Abstract
Background
On 7 and 11 July 2007, health officials in Texas and Indiana, respectively, reported 4 possible cases of type A foodborne botulism to the US Centers for Disease Control and Prevention. Foodborne botulism is a rare and sometimes fatal illness caused by consuming foods containing botulinum neurotoxin.
Methods
Investigators reviewed patients’ medical charts and food histories. Clinical specimens and food samples were tested for botulinum toxin and neurotoxin-producing Clostridium species. Investigators conducted inspections of the cannery that produced the implicated product.
Results
Eight confirmed outbreak associated cases were identified from Indiana (n = 2), Texas (n = 3), and Ohio (n = 3). Botulinum toxin type A was identified in leftover chili sauce consumed by the Indiana patients and 1 of the Ohio patients. Cannery inspectors found violations of federal canned-food regulations that could have led to survival of Clostridium botulinum spores during sterilization. The company recalled 39 million cans of chili. Following the outbreak, the US Food and Drug Administration inspected other canneries with similar canning systems and issued warnings to the industry about the danger of C. botulinum and the importance of compliance with canned food manufacturing regulations.
Conclusions
Commercially produced hot dog chili sauce caused these cases of type A botulism. This is the first US foodborne botulism outbreak involving a commercial cannery in >30 years. Sharing of epidemiologic and laboratory findings allowed for the rapid identification of implicated food items and swift removal of potentially deadly products from the market by US food regulatory authorities.
Keywords: botulism, commercial canning, outbreak, foodborne botulism
Foodborne botulism is a rare, neuroparalytic illness caused by consumption of a botulinum neurotoxin (BoNT) produced by the bacterium Clostridium botulinum. These bacteria are found in the environment as dormant spores but can germinate and produce toxin in foods under permissive conditions of pH, water activity, anaerobic environment, and temperature. Human botulism is usually caused by toxin types A, B, or E, and, rarely, type F. BoNT binds irreversibly to the presynaptic membranes of peripheral neuromuscular and autonomic nerve junctions, causing a symmetric, descending flaccid paralysis. Death (<5% annually) occurs from respiratory muscle paralysis [1, 2].
Most cases of foodborne botulism in the United States are due to improperly handled (primarily home-preserved) foods [1–3]. Botulism attributed to commercially canned foods is rare. Proper commercial canning, owing to the controlled temperature and processing time, renders food commercially sterile (free of viable microorganisms, including those of public health significance such as spores of C. botulinum, capable of reproducing under normal nonrefrigerated conditions during storage and transport) [4]. A commercial canning process uses a closed vessel (known as a retort) to heat a product under pressure. Specific heating time and temperature in the retort depends on the product being sterilized. Manufacturers apply a code on all cans processed during a defined time period (sometimes described as a single retort), which identifies the production lot. During 1 week in July 2007, 4 cases of suspected botulism were reported to the Centers for Disease Control and Prevention (CDC) from 2 states (Texas and Indiana). Given the rarity of botulism in the continental United States, the appearance of 4 botulism cases in different geographic locations within the same week suggested that a distributed product could be involved. Until this outbreak, no US botulism cases were reported associated with underprocessing (failure to adequately sterilize) of commercial canned foods in >30 years [1]. We describe the investigation of this national foodborne botulism outbreak caused by a commercially canned product.
METHODS
Epidemiologic Investigation
Investigators conducted interviews with the first 4 patients and their families, collected food samples from their homes, and requested that clinical specimens be submitted for botulism testing. Patients’ food histories were reviewed for common items. Consultation documents for all suspect foodborne botulism cases reported to the CDC from 1 January 2007 to 17 July 2007 (date the outbreak was identified), were reviewed retrospectively to determine if any identified products were in common with those consumed by the patients in Texas and Indiana. In addition, a nationwide active case investigation was conducted prospectively from 17 July 2007 to 1 September 2007, to identify new cases possibly associated with this outbreak.
