Abstract
Background
Evidence is emerging that obesity and weight gain may affect the prognosis of several types of cancer. We investigated the impact of body mass index (BMI) as well as pre- and post-diagnosis weight changes on non-Hodgkin lymphoma (NHL) prognosis.
Methods
A cohort of 573 female incident NHL cases diagnosed during 1996–2000 in Connecticut was followed for a median of 7.8 years. Self-reported height and weight at three time points before and after diagnosis were collected. Hazard ratios (HR) and 95% confidence intervals (CI) were estimated using proportional hazard models adjusting for factors believed to be associated with overall survival of NHL.
Results
Underweight (BMI < 18.5; HR = 2.84; 95% CI = 1.12–7.15) before diagnosis was associated with poorer survival compared to being normal weight (18.5 <= BMI < 25). Pre-diagnosis weight loss (HR = 1.42; 95% CI = 1.02–1.97) and post-treatment weight loss (HR = 1.98; 95% CI = 1.14–3.45) and weight gain (HR = 1.85; 95% CI = 1.04–3.32) were associated with poorer survival.
Conclusion
NHL patients who were underweight, lost weight pre-diagnosis, or change weight after treatment were found to have a poorer survival.
Keywords: non-Hodgkin lymphoma, survival, body mass index, weight loss, weight gain
Introduction
Non-Hodgkin lymphoma (NHL) is the sixth most common cancer in the United States (1). The incidence of non-Hodgkin lymphoma has nearly doubled from 11.1 cases per 100,000 in 1975 to 20.2 cases per 100,000 in 2008 (2), making it one of the most rapidly rising cancers. It is estimated that 65,540 people will be diagnosed with non-Hodgkin lymphoma and 20,210 will die in the U.S. in the year 2010 (1).
The survival of patients with NHL is relatively poor, with only half surviving through five years after diagnosis. Clinical predictors for NHL survival include age, stage, performance status (a measure of how the disease affects the daily living abilities of the patient), extranodal involvement and lactate dehydrogenase (LDH) level. Very few studies have examined the impact of modifiable lifestyle factors on NHL survival. Adiposity, an individual characteristic that can be altered through lifestyle changes, has been shown to be related to risk of NHL (3–10). To the best of our knowledge, only one study has examined the relationship between body mass index (BMI) prior to diagnosis and NHL prognosis (11) and found worse survival in obese patients. Weight loss greater than 10% is regarded as one of the “B-symptoms” (systemic symptoms of fever, night sweats and weight loss that may be present in lymphoma patients) at diagnosis which has been shown to be associated with worse prognosis and shortened survival (12). Two clinical reports with small number of cases found that weight gain during treatment was associated with better survival among NHL patients using chemotherapy (13, 14). To our knowledge, no study has examined the effect of BMI and weight change at pre- and post-diagnosis on NHL prognosis and survival. In this study, we used anthropometric information at three time points before and after diagnosis to examine the relationship of BMI and weight change and overall survival among female NHL patients diagnosed from 1996 to 2000 in Connecticut.
Materials and Methods
Study Population
The study population has been described in detail elsewhere (15–17). In brief, a total of 1,122 potential female NHL cases aged between 21 and 84 years were identified between 1996 and 2000 through the Yale Comprehensive Cancer Center’s Rapid Case Ascertainment Shared Resource (RCA), a component of the Connecticut Tumor Registry (CTR). CTR is the oldest tumor registry in the U.S. and all hospitals and private pathology laboratories in CT are required by public health legislation to report incident cases to CTR. Among those cases, 167 died before they could be interviewed and 123 were excluded because of doctor refusal, previous diagnosis of cancer except non-melanoma skin cancer or inability to speak English. Out of 832 eligible cases, 601 gave written consent and completed an in-person interview. Participants had a similar race distribution with non-participants and were slightly older than nonparticipants (mean ages 67 vs. 62). Pathology slides or tissue blocks were obtained from the hospitals where the cases had been diagnosed and the specimens were reviewed by two independent study pathologists. All NHL cases were classified into histological subtypes according to the World Health Organization (WHO) classification system (18, 19).
Vital status for these NHL cases was abstracted at the CTR in mid 2008. Other abstracted follow-up information included date of death, cause of death, most recent follow-up date and type and date of treatments; B-symptom presence and tumor stage were also obtained from CTR record. Of the 601 cases, 13 were unable to be identified in the CTR system and 13 were found to have a history of cancer except non-melanoma skin cancer prior to the current diagnosis of NHL. Further excluding 3 cases with missing weight, yielded 573 NHL patients in the final analyses. Of these, 182 had diffuse large B-cell lymphoma (DLBCL); 133 had follicular lymphoma (FL); 63 had chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL); 38 had marginal zone B-cell lymphoma (MZBL); 42 had T/NK-cell lymphoma (T-cell) and the rest 115 had other subtypes.
This study was approved by the Human Investigation Committee at Yale University and the Connecticut Department of Public Health.
