performed with Prism 5.0 (GraphPad Software) to derive Emax and EC50 values. Statistically significant differences in these values between groups were determined by two-way ANOVA with planned comparisons between vehicle- and THC-treated mice performed within each genotype using the Bonferroni post-hoc test.
Results
D1R Antagonism Inhibits ΔFosB Induction by Repeated THC Administration.
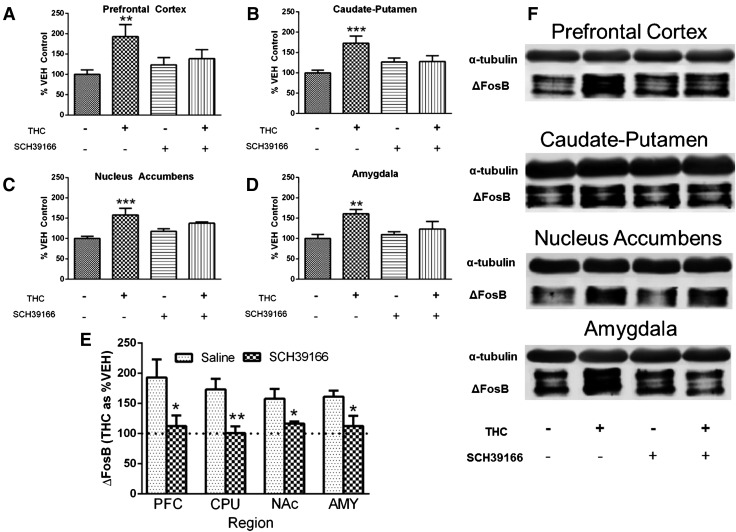
To determine whether D1R activation is required for ΔFosB induction by repeated THC administration, mice injected or not with D1R antagonist (SCH39166) were treated twice daily for 6 days with ramping doses of THC. SCH39166 or vehicle (saline) was administered 30 minutes before each THC or vehicle (1:1:18 ethanol/emulphor/saline) injection, which comprised four separate groups of mice treated as follows: saline/vehicle, saline/THC, D1R antagonist/vehicle, and D1R antagonist/THC. Repeated treatment with saline/THC induced ΔFosB expression as compared with saline/vehicle treatment whereas the combination of D1R antagonist/THC did not significantly increase ΔFosB expression compared with D1R antagonist/vehicle, based on planned comparisons in each region (Fig. 1).
Fig. 1.
SCH39166 inhibits ΔFosB induction by repeated THC treatment. Pretreatment with the D1R antagonist SCH39166 inhibited induction of ΔFosB by repeated THC treatment in the prefrontal cortex (A), caudate-putamen (B), nucleus accumbens (C), and amygdala (D). ΔFosB-ir is expressed as mean percentage of saline/vehicle-treated mice ± S.E.M. (n = 7–8 per group). (E) Comparisons of induction of ΔFosB by THC expressed as mean percentage of vehicle ± S.E.M. in saline- and SCH39166-pretreated mice. (F) Representative immunoblots. **P < 0.01, ***P < 0.001 compared with saline/vehicle mice (A–D); *P < 0.05 and **P < 0.01 compared with saline by Student’s t test (E). AMY, amygdala; CPU, caudate-putamen; NAc, nucleus accumbens; PFC, prefrontal cortex.
Repeated saline/THC treatment significantly increased ΔFosB expression by 93 ± 30% in the prefrontal cortex (significant main effect of THC treatment: F1,27 = 6.403, P < 0.05; Fig. 1A), 73 ± 17% in the caudate-putamen (significant interaction of THC treatment by D1R antagonist pretreatment: F1,27 = 7.98, P < 0.01; Fig. 1B), 58 ± 16% in the nucleus accumbens (significant main effect of THC treatment: F1,27 = 16.39, P < 0.001; Fig. 1C), and 61 ± 11% in the amygdala (significant main effect of THC treatment: F1,27 = 9.98, P < 0.01; Fig. 1D) compared with saline/vehicle treatment. The level of ΔFosB-ir did not differ between SCH39166/vehicle and SCH39166/THC treatment groups in any region examined.
These results suggest that THC did not increase ΔFosB expression in the presence of a D1R antagonist in these forebrain regions. To isolate the effect of THC itself in the SCH39166-treated groups, data were then normalized to the respective vehicle condition in both the saline- and SCH39166-treated mice. This normalization of the data, which controls for slight variations in ΔFosB expression in the presence of the antagonist alone, is shown in Fig. 1E. Comparison between saline/THC and D1R antagonist/THC groups using this calculation of the data shows that SCH39166 significantly attenuated the effect of THC on ΔFosB expression in every region examined.
To confirm these findings, we determined the effects of pretreatment with SCH23390, a more widely studied but less selective D1R antagonist. Repeated administration of THC significantly increased ΔFosB expression in the prefrontal cortex, caudate-putamen, nucleus accumbens, and amygdala of saline-pretreated mice but did not significantly affect ΔFosB expression after pretreatment with SCH23390 in the prefrontal cortex and caudate-putamen or amygdala (Supplemental Fig. 1). However, significant ΔFosB induction was observed in the nucleus accumbens of SCH23390-treated mice in both the THC- and vehicle-injected groups, indicating that SCH23390 alone increased ΔFosB expression in this region at the 1 mg/kg dose tested (Supplemental Fig. 1C).
When the data were normalized to control for the effects SCH23390 alone as described here, there was a statistically significant difference between the saline/THC and D1R antagonist/THC groups in every region examined except the amygdala (Supplemental Fig. 1E). Altogether, these results indicate a major role for D1R activation in the induction of ΔFosB expression in the forebrain by repeated THC administration.
FosB/ΔFosB-ir Nuclei Colocalize with Preprodynorphin-ir in Striatal Cells.
