1. Introduction
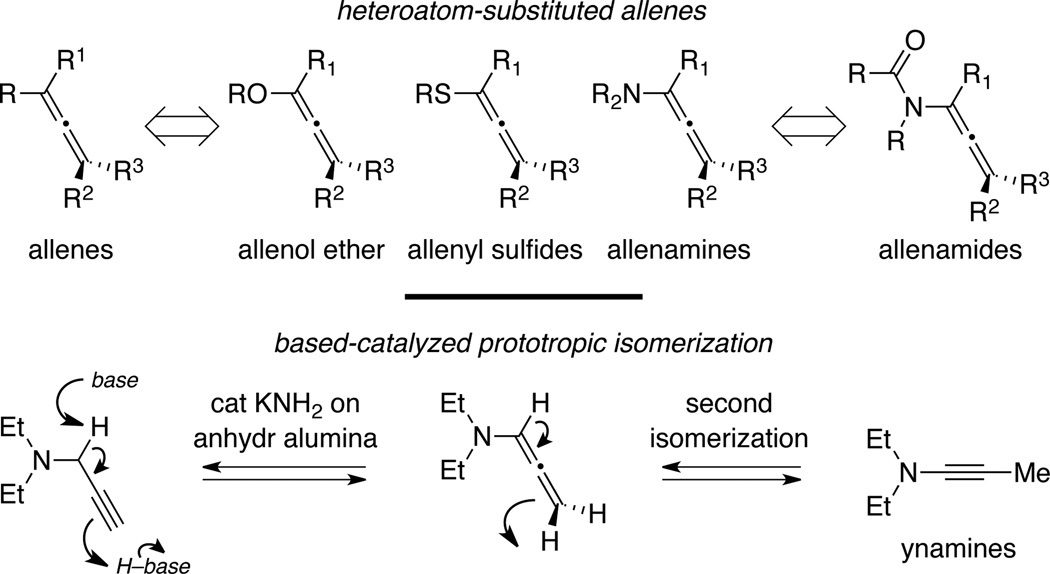
In the past four decades, allenes have progressively risen from an unenviable status of being a structural curiosity to becoming one of the most powerful and versatile synthetic building blocks in organic synthesis.1–3 Although the focal theme of this review is centered on chemistry of allenamides, a proper introduction would need to commence with allenamines. Allenamides are functionally derived from allenamines,4 which along with structurally related systems such as allenol ethers5 and allenyl sulfides,6 can be classified as heteroatom-substituted allenes. Allenamines have been known for more than forty years since the first documentation of their preparations and characterizations in 1968 by Viehe.7 It is noteworthy that Viehe was at the time developing a based-catalyzed isomerization of propargyl amines as a useful protocol for synthesizing ynamines (Scheme 1), which had just come onto the scene as a useful synthetic building block.8–10 Allenamines were postulated as an intermediate en route to ynamines in this prototropic isomerization that follows essentially the zipper-type mechanism.
Scheme 1.
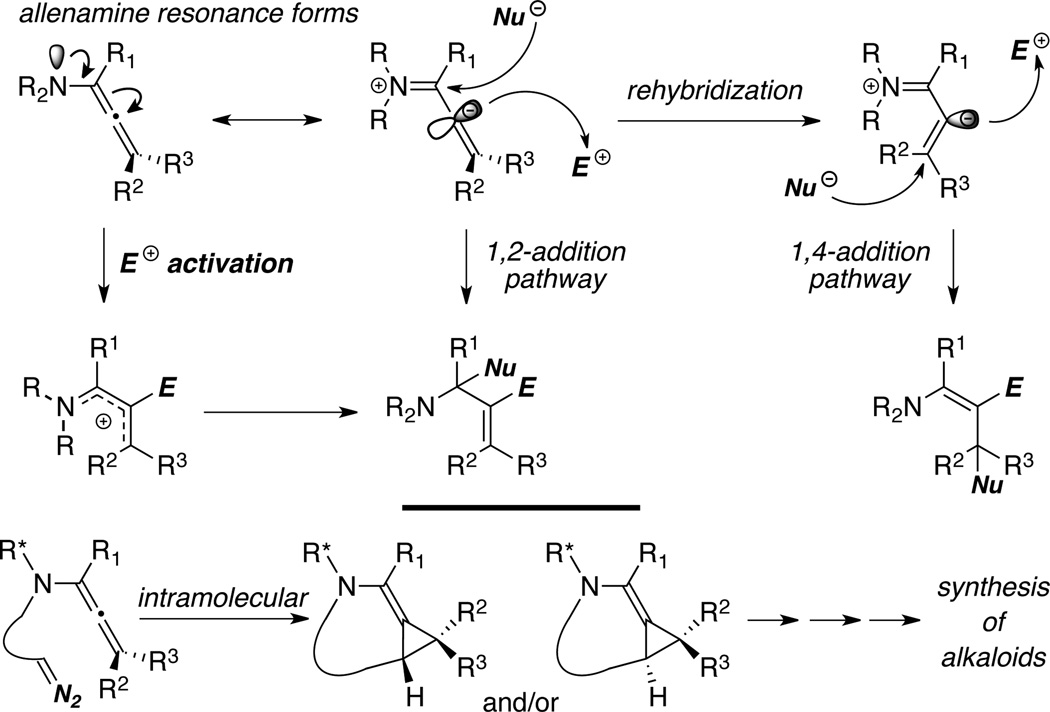
The π-donating ability of nitrogen atom renders allenamines more electron-rich than simple allenes, thereby predisposing them to electrophilic activations. An electronic bias can be exerted through delocalization of the nitrogen lone pair toward the allenic moiety as demonstrated in the resonance form of allenamines. Accordingly, highly regioselective transformations can be achieved with consecutive addition of electrophiles and nucleophiles (Scheme 2). In addition to aforementioned regiochemical control, allenamines also offer a number of other advantages over simple allenes. The trivalent nature of the nitrogen atom allows: (1) Tethering of a chirality-inducing unit for providing stereochemical induction; concomitantly with the inclusion of a coordinating unit to provide conformational rigidity; (2) a much greater flexibility in designing intramolecular reactions or tandem processes than with oxygen- or sulfur-substituted allenes; and last but not the least, (3) a novel entry to alkaloids if the nitrogen atom can be preserved throughout the entire transformative sequence. Moreover, intramolecular reaction manifolds as shown with a possible diastereoselective cyclopropanation reaction (Scheme 2) can greatly manifest these remarkable features, particularly the latter two. Therefore, while the chemistry of other heteroatom-substituted allenes is of high impact and value to organic synthesis, allenamines should prove to be more attractive for developing stereoselective methodologies as well as rapid assembly of structural complexity.1,2
Scheme 2.
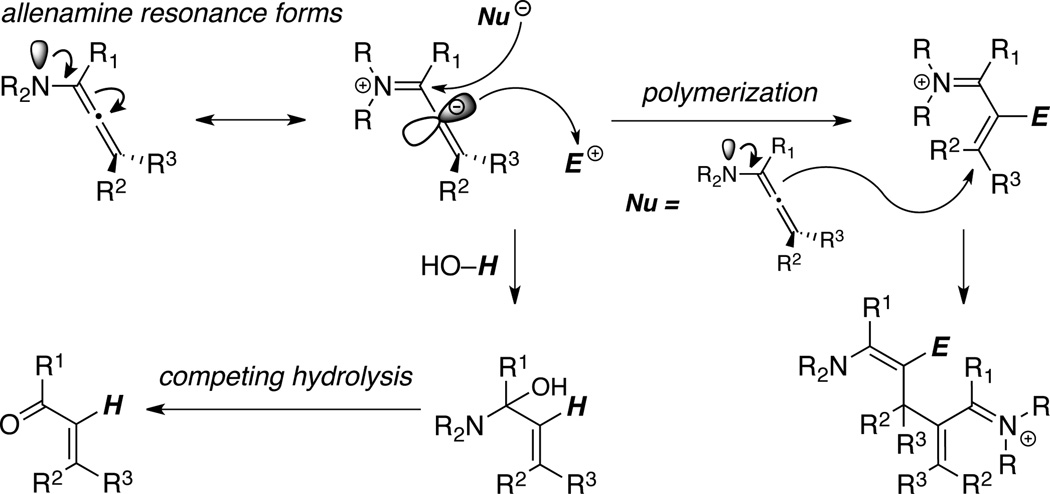
Without illustrating any specifics here on allenamine chemistry given all the comprehensive reviews,1,2 elegant precedents adopting allenamines in a range of transformations have indeed been documented to further support their synthetic potential and provoke interest from the synthetic community. Unfortunately, further developments had been severely thwarted because allenamines are also highly sensitive toward hydrolysis with a tendency to polymerize even at low temperatures (Scheme 3), thereby creating serious difficulties in their preparation and experimental handling.1,2 Consequently, the great potential of chemistry of nitrogen-substituted allenes could only be partially realized. Therefore, efforts to identify an allenamine-equivalent should be of high significance if it can strike the right balance between stability and reactivity.
Scheme 3.
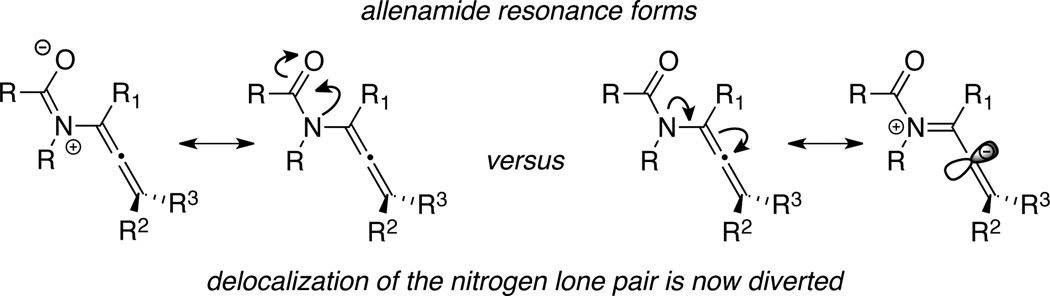
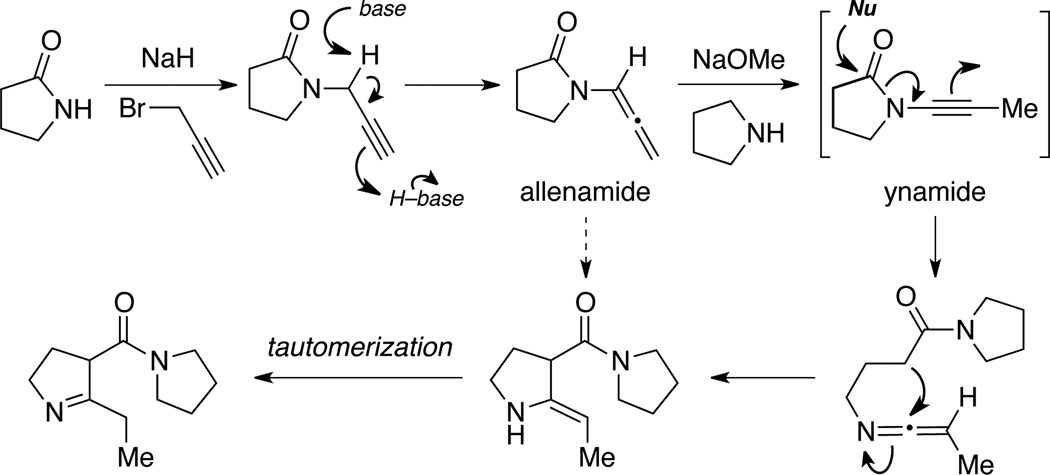
Toward this end, allenamides should represent ideal candidates as a stable allenamine-equivalent. Delocalization of the nitrogen lone-pair into the electron-withdrawing amido group should diminish its donating ability toward the allenic moiety, thereby leading to improved stability (Scheme 4). In short, the very simple fact that allenamides can champion an extra resonance form speaks volume of its superior stability over allenamines. It could be a great story if allenamides were a result of some clever design in search for a stable allenamine-equivalent. However, this is not true and the story is much less dramatic. Allenamides have co-existed along side of allenamines for all of the last four plus decades after Dickinson's first preparation and concise characterizations of 1,2-propadienyl-2-pyrrolidinone in 1967 (Scheme 5).11
Scheme 4.
Scheme 5.
In fact, Dickinson coined the term “allenamide” to describe 1,2-propadienyl-2-pyrrolidinone based the analogy of using enamides17 for Stork’s N-acylated enamines. To clarify reports by Cho12 and others,13 Dickinson concisely demonstrated that treatment of 2-pyrrolidinone with NaH and propargyl bromide had indeed led to the allenamide as the major and stable product also via the same prototropic isomerization pathway. Intriguingly, unlike Viehe’s work, allenamide did not undergo further isomerization to the respective ynamide, although with further treatment of NaOMe and pyrrolidine, ynamide was postulated as an intermediate en route to the N-acyl-pyrrolidine product. Nevertheless, this documentation of ynamide actually predated Viehe’s 1972 account,14 and chemistry of ynamides has indeed generated an immense amount of interest from the synthetic community in the last 15 years.15,16
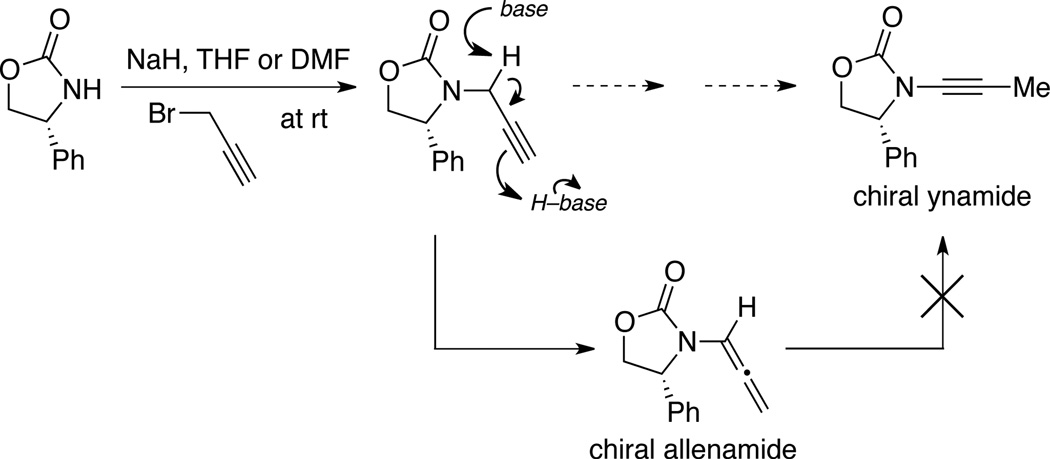
To align with the history, our foray into this field coincides with both of Viehe and Dickinson’s work. In search of a useful synthetic method to construct chiral ynamides 16 years ago,10 we found that based-catalyzed prototropic isomerization of propargyl amides reliably arrested at the allenic stage and gave none of the desired ynamides10 (Scheme 6) regardless of nature of the base used, temperature, and solvents (also see Schemes 24 and 25 vide infra). More importantly, to properly acknowledge a critical person in our entire endeavor in allenamide chemistry, I owe everything to the very first postdoctoral research fellow in my group, Dr. Lin-Li Wei [Ph.D. with Professor Teck-Peng Loh at National University of Singapore]. Dr. Wei, who was working on these isomerizations, pointed out that these allenamides that she had obtained could prove to be an excellent allenamine-equivalent, and evolve into highly versatile synthetic building blocks in organic synthesis.
Scheme 6.
Scheme 24.
Scheme 25.
Given the precedent, the ease of preparation, and stability, the most critical question would be whether these allenamides could possess sufficient reactivity. A survey of the literature indicates that although it was far from a blank page, allenamides have been much less explored relative to allenamines.4,18–20 Precise reasons are not very clear, but there were very few citations on synthesis and applications of allenamides before 1989. While few more reports appeared from late 1980’s to mid-1990’s, the real outburst in chemistry of allenamides came 16 years ago, just as we also became deeply involved in the development of allenamide chemistry. Such sustained emergence strongly suggests that allenamides have set the gold standard for balancing reactivity and stability. They are becoming proven allenamine-equivalents that can be employed in a diverse array of stereoselective and intramolecular reactions that were not possible with traditional allenamines. They represent the ideal platform for pushing the limit of synthetic potential of nitrogen-substituted allenes.
It is the purpose of this review to provide proper illustrations of the elegant chemistry involving allenamides that has come to pass, thereby eliciting a greater amount of interests from the synthetic community to create new allenamide chemistry. Lastly, this perspective that advancement of any field requires collective creativity and innovation from many people and not just a few individuals rings hollow here. On that note, although we are trying our very best to be comprehensive, it is likely that we have inadvertently missed some beautiful work for which we express our regret here in advance.
2. Preparation
2.1. Historical Examples
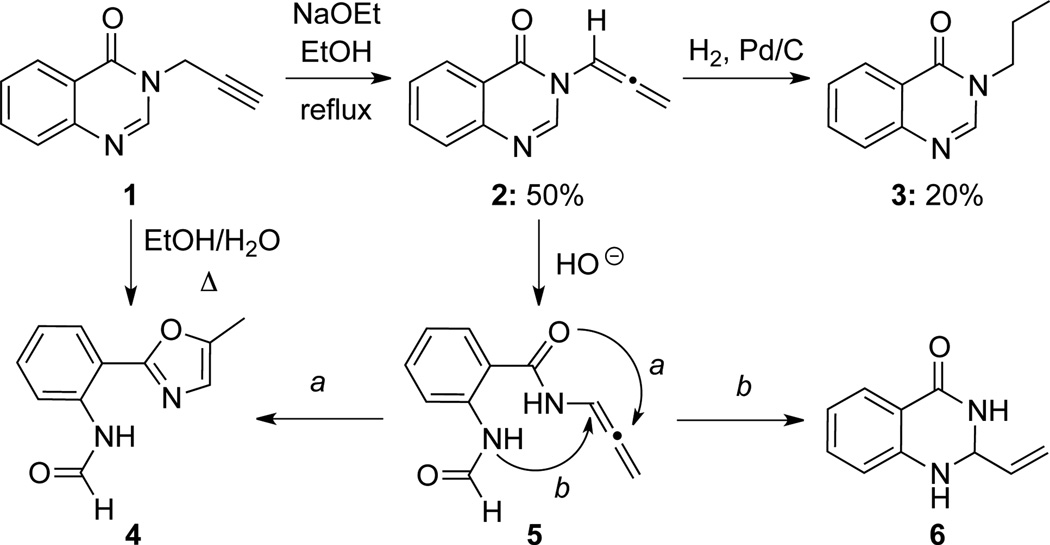
Besides Dickinson’s first allenamide synthesis11 (Scheme 5), Bogentoft21 reported another earlier example of allenamide synthesis (Scheme 7). Allenamide 2 was prepared in 50% yield via a base-induced isomerization of propargyl amide 1. Per-hydrogenation of allenamide 2 was also reported to give alkyl amide 3. In addition, under basic conditions, a mixture of oxazole 4 and oxo-quinazoline 6 could be obtained via C-O (pathway a) and C-N (pathway b) bond formation, respectively, from a ring-opened intermediate 5.
Scheme 7.
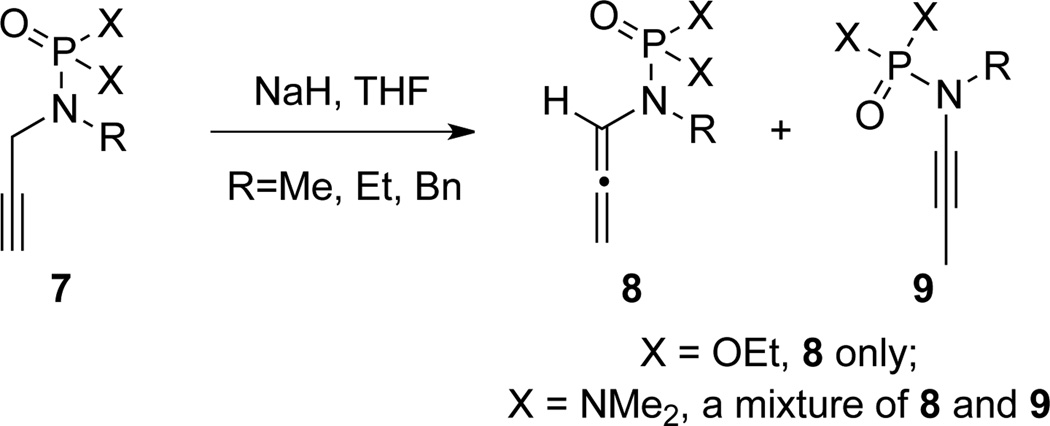
In 1976, Corbel22 achieved the first synthesis of an acyclic allenamide 8 also via the base induced isomerization of N-propargyl phosphoramidate [X = OEt]/phosphoramide [X = NMe2] 7 (Scheme 8). Ynamide 915 was found along with 8, as a mixture when X = NMe2.
Scheme 8.
2.2. Sigmatropic Rearrangement
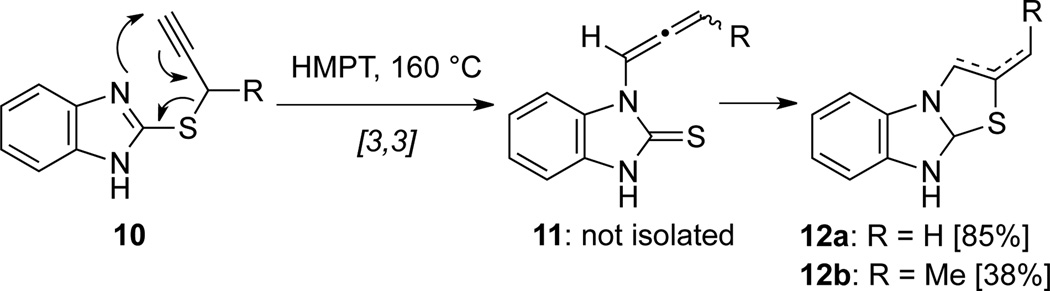
Balasubramanian’s23 syntheses of benzimidazolyl thiazoles 12 represents the first applications of [3,3]-sigmatropic-rearrangement in allenamide synthesis (Scheme 9). Although not isolated, allenamides 11 were postulated as intermediates to 12 through [3,3]-sigmatropic rearrangements of benzimidazolyl propargylic sulfides 10.
Scheme 9.
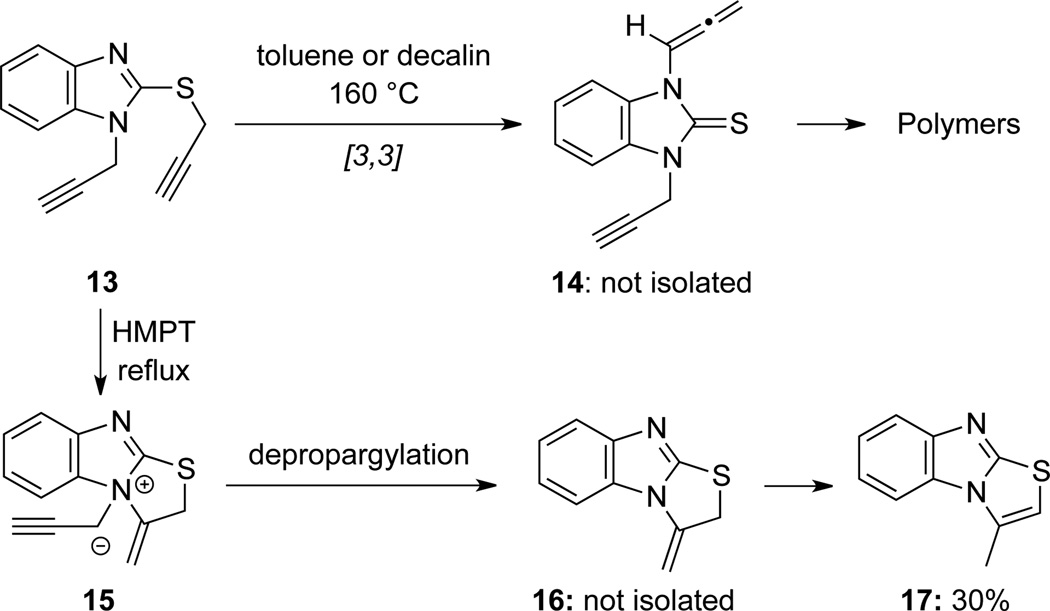
Balasubramanian24 also found that de-propargylation occurred when bis-propargyl thiol benzimidazole 13 was refluxed in HMPT to afford benzimidazolyl thiazole 17 (Scheme 10). However, when heated in non-polar solvents, polymerization products were observed, presumably through allenamide 14 resulting from the initial [3,3]-sigmatropic rearrangement, albeit not isolated.
Scheme 10.
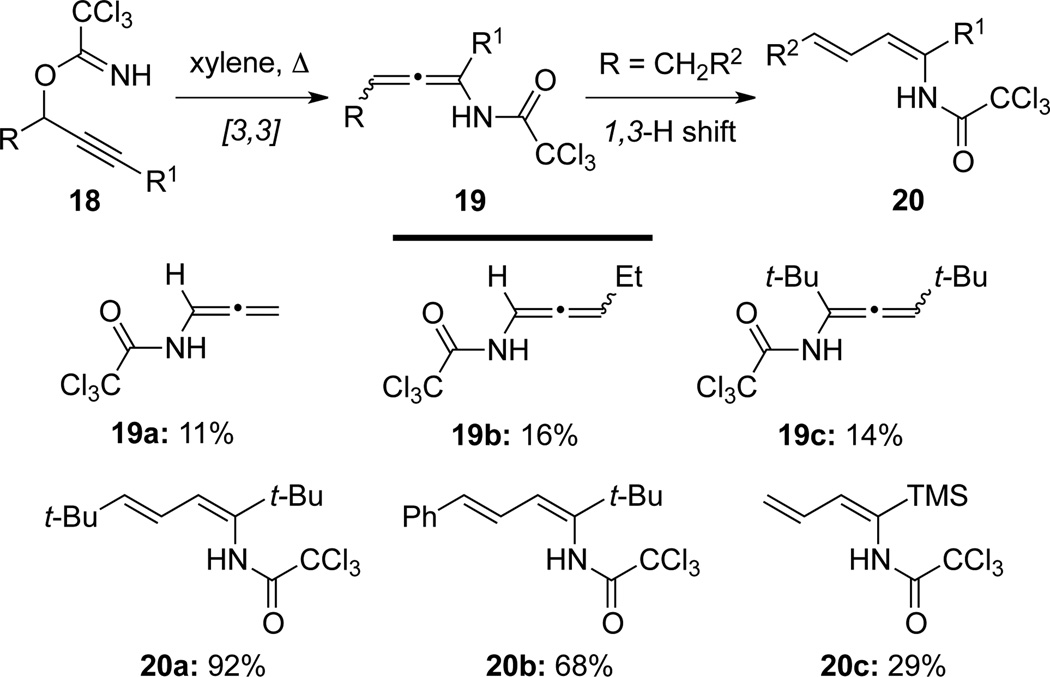
Overman25 discovered that allenamides 19, a secondary allenamide, could be isolated through an Overman-Claisen rearrangement of propargyl trichloroacetimidates 18 (Scheme 11). Allenamides 19 could be further isomerized to 1Z-3E-dienamides 20 in a highly stereoselective manner via 1,3-H shift from the γ-substituent.
Scheme 11.
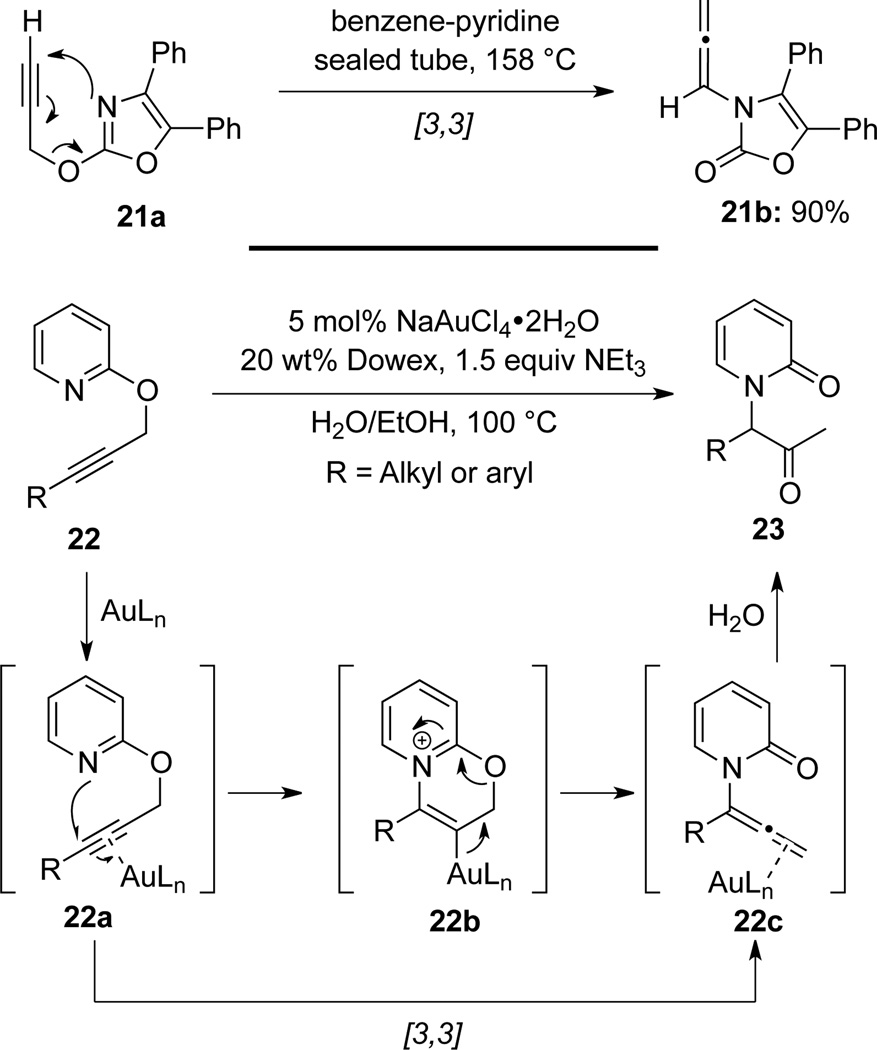
Padwa26a found that when heating oxazole 21a in a benzene-pyridine solution, 4-oxazolin-2-one derived allenamide 21b was obtained in high yield via an aza-Claisen rearrangement (Scheme 12). This very much reminiscences chiral allenamides later on reported by Hsung (see Scheme 6).
Scheme 12.
Very Recently, Anderson26b reported a related rearrangement in the synthesis of α-(N-2-pyridonyl)ketones 23 via an Au(III) catalyzed tandem amination-hydration reaction from propargyloxypyridines 22. The formation of allenamides 22c was the result of a formal aza-Claisen rearrangement of 22a.
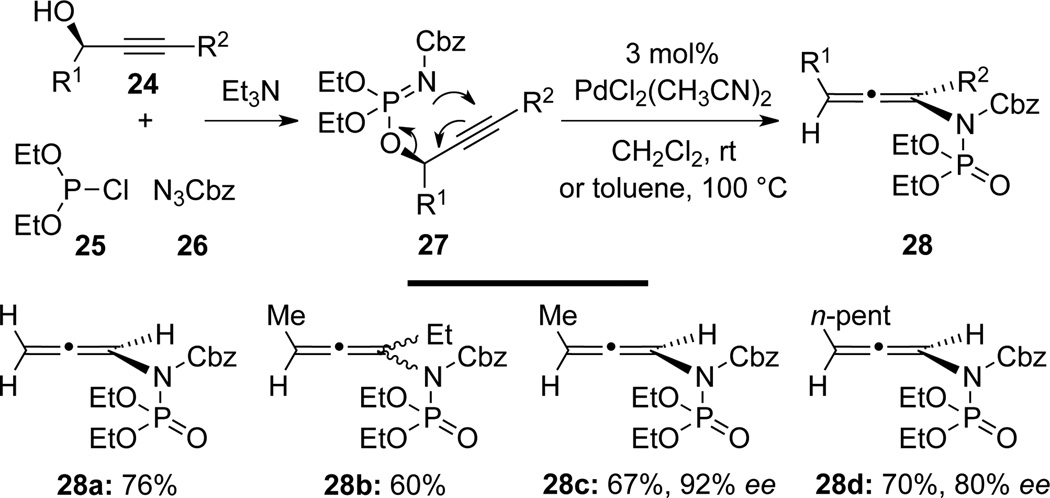
More recently, Mapp27 cleverly designed a facile synthesis of allenamides 28 via palladium-catalyzed [3,3]-sigmatropic rearrangement of propargyl phosphorimidates 27 generated from propargyl alcohols 24, chlorophosphite 25, and azide 26 (Scheme 13). With this method, an array of mono-, di-, and trisubstituted allenamides were obtained in good yields. When optically pure alcohols 24 [see c–d] were used as precursors, chiral allenamides 28 [see c–d] could be prepared with a high level of chirality transfer. It is noteworthy that formally with two electron-withdrawing substituents on the nitrogen atom, these are allen-imide equivalents.
Scheme 13.
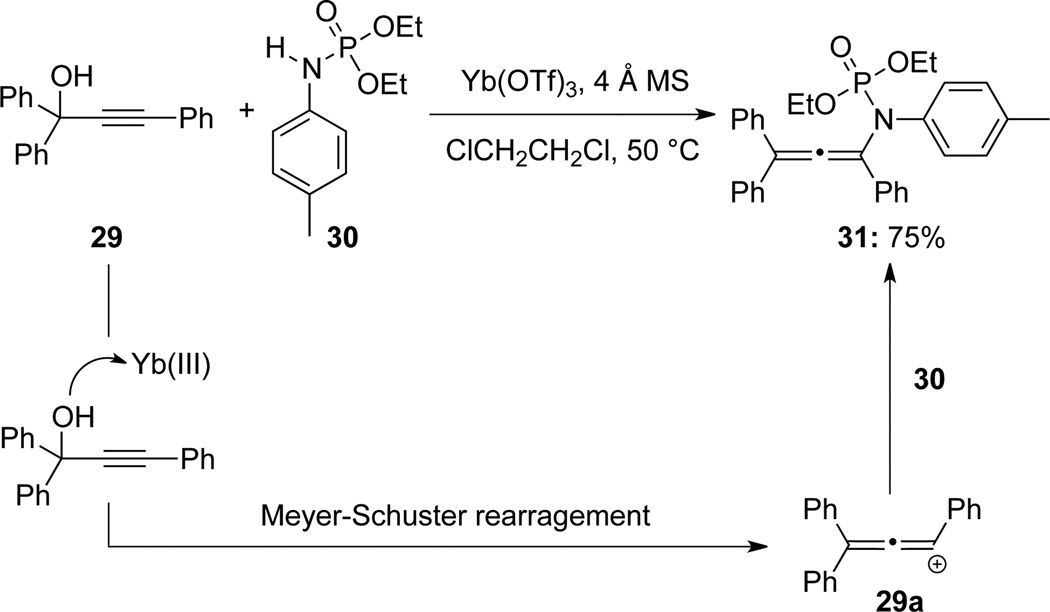
Lu and Wang28a reported the isolation of N-phosphoryl allenamides 31 from propargyl alcohol 29 through an Yb(III)-catalyzed Meyer-Schuster type rearrangement29a followed by trapping with N-tolyl phosphoroamidate 30 (Scheme 14).
Scheme 14.
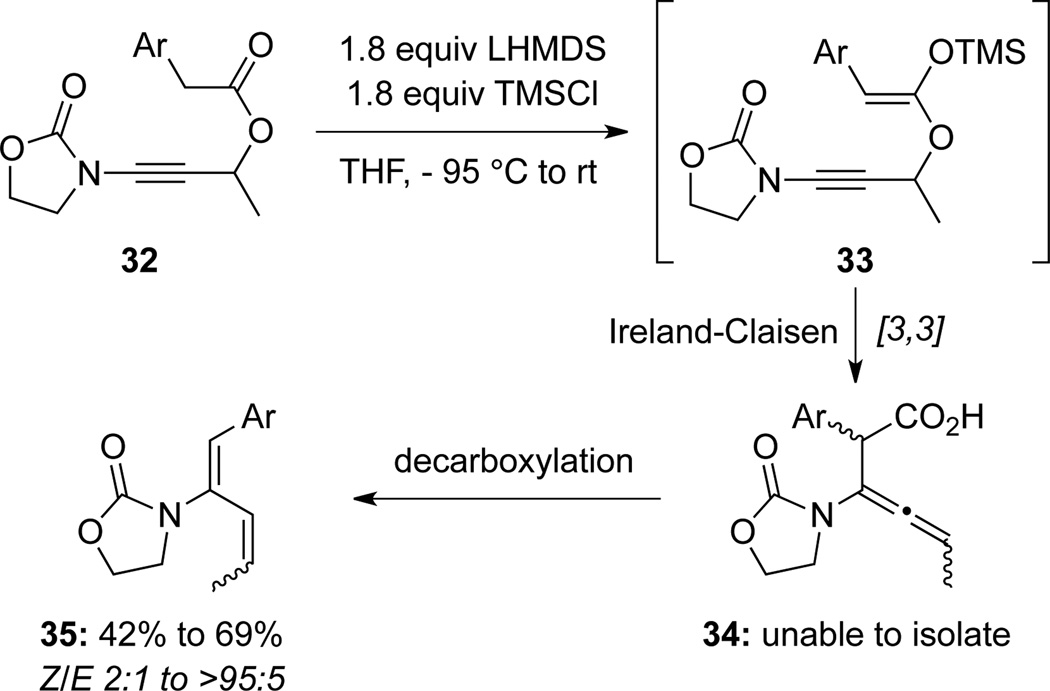
Very recently, Carbery30 attempted to prepare allenamides 34 via Ireland-Claisen rearrangements29b of ynamide15-derived silyl ketene acetal 33 (Scheme 15). However, they obtained Z-2-amido diene 35 only, presumably through a facile decarboxylation-isomerization sequence from allenamides 34 after the rearrangement.
Scheme 15.
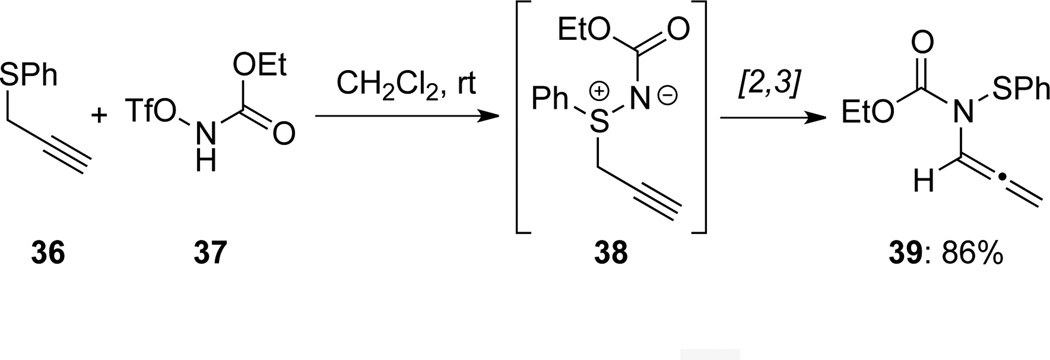
Tamura31 reported the first example of [2,3]-sigmatropic rearrangement of sulfimine 38, leading to allenamide 39 (Scheme 16). The rearranged precursor 38 was obtained by acylating the propargyl sulfide 36 with N-triflate carbamate 37.
Scheme 16.
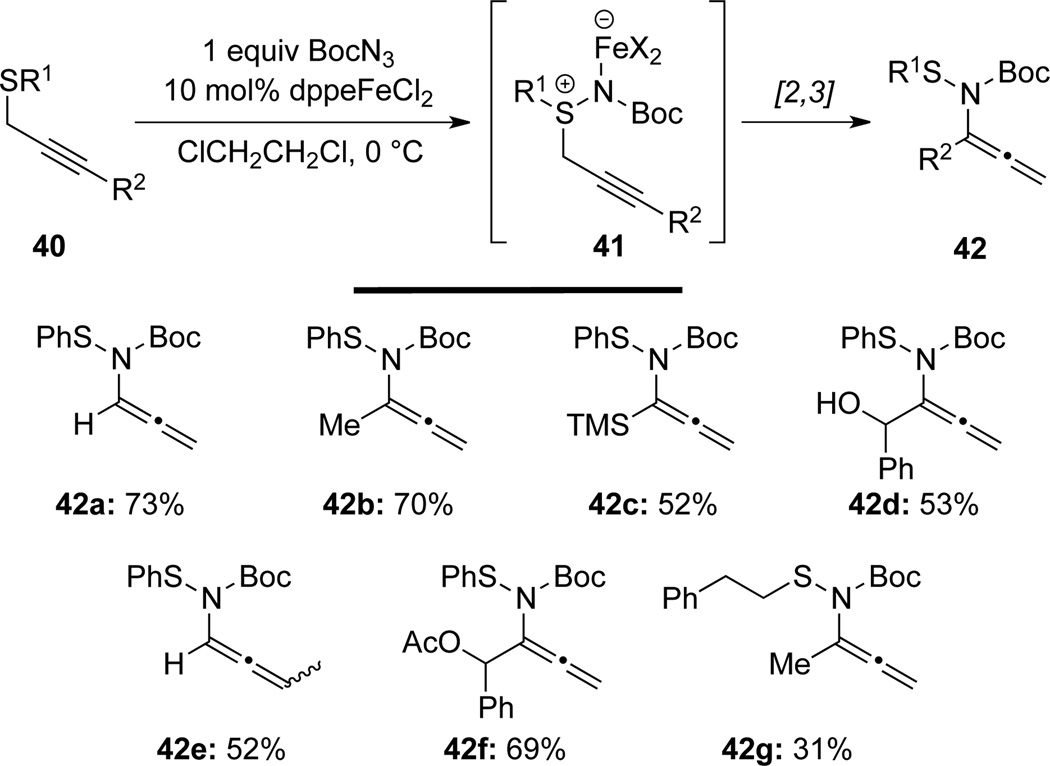
Van Vranken32 demonstrated that a range of allenamides 42 could be synthesized in moderate to high yields by a Fe(II)-catalyzed tandem S-imidation/[2,3]-sigmatropic rearrangement of the possible ironnitrene intermediates 41 (Scheme 17).
Scheme 17.
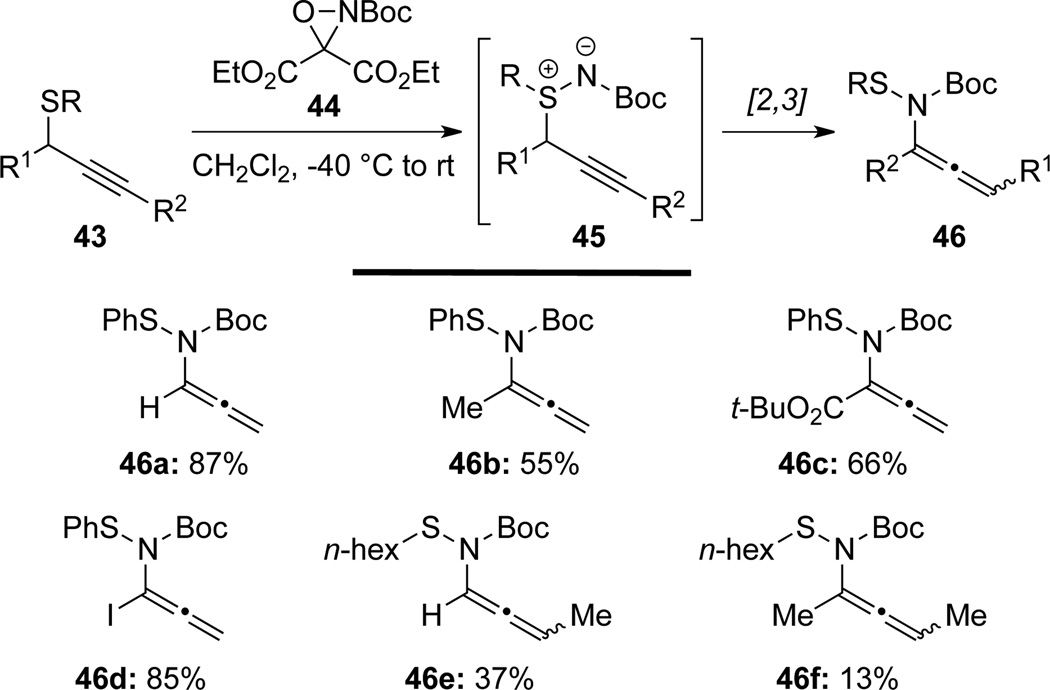
Armstrong33 showed that allenamides 46 could be prepared from propargyl sulfides 43 in an analogous manner but using oxaziridine 44 (Scheme 18). This reaction also proceeds through a related cascade Simidation/[2,3]-sigmatropic rearrangement sequence, under metal-free conditions with propargyl sulfimides 45 as a possible intermediate.
Scheme 18.
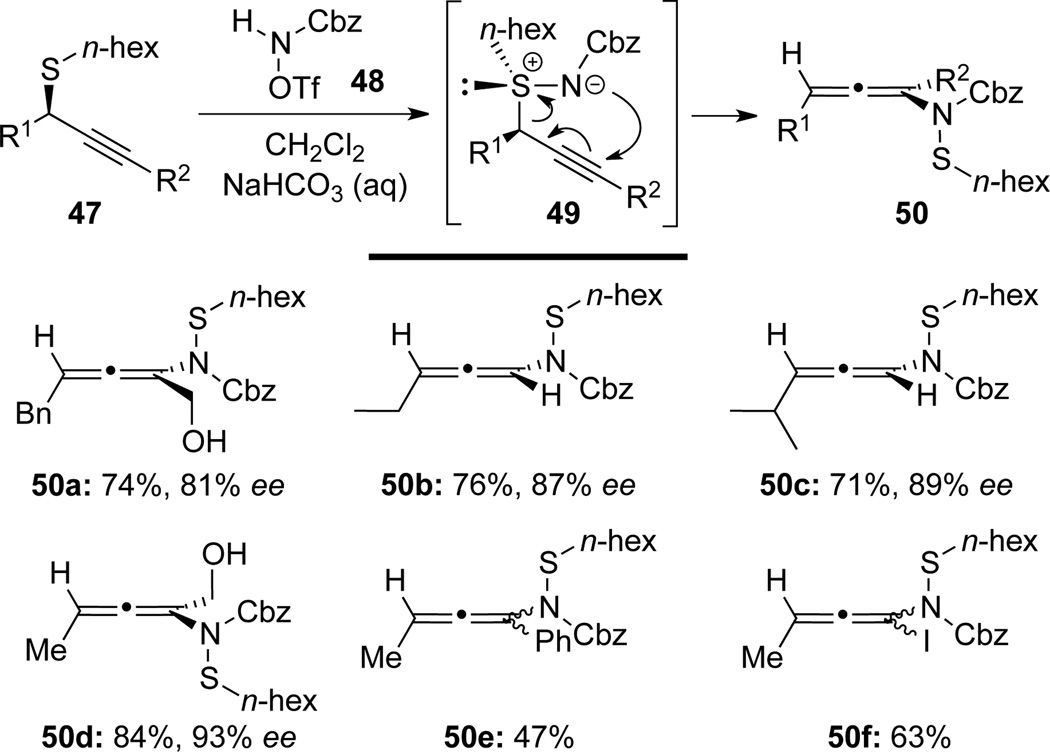
Armstrong34 later synthesized allenamides 50 in high yields via a [2,3]-sigmatropic rearrangement of propargyl sulfimides 49, which were prepared in situ from propargyl sulfides 47 and amidation agent 48 (Scheme 19). Notably, optically enriched allenamides 50 [see a-d] were also achieved with effective transfer of chirality information from enantiomerically enriched propargyl sulfimides 47 [see a–d].
Scheme 19.
2.3. Base-Induced Isomerization
2.3.1. Cyclic Propargyl Amides
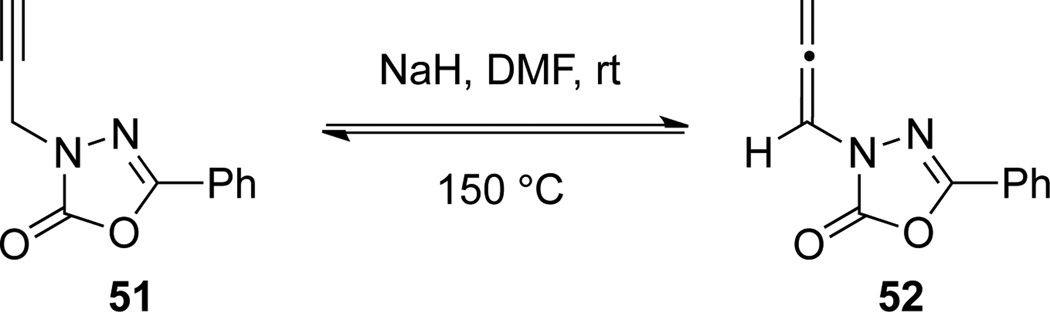
Base-induced isomerization of propargyl amides represents a highly atom-economical synthesis of allenamides. Padwa35a first discovered that propargyl amide 51 and allenamide 52 could be interconverted at high temperature under basic conditions when they were investigating flash vacuum pyrolysis of 52 (Scheme 20).
Scheme 20.
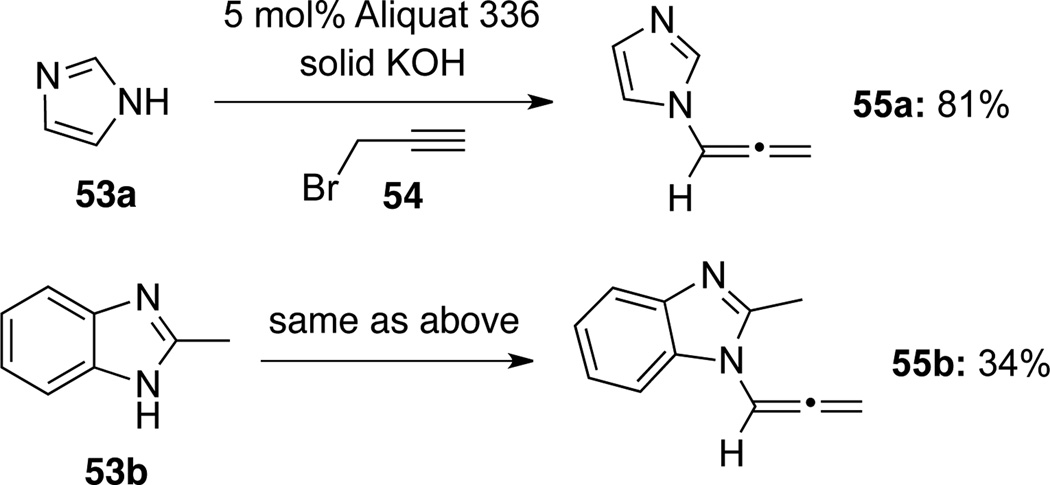
Galons35b reported another earlier example in which allenamides 55 were formed through a solid-liquid phase transfer catalyst-promoted reaction of imidazoles 53 with propargyl bromide 54 (Scheme 21).
Scheme 21.
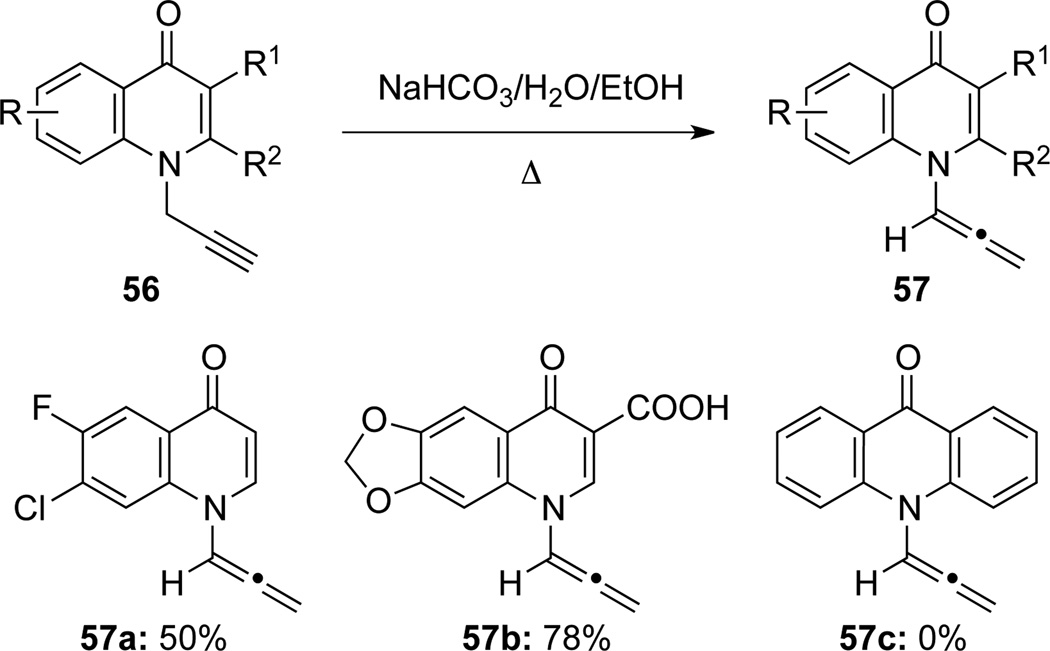
Radl36 employed the base-induced isomerization method to synthesize quinolone-derived allenamides 57 by using NaHCO3 in aq EtOH solution (Scheme 22). Intriguingly, the desired isomerization product 57c was not attainable from the respective N-propargyl acridone under this isomerization condition.
Scheme 22.
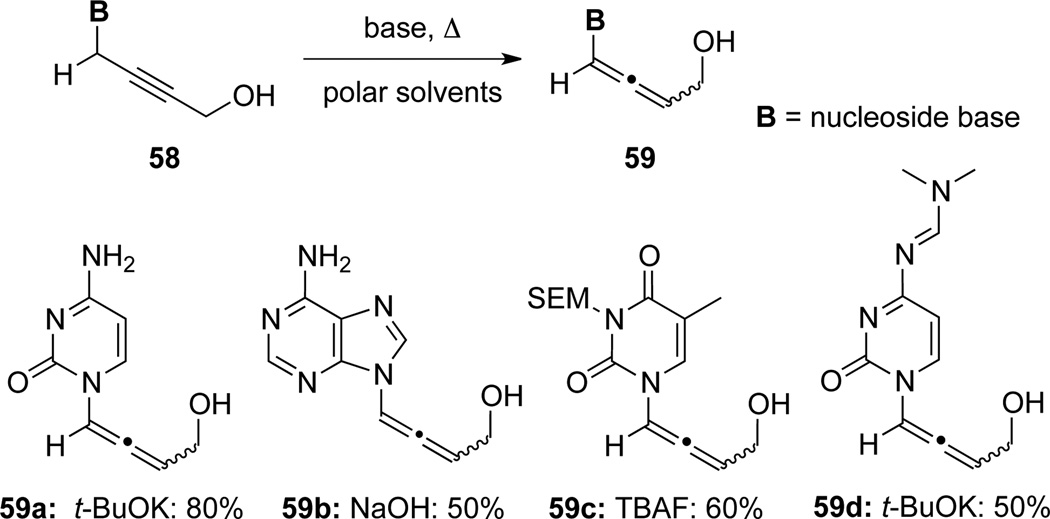
Zemlicka37 documented syntheses of a series of nucleoside derived allenyl alcohols 59 through the base promoted isomerization of the corresponding 2-butynols 58 (Scheme 23). These nucleoside-derived analogues possess potential cytotoxic and antiviral activities.38
Scheme 23.
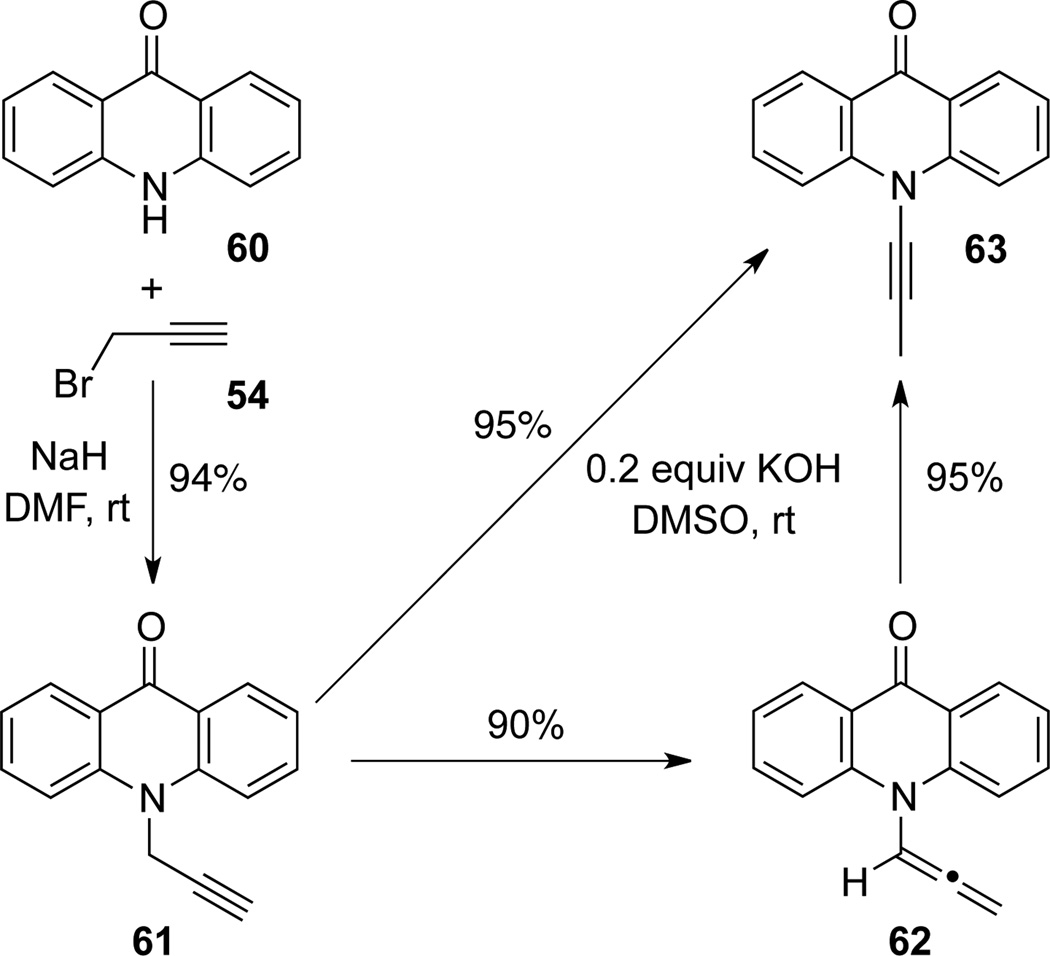
The N-propargylation-isomerization protocol for preparation of ynamide 6315 from acridone 60 have been documented from several research groups.39 By slightly modifying previous propargylationisomerization protocol,39a Hsung40 prepared ynamide 6315 from acridone 60 in two steps. However, they also found that by shortening the reaction time, allenamide 62 could be isolated in high yield as the isomerization intermediate41a (Scheme 24).