Exposure Assessment
Trained interviewers administered a standardized, structured questionnaire to obtain demographic information and known or suspected risk factors for NHL through in-person interviews. The median time between diagnosis and interview was 6.4 months. During the interview, patients were asked about their height without shoes, their usual weight prior to diagnosis (the question attempts to acquire the average weight 1–2 years before diagnosis without impacts from the disease), weight at the interview and the weight one year prior to the interview. The three weights were highly correlated (correlation coefficient 0.88–0.93 and p-values <0.0001). The usual weight prior to diagnosis (mean 154 lbs) was slightly higher than the weight at interview (mean 153 lbs) (p-value for paired t-test = 0.1845) and significantly lower than the weight one year prior to the interview (mean 157 lbs) (p-value for paired t-test <0.0001). Additional information on age, race, education, smoking, family history of cancer and other lifestyle factors before diagnosis was also obtained during the interview.
Statistical Analysis
BMI at each time point was calculated as weight in kilograms divided by squared height in meters (kg/m2), and classified into four categories according to the World Health Organization definition: underweight (<18.5 kg/m2); normal weight (18.5–24.99 kg/m2); overweight (25–29.99 kg/m2); obese (>=30 kg/m2). Weight changes between the time points were calculated and categorized as three groups: weight loss (<0 kg), no change (=0 kg) and weight gain (>0 kg). We are interested in relationships between five BMI or weight change exposures and overall survival of NHL. The five exposures include: baseline BMI defined as the usual BMI prior to diagnosis; post-diagnostic BMI before treatment which was calculated with the “weight at interview” among only the subjects interviewed before receiving any treatment (n=218); pre-diagnostic weight change which was calculated with the “weight one year prior to the interview - usual weight prior to diagnosis” among only the subjects interviewed within one year after diagnosis (n=518); post-diagnostic weight change before treatment which was calculated with “weight at interview-usual weight prior to diagnosis” among subjects who were interviewed before any treatment (n=218); post-diagnostic weight change after treatment which calculated with “weight at interview-usual weight prior to diagnosis” among subjects who were interviewed after a treatment (n=282). Survival analyses were conducted for NHL cases overall and the three major NHL subtypes (DLBCL, FL and CLL/SLL) using the Kaplan-Meier method, where deaths from any cause were events and subjects alive at the end of follow-up were right-censored. Follow-up time was calculated as the time between diagnosis and event/censoring. Log-rank tests were performed to detect the survival difference between categories. Hazard ratios (HR) and 95% confidence intervals (CI) were calculated by fitting Cox regression models, where normal weight or no weight change was reference group. Age (continuous), education (high school or less, some college, and college graduate or more), stage (I, II, III, IV, and unknown), presence of B-symptoms (yes, no/ unknown), initial treatment (none, radiation only, chemotherapy-based regimen, and other) and smoking (never/ever smoked >100 cigarette) were adjusted as confounding variables. A test for interaction of race (white or other) with BMI and weight change variables was conducted by adding the interaction terms into the model and no interaction effect was found, thus the analyses were not stratified by race. Adjustments for race and the time between diagnosis and interview did not result in material changes (>10%) for the observed associations when the analyses were performed in the entire cohort and thus were not included in the final models. For weight change variables, we further examined the interaction between baseline weight status and weight change for subjects that had a baseline BMI>=18.5. The assumption of proportionality of hazards (PH) was assessed by test of Schoenfeld residuals and it appeared to be met. Statistical analyses were performed using SAS, version 9.2 (SAS Institute, Cary, NC).
Results
Demographic characteristics for 573 NHL cases are presented in Table 1. Compare to all the female NHL cases diagnosed in Connecticut in 1996–2000 and aged 21–84, our sample has a similar race distribution (percentage of whites: 95.1% vs. 94.5%) and a younger age (percentage of age below 65: 52.5% vs. 42.8%) (20). The majority of these patients (60%) had stage I or II diseases and 6% had B-symptoms. The most common initial therapy was a chemotherapy-based regimen (52%), followed by observation (35%) and radiation (12%). For those who received treatment, the median time between diagnosis and treatment was 26 days. During the follow-up period, 252 patients died. Median follow-up time was 3.65 years for the deceased and 9.08 years for the survivors. At baseline before diagnosis, 20% of the patients were obese, 32% were overweight, 46% had normal weight, and the remaining 1% were underweight (Table 2). 19% of the patients lost weight and 34% gained weight in the year pre-diagnosis; among those who were interviewed before any treatment, 33% the lost weight and 32% gained weight after diagnosis; among those who were interviewed after receiving a treatment, 43% lost weight and 29% gained weight after treatment (Table 2).
Table 1.
Selected demographic characteristics of NHL cases, Connecticut, 1996–2000
| Characteristic | No. | % |
|---|---|---|
| Age at diagnosis | ||
| <=45 | 69 | 12.0 |
| 46–55 | 111 | 19.4 |
| 56–65 | 121 | 21.1 |
| 66–75 | 165 | 28.8 |
| >=76 | 107 | 18.7 |
| Race | ||
| White | 545 | 95.1 |
| Black | 18 | 3.1 |
| Other | 10 | 1.8 |
| Education | ||
| High School or Less | 246 | 42.9 |
| Some College | 190 | 33.2 |
| College graduate or more | 137 | 23.9 |
| Family History | ||
| None | 124 | 21.6 |
| Any other cancer | 441 | 77.0 |
| NHL | 8 | 1.4 |
| Cause of Death (total death=252) | ||
| Lymphoma | 149 | 59.1 |
| Other cancers | 23 | 9.1 |
| Cardiovascular disease | 26 | 10.3 |
| Respiratory disease | 5 | 2.0 |
| Nervous system disease | 4 | 1.6 |
| Infectious disease | 4 | 1.6 |
| Accident | 4 | 1.6 |
| Other & non-reported | 37 | 14.7 |
Table 2.