There are specific laboratory criteria to confirm botulism cases [1, 2, 5]. However, there are no established criteria for linking cases (confirmed or suspect) associated with an outbreak, particularly when the patients involved are not known to share a meal. In this investigation, a confirmed outbreak-associated case had to meet at least 1 of the following criteria: (1) Laboratory-confirmed botulism (defined as detecting BoNT and/or identifying a neurotoxin-producing Clostridium species in a clinical specimen), in a clinically diagnosed patient known to have consumed the implicated product (defined as the common food item identified in the initial 4 cases) and for whom remnants of the implicated food product were found to be contaminated with BoNT; (2) laboratory-confirmed botulism in a patient for whom there was strong evidence (such as a reliable patient or family member report) of consumption of the implicated food product within 10 days of illness onset; (3) clinically diagnosed botulism in a patient for whom remnants of the implicated product were found to be contaminated with BoNT; or (4) clinically diagnosed botulism in a patient for whom there was strong evidence (such as a reliable patient or family member report) of consumption of the implicated food product within 10 days of illness onset (epidemiologically linked case). We defined a suspected outbreak-associated case as a patient with clinical signs and symptoms suggestive of botulism with a possible history of consuming the implicated food within 10 days of illness onset.
Traceback and Investigation of the Cannery
Product information, including production lot codes and “best-by” dates, was collected for the sole food item that all patients reported eating. Investigators inspected the cannery where the implicated food item was processed. Investigators examined processing records and equipment, interviewed plant workers, and collected product samples for testing [6].
Laboratory Investigation
Clinical specimens and leftover food were tested by state public health laboratories or by the CDC. Standard procedures were used to test serum and stool specimens for BoNT. Additionally, stool specimens were tested for neurotoxin-producing Clostridium species. [5]. Food items were screened for BoNT by enzyme-linked immunosorbent assay (ELISA) [7]; confirmatory testing of ELISA-positive samples was done by mouse bioassay (MBA) [5]. US Food and Drug Administration (FDA) laboratories inspected intact cans collected from the manufacturer and tested the contents for BoNT by ELISA and MBA [8].
RESULTS
Epidemiologic Investigation
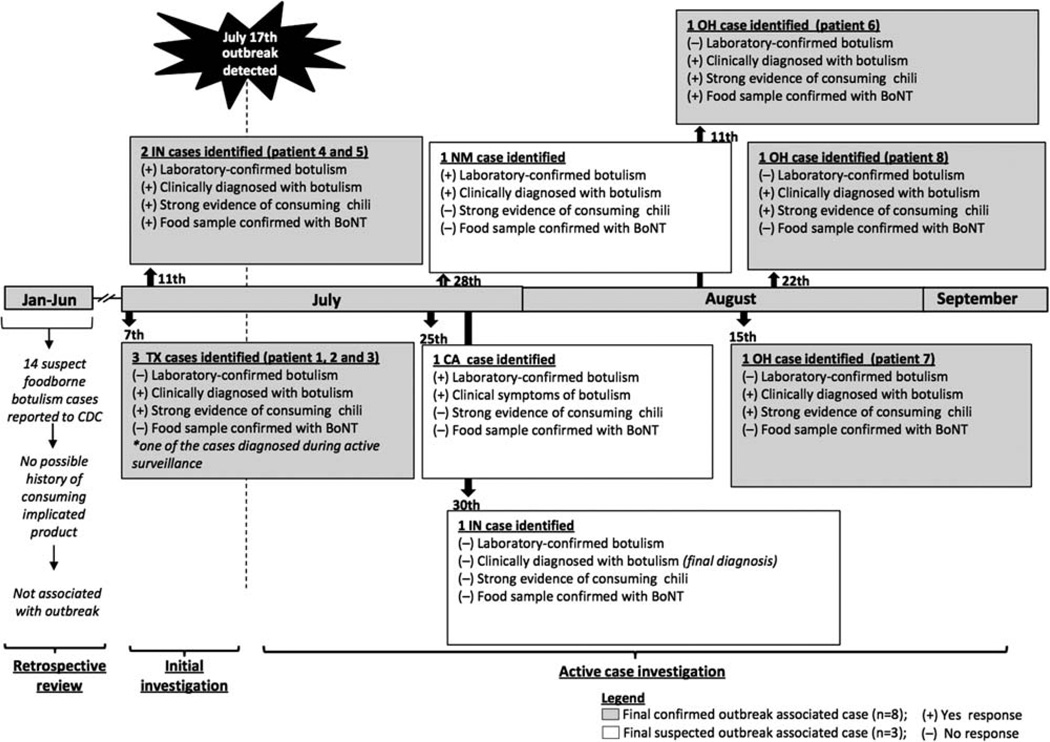
