Abstract
Purpose
Matrix metalloproteinase (MMP) 14 has been shown to promote angiogenesis, but the underlying mechanisms are poorly understood. In this study, we investigated exosomal transport of MMP14 and its target, MMP2, from corneal fibroblasts to vascular endothelial cells as a possible mechanism governing MMP14 activity in corneal angiogenesis.
Methods
We isolated MMP14-containing exosomes from corneal fibroblasts by sucrose density gradient and evaluated exosome content and purity by Western blot analysis. We then investigated exosome transport in vitro from corneal fibroblasts to two populations of vascular endothelial cells, human umbilical vein endothelial cells (HUVECs) and calf pulmonary artery endothelial cells (CPAECs). Western blot analysis and gelatin zymography were used to determine levels of MMP14 and MMP2, respectively, in exosomal fractions derived from cultured wild-type, MMP14 enzymatic domain-deficient (MMP14Δexon4), and MMP14-null corneal fibroblasts.
Results
Matrix metalloproteinase 14–containing exosomes isolated from corneal fibroblasts were readily taken up in vitro by HUVECs and CPAECs. We found that MMP14 was enriched in exosomal fractions of cultured corneal fibroblasts. Moreover, loss of the MMP14 enzymatic domain resulted in accumulation of pro-MMP2 protein in exosomes, whereas MMP2 was nearly undetectable in exosomes of MMP14-null fibroblasts.
Conclusions
Our results indicate that exosomes secreted by corneal fibroblasts can transport proteins, including MMP14, to vascular endothelial cells. In addition, recruitment of MMP2 into corneal fibroblast exosomes is an active process that depends, at least in part, on the presence of MMP14. The role of exosomal MMP14 transport in corneal angiogenesis has important implications for therapeutic applications targeting angiogenic processes in the cornea.
Keywords: exosomes, MMP14, angiogenesis, metalloproteinases, MMP2
Exosomes of corneal fibroblasts contain high levels of enzymatically active MMP14 that deliver their contents to target cells. Deletion of the MMP14 enzymatic domain leads to an increase in pro-MMP2 accumulation in exosomes, whereas absence of MMP14 results in near-complete failure of MMP2.
Matrix metalloproteinases (MMPs) are a large family of zinc-dependent endopeptidases that are crucial to extracellular matrix (ECM) remodeling.1 Matrix metalloproteinases function in the modification of essentially all components of the ECM, including collagens, proteoglycans, and fibronectins. They are also involved in modulating the activity of signaling molecules and play important roles in both normal physiology and pathological processes including neovascularization and tumor metastasis.2–5 As such, MMPs are important potential targets for controlling such processes and in treating of a variety of pathological conditions.
Matrix metalloproteinase 14 (also known as membrane type 1 MMP, MT1-MMP) in particular is involved in many processes including wound healing, angiogenesis, inflammation, and cancer invasion and metastasis.6–15 The importance of MMP14 is demonstrated by the deleterious effects resulting from its absence. For example, MMP14 knockout mice display an extremely disfigured phenotype as a result of inadequate collagen turnover and bone remodeling, which lead to marked deceleration in postnatal growth and, ultimately, premature death.11,12,16 Matrix metalloproteinase 14 is known to be required for proteolysis of the ECM during the normal growth of blood vessels (angiogenesis), which allows new endothelial cells to migrate and invade tissues.16–18 Proteolysis of the ECM is normally a highly regulated process, with MMP14 localized to discrete regions of the cell membrane.17,19 However, this regulation is disrupted in tumors, resulting in widespread ECM remodeling by MMP14 and its targets, which allows the tumor to grow quickly and in a highly disorganized fashion, gaining access to the body's vasculature and leading to metastasis.10 In previous studies, we demonstrated that MMP14 potentiates the angiogenic processes (including vessel invasion, increased levels of vascular endothelial growth factor [VEGF], and phosphorylation of signaling molecules) involved in fibroblast growth factor (FGF)2-induced corneal neovascularization in corneal fibroblasts in culture and in the mouse eye.7–9,20,21 Matrix metalloproteinase 14 also activates MMP2, which further facilitates breakdown of ECM in the cornea.22,23
Knowledge of the mechanisms underlying MMP14's actions in angiogenesis is needed for the development of therapeutics that target angiogenic processes. In the present study, we investigated whether exosomal transport between cells facilitates MMP14 activity in the cornea. Exosomes are composed of a common, characteristic set of membrane and cytosolic molecules, including tumor susceptibility gene (TSG)101, integrin β1 (ITGB1), and cytoskeletal proteins. The biochemically active components of the exosome membrane can include receptors, adhesion molecules, transporters, and enzymes, including MMPs.24–28 We show that MMP14 and MMP2 are concentrated and enzymatically active in exosomes derived from wild-type (WT) corneal fibroblasts. We then use genetically modified cell lines to show that MMP14-containing exosomes secreted by fibroblasts are readily taken up by endothelial cells, but not as effectively taken up by fibroblasts. The mechanisms responsible for this difference are unknown but appear to be cell type specific. Finally, we show that MMP14 is necessary for MMP2 sequestration and activation in exosomes secreted by fibroblasts. Considering recent studies suggesting that exosomes are likely vehicles for cell-to-cell signaling and transfer of material between cells,29–39 clarifying the roles of MMP14 and MMP2 in exosomal transport will have significant implications for understanding the mechanisms of angiogenesis.
