Abstract
Selective attention to grapheme-phoneme mappings during learning can impact the circuitry subsequently recruited during reading. Here we trained literate adults to read two novel scripts of glyph words containing embedded letters under different instructions. For one script, learners linked each embedded letter to its corresponding sound within the word (grapheme-phoneme focus); for another, decoding was prevented so entire words had to be memorized. Post-training, ERPs were recorded during a reading task on trained and untrained but decodable (transfer) words. We found reaction-time patterns suggesting alphabetic decoding of both trained and transfer words and a left-lateralized, late ERP modulation when decoding transfer as opposed to trained visual words of the grapheme-phoneme focus script. A left-lateralized N170 topography was observed only for the grapheme-phoneme script. Collectively, findings underscore the impact of selective attention to grapheme-phoneme mappings on reading expertise acquisition with implications for recognition of trained words and self-teaching of decodable words.
Keywords: reading expertise, decoding, artificial orthography, attention, phonology, grapheme-phoneme, N170
Graphical abstract
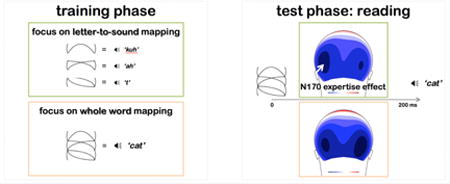
Success in early reading acquisition depends on a learner's ability to master the association between spoken words and their corresponding visual word forms. When learning these mappings, a student may attend to individual letters and link them to sounds within a word, thus focusing on sublexical grapheme-phoneme mappings. Alternatively, the budding reader might attend to larger grain sizes, such as letter clusters, onsets, rimes, or even whole words (Ziegler & Goswami, 2005). Reading development theories concur that gaining robust grapheme-phoneme connections is vital for achieving reading proficiency (Ehri, 1991; Frith, 1985; Gough & Juel, 1991). In addition to promoting the refinement of known word representations (Perfetti, 1991), proficiency in manipulating attained grapheme-phoneme associations serves as a crucial self-teaching device, which enables beginning readers to decode novel words that they have not encountered previously (Share & Stanovich, 1995). Overall, relative to approaches that promote memorization of the spelling patterns of entire words, sublexical phonics-based strategies yield superior reading acquisition outcomes according to behavioral cognitive psychology meta-analyses (e.g., Rayner, Foorman, Perfetti, Pesetsky, & Seidenberg, 2001) and systematic investigations of curriculum effects (e.g., Ehri, Nunes, Stahl, & Willows, 2001).
Reading instruction has valuable potential for directing a student's attention to representations at grain sizes that bolster the acquisition of reliable alphabetic and word knowledge (McCandliss, Beck, Sandak, & Perfetti, 2003). The need for specific attentional guidance during learning is highlighted by the fact that, upon viewing a word, this gamut of grain sizes is available – all at once – to a beginning reader. Additionally, although grapheme-phoneme decoding is typically advantageous, skilled reading of exception words, which cannot be decoded, requires fluent switching between grain sizes. Given the central role of grapheme-phoneme conversion for masterful reading, intentionally directing attention to sublexical specifically subsyllabic mappings might be an essential component of a learner's emerging decoding skills (McCandliss & Yoncheva, 2011). The brain mechanisms of this process remain unexplored yet are pivotal to understanding how instructional strategies can best be harnessed to support the development of the perceptual expertise for reading.
Skilled reading engages specialized brain processes allowing rapid categorization of orthographic input as language. The earliest print-sensitive response indexed in the event-related potential (ERP) is the N170 visual word effect, i.e., greater occipito-temporal negativity within 200 ms of viewing a word relative to a visually matched control stimulus. This N170 response temporally coincides with the time-window that reflects initial word recognition in eye movement studies (for review, see Rayner & Pollatsek, 1989). The educational experience of learning to read one's own language is crucial for the tuning of the N170 effect to the specific properties of one's native script. Language-specific effects in the perceptual expertise for visual words have been reported in literate adults when reading a known relative to an unfamiliar script in another language (Wong, Gauthier, Woroch, DeBuse, & Curran, 2005). Similarly, specific educational impact is evident in pre-literate children who progress through their literacy training to eventually exhibit native script sensitivity, characteristically left-lateralized as in skilled adult readers (Brem et al., 2009). Studies examining the visual word N170 response throughout reading skill accruement often report accompanying modulations of later ERP components (Brem et al., 2006, 2009; Maurer, Blau, Yoncheva, & McCandliss, 2010). These include the N400 component, thought to reflect deeper semantic processing as commonly revealed by comparing expected with unexpected sentence endings (Kutas & Federmeier, 2011), and the Late Positivity Complex (LPC) family of components often attributed to domain-general engagement of learning, attention, and memory functions (Polich, 2007). In the context of reading, LPC effects can be sensitive to old/new word recognition (Friedman, 1990) and explicit/implicit memorization effects frequently observed under repeated word presentation (Rugg et al., 1998). Either or both phenomena might be relevant to emergent decoding skills.
Orthographic (e.g., Dehaene & Cohen, 2011) as well as phonological processes (e.g., Vigneau et al., 2006) are predominantly left-lateralized in fluent readers. Moreover, in the same individual the left lateralization of reading-related activations is tightly coupled with the left lateralization of activations linked to speech perception (Pinel & Dehaene, 2010). Visual and spoken word lateralization are also systematically influenced by both genetic and environmental factors (Pinel et al., 2014). Importantly, selective attention to language can engage this left-lateralized integrated network in a purely top-down fashion in skilled readers: in the absence of visual input, phonological computations recruit the visual word form area yet acoustic non-linguistic judgments on this same word pair do not (Yoncheva, Zevin, Maurer, & McCandliss, 2010). Furthermore, selective attention to phonology can shape perception modulating processes as early as online stimulus encoding via transient, temporally specific engagement of orthographic and phonological regions within the left hemisphere (Yoncheva, Maurer, Zevin, & McCandliss, 2014). The exact relation between such modulations and focus on grapheme-phoneme mappings during learning beckons direct investigation.
The current study: approach, questions, and hypotheses
Motivated by accumulating evidence that selective attention to grapheme-phoneme mappings dynamically recruits left-lateralized linguistic processes, the present ERP study addressed questions central to a beginner's grapheme-phoneme (GP) decoding during reading. Early reading acquisition was modeled in a well-controlled paradigm training literate, adult English speakers to read artificial scripts. This manipulation allowed us to investigate – in relative isolation from other, typically confounded factors (e.g., ongoing print exposure, stimulus properties, training time, and a learner's preexisting attentional biases) – how attentional focus shapes subsequent reading responses. Two artificial scripts were created: a GP script, in which each grapheme mapped consistently onto a single phoneme, and another, whole-word (WW) script, in which the pairing between individual graphemes and corresponding phonemes was arbitrary across training trials. Any putative incidentally acquired knowledge of particular letter-sound associations in the WW script was therefore not generalizable to decoding transfer (not previously trained) words, forcing learners to further attend to WW-level mappings. Training was implemented by concurrent presentation of frequent, single-syllable spoken English words and their corresponding artificial script representations. Following training, each learner completed a reading verification task while we recorded their behavioral and ERP responses.
First, we tested the hypothesis that GP decoding engages left-lateralized processes and examined the time-course of this visual word ERP modulation. The theoretical focus of this paper, the left-lateralized N170 effect, is linked more closely to overall script familiarity rather than knowledge of particular visual word forms (Yoncheva, Blau, Maurer, & McCandliss, 2010); therefore we anticipated decoding-related ERP modulations to manifest after the initial perceptual categorization indexed by the N170 component, while active decoding processes were at play, e.g., during the N400 or the LPC ERP components. Second, we examined how attending to GP mappings during learning biases a learner's subsequent reading. Response latencies were studied first focusing on trials where a visual word did not match the concurrent single-syllable auditory word. Contrasting latencies during verification of syllable onsets (i.e., initial consonant) relative to syllable rimes (i.e., central vowel and final consonant) provided a behavioral assay likely reflective of decoding processes. To more directly link observed effects to the impact of attentional focus on GP grain sizes, behavioral and visual ERP responses to words trained under GP focus were contrasted with responses to words trained under WW focus, since the two training conditions were equated for total exposure and learning time. We expected to find reading expertise effects, indexed by a left-lateralized N170 response, only when the same student read the script learned under selective focus to GP mappings and not under WW focus. Capitalizing on high-density recordings to capture ERP topographic differences and on Bayesian statistics to temporally localize attentional effects, we carried out data-driven analyses unconstrained by a priori assumptions and examined the duration and persistence of effects throughout and following the N170 component.
