Abstract
Ovarian cancer is very treatable in the early stages of disease; however, it is usually detected in the later stages, at which time, treatment is no longer as effective. If discovered early (Stage I), there is a 90% chance of five-year survival. Therefore, it is imperative that early-stage biomarkers are identified to enhance the early detection of ovarian cancer. Cancer-testis antigens (CTAs), such as Per ARNT SIM (PAS) domain containing 1 (PASD1), are unique in that their expression is restricted to immunologically restricted sites, such as the testis and placenta, which do not express MHC class I, and cancer, making them ideally positioned to act as targets for immunotherapy as well as potential biomarkers for cancer detection where expressed. We examined the expression of PASD1a and b in a number of cell lines, as well as eight healthy ovary samples, eight normal adjacent ovarian tissues, and 191 ovarian cancer tissues, which were predominantly stage I (n = 164) and stage II (n = 14) disease. We found that despite the positive staining of skin cancer, only one stage Ic ovarian cancer patient tissue expressed PASD1a and b at detectable levels. This may reflect the predominantly stage I ovarian cancer samples examined. To examine the restriction of PASD1 expression, we examined endometrial tissue arrays and found no expression in 30 malignant tumor tissues, 23 cases of hyperplasia, or 16 normal endometrial tissues. Our study suggests that the search for a single cancer-testes antigen/biomarker that can detect early ovarian cancer must continue.
Keywords: biomarker, ovarian cancer, PASD1, human, cancer-testis antigen
Introduction
Ovarian cancer is mainly found in women aged over 50 years, although it can occur at any age. Around 225,500 women worldwide are diagnosed with ovarian cancer every year, and there are about 140,200 associated deaths.1 There are different types of ovarian cancers classified by the type of cell from which the cancer originates, including epithelial, germ cell, and stromal ovarian cancers, with epithelial ovarian cancer (EOC) being the most common type. Ovarian cancer is mostly diagnosed in the late stages of the disease, at which time, treatment is not as effective, and the cancer may have spread beyond the ovaries. However, if ovarian cancer is diagnosed in the early stages of the disease, there is a 90% survival rate.2 Currently, the marker carbohydrate antigen 125 (CA125) is used to confirm a diagnosis of ovarian cancer; however, CA125 is found most frequently in most stage III and stage IV tumors and/or serous tumors. These tumors are likely to develop from serous tubal intraepithelial carcinoma precursor lesions in the fallopian tubes,3,4 which then coat the ovary and fall into the abdominal cavity, and as such, are unlikely to be found at stage I of the disease. In contrast, clear cell/endometroid tumors arise in the ovary5 and can be detected in the early stages of the disease,6 although this is still rare.7 Recently, a combination of serum CA125, serum HE4, and age was used to triage women with suspected ovarian cancer and was found to have a clinically relevant sensitivity for discriminating benign from malignant ovarian disease.8
Cancer-testis antigens (CTAs) provide attractive targets in the pursuit of effective cancer immunotherapy. Their unique properties of only being expressed in cancer cells and not in healthy tissues, apart from immune-privileged sites such as the testis,9 means that they have the potential to also act as sensitive biomarkers for cancer. Per ARNT SIM (PAS) domain containing 1 (PASD1) was identified in diffuse large B-cell lymphoma10 and acute myeloid leukemia11 using the serological identification of antigens by recombinant expression cloning also known as the SEREX technique.12 PASD1 expression was subsequently described in multiple myeloma.13 PASD1 shows most similarity to the CLOCK gene in mice and was recently found to have a role in blocking circadian rhythms in human cancer cells.14
However, few CTAs have been identified as being frequently expressed in ovarian cancer (Table 1) and few investigations have examined PASD1 expression in solid tumors.10,15 We had hoped to find a new biomarker for early-stage ovarian cancer, and to do this, we examined PASD1 protein expression in ovarian cancer, and endometrial tissue arrays (to show specificity of the expression), through immunolabeling.
Table 1.
Overview of the expression of CTAs in ovarian cancer.
