Abstract
The interest in screening lysosomal storage disorders (LSDs) in newborns and high-risk population has increased in the last years due to the availability of novel treatment strategies coupled with the development of diagnostic techniques. We report the development of a short-incubation mass spectrometry-based protocol that allows the detection of Gaucher, Niemann-Pick A/B, Pompe, Fabry and mucopolysaccharidosis type I disease within four hours including sample preparation from dried blood spots. Optimized sample handling without the need of time-consuming offline preparations such as liquid-liquid and solid-phase extraction, allows the simultaneous quantification of five lysosomal enzyme activities using a cassette of substrates and deuterated internal standards. In a first clinical evaluation, we tested 825 unaffected newborns and 16 patients with LSDs using a multiplexed, multidimensional UHPLC-tandem mass spectrometer. All affected patients were identified accurately and could be differentiated from non-affected newborns. In comparison to previously published two-day assays, which included an overnight incubation, this protocol enabled the detection of lysosomal enzyme activities from sample to first result within half a day. Due to high grade of automation and simplified sample preparation, this assay could be used for urgent clinical diagnostics needed for suspected patients admitted to a hospital, as well as for routine newborn and high-risk population screening.
Keywords: short-incubation, lysosomal storage disorders, multiplex assay, tandem mass spectrometry, high-throughput, newborn screening, TurboFlow chromatography
INTRODUCTION
Lysosomal storage disorders (LSDs) result in the accumulation of macromolecular substrates that would normally be degraded by enzymes involved in lysosomal metabolism. These diseases have a progressive course, and might occur at any age affecting a number of different tissues and organ systems.1 New impetus for the development of diagnostic techniques was acquired by the availability of novel treatment strategies including enzyme replacement, stem cell transplantation and substrate reduction.2 However, clinical diagnostics of LSDs is still a technological challenge, time-consuming, and additional personnel and equipment is needed. In addition to fluorescent methods using 4-methylumbelliferone, efforts have been made to use tandem mass spectrometry (MS/MS) as the method of choice particularly for high-throughput analysis in routine newborn screening laboratories.3 In this context it is mandatory to achieve high laboratory standards in terms of technical proficiency and reproducibility of results; hence quality control materials provided by the Newborn Screening Quality Assurance Program at the Centers for Disease Control and Prevention (CDC, Atlanta, GA) are available.4
Protocols for analyzing lysosomal enzyme activities continuously evolved, and were refined and optimized, but the complexity of sample preparation prior to mass spectrometry including liquid-liquid extraction (LLE), solid phase extraction (SPE), and the handling of hazardous organic compounds such as ethyl acetate, still remains.5, 6 Novel aspects such as online multi-dimensional chromatography prior to flow injection analysis facilitate ease-of-use sample introduction and increased speed of analysis.7, 8 Our research group previously reported the use of TurboFlow™ (short for Turbulent Flow Chromatography) for online sample clean-up to remove matrix interferences such as salts, proteins and detergents for the analysis of lysosomal enzyme activities in DBS.9 Subsequently, purified analytes of interest that were removed from potential matrix interferences were transferred from a TurboFlow column to an analytical column for ultra high performance liquid chromatography (UHPLC) separation prior to MS/MS analysis in order to separate enzymatic products from residual substrate. This simplified protocol has recently been evaluated in a comprehensive pilot screening of more than 8,500 newborns to demonstrate the technical feasibility and robustness.10
Nonetheless, for future implementation of high-throughput LSD assays in routine clinical diagnostics, sample handling and mass spectrometric analysis has to be simplified; specifically, sample pretreatment, speed of analysis and finally detection must become more integrated.11
The aim of this study was to develop a mass spectrometry based protocol to eliminate long incubation times of more than 16 to 20 hours (two-day protocols with an overnight incubation), and to optimize and modify sample-handling prior to MS/MS analysis, to provide rapid and accurate detection of lysosomal enzyme activities within several hours.