Materials and Methods
1. Participants
Native English-speaking, right-handed (Oldfield, 1971) volunteers were recruited for the study. Each participant was neurologically healthy with normal hearing and normal, or corrected-to-normal, vision, and was screened for reading disability and prior knowledge of logographic scripts, such as Chinese. To ensure that every subject attended to the training and showed learning progress, accuracy greater than 85% on the average of the two training conditions in the final test of trained words on day 1 was required to invite participants for the second day session, which included the EEG recording (two subjects did not reach criterion). Further, two additional subjects did not meet the ERP data quality criterion detailed in 3.2. ERP pre-processing. Data from 16 participants (mean age: 21.7 years, range: 18 - 31; 8 women) are reported here. Ethical approval was granted by the Institutional Review Board of Vanderbilt University. All subjects were fully briefed and provided written informed consent.
2. Training in artificial orthography
2. 1. Procedure
Each participant learned to associate sets of auditory words with corresponding visual characters under two training conditions: grapheme-phoneme (GP) and whole-word (WW). The training in both conditions was identical except for the different instruction slide in the beginning of the training phase prescribing the use of one of the two strategies: GP (focus on the embedded letter-figures and associate each of the three sounds of the word to a given letter-figure within the glyph) and WW (focus on the whole glyph and link it to the spoken word) (Figure 1).
Figure 1.
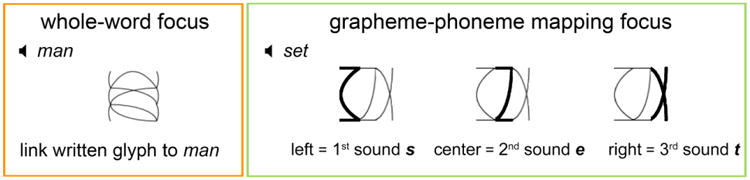
Manipulating attentional focus during training. Each student learned a set of English words focusing on grapheme-phoneme (GP) mappings, and another, in a different script, focusing on whole-word (WW) mappings. Learning conditions and time were identical for the training conditions apart from the instruction slide at the onset of training.
Training took place over two consecutive days, 24 hours apart. On each day, participants were trained on one word list per condition. Training was broken into three alternating training blocks per condition. Each block contained seven repetition of a word. After every training block, participants were tested in a two-alternative forced choice whether the visual word character matched with an auditory word. If the training was in the GP condition, the participant was additionally tested on a transfer list that assessed the accrual of alphabetic knowledge, as indexed by ability to decode transfer words composed of known letter-figures within a character. At the end of the second day, after the six sessions, the participants completed the EEG reading verification task experiment.
At the end of the EEG experiment, participants answered a series of questions probing their adoption of a GP or WW strategy throughout learning. For the WW (control) condition, subjects also elaborated on their personal approach to memorizing the entire characters (e.g., imagining how the shape of the visual character might remind them of the spoken word's meaning). None of these whole-word memorization strategies involved alphabetic knowledge. All subjects reported compliance with the prescribed strategy.
2. 2. Stimuli
Single-syllable, consonant-vowel-consonant English words were created using six consonants (b, d, k, n, s, t) and four vowels (a, u, e, i). The embedded letter-like figures were novel black line drawings on white background, and each character subtended 2.4° horizontal by 2.6° vertical visual angle. The spoken words (average duration = 600 ms; SD = 55 ms) were recorded by a female native English speaker.
2. 3. Word list selection
Each student was trained on 4 lists of English words associated with glyphs. The 4 lists of spoken English words were used to algorithmically generate WW and GP glyph stimuli by converting the initial consonant, vowel, and final consonant into individual glyph segments. This allowed us to counterbalance the four word lists across training condition (GP, WW). At the orthographic level of analysis, the letter position distribution and combination of units were equivalent between the GP and WW sets of glyphs. However, at the level of mapping of orthographic units to phonological representations, the sets differed to preclude possible learning of grapheme-phoneme mappings during the WW training condition. Unlike the GP glyphs, the mapping between WW glyphs and spoken English words was arbitrary, i.e., each “letter” was paired with different sounds during training. For each letter in the GP glyph set, an analogous letter existed in the WW glyph set, and it appeared with equal frequency in each position and within each bigram across the two sets, thereby preserving the statistical letter probabilities at the orthographic level across the two training scripts.
On each of the two training days, a student was trained on two lists that were unique to that training day: one presented within the GP condition and another presented within the WW condition. This also enabled counterbalancing the 4 lists across assignment to training session (first day, second day) and assignment of list to position within training session (first, second).
Each word list consisted of 12 unique words. Every list contained six consonants (appearing in the first and last positions twice in each list) and four vowels (each appearing three times in a list). Two additional six-word transfer lists each with equated letter frequency in each position) were used to monitor progress on using the GP rules acquired during training to decode untrained words. Transfer lists were counterbalanced across the 16 participants. To create mismatch trials, each word in the list was paired with a corresponding foil from the same list, designed to differ at one of two levels of orthographic/phonological position within the syllable: the onset unit (i.e., first consonant) or the rime unit (i.e., the central vowel and final consonant). Thus, mismatch trials either shared onset and differed in rime, or conversely, differed in onset and shared sounds in rime.
3. EEG
3. 1. EEG Reading verification task
The reading verification task was a two-alternative forced choice whether the presented visual word character matched with the auditory word (Figure 2). A trial commenced with fixation (mean duration 750 ms) followed by the presentation of a visual character (mean duration 2000 ms). Next, an auditory word (mean duration 600 ms) was presented at a normally distributed jitter ranging from 667 to 1000 ms after the onset of the visual character. After an average of 3000 ms (2500 – 3500 ms), during which participants could respond, the next trial began. Three types of visual characters were presented in mini-blocks: GP-trained, WW-trained, and GP-transfer (unfamiliar but decodable) words. Each training day contributed an equal number of trained words, resulting in mini-blocks composed of twelve unique words repeated twice, once as a target and once as a foil. Conditions were presented with equal probability in each position over five consecutive mini-blocks. If desired, participants could take breaks between the mini-blocks. Overall, the task lasted approximately 60 minutes. EEG was recorded and ERPs time-locked to onset of the visual character are reported.
Figure 2.
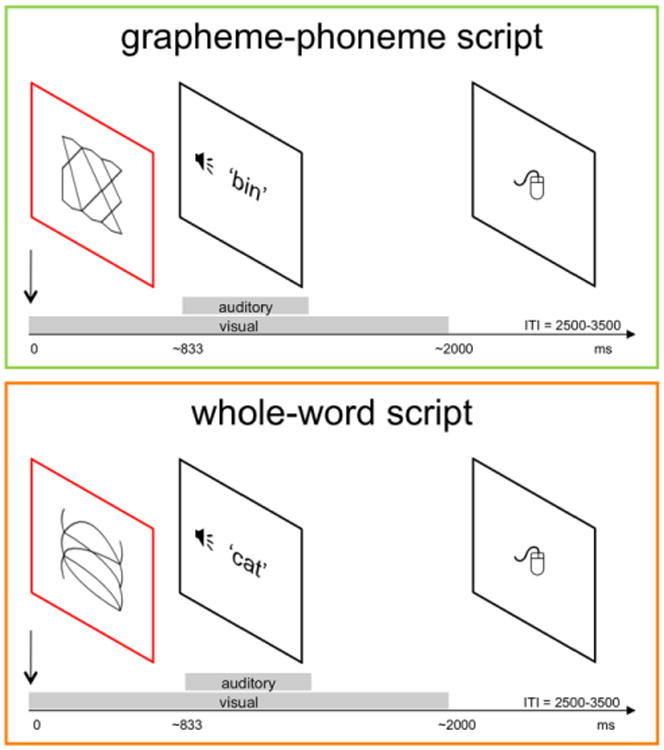
EEG reading verification task. On each trial learners indicated whether or not the written glyph and the spoken word matched. Short blocks of words trained under GP and WW focus were presented. Alphabetic transfer words, i.e., unfamiliar but decodable based on learned GP mappings, were tested in separate blocks. ERPs time-locked to the onset of the visual word were analyzed.