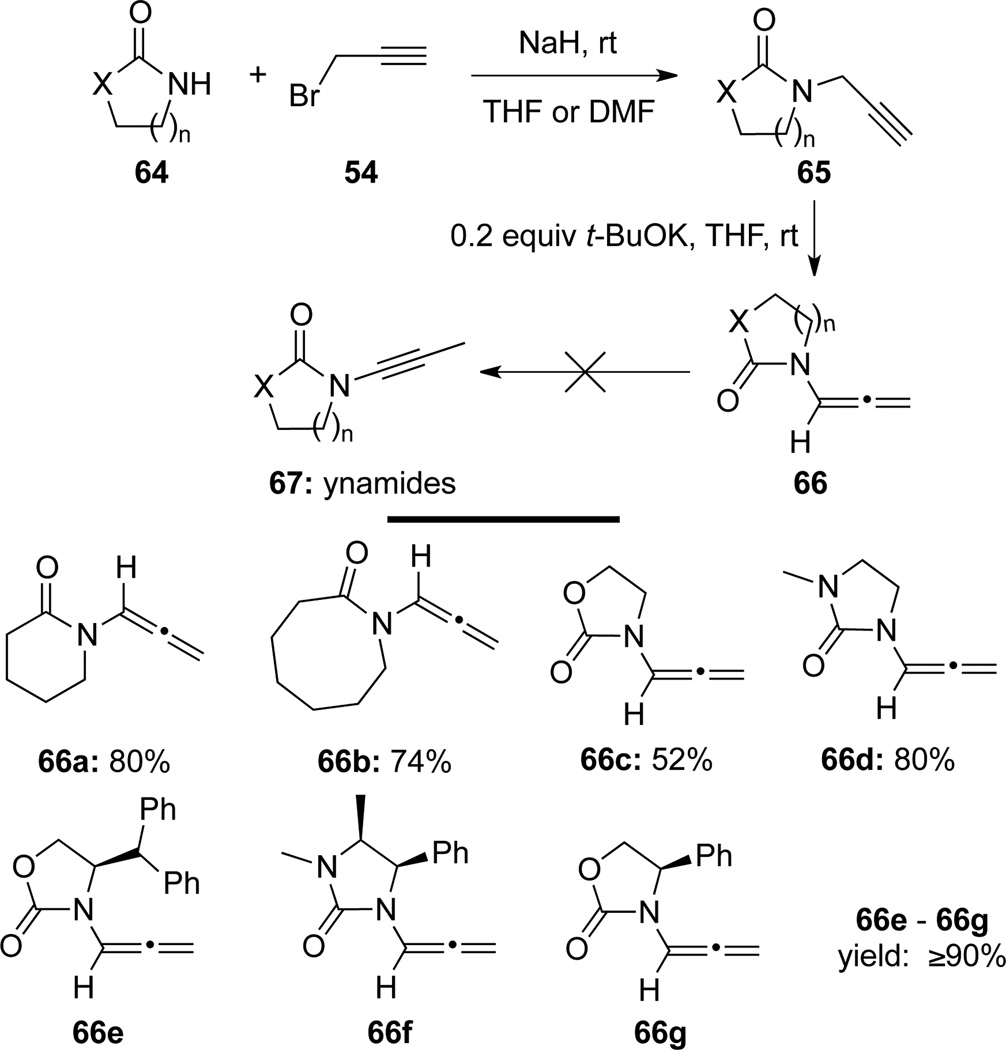
When extending this base-induced isomerization sequence to access a broader scope of ynamides 67,15 Hsung41a found that with t-BuOK as the base at room temperature, isomerization of propargyl amides 65 only provided allenamides 66 (Scheme 25). Consequently, a facile two-step protocol was established to prepare cyclic allenamides 66 from amides 64:41 (i) alkylation of amides 64 with propargyl bromide 54; and (ii) base-induced isomerization to allenamides 66. With this protocol, chiral allenamides 66e–g were accessible for the first time, and a large scale synthesis of chiral allenamide 66g was later documented.41c
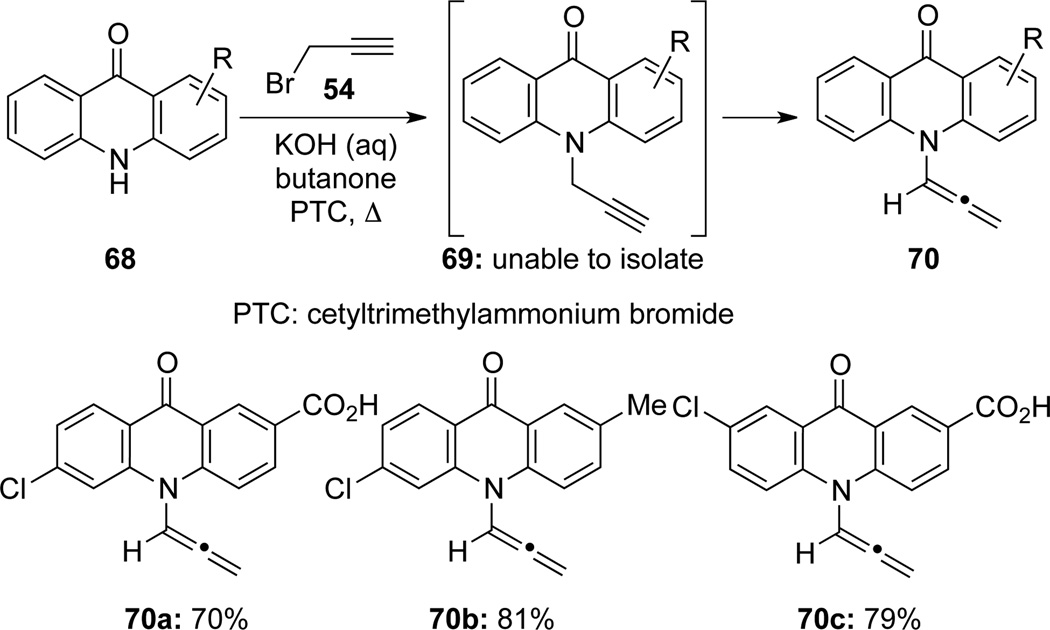
Pellón42 found that this two-step protocol could be rendered in one-pot by heating the mixture of acridones 68 and propargyl bromide 54 in KOH aqueous/butanone solution with cetyltrimethylammonium bromide as the phase transfer catalyst (PTC) (Scheme 26). Without isolating the N-propargylated intermediate 69, acridone-derived allenamides 70 were isolated in good yields. It is noteworthy that these allenamides could not be prepared under Radl’s conditions (Scheme 22).36
Scheme 26.
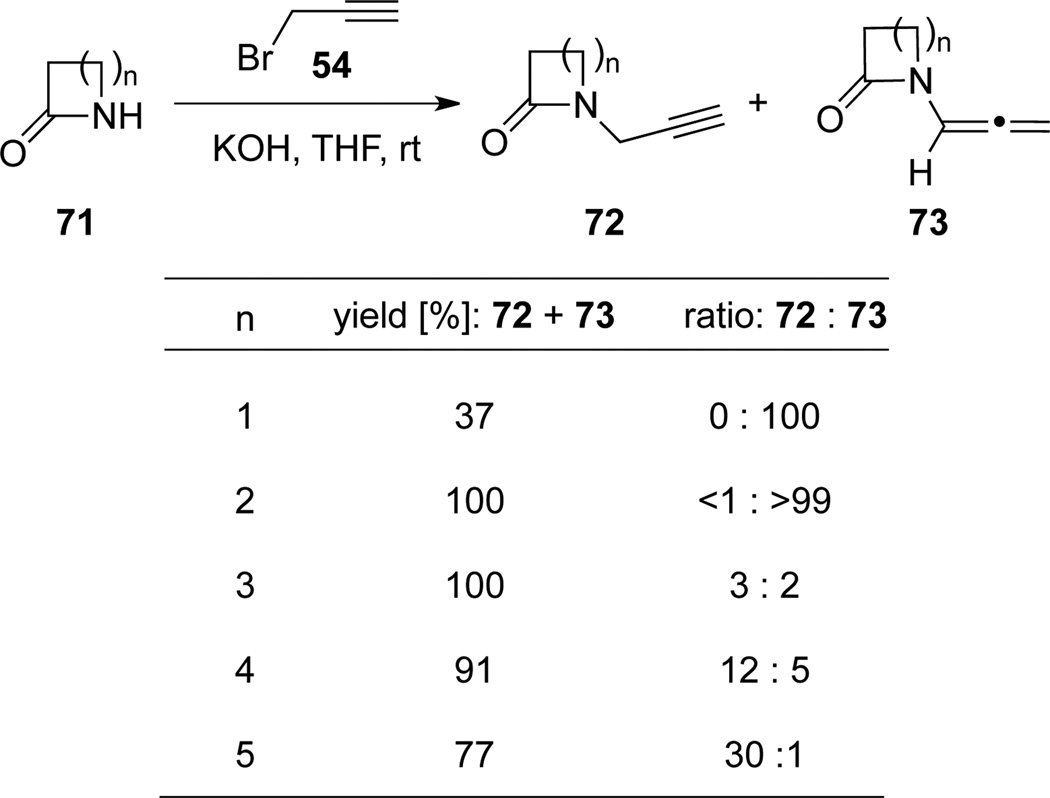
Plumet43 also examined the base-induced two-step/one-pot operation using lactams 71 with different ring sizes (n = 1–5) (Scheme 27). They found that as the ring size increases, it is more difficult to obtain the desired isomerized products 73.
Scheme 27.
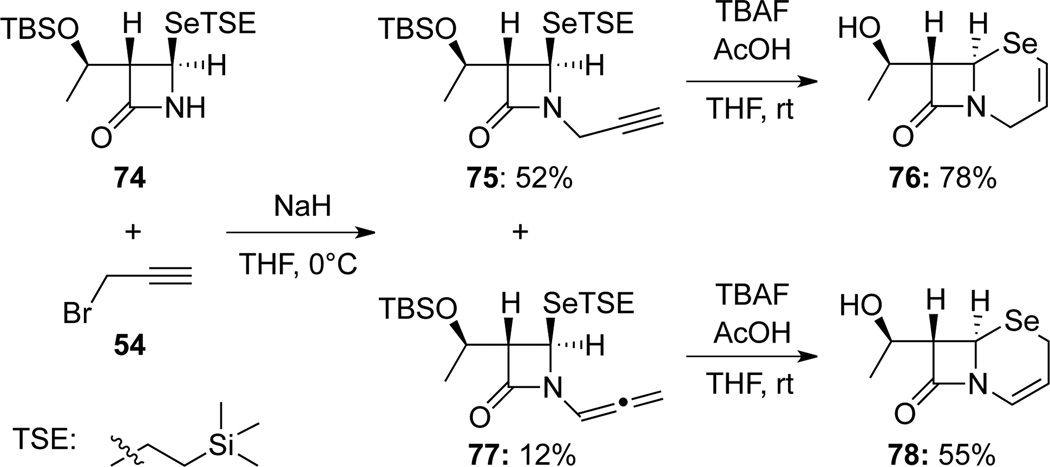
Ishihara44 reported an N-alkylation of selenium-containing β-lactams 74 in their synthesis of a key intermediates en route to antibacterial agents (Scheme 28). A small amount of allenamide 77 was isolated in addition to propargyl amide 75. Both propargyl amide 75 and allenamide 77 could be cyclized to give selenacephem 76 and 78, respectively.
Scheme 28.
2.3.2. Acyclic Propargyl Amides
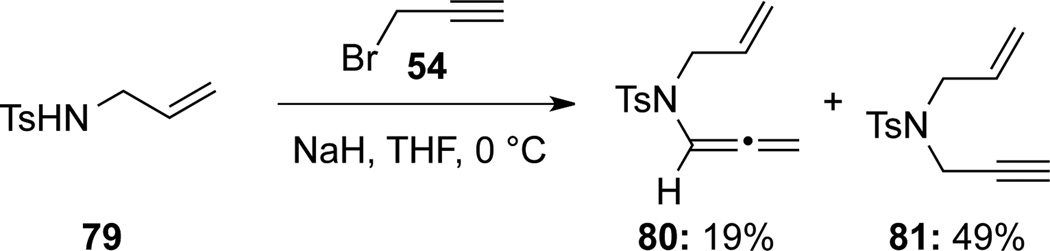
While propargylating N-allylsulfonamide 79, Meijere45 obtained a mixture of allenamide 80 and propargyl amide 81 (Scheme 29).
Scheme 29.
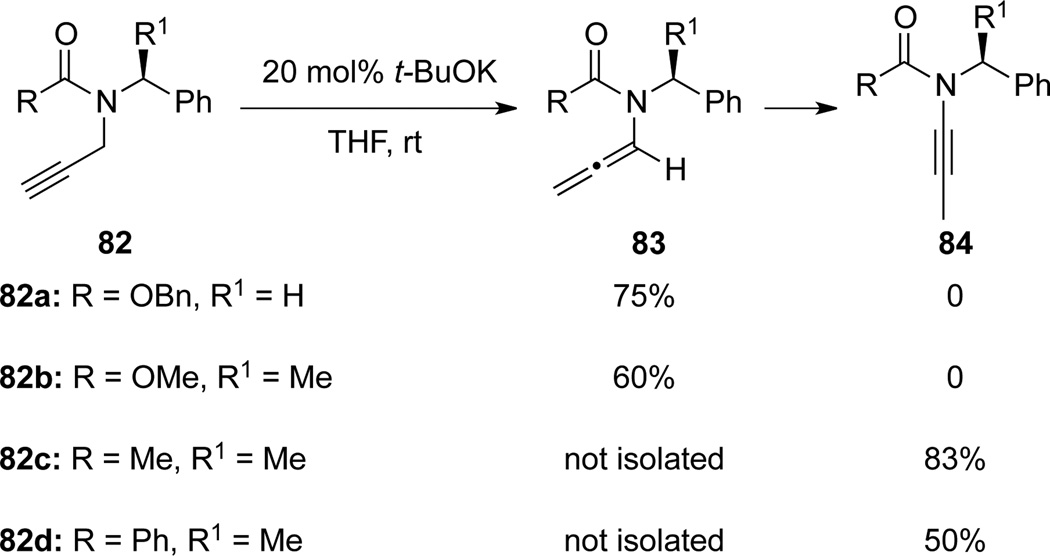
Hsung46 examined base-induced isomerizations of acyclic propargyl amides 82 and reported the first successful isomerization of chiral propargyl amides to ynamides 84 (Scheme 30).15 Intriguingly, under the same reaction conditions, unlike propargyl amides 82c and 82d, urethanes such as 82a and 82b stopped at the first isomerization step to form allenamides 83a and 83b. Reasons for these contrasts are not clear at this point.
Scheme 30.
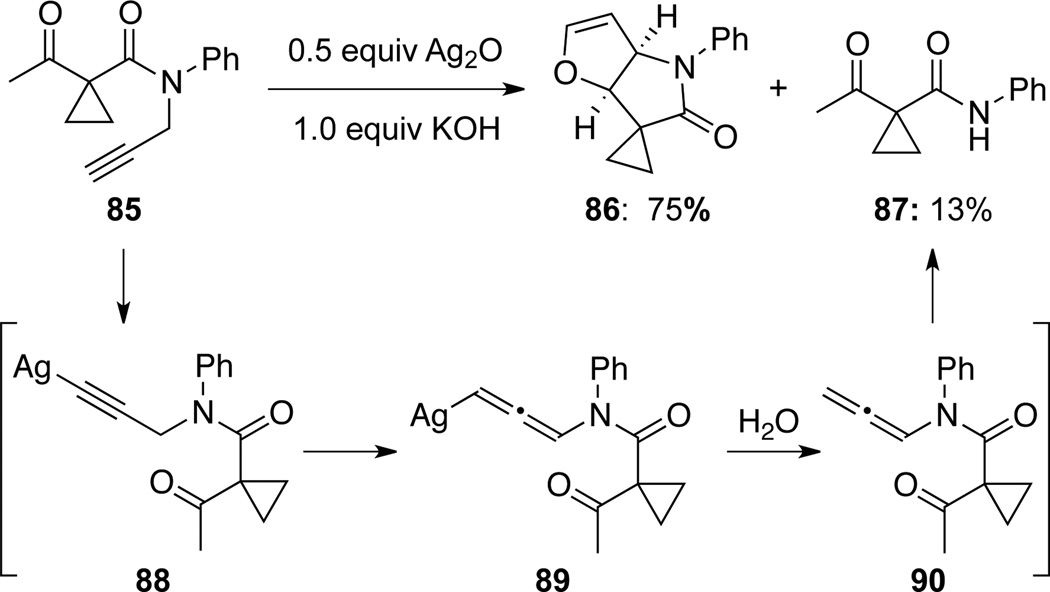
The base-promoted propargyl-allenamide isomerization was also observed in several metal-catalyzed reactions. Zhang and Liu47 found that besides their expected formal cycloaddition product lactam 86, the depropargylated amide 87 was also observed, probably via isomerization of Ag-acetylide 88 to allenamide 90 followed by a facile hydrolysis during quenching and work-up (Scheme 31). Although allenamide 90 was not isolated, it represents the most plausible intermediate that can explain the loss of the propargyl substituent.
Scheme 31.
2.4. Elimination
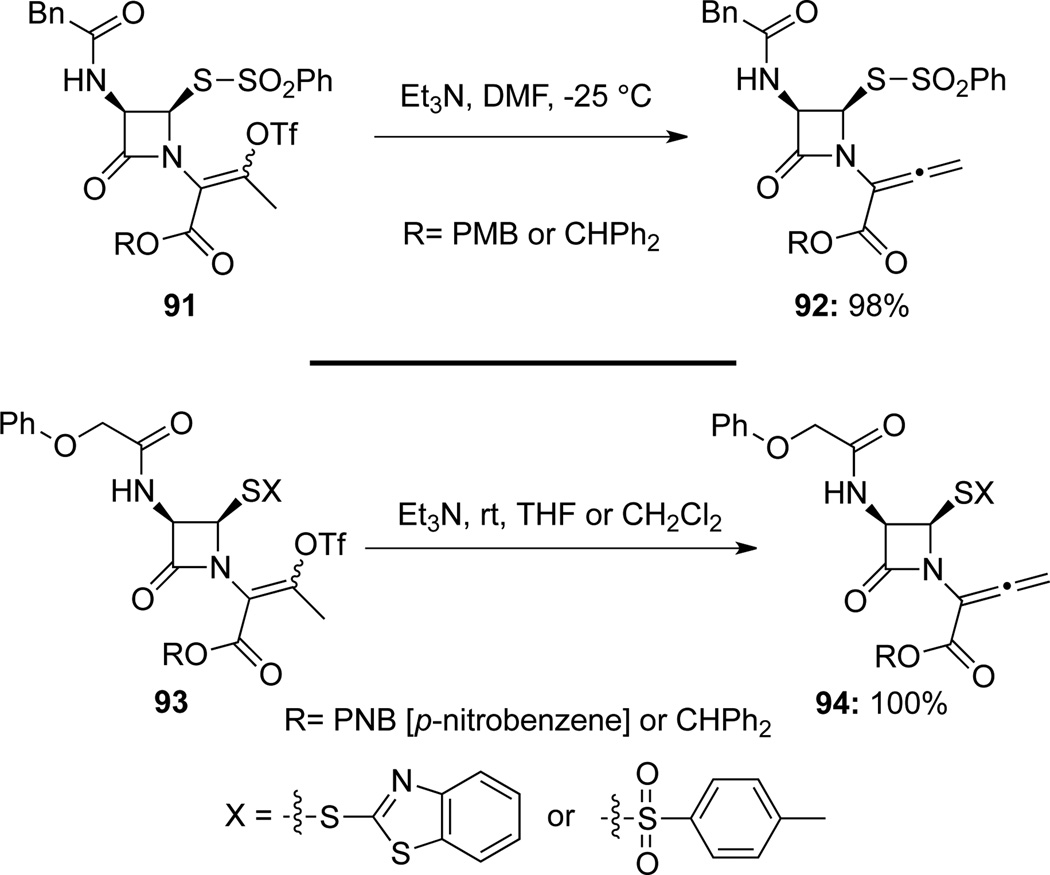
Tanaka48a found that lactam-derived allenamides 92 could be obtained in high yields from enol triflates 91 through a Et3-promoted E2-elimination (Scheme 32). A similar Et3N-assisted elimination protocol was also applied in Farina’s48b synthesis of lactam-derived allenamides 94 from their corresponding enol triflates 93.
Scheme 32.
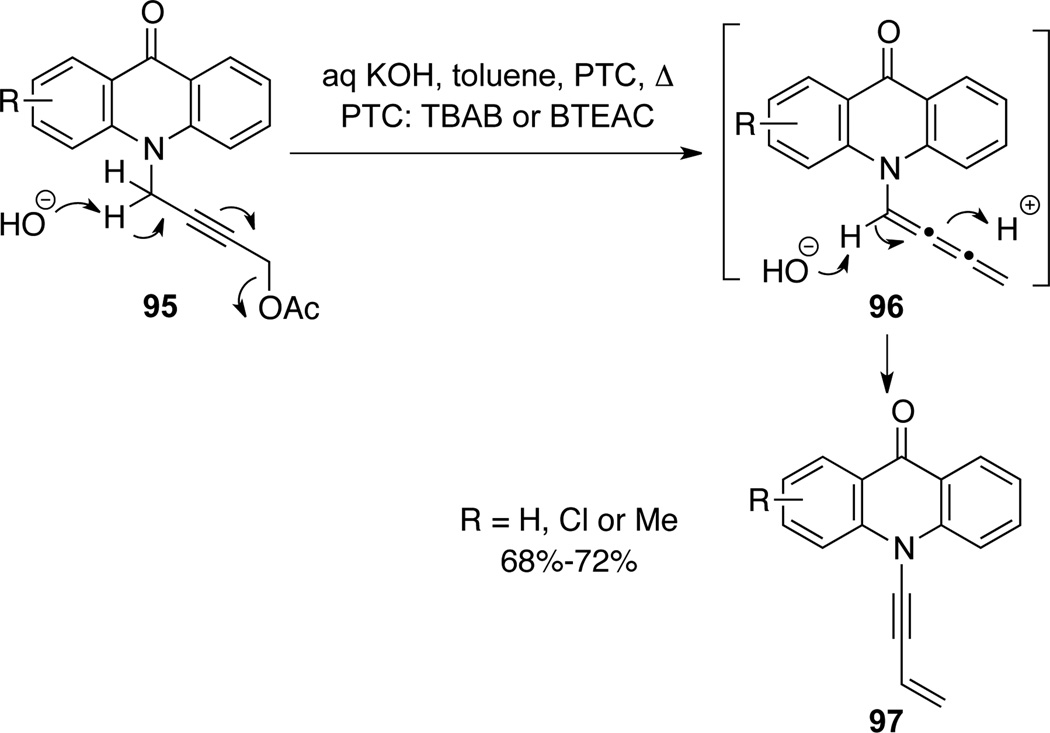
Majumdar49a reported preparation of N-enyne acrydone 97 under PTC conditions and cumulene-type allenamides 96 were proposed as intermediates (Scheme 33).
Scheme 33.
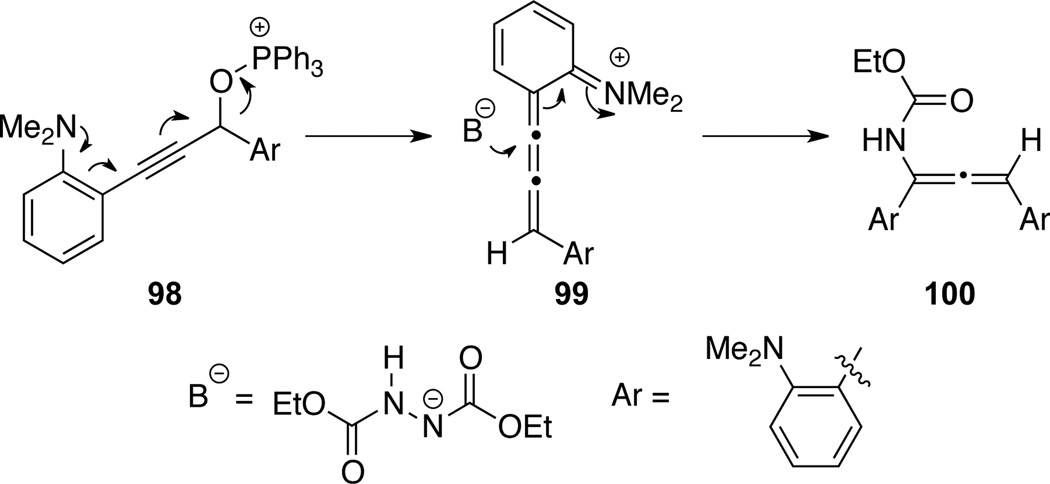
More recently, Fallis49b suggested that allenamide 100 [another secondary allenamide] was formed from aniline substituted propargyl phosphonium ether 98 via an initial intramolecular elimination of triphenylphosphine oxide followed by trapping the cumulene intermediate 99 (Scheme 34).
Scheme 34.
2.5. Amino-Cyclization
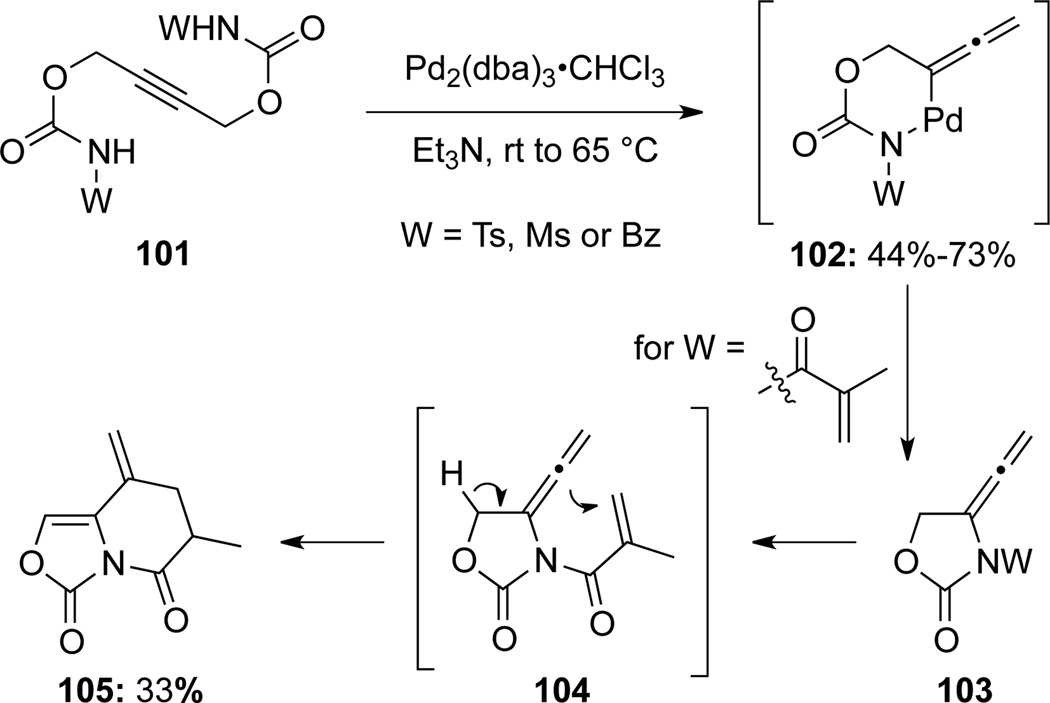
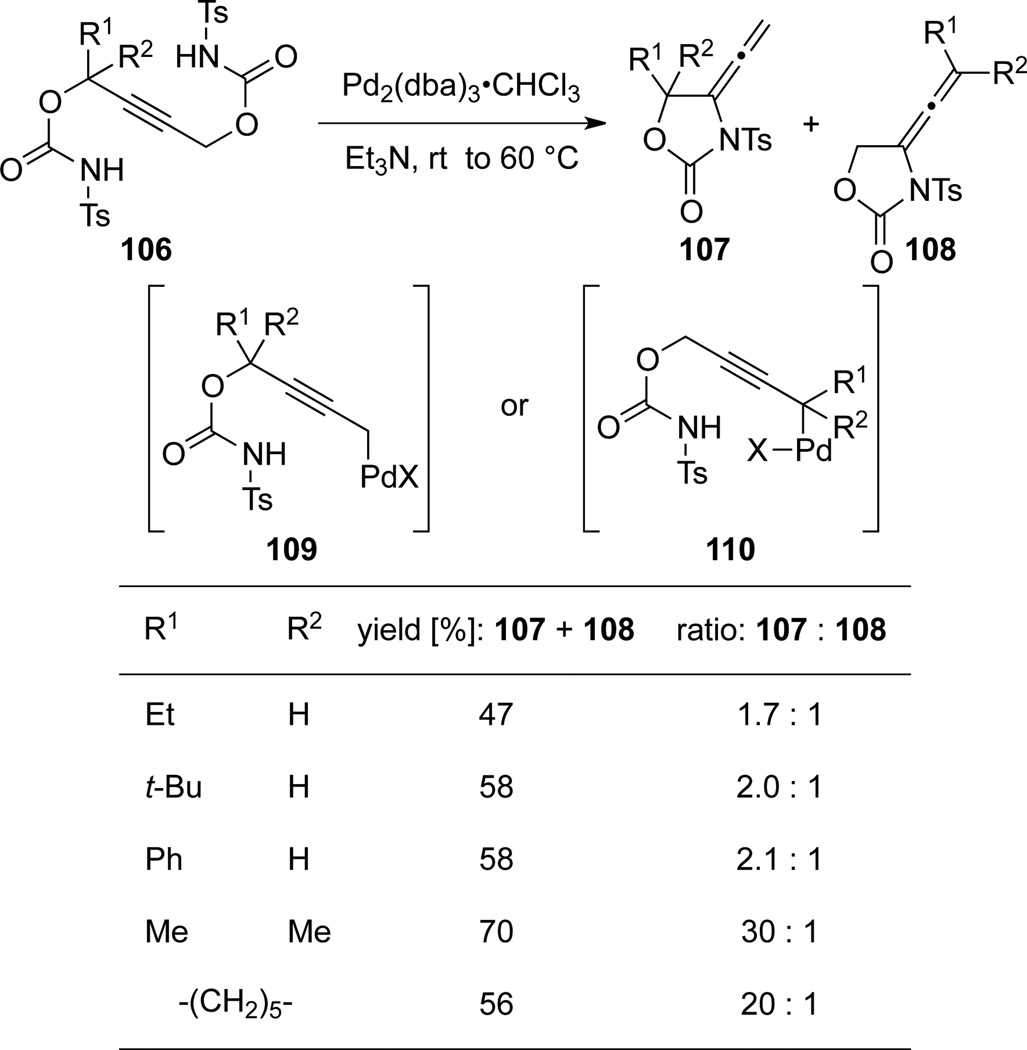
Tamaru50 reported a Pd-catalyzed intramolecular cyclization of propargyl biscarbamates 101 to generate allenamides 103 that could further cyclize to 105 (Scheme 35). The unsymmetrical biscarbamates 106 afforded a mixture of different allenamides 107 and 108, from adducts 109 and 110, respectively. Ratios of 107 and 108 with different substituents suggested that the oxidative addition of Pd(0) prefers the less hindered propargyl carbon as shown in 109 (Scheme 36).
Scheme 35.
Scheme 36.
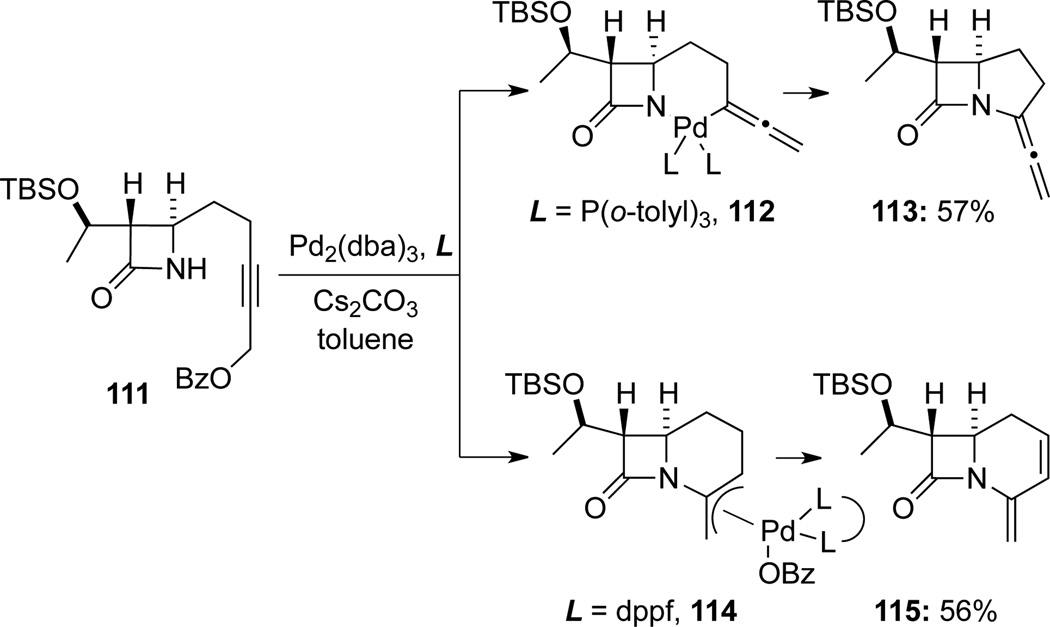
Mori51a demonstrated that aminocyclization of β-lactam containing propargyl benzoate 111 could deliver allenamide 113 or enamide 115 in a selective manner depending on the ligand used (Scheme 37). When a monodentate ligand such as P-(o-tolyl)3 was used, allenamide 113 with a carbapenam skeleton was isolated as the major product via the palladium intermediate 112. However, when the bidentate ligand such as dppf was used, enamide 115 was isolated as the sole elimination product from the palladium π-allyl complex 114.
Scheme 37.
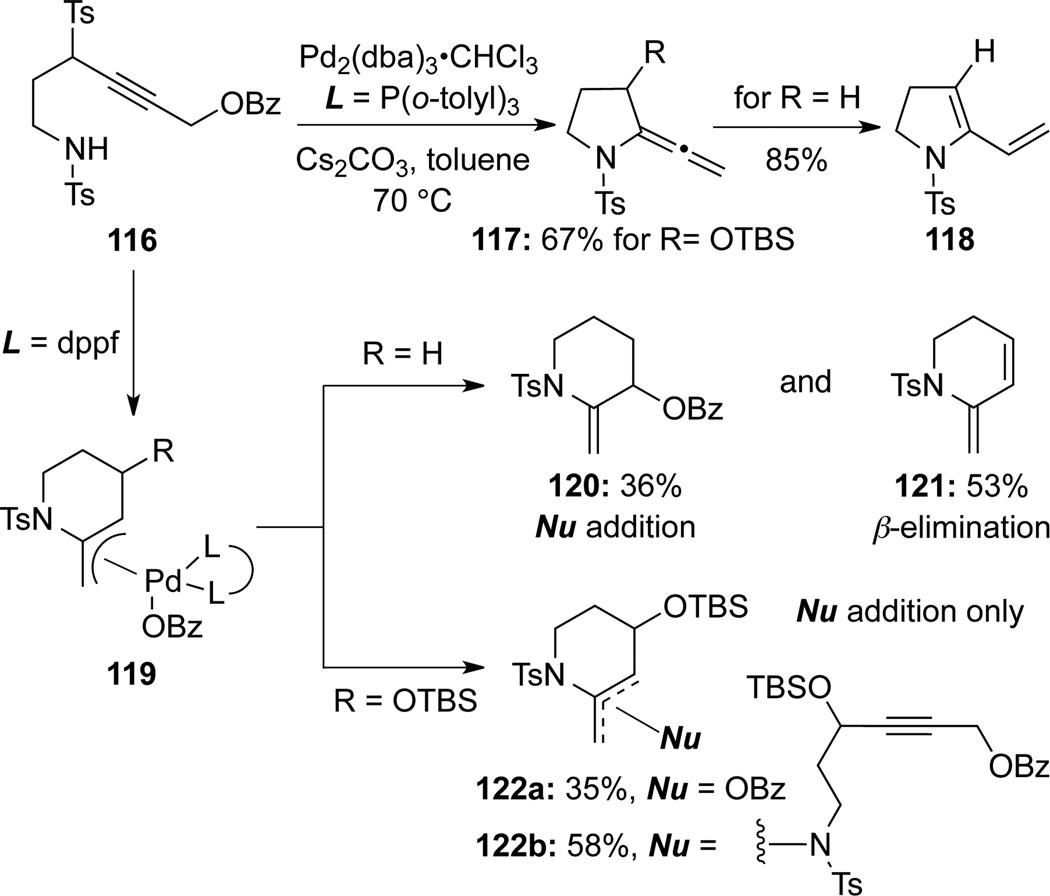
Mori51b later reported this Pd(0)-catalyzed intramolecular cyclization of propargyl ether-containing amides using general N-sulfonyl substituted allenamides 117 (Scheme 38). When using the monodentate ligand P-(o-tolyl)3, for R = OTBS, allenamides 117 could be isolated; However, in the case when R = H, 117 further isomerized to dienamide 118. With dppf serving as ligand, depending on the substrates, enamides 120–122 could be obtained via nucleophilic addition and/or β-elimination pathways from palladium π-allyl complex 119 depending up substitutions (R = H versus OTBS).
Scheme 38.
2.6. Trost-Hsung N-Allenylations
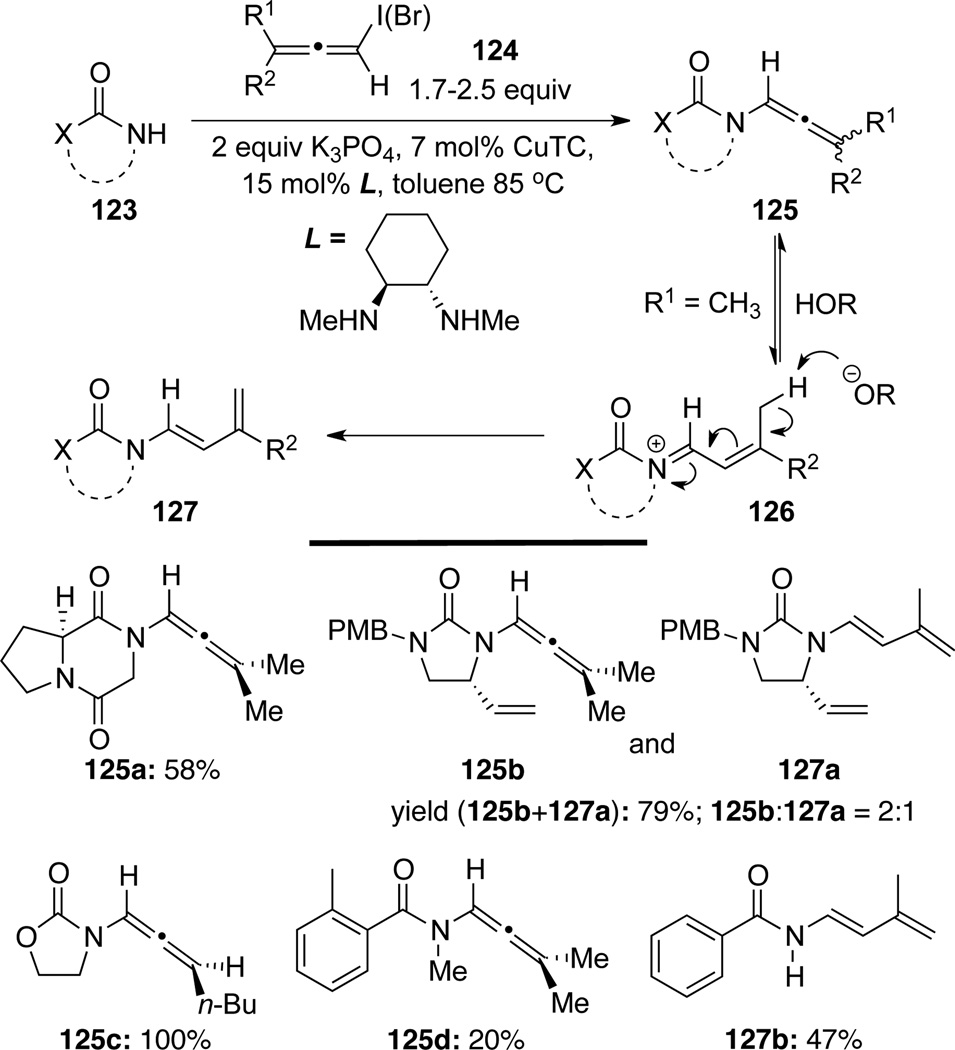
Trost52 reported the synthesis of cyclic and acyclic allenamides 125 by a Cu(I)-catalyzed C-N bond formation between allenyl halides 124 and various amides 123 (Scheme 39). Dienamides 127 were occasionally isolated, which likely resulted from isomerization (or 1,3-H shift) of the initially formed allenamides 125.
Scheme 39.
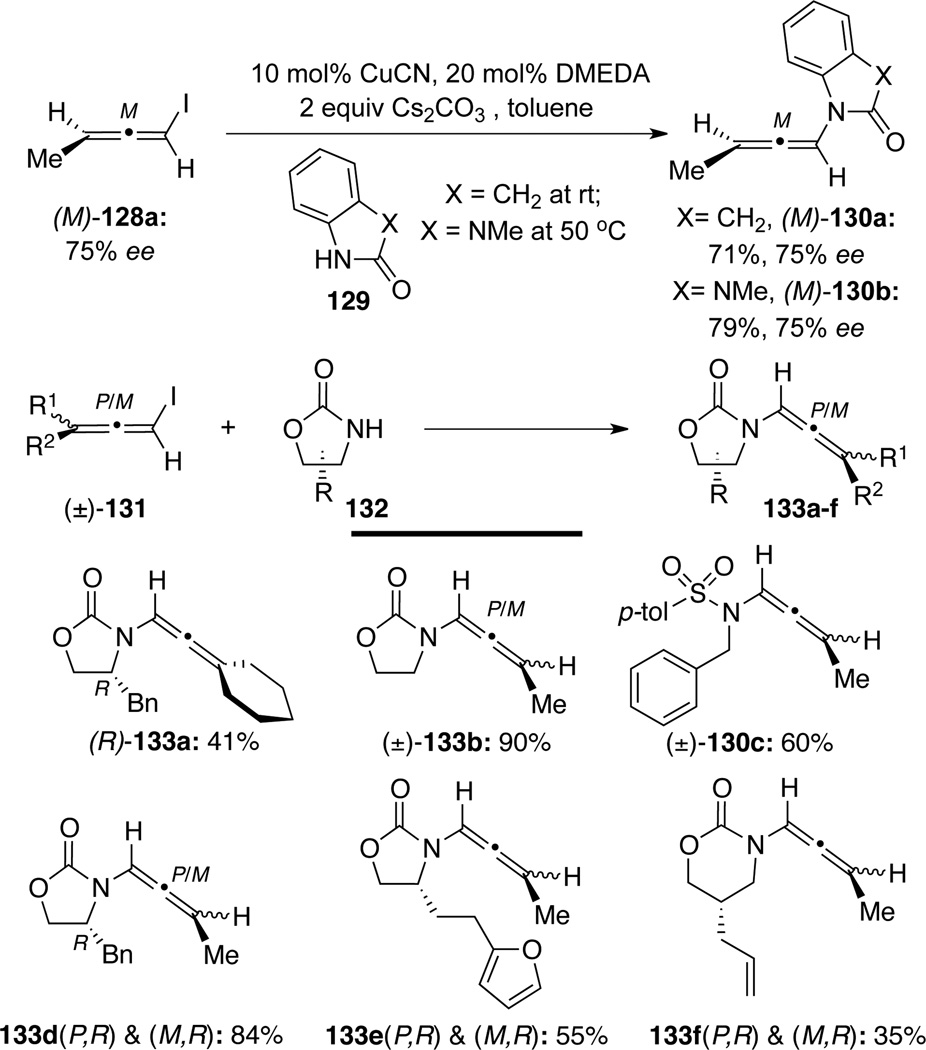
At the same time, Hsung53 also independently documented a related N-allenylation (Scheme 40). More critically, various chiral allenamides 130 were synthesized via Cu(I)-catalyzed stereospecific amidation of optically enriched allenyl halides such as (M)-128a [the (P)-enantiomer also led to the same result – enriched (P)-130a and (P)-130b, although not shown here].54 Under these reaction conditions, chirality information of optically enriched allenyl halides was transferred with high fidelity.
Scheme 40.
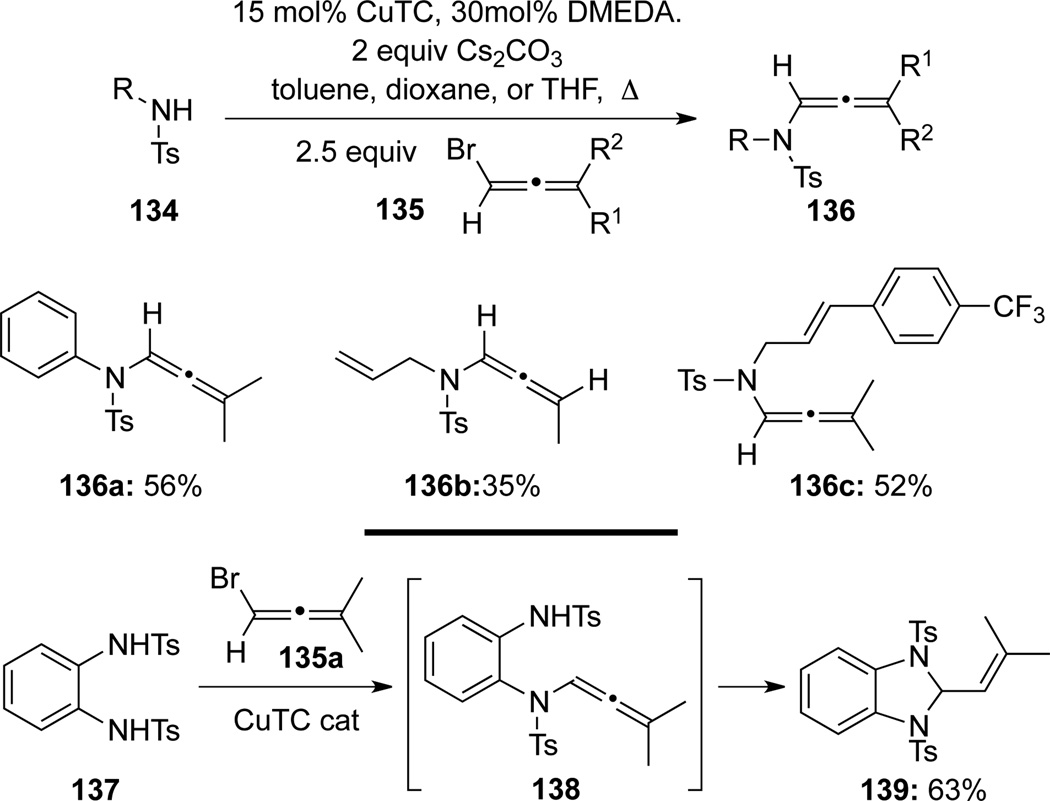
Recently, Bäckvall55 disclosed similar protocols in which a series of N-sulfonyl substituted allenamides 136 were synthesized using bromoallenes 135 and sulfonamides 134 (Scheme 41). In the coupling between N,N′-ditosyl-1,2-diaminobenzene 137 and bromoallene 135a, cyclized product 139 was obtained in 63% yield via the allenamide intermediate 138, thereby representing an intramolecular hydroamination of allenamides.
Scheme 41.
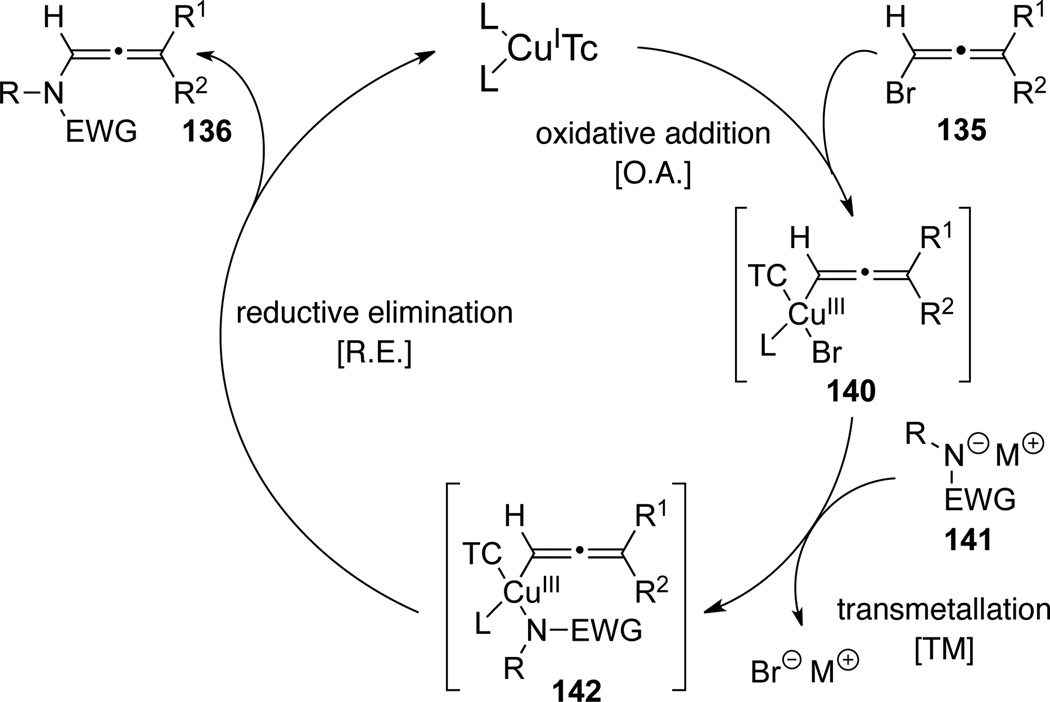
A general mechanism of this Cu(I)-catalyzed cross-coupling reaction is shown in Scheme 42. Overall, the catalytic cycle starts from oxidative addition of Cu(I) onto haloallenes 135 to form Cu(III) intermediates 140, which could then undergo transmetallation with deprotonated amides to form 142. The subsequent reductive elimination would afford allenamides 136. From Hsung’s work, this proposed catalytic cycle implies that the optical integrity of the allenic copper(III) intermediates 140 and 142 could be preserved throughout the amidation or the N-alkenylation process.
2.7. Suzuki-Miyaura Cross-Coupling
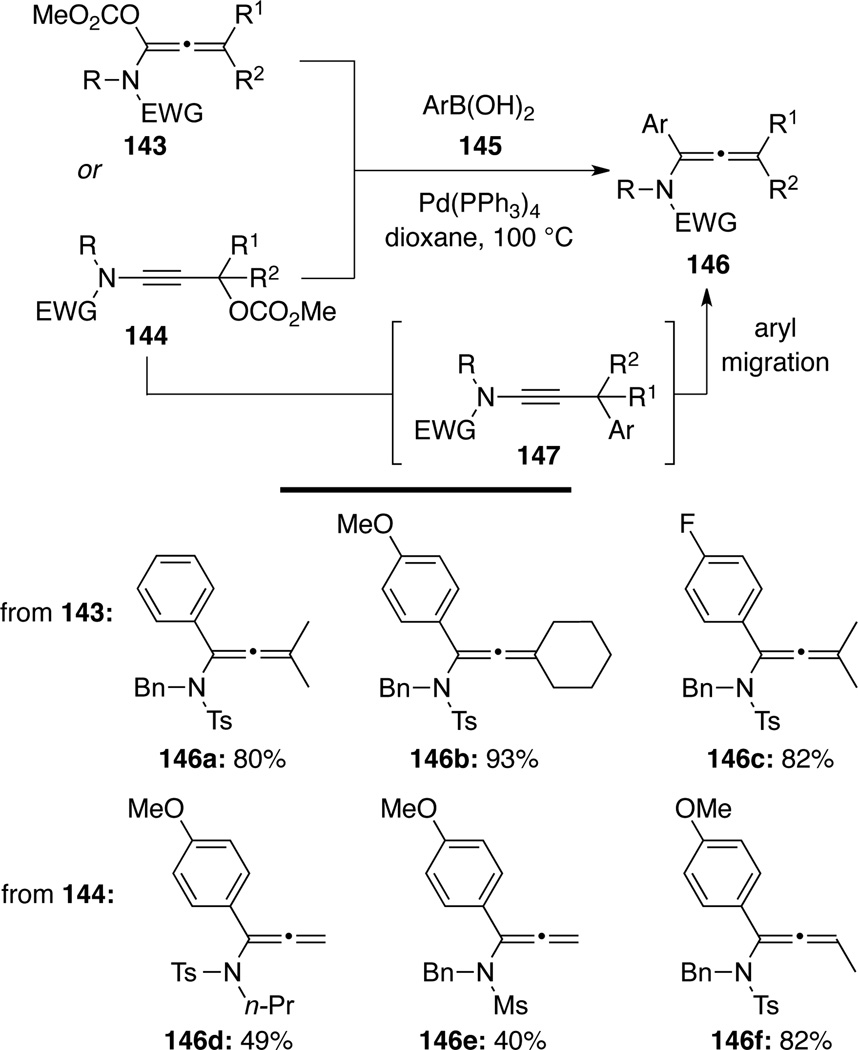
Cao and Lai56 reported α-arylated allenamides 146 through the Suzuki-Miyaura cross-coupling57 between 1-alkoxycarbonyloxy allenamides 143 and arylboronic acids 145 (Scheme 43). Allenamides 146 could also be produced through couplings of 3-alkoxycarbonyloxy ynamides 144 with arylboronic acids 145. A 1,3-aryl migration of the initially generated bulky ynamide intermediate 147 was proposed.
Scheme 43.
3. Reactions of Allenamides
3.1. Deprotonations
3.1.1. α-Deprotonation
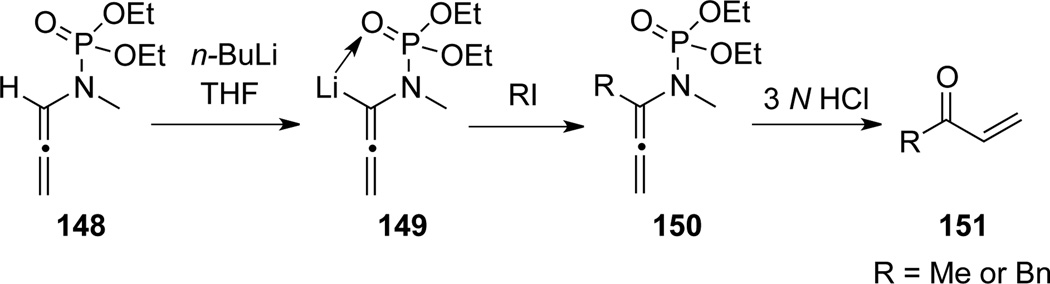
Corbel22 documented the first example of α-deprotonation of allenamide 148 to generate the lithiated allenamide 149, which was trapped by electrophiles to afford α-substituted allenamides 150 (Scheme 44). Subsequent hydrolysis of 150 resulted in the corresponding unsaturated ketones 151.
Scheme 44.
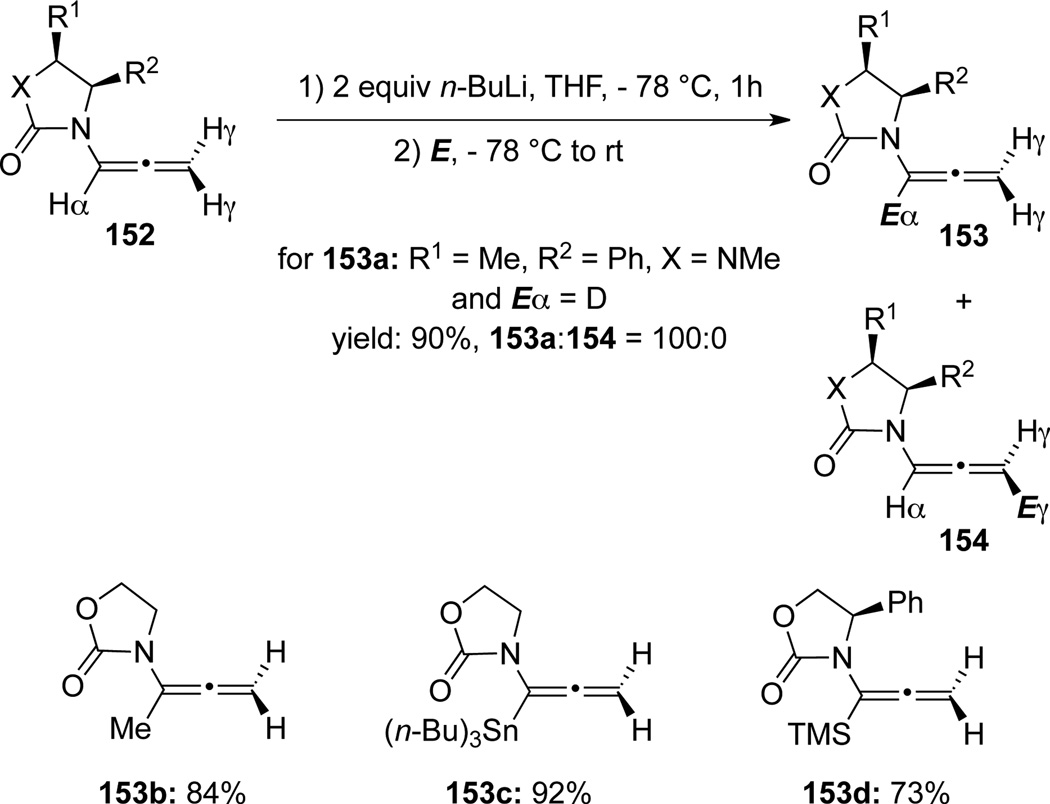
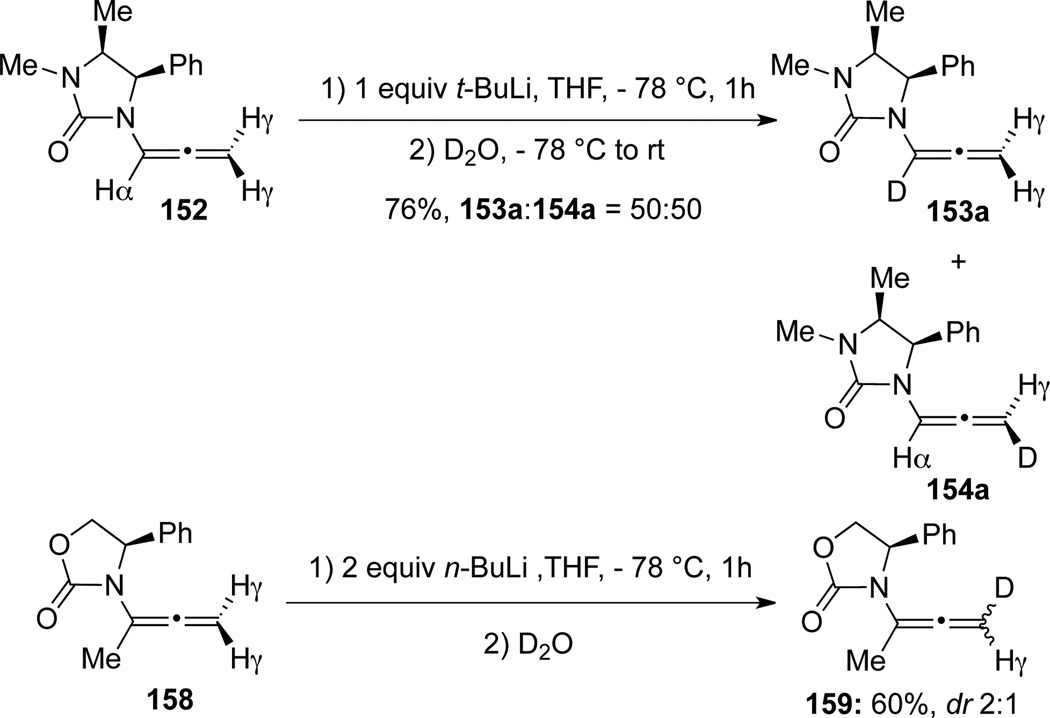
Hsung58 later reported a regioselective α-deprotonation of allenamides 152 using n-BuLi (Scheme 45). The one-pot deprotonation/alkylation sequence can be widely applicable to access a series of α-substituted allenamides 153, γ-deprotonation product 154 was not observed.
Scheme 45.
3.1.2. γ-Deprotonation
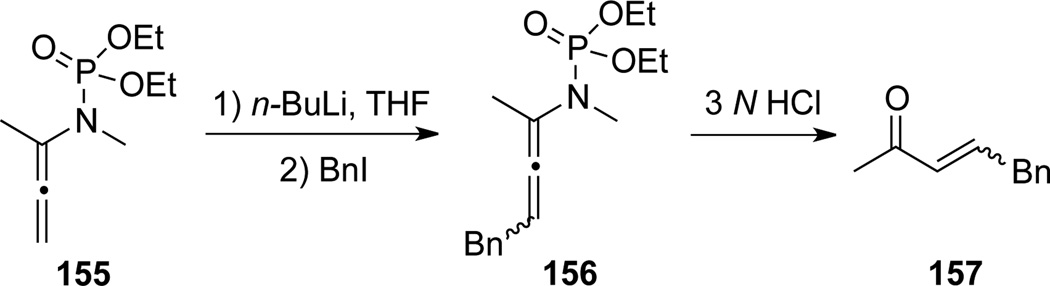
Corbel22 demonstrated that trisubstituted allenamide 156 could be obtained from α-substituted allenamide 155 through a γ-deprotonation/trapping sequence (Scheme 46). Allenamide 156 could also undergo hydrolysis to afford substituted unsaturated ketone 157.
Scheme 46.
Hsung58 reported that when using t-BuLi as the base, the protonation had no α/γ selectivity as revealed after D2O quench, leading to a 50:50 mixture of 153 and 154 (Scheme 47). However, the deprotonation/D2O trapping sequence of allenamide 158 with the α-position blocked would occur at γ-position to afford 159 with moderate diastereoselectivity.
Scheme 47.
