Abstract
Setting: Four public district hospitals offering asthma treatment in Gazeera State, Sudan. Incomplete recording of patient data directly affects the quality of asthma care and the evaluation of asthma management programmes.
Objective: To assess the completeness of filling out of treatment cards and accuracy of calculating peak expiratory flow (PEF) for confirming diagnosis and grading severity of asthma.
Design: Cross-sectional audit of asthma treatment cards from asthma centres, 2006–2012.
Results: Of 959 patient cards assessed, completeness ranged from 47% to 98%. Six of 13 variables had an unsatisfactory grade of completeness (<80% complete). Calculated PEF was indicated in 885 (92%) cards, but was correct in only 609 (69%). PEF variability was recorded in 835 (87%) cards, but was correctly calculated in 442 (53%). A scheduled follow-up visit was attended by only 359 (37%) patients, indicating 63% loss to follow-up. Contact telephone numbers were missing from 453 (47%) cards.
Conclusion: This is the first study in Africa to assess the data completeness and integrity of asthma patient cards, identifying important shortcomings. This affects quality of management of asthma patients and programme evaluation. Steps to rectify this situation are urgently needed.
Keywords: operational research, quality, chronic disease, electronic medical records
Abstract
Contexte : Quatre hôpitaux publics de district offrant un traitement de l'asthme dans l'état de Gazeera, Soudan. La saisie incomplète des données relatives aux patients affecte directement la qualité des soins de l'asthme et l'évaluation des programmes de prise en charge.
Objectif : Evaluer l'exhaustivité du remplissage des cartes de traitement et l'exactitude des calculs de débit expiratoire de pointe (DEP) pour la confirmation du diagnostic et l'estimation du degré de gravité de l'asthme.
Schéma : Audit transversal des cartes de traitement de patients asthmatiques dans les centres de prise en charge, de 2006 à 2012.
Résultats : Sur 959 cartes de patients évaluées, l'exhaustivité variait de 47% à 98%. Six variables sur 13 n'étaient pas correctement relevées (<80% d'exhaustivité). Le DEP calculé était indiqué sur 885 (92%) cartes, mais n'était juste que sur 609 (69%) cartes. La variabilité du DEP était notée sur 835 (87%) cartes mais était correctement calculée sur seulement 442 (53%). Seuls 359 (37%) patients ont assisté à leur consultation de contrôle, ce qui signifie que 63% ont été perdus de vue. Il manquait un numéro de téléphone de contact sur 453 (47%) cartes.
Conclusion : Cette première étude africaine d'évaluation de l'exhaustivité des données et de l'intégrité des cartes de traitement des patients asthmatiques a identifié des lacunes importantes. Celles-ci affectent la qualité de la prise en charge des patients asthmatiques et l'évaluation des programmes. Il est urgent de prendre des mesures afin de rectifier ces problèmes.
Abstract
Marco de referencia: Cuatro hospitales distritales del sector público que suministran tratamiento del asma en el estado de Gazeera en Sudán. El registro incompleto de los datos de los pacientes menoscaba directamente la calidad del tratamiento del asma y la evaluación de los programas de atención.
Objetivo: Evaluar el carácter integral del llenado de las tarjetas de tratamiento y la exactitud del cálculo del flujo espiratorio máximo (FEM) al confirmar el diagnóstico de asma y evaluar su gravedad.
Método: Se examinaron las tarjetas de tratamiento de los pacientes asmáticos en los centros especializados del 2006 al 2012.
Resultados: De las 959 tarjetas de tratamiento examinadas, entre 47% y 98% contaban con la información completa de los pacientes. Seis de las 13 variables presentaban un grado de integridad deficiente (menos de 80% de compleción). En 885 tarjetas se había consignado el FEM (92%), pero el cálculo era correcto en solo 609 casos (69%). La variabilidad del FEM se registró en 835 tarjetas (87%), pero su cálculo fue correcto solo en 442 (53%). Solo 359 pacientes (37%) acudieron a una cita de control programada, lo cual corresponde a 63% de pérdidas durante el seguimiento. En 453 tarjetas (47%) faltaba un número telefónico de contacto.