| ANTIGENS | TECHNIQUE USED | POSITIVE EXPRESSION | TYPE OF OC | POSITIVE AT STAGES OF OC | REFERENCES |
|---|---|---|---|---|---|
| Sperm-associated antigen 9 (SPAG9) | RT-PCR, RNA in situ hybridization, IHC | mRNA and protein expression detected in a total of 18/20 tissue samples, antibodies detected in a total of 20/30 patient sera | Epithelial | I = 1/1; Ib = 2/2; Ic = 1/1 II = 1/1; III =4/5; IIIa = 1/1; IIIc = 1/1; IV = 7/8 |
25 |
| Transcripts detected in 15/17 and protein expression observed in 11/19 patient samples in serous | Serous | ||||
| Transcripts present in 1/1 and protein expression in 2/2 samples in clear cell | Clear cell | ||||
| 2/2 samples expressed transcripts and 3/3 expressed the protein in mucinous | Mucinous | ||||
| OY-TES-1 | RT-PCR, IHC, ELISA | Expressed in a total of 69/100 ovarian tumours: which included: 43/100 positive samples in papillary serous | Papillary serous | Ia = 3/60, Ib = 1/60 Ic = 1/60, IIc = 3/60 IIIa = 4/60, IIIb = 1/60 IIIc = 43/60, IV = 3/60 |
26 |
| 3/100 positive samples in clear cell | Clear cell | ||||
| 4/100 positive samples in endometrioid | Endometrioid | ||||
| 1/100 positive samples in mucinous | Mucinous | ||||
| PIWI proteins | IHC | piwiI1, piwil2, piwil3, piwil4 expression significantly enhanced in primary tumour and metastatic tissues in EOC | EOC | NK | 40 |
| LAGE-1 and NY-ESO-1 | RT-PCR, IHC | NY-ESO-1 and/or LAGE-1 mRNA present in 42/107 samples, 11/37 positive patients also expressed antibodies | EOC | Ic = 1/32, IIIa = 1/32, IIIc = 27/32, IV = 3/32 | 27 |
| NY-ESO-1 positive in 10/53 patient samples | Serous | I–11 =2/14, III–IV = 8/39 | 41 | ||
| MAGE family | IHC, RT-PCR, ELISA | MAGE-A4 present in 30/53 tumour samples | Serous | I–II = 7/14, III–IV = 23/39 | 41 |
| MAGE-1 expressed in total of 15/27 samples which included: 10/14 patient samples | Serous | N/A | 42 | ||
| MAGE-4 protein expressed in total of 13/60 samples including: 6/25 positive samples | Serous | I = 2/16 II = 0/4 III = 10/29 IV = 0/4 |
43 | ||
| 3/14 positive samples | Mucinous | ||||
| 2/8 positive samples | Endometrioid | ||||
| BAGE mRNA detected in 15/27 samples | Ascites from peritoneal washings | NK | 44 | ||
| Sperm protein 17 (sp17) | RT-PCR, Northern blot | Transcripts detected in a total of 15/18 tumours by RT-PCR and in 17/25 ovarian tumours by Northern Blot which included: 7/11 positive samples | Papillary serous or mixed | NK | 28 |
| 4/8 positive samples | Endometrioid | ||||
| 2/2 positive samples | Clear cell | ||||
| SSX | RT-PCR, ELISA | SSX1 found expressed in 3/118 patient samples | EOC | SSX1: IIIc = 3/3 | 29 |
| SSX2 found expressed in 12/122 patient samples | SSX2: Ia = 1/12, | ||||
| SSX4 detected in 19/120 patient samples | IIIc = 10/12, IV = 1/12 | ||||
| Aberrant expression of these antigens found in 31/120 patient sera | SSX4: IIIc = 18/19, IV = 1/19 | ||||
| SSX4 found to be expressed in 6/12 tumour samples | NK | NK | 45 | ||
| A-kinase anchoring protein 3 (AKAP3) | One step RT-PCR | mRNA expression demonstrated in a total of 43/74 ovarian cancer specimens including 36/43 | Serous | Ia = 1/43 IIc = 2/43 IIIc = 31/43 IV = 7/43 |
30 |
| 4/43 positive samples | Endometrioid | ||||
| 1/43 positive samples | Clear cell | ||||
| SCP-1 (HOM-TES-1) | RT-PCR | SCP-1 mRNA expression detected in 15/100 tumour samples | EOC | Ia = 1/15, IIIc = 13/15, IV = 1/15 | 46 |
Abbreviations: ELISA, enzyme-linked immunosorbant assay; EOC, epithelial ovarian cancer; IHC, immunohistochemistry; NA, not applicable; NK, not known; PIWI, P-element-induced wimpy testis; RT-PCR, real-time polymerase chain reaction.
Materials and Methods
Cell lines and their preparation for immunolabeling
Cells were grown in RPMI 1640 media (Sigma) containing 10% foetal bovine serum (Thermo Fisher) and 1% penicillin and streptomycin (Thermo Fisher), in a humidified incubator at 37°C, and in 5% CO2. Cells were harvested, counted, and diluted in PBS (Fisher) to 5 × 106 per mL. Cells were recentrifuged for eight minutes at 1200 × g. Supernatant was removed, and the cell pellet was resuspended in 1 mL PBS. Glass slides (Fisher Scientific) were used, and 10 μL of cell solution was spotted on each microscope slide. The slides were air dried for at least four hours, double wrapped in Saran wrap, and stored at −20°C.
Tissue microarrays (TMAs)
The ovarian TMAs (OV2084 Biomax) constitute high-density ovary carcinoma and adjacent normal ovary tissue sections, including 165 stage I, 14 stage II, five stage III, and four stage IV ovary cancer samples, as well as eight adjacent normal tissues and eight normal tissues, single core per case. Ovarian cancer TMAs were tested in duplicate for the expression of each antigen and to test the controls. The endometrium carcinoma TMA (UT801 Biomax) incorporated 30 cases of endometrial adenocarcinoma, 23 of endometrial hyperplasia, six of metastastic disease, five of endometrial inflammation, 10 adjacent normal tissues, and six normal tissues of the uterus, single core per case. The endometrium carcinoma tissue array was only tested once, and we only examined PASD1b, and not PASD1a, expression.
Antibodies
Immunolabeling for each of the antigens was performed using antihuman primary antibodies; the monoclonal antibody PASD1-1 that recognizes the region common to the PASD1a and PASD1b proteins (clone 2ALCC136) between aa 195–474 and PASD1-2 (clone 2ALCC128), a monoclonal antibody that is specific for aa 540–773 present only in the longer PASD1b protein.10,16 CA125 clone OC125 (90 μg/mL, AbD Serotec), the current industry standard used to confirm a diagnosis for ovarian cancer, was used for comparison.
Dewaxing of tumour microarrays (TMAs) prior to immunolabeling
All TMAs were obtained from US Biomax, Inc. TMAs were de waxed in citroclear (TCS Biosciences) twice for five minutes, placed in 100% ethanol twice for five minutes, and then once in 50% ethanol for five minutes. The arrays were washed with tap water, and antigen retrieval was performed in Tris/EDTA buffer pH9 (Sigma) in a microwave (850 W) for 11 minutes at full power. Following cooling, the arrays were washed in TBS. Peroxide block (Dako) was added for five minutes, the tissue arrays were washed again in TBS, and the excess TBS was carefully removed from the periphery of the buttons using absorbent tissues.