EXPERIMENTAL SECTION
Materials and enzymatic assay
Substrates and internal standards were provided by the Newborn Screening Translation Research Initiative, Centers for Disease Control and Prevention, Atlanta, GA, USA. All other chemicals, HPLC-grade acetonitrile, isopropanol, acetone were purchased by Merck Chemicals; formic acid and trifluoroacetic acid from Sigma Aldrich Co. LLC, St. Louis, MO, USA; Cyclone-P™ (0.5×50mm) TurboFlow-columns and Hypersil Gold C8 (1.9µ, 50×2.1mm) columns were purchased from Thermo Fisher Scientific Inc., MA, USA; and microplates 96/U, microplates 96/F and deep well plates by Eppendorf AG, Hamburg, Germany.
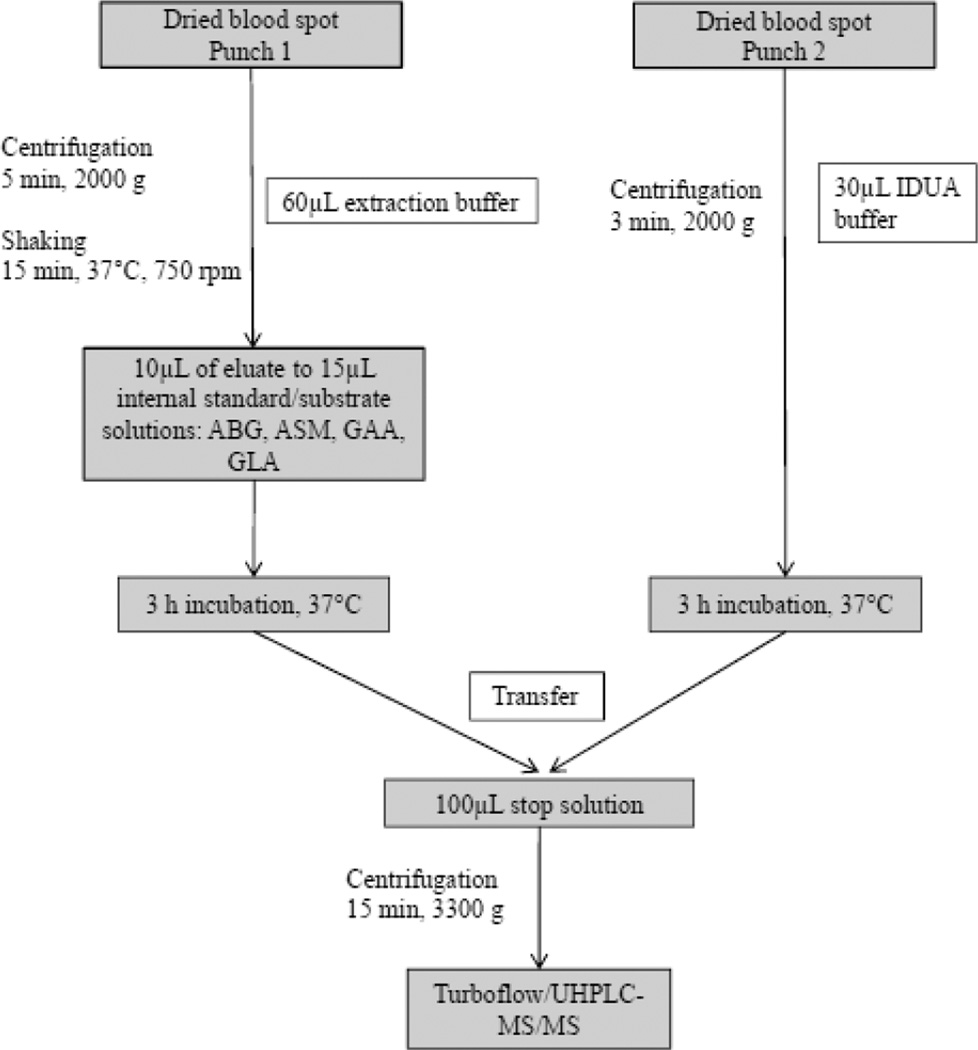
3.2-mm (1/8-inch) punches from DBS of 16 known patients with LSDs and 825 of non-affected newborns were tested in parallel with a new short (three hours) incubation protocol and compared to a previously published method with an overnight incubation of 16 to 20 hours.10 For the detection of all five lysosomal enzyme activities two punches from a DBS card in two separate 96well -plates were needed (Figure 1). Punch 1 was used for the detection of acid β–glucocerebrosidase (ABG; Gaucher disease), acid sphingomyelinase (ASM; Niemann-Pick A/B disease), α–glucosidase (GAA; Pompe disease) and α–galactosidase; GLA; Fabry disease), respectively. Punch 2 was used for α–L–iduronidase (IDUA; mucopolysaccharidosis type I) analysis. The first punch was diluted with 60µl extraction buffer (20 mmol/L sodium phosphate; pH 7.1), and aliquots of 10µL were used to perform separate incubations for ABG, ASM, ABG, GAA and GLA in specific buffer systems containing a cassette of enzyme specific substrates and internal standards. The second punch was directly incubated with 30µl specific IDUA buffer. Incubation was performed at 37°C for three hours. The incubation was stopped by adding 100µL stopping solution (80% acetonitrile plus 0.2% formic acid). All aliquots were transferred in a new deep-well plate covered with aluminum foil and centrifuged at 3000g for 15 min prior to mass spectrometry analysis.