Adjusted Hazard Ratios (HRs) for death in relation to BMI categories and weight change status for NHL cases, Connecticut, 1996–2000
| NHL overall
|
DLBCL
|
FL
|
CLL/SLL
|
|||||
|---|---|---|---|---|---|---|---|---|
| No. deaths/cases | HR | No. deaths/cases | HR | No. deaths/cases | HR | No. deaths/cases | HR | |
| Baseline BMI | ||||||||
| <18.5 | 5/6 | 2.84(1.12–7.15) | 0 / 0 | -- | 3 / 3 | 18.72(4.74–73.95) | 0 / 1 | -- |
| 18.5–24.9 | 111 / 266 | 1 | 36 / 80 | 1 | 18 / 61 | 1 | 20 / 36 | 1 |
| 25–30 | 78 / 185 | 0.98(0.73–1.31) | 27 / 65 | 0.70(0.41–1.20) | 16 / 39 | 1.29(0.63–2.66) | 9 / 18 | 2.43(0.92–6.42) |
| >=30 | 58 / 116 | 1.38(0.99–1.93) | 21 / 37 | 1.77(0.98–3.21) | 12 / 30 | 1.23(0.54–2.80) | 4 / 8 | 0.91(0.22–3.81) |
| BMI before treatment§ | ||||||||
| <18.5 | 6 / 9 | 0.98(0.39–2.43) | 0 / 1 | -- | 4 / 4 | 5.79(1.13–29.61) | 0 / 2 | -- |
| 18.5–24.9 | 48 / 93 | 1 | 8 / 17 | 1 | 6 / 19 | 1 | 16 / 24 | 1 |
| 25–30 | 22 / 67 | 0.55(0.33–0.92) | 1 / 6 | -- | 11 / 28 | 0.80(0.24–2.65) | 4 / 9 | 1.53(0.41–5.74) |
| >=30 | 17 / 49 | 0.62(0.34–1.12) | 1 / 7 | -- | 3 / 13 | 0.56(0.11–2.99) | 0 / 6 | -- |
| Pre-diagnostic weight change* | ||||||||
| <0 | 57 / 99 | 1.42(1.02–1.97) | 13 / 24 | 1.02(0.51–2.02) | 11 / 19 | 1.34(0.62–2.93) | 9 / 12 | 6.63(1.59–27.62) |
| 0 | 107 / 243 | 1 | 37 / 86 | 1 | 20 / 55 | 1 | 15 / 27 | 1 |
| >0 | 73 / 176 | 1.04(0.77–1.41) | 28 / 57 | 1.23(0.73–2.06) | 14 / 45 | 0.60(0.27–1.32) | 9 / 21 | 2.67(0.85–8.45) |
| Post-diagnostic weight change before treatment# | ||||||||
| <0 | 43 / 76 | 1.45(0.84–2.50) | 6 / 15 | 1.64(0.18–14.76) | 7 / 15 | 1.36(0.40–4.65) | 12 / 17 | 1.34(0.34–5.31) |
| 0 | 24 / 65 | 1 | 2 / 6 | 1 | 6 / 20 | 1 | 7 / 13 | 1 |
| >0 | 26 / 77 | 0.99(0.56–1.76) | 2/ 10 | -- | 11 / 29 | 0.92(0.29–2.93) | 1 / 11 | -- |
| Post-diagnostic weight change after treatment$ | ||||||||
| <0 | 67 / 126 | 1.98(1.14–3.45) | 31 / 55 | 1.42(0.61–3.31) | 6 / 15 | 1.17(0.20–6.95) | 5 / 8 | 14.77(0.36–607.47) |
| 0 | 17 / 64 | 1 | 8 / 23 | 1 | 3 / 15 | 1 | 1 / 5 | 1 |
| >0 | 37 / 92 | 1.85(1.04–3.32) | 16 / 39 | 1.44(0.60–3.49) | 8 / 25 | 1.22(0.25–5.83) | 5 / 5 | 1.01(0.04–22.69) |
Models were adjusted for age (continuous), education (high school or less, some college, and college graduate or more), stage (I, II, III, IV, and unknown), B-symptom (yes, no/unknown), initial treatment (none, radiation only, chemotherapy-based regimen, and other) and smoking (never, ever).
BMI at interview, only cases interviewed before treatment (n=218) were included.
Weight one year prior to interview-usual weight, only cases interviewed within one year after diagnosis (n=518) were included.
Weight change = weight at interview - usual weight, only cases interviewed before treatment (n=218) were included.
Weight change = weight at interview - usual weight, only cases interviewed after treatment (n=282) were included.