A total of 25 possible cases of foodborne botulism were reported to the CDC from 1 January through 1 September 2007, of which 11 were initially considered as suspected outbreak-associated cases (Figure 1). Of these 11, 2 patients (1 in California, 1 in New Mexico) had clinical signs suggestive of botulism but could not be definitively linked to the implicated product through interviews or product collection from the homes. One case in Indiana was originally reported as a suspected outbreak-associated case, but a follow-up medical records review determined that the patient’s clinical symptoms were not consistent with botulism. Eight met our definition of a confirmed outbreak-associated case, and all were identified in 3 states between 7 July and 22 August 2007 (Table 1). Patient and family interviews provided strong evidence that all 8 persons consumed Castleberry hot dog chili sauce the day before illness onset.
Figure 1.
Timeline of events and dates of reported suspect foodborne botulism cases, 1 January–1 September 2007. Abbreviations: BoNT, botulinum neurotoxin; CDC, Centers for Disease Control and Prevention.
Table 1.
Patient Characteristics, Time From Symptom Onset to Specimen Collection, and Laboratory Results
| Serum Specimen | Stool Specimen | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient | State | Age, y | Symptom Onset Date (2007) |
Symptom Onset to Clinical Diagnosis, d |
Symptom Onset to Serum Collection, d |
Toxin Detected |
Symptom Onset to Stool Collection, d |
Toxin Detected |
Culture Results |
| 1a,b | Texas | 39 | 29 June | 21 | 9 | No | 9 | No | Neg |
| 2a | Texas | 12 | 29 June | 8 | 9 | No | 9 | No | Neg |
| 3a | Texas | 13 | 29 June | 8 | 9 | No | 9 | No | Neg |
| 4c | Indiana | 63 | 7 July | 4 | 3 | Yes | 4 | No | Neg |
| 5c | Indiana | 58 | 7 July | 4 | 3 | Yes | 18 | No | Posd |
| 6e | Ohio | 37 | 7 August | 6 | 4 | No | 6 | No | Neg |
| 7a | Ohio | 39 | 30 June | 9 | 25 | No | 25 | No | Neg |
| 8a | Ohio | 39 | 7 July | 18 | 18 | No | 18 | No | Neg |
Clinically diagnosed botulism in a patient for whom there was strong evidence (such as a reliable patient or family member report) of consumption of the implicated food product within 10 days of illness onset (epidemiologically linked case).
Patient was initially misdiagnosed with exhaustion but confirmed with clinical botulism upon a more thorough review of medical history later on in the outbreak investigation.
Laboratory-confirmed botulism (defined as detection of botulinum neurotoxin and/or identification of a neurotoxin-producing Clostridium species in a clinical specimen), in a clinically diagnosed patient known to have consumed the implicated product, and for whom remnants of the implicated food product were found to be contaminated with botulinum neurotoxin.
Clostridium botulinum detected in culture using standard methods [5].
Clinically diagnosed botulism in a patient for whom remnants of the implicated product were found to be contaminated with botulinum neurotoxin.
The illness onset dates ranged from 29 June to 7 August 2007. The median age of patients was 39 years; 3 were female. The median number of days from illness onset to clinical diagnosis was 8 (range, 4–21). All 8 were hospitalized; 7 required mechanical respiratory support. Clinical symptoms in all patients were typical of botulism (Table 2). Five patients (63%) received bivalent AB and monovalent E botulinum antitoxin. No deaths were reported.
