Abstract
Objective
Hedgehog(Hh) signaling has recently been associated with cartilage degradation in osteoarthritis(OA). As interleukin-1β(IL-1β) is a critical mediator of OA pathogenesis, here we determined whether IL-1β induces the expression of sonic hedgehog(SHH) and its regulation by microRNAs in human chondrocytes.
Methods
SHH protein expression in human OA-cartilage and in an animal model of OA was determined by immunohistochemistry and immunofluorescence respectively. Gene and protein expression in IL-1β or SHH-stimulated chondrocytes was determined by TaqMan assays and immunoblotting respectively. Effect of overexpression of miR-602 and miR-608 or their anatgomirs on SHH expression was evaluated by transient transfections of human chondrocytes and HEK-293 cells. Role of signaling pathways was evaluated using small molecule inhibitors. Binding of miRNAs with the putative “seed sequence” in the SHH mRNA was validated with a SHH luciferase reporter assay.
Results
Expression of SHH, PTCH-1, GLI-1, HHIP, MMP-13, and COL10A1 was high in damaged OAcartilage. Expression of SHH was inversely correlated with the expression of miR-608 in damaged cartilage and in IL-1β-stimulated chondrocytes. Transfection with miR-608 or miR-602 mimics inhibited the reporter activity and mutation of the miRNAs “seed sequences” abolished the repression of reporter activity. Overexpression of miR-602 or miR-608 inhibited the expression of SHH mRNA and protein and this was abrogated by antagomirs. Stimulation with SHH-protein up-regulated the MMP-13 expression and inhibition of Hh signaling blocked MMP-13 expression in OA chondrocytes.
Conclusions
miR-602 and miR-608 are important regulators of SHH expression in chondrocytes and their suppression by IL-1β may contribute to the enhanced expression of SHH and MMP-13 in OA.
Keywords: Osteoarthritis, Chondrocytes, SHH, MMP-13, miR-602, miR-608
INTRODUCTION
Osteoarthritis (OA) is a leading cause of pain and disability worldwide among the aged (1). Although focal cartilage degradation is a hall mark of OA but the disease also involves synovial inflammation, osteophyte formation and sub-chondral bone sclerosis in OA joints (2). Considerable data now implicate pro-inflammatory cytokines produced by the synovium and activated chondrocytes in disease pathogenesis. Interleukin-1β (IL-1β) plays a key role in OA pathogenesis (3), co-localizes with MMP-13 to the site of cartilage degradation in OA joints (4) and also alters microRNAs (miRNAs) expression in chondrocytes (5). MicroRNAs are a class of small non-coding RNAs that are evolutionary conserved and function as regulator of gene expression at posttranscriptional level through perfect or imperfect complementarity with the seed sequences in the 3’UTR of target mRNA resulting in translation repression or mRNA cleavage (6). However, “seed sequence” present in the 5’UTR and in the coding region of mRNAs (CDS) are also targets of miRNAs in order to regulate gene expression (7, 8). Role of miRNAs in maintaining cartilage homeostasis and their deregulated expression has recently been linked with OA pathogenesis (9-12).
Hedgehog (Hh) signaling plays a crucial role in the skeletal development and regulates normal chondrocytes growth and differentiation during development and after birth (13, 14). Sonic hedgehog (SHH) and Indian hedgehog (Ihh) are the major ligands in chondrocytes and their binding to Patched (PTCH1) activates seven trans-membrane domain protein-Smoothened (SMO). Activated SMO releases GLI-1 from cytoplasmic sequestration which then translocate to the nucleus (15) and binds to the consensus 9-base pair DNA elements (5’-GACCACCCA-3’) within the target genes (15-17). Recent investigations showed that higher levels of Ihh signaling contributes to more severe OA like phenotype by decreasing articular cartilage thickness and enhanced proteoglycan loss coupled with increased expression of matrix metalloproteinase (MMP)-13 and aggrecanase ADAMTS-5, while inhibiting Ihh signaling attenuate the severity of OA (18,19). Elevated levels of Ihh in the joint synovial fluid have also been linked to early cartilage lesions (20).
SHH has been reported to play a critical role in the control of chondrogenic differentiation (21) but its expression and regulation is not well studied in OA. In the present study, we demonstrate that SHH mRNA is expressed in damaged human OA cartilage and in an animal model of OA and is induced by IL-1β in human OA chondrocytes. We also show that expression of miR-602 and miR-608, which target SHH mRNA, was low in damaged OA cartilage compared to smooth cartilage and their overexpression inhibited the SHH mRNA and protein expression in human OA chondrocytes. Furthermore, IL-1β is a potent inducer of SHH expression and down-regulates miR-608 expression and inhibition of SHH-activated signaling suppressed the expression of MMP-13 in OA chondrocytes. Importantly, regulation of SHH expression is apparently mediated by the miRNA target sites located in the coding region of SHH mRNA and are sufficient to inhibit reporter gene expression in human OA chondrocytes.
MATERIALS & METHODS
Cartilage Specimens and chondrocytes culture
Permission to use discarded and de-identified human cartilage was obtained from the Institutional Review Board (IRB) of Northeast Ohio Medical University (IRB13-275) and Summa Health System prior to the initiation of the studies which also determined that no informed consent was required. OA was diagnosed according to the American College of Rheumatology criteria (22, 23). Cartilage samples were obtained from 46 patients (28 females and 18 males; average age 63.7 years ± 8.7 SD) with OA who underwent total hip or knee joint arthroplasty at Crystal Clinic, Akron, OH. Specimens were washed with sterile PBS, stained with India ink and portions of the cartilage with smooth and damaged articular surface were used for histology and immunohistochemistry and chondrocytes were prepared by the enzymatic digestion of cartilage and cultured as previously described (12). Smooth or damaged cartilage was used for the results shown in Figure 1A-1E and chondrocytes derived from smooth or damaged cartilage were used in experiments shown in Figure 4A-B.
Figure 1.
Expression of Sonic hedgehog (SHH), its signaling targets and MMP-13 in OA. A, Articular cartilage stained with India ink and representative histological Safranin-0-fast green sections comparing smooth cartilage(left) and damaged cartilage(right) of human OA patients. B, Immunohistochemical localization of SHH expression in smooth and damaged cartilage. Left upper and lower sections are IgG-isotype controls. Right upper and lower sections were probed with a SHH-specific antibody. C, Representative histopathology and immunofluorescence for SHH expression in Sham and 8 week post ACLT rabbit cartilage. Upper left and riqht sections shows Safranin-0-fast qreen staininq and lower sections show SHH expression. D and E, Expression of SHH, its signaling targets (PTCH1, GLI1and HHIP) and known markers of OA (MMP-13 and COL10A1) in human OA cartilage. F and G, Modulation of SHH regulates MMP-13 expression in human chondrocytes. Expression of SHH signaling targets and OA markers in human chondrocytes treated with recombinant SHH (5µg/ml) or with SANT-1(5µM). Un-stimulated chondrocytes were used as controls and expression of β-actin was used as an endogenous-control. The level of expression was arbitrarily defined as ‘1’ in smooth cartilage. The error bars are 95% confidence intervals (P<0.05).