Conclusión: El presente fue el primer estudio de evaluación de la compleción y la integridad de los datos de las tarjetas de tratamiento del asma en África y reveló carencias considerables. Esta situación deteriora la calidad del tratamiento de los pacientes con asma y la evaluación de los programas. Es necesario adoptar con urgencia medidas encaminadas rectificar estas deficiencias.
Asthma is an airway inflammatory disease characterised by recurrent attacks of breathlessness and wheezing, and is caused by hypersensitivity of nerve endings in the bronchial tree of the lungs.1 Asthma affects 325 million people around the globe; one in 250 deaths worldwide is attributed to this condition.2 Most asthma-related deaths are preventable with effective treatment. The disease is a growing problem in Africa, and prevalence rates range from 9% to 20%.2 In Sudan, an East African country, asthma is the third major cause of hospitalisation after pneumonia and malaria, and public hospitals have seen a striking increase in the number of emergency visits by asthma patients, from 20 000 in 1998 to 106 000 in 2004 (a more than five-fold increase).3
Since 2006, the Epidemiological Laboratory (EPI-LAB), a non-governmental, non-profit research centre based in Khartoum, Sudan, has been implementing an asthma control programme in Khartoum and Gazeera States in collaboration with the Ministry of Health, the World Health Organization (WHO) and the International Union Against Tuberculosis and Lung Disease (The Union).4
Asthma severity is judged by the degree of impairment of airflow through the airways, assessed by measuring the peak expiratory flow (PEF), i.e., the maximum amount of air that can be expelled from the lung per minute. PEF can be measured in any clinical setting by using a simple hand-held device, called a peak flow meter. One of the cornerstones of the assessment of asthma severity and disease management is the correct and complete recording of patient data (including PEF) on patient master cards.5 Complete recording of such data is also vital for quarterly cohort reporting and for evaluating overall programme performance.5
No formal assessment of asthma patient monitoring has been made at programme level in Sudan. Anecdotal evidence suggests that patient cards are not being filled out correctly, and this has patient and programmatic implications.
We assessed 1) the completeness of filling out treatment cards and 2) the accuracy of PEF calculations for confirming asthma diagnosis and grading severity in four public health centres offering asthma treatment in Gazeera State, Sudan.
METHODS
Study design
A cross-sectional audit of asthma treatment cards.
Study setting
Sudan is a low-income country in East Africa with an estimated population of 27 million. Gazeera State in central Sudan covers an area of 27 549 million km2, and has a population of 3.5 million. The state is divided into seven districts, with one teaching hospital, seven district hospitals and 45 health centres. Each of the district hospitals has a health centre that offers services dedicated to asthma, supported by EPI-LAB. The present study involved four EPI-LAB asthma centres located at the four largest district hospitals, Kamleen, Hashesa, Mangel and Madani.
Study population
All asthma treatment cards for the period June 2006 to December 2012 were included in the study, which was conducted between March and December 2013.
Asthma EPI-LAB centres
The EPI-LAB centres are staffed by medical officers and general practitioners who provide asthma care according to The Union asthma guidelines.6 Briefly, patients who arrive at an asthma health centre are registered and given an asthma patient card before receiving any treatment. The asthma cards are filled out directly by the attending clinician after the patient has undergone clinical assessment. The cards are kept in lockable filing cabinets at each centre and are readily accessible. There are no dedicated nurses in the centres.
Clinical status and PEF are used to assess lung function as shown in Figure 1. Management is then tailored according to disease severity. Patients are required to return for follow-up visits at least once every 3 months. At each follow-up, asthma severity is re-assessed and treatment is adjusted accordingly. All health staff involved in asthma control in the four district hospitals have undergone training in various aspects of asthma management. Quarterly visits and refresher training are provided by both EPI-LAB and the Ministry of Health. There is a dedicated EPI-LAB asthma coordinator and a Gazeera State asthma coordinator who collaborate to ensure regular supervision.
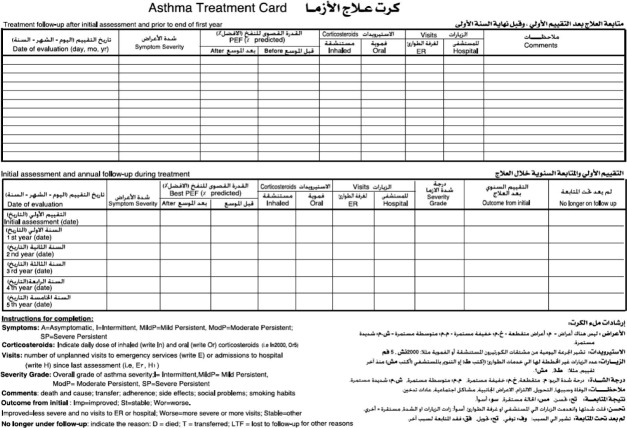
FIGURE 1.