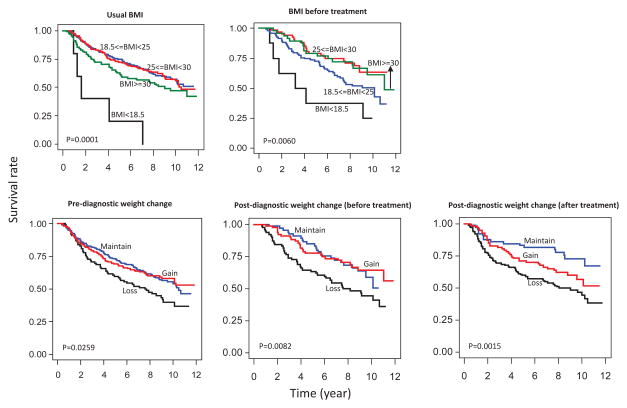
NHL patients who were underweight or obese at baseline had poorer survival compared to normal weight and overweight subjects (Figure 1). NHL patients who were overweight or obese before treatment had the best survival, followed by normal-weight subjects, and underweight patients before treatment had the worst survival (Figure 1). Those who lost weight either before or after diagnosis, including both before and after treatment, all had the worst survival compared to those who maintained their weight (Figure 1). Similar patterns were also seen for the three major subtypes, although the differences were not significant partly due to small sample size in the subtype analyses (figures not shown).
Figure 1.
Kaplan-Meir survival curves for NHL cases in Connecticut women and log-rank P-values
After adjustment for confounding variables, NHL patients who were underweight - at baseline had a higher risk of death compared to normal weight subjects and NHL patients who were overweight before treatment had a lower risk of death compared to normal weight subjects (Table 2). Weight loss before diagnosis was associated with 42% increased rate of death compared to subjects with no weight change (Table 2). After treatment, weight loss and weight gain were both associated with a higher risk of death (Table 2). When analyzed by NHL subtype, a higher risk of death was observed among FL patients who were underweight at baseline and who were underweight before treatment. Losing weight in the year before diagnosis was associated with a higher risk of death among CLL/SLL patients (Table 2).
No statistically significant interaction was found between baseline weight status and weight change pre- or post- diagnosis. However, the increased risk of death associated with pre-diagnostic weight loss was mainly seen in overweight subjects, and the increased risk of death associated with post-diagnostic weight change after treatment was mainly seen in normal weight subjects (Table 3).
Table 3.
Adjusted Hazard Ratios (HRs) for death in relation to weight change by baseline weight status for NHL cases, Connecticut, 1996–2000
| Normal weight (18.5<=BMI<25) | Overweight (25<=BMI<29.9) | Obese (BMI>=30) | ||||
|---|---|---|---|---|---|---|
| No. of deaths/cases | HR | No. of deaths/cases | HR | No. of deaths/cases | HR | |
| Pre-diagnostic weight change* | P-interaction = 0.1800 | |||||
| <0 | 21 / 41 | 1.28(0.76–2.13) | 23 / 33 | 2.51(1.38–4.55) | 13 / 25 | 0.88(0.44–1.75) |
| 0 | 55 / 126 | 1 | 22 / 63 | 1 | 26 / 50 | 1 |
| >0 | 27 / 74 | 1.08(0.67–1.72) | 30 / 69 | 1.19(0.68–2.08) | 15 / 31 | 1.05(0.54–2.04) |
| Post-diagnostic weight change before treatment# | P-interaction = 0.1475 | |||||
| <0 | 20 / 31 | 0.94(0.44–2.03) | 14 / 30 | 2.33(0.84–6.44) | 7 / 12 | 2.81(0.73–10.81) |
| 0 | 13 / 31 | 1 | 6 / 23 | 1 | 4 / 10 | 1 |
| >0 | 11 / 35 | 0.76(0.33–1.73) | 12 / 25 | 2.38(0.86–6.61) | 3 / 17 | 0.52(0.11–2.40) |
| Post-diagnostic weight change after treatment$ | P-interaction = 0.0766 | |||||
| <0 | 29 / 58 | 3.49(1.42–8.55) | 21 / 38 | 2.52(0.72–8.73) | 16 / 29 | 0.60(0.23–1.54) |
| 0 | 6 / 32 | 1 | 3 / 18 | 1 | 7 / 13 | 1 |
| >0 | 20 / 49 | 3.52(1.39–8.91) | 10 / 28 | 1.97(0.53–7.29) | 7 / 12 | 0.67(0.23–1.97) |
Models were adjusted for age (continuous), education (high school or less, some college, and college graduate or more), stage (I, II, III, IV, and unknown), B-symptom (yes, no/unknown), initial treatment (none, radiation only, chemotherapy-based regimen, and other) and smoking (never, ever).
Weight change = weight one year prior to interview - usual weight. Only cases interviewed within one year after diagnosis (n=518) were included.
Weight change = weight at interview - usual weight. Only cases interviewed before treatment (n=218) were included.
Weight change = weight at interview - usual weight. Only cases interviewed after treatment (n=282) were included.
Discussion
This study evaluated the relationships of BMI and weight change at different time points and survival of a cohort of recently diagnosed female non-Hodgkin lymphoma patients in Connecticut women. We found that prior to diagnosis, having a baseline BMI that was underweight was associated with poorer survival after accounting for clinical and demographic variables, and weight loss in the year pre-diagnosis was also associated with poorer survival. After diagnosis, being overweight before treatment was associated with better survival, and weight change after treatment (either weight loss or weight gain) was associated with poorer survival.