3. 2. EEG recording and preprocessing
The 128-channel EEG was acquired using a Hydrocel Geodesic Sensor Net (Electrical Geodesics Inc., Eugene, Oregon) referenced to the vertex electrode. Data were sampled at 1000 Hz/channel with calibrated technical zero baselines and a low-pass filter set at 0.1 Hz. Electrode impedances were maintained below 50 kΩ. Spline interpolation was applied to channels with excessive artifacts (on average 11.6 channels per subject), followed by eye blink correction in BESA 5.3 Research software (Berg & Scherg, 1994). EEG data were then digitally band-pass filtered (0.1-30 Hz, 24dB/oct), epoched from -200 ms pre-stimulus (visual character) to 800 ms post-stimulus. Artifacts exceeding ±100μV in any channel were automatically rejected. Single-subject averaging was done separately for each condition on correct trials only. In Brain Vision Analyzer 2.0, ERPs were transformed to the average reference (Lehmann & Skrandies, 1980), and grand averages and Global Field Power (GFP) were computed separately for each condition and - for the purposes of adaptive segmentation and quantitative ERP quality control - collapsed across all conditions. Any individual that had did not produce a GFP peak N1 response that was equal to or greater than nine times the baseline GFP response was excluded from further analysis.
3. 3. ERP analyses
The analysis strategy involved a data-driven approach sensitive to topographic differences, including lateralization (Yoncheva, Blau, et al., 2010; Yoncheva et al., 2014). First, we identified time-intervals where significant differences between conditions of interest occurred, indicating differential perceptual and/or cognitive processing (i.e., segmentation). Then, ERPs were averaged across these time-intervals, and the resulting segments were directly contrasted between conditions with respect to map strength, topography, and latency.
3. 3. 1. Segmentation
Topographic bootstrapping tests (topographic analysis of variance, TANOVA (Strik, Fallgatter, Brandeis, & Pascual-Marqui, 1998)) on raw ERP maps were conducted because TANOVA detects systematic topographic differences and overall amplitude variations between the contrasted conditions. To this end, global dissimilarity for each time-point was computed (Lehmann & Skrandies, 1980; Strik et al., 1998), and subsequently a probability distribution was created (randomization test with 5000 resamplings (Manly, 1991)). Finally, a z-score of the original dissimilarity in relation to its respective distribution was calculated. The multiple comparisons problem was addressed by an unified algorithm, which first fitted an empirical null model and then estimated tail, area-based and local, density-based false discovery rates based on a modified Grenander-density estimator (Strimmer, 2008a). This approach was chosen for several reasons. First, the empirical model fitting deals with the time-sample correlations inherent to the time domain (Efron, 2007). Additionally, the truncation point for model fitting is selected to minimize false non-discovery rate, i.e., type II error, increasing leverage in interpreting both significant and non-significant findings (Efron, 2004). Finally, the estimated local false discovery rate represents the empirical Bayesian posterior probability of the null hypothesis, which is readily interpretable unlike some variants of corrected p-values. The fdrtool algorithm, strimmerlab.org/software/fdrtool (Strimmer, 2008b), as part of the R package archive from CRAN (R Development Core Team, 2007) was used for this analysis with input z-scores for each time-point, separately for each condition of interest. Statistical significance was set at local fdr p<0.05. This procedure was previously applied to other independent ERP data sets (Yoncheva, Maurer, Zevin, & McCandliss, 2013; Yoncheva et al., 2014). Finally, to address which robust ERP components were modulated by experimental manipulation as revealed by TANOVA differences, an adaptive segmentation based on GFP minima was also performed (Lehmann & Skrandies, 1980) indicating that 72-144 ms corresponded to the P1; 144-306 ms corresponded to the N1, while late positivity complex (LPC) components spanned 306-375 and 375-581 ms.
Given our two central questions, separate segmentation was carried out for each condition of interest.
3. 3. 1. 1 Effects of alphabetic decoding
To isolate when reading not previously trained but decodable words modulated the ERP response, a raw-map TANOVA contrasting GP-transfer with GP-trained words was adopted in the 0-500 ms range. Significant differences were found in three time-intervals: 324-336 ms; 363-387 ms and 399-453 ms (local fdr p<0.05, fitting parameters: η0=0.179 with SD=0.32). These intervals were used as time-windows for LPC segmentation. Due to our a priori interest in lateralization, for these LPC modulations we focus on positive centroid positions on the x-axis.
3. 3. 1. 2 Effects of attentional training focus
To isolate when words trained under GP focus were differentially processed from ones trained under WW focus, a direct raw-map TANOVA contrasting GP-trained with WW-trained words was performed for each time-point in the 0-500 ms range. Significant differences were found only from 197 to 222 ms (local fdr p<0.05, fitting parameters: η0=0.946 with SD=1.28). This time-interval was characterized by an N170 topography and concurrent with the N1 component, and thus was selected as the N170 segmentation interval.
3. 3. 2. Map strength, topography, and latency summary measures
Within each TANOVA-selected time-segment individual's potentials were averaged to produce a mean map for each condition. The topography of each mean map was reduced to six descriptors using 3D centroids (averaged, voltage-weighted electrode positions of positive and negative map areas (Brandeis, Vitacco, & Steinhausen, 1994) in Talairach space). Between-condition position differences of the negative centroids specifically on the x-axis (left-right) were reported to test the hypothesis that attentional focus modulates N170 lateralization. Map strength was indexed by its GFP (i.e., the spatial standard deviations of the voltages in that map) (Lehmann & Skrandies, 1980). In line with our whole-map analysis approach, latencies were determined via topographical component recognition (TCR) (Brandeis, Naylor, Halliday, Callaway, & Yano, 1992; methodological strengths discussed on page 273 in Luck, 2014). The TCR approach has been successfully used across different investigations of N170 lateralization (Brem et al., 2006; Maurer, Zevin, & McCandliss, 2008). First, a template map was created by computing the grand-averaged map within TANOVA-selected segments, averaged across conditions from potentials that have been GFP-normalized in order to prevent bias by stronger maps. Then, within the boundaries of the relevant ERP component, the topographically most similar time-point in individual's ERP (as determined by maximal correlation) was selected as the latency for that condition.
3. 3. 3. Statistical analyses
Analyses of GFP, centroid locations, and latencies were conducted in SPSS v18. Multivariate analyses of variance (MANOVA) for repeated measures with within-subject factor learning condition (WW-, GP-trained characters) were performed, as well as planned comparisons separately of GP-trained with GP-transfer words. The centroid analyses included polarity (positive, negative centroid) as an additional factor, and the three location dimensions of the centroids (x-, y-, and z-axes) were treated as multivariate dependent measures. Polarity is only reported when it interacts with other factors. Effects on the x-axis indicate lateralization effects: negative x-values correspond to left lateralization.
Finally, to facilitate comparison with conventional ERP analyses, the grand-average waveforms time-locked to visual word onset, shown, separately for GP-trained, GP-transfer, and WW-trained words are presented. Nine non-overlapping channel clusters are created by selecting the approximate 10-10 equivalents of hallmark channels (Luu & Ferree, 2000), finding their immediate neighbors, and averaging ERP potentials within the cluster. The resulting clusters are: “FC5” (E28 and E20, E24, E27, E29, E34, E35); “FCz” (E6 and E5, E7, E12, E13, E106, E112); “FC6” (E117 and E110, E111, E116, E118, E123, E124); “CP5” (E47 and E41, E42, E46, E51, E52); “CPz” (E55 and Cz, E31, E54, E79, E80); “CP6” (E98 and E92, E93, E97, E102, E103); “PO7” (E65 and E58, E59, E64, E66, E69, E70); “POz” (E72 and E62, E67, E71, E76, E7); “PO8” (E90 and E83, E84, E89, E91, E95, E96).”
4. Behavioral data analyses
To assess overall processing difficulty across tasks, accuracy and reaction times (RT; 5% trimmed mean RTs on correct trials reported in ms from spoken word onset) were computed for each condition. First, echoing the ERP analyses, planned comparisons were performed contrasting GP-trained with GP-transfer words (alphabetic decoding effect) and contrasting GP-trained with WW-trained words (attentional training focus effect). Additionally, to directly assess the potential contribution of task difficulty (average of z-scored RTs and error rates) as a mediator of the differential N170 lateralization for GP relative to WW training, a bootstrap Sobel test followed the main analysis (Sobel, 1982).