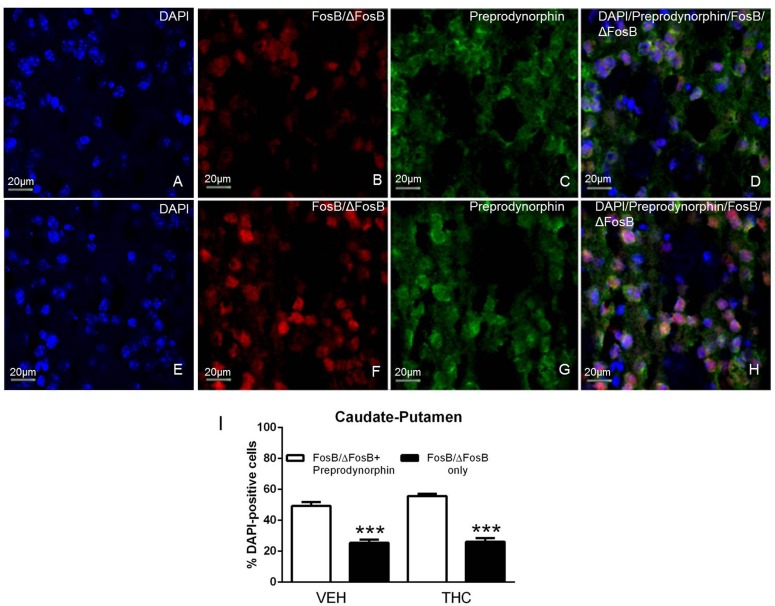
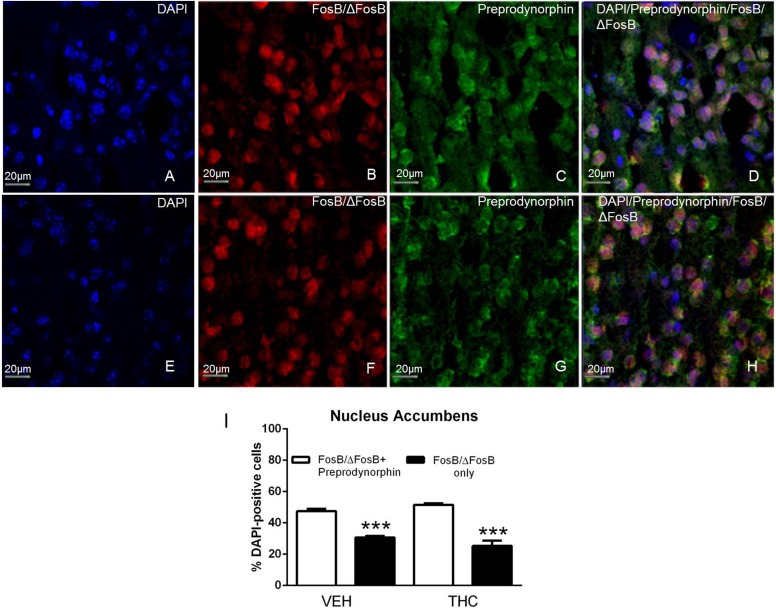
The finding that D1R antagonists inhibited THC-mediated ΔFosB induction indicates that ΔFosB is present in D1R-expressing MSNs after repeated THC administration. To determine localization of ΔFosB in D1R-expressing MSNs, we performed dual immunohistochemistry using antibodies that recognize FosB/ΔFosB and preprodynorphin, which colocalizes with D1R in MSNs (Gerfen et al., 1990). DAPI was used to identify cell nuclei using the blue channel, FosB/ΔFosB-ir was visualized using the red channel, and preprodynorphin-ir was visualized using the green channel (Figs. 2 and 3). FosB/ΔFosB-ir appeared to be localized in cell nuclei, which was confirmed by DAPI staining (Figs. 2, A and E, and 3, A and E). FosB/ΔFosB-ir cell nuclei were seen in the caudate-putamen (Fig. 2, B and F) and nucleus accumbens (Fig. 3, B and F) in brains from both vehicle- and THC-treated mice. Preprodynorphin-ir was observed in the soma of cells in the caudate-putamen (Fig. 2, C and G) and nucleus accumbens (Fig. 3, C and G). The three images were overlaid to show the localization of DAPI, FosB/ΔFosB-ir, and preprodynorphin-ir in the caudate-putamen (Fig. 2, D and H) and nucleus accumbens (Fig. 3, D and H).
Fig. 2.
FosB/ΔFosB colocalizes with preprodynorphin in the caudate-putamen. Representative images (magnification, 40×) showing DAPI (A and E), FosB/ΔFosB-ir (B and F), and preprodynorphin-ir (C and G) in the caudate-putamen of mice that received repeated vehicle (top row) or THC (bottom row) treatment. FosB/ΔFosB-ir was localized to the nucleus, as demonstrated with DAPI staining, and preprodynorphin-ir was localized to the cell body. Merged images show colocalization of FosB/ΔFosB-ir (red), preprodynorphin-ir (green), and DAPI (blue) (D and H). Cell counts showed that FosB/ΔFosB-ir cells that were also positive for preprodynorphin-ir (white bars) were in the majority relative to cells positive for FosB/ΔFosB-ir only (black bars) in both vehicle- and THC-treated mice (I). Data are mean counts ± S.E.M. (n = 4 per group). ***P < 0.001 compared with FosB/ΔFosB-ir only cells. VEH, vehicle.
Fig. 3.
FosB/ΔFosB colocalizes with preprodynorphin in the nucleus accumbens. Representative images (magnification, 40×) showing DAPI (A and E), FosB/ΔFosB-ir (B and F), and preprodynorphin-ir (C and G) in the nucleus accumbens of mice that received repeated vehicle (top row) or THC (bottom row) treatment. FosB/ΔFosB-ir was localized to the nucleus, as demonstrated with DAPI staining, and preprodynorphin-ir was localized to the cell body. Merged images show colocalization of FosB/ΔFosB-ir (red), preprodynorphin-ir (green), and DAPI (blue) (D and H). Cell counts showed that FosB/ΔFosB-ir cells that were also positive for preprodynorphin-ir (white bars) were in the majority relative to cells positive for FosB/ΔFosB-ir only (black bars) in both vehicle- and THC-treated mice (I). Data are mean counts ± S.E.M. (n = 4 per group). ***P < 0.001 compared with FosB/ΔFosB-ir only cells. VEH, vehicle.
The number of DAPI-positive nuclei was first counted to determine the total number of striatal neurons. Images of FosB/ΔFosB-ir and preprodynorphin-ir were then merged, and the number of dual labeled FosB/ΔFosB-ir + preprodynorphin-ir cells were counted. Cell counts revealed that approximately half of DAPI-positive cells contained FosB/ΔFosB-ir + preprodynorphin-ir in the caudate-putamen (49 ± 3%, Fig. 2I) and nucleus accumbens (47 ± 2%, Fig. 3I) of repeated vehicle-treated mice. After repeated THC administration, the percentage of DAPI positive cells that contained FosB/ΔFosB-ir + preprodynorphin-ir was 55 ± 2% in caudate-putamen (Fig. 2I) and 52 ± 1% in nucleus accumbens (Fig. 3I).
These results support the idea that FosB/ΔFosB-ir is expressed in D1R/dynorphin-positive neurons in the caudate-putamen and nucleus accumbens and that this expression pattern is not altered after repeated THC administration. Two-way ANOVA comparing colocalization and treatment determined that there was a significant main effect of colocalization (F1,12 = 150.60, P < 0.001) in caudate-putamen and a statistically significant interaction (F1,12 = 7.67, P < 0.05) and main effect of colocalization (F1,12 = 178.00, P < 0.001) in nucleus accumbens. Bonferroni post-hoc tests show that the number of FosB/ΔFosB-ir + preprodynorphin-ir cells was significantly greater than FosB/ΔFosB-ir only cells in both regions after both saline and THC administration.
