Abstract
Background
Some non-antibiotic drugs, such as the phenothiazine antipsychotic agents, may have antimicrobial activity.
Materials and Methods
We sought to determine the in vivo antimicrobial effects of the phenothiazine thioridazine in two mouse models of Staphylococcus aureus skin infection.
Results
Thioridazine significantly suppressed dissemination from skin to spleen and kidney after inoculation of the skin surface. However, the drug did not affect infection parameters in the skin itself. Thioridazine did suppress the size of abscesses produced when the bacteria were injected intradermally. On the other hand, using the cutaneous abscess model we were not able to demonstrate synergistic activity between thioridazine and the β-lactam drug cefazolin against methicillin-resistant S. aureus, as previously demonstrated in vitro.
Conclusion
The phenothiazine drug thioridazine has in vivo antimicrobial activity against certain S. aureus skin infections, although the previously-demonstrated reversal of methicillin resistance by this agent may not be readily evident in vivo.
Keywords: Phenothiazines, thioridazine, Staphylococcus aureus, cutaneous infections, mouse model
Because of increasing antibiotic resistance and the great expense of developing new drugs for clinical use, there is a continuing need for new antimicrobial agents. One way to address this problem is to examine drugs used to treat other medical conditions to see if they might have useful antimicrobial activity. In fact, a variety of so-called non-antibiotic drugs have been studied in this way and found to have such activities (1, 2). Of these, agents of the phenothiazine class of antipsychotic drugs would seem to have the most promise, especially against staphylococci (3–6), salmonella (7, 8), and mycobacteria (9). Most of the extensive previous work on the antimicrobial activity of phenothiazines has been performed in vitro, and because of the unusual pharmacokinetics of these drugs (as discussed below), it may be difficult to predict from this information how effective they might be in vivo. On the other hand, these drugs have been used for over 50 years to treat psychiatric disorders and although they have significant toxicities, they are currently available and there is a great deal of experience with their clinical use.
Phenothiazines have only modest antimicrobial activity against bacteria in suspension, but are markedly concentrated within phagocytic cells and are much more effective against intracellular organisms (3). These drugs intercalate into bacterial DNA (3), cause ultra-structural changes in bacteria (10), interrupt transport of small ions across bacterial cell membranes (11, 12), and reduce expression of selected cell wall proteins thereby weakening cell walls (13). Phenothiazines also potently inhibit efflux pumps in both human (14, 15) and microbial (14, 16) cells. These drugs also appear to suppress antimicrobial resistance in both gram-negative bacteria (17) and methicillin-resistant staphylococci (MRSA) (3, 7, 18).
Although blood and tissue levels of phenothiazines have been studied in humans and mice (19, 20), it is not really known what their levels might be in the individual cells of living hosts. Therefore, although this drug class would seem to have promise for treating certain infectious agents, their activity would likely be restricted to intracellular infections. Phenothiazines have not been studied in animal models of staphylococcal infections. Since Staphylcoccus aureus has the potential to act as an intracellular pathogen (21–23), these drugs may be useful for in vivo infections with this organism. In addition, they might also act to reverse resistance in MRSA and allow for treatment of such infections with standard β-lactams. Most of the previous work on in vitro phenothiazine activity was performed with thioridazine, and this drug was chosen for the present in vivo study. These experiments were undertaken to evaluate the effect of thioridazine against staphylococcal infections in two cutaneous models in mice, and to determine if this drug might alter antibiotic resistance of MRSA to allow for treatment of this organism with cefazolin.
Materials and Methods
Organisms
The inoculations were carried out with 107 CFU of S. aureus ATCC strain 25923 (a methicillin-sensitive strain) and ATCC BAA-1680 (a USA300 methicillin-resistant strain) (American Type Culture Collection, Manassas, VA, USA). The organisms were cultured overnight in tryptic soy broth and then washed three times in sterile water before use.