3.2. Addition Reactions
3.2.1. Hydroalkoxylation
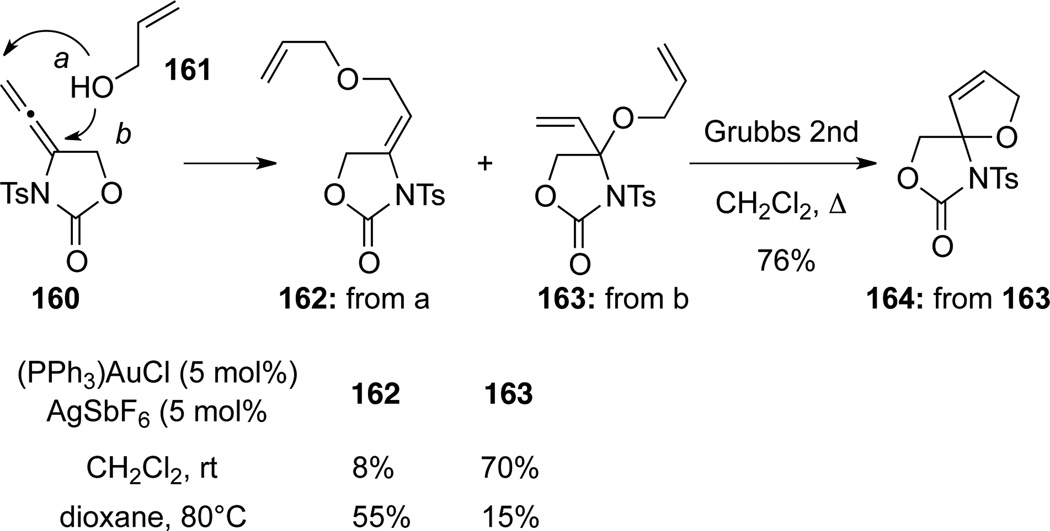
Horino59 found that hydroalkoxylations of allenamide 160 with alcohol 161 could take place at both γ (pathway a) and α (pathway b) positions to afford distal addition product 162 and proximal addition product 163, respectively (Scheme 48). With an Au(I) catalyst, hydroxylation of 160 could be achieved more selectively at the α position to give 163 in high yield. Diene 163 was subsequently used to construct spiro dihydrofuran 164 through Ring-closing metathesis (RCM). In contrast, the distal addition product 162 was more favored under thermal conditions.
Scheme 48.
3.2.2. Hydro-Hydroxyalkylation
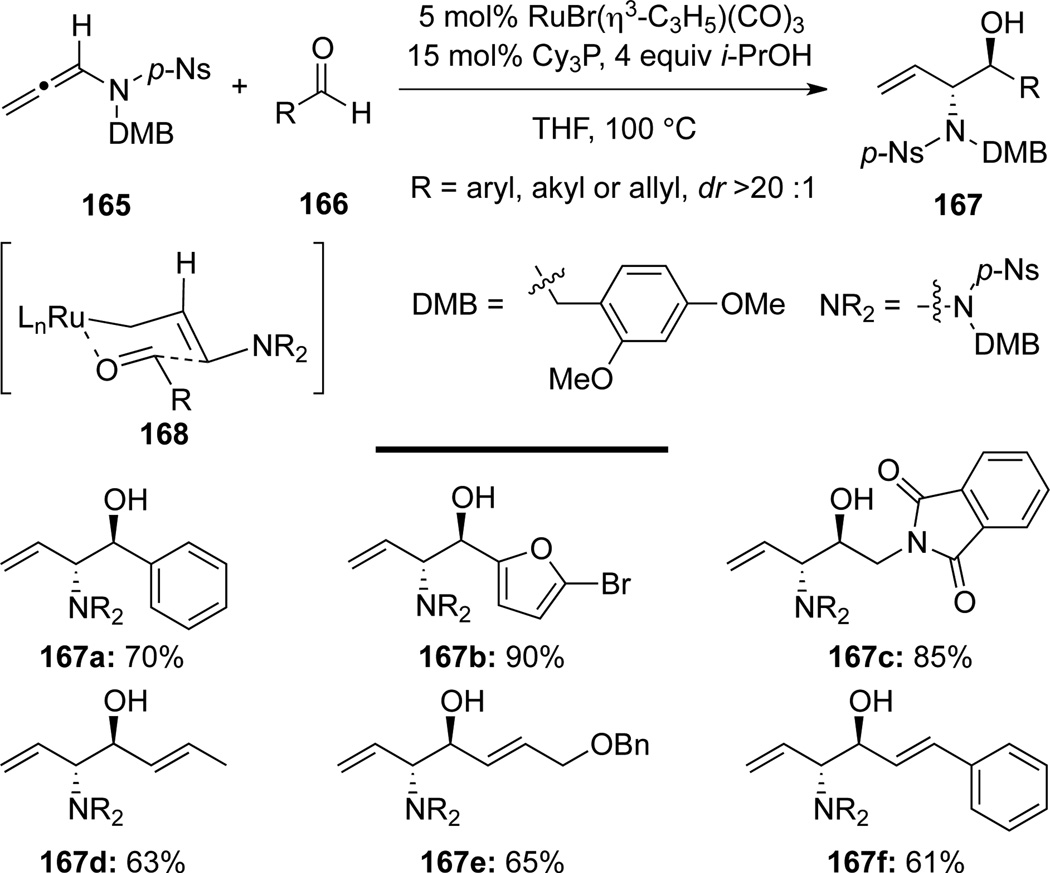
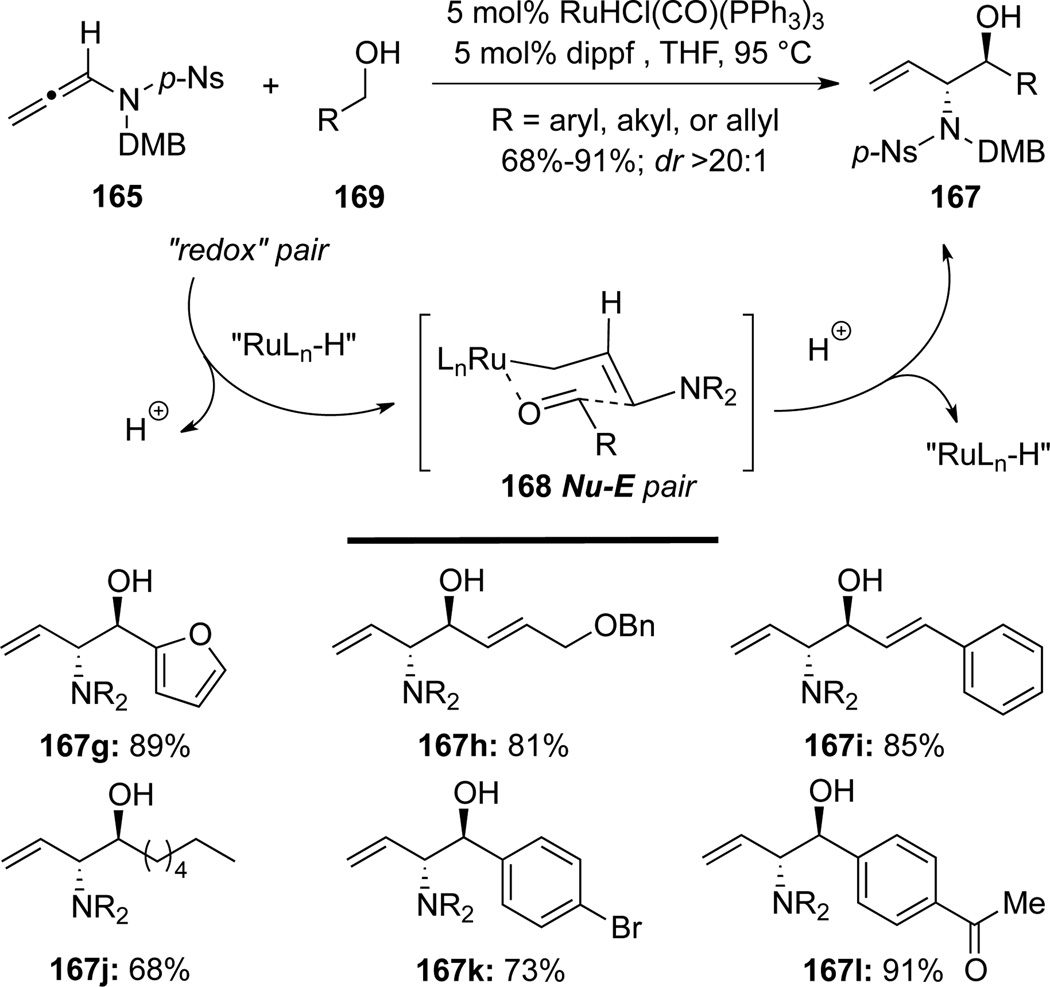
Krische60a cleverly designed an equivalent of aminoallylations of aldehydes via a Ru(II)-catalyzed stereoselective hydro-hydroxyalkylation of allenamide 165 to construct anti-1,2-amidoalcohols 167 (Scheme 49). More specifically, aminoallylations of aldehydes 166 with allenamide 165 were achieved in a highly stereoselective manner via the closed chair-like transition state 168. The hydrometalation of allenamides was achieved through the use of Ru(II)-catalyst with Cy3P serving as the ligand and i-PrOH as the hydrogen source.
Scheme 49.
Krische60b further evolved this highly anti-selective hydro-hydroxyalkylation of allenamide 165 to prepare an array of anti-1,2-amidoalcohols 167 through the use of simple alcohols 169 (Scheme 50). The anti selectivity could be rationalized possibly via the same chair-like transition state formed through “Nu-E” pair 168, which involved complexation of the hydro-ruthenated allenamide intermediate with the aldehyde generated in situ from respective alcohols 169 via hydrogen transfer.
Scheme 50.
3.2.3. Hydroamination
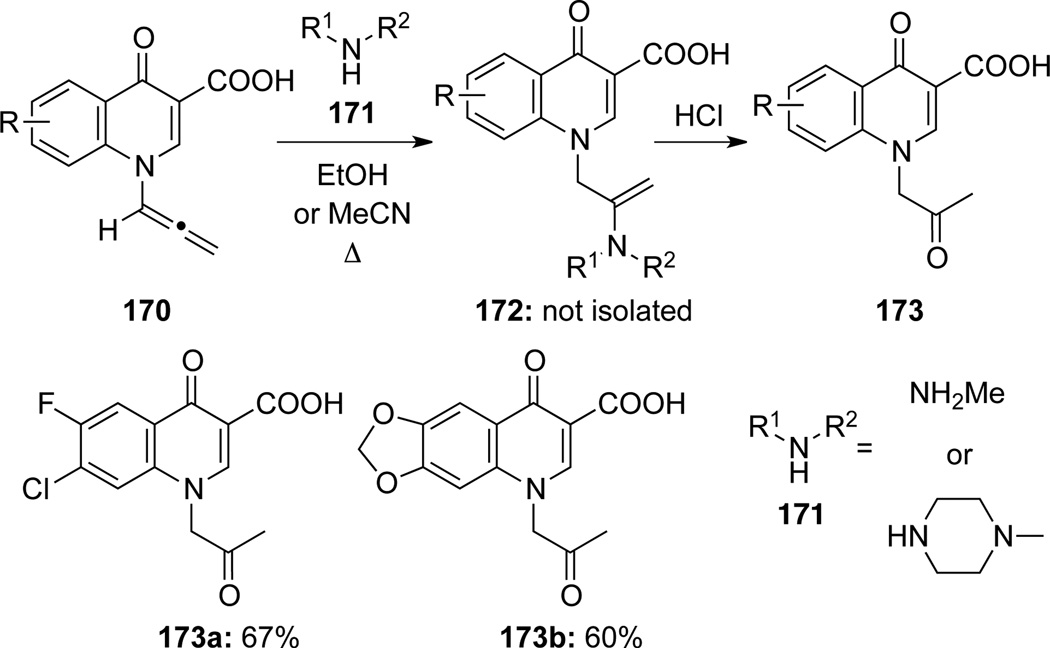
Radl61 reported the preparation of a series of N-2-oxo-propyl-4-quinolones 173 from allenamides 170 via an initial hydroamination reaction using primary or secondary amines 171. Subsequent hydrolysis of the enamine intermediate 172 [not isolated] during the work-up would lead to 173 (Scheme 51). It is noteworthy that the regioselectivity of this hydroamination appears to be opposite from what one would have been predicted based on the general resonance structure of allenamides (see Scheme 2). That is, the nucleophilic amino group landed on the more electron rich central allenic carbon. This could be regarded as an umpolung addition.
Scheme 51.
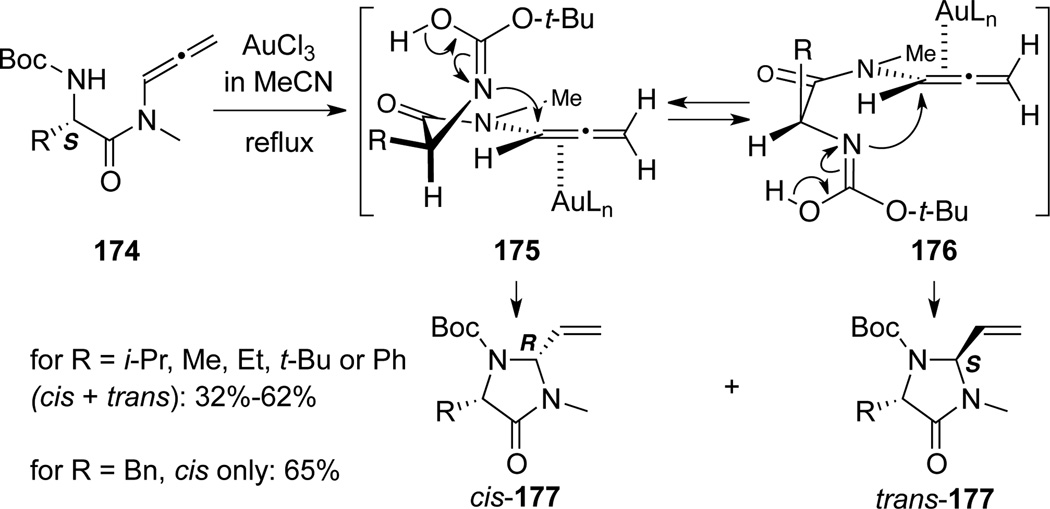
Broggini62 reported an Au(III)-catalyzed intramolecular hydroamination of allenamides 174, which afforded a mixture of cis- and trans 2-vinylimidazolidines 177 through the 5-exo-trig transition states 175 and 176, respectively. Intriguingly, when R = Bn, only cis-177 was isolated in good yield (Scheme 52). Although authors did not provide details, cis- and trans 2-vinylimidazolidines 177 likely came from cyclizations through Au(II)-complexes 175 and 176, respectively [carbamate tautomers were drawn to show clarity].
Scheme 52.
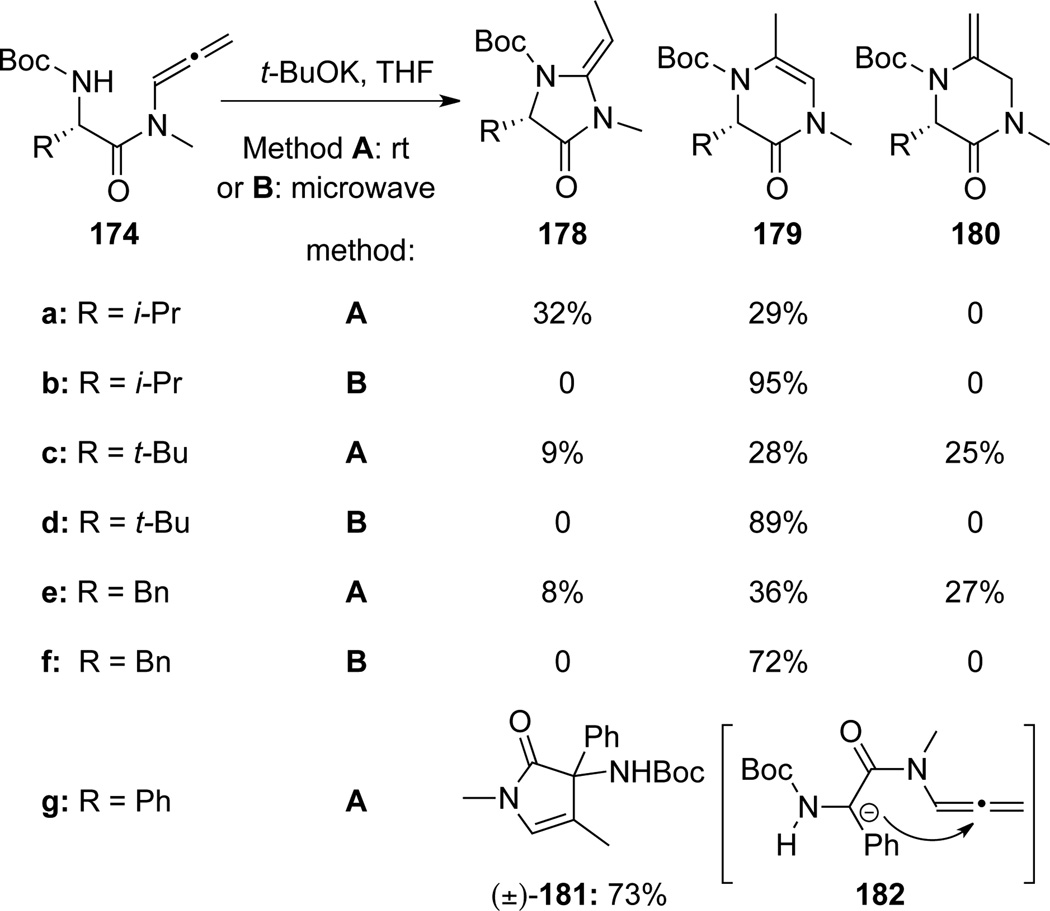
Broggini63 also found that allenamides 174 could be subjected to base promoted intramolecular hydroamination, leading to heterocycles 178, 179 and/or 180 (Scheme 53). In most cases, the cyclization occurs effectively with microwave irradiation at the central allenic carbon to afford 179, thereby representing another umpolung addition. However, no hydroamination products were observed when R = Ph. Instead, at room temperature 181 was obtained via C-C bond formation through the transition state 182.
Scheme 53.
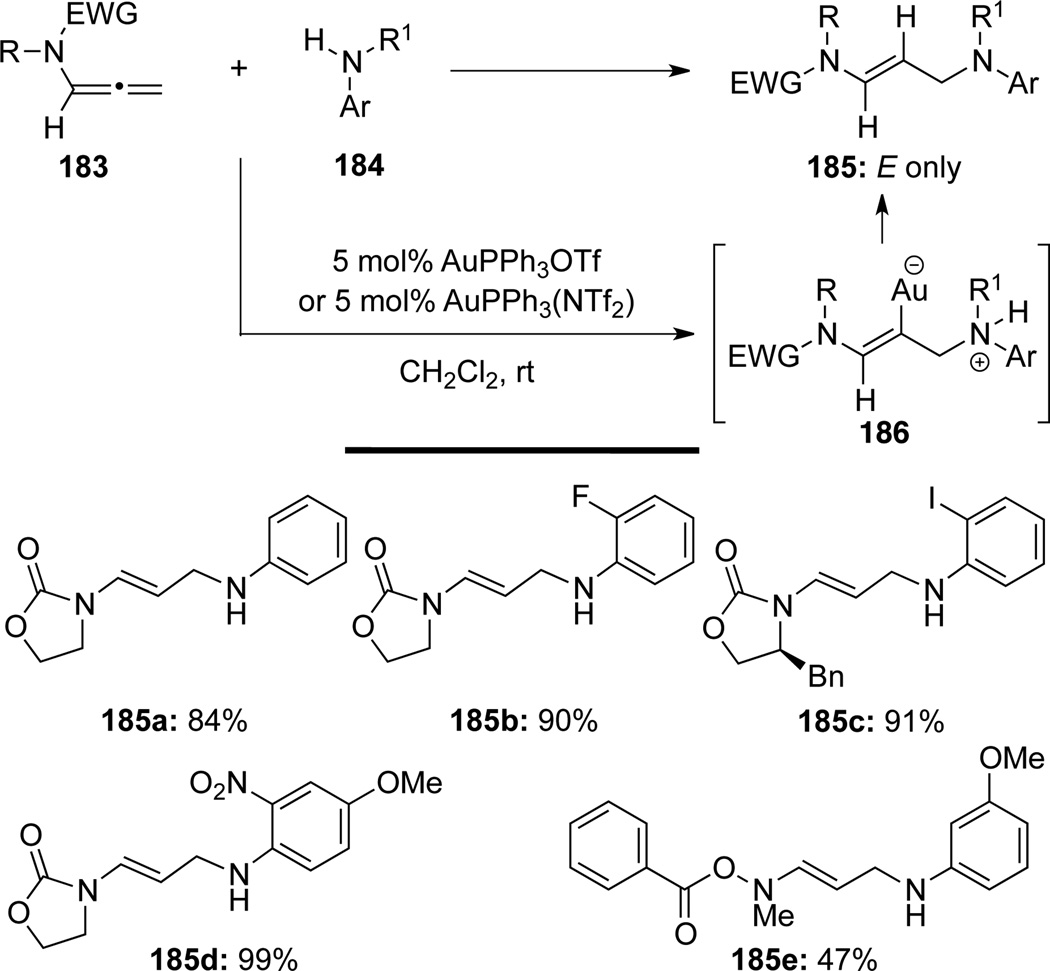
In a clever design of enamide synthesis, Kimber64 reported stereoselective hydroaminations of allenamides 183 with anilines 184 under Au(I)-catalyzed conditions to afford E-enamides 185 via intermediates 186 (Scheme 54).
Scheme 54.
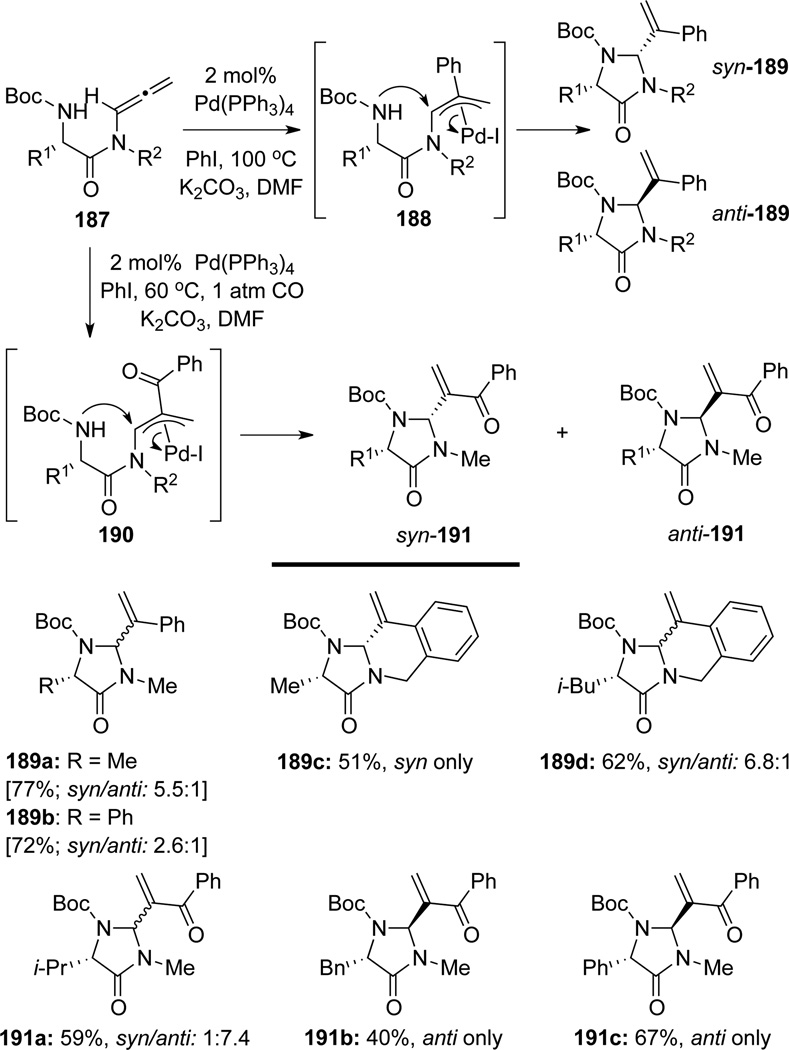
Broggini65 evolved their hydroamination method into the palladium-catalyzed intramolecular carboamination of allenamides for the synthesis of 4-imidazolidinones 189 and 191 (Scheme 55). In this work, they employed optically enriched a-amino allenamides 187, and the reaction proceeded through a palladium-catalyzed carbopalladation–5-exo-dig amination process via palladium π-allyl intermediates 188 or 190 [carbonylated]. It is noteworthy that this cascade worked well for intramolecular carbopalladation of allenamides en route to tricyclic fused-ring imidazolidinones such as 189c and 189d.
Scheme 55.
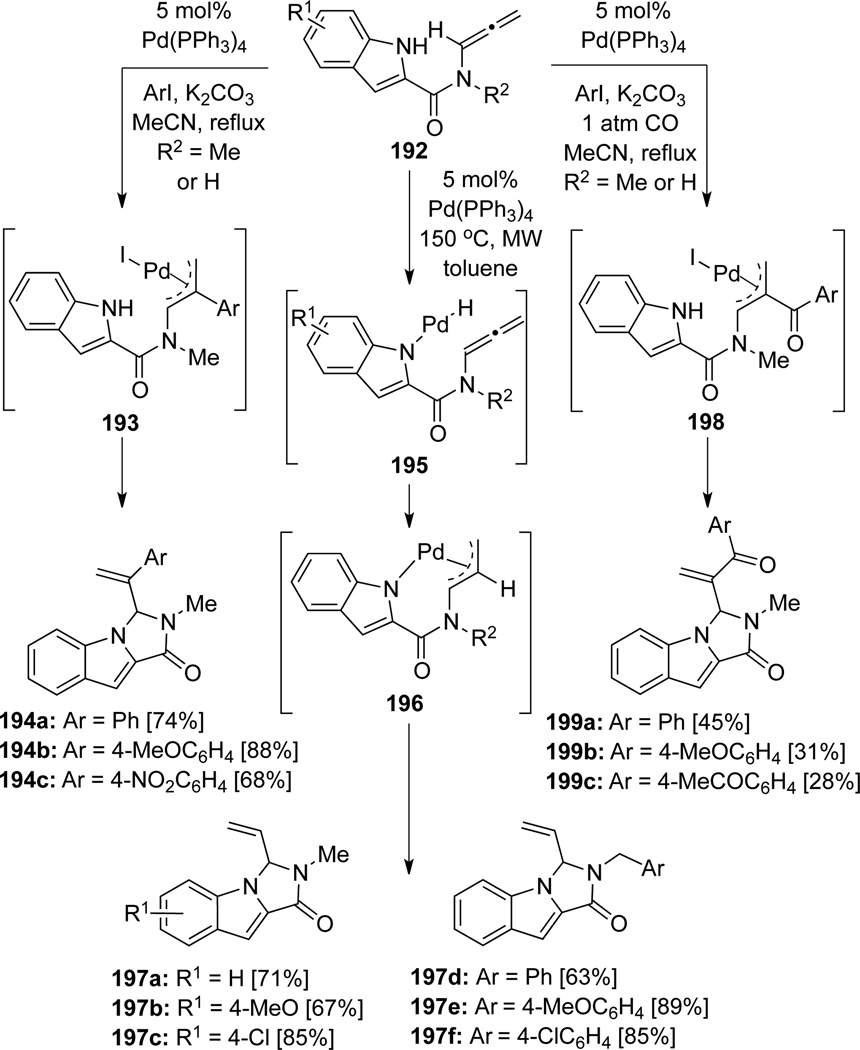
Broggini66 further developed their carboamination and microwave-assisted hydroamination work using allenamides 192 that contains an indolyl unit (Scheme 56). Using 192, Styryl-substituted indoloimidazoles derivatives 194 were synthesized through the intermediacy of palladium π-allyl complex 193 in which the trapping exclusively occurred at the internal allenic carbon by the indole nitrogen. In the presence of CO, this cyclization cascade could afford indoloimidazoles 199. In addition, the authors found that the use of microwave was crucial in dictating the hydroamination pathway of 192. In particular, under the microwave activation, oxidative addition of the indolyl NH bond took place to give the Pd(II)-complex 195. A subsequent hydride transfer or hydro-palladation would lead to the palladium π-allyl intermediate 196 and streamline the reaction pathway toward vinyl indoloimidazoles 197.
Scheme 56.
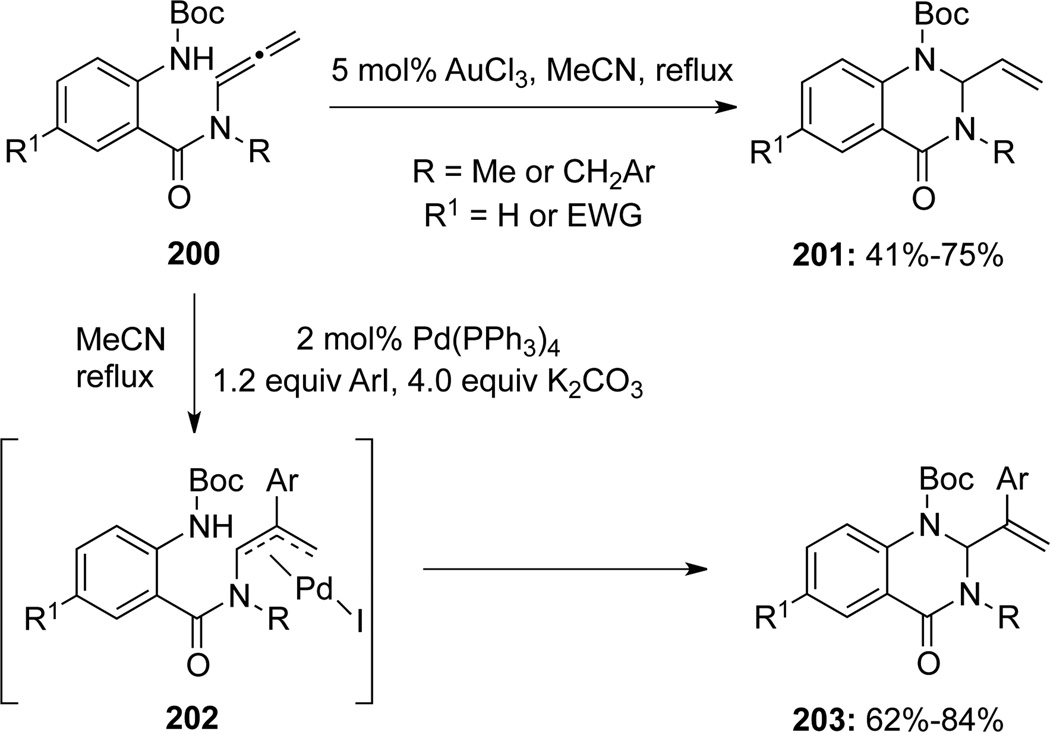
More recently, Broggini67 documented an Au(III)-catalyzed intramolecular hydroamination of allenamides 200 to give 2-vinyl-4-quinazolinones 201 (Scheme 57). In addition, when switching to Pd(0) catalyst and along with ArI, the carboamination products 2-(α-styryl)quinazolin-4-one derivatives 203 could again be produced with good yields.
Scheme 57.
Again, Bäckvall55 also has an example already shown in Scheme 41 (See 137→138→139) in Section 2.6.
3.2.4. Hydroarylation
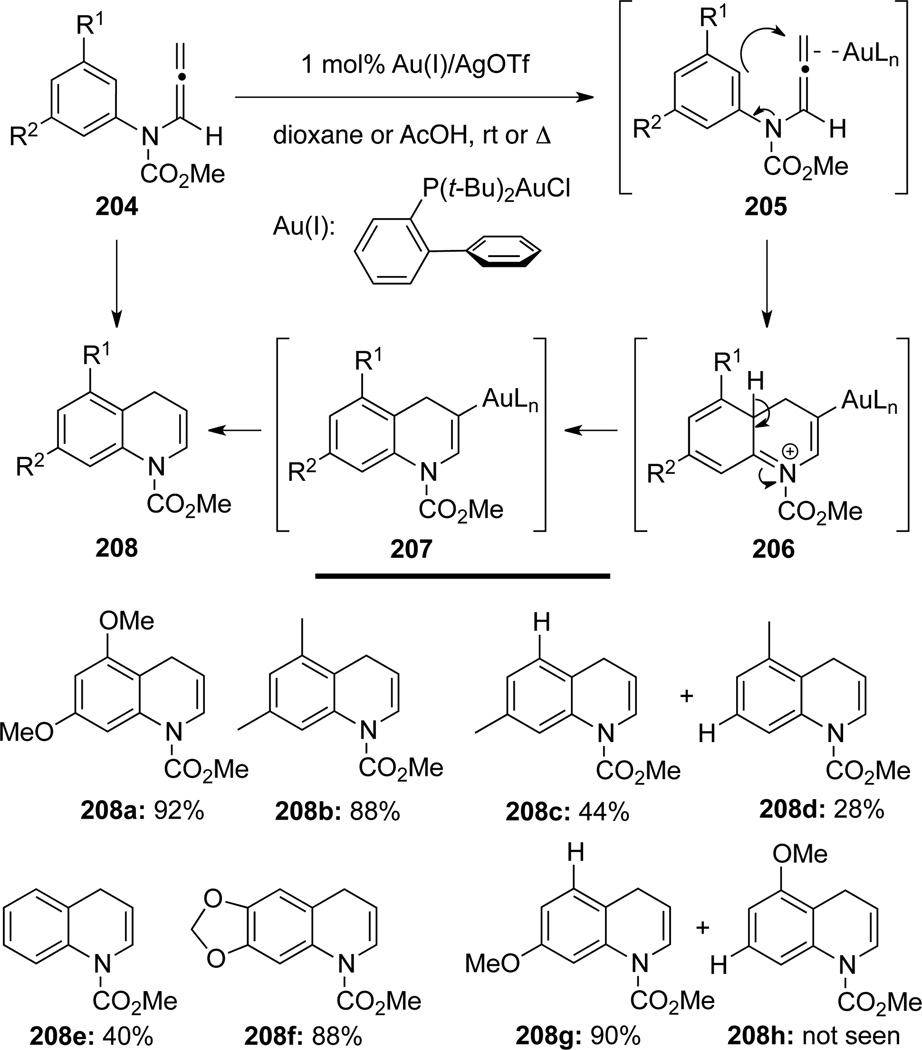
Oishi, Fujii and Ohno68 developed the first gold-catalyzed intramolecular hydroarylation of allenamides 204 for the formation of dihydroquinolines 208 (Scheme 58). Mechanistically, the Au(I)-coordinated allenamides 205 could undergo cyclization to form the vinyl-gold cation complex 206 via an intramolecular SE-Ar reaction. Upon deprotonation-rearomatization, the neutral vinyl gold complex 207 could undergo protodemetalation to form the dihydroquinoline products 208. For the unsymmetrical arenes, the regioselectivity was found to be ranging from moderate to excellent (see 208c/208d and 208g/208h).
Scheme 58.
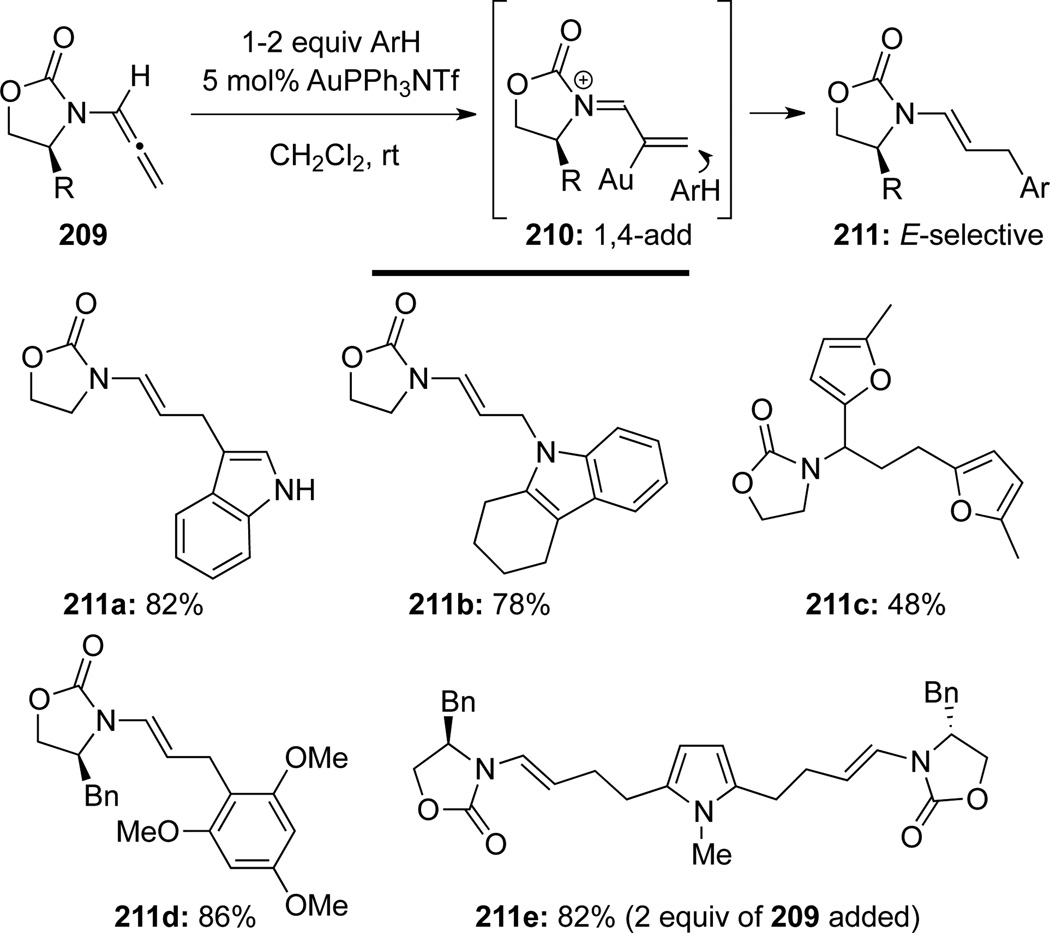
Kimber69 reported a highly stereoselective hydroarylation of allenamides 209 to obtain a series of achiral and chiral oxazolidinone-derived E-enamides 211 via the 1,4-addition to N-acyl iminium intermediates 210 (Scheme 59). This gold-catalyzed hydroarylation took place relatively faster than their Pd- and Ru-catalyzed counterparts. Bis-hydroarylation products could be obtained in some cases. For example, 209 reacted with furan twice through both 1,4- and 1,2-hydroarylation to give 211c. In another case, bis-hydroarylation afforded 211e when 2 equiv of 209 were used.
Scheme 59.
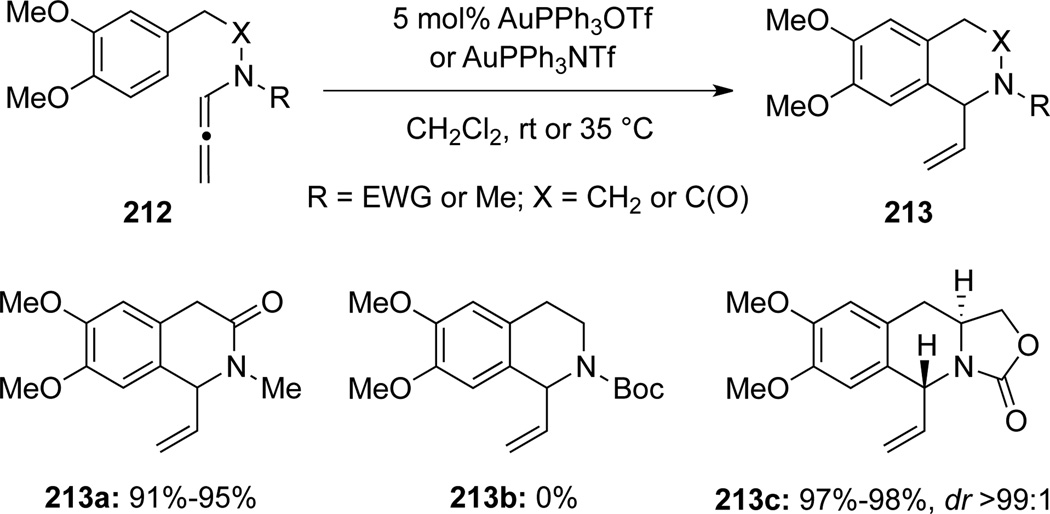
Kimber70 also unveiled an Au(I)-catalyzed intramolecular hydroarylation of allenamides 212 for the construction of α-vinyl substituted tetrahydro isoquinolines 213 (Scheme 60). When R = Boc, the hydroarylation reaction was impeded presumably due to the steric hindrance of the bulky Boc group. On the other hand, these hydroarylations can be highly diastereoselective as shown with 213c, which was isolated as a single diastereomer.
Scheme 60.
See Section 3.6.6. [3 + 3] Formal Cycloadditions for another example.
3.2.5. Hydroalkenylation
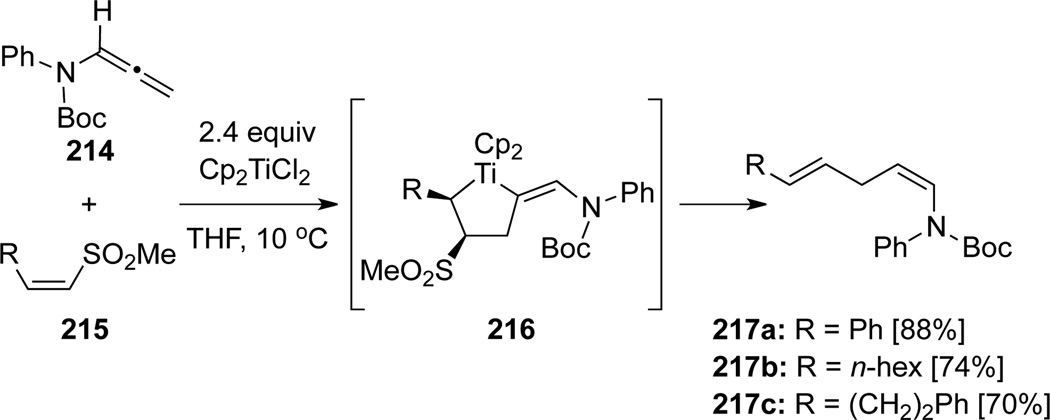
Takeda71 reported a titanocene(II)-promoted cross-coupling of allenamide 214, which could generate 1,4-dienes 217 regioselectively via the formation of the 2-alkylidenetitanacyclopentane complex 216 (Scheme 61). This process formally constitutes a hydroalkenylation [or hydrovinylation] of allenamides.
Scheme 61.
3.2.6. Hydroacylation
See Section 3.3 Aldol Reactions.
3.2.7. Cyano- and Boro-Acylations
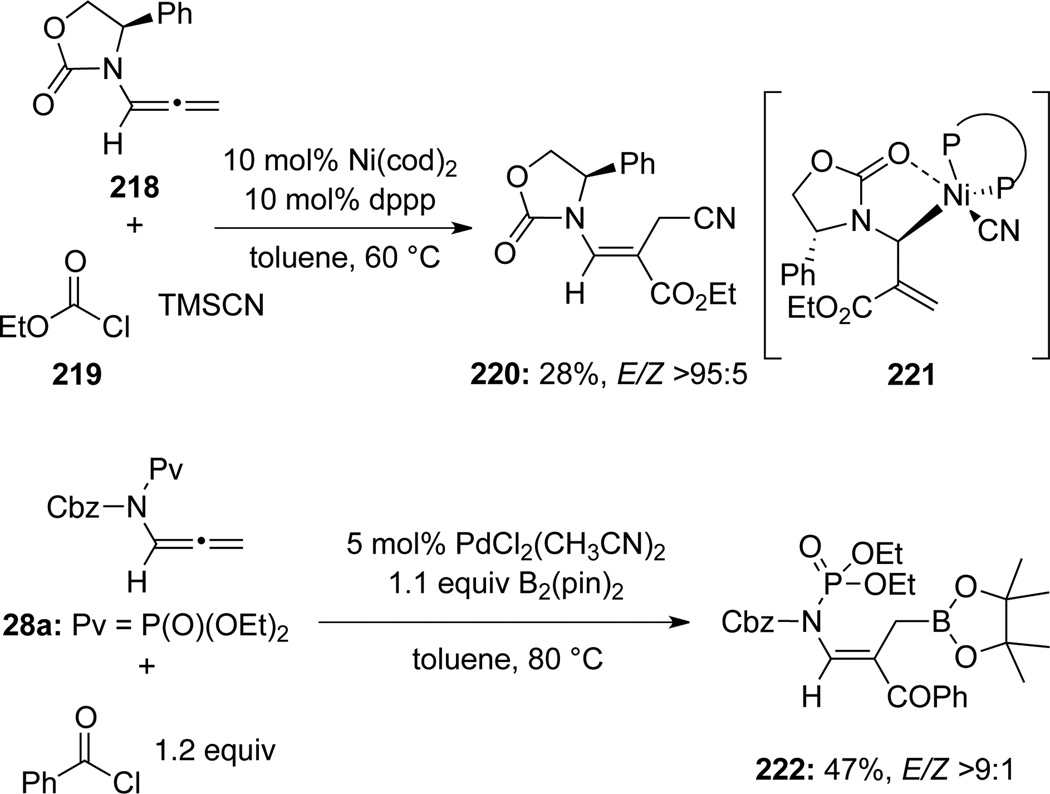
Nakao and Hiyama72 discovered that with a Ni(0) catalyst, cyanoesterification of allenamide 218 could take place to afford highly functionalized E-enamide 220 with moderate yield as well as high regio-and stereoselectivity via the Ni(II)-complex 221 (Scheme 62). The process constitutes a three-component coupling protocol with cyanoformate being generated in situ from chloroformate ester 219 and TMS-CN. Mapp27 also demonstrated that their de novo allenamide 28a (see Scheme 13) could be employed in a palladium-catalyzed regio- and stereoselective boro-acylation of the terminal allenic olefin, leading to enamide 222 (Scheme 62).
Scheme 62.
3.2.8. Hydrostannylation
See Section 3.4.2. Palladium Catalyzed Cyclizations.
3.2.9. Silylstannylation
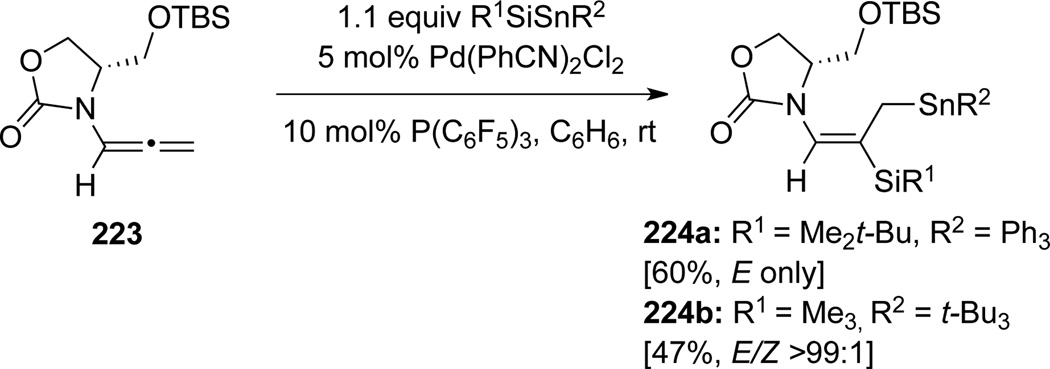
Rajanbabu73 found that highly functionalized E-silylenamides 224 could be obtained from allenamide 223 via a Pd-catalyzed silylstannylation in an excellent regio- and stereoselective manner (Scheme 63).
Scheme 63.
3.3. Aldol Additions
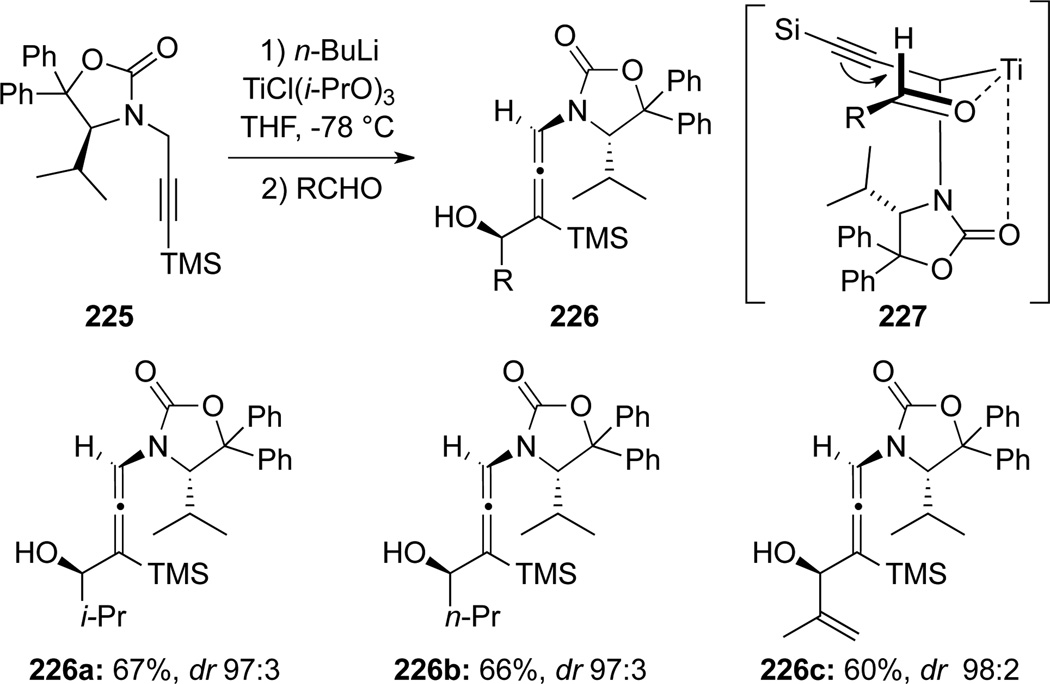
Seebach74 first demonstrated that in the presence of TiCl(i-PrO)3, lithiated chiral propargyl amides could undergo 1,2-additions to aldehydes in a stereoselective manner (Scheme 64). When chiral propargyl amide 225 was treated with n-BuLi and TiCl(i-PrO)3, and subsequently added to various aldehydes, an array of de novo γ,γ-disubstituted chiral allenamides 226 were obtained with high stereoselectivity. The excellent selectivity could be rationalized through a highly organized chair-like transition state shown in the propargylic titanium complex 227 after the transmetallation.
Scheme 64.
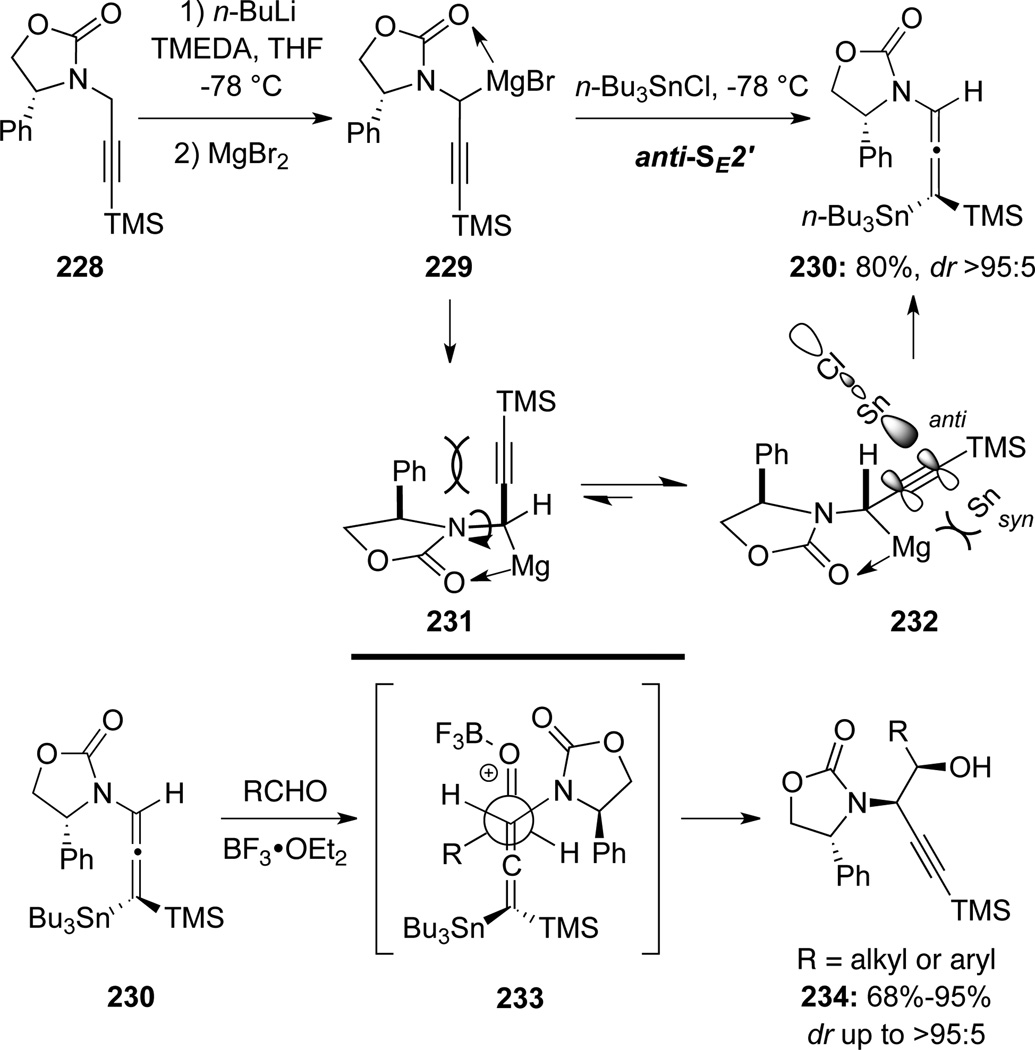
Hegedus75a found that transmetallating deprotonated chiral N-propargyl-oxazolidinones with MgBr2 could produce de novo optically active γ-stannylated allenamide 230 through a highly stereoselective anti-SE2′ process (Scheme 65). The presence of MgBr2 favors coordinated intermediates but with transition state 232 being preferred over 231, which suffers from syn-pentane like steric interaction. These authors subsequently developed an elegant application of these novel stannylated allenamides. Specifically, a BF3-OEt2 catalyzed aldol reaction between γ-stannylated allenamide 230 and various aldehydes afforded an array of β-hydroxylpropargyl amides 234 with high syn-stereoselectivity through the preferred open transition state 233.
Scheme 65.
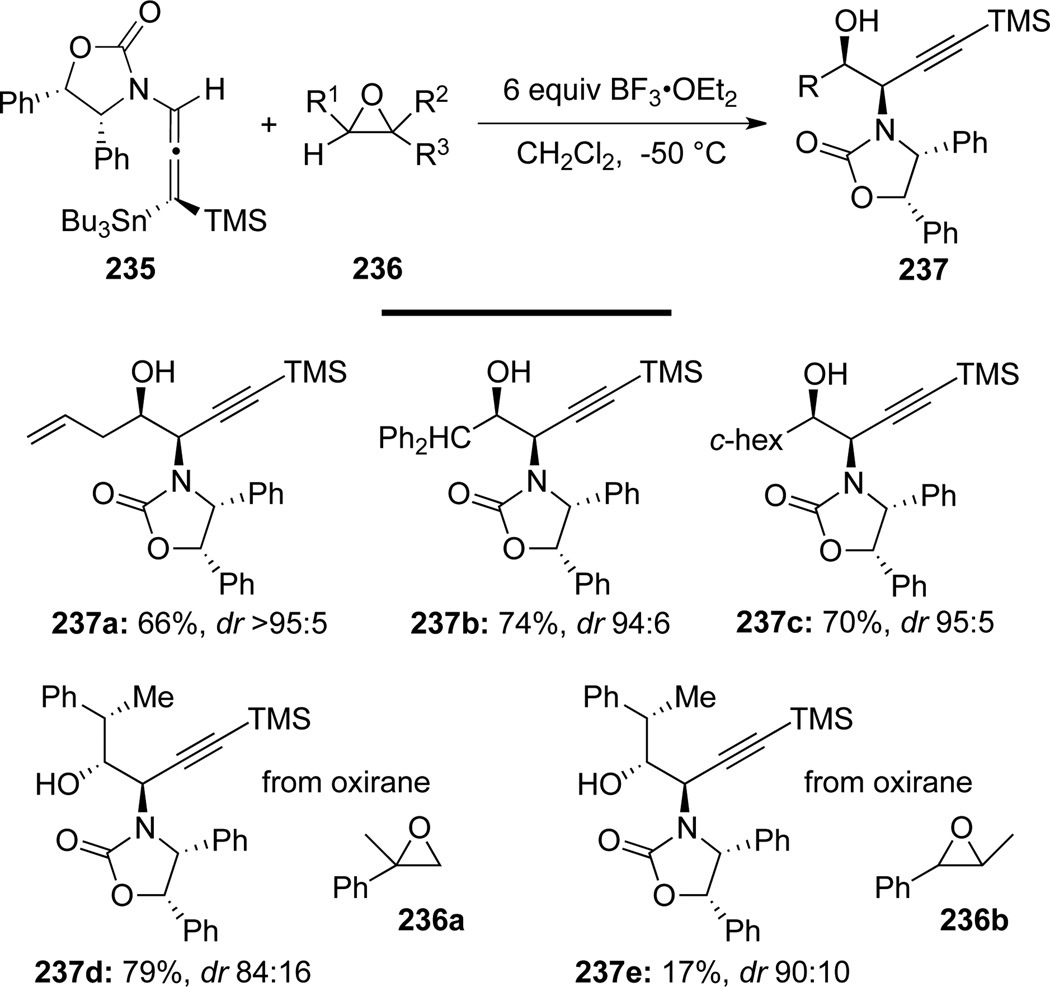
Hegedus75b also found that in the presence of BF3-OEt2, the readily available oxiranes 236 could be the precursor in place of aldehydes (through a Lewis acid assisted alkyl, aryl or hydride-shift) for the aldol addition to chiral γ-stannylated allenamide 235 (Scheme 66). These reactions likely proceed through a similar open transition state as shown in 233 (Scheme 65), leading to β-hydroxyl propargyl amides 237 with high syn-stereoselectivity.
Scheme 66.
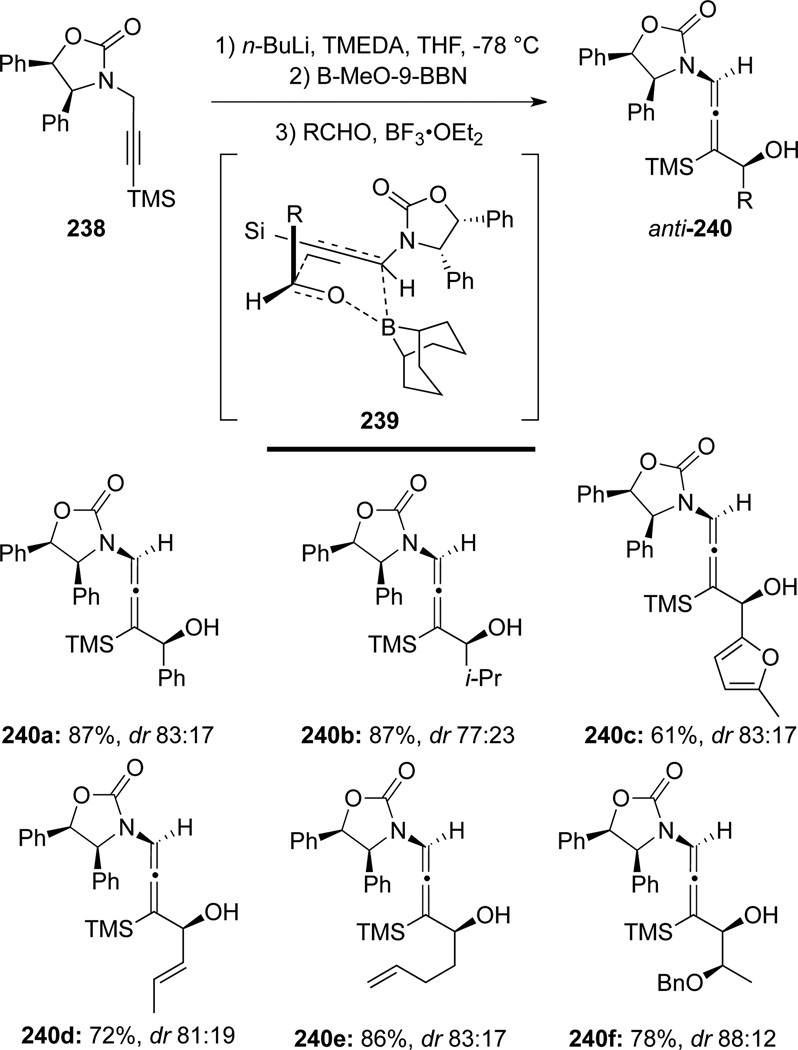
Hegedus76 later disclosed that optically active γ,γ-disubstituted allenamides 240 could be synthesized (Scheme 67). However, in this case, with the aid of 9-BBN derived organoboranes, high anti-stereoselectivity was achieved through the transition state shown in 239. This method proves to be an excellent stereochemical complement to Seebach’s work.74
Scheme 67.