Secondly, to link more directly grain size influences on decoding spoken words trained under GP focus to alphabetic decoding, a finer-grain RT examination was carried out based on the relationship between the visual and the auditory word within a trial. This analysis capitalized on the trial design, which manipulated the position of the initial disambiguating information for deciding whether or not the visual word corresponded to the single-syllable spoken word. Initial mismatch could occur at the onset unit of the syllable (e.g., /t-i-n/ vs. /k-i-d/) or at the rime unit (i.e., /k-æ-t/ vs. /k-i-d/). Trial types based on whether the onset within a ‘no’ trial was the same or different were collapsed and contrasted directly within each condition (GP-trained, GP-transfer, WW-trained). Our trial types could not be segregated to uniquely inform second relative to third letter mismatches within the rime unit of the syllable due to practical constraints. Namely, foils were engineered to be English words that were being taught in the same training session and the number of combinations of six consonants (first and third position) and four vowels (always second position), which create frequent English words, is inherently limited. Additionally, within each of the 3 training conditions, we contrasted ‘yes’ with ‘no’ trials. Within ‘no’ trials, higher RTs for shared onset than for different onset would indicate an impact of grain sizes on RTs and plausibly underlying alphabetic decoding. Such onset verification effect is expected most notably for GP-transfer words, but also for GP-trained words, if participants still focus on the GP mappings acquired throughout the learning. WW-trained words should produce equivalent RTs. Directly contrasting ‘yes’ with ‘no’ trials for the GP-trained and WW-trained words would help inform whether differential facilitation for trained exemplars is produced by the two attentional focus training conditions. For WW trained words, endorsements of familiar words are expected to be faster than rejections.
Results
1. Behavioral results
Performance in the reading verification task for words trained under WW focus was more accurate (errors: 5.1% ± 1.3 vs. 12.4%±1.5: t(15)=8.58, p<0.001) and faster (1022.5ms±59.9 vs. 1288.7ms±67.0: t(15)=5.54 p<0.001) than for words trained under GP focus, in line with previous short-term training results (Yoncheva, Blau, et al., 2010).
Importantly, accuracy for transfer words was much greater than chance (87.2%±2.4 vs. 50%: t(15)=15.37, p<0.001). Further, despite slower reaction times (1372.1ms±64.3 vs. 1288.7ms±67.0: t(15)=4.9, p<0.001), the equivalent error rate for GP-transfer relative to GP-trained words (12.8%±2.4 vs. 12.4%±1.5: t(15)=0.32, p=0.76) suggested that participants were actively engaged in GP decoding when reading GP words, be it not previously trained or familiar ones (Table 1).
Table 1.
Behavioral performance (RTs and error rates) on the reading verification task based on training condition.
| GP-trained | WW-trained | GP-transfer | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| Mean | SEM | Mean | SEM | Mean | SEM | |
| Reaction Times (ms) | 1288 | 67 | 1022 | 60 | 1372 | 64 |
| Errors (%) | 12.4 | 1.5 | 5.1 | 1.3 | 12.8 | 2.4 |
A finer-grain analysis directly assessing how the position of letter-sound mismatch impacts response latencies revealed modulation patterns that notably differed between the two scripts while remaining broadly consistent within the GP script. When decoding GP-transfer words, participants were faster to reject foils where the mismatch was at syllable onset rather than syllable rime (t(15)=2.29, p<0.05). An analogous trend was detected when decoding GP-trained words (t(15)=1.31, p=0.1). It was confirmed that for the WW script, a mismatch at onset relative to rime did not affect response latencies (t(15)=0.06, p=0.5). Notably, for the WW script, ‘yes’ trials were faster than ‘no’ trials (t(15)=-4.57, p<0.0005), as expected for over-trained exemplars. In contrast, latencies to reject GP-transfer foils showed the opposite pattern and were greater than these to endorse a match, t(15)=2.30, p<0.05. GP-trained words showed no difference between ‘yes’ and ‘no’ trials, t(15)=0.75, p=0.5 (Figure 3).
Figure 3.
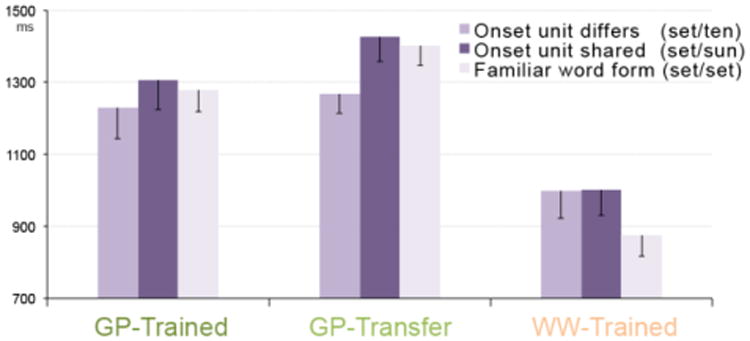
Behavioral performance in the reading verification task based on visual/auditory stimulus pairing within a trial, separately for each attentional condition. Reaction times reported from the onset of the spoken word. On mismatch trials, we manipulated whether the onset syllable unit was shared (and the rime unit differed) or whether the onset syllable unit differed between the visual and the spoken word. Match trials presented familiar words. Onset verification was significantly faster relative to rime verification for GP-transfer words, as well as GP-trained words. WW-trained words show an RT advantage of word familiarity.
2. ERP results
2. 1. Differences between GP-trained and GP-transfer in consecutive ERP maps
Differential ERP responses over time based on visual word familiarity were evaluated using a topographic analysis of variance (TANOVA). Significant differences based on TANOVA were observed at 324-336 ms (termed here LPC1); 363-387 ms (LPC2) and 399-453 ms (LPC3). All three were characterized by LPC topography.
2.1.1. Topographic centroid effects
Left-lateralization of the positive centroid in the GP-transfer condition was tested in each of the three LPC segments (LPC1: t(15)= -0.92, p=0.19; LPC2: t(15)=-0.11, p=0.46; LPC3: t(15)=-1.63, p=0.06). Further investigation of the LPC3, the component showing the strongest left-lateralization for GP-transfer words, demonstrated that relative to WW-trained words, GP-transfer topographies were significantly more left-lateralized (t(15)= -2.44, p<0.05) (Figure 4). This held also for GP-trained words relative to WW-trained words during the LPC3 (t(15)=1.91, p<0.05). For the other two components (LPC1 and LPC3), there was no difference between GP-transfer and WW-trained (tmax=0.94, pmin>0.4) and no difference between GP-trained and WW-trained (tmax=1.54, pmin>0.15).
Figure 4.
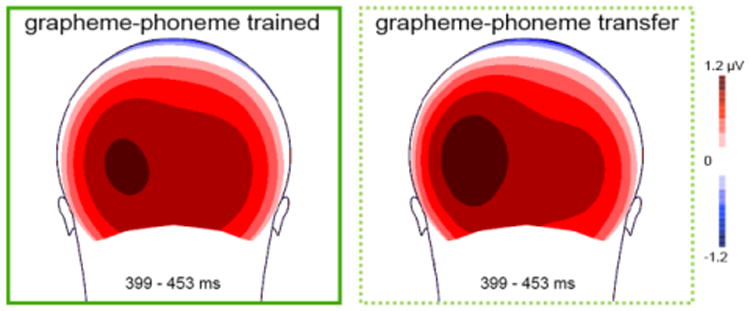
Decoding effects in visual word ERPs. Decoding visual words that have not been explicitly trained (GP alphabetic transfer words) elicits a more left-lateralized ERP response during the late positivity complex (399 – 453 ms) relative to reading trained words written in the same GP script as seen in grand-averaged voltage maps.
2.1.2 GFP
GFP for GP-trained and GP-transfer words were generally comparable (LPC1: t(15)=1.92 p=0.074; LPC2: t(15)=0.39 p=0.70; LPC3: t(15)=1.13 p=0.28).
2.1.3. Latency
No differences were found between GP-trained and GP-transfer latencies (LPC1: t(15)=-0.64 p=0.53; LPC2: t(15)=1.26 p=0.23; LPC3: t(15)=0.43 p=0.97).