Side one asthma treatment card, Gazera State, Sudan, 2006–2012.
Completeness of treatment cards
Completeness of data was verified for the following indicators from the patient card (Figures 1 and 2): age, sex (used to calculate predicted PEF), contact details (patient, neighbour, next of kin, telephone numbers), predicted PEF, PEF measured before and after bronchodilator use, the calculated PEF percentage, confirmation of diagnosis and severity and treatment prescribed at the initial visit. For the follow-up visits, data collected included whether the patient had attended the visit and data on severity, treatment prescribed and emergency visits/hospitalisation.
FIGURE 2.
Side two asthma treatment card, Gazera State, Sudan, 2006–2012.
Accuracy of PEF calculations for grading severity and confirming diagnosis
The highest value (obtained before and after bronchodilator use) in relation to the predicted PEF (the PEF of a given person in relation to age and sex), termed the ‘best PEF’, was used to determine asthma severity and the line of management. PEF variability was used to confirm asthma diagnosis. The method used to calculate this is shown in Figure 1.
Based on the PEF measures entered on the treatment cards by the health care provider, we re-calculated the best PEF (used to grade severity) and PEF variability (required to confirm the asthma diagnosis) to evaluate the accuracy of the PEF calculations.
Data collection and analysis
Data were collected from the asthma treatment cards and electronically captured using EpiData software (version 3.1, EpiData Association, Odense, Denmark). Data entry was validated by comparing data from 10% of the patient cards that have been randomly selected. The completeness of data was graded according to the following pre-defined criteria: good (filled out in >95% of cases); satisfactory (80–95% complete); unsatisfactory (<80% complete). Correctness of PEF calculation was graded using the same cut-offs. These cut-offs were set in-house as there is currently no reference standard. Descriptive analysis was performed by calculating frequencies and percentages.
Ethics approval
Ethics approval was obtained from the EPI-LAB Ethics Committee of the University of Science and Technology, Khartoum, Sudan. This study met the Médecins Sans Frontières (Geneva, Switzerland) Ethics Review Board-approved criteria for analysis of routinely collected programme data. It also satisfied the requirements of the Ethics Advisory Group of The Union, Paris, France.
RESULTS
During the study period, a total of 959 registered asthma patient cards were assessed for completeness (Table 1). The completeness of recording for the nine variables assessed at the first patient visit ranged from 47% to 98%. Three of the nine variables had an unsatisfactory grade (<80%) of completeness. Although unrecorded information was the main problem, 99 (10%) cards had at least one variable with unreadable information. Only 453 (47%) treatment cards had a telephone contact number. The PEF calculated was indicated on 885 (92%) patient cards and was graded as satisfactory. However, the calculation was correct in only 609 (69%) cases. Errors in calculation led to failure to diagnose 15 patients as severe asthma (underdiagnosis).
TABLE 1.
Completeness of baseline key information on asthma treatment cards, Gazeera State, Sudan, 2006–2012 (n = 959)
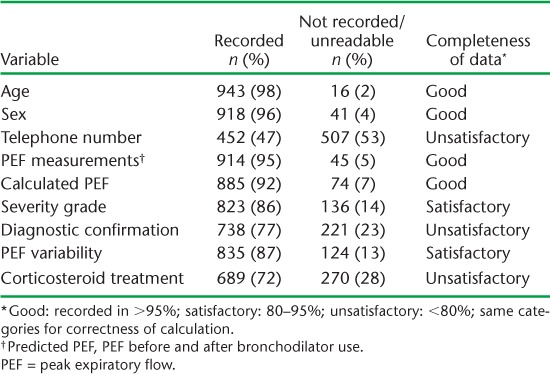
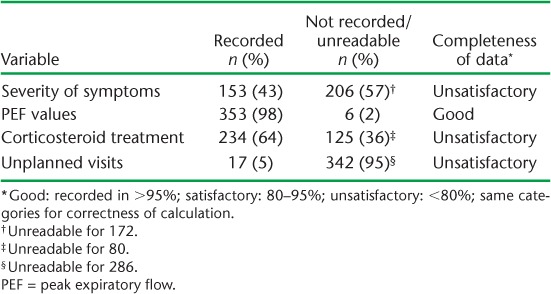
Similarly, although PEF variability was indicated satisfactorily in 835 (87%) patient cards, the calculation was correct in only 442 (53%). Table 2 shows the completeness of follow-up data. A scheduled follow-up visit was attended by only 359 (37%) patients, indicating 63% loss to follow up. The four variables assessed for completeness of recording at the follow-up visit ranged from 5% to 98%. Three (75%) of the four variables had an unsatisfactory grade of completeness. Unreadable notes were common, with 299 (83%) cards having at least one variable with unreadable information.