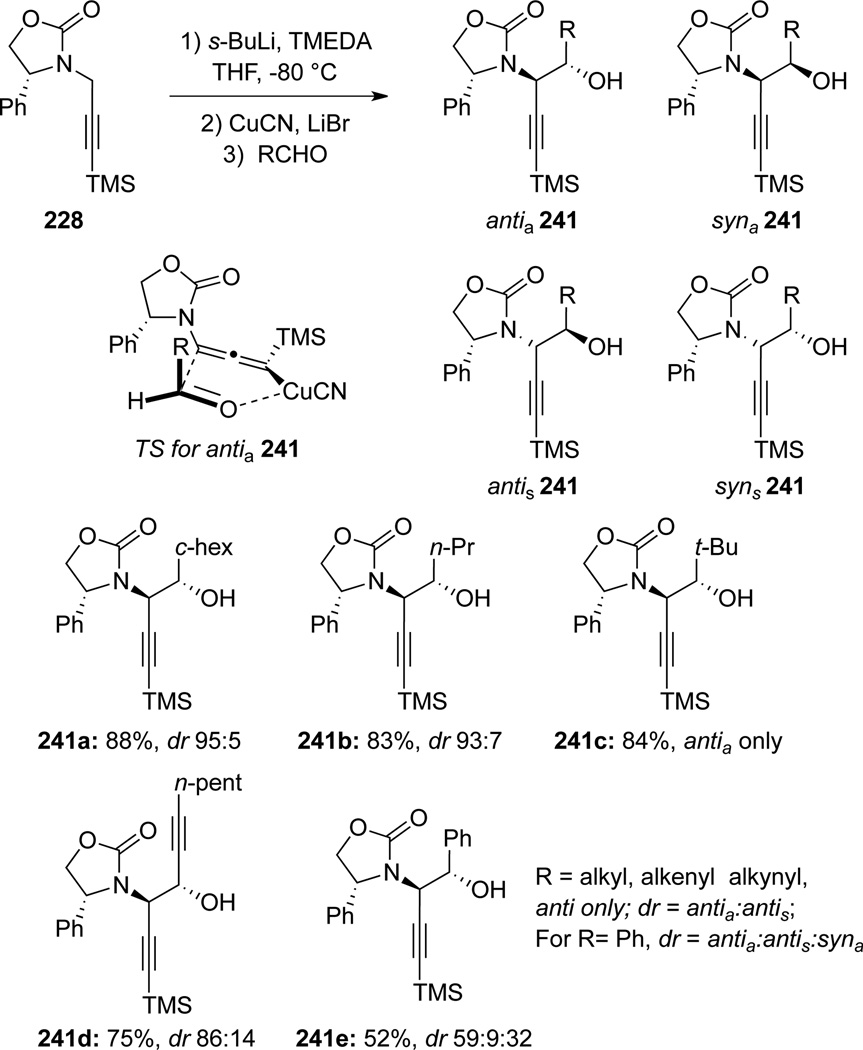
Vrancken and Mangeney77 found that lithiated allemamides generated from the propargyl amide 228 could undergo aldol reactions in the presence of CuCN to afford anti-homopropargyl amino alcohols 241 with high yields and diastereoselectivities (Scheme 68). In most cases, the addition proceeds through the favored transition state antia241.
Scheme 68.
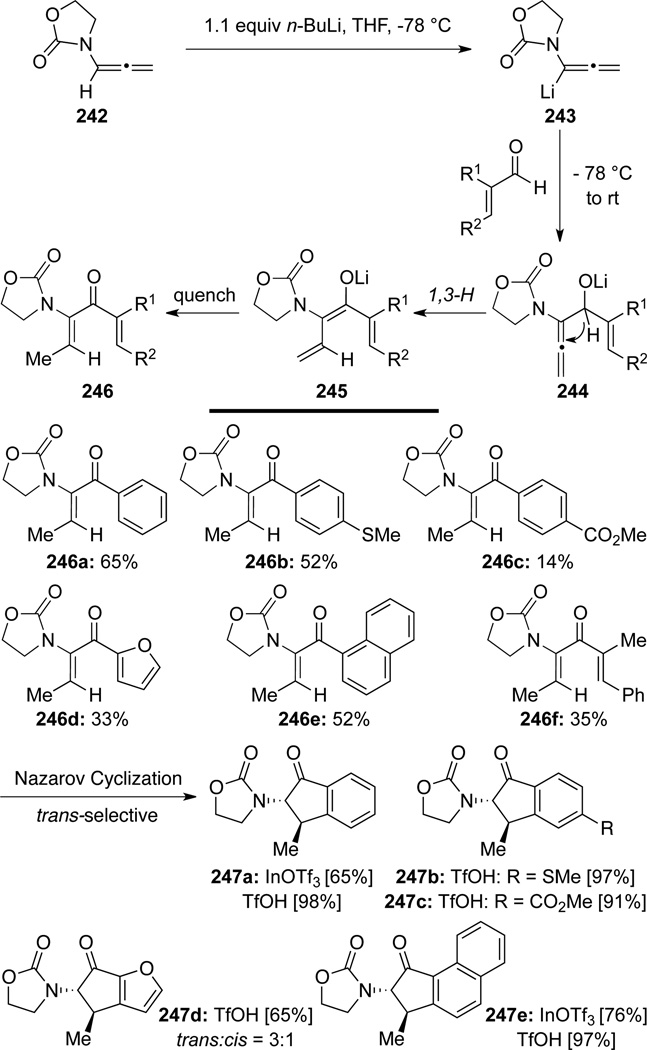
West78 found that when lithiated allenamides 243 reacted with unsaturated aldehydes, the resulting aldol product would undergo a 1,3-H shift to afford 2-amido-1,4-pentadiene-3-ones 246 (Scheme 69). While this work is being classified under aldol reaction, this beautiful tandem sequence of aldol addition–1,3-H-shift sequence constitutes a formal hydroacylation process of allenamides, and is indeed also related to Section 3.7. Tandem Cascades via Isomerizations. Subsequently, West carried out Lewis or Brønsted acid-promoted Nazarov cyclization employing these 2-amido-1,4-pentadiene-3-ones 246. Specifically, a reaction with 0.2 equiv of In(OTf)3 or 5.0 equiv of TfOH was found to be highly effective, leading to 2-amido-1-indanones 247 in good yields and high diastereoselectivity in most cases.
Scheme 69.
3.4. Cyclizations
We note here that some of the intramolecular hydroamination work featured in Section 3.2.3. Hydroamination (see Schemes 52, 53, and 55), and intramolecular hydroarylation reports showcased in Section 3.2.4. Hydroarylation (see Schemes 58 and 60) also involved cyclizations.
3.4.1. Non-Transition Metal-Mediated
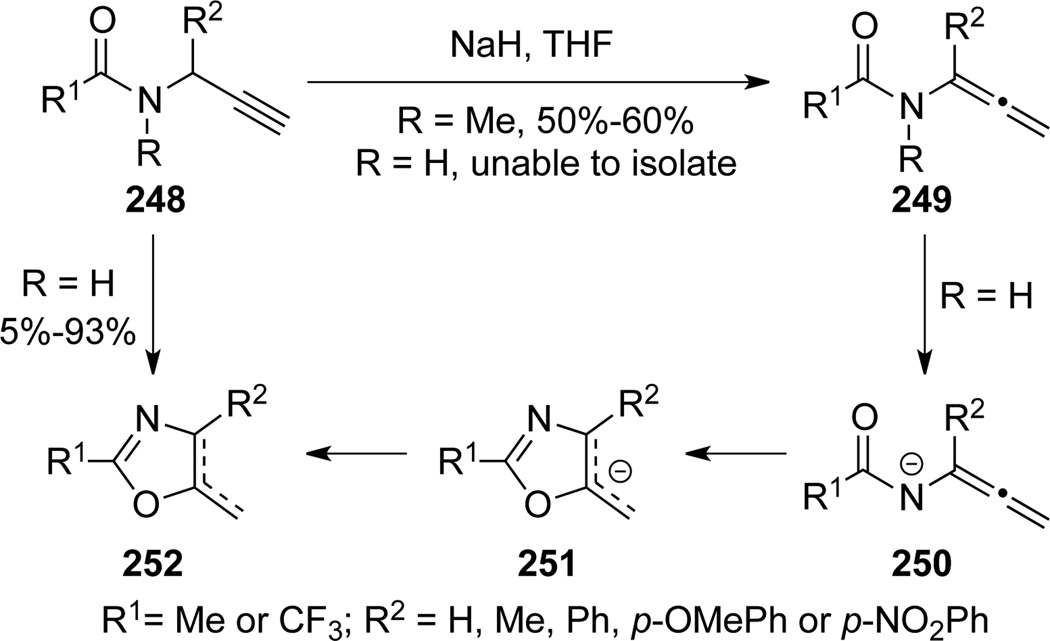
Hacksell79 reported the first base-promoted cyclization involving allenamide intermediates in their effort to achieve N-methylation of propargyl amides 248 (Scheme 70). With NaH, methylated (when R = Me) propargyl amides isomerized to allenamides 249 in moderate yields. When R = H, allenamides 250 were believed to be the intermediate en route to the cyclized oxazoles 252.
Scheme 70.
Tanaka and Torii48a synthesized a series of antibiotics 3-norcephems 255 from α-acylated β-lactam derived allenamides 253 (Scheme 71). In their work, the key step involved a CaCl2-assisted 1,4-addition of nucleophiles to the central allenic carbon of 253 as shown in the complex 254. Although this could be considered also as an umpolung phenomenon, with the α-acyl group in 253, this is more in line with a classical 1,4-addition. An ensuing cyclization via a vinylogous enolate addition would furnish 255.
Scheme 71.
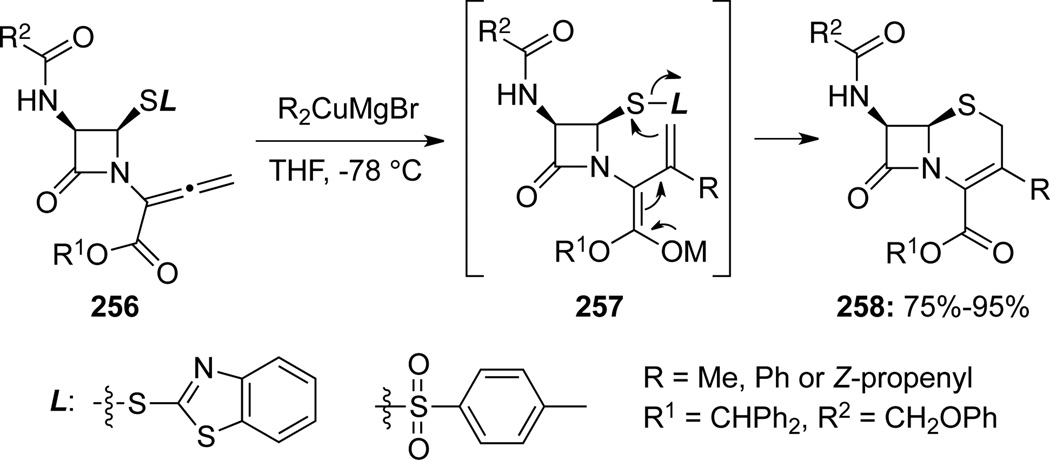
Kant and Farina80 reported a related conjugate addition of organocuprates to allenamides 256 (Scheme 72). Subsequent cyclization through intermediates 257 would give cephams 258.
Scheme 72.
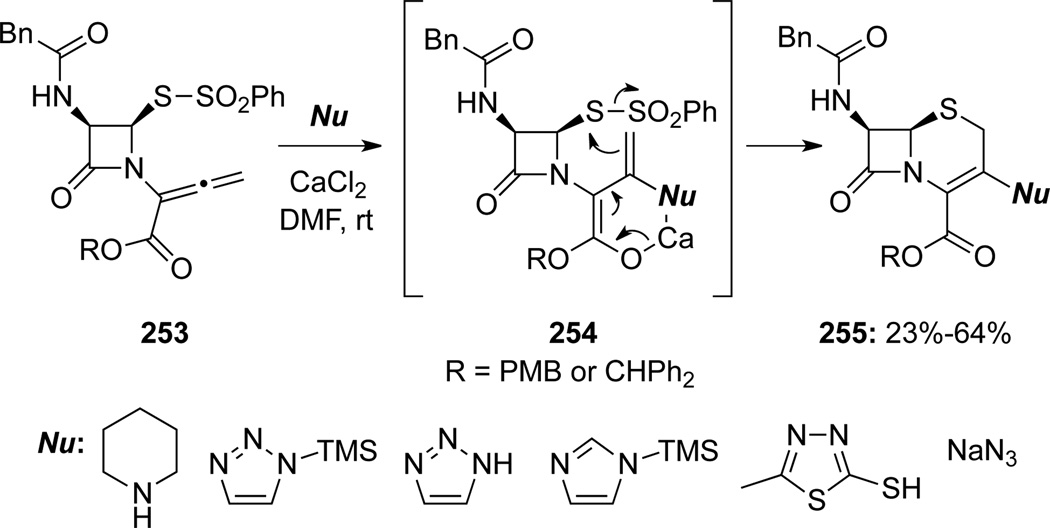
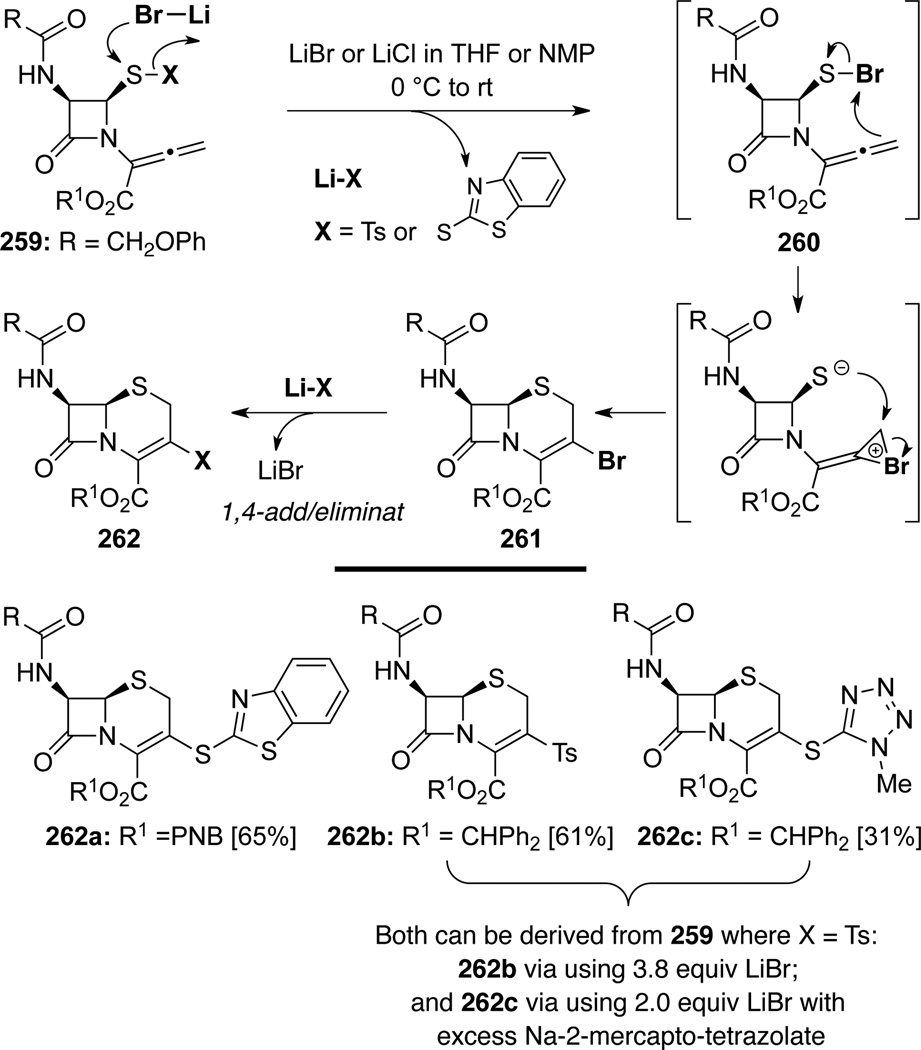
Farina and Kant48b also uncovered that LiBr or LiCl could achieve the same purpose (Scheme 73). Mechanistically, this transformation should be similar to those in Schemes 71 and 72. With X = Ts or SAr as a leaving group, LiBr (or LiCl) would attack the more positively charged sulfur moiety to form a sulfenyl bromide 260 and lithium sulfinate Li-Ts (or LiSAr if X = SAr). The sulfenyl bromide motif in 260 could now electrophilically activate the terminal allenic double bond in an intramolecular manner leading to a bromonium ion intermediate, and an ensuing nucleophilic addition of the sulfide anion would lead to 261. Subsequently, a 1,4-addition of Li-Ts (or LiSAr) followed by elimination of LiBr would afford cephams 262.
Scheme 73.
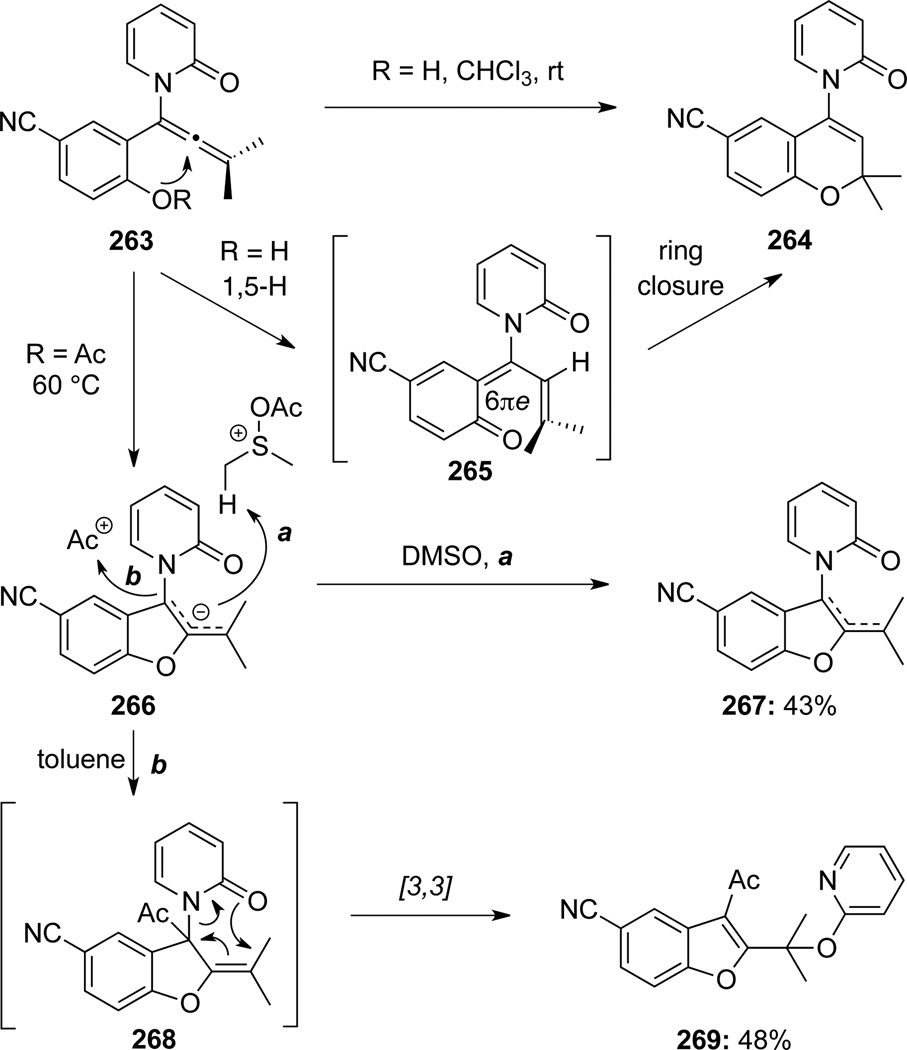
Gericke81 documented that cyclizations of 2-pyridone substituted allenamides 263 took place in different pathways depended on R groups and/or reaction conditions (Scheme 74). In chloroform at room temperature and when R = H, 263 was transformed to chromene 264 through a 6π-electron pericyclic ring-closure of the 1-oxatriene intermediate 265, which was generated through an initial 1,5-H shift. At 60 °C when R = Ac, allenamide 263 would cyclize to first give the allylic anion intermediate 266. This allylic anion could then lead to benzofuran 267 or 269 through either abstracting a proton from DMSO when DMSO is the solvent (see a); or when in toluene, trapping of the Ac+ cation (see b) followed by [3,3]-sigmatropic rearrangement.
Scheme 74.
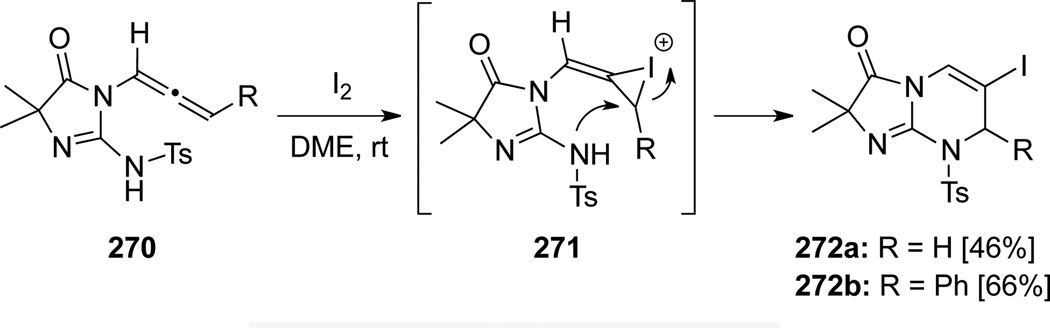
Noguchi82 demonstrated that iodine could promote the cyclization of allenamides 270 via a 6-endo-trig pathway (Scheme 75). Authors proposed that the iodonium intermediates 271 were formed selectively at the terminal olefin to deliver bicyclic guanidines 272.
Scheme 75.
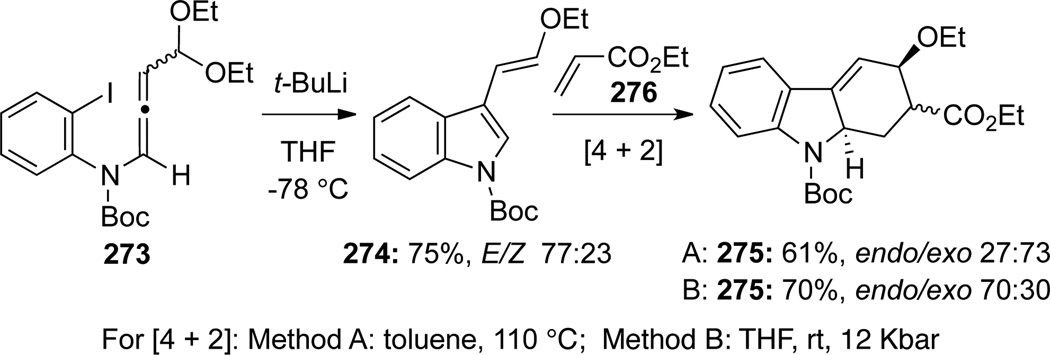
Maddaluno83 reported the synthesis of 3-(2-ethoxy)vinyl indole 274 from allenamide 273 (Scheme 76). The E-isomer was obtained as the major product through an initial aryl lithium addition onto the central allenic carbon followed by elimination of an OEt group. The E-isomer 274 could serve as an excellent 1,3-diene to undergo thermal or high-pressure [4 + 2] cycloaddition with ethyl acrylate 276 to give tetrahydro-carbazoles 275.
Scheme 76.
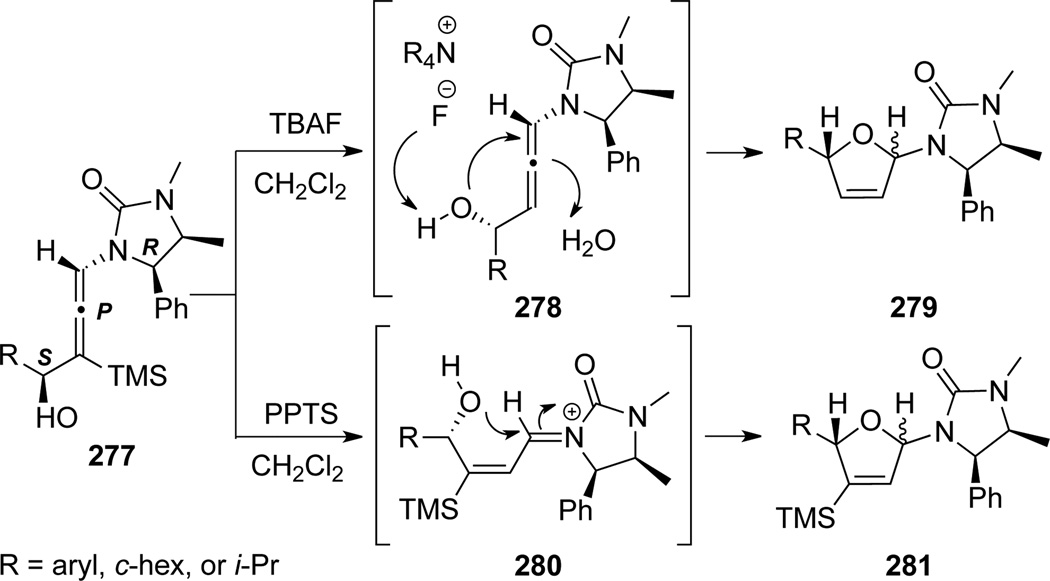
Hsung84 found that depending on the reaction conditions, a series of urea derived γ-substituted chiral allenamides 277 could undergo cyclization to afford substituted dihydrofurans 279 when using TBAF or 281 when using PPTS (Scheme 77).
Scheme 77.
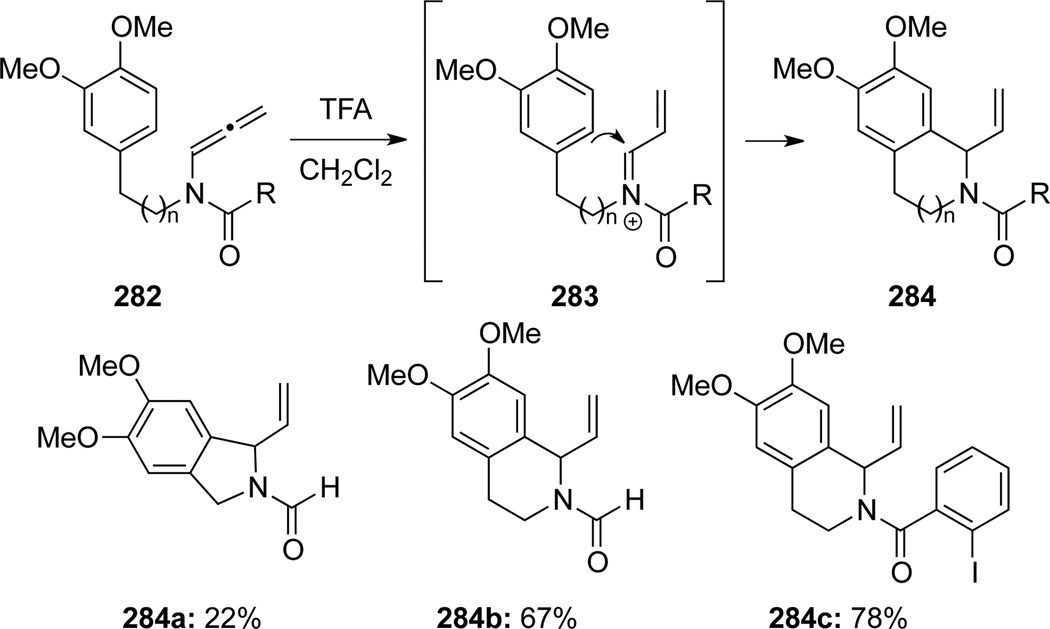
Vázquez85 illustrated that with TFA, allenamides 282 could be cyclized to give isoquinolines 284 via a Pictet-Spengler type cyclization onto N-acyl iminium ion 283 (Scheme 78).
Scheme 78.
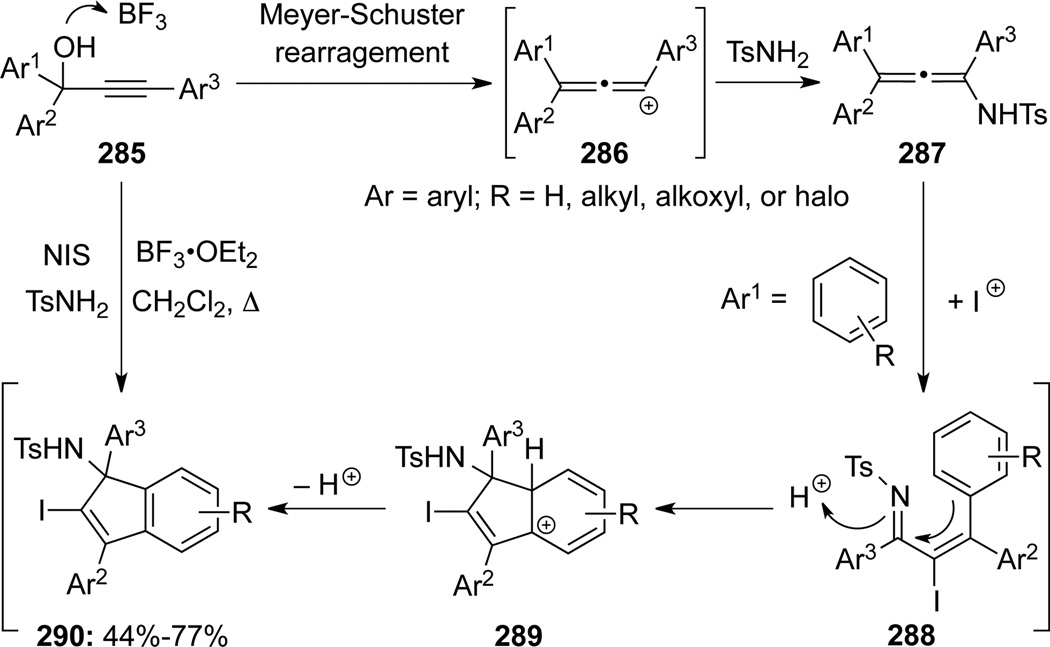
Wang and Lu28c demonstrated that haloindenyl sulfononamides 290 could be synthesized through a BF3-OEt2-catalyzed tandem sequence commencing from propargyl alcohols 285, sulfonamides, and NIS (Scheme 79). Although not isolated, the allenyl cations 288 was first formed via Meyer-Schuster rearrangement, and trapping of 288 with sulfonamides would lead to allenamides 287, which could then undergo subsequent iodonium promoted cyclization.
Scheme 79.
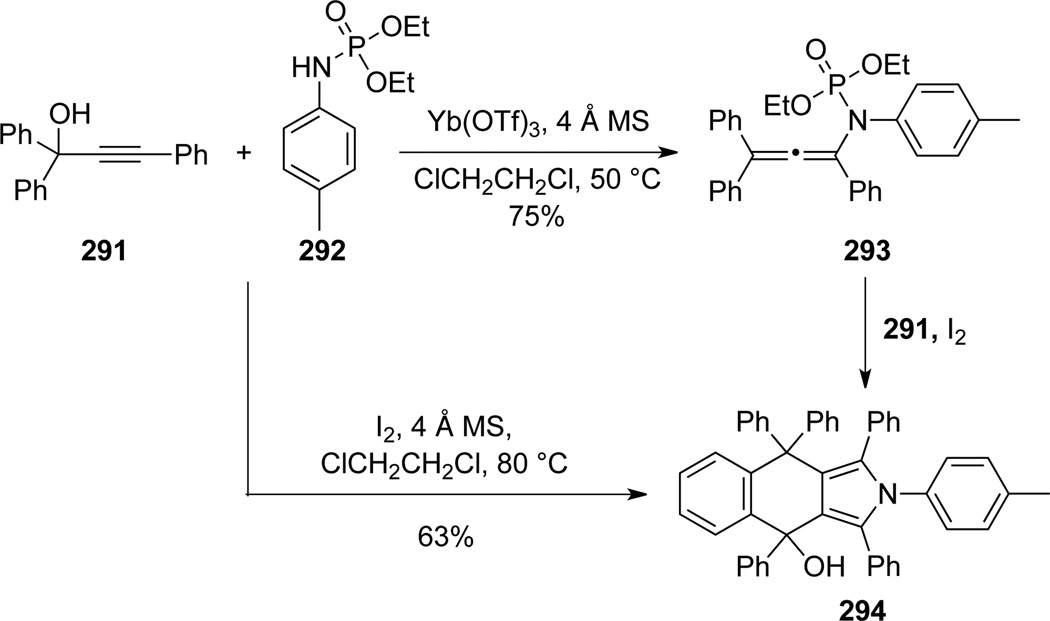
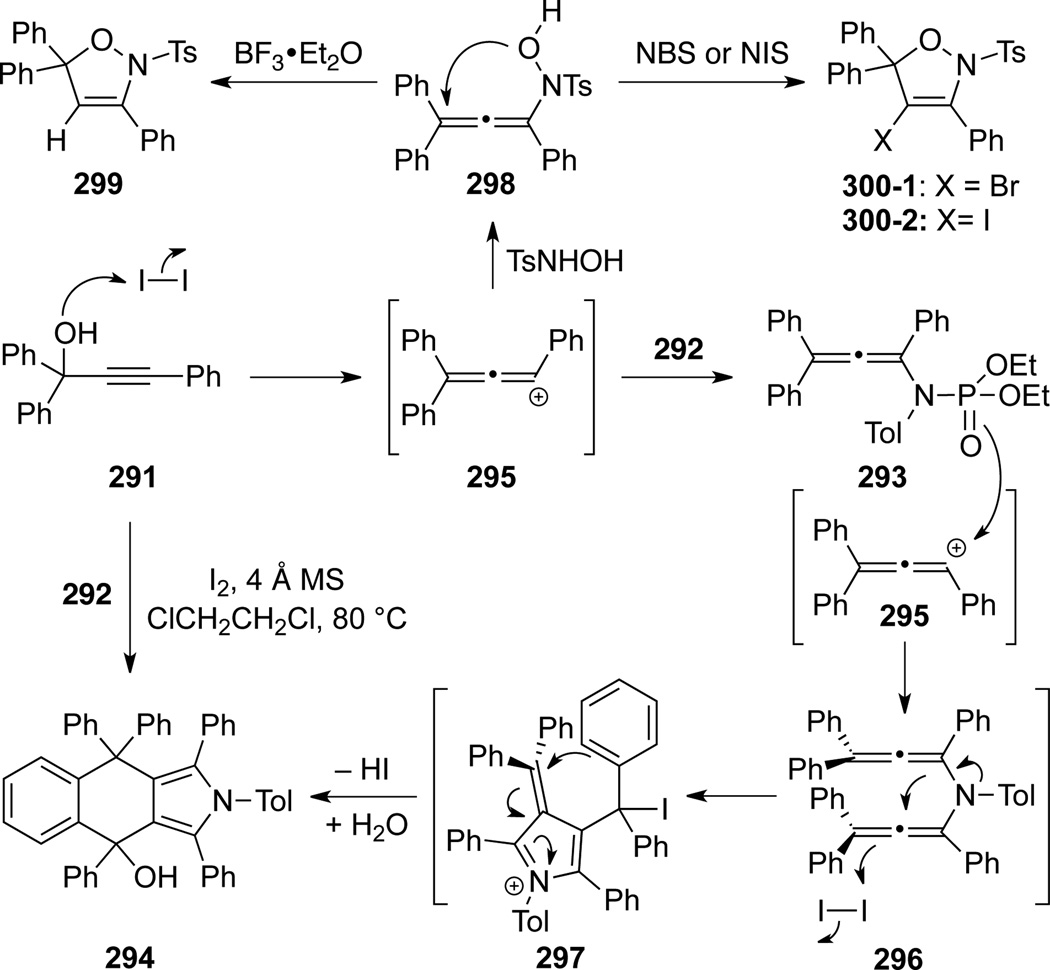
Wang and Lu28a also reported that phosphoryl substituted allenamide 293, prepared from propargyl alcohol 291 through a Yb(III)-catalyzed Meyer-Schuster rearrangement and trapping with phosphoramidate 292, could also undergo related cyclizations to give pyrrole 294 (Scheme 80). Intriguingly, iodine could promote the entire sequence en route to 294. The formation of this structurally interesting pyrrole 294 is proposed in Scheme 81 (see 293→296→297→294). In addition, authors found that when 295 reacted with N-tosyl hydroxylamine, allenamide 298 could be formed and further cyclize to give 2,5-dihydroisoxazole 299 or 4-halo-2,5-dihydroisoxazole 300, respectively.28b
Scheme 80.
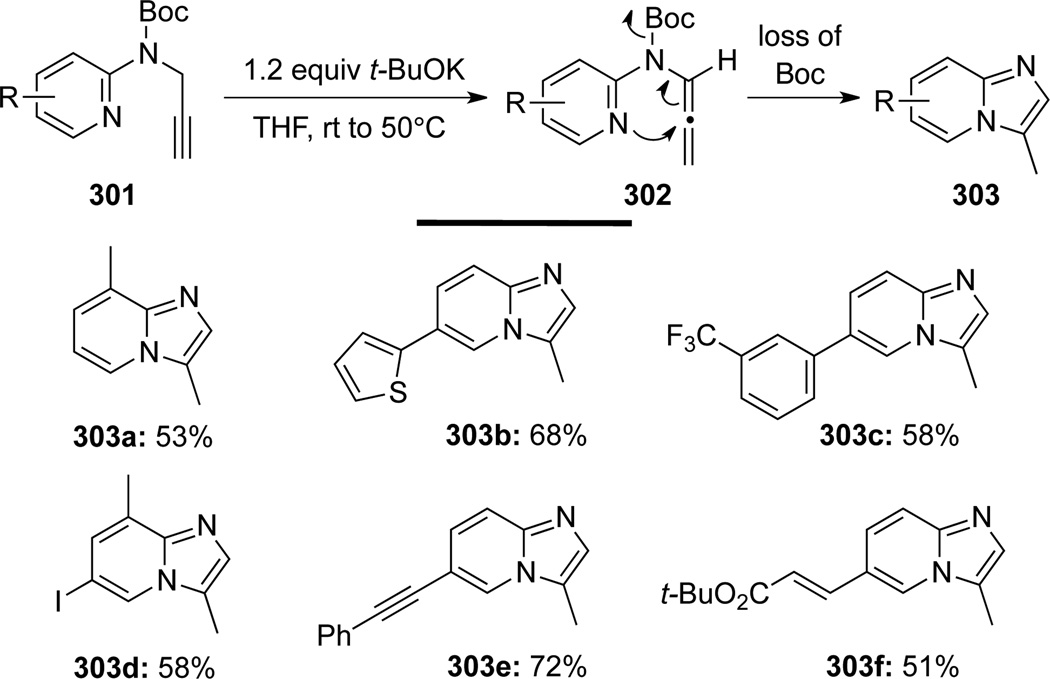
Savic86 discovered that upon treatment with t-BuOK, Boc protected propargyl aminopyridines 301 could be cyclized to imidazo[1,2-a]pyridine derivatives 303 (Scheme 82). Authors suggest that allenamides 302 are likely the key intermediate involved in these cyclization reactions.
Scheme 82.
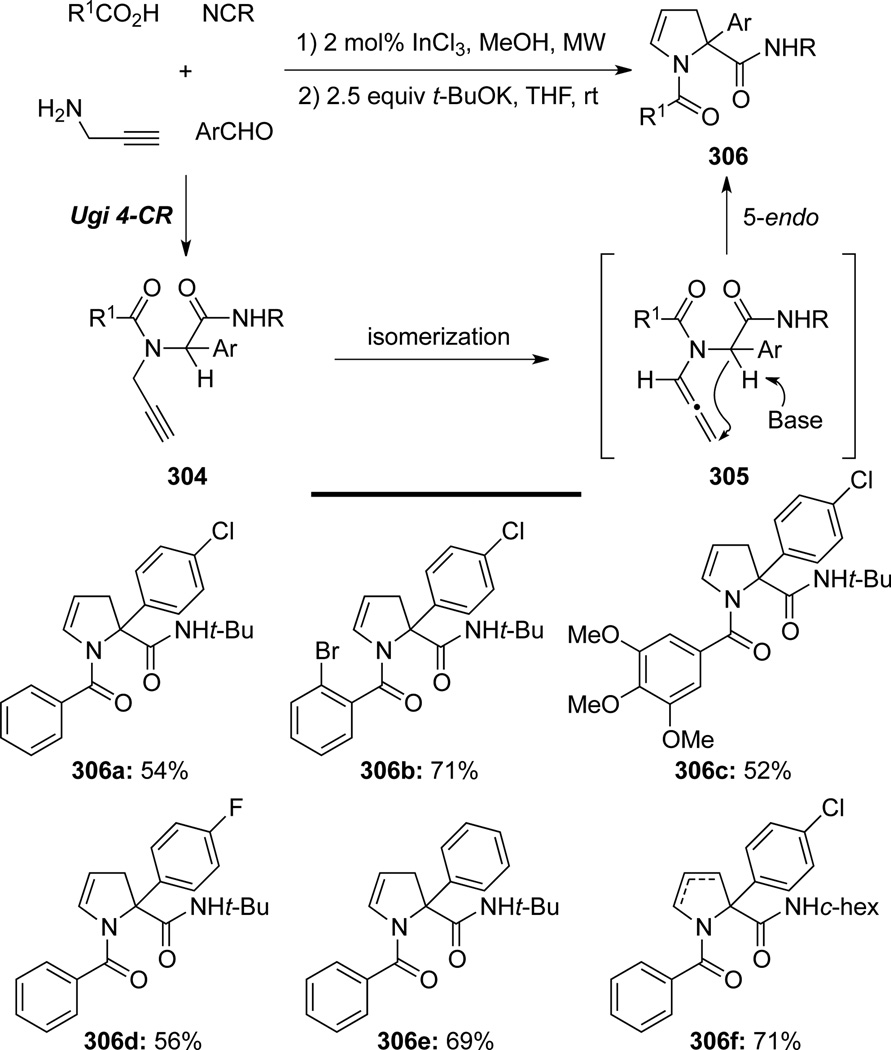
Miranda87 reported a two-step operation to access 2,3-dihydropyrroles 306 involving a base-promoted cyclization of allenamide intermediates 305 (Scheme 83). A Ugi four-component reaction (Ugi 4-CR)88 was conducted with In(III) catalyst to afford propargyl amide 304. A subsequent base-promoted isomerization would generate allenamide intermediates 305, which subsequently underwent 5-endotrig cyclization to produce 2,3-dihydropyrroles 306.
Scheme 83.
3.4.2. Palladium Catalyzed Cyclizations
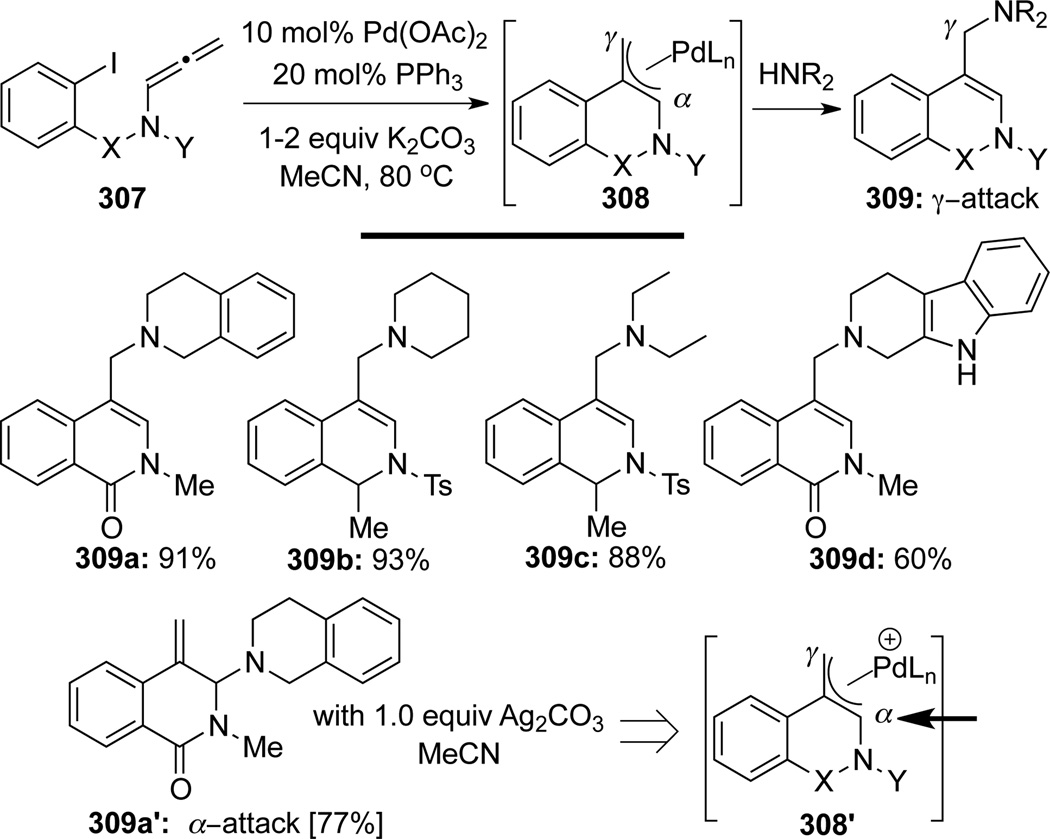
Grigg89 first reported a de novo palladium-catalyzed intramolecular carbopalladation/cyclization–anion capture cascade process using allenamides 307 (Scheme 84). After oxidative addition of Pd(0) to the aryl iodide, a selective carbopalladation/cyclization onto the central allenic carbon would first generate the Pd-π-allyl complex 308. This π-allyl species could be captured regioselectively by amines in the presence of K2CO3 at the sterically less hindered γ-allenic position (γ-attack), leading to allylic amines 309. Although α-attack was confirmed by NMR, an equilibration appeared to take place under the reaction conditions to favor the more stable allylic amines. Intriguingly, the use of Ag2CO3 can alter this regioselectivity to favor in the α-attack as shown in vinyl aminal 309a’. Authors believed that with Ag2CO3, the reaction proceeds through the cationic π-ally complex 308’, which would favor an α-attack and impede any ensuing equilibration.
Scheme 84.
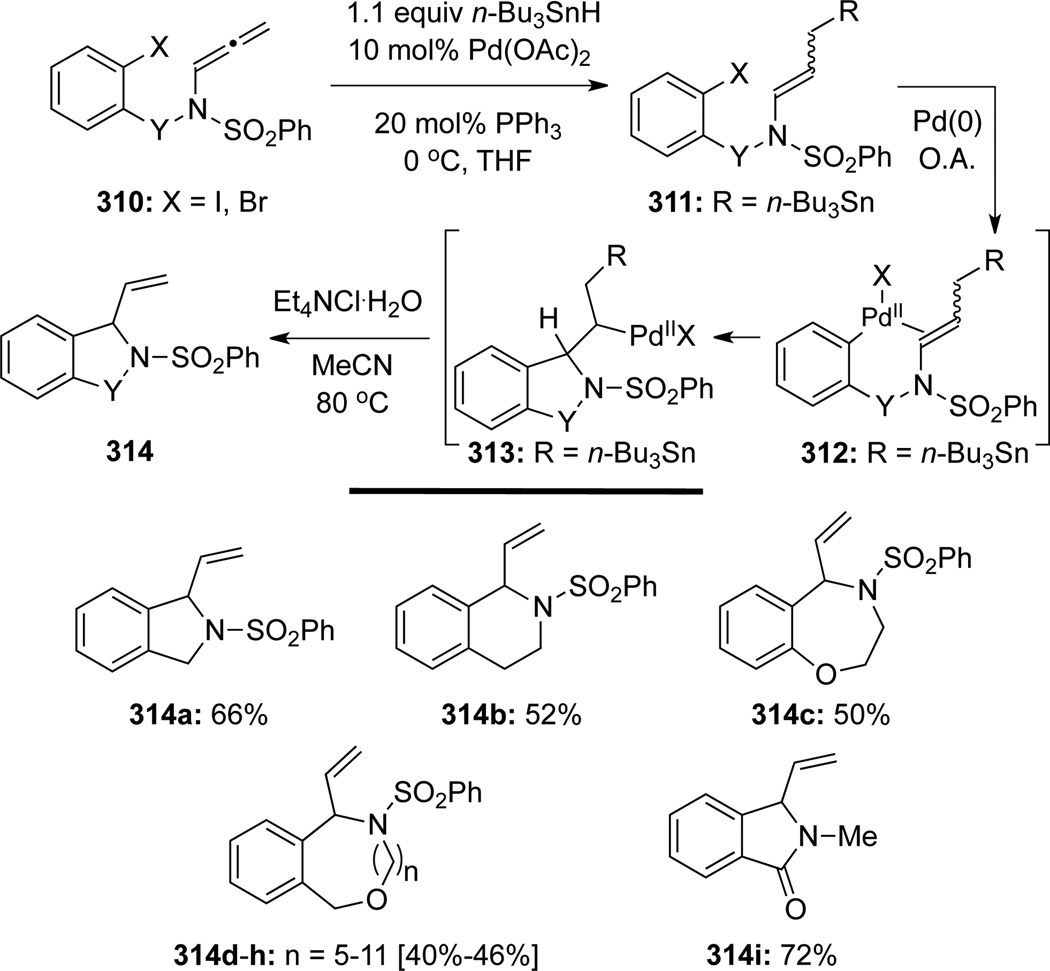
In the related discovery of the Pd-catalyzed carbopalladation/cyclization-anion capture methodology, Grigg90 showcased a palladium-catalyzed hydrostannylation–carbopalladation/cyclization cascade to afford small (5-7) and large (11-17) nitrogen-heterocycles, in which cyclization occurred at the α-allenic carbon (Scheme 85). A series of allenamides 310 could be subjected to highly regioselective palladium [Pd(0) or Pd(II) could be operative]-catalyzed hydrostannylations to afford allylstannanes 311 as a mixture of E/Z isomers. Both E/Z isomeric allylstannanes could undergo an ensuing sequence of oxidative addition, carbopalladation/cyclization, and elimination promoted by Pd(0). It is noteworthy that the elimination of n-Bu3SnPdX was faster than β-hydride (HPdX) elimination, thereby leading to the final products 314 in moderate to good yields.
Scheme 85.
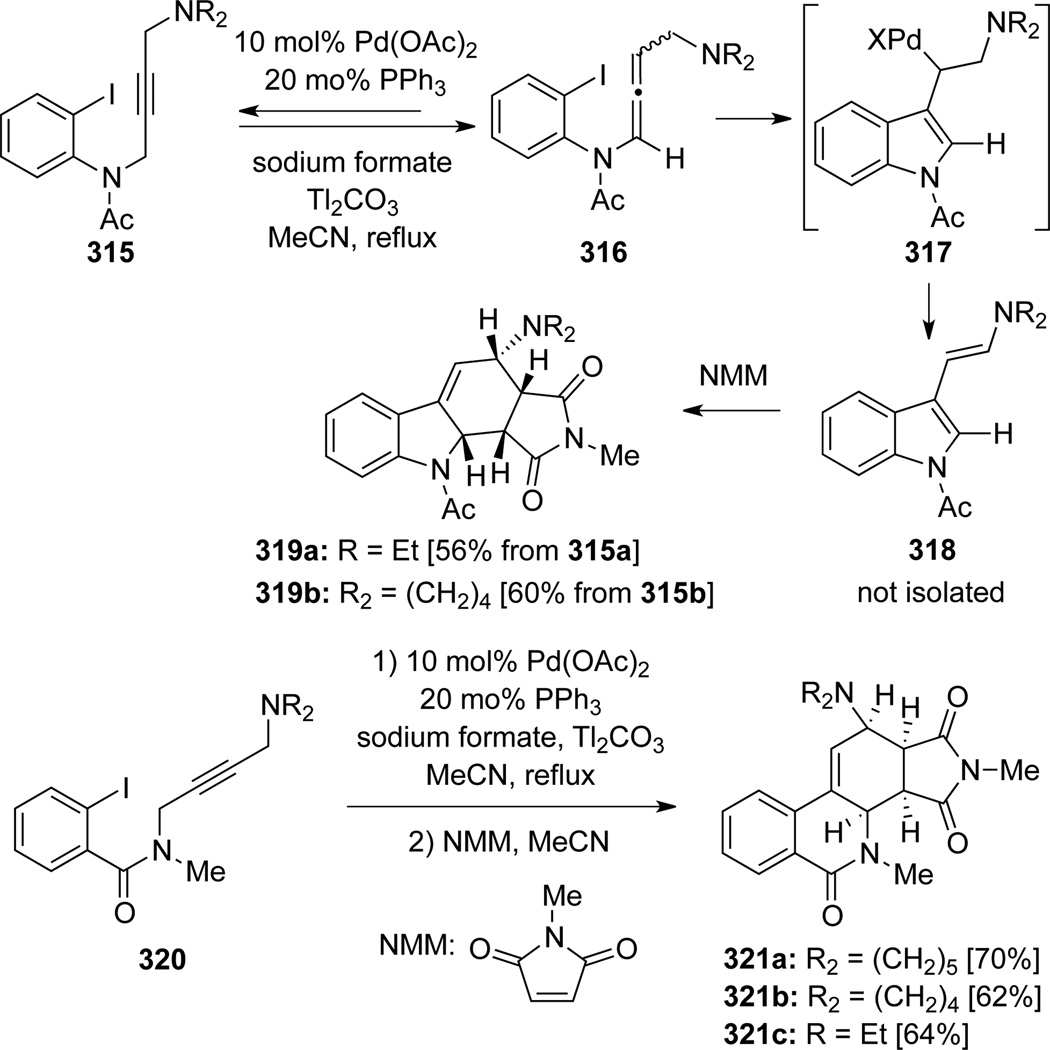
Grigg91 also reported an impressive poly-component cascade involving a wide range of regio- and stereoselective 5-, or 6-exo-dig cyclization (Scheme 86). Commencing from propargyl amides 315, a Tl2CO3 promoted isomerization would first lead to allenamides 316. Subsequent oxidative addition and carbopalladation/cyclization onto the central allenic carbon would afford the formation of an unstable enaminoindoles 318, which could be trapped through Diels-Alder cycloadditions with N-methylmaleimide (NMM) in refluxing CH3CN to yield endo-cycloadducts 319. Three examples from propargyl amides 320 to polycyclic products 321 were reported without isolation of the diene intermediate.
Scheme 86.
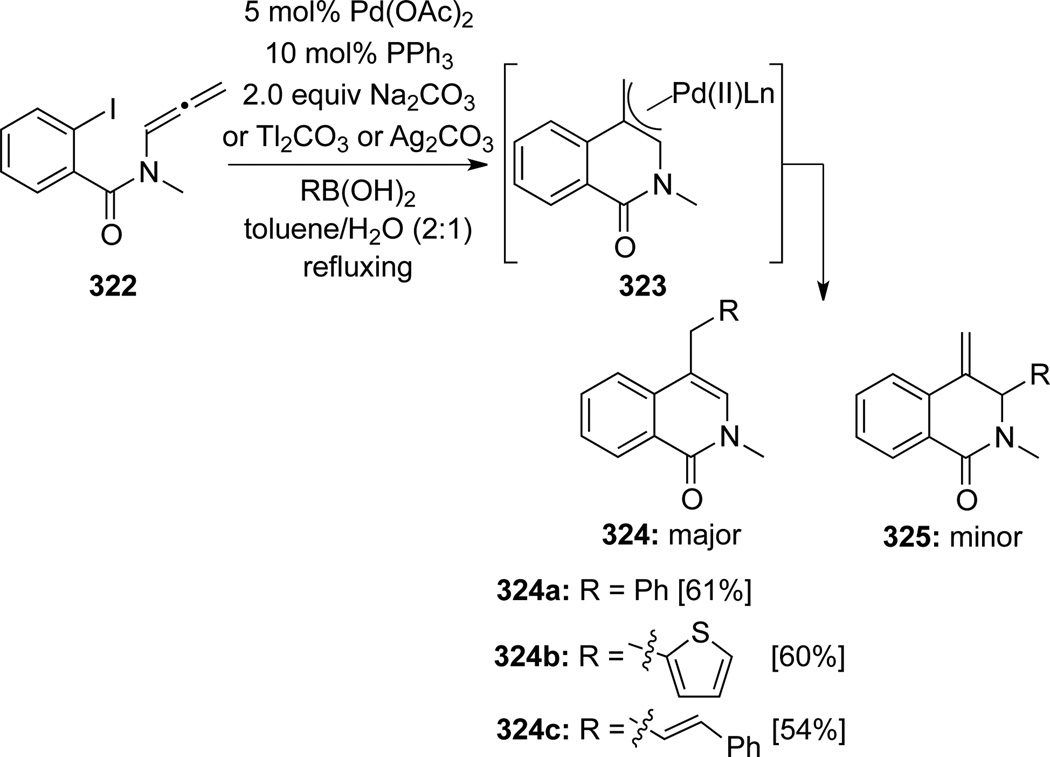
Grigg92 also adopted organoboron reagents as anion transfer reagents in their palladium-catalyzed cyclization–anion capturing cascade (Scheme 87). Again, after carbopalladation/cyclization, the resulting palladium π-allyl complexes 323 could undergo transmetallation with boronic aids to give isoquinolones 324a–c after reductive elimination. Although minor isomer 325 was also found, the Suzuki-Miyaura type cross-coupling was overall highly regioselective and favored predominantly at the less hindered γ-allenic carbon regardless of the base used.
Scheme 87.
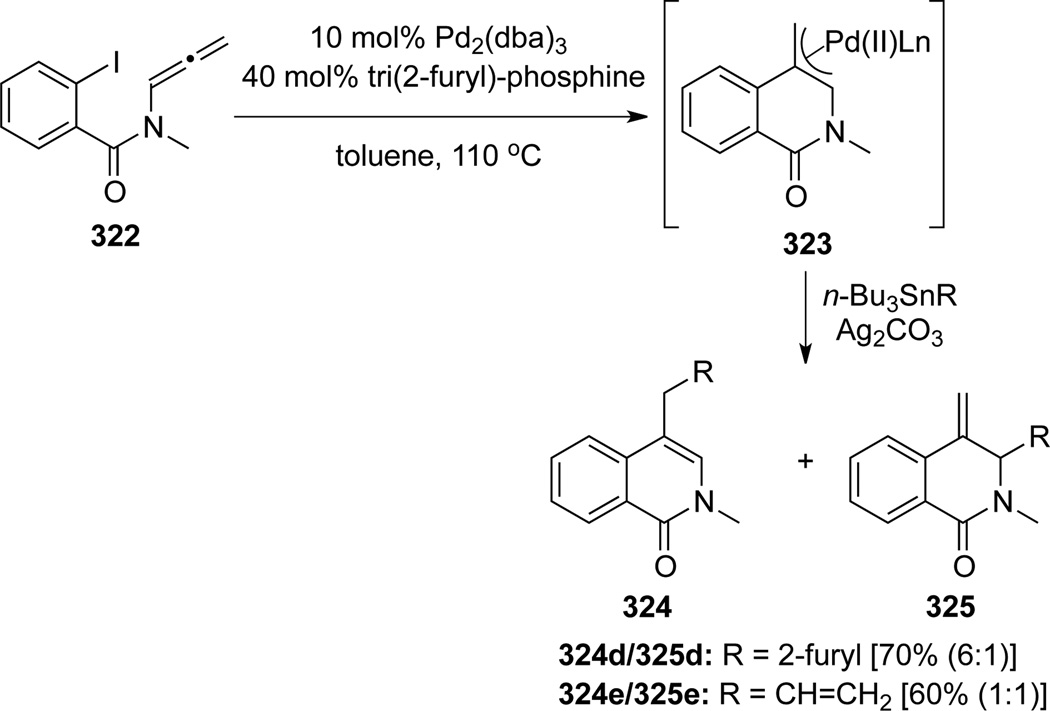
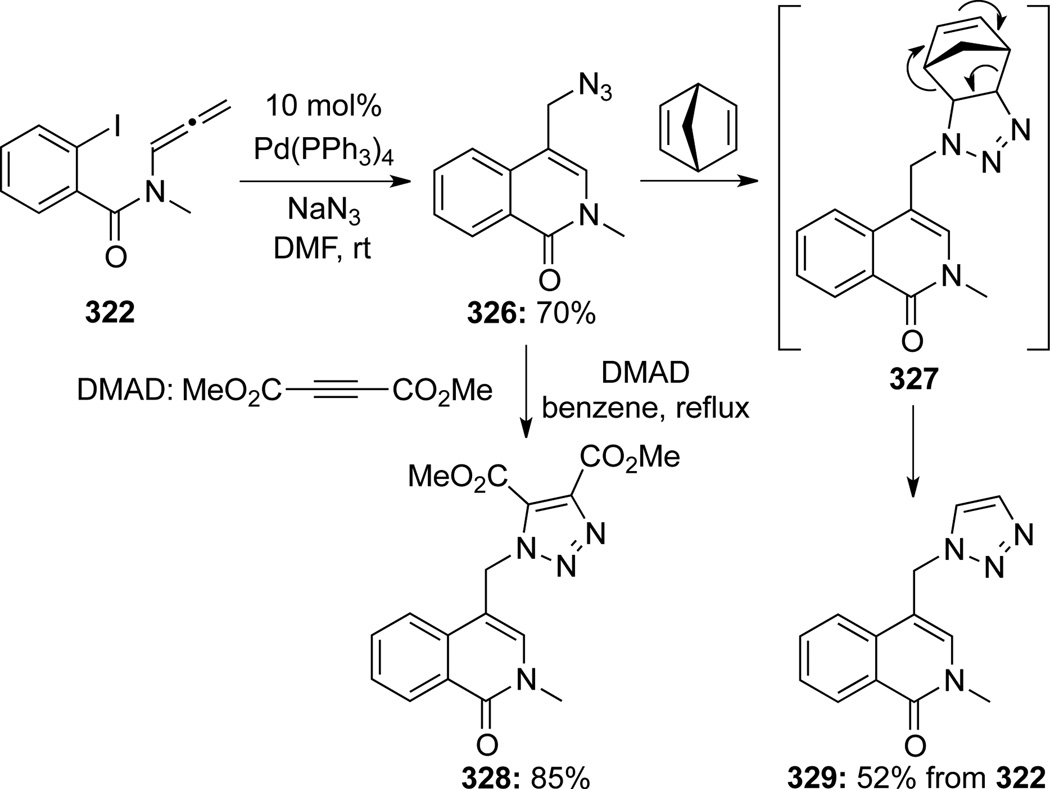
Organostannanes could also be utilized as anion capture reagents in a Stille-type cross-coupling to give isoquinolones 324d–e, although regiochemistry here was not as good93 (Scheme 88). Again intriguing here, Ag2CO3 appears to have no significant bearing on the regioselectivity (see Scheme 84). When NaN3 was used as the capture reagent, azides 32694 could be attained (Scheme 89). Upon treatment with DMAD in benzene, an ensuing 1,3-dipolar cycloaddition could take place to give triazole 328. A one-pot cascade using norbornadiene as the dipolarophile was also explored and triazole 329 was obtained after a retro Diels-Alder reaction of the initially generated triazoline cycloadduct 326.