Figure 4.
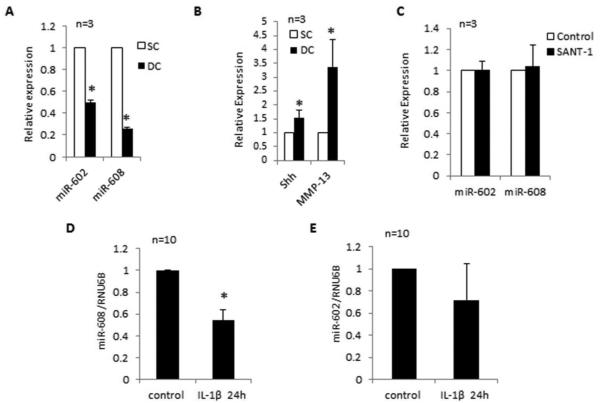
Correlation between SHH and miR-602 and miR-608 expression in OA chondrocytes. A and B, Real-time PCR analyses of the constitutive expression of SHH mRNA, MMP-13 mRNA and of miR-602 and miR-608 predicted to target SHH mRNA in OA chondrocytes. C, Inhibition of SHH signaling has no effect on miR-602 and miR-608 expression in chondrocytes treated with Smoothend (SMO) antagonist, SANT-1(5 µM) for 24h. D and E, IL-1β (10ng/ml) inhibited the expression of miR-608 but not of miR-602 in OA chondrocytes. For all the experiments un-stimulated chondrocytes were used as controls and expression of RNU6B was used as an endogenous-control. Data from individual patients is shown. The level of expression was arbitrarily defined as ‘1’ in control samples. The error bars are 95% confidence intervals (P<0.05).
Chondrocyte treatments and preparation of microRNAs
OA chondrocytes were serum starved overnight and then stimulated with IL-1β (10ng/ml; R & D Systems, St Paul, MN) or recombinant human SHH protein (5µg/ml; R & D Systems) or Hh signaling inhibitor SANT-1 (SMO antagonist, EMD Millipore, Billerica, MA) or NF-κB inhibitors SC514 (100µM) (TOCRIS Biosciences, Minneapolis, MN), MG132 (100µM) (EMD Millipore, Germany) and Parthenolide (50µM) (A.G. Scientific, San Diego, CA) or JNK II inhibitor (10µM) (EMD Millipore, Germany), ERK inhibitor (PD98059;50µM) (EMD Millipore) and p38 inhibitor (SB202190; 50µM) (A.G. Scientific) for indicated time and total RNA containing miRNAs fraction was prepared using the Qiagen miReasy kit (Qiagen, Chatsworth, CA). For some studies total RNA was prepared directly from smooth and damaged cartilage samples. Briefly, cartilage pieces were grounded to a fine powder in liquid nitrogen using Freezer mill (SPEX, Metuchen, NJ) and then processed to purify the RNA as above (Qiagen).
Quantitative RT-PCR analysis
Genomic DNA-free total RNA (500ng) was reverse-transcribed using QuantiTect Reverse Transcription Kit (Qiagen) according to the manufacturer’s protocol. The mRNA expression of SHH, PTCH-1, GLI-1, HHIP, MMP-13 and COL10A1 was quantified using the TaqMan Gene Expression Assays with β-actin mRNA expression as control (Life Technologies, Carlsbad, CA). Expression of miR-602 and miR-608 was quantified using TaqMan microRNA Assays with RNU6B as control (Life Technologies). Quantification of relative expression levels was determined by ΔΔCt method as previously described (5, 12).
Luciferase reporter assay
The pMIR-REPORT miRNA Expression Reporter Vector System (LifeTechnologies) was used to construct the reporter plasmid containing only the coding region of SHH mRNA (NM_000193.2). Briefly, total RNA (1µg) was reverse transcribed into cDNA and a fragment of the SHH coding region was amplified by PCR using forward primer SHH_F1HindIII (position 814-831) 5’-GTCAAAGCTTCGCACCTGCTCTTTGTGG-3’ and the reverse primer (position 1323-1343) SHH_R1SpeI 5’-GTCAACTAGTCCAGGTGCCTATTTG GTAGAG-3’. The amplified DNA fragment was purified (Qiagen) and was digested with Hind III and Spe I restriction endonucleases (New England Biolabs, Ipswich, MA) in buffer 4 overnight, gel purified, and ligated to the luciferase reporter gene in the Hind III–Spe I–digested vector to generate pMIR-REPORT-Luc-SHH plasmid while the pMIR-REPORT-mutant-Luc-SHH plasmid contained the miR-602 and miR-608 seed sequences in the reverse orientation. PCR primers used were the same as above but the location of restriction enzyme sites for Hind III and Spe I were altered. Following standard protocols, LPS-free plasmid DNA was prepared and OA chondrocytes were co-transfected with 1µg of reporter plasmid, 500ng of Renilla luciferase control vector (Promega Madison, WI) and 100nM of pre-miR-602 or pre-miR-608 or negative control miRNAs (LifeTechnologies) using Amaxa Nucleofection System (Lonza). Cell lysates were prepared 48h after transfection and luciferase activity was assayed using a Dual Luciferase® Reporter Assay kit (Promega) using Synergy H1 hybrid multi-mode plate reader (BioTek, Winooski, VT). Firefly luciferase activity was normalized to Renilla luciferase activity. Each experiment was repeated 3 times and each assay was performed in triplicate.
Transfection of HEK-293 cells with the SHH expression plasmid and miRNAs mimic and inhibitor
HEK-293 cells were transfected with SHH expression plasmid (1µg) (Origene, Rockville, MD), pre-miRs or negative control pre-miRNAs (LifeTechnologies) using TransIT®-293 transfection reagent (Mirus, Madison, WI) in a 6-well plate. Total RNA containing miRNA fraction and cell lysate were prepared after 48h of transfection.
Modulation of SHH expression by miRNA mimics and inhibitors in human OA chondrocytes
Human OA chondrocytes from damaged cartilage were transfected with SHH reporter plasmid (1µg) and/or pre-miRs (100nM) or antagomirs (150nM) and with negative control pre-miRNAs (LifeTechnologies) using HiPerFect transfection reagent (Qiagen). Chondrocytes were harvested 48h after transfection and SHH mRNA expression and protein production was determined using TaqMan assays and Western Immunobloting respectively.