Defrosting of frozen cell lines spotted onto glass slides prior to immunolabeling
Slides of frozen cells that had been spotted onto glass slides were taken from storage at −20°C and defrosted for 20 minutes at room temperature before removing the Saran wrap. A diamond pen (Fisher) was used to mark around the area of the slide to be stained (the “button”). The cells were fixed by inserting the slides into a Coplin jar containing 100% 50 mL cold methanol (Fisher) on ice for 15 minutes. The slides were removed from the fixative and placed horizontally into a moisture chamber face up. They were washed three times in TBS (Sigma), and the excess TBS was carefully removed from the periphery of each of the buttons using tissues.
Immunolabeling antigens in cell lines and TMAs
A peroxidase block from the Envision Kit (Dako) was added to the cells for five minutes. The appropriate primary antibodies were diluted in TBS, and 40 μL of the antibody solution added to each button of cells. Actin staining was used as a positive control to demonstrate that the assay and its component reagents were working. The slides were incubated with primary antibody for one hour at room temperature, and then washed three times with TBS. The secondary antibody was supplied as part of the Envision+ System HRP (DAB) kit (Dako) and was a HRP conjugated anti-mouse IgG antibody which was added and incubated for 30 minutes at room temperature. Following washes with TBS, 20–30 μL of DAB substrate from the Envision kit was added to each cell button, incubated for five minutes, and then washed gently with water. A total of 40 μL of 1:5 dilution Mayer’s hematoxylin: Lillie’s Modification (Dako) was added to each button as a counterstain and washed with copious amounts of tap water to remove all excess stain. Slides were mounted in Faramount Aqueous Mounting Medium (Dako) and imaged using the virtual microscopy system Olympus Dotslide at 40× magnification at the University of Southampton. Samples were scored depending on the intensity of the labeling with 0 and 1 being classed as negative and 2, 3, and 4 considered as positive.
Statistical analysis
A one-tailed t-test analysis was undertaken to compare the distribution of the scoring across the different tissue subtypes; in addition, chi2 tests were performed on subgroups of tissues, including those from different stages of ovarian cancer and healthy donors.
Results
PASD1 protein expression in cancer cell lines
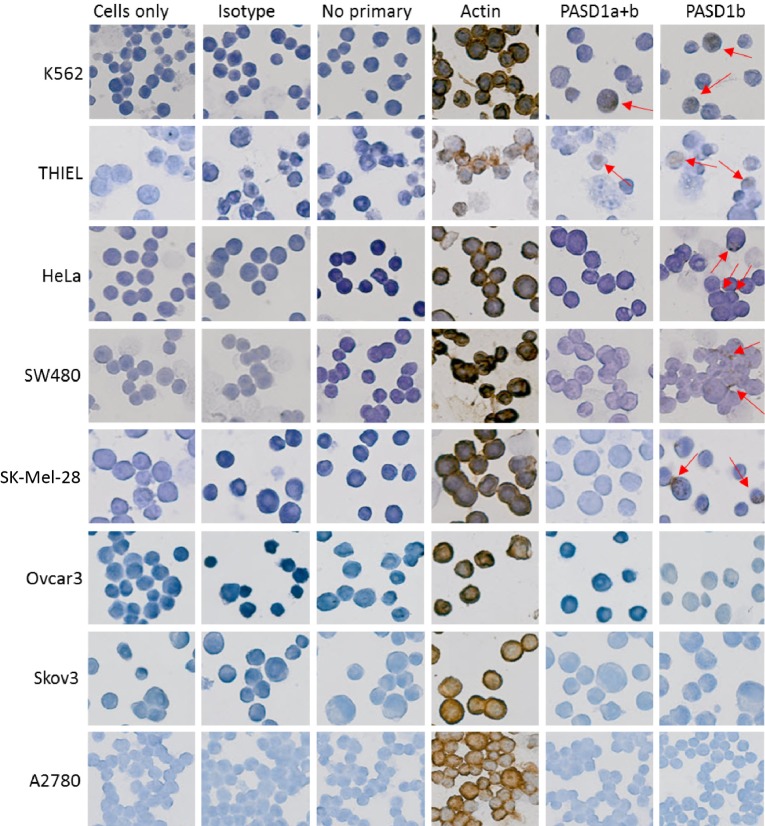
Although the expression of PASD1 protein was detected by immunolabeling chronic myeloid leukemia, K562,17 multiple myeloma, THIEL (a gift from Prof. Diehl, University of Cologne, Germany), cervical cancer HeLa,18 colorectal cancer, SW480,19 and melanoma Sk-Mel-2820 cell line, PASD1 was not detected in the ovarian cancer cell lines: Skov3,21 Ovcar3,22 and A278023 (Fig. 1).16
Figure 1.
PASD1 was found to be expressed in a number of human cancer cell lines. PASD1 expression was identified in K562, THIEL, HeLa, SW480, and Sk-Mel-28 cells; however, it was not found in the Ovcar3, Skov3, and A2780 ovarian cancer cell lines. Cells only and isotype controls were used as negative controls while no primary was used to determine background staining. Actin was used as a positive control. Red arrows indicate the brown deposition that identifies the subcellular localization of the immunolabeled target protein.
PASD1 protein expression in normal and normal adjacent tissues
The PASD1-1 antibody that immunolabeled PASD1a and b scored only 0 in normal adjacent ovarian tissue (NAT) and 0–1 in healthy ovarian tissue, whereas the PASD1-2 monoclonal antibody that immunolabels PASD1b had immunolabeling scores ranging 0–1 for healthy tissue and 0–2 for normal adjacent tissue (Table 2A and Fig. 2). Immunolabeling of normal endometrial tissue with the anti-PASD1b antibody was not observed (Table 2C), although CA125 was found to immunolabel some normal endometrial tissues.