DARPP-32 Modulates Induction of ΔFosB in the Striatum after Acute THC Administration.
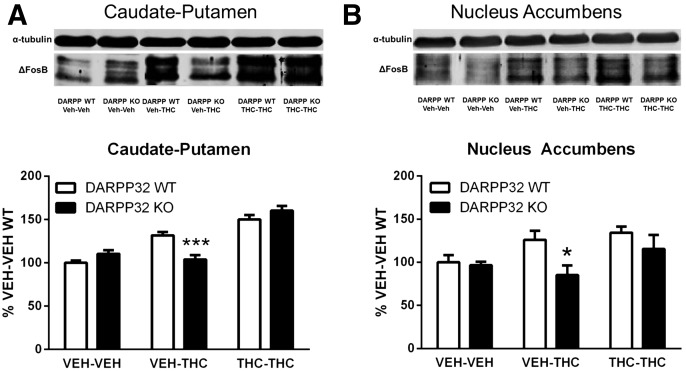
The inhibitory effects of D1R antagonists on THC-induced ΔFosB expression and colocalization of ΔFosB with preprodynorphin in the striatum of mice treated repeatedly with THC indicates a role for the D1R signaling pathway in the induction of striatal ΔFosB expression by THC. DARPP-32 acts within this pathway to integrate converging signals with the net effect of amplifying D1R-mediated cAMP signaling (Greengard, 2001a; Nairn et al., 2004). Therefore, to determine whether DARPP-32 is involved in acute or repeated THC-induced ΔFosB expression, DARPP-32 KO mice were injected repeatedly with THC or vehicle for 13 days; on day 14, mice in each group received an acute injection of THC or vehicle and were tested as described here.
This experimental design generated three groups of mice of each genotype: repeated vehicle + acute vehicle (vehicle), repeated vehicle + acute THC (acute THC), and repeated THC + acute THC (repeated THC). Based on planned comparisons between genotypes, there was a statistically significant effect of acute THC administration (70 mg/kg) on ΔFosB expression in both the caudate-putamen (P < 0.001) and nucleus accumbens (P < 0.05).
In caudate-putamen, two-way ANOVA revealed a significant interaction of treatment by genotype (F2,36 = 12.40, P < 0.001) (Fig. 4A). In wild-type (WT) mice, acute THC administration increased ΔFosB expression by 32 ± 4% relative to vehicle-treated WT mice, whereas there was no change in ΔFosB expression after acute THC administration in DARPP-32 KO mice (Fig. 4A).
Fig. 4.
Effect of genetic deletion of DARPP-32 on ΔFosB expression in the striatum of mice treated acutely or repeatedly with THC. Genetic deletion of DARPP-32 attenuated ΔFosB expression measured after acute THC (VEH-THC) but not repeated THC (THC-THC) or vehicle (VEH-VEH) administration in the caudate-putamen (A) and nucleus accumbens (B). Representative immunoblots are shown above each graph. Data are expressed as mean percentage of VEH-VEH–treated WT mice ± S.E.M. (n = 7 per group). *P < 0.05 and ***P < 0.001 compared with WT control within the same treatment condition.
In the nucleus accumbens, there was a significant main effect of both treatment (F2,36 = 13.71 P < 0.0001) and genotype (F1,36 = 12.04, P < 0.05), but no significant interaction (Fig. 4B). After acute THC treatment, ΔFosB-ir increased 26 ± 10% in WT mice but decreased 15 ± 11% in DARPP-32 KO mice compared with vehicle-vehicle treated controls such that there was a statistically significant genotype difference after acute THC administration (P < 0.05; Fig. 4B). In contrast, there were no statistically significant genotype differences in either region after repeated THC administration.
In summary, these results indicate that deletion of DARPP-32 blocked ΔFosB induction by acute THC administration in the caudate-putamen and nucleus accumbens, but it did not significantly alter ΔFosB expression in either region after repeated THC administration.
DARPP-32 KO Mice Exhibit Enhanced THC-Mediated Locomotor Suppression and Enhanced Tolerance to This Effect after Repeated THC Administration.
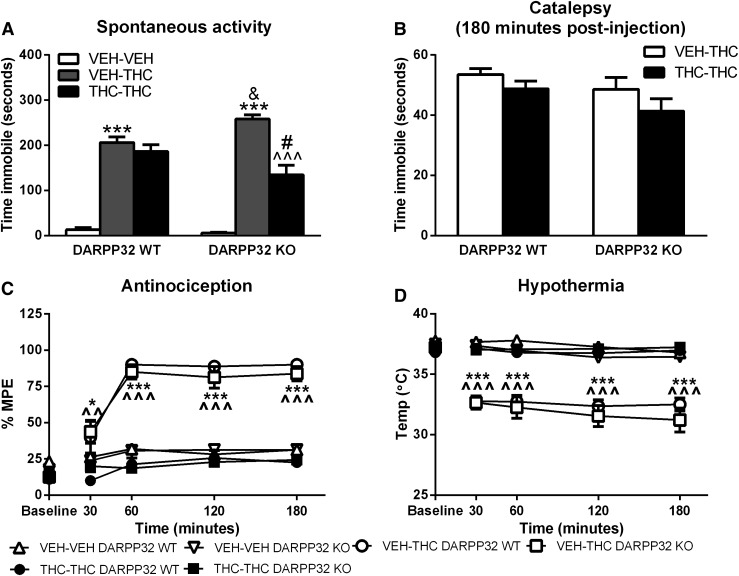
The effect of genetic ablation of DARPP-32 on striatal ΔFosB induction by acute but not repeated THC suggests that this regulatory protein might modulate the acute behavioral effects of THC. The effects of acute and repeated THC administration on locomotor activity, bar immobility, rectal temperature, and warm-water tail withdrawal were therefore assessed in DARPP-32 KO mice and WT littermates using the same treatment groups as described for ΔFosB immunoblots.
For spontaneous activity, there was a statistically significant genotype by treatment interaction (F2,42 = 8.502, P < 0.001). Mice spent very little time immobile after receiving acute vehicle treatment, and there was no significant difference between WT and DARPP-32 KO mice (Fig. 5A). After acute THC administration, mice spent more time immobile compared with acute vehicle-treated mice regardless of genotype (P < 0.001; Fig. 5A); however, DARPP-32 KO mice exhibited significantly greater THC-induced immobility compared with WT mice (P < 0.05; Fig. 5A). In WT mice, THC-mediated locomotor suppression did not differ between repeated vehicle-treated versus repeated THC-treated mice (Fig. 5A). However, significantly less THC-mediated locomotor suppression was measured in DARPP-32 KO mice treated repeatedly with THC compared with their respective vehicle-treated controls (P < 0.001; Fig. 5A). This result suggests that tolerance developed to THC-mediated locomotor suppression in DARPP-32 KO but not in WT mice.
