Abstract
Alcohol is one of the most prevalent addictive substances in the world. Withdrawal symptoms result from abrupt cessation of alcohol consumption in habitual drinkers. The emergence of both affective and physical symptoms produces a state that promotes relapse. Mice provide a preclinical model that could be used to study alcohol dependence and withdrawal while controlling for both genetic and environmental variables. The use of a liquid ethanol diet offers a reliable method for the induction of alcohol dependence in mice, but this approach is impractical when conducting high throughput pharmacological screens or when comparing multiple strains of genetically engineered mice. The goal of this study was to compare withdrawal associated behaviors in mice chronically treated with a liquid ethanol diet vs. mice treated with a short-term ethanol treatment that consisted of daily ethanol injections containing the alcohol dehydrogenase inhibitor, 4-methylpyrazole. Twenty-four hours after ethanol treatment, mice were tested in the open field arena, the elevated plus maze, the marble burying test or for changes in somatic signs during spontaneous ethanol withdrawal. Anxiety-like and compulsive-like behavior, as well as physical signs, were all significantly elevated in mice undergoing withdrawal, regardless of the route of ethanol administration. Therefore, a short-term ethanol treatment can be utilized as a screening tool for testing genetic and pharmacological agents before investing in a more time consuming ethanol treatment.
Keywords: alcohol, withdrawal, anxiety, compulsive behavior, somatic signs, mouse
Introduction
Alcohol abuse and dependence are a significant concern within the United States and many other Countries (Carlson et al., 2012). In the United States, the rate of alcohol dependence is double that of other drugs of abuse, with more than 80 million individuals meeting the criteria for dependence (Carlson et al., 2012). Withdrawal symptoms represent a significant obstacle to successful alcohol cessation and promote drug relapse. They include increased anxiety, agitation, hypervigilance, insomnia, irritability, tremors and, in the most severe cases, seizures occurring within 8–24 hours after ethanol ingestion. (Hall & Zador, 1997).
Current preclinical models used to induce alcohol dependence in both rats and mice include involuntary/forced exposure to ethanol through vapor, intragastric intubation, or liquid diet (Braconi et al., 2010; Fidler, Clews, & Cunningham, 2006; Fidler et al., 2012; Gilpin, Misra, & Koob, 2008; Gilpin et al., 2009; Kurokawa, Mizuno, & Ohkuma, 2013; Macey, Schulteis, Heinrichs, & Koob, 1996; O’Dell, Roberts, Smith, & Koob, 2004). Each procedure has advantages and disadvantages. Ethanol vapor produces very stable blood ethanol concentrations for specific durations of time and temporal patterns (O’Dell et al., 2004), but the equipment is expensive and requires a suitable amount of space. Intragastic intubation is a short-duration procedure but can produce excessively elevated blood ethanol concentrations resulting in increased mortality rates (Majchrowicz, 1975). Ethanol liquid diets delivered as the only nutritional source to the animal produce high volume intakes and withdrawal symptoms upon ethanol cessation (Lieber & DeCarli, 1982). The liquid diet paradigms are more time consuming and require dedicated space since each experimental subject is single housed. In addition, the diet is consumed throughout the day and this leads to increased variability in blood ethanol concentrations and behavior within groups, which requires larger animal numbers for accurate comparisons. Given the increasing number of genetically modified mouse models available for the identification of potential therapeutic approaches to ethanol dependence, it would be advantageous to have an inexpensive, short-duration preclinical model that can be used for drug screens before investing in a costly and time-consuming ethanol treatment.
The goal of this study was to survey an array of alcohol withdrawal symptoms in a short-term ethanol treatment paradigm that included an alcohol dehydrogenase inhibitor to prolong the half-life of ethanol in the plasma. Such paradigm has been successfully employed in mice to induce handling-induced seizures and anxiety-like behavior during withdrawal (Farook et al., 2008; Farook, Morrell, Lewis, Littleton, & Barron, 2007). Our results indicate that ethanol cessation after a short course of intraperitoneal (ip) injections can produce withdrawal symptoms comparable to those observed after a six week ethanol diet. The study also expands the battery of behavioral tests that can be used to detect ethanol withdrawal symptoms in mice to include compulsive-like and physical symptoms.
Materials and Methods
Animals
C57BL/6J mice of both sexes were used throughout the experiments. C57BL/6J is an inbred strain commonly used for behavioral studies because mice exhibit average behavior in most behavioral tasks during baseline conditions (Brooks, Pask, Jones, & Dunnett, 2005; Crawley et al., 1997). In addition, C57BL/6J is a relatively good breeder, easily available from the Jackson Laboratory, and is widely used as a background strain for many genetically engineered mouse models. Mice were no older than three months of age at the beginning of the ethanol treatments. All procedures complied with the directives of the Institutional Animal Care and Use Committee (IACUC) and the Center for Comparative Medicine. Weaning was performed 21 days after birth, and same sex littermates were housed in cages containing one cm of corn cob bedding with a maximum of five animals. The animals had ad libitum access to water and food pellets (Labdiet 5001, PMI®, Brentwood, MO), and were maintained on a 12-h light/dark cycle (lights on 7AM and off at 7PM). Ambient light level in the housing rooms was 350 luxes, while the average light on the housing selves, where cages are kept, was 80 luxes.
Chronic Alcohol Treatment
Chronic Injections
We adopted the chronic ethanol treatment described by Farook et al (2007). Mice were injected daily with either 2 g/kg (20% w/v) ethanol or saline for a minimum of nine days. Both ethanol and control solutions contained 9 mg/kg 4-methylpyrazole (4MP), an alcohol dehydrogenase inhibitor that helps to achieve elevated blood ethanol levels for longer duration (Farook et al., 2007). All behavioral paradigms were performed during spontaneous ethanol withdrawal (24 hours after the last injection), during the lights-on phase of the light/dark cycle (12PM–5:30PM). All treatments were performed in the housing room, under a hood (73 luxes). During experiments, ambient light level was kept at 11 luxes, unless otherwise stated. All animals were acclimated to the behavior room for a minimum of 45 minutes before testing. Multiple testing was planned so that mice received their respective treatment immediately after each behavioral test and during the following day before being tested in another behavioral paradigm. Behavioral tasks were performed in the order of increasing stress: open field arena, elevated plus maze, marble burying, and somatic signs. Mice were weighed daily throughout the treatment and withdrawal testing, and no significant differences were observed between treatment groups (data not shown).