Materials and Methods
Antibodies
A polyclonal antibody against mouse MMP14 was generated as previously described.9 Antibodies to TSG101 (AbCam, Cambridge, MA, USA), Mitogen-activated protein kinase (MAPK) (ERK; Santa Cruz Biotechnology, Dallas, TX, USA), COX4 (Santa Cruz Biotechnology), ITGB1 (Santa Cruz Biotechnology), actin (Cell Signaling Technology, Danvers, MA, USA), and MMP2 (Calbiochem/Millipore, Billerica, MA, USA) were purchased from the indicated commercial sources, and 1:1000 working dilutions were prepared for all antibodies.
Isolation of Membrane Components From Corneal Fibroblasts
Corneal fibroblasts (5 × 107) were obtained via a previously described method7 and cultured in Dulbecco's modified Eagle's medium (DMEM; Invitrogen, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS) overnight at 37°C in 5% CO2 to render the cells quiescent. Cell pellets were lysed in a buffer containing 25 mM HEPES [(4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid)], 150 mM NaCl, 1 mM EDTA (ethylenediaminetetraacetic acid), 1% nonyl phenoxypolyethoxylethanol (NP-40), protease inhibitors (1 mM PMSF; phenylmethylsulfonyl fluoride), and 2 mM protease inhibitor cocktail for 90 minutes at 4°C. The lysed cells were then subjected to homogenization and clarified by centrifugation at 16,000g for 15 minutes. The supernatant was collected as cell lysate.
Exosome Isolation From Mouse Corneal Fibroblasts
Exosomes were isolated using a sucrose density gradient. Wild-type mouse corneal fibroblasts (5 × 107 cells) were seeded onto a 150-mm culture dish with DMEM supplemented with 10% FBS. The next day the cells were washed with phosphate-buffered saline (PBS) and cultured in 1% ultracentrifuged FBS (prepared by ultracentrifugation at 100,000g for 18 hours to exclude bovine exosomes). The conditioned medium was collected and centrifuged at 1500 rpm for 10 minutes and 3000 rpm for 30 minutes to remove cellular debris. The supernatant was then filtered through a 0.45-μm membrane and concentrated using a Millipore concentrator tube (Calbiochem/Millipore) with 100 K MWCO filter. The concentrated conditioned medium was ultracentrifuged at 100,000g for 2 hours. The resulting pellet was resuspended in a 1:200 dilution of Proteinase Inhibitor Cocktail III (Calbiochem/Millipore) in PBS. The pellet was adjusted to 40% sucrose and overlaid with 30% and 5% sucrose. Buoyant-density centrifugation was performed at 100,000g for 18 hours at 4°C in a Beckman SW40Ti or SW60Ti rotor (Beckman Coulter, Inc., Pasadena, CA, USA). Eleven fractions were collected from the top of the gradient.
Marker Protein Analysis
The proteins of the cell lysate and the exosome preparation were separated by 4–20% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) under nonreducing conditions unless stated otherwise and were transferred to polyvinylidene difluoride (PVDF) membranes (20 μg exosomes per lane, except in the gel shown in Fig. 4A, which was loaded with 2 μg exosomes per lane). Reducing conditions, when used, consisted of treatment with 100 mM β-mercaptoethanol solution followed by boiling for 10 minutes. Blocking was performed using 5% milk and 3% BSA. The membranes were incubated overnight with the appropriate primary antibody to identify membrane protein markers (MMP14 and ITGB1), exosome marker (TSG101), a mitochondrial protein marker (COX4), and cytosolic protein markers (actin and nonphosphorylated ERK or MAPK). For nonreducing conditions, cell lysate and isolated exosomal proteins were analyzed as in reducing conditions but in the absence of β-mercaptoethanol. The PVDF membrane was incubated with horseradish peroxidase–conjugated or IRDye-conjugated secondary antibody. Protein bands were detected by an enhanced chemiluminescence or Li-Cor Odyssey system (Lincoln, NE, USA).
Figure 4.