Figure 1.
Optimized workflow for the analyses of up to five lysosomal enzyme activities simultaneously from dried blood spots within four hours.
ABG, acid β–glucocerebrosidase; ASM, acid sphingomyelinase; GAA, α–glucosidase; GLA, α–galactosidase; IDUA, α–L–iduronidase.
TurboFlow/UHPLC-MS/MS analysis
We used a previously described online-sample clean-up UHPLC method for sample analysis.9, 10 A Transcend™ TLX-2 UHPLC system with quaternary pumps was used (Thermo Fisher Scientific). The mobile phases were; A = 0.1% formic acid, 0.01% TFA in water, B = 0.1% formic acid, 0.01% TFA in acetonitrile, C = 45:45:10 isopropanol/acetonitrile/acetone. The sample injection volume was 10 µL. The Transcend system employs two TurboFlow-HPLC channels, each with two six-port valves configured in focusing mode (recovered analytes from the TurboFlow column were transferred and focused on a subsequent UHPLC column for further separation).12 Samples were separated from matrix components during the loading step by TurboFlow chromatography using a Cyclone-P column with aqueous mobile phase. After buffer-salts, proteins and DBS residuals were rinsed away, the valves were switched and the extracted analytes were back-flushed off the TurboFlow column by the contents of a 200µl eluting loop filled with 20:80 mobile phase A/mobile phase B and focused on a Hypersil Gold C8 UHPLC column. All analytes were separated using a linear gradient from 0% to 100% B in 40 seconds.10 The columns were then washed and re-equilibrated for the next injection. The system was operated by Aria™ Software V 1.6.3 (Thermo Fisher Scientific). The total run time for one TurboFlow/UHPLC experiment was 4 min per channel. MS/MS data acquisition started 2.15 min after injection and continued for 90 seconds until all analyte signals were recorded. MS/MS analysis was performed on a TSQ Quantum Ultra™ (Thermo Fisher Scientific) equipped with an HESI-II heated electrospray probe and operated by Xcalibur™ V 2.1.0.1139 (Thermo Fisher Scientific). We used selected-reaction monitoring (SRM) transitions for the five products and their respective internal standards. The amount of product was calculated from the ion abundance ratio of the product to internal standard for a sample multiplied by the amount of added internal standard, divided by the incubation time.
Statistical Analysis
All mass spectrometry data were analyzed with LCquan™ 2.6.0.1128 (Thermo Fisher Scientific). We used SPSS version 16·0 (SPSS Inc,, Chicago, Ill, USA) for data analysis.