2. 2. Differences between GP and WW-trained words in consecutive ERP maps
Differential ERP responses over time based on training focus were evaluated using a topographic analysis of variance (TANOVA). Processing visual words trained under GP as opposed to WW focus differed (fdr p<0.05) in the 197-222 ms time-range, which was characterized by an N170 topography. Further, an adaptive segmentation approach (collapsed over training strategy, thus reflecting the robust N1 ERP response associated with visual word processing) corroborated that the 197-222 ms segment fell within the boundaries of the N1 component. Hence, this N170 segment was selected for further investigation.
2.2.1. Topographic centroid effects
Centroid distribution differed between WW-trained and GP-trained words (multivariate MANOVA: polarity by training F(3,13) = 6.098, p < 0.01; x-axis univariate: polarity by training F(3,13) = 4.707, p < 0.05) with the negative centroid along the x-axis (i.e., left-right) being more negative for GP-trained than for WW-trained words, indicating a more left-lateralized N170 negativity for GP-trained words (Figure 5). The Sobel test indicated that task difficulty was not a significant mediator of the differential – by training condition – N170 lateralization (z=0.16, p=0.85).
Figure 5.
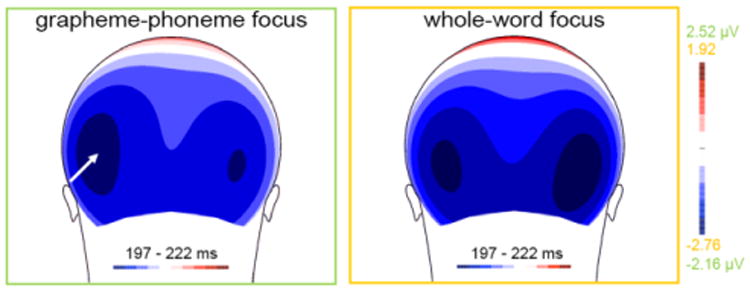
Training focus modulates N170 lateralization during subsequent reading. Reading words trained under GP focus elicits a significantly more left-lateralized N170 topography relative to words trained under WW focus as evident over the 197 – 222 ms N170 interval in the grand-averaged voltage maps for each training condition.
2.2.2. GFP
Reading GP-trained words evoked smaller GFP than reading WW-trained words (1.55 μV±0.2 vs. 1.81±0.2 μV: t(15)=2.92 p<0.05).
2.2.3. Latency
No significant differences were found between GP- and WW-trained word latencies (t(15)=1.08, p=0.30).
2. 3. Planned comparison: N170 of GP-trained and GP-transfer words
No significant whole-map differences between GP-trained and GP-transfer words were present in the N170 interval. Nevertheless, since a robust N170 response was notable in both conditions, the positions of the negative centroids on the x-axis were directly compared between the two conditions. N170 lateralization was equivalent for GP-trained and GP-transfer words (t(15)=1.22 p=0.24). No N170 latency differences were detected (t(15)=0.73 p=0.48). GFP when reading GP-trained and GP-transfer words was also comparable (t(15)=0.43 p=0.67).
Figure 1 illustrates the lack of decoding effect (GP-trained/GP-transfer contrast) during the N170 interval at the waveform level, particularly at left relative to right posterior sites (PO7 and PO8 respectively).
Discussion
Selective attention to grapheme-phoneme (GP) mappings throughout training in an artificial script drastically biased behavioral and ERP responses in later tests. Specifically, reaction time patterns showed evidence of differential onset relative to rime verification when reading GP words suggesting that alphabetic decoding was readily engaged upon subsequent encounters of the GP script. Notably, decoding visual words that were not previously trained, as opposed to familiar words in the same GP script, produced a left-lateralized ERP modulation of the late positivity complex (LPC) response. Finally, despite identical stimulus exposure and training times for the two scripts, the left-lateralized N170 perceptual expertise for visual words was only observed when reading words that had been trained under GP attentional focus and not under WW focus.
Decoding familiar visual word forms
Greater left lateralization when decoding words in the GP script that were not previously trained relative to familiar ones was found in a time-window characterized by whole-map divergence (fdr p<0.05) based on familiarity. The LPC topography characterizing this segment would be broadly consistent with domain-general modulations of post-perceptual processes (Polich, 2007). For instance, differential LPC engagement might be associated with differential memory strength for entire words relative to constituent letters (Friedman, 1990). Furthermore, decoding transfer words might engage explicit recall of grapheme-phoneme mappings to a greater extent than decoding trained words. In the context of reading expertise development, recent studies seem to show additional later ERP modulations of visual word processing at least under implicit reading conditions (Brem et al., 2006, 2009). Such observations might speculatively be related to differential automaticity of the emergent decoding skills that are not specifically needed for task performance. This interpretation dovetails with our previous findings of training-induced ERP changes that showed an LPC-like modulation in addition to the N170 learning effect, when probed in a phonologically shallow implicit reading task (Maurer et al., 2010).
Interestingly, the modulated time-window showed a significantly right-lateralized topography in the WW script suggesting lack of engagement of left-lateralized linguistic processes to any recently trained, control script. Notably, the decoding ERP effect was not merely a sustained modulation of lateralization carried over from the N170 effect but seemed to reflect a distinct engagement of decoding processes. Not inconsistent with a growing body of studies mapping phonological to orthographic influences (Pattamadilok, Knierim, Kawabata Duncan, & Devlin, 2010; Pinel et al., 2014), we recently suggested that attentional influences may act on the early integration of grapheme and phoneme representations. Specifically, an fMRI study revealed that a left-lateralized network, including the visual word form area, was differentially engaged when focusing attention on phonological as opposed to general acoustic features (Yoncheva, Zevin, et al., 2010). This was the case when contrasting two equally difficult tasks performed on identical auditory stimuli (combining speech and tones), thus isolating the impact of attentional focus on phonology, and demonstrating how such focus impacts regions associated with orthography, even in the absence of any visual stimulation. In a parallel paradigm, ERP responses during stimulus encoding were shown to be modulated by intentionally focusing on phonological distinctions within spoken words (Yoncheva et al., 2014). In both the fMRI and the ERP studies, these top-down attentional effects were observed without visual word presentation. In light of these convergent findings, the current left-lateralized engagement may reflect aspects of acquired print-to-speech associations, rather than mere attentional biases on phonological processing.
Behavioral response latency analyses at a finer grain size scale (Ziegler & Goswami, 2005) provided a more direct link to decoding processes, inaccessible in aggregate RTs. The central question of the current experiment involved isolating selective attention, which required task demands, i.e., reading verification, to be kept identical across all attentional focus conditions. However, we manipulated, within the constraints of a limited set of frequent English words, the visual-auditory stimulus pairing in the ‘no’ trials to allow examining the effect of onset relative to rime verification on behavioral performance. When rejecting mismatching pairs in the GP script, faster RTs were observed when the first sound was the different from, rather than the same as, the first letter. Importantly, this onset/rime verification auditory RT effect was present for both transfer and trained words, albeit as a non-significant trend for trained items. In contrast, the manipulation of grain size in stimulus pairing produced no effect on behavioral performance for the WW script. The finding that onset match/mismatch biased responses within the GP script, but elicited no differences when reading the WW script suggests involvement of active GP decoding when processing the GP script. Secondly, in the WW script, latencies to endorse ‘yes’ trials were found to be much faster than ones to reject mismatching ‘no’ trials. Despite identical training times for the two scripts, no such RT advantage was found for processing the well-rehearsed, over-learned GP stimulus exemplars. Thus, the overall behavioral pattern of GP trained items echoed more closely that of GP transfer words, rather than WW trained words. This provides complementary evidence that even after extensive training, reading trained words in the GP script can evoke active decoding processes. The present GP visual ERP effects also likely reflect these trained patterns of selective attention to GP mappings.