Blood ethanol concentrations (BECs) were measured using a separate group of mice. Mice were treated with saline + 4MP or ethanol (2g/kg) + 4MP for 9 days. On the last day of treatment, blood was collected 1, 4, and 6 hours after ethanol injections. Blood plasma was analyzed using the colorimetric ethanol assay kit from Sigma-Aldrich™ (St Louis, MO). One hour after ethanol injection BEC was 194.88 mg/dl (STE ±11.13, n=6 per time point) and was reduced by 40% and 79%, 4 hours and 6 hours later, respectively.
Liquid Ethanol Diet
A separate group of mice was exposed to an ethanol liquid diet for six weeks. Liquid diets have extensively been used in rodents to produce ethanol dependence and physiological changes associated with chronic ethanol use (Gilpin et al., 2008; Macey et al., 1996; Nan et al., 2013; Umathe, Bhutada, Dixit, & Shende, 2008; Verleye, Heulard, & Gillardin, 2009; Zhang et al., 2013). Mice were single-housed with a nestlet, and provided for one week with a basic diet consisting of the chocolate flavored high protein nutritional drink, Boost® (Nestlé Health Science, Switzerland), supplemented with 3g/L vitamin mixture (MP Biomedicals, LLC, Solon, OH) and 5g/L mineral mix (ICN Biomedicals, Inc., Aurora, OH) (Gilpin et al., 2008; Verleye et al., 2009). After an habituation period to the liquid diet, mice were divided into two groups based on age, sex, and weight. The first group received the basic diet containing 4% vol/vol ethanol for six weeks (Nan et al., 2013; Verleye et al., 2009; Zhang et al., 2013). The second, yoked-control, group continued to receive the basic diet supplemented with an iso-coloric amount of sucrose in order to account for the increased calories associated with ethanol consumption. Ethanol-treated mice received unlimited amount of liquid diet throughout the day while control mice received a specific amount of control diet based on the average intake of the ethanol group on the previous day. All diets were made and replaced daily two hours after lights out (under red lights not to disrupt the light cycle). All mice had unlimited access to drinking water. Animal weights and amount of diet consumed were recorded daily. Mice consumed on average 17.4 g/kg (±0.55 g/kg) ethanol during the last two weeks of the six-week treatment. It should be noted that female mice on average consume significantly more ethanol than male mice (Fsex[1,14]=6.51. p=0.023), 18.2 g/kg (±0.5 g/kg) and 16.6 g/kg (±0.39 g/kg) respectively. Similar sex differences in ethanol consumption have been observed in rodents exposed to liquid ethanol diet, continuous two-bottle choice, intermittent two-bottle choice and drinking in the dark paradigms (Hwa et al., 2011; McCall et al., 2013; Piano, Carrigan, & Schwertz, 2005; Trujillo, Do, Grahame, Roberts, & Gorman, 2011).
Mice were tested during spontaneous ethanol withdrawal, 24 hours after removal of the ethanol diet. To that end, on the last day of liquid diet treatment, mice received fresh diet for a minimum of 3 hours before the ethanol diet was replaced with control diet. All behavioral testing was performed during the dark phase of the cycle (12AM–5:30AM) using the same ambient light levels (11 luxes) used for behavioral testing in ethanol-injected mice. Mice were acclimated to the behavior room for a minimum of 45 minutes before testing. Immediately after testing, ethanol diet was reinstated for a minimum of three days before subsequent behavioral testing. Behavioral testing was performed in the same order as the chronic ethanol injection treatment paradigm: open field arena, elevated plus maze, marble burying test and somatic signs.
BECs were measured using a separate group of mice treated with either control or ethanol diet for 6 weeks. On the last day of treatment, mice were given access to fresh ethanol diet for 3 hours, followed by replacement of the ethanol diet with the control diet. Blood samples were collected immediately and 6 hours after the diet switch. Blood plasma was analyzed using the colorimetric ethanol assay kit from Sigma-Aldrich™ (St Louis, MO). One hour after ethanol injection BEC was 142.72 mg/dl (STE ±20.65, n=8 per time point) and was reduced by 77% 6 hours later.
Open Field Arena
Mice experiencing ethanol withdrawal were placed in a clear Plexiglas® arena (40 × 40 × 40 cm), and locomotor activity was measured for 30 min. For analysis purposes, the arena was divided into two zones, the center (20 × 20 × 20 cm), and the outer peripheral zone. Anymaze™ software (Stoeling Co, Wood Dale, IL) was used to track the distance traveled within the two zones. The total distance traveled throughout the arena was used as a measure of locomotor behavior and the center ratio (distance traveled in the center zone/total distance traveled) was calculated as a measure for anxiety-like behavior (Gangitano, Salas, Teng, Perez, & De Biasi, 2009; Salas et al., 2008; Salas, Orr-Urtreger, et al., 2003; Salas, Pieri, Fung, Dani, & De Biasi, 2003). A digital lux meter was used to measure light levels of either 11 or 80 luxes for behavioral testing.
Elevated Plus Maze
Withdrawal-induced changes in anxiety-like behaviors were also measured in the elevated plus maze (EPM) for 10 minutes. Briefly, mice were placed into a plus shaped maze with two corridors (25 × 7 cm) with black, 15 cm high walls, and two corridors with no walls connected by a (7 × 7 cm) square. The maze is elevated 50 cm above the floor (Gangitano et al., 2009; Salas et al., 2008; Salas, Pieri, et al., 2003). The Anymaze™ software was used to track the movement of the mouse throughout the maze. The time spent in the open arms and the entry ratio (open entries/total entries) was used as measure of anxiety-like behavior. The total number of entries into the open and closed arms was used as a measure of locomotion.
Marble Burying Test
Ethanol withdrawal-induced compulsive-like behavior was measured using the marble burying test (Umathe et al., 2008). Mice were placed for 30 min in a cage (26.5 cm × 15.5 cm × 12.5 cm) that contained 5 cm of corn cob bedding with 20 marbles (13 mm diameter) evenly spaced throughout the cage. A minimum of 2/3 of the marble needed to be covered in bedding, <4 mm of the marble above the bedding, to be counted as buried. Compulsive-like behavior was scored based on the number of marbles buried, with a higher number of marbles buried signaling increased compulsive-like behavior.
Somatic Signs of Ethanol Withdrawal
Somatic (i.e. physical) signs were monitored for 20 min, twenty-four hours after cessation of ethanol treatment. We monitored changes in shaking, scratching, grooming (including paw tremors, paw licks and genital licking), and chewing (including swallowing and licking) behaviors, which are classically used to measure nicotine, morphine, and cocaine withdrawal (Malin et al., 2000; Muldoon et al., 2014; Salas, Main, Gangitano, & De Biasi, 2007; Salas, Pieri, & De Biasi, 2004; Salas, Sturm, Boulter, & De Biasi, 2009). In addition to these symptoms, we recorded physical signs previously reported in rats during alcohol withdrawal: vocalizations, tail rattling, cage scratching, head nodding and writhing behavior (Macey et al., 1996; Majchrowicz, 1975; Uzbay, Erden, Tapanyigit, & Kayaalp, 1997). Those signs occurred frequently but were exclusively observed during withdrawal.