RESULTS
Development of a short-incubation protocol
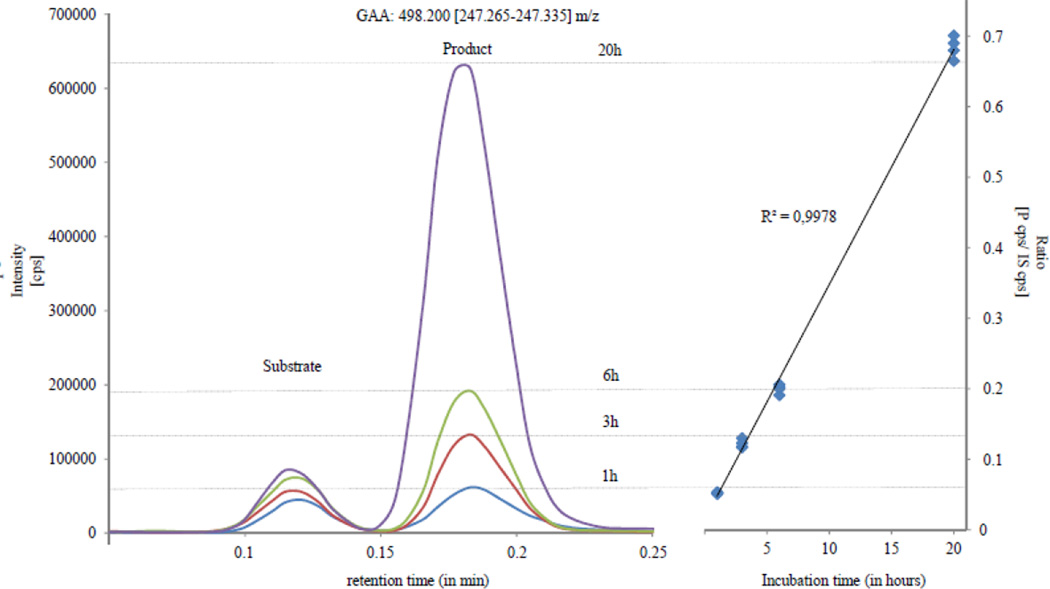
We have optimized the workflow for all pre-analytical steps in terms of sample preparation prior to mass spectrometry analysis. This included centrifugation, sample shaking and liquid handling steps (Figure 1). Using these optimizations, sample preparation time before and after incubation could be reduced to approximately 30 min each (for up to 90 samples plus quality control materials). Different incubation times of one, three, six and 20 hours were tested. Representative data for GAA are displayed in Figure 2, including different incubation times and their respective peak intensities of product versus product/internal-standard ratios. An incubation time of three hours was chosen and revealed coefficients of variation (CVs) of CDC quality control material below 11% (Table 3), and all affected patients could be differentiated from normal newborns (Table 1 and 2). Subsequently, the samples were injected into a multiplexed, multidimensional TurboFlow/UHPLC-MS/MS system. TurboFlow methodology enables the removal of salts, buffers, detergents, residual proteins, endogenous metabolites and significant amounts of un-reacted substrate prior to flow injection MS/MS analysis without the need for time-consuming offline sample preparation such as LLE and SPE. The total time for analysis was four minutes per sample, and with the use of staggered injections (dual-channel TLX-2 system with two TurboFlow and two analytical columns), the effective analysis time per sample was finally reduced to 2 min. In summary, pre-sample treatment (approximately one hour), short-incubation (three hours) and TurboFlow/UHPLC-MS/MS (two min per sample), provided the first results after four hours. Using this protocol, one 96-well plate with around 90 samples including quality controls could be analyzed within seven hours.
Figure 2.
Exemplary data for GAA including different incubation times and their respective peak intensities of product versus product to internal standard ratios.
GAA, α–glucosidase; cps, counts per second; P, product; S, substrate
Table 3.
Quality control samples.
| CDC quality controls (low/medium/high; mean)1 |
Mean (n=20) |
%CV | Slope (novel protocol vs. CDC) | R2 | |
|---|---|---|---|---|---|
| GBA | 0.55 | 0.63 | 7.4 | 1.045 | 0.988 |
| 5.11 | 4.13 | 7.6 | |||
| 9.69 | 9.27 | 6.0 | |||
| ASM | 0.25 | 0.33 | 10.6 | 1.030 | 0.993 |
| 1.60 | 1.45 | 7.0 | |||
| 3.02 | 3.00 | 10.5 | |||
| GAA | 0.75 | 0.82 | 9.5 | 0.850 | 0.998 |
| 6.62 | 7.15 | 6.1 | |||
| 12.89 | 15.06 | 3.3 | |||
| GLA | 0.71 | 0.92 | 9.1 | 1.244 | 0.974 |
| 5.74 | 6.00 | 6.2 | |||
| 11.05 | 9.04 | 10.4 | |||
| IDUA | 0.29 | 0.96 | 10.9 | 1.032 | 0.980 |
| 4.50 | 3.81 | 11.0 | |||
| 9.73 | 9.84 | 7.9 |
in µmol/L/h; Center for Disease Control and Prevention (CDC) batch Set 5; September 2010; all data were background substracted using blank samples (filter paper only)
ABG, β-Glucocerebrosidase; ASM, acid-Sphingomyelinase; GAA α-Glucosidase; GLA, alpha-Galactosidase; IDUA, Iduronidase Alpha-L; CV, coefficient of variation
Table 1.