Table 2.
Immunostaining of tissue arrays for PASD1a and PASD1b protein expression.
| (A) | HEALTHY OVARIAN TISSUE | NORMAL ADJACENT OVARIAN TISSUE | I | Ia | Ib | Ic | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Staining intensity | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 |
| Cells only | 8 | 8 | 32 | 59 | 1 | 41 | 32 | |||||||||||||||||
| Actin | 0 | 2 | 5 | 1 | 0 | 8 | 1 | 18 | 4 | 9 | 4 | 33 | 16 | 6 | 2 | 22 | 12 | 5 | 2 | 18 | 10 | 2 | ||
| CA125 | 7 | 1 | 5 | 3 | 18 | 9 | 4 | 42 | 14 | 3 | 1 | 22 | 16 | 3 | 23 | 7 | 1 | |||||||
| PASD1a + b | 5 | 2 | 8 | 29 | 3 | 58 | 2 | 39 | 1 | 1 | 32 | |||||||||||||
| PASD1b | 6 | 1 | 2 | 5 | 1 | 25 | 7 | 47 | 12 | 37 | 4 | 29 | 3 | |||||||||||
| (B) | II | IIb | IIc | III | IIIc | IV | SKIN CANCER | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Staining intensity | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 |
| Cells only | 11 | 2 | 1 | 3 | 2 | 4 | 1 | |||||||||||||||||||||
| Actin | 0 | 4 | 6 | 1 | 0 | 1 | 1 | 1 | 2 | 1 | 3 | 0 | 1 | 2 | 1 | 1 | ||||||||||||
| CA125 | 6 | 4 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 2 | 1 | ||||||||||||||||
| PASD1a + b | 10 | 1 | 2 | 1 | 3 | 2 | 1 | 4 | 1 | |||||||||||||||||||
| PASD1b | 11 | 1 | 1 | 1 | 3 | 3 | 4 | 1 | ||||||||||||||||||||
| (C) | CELLS ONLY | CA125 | PASD1b | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Staining score | 0 | 1 | 2 | 3 | 4 | 0 | 1 | 2 | 3 | 4 | 0 | 1 | 2 | 3 | 4 |
| Malignant tumour | 30 | 17 | 4 | 9 | 30 | ||||||||||
| Hyperplasia | 23 | 1 | 11 | 11 | 23 | ||||||||||
| Metastasis | |||||||||||||||
| Abdominal cavity | 2 | 1 | 1 | 1 | |||||||||||
| Fibrofatty tissue | 1 | 1 | 1 | ||||||||||||
| Lymph node | 1 | 1 | 1 | ||||||||||||
| Pelvic cavity | 1 | 1 | 1 | ||||||||||||
| Ovary | 1 | 1 | 1 | ||||||||||||
| Inflammation | 5 | 4 | 1 | 5 | |||||||||||
| Normal endometrial tissue | 16 | 8 | 2 | 4 | 2 | 16 | |||||||||
Notes: Expression of PASD1a and b in (A) healthy tissue, normal tissue adjacent to ovarian cancer cells and stage I ovarian cancer (B) stage II, III and IV ovarian cancer and (C) endometrial tissues were investigated. CA125 was used as an industry standard comparator. Staining intensity is indicated by the colour of the cells as follows 0–1 was considered to be negative staining, 2–4 was considered to be positive immunolabelling with 2 being moderate levels of protein, 3 high levels and 4 very high levels of protein detected. Actin was used as the positive control to confirm the immunostaining protocol was working and provide a staining intensity comparator and cells only provided a control for background staining with haematoxylin. Scoring was also carried out for healthy ovarian tissue and normal adjacent tissue. A small number of tissue cores were missing from the MTAs following immunolabelling and so data on these samples is absent from the table. Melanoma (skin cancer) tissue on each TMA was used as a positive control for PASD1 immunolabelling.
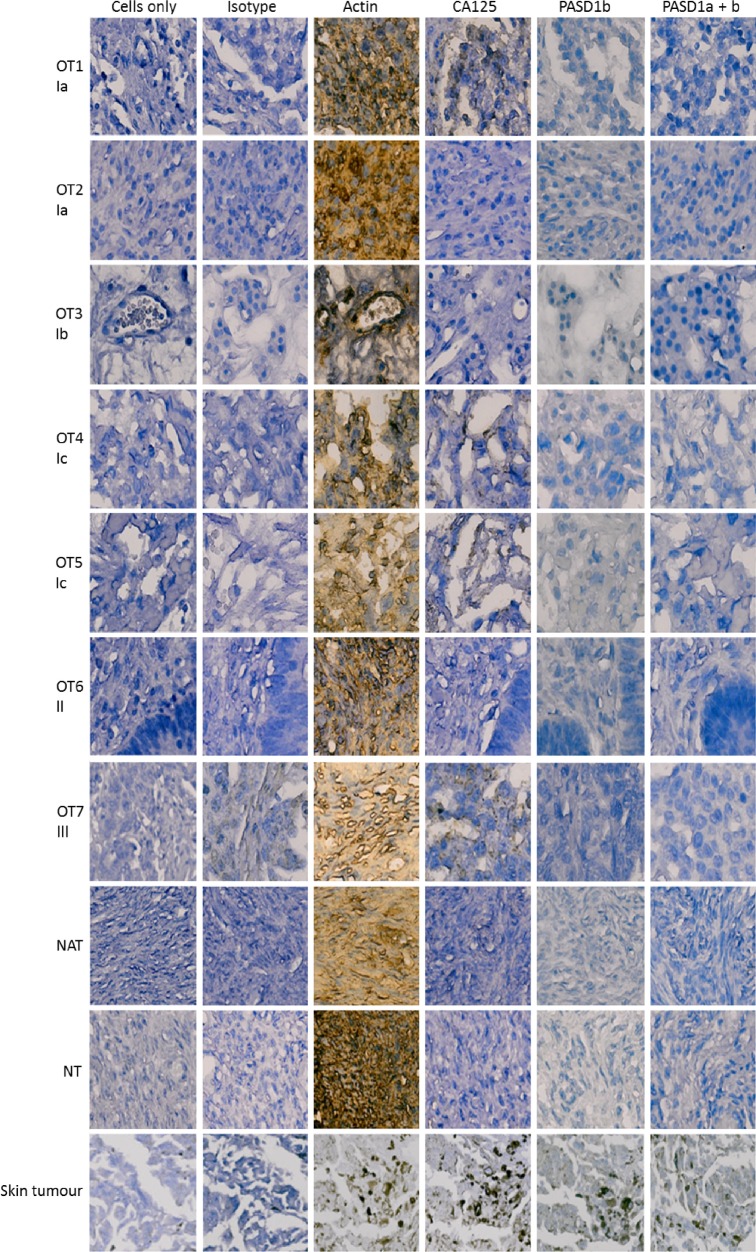
Figure 2.