Data analysis and statistics
Data were examined by two-tailed student T-Test or ANOVA, when appropriate. The Newman-Keuls post-hoc test was used for specific comparisons. Behavioral data were examined for the effect of sex on withdrawal behaviors using a two-way ANOVA. There were no significant interactions between sex and ethanol withdrawal or main effect of sex in any of the behavioral test for either treatment paradigm (N’s for female mice are 5–8 per experimental condition).
Results
Ethanol withdrawal produces an increase in anxiety-like behavior
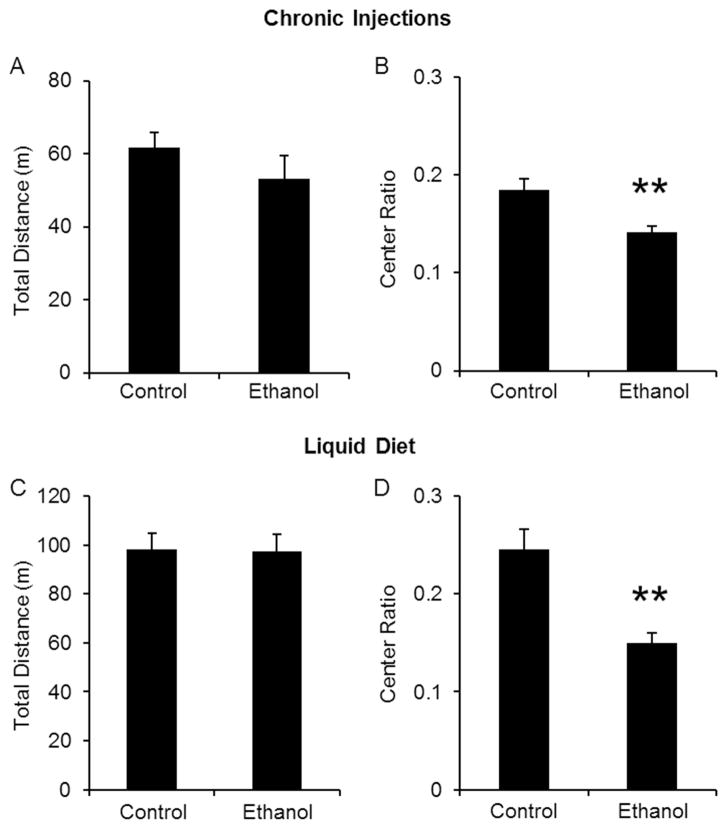
We chose the open field arena (OFA) and the elevated plus maze (EPM) to measure potential changes in anxiety-like behavior induced by ethanol withdrawal because increases in anxiety are commonly reported after alcohol cessation (Hall & Zador, 1997). All behavioral testing was conducted during spontaneous ethanol withdrawal, 24 hours after the last ethanol injection or removal of ethanol liquid diet. Since most anxiety tests are based on locomotor behavior, we first determined whether ethanol withdrawal produces changes in locomotion in the OFA. Mice undergoing withdrawal from either ethanol injections or liquid diet did not display significant differences in total distance traveled compared to their respective control groups (Fig 1A, C). Overall, mice treated with the liquid diet (regardless of treatment) displayed a higher level of locomotion compared to chronically injected mice. This effect is most likely due to testing at different times within the light/dark cycle, as mice have a natural diurnal difference in activity levels (Deimling & Schnell, 1980; Mitler, Lund, Sokolove, Pittendrigh, & Dement, 1977). While locomotion did not change, ethanol withdrawal induced an increase in anxiety-like behavior, as measured by the significant decrease in the center ratio in both types of ethanol treatments (Fig 1B, D).
Figure 1. Ethanol withdrawal induces anxiety-like behavior without affecting locomotion in the open field arena.
Mice were treated chronically with either control and ethanol injections (A, B) or liquid diet (C, D). Locomotor behavior was not altered by ethanol withdrawal (A, C) but produced a significant decrease in the center ratio (distance traveled in center zone/total distance traveled; B and D). **p<0.05 compared to control-treated mice as measured by student t-test. N’s are 13, 9 for injections and 13, 13 for liquid diet.
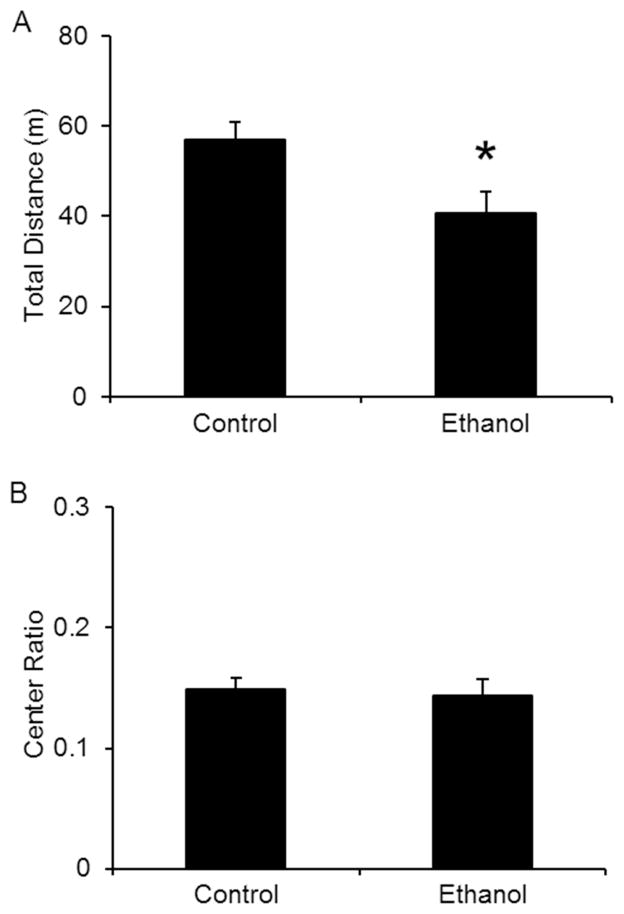
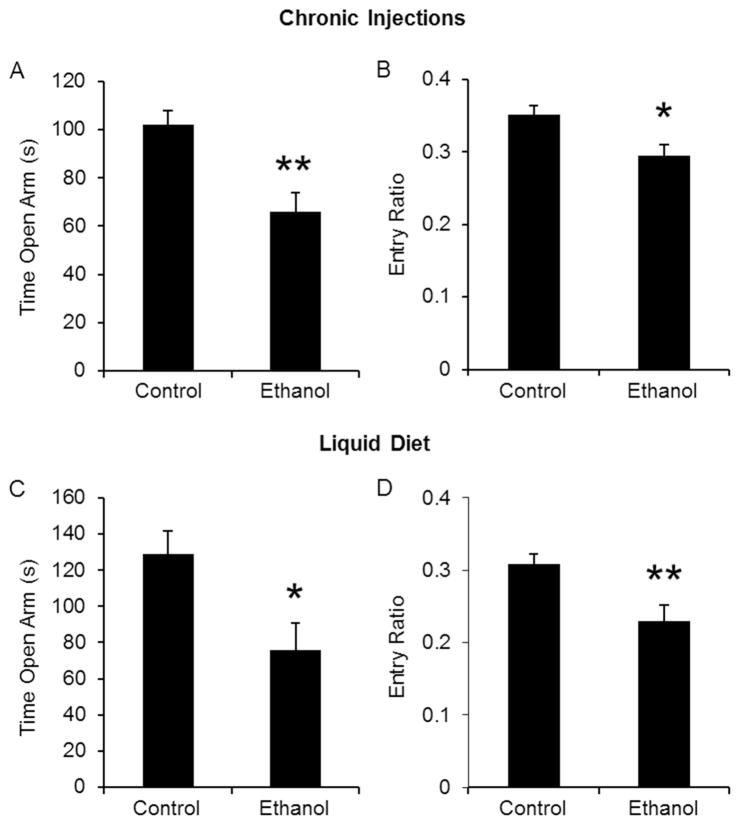