Fig. 5.
THC-mediated in vivo responses in repeated vehicle- and THC-treated WT and DARPP-32 KO mice: locomotor suppression (A), catalepsy (B), antinociception (C), and hypothermia (D). DARPP-32 KO mice exhibited greater acute THC-mediated locomotor suppression and enhanced tolerance to this response after repeated THC administration. Measures are expressed as mean ± S.E.M. (n = 8 per group), and statistical analysis was conducted as described in Materials and Methods. *P < 0.05, ***P < 0.001 compared with their respective genotype for repeated vehicle-treated mice; ^^P < 0.01, ^^^P < 0.001 compared with their respective genotype for repeated THC-treated mice; &P < 0.05 compared with repeated vehicle-treated WT mice; #P < 0.05 compared with repeated THC-treated WT mice. VEH, vehicle.
Catalepsy, measured as immobility in the bar test, was assessed at 180 minutes after injection because mice also exhibited hyperreflexia at earlier time points (unpublished data). Mice that received only vehicle treatments did not exhibit catalepsy. There was no significant difference in immobility between DARPP-32 KO and WT mice based on two-way ANOVA (Fig. 5B). THC-mediated bar immobility did not differ between repeated vehicle- versus THC-treated mice for either genotype.
THC-mediated antinociception did not differ between WT and DARPP-32 KO mice across the 3-hour test duration (Fig. 5C). For antinociception, there was a significant interaction of time by treatment (F8,164 = 78.00, P < 0.001) but no significant interactions with regard to genotype. Based on the results of the three-way ANOVA, a two-way ANOVA was performed to test the effect of treatment by time. A significant interaction of treatment by time was found (F20,164 = 17.96, P < 0.001). The baseline measures were not different between any groups. Acute THC administration produced a similar magnitude of antinociception in mice of both genotypes. THC-mediated antinociception was attenuated in repeated THC-treated mice, and it was similar in magnitude to mice that received vehicle-vehicle treatment. THC-mediated antinociception was significantly reduced in repeated THC- compared with repeated vehicle-treated mice at all time points in mice of both genotypes (Fig. 5C).
THC-mediated hypothermia was similar in magnitude in WT and DARPP-32 KO mice throughout the testing period (Fig. 5D). For hypothermia, there was a significant interaction of time by treatment (F8,164 = 48.74, P < 0.001) but no interactions with genotype. Two-way ANOVA confirmed a significant interaction of time by genotype (F20,164 = 20.71, P < 0.001). Less hypothermia was measured in repeated THC-treated compared with repeated vehicle-treated mice of both genotypes (Fig. 5D).
These results indicate that deletion of DARPP-32 did not affect THC-mediated catalepsy, antinociception, or hypothermia, or the development of tolerance to THC-mediated antinociception or hypothermia after repeated THC treatment.
Deletion of DARPP-32 Does Not Affect CB1R Desensitization in the Striatum.
Cannabinoid-mediated G-protein activity was assessed in the striatum of WT and DARPP-32 KO mice after repeated THC or vehicle treatment to determine whether enhanced THC-mediated locomotor activity and tolerance were associated with altered CB1R-mediated G-protein activity and/or desensitization. Basal [35S]GTPγS binding in the absence of agonist in both nucleus accumbens and caudate-putamen appeared to be highest in THC-treated DARPP-32 KO mice (data not shown). Two-way ANOVA revealed a main effect of genotype on basal [35S]GTPγS binding in nucleus accumbens (F1,16 = 5.63, P = 0.030) and caudate-putamen (F1,20 = 5.08, P = 0.036). However, there was no significant main effect of THC on basal [35S]GTPγS binding, nor was there an interaction between genotype and treatment in either region.
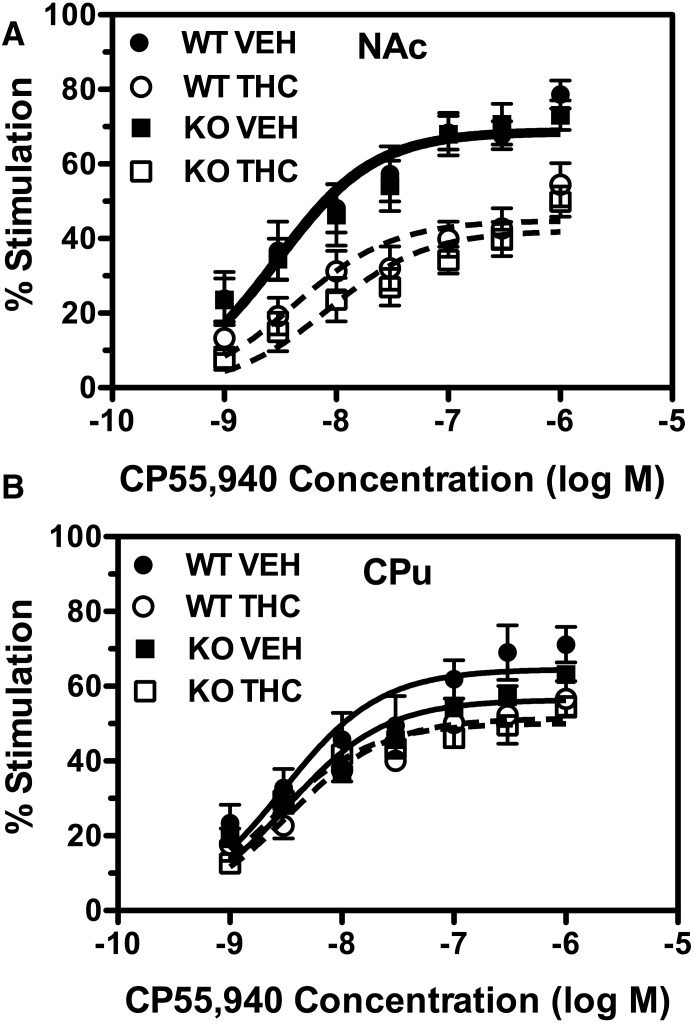
CP55,940-stimulated [35S]GTPγS binding was then compared between WT and DARPP-32 KO mice (Fig. 6). Decreased CP55,940-stimulated G-protein activation was evident in the nucleus accumbens after repeated THC treatment, with only a modest apparent effect of THC in the caudate-putamen. Emax and EC50 values for CP55,940-stimulated [35S]GTPγS binding were calculated from concentration-effect curves (Table 1). CP55,940-stimulated [35S]GTPγS binding did not significantly differ between WT and DARPP-32 KO mice in the caudate-putamen or nucleus accumbens because there was no main effect of genotype on CP55,940 Emax or EC50 values in either region by two-way ANOVA.
Fig. 6.