Western Immunobloting
Chondrocytes were lysed in RIPA lysis buffer with complete protease inhibitor cocktail (Roche, Indianapolis, IN) and the lysate protein was resolved by SDS-PAGE (10% resolving gel with 4% stacking) and transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA). Membranes were blocked with non-fat dry milk powder in Tris buffered saline containing 0.1% Tween-20 (TBS-T), and probed with 1:1000 diluted polyclonal or monoclonal antibodies specific for SHH (Millipore, Billerica, MA) or Anti-DDK (Origene) or β-actin (Santa Cruz Biotechnology, Santa Cruz, CA). Immuno-reactive proteins were visualized using HRP-linked secondary antibodies and enhanced chemiluminescence (GE Healthcare, Milwaukee, WI). Images were captured using the Syngene Pxi-Imaging System (Syngene, Frederick, MD), and analyzed using the UN-SCAN-IT software (Silk Scientific Corporation, Idaho, UT). Each band was scanned three times with background correction and the values (pixels/band) were averaged and expressed as Mean ± SD.
Immunohistochemistry and immunofluorescence staining
Full thickness cartilage pieces were taken from damage and smooth area of knee cartilage. Samples were fixed in 4% paraformaldehyde and decalcified using 10% EDTA. Dehydrated samples were embedded in paraffin and 5µm thick sections were cut. Representative sections from human or rabbit cartilage were stained with Safranin-O and counterstained with Fast Green.
To detect SHH expression, cartilage sections were collected on SuperFrost® Plus glass slides (Thermo-Fisher, Hampton, NH), de-paraffinized and rehydrated and antigen retrieval was performed by heating slides for 10 min at 95ºC in 0.01M sodium citrate in Decloaking chamber (Biocare Medical, Concord, CA). Endogenous peroxide was blocked by treating the sections with 0.1% H2O2 (Sigma-Aldrich, St Louis, MO) in water for 5 min. After washing with 1xPBS, non-specific binding was blocked by incubation with normal horse serum provided in the kit. The sections were incubated with 1:200 diluted primary SHH antibody (Millipore) at 4ºC overnight. The negative control sections were incubated with IgG isotype control. Thereafter, sections were treated sequentially with biotinylated secondary antibody and streptavidin-peroxidase conjugate, and then were developed using DAB substrate kit for peroxide (R.T.U. Vectastain® Universal ABC kit, Vector Laboratories, Burlingame, CA) and counterstained with Safranin-O/Fast Green.
Induction of OA in Rabbits
To induce OA in rabbits, anterior cruciate ligament transection (ACLT) surgery was performed. Analgesic (buprenorphine hydrochloride, 0.03mg/kg injected intramuscularly every 6h) was administered preoperatively and for 3 days following surgery. Antibiotic (Convenia, 8mg/kg injected subcutaneously) was also administered postoperatively. Anesthesia for the surgical procedure was maintained with a mixture of isoflurane in oxygen. The limb was clipped and prepared for surgery in a standard manner. A medial arthrotomy was performed on the left femoropatellar joint to permit transection of the ACL. Routine skin incision closure was performed. Cartilage samples were harvested 8 weeks after surgery and processed as above. Slides were incubated with anti-SHH antibody (Millipore) as above followed by incubation with Alexa Flour 594 (1:100) conjugated secondary antibody (LifeTechnologies). Slides were photographed using Nikon Eclipse Ti microscope (Nikon, Melville, NY).
Statistical analyses
All experiments were performed using chondrocytes or cartilage from indicated number of patients as given in figure legends. Values shown are Mean ± SE unless stated otherwise. Comparisons were performed using Origin 8.1 software package (one paired two tailed t-test with one way ANOVA) with P<0.05 as significant.
RESULTS
Expression of SHH, its signaling targets and of known markers of OA was upregulated in OA cartilage
To determine whether SHH signaling is activated in human OA cartilage, we studied the expression of SHH, PTCH-1, GLI-1 and HHIP. Our results showed higher expression of SHH mRNA and protein in human damaged OA cartilage and also in the cartilage of rabbits showing proteoglycan loss post-ACLT surgery as evidenced by immunohistochemical and immunofluorescence staining (Figure 1A-C). Our results also demonstrate that expression of SHH and its signaling targets was low in smooth human cartilage (Figure 1B-D) while their expression was significantly higher in damaged cartilage from the same OA patients (Figure 1D; P<0.05). Interestingly, there was also enhanced expression of MMP-13 (~3-fold) and COL10A1 (~4.8-fold) mRNAs in damaged cartilage (Figure 1E; P<0.05) and correlated with high expression of SHH and its signaling targets suggesting a role of SHH signaling in their expression in OA.
Activation of SHH signaling up-regulates MMP-13 expression in OA chondrocytes under pathological conditions
To elucidate the role of SHH in OA pathogenesis, we examined the MMP-13 expression in human OA chondrocytes and cartilage explants after stimulation for 24h in vitro with either human recombinant SHH protein or vehicle control. We also used the SMO antagonist SANT-1 to determine whether inhibition of SHH signaling modulates MMP-13 expression in IL-1β-stimulated OA chondrocytes. Human cartilage explants treated with recombinant SHH protein showed activation of SHH signaling as evidenced by enhanced expression of PTCH-1 (~106%), GLI-1 (~173%) and HHIP (~98%) as well as of osteoarthritic markers MMP-13 (~114%) and COL10A1 (~60%) (Figure-1F; P<0.05). Interestingly, treatment with SANT-1 significantly inhibited the SHH-induced expression of SHH, PTCH-1, GLI-1, HHIP and MMP-13 mRNAs but the effect on COL10A1 expression was minimal (Figure-1G; P<0.05) in human OA chondrocytes. In addition, a significant decrease in the expression of SHH, and MMP-13 was also observed in human OA chondrocytes treated with SANT-1 and then stimulated with IL-1β suggesting an important role of Hh signaling activation in the regulation of OA marker genes MMP-13 and COL10A1 in human chondrocytes stimulated with IL-1β (Figure-2E-G; P<0.05).
Figure 2.
Effect of IL-1β on the expression of SHH and MMP-13 in OA chondrocytes. A, B and C, SHH and PTCH-1 mRNA, and SHH protein expression after IL-1β treatment was determined using TaqMan assays and Western immunoblotting, respectively. D, MMP-13 mRNA expression in IL-1β-stimulated and control chondrocytes was determined by TaqMan assays. E, F and G, OA chondrocytes were pretreated with SANT-1 for 2h and then stimulated with IL-1β (10ng/ml) for 24h. Expression of SHH, MMP-13 and COL10A1 mRNA was determined using TaqMan assays. H and I, Inhibition of NF-кB and MAPKs enhanced SHH expression in OA chondrocytes. Chondrocytes were pretreated with NF-кB inhibitors (SC514, MG132 and Parthenolide) or MAPKs inhibitors (SB202190, PD98059 and JNKII inhibitor) for 2h and then stimulated with IL-1β (10ng/ml) for 24h. For all the experiments un-stimulated chondrocytes were used as controls and expression of β-actin was used as an endogenous-control. Each experiment was performed in duplicate with the indicated number of patient samples. The error bars are 95% confidence intervals (P<0.05).