In a second set of experiments, which was conducted only in mice receiving ethanol injections, we changed the anxiety-provoking levels of the OFA test by increasing the intensity of the ambient light in the testing room (from 11 luxes to 80 luxes). When mice were tested in those two conditions, it became apparent that the increased luminosity produced a significant locomotor deficit in mice undergoing ethanol withdrawal (Fig 2A), and the difference in the center ratio between control and ethanol treated mice disappeared (Fig 2B). As both the OFA and the EPM tests of anxiety-like behavior depend on locomotion, the lower ambient light level condition was subsequently used in the EPM and all other behavioral tests utilized. Similar to the OFA results, ethanol-treated mice undergoing withdrawal exhibited an increase in anxiety-like behavior in the EPM without changes in the total number of entries (data not shown). Total time spent in the open arms and entry ratio were significantly reduced compared to control-treated mice (Fig 3). Overall, chronic ethanol injection treatment was as effective as the liquid ethanol diet at producing changes in anxiety-like behavior during withdrawal.
Figure 2. Increased luminosity during anxiety tests produces changes in locomotion in mice undergoing ethanol withdrawal.
Control and ethanol injected mice were tested in the OFA, 24 hours after the last injection. At high light levels, the total distance traveled was significantly decreased during withdrawal when compared to control-treated mice (A). In this condition, anxiety, measured as the center ratio, was similar in control vs. ethanol-treated mice (B). *p<0.05 compared to control treated mice as measured by student t-test. N’s are 14 and 10.
Figure 3. Ethanol withdrawal induces anxiety-like behavior in the elevated plus maze.
Mice were exposed to ethanol injections (A, B) or ethanol liquid diet (C, D). During withdrawal, mice were placed in the EPM for 10 min. The total time spent in the open arms (A, C) and the entry ratios (B, D) were significantly reduced during ethanol withdrawal. *p<0.05 compared to control-treated mice as measured by student t-test. N’s are 13, 9 for injections and 13, 13 for liquid diet.
Ethanol withdrawal increases compulsive-like behavior
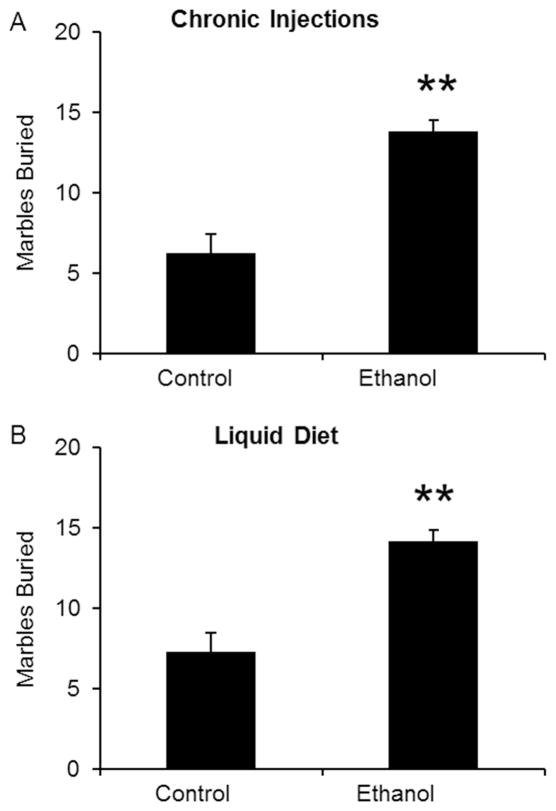
In addition to measuring anxiety-like behavior, mice were also tested for changes in compulsive-like behavior in the MBT. Mice undergoing withdrawal were placed in a cage containing 20 marbles for 30 minutes. Ethanol withdrawal produced a significant increase in the number of marbles buried in both ethanol injection (Fig 4A) and ethanol diet groups (Fig 4B). Therefore, marble burying can be adopted as an additional measure of ethanol withdrawal symptoms in mice.
Figure 4. Ethanol withdrawal produces increases in compulsive-like behavior.
Mice experiencing ethanol withdrawal were tested in the marble burying paradigm for 30 min. Marbles were considered buried if they were 2/3 covered with bedding. Mice treated with ethanol injections (A) and liquid ethanol diet (B) buried more marbles, suggesting that they were experiencing an increase in compulsive-like behavior during ethanol withdrawal. **p<0.01 compared to control treated mice as measured by student t-test. N’s are 13, 12 for injections and 13, 13 for liquid diet.