Deletion of DARPP-32 does not affect CB1R-mediated G-protein activation or desensitization after repeated THC administration. Data are mean percentage of stimulation of [35S]GTPγS binding by varying concentrations of CP55,940 ± S.E.M. (n = 5 per group) in membranes prepared from nucleus accumbens (NAc) (A) or caudate-putamen (CPu) (B). VEH, vehicle.
TABLE 1.
Emax and EC50 values of CP55,940-stimulated [35S]GTPγS binding
Data are mean Emax and EC50 values ± S.E.M. (n = 5), derived from nonlinear regression analysis of the CP55,940 concentration-effect curves shown in Fig. 6.
| DARPP-32 WT |
DARPP-32 KO |
|||
|---|---|---|---|---|
| Emax | EC50 | Emax | EC50 | |
| %Stim. | nM | %Stim. | nM | |
| Nucleus accumbens | ||||
| Vehicle | 72.7 ± 3.1 | 5.8 ± 3.7 | 71.5 ± 5.6 | 5.0 ± 1.5 |
| THC | 45.6 ± 4.4a | 8.1 ± 3.6 | 41.0 ± 3.3a | 15.6 ± 8.7 |
| Caudate-putamen | ||||
| Vehicle | 66.4 ± 3.9 | 5.5 ± 3.1 | 63.3 ± 5.1 | 5.4 ± 3.8 |
| THC | 49.3 ± 3.3 | 3.7 ± 1.5 | 51.1 ± 8.2 | 2.9 ± 0.8 |
P < 0.001 different from vehicle-treated mice of the same genotype.
The effect of repeated THC-treatment on CP55,940-stimulated [35S]GTPγS binding was then assessed in WT and DARPP-32 KO mice. A main effect of THC on CP55,940 Emax values was found in the nucleus accumbens (F1,16 = 46.61, P < 0.0001), but there was no main effect of genotype, nor was there a significant interaction between genotype and treatment, indicating that THC treatment produced similar CB1R desensitization in mice of both genotypes. Indeed, planned comparison by Bonferroni post-hoc test showed a significant decrease in CP55,940 Emax values in THC-treated relative to vehicle-treated mice in both WT and DARPP-32 KO mice. In contrast, there were no significant differences in CP55,940 EC50 values between genotypes or treatment groups. In the caudate-putamen, a main effect of repeated THC was found (F1,19 = 6.66, P = 0.018), but there was no main effect of genotype, nor was there an interaction between genotype and treatment. However, planned comparisons by Bonferroni post-hoc test showed no significant effect of THC on CP55,940 Emax values in either WT or DARPP-32 KO mice. Likewise, CP55,940 EC50 values were unaffected by either treatment or genotype in this region.
These data suggest that repeated THC treatment induced greater CB1R desensitization in the nucleus accumbens relative to the caudate-putamen, but genetic deletion of DARPP-32 did not affect desensitization of CB1R-mediated G-protein activation by repeated THC treatment in either region.
Discussion
This study showed that D1Rs play a major role in CB1R-mediated ΔFosB induction in the forebrain because treatment with THC in the presence of D1R antagonists did not significantly increase ΔFosB expression above levels produced by the D1R antagonist alone. Previous studies had shown that D1R antagonism or genetic deletion attenuated morphine- or cocaine-mediated induction of ΔFosB and Fos proteins in the striatum (Nye et al., 1995; Zhang et al., 2002; Muller and Unterwald, 2005). These results indicate that THC and other abused drugs share a common mechanism of ΔFosB induction by D1R activation in the striatum, which probably involves dopamine because THC and other drugs increase dopamine release (Di Chiara and Imperato, 1988; Tanda et al., 1997).
We also reported that THC induced ΔFosB in the prefrontal cortex and amygdala (Perrotti et al., 2008; Lazenka et al., 2014b), and we now show that ΔFosB induction involves D1Rs. Morphine-mediated induction of ΔFosB in the frontal cortex was not affected by the D1R antagonist SCH23390 (Muller and Unterwald, 2005). Thus, although D1Rs are a common mechanism for ΔFosB induction by abused drugs in the striatum, the mechanisms underlying this response might be drug-specific in other regions. CB1R-mediated dopamine release is a likely mechanism for D1R-dependent ΔFosB induction in cortex and amygdala because cannabinoids enhance the firing of meso-prefrontal dopamine neurons (Diana et al., 1998) and enhance dopamine in the prefrontal cortex (Pistis et al., 2002). Midbrain dopamine neurons also project to the basolateral amygdala (Fattore et al., 2010; Robison and Nestler, 2011). Thus, CB1R-mediated dopamine release could be a common mechanism for THC-induced ΔFosB in the prefrontal cortex, amygdala, and striatum.
Our present study used both SCH39166 and SCH23390 to assess the role of D1Rs. SCH39166 is >275-fold and >80-fold more selective for D1Rs over dopamine D2Rs and serotonin type-2 receptors (5-HT2Rs), respectively, whereas SCH23390 is only ∼30-fold more selective for D1Rs than 5-HT2Rs (Chipkin et al., 1988). Nonetheless, both antagonists inhibited THC-stimulated ΔFosB expression, although SCH23390 also induced ΔFosB in the nucleus accumbens, possibly due to 5-HT2R activity. Although D1R activation was required for maximal ΔFosB induction by repeated THC, these findings do not rule out a minor contribution of D1R-independent mechanisms. For instance, we previously reported that a small fraction of THC-induced ΔFosB is also coexpressed with CB1Rs in the striatum (Lazenka et al., 2014b), suggesting that ΔFosB induction could be a cell-autonomous downstream consequence of CB1R activation in some cells.
CB1Rs are located on both D1R/dynorphin and D2R/enkephalin MSNs in the striatum (Hohmann and Herkenham, 2000), but pharmacological and anatomical results showed that THC-induced ΔFosB was primarily expressed in D1R/dynorphin striatal MSNs. Consistent with this finding, a recent study showed that several abused drugs, including THC, induced ΔFosB in D1R MSNs of the caudate-putamen and nucleus accumbens (Lobo et al., 2013). Transgenic overexpression of ΔFosB in D1R MSNs enhanced the rewarding properties of other abused drugs in conditioned place preference (CPP) (McClung et al., 2004). However, cannabinoids have been reported to produce both CPP and conditioned place aversion (Valjent and Maldonado, 2000). Similarly, cannabinoids have been reported to both facilitate (Gardner et al., 1988) and depress (Wiebelhaus et al., 2015) intracranial self-stimulation. Thus, the effects of cannabinoids on measures of reward vary depending on study parameters, which complicates attempts to determine the effect of ∆FosB on THC-mediated reward. However, prior THC exposure could enhance the effects of other rewarding drugs by inducing ΔFosB. For example, repeated THC treatment produced cross-sensitization to opioids and amphetamine (Cadoni et al., 2001; Lamarque et al., 2001) and enhanced nicotine self-administration (Panlilio et al., 2013).
