Abstract
GBV-C, a pan-lymphotropic flavivirus capable of persistent infection, is associated with prolonged survival and reduced T cell activation in HIV-infected subjects. GBV-C was associated with reduced CD56brt/CD16− NK cell and monocyte activation, and a trend towards reduced B cell activation by measuring cell surface activation markers or HIV entry coreceptors. The GBV-C association was independent of HIV VL. Thus, GBV-C may influence non-T cell immune activation in individuals with HIV infection.
Keywords: GBV-C, HIV, B lymphocyte, NK cell, Monocyte, Activation
Research Letter
HIV disease progression is predicted both by the level of viral replication measured by plasma HIV viral load (VL), and by the extent of chronic immune activation as measured by T cell expression of cell surface activation markers (reviewed in [1]). Although HIV-infected individuals treated with combination antiretroviral therapy (cART) demonstrate a reduction in markers of immune activation following treatment, the level of activation remains elevated compared to HIV uninfected people [1]. The extent of immune activation in treated HIV-infected people correlates with CD4 recovery, morbidity, and mortality, thus understanding factors that influence chronic immune activation in HIV-infected people may provide insight into novel therapeutic interventions to reduce morbidity and mortality.
GB virus C (GBV-C) is a pan-lymphotropic flavivirus that commonly infects humans and is capable of persistent infection [2]. Due to shared modes of transmission, up to 39% of HIV-infected subjects are actively co-infected with GBV-C (reviewed in [3]). Most, though not all, clinical studies demonstrate an association between persistent GBV-C infection and prolonged survival in HIV-infected humans (also reviewed in [3]). Consistent with this, several studies found an association between GBV-C viremia and reduced expression of the HIV entry co-receptors CCR5 [4–6] and CXCR4 [7] on CD4+ and CD8+ lymphocytes in HIV-infected people. Although these entry co-receptors are not typically considered markers of T cell activation, both receptors are upregulated following T cell activation. Furthermore, studies have demonstrated an association between GBV-C viremia and reduced levels of more typical markers of activated T cells including CD25, CD38, HLA-DR, and CD69 [5, 6, 8]. Finally, CD4+ and CD8+ T cell proliferation is reduced in GBV-C viremic subjects compared to those without GBV-C based on expression of the proliferation marker Ki67 [6]. Together these findings suggest a relationship between GBV-C infection and reduced T cell activation in HIV-infected people, and raise the possibility that this may contribute to the beneficial association between GBV-C viremia and survival in HIV-infected people [3].
GBV-C is present in and produced by human B cells [2]. In addition, GBV-C RNA was recently detected in highly purified NK cells and monocytes obtained from infected subjects (Chivero, et al., unpublished). To date, no studies have reported interactions between GBV-C and the activation status of B cells, NK cells or monocytes in HIVinfected people. To address this, we analyzed PBMCs from a previously described cohort of HIV-infected individuals [6] for expression of activation markers on B cells (CD86), total NK cells (CD69), NK cell subsets (CD56, CD16), and monocytes (CCR5) by flow cytometry (percent positive or mean fluorescent intensity [MFI]). A minimum of 35,000 events were acquired for each analysis.
Subjects were evaluated based on their HIV treatment status. Those not on therapy (HIV viremic or HV) and those on cART with documented suppression of HIV VL (< 48 copies/mL; HIV suppressed or HS) for greater than 6 months were evaluated. GBV-C viremia was assessed by real-time RT-PCR as described previously [9]. There were 10 subjects with HIV viremia and GBV-C viremia (HVG+), 21 with HIV viremia but without GBV-C coinfection (HVG-), and 28 subjects with suppressed HIV VL, 14 with and 14 without GBV-C viremia (HSG+ and HSG− respectively). All subjects provided written informed consent and the study was approved by the University of Iowa Institutional Review Board.