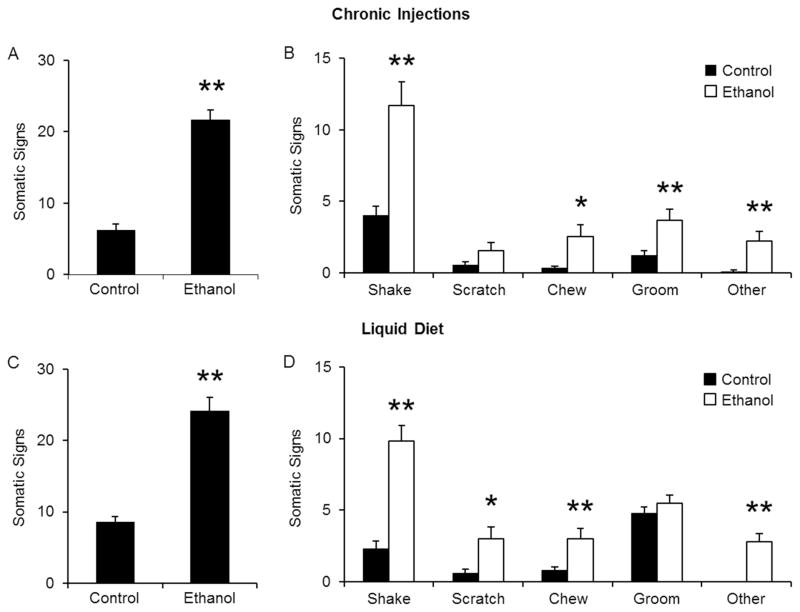
Ethanol withdrawal induces increases in somatic signs
In another series of experiments, we determined whether the emergence of physical symptoms of withdrawal could be used to evaluate the alcohol withdrawal syndrome. The following physical signs were measured for 20 minutes: shaking, scratching, grooming, chewing, tail rattling, vocalization, writhing and cage scratching. While control-treated mice had normal levels of somatic behavior, ethanol withdrawal was associated with a significant increase in the total number of physical symptoms. Such increase was observed in both types of treatment regimens (Fig 5A and C). In ethanol-injected mice, the number of shakes, chews, and grooms were significantly elevated during withdrawal. Scratching behavior was also increased, but did not reach significance. Other signs (tail rattling, vocalizations, writhing, and cage scratching) were also observed in some, but not all, ethanol-treated mice during withdrawal, and so, incidence count data were combined. “Other” signs were hardly ever observed in control-treated mice (Fig 5B). A different pattern of changes in individual somatic signs were observed in mice treated with ethanol liquid diet. Shaking, scratching, chewing and “other” signs were significantly increased during withdrawal, but grooming behavior was not affected (Fig 5C).
Figure 5. Ethanol withdrawal increases somatic signs.
Mice were observed for 20 min for the following signs: shaking, scratching, chewing, grooming, tail rattling, vocalizations, writhing, and cage scratching. Ethanol withdrawal produced a significant increase in the total number of somatic signs in both injection-treated (A) and liquid diet-treated mice (C). Changes were also observed when individual signs were examined (B, D). *p<0.05, **p<0.01 compared to control treated mice as measured by student t-test. N’s are 13, 12 for injections and 13, 13 for liquid diet.
Discussion
The main finding of this report is that a relatively short ethanol injection regimen, when combined with an alcohol dehydrogenase inhibitor, can produce withdrawal-associated behaviors that are similar to those observed upon withdrawal from long-term treatment of mice with ethanol liquid diet (Kliethermes, 2005; Umathe et al., 2008; Uzbay et al., 1997). In addition to replicating previously reported increases in anxiety-like behavior in the EPM during ethanol withdrawal after chronic ethanol injections (Farook et al., 2007), our study also demonstrated that our shot-term alcohol treatment can produce increased anxiety-like behavior in the OFA. The results also point to the fact that the light conditions under which anxiety tests are conducted can influence the outcome and interpretation of results (Bouwknecht & Paylor, 2008; Kliethermes, 2005). The OFA and EPM are exploration-driven paradigms that measure the tendency of a mouse to stay in a relatively “safe” area (the peripheral zone of the OFA and the closed arms of the EPM) vs. a more aversive area (the center of the OFA and the open arms of the EPM). When we tested mice in low vs. high ambient light levels we found that in low light conditions, locomotor behavior is not affected and significant changes in anxiety-like behavior can be detected during ethanol withdrawal. When luminosity is increased to amplify the aversive properties of the OFA, locomotor behavior is impaired in mice undergoing ethanol withdrawal, making conclusions about anxiety-like behavior difficult to interpret. Our results could in part help to explain the variability of anxiety-like behavioral outcomes that have been reported in these behavioral paradigms, even when rodents were exposed to ethanol through vapor or liquid diet (Kliethermes, 2005).
Obsessive-compulsive disorder is an anxiety disorder often evident in alcohol dependent patients undergoing withdrawal (Lima, Pechansky, Fleck, & De Boni, 2005; Neziroglu, Yaryura Tobias, Lemli, & Yaryura, 1994; Suzuki, Muramatsu, Takeda, & Shirakura, 2002). Elevated compulsive-like behavior has been shown to occur in male mice during ethanol withdrawal in the MBT (Umathe et al., 2008). Both our short-term ethanol injection treatment and liquid ethanol diet were sufficient to produce increases in compulsive-like behavior during withdrawal. Our results are comparable to previous findings in the MBT (Umathe et al., 2008). In addition, we show that this behavioral test can be used with both male and female mice. This piece of information was not provided in Umathe et al., 2008, which only examined male mice.
Somatic symptoms are an important component of ethanol withdrawal in humans. Physical signs include tremors, changes in heart rhythm and blood pressure, sweating, and tactile disturbances reported as itching, burning and numbness sensations (Hall & Zador, 1997; Jaeger, Lohr, & Pankratz, 2001; McKinley, 2005; Riddle, Bush, Tittle, & Dilkhush, 2010). Together with affective changes, physical symptoms have a predictive value for the severity of alcohol withdrawal and the subsequent therapeutic approaches (Jaeger et al., 2001; Wetterling et al., 1997). In rodents, physical signs of withdrawal have been observed after cession of various drugs of abuse, including nicotine, morphine, and cocaine (Erami et al., 2012; Malin et al., 2000; Muldoon et al., 2014; Salas et al., 2007; Salas et al., 2004; Salas et al., 2009). Somatic signs of ethanol withdrawal have also been reported in rats. These signs include vocalizations, tail rigidity, tail tremors, wet dog shakes, teeth chattering, and sniffling (Braconi et al., 2010; Economidou et al., 2011; Majchrowicz, 1975; Uzbay et al., 1997). We chose to measure signs that are commonly observed in mice undergoing nicotine and morphine withdrawal in addition to the aforementioned signs. Those signs include wet dog shakes, scratching, grooming (including repetitive paw licking and genital licking), chewing, jumping and cage scratching (Erami et al., 2012; Salas et al., 2004). Both ethanol injection and ethanol diet produced a similar increase in total somatic signs during withdrawal, although each treatment led to a different pattern of signs.