This study also examined the role of DARPP-32 in ∆FosB induction by THC in the striatum. Previous studies showed that cannabinoids promote DARPP-32 phosphorylation at T34 in D1R and D2R MSNs (Andersson et al., 2005; Borgkvist et al., 2008). D1Rs stimulate PKA-mediated DARPP-32 phosphorylation at T34, which inhibits protein phosphatase 1 and prolongs PKA-mediated phosphorylation (Greengard, 2001b; Nairn et al., 2004). D2R-containing MSNs express adenosine A2A receptors, which stimulate PKA and DARPP-32 phosphorylation at T34, thereby enhancing PKA activity (Greengard, 2001b; Nairn et al., 2004). Our results suggest that THC-induced dopamine release activates D1Rs, which induces ∆FosB via a mechanism that depends in part on DARPP-32–mediated signaling. Genetic deletion or point mutation of DARPP-32 at T34 also attenuated cocaine-mediated ΔFosB induction in the striatum and nucleus accumbens (Hiroi et al., 1999; Zachariou et al., 2006b). Genetic deletion of DARPP-32 attenuated acute THC-induced expression of ΔFosB in caudate-putamen and nucleus accumbens, but had no significant effect in either region following repeated THC administration.
ΔFosB induction is generally associated with repeated drug exposure because induction is small in magnitude after a single drug dose, but ΔFosB is stable and accumulates with repeated administration (McClung et al., 2004). However, previous studies also showed induction of ΔFosB mRNA or protein after initial drug administration (Chen et al., 1998; Chocyk et al., 2006; Nestler, 2008). In the current study, mice received a maximally effective dose of THC, which was sufficient to induce ΔFosB acutely. Our results in both nucleus accumbens and caudate-putamen suggest that activation of DARPP-32 plays a role in acute induction of ΔFosB by THC but is not necessary for induction after repeated THC administration. Assuming that DARPP-32 acts as an amplifier of D1R signaling and ΔFosB levels accumulate over time with each THC injection, it is perhaps not surprising that amplification of the D1R signal by DARPP-32 plays a more critical role in ΔFosB induction by acute than repeated THC administration.
The effects of ΔFosB induction have typically been assessed after long-term (weeks) expression. However, a recent study reported that short-term (3 to 4 days) virally mediated expression of ΔFosB targeted to D1R MSNs in the nucleus accumbens decreased the excitatory strength of glutamatergic synapses, increased immature synaptic spines, and enhanced cocaine-induced CPP and locomotor sensitization (Grueter et al., 2013). Regulation of target genes by ΔFosB might also differ between short-term and long-term expression. Transgenic overexpression of ΔFosB for 1 to 8 weeks revealed differential regulation between the 1- and 8-week time points (McClung and Nestler, 2003). These findings suggest that acute THC-induced ΔFosB expression might have different functional consequences from those of repeated administration.
The in vivo effects of THC were also assessed in DARPP-32 KO mice. THC-induced locomotor suppression was enhanced in DARPP-32 KO relative to WT mice. The D1R/direct pathway is generally associated with initiation of movement and motor activation whereas the D2R/indirect pathway is associated with motor suppression and catalepsy (Lenz and Lobo, 2013). However, CB1Rs are localized postsynaptically on D1R/dynorphin and D2R/enkephalin MSNs, and presynaptically on glutamatergic corticostriate synapses (Ferre et al., 2010), suggesting more complex regulation of motor activity. Ferre et al. (2010) proposed that postsynaptic CB1Rs on both D1R and D2R MSNs mediate catalepsy whereas presynaptic CB1Rs on glutamate afferents to D1R MSNs mediate motor depressant and rewarding effects of cannabinoids. Our results in DARPP-32 KO mice suggest that the loss of stimulatory DARPP-32 signaling in D1R-positive MSNs enhances the inhibitory influence of presynaptic CB1Rs, thereby enhancing THC-induced hypolocomotion. Andersson et al. (2005) reported that CP55,940-induced catalepsy was reduced in mice lacking DARPP-32 or with a point mutation of T34, as well as in mice with deletion of D2 or A2A receptors, and concluded that CB1R-mediated catalepsy involved phosphorylation of DARPP-32 on T34 in D2R MSNs.
Our previous studies showed that ΔFosB inhibits CB1R desensitization in the caudate-putamen (Lazenka et al., 2014a,b). Because DARPP-32 regulates signaling in striatal MSNs, we assessed CB1R desensitization and tolerance in DARPP-32 KO mice. Greater CB1R desensitization was found in the nucleus accumbens than caudate-putamen, consistent with an inhibitory effect of THC-induced ΔFosB expression on desensitization in caudate-putamen. Tolerance to THC-mediated hypolocomotion was enhanced in DARPP-32 KO mice, but CB1R-mediated G-protein activity did not differ between WT and DARPP-32 KO mice. This result is consistent with a possible role for ΔFosB in attenuating CB1R desensitization because similar ΔFosB expression was induced by repeated THC in both WT and DARPP-32 mutant mice.
The most likely explanation for the different effect of DARPP-32 on tolerance versus CB1R desensitization is that effects on tolerance occur downstream of CB1R–G-protein interactions. PKA inhibitors reduced tolerance to in vivo effects of THC (Bass et al., 2004), supporting the possibility that deletion of DARPP-32 could regulate tolerance to hypolocomotion by regulating PKA. Moreover, CB1Rs are expressed by multiple neuronal elements in the striatum, and effects on specific receptor populations could be obscured by the use of gross dissection.
These studies demonstrate a neurochemical commonality in the striatum between THC and other abused drugs, including cocaine and morphine, because ΔFosB induction is largely mediated by activation of D1Rs. The role of DARPP-32 in CB1R signaling in the striatum is complex and might reflect the localization of CB1Rs on D1R and D2R MSNs as well as glutamatergic terminals. Nevertheless, deletion of DARPP-32 attenuated acute THC-mediated ΔFosB induction in the striatum and enhanced THC-mediated locomotor suppression and tolerance, suggesting a role for dopamine-mediated signaling in the acute and chronic effects of THC.