Single-letter processing seems to elicit differential responses relative to letter strings in skilled readers (Coch, Hart, & Mitra, 2008; James, James, Jobard, Wong, & Gauthier, 2005; Stevens, McIlraith, Rusk, Niermeyer, & Waller, 2013). Intriguingly, it is the relative pattern of lateralization of the N170 effect that differs between single letter and letter string viewing (Stevens et al., 2013). Moreover, elegant developmental work suggests that the type of experience through which reading knowledge is accrued (e.g., writing or viewing) can powerfully modulate the circuitry subsequently engaged in reading (Butler, James, & James, 2011; James, 2010). Finally, far from being static, along its protracted development a learner's performance typically shows differential characteristics, proposed to reflect distinct reading phases relevant to the degree to which alphabetic processing has been consolidated (e.g., amalgamation theory Ehri, 1991), which naturally interacts with propensity to focus on different grain sizes. Our current visual ERP and behavioral findings further highlight the importance of delineating the factors relevant to decoding as opposed to whole word familiarity in order to ensure that the budding reader's attention is focused on the appropriate grain sizes.
Training focus impacts subsequent reading expertise recruitment
Under identical task demands – reading verification – and following identical training time, whole-map topographic ERP differences (fdr p<0.05) between WW and GP training conditions were observed only during the time interval coinciding with the typical engagement of the N170 perceptual expertise for reading. The only difference between the two conditions was that the same learner was prevented from using a GP strategy when training on the WW script. Notably, the current within-subjects design controls for relevant differences among learners, which might generate biases in the degree of a student's engagement in one strategy (e.g., GP) relative to another (e.g., WW) during learning (for an example of such effects in extreme preference readers, see Wise, Yoncheva, & McCandliss, 2011). Additionally, the subjects we report on had no prior knowledge of logographic scripts, which minimized potential meaningful inter-individual learning differences due to propensity or skill to focus on WW as opposed to GP strategy.
The functional asymmetry of visual word processing in skilled readers holds interest from different theoretical perspectives. After assessing genetic and environmental factors, Pinel and colleagues have argued that an individual's lateralization of spoken language shapes the extent to which their responses to visual words are more strongly left-lateralized (Pinel et al., 2014). Further, Seghier and Price used fMRI to delineate sub-regions of left ventral occipito-temporal cortex, demonstrating spatial segregation of sub-regions predominantly activated by semantic as opposed to visual properties of written words (Seghier & Price, 2011). We have theorized that selective attention to phonology drives the development of the left-lateralized reading expertise effect (McCandliss & Yoncheva, 2011) and have supported this idea with empirical demonstrations that attentional focus on language can co-activate phonological and left-lateralized orthographic circuits (Yoncheva et al., 2014; Yoncheva, Zevin, et al., 2010). Most theories concur that examining the relative strength of hemispheric contributions to reading-relevant brain responses is more fruitful than merely ascribing processes to each hemisphere in isolation from the other (McCandliss & Yoncheva, 2011; Pinel & Dehaene, 2010; Seghier & Price, 2011). Indeed, relative lateralization assays have provided valid correlates of reading processing (Brem et al., 2009; Maurer et al., 2010, 2008; Yoncheva, Blau, et al., 2010) and have collectively suggested that the degree of N170 lateralization may be influenced by training focus, task demands, and familiarity with script. Against this backdrop, our investigation highlights the unique contribution of a specific process – selective attention to GP mappings – as key to the recruitment of the predominantly left-lateralized language network.
Short-term training lasting only two days was sufficient to produce robust ERP differences when learners read a novel script. An analogous pattern of ERP and behavioral results has been shown even following a twenty-minute learning session (Yoncheva, Blau, et al., 2010). Such findings are consistent with recent demonstrations of lateralization effects in the fusiform gyrus elicited by differential alphabetic as opposed to logographic training over eight consecutive days (Mei et al., 2013). Likewise, modulations in activity of the visual word form area were evident after a three-day language training (Song, Bu, Hu, Luo, & Liu, 2010). Undoubtedly, perceptual expertise for reading develops progressively over years of exercising and honing one's decoding skills (Brem et al., 2006). Gaining perceptual expertise for laboratory-trained objects, e.g., Greebles, also requires multiple hours of practice (reviewed in Bukach, Gauthier, & Tarr, 2006). Therefore, the short time-scale of such adult training study findings on the order of minutes or days might appear puzzling. The current fast emerging left-lateralized N170 effects are explained well by an assimilation learning account (Nelson, Liu, Fiez, & Perfetti, 2009), which would suggest that skilled readers recruit their specialized network mediating perceptual expertise for visual linguistic information during novel learning and subsequent reading activities that notably pose the same demands as reading in the native language. The finding that only GP focus training produced novel script assimilation into literate adult's reading expertise lends further support to the idea that selective attention to GP mappings in particular is central in biasing which brain networks get trained, and consequently honed, as a student progresses with training.
The ERP modulation we found based on prior attentional carry-over notably manifested as a greater left-lateralization of the N170 topography to the alphabetic GP script in contrast with the more right-lateralized N170 to the WW trained script. To further refine the interpretation of the current ERP findings as reflecting attentional focus on grapheme-phoneme mappings, below we differentiate this construct from more general, perceptually driven forms of attention known to bias early ERPs. First, visuo-spatial attention are commonly characterized by retinotopic organization and a latency corresponding to the P100 component of the visual ERP (Woldorff et al., 1997). All current effects took place after this time-window, and this was the case irrespective of whether attention was putatively directed left-to-right (current findings) or top-to-bottom (Yoncheva, Blau, et al., 2010). Secondly, hierarchically organized figures might engage focus on local as opposed to global stimulus features. However, specific predictions regarding the timing and direction of ERP lateralization differences remain complex given its sensitivity to experimental context (Beaucousin et al., 2011; Hübner, 2014). Finally, the current ERP findings could be potentially considered along the learning trajectory from controlled, attention-demanding processing to automatized processing (Schneider & Shiffrin, 1977). After only two days of training with room left to improve performance, and given that acquisition of perceptual expertise takes many hours over multiple sessions of training (reviewed in Bukach et al., 2006) it is reasonable to assume that across all conditions subjects were still in the early stages of learning well before automatic, attention-free processing. Together, these considerations support the notion that the current findings tap into a distinct, domain-specific type of attention to linguistic information.
A left-lateralized N170 response during reading verification of GP words was observed relative to the more right-lateralized N170 response for the WW script despite superior behavioral performance when reading the WW script. This opposing pattern of ERP and behavioral results mirrors directly our previous findings (Yoncheva, Blau, et al., 2010). Such correspondence might raise the question of whether the lateralization of the ERP effect reflects mere domain-general task difficulty differences in processing the two scripts. A direct test demonstrated that task difficulty (reaction times or error rates) was not a significant mediator of the effect of training condition on lateralization. Collectively, these findings indicate that the left lateralization of the N170 response robustly evoked by the GP script cannot be reduced to task difficulty differences and instead indexes the GP focus exercised during learning.
Conclusion
Collectively, the current results demonstrate how the left-lateralized networks that sub-serve phonological processing get engaged under different training focus conditions and differential need for active decoding during reading. Since the whole gamut of grain sizes – from graphemes to entire words – is typically present during exposure to print, focusing beginning learners' attention is invaluable to ensure that their emerging decoding skills are best supported and that perceptual expertise for visual words is fluently integrated with left-lateralized phonological regions. Notably, we show that the footprints of instruction are evident well beyond training. The present findings underscore the role of selective attention to grapheme-phoneme mappings during training in reading expertise acquisition with implications not only for recognizing trained visual words but also for self-teaching of unfamiliar but decodable words.
Supplementary Material
Supplementary Figure 1. Individual plots of the grand-averaged waveforms time-locked to the onset of the visual word at each of the 128 electrodes separately for every experimental condition (GP-trained, WW-trained and GP- transfer words).
Figure 6.