The ethanol withdrawal syndrome, like withdrawal from other drugs of abuse, is characterized by symptoms that range in severity and follow distinct temporal patterns. Within six hours of alcohol cessation, patients may experience symptoms such as increased anxiety, depressed mood, sweating, hyperthermia, hypertension, tachycardia, tremors, and nausea. Seizures and delirium take up to a couple of days to develop (Saitz, 1998). Physical signs are generally short-lived, lasting no longer than a few days and are characterized as acute withdrawal. Increased anxiety, depressed mood, and sleep disturbances can last weeks and even years after the presence of the physical withdrawal signs has abated (Heilig, Egli, Crabbe, & Becker, 2010). Such long lasting negative affect (increased anxiety and depressed mood) is also described as protracted withdrawal and has been cited by many studies as a contributor to alcohol relapse (Bradizza, Stasiewicz, & Paas, 2006; Swan, Ward, Carmelli, & Jack, 1993; West, Hajek, & Belcher, 1989). Although our two distinct treatment paradigms produced similar acute withdrawal symptoms, it is possible that protracted symptoms could be observed in the liquid ethanol diet but not the short-term ethanol injection paradigm.
This study utilized C57BL/6J mice as experimental subjects. Our decision to focus on this particular inbred strain was based on its wide use as a background strain for genetically engineered mice and its ability to perform a wide range of behavioral tasks (Crawley et al., 1997). C57BL/6J mice drink significantly more ethanol than other inbred strains (Belknap, Crabbe, & Young, 1993; Yoneyama, Crabbe, Ford, Murillo, & Finn, 2008). However, they are relatively resistant to handling-induced convulsions, a commonly examined symptom of alcohol abstinence (Crabbe, Young, & Kosobud, 1983; Metten & Crabbe, 1994). When compared to C57BL/6J, the outbred strain Swiss Webster has similar baseline anxiety-like behavior in the EPM and the light/dark box (Crawley et al., 1997; van Gaalen & Steckler, 2000) and similar, low sensitivity to handling-induced convulsions during ethanol withdrawal (Metten & Crabbe, 1994)(Farook et al., 2008; Farook et al., 2007). The DBA2/A inbred strain consumes less alcohol but displays higher handling-induced convulsions scores during alcohol withdrawal when compared to the C57BL/6J strain (Belknap et al., 1993; Yoneyama et al., 2008). However, both strains exhibit significant increases in anxiety-like behavior in the EPM during ethanol withdrawal (Finn, Gallaher, & Crabbe, 2000). Not surprisingly, C57BL/6J and DBA2/A inbred strains are considered to be “good” and “moderate” performers, respectively, in behavioral tasks that examine anxiety-like behavior (Crawley et al., 1997). Overall, these observations suggest that the potential for strain-specific withdrawal symptoms using either of the ethanol treatment paradigms presented in this paper will most likely be influenced by the strain’s baseline behavior during a specific behavioral test.
In conclusion, we have shown that a short-term regimen of daily ethanol injections containing 4MP is sufficient to increase ethanol withdrawal-associated behaviors without affecting the rewarding properties of ethanol. In addition, we showed that anxiety-like behavior during withdrawal is affected by the ambient light levels in the testing environment. Similar to other drugs of abuse, ethanol withdrawal also produces increases in physical signs. The proposed treatment paradigm provides various advantages when screening different mutant mouse lines and pharmaceutical agents. The advantages include the ability to group house mice, the use of a smaller number of mice -as they can be examined in several behavioral tests-, and the ability to study withdrawal at precise time points after ethanol cessation.
Highlights.
Mice were treated with daily injections of ethanol + 4-methylpyrazole, or ethanol in a liquid diet.
Withdrawal was compared between the two types of treatments.
Withdrawal from daily injections or liquid diet produced similar results.
Withdrawal increased anxiety-like and compulsive-like behaviors, and somatic signs.
Abbreviations
- ip
intraperitoneal
- 4MP
4-methylpyrazole
- OFA
open field arena
- EPM
elevated plus maze
- MBT
marble burying test
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Belknap JK, Crabbe JC, Young ER. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology (Berl) 1993;112(4):503–510. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- Bouwknecht JA, Paylor R. Pitfalls in the interpretation of genetic and pharmacological effects on anxiety-like behaviour in rodents. Behav Pharmacol. 2008;19(5–6):385–402. doi: 10.1097/FBP.0b013e32830c3658. [DOI] [PubMed] [Google Scholar]
- Braconi S, Sidhpura N, Aujla H, Martin-Fardon R, Weiss F, Ciccocioppo R. Revisiting intragastric ethanol intubation as a dependence induction method for studies of ethanol reward and motivation in rats. Alcohol Clin Exp Res. 2010;34(3):538–544. doi: 10.1111/j.1530-0277.2009.01119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradizza CM, Stasiewicz PR, Paas ND. Relapse to alcohol and drug use among individuals diagnosed with co-occurring mental health and substance use disorders: a review. Clin Psychol Rev. 2006;26(2):162–178. doi: 10.1016/j.cpr.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Brooks SP, Pask T, Jones L, Dunnett SB. Behavioural profiles of inbred mouse strains used as transgenic backgrounds. II: cognitive tests. Genes Brain Behav. 2005;4(5):307–317. doi: 10.1111/j.1601-183X.2004.00109.x. [DOI] [PubMed] [Google Scholar]
- Carlson RW, Kumar NN, Wong-Mckinstry E, Ayyagari S, Puri N, Jackson FK, et al. Alcohol withdrawal syndrome. Crit Care Clin. 2012;28(4):549–585. doi: 10.1016/j.ccc.2012.07.004. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Jr, Young ER, Kosobud A. Genetic correlations with ethanol withdrawal severity. Pharmacol Biochem Behav. 1983;18(Suppl 1):541–547. doi: 10.1016/0091-3057(83)90233-2. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, et al. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology (Berl) 1997;132(2):107–124. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- Deimling MJ, Schnell RC. Circadian rhythms in the biological response and disposition of ethanol in the mouse. J Pharmacol Exp Ther. 1980;213(1):1–8. [PubMed] [Google Scholar]