Scheme 88.
Scheme 89.
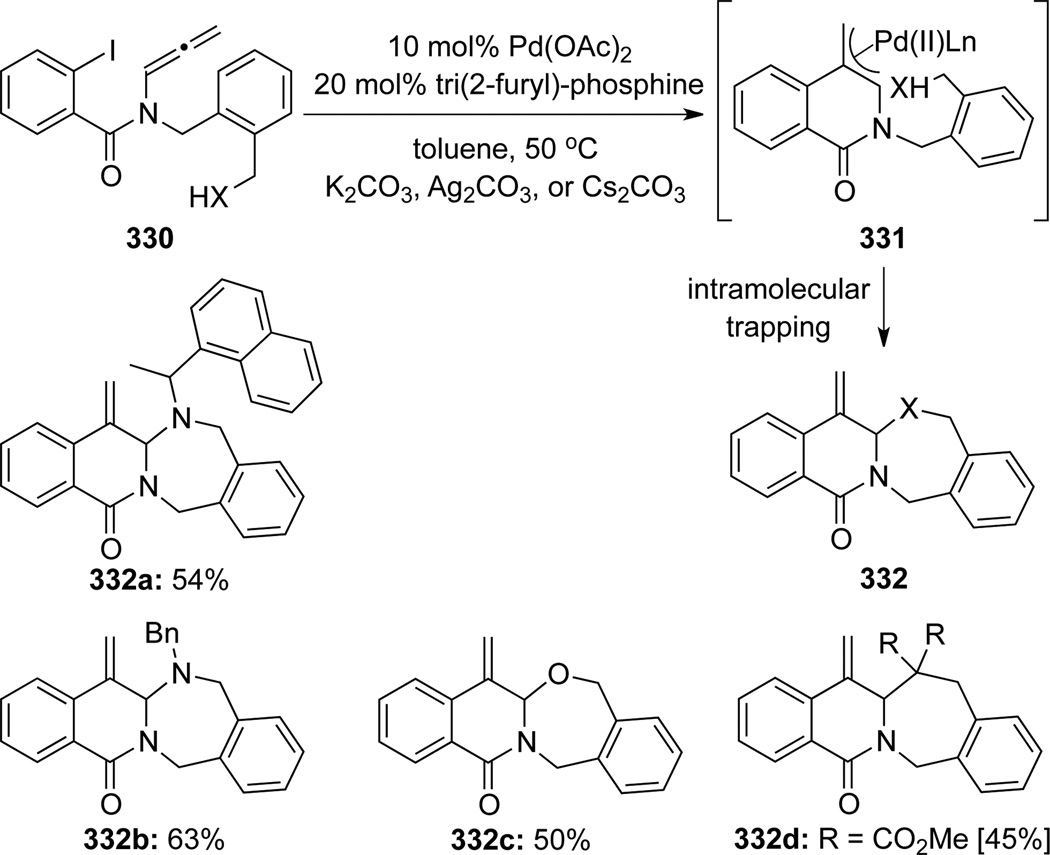
Grigg95 subsequently rendered the anion capture part of their intramolecular cascade (Scheme 90). This process was initiated also through the same carbopalladation/cyclization and the formation of palladium π-allyl complexes 331 but followed by their interception, via an intramolecular nucleophilic addition, leading to polycyclic isoquinolones 332.
Scheme 90.
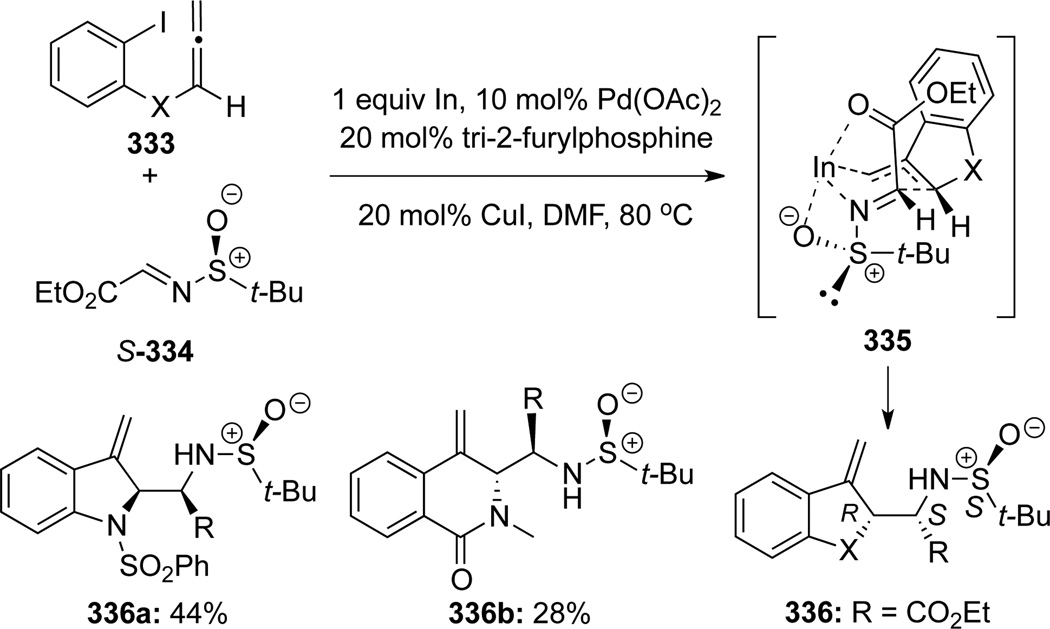
Grigg96 reported another novel cascade process entailing a highly regio-and stereoselective Pd/In bimetallic mediated cyclization–allylation reaction (Scheme 91). Transmetallation of the palladium π-allyl intermediate with indium followed by allylation of chiral sulfonamide S-344 afforded esters 336 with two contiguous chiral centers generated with complete stereochemical control. The major diastereomer could be rationalized through the more favored transition state 335 in which the coordination to In metal likely involves both the sulfoxide and carbonyl oxygen atoms.
Scheme 91.
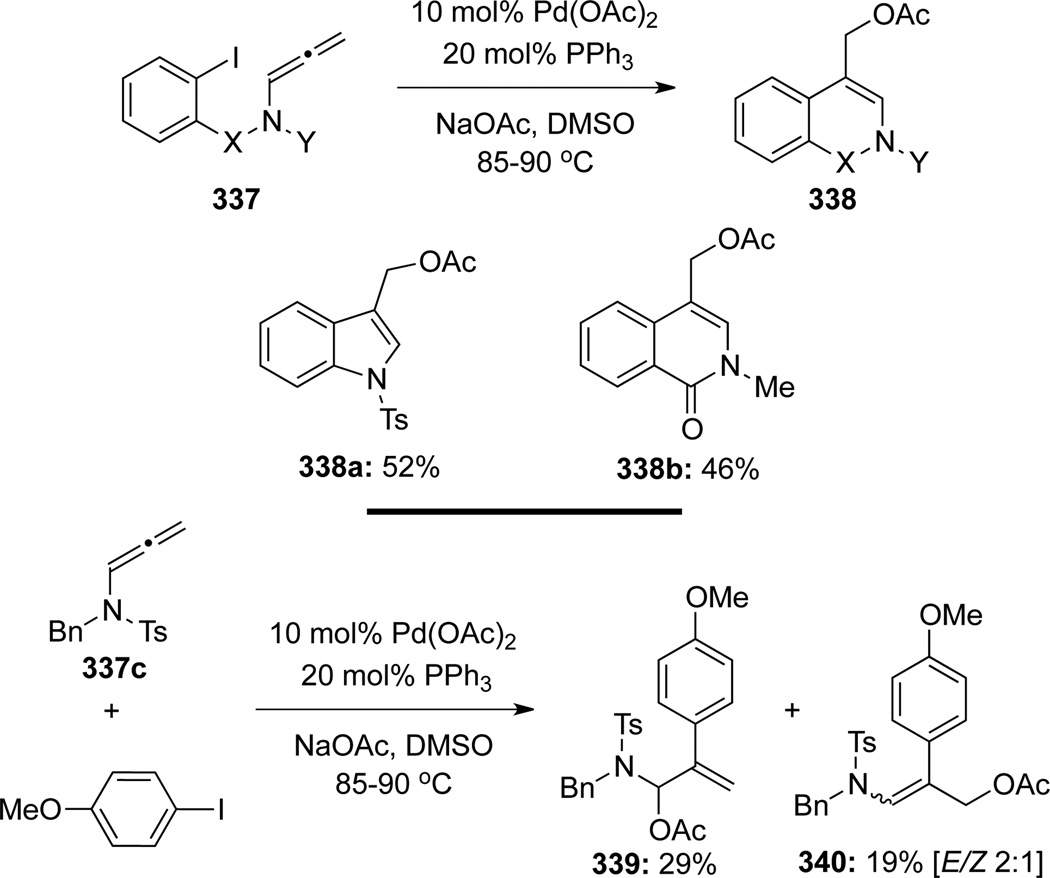
Savic97 showed their work on the palladium-catalyzed carbopalladation/cyclization–anion capture cascade but with the acetate anion as a capturing nucleophile (Scheme 92). The intramolecular version of this reaction proceeded quite well with allenamides 337 to give desired cyclized products 338. However, while the intermolecular version with allenamide 337c was also successful, a mixture of regioisomeric acetates 339 and 340 was obtained.
Scheme 92.
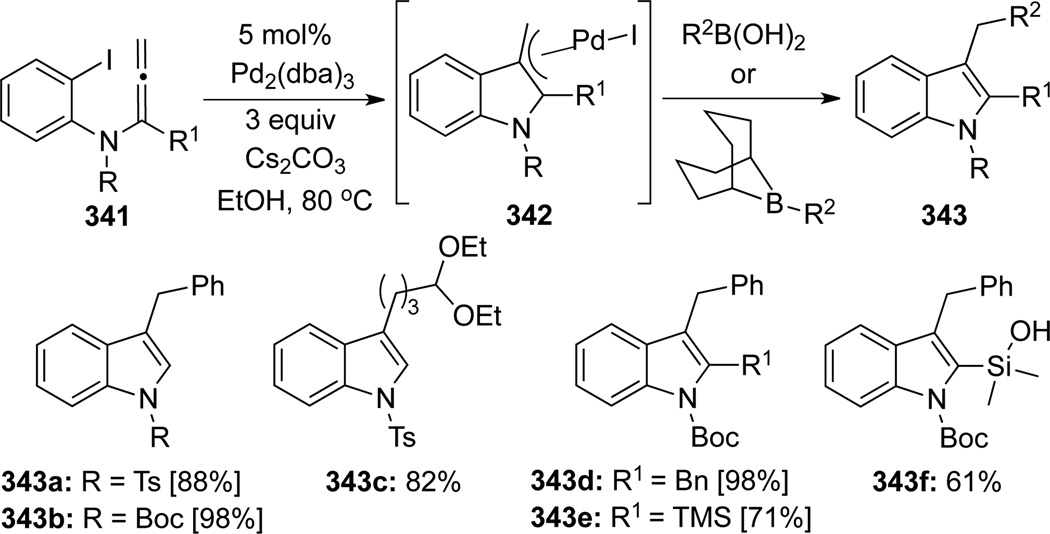
Fuwa and Sasaki98 unveiled a clever approach for the synthesis of 2,3-disubstituted indole derivatives based on the intramolecular carbopalladation/cyclization–anion capture strategy (Scheme 93). Allenamides 341 bearing a substituent at the α-position were found to undergo a facile carbopalladation/cyclization to generate the palladium π-allyl intermediate 342, which in turn could be cross-coupled with a wide range of orgnanoboron species to give a variety of 2,3-disubstituted indoles 343.
Scheme 93.
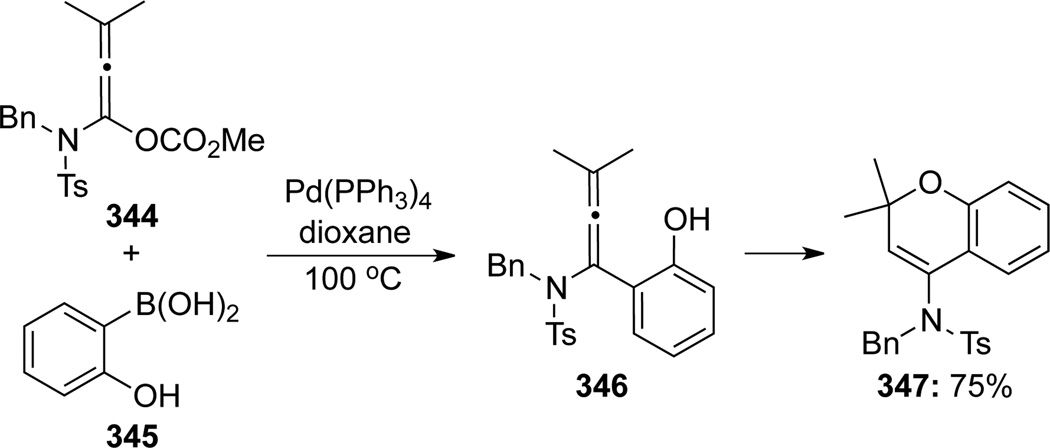
Lai57 also reported an example of the Pd-catalyzed carbopalladation/cyclization cascade while attempting to construct tetrasubstituted allenamides via Suzuki-Miyaura coupling (Scheme 94). When allenamide 344 reacted with (2-hydroxyphenyl)boronic acid 345, the cyclized amido-chromene product 347 was obtained instead of the α-aryl-substituted allenamide 346, although 346 likely had served as an intermediate.
Scheme 94.
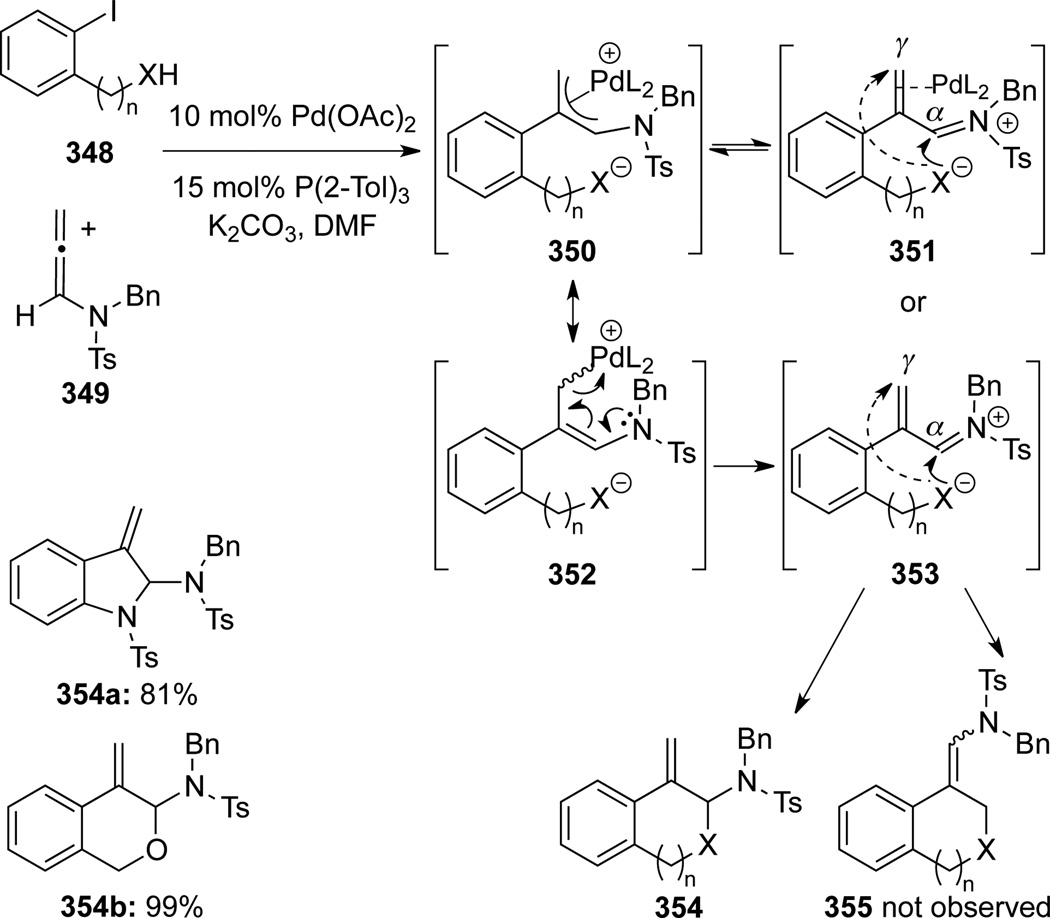
Hiroya99 published a Pd-catalyzed annulation reaction of allenamide 349 with aryl iodides 348 (Scheme 95). In this work, the Pd-π-allyl species 350 readily underwent an intramolecular nucleophilic attack by an oxygen- or nitrogen-based nucleophile to give the annulated product 354. The product via α-attack in a 5- or 6-exo-trig manner was obtained exclusively regardless of the bulkiness of the allenic substituents. Authors proposed that the nitrogen stabilization could render the α-allenic carbon more electropositive than the γ-allenic carbon, thereby favoring the α-attack. In cases such as 354a, it is also a matter of 5-exo-trig α-attack versus 5-endo-trig γ-attacked.
Scheme 95.
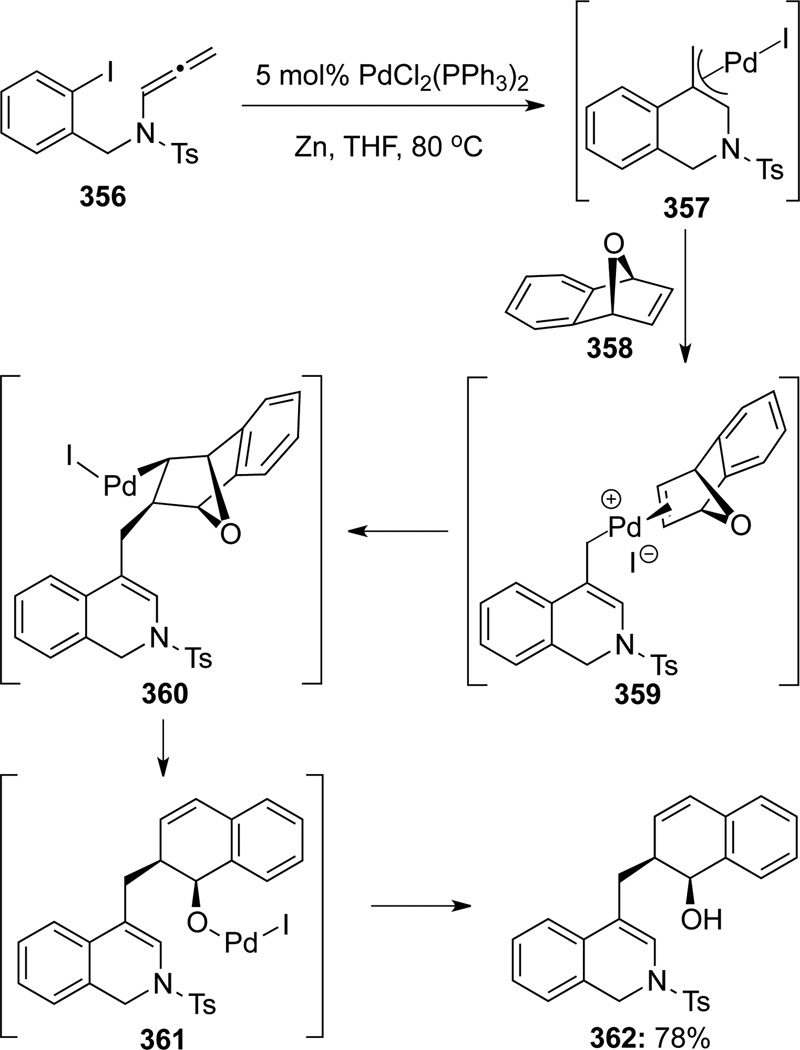
Cheng100 described their Pd-catalyzed cyclization–capture cascade utilizing the bicyclic alkene 358 for the terminating process (Scheme 96). The palladium π-allyl complex 357 generated from allenamide 356 through the carbopalladation/cyclization sequence could be cross-coupled with oxa-norbornadiene 358, leading to 1,2-dihydroisoquinoline 362 in 78% yield. Authors proposed that the coordination of 358 to the palladium π-allyl complex 357 favored the exo face, and that an ensuing migratory insertion and β-oxy syn-elimination would lead to the palladium-oxo complex 361 before the Zn-reduction.
Scheme 96.
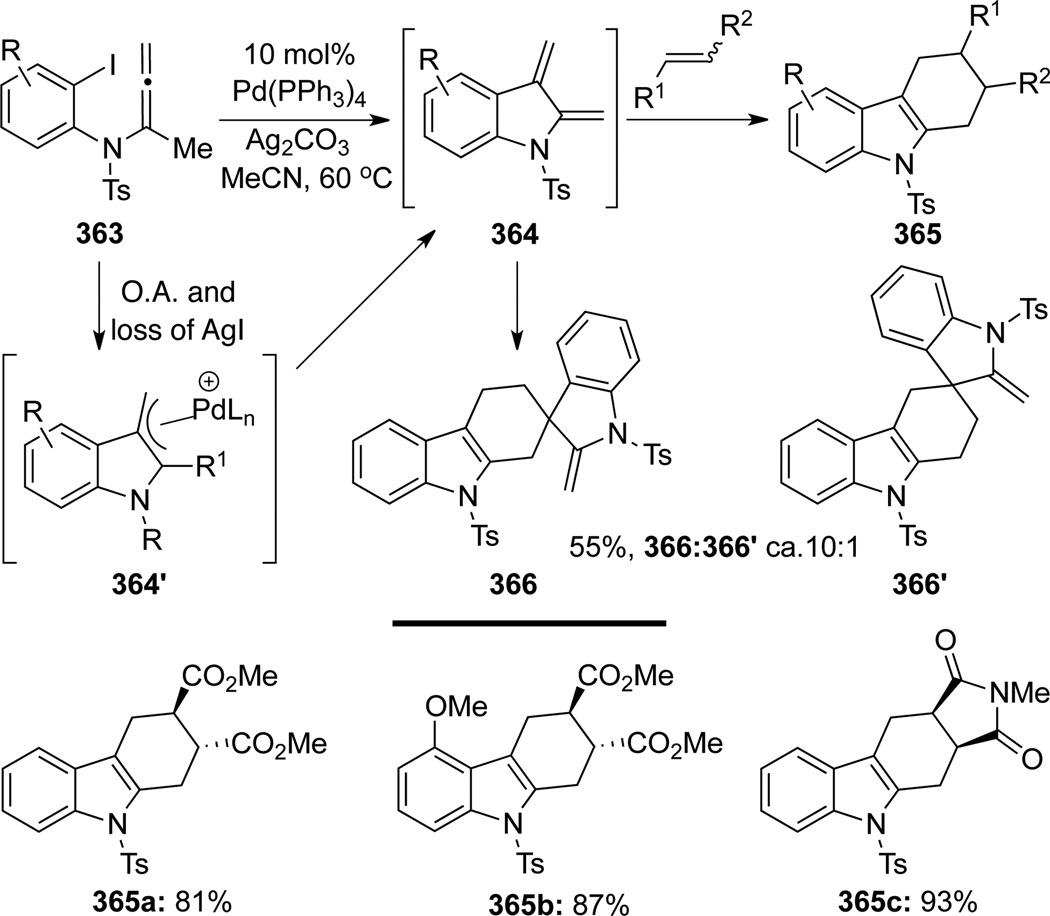
Following their successful synthesis of substituted indoles, Fuwa and Sasaki101 evolved their strategy further to access indole-2,3-quinodimethanes 364 from allenamide 363 through the intermediacy of the cationic palladium π-allyl complex 364’ (Scheme 97). Indole-2,3-quinodimethanes 364 could be trapped efficiently in situ through Diels-Alder cycloaddition with an external dienophile to afford tetrahydrocarbazoles 365 in excellent yields. In the absence of an external dienophile, homo-dimers 366/366’ were isolated through a regioselective Diels-Alder cycloaddition in favor of 366.
Scheme 97.
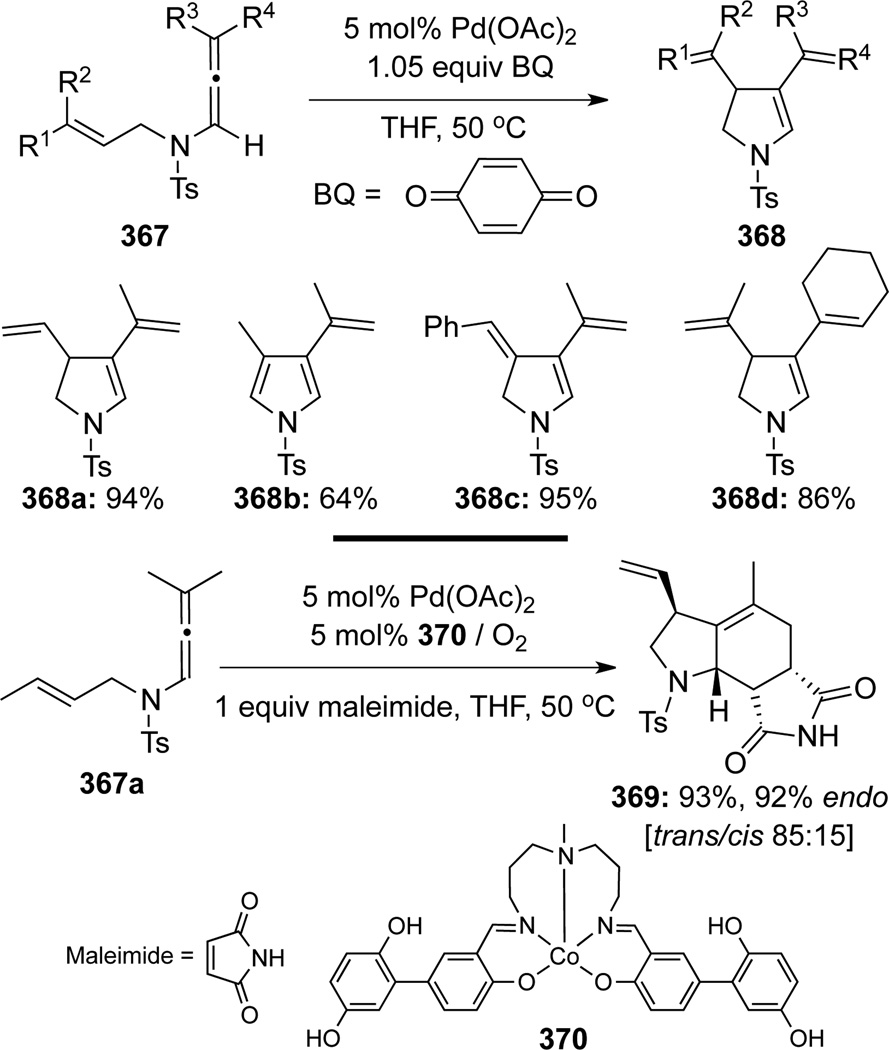
Bäckvall102 recently published a novel Pd(II)-catalyzed oxidative carbocyclization methodology (Scheme 98). Under these conditions, allenamides 367 could cyclize to afford the corresponding dihydropyrrole derivatives 368. When maleimide was employed as a dienophile with the Co-salen type complex 370 as co-catalyst, a tandem oxidative cyclization/Diels-Alder reaction occurred to give the polycyclic endo-cycloadduct 369.
Scheme 98.
3.4.3. Rhodium Catalyzed Cyclizations
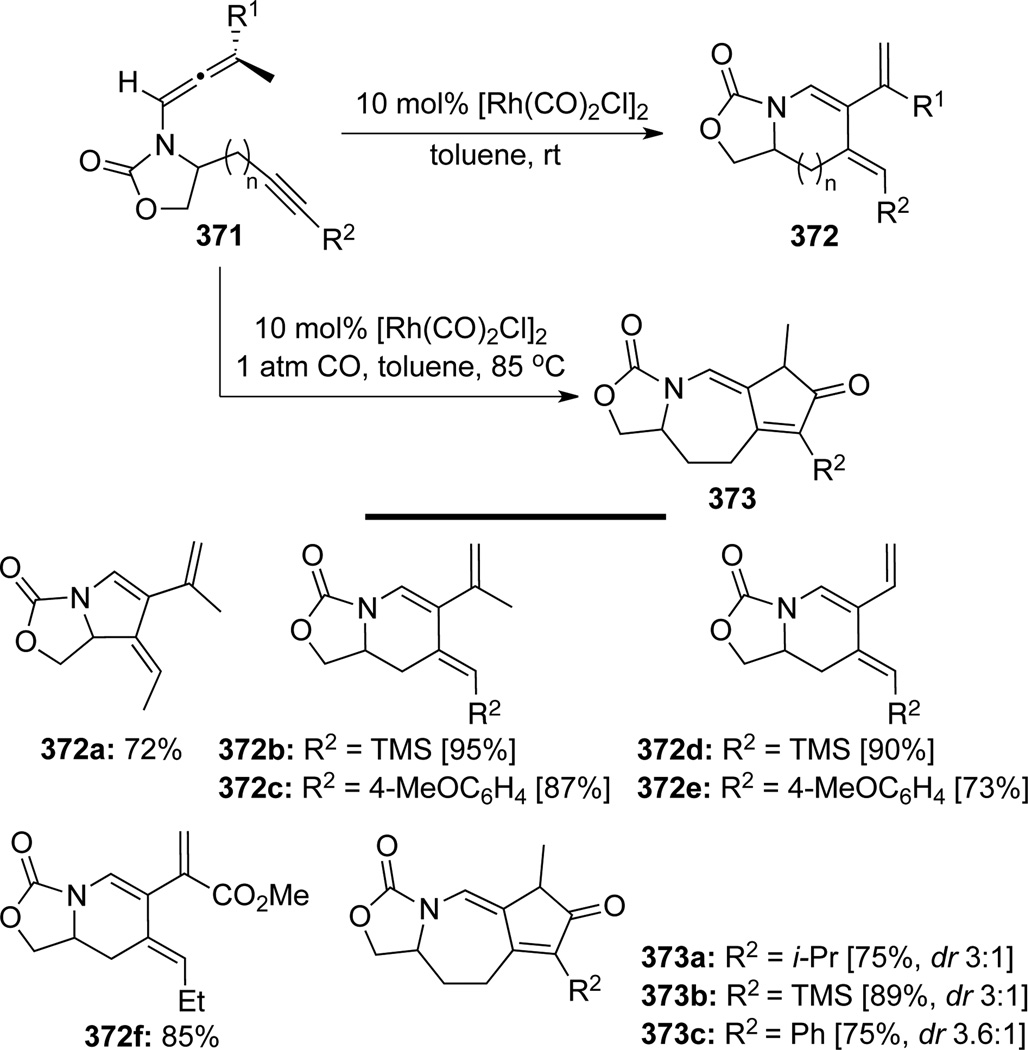
Brummond103 first demonstrated a rhodium(I)-catalyzed allenic Alder-ene reaction employing alkynyl allenamides 371 (Scheme 99). A variety of cross-conjugated triene-containing heterocycles 372 were obtained in good to excellent yields. It was also demonstrated that when changing the atmosphere from argon to carbon monoxide, the reaction generated complex tricycles 373 in a Pauson-Khand manner with moderate diastereoselectivity.
Scheme 99.
3.4.4. Gold Catalyzed Cyclizations
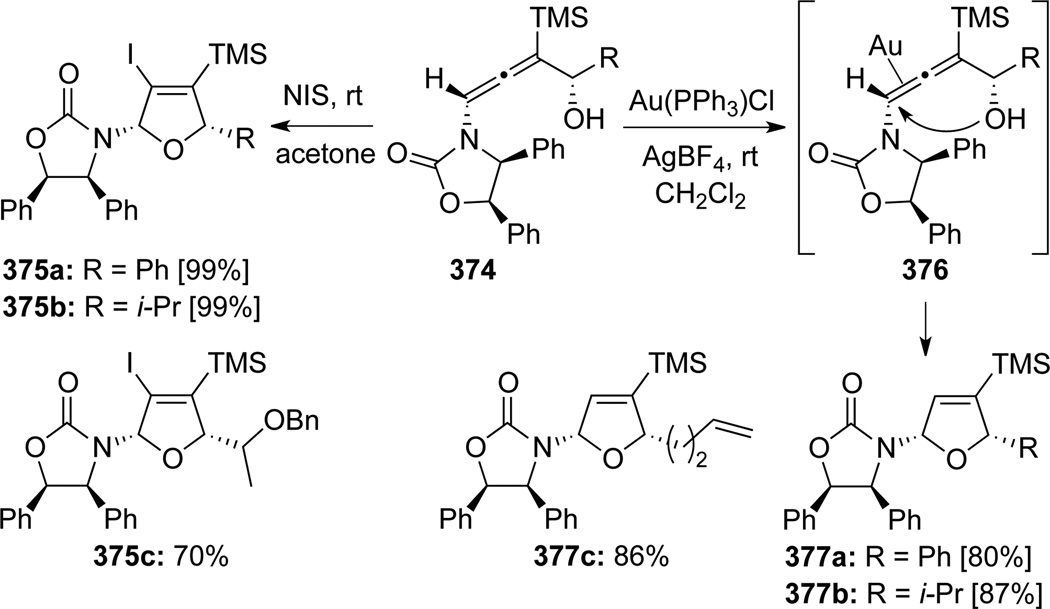
Hegedus104 published an efficient Au-catalyzed cyclization of chiral γ-substituted allenamides 374 (Scheme 100). An impressive series of highly functionalized cis-dihydrofurans 377 were synthesized as a single diastereomer. Authors also reported an alternative electrophilic activation method for cyclizing allenamides 374 that utilized NIS, leading to 375 with a handle for further elaboration at C-3 position of the furan ring.
Scheme 100.
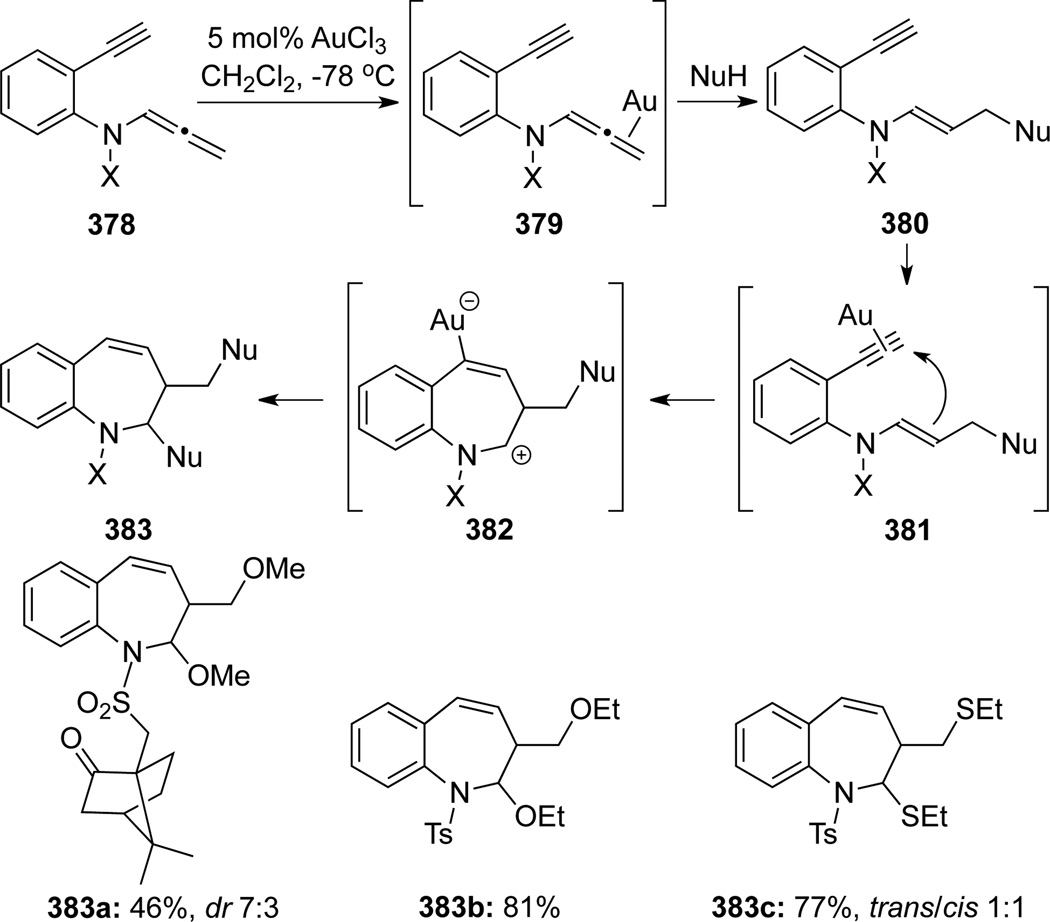
Pérez-Castells105 reported an Au-catalyzed cyclization and a subsequent gold-promoted nucleophile trapping for the formation of benzazepines 383 (Scheme 101). In this reaction, authors proposed that the gold first coordinated with the allene rather than the alkyne group as shown in 379, although both coordination events are likely taking place and in rapid equilibrium. An ensuing nucleophilic attack accounted for the formation of enamides 380, and at subsequent gold promoted cyclization onto the alkyne would occur (see 381→382→383). It was also found that if the N-substituent X were less electron-withdrawing than sulfonyl groups, this cyclization did not take place.
Scheme 101.
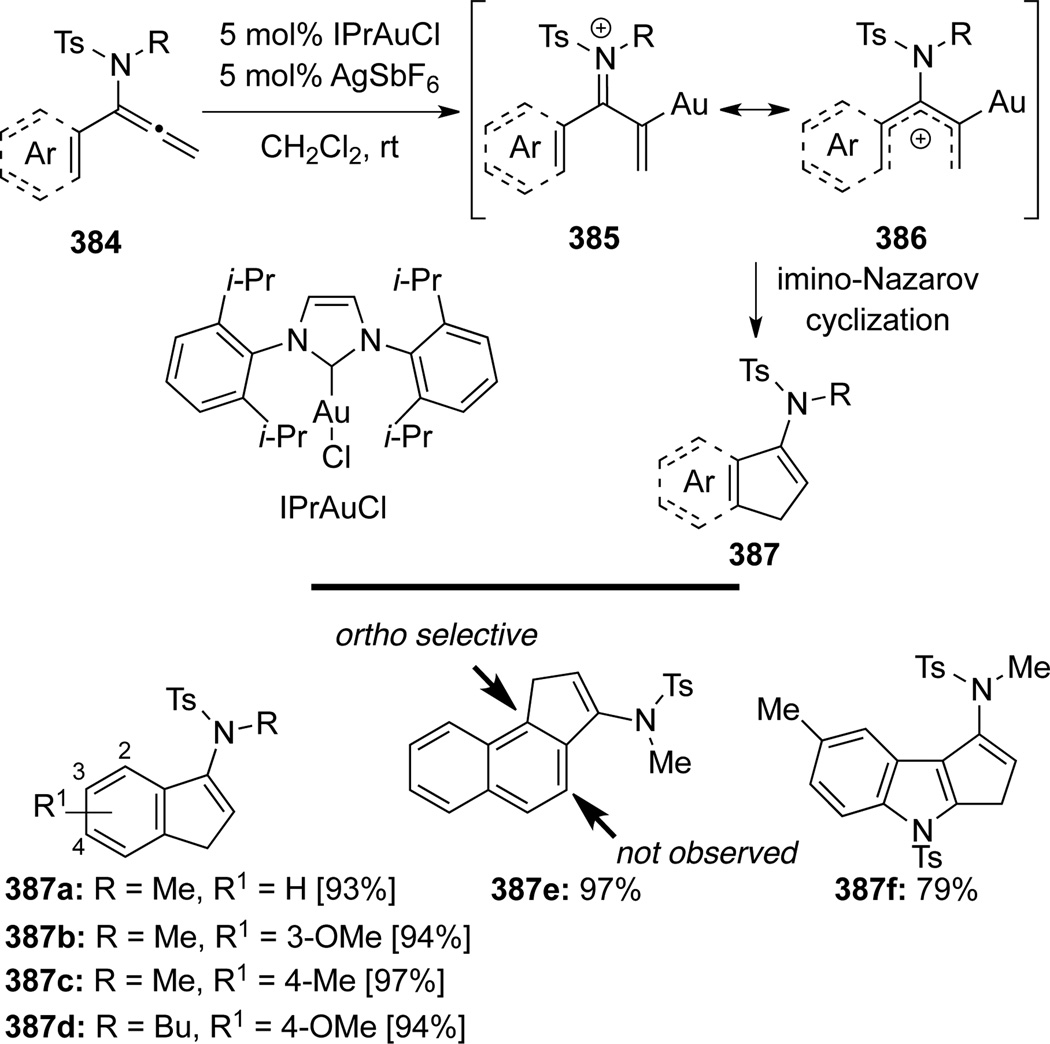
Hsung106 published an Au-catalyzed imino-Nazarov cyclization of α-aryl substituted allenamides 384 (Scheme 102). This regioselective cascade provided an efficient synthetic method in constructing aromatic ring-fused cyclopentenamides 387. Authors suggested that the electronic preference accounted for the observed regioselectivity.
Scheme 102.
3.4.5. Radical Cyclizations
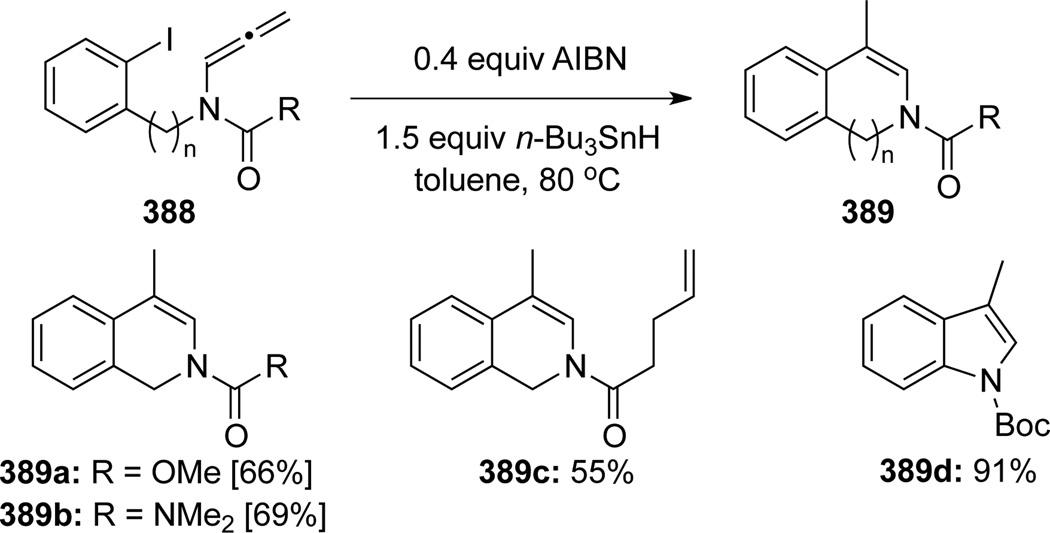
Hsung107 reported the first radical cyclization of allenamides (Scheme 103). This cyclization reaction was highly regioselective for the central allenic carbon in 388, leading to isoquinolines and isoindoles 389 in good to excellent yields. Authors suggested that the electronic nature of the allenamide nitrogen atom has no impact on the regioselectivity.
Scheme 103.
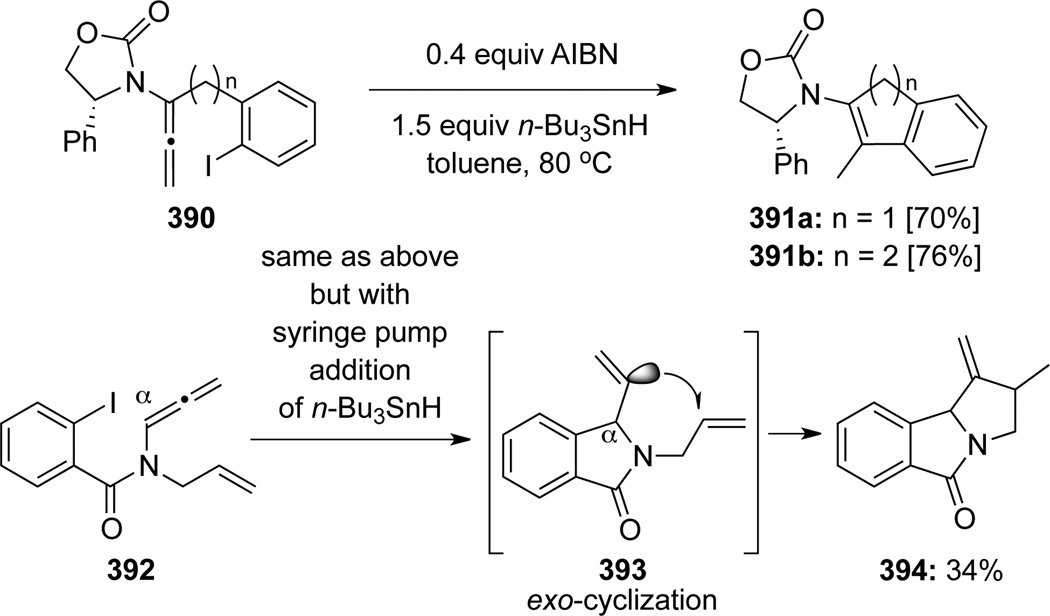
In addition, when chiral allenamides 390 with the aryl iodide group tethered through the α-carbon of the allenamide were employed, the radical cyclization afforded exclusively the central-cyclized indene products 391 (Scheme 104). Authors also described a tandem radical cyclization using allenamide 392 to give tricycle 394. An initial exo-cyclization onto the α-allenic carbon of 393 appeared to be prerequisite for completing the tandem process.
Scheme 104.
3.4.6. Garratt-Braverman Cyclization
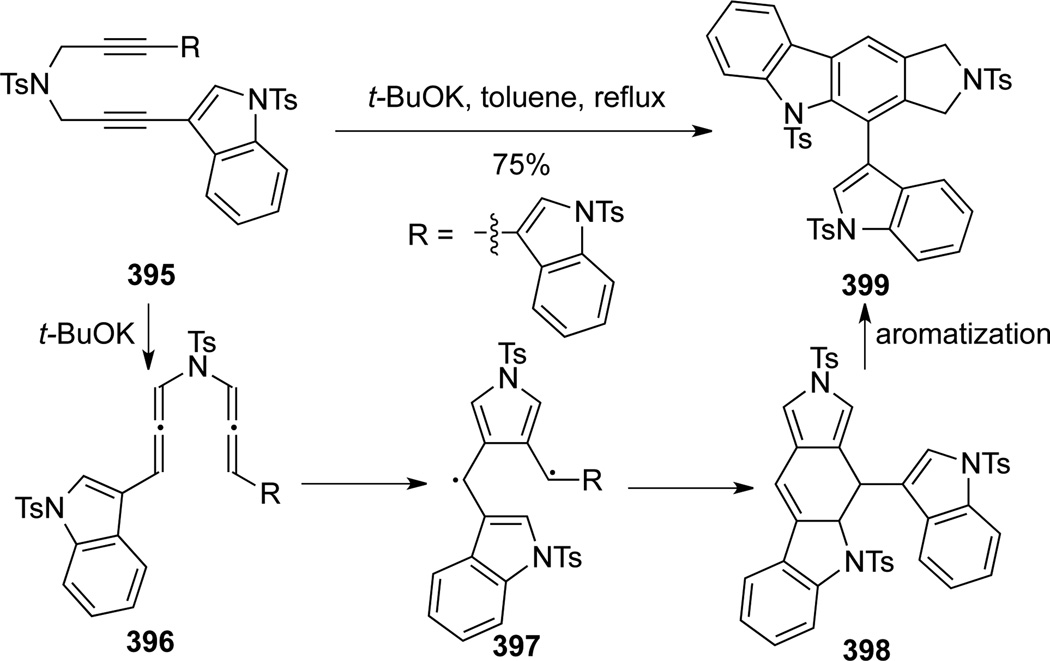
Garratt-Braverman cyclization,108 a biradical generating process, represents a powerful tool for new CC bond formation.108c Recently, Basak109 demonstrated successful construction of indolyl carbazole 399 through Garratt-Braverman cyclization of bis-allenamide 396 (Scheme 105). When refluxed in toluene in the presence of t-BuOK, amide 395 underwent isomerization to bis-allenamide 396, and the subsequent intramolecular cyclization generated the biradical pyrrole intermediate 397. The final product indolyl carbazole 399 was produced through the self-quenching/cyclization followed by aromatization.
Scheme 105.
3.4.7. Electrocyclization(EC)
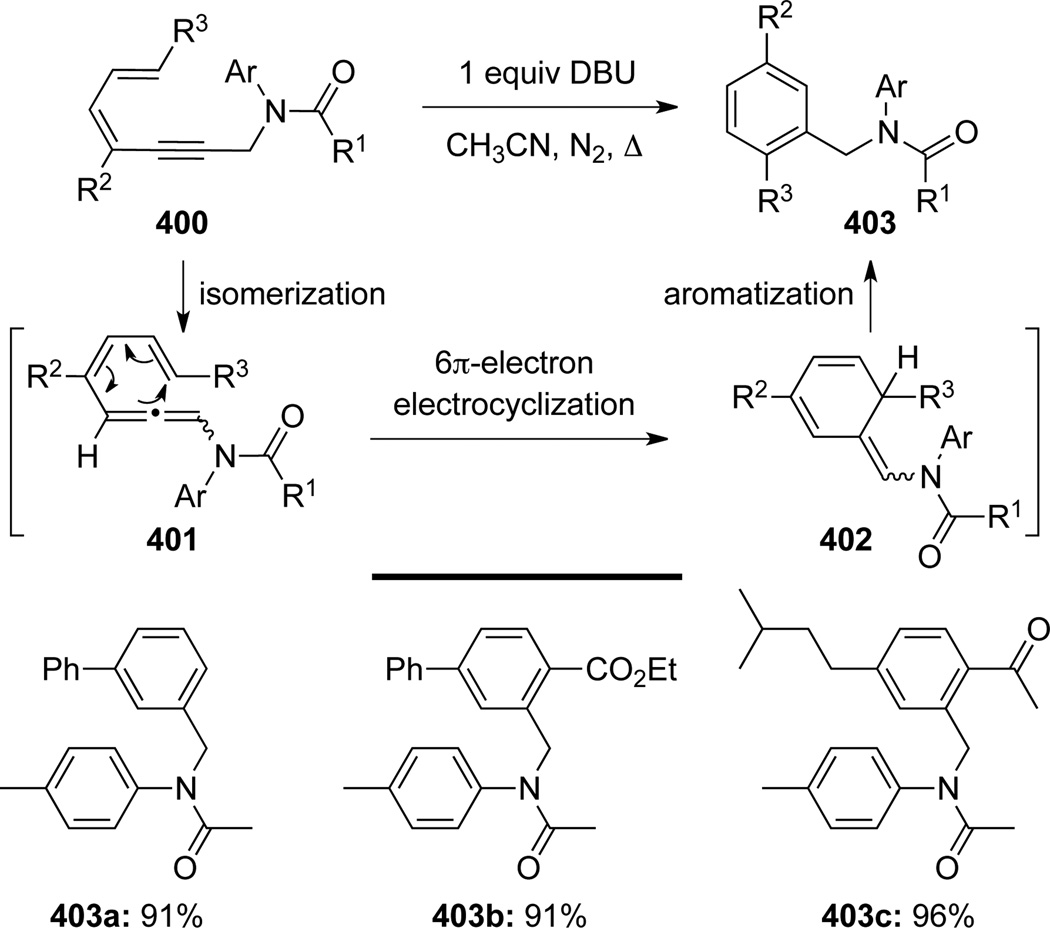
Besides Garratt-Braverman cyclization, allenamides 401 could also be utilized to construct 6-membered aromatics through electrocyclization (Scheme 106). Zhou110 synthesized a series of polyfunctional benzenes 403 from diene-propargyl amides 400. They proposed that allenamides 401 were the precursor subject to the 6π EC reaction to afford cyclized intermediates 402. In the presence of DBU, 402 isomerized to desired products 403 via aromatization.
Scheme 106.
3.5. Ring-Closing Metathesis
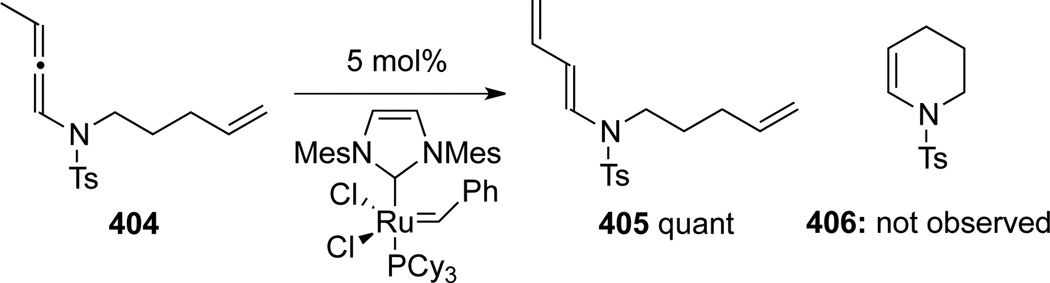
Hiemstra and Rutjes111 attempted to date the only ring-closing metathesis reaction involving allenamides (Scheme 107). However, when using allenamide 404, dienamide 405 was isolated as a result of the Ru-catalyzed isomerization (or 1,3-H shift), and the desired RCM product 406 was not observed.
Scheme 107.
3.6. Cycloadditions
3.6.1. [2 + 1] Cycloadditions
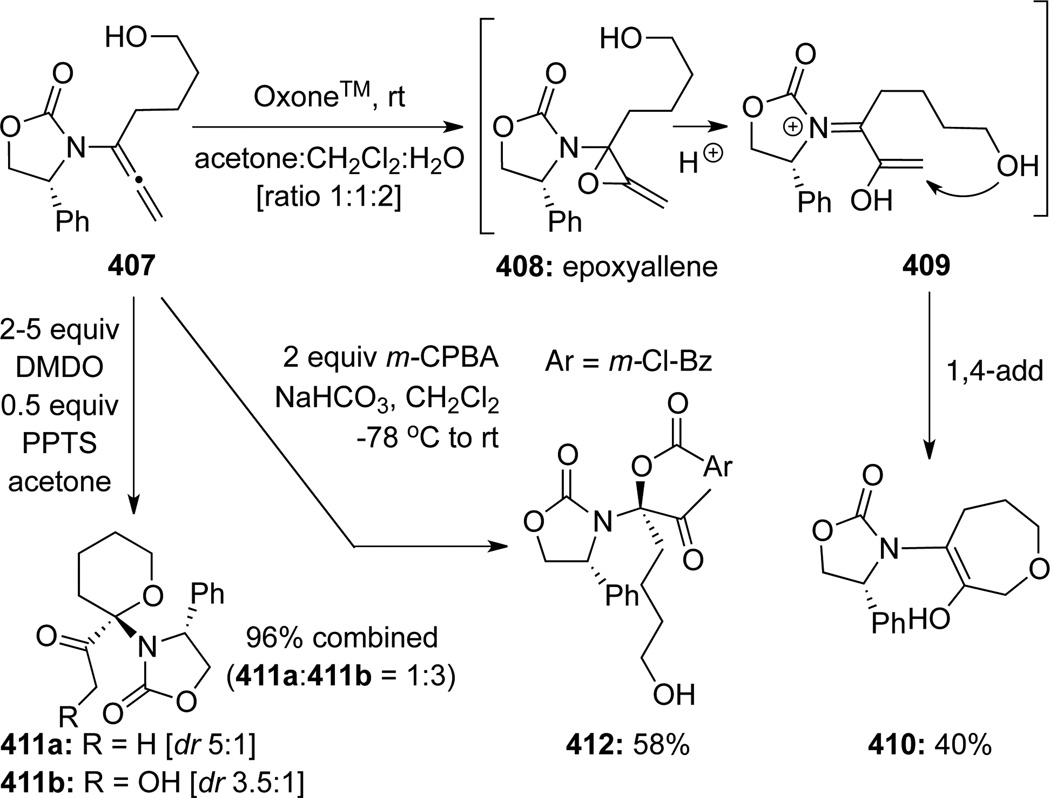
Hsung112 reported the first detailed studies on epoxidations of chiral allenamides including 1H NMR studies of these reactions (Scheme 108). When Oxone™ was used, isolation of cyclic ether 410 suggested that a more acidic medium during the oxidation can promote rapid ring-opening of allene oxide or epoxyallene 408 to provide the α, β-unsaturated N-acyl iminium intermediate 409. A subsequent intramolecular 1,4-addition would generate ether 410. The DMDO epoxidation of 407 in the presence of PPTS led to the formation of a mixture of pyrans 411a and 411b, which represent a 1,2-addition process to the N-acyl iminium intermediate related to 409 derived 408. Isolation of the major pyran 411b also implies double epoxidation of 407 or possible presence of a 2-amido-1,4-dioxasprio[2.2]pentane intermediate [not shown]. Treatment of allenamide 407 with buffered m-CPBA led to highly stereoselective formation of α-keto-N,O-acetal 412, thereby suggesting a very rapid in situ trapping of 408 by the m-chlorobenzoate anion.
Scheme 108.