Table 2.
Clinical Characteristics of the 8 Outbreak-Associated Cases
| Characteristic | No. (%) |
|---|---|
| Clinical severity | |
| Hospitalized | 8 (100) |
| Required mechanical ventilatory support | 7 (88) |
| Received botulinum antitoxina | 5 (63) |
| Neurologic symptoms reported | |
| Dysphagia | 7 (88) |
| Blurred vision | 5 (63) |
| Slurred speech | 7 (88) |
| Double vision | 5 (63) |
| Muscle weakness | 8 (100) |
| Gastrointestinal symptoms | |
| Abdominal pain | 1 (13) |
| Diarrhea | 2 (25) |
| Nausea | 3 (38) |
| Vomiting | 1 (13) |
Of the 3 patients who did not receive antitoxin, 2 (patients 1 and 8) were diagnosed with botulism weeks after illness onset and no circulating botulinum neurotoxin was found in the serum tested. The third (patient 7) was reported to the Centers for Disease Control and Prevention a month after illness onset and no circulating botulinum neurotoxin was found in the serum tested.
The 3 Texas patients shared a meal the day before illness onset, which included Castleberry hot dog chili sauce. No food remnants were available for laboratory testing from an opened can found in the home. However, an unopened can of this product with an identical best-by date, purchased at the same time as the discarded can, was found in the patients’ home.
Because the severity of illness prevented interviews, investigators collected 25 food specimens from the home of the 2 Indiana patients, including an unlabeled, sealed plastic bag of leftover chili. Empty cans of 2 brands of commercially manufactured chili, one of which was Castleberry hot dog chili sauce, were found in the couple’s recycling bin. When their clinical condition permitted, the patients confirmed that the leftover chili recovered from their home was a mixture of product from both cans. The CDC informed the FDA on 16 July 2007 of the epidemiologic link between illness and consuming Castleberry hot dog chili sauce.
The 3 Ohio patients were identified during the active case investigation and were not related to one another (Figure 1). Interviews revealed strong evidence of consuming Castleberry hot dog chili sauce before illness onset. Leftover chili sauce was recovered from the home of one of the patients, and an unopened can of the Castleberry product was recovered from the home of the second patient. No leftover food or can was found in the third patient’s home.
Laboratory Investigation
Serum and stool specimens from all 8 patients were tested; however, only specimens collected from the Indiana patients (patients 4 and 5) were positive (Table 1). Botulinum toxin was detected in sera of both Indiana patients, which were collected 3 days from symptom onset. Serotype A was identified in the serum from patient 4. Clostridium botulinum type A was isolated from the stool specimen of patient 5.
On 16 July, the CDC identified BoNT type A in the leftover chili mixture obtained from the home of the 2 Indiana patients (Table 3). All other foods recovered from the home were negative for BoNT. Further tests of this food product showed a BoNT level of approximately 4000 LD50/g. (Mouse intraperitoneal lethal dose 50 [LD50] per gram is the dose required to kill half the members of a tested population of mice per gram of sample [8]; the CDC determined an approximate LD50 by humane euthanasia at symptom onset in accordance with an approved animal use protocol.) The Ohio Department of Health identified BoNT type A toxin in leftover chili sauce retrieved from the home of patient 6 (Table 3). Unopened cans of the same lots consumed by the patients, as well as an opened empty can, were negative for BoNT.
Table 3.
Laboratory Results and Production Dates of Samples of Castleberry Hot Dog Chili Sauce Collected From Patients’ Homes and the Canning Facility
| Food Collected | Production Date (2007) |
Collection Site | Toxin Detected |
Toxin Concentration (LD50/g) |
|---|---|---|---|---|
| Unopened can | 7 May | Home of patients 1–3 from Texas | No | … |
| Opened empty cana | 8 May | Home of patients 4–5 from Indiana | No | … |
| Leftover chili mixturea | … | Home of patients 4–5 from Indiana | Yes | ~4000 |
| Unopened can | 8 May | Home of patient 8 from Ohio | No | … |
| Leftover chili | … | Home of patient 6 from Ohio | Yes | Not determined |
| 20 unopened swollen cans | 8 May | Canning facility | Yes | Not determined |
| 3 unopened swollen cans | 8 May | Canning facility | No | … |
Abbreviation: LD50, mouse intraperitoneal lethal dose 50/g, or the dose required to kill half the members of a tested population of mice per gram of sample.