Expression of PASD1 in ovarian cancer TMAs. Images show the PASD1 staining of representative ovarian cancer samples at various stages of the disease. Each sample is identified by a unique sample identifier, ie, OT1, OT2, OT3, etc, followed by disease stage as indicated by a roman numeral and an alphabetical letter, ie, Ia, Ib, etc. PASD1 expression was predominantly absent from the ovarian cancer tissues tested. CA125 was used as a comparator as it is the industry standard for the confirmation of a diagnosis of ovarian cancer. Cells only and isotype controls were used as negative controls and actin as a positive control. The single melanoma (skin tumor) sample on each TMA was used as a positive control for immunolabeling with the PASD1 antibodies. Skin tumor samples expressed higher levels of actin, CA125, and PASD1 but did not immunolabel when incubated with isotype control antibody. NATs and normal ovarian tissues (NTs) were also tested and were predominantly negative except for actin expression.
PASD1 protein expression in ovarian cancer tissues and endometrial arrays
We found no significant expression of PASD1a or b in ovarian cancer tissue samples (Fig. 2 and Table 2A & B). A comparison of I vs II/III/IV was not significant for PASD1a + b (P = 0.564) or PASD1b (P = 0.492) Both of the PASD1 variants were scored at 0 and 1 (classed as negative in our scoring system), and only one sample had a score of 2 (scores of 2–4 were classed as positive). There was very little background staining for both of the antigens, although one core of NAT scored positively for PASD1b. We found no expression of PASD1b in endometrial tissues (Table 2C). In contrast, CA125 expression was identified in 12/165 stage I, 1/15 stage II (P = 0.576), 0/3 stage IIIc, and 0/4 stage IV tumors. These frequencies of expression were not significant when compared to the normal tissue (P = 0.536, 0.576, 1, and 1, respectively).
The single core of malignant melanoma skin tissue on each TMA was positive following immunolabeling with either of the PASD1 antibodies, providing a positive control for PASD1 staining.
Discussion
The aim of our study was to investigate the expression of PASD1 protein expression in early-stage ovarian cancer through the use of TMAs. To optimize staining with the PASD1a and PASD1b antibodies, we identified PASD1 protein expression in leukemia (K562), multiple myeloma (THIEL), cervical cancer (HeLa), colorectal cancer (SW480), and a melanoma cell line (SK-Mel-28). We confirmed the previously published data,10,11,13 including the study by Liggins et al,10 who had found PASD1 expression in K562, HeLa, SW480, and G361 (melanoma) cell lines. The staining we observed was cytoplasmic and nuclear as described previously.16 However, PASD1 expression was not detected in the ovarian cancer cell lines: Skov3, Ovcar3, and A2780. Liggins et al10 discovered some transcript expression of PASD1 in three ovarian cancer tumor tissues; however, this was quite weak when compared to the other solid tumor tissues tested such as the kidney and prostate. We did see some staining that achieved a score of 2 for PASD1b (1/8) with NAT but there is some evidence that PASD1 mRNA may be present in histologically normal tissues signaling the potential of the cells to become cancerous.10,24
The expression of a number of other CTAs have been examined in ovarian cancer (summarized in Table 1), and some of these antigens have shown a frequency of expression, which make them promising targets for immunotherapy clinical trials. These include Spag9 whose mRNA and protein expression was detected in 18/20 tissue samples, while Spag9 transcripts were detected in 15/17 and protein expression observed in 11/19 serous ovarian cancer samples.25 OY-TES-1 was found to be predominantly expressed in papillary serous patient samples (43/100) along with 26 other patient samples in the cohort analyzed.26 NY-ESO-1 and/or LAGE-1 mRNA transcripts were found in 42/107 EOC samples, most of these transcripts were detected in samples from patients with stage IIIc disease.27 In papillary serous or mixed ovarian cancer tumors, Sperm protein 17 (Sp17) transcripts were detected by reverse transcription-polymerase chain reaction (RT-PCR) in 15/18 samples and by Northern blot in 17/25 samples.28 Of note, synovial sarcoma X 4 (SSX4) was detected in 19/120 EOC mostly stage IIIc patient samples,29 while A-kinase anchoring protein 3 (AKAP3) mRNA expression was found in a total of 43/74 ovarian cancer specimens, including 36/43 serous samples most of which were stage IIIc.30 In contrast, when we examined ovarian cancer tissue for PASD1 expression, we found infrequent expression that may reflect the predominantly early-stage disease samples we examined.