Enzyme activities of affected patients according to their incubation time.
| Enzyme activity 20h incubation (µmol/L/h) |
Enzyme activity 3h incubation (µmol /L/h) |
Patient statistics 20h incubation (µmol /L/h) |
Patient statistics 3h incubation (µmol /L/h) |
P value 20h vs. 3h |
|
|---|---|---|---|---|---|
| Gaucher Patient 1 | 1.2 | 1.5 | Mean: 0.8 Std.: 0.4 Max: 1.2 Min: 0.2 |
Mean:1.1 Std.: 0.4 Max: 1.5 Min: 0.5 |
0.2 |
| Gaucher Patient 2 | 0.8 | 1.3 | |||
| Gaucher Patient 3 | 0.9 | 1.1 | |||
| Gaucher Patient 4 | 0.7 | 1.0 | |||
| Gaucher Patient 5 | 0.2 | 0.5 | |||
| Fabry Patient 1 (m) | 0.3 | 0.5 | Mean: 1.1 Std.: 0.9 Max: 2.2 Min: 0.3 |
Mean: 1.0 Std.: 1.8 Max: 1.9 Min: 0.4 |
0.9 |
| Fabry Patient 2 (m) | 0.7 | 0.5 | |||
| Fabry Patient 3 (m) | 0.4 | 0.4 | |||
| Fabry Patient 4 (f) | 2.2 | 1.8 | |||
| Fabry Patient 5 (f) | 2.0 | 1.9 | |||
| Pompe Patient 1 | 0.6 | 0.2 | Mean: 0.5 Std.: 0.2 Max: 0.6 Min: 0.2 |
Mean: 0.4 Std.: 0.3 Max: 0.7 Min: 0.1 |
0.6 |
| Pompe Patient 2 | 0.2 | 0.1 | |||
| Pompe Patient 3 | 0.6 | 0.7 | |||
| Pompe Patient 4 | 0.4 | 0.4 | |||
| Niemann Pick A/B Patient | 0.1 | 0.5 | - | - | - |
| Mucopolysaccharidosis type I Patient | 0.1 | 0.1 | - | - | - |
m, male; f, female.
Table 2.
Enzymatic activities (µmol/L/h) of 825 neonates determined by three-hour incubation and Turboflow chromatography method.
| ABG |
ASM |
GAA |
GLA |
IDUA |
|
|---|---|---|---|---|---|
| n | n | n | n | n | |
| mean | 19.6 | 4.4 | 18.6 | 6.4 | 12.4 |
| percentile 0.5% | 6.97 | 1.06 | 5.63 | 2.60 | 3.27 |
| percentile 1.0% | 7.99 | 1.12 | 6.90 | 2.68 | 3.87 |
| percentile 25% | 15.41 | 2.34 | 13.55 | 4.35 | 9.21 |
| median | 18.78 | 3.80 | 17.17 | 5.52 | 11.73 |
| percentile 75% | 23.06 | 5.67 | 22.49 | 7.38 | 14.77 |
| percentile 99% | 39.30 | 13.41 | 43.52 | 18.88 | 27.18 |
| percentile 99.5% | 41.49 | 13.73 | 50.31 | 22.24 | 31.12 |
| min | 5.19 | 1.06 | 4.75 | 2.54 | 2.67 |
| max | 44.02 | 21.34 | 69.25 | 60.02 | 62.72 |
ABG, β-Glucocerebrosidase; ASM, acid-Sphingomyelinase; GAA α-Glucosidase; GLA, α -Galactosidase; IDUA, Iduronidase α-L; nbs, newborns;
Maximum enzyme activity observed among the affected patients divided by the mean activity measured for newborns.