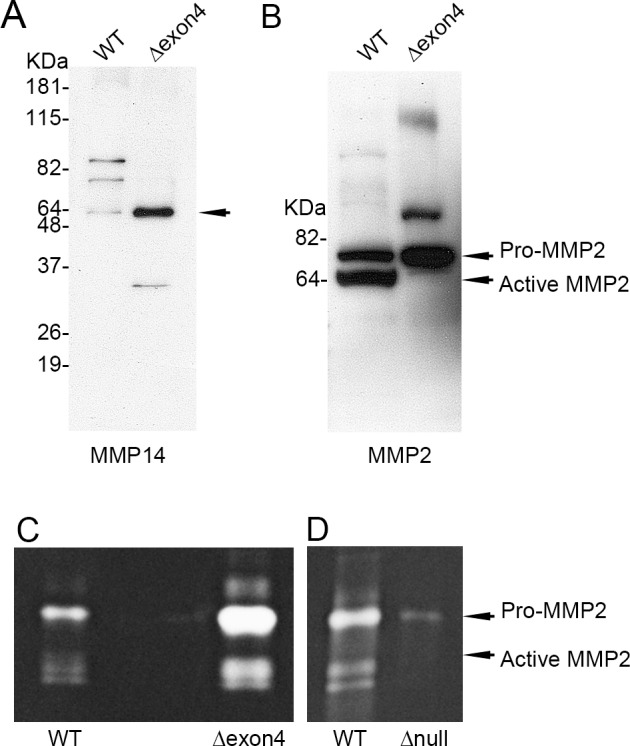
Localization of MMP14 and MMP2 in exosomes in MMP14Δexon4 and MMP14-null corneal fibroblasts. (A) Matrix metalloproteinase 14 detection by Western blotting in WT and MMP14Δexon4 corneal fibroblast–derived exosomes. Matrix metalloproteinase 14 was present at concentrated levels in MMP14Δexon4 exosomes. (B) Matrix metalloproteinase 2 detection by Western blotting in WT and MMP14Δexon4 corneal fibroblast–derived exosomes. Although pro-MMP2 was present at higher levels in MMP14Δexon4 exosomes than in WT, active MMP2 was nearly undetectable. (C) Matrix metalloproteinase 2 enzymatic activity assayed by zymography in WT and MMP14Δexon4 corneal fibroblast–derived exosomal fractions. Although pro-MMP2 was, again, present at much higher concentrations in the MMP14Δexon4 exosomes, an elevated level of active MMP2 was also observed. (D) Matrix metalloproteinase 2 enzymatic activity assayed by zymography in WT and MMP14-null corneal fibroblast–derived exosomes. This gel was loaded with twice the volume of exosomes as in (C), which demonstrates that although MMP2 was present in MMP14-null exosomes, its expression was reduced to near-undetectable levels.
Exposure of CPAECs, HUVECs, or Normal Corneal Fibroblasts to Exosomes Containing MMP14-YPet or Coculture With MMP14-YPet–Expressing Cells
Wild-type corneal fibroblasts were infected with a retrovirus containing MMP14-YPet. Briefly, pCMMV-MMP14-YPet plasmid was transfected into Phoenix virus packaging cells (American Type Culture Collection [ATCC], Manassas, VA, USA). Secreted virus containing MMP14-YPet was used to infect corneal fibroblasts. Secreted exosomes were isolated from the culture medium of these MMP14-YPet–expressing cells for the reported experiments.
Cover glasses in the wells of a six-well plate were seeded with human umbilical vein endothelial cells (HUVECs), calf pulmonary artery endothelial cells (CPAECs), or normal corneal fibroblasts. For direct exposure to MMP14-YPet–containing exosomes, 1 mL culture medium containing 2 μg isolated exosomes was added to each sample of cultured cells. For coculture with MMP14-YPet–expressing cells, transwells containing corneal fibroblasts overexpressing MMP14-YPet were added to the culture wells. The plate was then incubated for 4 hours, allowing for diffusion of the exosomes to the cells in the bottom dishes. Fluorescence emitted by the exosomes was then observed by confocal microscopy.
Generation of Immortalized MMP14 Corneal Fibroblast Cell Lines
MMP14Δexon4 immortalized mouse corneal cell lines were generated according to methods previously described.8 Briefly, the entire mouse corneal stroma was excised from a MMP14Δexon4 cornea and incubated with DMEM containing 3.3 mg/mL collagenase type II (Sigma-Aldrich Corp., St. Louis, MO, USA) at 37°C with shaking for 90 minutes. Isolated keratocytes were grown in DMEM supplemented with 10% fetal calf serum (HyClone Laboratories, Inc., Logan, UT, USA) at 37°C in a 5% CO2 humidified atmosphere. Subconfluent stromal fibroblasts were supplemented with a mixture containing polybrene (4 μg/mL) and an equal volume of pZIPTEX virus (containing SV40T antigen). Matrix metalloproteinase 14–null corneal fibroblast cell lines were generated according to a protocol similar to that used for generation of MMP14Δexon4 immortalized mouse corneal cell lines.
Gelatin Zymography
Proteins from the exosome preparations obtained from WT corneal fibroblasts, MMP14Δexon4, and MMP14-null cell lines were separated on 4% to 16% zymogram gels (2 μg exosomes per lane) to analyze the status of the proenzyme and active forms of MMP2. The gels were washed twice with 50 mM Tris/HCl, 5 mM CaCl2, 1 M ZnCl2, and 2.5% Triton X-100 (pH 7.6). After one wash in buffer without Triton, the gel was incubated overnight at 37°C in wash buffer containing 1% Triton X-100. Proteins were visualized using Coomassie brilliant blue. The gel was fixed and destained using 10% acetic acid in 10% methanol.
Results
MMP14 Is Highly Concentrated in Exosomes Secreted by Corneal Fibroblasts
To determine whether MMP14 was localized in corneal fibroblasts and corneal fibroblast-secreted exosomes, we collected cells and conditioned media from corneal fibroblasts cultured in 10% FBS. The conditioned media were subjected to ultracentrifugation, filtration, and sucrose gradient ultracentrifugation. Samples were analyzed for the diagnostic presence of TSG101 (an exosomal marker; Fig. 1A) and MMP14 (Fig. 1B). Both TSG101 and MMP14 were present at high levels in fractions 9 and 10, indicating the presence of MMP14 in the exosomal fractions. Fractions 1 and 2 did not contain detectable levels of MMP14 or TSG101 (data not shown).
Figure 1.