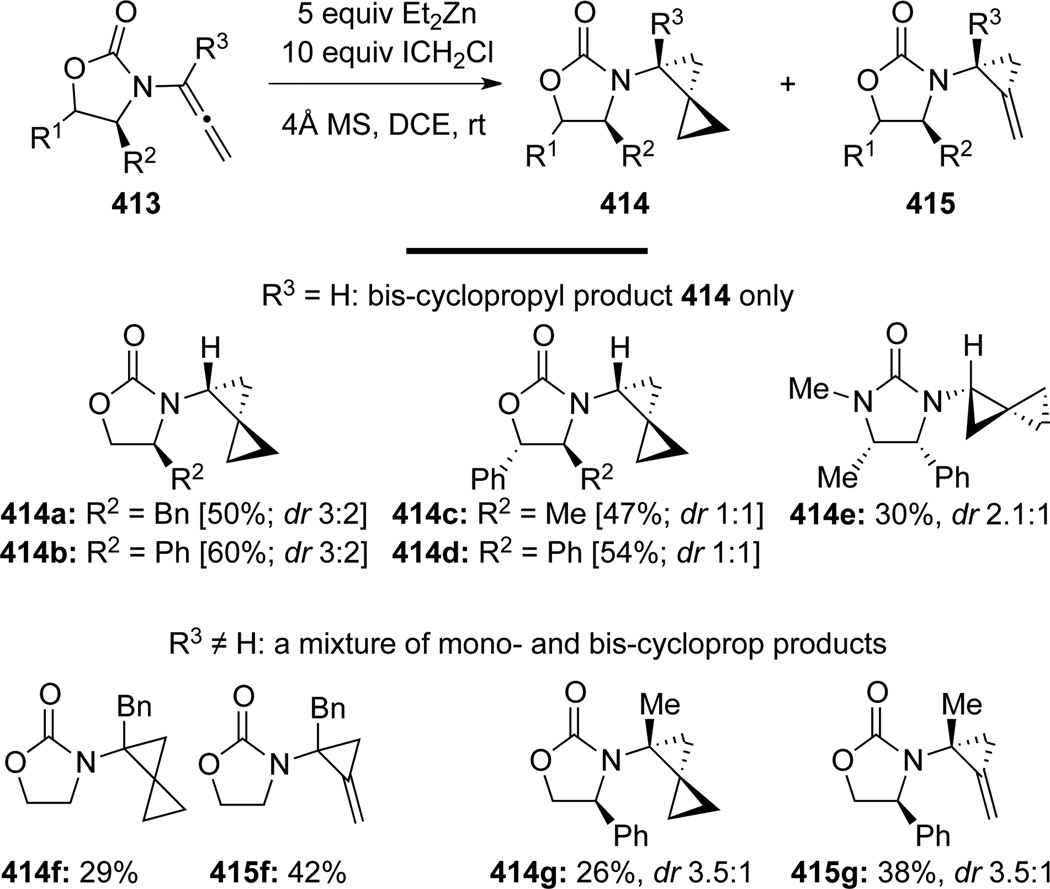
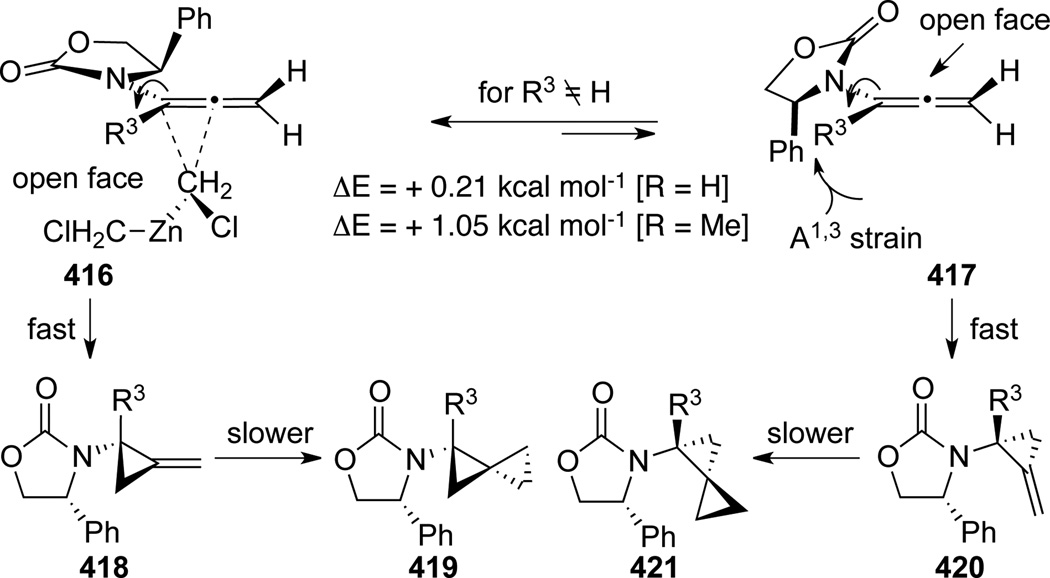
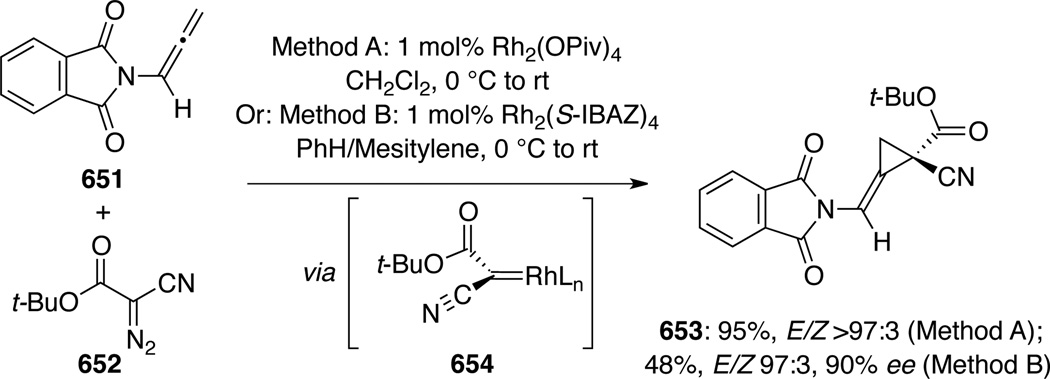
Hsung113 published a detailed account of Simmons-Smith cyclopropanation of allenamides to generate amido-spiro[2,2]pentanes (Scheme 109). For α-unsubstituted allenamides 413, only biscyclopropanation products 414 were observed, while α-substituted allenamides resulted in mixtures of mono- and bis-cyclopropanation products 415 and 414. Diastereoselectivity was modest but a conformational analysis was proposed in Scheme 110 to account for the lack of selectivity. The preference for the two conformers 416 and 417 is small, and because the first cyclopropanation to 416 and 417 took place very rapidly, there was very little facial selectivity, as both 416 and 417 could participate cyclopropanation via their respective open face. However, the selectivity increases modestly when R3 ≠ H because 416 becomes more favored as the conformer 417 suffers from enhanced A1,3 strain.
Scheme 109.
Scheme 110.
3.6.2. [2 + 2] Cycloadditions
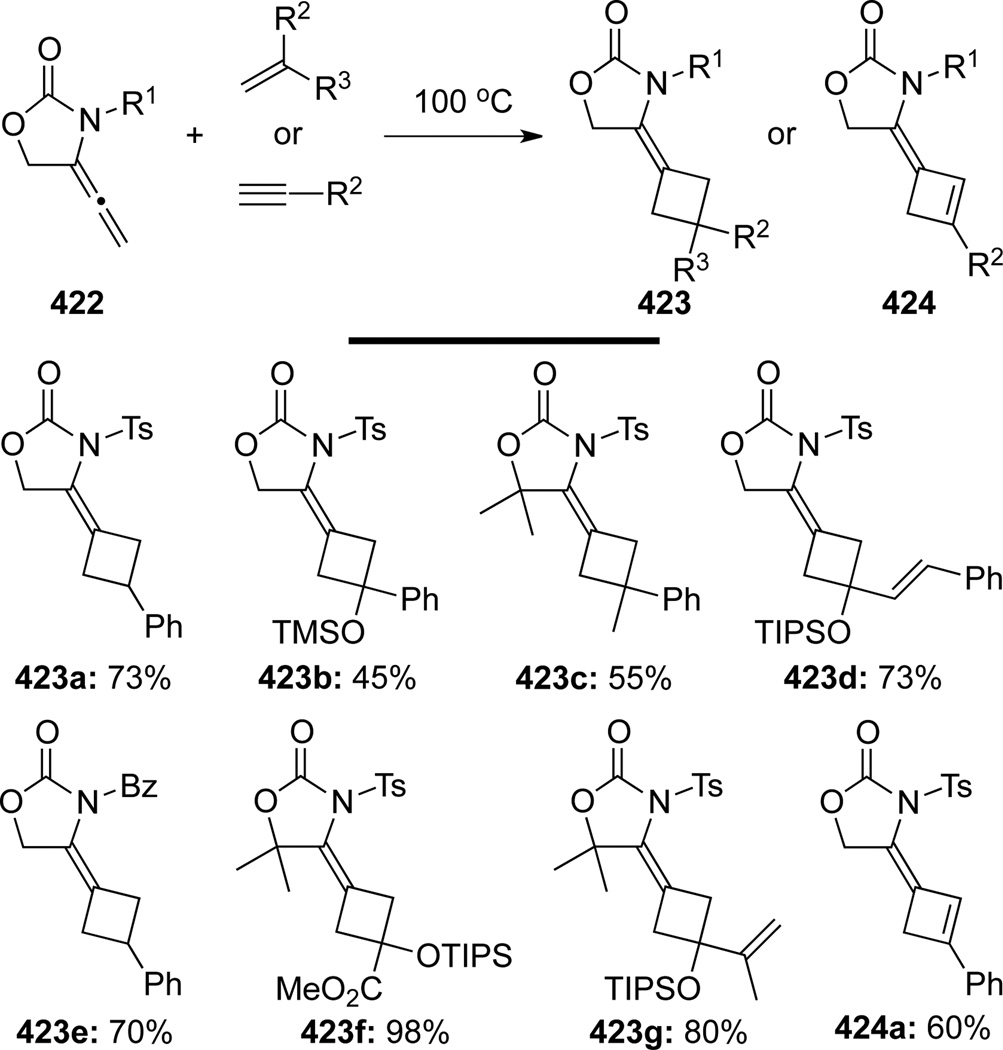
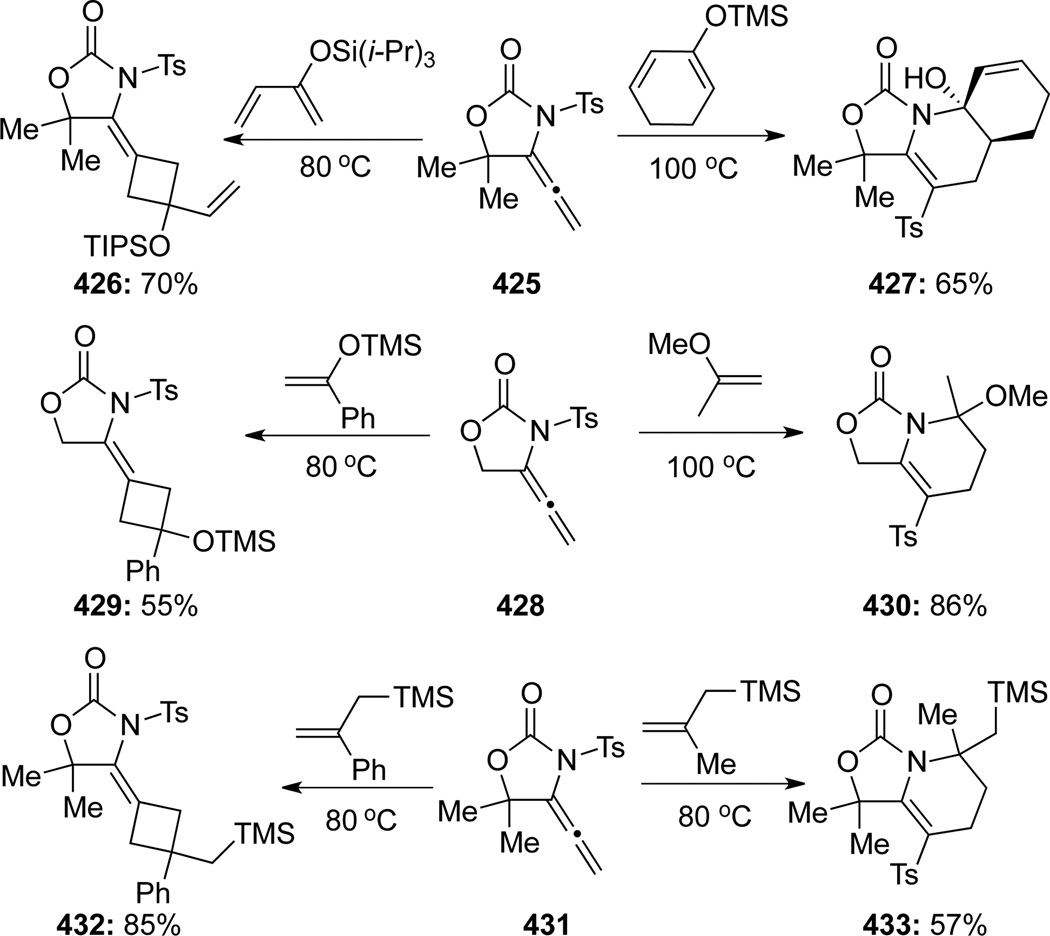
Studies of [2 + 2] cycloadditions were carried out by several research groups, among them Tamaru114a was the earliest in reporting their thermal [2 + 2] cycloaddition of allenamides. Various allenamides 422 underwent [2 + 2] cycloaddition reactions chemoselectively at the allenic β, γ-position with alkenes and alkynes to furnish cyclobutanes 423 and cyclobutenes 424 regio- and stereoselectively114b (Scheme 111). This methodology is general for alkenes bearing not only electron-withdrawing and conjugating groups but also electron-donating groups with complete retention of the alkene double bond geometries. However, the reaction is limited to terminal alkenes and alkynes, as internal alkynes, alkenes, and 1,3-dienes were unreactive.
Scheme 111.
In detailed study, Tamaru115 documented an interesting dichotomy between inverse demand hetero-[4 + 2] and [2 + 2] cycloadditions of allenamides (Scheme 112). Depending upon the alkene substitution pattern, allenamides can react with siloxydienes, enol ethers, and allylsilanes to either undergo [2 + 2] cycloadditions to exclusively provide cyclobutane derivatives, or a hetero-[4 + 2] cycloaddition pathway with a vinyl N-acyl imine intermediate resulting from an initial 1,3-Ts shift. While details of this excellent and thorough investigation will not be digested here and can be found in the original paper, see Section 3.6.5 for discussions on the 1,3-Ts-shift–inverse demand hetero-[4 + 2] cycloaddition cascade.
Scheme 112.
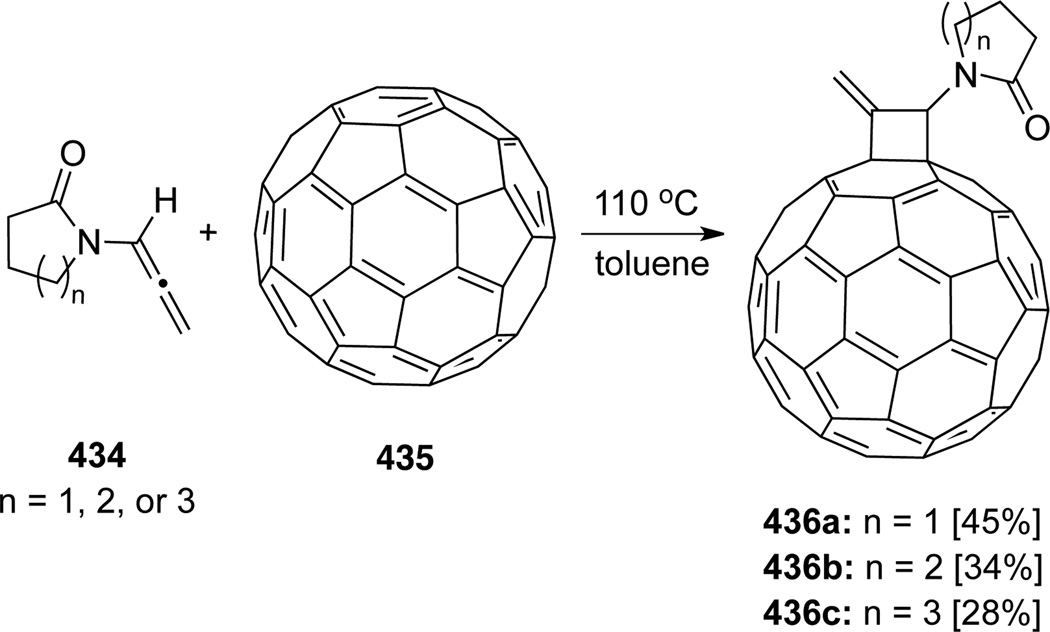
Nair116 published a unique [2 + 2] cycloaddition of allenamides 434 with [60]fullerene 435 to afford novel cyclobutane annulated fullerene derivatives 436 (Scheme 113). The reaction took place selectively on the internal double bond of allenamides, which had not been observed previously.
Scheme 113.
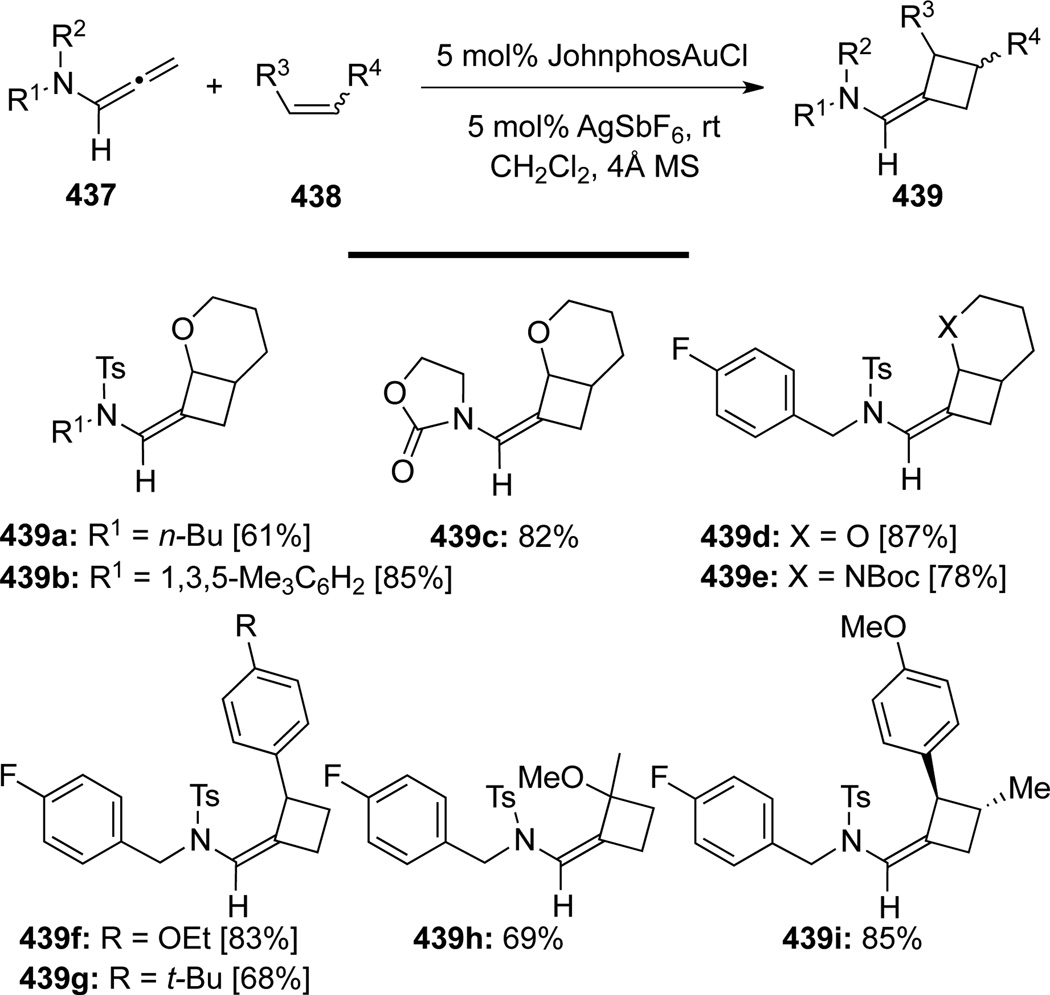
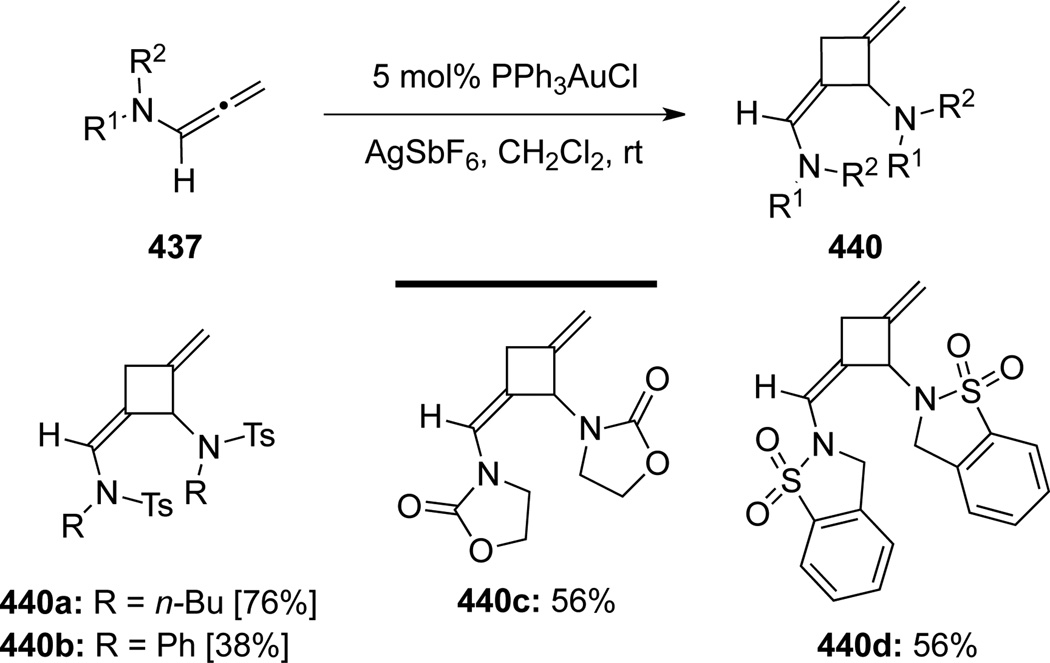
Chen117 reported the first publication of Au-catalyzed [2 + 2] cycloadditions of allenamides (Scheme 114). Their methodology provided densely functionalized cyclobutane adducts 439 using a wide range of allenamides 437 with electron-rich olefins 438. The cycloaddition was completely regio- and stereoselective, although α- or γ-substituted allenamides did not give desired products possibly due to the steric hindrance. The author also reported dimerization of starting allenamides 437 under the Au-catalysis in the absence of alkenes, leading to a series of dimerization products 440 (Scheme 115).
Scheme 114.
Scheme 115.
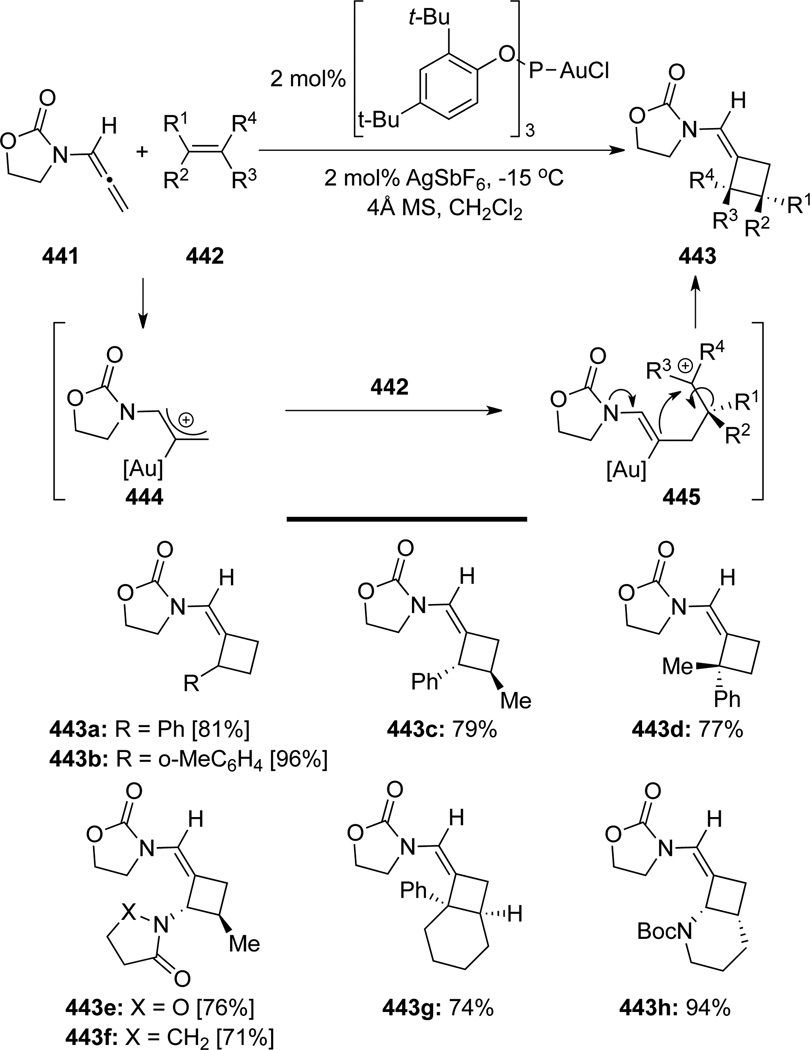
Mascareñas118 reported a highly regioselective Au-catalyzed intermolecular [2 + 2] cycloaddition of allenamide 441 with various alkenes 442 for the synthesis of cyclobutanes 443 (Scheme 116). Most critically, these authors found that when using enamides as reacting partners, both (E) and (Z) isomers of enamides 442e and 442f generated single trans-stereoisomer as shown in 443e and 443f, respectively, thereby suggesting a stepwise cationic pathway. Intermolecular nucleophilic interception of the Au-allyl cation species 444 by the alkene would form a second cationic intermediate 445. The formation of the more stabilized benzylic or N-acyl iminium cation (when using cyclic or acyclic enamides) should lead to the observed regioselectivity, while rotation around the σC-C bond would result in the loss of original alkene stereochemical information. A final allenamide nitrogen atom-assisted ring-closing process followed by elimination and regeneration of the Au complex would furnish [2 + 2] adduct 443.
Scheme 116.
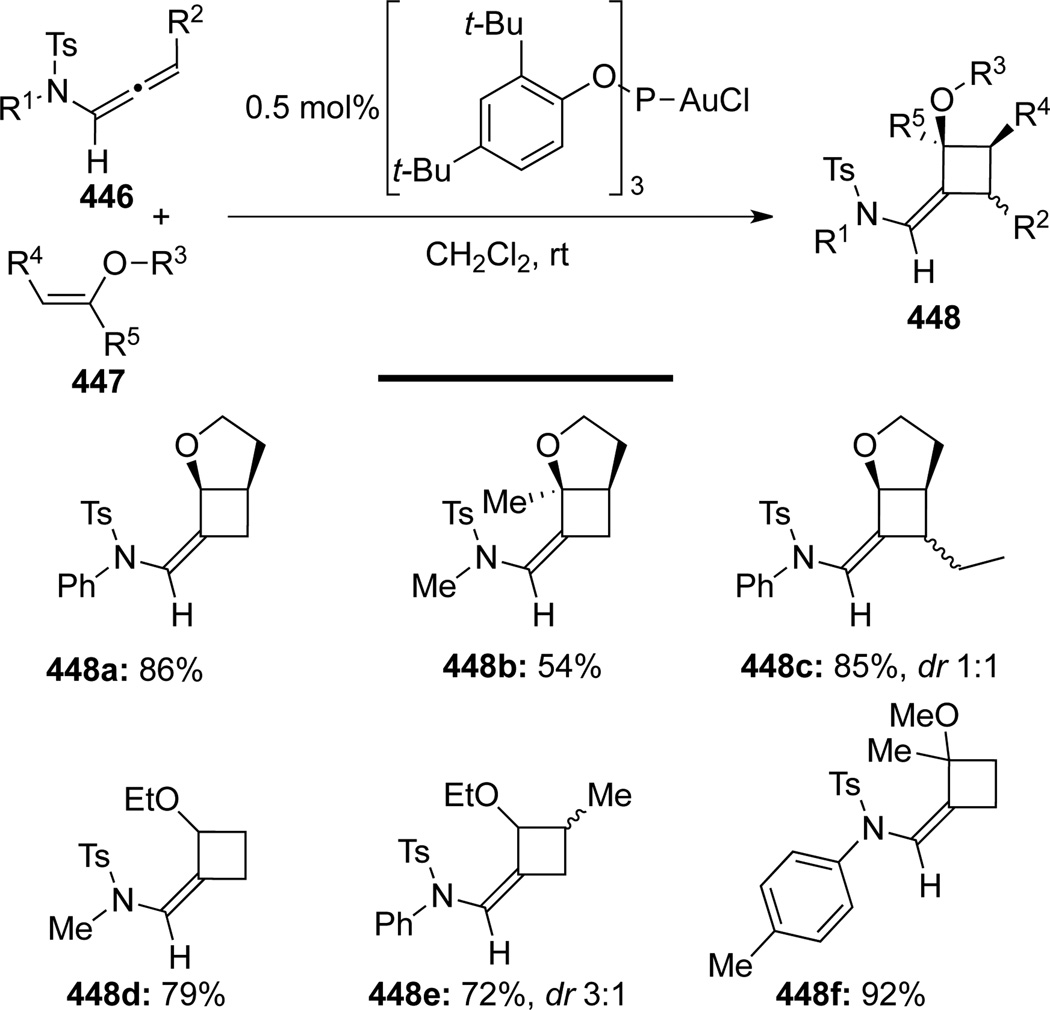
González119 independently published their Au-catalyzed [2+2] cycloaddition of N-tosyl allenamides 446 with enol ethers 447 (Scheme 117) at the same time. Although the same gold complex was used as in Mascareñas’s report, catalyst loading was much lower, and more importantly, γ-substituted allenamides worked very well in this system to generate the cycloadduct such as 448c.
Scheme 117.
3.6.3. [2 + 2 + 1] Cycloadditions
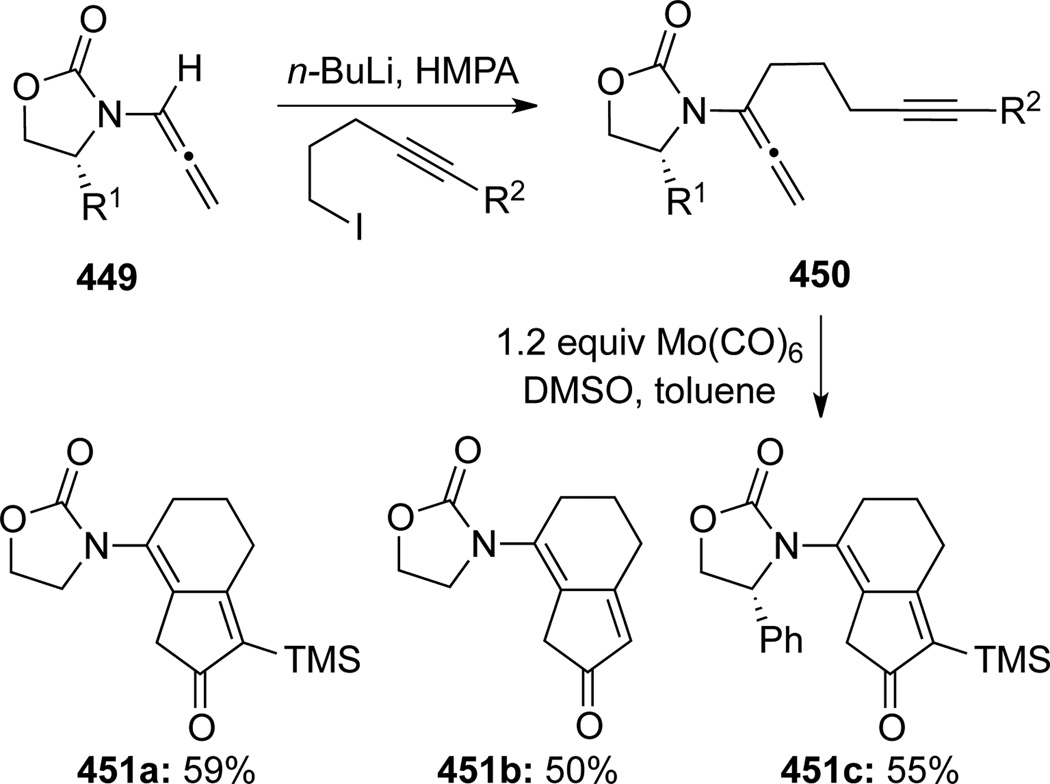
Hsung58 first published a regioselective α-deprotonation and functionalization of electron-deficient allenamides 449, and application of the resulting α-substituted allenamides in an intramolecular Pauson-Khand-type cycloaddition (Scheme 118). The functionalized allenamides 450 were treated with Mo(CO)6 to provide the desired [2 + 2 + 1] cycloaddition products 451 regioselectively in favor of the terminal olefin. As the first examples of Pauson-Khand-type reactions involving nitrogensubstituted allenes, these authors accounted the regioselectivity as a result of the Mo metal complexing to the less sterically congested terminal olefin of the allene.
Scheme 118.
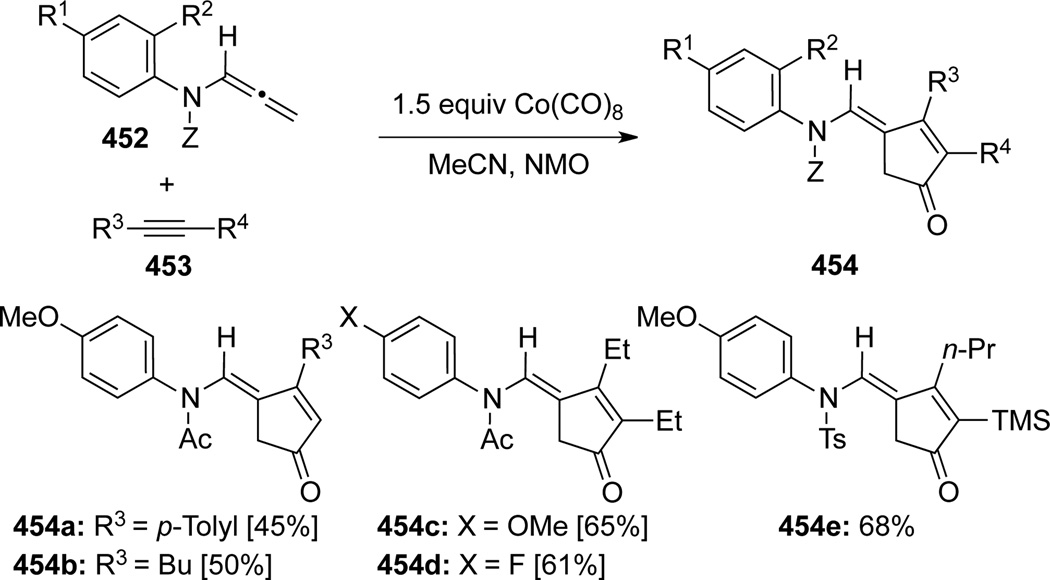
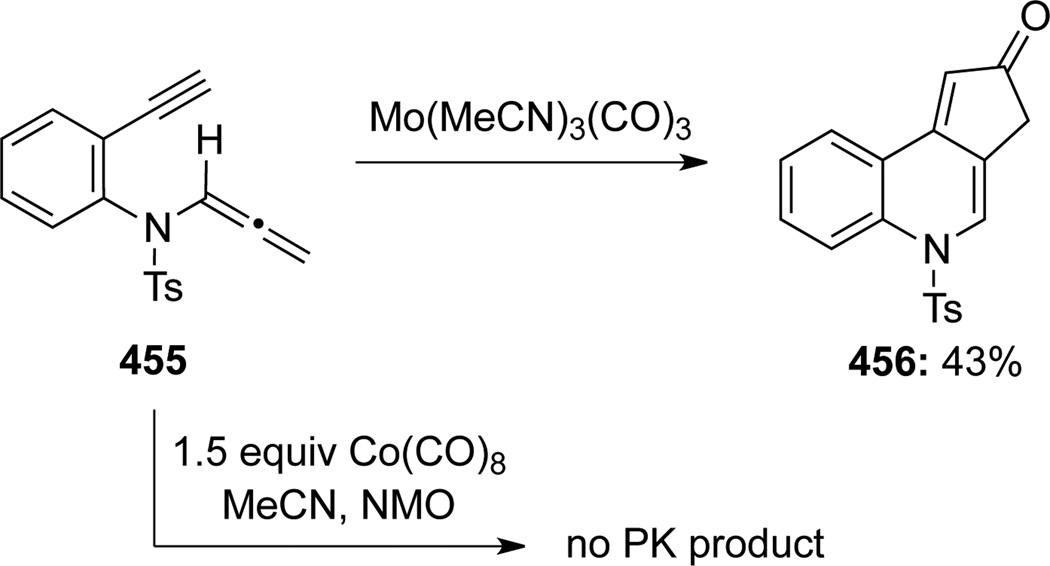
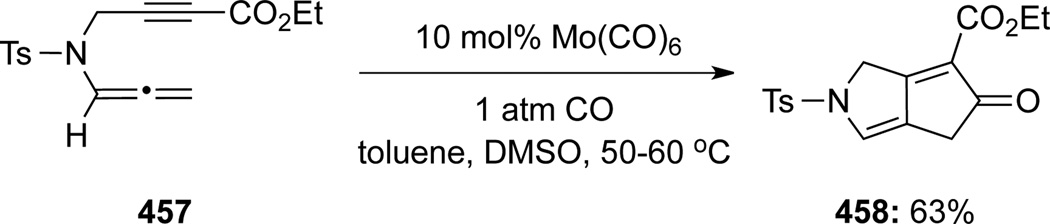
Pérex-Castells120 reported the first intermolecular Pauson-Khand reaction of allenamides 452 promoted with Co(CO)8 (Scheme 119). These reactions were both regio- and stereoselective with several alkynes 453 to give functionalized cyclopentenones 454 bearing an exo-cyclic E-enamide. Subsequently, Pérez-Castells121 unveiled a Mo(MeCN)3(CO)3 intramolecular Pauson-Khand type reaction of allenamide 455, leading to tricycle 456 in 43% yield, although the original Co-conditions were not effective (Scheme 120). In it noteworthy that Oh122 had earlier also demonstrated a molybdenumcatalyzed [2 + 2 + 1] cycloaddition of allenamide 457 to produce compound 458 (Scheme 121).
Scheme 119.
Scheme 120.
Scheme 121.
3.6.4. [3 + 2] Cycloadditions
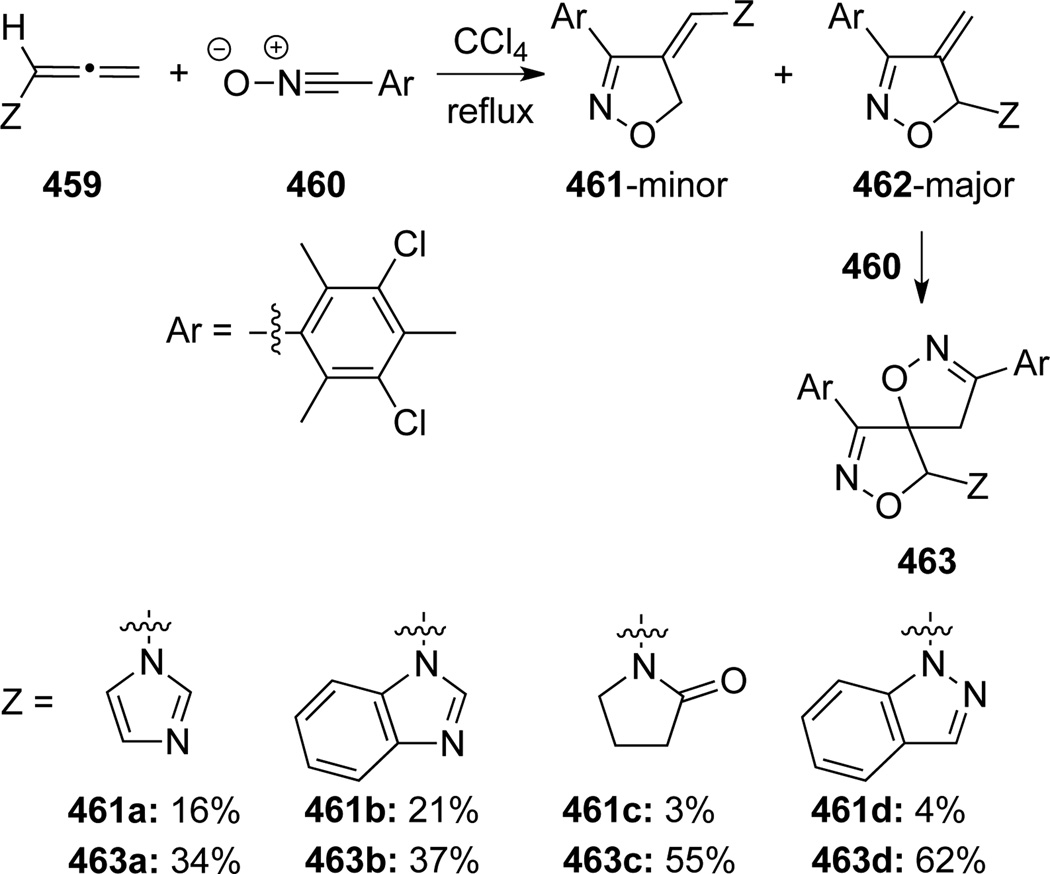
Broggini and Zecchi123 documented the first [3+2] cycloaddition in which a series of allenamides 459 were treated with benzonitrile oxide 460 under thermal conditions (Scheme 122). The reaction occurred predominantly at the internal double bond of allenamides to give dihydroisoxazoles 462 with an exo-cyclic olefin as the major product, which reacted readily with excess amount of benzonitrile oxide 460 to provide bis-spiro-oxazoles 463. In addition, unlike 462, the minor cycloadduct 461 was inert toward a second cycloaddition due to the sterics.
Scheme 122.
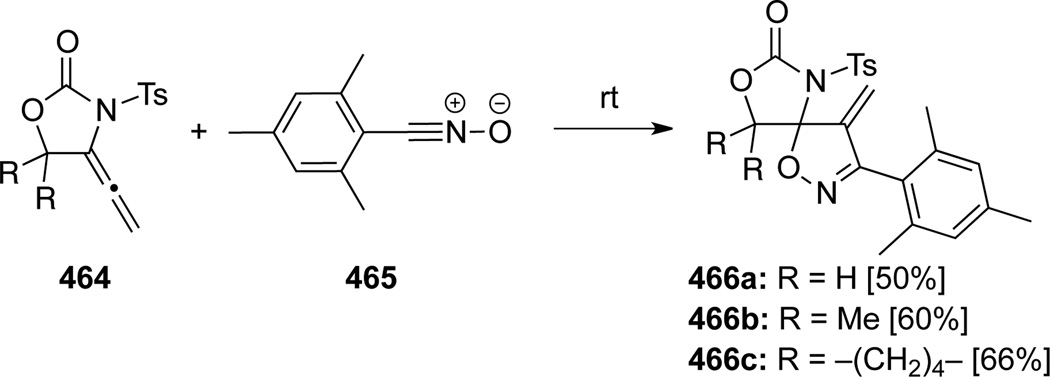
Tamaru114b also reported hetero-[3 + 2] cycloadditions of allenamides 464 with nitrile oxide 465 (Scheme 123). The reaction also took place on the internal double bond to afford diazadioxaspirocycles 466.
Scheme 123.
Barluenga124 published the first Rh-catalyzed carbo-[3 + 2] cycloaddition of allenamides 467 with chromium alkenyl(methoxy)carbine complexes 468 (Scheme 124). Most cyclization reactions took place with complete chemo-, regio-, and diastereoselectivities, leading to a broad range of functionalized amidocyclopentenes 469 in good to excellent yields. A tentative reaction pathway for the cycloaddition involved the Fischer-type rhodium(I)-carbene complexes 470 generated via a chromium-rhodium exchange. CO appears to be critical in this cycloaddition, as it not only improved the efficiency of the catalytic reaction by favoring the transmetallation step, but also allowed almost quantitatively recovery of Cr(CO)6.
Scheme 124.
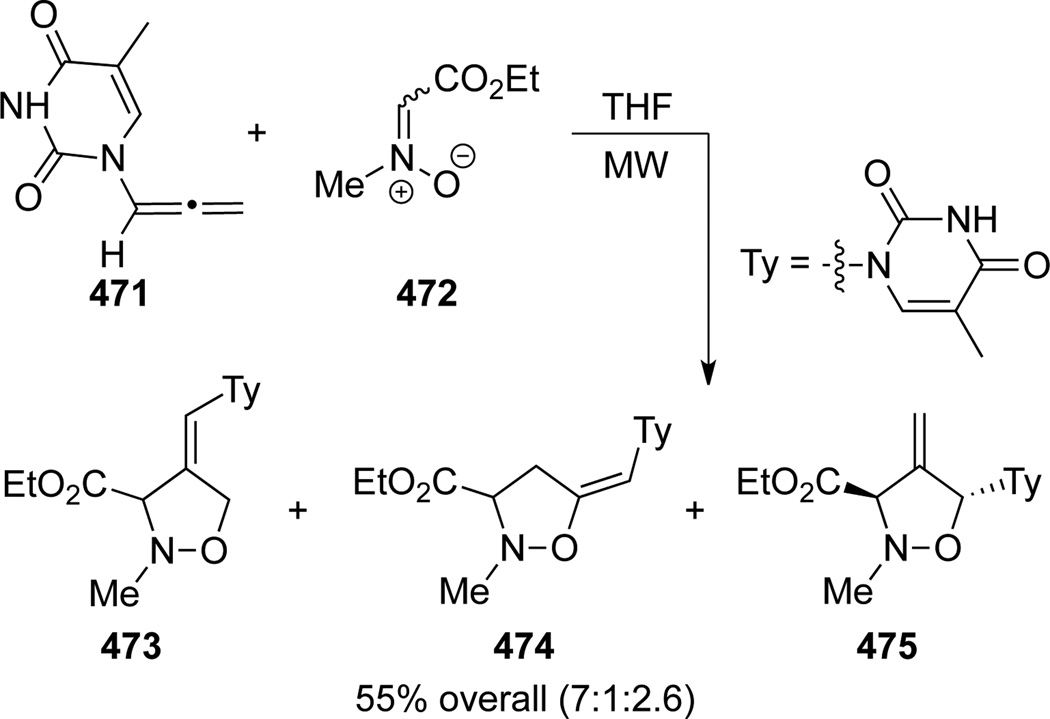
Piperno and Romeo125 reported an example of thermal [3 + 2] cycloaddition of pyrimidine-dione containing allenamide 471 with nitrone 472 under conditions using microwave irradiation, leading to a mixture of iso-oxazolidines 473, 474 and 475 (Scheme 125). The cycloadducts 473 and 474 represent the two possible regiochemical addition of nitrone onto the terminal allenic double bond with a ratio of 7:1 in favor of the former, while the stereochemistry of iso-oxazolidine 475 was assigned by NOE as trans.
Scheme 125.
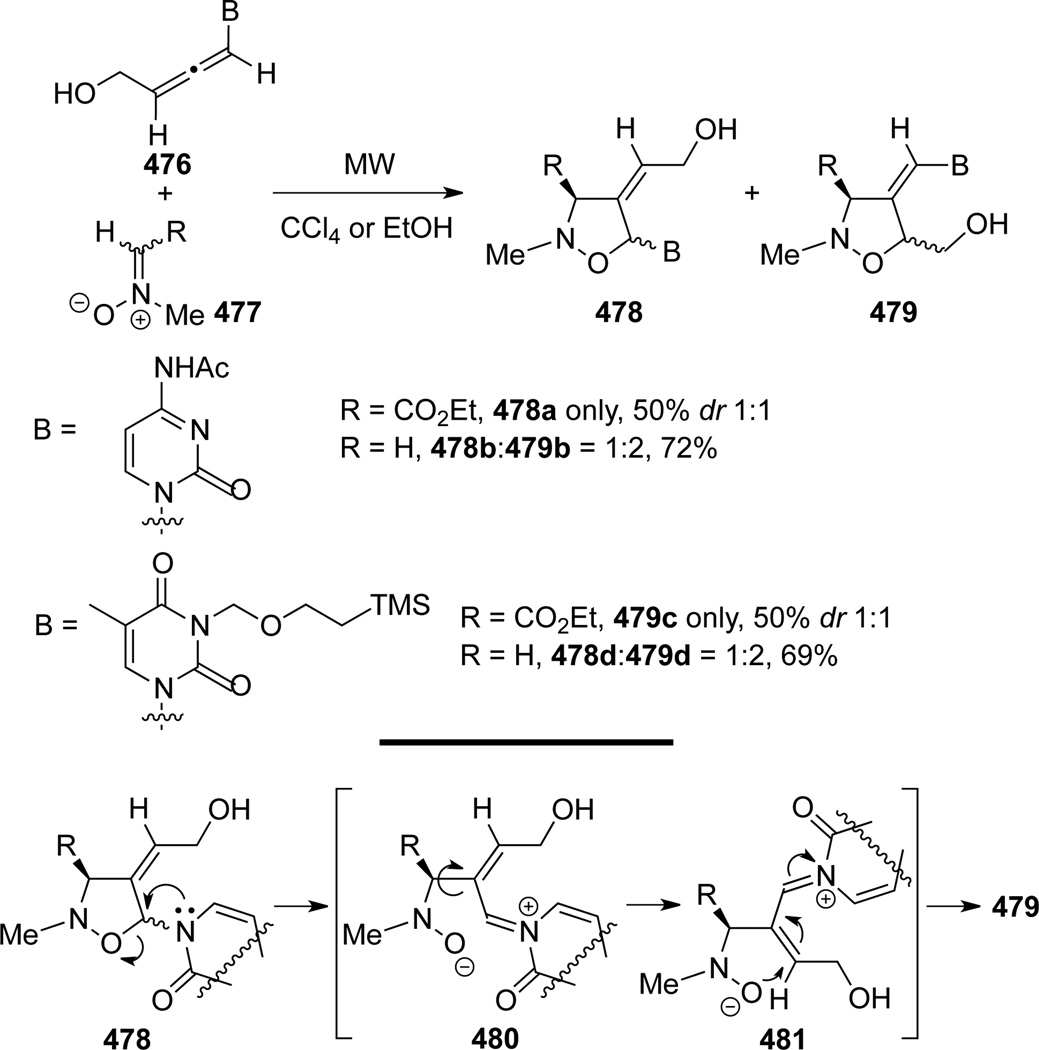
Piperno, Romeo, and Rescifina126 later published another microwave assisted [3 + 2] cycloaddition of allenamides 476 with nitrones 477 (Scheme 126). In this work, both cycloadducts 478 and 479 were observed, representing cycloadditions onto the internal and terminal allenic double bonds, respectively. The cycloadduct 479 was believed to be a result of thermal equilibration from 478 through intermediates 480 and 481.
Scheme 126.
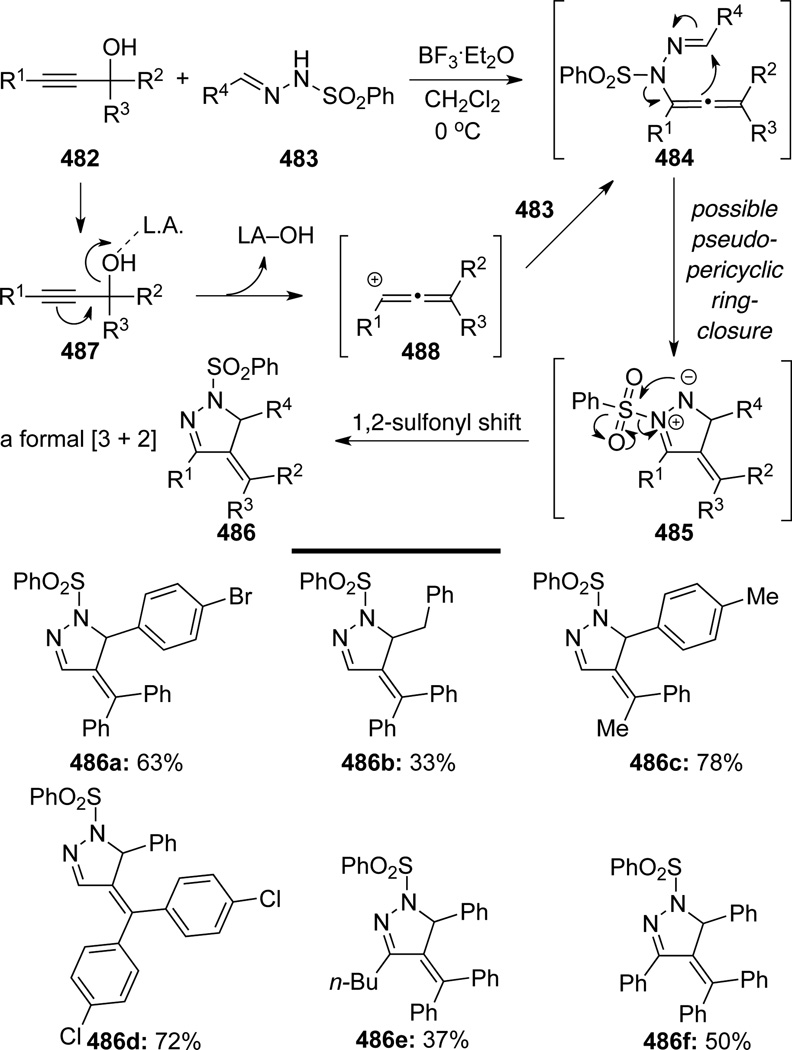
Wang and Lu127 reported an efficient strategy for the synthesis of functionalized pyrazoles 486 from propargyl alcohol and N-sulfonyl-hydrazone (Scheme 127). N-Sulfonyl allenamide 484 was postulated as the key intermediate for this impressive tandem cascade as mapped in Scheme 127, featuring a pseudo-pericyclic (2n+4π) ring-closure of 484 and representing ultimately a formal [3 + 2] cycloaddition process. Various propargyl alcohols 482 and hydrazones 483 were applicable to give products 486, although lower yields were obtained for hydrazones derived from aliphatic aldehydes.
Scheme 127.
3.6.5. [4 + 2] Cycloadditions
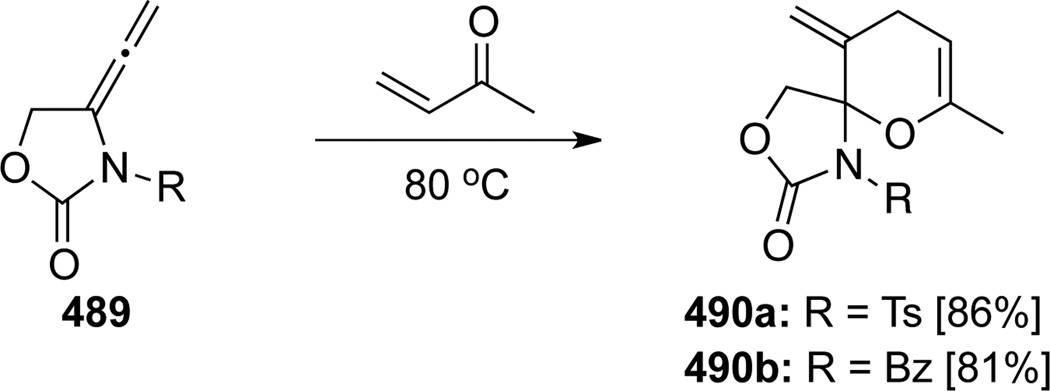
Tamaru50 examined the first inverse electron-demand hetero-[4 + 2] cycloaddition reaction of allenamides 489 with MVK under thermal conditions (Scheme 128). The cycloaddition took place selectively at the internal double bond, leading to the desired spiro N,O-acetal products 490 in excellent yields.
Scheme 128.
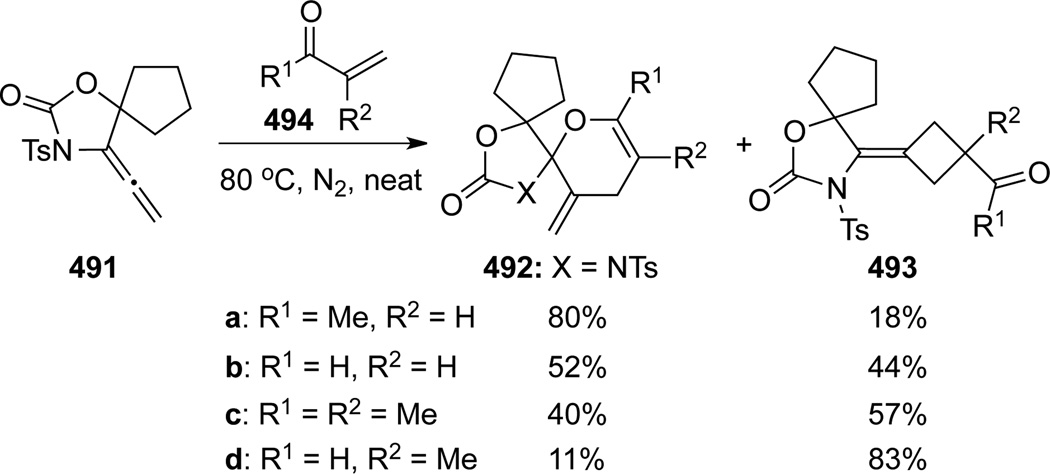
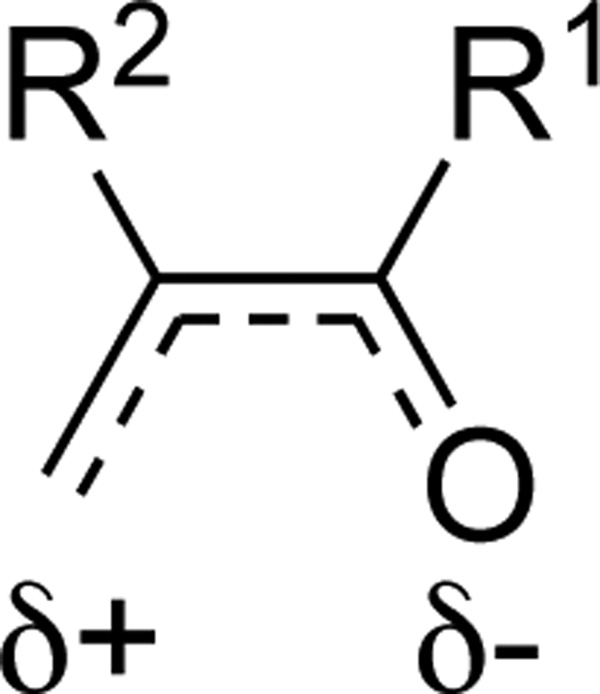
These authors also conducted a detailed study of this [4 + 2] cycloaddition using allenamides 491 with a series of heterodienes (Scheme 129).114b While a competing [2 + 2] cycloaddition product was also detected (vide supra), ratios of the [4 + 2] cycloadducts versus the corresponding [2 + 2] cycloadducts varied with different heterodienes. The reaction with MVK afforded the [4 + 2] product 492a in 80% yield, while the reaction with 2-methylacrolein predominantly gave the [2 + 2] addition product 493d. The product distribution could be qualitatively rationalized by the charge delocalization caused by different substitution patterns (Scheme 130), that is, the methyl group of MVK increases the electron density on O atom and promotes the [4 + 2] reaction while the methyl group of α-methylacrolein decreases the positive charge on the terminal CH2 and retards the [4 + 2] reaction. Relative activation energies were calculated and changed just as expected (–3.0, 0.0, 9.3 12.7 for 492a–d respectively with acrolein as the standard). Detailed investigation can be found in the origin paper.
Scheme 129.
Scheme 130.
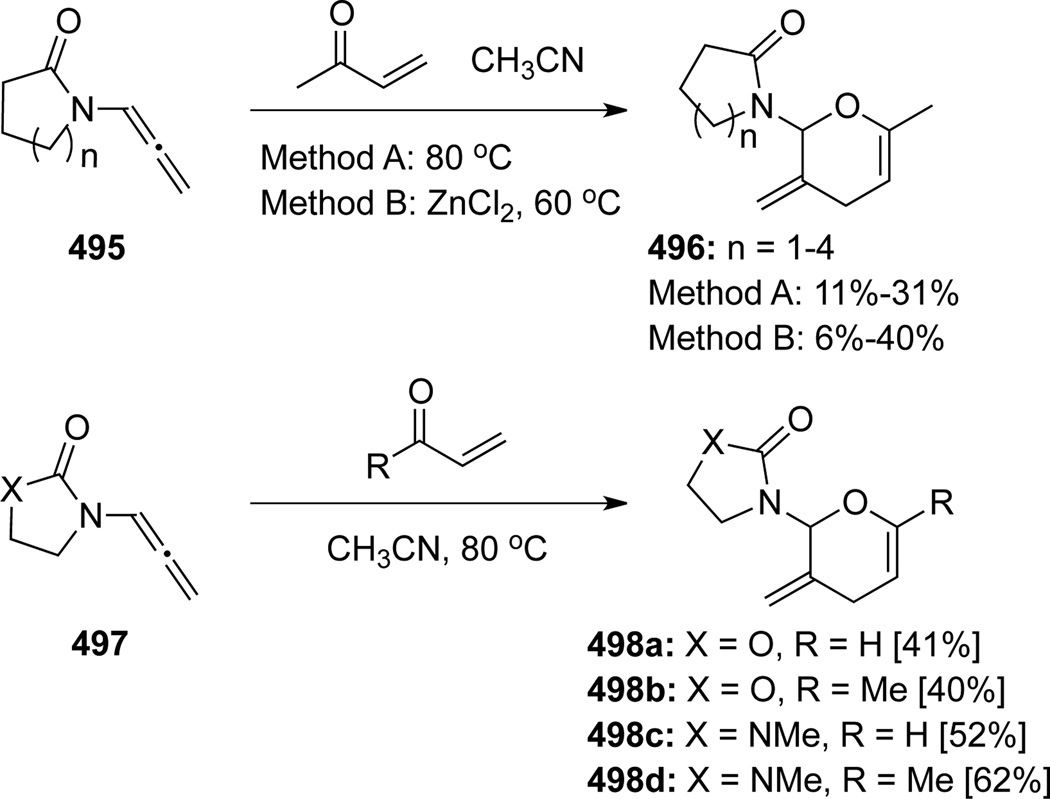
Hsung41b also disclosed an inverse electron-demand hetero-[4 + 2] cycloaddition reaction of allenamides 495 with acrolein or MVK (Scheme 131). The reaction of allenamides 495 containing a lactam moiety could proceed under both thermal and Lewis acid conditions with the five-membered lactam allenamide (n = 1) giving the best results. Further studies revealed that allenamides 497 containing an oxazolidinone or imidazolidinone moiety proved to be more reactive.
Scheme 131.