Animals and treatments
C57BL/6 mice were obtained from Charles Rivers Laboratories (Wilmington, MA, USA). The animals were female of 8–14 weeks of age, and were described by the supplier as being free of specific pathogens (including S. aureus). Thioridazine and cefazolin were obtained from Sigma (St. Louis, MO, USA). The drugs were dissolved in normal saline and given by intraperitoneal injection (for thioridazine) or subcutaneous injection over the upper back (for cefazolin). Thioridazine was given 24 and 1 h before inoculation with S. aureus, and on the morning of each subsequent day; doses used ranged from 3–30 mg/kg for each administration. This regimen was used because of the propensity of this drug to accumulate intracellularly in host cells. Cefazolin was given on the morning following inoculation with S. aureus and on the morning of each subsequent day; doses used were either 25 or 50 mg/kg for each administration. The drug doses were chosen from the results of preliminary experiments showing evidence of efficacy in the two models used; however, for studies of combination therapy the cefazolin regimen was purposely designed to be sub-optimal. The mice for these studies were housed in a separate BSL 2-enhanced section of the Veterinary Medical Unit at the Milwaukee VA Medical Center. The experimental procedures were approved by the appropriate committees at the Milwaukee Veterans Affairs Medical Center, 8033-17.
S. aureus inoculations
The skin was shaved with an electric razor and then disinfected with iodine, washed with alcohol followed by saline, and dried with gauze. For epicutaneous inoculation (24, 25), the skin surface was prepared by gentle tape-stripping seven times with Transpore tape (approximately 27 mm in width, 3M, Minneapolis, MN, USA). This technique was found to cause minimal damage to the epidermis and dermis (24). An inoculum of 107 S. aureus CFU in 0.025 ml of saline was added to 4 mm filter paper discs placed on prepared skin of both flanks; the sites were covered with 1.0 cm2 pieces of plastic sheet and then overwrapped with dressings of Transpore tape and Nexcare waterproof tape (3M). To produce cutaneous abscesses, the same inoculum of S. aureus was injected intradermally into the flank sites.
Monitoring of infections
After 24 h, the occlusive dressings over the epicutaneous inoculation sites were removed and the skin washed four times with saline-soaked gauze pads. At various times after inoculation, the animals were killed and skin from one epicutaneous inoculation site was removed for histology. Paraffin sections were prepared and stained with tissue gram stains, with analysis as discussed below. Cultures were performed on skin scrapings from the other flank site or homogenates of the spleen or one kidney. In this model system (epicutaneous inoculations) spleen and kidney cultures are positive for the majority of animals at 24 h after inoculation (24, 25). The two organs were homogenized in 1 ml of saline and 0.1 ml of the homogenate was cultured on tryptic soy agar, with results recorded as CFU per ml. For skin scrapings, the entire inoculation site was sampled by abrasion with a scalpel blade until a glistening surface could be seen; the material removed was vortexed in 1 ml of saline and then cultured for CFU determinations.
The size of cutaneous abscesses resulting from intradermal inoculation was determined at day 3. In preliminary studies, we found that the abscesses generally reached their greatest size at this time, and regressed afterward. At three days the animals were killed and the skin containing the inoculation site removed. Abscess size (mm2) was measured in two directions on the inner surface of the skin, and the two measurements averaged.
Sections of S. aureus inoculated skin were examined in a blinded fashion for bacteria and cutaneous changes at a magnification of ×400 under light microscopy as previously described (24). For the purposes of this study, the epidermis was defined as the epidermal keratinocyte layers above the dermal-epidermal junction, but excluding the stratum corneum and crusts. In ten random fields in each section, the site of bacteria was determined to be in the stratum corneum or crusts, the epidermis, the dermis, or combination thereof. Mouse skin structure is generally similar to that of humans except for a thinner epidermis and the grouping of hair follicles together as actively growing (anagen) or resting (telogen).
Cutaneous damage was also assessed for each linear high power ×400 field across the section's entire epidermis as absent epidermis, neutrophil infiltration into the keratinocyte layers, or complete dermal necrosis. The latter represented conversion of the entire dermis into a discolored amorphous crust (24). Dermal necrosis was assessed at 48 h after inoculation (when this process first becomes manifested), whereas the other two parameters were assessed at 24 h after inoculation.
Statistics
Data were expressed as the mean±SE, with statistics carried out in the GraphPad Prism 5.0 for Macintosh statistical package using either unpaired t-tests or ANOVA and Bonferroni’s test for multiple comparisons. Generally at least 4–6 mice per time-point were studied in 2–4 experiments (each consisting of animals inoculated in a similar manner on a single day). Statistical significance was taken as p<0.05.