Body mass index prior to diagnosis was linked to NHL prognosis and survival in another population-based study, in which Geyer et al. (11) followed 1189 NHL patients diagnosed in the U.S during 1998–2000 through 2007 and found that being obese (BMI≥30) one year before diagnosis was associated with 1.32 times the risk of death compared to normal weight. We observed a comparable borderline significant hazard ratio of 1.38 observed for baseline obesity before diagnosis.
Obesity probably affects cancer progression and prognosis through multiple biological mechanisms. For example, obesity is a proinflammatory state, and this promotes tumor growth (21)(22). Obesity is associated with altered immune function (23) which is implicated in lymphomagenesis. Moreover, obesity is known to induce insulin resistance and elevated insulin levels, and insulin stimulates cell proliferation (24). Excess adipose tissue produces estrogen, which stimulates tumor growth and progression (25). Obesity is also associated with altered production of adipokines and other cytokines (e.g., adiponectin, leptin, IL-6 and tumor necrosis factor-α), and these factors can affect cell proliferation and cell survival (21).
We observed a high risk of death among patients who were underweight at baseline. Underweight people may have poor physical stamina, a weak immune system due to malnutrition and increased risk of comorbidities. They are also at risk of overdosing and greater toxicity from chemotherapy (26). Previous studies have linked being underweight to both overall and cancer-specific mortality (27–29), however, to our knowledge our study is the first to show poorer survival in underweight NHL patients. Although the associations observed here were extreme, our result should be viewed with caution since the number of underweight subjects was very small.
Studies examining the impact of overweight/obesity at the time of treatment on outcomes in lymphoma patients have been inconsistent (30), with some studies finding overweight/obesity associated with worse outcomes (31), some finding no association (32, 33), and some finding it associated with improved outcomes (34). In our study, we did not have the weight at time of treatment. However, in analysis that limited subjects to only those who were interviewed before receiving any treatments, we were able to explore the relationship between post-diagnostic BMI before treatment and overall survival. We found that subjects who were overweight had significantly lower risk of death compared to normal weight subjects, and obese subjects did not show a worse survival. Our results support the argument that obesity alone does not predict poorer long-term treatment outcomes. Our findings need to be replicated in other populations.
Weight loss was associated with poorer survival compared to weight maintenance in our study. Although weight loss among overweight and obese people has been shown to reduce blood pressure and blood glucose and improve lipid profile and insulin sensitivity (35, 36), unintentional weight loss has been linked to higher mortality risk (37) and can be a sign of many diseases including cancer (38)(39)(40). Furthermore, studies have shown that weight loss is associated with a decrease in the function aspects of the immune system (23, 41, 42). Currently immune dysregulation is the only established risk factor for NHL, and decreased immune function may also cause a worse prognosis among NHL patients. However, our study cannot exclude the effect of reverse causality on the observed associations between weight loss and poorer survival. This bias of reverse causality may obscure relationships between obesity, weight change and mortality (43). The ability to differentiate intentional weight loss from weight loss due to health problems would help in evaluating the impact of reverse causality. Further research will be important to elucidate the true effect of weight loss on survival after cancer diagnosis.
Recent studies have shown that post-diagnosis weight change, both gain and loss, may be associated with a worse prognosis for patients with various cancer types such as breast cancer (44–46), gastrointestinal cancer (45) and lung cancer (45). Similarly, in patients who already received treatment, we observed that both weight gain and weight loss were associated with reduced survival among NHL patients. Chemotherapy (47), psychological stress (48), and modifications in dietary intake and physical activities after diagnosis all could possibly disturb energy balance and cause weight variations. Two clinical studies from Taiwan and Serbia have examined the relationship between weight change during chemotherapy and survival (13, 14), and both found weight gain during the 18-week treatment regimen was associated with improved survival. However, these studies included a small number of cases (138 in the Taiwan study and 30 in the Serbia study) and did not adjust for other confounding variables or consider NHL subtypes. The Taiwan study did not differentiate weight loss and weight maintenance when making comparisons to weight gain. These clinical studies suggested a beneficial effect of weight gain during chemotherapy, after adjusting for potential demographic and clinical confounding variables. In contrast our results suggest that a stable weight might be best for cancer survivors. This difference may be due to combining weight losers and weight maintainers in the Taiwan study. Like our studies, none of the studies reviewed here can exclude the effect of reverse causality on the observed associations between weight loss and poorer survival.
The strengths of our study include a population-based sample, nearly complete follow-up, availability of several important clinical features such as disease stage and treatment information, and weight information at multiple time points around diagnosis. Self reported anthropometric measurements were a potential limitation of our study. Although some studies (49) suggest that self-reported current anthropometric measures are highly correlated with measured values and therefore appropriate for epidemiologic studies, there are also studies showing that underreporting weight is associated with obesity in women (50) and this could bias our results. Differential recall of weight based on cancer severity is possible too and could result in an underestimation of the harmful effect of obesity and an overestimation of the effect of underweight. Moreover, our results would have had a clearer clinical meaning if the reference time point of the weight assessment questions had been clearly defined as diagnosis or treatment. We had limited power for subtype analysis although notable patterns for NHL subtypes were still observed. We did not collect information on comorbidity and post-diagnostic lifestyle change such as dietary modification, thus were not able to control for the possible confounding effects from it. Finally, the generalizability of our study findings is limited since the population was restricted to women in Connecticut and the study sample included few ethnic minorities. Our findings may not apply to the very severe NHL cases, since 26% of the eligible cases were not enrolled in the study because of the severity of the disease (either they died before they could be interviewed or refused by physicians).