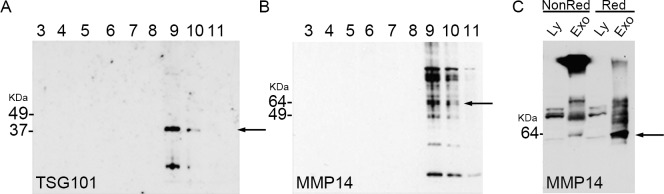
Matrix metalloproteinase 14 was highly concentrated in exosomes secreted by corneal fibroblasts. (A, B) Exosomal proteins were separated into 11 fractions by sucrose density gradient centrifugation and analyzed via Western blotting for the presence of MMP14 and the exosomal marker TSG101. (A) Tumor susceptibility gene 101 detected by immunoblotting (arrow) identified fractions 9 and 10 as the exosomal fractions. (B) Matrix metalloproteinase 14 (arrow) was present in the exosomal fractions 9 and 10. (C) Nonreduced and reduced cellular lysates contained low-level amounts of MMP14. Reduced exosomes showed the greatest amount of MMP14 (present as free monomer) compared to that in nonreduced exosomes (arrow). Ly, cell lysate; Exo, exosomal protein; NonRed, nonreduced conditions; Red, reduced conditions.
We then examined MMP14 in the cell lysate and exosomal fractions in both nonreducing and reducing conditions. In nonreducing conditions, MMP14 in exosomes was aggregated in a high molecular weight position, indicating that it had formed disulfide bonds with other molecules. In reducing conditions, exosomal MMP14 was primarily observed as a monomer with a molecular weight of 60 kDa (Fig. 1C). In cell lysate, MMP14 was present at much lower levels, primarily as a monomer, in both reducing and nonreducing conditions. These results indicate that in exosomes, MMP14 exists primarily in multimers or complexed with other molecules, whereas in cell lysates, MMP14 exists primarily as a monomer.
MMP14 Is More Concentrated in Exosomes Than in Cell Lysate
To confirm that our exosomal samples were pure (i.e., contained little to no cell lysate), we performed Western blot analysis using cell lysate and exosomal markers. MAPK (Fig. 2A; an extracellular signal regulated kinase), COX4 (Fig. 2B; a cytochrome c oxidase subunit and an inner mitochondrial protein), and actin (Fig. 2C) are general cellular proteins. In contrast, ITGB1 (Fig. 2D; a fibronectin receptor) is a marker of cell membranes and thus expected to be in both lysate and exosomal fractions, because exosomal vesicular membranes are derived from parent cell membranes. TSG101 (Fig. 2E) is an exosomal marker. Cell lysate fractions contained all general cellular proteins at levels that were consistently higher than those in exosomes. In contrast, the exosomal fraction was enriched in ITGB1 and TSG101 compared to the lysate. These results indicate that our exosomal samples were highly pure and support the presence of a specialized profile of markers, including TSG101, MMP14, and ITGB1, in exosomes at concentrations well above those in lysate of corneal fibroblasts.
Figure 2.
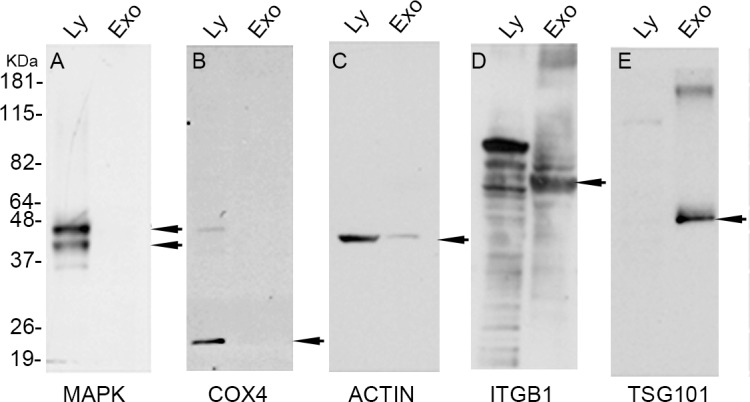
Cellular lysates and exosomal fractions contained different levels of component proteins. Cellular lysates and exosomal fractions were western blotted with (A) Anti-MAPK, (B) Anti-Cox4, (C) anti-Actin, (D) anti-ITGB1 and (E) anti-TSG101 antibodies. The cellular lysate fraction contained high levels of MAPK, COX4, ITGB1, and actin and an undetectable level of TSG101. In contrast, the exosomal fraction contained undetectable levels of MAPK and COX4, low levels of actin, and high levels of ITGB1 and TSG101. Ly, cell lysate; Exo, exosomal protein.
Exosomes Transfer MMP14 to Endothelial Cells
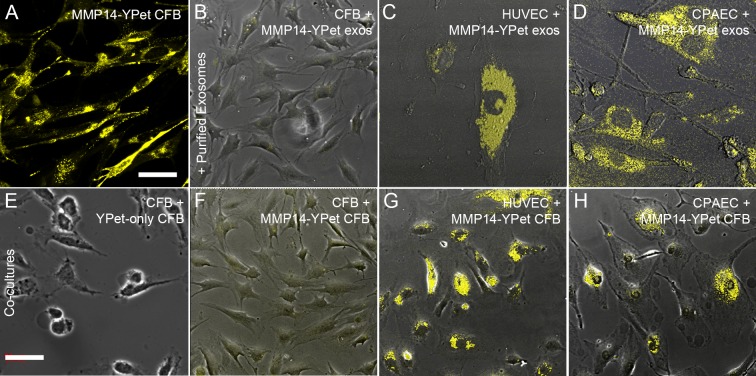
Wild-type corneal fibroblasts were infected with a retrovirus containing an MMP14-YPet fusion molecule. Secreted exosomes were isolated from the culture medium of these MMP14-YPet–expressing cells (Fig. 3A). Exosomes containing MMP14-YPet were added to untransfected WT corneal fibroblasts (Fig. 3B), HUVECs (Fig. 3C), and CPAECs (Fig. 3D). Cultures were assayed for YPet expression as an indicator of cellular uptake of exosomes and their contents. We found that all three cell lines expressed YPet and that endothelial cells (HUVECs and CPAECs) expressed higher levels of YPet than corneal fibroblasts, indicating possible cell type–specific differences in the ability of cells to absorb or accept exosomes containing MMP14-YPet. The in vitro fusion of MMP14-YPet–containing exosomes to recipient cells showed that MMP14-YPet can be transferred from purified exosomes to vascular endothelial cells.