Results
For infections produced by epicutaneous inoculation with S. aureus, treatment with the phenothiazine thioridazine produced reductions in the number of bacterial CFU obtained in cultures of spleen and kidney, but not skin, compared to animals not treated with this drug (Figure 1). There appeared to be a dose-response relationship, with the greatest effects occurring at doses of 30 mg/kg compared to those at lower doses. Lack of effect of this drug treatment on total CFU in skin cultures was also reflected in histological studies of bacterial invasion and cutaneous damage in the infections (Table I). Numbers of bacteria seen in the various skin layers (stratum corneum, epidermal keratinocyte layer, and dermis were similar in treated and untreated animals. The types of cutaneous damage produced by these experimental infections (neutrophil infiltration into the epidermis, loss of epidermis, and dermal necrosis) were also similar between treated and untreated animals. Therefore, in infections produced by applying the bacteria to the skin surface, thioridazine significantly suppressed staphylococcal dissemination to deep organs, but did not really affect the local cutaneous infection or resulting damage.
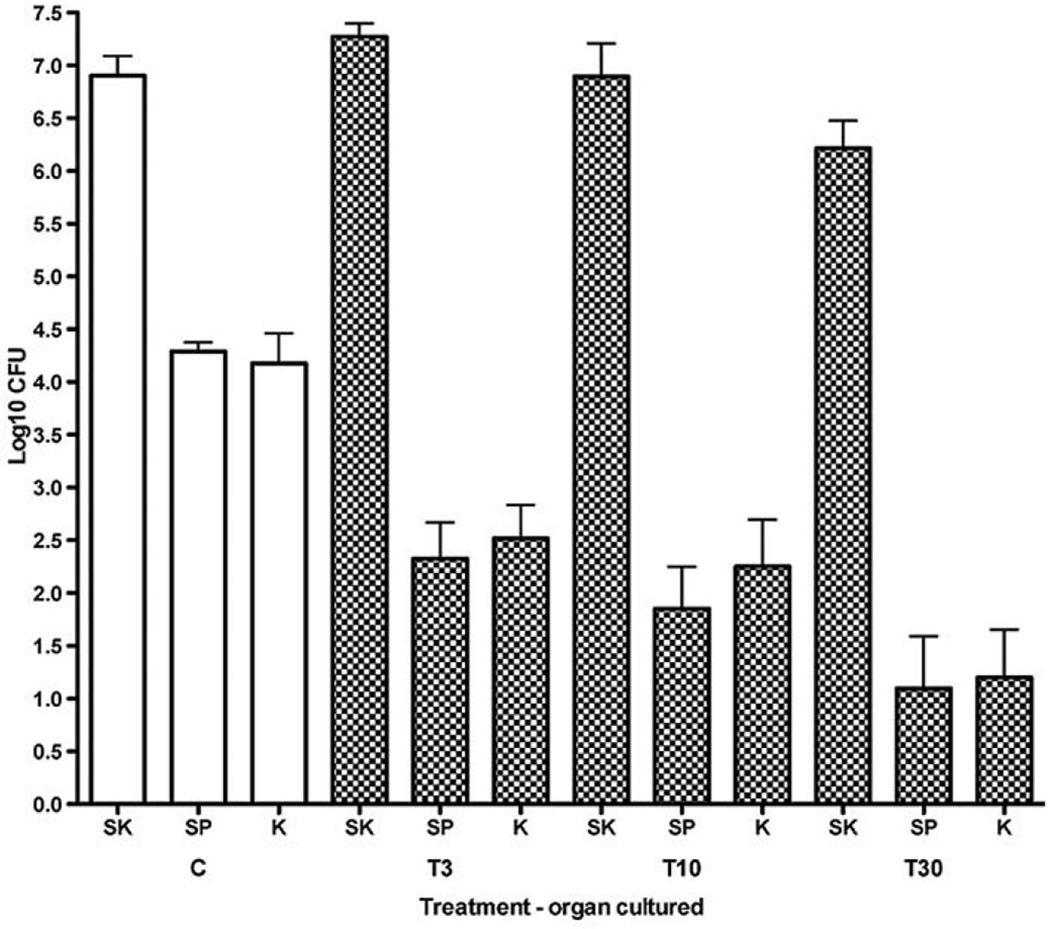
Figure 1.