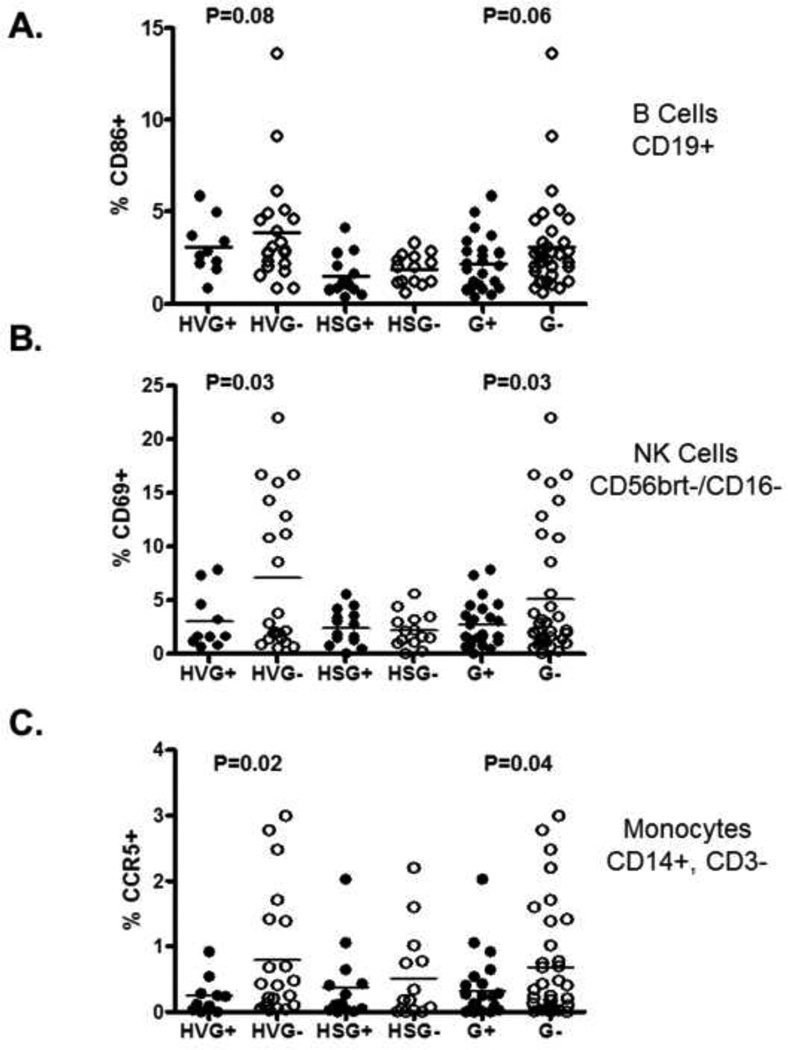
Subjects with GBV-C viremia had a trend towards reduced levels of B cell activation (CD86+) as compared to those without GBV-C (p=0.06), and this was largely driven by those who were not receiving cART (Fig. 1A). Because there was heterogeneity in the variance, a Kruskall-Wallace (KW) non-parametric test was used for each cell type to examine if there are differences between the 4 groups defined by HIV viremia (HV or HS) and GBV-C viremia (G+ or G−) as described previously [6]. When the KW test was significant, nominal (unadjusted) p-values are reported.
Figure.
GBV-C viremia is associated with reduced B cell and NK cell activation, and CCR5 expression on monocytes. The percent of B cells (A), NK subset (CD56 bright, CD16−), and monocytes (CD14+, CD3−) expressing activation markers CD86, CD69, and CCR5 respectively were assessed by flow cytometry. HV = HIV viremic (not on therapy), HS = HIV suppressed (on therapy with documented HIV VL <48 copies/mL for > 6 months), G+ = GBV-C viremic, G− = no GBV-C RNA detected. G+ and G− represent those with and without detectable HIV VL.
Among total NK cells, there was a trend towards reduced surface expression of the activation marker CD69 in GBV-C viremic subjects (not shown). However, among the CD56brt/CD16− NK cell subset, significantly less activation was observed in those with GBV-C viremia and in the non-cART treated subjects (p=0.03, Fig. 1B). NK cell activation marker expression was low in all subjects with GBV-C viremia, independent of HIV therapy, and in those without GBV-C viremia in whom HIV viremia was suppressed by cART. However, among treatment-naïve HIV-infected subjects, activation markers were more widely distributed in those without GBV-C viremia (Fig. 1A). There was no correlation between NK cell activation in this group and HIV VL, indicating that factors other than HIV viremia may influence NK cell activation marker expression. Although CD56brt/CD16− NK cells represent a minority of peripheral blood NK cells, they are numerically the majority of NK cell subset in secondary lymphoid tissues and are abundant cytokine producers [10]. The association between GBV-C viremia and reduced NK cell activation was not observed in subjects with suppressed HIV viral load (VL). Nevertheless, the main effect of GBV-C (G+ or G−) was independent of HIV viral load (HV and HS) using an additive linear model as described previously [6]. The surface expression of CCR5 on monocytes was significantly lower among GBV-C viremic subjects compared to those without GBV-C viremia (Fig. 1C) and this too was independent of HIV VL in the additive linear model [6]. GBV-C viremia was also associated with a reduced MFI of activation marker expression on B cells, NK cells and monocytes, and groups that were statistically different by measuring the percent positive cells (Fig. 1) remained significant when MFI values were compared (p<0.05; data not shown).
This study is the first to identify an association between GBV-C viremia and reduced levels of activation of B cells, the CD56brt/CD16− subset of NK cells, and CCR5 expression on monocytes. Although data on the effects of HIV on NK cell, B cell and monocyte activation are limited, HIV increases microbial translocation resulting in the exposure of these cells to endotoxin and other cytokines, resulting in global immune activation [1]. These data demonstrate that GBV-C viremia is associated with a significant reduction in NK and monocyte activation markers, and with a trend towards reducted B cell activation. Since activation of these cells contributes to immune activation overall, the association between GBV-C infection and reduced activation of these cells likely contributes to a modest reduction in the global immune activation associated with HIV infection. The fact that this reduction in immune activation observed in GBV-C viremic subjects was modest is consistent with the observation that GBV-C infected people do not have clinical evidence of immune compromise.