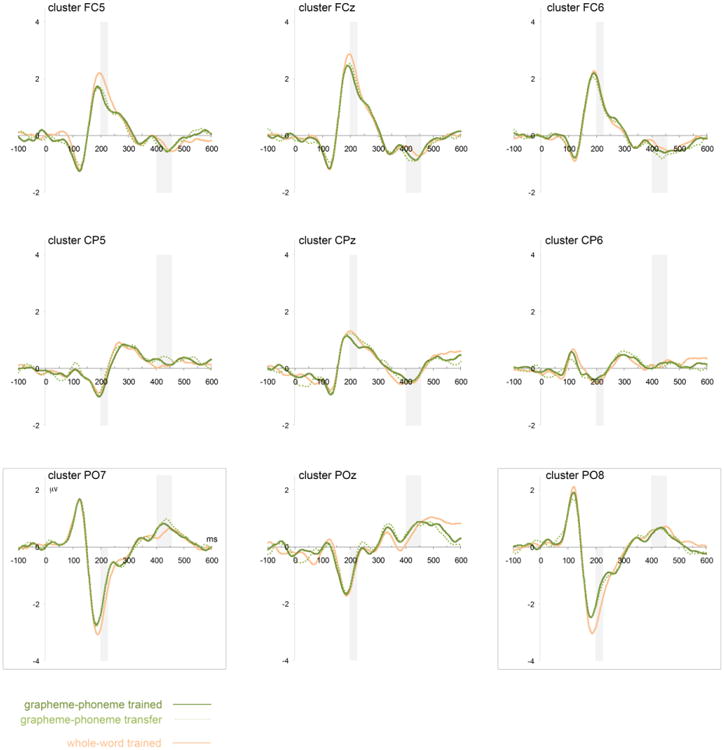
Grand-average waveforms time-locked to the onset of the visual word within the reading verification task at nine channel clusters. Three conditions are shown separately: words that have been previously trained in the GP script (GP-trained, solid dark green), words that have not been previously trained in the GP script but are decodable based on GP-mappings (GP-transfer, dotted green), and words that have been trained in the WW script (WW-trained, solid gold). The 9 sites cover approximately the 10-10 equivalents of fronto-central (top row), centro-parietal (middle row), and occipito-parietal (bottom row) in the left hemisphere (odd numbered channels, leftmost column), midline, and the right hemisphere (even numbered channels, rightmost column). Gray vertical rectangles illustrate significant intervals (fdr p<0.05) based on the whole-map TANOVA analyses. Highlighted are the two sites – PO7 and PO8 – in which the whole-map the effect of attentional focus during learning (contrasting GP-trained with WW-trained words in the N170 segment) and the effect of decoding (contrasting GP-trained with GP-transfer words in the LPC segment) are most prominent. Notable also is the lack of differences between GP-trained and GP-transfer word processing during the N170 interval.
Highlights.
Training shapes N170 and later LPC responses to visual words in a new writing system
When training biases attention to graphemes vs whole words ERP lateralization changes
Focusing on phonemes during training leads to a more left lateralized visual N170 ERP
Alphabetic training generalizes to decoding novel words and N170 lateralization
N170 lateralization is not mediated by variations in training condition's difficulty
Acknowledgments
This research was partially supported by grant T32MH067763. We thank the anonymous reviewers for constructive feedback on a previous version of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beaucousin V, Cassotti M, Simon G, Pineau A, Kostova M, Houdé O, Poirel N. ERP evidence of a meaningfulness impact on visual global/local processing: when meaning captures attention. Neuropsychologia. 2011;49(5):1258–66. doi: 10.1016/j.neuropsychologia.2011.01.039. [DOI] [PubMed] [Google Scholar]
- Berg P, Scherg M. A multiple source approach to the correction of eye artifacts. Electroencephalography and Clinical Neurophysiology. 1994;90(3):229–241. doi: 10.1016/0013-4694(94)90094-9. [DOI] [PubMed] [Google Scholar]
- Brandeis D, Naylor H, Halliday R, Callaway E, Yano L. Scopolamine effects on visual information processing, attention, and event-related potential map latencies. Psychophysiology. 1992;29:315–336. doi: 10.1111/j.1469-8986.1992.tb01706.x. [DOI] [PubMed] [Google Scholar]
- Brandeis D, Vitacco D, Steinhausen HC. Mapping brain electric micro-states in dyslexic children during reading. Acta Paedopsychiatrica. 1994;56:239–247. [PubMed] [Google Scholar]
- Brem S, Bucher K, Halder P, Summers P, Dietrich T, Martin E, Brandeis D. Evidence for developmental changes in the visual word processing network beyond adolescence. NeuroImage. 2006;29:822–837. doi: 10.1016/j.neuroimage.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Brem S, Halder P, Bucher K, Summers P, Martin E, Brandeis D. Tuning of the visual word processing system: Distinct developmental ERP and fMRI effects. Human Brain Mapping. 2009;30(6):1833–1844. doi: 10.1002/hbm.20751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukach CM, Gauthier I, Tarr MJ. Beyond faces and modularity: the power of an expertise framework. Trends in Cognitive Sciences. 2006;10:159–166. doi: 10.1016/j.tics.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Butler AJ, James TW, James KH. Enhanced multisensory integration and motor reactivation after active motor learning of audiovisual associations. Journal of Cognitive Neuroscience. 2011;23(11):3515–28. doi: 10.1162/jocn_a_00015. [DOI] [PubMed] [Google Scholar]
- Coch D, Hart T, Mitra P. Three kinds of rhymes: An ERP study. Brain and Language. 2008;104(3):230–243. doi: 10.1016/j.bandl.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Cohen L. The unique role of the visual word form area in reading. Trends in Cognitive Sciences. 2011;15(6):254–62. doi: 10.1016/j.tics.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Efron B. Large-scale simultaneous hypothesis testing: the choice of a null hypothesis. Journal of the American Statistical Association. 2004;99:96–104. [Google Scholar]
- Efron B. Correlation and large-scale simultaneous significance testing. Journal of the American Statistical Association. 2007;102:93–103. [Google Scholar]
- Ehri LC. Learning to read and spell words. In: Rieben L, Perfetti CA, editors. Learning to read: basic research and its implications. Hillsdale, NJ: L. Erlbaum Associates; 1991. pp. 57–73. [Google Scholar]
- Ehri LC, Nunes SR, Stahl SA, Willows DM. Systematic phonics instruction helps students learn to read: Evidence from the national reading panel's meta-analysis. Review of Educational Research. 2001;71:393–447. [Google Scholar]
- Friedman D. ERPs during continuous recognition memory for words. Biological Psychology. 1990;30(1):61–87. doi: 10.1016/0301-0511(90)90091-a. [DOI] [PubMed] [Google Scholar]
- Frith U. Beneath the surface of developmental dyslexia. In: Patterson K, Marshall J, Coltheart M, editors. Surface dyslexia: Neuropsychological and cognitive studies of phonological reading. London: Erlbaum; 1985. pp. 301–330. [Google Scholar]
- Gough P, Juel C. The first stages of word recognition. In: Rieben L, Perfetti CA, editors. Learning to read: basic research and its implications. Hillsdale, NJ: L. Erlbaum Associates; 1991. pp. 47–56. [Google Scholar]
- Hübner R. Does attentional selectivity in global/local processing improve discretely or gradually? Frontiers in Psychology. 2014;5:61. doi: 10.3389/fpsyg.2014.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James KH. Sensori-motor experience leads to changes in visual processing in the developing brain. Developmental Science. 2010;13(2):279–88. doi: 10.1111/j.1467-7687.2009.00883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James KH, James TW, Jobard G, Wong ACN, Gauthier I. Letter processing in the visual system: different activation patterns for single letters and strings. Cognitive, Affective & Behavioral Neuroscience. 2005;5:452–466. doi: 10.3758/cabn.5.4.452. [DOI] [PubMed] [Google Scholar]
- Kutas M, Federmeier KD. Thirty years and counting: finding meaning in the N400 component of the event-related brain potential (ERP) Annual Review of Psychology. 2011;62:621–47. doi: 10.1146/annurev.psych.093008.131123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann D, Skrandies W. Reference-free identification of components of checkerboard-evoked multichannel potential fields. Electroencephalography and Clinical Neurophysiology. 1980;48:609–621. doi: 10.1016/0013-4694(80)90419-8. [DOI] [PubMed] [Google Scholar]
- Luck SJ. An Introduction to the Event-Related Potential Technique. 2nd. MIT Press; 2014. [Google Scholar]
- Luu P, Ferree T. Determination of the Geodesic Sensor Nets' average electrode positions and their 10-10 international equivalents. Eugene, OR: Electrical Geodesics, Inc; 2000. [Google Scholar]
- Manly BFJ. Randomization and Monte Carlo methods in biology. Chapman and Hall; 1991. [Google Scholar]
- Maurer U, Blau V, Yoncheva Y, McCandliss BD. Development of Visual Expertise for Reading: Rapid Emergence of Visual Familiarity for an Artificial Script. Developmental Neuropsychology. 2010;35(4) doi: 10.1080/87565641.2010.480916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer U, Zevin JD, McCandliss BD. Left-lateralized N170 effects of visual expertise in reading: evidence from Japanese syllabic and logographic scripts. Journal of Cognitive Neuroscience. 2008;20:1878–1891. doi: 10.1162/jocn.2008.20125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCandliss BD, Beck IL, Sandak R, Perfetti CA. Focusing attention on decoding for children with poor reading skills: design and preliminary tests of the word building intervention. Scientific Studies of Reading. 2003;7:75–104. [Google Scholar]