Effect of thioridazine treatment on dissemination of bacteria to spleen and kidney after epicutaneous application of S. aureus onto the flank skin of mice. Values represent log10 CFU per ml (mean±SE) in skin (SK), spleen (SP), and kidney (K) at 24 h after inoculation onto flank skin of 107 CFU of S. aureus (ATCC 25923). Open bars represent control animals (C), and checked bars represent animals treated with intraperitoneal injections of thioridazine at the indicated doses at 24 and one hour before inoculation with bacteria (for example, T3=doses of 3 mg/kg). Note that numbers of CFU in the skin were not significantly affected by thioridazine treatment. However, CFU in spleen and kidney were significantly reduced in most cases compared to those from untreated animals (p-values by ANOVA and Bonferroni’s tests were as follows: T30-SP p<0.001; T30-KD p<0.001; T10-SP p<0.01; T10-KD p<0.05; T3-SP p<0.01; T3-KD p>0.05). Therefore, thioridazine treatment suppressed dissemination to distant organs, but did not reduce bacterial numbers in the local skin infections.
Table I.
Lack of effect of thioridazine on local bacterial invasion and cutaneous damage after epicutaneous inoculation of 107 S. aureus onto flank skin of mice. Values represent the mean±SE percentage of microscopic fields with the indicated characteristics in control and thioridazine-treated mice. The latter were given 10 mg/kg of the drug intraperitoneally 24 and 1 h before epicutaneous inoculation with S. aureus (ATCC 25923) on the flank. Values for invasion of bacteria or for presence of either neutrophils in the epidermis or absence of the latter layer were determined at 24 h after inoculation; values for dermal necrosis were determined in a separate set of animals at two days after inoculation (when this process becomes manifested). For these determinations, the epidermis was defined as the layers of keratinocytes, not counting the stratum corneum or crusts. The data were collected from 5–6 mice per point studied in 2–4 experiments. Note that thioridazine treatment did not affect cutaneous invasion by the bacteria or damage to the infected skin.
| Parameter tested | Control mice |
Thioridazine-treated mice |
|---|---|---|
| Bacteria in stratum corneum | 83.0±27.9 | 83.3±12.3 |
| Bacteria in epidermis | 54.0±27.6 | 52.2±29.5 |
| Bacteria in dermis | 29.0±25.4 | 23.3±20.0 |
| Neutrophils in epidermis | 38.1±21.8 | 39.7±23.2 |
| Epidermis absent | 10.9±16.9 | 11.2±13.9 |
| Dermal necrosis | 52.2±34.1 | 53.1±27.3 |
The effect of thioridazine on cutaneous staphylococcal infections was also studied for deeper infections produced by injecting the same inoculum intra-dermally. With this type of infection, thioridazine treatment did indeed suppress the size of the abscesses at the 10 mg/kg dose using both methicillin-sensitive (25923) and resistant (BAA-1680) strains (Figure 2). Dissemination to spleen and kidney was not evaluated with the intradermal model because preliminary studies demonstrated that secondary infections of the deep organs were not as frequent or consistent as with epicutaneous inoculation (data not shown).