TABLE 2.
Completeness of key follow-up information on asthma treatment cards, Gazeera State, Sudan, 2006–2012 (n = 359)
DISCUSSION
This is the first study in Africa to assess the integrity of asthma-related data recorded on patient cards. In four district hospitals in Sudan, about 6/13 variables on the patient cards were deemed unsatisfactory in terms of data completeness. In addition, correctness of PEF calculations was only 53% for confirming an asthma diagnosis and 69% for ‘best PEF’, which is vital for judging disease severity and the line of management. Incomplete data recording directly affects the quality of patient care and and seriously compromises the ability to evaluate programme performance. It thus has important implications.
The strengths of this study are that the study sample was large, the majority of the variables included in the patient card were assessed for completeness, and data came from district hospital settings and thus likely reflected ground reality. Data entry was performed by trained data entry staff and validated. In addition, we followed STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines for observational studies, including ethics.7,8 A study limitation is that we did not specifically investigate the possible reasons for incomplete data recording or assess for varying trends over the approximately 6-year study period. Furthermore, the cutoffs used to classify data completeness were set in-house, as there is no reference standard. The standards we have used might thus be deemed too high or low.
A number of study findings merit discussion. First, 6/10 patients didn't return for the scheduled follow-up visit at month 3 and were lost to follow-up. The fact that patient contact information was recorded in only 47% of patient cards is thus of operational concern, as this hampers attempts to trace such patients. The importance of recording contact information should be emphasised to patient registration clerks. Another study from Sudan, focusing on severe cases, revealed a loss to follow-up of 56%,9 and another from Benin reported 36% loss to follow-up.10 These findings suggest the need for more effective tracing strategies for asthma patients on follow-up.
Second, an issue of serious concern is the high error rate of PEF calculations. Anecdotal evidence from site visits revealed that none of the centres had functional calculators. This may have led to busy clinicians resorting to manual calculations, which are more prone to error. A possible solution is to introduce real-time computer or touch-screen electronic patient cards, as is currently being used for chronic disease management in health facilities in Malawi11,12 and Jordan.13 PEF calculations could then be automated, which would limit errors. Electronic patient cards (EPC) would also address the problem of illegible writing, a common problem identified in this study. The EPC can be preset to ensure compulsory and complete data entry. Such a system can also allow bar coding on patient identity cards, which would provide the added advantage of allowing quick access to individual patient information at any of the asthma centres to which a registered patient may present. Specific training to improve the understanding and recording of PEF calculations is required.
Third, only 7/10 patient cards recorded whether or not corticosteroid treatment had been prescribed. Such information is vital to be able to project drug consumption and avoid stock ruptures. This is particularly relevant for corticosteroids, which are essential for those with severe asthma in whom morbidity and mortality are likely to be highest.
Finally the asthma centres face staffing shortages, with only one attending general practitioner and no dedicated nurses or support staff. Even the general practitioners, although theoretically dedicated to asthma centres, cover several additional clinical responsibilities in various hospital wards. The storage infrastructure for patient cards also varied between centres; this may affect organisation and access to cards. Anecdotal evidence suggests that there are delays associated with finding cards before patients can be seen by clinicians. Undue delays that add to patient waiting times may affect the acceptability of a centre and influence loss to follow-up rates. This merits specific evaluation. The various short-comings highlight the need for rigorous quarterly supervision and targeted training, with particular attention to data quality.