A recent study found that the GBV-C envelope glycoprotein E2 interferes with both IL-2 receptor signaling and with T cell receptor-mediated activation [8]. GBV-C E2 protein did not interfere with the function of non-stimulated cells, but did reduce activation significantly in CD4+ and CD8+ T cells following stimulation through the T cell receptor. Thus, GBV-C appears to reduce or fine tune immune activation but does not lead to a complete blockage. If the association between GBV-C viremia and reduced NK cell, monocyte and possibly B cell activation is confirmed in additional studies, this would provide additional evidence that GBV-C infection reduces global immune activation in HIV infected people. Furthermore, identification of the mechanisms by which GBV-C might influence immune activation may identify novel approaches of therapy interventions.
Acknowledgements
We thank our patients for participation and Wendy Sauter for obtaining specimens and clinical assistance.
Support for this work was provided by Merit Review grants from the Department of Veterans Affairs (1-2I01BX000207 [JTS], 1I01BX001241 [JX]), an RO1 grant from the National Institute of Allergy and Infectious Diseases/NIH AI058740 [JTS]), and with assistance from the John B. Pendleton Charitable Trust and the Flow Cytometry Core facility at Rush University.
Footnotes
Contributions: J.T.S., S.N.D., and A.L.L. designed the study, analyzed data and wrote the manuscript. J.M., D.K., and J.X. assisted in the design and analysis of the laboratory studies, conducted the experiments, and critically reviewed the manuscript.
Conflicts of interest: None
References
- 1.Klatt NR, Funderburg NT, Brenchley JM. Microbial translocation, immune activation, and HIV disease. Trends Microbiol. 2013;21:6–13. doi: 10.1016/j.tim.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.George SL, Varmaz D, Stapleton JT. GB virus C replicates in primary T and B lymphocytes. J Infect Dis. 2006;193:451–454. doi: 10.1086/499435. [DOI] [PubMed] [Google Scholar]
- 3.Zhang W, Chaloner K, Tillmann HL, Williams CF, Stapleton JT. Effect of early and late GBV-C viremia on survival of HIV infected individuals: A meta-analysis. HIV Med. 2006;7:173–180. doi: 10.1111/j.1468-1293.2006.00366.x. [DOI] [PubMed] [Google Scholar]
- 4.Nattermann J, Nischalke HD, Kupfer B, Rockstroh J, Hess L, Sauerbruch T, et al. Regulation of CC chemokine receptor 5 in Hepatitis G virus infection. AIDS. 2003;17:1457–1462. doi: 10.1097/00002030-200307040-00006. [DOI] [PubMed] [Google Scholar]
- 5.Maidana Giret MT, Silva TM, Sauer MM, Tomiyama H, Levi JE, Bassichetto KC, et al. GBV-C infection modulates T cell activation in recently HIV-infected subjects and is independent of HIV-1 viral load. AIDS. 2009;23:2277–2287. doi: 10.1097/QAD.0b013e32832d7a11. [DOI] [PubMed] [Google Scholar]
- 6.Stapleton JT, Chaloner K, Martenson JA, Zhang J, Klinzman, Xiang J, et al. GB virus C infection is associated with altered lymphocyte subset distribution and reduced T cell activation and proliferation in HIV-infected individuals. PLoS One. 2012;7:e50563. doi: 10.1371/journal.pone.0050563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwarze-Zander C, Neibecker M, Othman S, Tural C, Clotet B, Blackard JT, et al. GB virus C coinfection in advanced HIV type-1 disease is associated with low CCR5 and CXCR4 surface expression on CD4(+) T-cells. Antivir Ther. 2010;15(5):745–752. doi: 10.3851/IMP1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhattarai N, McLinden JH, Xiang J, Kaufman TM, Stapleton JT. GB Virus C Envelope Protein E2 Inhibits TCR-Induced IL-2 Production and Alters IL-2-Signaling Pathways. J Immunol. 2012;189:2211–2216. doi: 10.4049/jimmunol.1201324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rydze RT, Bhattarai N, Stapleton JT. GB virus C infection reduces reactivation of latent HIV and protects against T cell depletion in patients on antiretroviral therapy. Antiviral Ther. 2011;17:1271–1279. doi: 10.3851/IMP2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polis A, Michel T, Theresine M, Andres E, Hentges F, Zimmer J. CD56bright natural killer (NK) cells: an important NK cell subset. Immunology. 2009;126:458–465. doi: 10.1111/j.1365-2567.2008.03027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]