Although PASD1 does show some expression in some solid cancer samples and cell lines, expression has still predominately been identified in hematological cancers such as leukemia and lymphomas.10,11 PASD1 is located on Xq28, and mutations in the q28 region have been linked with increased risk of lymphoma and leukemia,31 indicating a potentially important role for PASD1 as a target for immunotherapy for blood cancers. In addition, PASD1 expression has not been found in any of the 78 basal cell carcinoma samples and 15 normal skin samples using semiquantitative RT-PCR.15
CA125 was expressed more frequently than PASD1 in our study of ovarian cancer samples; however, CA125 has been proven to have low specificity and sensitivity, and inconsistent expression patterns among different patients. In our study, we found CA125 expression in normal endometrial tissue and tissues with inflammation and reports of expression in endometrial cell growth and deciduation have been published previously.32 CA125 is mainly used as a marker to detect disease relapse and for monitoring treatment efficacy rather than primary ovarian cancer;33 however, it may be more effective as a marker for predicting advanced stage disease.34 CA125 has also been found in endometrial cancer where raised CA125 correlated with worse overall disease-specific survival (66.1 vs 87.8 months, P = 0.021).35
A new PASD1 immune response was described in a melanoma patient who achieved complete remission after irradiation as part of an abscopal effect.36 Similarly, we found PASD1 expression in the skin tumor sample on each ovarian cancer array providing a positive control, indicating that the PASD1 antibodies were working on the paraffin-embedded tissues analyzed in this study. Previously, PASD1 expression has been demonstrated in more advanced tumor stages, in some solid tumor cell lines Hn5 (head and neck),11 SW480 (colorectal adenocarcinoma),10 and H1299 (lung cancer).37 Twenty-five of 68 solid tumor tissues expressed PASD1.10 PASD1 has also been found to be expressed in 22/25 cell lines derived from 21 B- and four T-cell malignancies by RT-PCR.38 We now add to this knowledge showing that PASD1 is not expressed in ovarian cancer, particularly at the early stages of the disease. This adds to a growing list of solid tumors (78 basal cell carcinomas),15 which do not appear to express PASD1 at notable levels. We also found that PASD1 was not expressed in healthy or inflamed endometrial tissues indicating a high level of specificity of expression, consistent with its description as a CTA. Other groups have found PASD1 to be expressed in the nuclei of a subpopulation of spermatogonia near the basal membrane in the testicular tubules.16 A feature typical of CTAs with the most restricted expression in healthy tissues.
We believe that it is important to publish well-controlled null data39 so that other groups do not need to repeat these studies and so the true expression of antigens like PASD1 can become clear to the research community. Our search for a CTA that is expressed in ovarian tissues but not healthy tissues continues.
Acknowledgments
We would like to thank Professor Alison Banham and Mrs Linden Lyne for providing the PASD1 antibody.
Abbreviations
- CA125
carbohydrate antigen 125
- CTA
cancer-testis antigens
- ELISA
enzyme-linked immunosorbent assay
- EOC
epithelial ovarian cancer
- IHC
immunohistochemistry
- PASD1
Per ARNT SIM (PAS) domain containing 1
- RT-PCR
reverse transcription-PCR
- TMA
tissue microarrays
Footnotes
ACADEMIC EDITOR: Graham Ball, editorial board member
PEER REVIEW: Eleven peer reviewers contributed to the peer review report. Reviewers’ reports totaled 3,173 words, excluding any confidential comments to the academic editor.
FUNDING: This work was funded by Research Investment Program funding at the University of Bedfordshire. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions were made by an independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: GK, BG. Analyzed the data: GK, SEB, KIM, BG. Wrote the first draft of the manuscript: GK. Contributed to the writing of the manuscript: GK, KIM, BG. Agree with the manuscript results and conclusions: GK, SEB, KIM, BG. Jointly developed the structure and arguments for the paper: GK, KIM, BG. Made critical revisions and approved the final version: KIM, BG. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Target Ovarian Cancer Information about Ovarian Cancer. 2013. [Accessed July 15 2014]. http://www.targetovariancancer.org.uk/landing_page.asp?section=47§ionTitle=Information+about+ovarian+cancer.