In conclusion, we have identified important shortcomings in the integrity and completeness of recorded asthma patient data in Sudan. This has a bearing on the quality of asthma patient management as well as on the evaluation of programme performance; steps to rectify this situation are urgently needed.
Acknowledgments
This research was supported through an operational research course that was jointly developed and run by the Operational Research Unit (Luxembourg), Médecins Sans Frontières (MSF), Brussels-Luxembourg, The Centre for Operational Research, International Union Against Tuberculosis and Lung Disease (The Union), Paris, France, and The Union South-East Asia Regional Office, New Delhi, India.
Additional support for running the course was provided by the Centre for International Health, University of Bergen, Bergen, Norway; the University of Nairobi, Nairobi, Kenya and Partners In Health, Kigali, Rwanda. The course was conducted under the umbrella of the World Health Organization (WHO-TDR) SORT-IT programme (Structured Operational Research and Training Initiative) for capacity building in low- and middle-income countries. The Epidemiological Laboratory, Khartoum, Sudan, supported this study by providing manpower, transportation and logistics assistance for data collection and management. The Ministry of Health, Wad Madani, Gazeera State also provided invaluable support by providing access to health centre records and database. Funding for the course was provided by MSF Luxembourg, Brussels Operational Centre, Luxembourg; the Bloomberg Philanthropies, New York, NY, USA; and the Department for International Development, London, UK. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflicts of interest: none declared.
References
- 1.International Union Against Tuberculosis and Lung Disease. The Global Asthma Report 2011. Paris, France: International Union Against Tuberculosis and Lung Disease; 2011. [Google Scholar]
- 2.van Gemert F, van der Molen T, Jones R, Chavannes N. The impact of asthma and COPD in sub-Saharan Africa. Prim Care Respir J. 2011;20:240–248. doi: 10.4104/pcrj.2011.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Federal Ministry of Health. Annual Statistical Report. Khartoum, Sudan: Federal Ministry of Health; 2007. [Google Scholar]
- 4.El Sony A. The burden of HIV co-infection to health services in Sudan. Oslo, Norway: University of Oslo; 2005. [PhD Thesis] [Google Scholar]
- 5.Aït-Khaled N, Enarson D A, Bencharif N et al. Treatment outcome of asthma after one year follow-up in health centres of several developing countries. Int J Tuberc Lung Dis. 2006;10:911–916. [PubMed] [Google Scholar]
- 6.International Union Against Tuberculosis and Lung Disease. Management of asthma: a guide to the essentials of good clinical practice. Paris, France: International Union Against Tuberculosis and Lung Disease; 2008. [PubMed] [Google Scholar]
- 7.von Elm E, Altman D G, Egger M et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLOS MED. 2007;4:e296. doi: 10.1371/journal.pmed.0040296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edginton M, Enarson D, Zachariah R et al. Why ethics is indispensable for good-quality operational research. Public Health Action. 2012;2:21–22. doi: 10.5588/pha.12.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El Sony A I, Chiang C-Y, Malik E et al. Standard case management of asthma in Sudan: a pilot project. Public Health Action. 2013;3:247–252. doi: 10.5588/pha.13.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ade G, Gninafon M, Tawo L et al. Management of asthma in Benin: the challenge of loss to follow-up. Public Health Action. 2013;3:76–80. doi: 10.5588/pha.12.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allain T J, van Oosterhout J J, Douglas G P et al. Applying lessons learnt from the ‘DOTS’ Tuberculosis Model to monitoring and evaluating persons with diabetes mellitus in Blantyre, Malawi. Trop Med Int Health. 2011;16:1077–1084. doi: 10.1111/j.1365-3156.2011.02808.x. [DOI] [PubMed] [Google Scholar]
- 12.Douglas G P, Gadabu O J, Joukes S et al. Using touchscreen electronic medical record systems to support and monitor national scale-up of antiretroviral therapy in Malawi. PLOS MED. 2010;7:pii, e1000319. doi: 10.1371/journal.pmed.1000319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khader A, Farajallah L, Shahin Y et al. Cohort monitoring of persons with hypertension: an illustrated example from a primary healthcare clinic for Palestine refugees in Jordan. Trop Med Int Health. 2012;17:1163–1170. doi: 10.1111/j.1365-3156.2012.03048.x. [DOI] [PubMed] [Google Scholar]