Abstract
Prevention of mother-to-child transmission ‘Option B+’ originated in Malawi in 2011 to prevent new infections in infants exposed to the human immunodeficiency virus (HIV). We assessed 12-month programme retention and HIV testing uptake among infants born to HIV-infected mothers from September 2011 to June 2012 in Thyolo District Hospital. Of 513 infants, 368 (71.7%) remained in care at 12 months. Altogether, 412 (80.3%) underwent HIV DNA polymerase chain reaction testing, with 267 (52.0%) tested at 6–12 weeks, and 255 (49.7%) underwent rapid HIV testing, with 144 (28.1%) tested at 12 months. Eighty-eight (17.2%) infants had both tests as scheduled. Measures are needed to improve adherence to national testing protocols.
Keywords: HIV guidelines, children's health, HIV testing, operational research, PMTCT
Abstract
L'option B+ de la prévention de la transmission mère-enfant a débuté au Malawi en 2011 afin de prévenir de nouvelles infections chez les enfants exposés au virus de l'immunodéficience humaine (VIH). Nous avons évalué un programme de 12 mois de rétention et de réalisation du test VIH parmi les bébés nés de mères VIH-positives de septembre 2011 à juin 2012 à l'hôpital de district de Thyolo. Sur 513 nourrissons, 368 (71,7%) sont restés en soins pendant 12 mois. Au total, 412 bébés (80,3%) ont bénéficié d'une recherche de VIH par ADN-PCR ; 267 (52%) ont été testés entre 6 et 12 semaines et 255 (49,7%) ont eu un test rapide, dont 144 (28,1%) testés à 12 mois. Quatre-vingt-huit bébés (17,2%) ont eu deux tests comme prévu. Il est nécessaire d'améliorer l'adhésion aux protocoles nationaux de dépistage.
Abstract
En el 2011 se puso en marcha en Malawi la estrategia Opción B+ de prevención de la transmisión maternoinfantil, con el fin de evitar nuevas infecciones por el virus de la inmunodeficiencia humana (VIH) en los lactantes expuestos. Se evaluó durante un período de 12 meses, entre septiembre del 2011 y junio del 2012, la fidelización al programa y la práctica de la prueba diagnóstica del VIH a los lactantes de madres infectadas por el VIH en el Hospital Distrital de Thyolo. De los 513 lactantes expuestos atendidos durante el período del estudio, 368 continuaban en el programa a los 12 meses (71,7%). En total, se practicó la prueba del VIH mediante la reacción en cadena de la polimerasa (PCR-ADN) a 412 lactantes (80,3%); en 267 niños la prueba se realizó entre las 6 y las 12 semanas de edad (52,0 %). La prueba serológica rápida del VIH se practicó en 255 lactantes (49,7%) y en 144 de estos casos a los 12 meses (28,1%). Ochenta y ocho niños recibieron ambas pruebas, en conformidad con las pautas del programa. Es preciso adoptar medidas encaminadas a mejorar el cumplimiento de los protocolos nacionales en materia de pruebas diagnósticas del VIH.
In 2012, 260 000 new cases of human immunodeficiency virus (HIV) infection in children occurred in sub-Saharan Africa, primarily due to mother-to-child transmission of HIV.1 However, childhood HIV infection can be prevented by the implementation of prevention of mother-to-child transmission (PMTCT) strategies.
PMTCT ‘Option B+’ originated in Malawi in July 2011.2–5 With Option B+, all pregnant and breastfeeding women are offered HIV testing and counselling, and those who are HIV-infected are offered life-long antiretroviral treatment (ART) with tenofovir/lamivudine/efavirenz.2,6 HIV-exposed infants receive nevirapine from birth until 6 weeks of age.2,6 National guidelines recommend that all HIV-exposed infants undergo HIV DNA polymerase chain reaction (PCR) testing 6–8 weeks after birth and rapid HIV testing (RHT) at 12–24 months of age, and that those with HIV infection be started on ART immediately.4,6
Option B+ was endorsed by the World Health Organization (WHO) in 2012 and included in the 2013 WHO ART guidelines.3 By June 2013, 588 health facilities were offering Option B+ in Malawi.7
A seven-fold increase in the number of women initiated on ART in the first year of Option B+ in Malawi was documented, with 77% retained in care at 12 months.8 More evidence, however, is needed on the management of HIV-exposed infants under routine conditions of Option B+ implementation.2,3,9 The aim of this study was to evaluate programme retention and uptake and timing of HIV testing among infants born to HIV-infected mothers enrolled in Option B+ at Thyolo District Hospital (TDH).