- Economidou D, Cippitelli A, Stopponi S, Braconi S, Clementi S, Ubaldi M, et al. Activation of brain NOP receptors attenuates acute and protracted alcohol withdrawal symptoms in the rat. Alcohol Clin Exp Res. 2011;35(4):747–755. doi: 10.1111/j.1530-0277.2010.01392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erami E, Azhdari-Zarmehri H, Rahmani A, Ghasemi-Dashkhasan E, Semnanian S, Haghparast A. Blockade of orexin receptor 1 attenuates the development of morphine tolerance and physical dependence in rats. Pharmacol Biochem Behav. 2012;103(2):212–219. doi: 10.1016/j.pbb.2012.08.010. [DOI] [PubMed] [Google Scholar]
- Farook JM, Krazem A, Lewis B, Morrell DJ, Littleton JM, Barron S. Acamprosate attenuates the handling induced convulsions during alcohol withdrawal in Swiss Webster mice. Physiol Behav. 2008;95(1–2):267–270. doi: 10.1016/j.physbeh.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farook JM, Morrell DJ, Lewis B, Littleton JM, Barron S. Topiramate (Topamax) reduces conditioned abstinence behaviours and handling-induced convulsions (HIC) after chronic administration of alcohol in Swiss-Webster mice. Alcohol Alcohol. 2007;42(4):296–300. doi: 10.1093/alcalc/agm047. [DOI] [PubMed] [Google Scholar]
- Fidler TL, Clews TW, Cunningham CL. Reestablishing an intragastric ethanol self-infusion model in rats. Alcohol Clin Exp Res. 2006;30(3):414–428. doi: 10.1111/j.1530-0277.2006.00046.x. [DOI] [PubMed] [Google Scholar]
- Fidler TL, Powers MS, Ramirez JJ, Crane A, Mulgrew J, Smitasin P, et al. Dependence induced increases in intragastric alcohol consumption in mice. Addict Biol. 2012;17(1):13–32. doi: 10.1111/j.1369-1600.2011.00363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn DA, Gallaher EJ, Crabbe JC. Differential change in neuroactive steroid sensitivity during ethanol withdrawal. J Pharmacol Exp Ther. 2000;292(1):394–405. [PubMed] [Google Scholar]
- Gangitano D, Salas R, Teng Y, Perez E, De Biasi M. Progesterone modulation of alpha5 nAChR subunits influences anxiety-related behavior during estrus cycle. Genes Brain Behav. 2009;8(4):398–406. doi: 10.1111/j.1601-183X.2009.00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Misra K, Koob GF. Neuropeptide Y in the central nucleus of the amygdala suppresses dependence-induced increases in alcohol drinking. Pharmacol Biochem Behav. 2008;90(3):475–480. doi: 10.1016/j.pbb.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Smith AD, Cole M, Weiss F, Koob GF, Richardson HN. Operant behavior and alcohol levels in blood and brain of alcohol-dependent rats. Alcohol Clin Exp Res. 2009;33(12):2113–2123. doi: 10.1111/j.1530-0277.2009.01051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall W, Zador D. The alcohol withdrawal syndrome. Lancet. 1997;349(9069):1897–1900. doi: 10.1016/S0140-6736(97)04572-8. [DOI] [PubMed] [Google Scholar]
- Heilig M, Egli M, Crabbe JC, Becker HC. Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked? Addict Biol. 2010;15(2):169–184. doi: 10.1111/j.1369-1600.2009.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa LS, Chu A, Levinson SA, Kayyali TM, DeBold JF, Miczek KA. Persistent escalation of alcohol drinking in C57BL/6J mice with intermittent access to 20% ethanol. Alcohol Clin Exp Res. 2011;35(11):1938–1947. doi: 10.1111/j.1530-0277.2011.01545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger TM, Lohr RH, Pankratz VS. Symptom-triggered therapy for alcohol withdrawal syndrome in medical inpatients. Mayo Clin Proc. 2001;76(7):695–701. doi: 10.4065/76.7.695. [DOI] [PubMed] [Google Scholar]
- Kliethermes CL. Anxiety-like behaviors following chronic ethanol exposure. Neurosci Biobehav Rev. 2005;28(8):837–850. doi: 10.1016/j.neubiorev.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Kurokawa K, Mizuno K, Ohkuma S. Dopamine D1 receptor signaling system regulates ryanodine receptor expression in ethanol physical dependence. Alcohol Clin Exp Res. 2013;37(5):771–783. doi: 10.1111/acer.12036. [DOI] [PubMed] [Google Scholar]
- Lieber CS, DeCarli LM. The feeding of alcohol in liquid diets: two decades of applications and 1982 update. Alcohol Clin Exp Res. 1982;6(4):523–531. doi: 10.1111/j.1530-0277.1982.tb05017.x. [DOI] [PubMed] [Google Scholar]
- Lima AF, Pechansky F, Fleck MP, De Boni R. Association between psychiatric symptoms and severity of alcohol dependence in a sample of brazilian men. J Nerv Ment Dis. 2005;193(2):126–130. doi: 10.1097/01.nmd.0000152818.38001.2c. [DOI] [PubMed] [Google Scholar]
- Macey DJ, Schulteis G, Heinrichs SC, Koob GF. Time-dependent quantifiable withdrawal from ethanol in the rat: effect of method of dependence induction. Alcohol. 1996;13(2):163–170. doi: 10.1016/0741-8329(95)02030-6. [DOI] [PubMed] [Google Scholar]
- Majchrowicz E. Induction of physical dependence upon ethanol and the associated behavioral changes in rats. Psychopharmacologia. 1975;43(3):245–254. doi: 10.1007/BF00429258. [DOI] [PubMed] [Google Scholar]
- Malin DH, Moon WD, Moy ET, Jennings RE, Moy DM, Warner RL, et al. A rodent model of cocaine abstinence syndrome. Pharmacol Biochem Behav. 2000;66(2):323–328. doi: 10.1016/s0091-3057(00)00181-7. [DOI] [PubMed] [Google Scholar]
- McCall NM, Sprow GM, Delpire E, Thiele TE, Kash TL, Pleil KE. Effects of sex and deletion of neuropeptide Y2 receptors from GABAergic neurons on affective and alcohol drinking behaviors in mice. Front Integr Neurosci. 2013;7:100. doi: 10.3389/fnint.2013.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinley MG. Alcohol withdrawal syndrome overlooked and mismanaged? Crit Care Nurse. 2005;25(3):40–42. 44–48. quiz 49. [PubMed] [Google Scholar]
- Metten P, Crabbe JC. Common genetic determinants of severity of acute withdrawal from ethanol, pentobarbital and diazepam in inbred mice. Behav Pharmacol. 1994;5(4 And 5):533–547. doi: 10.1097/00008877-199408000-00014. [DOI] [PubMed] [Google Scholar]