Supplementary Material
Abbreviations
- ANOVA
analysis of variance
- CB1R
cannabinoid CB1 receptor
- CP55,940
(−)-cis-3-[2-hydroxy-4-(1,1-dimethylheptyl)phenyl]-trans-4-(3-hydroxypropyl)cyclohexanol
- CPP
conditioned place preference
- DAPI
4ʹ,6-diamidino-2-phenylindole
- DARPP-32
dopamine- and cAMP-regulated phosphoprotein Mr 32 kDa
- D1R
dopamine D1 receptor
- D2R
dopamine D2 receptor
- ΔFosB
Δ FBJ murine osteosarcoma viral oncogene homolog B
- -ir
immunoreactive
- KO
knockout
- MSN
medium spiny neuron
- PBS
phosphate-buffered saline
- PKA
protein kinase A
- SCH23390
(R)-(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride
- SCH39166
(6aS-trans)-11-chloro-6,6a,7,8,9,13-b-hexahydro-7-methyl-5H-benzo[d]naphth[2,1-b]azepi-n-12-ol hydrobromide
- [35S]GTPγS
guanosine 5′-O-(3-[35S]thio)triphosphate
- THC
Δ9-tetrahydrocannabinol
- WT
wild-type
Authorship Contributions
Participated in research design: Lazenka, Lichtman, Selley, Sim-Selley
Conducted experiments: Lazenka, Tomarchio.
Contributed new reagents or analytic tools: Greengard, Flajolet.
Performed data analysis: Lazenka, Tomarchio, Selley, Sim-Selley.
Wrote or contributed to the writing of the manuscript: Lazenka, Lichtman, Greengard, Flajolet, Selley, Sim-Selley.
Footnotes
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grants R01-DA030404, P01-DA10044, F31-DA030227] and the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grant R01-NS070715]. Microscopy was performed at the Virginia Commonwealth University Department of Anatomy and Neurobiology Microscopy Facility supported by the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grant P30-NS047463] and the National Institutes of Health National Cancer Institute [Grant P30-CA016059].
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Andersson M, Usiello A, Borgkvist A, Pozzi L, Dominguez C, Fienberg AA, Svenningsson P, Fredholm BB, Borrelli E, Greengard P, et al. (2005) Cannabinoid action depends on phosphorylation of dopamine- and cAMP-regulated phosphoprotein of 32 kDa at the protein kinase A site in striatal projection neurons. J Neurosci 25:8432–8438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass CE, Welch SP, Martin BR. (2004) Reversal of delta 9-tetrahydrocannabinol-induced tolerance by specific kinase inhibitors. Eur J Pharmacol 496:99–108. [DOI] [PubMed] [Google Scholar]
- Borgkvist A, Marcellino D, Fuxe K, Greengard P, Fisone G. (2008) Regulation of DARPP-32 phosphorylation by Δ9-tetrahydrocannabinol. Neuropharmacology 54:31–35. [DOI] [PubMed] [Google Scholar]
- Bradford MM. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. [DOI] [PubMed] [Google Scholar]
- Cadoni C, Pisanu A, Solinas M, Acquas E, Di Chiara G. (2001) Behavioural sensitization after repeated exposure to Δ 9-tetrahydrocannabinol and cross-sensitization with morphine. Psychopharmacology (Berl) 158:259–266. [DOI] [PubMed] [Google Scholar]
- Chen J, Kelz MB, Zeng G, Sakai N, Steffen C, Shockett PE, Picciotto MR, Duman RS, Nestler EJ. (1998) Transgenic animals with inducible, targeted gene expression in brain. Mol Pharmacol 54:495–503. [DOI] [PubMed] [Google Scholar]
- Chipkin RE, Iorio LC, Coffin VL, McQuade RD, Berger JG, Barnett A. (1988) Pharmacological profile of SCH39166: a dopamine D1 selective benzonaphthazepine with potential antipsychotic activity. J Pharmacol Exp Ther 247:1093–1102. [PubMed] [Google Scholar]
- Chocyk A, Czyrak A, Wedzony K. (2006) Acute and repeated cocaine induces alterations in FosB/ΔFosB expression in the paraventricular nucleus of the hypothalamus. Brain Res 1090:58–68. [DOI] [PubMed] [Google Scholar]
- Colby CR, Whisler K, Steffen C, Nestler EJ, Self DW. (2003) Striatal cell type-specific overexpression of ΔFosB enhances incentive for cocaine. J Neurosci 23:2488–2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. (1988) Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA 85:5274–5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana M, Melis M, Gessa GL. (1998) Increase in meso-prefrontal dopaminergic activity after stimulation of CB1 receptors by cannabinoids. Eur J Neurosci 10:2825–2830. [DOI] [PubMed] [Google Scholar]
- Fattore L, Melis M, Fadda P, Pistis M, Fratta W. (2010) The endocannabinoid system and nondrug rewarding behaviours. Exp Neurol 224:23–36. [DOI] [PubMed] [Google Scholar]
- Ferré S, Lluís C, Justinova Z, Quiroz C, Orru M, Navarro G, Canela EI, Franco R, Goldberg SR. (2010) Adenosine-cannabinoid receptor interactions. Implications for striatal function. Br J Pharmacol 160:443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald ML, Shobin E, Pickel VM. (2012) Cannabinoid modulation of the dopaminergic circuitry: implications for limbic and striatal output. Prog Neuropsychopharmacol Biol Psychiatry 38:21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. (2008) The Mouse Brain in Stereotaxic Coordinates, Academic Press, San Diego, CA. [Google Scholar]
- Gardner EL, Paredes W, Smith D, Donner A, Milling C, Cohen D, Morrison D. (1988) Facilitation of brain stimulation reward by delta 9-tetrahydrocannabinol. Psychopharmacology (Berl) 96:142–144. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Jr, Sibley DR. (1990) D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science 250:1429–1432. [DOI] [PubMed] [Google Scholar]
- Greengard P. (2001a) The neurobiology of dopamine signaling. Biosci Rep 21:247–269. [DOI] [PubMed] [Google Scholar]
- Greengard P. (2001b) The neurobiology of slow synaptic transmission. Science 294:1024–1030. [DOI] [PubMed] [Google Scholar]
- Grueter BA, Robison AJ, Neve RL, Nestler EJ, Malenka RC. (2013) ∆FosB differentially modulates nucleus accumbens direct and indirect pathway function. Proc Natl Acad Sci USA 110:1923–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroi N, Fienberg AA, Haile CN, Alburges M, Hanson GR, Greengard P, Nestler EJ. (1999) Neuronal and behavioural abnormalities in striatal function in DARPP-32-mutant mice. Eur J Neurosci 11:1114–1118. [DOI] [PubMed] [Google Scholar]
- Hohmann AG, Herkenham M. (2000) Localization of cannabinoid CB(1) receptor mRNA in neuronal subpopulations of rat striatum: a double-label in situ hybridization study. Synapse 37:71–80. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, et al. (2002) International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev 54:161–202. [DOI] [PubMed] [Google Scholar]
- Lamarque S, Taghzouti K, Simon H. (2001) Chronic treatment with Δ(9)-tetrahydrocannabinol enhances the locomotor response to amphetamine and heroin. Implications for vulnerability to drug addiction. Neuropharmacology 41:118–129. [DOI] [PubMed] [Google Scholar]