Figure 3.
Exosomes fused more readily with endothelial cells than with corneal fibroblasts. (A–H) Images are overlaid brightfield and fluorescent micrographs showing YPet expression. (A) After transfection with MMP14-YPet plasmid, cultured corneal fibroblasts expressed high levels of YPet in the cell membranes and cytoplasm. (B–D) Images of cells incubated with exosomes isolated from MMP14-YPet–transfected corneal fibroblasts. (B) Corneal fibroblasts did not efficiently absorb or accept MMP14-YPet–containing exosomes. (C, D) Cultured HUVECs and CPAECs readily accepted or absorbed the exosomes and exhibited high levels of YPet expression. (E–H) Cells were cocultured with either YPet-expressing corneal fibroblasts (E) or MMP14-YPet–expressing corneal fibroblasts (F–H). Cells expressing YPet-only failed to transfer fluorescent signal to recipient cells (E). Cells expressing MMP14-YPet did not efficiently transfer MMP14-YPet to recipient corneal fibroblasts (F), but very efficiently transferred MMP14-YPet to both HUVECs (G) and CPAECs (H). Note: Top row and bottom row use different scales; scale bars: 32.06 μm (A–D), 71.41 μm (E–H).
Next, we examined the cell-to-cell transfer of MMP14 via exosomes in a coculture assay. Corneal fibroblasts transfected with MMP14-YPet were cocultured with WT corneal stromal fibroblasts (Fig. 3F) or vascular endothelial cells (HUVECs, Fig. 3G, and CPAECs, Fig. 3H). No direct cell-to-cell contact was possible. The results of these experiments showed that MMP14-YPet secreted by corneal fibroblasts was readily transferred to the two types of vascular endothelial cells but, again, not as effectively transferred to corneal stromal fibroblasts. In order to determine whether MMP14-YPet was taken up by exosomes, transferred into target cells via exosomes, and subsequently moved into the cell membrane, we conducted a control experiment in which we transfected cells with YPet and cocultured these cells with corneal fibroblasts (Fig. 3E), HUVECs, and CPAECs (data not shown). No YPet was seen in the cells or cell membranes for any of the three cell types.
Localization of MMP14 in Exosomes in MMP14Δexon4 Corneal Fibroblasts
The mechanisms involved in MMP14 localization to and retention in exosomes remain unknown. In WT cells, MMP14 was concentrated in exosomes at much higher levels than in cell lysate (Fig. 2), suggesting that it is actively sequestered or trafficked into exosomes. To further investigate the mechanisms underlying MMP14 activity, we generated a line of MMP14Δexon4 cells in which exon 4, which contains the enzymatic domain, was deleted. The enzymatic domain is necessary to catalyze pro-MMP2 into active MMP2,23 which is a major contributor to ECM remodeling. Despite the absence of the enzymatic domain, we found that MMP14 was still localized to exosomes in this mutant cell line (Fig. 4A), indicating that the enzymatic domain is not required for sequestration. We hypothesized that MMP14 may be more effectively sequestered into exosomes in MMP14Δexon4 cells, but when we used a second MMP14 antibody that targeted a different region of the protein, we did not observe this particular result. However, the antibody confirmed that MMP14 is successfully sequestered in these cells.
Increased MMP2 in Exosomes in MMP14Δexon4 Corneal Fibroblasts
Our next aim was to analyze the presence of MMP14 in relation to MMP2 uptake or activity in exosomes using Western blot analysis and gelatin zymography. Western blot analysis showed elevated levels of pro-MMP2 in MMP14Δexon4 exosomes but near absence of active MMP2 (Fig. 4B), whereas gelatin zymography showed elevated levels of pro-MMP2 and a substantial presence of active MMP2 (Fig. 4C). These divergent results suggest that the antibody used in the Western blot analysis may be less sensitive to the active epitope of the MMP2 protein. Using gelatin zymography, we next showed that exosomes derived from MMP14-null corneal fibroblasts contained pro-MMP2 but at nearly undetectable levels (more exosomal pro-MMP2 protein was detected in MMP14Δexon4 exosomes; compare Figs. 4C, 4D) and that active MMP2 was completely absent (Fig. 4D). It appears that whereas MMP14Δexon4 exosomes contain much higher levels of pro-MMP2 than do WT cells, exosomes of MMP14-null cells contain very little pro-MMP2 and virtually no active MMP2. These data reveal two critical points regarding MMP2 sequestration in corneal fibroblast exosomes: (1) Normal MMP2 sequestration is dependent, at least in part, on the presence of MMP14 in the cell, and (2) removal of the enzymatic domain from MMP14 does not impair the sequestration of MMP2 into exosomes, but instead increases it. This increase may be due to either a change in the mechanism by which MMP2 is sequestered or, more likely, to increased levels of pro-MMP2 in the cell resulting from a lack of MMP14, its major endogenous catalyzer.