ASPECTS OF INTEREST
This was a retrospective study of routinely collected programme data. At TDH, the Ministry of Health (MoH) introduced Option B+ in October 2011, with support from Médecins Sans Frontières (MSF). Information on infants enrolled in Option B+ was abstracted from patient cards and entered into an MS Access® (Microsoft Corp, Redmond, WA, USA) database. All infants born at TDH to women in the Option B+ programme from October 2011 to June 2012 were included in the study. A descriptive analysis was performed using EpiData version 2.2.2.182 (EpiData Association, Odense, Denmark) to assess programme retention at 12 months and the uptake and timing of HIV testing.
Ethics approval for the study was granted by the Malawi National Health Sciences Research Committee, the MSF Ethics Review Board, and the Ethics Advisory Group of the International Union Against Tuberculosis and Lung Disease, Paris, France.
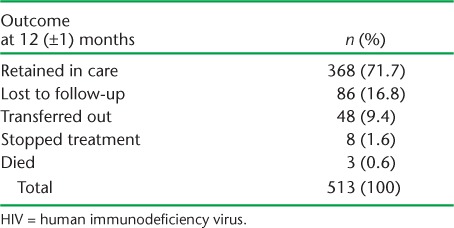
There were 513 infants, of whom 277 (53.9%) were female. Programme retention at 12 (±1) months is shown in Table 1. Altogether, 368 (71.7%) infants were retained in care at TDH, 86 (16.8%) were lost to follow-up, 48 (9.4%) were transferred out and 4 (0.8%) died.
TABLE 1.
12-month outcomes in children born to HIV-infected mothers in Thyolo District Hospital, Malawi, October 2011–June 2012
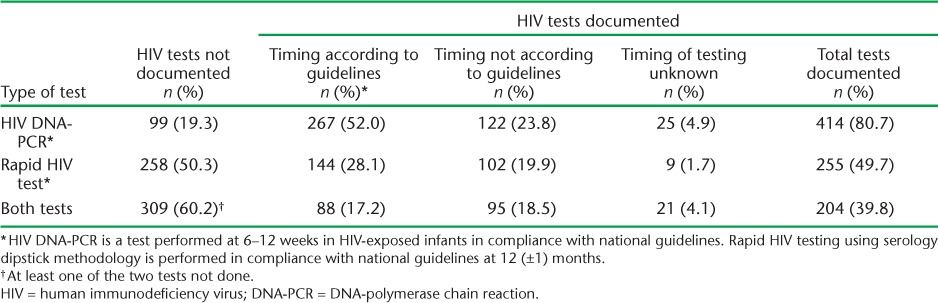
Uptake of HIV testing is shown in Table 2. Of the 513 infants, 412 (80.7%) had a record of PCR testing, with 267 (52.0%) tested at 6–12 weeks; 255 (49.7%) had a record of RHT, with 144 (28.1%) tested at 12 (±1) months. Eighty-eight (17.2%) infants received both tests as specified in the national guidelines.
TABLE 2.
HIV testing performed according to national guidelines in children born to HIV-infected mothers in Thyolo District Hospital, Malawi, October 2011–June 2012
Twenty-one (4.1%) infants were documented as HIV-infected, and all had been started on ART by the age of 12 months. Of the 21 infants, HIV infection was diagnosed by PCR in 13 (2.5%) and by RHT in eight (1.5%).
DISCUSSION
This is the first study to report on 12-month programme retention and adherence to HIV testing recommendations in infants enrolled in Option B+ in TDH. Over 70% of children remained in care at 12 months, but <20% of infants were recorded as having received both HIV tests at specified times. There is therefore room for improvement in the uptake of PCR testing at 6–12 weeks and RHT at 12 months. An ongoing Option B+ programme evaluation in Thyolo District found that between April and June 2013, 80% of infants born to HIV-infected mothers who had enrolled in Option B+ during pregnancy had undergone PCR testing between 5 and 13 weeks.5
More emphasis is needed to address the programmatic challenges that prevent health providers from offering HIV testing to infants according to protocol. This includes building human resource capacity, addressing health staff and testing kit shortages, ensuring that no opportunities for testing are missed, that all tests are recorded on the infant′s clinic card at the time of sample collection, and follow-up of unreported test results with the laboratory. In addition, qualitative research would help to identify reasons for lack of adherence to HIV testing protocols so that current deficiencies can be addressed and to understand mothers′ barriers to accessing Option B+.
A limitation of this study is the completeness of routinely collected programme data: 236 results of PCR testing at 6–8 weeks were not captured. It was not possible to ascertain whether infants without PCR results were not tested, were tested but the result not received, or were tested and the result was received but not documented. In addition, the HIV status of the 125 infants who were transferred to another facility or lost to follow-up before the age of 12 months was not documented. Registers to log infant PCR results were introduced nationally by the MoH at the beginning of 2013. As these registers only record test results and do not identify infants due for PCR testing or those awaiting test results, additional measures are needed to ensure that all HIV-exposed infants are tested.