Stimulation with IL-1β induces upregulation of SHH in OA chondrocytes
As IL-1β plays a crucial role in OA pathogenesis, we determined whether IL-1β-induces SHH expression in human OA chondrocytes. Our results demonstrate that stimulation with IL-1β is sufficient to induce high levels of SHH mRNA and protein expression in OA chondrocytes (Figure-2A and C; P<0.05) and this also correlates with increased expression of the matrix degrading enzyme MMP-13 (Figure-2D; P<0.05). Among the examined downstream targets of SHH signaling molecules, expression of PTCH-1 was significantly upregulated in IL-1β-stimulated OA chondrocytes (Figure-2B; P<0.05) while that of HHIP and GLI-1 was not significantly affected (data not shown). These results suggest that stimulation with IL-1β plays a major role in the induction and expression of high levels of SHH and its receptor PTCH-1 in OA.
Regulation of SHH expression by NF-kB and MAPKs pathways
To investigate whether IL-1β-mediated activation of NF-κB or MAPK signaling pathways regulate SHH expression, we used small molecule inhibitors of NFκB and MAPK. Treatment with NFκB or MAPK inhibitors did not suppress but enhanced the SHH expression in IL-1β-stimulated OA chondrocytes. This indicated that activated NF-κB and MAPK pathways are not involved in the up-regulation of SHH mRNA expression seen in IL-1β-stimulated OA chondrocytes (Figure-2H-I). Furthermore, these results suggests that activation of NF-κB and MAPK signaling may be involved in inhibiting SHH expression possibly by inducing the expression of a SHH suppressor molecule. However, this needs to be investigated further.
Coding region of SHH mRNA contains binding sites for miR-602 and miR-608
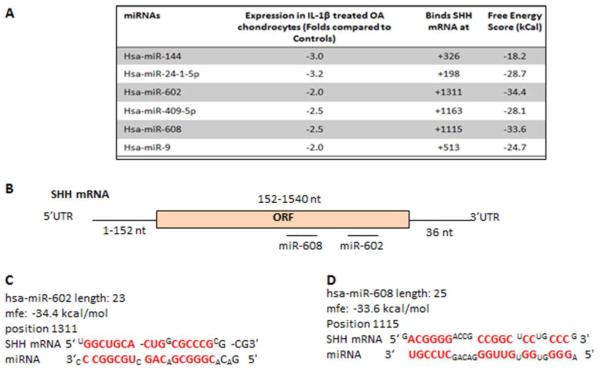
To determine whether the expression of SHH is subject to post-transcriptional regulation by miRNAs, we first determined whether SHH mRNA is targeted by known miRNAs. The ORF of the SHH mRNA (NM_000193) is 1692 bases long but the 3’UTR is quite short (contains only 36 bases). Using Target ScanS no conserved miRNA target sites were identified in the 3’UTR of SHH mRNA, but RNAhybrid (http://bibiserv.techfak.uni-bielefeld.de/rnahybrid/submission.html) identified several miRNAs with potential target sites in the coding region of SHH mRNA (Figure-3A). Out of the predicted miRNAs, miR-602 (−34.4 kCal) and miR-608 (−33.6 kCal) with seed-matched sequence in the coding region of SHH mRNA were selected for further studies based on their free energy score and expression patterns (see below). Predicted duplex of selected miRNAs with seed-matched sites in the coding region of human SHH mRNA are shown in Figure 3B-D. These results suggested that the identified miRNAs can bind the respective seed-matched sequences present in the coding region of SHH mRNA.
Figure 3.
Seed sequence of selected miRNAs present in the coding region of SHH mRNA. A, Predicted miRNAs identified using miRGen, their free energy scores, binding sites in SHH mRNA coding region and expression patterns miRNA in IL-1β stimulated OA chondrocytes for 6h. B-D, Seed-matched sequences for miR-602 and miR-608 in the coding region of SHH mRNA were identified by miRGen and computer aided prediction of interactions with sites in the SHH mRNA coding region were derived using RNA hybrid and RegRNA computational algorithms.
Correlation between SHH expression and expression of miR-602 and miR-608 in OA chondrocytes
To determine whether miR-602 and miR-608 expression levels differ between damaged and smooth cartilage in OA, we compared their expression levels in chondrocytes obtained from smooth and damaged cartilage of OA patients. Our results showed a differential expression of miR-602 and miR-608 in chondrocytes obtained from damaged and smooth cartilage (Figure-4A; P<0.05). Also expression levels of SHH and MMP-13 were significantly higher (~52% and ~236%, respectively) in chondrocytes obtained from damaged cartilage compared to the levels seen in chondrocytes obtained from smooth cartilage (Figure 4B; P<0.05). When SHH signaling was inhibited by SANT-1 no effect on the expression of miR-602 and miR-608 was seen (P>0.05; Figure-4C). We then determined the effect of IL-1β-stimulation on the expression of miR-602 and miR-608. Interestingly, IL-1β-stimulated OA chondrocytes showed a significant decrease in the expression of miR-608 (46%) (Figure-4D; P<0.05) and had high levels of SHH mRNA but correlation between the expression of miR-602 and SHH expression was observed (Figure-4E; P>0.05) indicating that miR-602 expression level may not be a factor in regulating SHH expression in IL-1β-stimulated human chondrocytes in vitro. However, miR-602 inhibited the activity of the reporter enzyme and SHH mRNA and protein expression in other experiments (see below) which suggests that stimulation with IL-1β may be suppressing some factor(s) required for the interaction of miR-602 and the “seed sequence” in SHH mRNA.