Discussion
In the present study, we found that MMP14 expression is greater in exosomes of corneal fibroblasts than in cell lysate. Our results also demonstrate that corneal fibroblasts can transfer MMP14 protein via exosomes to other cell types, including vascular endothelial cells, which readily take up these exosomes and their contents. Finally, we found that deletion of the MMP14 enzymatic domain leads to an increase in pro-MMP2 accumulation in exosomes, whereas absence of MMP14 results in near-complete failure of MMP2 to localize to exosomes. Our study yields the following significant findings: (1) MMP14 is critically involved in MMP2 localization to exosomes in corneal fibroblasts; (2) exosomes may represent a mechanism for the localization of MMP14 and MMP2 enzymatic activity in the extracellular space; and (3) intercellular transfer of MMP14 and MMP2 between fibroblasts and endothelial cells may occur during angiogenesis and other biological processes.
Research groups including ours have established the ability of exosomes to transfer materials between cells, and this transfer method has implications for processes beyond angiogenesis. For example, enzymatically active MMP14 has been identified in exosomes secreted by fibrosarcoma and melanoma cells,40 and exosomes may provide a mechanism for intercellular transfer of material in these cell types during the growth and spread of ovarian cancers.41 However, the mechanisms that control exosomal transport of materials remain unclear. Ligand receptor binding, attachment, and fusion with the target cell membrane or internalization via endocytosis are all posited as possible modes of interaction between exosomes and target cells.34,36 Regardless of the precise mechanism, exosomes likely require selective targeting of recipient cells to execute the level and specificity of appropriate intercellular transfer and communication currently hypothesized. Through the use of YPet-tagged MMP14, we demonstrated the ability of exosomes to deliver MMP14 to other cells and showed that the efficiency of this delivery varies between different cell types (endothelial cells appeared to accept MMP14-containing exosomes more efficiently than corneal fibroblasts). The differential effects observed for various cell types support the concept of target-specific exosomal activity.
Our data suggest a possible mechanism of MMP14 action in corneal vascularization. We previously demonstrated that MMP14 potentiates multiple processes in FGF2-induced corneal neovascularization,9 with neovascularization requiring MMP14 activity in the extracellular space to degrade ECM to create routes for endothelial cells to directionally proliferate, migrate, and establish new vessels. However, endothelial cells themselves do not naturally produce substantial amounts of MMP14. Interestingly, corneal fibroblasts produce higher levels of MMP14 that resides in their cell membranes and, as our findings show, in the exosomes they secrete (Figs. 1, 2). We show that these MMP14-containing exosomes, secreted by fibroblasts, may be the source of endothelial MMP14.
Matrix metalloproteinase 2 is synthesized and secreted as pro-MMP2 in a complex with tissue inhibitor of metalloproteinase-2 (TIMP2). Pro-MMP2 is cleaved by MMP14, releasing activated MMP2. Activated MMP2 degrades basement membrane collagen (type IV), elastin, and several other ECM molecules, including interstitial collagen types I, II, and III, and is associated with ECM remodeling in wound healing, angiogenesis, and tumor invasion.42 Our finding that MMP2 is carried by exosomes and released by limbal endothelial cells into the extracellular space reveals a possible mechanism by which MMP14 and MMP2 may facilitate the breakdown of ECM in a directional manner toward a wound. Thus, MMP2 may interact with MMP14 in exosomes released by corneal fibroblasts. Therefore, exosomes provide a vehicle by which cells can release MMP14 to directionally modulate endothelial activity and neovascularization.
Our investigation of the roles of MMP14 and MMP2 in exosomes builds upon our understanding of their relationship in the plasma membrane. At the cell surface, MMP14 functions as a receptor for both pro-MMP2 and TIMP2. Matrix metalloproteinase 14 forms a membrane-bound homodimer; one dimerized MMP14 monomer unit also binds TIMP2, which recruits pro-MMP2. The recruited pro-MMP2 is then in position to interact with the other dimerized MMP14.17 The propeptide of MMP2 is cleaved by MMP14, resulting in an intermediate form, which is subsequently converted to fully active MMP2 through an autocatalytic cleavage. Active MMP2 is then released into the extracellular space.18,23 Our experiments using MMP14Δexon4 cell cultures show that the dependence of MMP2 activation on MMP14 enzymatic activity is maintained in exosomes. Wild-type corneal fibroblasts contained significant amounts of active MMP2 in exosomes, whereas the loss of MMP14 enzymatic ability led to pro-MMP2 accumulation in exosomes. The finding that MMP14-null corneal fibroblasts produced exosomes with virtually undetectable amounts of pro-MMP2 and no active MMP2 indicates that MMP14 is critically involved in the recruitment of MMP2 to exosomes, although this process does not seem to require the MMP14 enzymatic domain. The interaction of MMP14 in the exosomal packaging of MMP2 is a novel finding and further suggests that exosomes play a key role in the ability of MMP14 to achieve downstream effects in non–MMP14-producing cells. Future research is needed to determine how MMP14 recruits, modifies, and directs MMP2 into and out of exosomes.