- 3.Salvador S, Rempel A, Soslow RA, Gilks B, Huntsman D, Miller D. Chromosomal instability in fallopian tube precursor lesions of serous carcinoma and frequent monoclonality of synchronous ovarian and fallopian tube mucosal serous carcinoma. Gynecol Oncol. 2008;110(3):408–417. doi: 10.1016/j.ygyno.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 4.Dietl J. Revisiting the pathogenesis of ovarian cancer: the central role of the fallopian tube. Arch Gynecol Obstet. 2014;289(2):241–246. doi: 10.1007/s00404-013-3041-3. [DOI] [PubMed] [Google Scholar]
- 5.McMeekin DS, Burger RA, Manetta A, DiSaia P, Berman ML. Endometrioid adenocarcinoma of the ovary and its relationship to endometriosis. Gynecol Oncol. 1995;59(1):81–86. doi: 10.1006/gyno.1995.1271. [DOI] [PubMed] [Google Scholar]
- 6.Ledermann JA, Raja FA, Fotopoulou C, Gonzalez-Martin A, Colombo N, Sessa C, ESMO Guidelines Working Group Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(suppl 6):vi24–vi32. doi: 10.1093/annonc/mdt333. [DOI] [PubMed] [Google Scholar]
- 7.Maringe C, Walters S, Butler J, et al. ICBP Module 1 Working Group Stage at diagnosis and ovarian cancer survival: evidence from the International Cancer Benchmarking Partnership. Gynecol Oncol. 2012;127(1):75–82. doi: 10.1016/j.ygyno.2012.06.033. [DOI] [PubMed] [Google Scholar]
- 8.Karlsen MA, Høgdall EV, Christensen IJ, et al. A novel diagnostic index combining HE4, CA125 and age may improve triage of women with suspected ovarian cancer—an international multicenter study in women with an ovarian mass. Gynecol Oncol. 2015 doi: 10.1016/j.ygyno.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 9.Chen YT, Scanlan MJ, Sahin U, et al. A testicular antigen aberrantly expressed in human cancers detected by autologous antibody screening. Proc Natl Acad Sci U S A. 1997;94(5):1914–1918. doi: 10.1073/pnas.94.5.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liggins AP, Brown PJ, Asker K, Pulford K, Banham AH. A novel diffuse large B-cell lymphoma-associated cancer testis antigen encoding a PAS domain protein. Br J Cancer. 2004;91(1):141–149. doi: 10.1038/sj.bjc.6601875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guinn BA, Bland EA, Lodi U, et al. Humoral detection of leukaemia-associated antigens in presentation acute myeloid leukaemia. Biochem Biophys Res Commun. 2005;335(4):1293–1304. doi: 10.1016/j.bbrc.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 12.Sahin U, Türeci O, Schmitt H, et al. Human neoplasms elicit multiple specific immune responses in the autologous host. Proc Natl Acad Sci U S A. 1995;92(25):11810–11813. doi: 10.1073/pnas.92.25.11810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sahota SS, Goonewardena CM, Cooper CD, et al. PASD1 is a potential multiple myeloma-associated antigen. Blood. 2006;108(12):3953–3955. doi: 10.1182/blood-2006-04-014621. [DOI] [PubMed] [Google Scholar]
- 14.Michael AK, Harvey SL, Sammons PJ, et al. Cancer/testis antigen PASD1 silences the circadian clock. Mol Cell. 2015;58(5):743–754. doi: 10.1016/j.molcel.2015.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghafouri-Fard S, Abbasi A, Moslehi H, et al. Elevated expression levels of testis-specific genes TEX101 and SPATA19 in basal cell carcinoma and their correlation with clinical and pathological features. Br J Dermatol. 2010;162(4):772–779. doi: 10.1111/j.1365-2133.2009.09568.x. [DOI] [PubMed] [Google Scholar]
- 16.Cooper CD, Liggins AP, Ait-Tahar K, Roncador G, Banham AH, Pulford K. PASD1, a DLBCL-associated cancer testis antigen and candidate for lymphoma immunotherapy. Leukemia. 2006;20(12):2172–2174. doi: 10.1038/sj.leu.2404424. [DOI] [PubMed] [Google Scholar]
- 17.Lozzio CB, Lozzio BB. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975;45(3):321–334. [PubMed] [Google Scholar]
- 18.Scherer WF, Syverton JT, Gey GO. Studies on the propagation in vitro of poliomyelitis viruses. IV. Viral multiplication in a stable strain of human malignant epithelial cells (strain HeLa) derived from an epidermoid carcinoma of the cervix. J Exp Med. 1953;97(5):695–710. doi: 10.1084/jem.97.5.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leibovitz A, Stinson JC, McCombs WB, III, McCoy CE, Mazur KC, Mabry ND. Classification of human colorectal adenocarcinoma cell lines. Cancer Res. 1976;36(12):4562–4569. [PubMed] [Google Scholar]
- 20.Carey TE, Takahashi T, Resnick LA, Oettgen HF, Old LJ. Cell surface antigens of human malignant melanoma: mixed hemadsorption assays for humoral immunity to cultured autologous melanoma cells. Proc Natl Acad Sci U S A. 1976;73(9):3278–3282. doi: 10.1073/pnas.73.9.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fogh J. Human Tumor Cells In Vitro. New York, NY: Plenum Press; 1975. [Google Scholar]
- 22.Hamilton TC, Young RC, McKoy WM, et al. Characterization of a human ovarian carcinoma cell line (NIH:OVCAR-3) with androgen and estrogen receptors. Cancer Res. 1983;43(11):5379–5389. [PubMed] [Google Scholar]
- 23.Hamilton TC, Young RC, Ozols RF. Experimental model systems of ovarian cancer: applications to the design and evaluation of new treatment approaches. Semin Oncol. 1984;11(3):285–298. [PubMed] [Google Scholar]
- 24.Ait-Tahar K, Liggins AP, Collins GP, et al. Cytolytic T-cell response to the PASD1 cancer testis antigen in patients with diffuse large B-cell lymphoma. Br J Haematol. 2009;146(4):396–407. doi: 10.1111/j.1365-2141.2009.07761.x. [DOI] [PubMed] [Google Scholar]