Over-expression of miR-602 and miR-608 inhibited the activity of pMIR-REPORT-Luc-SHH reporter vector in vitro
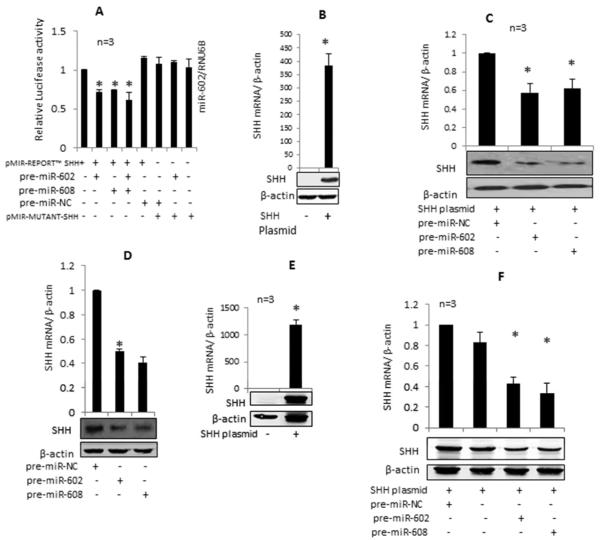
Chondrocytes were transiently co-transfected with the pMIR-REPORT-SHH luciferase reporter construct or the mutant reporter construct and pre-miR-602/pre-miR-608/negative control pre-miRNAs (100 nM) and the internal control plasmid pRL-SV-40. Co-transfection of SHH reporter construct individually with either pre-miR-602 or pre-miR-608 or in combination decreased the luciferase activity by ~28%, ~26% and ~39% respectively (Figure-5A; P<0.05) compared to the chondrocytesco-transfected with SHH reporter construct and the negative control pre-miRNA. However, co-transfection of pMIR-REPORT-mutant-SHH reporter vector with pre-miR-602 or pre-miR-608 had no significant effect on luciferase activity (Figure-5A). Since the reporter vector contained only the coding region of SHH mRNA, these results demonstrated that miR-602 and miR-608 bind the “seed sequence” present in the coding region of SHH mRNA.
Figure 5.
miR-602 and miR-608 inhibit the SHH mRNA 3'UTR reporter activity and overexpression of miR-602 and miR-608 inhibit SHH expression in human OA chondrocytes and in HEK-293 cells transfected with SHH expression plasmid. A. Luciferase activity in human OA chondrocytes transfected with the pMIR-REPO RT™-Luc-SHH CDS construct or pMIR-REPORT-mumnt-Luc-SH H construct with pre-miR-602, pre-miR-608 and pre-miR-negative control (NC); B. Overexpression of SHH mRNA and protein in human OA chondrocytes transfected with SHH expression plasmid; C, D. Inhibition of plasmid-mediated and endogenous and SHH mRNA and protein expression in human OA chondrocytes transfected with pre-miR-602, pre-miR-608 respectively. E. Enhanced expression of SHH mRNA and protein in HEK-293 cells after 48h of transfection. F, Down regulation of SHH mRNA and protein expression in HEK-293 cells transfected with SHH expression plasmid, pre-miR602 and pre-miR608. Expression of mRNA and miRNAs was determined using TaqMan assays. Gene expression of RNU6B/β-actin was used as an endogenous control. Each experiment was performed in duplicate using three different patient samples. SHH protein expression was determined by Western immunoblottinq. The error bars are 95% confidence intervals (P<0.05).
miR-602 and miR-608 regulates the expression of SHH in vitro
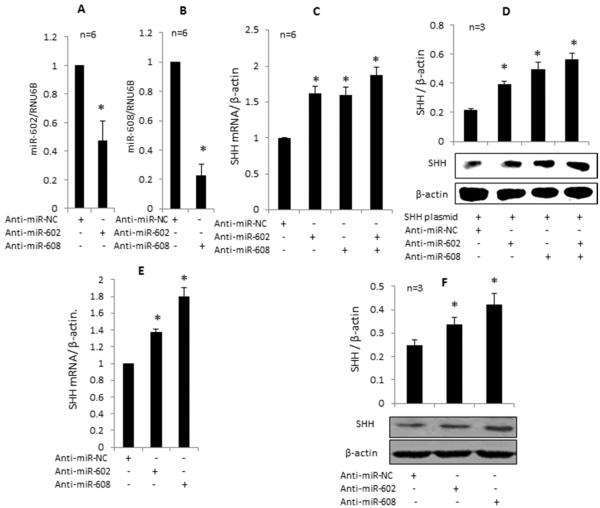
For these studies, human OA chondrocytes or HEK-293 cells were transfected with SHH expression plasmid and/or selected pre-miRNAs or their antagomirs and effect on SHH mRNA and protein expression was determined. Significant over expression of SHH mRNA and protein was seen in OA chondrocytes transfected with the SHH expression plasmid which was inhibited significantly by the overexpression of miR-602 and miR-608 (Figure-5B and C, p<0.05). Overexpression of miR-602- and miR-608 in OA chondrocytes also significantly inhibited the IL-1β-induced SHH mRNA and protein expression (Figure-5D, p<0.05). Transfection with miR-602 and miR-608 antagomirs led to a significant down regulation of these miRNAs in OA chondrocytes (Figure-6A and 6B; P<0.05) and upregulation of SHH mRNA (Figure-6C, p<0.05). Over expression of miR-602 or miR-608 in HEK-293 cells transfected with the SHH expression plasmid showed ~43% and ~38% inhibition of SHH mRNA expression respectively compared to cells co-transfected with the SHH plasmid and negative control miRNAs (Figure-5E and F; P<0.05). These results were consistent with the decrease observed in SHH protein expression in OA chondrocytes transfected with the SHH plasmid with overexpression of pre-miR-602 or pre-miR-608 (Figure-5B, C). Transfection of OA chondrocytes with respective antagomirs showed an approximately 60% increase in SHH mRNA and protein expression (Figure-6C and 6D; P<0.05). This effect was further enhanced when miR-602 and miR-608 inhibitors both were used in combination. Overexpression of miR-602 and miR-608 suppressed the IL-1β-induced endogenous SHH mRNA and protein expression in OA chondrocytes (Figure-5E) while overexpression of antagomirs enhanced the SHH expression (Figure 6E) further confirmed that both miR-602 and miR-608 may be involved in the post-transcriptional regulation of SHH expression in OA chondrocytes.
Figure 6.