Clinical evaluation
We analyzed a total of 16 patients with known LSDs (four patients with Pompe, five with Gaucher, five with Fabry, one with Niemann-Pick A/B, and one with MPS I disease) for clinical evaluation. We compared the short-incubation with our previous developed reference protocol (16-20 hours incubation).10 In Table 1, a detailed overview of all single enzyme activities using short versus the long incubation time is displayed, and we did not observe any statistical difference between both protocols in this first clinical setup. In addition, we analyzed all five lysosomal enzyme activities in 825 normal non-affected newborns (Table 2). All affected patients could be differentiated from normal newborns in this first pilot study by using the 0.5th percentile of normal newborns as a preliminary cut-off value. Certainly, further studies with a larger number of newborns for cut-off determination are needed. For evaluation, we analyzed quality control materials provided by the CDC. Our results were compared successfully with those provided by the CDC with high coefficients of determination ranging from 0.974 to 0.998 (Table 3). The CVs were in the range from 3.3 to 11.0% (Table 3).
We concluded that this time saving protocol could be used for different clinical areas including selective metabolic screening for suspected patients at risk in a hospital, as well as for newborn or high-risk population screening.
DISCUSSION AND CONCLUSION
Currently, routine newborn screening for LSDs has been introduced for Pompe disease in Taiwan13 and for Krabbe disease in the State of New York.14 The Austrian Newborn Screening center15 and others, e.g. in Washington State6, have successfully started pilot studies using multiplexed MS/MS screening assays.16–18 The aim of this study was to develop a mass spectrometry based protocol for the rapid and accurate detection of lysosomal enzyme activities. Previously published protocols used 16-20 hours incubation time.3, 5, 10 We report the simplification and optimization of pre-analytical sample preparation to decrease the incubation time from 16–20 to three hours.
We used TurboFlow technology for sample clean-up prior to flow injection MS/MS analysis. The proof of concept using this technology was published recently by our research group.9 Moreover, in a first comprehensive clinical evaluation we could demonstrate that the technology is robust and accurate to detect patients with diminished lysosomal enzyme activity and to differentiate them from normal newborns.10 We took advantage of online sample clean-up using multidimensional chromatography that eliminates time-consuming and laborious protocol steps such as LLE and SPE, the use of organic compounds such as ethyl acetate3, 5, 19, and reduces the need for large amounts of consumables. Benefits of using TurboFlow columns were described in a large number of varied analytical environments, drug discovery and pharmacokinetics, metabolite profiling, and clinical applications.12, 20, 29 The combination of both a TurboFlow and an analytical columns, improved the multiplexed enzymatic assay by separating enzymatic products from residual substrates. This is of importance because previously published LSD assays reported the potential interference of the enzyme product signal from excess substrate due to in-source fragmentation.7 The higher resolving power of UHPLC, completely eliminates such interferences while keeping total analysis time to two minutes per sample and facilitates the expansion of the screening panel.
However, one drawback of using MS/MS-based assays for LSDs was the long enzyme incubation time of more than 16 hours that required two-day protocols (with an overnight incubation). We optimized and adapted our previously published protocol and modified the work-flow (Figure 1). This allows the reduction of DBS incubation time with a cassette of substrates and deuterated internal standards from 16 to three hours. Sista and co-workers reported the use of a digital microfluidic platform to perform multiplexed enzymatic analysis using fluorescence with 4-methylumbelliferone within two hours on a small set of samples. However, it was restricted to two LSDs (Pompe and Fabry disease), and still needed evaluation.21 Conventional fluorescence methods usually include incubation times of more than three to 20 hours depending on the respective lysosomal enzyme and sensitivity of the assay.22
One limitation of the current protocol is the use of several buffer systems for different enzymes, and the requirement of a second DBS punch for IDUA due to low enzyme activity. In addition, the lysosomal enzyme activity for galactocerebrosidase (GALC; Krabbe disease) was also reported to be very low9, 23, and thus it was not possible to include this LSD in the current assay. However, novel buffer systems for the combined incubation of more than 6 or 9 enzymes simultaneously are on the horizon including substrates for mucopolysaccharidosis type II, IVA and VI 24–27, and were presented recently by Gelb and his research group.28 These new buffer systems might allow the incubation of several enzymes in one reaction vial, and might also be used for reducing the incubation time.