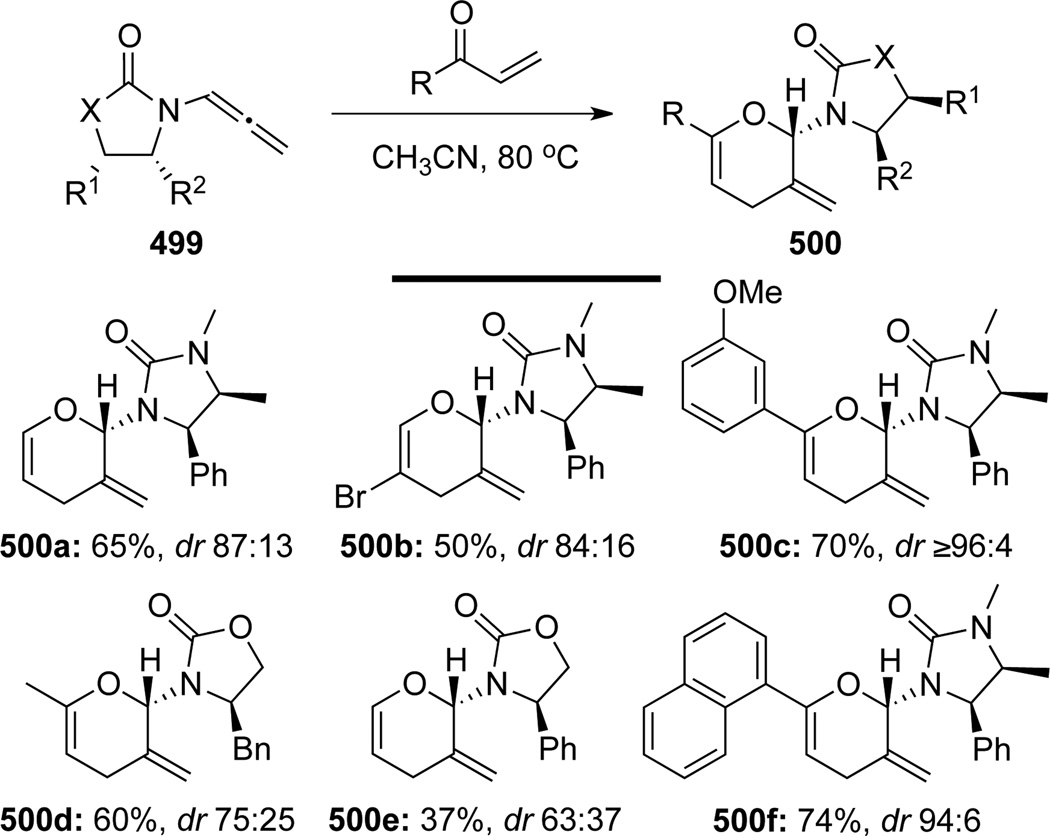
Hsung128 further reported the first stereoselective version of this hetero-[4 + 2] cycloaddition reaction with chiral allenamides 499 (Scheme 132). A series of heterodienes as well as chiral allenamides were examined, affording pyranyl heterocycles 500 in good yields with selectivity being as high as ≥96:4.
Scheme 132.
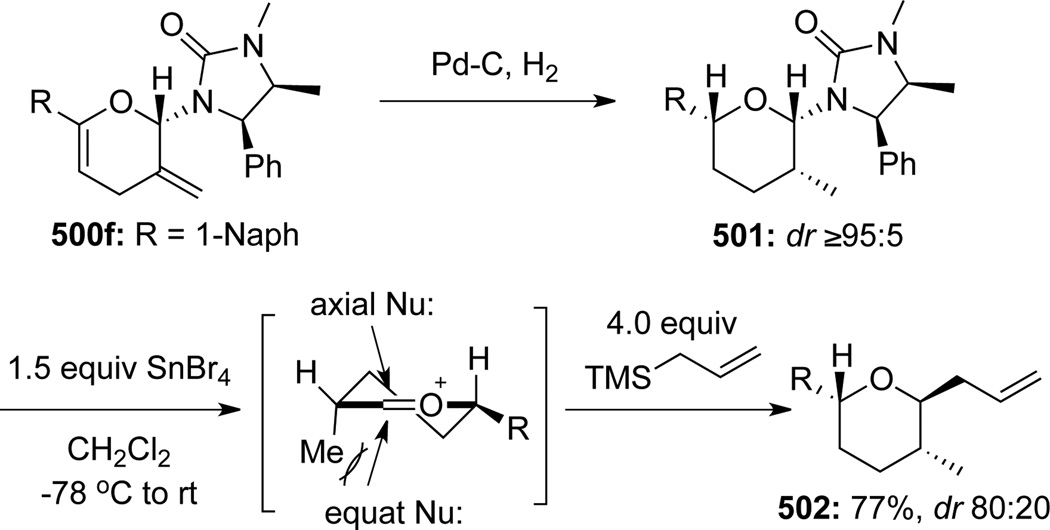
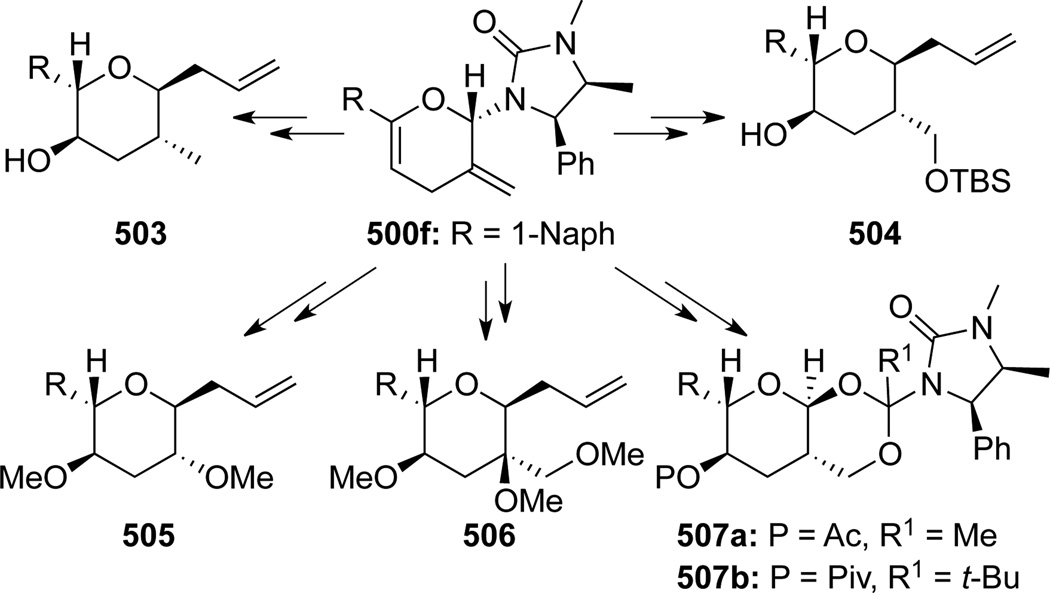
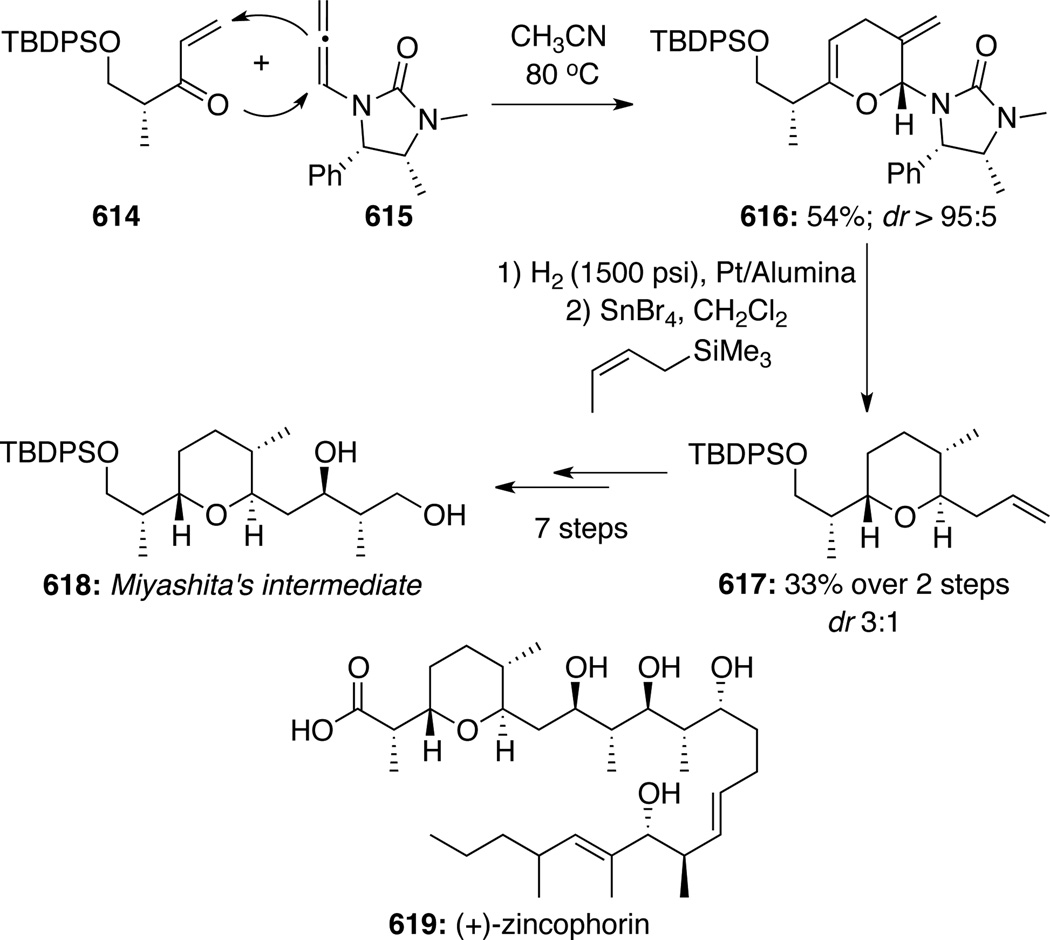
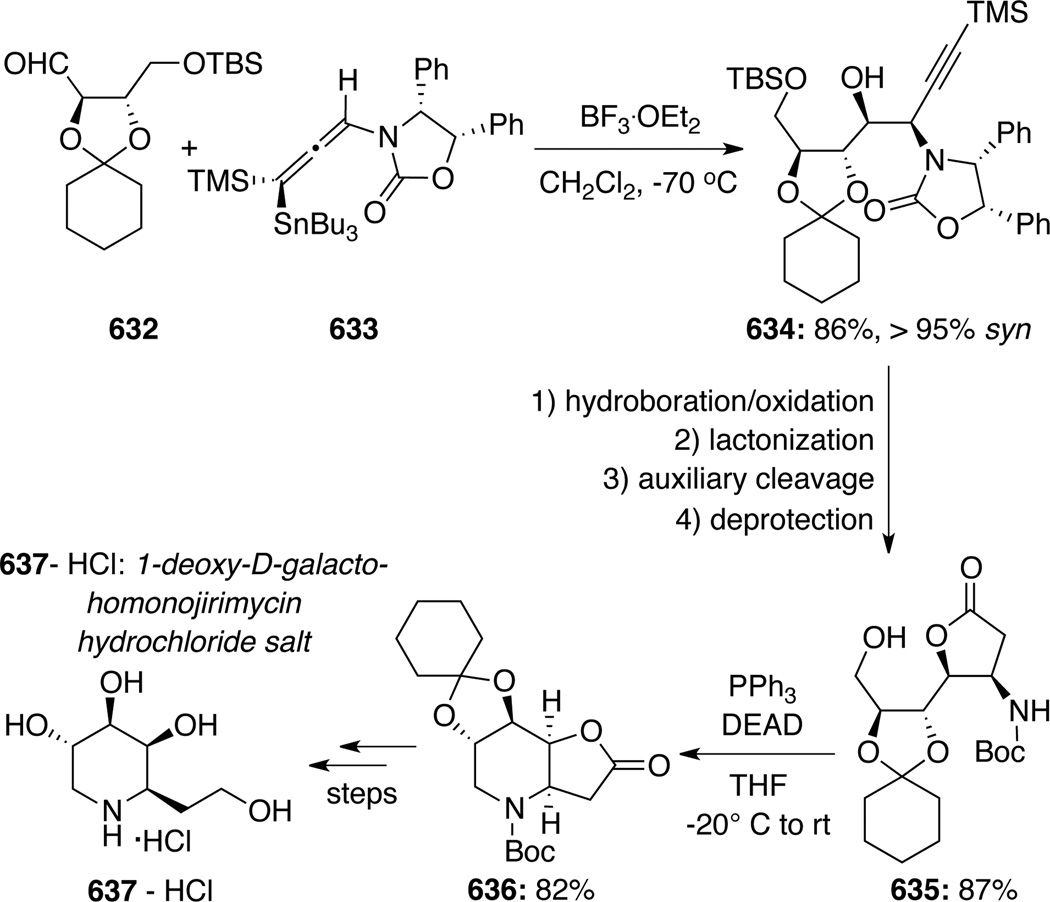
To illustrate synthetic utilities of these pyranyl cycloadducts, Hsung129 developed the first Lewis acid catalyzed stereoselective removal of the anomeric chiral urea group concomitant with allylation to give pyrans 502 (Scheme 133). Based on this methodology, an array of highly functionalized and structurally interesting tetrahydropyrans was assembled in stereoselective manner (Scheme 134). These studies eventually led to the formal synthesis of natural product (+)-zincophorin (vide infra).
Scheme 133.
Scheme 134.
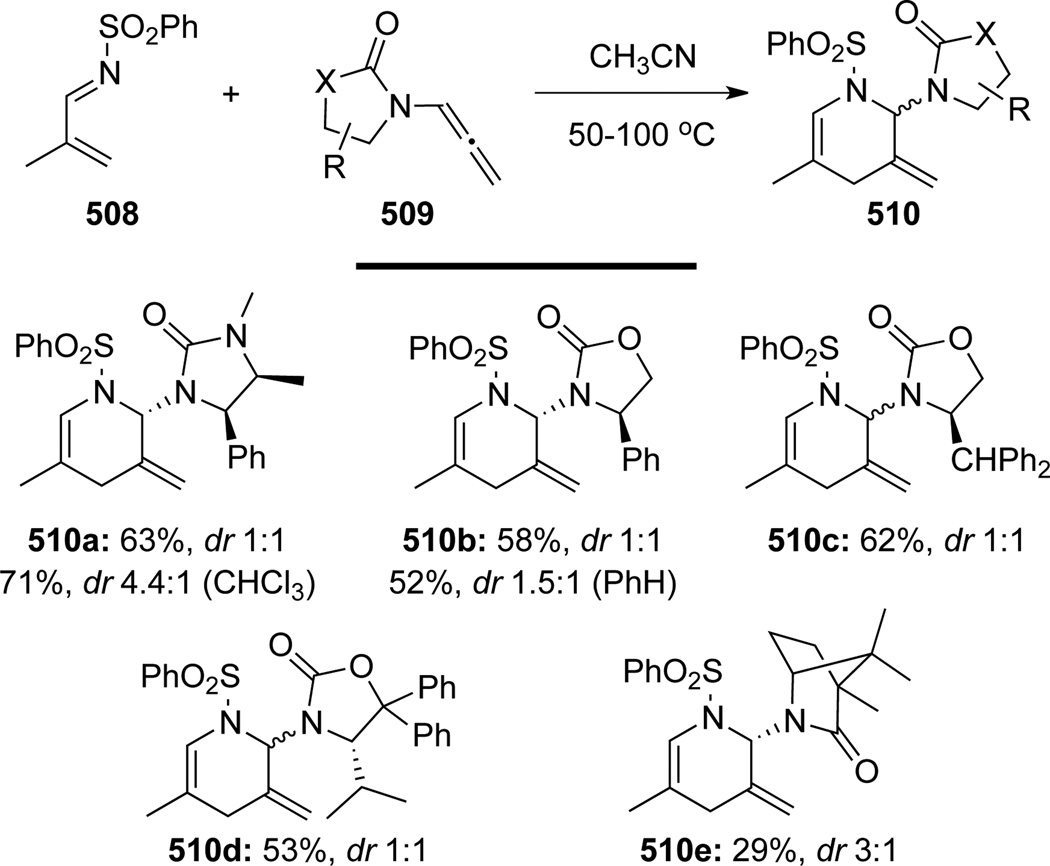
Hsung130 also described the inverse electron-demand aza-[4 + 2] cycloaddition reaction of chiral allenamides 509 employing phenylsulfonyl protected 1-azadiene 508 (Scheme 135). Despite the moderate yield and selectivity, this aza-[4 + 2] reaction demonstrated the synthetic utility of chiral alleneamides for the synthesis of aza-glycoside related heterocycles.
Scheme 135.
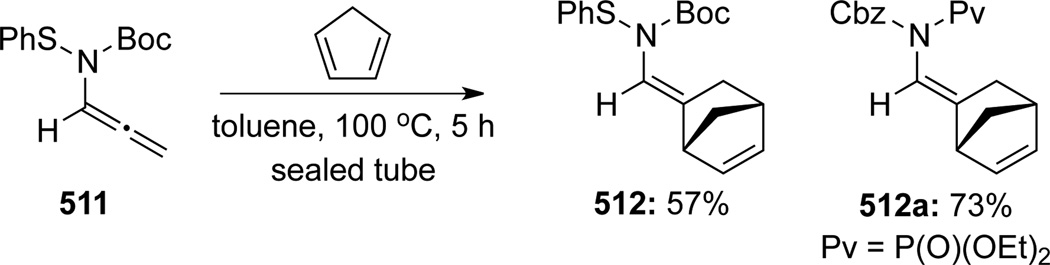
Van Vranken32 was the first to demonstrate that an allenamide could actually participate in a normal electron-demand [4 + 2] cycloaddition reaction with the external double bond of the allene motif serving as the corresponding dienophile. These authors reported an example of such reaction with allenamide 511, which afforded cycloadduct 512 containing an enamide motif in 57% yield (Scheme 136). It is noteworthy that Mapp27 also reported a similar reaction with N-phosporamidate substituted allenamide, which was synthesized using the method they had developed (See cycloadduct 512a and Scheme 17)
Scheme 136.
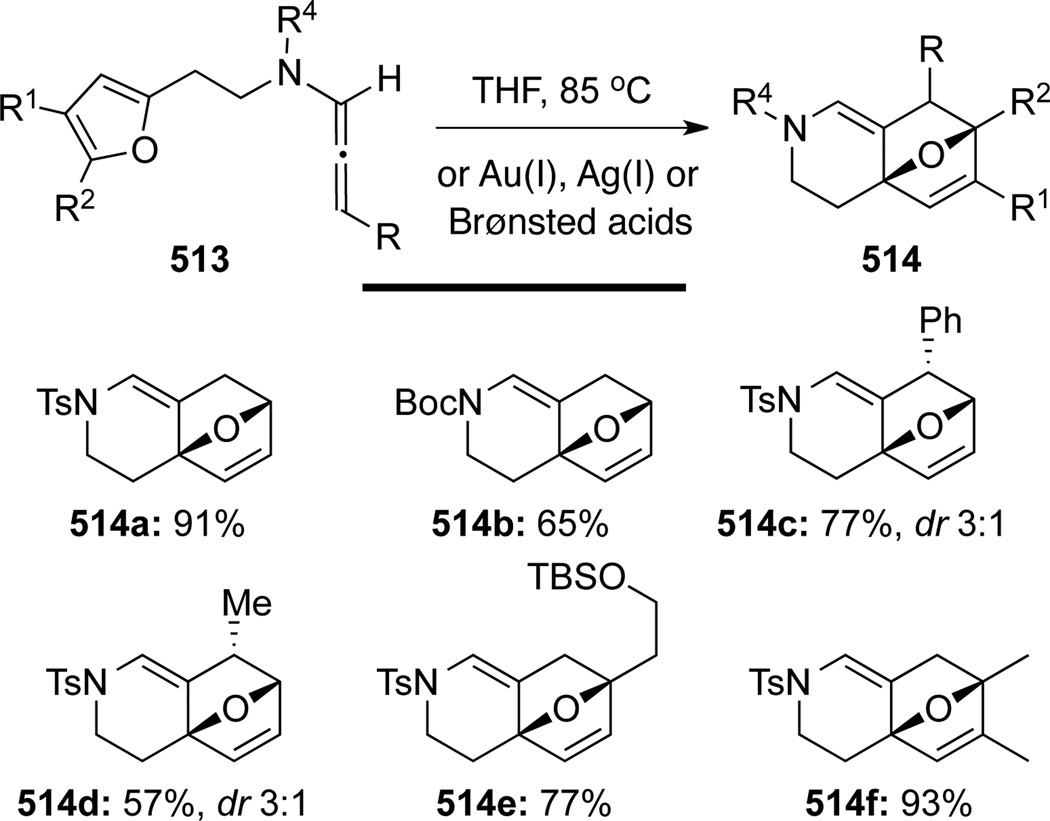
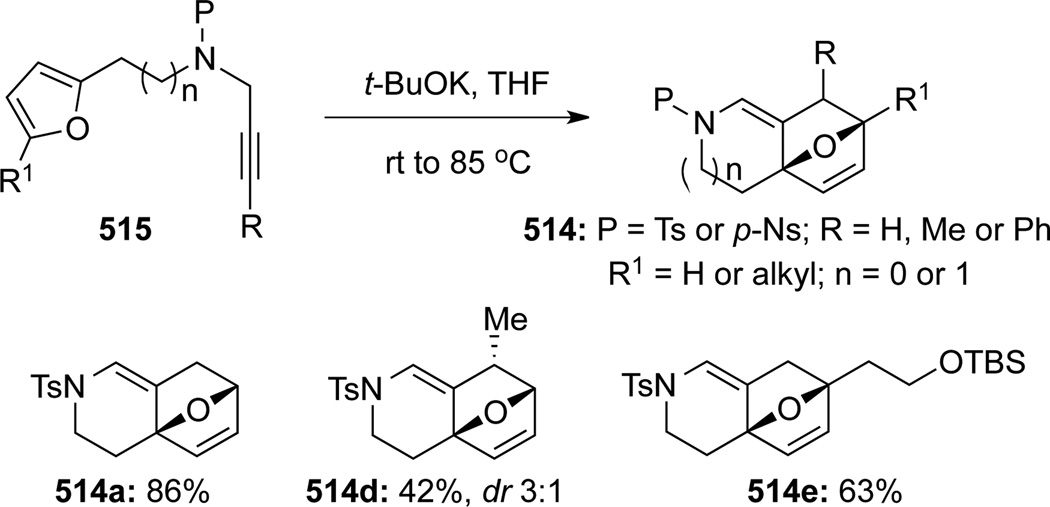
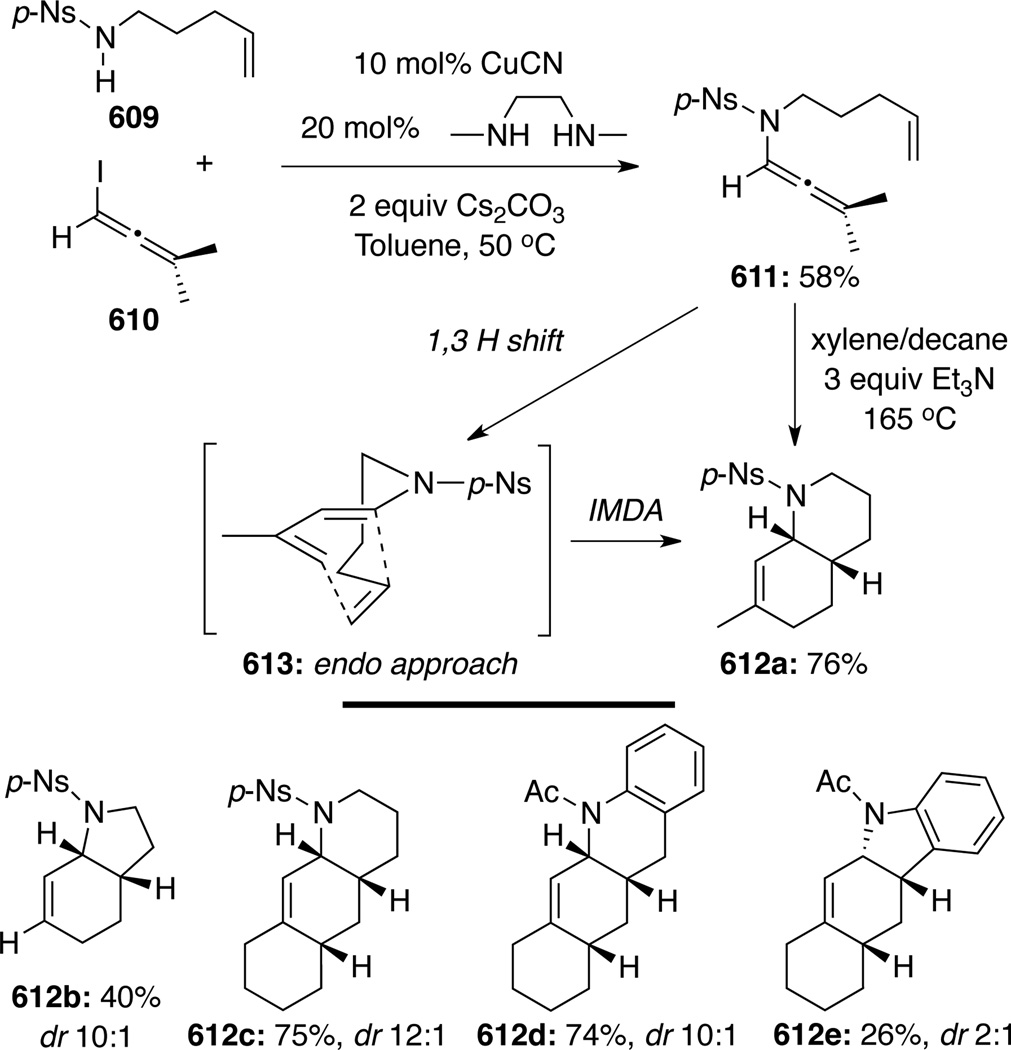
Hsung131 later showed that a stereoselective intramolecular normal electron demand [4 + 2] cycloaddition of allenamides 513 tethered to a diene motif could be achieved in good to excellent yields (Scheme 137). While Brønsted acids or transition metals including noble metals AuCl, AgBF4 and AgSbF6 could also catalyze this cycloaddition, and that this work predates elegant and high profiled efforts by Mascareñas and co-workers (See below), they were not necessary as thermal conditions was sufficient to give comparable results. Based on these efforts, the authors developed a tandem isomerization-[4 + 2] cycloaddition sequence using propargyl amides 515, leading to a rapid assembly of structural complexity as demonstrated in 514 (Scheme 138).
Scheme 137.
Scheme 138.
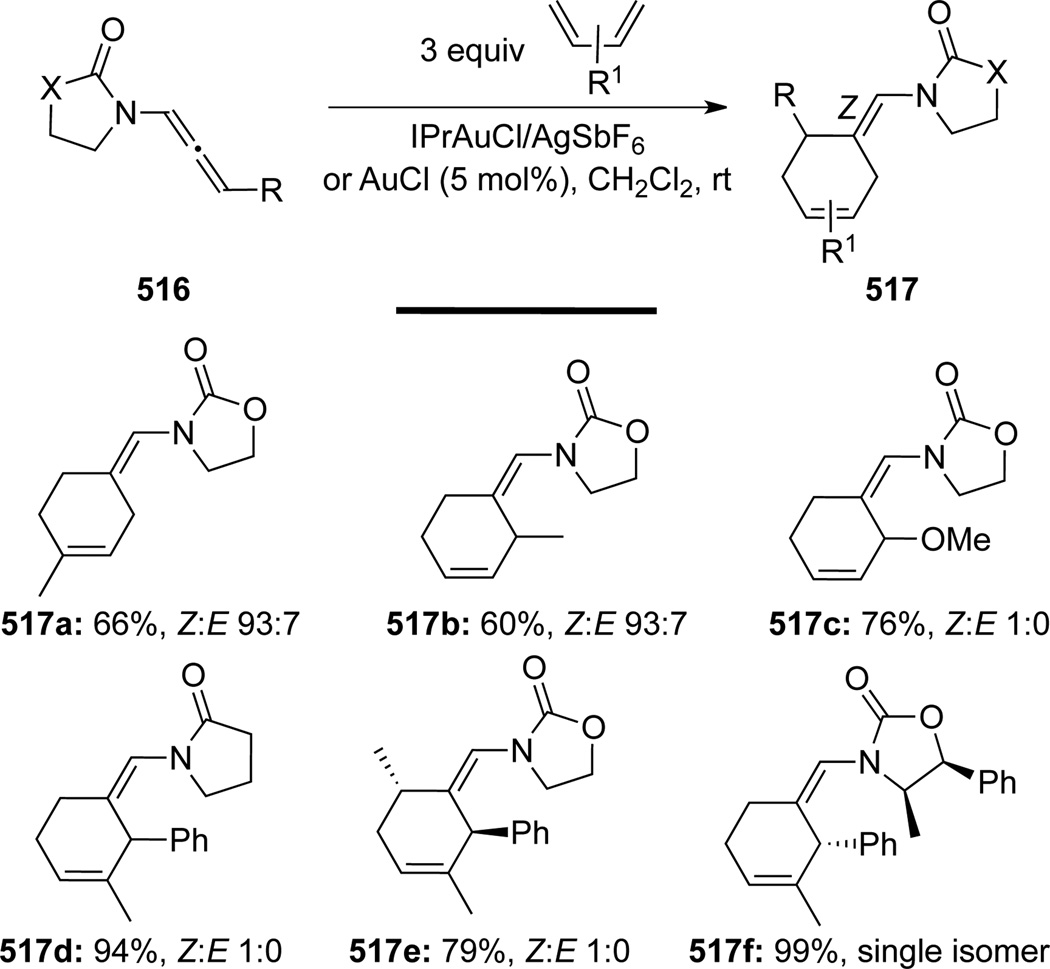
López and Mascareñas132 unveiled an intermolecular [4 + 2] reaction of allenamides 516 with a series of acyclic conjugated dienes to afford 517 in moderate to excellent yields and high Z selectivities (referring to the enamide double bonds) (Scheme 139). A preliminary reactivity screening revealed that similar reactions with either isolated allenes or allenol ethers instead of allenamides led to only complex mixtures, thereby further confirming the superiority of allenamides in these reactions. AuCl was proved to be a suitable catalyst for this cycloaddition reaction with cationic IPrAuCl/AgSbF6 providing better results in some cases.
Scheme 139.
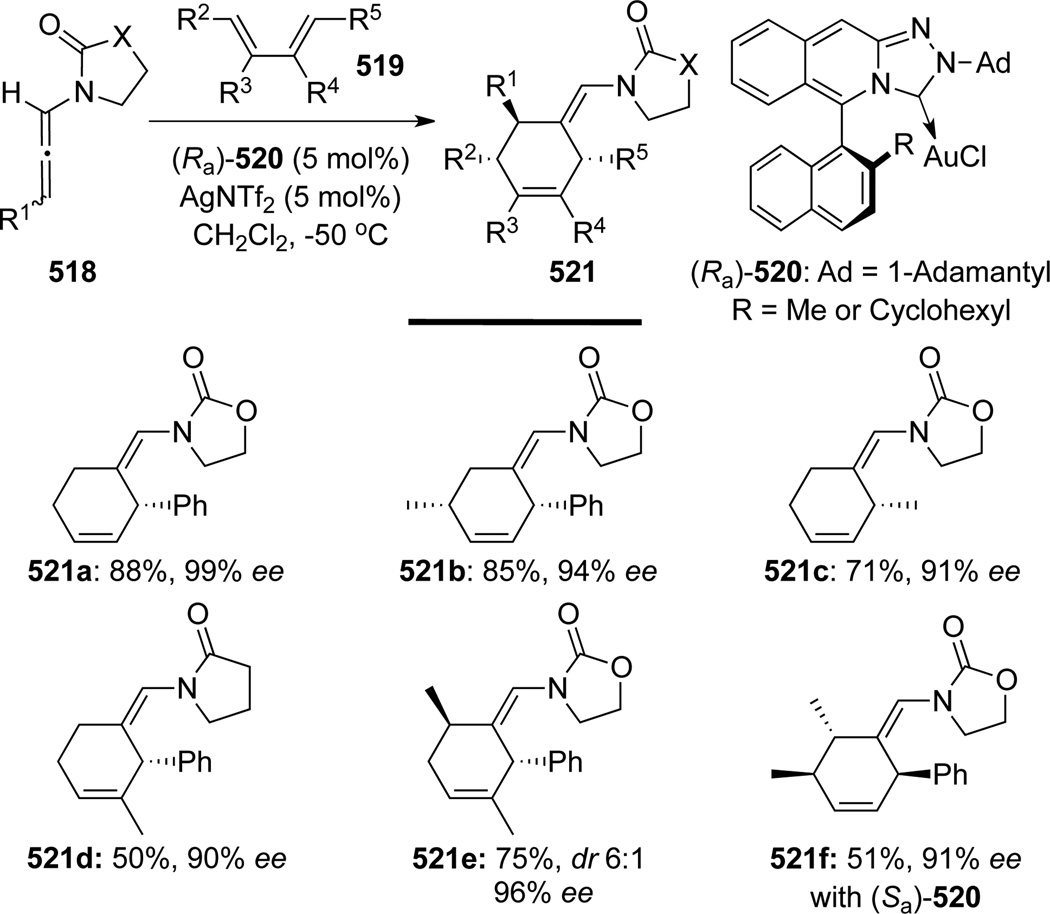
Mascareñas and co-workers133 then reported an impressive enantioselective version of this [4 + 2] reaction (Scheme 140). High enantioselectivities (usually >90% ee) were obtained using chiral Au(I) complex 520 containing axially chiral triazoloisoquinolin-3-ylidene ligands. This method represented the first example of a highly enantioselective intermolecular [4 + 2] cycloaddition of allenamides.
Scheme 140.
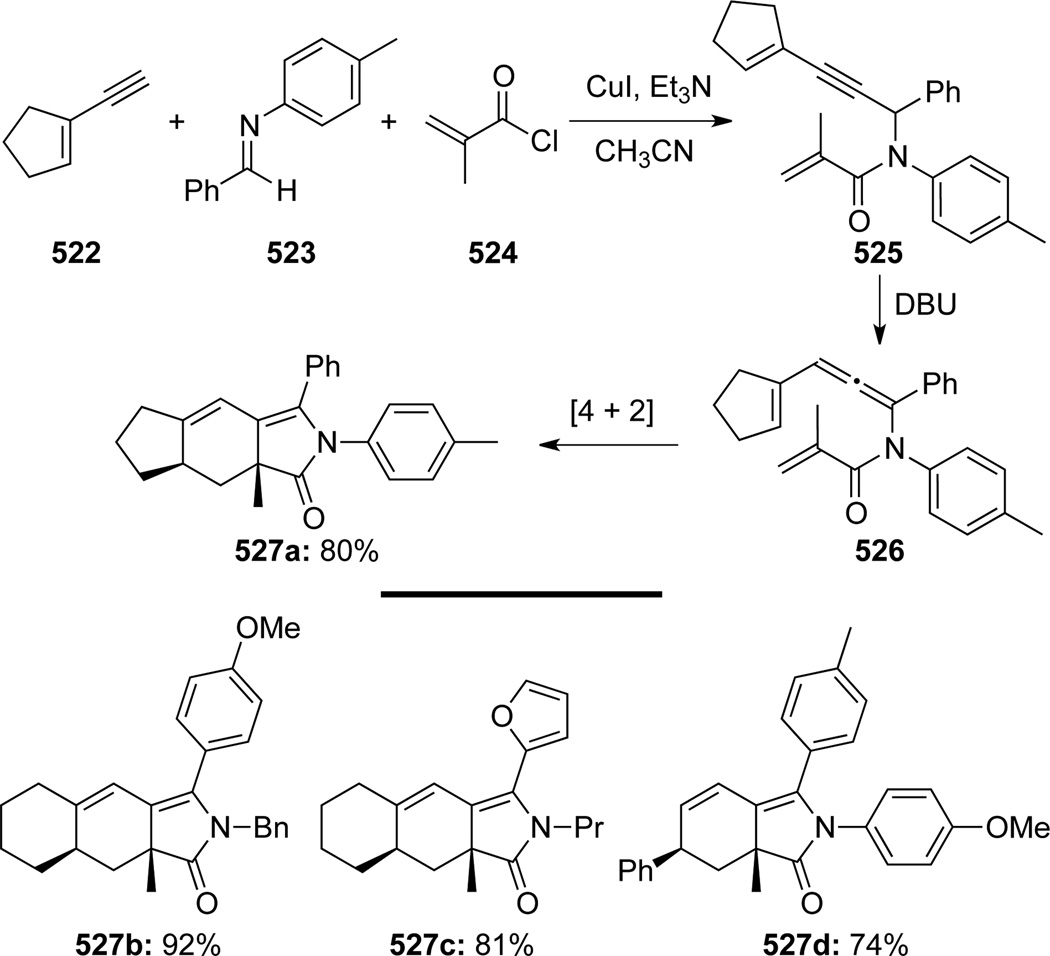
Huang134 reported a facile synthesis of highly substituted tetrahydro-1H-isoindolones from conjugated vinylic alkynes, imines and α, β-unsaturated enoic acid chlorides (Scheme 141). This reaction sequence proceeded through a CuI-catalyzed addition of alkyne 522 to imine 523 followed by amidation to give enyne 525. Enyne 525 could then undergo a cascade of DBU induced propargyl amide-allenamide isomerization and intramolecular [4 + 2] cycloaddition, leading to the desired product 527a in good yield. Although sufficiently stable, isolation of the pure 525 was not necessary and the sequence worked very well in one pot.
Scheme 141.
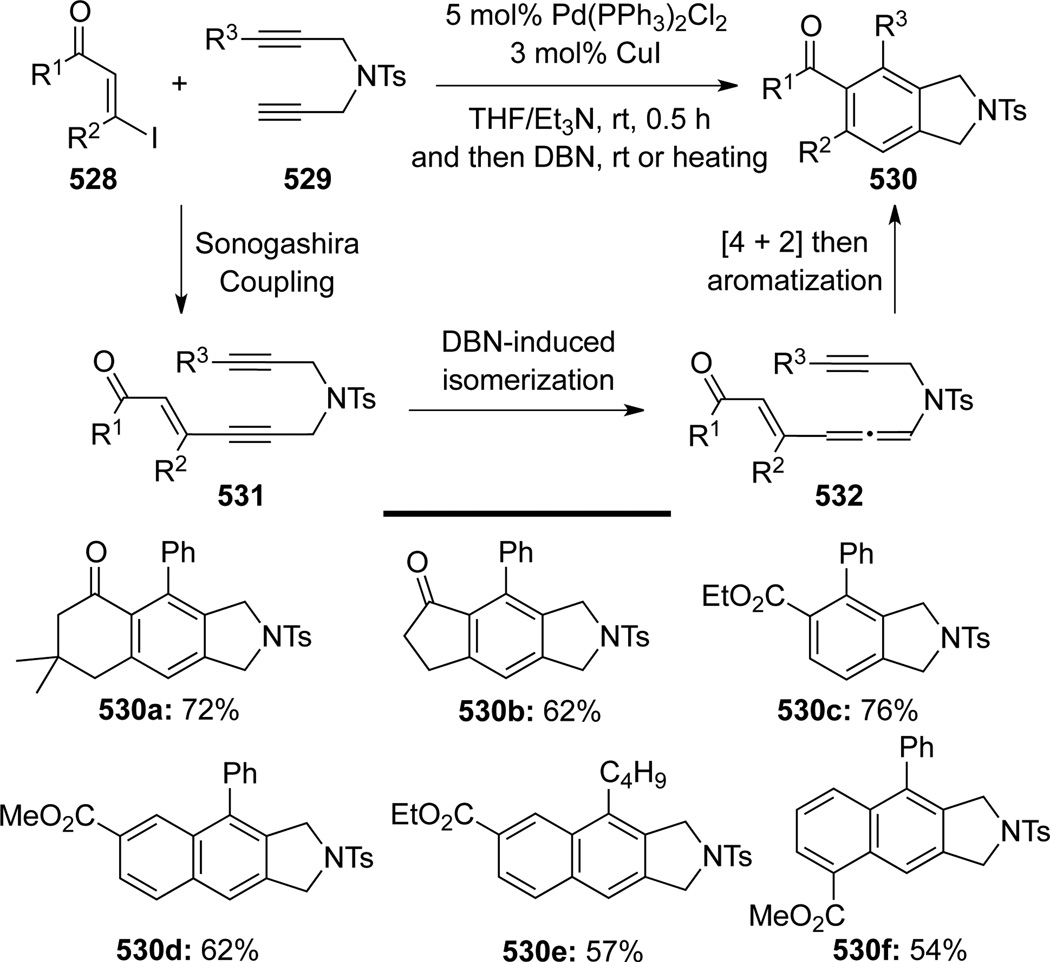
Wu and Huang135 disclosed a convenient one-pot synthesis of substituted isoindolines (Scheme 142). DBN-induced isomerization of bis-propargyl amides 531, which were generated via Pd-catalyzed Sonogashira-type cross-coupling of β-iodoenones 528 (or electronic deficient iodobenzenes, see 530df) with bis-propargyl amides 529, would afford allenamide intermediates 532 that could further undergo an intramolecular [4 + 2] cycloaddition/aromatization to deliver 530 in moderate to good yields.
Scheme 142.
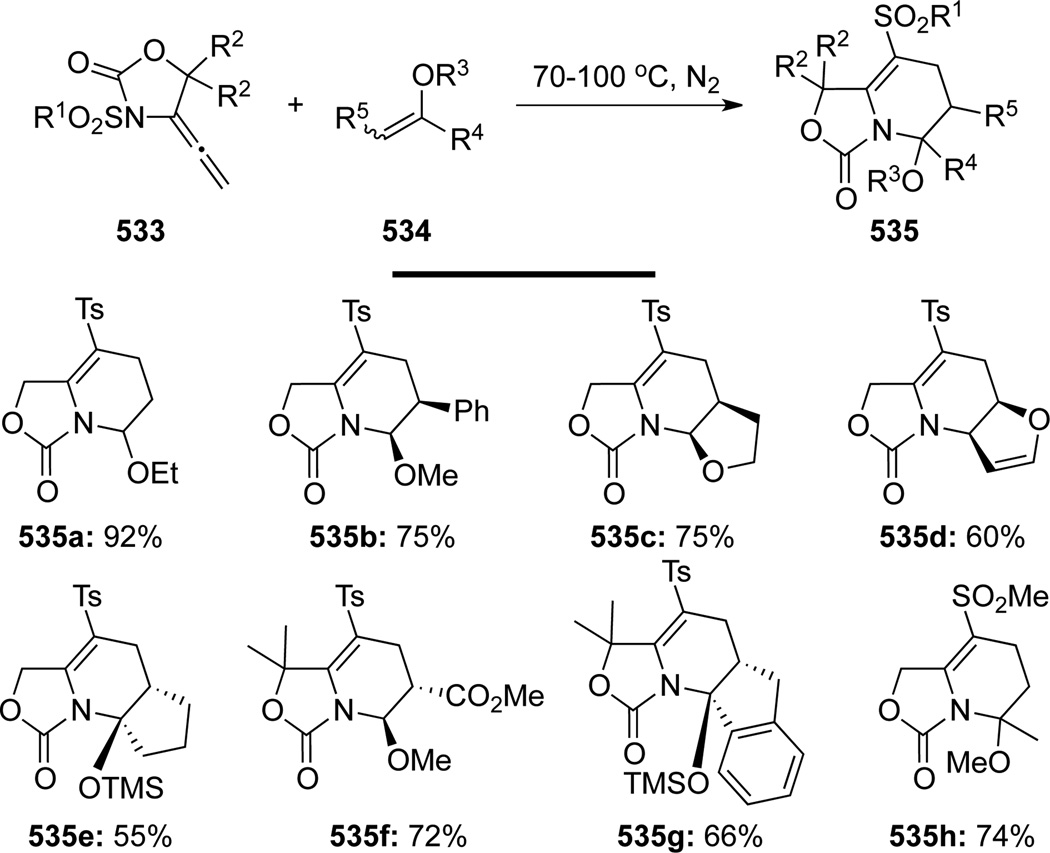
Tamaru136,115 uncovered an unusual thermal 1,3-Ts-shift–hetero-[4 + 2] cycloaddition cascade employing allenamides 533 (Scheme 143). In the presence of a large excess of enol ethers 534, the Ts group underwent N-to-C 1,3-migration concomitant with an ensuing hetero-[4 + 2] cycloaddition. The reaction was highly stereoselective with complete retention of configurations in the starting alkenes in which Z-enol ethers gave cis-products, while E-enol ethers afforded trans-products. However, the recovered enol ethers revealed that disubstituted acyclic Z-enol ethers had isomerized to give a Z/E mixture under the reaction conditions, while no isomerization took place without allenamides.
Scheme 143.
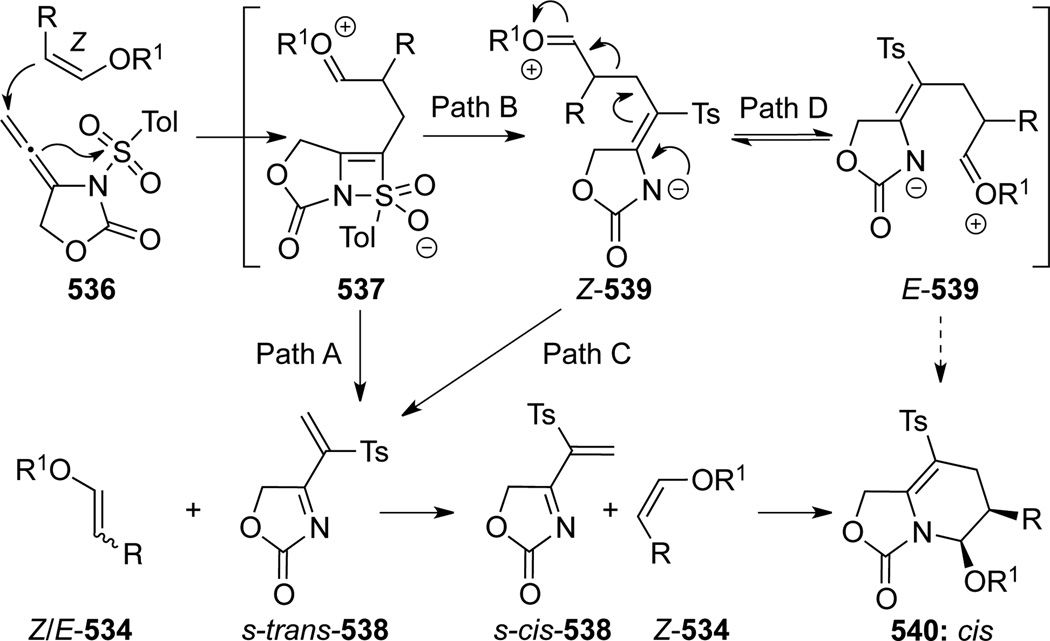
Consequently, authors suggested a unique enol ether-promoted 1,3-Ts-migration mechanism through the intermediacy of zwitter ion 537 to deliver 1-azadiene s-trans-538 with the loss of geometrical integrity of enol ether as shown in path A and/or pathway B-to-C (Scheme 144). The ultimate cycloaddition of reactive s-cis-538 with more reactive Z-enol ethers would afford the desired adducts 540 in a stereospecific manner. Although a direct cyclization pathway from E-539 after equilibrating from Z-539 (Path B-to-D) is a distinct possibility, the fact that cycloadduct 540 was isolated exclusively as cis ruled out this possibility, this is less likely because such cyclization would lose the original stereochemical information of the enol ether.
Scheme 144.
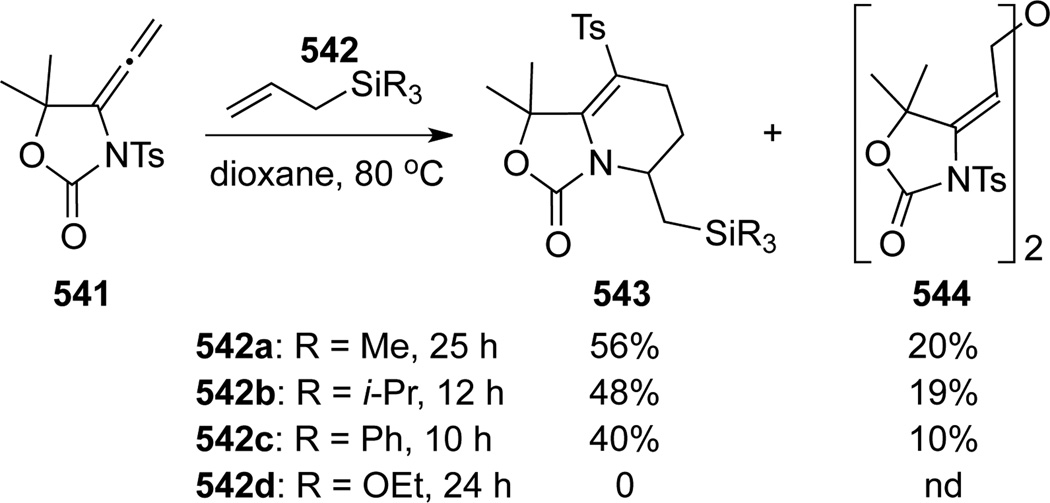
Tamaru137,115 further studied the reaction of allenamide 541 with allylsilanes 542 to achieve a better understanding of this unique 1,3-sulfonyl rearrangement (Scheme 145). Under thermal conditions, the desired [4 + 2] adducts 543a–c were obtained with allylsilanes 542a–c. A small amount of 544 was also isolated as a side product, but its mechanism was not clear. Authors again suggested that the nucleophilicity of silanes was not the key factor, and instead they proposed that the Lewis acidity of silicon might be critical for promoting the sulfonyl shift.
Scheme 145.
3.6.6. [3 + 3] Formal Cycloadditions
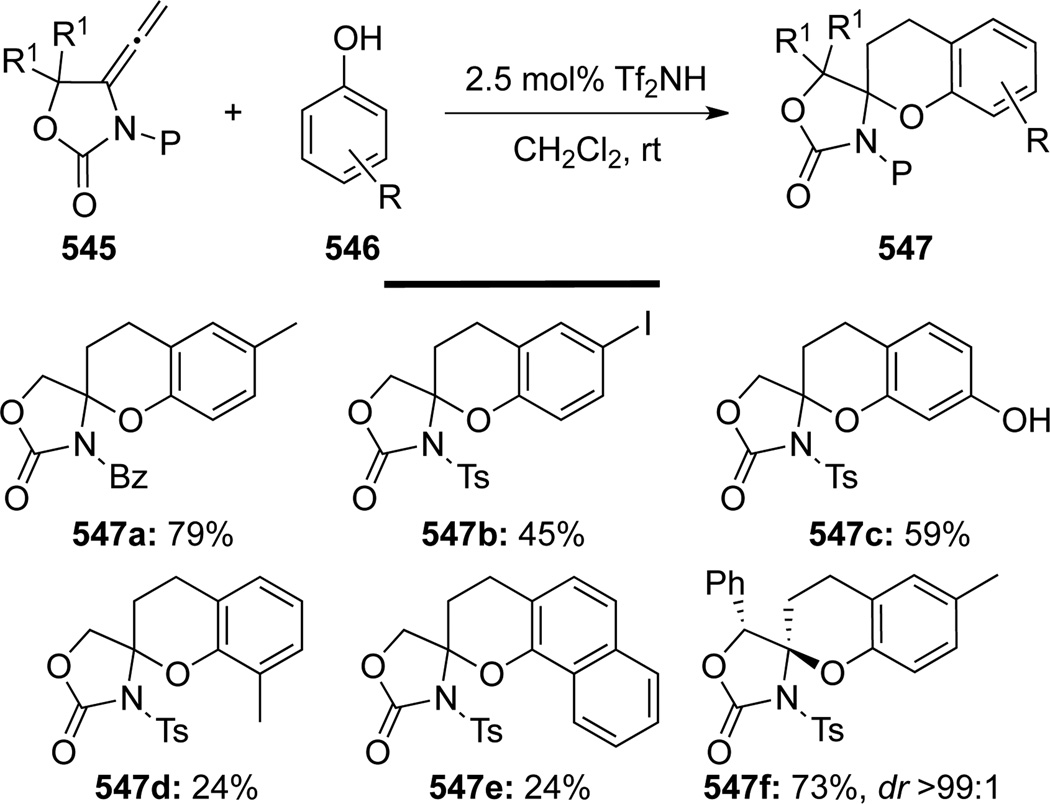
Kuroda138 unveiled the only example of [3 + 3] formal cycloadditions or annulations139 of allenamides 545 with phenols 546 (Scheme 146). These annulations could be carried out under mild conditions in the presence of a catalytic amount of Tf2NH, leading to a series of highly functionalized chromanes 547. This methodology also represents a formal hydroarylation at the terminal carbon of the allenic double bond without any metal catalyst.
Scheme 146.
3.6.7. [4 + 3] Cycloadditions
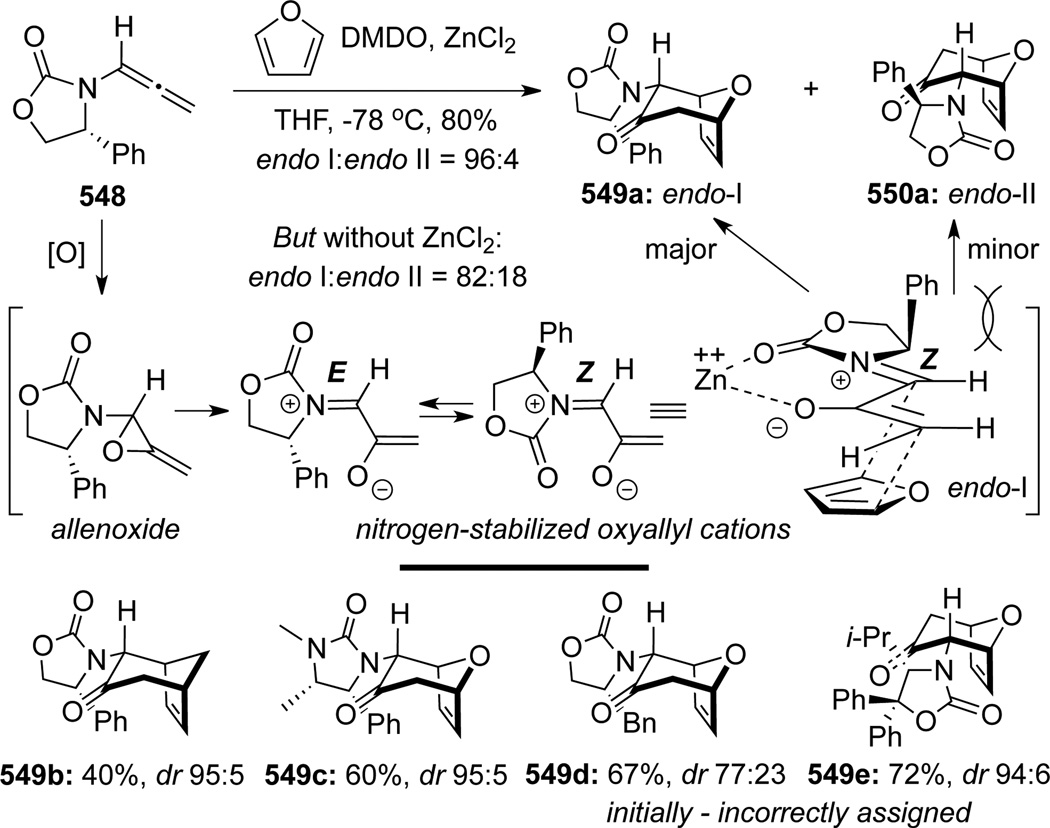
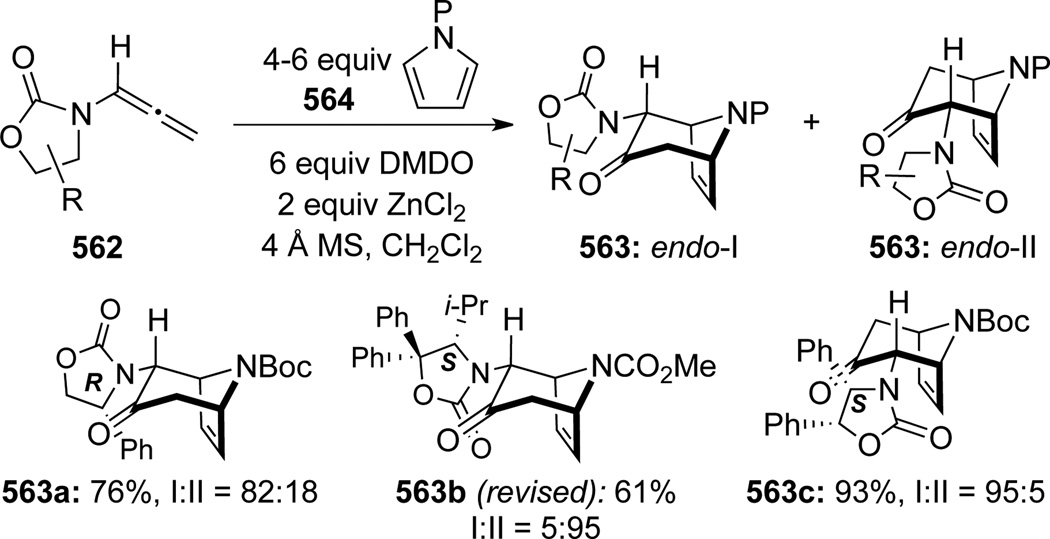
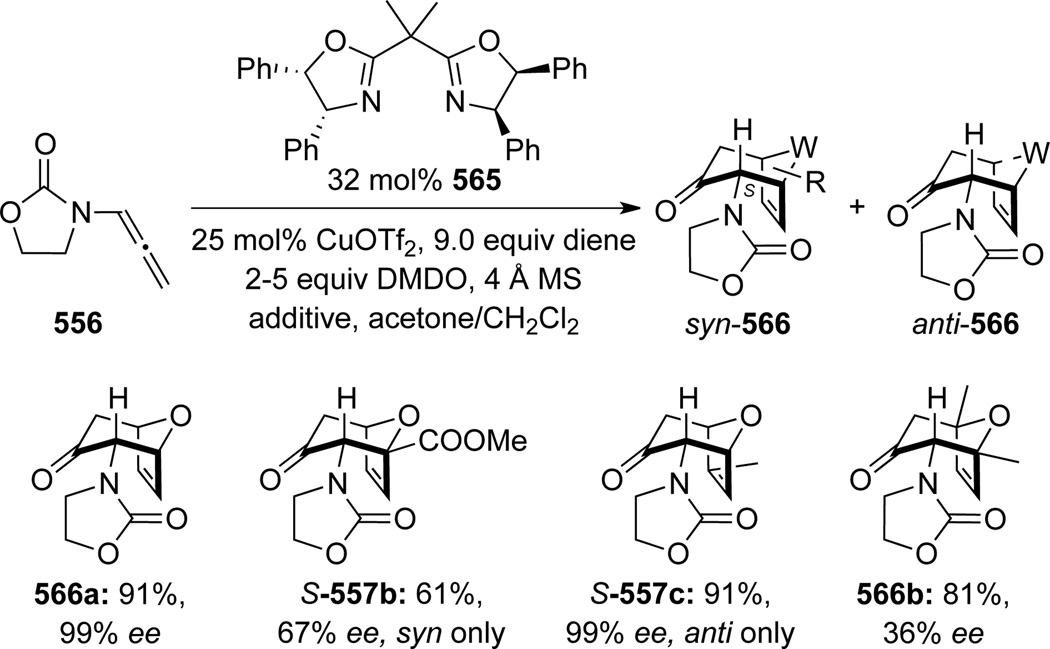
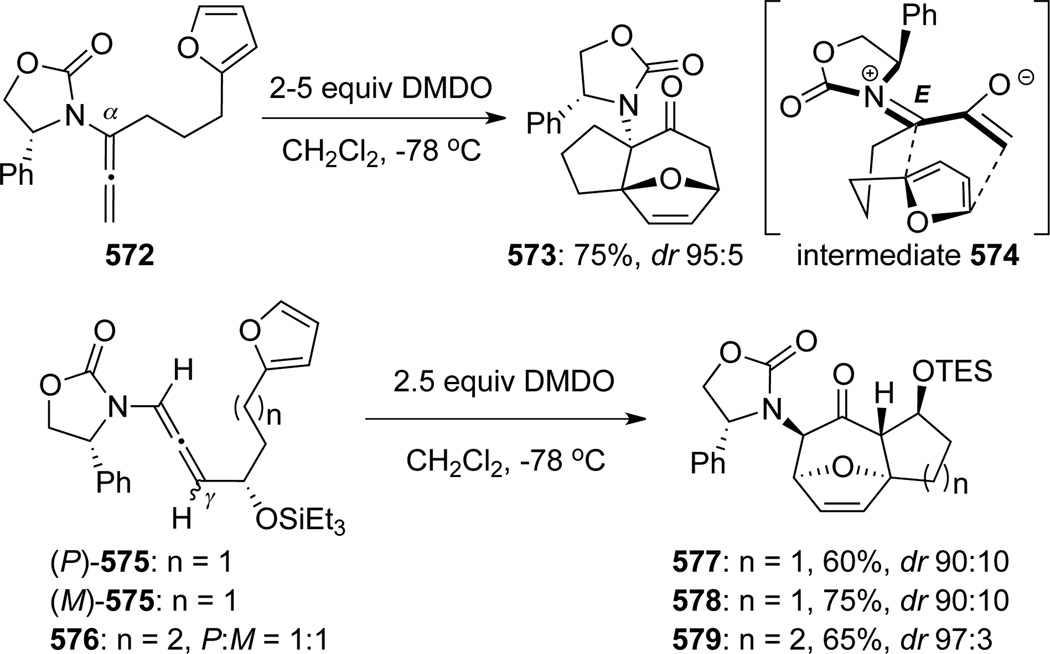
In 2001, Hsung140 uncovered a novel cascade consisting of epoxidation of chiral allenamides 548 with DMDO followed by [4 + 3] cycloadditions141 of various dienes, leading to oxabicyclo[3.2.1]octanes 549 (Scheme 147). The reaction was initially believed to proceed through the Z-isomer of nitrogen-stabilized oxyallyl cations, which were generated through ring-opening of the allenoxide intermediate (see Scheme 108). With the Z-oxyallyl cations being operative and sterics serving as the key and the only stereochemical controlling element, approaching of the diene from the less congested top face [called endo-I here for consistency] would lead to the major endo-I cycloadduct as assigned for 549a. Improvement of the overall stereoselectivity when using ZnCl2 further deepens the faith in such a claim because the zinc cation can lock up the oxyallyl cation in a bidentate manner to further enhance the facial differentiation.