Leftover chili mixture contained the implicated product from the open empty can also recovered from the patients’ home.
Traceback and Investigation of the Cannery
The date/time stamp on the can labels recovered from the Texas (7 May 2001; 9:41 pm), Indiana (8 May 2007; 2:23 am), and Ohio (8 May 2007; 1:41 am) patients’ homes indicated that all 3 cans were produced within a 5-hour window between 9:41 pm on 7 May 2007 and 2:23 am on 8 May 2007 (Table 3). This information suggested that the cans came from the same production run. The time/date on the can labels recovered from the Indiana and Ohio homes were different by <1.5 minutes, suggesting that these cans were produced in 2 separate retorts on the same production line. All labels indicated that the cans were produced at the company’s cannery in Georgia.
On 18 July 2007, the manufacturer issued a nationwide voluntary recall (39 million cans) of all brands of canned hot dog chili sauce with best-by dates from 30 April through 22 May 2009, which included the same lots as the cans collected from the initial 4 patients’ homes. The recall was further expanded, on 19 and 21 July 2007, to include all products manufactured on the same production line for a period of 2 years. Approximately 111 million cans were recalled during this investigation, of which 39 million were cans of hot dog chili sauce. The company permanently closed its Georgia cannery in November 2008.
FDA officials conducted a lengthy investigation, from 17 July to 10 August 2007, of the cannery that produced Castleberry hot dog chili sauce and identified violations of federal canned-food regulations that might have permitted spores of C. botulinum to survive the canning process. Two different retorts (separate heating vessels supplied through a single production line) used to sterilize cans of the implicated product showed signs of improper maintenance and operation. A malfunctioning cooling water valve, faulty alarm indicator lights, and improperly calibrated temperature monitoring devices were observed. Either of these issues may have resulted in underprocessing of the implicated canned products. Additionally, records indicated that proper system monitoring may not have been performed during the cooking process, so the exact cause of underprocessing could not be determined.
As part of the plant investigation, FDA officials tested 23 swollen cans of Castleberry hot dog chili sauce produced on May 8 in the same retorts as the cans associated with the Indiana, Texas, and Ohio cases (Table 3); 20 (90%) were positive for BoNT type A.
DISCUSSION
In this investigation, 8 confirmed outbreak-associated cases were identified through the retrospective review and active case investigation. The implicated food vehicle was commercially canned chili sauce. Chili recovered from 2 of the patients’ home was contaminated with approximately 4000 LD50/g of BoNT type A. Because as little as 10 000 LD50 may be sufficient to cause death in humans, consuming approximately 3 g of this food could have been fatal [9, 10]. Improper maintenance and/or operation of 2 retorts within the cannery likely contributed to the underprocessing event that allowed C. botulinum spores to survive the canning process [4, 11].
As a result of this investigation, approximately 39 million cans of chili sauce (marketed under different brand names) were recalled. This was later expanded to other canned products, resulting in the recall of approximately 111 million cans. Regulatory authorities issued a warning to manufacturers about the requirement for compliance to federal regulations concerning canned foods including assuring proper maintenance and monitoring procedures.