- McCandliss BD, Yoncheva Y. Integration of left-lateralized neural systems supporting skilled reading. In: Benasich AA, Fitch RA, editors. Developmental Dyslexia: Early Precursors, Neurobehavioral Markers and Biological Substrates (The Extraordinary Brain Series) Baltimore, MD: Brookes Publishing Co; 2011. pp. 325–339. [Google Scholar]
- Mei L, Xue G, Lu ZL, He Q, Zhang M, Xue F, et al. Dong Q. Orthographic transparency modulates the functional asymmetry in the fusiform cortex: An artificial language training study. Brain and Language. 2013;125:165–172. doi: 10.1016/j.bandl.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson JR, Liu Y, Fiez J, Perfetti CA. Assimilation and accommodation patterns in ventral occipitotemporal cortex in learning a second writing system. Human Brain Mapping. 2009;30(3):810–820. doi: 10.1002/hbm.20551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pattamadilok C, Knierim IN, Kawabata Duncan KJ, Devlin JT. How does learning to read affect speech perception? Journal of Neuroscience. 2010;30(25):8435–8444. doi: 10.1523/JNEUROSCI.5791-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfetti CA. Representations and awareness in the acquisiton of reading competence. In: Rieben L, Perfetti CA, editors. Learning to read: basic research and its implications. Hillsdale, NJ: L. Erlbaum Associates; 1991. pp. 33–44. [Google Scholar]
- Pinel P, Dehaene S. Beyond hemispheric dominance: brain regions underlying the joint lateralization of language and arithmetic to the left hemisphere. Journal of Cognitive Neuroscience. 2010;22(1):48–66. doi: 10.1162/jocn.2009.21184. [DOI] [PubMed] [Google Scholar]
- Pinel P, Lalanne C, Bourgeron T, Fauchereau F, Poupon C, Artiges E, et al. Dehaene S. Cerebral Cortex. Vol. 1991. New York, N.Y.: 2014. Genetic and Environmental Influences on the Visual Word Form and Fusiform Face Areas; pp. 1–16. [DOI] [PubMed] [Google Scholar]
- Polich J. Updating P300: an integrative theory of P3a and P3b. Clinical Neurophysiology : Official Journal of the International Federation of Clinical Neurophysiology. 2007;118(10):2128–48. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. R Foundation for Statistical Computing; Vienna, Austria: 2007. R: A language and environment for statistical computing. Retrieved from http://www.r-project.org. [Google Scholar]
- Rayner K, Foorman BR, Perfetti CA, Pesetsky D, Seidenberg MS. How psychological science informs the teaching of reading. Psychological Science. 2001;2(2):31–74. doi: 10.1111/1529-1006.00004.. [DOI] [PubMed] [Google Scholar]
- Rayner K, Pollatsek A. The psychology of reading. London, United Kingdom: Prentice-Hall International; 1989. [Google Scholar]
- Rugg MD, Mark RE, Walla P, Schloerscheidt AM, Birch CS, Allan K. Dissociation of the neural correlates of implicit and explicit memory. Nature. 1998;392(6676):595–598. doi: 10.1038/33396. [DOI] [PubMed] [Google Scholar]
- Schneider W, Shiffrin RM. Controlled and automatic human information processing: I. Detection, search, and attention. Psychological Review. 1977;84:1–66. [Google Scholar]
- Seghier ML, Price CJ. Explaining Left Lateralization for Words in the Ventral Occipitotemporal Cortex. Journal of Neuroscience. 2011;31(41):14745–14753. doi: 10.1523/JNEUROSCI.2238-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Share DL, Stanovich KE. Cognitive processes in early reading development: accommodating individual differences into a model of acquisition. Issues in Education. 1995;1:1–57. [Google Scholar]
- Sobel M. Asymptotic intervals for indirect effects in structural equations models. In: Leinhart S, editor. Sociological methodology. San Francisco, CA: Jossey-Bass; 1982. pp. 290–312. [Google Scholar]
- Song Y, Bu Y, Hu S, Luo Y, Liu J. Short-term language experience shapes the plasticity of the visual word form area. Brain Research. 2010;1316:83–91. doi: 10.1016/j.brainres.2009.11.086. [DOI] [PubMed] [Google Scholar]
- Stevens C, McIlraith A, Rusk N, Niermeyer M, Waller H. Relative laterality of the N170 to single letter stimuli is predicted by a concurrent neural index of implicit processing of letter names. Neuropsychologia. 2013;51(4):667–74. doi: 10.1016/j.neuropsychologia.2012.12.009. [DOI] [PubMed] [Google Scholar]
- Strik WK, Fallgatter AJ, Brandeis D, Pascual-Marqui RD. Three-dimensional tomography of event-related potentials during response inhibition: evidence for phasic frontal lobe activation. Electroencephalography and Clinical Neurophysiology. 1998;108:406–413. doi: 10.1016/s0168-5597(98)00021-5. [DOI] [PubMed] [Google Scholar]
- Strimmer K. A unified approach to false discovery rate estimation. BMC Bioinformatics. 2008a;9:303. doi: 10.1186/1471-2105-9-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strimmer K. Fdrtool: a versatile R package for estimating local and tail area-based false discovery rates. Bioinformatics. 2008b;24(12):1461–1462. doi: 10.1093/bioinformatics/btn209. [DOI] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Herve PY, Duffau H, Crivello F, Houde O, et al. Tzourio-Mazoyer N. Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. NeuroImage. 2006;30:1414–1432. doi: 10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Wise J, Yoncheva Y, McCandliss BD. Effects of preference and strategy on learning to read an artificial script. Indiana University Undergraduate Journal of Cognitive Science. 2011;6:38–47. [Google Scholar]
- Woldorff MG, Fox PT, Matzke M, Lancaster JL, Veeraswamy S, Zamarripa F, et al. Jerabek P. Retinotopic organization of early visual spatial attention effects as revealed by PET and ERPs. Human Brain Mapping. 1997;5:280–286. doi: 10.1002/(SICI)1097-0193(1997)5:4<280::AID-HBM13>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Wong ACN, Gauthier I, Woroch B, DeBuse C, Curran T. An early electrophysiological response associated with expertise in letter perception. Cognitive, Affective & Behavioral Neuroscience. 2005;5(3):306–318. doi: 10.3758/cabn.5.3.306. [DOI] [PubMed] [Google Scholar]
- Yoncheva Y, Blau V, Maurer U, McCandliss BD. Attentional focus during learning impacts N170 responses to an artificial script. Developmental Neuropsychology. 2010;35(4):423–445. doi: 10.1080/87565641.2010.480918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoncheva Y, Maurer U, Zevin JD, McCandliss BD. Effects of rhyme and spelling patterns on auditory word ERPs depend on selective attention to phonology. Brain and Language. 2013;124(3):238–243. doi: 10.1016/j.bandl.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoncheva Y, Maurer U, Zevin J, McCandliss B. Selective attention to phonology dynamically modulates initial encoding of auditory words within the left hemisphere. NeuroImage. 2014;97:262–270. doi: 10.1016/j.neuroimage.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoncheva Y, Zevin JD, Maurer U, McCandliss BD. Auditory selective attention to speech modulates activity in the visual word form area. Cerebral Cortex. 2010;20(3):622–32. doi: 10.1093/cercor/bhp129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler JC, Goswami U. Reading acquisition, developmental dyslexia and skilled reading across languages: a psycholinguistic grain size theory. Psychological Bulletin. 2005;131:3–29. doi: 10.1037/0033-2909.131.1.3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Individual plots of the grand-averaged waveforms time-locked to the onset of the visual word at each of the 128 electrodes separately for every experimental condition (GP-trained, WW-trained and GP- transfer words).