- Mitler MM, Lund R, Sokolove PG, Pittendrigh CS, Dement WC. Sleep and activity rhythms in mice: a description of circadian patterns and unexpected disruptions in sleep. Brain Res. 1977;131(1):129–145. doi: 10.1016/0006-8993(77)90033-6. [DOI] [PubMed] [Google Scholar]
- Muldoon PP, Jackson KJ, Perez E, Harenza JL, Molas S, Rais B, et al. The alpha3beta4* nicotinic ACh receptor subtype mediates physical dependence to morphine: mouse and human studies. Br J Pharmacol. 2014;171(16):3845–3857. doi: 10.1111/bph.12741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nan YM, Kong LB, Ren WG, Wang RQ, Du JH, Li WC, et al. Activation of peroxisome proliferator activated receptor alpha ameliorates ethanol mediated liver fibrosis in mice. Lipids Health Dis. 2013;12:11. doi: 10.1186/1476-511X-12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neziroglu FA, Yaryura Tobias JA, Lemli JM, Yaryura RA. Demographic study of obsessive compulsive disorder. Acta Psiquiatr Psicol Am Lat. 1994;40(3):217–223. [PubMed] [Google Scholar]
- O’Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol Clin Exp Res. 2004;28(11):1676–1682. doi: 10.1097/01.alc.0000145781.11923.4e. [DOI] [PubMed] [Google Scholar]
- Piano MR, Carrigan TM, Schwertz DW. Sex differences in ethanol liquid diet consumption in Sprague-Dawley rats. Alcohol. 2005;35(2):113–118. doi: 10.1016/j.alcohol.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Riddle E, Bush J, Tittle M, Dilkhush D. Alcohol withdrawal: development of a standing order set. Crit Care Nurse. 2010;30(3):38–47. doi: 10.4037/ccn2010862. quiz 48. [DOI] [PubMed] [Google Scholar]
- Saitz R. Introduction to alcohol withdrawal. Alcohol Health Res World. 1998;22(1):5–12. [PMC free article] [PubMed] [Google Scholar]
- Salas R, Main A, Gangitano D, De Biasi M. Decreased withdrawal symptoms but normal tolerance to nicotine in mice null for the alpha7 nicotinic acetylcholine receptor subunit. Neuropharmacology. 2007;53(7):863–869. doi: 10.1016/j.neuropharm.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas R, Main A, Gangitano DA, Zimmerman G, Ben-Ari S, Soreq H, et al. Nicotine relieves anxiogenic-like behavior in mice that overexpress the read-through variant of acetylcholinesterase but not in wild-type mice. Mol Pharmacol. 2008;74(6):1641–1648. doi: 10.1124/mol.108.048454. [DOI] [PubMed] [Google Scholar]
- Salas R, Orr-Urtreger A, Broide RS, Beaudet A, Paylor R, De Biasi M. The nicotinic acetylcholine receptor subunit alpha 5 mediates short-term effects of nicotine in vivo. Mol Pharmacol. 2003;63(5):1059–1066. doi: 10.1124/mol.63.5.1059. [DOI] [PubMed] [Google Scholar]
- Salas R, Pieri F, De Biasi M. Decreased signs of nicotine withdrawal in mice null for the beta4 nicotinic acetylcholine receptor subunit. J Neurosci. 2004;24(45):10035–10039. doi: 10.1523/JNEUROSCI.1939-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas R, Pieri F, Fung B, Dani JA, De Biasi M. Altered anxiety-related responses in mutant mice lacking the beta4 subunit of the nicotinic receptor. J Neurosci. 2003;23(15):6255–6263. doi: 10.1523/JNEUROSCI.23-15-06255.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas R, Sturm R, Boulter J, De Biasi M. Nicotinic receptors in the habenulo-interpeduncular system are necessary for nicotine withdrawal in mice. J Neurosci. 2009;29(10):3014–3018. doi: 10.1523/JNEUROSCI.4934-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Muramatsu T, Takeda A, Shirakura K. Co-occurrence of obsessive-compulsive personality traits in young and middle-aged Japanese alcohol-dependent men. Alcohol Clin Exp Res. 2002;26(8):1223–1227. doi: 10.1097/01.ALC.0000023985.20126.DC. [DOI] [PubMed] [Google Scholar]
- Swan GE, Ward MM, Carmelli D, Jack LM. Differential rates of relapse in subgroups of male and female smokers. J Clin Epidemiol. 1993;46(9):1041–1053. doi: 10.1016/0895-4356(93)90172-w. [DOI] [PubMed] [Google Scholar]
- Trujillo JL, Do DT, Grahame NJ, Roberts AJ, Gorman MR. Ethanol consumption in mice: relationships with circadian period and entrainment. Alcohol. 2011;45(2):147–159. doi: 10.1016/j.alcohol.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umathe S, Bhutada P, Dixit P, Shende V. Increased marble-burying behavior in ethanol-withdrawal state: modulation by gonadotropin-releasing hormone agonist. Eur J Pharmacol. 2008;587(1–3):175–180. doi: 10.1016/j.ejphar.2008.03.035. [DOI] [PubMed] [Google Scholar]
- Uzbay IT, Erden BF, Tapanyigit EE, Kayaalp SO. Nitric oxide synthase inhibition attenuates signs of ethanol withdrawal in rats. Life Sci. 1997;61(22):2197–2209. doi: 10.1016/s0024-3205(97)00922-3. [DOI] [PubMed] [Google Scholar]
- van Gaalen MM, Steckler T. Behavioural analysis of four mouse strains in an anxiety test battery. Behav Brain Res. 2000;115(1):95–106. doi: 10.1016/s0166-4328(00)00240-0. [DOI] [PubMed] [Google Scholar]
- Verleye M, Heulard I, Gillardin JM. The anxiolytic etifoxine protects against convulsant and anxiogenic aspects of the alcohol withdrawal syndrome in mice. Alcohol. 2009;43(3):197–206. doi: 10.1016/j.alcohol.2009.02.003. [DOI] [PubMed] [Google Scholar]
- West RJ, Hajek P, Belcher M. Severity of withdrawal symptoms as a predictor of outcome of an attempt to quit smoking. Psychol Med. 1989;19(4):981–985. doi: 10.1017/s0033291700005705. [DOI] [PubMed] [Google Scholar]
- Wetterling T, Kanitz RD, Besters B, Fischer D, Zerfass B, John U, et al. A new rating scale for the assessment of the alcohol-withdrawal syndrome (AWS scale) Alcohol Alcohol. 1997;32(6):753–760. doi: 10.1093/oxfordjournals.alcalc.a008326. [DOI] [PubMed] [Google Scholar]
- Yoneyama N, Crabbe JC, Ford MM, Murillo A, Finn DA. Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol. 2008;42(3):149–160. doi: 10.1016/j.alcohol.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang RH, Gao JY, Guo HT, Scott GI, Eason AR, Wang XM, et al. Inhibition of CYP2E1 attenuates chronic alcohol intake-induced myocardial contractile dysfunction and apoptosis. Biochim Biophys Acta. 2013;1832(1):128–141. doi: 10.1016/j.bbadis.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]