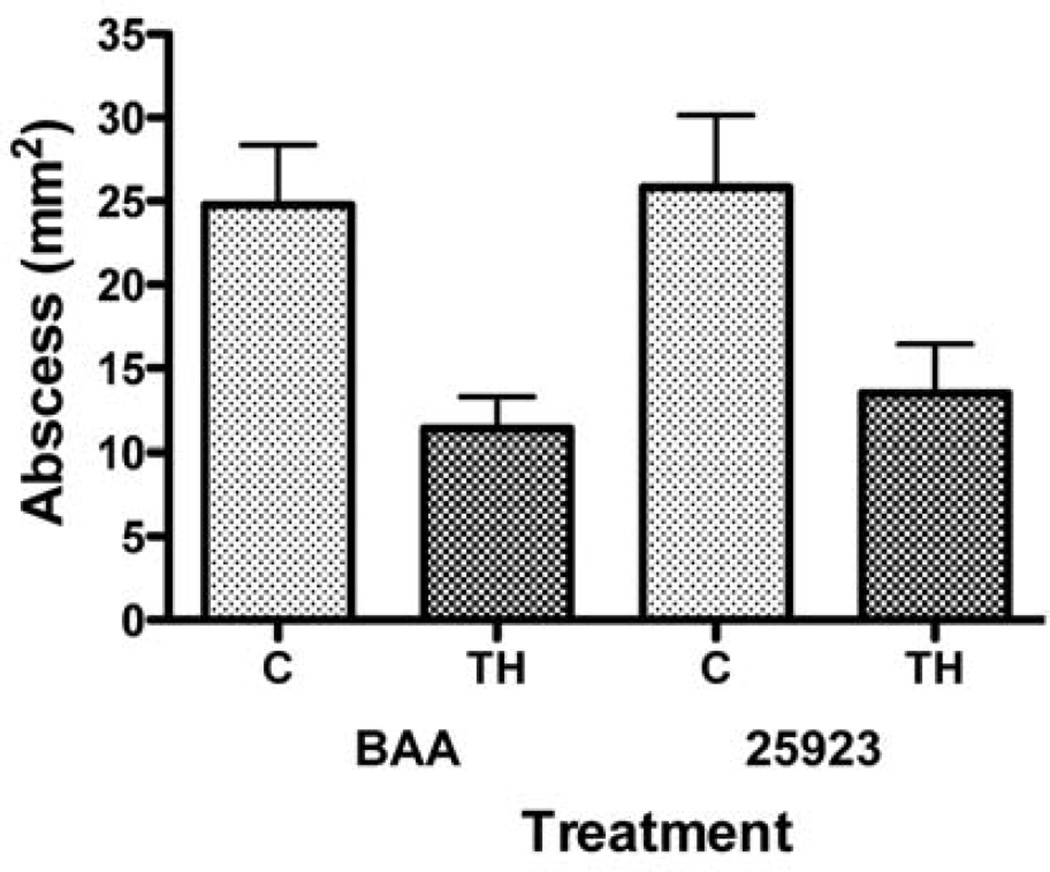
Figure 2.
Effect of thioridazine treatment on the size of cutaneous staphylococcal abscesses in mice. Values represent size of abscesses (as area in mm2) after injection of 107 CFU of S. aureus (either ATCC 25923 or BAA-1680) intradermally into the flank skin of mice, three days previously. Note that the sizes of abscesses in thioridazine-treated (TH) mice versus control mice (C) were reduced for both the BAA 1680 (BAA) strain (p<0.0496) and the 25923 strain (p<0.0026) as shown by the unpaired t-test.
The intradermal inoculation model and the BAA-1680 MRSA strain were used to test the possibility that thioridazine might be able to suppress methicillin resistance in vivo and allow for treatment of the resistant staphylococci with cefazolin. Several doses of each drug were evaluated to find those that produced minimal effects by themselves, and they were then combined to evaluate the possibility of synergistic effects (Table II). However, the latter were not found for any combinations of the two drugs.
Table II.
Effects of combining thioridazine (THZ) and cefazolin (CEF) on the size of experimental cutaneous staphylococcal abscesses in mice. Numbers after each drug designation represent doses (in mg/kg) given intraperitoneally (for THZ) or subcutaneously (for CEF) on the day before inoculation, one hour before inoculation with S. aureus (ATCC BAA-1680), and each day after until sacrifice for THZ or each day after inoculation until sacrifice for CEF. Animals were sacrificed on day 3 after inoculation and abscess sizes determined then. A positive effect of the combination was taken as an abscess size significantly smaller than that obtained with either drug alone. Note that none of the combinations produced significantly smaller abscesses than those obtained with either drug alone.
| Treatment | Abscess size | Effect |
|---|---|---|
| Control | 32.2±4.8 | – |
| THZ3 | 27.9±7.2 | – |
| THZ10 | 13.3±2.0 | – |
| CEF25 | 52.8±10.5 | – |
| CEF50 | 14.5±4.5 | – |
| THZ3+CEF25 | 36.1±5.8 | None |
| THZ3+CEF50 | 13.3±3.7 | None |
| THZ10+CEF 25 | 22.4±6.2 | None |
| THZ10+CEF 50 | 12.7±4.8 | None |
Discussion
The results of the present study demonstrate that the phenothiazine antipsychotic drug thioridazine, indeed has antimicrobial activity in two mouse models of S. aureus skin infection. The drug significantly suppressed dissemination from the skin to spleen and kidney in a model system employing application of the organisms to the skin surface. However, infection parameters in the skin itself, including total numbers of organisms present in skin cultures, invasion into deeper layers of the skin, and damage to skin structures were not affected by thioridazine. This drug did suppress the size of abscesses produced when the bacteria were injected intradermally. On the other hand, using the cutaneous abscess model, we were not able to show that thioridazine might suppress methicillin resistance in MRSA and allow for synergistic activity of the combination of cefazolin and thioridazine against infections produced with a USA300 strain of MRSA.