Our study found that being underweight or obese at baseline and weight loss before or after diagnosis was associated with reduced overall survival of NHL. Our findings highlight the importance of maintaining a healthy body weight before and after developing NHL and avoiding weight fluctuations near the time of treatment. Our results warrant replication from studies of larger populations that include men and other racial and ethnic groups and a more detailed assessment regarding the intent of weight change.
Acknowledgments
Dr. Han was supported by American Institute for Cancer Research Marilyn Gentry Fellowship. This research was funded by grant CA62006 from the National Cancer Institute (NCI) and by Hull Argall & Anna Grant 22067A from the Yale Cancer Center. This research was approved by the DPH HIC. Certain data used in this study were obtained from the Connecticut Department of Public Health. The authors assume full responsibility for analyses and interpretation of these data.
References
- 1.Altekruse SF, kosary CL, krapcho M, neyman N, aminou R, waldron W, ruhl J, howlader N, tatalovich Z, cho H, mariotto A, eisner MP, lewis DR, cronin K, chen HS, feuer EJ, stinchcomb DG, edwards BK. SEER cancer statistics review, 1975–2007. national cancer institute; bethesda, MD: http://seer.cancer.gov/csr/1975_2007/, based on november 2009 SEER data submission, posted to the SEER web site, 2010. [Google Scholar]
- 2.national cancer institute; [accessed on 7-22-2011]. Fast stats: An interactive tool for access to SEER cancer statistics. surveillance research program. http://seer.cancer.gov/faststats. [Google Scholar]
- 3.Skibola CF, Holly EA, Forrest MS, Hubbard A, Bracci PM, et al. Body mass index, leptin and leptin receptor polymorphisms, and non-hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev. 2004;13:779–86. [PubMed] [Google Scholar]
- 4.Cerhan JR, Bernstein L, Severson RK, Davis S, Colt JS, et al. Anthropometrics, physical activity, related medical conditions, and the risk of non-hodgkin lymphoma. Cancer Causes Control. 2005;16:1203–14. doi: 10.1007/s10552-005-0358-7. [DOI] [PubMed] [Google Scholar]
- 5.Pan SY, Mao Y, Ugnat AM. Physical activity, obesity, energy intake, and the risk of non-hodgkin’s lymphoma: A population-based case-control study. Am J Epidemiol. 2005;162:1162–73. doi: 10.1093/aje/kwi342. [DOI] [PubMed] [Google Scholar]
- 6.Willett EV, Skibola CF, Adamson P, Skibola DR, Morgan GJ, et al. Non-hodgkin’s lymphoma, obesity and energy homeostasis polymorphisms. Br J Cancer. 2005;93:811–6. doi: 10.1038/sj.bjc.6602762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiu BC, Soni L, Gapstur SM, Fought AJ, Evens AM, et al. Obesity and risk of non-hodgkin lymphoma (united states) Cancer Causes Control. 2007;18:677–85. doi: 10.1007/s10552-007-9013-9. [DOI] [PubMed] [Google Scholar]
- 8.Larsson SC, Wolk A. Obesity and risk of non-hodgkin’s lymphoma: A meta-analysis. Int J Cancer. 2007;121:1564–70. doi: 10.1002/ijc.22762. [DOI] [PubMed] [Google Scholar]
- 9.Lim U, Morton LM, Subar AF, Baris D, Stolzenberg-Solomon R, et al. Alcohol, smoking, and body size in relation to incident hodgkin’s and non-hodgkin’s lymphoma risk. Am J Epidemiol. 2007;166:697–708. doi: 10.1093/aje/kwm122. [DOI] [PubMed] [Google Scholar]
- 10.Kanda J, Matsuo K, Suzuki T, Hosono S, Ito H, et al. Association between obesity and the risk of malignant lymphoma in japanese: A case-control study. Int J Cancer. 2010;126:2416–25. doi: 10.1002/ijc.24955. [DOI] [PubMed] [Google Scholar]
- 11.Geyer SM, Morton LM, Habermann TM, Allmer C, Davis S, et al. Smoking, alcohol use, obesity, and overall survival from non-hodgkin lymphoma: A population-based study. Cancer. 2010;116:2993–3000. doi: 10.1002/cncr.25114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han X, Kilfoy B, Zheng T, Holford TR, Zhu C, et al. Lymphoma survival patterns by WHO subtype in the united states, 1973–2003. Cancer Causes Control. 2008;19:841–58. doi: 10.1007/s10552-008-9147-4. [DOI] [PubMed] [Google Scholar]
- 13.Chin YH, Liu JM, Tai JJ, Chuang MS, Ho YL, et al. The significance of body weight change in non-hodgkins lymphoma. Anticancer Res. 1999;19:5607–5610. [PubMed] [Google Scholar]
- 14.Stanisavljevic NS, Marisavljevic DZ. Weight and body composition changes during R-CHOP chemotherapy in patients with non-hodgkin’s lymphoma and their impact on dose intensity and toxicity. J BUON. 2010;15:290–296. [PubMed] [Google Scholar]