Effect of miRNA anatagomirs on miR-602, miR-608 and on SHH mRNA and protein express ion in human QA chondrocytes. A and B. Qverexpression of miR-602 and miR-608 antagomirs downregulated the endogenous expression of miR-602 and miR-608 in human QA chondrocytes. C. Qverexpression of miR-602 and miR-608 antagomirs alone or in combination in human QA chondrocytes obtained from the damaged cartilage enhanced the expression of SHH mRNA and protein; D. Qverexpression of miR-602 and miR-608 antagomirs alone or in combination in human QA chondrocytes obtained from the damaged cartilage enhanced the expression of SHH mRNA and protein from the transfected SHH expression plasmid. E, F. Qverexpression of miR-602 and miR-608 anatagomi rs in human QA chondrocytes also boosted the endogenous SHH mRNA and protein expression respectively. Expression of SHH mRNA was determined by TaqMan assay, and expression of β-actin or RNU6B was used as an endogenous control. SHH protein expression was determined by Western immunoblotting. Each experiment was performed in duplicate using six different samples for the data shown in A-C and three different patient samples for the data shown in D-F. The error bars are 95% confidence intervals (P<0.05). NC, neqative control
DISCUSSION
Hh protein family plays a crucial role during embryonic development by governing skeletal growth through the development of chondrocytes and promoting endochondral ossification (13). Ihh is known to be expressed in prehypertrophic chondrocytes and coordinates their proliferation and maturation during the development via negative regulation of parathyroid related protein (PTHrP) (24). Due to activated Hh signaling, OA chondrocytes acquire phenotype of growth plate chondrocytes including an increase in catabolic agent’s expression and inappropriate hypertrophic differentiation (25). Expression of Hh signaling genes was highly up-regulated in cartilage of OA patients and in an animal model of OA (18). Recent studies positively correlated Hh signaling with the OA progression and osteophyte formation (18,19,26,27). High level expression of matrix degrading enzymes MMP-13 and ADAMTS-5 is a hall mark of OA (28,29) and activated Hh signaling can up-regulate their expression via transcription factor RUNX-2 in OA chondrocytes (18,30). Over expression of SHH is also known to affect chondrocyte differentiation and growth plate organization in transgenic mice (21). We examined the post transcriptional regulation of SHH expression by miRNAs in OA chondrocytes and found that its expression was regulated directly by miR-602 and miR-608. Our data also provide the evidence linking miR-602 and miR-608 to OA pathogenesis by demonstrating their expression and modulation by IL-1β in human OA chondrocytes.
Our results show loss of proteoglycan and higher expression of SHH, PTCH-1, GLI-1 HHIP and increased MMP-13 and COL10A1 expression in damaged cartilage. Enhanced expression of SHH with the loss of proteoglycans was also seen in the arthritic joints of rabbits with post-ACLT-surgery and these results are consistent with results reported previously in a mouse model of OA (18). Treatment of OA chondrocytes with human recombinant SHH protein resulted in enhanced expression of PTCH-1, GLI-1, HHIP and MMP-13 indicating that activation of SHH-induced signaling is an important regulator of MMP-13 expression in OA. On the other hand OA chondrocytes treated with the small molecule inhibitor SANT-1 showed inhibition of IL-1β-induced expression of PTCH-1 and MMP-13. Our results are consistent with published studies reporting enhanced expression and production of matrix degrading proteases after treatment with SHH ligand in cancer (31,32). It is important to note that SANT-1 is a cell-permeable high affinity antagonist of Smoothened (SMO) activity which in turn inhibits Sonic hedgehog (Shh). Therefore it is not possible to completely rule out a role for IHh in the effect of IL-1 on MMP13 and COL10A1 expression in human chondrocytes.
Physiological role of miRNAs in the maintenance of joint homeostasis and degeneration in OA is an active area of investigation (see ref 10). Role of miRNA-140 in cartilage formation and maintenance via histone deacetylase-4 suppression (a known repressor of RUNX-2) has been shown (33). MiR-148a has been shown to promote cartilage production and inhibit cartilage degradation in OA chondrocytes (34). Ihh is a mechnoresponsive gene in chondrocytes and shown to be up regulated in OA owing to abnormal forces on the joints (35). A recent study identified miR-365 as a mechanosensitive miRNA whose expression was higher in pre-hypertrophic chondrocytes and coincides with Ihh expression, supporting the role of miRNAs in chondrocytes differentiation and hedgehog signaling (36). Other studies (5,11,12,37) have also shown the regulation of OA marker genes by miRNAs indicating deregulation of post-transcriptional machinery as an important factor in OA pathogenesis.
The 3’ un-translated region of SHH mRNA (NM_000193) is fairly short and no conserved target sites in the 3’UTR were identified using TargetScanS. However, potential target sites of several miRNAs in the coding region of SHH mRNA were identified using the RNA hybrid bioinformatics tool. Based on the criteria employed by us previously (5,12) miR-602 and miR-608 were selected to experimentally determine whether they interact with the identified sites in the coding region of SHH mRNA. Regulation of mRNA expression through the binding sites in the coding region is not without precedent as splice variants of DNMT have been shown to be regulated by miR-148 via a conserved site present in the coding region (38). Similarly, miR-34a has been reported to target human hepatocytes nuclear factor 4 and MDM-4 by a functional site in the coding region of these mRNAs (7,39).
When human chondrocytes were co-transfected with a reporter vector and miR-608 and miR-602 mimics, a significant reduction in the luciferase activity was observed (~39%, P<0.05) but no significant change in luciferase activity was detected by co-transfection of the mutant-SHH reporter and either miR-602 or miR-608 mimics. These data confirm the direct binding of miR-602 and miR-608 with the target sites identified in the coding region of human SHH mRNA. Furthermore, transfection of OA chondrocytes with respective inhibitors induced significant silencing of miR-602 and miR-608 expression and marked up-regulation of SHH mRNA and protein expression in OA chondrocytes. However, pharmacological blockage of SHH signaling did not change the expression level of miR-602 and miR-608 in OA chondrocytes indicating that expression of miR-602 and miR-608 in OA is not regulated by SHH expression. Down regulation of miR-608 expression and enhanced production of SHH protein observed in human OA chondrocytes stimulated with IL-1β suggests a direct link between the expression of miR-608 and the expression of SHH in OA chondrocytes.
IL-1β is a key player in OA pathogenesis (3,40) and up-regulates MMP-13 expression in cartilage (41). Our results showed for the first time that IL-1β is a potent inducer of SHH and its receptor PTCH-1’s expression in OA chondrocytes. Enhanced expression of SHH in IL-1β-stimulated OA chondrocytes treated with the NF-κB and MAPK inhibitors is a novel finding and strongly indicates that activation of NFκB or MAPK signaling negatively regulates the expression of SHH in OA chondrocytes. Potential of this finding for developing new OA therapies need to be explored further.
miR-602 and miR-608 are located on different chromosomes but their lower expression have been reported in cancer (20,42,43) and in chordoma malignancy cells (20). On the other hand, higher expression of miR-602 has been reported in germinal vesicle and meiosis II stage during human oocyte development indicating a possible regulatory role in meiosis (44). Chordomas are rare malignant tumors that originate from the notochord remnants and occur in the skull base, spine and sacrum. MiR-608 has recently been identified as novel tumor suppressive miRNA that regulate malignancy in chordoma via direct targeting of deregulating oncogenes EGFR and Bcl-xL (43). MiR-608 has also been suggested to inhibit cell survival and induces apoptosis in chordoma malignancy and in lung adenocarcinoma cells (20, 43). However, role of miR-602 and miR-608 as a regulator of SHH in OA cartilage has not been reported. Here we showed that SHH induces the expression of MMP-13 and that inhibition of SHH signaling blocked MMP-13 expression in OA chondrocytes. Furthermore, SHH expression was inhibited by miR-602 and miR-608 in OA chondrocytes suggesting that physiological or therapeutic up regulation of miR-602 and miR-608 may be useful in blocking MMP-13 expression in OA. Further, in vivo studies using miRNA-602 and miR-608 could provide information on their efficacy using optimal miRNA delivery method.