As discussed above, phenothiazines accumulate within mammalian cells and this appears to be needed in order to achieve levels high enough to actually kill microorganisms. For example, peak thioridazine concentrations in serum of humans generally range from 0.13 to 0.52 µg/ml (20) and peak chlorpromazine blood levels in mice (after 25–50 mg/kg i.p.) range from 0.31 to 0.87 µg/ml (19). Minimal inhibitory concentration (MIC) and minimal bactericidal concentration (MBC) values for thioridazine against S. aureus (including MRSA) in suspension, have been shown to be 20 and 40–60 µg/ml respectively, but the latter decreases to approximately 0.1 µg/ml when the bacteria have been ingested by macrophages (3). Mammalian lung tissue accumulates phenothiazines (26), but levels in other tissues are quite variable (19) and in individual cells could be much different. In addition, the synergy between phenothiazines and oxacillin has been shown to occur at much less than the MIC/MBCs of phenothiazines for bacteria in suspension (27). Because of the complicated pharmacokinetics of phenothiazines, we cannot actually predict the extent of antimicrobial activity these drugs might have for actual infections without performing in vivo studies in experimental infection models. Because S. aureus probably causes both extracellular and intracellular infections, and because the phenothiazines would be expected to affect only the latter, we might expect the type of mixed result that we found in the present study.
If phenothiazines can kill intracellular staphylococci, they could have a place in treatment of clinical infections with these organisms. Most antibiotics, including the β-lactams, are significantly less effective in intracellular, killing as opposed to extracellular S. aureus (28). Antibiotic efflux pumps of macrophages appear to actively excrete β-lactam antibiotics from intracellular sites (29, 30). S. aureus itself also has efflux pumps that may contribute to antibiotic resistance of this organism (16). Inhibition of these pumps by phenothiazines might be expected to help in the treatment of infections with this organism by other antibiotics. In addition, reversal of antibiotic resistance of various kinds has been shown for phenothiazines (3, 7, 17, 18, 27); however, this work was performed in vitro and it might not translate to the in vivo situation. Indeed, we did not demonstrate synergy between thioridazine and cefazolin against MRSA in our experiments. It may be that the phenothiazine levels needed to reverse antibiotic resistance to methicillin for our MRSA strain were too high to be attained in our in vivo infections.
Phenothiazines have shown significant toxicity over the many decades in which they were used as major therapy for psychiatric disorders. In fact, knowledge of this toxicity retarded their development as antimicrobial agents at a time when safer antibiotic agents were becoming available. Thioridazine, the agent used in the present study, was withdrawn from the market by its major manufacturer in 2005; however, generic versions of the drug are still being manufactured and are available for clinical use. Other agents in this class are available; in fact, thioridazine (Mellaril), chlorpromazine (Thorazine), and trifluoperazine (Stelazine) are all on the formulary at the Milwaukee VA Medical Center at the present time. Therefore, the role of these drugs in anti-infective therapy against resistant organisms, or in difficult infections, should perhaps be evaluated further in appropriate studies. Treatment of infections with these agents would likely require a much shorter course of therapy and less toxicity than would treatment of a chronic psychiatric illness. As our studies demonstrate, phenothiazines may have promise for certain S. aureus infections. For difficult infections with this organism, particularly those in which the bacteria may have attained a chronic intracellular foothold, addition of a phenothiazine agent to standard antibiotic therapy might improve clinical outcomes. Less toxic phenothiazine derivatives are also being developed as new antimicrobial agents. However, these agents would not share the advantage that standard phenothiazines have of previous clinical experience and ready availability.
Acknowledgements
This work was supported by the Department of Veterans Affairs and by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1RR031973.