Given the number of distributed products recalled and the number of cans which tested positive for BoNT, the number of cases reported to the CDC was surprisingly low. CDC botulism consultations since this event have not identified any other associations with commercially canned chili products (CDC, unpublished data). Unopened cans collected from patients’ homes of the same lot number consumed were negative for BoNT, suggesting that not all cans of a single lot were underprocessed. This variation in the number of contaminated cans may be due in part to the nature of the equipment malfunction within the cannery. A single production line supplied several retorts in this facility. It is possible that only a few loads were underprocessed, or that temperature variances within a single malfunctioning retort resulted in a small fraction of cans within each load being insufficiently sterilized. Although the exact cause of the failure cannot be determined with certainty, both scenarios imply that only a percentage of the total cans processed on the malfunctioning production line(s) were inadequately sterilized. If the bulk of cans processed during the recall period were properly heated during manufacturing, it would mean that the majority of individuals who consumed the implicated product were not exposed. Public notices of the recall and descriptions of the botulism outbreak through various news media may also have limited exposure (CDC, unpublished data).
Another possible cause for the low number of cases in this outbreak is the improper diagnosis of botulism. Clinicians’ unfamiliarity with botulism in the United States and disease symptoms that can be easily confused for other more common illnesses such as stroke and Guillain-Barré syndrome often result in a slow or inaccurate diagnosis, particularly if single cases occur in diverse geographical areas [2, 12]. Even for this outbreak, symptom onset to clinical diagnosis took as long as 21 days. In addition, there may have been patients with subclinical symptoms who did not seek medical attention and thus were not identified in the outbreak investigation [13, 14]. Finally, BoNT is readily destroyed by heat. Because hot dog chili sauce is usually consumed only after heating, consumers may be more likely to thoroughly heat this product, which would render it safe to eat [1].
In the US, foodborne botulism is usually associated with home-processed foods, such as canned, pickled, smoked, or fermented foods. From 1950 through 2006, US local and state health departments reported 413 events (sporadic or outbreaks of ≥2 cases) of foodborne botulism in which a food item was implicated [1, 3, 5]. Of these 413 events, 376 (91%) were linked to home-processed foods and 37 (9%) to commercially processed and prepared foods, including foods prepared in restaurants.
In the first half of the 20th century, botulism was caused by commercially canned foods, including several outbreaks associated with black olives (heat preserved in glass jars) in 1919 and the early 1920s [15, 16]. Subsequent research on the heat resistance of spore-forming bacteria by E. C. Dickson at Stanford University and K. F. Meyer at the University of California, technological advancements in canning processes by the National Canners Association, and the implementation in 1976 of federal regulations for low-acid and acidified canned food that required the use of scientifically validated thermal processes and other canned food manufacturing controls have led to safer canned food products [11, 17–20]. Such measures resulted in a significant decrease of botulism cases linked to commercial processed foods with 62 reported outbreaks before 1976 but only 3 reported outbreaks after (including this outbreak) [18, 21–23].
Prior to this outbreak, only 8 US commercial canning events linked to human cases of botulism had been reported to the CDC since 1950 (Table 4) [21, 22, 24–27]. Three of these events occurred in persons residing outside the United States (Canada, Belgium, and England). Inadequate equipment maintenance and operation as well as faulty machinery were the implicated causes of many of these botulism contamination events [11, 18]. The last such failure that caused illness in the United States occurred in 1974 [26]. Two elderly persons obtained botulism from eating commercially canned beef stew; 1 patient died. An investigation of the manufacturing facility identified 2 major deficiencies that may have caused underprocessing of a very limited number of cans. Improper retort venting may have produced air pockets in the retort vessel, which could insulate some cans from reaching proper temperature. Alternatively, a 2-way production line may have allowed some cans to escape heat treatment [11, 26].
Table 4.
Previously Reported US Commercial Canning Events Linked to Human Illness Since 1950
| Date | Food | Toxin Type | Cases | Death | Canning Deficiencies Identified | Reference |
|---|---|---|---|---|---|---|
| 1963 | Tuna fish | E | 3 | 2 | Unknown | [24] |
| 1963 | Liver paste | A | 2 | 0 | Unknown | [24] |
| 1971 | Vichyssoise | A | 2 | 1 | Unknown | [24] |
| 1973 | Peppersa | B | 8 | 0 | Unknown | [25] |
| 1973b | Mushroomsa | B | 1 | 0 | Unknown | [27] |
| 1974 | Beef stew | A | 2 | 1 | Underprocessing: deficiency in venting the retorts; may have accidentally escaped retorting | [26] |
| 1978c | Salmon | E | 4 | 2 | Contamination during processing: possible defect in tin during cooling after heat treatment | [21] |
| 1982d | Salmon | E | 2 | 1 | Contamination during can sealing: can reforming machine accidentally punched holes in cans | [22] |
Commercially canned glass jar.