Scheme 147.
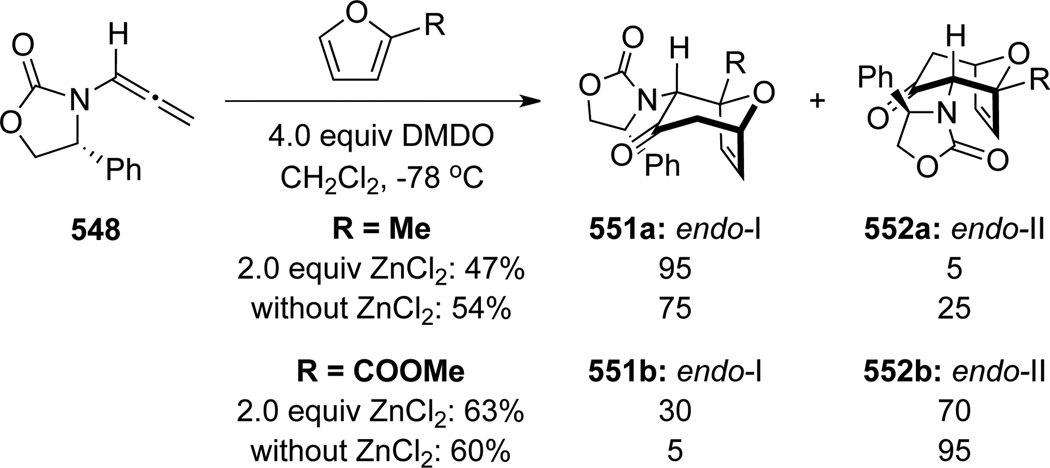
Hsung142 later found a reversal of diastereoselectivity in the [4 + 3] cycloaddition of allenamides with 2-substituted furan. While DMDO oxidation of allenamide 548 in the presence of 2-methyl furan and ZnCl2 afforded 551a as the major product, the corresponding cycloaddition reaction with methyl 2-furoate afforded the endo-II type adduct 552b more favorably (Scheme 148). These results collectively aroused suspicious of the original stereochemical understanding of this cycloaddition, and more critically, initiated a long-term collaborative effort to understand the regiochemical outcome of this cycloaddition regiochemically, which has been devoid of within the literature.
Scheme 148.
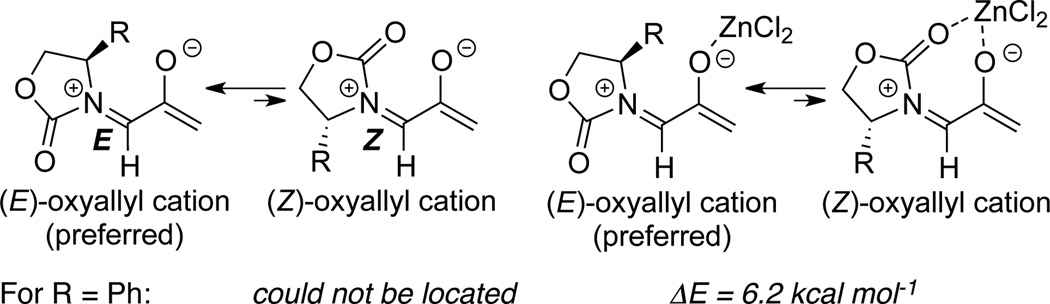
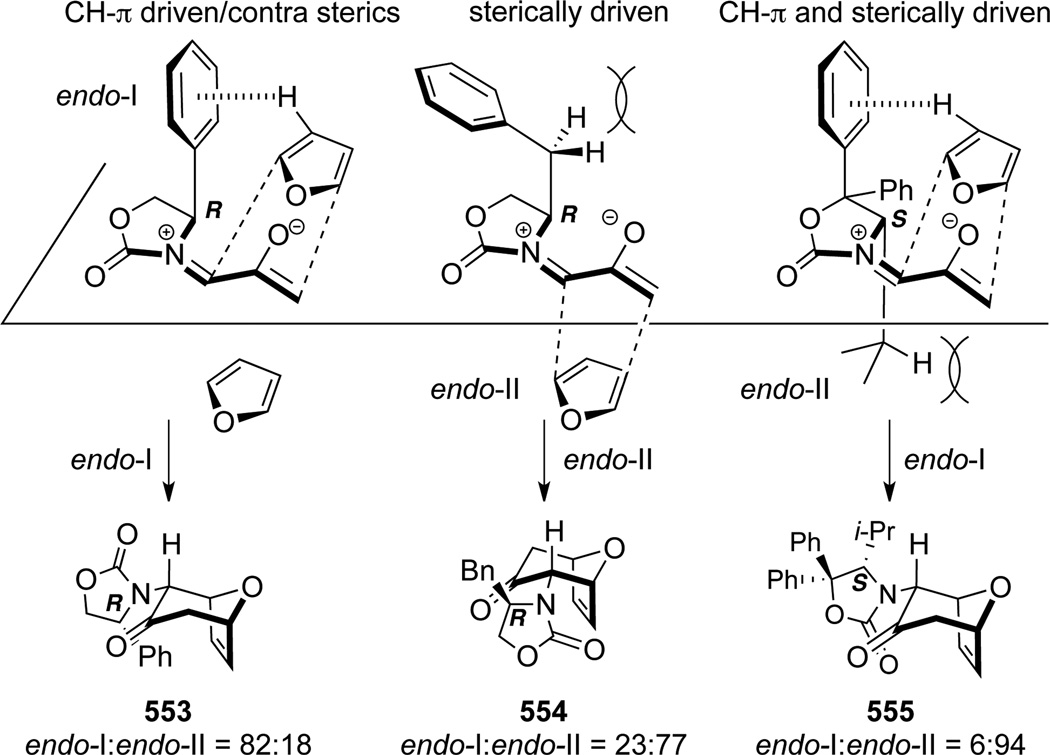
Consequently, Krenske, Houk, and Hsung143 conducted a detailed mechanistic study of this nitrogenstabilized oxyallyl cation [4 + 3] cycloadditions to provide a rationale. DFT calculations revealed that the E-oxyallyl cations were actually the preferred species regardless of the presence of ZnCl2, and there was no bidentate coordination (Scheme 149). Furthermore, when phenyl-substituted Evans auxiliary was employed as the chiral auxiliary as shown in allenamide 548, the approach of dienes preferred the sterically more hindered face of the oxyallyl cation! This contrasteric approach was attributed to a stabilizing CH-π interaction between the C3-H on furan and the phenyl substituent as in 553 (Scheme 150).
Scheme 149.
Scheme 150.
On the other hand, when no CH-π interaction could be achieved, as with the Bn-substituted Evans auxiliary, reaction did indeed proceed from the sterically less hindered face of the E-oxyallyl cation to provide the endo-II cycloadduct 554 as the major product. Steric preference and CH-π interaction could also operate in sync to give 555 (note the S-stereochemistry) in excellent diastereoselectivity. These new mechanistic insights provided corrections to the stereochemical assignment of 549d and 549e (see Scheme 147), which should in fact be as shown in 554 and 555, respectively.
Based on the new theoretical evidence, Krenske, Houk, and Hsung144 subsequently disclosed a systematic study of the [4 + 3] cycloaddition reaction of achiral allenamide 556 with unsymmetrically substituted furans and established the first cohesive model for the regiochemistry of [4 + 3] cycloadditions based on both theory and experiments (Scheme 151). For reactions with 2-substituted or 2,3-disubstituted furans, syn selectivity was favored, while anti was favored for cycloadditions with 3-substituted furans. DFT Calculations predicted the correct major products and brought forth the first cohesive mechanistic model based on the theoretic premise that (a) these nitrogen-stabilized oxyallyl cations are actually ambiphlic with enolate like-character in their HOMO; and (b) while asynchronously concerted, ω-bond formation occurs first (Scheme 152). Consequently, with electron-withdrawing ester groups, the regiochemical preference was consistent with electronic effects, mimicking a conjugate addition, while the observed regioselectivities for methylfurans likely a result of the preference for least hindered ω-bond formation.
Scheme 151.
Scheme 152.
Krenske, Houk, and Hsung145 further studied both regio- and stereoselectivity of [4 + 3] cycloadditions of 2 or 3-monosubstituted furans with chiral allenamides 548 (Schemes 153). For brevity and clarity, only a few examples of C-3-substituted cases are shown here, while details can be referred back to the original publication. Overall, the regiochemical model for achiral allenamides is consistent with these experimentally observed regioselectivities, and the new stereochemical model could be again adopted for the observed endo-I/II selectivities.
Scheme 153.
Krenske, Houk, and Hsung146 recently also disclosed their investigations of regio- and stereoselectivity issues of [4 + 3] cycloadditions of chiral allenamide 548 with a series unsymmetrically disubstituted furans (Scheme 154). The reaction was found to give syn-endo-II products 560 predominantly. DFT calculations were again performed to rationalize the experimental outcomes. Interestingly, in the calculated TS-561, the original proposed CH-π interaction was not found.
Scheme 154.
Song and Hsung147 demonstrated that these nitrogen-stabilized oxyallyl cations are also suitable for [4 + 3] cycloadditions with protected pyrroles 564 to construct tropanone systems (Scheme 155). This cycloaddition reaction showed similar stereoselectivities as those with furans.
Scheme 155.
Hsung148 unveiled an enantioselective [4 + 3] cycloaddition reaction149 using Lewis acid catalysts (Scheme 156). Reactions of allenamides 556 with DMDO and various dienes in the presence of catalytic CuOTf2 and C2-symmetric bisoxazoline 565 afforded cycloadducts 566 in good yields and up to 99% ee. This asymmetric reaction with unsymmetrical furans also provided regioselectivities consistent with what have been discussed above.
Scheme 156.
Hsung150 also reported a stereoselective intramolecular [4 + 3] cycloaddition reaction of allenamides 567 or 570 with N-tethered dienes through the intermediacy of the E-oxyallyl cation 569 (Schemes 157 and 158). A series of N-heterocyclic products 568 and 571, including tethers to form 7- and 8-membered rings (571g and 568d), was obtained in good yields and exo selectivity was actually observed. The tolerance of longer tethering length reaffirms the advantage of nitrogen-stabilized oxyallyl cations because of the conformationally rigid trivalent nature of the nitrogen atom and its aptitude to overcome greater entropy for intramolecular cycloadditions.
Scheme 157.
Scheme 158.
Hsung151 further extended this diastereoselective intramolecular [4 + 3] cycloaddition to disubstituted chiral allenamides with the diene motif being tethered at either α- or γ-allenic carbon (Scheme 159). High endo-selectivity was observed for these reactions, and interestingly, for γ-tethered allenamides 575 and 576, the axial chirality of allenamides did not have an impact on the overall stereoselectivity of the cycloaddition.
Scheme 159.
3.7. Tandem Cascades via Isomerizations
3.7.1. α-Isomerization
Hsung152a reported a series of studies on regio- and stereoselective isomerizations of allenamides with first of which leading to the preparation of de novo 2-amido-dienes and a tandem isomerization-6p-electron electrocyclic ring-closure (Scheme 160). It was found that under either thermal conditions (condition A) or Brønsted acidic conditions (condition B), allenamides 580 underwent effective isomerization, or 1,3-H shift, to give de novo 2-amido-dienes 581. This 1,3-H shift was found to be highly regio- and stereoselective, as products 581 were obtained with >20:1 E/Z ratios. It is noteworthy that related 1,3-H shift has been documented by others78,152b (also see Scheme 69). Recently, Das152c reported a computational study on such 1,3-H shift. Details of their study will not be discussed here.
Scheme 160.
The excellent E-selectivity attained provided a platform for a pericyclic transformation, as allenamide 582 underwent isomerization to give 3-amido-triene 583 in 89% yield, and subsequently, a thermal 6π-electron electrocyclic ring-closure of 583 gave cyclic diene 584. Alternatively, cyclic diene 584 could also be obtained directly from allenamide 582 under thermal condition in a tandem sequence, albeit with lower overall yield.
Hsung153 subsequently expanded the substrate scope of this 1,3-H shift for the preparation of 3-amidotrienes. Under acidic conditions, allenamides 585 can be efficiently transformed into 3-amido-trienes 586 in good to excellent yields (Scheme 161). Allenamides such as 587 with both α- and γ-substitutions were also examined. The 1,3-H shift in this case was found to be completely regioselective and took place exclusively from the α-position, affording highly substituted (E)-3-amido-trienes 588a–b.
Scheme 161.
Hsung154 also found that heating allenamide 589 led to two ring-closure products: the desired cyclic 2-amido diene 591 and the unexpected 1-amido diene 592 in 1:4.5 ratio (Scheme 162). The formation of 592 implied the presence of 1,3,5-hexatriene 593, resulting from an antarafacial 1,7-H shift from the initial triene 590. It is noteworth that the ring-closure of 1,3,5-hexatriene 593 is highly diastereoselective. Diene 592 could also be obtained from 589 via a two-step sequence, with the initial 1,3-H shift promoted by an acid.
Scheme 162.
An application154 of this 1,3-H-1,7-H shift in tandem with [4+2] cycloaddition was explored (Scheme 163). Reactions of allenamides 594, containing a Z-allyl group, led to the desired tricyclic products 596a and 596b as single isomers through a highly stereoselective [4 + 2] cycloaddition of cyclic 1-amido-diene intermediate 595 after the 1,3-H-1,7-H shift. In contrast, the 1,7-shift was suppressed in reactions of allenamide 597, containing an E-allyl group. In this case, tricyclic products 599a and 599b were obtained in excellent yield and diastereoselectivity. This reaction can be carried out thermally in a triple tandem manner commencing from 597. These tandem processes provide a rapid assembly of complex tricycles from simple allenamides, demonstrating their tremendous synthetic potential.
Scheme 163.
Hsung155 later described a diastereoselective 6π-electrocyclic ring-closure employing halogensubstituted 3-amidotrienes via a 1,6-remote asymmetric induction (Scheme 164). This process utilized electrophilic halogenations of α-substituted allenamides 600, and 2-halo-3-amido-di- and -trienes 601 were prepared in synthetically useful overall yields with E-stereoselectivity under optimized conditions. These reactions were thought to proceed through N-acyl iminium ions 602.
Scheme 164.
The successful de novo synthesis of these chiral 2-halo-3-amidotrienes enabled the diastereoselective 6 π-electron electrocyclizations via a challenging remote 1,6-asymmetric induction155 (Scheme 165). While simple thermal conditions failed, addition of AlMe3 effectively promoted the electrocyclization of 603, leading to the desired cyclic products 604 in good to excellent yields with overall good diastereoselectivity. A potential model is proposed as shown in TS-604 to rationalize the selectivity.
Scheme 165.
3.7.2. γ-Isomerization
Hsung152a also examined isomerizations or 1,3-H shift of allenamides from the γ-position for the preparation of 1-amido-dienes (Scheme 166). Authors found that 1,3-H shift of two types of γ-substituted allenamides with a cyclohexylidene group 605 and those with an iso-propylidene group 607 led to 1-amidodienes 606 or 608, respectively, as E-enamides exclusively. It was observed that acidic conditions appear to be more effective than thermal conditions. The thermal 1,3-H shift required higher temperatures and/or longer reaction times than those of α-isomerizations. It is noteworthy again that related isomerization or 1,3-H shift from the γ-position has been documented by others25,52,111,152b (see Schemes 11, 19, and 107).
Scheme 166.
Hsung156 utilized the 1,3-H shift from the γ-position of allenamide for Oppolzer-type intramolecular Diels-Alder cycloadditions (Scheme 167). Fully functionalized allenamide 611 could be prepared from the copper catalyzed coupling between amide 609 and 1-iodoallene 610. The authors found that allenamide 611 underwent effective base-mediated 1,3-H shift (see Trost’s similar isomerization52 in Scheme 39) and Diels-Alder cycloaddition to give cycloadduct 612a in excellent yield. The cycloaddition was believed to proceed via an endo-transition state as shown in 613, leading to rapid assembly of a diverse array of N-heterocyclic manifolds.
Scheme 167.
4. Natural Product Synthesis
Hsung157 documented a successful application of allenamide chemistry in the formal total synthesis of (+)-zincophorin 619 via an inverse electron-demand hetero-[4 + 2] cycloaddition of chiral allenamide 615 (Scheme 168). Heterodiene 614 and chiral allenamide 615 underwent highly stereoselective cycloaddition to afford pyran 616 as a single isomer. Pyran 616 underwent high-pressure hydrogenation of 616, which was not trivial, and the ensuing SnBr4-promoted crotylation with concomitant removal of the chiral urea group gave the key intermediate 617. It is noteworthy that in this entire sequence, the chiral auxiliary of allenamide 615 serves to provide stereochemical control for the cycloaddition and hydrogenation, and in return, the new stereocenters would dictate the selectivity of the final crotyalation and removal (and recovery) of the auxiliary. Pyran 617 was subsequently transformed into 618, which spectroscopically matched Miyashita’s advanced intermediate,158 thereby constituting a formal total synthesis of (+)-zincophorin.
Scheme 168.
Hsung159 applied the highly stereoselective tandem sequence consisting of an allenic 1,3-H shift, 6π-electron pericyclic ring-closure and an intramolecular Diels-Alder cycloaddition as an approach toward the BCD-ring of atropurpuran (Scheme 169). Fully functionalized allenamide 622 was prepared from allylic bromide 620 and lithiated allenamide 621. The desired 1,3-H shift was very fast and occurred with a catalytic amount of acid. The ring-closure of 623 and Diels-Alder cycloaddition took place in the presence of Lewis acid and high temperature to furnish the endo cycloadduct 626 as the desired product, although dr was very modest. A series of standard but stereoselective transformation using cycloadduct 627 led to 631, which was unambiguously assigned to match the carbon skeleton of the BCD-ring of atropurpuran (Scheme 170).
Scheme 169.
Scheme 170.
Hegedus160 reported the first exercise of utilizing allenamides in natural product synthesis through achieving a synthesis of 1-deoxy-D-galactohomonojirimycin using an optically active allenamide 633 (Scheme 171). The Lewis acid catalyzed addition of 633 to aldehyde 632 produced aminotetraol 634 in excellent yield with > 95% syn selectivity. After subsequent transformations including hydroboration/oxidation of silyl-alkyne, lactonization, auxiliary cleavage and deprotection of alcohol, amino-alcohol 635 was obtained in excellent overall yield. Mitsunobu cyclization of the alcohol 635 gave desired bicyclic amino lactone 636, which upon reduction and deprotection furnished the hydrochloride salt of 1-deoxy-D-galactohomonojirimycin 637.
Scheme 171.
Vázquez and Domίnguez85 described a novel approach to the tetracyclic unit of protoberberine using allenamide chemistry (Scheme 172). Allenamide 638 was subjected to acid catalyzed intramolecular electrophilic aromatic substitution via the N-acyl iminium ion 639. The desired cyclization product 640 was obtained in good yield, and a subsequent 6-exo Heck reaction of 640 furnished methylene protoberberine 641.
Scheme 172.
Lastly, Hsung147 illustrated an approach to parvineostemonine using a highly stereoselective [4 + 3] cycloaddition reaction of nitrogen-stabilized oxyallyl cation derived from DMDO-epoxidation of allenamide 642 with N-Boc-pyrrole (Scheme 173). The [4+3] cycloadduct 643 was obtained in excellent yield and essentially as a single diastereomer. Reduction of 643 followed by acid promoted removal of then Boc group and N-allylation gave the tropanone intermediate 644. Allylations of 644 would furnish the bis-allyl intermediate 645, which was subjected to ring-closing metathesis condition to give aza-tricycle 646 containing the ABC-core of parvineostemonine.
Scheme 173.
5. Most Recent Development
In addition to attempts to prepare allenamides from ynamide-derived15 propargyl ester via Ireland-Claisen rearrangement30 (see Scheme 15), Carbery161 recently found that ynamide-derived15 propargyl esters 32 could undergo an Au-catalyzed [3,3]-sigmatropic rearrangement to form allenamide intermediates 648, which could be subjected to 1H-indole 647 trapping/proto-deauration sequence to afford γ-indolyl α-acyloxyenamides 650 (Scheme 174).
Scheme 174.
Charette162 recently reported a Rh(II) catalyzed cyclopropanation between N-phthaloyl derived allenamide 651 and α-cyano diazo ester 652 to afford diacceptor alkylidene cyclopropyl amide 653 (Scheme 175). This cyclopropanation took place regioselectively at the less-hindered terminal double bond with high E/Z ratio and high ee when using Rh2(S-IBAZ)4 as the catalyst through carbenoid 654.
Scheme 175.
Conclusion
It is clear that the field of allenamide chemistry has received an increasingly growing attention in the past 15 years. This review has showcased these elegant developments from many dozens of research groups around the world. These innovative transformations have rendered allenamide a highly versatile building in organic synthesis and led to diverse array of carbo- and heterocyclic structures that can serve as platforms for further transformations. There is no doubt the level of interest in allenamides from the synthetic community is immensely high, and there is a tremendous amount of momentum to continue demonstrating the synthetic utility and applications of allenamides and taking their chemistry to the highest level of visibility. We hope this review can serve as another starting point for the allenamide chemistry.
Scheme 42.
Scheme 81.
Biographies

Ting Lu obtained her B.S. in Chemistry from Nankai University in China in 2002. She then went to National University of Singapore to obtain her M.S. degree in Materials Science in 2005. After that, she joined Professor Richard Hsung’s research group in University of Minnesota-Twin Cities and then to University of Wisconsin-Madison, where she studied on the methodology development in cyclopropanations of enamides and allenamides, and got her Ph.D. degree in Organic Chemistry in 2009. Now she is working on new catalytic systems for the production of bio-renewable chemicals from biomass in Institute of Bioengineering and Nanotechnology, Agency for Science, Technology and Research, Singapore.

Zhenjie Lu obtained her B.S degree in chemistry in 1996 from the East China University of Science and Technology in Shanghai, China. She started as a graduate student at Michigan State University under Professor Bill Wulff’s direction in 2002 as a graduate student and fished her Ph.D. degree in 2008. Her research involved studies on the optimization and synthetic application of catalytic asymmetric aziridination reactions.

Zhi-Xiong Ma received a B.S. degree in chemistry from University of Science and Technology of China (USTC) in 2005. He carried out doctoral research under the supervision of Professor Gang Zhao at Shanghai Institute of Organic Chemistry (SIOC), CAS and obtained his Ph.D. degree in late 2010. Currently he is conducting postdoctoral research with Professor Richard Hsung at University of Wisconsin-Madison. His research interests include visible-light photoredox catalysis and natural product synthesis.

Yu Zhang received his B.S degree in chemistry in 1996 from East China University of Science and Technology in Shanghai, China. In 2000 he joined Professor Bill Wulff’s group as a graduate student at Michigan State University. He obtained his Ph.D. degree in 2006 after working in the area of mechanism and methodology development of catalytic asymmetric aziridination. After spending one year at University of Maryland working for Professor Michael P. Doyle, from 2007 to 2009 he was a postdoctoral scholar at the University of Wisconsin at Madison, working with Professor Richard Hsung in the area of ynamide chemistry and natural product synthesis. He is currently a research scientist at Dow AgroSciences LLC.

Richard P. Hsung obtained his B.S. in Chemistry and Mathematics from Calvin College in Grand Rapids, MI. He then attended The University of Chicago and received his M.S. and Ph.D. degrees in Organic Chemistry, respectively, under the supervision of Professors Jeff Winkler and Bill Wulff. After pursuing a postdoctoral stay with Professor Larry Sita in Chicago and NIH-postdoctoral work with Professor Gilbert Stork at Columbia University, he moved to University of Minnesota-Twin Cities as an Assistant Professor in 1997 and was promoted to Associate Professor in 2002. He was promoted to Professor and moved to University of Wisconsin-Madison in 2006. He was a recipient of the Camille Dreyfus Teacher-Scholar Award and the National Science Foundation Career Award. He has coauthored over 200 publications, delivered over 200 invited lectures, and supervised over 150 students and postdoctoral fellows with research interests in developing cycloaddition and annulation approaches to natural product syntheses and stereoselective methods using allenamides, ynamides, enamides, and cyclic acetals.
References
- 1.For a compendium on chemistry of allenes, see: Krause N, Hashmi ASK. Modern Allene Chemistry. Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA; 2004. Vol. 1 and Vol. 2.
- 2.For earlier reviews see: Sainsbury M. Rodd's Chemistry of Carbon Compounds. Oxford: Elsevier; 1991. pp. 115–155. Schuster HE, Coppola GM. Allenes in Organic Synthesis. New York: John Wiley and Sons; 1984.
- 3.For other general reviews on allenes, see: Brummond KM, Kent JL. Tetrahedron. 2000;56:3263. Ma S. Chem. Rev. 2005;105:2829. doi: 10.1021/cr020024j.
- 4.Saalfrank RW, Lurz CJ. In: Methoden Der Organischen Chemie (Houben-Weyl) Kropf H, Schaumann E, editors. Stuttgart: Georg Thieme Verlag; 1993. pp. 3093–3102. [Google Scholar]
- 5.For leading reviews on chemistry of allenol ethers: Brasholz M, Reissig H-U, Zimmer R. Acc. Chem. Res. 2009;42:45. doi: 10.1021/ar800011h. Zimmer R. Synthesis. 1993:165.
- 6.For a leading review on chemistry of allenyl sulfides: Hayashi Y, Narasaka K. In: Advances in Cycloaddition. Lautens M, editor. London: JAI Press Ltd.; 1997. pp. 41–86.
- 7.Hubert AJ, Viehe HG. J. Chem. Soc. C. 1968:228. [Google Scholar]
- 8.(a) For the earliest documentations, see: Bode J. Ann. 1892;267:268–286. (b) For a correction of this claim see: Klages F, Drerup E. Ann. 1941;547:65.
- 9.For practical synthesis of ynamines, see: Zaugg HE, Swett LR, Stone GR. J. Org. Chem. 1958;23:1389. Wolf V, Kowitz F. Ann. 1960;638:33. Viehe HG. Angew Chem. Int. Ed. Engl. 1963;2:477.
- 10.For reviews on chemistry of ynamines, see: Hsung RP, Zificsak CA, Mulder JA, Rameshkumar C, Wei L-L. Tetrahedron. 2001;57:7575–7606. Himbert G. In: Methoden Der Organischen Chemie (Houben-Weyl) Kropf H, Schaumann E, editors. Stuttgart: Georg Thieme Verlag; 1993. pp. 3267–3443. Collard-Motte J, Janousek Z. Topics in Current Chem. 1986;130:89. Pitacco G, Valentin E. Chemistry of Functional Groups. Chapter 15. 1979:623–714. Brandsma L, Verkruijse HD. Synthesis of Acetylenes, Allenes and Cumulenes. Amsterdam: Elsevier; 1981. Brandsma L, Verkruijsse HD. Stud. Org. Chem.: Amsterdam. 1981;8 Ficini J. Tetrahedron. 1976;32:448. Viehe HG. Chemistry of Acetylenes. Chapter 12. New York: Marcel Dekker; 1969. pp. 861–912. Viehe HG. Angew Chem. Int. Ed. Engl. 1967;6:767.
- 11.Dickinson WB, Lang PC. Tetrahedron Lett. 1967;8:3035. [Google Scholar]
- 12.Cho AK, Haslett WL, Jenden D. Biochem. Biophys. Res. Commun. 1961;5:276. doi: 10.1016/0006-291x(61)90162-0. [DOI] [PubMed] [Google Scholar]
- 13.(a) Bebbington A, Shakeshaft D. J. Med. Chem. 1965;8:274. doi: 10.1021/jm00326a037. [DOI] [PubMed] [Google Scholar]; (b) Archibald JL. J. Med Chem. 1965;8:390. doi: 10.1021/jm00327a026. [DOI] [PubMed] [Google Scholar]
- 14.(a) Janousek Z, Collard J, Viehe HG. Angew. Chem. Int. Ed. Engl. 1972;11:917. [Google Scholar]; (b) Goffin E, Legrand Y, Viehe HG. J. Chem. Res. (S) 1977:105. [Google Scholar]
- 15.For leading reviews on chemistry of ynamides, see: DeKorver K, Li H, Lohse A, Hayashi R, Lu Z, Zhang Y, Hsung R. Chem. Rev. 2010;110:5064. doi: 10.1021/cr100003s. Evano G, Coste A, Jouvin K. Angew. Chem. Int. Ed. 2010;49:2840. doi: 10.1002/anie.200905817.
- 16.Also see: (a) Reference 10a. Katritzky AR, Jiang R, Singh SK. Heterocycles. 2004;63:1455. Mulder JA, Kurtz KCM, Hsung RP. Synlett. 2003:1379. Ackermann L, Potukuchi HK. Org. Biomol. Chem. 2010;8:4503. doi: 10.1039/c0ob00212g.
- 17.For reviews on chemistry of enamides, see: Carbery DR. Org. Biomol. Chem. 2008;9:3455. doi: 10.1039/b809319a. Rappoport Z. The Chemistry of Enamines in The Chemistry of Functional Groups. New York: John Wiley and Sons; 1994. Also see: Overman LE. Acc. Chem. Res. 1980;13:218. Petrzilka M. Synthesis. 1981:753. Campbell AL, Lenz GR. Synthesis. 1987:421. Krohn K. Angew. Chem. Int. Ed. Engl. 1993;32:1582. Enders D, Meyer O. Liebigs Ann. 1996:1023.
- 18.Wei L-L, Xiong H, Hsung RP. Acc. Chem. Res. 2003;36:773. doi: 10.1021/ar030029i. [DOI] [PubMed] [Google Scholar]
- 19.Tracey MR, Hsung RP, Antoline JA, Kurtz KCM, Shen L, Slafer BW, Zhang Y. In: Science of Synthesis, Houben-Weyl Methods of Molecular Transformations. Weinreb Steve M., editor. Stuttgart, Germany: Georg Thieme Verlag KG; 2005. Chapter 21.4. [Google Scholar]
- 20.Also see: Standen PE, Kimber MC. Curr. Opin. Drug Discov. & Devel. 2010;13:645. Deagostino A, Prandi C, Tabasso S, Venturello P. Molecules. 2010;15:2667. doi: 10.3390/molecules15042667.
- 21.Bogentoft C, Ericsson Ö, Stenberg P, Danielsson B. Tetrahedron Lett. 1969;10:4745. [Google Scholar]
- 22.Corbel B, Paugam J-P, Dreux M, Savignac P. Tetrahedron Lett. 1976;17:835. [Google Scholar]
- 23.Balasubramanian KK, Venugopalan B. Tetrahedron Lett. 1974;15:2643. [Google Scholar]
- 24.Balasubramanian KK, Venugopalan B. Tetrahedron Lett. 1974;15:2645. [Google Scholar]
- 25.(a) Overman LE, Charles KM, Clizbe LA. Tetrahedron Lett. 1979;20:599. [Google Scholar]; (b) Overman LE, Clizbe LA, Freerks RL, Marlowe CK. J. Am. Chem. Soc. 1981;103:2807. [Google Scholar]
- 26.(a) Padwa A, Cohen LA. J. Org. Chem. 1984;49:399. [Google Scholar]; (b) Romero NA, Klepser BM, Anderson CE. Org. Lett. 2012;14:874. doi: 10.1021/ol203398e. [DOI] [PubMed] [Google Scholar]
- 27.Danowitz AM, Taylor CE, Shrikian TM, Mapp AK. Org. Lett. 2010;12:2574. doi: 10.1021/ol1007845. [DOI] [PubMed] [Google Scholar]
- 28.(a) Yin G, Zhu Y, Zhang L, Lu P, Wang Y. Org. Lett. 2011;13:940. doi: 10.1021/ol102992n. [DOI] [PubMed] [Google Scholar]; (b) Zhu Y, Yin G, Sun L, Lu P, Wang Y. Tetrahedron. 2012;68:10194. [Google Scholar]; (c) Zhu Y, Yin G, Hong D, Lu P, Wang Y. Org. Lett. 2011;13:1024. doi: 10.1021/ol103074d. [DOI] [PubMed] [Google Scholar]
- 29.Related [3,3] sigmatropic rearrangements, see: Swaminathan S, Narayan KV. Chem. Rev. 1971;71:429. Ireland RE, Mueller RH. J. Am. Chem. Soc. 1972;94:5897. doi: 10.1021/ja00765a079.
- 30.Heffernan SJ, Carbery DR. Tetrahedron Lett. 2012;53:5180. [Google Scholar]
- 31.Tamura Y, Ikeda H, Mukai C, Morita I, Ikeda M. J. Org. Chem. 1981;46:1732. [Google Scholar]
- 32.Bacci JP, Greenman KL, Van Vranken DL. J. Org. Chem. 2003;68:4955. doi: 10.1021/jo0340410. [DOI] [PubMed] [Google Scholar]
- 33.Armstrong A, Cooke RS, Shanahan SE. Org. Biomol. Chem. 2003;1:3142. doi: 10.1039/b307722e. [DOI] [PubMed] [Google Scholar]
- 34.Armstrong A, Emmerson DPG. Org. Lett. 2009;11:1547. doi: 10.1021/ol900146s. [DOI] [PubMed] [Google Scholar]
- 35.(a) Padwa A, Caruso T, Nahm S, Rodriguez A. J. Am. Chem. Soc. 1982;104:2865. [Google Scholar]; (b) Galons H, Bergerat I, Combet-Farnoux C, Miocque M, Decodts G, Bram G. J. Chem. Soc., Chem. Comm. 1985:1730. [Google Scholar]
- 36.Radl S, Kovarova L, Holubek J. Collect. Czech. Chem. Commun. 1991;56:439. [Google Scholar]
- 37.(a) Phadtare S, Zemlicka J. J. Am. Chem. Soc. 1989;111:5925. [Google Scholar]; (b) Phadtare S, Zemlicka J. J. Org. Chem. 1989;54:3675. [Google Scholar]; (c) Jones BCNM, Silverton JV, Simons C, Megati S, Nishimura H, Maeda Y, Mitsuya H, Zemlicka J. J. Med. Chem. 1995;38:1397. doi: 10.1021/jm00008a018. [DOI] [PubMed] [Google Scholar]
- 38.Zemlicka J. Biochim. Biophys. Acta. 2002;1587:276. doi: 10.1016/s0925-4439(02)00090-x. [DOI] [PubMed] [Google Scholar]
- 39.(a) Katritzky AR, Ramer WH. J. Org. Chem. 1985;50:852. [Google Scholar]; (b) Galy GP, Elguero J, Vincent EJ, Galy AM, Barbe J. Synthesis. 1979:944. [Google Scholar]; (c) Mahamoud A, Galy JP, Vincent EJ, Barbe J. Synthesis. 1981:917. [Google Scholar]; (d) Reschi J, Salehi-Artimani RA. J. Heterocyclic Chem. 1989;26:1083. [Google Scholar]
- 40.Hsung RP, Zificsak CA, Wei L-L, Douglas CJ, Xiong H, Mulder JA. Org. Lett. 1999;1:1237. [Google Scholar]
- 41.(a) Wei L-L, Mulder JA, Xiong H, Zificsak CA, Douglas CJ, Hsung RP. Tetrahedron. 2001;57:459. [Google Scholar]; (b) Wei L-L, Xiong H, Douglas CJ, Hsung RP. Tetrahedron Lett. 1999;40:6903. [Google Scholar]; (c) Xiong H, Tracey MR, Grebe TP, Mulder JA, Hsung RP, Wipf P, Smotryski J. Organic Syn. 2004;81:147. [Google Scholar]
- 42.Xuárez L, Pellón RF, Fascio M, Montesano V, D'Accorso N. Heterocycles. 2004;63:23. [Google Scholar]
- 43.Fenández I, Monterde MI, Plumet J. Tetrahedron Lett. 2005;46:6029. [Google Scholar]
- 44.Garud DR, Ando H, Kawai Y, Ishihara H, Koketsu M. Org. Lett. 2007;9:4455. doi: 10.1021/ol701761t. [DOI] [PubMed] [Google Scholar]
- 45.Van Boxtel LJ, Körbe S, Noltemeyer M, De Meijere A. Eur. J. Org. Chem. 2001:2283. [Google Scholar]
- 46.Huang J, Xiong H, Hsung RP, Rameshkumar C, Mulder JA, Grebe TP. Org. Lett. 2002;4:2417. doi: 10.1021/ol020097p. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Z, Zhang Q, Ni Z, Liu Q. Chem. Comm. 2010;46:1269. doi: 10.1039/b917856b. [DOI] [PubMed] [Google Scholar]
- 48.(a) Tanaka H, Kameyama Y, Sumida S, Yamada T, Tokumaru Y, Shiroi T, Sasaoka M, Taniguchi M, Torii S. Synlett. 1991:888. [Google Scholar]; (b) Farina V, Kant J. Tetrahedron Lett. 1992;33:3559. [Google Scholar]
- 49.(a) Majumdar KC, Ghosh SK. Synthetic Commun. 1994;24:217. [Google Scholar]; (b) Clay MD, Fallis AG. Angew. Chem. Int. Ed. 2005;44:4039. doi: 10.1002/anie.200500484. [DOI] [PubMed] [Google Scholar]
- 50.Kimura M, Wakamiya Y, Horino Y, Tamaru Y. Tetrahedron Lett. 1997;38:3963. [Google Scholar]
- 51.(a) Kozawa Y, Mori M. Tetrahedron Lett. 2001;42:4869. [Google Scholar]; (b) Kozawa Y, Mori M. Tetrahedron Lett. 2002;43:1499. [Google Scholar]
- 52.Trost BM, Stiles DT. Org. Lett. 2005;7:2117. doi: 10.1021/ol050395x. [DOI] [PubMed] [Google Scholar]
- 53.Shen L, Hsung RP, Zhang Y, Antoline JE, Zhang X. Org. Lett. 2005;7:3081. doi: 10.1021/ol051094q. [DOI] [PubMed] [Google Scholar]
- 54.Tang Y, Shen L, Dellaria BJ, Hsung RP. Tetrahedron Lett. 2008;49:6404. doi: 10.1016/j.tetlet.2008.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Persson AKÅ, Johnston EV, Bäckvall J-E. Org. Lett. 2009;11:3814. doi: 10.1021/ol901294j. [DOI] [PubMed] [Google Scholar]
- 56.Cao J, Kong Y, Deng Y, Lai G, Cui Y, Hu Z, Wang G. Org. Biomol. Chem. 2012;10:9556. doi: 10.1039/c2ob26727f. [DOI] [PubMed] [Google Scholar]
- 57.Miyaura N, Suzuki A. J. Chem. Soc. Chem. Comm. 1979:866. [Google Scholar]
- 58.Xiong H, Hsung RP, Wei L-L, Berry CR, Mulder JA, Stockwell B. Org. Lett. 2000;2:2869. doi: 10.1021/ol000181+. [DOI] [PubMed] [Google Scholar]
- 59.Horino Y, Takata Y, Hashimoto K, Kuroda S, Kimurab M, Tamaru Y. Org. Biomol. Chem. 2008;6:4105. doi: 10.1039/b808921c. [DOI] [PubMed] [Google Scholar]
- 60.(a) Skucas E, Zbieg JR, Krische MJ. J. Am. Chem. Soc. 2009;131:5054. doi: 10.1021/ja900827p. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Zbieg JR, McInturff EL, Krische MJ. Org. Lett. 2010;12:2514. doi: 10.1021/ol1007235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Radl S, Kovarova L. Collect. Czech. Chem. Commun. 1991;56:2413. [Google Scholar]
- 62.Manzo AM, Perboni AD, Broggini G, Rigamonti M. Tetrahedron Lett. 2009;50:4696. [Google Scholar]
- 63.Broggini G, Galli S, Rigamonti M, Sottocornola S, Zecchi G. Tetrahedron Lett. 2009;50:1447. [Google Scholar]
- 64.Hill AW, Elsegood MRJ, Kimber MC. J. Org. Chem. 2010;75:5406. doi: 10.1021/jo101035n. [DOI] [PubMed] [Google Scholar]
- 65.Beccalli EM, Broggini G, Clerici F, Galli S, Kammerer C, Rigamonti M, Sottocornola S. Org. Lett. 2009;11:1563. doi: 10.1021/ol900171g. [DOI] [PubMed] [Google Scholar]
- 66.Beccalli EM, Bernasconi A, Borsini E, Broggini G, Rigamonti M, Zecchi G. J. Org. Chem. 2010;75:6923. doi: 10.1021/jo101501u. [DOI] [PubMed] [Google Scholar]
- 67.Broggini G, Borsini E, Fasana A, Poli G, Liron F. Eur. J. Org. Chem. 2012:3617. [Google Scholar]
- 68.Watanabe T, Oishi S, Fujii N, Ohno H. Org. Lett. 2007;9:4821. doi: 10.1021/ol702179n. [DOI] [PubMed] [Google Scholar]
- 69.Kimber MC. Org. Lett. 2010;12:1128. doi: 10.1021/ol1001494. [DOI] [PubMed] [Google Scholar]
- 70.Singh S, Elsegood MRJ, Kimber MC. Synlett. 2012;23:565. [Google Scholar]
- 71.Oishi S, Hatano K, Tsubouchi A, Takeda T. Chem. Comm. 2011;47:11639. doi: 10.1039/c1cc14765j. [DOI] [PubMed] [Google Scholar]
- 72.Hirata Y, Inui T, Nakao Y, Hiyama T. J. Am. Chem. Soc. 2009;131:6624. doi: 10.1021/ja9010282. [DOI] [PubMed] [Google Scholar]
- 73.Kumareswaran R, Shin S, Gallou I, Rajanbabu TV. J. Org. Chem. 2004;69:7157. doi: 10.1021/jo049010z. [DOI] [PubMed] [Google Scholar]
- 74.Gaul C, Seebach D. Helv. Chim. Acta. 2002;85:963. [Google Scholar]
- 75.(a) Ranslow PBD, Hegedus LS, de los Rίos C. J. Org. Chem. 2004;69:105. doi: 10.1021/jo0302861. [DOI] [PubMed] [Google Scholar]; (b) de los Rίos C, Hegedus LS. J. Org. Chem. 2005;70:6541. doi: 10.1021/jo050813b. [DOI] [PubMed] [Google Scholar]
- 76.Hyland CJT, Hegedus LS. J. Org. Chem. 2005;70:8628. doi: 10.1021/jo051385c. [DOI] [PubMed] [Google Scholar]
- 77.Alouane N, Bernaud F, Marrot J, Vrancken E, Mangeney P. Org. Lett. 2005;7:5797. doi: 10.1021/ol0522743. [DOI] [PubMed] [Google Scholar]
- 78.Wu Y-K, Niu T, West FG. Chem. Comm. 2012;48:9186. doi: 10.1039/c2cc34644c. [DOI] [PubMed] [Google Scholar]
- 79.Nilsson BM, Hacksell U. J. Heterocyclic Chem. 1989;26:269. [Google Scholar]
- 80.Kant J, Farina V. Tetrahedron Lett. 1992;33:3563. [Google Scholar]
- 81.Gericke R, Lues I. Tetrahedron Lett. 1992;33:1871. [Google Scholar]
- 82.Noguchi M, Okada H, Watanabe M, Okuda K, Nakamura O. Tetrahedron. 1996;52:6581. [Google Scholar]
- 83.Le Strat F, Maddaluno J. Org. Lett. 2002;4:2791. doi: 10.1021/ol026351v. [DOI] [PubMed] [Google Scholar]
- 84.Berry CR, Hsung RP, Antoline JE, Petersen ME, Rameshkumar C, Nielson JA. J. Org. Chem. 2005;70:4038. doi: 10.1021/jo0503411. [DOI] [PubMed] [Google Scholar]
- 85.Navarro-Vázquez A, Rodrίguez D, Martίnez-Esperón MF, Garcίa A, Saá C, Domίnguez D. Tetrahedron Lett. 2007;48:2741. [Google Scholar]
- 86.Husinec S, Markovic R, Petkovic M, Nasufovic V, Savic V. Org. Lett. 2011;13:2286. doi: 10.1021/ol200508x. [DOI] [PubMed] [Google Scholar]
- 87.Polindara-García LA, Miranda LD. Org. Lett. 2012;14:5408. doi: 10.1021/ol3024727. [DOI] [PubMed] [Google Scholar]
- 88.Domling A, Ugi I. Angew. Chem. Int. Ed. 2000;39:3168. doi: 10.1002/1521-3773(20000915)39:18<3168::aid-anie3168>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 89.Grigg R, Sridharan V, Xu L-H. J. Chem. Soc. Chem. Comm. 1995:1903. [Google Scholar]
- 90.Grigg R, Sansano JM. Tetrahedron. 1996;52:13441. [Google Scholar]
- 91.Grigg R, Loganathan V, Sridharan V, Stevenson P, Sukirthalingam S, Worakun T. Tetrahedron. 1996;52:11479. [Google Scholar]
- 92.Grigg R, Sansano JM, Santhakumar V, Sridharan V, Thangavelanthum R, Thornton-Pett M, Wilson D. Tetrahedron. 1997;53:11803. [Google Scholar]
- 93.Fretwell P, Grigg R, Sansano JM, Sridharan V, Sukirthalingam S, Wilson D, Redpath J. Tetrahedron. 2000;56:7525. [Google Scholar]
- 94.(a) Gardiner M, Grigg R, Sridharan V, Vicker N. Tetrahedron Lett. 1998;39:435. [Google Scholar]; (b) Gardiner M, Grigg R, Kordes M, Sridharan V, Vicker N. Tetrahedron. 2001;57:7729. [Google Scholar]
- 95.Grigg R, Köppen I, Rasparini M, Sridharan V. Chem. Comm. 2001:964. [Google Scholar]
- 96.Grigg R, McCaffrey S, Sridharan V, Fishwick CWG, Kilner C, Korn S, Bailey K, Blacker J. Tetrahedron. 2006;62:12159. [Google Scholar]
- 97.Husinec S, Petkovic M, Savic V, Simic M. Synthesis. 2012;44:399. [Google Scholar]
- 98.Fuwa H, Sasaki M. Org. Biomol. Chem. 2007;5:2214. doi: 10.1039/b707338k. [DOI] [PubMed] [Google Scholar]
- 99.Inamoto K, Yamamoto A, Ohsawa K, Hiroya K, Sakamoto T. Chem. Pharm. Bull. 2005;53:1502. doi: 10.1248/cpb.53.1502. [DOI] [PubMed] [Google Scholar]
- 100.Parthasarathy K, Jeganmohan M, Cheng C-H. Org. Lett. 2006;8:621. doi: 10.1021/ol0527936. [DOI] [PubMed] [Google Scholar]
- 101.Fuwa H, Tako T, Ebine M, Sasaki M. Chem. Lett. 2008;37:904. [Google Scholar]
- 102.Persson AKÅ, Bäckvall J-E. Angew. Chem. Int. Ed. 2010;49:4624. doi: 10.1002/anie.201000726. [DOI] [PubMed] [Google Scholar]
- 103.Brummond KM, Yan B. Synlett. 2008:2303. [Google Scholar]
- 104.Hyland CJT, Hegedus LS. J. Org. Chem. 2006;71:8658. doi: 10.1021/jo061340r. [DOI] [PubMed] [Google Scholar]
- 105.González-Gómez A, Domínguez G, Pérez-Castells J. Eur. J. Org. Chem. 2009:5057. [Google Scholar]
- 106.Ma Z-X, He S, Song W, Hsung RP. Org. Lett. 2012;14:5736. doi: 10.1021/ol302743k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shen L, Hsung RP. Org. Lett. 2005;7:775. doi: 10.1021/ol047572z. [DOI] [PubMed] [Google Scholar]
- 108.(a) Braverman S, Segev D. J. Am. Chem. Soc. 1974;96:1245. [Google Scholar]; (b) Garratt PJ, Neoh SB. J. Am. Chem. Soc. 1975;97:3255. [Google Scholar]; (c) Mondal S, Mitra T, Mukherjee R, Addy PS, Basak A. Synlett. 2012;23:2582. [Google Scholar]
- 109.Mukherjee R, Basak A. Synlett. 2012;23:877. [Google Scholar]
- 110.Zhou H, Xing Y, Yao J, Lu Y. J. Org. Chem. 2011;76:4582. doi: 10.1021/jo2004555. [DOI] [PubMed] [Google Scholar]
- 111.Kinderman SS, Van Maarseveen JH, Schoemaker HE, Hiemstra H, Rutjes FPT. Org. Lett. 2001;3:2045. doi: 10.1021/ol016013e. [DOI] [PubMed] [Google Scholar]
- 112.Rameshkumar C, Xiong H, Tracey MR, Berry CR, Yao LJ, Hsung RP. J. Org. Chem. 2002;67:1339. doi: 10.1021/jo011048d. [DOI] [PubMed] [Google Scholar]
- 113.Lu T, Hayashi R, Hsung RP, DeKorver KA, Lohse AG, Song Z, Tang Y. Org. Biomol. Chem. 2009;7:3331. doi: 10.1039/b908205k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.(a) Kimura M, Horino Y, Wakamiya Y, Okajima T, Tamaru Y. J. Am. Chem. Soc. 1997;119:10869. [Google Scholar]; (b) Horino Y, Kimura M, Tanaka S, Okajima T, Tamaru Y. Chem.—Eur. J. 2003;9:2419. doi: 10.1002/chem.200304586. [DOI] [PubMed] [Google Scholar]
- 115.Kimura M, Horino Y, Mori M, Tamaru Y. Chem.—Eur. J. 2007;13:9686. doi: 10.1002/chem.200700691. [DOI] [PubMed] [Google Scholar]
- 116.Nair V, Sethumadhavan D, Nair SM, Shanmugam P, Treesa PM, Eigendorf GK. Synthesis. 2002:1655. [Google Scholar]
- 117.Li X-X, Zhu L-L, Zhou W, Chen Z. Org. Lett. 2012;14:436. doi: 10.1021/ol202703a. [DOI] [PubMed] [Google Scholar]
- 118.Faustino H, Bernal P, Castedo L, López F, Mascareñas JL. Adv. Syn. Cat. 2012;354:1658. [Google Scholar]
- 119.Suárez-Pantiga S, Hernández-Díaz C, Piedrafita M, Rubio E, Gonzáleza JM. Adv. Synth. Catal. 2012;354:1651. [Google Scholar]
- 120.Anorbe L, Poblador A, Dominguez G, Pérez-Castells J. Tetrahedron Lett. 2004;45:4441. [Google Scholar]
- 121.González-Gómez Á, Añorbe L, Poblador A, Domínguez G, Pérez-Castells J. Eur. J. Org. Chem. 2008:1370. [Google Scholar]
- 122.Gupta AK, Park DI, Oh CH. Tetrahedron Lett. 2005;46:4171. [Google Scholar]
- 123.Broggini G, Bruché L, Zecchi G. J. Chem. Soc. Perkin 1: 1990:533. [Google Scholar]
- 124.Barluenga J, Vicente R, López LA, Tomas M. J. Am. Chem. Soc. 2006;128:7050. doi: 10.1021/ja0586788. [DOI] [PubMed] [Google Scholar]
- 125.Piperno A, Rescifina A, Corsaro A, Chiacchio MA, Procopio A, Romeo R. Eur. J. Org. Chem. 2007;9:1517. [Google Scholar]
- 126.Chiacchio U, Corsaro A, Iannazzo D, Piperno A, Romeo G, Romeo R, Saita MG, Rescifina A. Eur. J. Org. Chem. 2007:4758. [Google Scholar]
- 127.Zhu Y, Wen S, Yin G, Hong D, Lu P, Wang Y. Org. Lett. 2011;13:3553. doi: 10.1021/ol201203g. [DOI] [PubMed] [Google Scholar]
- 128.Wei L-L, Hsung RP, Xiong H, Mulder JA, Nkansah NT. Org. Lett. 1999;1:2145. [Google Scholar]
- 129.(a) Berry CR, Rameshkumar C, Tracey MR, Wei L-L, Hsung RP. Synlett. 2003:791. [Google Scholar]; (b) Rameshkumar C, Hsung RP. Synlett. 2003:1241. [Google Scholar]
- 130.Berry CR, Hsung RP. Tetrahedron. 2004;60:7629. [Google Scholar]
- 131.Lohse AG, Hsung RP. Org. Lett. 2009;11:3430. doi: 10.1021/ol901283m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Faustino H, López F, Castedo L, Mascareñas JL. Chem. Sci. 2011;2:633. [Google Scholar]
- 133.Francos J, Grande-Carmona F, Faustino H, Iglesias-Sigüenza J, Díez E, Alonso I, Fernández R, Lassaletta JM, López F, Mascareñas JL. J. Am. Chem. Soc. 2012;134:14322. doi: 10.1021/ja3065446. [DOI] [PubMed] [Google Scholar]
- 134.Cao J, Huang X. Org. Lett. 2010;12:5048. doi: 10.1021/ol102235t. [DOI] [PubMed] [Google Scholar]
- 135.Zhu S, Cao J, Wu L, Huang X. J. Org. Chem. 2012;77:1049. doi: 10.1021/jo301437k. [DOI] [PubMed] [Google Scholar]
- 136.Horino Y, Kimura M, Wakamiya Y, Okajima T, Tamaru Y. Angew. Chem. Int. Ed. 1999;38:121. [Google Scholar]
- 137.Horino Y, Kimura M, Naito M, Tanaka S, Tamaru Y. Tetrahedron Lett. 2000;41:3427. [Google Scholar]
- 138.Hashimoto K, Horino Y, Kuroda S. Heterocycles. 2010;80:187. [Google Scholar]
- 139.Reviews about [3 + 3]: Buchanan GS, Feltenberger JB, Hsung RP. Curr. Org. Synth. 2010;7:363. doi: 10.2174/157017910791414490. Hsung RP, Kurdyumov AV, Sydorenko N. Eur. J. Org. Chem. 2005;1:23. Hsung RP, Cole KP. In: Strategies and Tactics in Organic Synthesis. Harmata M, editor. Vol. 4. Oxford, UK: Elsevier Science, Pergamon; 2004. p. 41. Hsung RP, Wei LL, Sklenicka HM, Shen HC, McLaughlin MJ, Zehnder LR. Trends Heterocycl. Chem. 2001;7:1.
- 140.Xiong H, Hsung RP, Berry CR, Rameshkumar C. J. Am. Chem. Soc. 2001;123:7174. doi: 10.1021/ja0108638. [DOI] [PubMed] [Google Scholar]
- 141.Recent reviews: Lohse AG, Hsung PR. Chem.-Eur. J. 2011;17:3812. doi: 10.1002/chem.201100260. Harmata M. Chem. Commun. 2010;46:8904. doi: 10.1039/c0cc03621h. Harmata M. Chem. Commun. 2010;46:8886. doi: 10.1039/c0cc03620j. Examples of amido-stablized: Magee DI, Godineau E, Thornton PD, Walters MA, Sponholtz DJ. Eur. J. Org. Chem. 2006:3667. Xiong H, Hsung RP, Shen L, Hahn JM. Tetrahedron Lett. 2002;43:4449. Walters MA, Arcand HR. J. Org. Chem. 1996;61:1478. Walters MA, Arcand HR, Lawrie DJ. Tetrahedron Lett. 1995;36:23.
- 142.Antoline JE, Hsung RP. Synlett. 2008:739. [Google Scholar]
- 143.Krenske EH, Houk KN, Lohse AG, Antoline JE, Hsung RP. Chem. Sci. 2010;1:387. doi: 10.1039/C0SC00280A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lohse AG, Krenske EH, Antoline JE, Houk KN, Hsung RP. Org. Lett. 2010;12:5506. doi: 10.1021/ol1023745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Antoline JE, Krenske EH, Lohse AG, Houk KN, Hsung RP. J. Am. Chem. Soc. 2011;133:14443. doi: 10.1021/ja205700p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Du Y, Krenske EH, Antoline JE, Lohse AG, Houk KN, Hsung RP. J. Org. Chem. 2012 doi: 10.1021/jo3011792. ASAP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Antoline JE, Hsung RP, Huang J, Song Z, Li G. Org. Lett. 2007;9:1275. doi: 10.1021/ol070103n. [DOI] [PubMed] [Google Scholar]
- 148.Huang J, Hsung RP. J. Am. Chem. Soc. 2005;127:50. doi: 10.1021/ja044760b. [DOI] [PubMed] [Google Scholar]
- 149.Reviews: Harmata M. Adv. Synth. Catal. 2006;348:2297. Hartung IV, Hoffmann HMR. Angew. Chem. Int. Ed. 2004;43:1934. doi: 10.1002/anie.200300622. Examples: Lo B, Lam S, Wong WT, Chiu P. Angew. Chem. Int. Ed. 2012;51:12120. doi: 10.1002/anie.201207427. Dai X, Davies HML. Adv. Synth. Catal. 2006;348:2449. (formal 4+3) Harmata M, Ghosh SK, Hong X, Wacharasindhu S, Kirchhoefer P. J. Am. Chem. Soc. 2003;125:2058. doi: 10.1021/ja029058z.
- 150.(a) Xiong H, Huang J, Ghosh SK, Hsung RP. J. Am. Chem. Soc. 2003;125:12694. doi: 10.1021/ja030416n. [DOI] [PubMed] [Google Scholar]; (b) Lohse AG, Hsung RP, Leider MD, Ghosh SK. J. Org. Chem. 2011;76:3246. doi: 10.1021/jo200147h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rameshkumar C, Hsung RP. Angew. Chem. Int. Ed. 2004;43:615. doi: 10.1002/anie.200352632. [DOI] [PubMed] [Google Scholar]
- 152.(a) Hayashi R, Hsung RP, Feltenberger JB, Lohse AG. Org. Lett. 2009;11:2125. doi: 10.1021/ol900647s. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Farmer ML, Billups WE, Greenlee RB, Kurtz AN. J. Org Chem. 1966;31:2885. [Google Scholar]; (c) Basak A, Gupta SN, Chakrabarty K, Das GK. Comput. Theor. Chem. 2013;1007:15. [Google Scholar]
- 153.Hayashi R, Feltenberger JB, Lohse AG, Walton MC, Hsung RP. Beil. J. Org. Chem. 2011;7:410. doi: 10.3762/bjoc.7.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hayashi R, Feltenberger JB, Hsung RP. Org. Lett. 2010;12:1152. doi: 10.1021/ol902821w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hayashi R, Walton MC, Hsung RP, Schwab J, Yu X. Org. Lett. 2010;12:5768. doi: 10.1021/ol102693e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Feltenberger JB, Hsung RP. Org. Lett. 2011;13:3114. doi: 10.1021/ol2010227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.(a) Song Z, Hsung RP. Org. Lett. 2007;9:2199. doi: 10.1021/ol070791a. [DOI] [PubMed] [Google Scholar]; (b) Song Z, Hsung RP, Lu T, Lohse AG. J. Org. Chem. 2007;72:9722. doi: 10.1021/jo7017922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Komatsu K, Tanino K, Miyashita M. Angew. Chem. Int. Ed. 2004;43:4341. doi: 10.1002/anie.200460434. [DOI] [PubMed] [Google Scholar]
- 159.Hayashi R, Ma Z-X, Hsung RP. Org. Lett. 2012;14:252. doi: 10.1021/ol203030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Achmatowicz M, Hegedus LS. J. Org. Chem. 2004;69:2229. doi: 10.1021/jo0303012. [DOI] [PubMed] [Google Scholar]
- 161.Heffernan SJ, Beddoes JM, Mahon MF, Hennessy AJ, Carbery DR. Chem. Comm. 2013 doi: 10.1039/c3cc00273j. [DOI] [PubMed] [Google Scholar]
- 162.Lindsay VNG, Fiset D, Gritsch PJ, Azzi S, Charette AB. J. Chem. Soc. 2013;135:1643. doi: 10.1021/ja3099728. [DOI] [PubMed] [Google Scholar]