In conclusion, our data demonstrate that both miR-602 and miR-608 bind the target sites present in the coding region of SHH mRNA and inhibit its expression in human chondrocytes at least in vitro. This data provides an explanation for our findings that expression of miR-602 and miR-608 was low in damaged cartilage where the expression of SHH was high, while their expression was high and expression of SHH was low in smooth cartilage. We also show that activation of SHH signaling induces the expression of MMP-13 and its inhibition abrogates MMP-13 expression in human chondrocytes. Thus, IL-1β-induced up-regulation of SHH expression and the activation of the SHH signaling shown here may be critical factors in upregulating the MMP-13 expression in OA. Our results suggest that expression of miR-608 and possibly miR-602 may be exploited in novel therapeutic strategies for the treatment of OA.
ACKNOWLEDGMENTS
This work was supported in part by grants from the National Institute of Health (RO1 AT-003267; RO1-AT-005520; RO1-AT-007373; R21-AR-064890) and funds from the Northeast Ohio Medical University.
Footnotes
AUTHORS CONTRIBUTIONS
All authors approved the final version of the manuscript. Tariq M. Haqqi has the full access to the data and takes responsibility of the integrity of the data presented in the manuscript.
Study design. Tariq M. Haqqi
Acquisition of the data and analysis. Nahid Akhtar, Tariq M. Haqqi, Mohammad S. Makki,
Analysis and interpretation of the data. Nahid Akhtar, Mohammad S. Makki,Tariq M. Haqqi
Manuscript preparation. Nahid Akhtar, Mohammad S. Makki, Tariq M. Haqqi
Statistical analysis. Nahid Akhtar, Mohammad S. Makki
CONFLICT OF INTEREST
The authors have no conflict of interest.
REFERENCES
- 1.Arden N, Nevitt MC. Osteoarthritis: epidemiology. Best Pract Res Clin Rheumatol. 2006;20(1):3–25. doi: 10.1016/j.berh.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Aigner T, Soder S, Gebhard PM, McAlinden A, Haag J. Mechanism of disease: role of chondrocytes in the pathoegensisof osteoarthritis-structure, chaos and senescence. Nature Clin Prect Rheumatol. 2007;3:391–99. doi: 10.1038/ncprheum0534. [DOI] [PubMed] [Google Scholar]
- 3.Blom AB, van der Kraan PM, van den Berg WB. Cytokine targeting in osteoarthritis. Curr Drug Targets. 2007;8:283–92. doi: 10.2174/138945007779940179. [DOI] [PubMed] [Google Scholar]
- 4.Tetlow LC, Adlam DJ, Woolley DE. Matrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage: associations with degenerative changes. Arthritis Rheum. 2001;44:585–94. doi: 10.1002/1529-0131(200103)44:3<585::AID-ANR107>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 5.Akhtar N, Rasheed Z, Ramamurthy S, Anbazhagan AN, Voss FR, Haqqi TM. MicroRNA-27b regulates the expression of matrix metalloproteinase 13 in human osteoarthritis chondrocytes. Arthritis Rheum. 2010;62(5):1361–71. doi: 10.1002/art.27329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 7.Mandke P, Wyatt N, Fraser J, Bates B, Berberich SJ, Markey MP. MicroRNA-34a modulates MDM4 expression via a target site in the open reading frame. PLoS One. 2012;7(8):e42034. doi: 10.1371/journal.pone.0042034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lytle JR, Yario TA, Steitz JA. Target mRNAs are repressed as efficiently by microRNAbinding sites in the 5’UTR as in the 3’UTR. Proc Natl Acad Sci U S A. 2007;104(23):9667–72. doi: 10.1073/pnas.0703820104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakasa T, Nagata Y, Yamasaki K, Ochi M. mini-review: microRNA in arthritis. Physiol Genomics. 2011;43(10):566–70. doi: 10.1152/physiolgenomics.00142.2010. [DOI] [PubMed] [Google Scholar]
- 10.Miyaki S, Asahara H. Macro view of microRNA function in osteoarthritis. Nat Rev Rheumatol. 2012;8(9):543–52. doi: 10.1038/nrrheum.2012.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le LT, Swingler TE, Clark IM. Review: The Role of MicroRNAs in Osteoarthritis and Chondrogenesis. Arthritis Rheum. 2013;65(8):1963–74. doi: 10.1002/art.37990. [DOI] [PubMed] [Google Scholar]
- 12.Akhtar N, Haqqi TM. MicroRNA-199a* regulates the expression of cyclooxygenase-2 in human chondrocytes. Ann Rheum Dis. 2012;71(6):1073–80. doi: 10.1136/annrheumdis-2011-200519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung UI, Schipani E, McMahon AP, Kronenberg HM. Indian hedgehog couples chondrogenesis to osteogenesis in endochondral bone development. J Clin Invest. 2001;107(3):295–304. doi: 10.1172/JCI11706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallet A. Hedgehog morphogen: from secretion to reception. Trends Cell Biol. 2011;21(4):238–46. doi: 10.1016/j.tcb.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15(23):3059–87. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- 16.Kinzler KW, Vogelstein B. The GLI gene encodes a nuclear protein which binds specific sequences in the human genome. Mol Cell Biol. 1990;10(2):634–42. doi: 10.1128/mcb.10.2.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu H, Lo HW. The Human Glioma-Associated Oncogene Homolog 1 (GLI1) Family of Transcription Factors in Gene Regulation and Diseases. Curr Genomics. 2010;11(4):238–45. doi: 10.2174/138920210791233108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin AC, Seeto BL, Bartoszko JM, Khoury MA, Whetstone H, Ho L. Modulating hedgehog signaling can attenuate the severity of osteoarthritis. Nat Med. 2009;15(12):1421–5. doi: 10.1038/nm.2055. [DOI] [PubMed] [Google Scholar]
- 19.Zhou J, Chen Q, Lanske B, Fleming BC, Terek R, Wei X, et al. Disrupting the Indian hedgehog signaling pathway in vivo attenuates surgically induced osteoarthritis progression in Col2a1-CreERT2; Ihhfl/fl mice. Arthritis Res Ther. 2014;16(1):R11. doi: 10.1186/ar4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Schiff D, Park D, Abounader R. MicroRNA-608 and microRNA-34a regulate chordoma malignancy by targeting EGFR, Bcl-xL and MET. PLoS One. 2014;9(3):e91546. doi: 10.1371/journal.pone.0091546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tavella S, Biticchi R, Schito A, Minina E, Di Martino D, Pagano A, et al. Targeted expression of SHH affects chondrocyte differentiation, growth plate organization, and Sox9 expression. J Bone Miner Res. 2004;19(10):1678–88. doi: 10.1359/JBMR.040706. [DOI] [PubMed] [Google Scholar]