Involved cases in Canada.
Involved cases in England.
Involved cases in Belgium.
CONCLUSIONS
A coordinated epidemiologic and laboratory investigation linked an outbreak of botulism to commercially produced hot dog chili sauce. Sharing of epidemiologic and laboratory findings with regulatory authorities enabled the rapid identification of implicated food items and the swift removal of contaminated products from the market, preventing additional illnesses. Although rare, deficiencies in the canning process are a major public health concern because of the severity of botulism, especially when the implicated canned product is widely distributed. Manufacturers must comply with canned food regulations and remain diligent to ensure that their products are safe. Such diligence should not only involve use of adequate canning technology but also proper use, maintenance, and monitoring of equipment.
Acknowledgments
We thank the County of San Diego Health and Human Services Agency (M. M. Ginsberg), Indiana State Department of Health (R. F. Teclaw), Texas Department of State Health Services (A. Cole and R. Drumgoole), Ohio Department of Health (Cuyahoga County Board of Health, Lorain County General Health District, Huron County General Health District), the Centers for Disease Control and Prevention (CDC; Ryan Fagan), and the US Department of Agriculture (USDA). We are especially grateful to the patients and family members, and the healthcare professionals involved in their care.
Financial support. This work was supported by the CDC; the US Food and Drug Administration; the USDA; and the state health departments of Texas, Indiana, and Ohio.
Footnotes
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the participating agencies.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Yu PA, Maslanka SE, Louis MESt, et al. Bacterial infections of humans. epidemiology and control. New York, NY: Springer Science; 2009. Botulism; pp. 159–176. [Google Scholar]
- 2.Sobel J, Maslanka S. Botulism. In: Longo DL, Kasper KL, Jameson JL, Fauci AS, Hauser SL, Loscalzo J, editors. Harrison’s principles of internal medicine. 18th ed. New York: McGraw-Hill; 2012. pp. 1200–1203. [Google Scholar]
- 3.Sobel J, Tucker N, Sulka A, et al. Foodborne botulism in the United States, 1990–2000. Emerg Infect Dis. 2004;10:1606–1611. doi: 10.3201/eid1009.030745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.US Food and Drug Administration. 21 CFR 113. Chapter I Food and Drug Administration. Subchapter B. Food for human consumption. Part 113. Thermally processed low-acid foods packaged in hermetically sealed container. [Accessed 28 October 2012]; Revised 1 April 2012. Available at: www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=113&showFR=1.
- 5.Centers for Disease Control and Prevention. Handbook for epidemiologist, clinicians, and laboratory workers. Atlanta, GA: Centers for Disease Control and Prevention; 1998. Botulism in the United States, 1899–1996. [Google Scholar]
- 6.US Food and Drug Administration. Guide to inspections of low acid canned food manufacturers. In: Low acid canned food manufacturers Part 1—administrative procedures/scheduled processes. Inspections, compliance, enforcement, and criminal Investigations. [Accessed 28 October 2012]; Revised 13 March 2009. Available at: www.fda.gov/ICECI/Inspections/InspectionGuides/ucm074992.htm.