Our results using the short-incubation assay for Gaucher, Niemann-Pick A/B, Pompe, Fabry and mucopolysaccharidosis type I disease from DBS were in close agreement with previously published standard incubation time (16–20 hours) in our clinical reference laboratory. The mean activities for ABG, ASM, GAA, GLA and IDUA using short-incubation protocol with 3 hour time were similar to those obtained using 16 to 20 hours incubation time. There was clear separation between normal non-affected newborn samples and confirmed affected samples for all five LSDs despite the much lower incubation time.
In conclusion, we successfully demonstrated and evaluated the performance of a multiplexed mass spectrometry-based assay to screen for Pompe, Fabry, Niemann-Pick A/B and Gaucher and mucopolysaccharidosis type I diseases using a short-incubation of three hours. This protocol can be used for selective metabolic screening for patients who are suspected to LSDs, and for newborn and high-risk population screening in both routine and research studies.
ACKNOWLEDGMENT
Support of this work by the Austrian mucopolysaccharidosis research and patient’s organization is gratefully acknowledged. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
REFERENCES
- 1.Beck M. J Inherit Metab Dis. 2001;24(Suppl 2):47–51. doi: 10.1023/a:1012463605992. discussion 45-46. [DOI] [PubMed] [Google Scholar]
- 2.Beck M. IUBMB Life. 2010;62:33–40. doi: 10.1002/iub.284. [DOI] [PubMed] [Google Scholar]
- 3.Li Y, Scott CR, Chamoles NA, Ghavami A, Pinto BM, Turecek F, Gelb MH. Clin Chem. 2004;50:1785–1796. doi: 10.1373/clinchem.2004.035907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Jesus VR, Zhang XK, Keutzer J, Bodamer OA, Muhl A, Orsini JJ, Caggana M, Vogt RF, Hannon WH. Clin Chem. 2009;55:158–164. doi: 10.1373/clinchem.2008.111864. [DOI] [PubMed] [Google Scholar]
- 5.Zhang XK, Elbin CS, Chuang WL, Cooper SK, Marashio CA, Beauregard C, Keutzer JM. Clin Chem. 2008;54:1725–1728. doi: 10.1373/clinchem.2008.104711. [DOI] [PubMed] [Google Scholar]
- 6.Duffey TA, Bellamy G, Elliott S, Fox AC, Glass M, Turecek F, Gelb MH, Scott CR. Clin Chem. 2010;56:1854–1861. doi: 10.1373/clinchem.2010.152009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shushan B. Mass Spectrom Rev. 2010;29:930–944. doi: 10.1002/mas.20295. [DOI] [PubMed] [Google Scholar]
- 8.la Marca G, Casetta B, Malvagia S, Guerrini R, Zammarchi E. Anal Chem. 2009;81:6113–6121. doi: 10.1021/ac900504s. [DOI] [PubMed] [Google Scholar]
- 9.Kasper DC, Herman J, De Jesus VR, Mechtler TP, Metz TF, Shushan B. Rapid Commun Mass Spectrom. 2010;24:986–994. doi: 10.1002/rcm.4496. [DOI] [PubMed] [Google Scholar]
- 10.Metz TF, Mechtler TP, Orsini JJ, Martin M, Shushan B, Herman JL, Ratschmann R, Item CB, Streubel B, Herkner KR, Kasper DC. Clin Chem. 2011;57:1286–1294. doi: 10.1373/clinchem.2011.164640. [DOI] [PubMed] [Google Scholar]
- 11.Annesley T, Majzoub J, Hsing A, Wu A, Rockwood A, Mason D. Clin Chem. 2009;55:1236–1239. doi: 10.1373/clinchem.2009.127522. [DOI] [PubMed] [Google Scholar]
- 12.Grant RP, Cameron C, Mackenzie-McMurter S. Rapid Commun Mass Spectrom. 2002;16:1785–1792. doi: 10.1002/rcm.784. [DOI] [PubMed] [Google Scholar]