Option B+ was conceived with the vision of eliminating new cases of HIV infection among children. Early results show adequate uptake and retention of pregnant and breastfeeding women in Option B+,8,9 and this study shows good results for programme retention among HIV-exposed infants. It is likely that HIV infections in infants will decrease and that follow-up of exposed infants will improve over time, as, by removing the gating CD4 step from the HIV care cascade, more mothers will receive ART.2,10 It is important to perform HIV testing according to schedule, to keep a record of all specimens sent for PCR testing to the Central Laboratory in Queen Elizabeth Hospital in Blantyre, and to follow up on missing results. It would also be useful to conduct further research to ascertain the reasons for the high proportion of infants with missing HIV test results, and the reasons for loss to follow-up.
Acknowledgments
The authors are greatly indebted to all persons from Médecins Sans Frontières (MSF) and from Thyolo District Hospital involved in collecting data in the field to make the preparation of this study possible.
This research was supported through an operational research course that was jointly developed and run by the Operational Research Unit (LUXOR), Médecins Sans Frontières, Brussels Operational Centre, Luxembourg; the Centre for Operational Research, International Union Against Tuberculosis and Lung Disease (The Union), Paris, France; and The Union South-East Asia Office, Delhi, India. Additional support for running the course was provided by the Institute for Tropical Medicine, Antwerp, Belgium; the Centre for International Health, University of Bergen, Bergen, Norway; the University of Nairobi, Nairobi, Kenya, and Partners In Health, Rwanda. This course is under the umbrella of the World Health Organization (WHO-TDR) SORT IT (Structured Operational Research and Training Initiative) programme for capacity building in low- and middle-income countries.
Funding for the course was provided by MSF Luxembourg, Brussels Operational Centre, Luxembourg, the Bloomberg Philanthropies and the Department for International Development (DFID), UK. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflict of interest: none declared.
References
- 1.UNAIDS. Global Report: UNAIDS report on the global AIDS epidemic 2013. Geneva, Switzerland: WHO; 2013. UNAIDS/JC2502/1/E. http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2013/gr2013/UNAIDS_Global_Report_2013_en.pdf Accessed April 2014. [Google Scholar]
- 2.World Health Organization. Programmatic update. Use of antiretroviral drugs for treating pregnant women and preventing HIV infection in infants. Geneva, Switzerland: WHO; April 2012. WHO/HIV/2012.6. http://www.who.int/hiv/pub/mtct/programmatic_update2012. Accessed April 2014. [Google Scholar]
- 3.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Recommendations for a public health approach. Geneva, Switzerland: WHO; June 2013. http://apps.who.int/iris/bitstream/10665/85321/1/9789241505727_eng.pdf Accessed April 2014. [PubMed] [Google Scholar]
- 4.Schouten E J, Jahn A, Midiani D et al. Prevention of mother-to-child transmission of HIV and the health-related Millennium Development Goals: time for a public health approach. Lancet. 2011;378:282–284. doi: 10.1016/S0140-6736(10)62303-3. [DOI] [PubMed] [Google Scholar]
- 5.Coulborn R M, Triviño Durán L, Metcalf C Preliminary findings of a PMTCT Option B+ program in a rural district in Malawi. Lilongwe, Malawi: Malawi Med J; 2013. Presented at Malawi College of Medicine, National AIDS Commission Research Dissemination Conference. Towards 2015 successes and challenges of health and HIV/AIDS research in the context of Millennium Development Goals, 22–23 Nov 2013. http://www.medcol.mw/mmj/?p=1823 Accessed April 2014. [Google Scholar]
- 6.Government of Malawi, Ministry of Health. Integrated HIV Program Report April – June 2013. Lilongwe, Malawi: MoH; 2013. [Google Scholar]
- 7.Ministry of Health, Malawi. Clinical management of HIV in children and adults. 1st ed. Lilongwe, Malawi: MoH; 2011. http://www.who.int/hiv/pub/guidelines/malawi_art.pdf Accessed April 2014. [Google Scholar]
- 8.Chimbwandira F, Mhango E, Makombe S et al. Impact of an innovative approach to prevent mother-to-child transmission of HIV — Malawi, July 2011–September 2012. Centers for Disease Control and Prevention. MMWR (Morb Mortal Wkly Rep) 2013;62:148–151. [PMC free article] [PubMed] [Google Scholar]
- 9.Tenthani L, Haas A, Tweya H et al. Retention in care under universal antiretroviral therapy for HIV-infected pregnant and breastfeeding women (‘Option B+’) in Malawi. AIDS. 2014;28:589–598. doi: 10.1097/QAD.0000000000000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.UNICEF/Business Leadership Council. A business case for Options B and B+. To eliminate mother to child transmission of HIV by 2015. New York, NY, USA: UNICEF; 2012. http://www.unicef.org/aids/files/DISCUSSION_PAPER.A_BUSINESS_CASE_FOR_OPTIONS_B.pdf Accessed April 2014. [Google Scholar]