- 22.Altman R, Alarcón G, Appelrouth D, Bloch D, Borenstein D, Brandt K, et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hip. Arthritis Rheum. 1991;34:505–14. doi: 10.1002/art.1780340502. [DOI] [PubMed] [Google Scholar]
- 23.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnotic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29:1039–49. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 24.Mak KK, Kronenberg HM, Chuang PT, Mackem S, Yang Y. Indian hedgehog signals independently of PTHrP to promote chondrocyte hypertrophy. Development. 2008;135(11):1947–56. doi: 10.1242/dev.018044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dreier R. Hypertrophic differentiation of chondrocytes in osteoarthritis: the developmental aspect of degenerative joint disorders. Arthritis Res Ther. 2010;12(5):216. doi: 10.1186/ar3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei F, Zhou J, Wei X, Zhang J, Fleming BC, Terek R, et al. Activation of Indian hedgehog promotes chondrocyte hypertrophy and upregulation of MMP-13 in human osteoarthritic cartilage. Osteoarthritis Cartilage. 2012;20(7):755–63. doi: 10.1016/j.joca.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruiz-Heiland G, Horn A, Zerr P, Hofstetter W, Baum W, Stock M, et al. Blockade of the hedgehog pathway inhibits osteophyte formation in arthritis. Ann Rheum Dis. 2012;71(3):400–7. doi: 10.1136/ard.2010.148262. [DOI] [PubMed] [Google Scholar]
- 28.Wang M, Sampson ER, Jin H, Li J, Ke QH, Im HJ, et al. MMP13 is a critical target gene during the progression of osteoarthritis. Arthritis Res Ther. 2013;15(1):R5. doi: 10.1186/ar4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glasson SS, Askew R, Sheppard B, Carito B, Blanchet T, Ma HL, et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434(7033):644–8. doi: 10.1038/nature03369. [DOI] [PubMed] [Google Scholar]
- 30.Tetsunaga T, Nishida K, Furumatsu T, Naruse K, Hirohata S, Yoshida A, et al. Regulation of mechanical stress-induced MMP-13 and ADAMTS-5 expression by RUNX-2 transcriptional factor in SW1353 chondrocyte-like cells. Osteoarthritis Cartilage. 2011;19(2):222–32. doi: 10.1016/j.joca.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 31.Chen JS, Huang XH, Wang Q, Huang JQ, Zhang LJ, Chen XL, et al. Sonic hedgehog signaling pathway induces cell migration and invasion through focal adhesion kinase/AKT signaling-mediated activation of matrix metalloproteinase (MMP)-2 and MMP-9 in liver cancer. Carcinogenesis. 2013;34(1):10–9. doi: 10.1093/carcin/bgs274. [DOI] [PubMed] [Google Scholar]
- 32.Yoo YA, Kang MH, Lee HJ, Kim BH, Park JK, Kim HK, et al. Sonic hedgehog pathway promotes metastasis and lymphangiogenesis via activation of Akt, EMT, and MMP-9 pathway in gastric cancer. Cancer Res. 2011 Nov. 15;71(22):7061–70. doi: 10.1158/0008-5472.CAN-11-1338. [DOI] [PubMed] [Google Scholar]
- 33.Tuddenham L, Wheeler G, Ntounia-Fousara S, Waters J, Hajihosseini MK, Clark I, Dalamy T. The cartilage specific microRNA-140 targets histone deacetylase 4 in mouse cells. FEBS Lettrs. 2006;580:4214–4217. doi: 10.1016/j.febslet.2006.06.080. [DOI] [PubMed] [Google Scholar]
- 34.Vonk LA, Kragten AH, Dhert WJ, Saris DB, Creemers LB. Overexpression of hsa-miR-148a promotes cartilage production and inhibits cartilage degradation by osteoarthritic chondrocytes. Osteoarthritis Cartilage. 2014;22(1):145–53. doi: 10.1016/j.joca.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 35.Ng TC, Chiu KW, Rabie AB, Hägg U. Repeated mechanical loading enhances the expression of Indian hedgehog in condylar cartilage. Front Biosci. 2006;11:943–8. doi: 10.2741/1850. [DOI] [PubMed] [Google Scholar]
- 36.Guan YJ, Yang X, Wei L, Chen Q. MiR-365: a mechanosensitive microRNA stimulates chondrocyte differentiation through targeting histone deacetylase 4. FASEB J. 2011;25(12):4457–66. doi: 10.1096/fj.11-185132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park SJ, Cheon EJ, Lee MH, Kim HA. MicroRNA-127-5p regulates matrix metalloproteinase 13 expression and interleukin-1β-induced catabolic effects in human chondrocytes. Arthritis Rheum. 2013;65(12):3141–52. doi: 10.1002/art.38188. [DOI] [PubMed] [Google Scholar]
- 38.Duursma AM, Kedde M, Schrier M, le Sage C, Agami R. miR-148 targets human DNMT3b protein coding region. RNA. 2008;14(5):872–7. doi: 10.1261/rna.972008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takagi S, Nakajima M, Kida K, Yamaura Y, Fukami T, Yokoi T. MicroRNAs regulate human hepatocyte nuclear factor 4alpha, modulating the expression of metabolic enzymes and cell cycle. J Biol Chem. 2010;285(7):4415–22. doi: 10.1074/jbc.M109.085431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daheshia M, Yao JQ. The interleukin 1beta pathway in the pathogenesis of osteoarthritis. J Rheumatol. 2008;35(12):2306–12. doi: 10.3899/jrheum.080346. [DOI] [PubMed] [Google Scholar]
- 41.Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7(1):33–42. doi: 10.1038/nrrheum.2010.196. [DOI] [PubMed] [Google Scholar]
- 42.Xiao W, Bao ZX, Zhang CY, Zhang XY, Shi LJ, Zhou ZT, et al. Upregulation of miR-31* is negatively associated with recurrent/newly formed oral leukoplakia. PLoS One. 2012;7(6):e38648. doi: 10.1371/journal.pone.0038648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Othman N, In LL, Harikrishna JA, Hasima N. Bcl-xL silencing induces alterations in hsa-miR-608 expression and subsequent cell death in A549 and SK-LU1 human lung adenocarcinoma cells. PLoS One. 2013;8(12):e81735. doi: 10.1371/journal.pone.0081735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu YW, Wang B, Ding CH, Li T, Gu F, Zhou C. Differentially expressed micoRNAs in human oocytes. J Assist Reprod Genet. 2011;28(6):559–66. doi: 10.1007/s10815-011-9590-0. [DOI] [PMC free article] [PubMed] [Google Scholar]