- 13.Chien YH, Chiang SC, Zhang XK, Keutzer J, Lee NC, Huang AC, Chen CA, Wu MH, Huang PH, Tsai FJ, Chen YT, Hwu WL. Pediatrics. 2008;122:e39–e45. doi: 10.1542/peds.2007-2222. [DOI] [PubMed] [Google Scholar]
- 14.Orsini JJ, Morrissey MA, Slavin LN, Wojcik M, Biski C, Martin M, Keutzer J, Zhang XK, Chuang WL, Elbin C, Caggana M. Clin Biochem. 2009;42:877–884. doi: 10.1016/j.clinbiochem.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 15.Mechtler TP, Stary S, Metz TF, De Jesus VR, Greber-Platzer S, Pollak A, Herkner KR, Streubel B, Kasper DC. Lancet. 2012;379:335–341. doi: 10.1016/S0140-6736(11)61266-X. [DOI] [PubMed] [Google Scholar]
- 16.Dajnoki A, Fekete G, Keutzer J, Orsini JJ, De Jesus VR, Chien YH, Hwu WL, Lukacs Z, Muhl A, Zhang XK, Bodamer O. Clin Chim Acta. 2010;411:1428–1431. doi: 10.1016/j.cca.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 17.Dajnoki A, Muhl A, Fekete G, Keutzer J, Orsini J, Dejesus V, Zhang XK, Bodamer OA. Clin Chem. 2008;54:1624–1629. doi: 10.1373/clinchem.2008.107722. [DOI] [PubMed] [Google Scholar]
- 18.Legini E, Orsini JJ, Hung C, Martin M, Showers A, Scarpa M, Zhang XK, Keutzer J, Muhl A, Bodamer OA. Clin Chim Acta. 2010 doi: 10.1016/j.cca.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 19.Duffey TA, Bellamy G, Elliott S, Fox AC, Glass M, Turecek F, Gelb MH, Scott CR. Clin Chem. 2010;56(12):1854–1861. doi: 10.1373/clinchem.2010.152009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harlan R, Clarke W, Di Bussolo JM, Kozak M, Straseski J, Meany DL. Clin Chim Acta. 2010;411:1728–1734. doi: 10.1016/j.cca.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 21.Sista RS, Eckhardt AE, Wang T, Graham C, Rouse JL, Norton SM, Srinivasan V, Pollack MG, Tolun AA, Bali D, Millington DS, Pamula VK. Clin Chem. 2011;57:1444–1451. doi: 10.1373/clinchem.2011.163139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Civallero G, Michelin K, de Mari J, Viapiana M, Burin M, Coelho JC, Giugliani R. Clin Chim Acta. 2006;372:98–102. doi: 10.1016/j.cca.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Brockmann K, Turecek F, Scott CR, Gelb MH. Clin Chem. 2004;50:638–640. doi: 10.1373/clinchem.2003.028381. [DOI] [PubMed] [Google Scholar]
- 24.Duffey TA, Sadilek M, Scott CR, Turecek F, Gelb MH. Anal Chem. 2010;82:9587–9591. doi: 10.1021/ac102090v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khaliq T, Sadilek M, Scott CR, Turecek F, Gelb MH. Clin Chem. 2010 doi: 10.1373/clinchem.2010.149880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang D, Eadala B, Sadilek M, Chamoles NA, Turecek F, Scott CR, Gelb MH. Clin Chem. 2005;51:898–900. doi: 10.1373/clinchem.2004.047167. [DOI] [PubMed] [Google Scholar]
- 27.Wang D, Wood T, Sadilek M, Scott CR, Turecek F, Gelb MH. Clin Chem. 2007;53:137–140. doi: 10.1373/clinchem.2006.077263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gelb MH, Scott CR. APHL Newborn Screening and Genetics Testing Symposium. 2011 [Google Scholar]
- 29.Herman J, Di Bussolo J. Turbulent-Flow LC-MS: Applications for Accelerating Pharmacokinetic Profiling and Metabolite Identification. In: Ramanathan R, editor. Mass Spectrometry in Drug Metabolism and Pharmacokinetics. Hoboken, New Jersey: John Wiley &Sons; 2009. [Google Scholar]