- 15.Morton LM, Holford TR, Leaderer B, Zhang Y, Zahm SH, et al. Alcohol use and risk of non-hodgkin’s lymphoma among connecticut women (united states) Cancer Causes Control. 2003;14:687–94. doi: 10.1023/a:1025626208861. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Holford TR, Leaderer B, Boyle P, Zahm SH, et al. Hair-coloring product use and risk of non-hodgkin’s lymphoma: A population-based case-control study in connecticut. Am J Epidemiol. 2004;159:148–54. doi: 10.1093/aje/kwh033. [DOI] [PubMed] [Google Scholar]
- 17.Han X, Zheng T, Foss FM, Ma S, Holford TR, et al. Alcohol consumption and non-hodgkin lymphoma survival. J Cancer Surviv. 2010;4:101–9. doi: 10.1007/s11764-009-0111-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.A clinical evaluation of the international lymphoma study group classification of non-hodgkin’s lymphoma. the non-hodgkin’s lymphoma classification project. Blood. 1997;89:3909–18. [PubMed] [Google Scholar]
- 19.Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, et al. World health organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: Report of the clinical advisory committee meeting-airlie house, virginia, november 1997. J Clin Oncol. 1999;17:3835–49. doi: 10.1200/JCO.1999.17.12.3835. [DOI] [PubMed] [Google Scholar]
- 20.Altekruse SF, Kosary CL, Krapcho M, Neyman N, Aminou R, Waldron W, Ruhl J, Howlader N, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Cronin K, Chen HS, Feuer EJ, Stinchcomb DG, Edwards BK. SEER cancer statistics review, 1975–2007. Bethesda, MD: National Cancer Institute; 2010. [Google Scholar]
- 21.van Kruijsdijk RC, van der Wall E, Visseren FL. Obesity and cancer: The role of dysfunctional adipose tissue. Cancer Epidemiol Biomarkers Prev. 2009;18:2569–78. doi: 10.1158/1055-9965.EPI-09-0372. [DOI] [PubMed] [Google Scholar]
- 22.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 23.Nieman DC, Henson DA, Nehlsen-Cannarella SL, Ekkens M, Utter AC, et al. Influence of obesity on immune function. J Am Diet Assoc. 1999;99:294–299. doi: 10.1016/S0002-8223(99)00077-2. [DOI] [PubMed] [Google Scholar]
- 24.Parekh N, Okada T, Lu-Yao GL. Obesity, insulin resistance, and cancer prognosis: Implications for practice for providing care among cancer survivors. J Am Diet Assoc. 2009;109:1346–1353. doi: 10.1016/j.jada.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sinicrope FA, Dannenberg AJ. Obesity and breast cancer prognosis: Weight of the evidence. J Clin Oncol. 2010 doi: 10.1200/JCO.2010.32.1752. [DOI] [PubMed] [Google Scholar]
- 26.Field KM, Kosmider S, Jefford M, Michael M, Jennens R, et al. Chemotherapy dosing strategies in the obese, elderly, and thin patient: Results of a nationwide survey. J Oncol Pract. 2008;4:108–113. doi: 10.1200/JOP.0832001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flegal KM, Graubard BI, Williamson DF, Gail MH. Excess deaths associated with underweight, overweight, and obesity. JAMA. 2005;293:1861–1867. doi: 10.1001/jama.293.15.1861. [DOI] [PubMed] [Google Scholar]
- 28.Flegal KM, Graubard BI, Williamson DF, Gail MH. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA. 2007;298:2028–2037. doi: 10.1001/jama.298.17.2028. [DOI] [PubMed] [Google Scholar]
- 29.Moore SC, Mayne ST, Graubard BI, Schatzkin A, Albanes D, et al. Past body mass index and risk of mortality among women. Int J Obes (Lond) 2008;32:730–739. doi: 10.1038/sj.ijo.0803801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Navarro WH, Loberiza FR. Obesity and lymphoma therapy: Not a bad combination after all. Leuk Lymphoma. 2010;51:1590–1591. doi: 10.3109/10428194.2010.512096. [DOI] [PubMed] [Google Scholar]
- 31.Tarella C, Caracciolo D, Gavarotti P, Argentino C, Zallio F, et al. Overweight as an adverse prognostic factor for non-hodgkin’s lymphoma patients receiving high-dose chemotherapy and autograft. Bone Marrow Transplant. 2000;26:1185–1191. doi: 10.1038/sj.bmt.1702692. [DOI] [PubMed] [Google Scholar]
- 32.Navarro WH, Loberiza FR, Jr, Bajorunaite R, van Besien K, Vose JM, et al. Effect of body mass index on mortality of patients with lymphoma undergoing autologous hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2006;12:541–551. doi: 10.1016/j.bbmt.2005.12.033. [DOI] [PubMed] [Google Scholar]