- 7.Maslanka SE, Luquez C, Raphael BH, et al. Utility of botulinum toxin ELISA A, B, E, F kits for clinical laboratory investigations of human botulism. Botulinum J. 2011;2:72–92. [Google Scholar]
- 8.Solomon HM, Lilly JT. Clostridium botulinum. In: Merker RI, editor. Bacteriological analytical manual online. Baltimore, MD: Center for Food Safety and Applied Nutrition, Food and Drug Administration; 2003. [Google Scholar]
- 9.Schantz EJ, Sugiyama H. Toxic proteins produced by Clostridium botulinum. J Agric Food Chem. 1974;22:26–30. doi: 10.1021/jf60191a033. [DOI] [PubMed] [Google Scholar]
- 10.Arnon SS, Schechter R, Inglesby TV, et al. Botulinum toxin as a biological weapon: medical and public health management. JAMA. 2001;285:1059–1070. doi: 10.1001/jama.285.8.1059. [DOI] [PubMed] [Google Scholar]
- 11.Pflug IJ. Science, practice, and human errors in controlling Clostridium botulinum in heat-preserved food in hermetic containers. J Food Prot. 2010;73:993–1002. doi: 10.4315/0362-028x-73.5.993. [DOI] [PubMed] [Google Scholar]
- 12.St Louis ME, Peck SH, Bowering D, et al. Botulism from chopped garlic: delayed recognition of a major outbreak. Ann Intern Med. 1988;108:363–368. doi: 10.7326/0003-4819-108-3-363. [DOI] [PubMed] [Google Scholar]
- 13.Villar RG, Shapiro RL, Busto S, et al. Outbreak of type A botulism and development of a botulism surveillance and antitoxin release system in Argentina. JAMA. 1999;281:1334–1340. doi: 10.1001/jama.281.14.1334. [DOI] [PubMed] [Google Scholar]
- 14.Sobel J, Malavet M, John S. Outbreak of clinically mild botulism type E illness from home-salted fish in patients presenting with predominantly gastrointestinal symptoms. Clin Infect Dis. 2007;45:e14–e16. doi: 10.1086/518993. [DOI] [PubMed] [Google Scholar]
- 15.Armstrong C. Botulism from eating canned ripe olives. Public Health Rep. 1919;34:2877–2905. [Google Scholar]
- 16.Anonymous. Botulism. Protective measures and cautions from the U. S. Bureau of Chemistry, Department of Agriculture. Public Health Rep. 1920;35:327–330. [Google Scholar]
- 17.Osheroff BJ, Slocum GG, Decker WM. Status of botulism in the United States. Public Health Rep. 1964;79:871–878. [PMC free article] [PubMed] [Google Scholar]
- 18.Lynt RK, Kautter DA, Read RBJ. Botulism in commercially canned foods. J Milk Food Technol. 1975;38:546–550. [Google Scholar]
- 19.Esty JR. The heat resistance of C. botulinus spores. Am J Public Health (N Y) 1923;13:108–113. doi: 10.2105/ajph.13.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gross CE, Vinton C, Stumbo CR. Bacteriological studies relating to thermal processing of canned meats; thermal death-time curve for spores of test putrefactive anaerobe in meat. Food Res. 1946;11:411–418. doi: 10.1111/j.1365-2621.1946.tb16369.x. [DOI] [PubMed] [Google Scholar]
- 21.Ball AP, Hoopkinson RB, Farrell ID, et al. Human botulism caused by Clostridium botulinum type E: the Birmingham outbreak. Q J Med. 1979;48:473–491. [PubMed] [Google Scholar]
- 22.Hayes AH., Jr The Food and Drug Administration’s role in the canned salmon recalls of 1982. Public Health Rep. 1983;98:412–415. [PMC free article] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention. Botulism associated with commercially canned chili sauce—Texas and Indiana, July 2007. MMWR Morb Mortal Wkly Rep. 2007;56:767–769. [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention. Botulism associated with commercially canned vichyssoise. MMWR Morb Mortal Wkly Rep. 1971;20:242. [Google Scholar]
- 25.Centers for Disease Control and Prevention. Botulism—West Virginia, Pennsylvania. MMWR Morb Mortal Wkly Rep. 1973;22:161–162. [Google Scholar]
- 26.Blake PA, Horwitz MA, Hopkins L, et al. Type A botulism from commercially canned beef stew. South Med J. 1977;70:5–7. doi: 10.1097/00007611-197701000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention. Botulism traced to commercially canned marinated mushrooms—Canada. MMWR Morb Mortal Wkly Rep. 1973;22:241–242. [Google Scholar]