- Lazenka MF, David BG, Lichtman AH, Nestler EJ, Selley DE, Sim-Selley LJ. (2014a) Delta FosB and AP-1-mediated transcription modulate cannabinoid CB₁ receptor signaling and desensitization in striatal and limbic brain regions. Biochem Pharmacol 91:380–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazenka MF, Selley DE, Sim-Selley LJ. (2014b) ΔFosB induction correlates inversely with CB₁ receptor desensitization in a brain region-dependent manner following repeated Δ⁹-THC administration. Neuropharmacology 77:224–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz JD, Lobo MK. (2013) Optogenetic insights into striatal function and behavior. Behav Brain Res 255:44–54. [DOI] [PubMed] [Google Scholar]
- Little PJ, Compton DR, Johnson MR, Melvin LS, Martin BR. (1988) Pharmacology and stereoselectivity of structurally novel cannabinoids in mice. J Pharmacol Exp Ther 247:1046–1051. [PubMed] [Google Scholar]
- Lobo MK, Nestler EJ. (2011) The striatal balancing act in drug addiction: distinct roles of direct and indirect pathway medium spiny neurons. Front Neuroanat 5:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo MK, Zaman S, Damez-Werno DM, Koo JW, Bagot RC, DiNieri JA, Nugent A, Finkel E, Chaudhury D, Chandra R, et al. (2013) ΔFosB induction in striatal medium spiny neuron subtypes in response to chronic pharmacological, emotional, and optogenetic stimuli. J Neurosci 33:18381–18395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JZ, Nomura DK, Vann RE, Walentiny DM, Booker L, Jin X, Burston JJ, Sim-Selley LJ, Lichtman AH, Wiley JL, et al. (2009) Dual blockade of FAAH and MAGL identifies behavioral processes regulated by endocannabinoid crosstalk in vivo. Proc Natl Acad Sci USA 106:20270–20275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupica CR, Riegel AC, Hoffman AF. (2004) Marijuana and cannabinoid regulation of brain reward circuits. Br J Pharmacol 143:227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA, Nestler EJ. (2003) Regulation of gene expression and cocaine reward by CREB and ΔFosB. Nat Neurosci 6:1208–1215. [DOI] [PubMed] [Google Scholar]
- McClung CA, Ulery PG, Perrotti LI, Zachariou V, Berton O, Nestler EJ. (2004) ΔFosB: a molecular switch for long-term adaptation in the brain. Brain Res Mol Brain Res 132:146–154. [DOI] [PubMed] [Google Scholar]
- Muller DL, Unterwald EM. (2005) D1 dopamine receptors modulate deltaFosB induction in rat striatum after intermittent morphine administration. J Pharmacol Exp Ther 314:148–154. [DOI] [PubMed] [Google Scholar]
- Nairn AC, Svenningsson P, Nishi A, Fisone G, Girault JA, Greengard P. (2004) The role of DARPP-32 in the actions of drugs of abuse. Neuropharmacology 47 (Suppl 1):14–23. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. (2008) Review. Transcriptional mechanisms of addiction: role of ΔFosB. Philos Trans R Soc Lond B Biol Sci 363:3245–3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nye HE, Hope BT, Kelz MB, Iadarola M, Nestler EJ. (1995) Pharmacological studies of the regulation of chronic FOS-related antigen induction by cocaine in the striatum and nucleus accumbens. J Pharmacol Exp Ther 275:1671–1680. [PubMed] [Google Scholar]
- Oleson EB, Cheer JF. (2012) A brain on cannabinoids: the role of dopamine release in reward seeking. Cold Spring Harb Perspect Med 2:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panlilio LV, Zanettini C, Barnes C, Solinas M, Goldberg SR. (2013) Prior exposure to THC increases the addictive effects of nicotine in rats. Neuropsychopharmacology 38:1198–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrotti LI, Weaver RR, Robison B, Renthal W, Maze I, Yazdani S, Elmore RG, Knapp DJ, Selley DE, Martin BR, et al. (2008) Distinct patterns of DeltaFosB induction in brain by drugs of abuse. Synapse 62:358–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickel VM, Chan J, Kash TL, Rodríguez JJ, MacKie K. (2004) Compartment-specific localization of cannabinoid 1 (CB1) and mu-opioid receptors in rat nucleus accumbens. Neuroscience 127:101–112. [DOI] [PubMed] [Google Scholar]
- Pistis M, Ferraro L, Pira L, Flore G, Tanganelli S, Gessa GL, Devoto P. (2002) Δ(9)-tetrahydrocannabinol decreases extracellular GABA and increases extracellular glutamate and dopamine levels in the rat prefrontal cortex: an in vivo microdialysis study. Brain Res 948:155–158. [DOI] [PubMed] [Google Scholar]
- Robison AJ, Nestler EJ. (2011) Transcriptional and epigenetic mechanisms of addiction. Nat Rev Neurosci 12:623–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim LJ, Selley DE, Childers SR. (1995) In vitro autoradiography of receptor-activated G proteins in rat brain by agonist-stimulated guanylyl 5′-[γ-[35S]thio]-triphosphate binding. Proc Natl Acad Sci USA 92:7242–7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda G, Pontieri FE, Di Chiara G. (1997) Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science 276:2048–2050. [DOI] [PubMed] [Google Scholar]
- Valjent E, Maldonado R. (2000) A behavioural model to reveal place preference to delta 9-tetrahydrocannabinol in mice. Psychopharmacology (Berl) 147:436–438. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Baler RD, Compton WM, Weiss SR. (2014) Adverse health effects of marijuana use. N Engl J Med 370:2219–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebelhaus JM, Grim TW, Owens RA, Lazenka MF, Sim-Selley LJ, Abdullah RA, Niphakis MJ, Vann RE, Cravatt BF, Wiley JL, et al. (2015) Δ9-tetrahydrocannabinol and endocannabinoid degradative enzyme inhibitors attenuate intracranial self-stimulation in mice. J Pharmacol Exp Ther 352:195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachariou V, Bolanos CA, Selley DE, Theobald D, Cassidy MP, Kelz MB, Shaw-Lutchman T, Berton O, Sim-Selley LJ, Dileone RJ, et al. (2006a) An essential role for ΔFosB in the nucleus accumbens in morphine action. Nat Neurosci 9:205–211. [DOI] [PubMed] [Google Scholar]
- Zachariou V, Sgambato-Faure V, Sasaki T, Svenningsson P, Berton O, Fienberg AA, Nairn AC, Greengard P, Nestler EJ. (2006b) Phosphorylation of DARPP-32 at threonine-34 is required for cocaine action. Neuropsychopharmacology 31:555–562. [DOI] [PubMed] [Google Scholar]
- Zhang D, Zhang L, Lou DW, Nakabeppu Y, Zhang J, Xu M. (2002) The dopamine D1 receptor is a critical mediator for cocaine-induced gene expression. J Neurochem 82:1453–1464. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.