- 33.Nikolousis E, Nagra S, Paneesha S, Delgado J, Holder K, et al. Allogeneic transplant outcomes are not affected by body mass index (BMI) in patients with haematological malignancies. Ann Hematol. 2010;89:1141–1145. doi: 10.1007/s00277-010-1001-6. [DOI] [PubMed] [Google Scholar]
- 34.Jones JA, Fayad LE, Elting LS, Rodriguez MA. Body mass index and outcomes in patients receiving chemotherapy for intermediate-grade B-cell non-hodgkin lymphoma. Leuk Lymphoma. 2010;51:1649–1657. doi: 10.3109/10428194.2010.494315. [DOI] [PubMed] [Google Scholar]
- 35.Anderson AS, Caswell S. Obesity management--an opportunity for cancer prevention. Surgeon. 2009;7:282–5. doi: 10.1016/s1479-666x(09)80005-x. [DOI] [PubMed] [Google Scholar]
- 36.Sjostrom L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–2693. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 37.French SA, Folsom AR, Jeffery RW, Williamson DF. Prospective study of intentionality of weight loss and mortality in older women: The iowa women’s health study. Am J Epidemiol. 1999;149:504–514. doi: 10.1093/oxfordjournals.aje.a009844. [DOI] [PubMed] [Google Scholar]
- 38.Lankisch P, Gerzmann M, Gerzmann JF, Lehnick D. Unintentional weight loss: Diagnosis and prognosis. the first prospective follow-up study from a secondary referral centre. J Intern Med. 2001;249:41–46. doi: 10.1046/j.1365-2796.2001.00771.x. [DOI] [PubMed] [Google Scholar]
- 39.Hernandez JL, Matorras P, Riancho JA, Gonzalez-Macias J. Involuntary weight loss without specific symptoms: A clinical prediction score for malignant neoplasm. QJM. 2003;96:649–655. doi: 10.1093/qjmed/hcg107. [DOI] [PubMed] [Google Scholar]
- 40.Weiser MA, Cabanillas M, Vu K, Tamm EP, Wallace MJ, et al. Diagnostic evaluation of patients with a high suspicion of malignancy: Comorbidities and clinical predictors of cancer. Am J Med Sci. 2005;330:11–18. doi: 10.1097/00000441-200507000-00003. [DOI] [PubMed] [Google Scholar]
- 41.Kelley DS, Daudu PA, Branch LB, Johnson HL, Taylor PC, et al. Energy restriction decreases number of circulating natural killer cells and serum levels of immunoglobulins in overweight women. Eur J Clin Nutr. 1994;48:9–18. [PubMed] [Google Scholar]
- 42.Scanga CB, Verde TJ, Paolone AM, Andersen RE, Wadden TA. Effects of weight loss and exercise training on natural killer cell activity in obese women. Med Sci Sports Exerc. 1998;30:1666–1671. doi: 10.1097/00005768-199812000-00002. [DOI] [PubMed] [Google Scholar]
- 43.Flanders WD, Augestad LB. Adjusting for reverse causality in the relationship between obesity and mortality. Int J Obes (Lond) 2008;32(Suppl 3):S42–6. doi: 10.1038/ijo.2008.84. [DOI] [PubMed] [Google Scholar]
- 44.Chen X, Lu W, Zheng W, Gu K, Chen Z, et al. Obesity and weight change in relation to breast cancer survival. Breast Cancer Res Treat. 2010;122:823–833. doi: 10.1007/s10549-009-0708-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martin L, Watanabe S, Fainsinger R, Lau F, Ghosh S, et al. Prognostic factors in patients with advanced cancer: Use of the patient-generated subjective global assessment in survival prediction. J Clin Oncol. 2010;28:4376–4383. doi: 10.1200/JCO.2009.27.1916. [DOI] [PubMed] [Google Scholar]
- 46.Thivat E, Therondel S, Lapirot O, Abrial C, Gimbergues P, et al. Weight change during chemotherapy changes the prognosis in non metastatic breast cancer for the worse. BMC Cancer. 2010;10:648. doi: 10.1186/1471-2407-10-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Freedman RJ, Aziz N, Albanes D, Hartman T, Danforth D, et al. Weight and body composition changes during and after adjuvant chemotherapy in women with breast cancer. J Clin Endocrinol Metab. 2004;89:2248–2253. doi: 10.1210/jc.2003-031874. [DOI] [PubMed] [Google Scholar]
- 48.Foreyt JP, Brunner RL, Goodrick GK, Cutter G, Brownell KD, et al. Psychological correlates of weight fluctuation. Int J Eat Disord. 1995;17:263–275. doi: 10.1002/1098-108x(199504)17:3<263::aid-eat2260170307>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 49.Weaver TW, Kushi LH, McGovern PG, Potter JD, Rich SS, et al. Validation study of self-reported measures of fat distribution. Int J Obes Relat Metab Disord. 1996;20:644–650. [PubMed] [Google Scholar]
- 50.Merrill RM, Richardson JS. Validity of self-reported height, weight, and body mass index: Findings from the national health and nutrition examination survey, 2001–2006. Prev Chronic Dis. 2009;6:A121. [PMC free article] [PubMed] [Google Scholar]