In summary, our study demonstrates that exosomes of corneal fibroblasts contain high levels of enzymatically active MMP14 and can deliver their contents to target cells. Although it is clear that MMP14 can modify exosomal levels of MMP2 and significantly impact the transport of MMP2 into exosomes, the exact timing and mechanism of this interaction remain uncertain. More research is needed to characterize the effects of MMP14 transport to recipient cells on physiological and pathological processes. Establishing the potential effects of modulating MMP14 levels will have significant implications for the development of therapeutics targeting angiogenesis in the cornea and other tissues as well as tumor metastasis in many cancers.
Acknowledgments
Supported by National Institutes of Health Grants EY10101 (DTA) and EY01792 and an unrestricted grant from Research to Prevent Blindness, New York, New York, United States. The authors alone are responsible for the content and writing of the paper.
Disclosure: K.-Y. Han, None; J. Dugas-Ford, None; M. Seiki, None; J.-H. Chang, None; D.T. Azar, Novartis (S), Association for Research in Vision and Ophthalmology (S), Chicago Ophthalmological Society (S), Chicago Medical Society (S)
References
- 1. Egeblad M,, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002; 2: 161–174. [DOI] [PubMed] [Google Scholar]
- 2. Klose A,, Zigrino P,, Mauch C. Monocyte/macrophage MMP-14 modulates cell infiltration and T-cell attraction in contact dermatitis but not in murine wound healing. Am J Pathol. 2013; 182: 755–764. [DOI] [PubMed] [Google Scholar]
- 3. Koenig GC,, Rowe RG,, Day SM,, et al. MT1-MMP-dependent remodeling of cardiac extracellular matrix structure and function following myocardial infarction. Am J Pathol. 2012; 180: 1863–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Klein T,, Bischoff R. Active metalloproteases of the A Disintegrin and Metalloprotease (ADAM) family: biological function and structure. J Proteome Res. 2011; 10: 17–33. [DOI] [PubMed] [Google Scholar]
- 5. Siefert SA,, Sarkar R. Matrix metalloproteinases in vascular physiology and disease. Vascular. 2012; 20: 210–216. [DOI] [PubMed] [Google Scholar]
- 6. Itoh Y,, Seiki M. MT1-MMP: an enzyme with multidimensional regulation. Trends Biochem Sci. 2004; 29: 285–289. [DOI] [PubMed] [Google Scholar]
- 7. Azar DT,, Casanova FH,, Mimura T,, Jain S,, Chang JH. Effect of MT1-MMP deficiency and overexpression in corneal keratocytes on vascular endothelial cell migration and proliferation. Curr Eye Res. 2008; 33: 954–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Azar DT,, Casanova FH,, Mimura T,, et al. Corneal epithelial MT1-MMP inhibits vascular endothelial cell proliferation and migration. Cornea. 2010; 29: 321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Onguchi T,, Han KY,, Chang JH,, Azar DT. Membrane type-1 matrix metalloproteinase potentiates basic fibroblast growth factor-induced corneal neovascularization. Am J Pathol. 2009; 174: 1564–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sounni NE,, Devy L,, Hajitou A,, et al. MT1-MMP expression promotes tumor growth and angiogenesis through an up-regulation of vascular endothelial growth factor expression. FASEB J. 2002; 16: 555–564. [DOI] [PubMed] [Google Scholar]
- 11. Zhou Z,, Apte SS,, Soininen R,, et al. Impaired endochondral ossification and angiogenesis in mice deficient in membrane-type matrix metalloproteinase I. Proc Natl Acad Sci U S A. 2000; 97: 4052–4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Holmbeck K,, Bianco P,, Caterina J,, et al. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999; 99: 81–92. [DOI] [PubMed] [Google Scholar]
- 13. Albrechtsen R,, Kveiborg M,, Stautz D,, et al. ADAM12 redistributes and activates MMP-14, resulting in gelatin degradation, reduced apoptosis and increased tumor growth. J Cell Sci. 2013; 126: 4707–4720. [DOI] [PubMed] [Google Scholar]
- 14. Shuman Moss LA, Jensen-Taubman S, Stetler-Stevenson WG. Matrix metalloproteinases: changing roles in tumor progression and metastasis. Am J Pathol. 2012; 181: 1895–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Deryugina EI,, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006; 25: 9–34. [DOI] [PubMed] [Google Scholar]
- 16. Irie K,, Komori K,, Seiki M,, et al. Impaired alveolization in mice deficient in membrane-type matrix metalloproteinase 1 (MT1-MMP). Med Mol Morphol. 2005; 38: 43–46. [DOI] [PubMed] [Google Scholar]
- 17. Frittoli E,, Palamidessi A,, Disanza A,, Scita G. Secretory and endo/exocytic trafficking in invadopodia formation: the MT1-MMP paradigm. Eur J Cell Biol. 2011; 90: 108–114. [DOI] [PubMed] [Google Scholar]