References
- 1.Kristiansen JE, Amaral L. The potential management of resistant infections with non-antibiotics. J Antimicrob Chemother. 1997;40:319–327. doi: 10.1093/jac/40.3.319. [DOI] [PubMed] [Google Scholar]
- 2.Kruszewska H, Zareba T, Tyski S. Search of antimicrobial activity of selected non-antibiotic drugs. Acta Pol Pharm. 2002;59:436–439. [PubMed] [Google Scholar]
- 3.Martins M, Bleiss W, Marko A, Ordway D, Viveiros M, Leandro C, Pacheco T, Molnar J, Kristiansen JE, Amaral L. Clinical concentrations of thioridazine enhance the killing of intracellular methicillin-resistant Staphylococcus aureus - an in vivo, ex vivo and electron microscopy study. Int J Exp Clin Pathophysiol Drug Res. 2004;18:787–794. [PubMed] [Google Scholar]
- 4.Mazumder R, Ganguly K, Dastidar SG, Chakrabarty NA. Trifluoperazine – A broad spectrum bactericide especially active on staphylococci and vibrios. Int J Antimicrob Agents. 2001;18:403–406. doi: 10.1016/s0924-8579(01)00324-7. [DOI] [PubMed] [Google Scholar]
- 5.Ordway D, Viveiros M, Leandro C, Arroz MJ, Amaral L. Intracellular activity of clinical concentrations of phenothiazines including thioridazine against phagocytosed Staphylococcus aureus. Int J Antimicrob Agents. 2002;20:34–43. doi: 10.1016/s0924-8579(02)00110-3. [DOI] [PubMed] [Google Scholar]
- 6.Ordway D, Viveiros M, Leandro C, Arroz MJ, Molnar J, Kristiansen JE, Amaral L. Chlorpromazine has intracellular killing activity against phagocytosed Staphylococcus aureus at clinical concentrations. J Infect Chemother. 2002;8:227–231. doi: 10.1007/s10156-002-0188-4. [DOI] [PubMed] [Google Scholar]
- 7.Amaral L, Viveiros M, Molnar J. Antimicrobial activity of phenothiazines. In Vivo. 2004;18:725–732. [PubMed] [Google Scholar]
- 8.Dasgupta A, Mukherjee S, Chaki S, Dastidar S, Hendricks O, Christensen JB, Kristiansen JE, Amaral L. Thioridazine protects the mouse from a virulent infection by Salmonella enterica serovar Tyhpimurium 74. Int J Antimicrob Agents. 2010;35:174–176. doi: 10.1016/j.ijantimicag.2009.09.027. [DOI] [PubMed] [Google Scholar]
- 9.Amaral L, Kristiansen JE, Viveiros M, Atouguia J. Activity of phenothiazines against antibiotic-resistant Mycobacterium tuberculosis – A review supporting further studies that may elucidate the potential use of thioridazine as anti-tuberculosis therapy. J Antimicrob Chemother. 2001;47:505–511. doi: 10.1093/jac/47.5.505. [DOI] [PubMed] [Google Scholar]
- 10.Kristiansen JE, Blom J. Effect of chlorpromazine on the ultrastructure of Staphylococcus aureus. Acta Path Microbiol Scand. 1981;89:399–405. [PubMed] [Google Scholar]
- 11.Chattopadhyay D, Mukherjee T, Pal P, Saha B, Bhadra R. Altered membrane permeability as the basis of bactericidal action of methdilazine. J Antimicrob Chemother. 1998;42:83–86. [PubMed] [Google Scholar]
- 12.Kristiansen JE. Experiments to illustrate the effect of chlorpromazine on the permeability of the bacterial cell wall. Acta Path Microbiol Scand. 1979;87:317–319. doi: 10.1111/j.1699-0463.1979.tb02445.x. [DOI] [PubMed] [Google Scholar]
- 13.Bonde M, Holland DH, Kolmos HJ, Kallippolitis BH, Klitgaard JK. Thioridazine affects transcription of genes involved in cell wall biosynthesis in methicillin-resistant Staphylococcus aureus. FEMS Microbiol Lett. 2011;318:168–176. doi: 10.1111/j.1574-6968.2011.02255.x. [DOI] [PubMed] [Google Scholar]
- 14.Amaral L, Engi H, Viveiros M, Molnar J. Comparison of multidrug resistant efflux pumps of cancer and bacterial cells with respect to the same inhibitory agents. In Vivo. 2007;21:237–244. [PubMed] [Google Scholar]