- 25.Garg M, Chaurasiya D, Rana R, et al. Sperm-associated antigen 9, a novel cancer testis antigen, is a potential target for immunotherapy in epithelial ovarian cancer. Clin Cancer Res. 2007;13(5):1421–1428. doi: 10.1158/1078-0432.CCR-06-2340. [DOI] [PubMed] [Google Scholar]
- 26.Tammela J, Uenaka A, Ono T, et al. OY-TES-1 expression and serum immunoreactivity in epithelial ovarian cancer. Int J Oncol. 2006;29(4):903–910. [PubMed] [Google Scholar]
- 27.Odunsi K, Jungbluth AA, Stockert E, et al. NY-ESO-1 and LAGE-1 cancer-testis antigens are potential targets for immunotherapy in epithelial ovarian cancer. Cancer Res. 2003;63(18):6076–6083. [PubMed] [Google Scholar]
- 28.Straughn JM, Jr, Shaw DR, Guerrero A, et al. Expression of sperm protein 17 (Sp17) in ovarian cancer. Int J Cancer. 2004;108(6):805–811. doi: 10.1002/ijc.11617. [DOI] [PubMed] [Google Scholar]
- 29.Valmori D, Qian F, Ayyoub M, et al. Expression of synovial sarcoma X (SSX) antigens in epithelial ovarian cancer and identification of SSX-4 epitopes recognized by CD4+ T cells. Clin Cancer Res. 2006;12(2):398–404. doi: 10.1158/1078-0432.CCR-05-1902. [DOI] [PubMed] [Google Scholar]
- 30.Sharma S, Qian F, Keitz B, et al. A-kinase anchoring protein 3 messenger RNA expression correlates with poor prognosis in epithelial ovarian cancer. Gynecol Oncol. 2005;99(1):183–188. doi: 10.1016/j.ygyno.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 31.Vineis P, Masala G, Costantini AS. Does a gene in the Xq28 region increase the risk of non-Hodgkin’s lymphomas? Working Group for the epidemiology of hematolymphopoietic malignancies in Italy. Ann Oncol. 1999;10(4):471–473. doi: 10.1023/a:1008359604717. [DOI] [PubMed] [Google Scholar]
- 32.Bischof P, Tseng L, Brioschi PA, Herrmann WL. Cancer antigen 125 is produced by human endometrial stromal cells. Hum Reprod. 1986;1(7):423–426. doi: 10.1093/oxfordjournals.humrep.a136445. [DOI] [PubMed] [Google Scholar]
- 33.Zhen S, Bian LH, Chang LL, Gao X. Comparison of serum human epididymis protein 4 and carbohydrate antigen 125 as markers in ovarian cancer: a meta-analysis. Mol Clin Oncol. 2014;2(4):559–566. doi: 10.3892/mco.2014.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim HS, Choi HY, Lee M, et al. Systemic inflammatory response markers and CA-125 levels in ovarian clear cell carcinoma: a two center cohort study. Cancer Res Treat. 2015 doi: 10.4143/crt.2014.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Myriokefalitaki E, Vorgias G, Vlahos G, Rodolakis A. Prognostic value of preoperative Ca125 and Tag72 serum levels and their correlation to disease relapse and survival in endometrial cancer. Arch Gynecol Obstet. 2015 doi: 10.1007/s00404-015-3675-4. In Press. [DOI] [PubMed] [Google Scholar]
- 36.Stamell EF, Wolchok JD, Gnjatic S, Lee NY, Brownell I. The abscopal effect associated with a systemic anti-melanoma immune response. Int J Radiat Oncol Biol Phys. 2013;85(2):293–295. doi: 10.1016/j.ijrobp.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hardwick N, Buchan S, Ingram W, et al. An analogue peptide from the cancer/testis antigen PASD1 induces CD8+ T cell responses against naturally processed peptide. Cancer Immun. 2013;13:16. [PMC free article] [PubMed] [Google Scholar]
- 38.Liggins AP, Lim SH, Soilleux EJ, Pulford K, Banham AH. A panel of cancer-testis genes exhibiting broad-spectrum expression in haematological malignancies. Cancer Immun. 2010;10:8. [PMC free article] [PubMed] [Google Scholar]
- 39.Guinn BA. The future of publishing scientific data: is it time to accept the wider publication of null data? EC Cancer. 2014;1:1–2. [Google Scholar]
- 40.Chen C, Liu J, Xu G. Overexpression of PIWI proteins in human stage III epithelial ovarian cancer with lymph node metastasis. Cancer Biomark. 2013;13(5):315–321. doi: 10.3233/CBM-130360. [DOI] [PubMed] [Google Scholar]
- 41.Yakirevich E, Sabo E, Lavie O, Mazareb S, Spagnoli GC, Resnick MB. Expression of the MAGE-A4 and NY-ESO-1 cancer-testis antigens in serous ovarian neoplasms. Clin Cancer Res. 2003;9(17):6453–6460. [PubMed] [Google Scholar]
- 42.Gillespie AM, Rodgers S, Wilson AP, et al. MAGE, BAGE and GAGE: tumour antigen expression in benign and malignant ovarian tissue. Br J Cancer. 1998;78(6):816–821. doi: 10.1038/bjc.1998.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kawagoe H, Yamada A, Matsumoto H, et al. Serum MAGE-4 protein in ovarian cancer patients. Gynecol Oncol. 2000;76(3):336–339. doi: 10.1006/gyno.1999.5701. [DOI] [PubMed] [Google Scholar]
- 44.Hofmann M, Ruschenburg I. mRNA detection of tumor-rejection genes BAGE, GAGE, and MAGE in peritoneal fluid from patients with ovarian carcinoma as a potential diagnostic tool. Cancer. 2002;96(3):187–193. doi: 10.1002/cncr.10622. [DOI] [PubMed] [Google Scholar]
- 45.Tureci O, Chen YT, Sahin U, et al. Expression of SSX genes in human tumors. Int J Cancer. 1998;77(1):19–23. doi: 10.1002/(sici)1097-0215(19980703)77:1<19::aid-ijc4>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 46.Tammela J, Jungbluth AA, Qian F, et al. SCP-1 cancer/testis antigen is a prognostic indicator and a candidate target for immunotherapy in epithelial ovarian cancer. Cancer Immun. 2004;4:10. [PubMed] [Google Scholar]