- 18. Osenkowski P,, Toth M,, Fridman R. Processing shedding, and endocytosis of membrane type 1-matrix metalloproteinase (MT1-MMP). J Cell Physiol. 2004; 200: 2–10. [DOI] [PubMed] [Google Scholar]
- 19. Murphy DA,, Courtneidge SA. The “ins” and “outs” of podosomes and invadopodia: characteristics formation and function. Nat Rev Mol Cell Biol. 2011; 12: 413–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Azar DT,, Chang JH,, Han KY. Wound healing after keratorefractive surgery: review of biological and optical considerations. Cornea. 2012; 31 (suppl 1): S9–S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Han KY,, Fahd DC,, Tshionyi M,, et al. MT1-MMP modulates bFGF-induced VEGF-A expression in corneal fibroblasts. Protein Pept Lett. 2012; 19: 1334–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sato H,, Kinoshita T,, Takino T,, Nakayama K,, Seiki M. Activation of a recombinant membrane type 1-matrix metalloproteinase (MT1-MMP) by furin and its interaction with tissue inhibitor of metalloproteinases (TIMP)-2. FEBS Lett. 1996; 393: 101–104. [DOI] [PubMed] [Google Scholar]
- 23. Lehti K,, Lohi J,, Valtanen H,, Keski-Oja J. Proteolytic processing of membrane-type-1 matrix metalloproteinase is associated with gelatinase A activation at the cell surface. Biochem J. 1998; 334 (pt 2): 345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chavez-Munoz C,, Kilani RT,, Ghahary A. Profile of exosomes related proteins released by differentiated and undifferentiated human keratinocytes. J Cell Physiol. 2009; 221: 221–231. [DOI] [PubMed] [Google Scholar]
- 25. Choi DS,, Kim DK,, Kim YK,, Gho YS. Proteomics, transcriptomics and lipidomics of exosomes and ectosomes. Proteomics. 2013; 13: 1554–1571. [DOI] [PubMed] [Google Scholar]
- 26. Olver C,, Vidal M. Proteomic analysis of secreted exosomes. Subcell Biochem. 2007; 43: 99–131. [DOI] [PubMed] [Google Scholar]
- 27. Simpson RJ,, Jensen SS,, Lim JW. Proteomic profiling of exosomes: current perspectives. Proteomics. 2008; 8: 4083–4099. [DOI] [PubMed] [Google Scholar]
- 28. Wubbolts R,, Leckie RS,, Veenhuizen PT,, et al. Proteomic and biochemical analyses of human B cell-derived exosomes. Potential implications for their function and multivesicular body formation. J Biol Chem. 2003; 278: 10963–10972. [DOI] [PubMed] [Google Scholar]
- 29. Regev-Rudzki N,, Wilson DW,, Carvalho TG,, et al. Cell-cell communication between malaria-infected red blood cells via exosome-like vesicles. Cell. 2013; 153: 1120–1133. [DOI] [PubMed] [Google Scholar]
- 30. Ochiya T,, Lotvall J. Exosome as a novel shuttle for innovation. Preface. Adv Drug Deliv Rev. 2013; 65: v. [DOI] [PubMed] [Google Scholar]
- 31. Bang C,, Thum T. Exosomes: new players in cell-cell communication. Int J Biochem Cell Biol. 2012; 44: 2060–2064. [DOI] [PubMed] [Google Scholar]
- 32. Camussi G,, Deregibus MC,, Bruno S,, Cantaluppi V,, Biancone L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int. 2010; 78: 838–848. [DOI] [PubMed] [Google Scholar]
- 33. Ludwig AK,, Giebel B. Exosomes: small vesicles participating in intercellular communication. Int J Biochem Cell Biol. 2012; 44: 11–15. [DOI] [PubMed] [Google Scholar]
- 34. Denzer K,, Kleijmeer MJ,, Heijnen HF,, Stoorvogel W,, Geuze HJ. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci. 2000; 113 (pt 19): 3365–3374. [DOI] [PubMed] [Google Scholar]
- 35. Valadi H,, Ekstrom K,, Bossios A,, Sjostrand M,, Lee JJ,, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007; 9: 654–659. [DOI] [PubMed] [Google Scholar]
- 36. Fevrier B,, Raposo G. Exosomes: endosomal-derived vesicles shipping extracellular messages. Curr Opin Cell Biol. 2004; 16: 415–421. [DOI] [PubMed] [Google Scholar]
- 37. Simons M,, Raposo G. Exosomes--vesicular carriers for intercellular communication. Curr Opin Cell Biol. 2009; 21: 575–581. [DOI] [PubMed] [Google Scholar]
- 38. Robbins PD,, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014; 14: 195–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Buzas EI,, Gyorgy B,, Nagy G,, Falus A,, Gay S. Emerging role of extracellular vesicles in inflammatory diseases. Nat Rev Rheumatol. 2014; 10: 356–364. [DOI] [PubMed] [Google Scholar]
- 40. Hakulinen J,, Sankkila L,, Sugiyama N,, Lehti K,, Keski-Oja J. Secretion of active membrane type 1 matrix metalloproteinase (MMP-14) into extracellular space in microvesicular exosomes. J Cell Biochem. 2008; 105: 1211–1218. [DOI] [PubMed] [Google Scholar]
- 41. Keller S,, Konig AK,, Marme F,, et al. Systemic presence and tumor-growth promoting effect of ovarian carcinoma released exosomes. Cancer Lett. 2009; 278: 73–81. [DOI] [PubMed] [Google Scholar]
- 42. Vu TH,, Werb Z. Matrix metalloproteinases: effectors of development and normal physiology. Genes Dev. 2000; 14: 2123–2133. [DOI] [PubMed] [Google Scholar]