- 15.Molnar J, Scabo D, Mandi Y, Mucsi I, Fischer J, Varga A, Konig S, Motohashi N. Multidrug resistance reversal in mouse lymphoma cells by heterocyclic compounds. Anticancer Res. 1998;18:3033–3038. [PubMed] [Google Scholar]
- 16.Kaatz GW, Moudgal VV, Seo SM, Kristiansen JE. Phenothiazines and thioxanthenes inhibit multidrug efflux pump activity in Staphylococcus aureus. Antimicrob Agents Chemother. 2003;47:719–726. doi: 10.1128/AAC.47.2.719-726.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan YY, Ong YM, Chua KL. Synergistic interaction between phenothiazines and antimicrobial agents against Burkholderia pseudomallei. Antimicrob Agents Chemother. 2007;51:623–630. doi: 10.1128/AAC.01033-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klitgaard JK, Skov MN, Kallipolitis BH, Kolmos HJ. Reversal of methicillin resistance in Staphylococcus aureus by thioridazine. J Antimicrob Chemother. 2008;62:1215–1221. doi: 10.1093/jac/dkn417. [DOI] [PubMed] [Google Scholar]
- 19.Fairchild RG, Greenberg D, Watts KP, Packer S, Atkins HL, Som P, Hannon SJ, Brill AB, Fand I, McNally WP. Chlorpromazine distribution in hamsters and mice bearing transplantable melanoma. Cancer Res. 1982;42:556–562. [PubMed] [Google Scholar]
- 20.Martensson E, Roos BE. Serum levels of thioridazine in psychiatric patients and healthy volunteers. Eur J Clin Pharm. 1973;6:181–186. doi: 10.1007/BF00558283. [DOI] [PubMed] [Google Scholar]
- 21.Ellington JK, Harris M, Webb L, Smith B, Smith T, Tan K, Hudson M. Intracellular Staphylococcus aureus. A mechanism for the indolence of osteomyelitis. J Bone Joint Surg. 2003;85:91–921. [PubMed] [Google Scholar]
- 23.Sendi P, Proctor RA. Staphylococcus aureus as an intracellular pathogen – the role of small colony variants. Trends Microbiol. 2009;17:54–58. doi: 10.1016/j.tim.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 24.Hahn BL, Onunkwo CC, Watts CJ, Sohnle PG. Systemic dissemination and cutaneous damage in a mouse model of staphylococcal skin infections. Microb Pathogen. 2009;47:16–23. doi: 10.1016/j.micpath.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Onunkwo CC, Hahn BL, Sohnle PG. Clearance of experimental cutaneous Staphylococcus aureus infections in mice. Arch Dermatol Res. 2010;302:372–382. doi: 10.1007/s00403-010-1030-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guth PS, Spirtes MA. The phenothiazine tranquilizers – biochemical and biophysical actions. Int Rev Neurobiol. 1964;7:231–278. doi: 10.1016/s0074-7742(08)60269-x. [DOI] [PubMed] [Google Scholar]
- 27.Hadji-Nejad S, Rahbar M, Mehrgan H. Synergy between phenothiazines and oxacillin against clinical isolates of methicillin-resistant Staphylococcus aureus. Trop J Pharm Res. 2010;9:243–249. [Google Scholar]
- 28.Barcia-Macay M, Seral C, Mingeot-Leclercq MP, Tulkens PM, Van Bambeke F. Pharmacodynamic evaluation of the intracellular activities of antibiotics against Staphylococcus aureus in a model of THP-1 macrophages. Antimicrob Agents Chemother. 2006;50:841–851. doi: 10.1128/AAC.50.3.841-851.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao CX, Silverstein SC, Neu HC, Steinberg TH. J774 macrophages secrete antibiotics via organic anion transporters. J Infect Dis. 2001;165:322–328. doi: 10.1093/infdis/165.2.322. [DOI] [PubMed] [Google Scholar]
- 30.Van Bambeke F, Michot JM, Tulkens PM. Antibiotic efflux pumps in eukaryotic cells – Occurrence and impact on antibiotic cellular pharmacokinetics, pharmacodynamics and toxicodynamics. J Antimicrob Chemother. 2003;51:1067–1077. doi: 10.1093/jac/dkg225. [DOI] [PubMed] [Google Scholar]