Abstract
Objective
We investigated the hypothesis that rimonabant, a cannabinoid antagonist/inverse agonist, would increase anxiety in healthy subjects during a simulation of the public speaking test.
Methods
Participants were randomly allocated to receive oral placebo or 90 mg rimonabant in a double-blind design. Subjective effects were measured by Visual Analogue Mood Scale. Physiological parameters, namely arterial blood pressure and heart rate, also were monitored.
Results
Twelve participants received oral placebo and 12 received 90 mg rimonabant. Rimonabant increased self-reported anxiety levels during the anticipatory speech and performance phase compared with placebo. Interestingly, rimonabant did not modulate anxiety prestress and was not associated with sedation, cognitive impairment, discomfort, or blood pressure changes.
Conclusions
Cannabinoid-1 antagonism magnifies the responses to an anxiogenic stimulus without interfering with the prestress phase. These data suggest that the endocannabinoid system may work on-demand to counteract the consequences of anxiogenic stimuli in healthy humans.
Keywords: anxiety, public speaking test, rimonabant, SR141716, CB1 receptor
INTRODUCTION
Cannabis sativa induces multiple subjective effects including pleasure, relaxation, and anxiety relief (Hall and Solowij, 1998; Hall and Degenhardt, 2009; Zuardi et al., 2010). The growing understanding of endocannabinoid function raised interest in this system as a target for new drugs. In addition to synthetic cannabinoids (CBs), several CB1 receptor antagonists were developed, from which SR141716 (rimonabant) is the prototype (Engeli, 2012; Kirilly et al., 2012).
Studies focusing on anxiety-related responses revealed that delta-9-tetrahydrocannabinol (THC) and other CBs induce complex effects, depending on dose, environment, and subjects’ previous experience (Viveros et al., 2005; Moreira and Wotjak, 2010). Endocannabinoid hydrolysis inhibitors generally induce anxiolytic effects (Kathuria et al., 2003; Patel and Hillard, 2006; Moreira et al., 2008; Haller et al., 2009). On the other hand, CB1 antagonists tend to increase anxiety-like behaviors, particularly in animals exposed to a highly aversive environment (Haller et al., 2004; Patel and Hillard, 2006), indicating that the endocannabinoid system may inhibit anxiety and fear responses, working on-demand to counteract consequences of highly-aversive stimuli (Moreira and Wotjak, 2010; Riebe et al., 2012). The limited available data emerged mainly after clinical investigation with rimonabant, which was removed from the market due to psychiatric side effects characterized by feelings of anxiety and depression (Christensen et al., 2007; Moreira and Crippa, 2009). Rimonabant's clinical profile suggests that blocking endocannabinoid actions increases anxiety, but this observation is confounded by the fact that psychiatric disorders are frequent comorbidities in the obesity (McIntyre et al., 2012). Therefore, in order to establish the role of CB1 receptors in anxiety modulation in humans, experimental studies are needed.
We evaluated the hypothesis that rimonabant would increase anxiety in healthy humans exposed to the simulation of the public speaking test. This model induces anxiety and is sensitive to both anxiolytic and anxiogenic drugs (McNair et al., 1982; Guimaraes et al., 1987; Bergamaschi et al., 2011). We analyzed drug effects at baseline and during the test, in order to investigate whether CB1 blockade selectively modified responses under high-anxiety levels.
METHODS
Subjects
Healthy participants were selected through a screening procedure described in the next section. Subjects were randomly allocated to receive placebo or 90 mg rimonabant in a double-blind study design. Groups were matched according to gender, age, years of education, socioeconomic status, body mass index, fear of public speaking [social phobia inventory; (Connor et al., 2000)] and general trait anxiety {Beck anxiety inventory [BAI]; (Beck et al., 1988; Cunha, 2001)}. No subject had a history of head trauma, neurological and psychiatric illness, substance abuse or major medical illnesses, and general medical condition based on a semi-standardized medical questionnaire and physical examination. Participants were all non-tobacco smokers and had not taken any prescribed medication for at least 3 months prior to the study. Subjects self-reported no cannabis or any other illegal drug use in their life. Women were required to have a negative pregnancy test prior to admission. Subjects provided a written informed consent after being fully informed about research procedures that were approved by the local institutional review board (HCRP No. 12407/2009), in accordance with the Declaration of Helsinki.
Screening procedure and clinical assessment
Participants, recruited by telephone and printed advertisements, were screened through self-assessment diagnostic instruments. Participants who scored BAI <10, fast alcohol screening test <3 (Hodgson et al., 2002; Meneses-Gaya et al., 2010), and Patient Health Questionnaire-9 < 10 (Lowe et al., 2004; de Lima Osorio et al., 2009), were invited to attend an interview for diagnosis absence through the full structured clinical interview for the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, clinical version (First et al., 1997), translated into Portuguese (Del-Ben et al., 2001), by one examiner familiar with the instrument.
Drug preparation
Rimonabant (90 mg; Acomplia®, Sanofi-Aventis, Brazil) or wheat flour (placebo) were administered inside an identical gelatin capsules. Rimonabant dose was selected based on previous studies demonstrating that this was the minimum required dose to acutely block the CB1 receptor (Huestis et al., 2001; Gorelick et al., 2006), and participants who received rimonabant only reported no significant physiological or psychological effects compared with other groups, attesting that this 90 mg dose is safe in humans in controlled clinical study.
Psychological and physiological measurements
The state-anxiety level and other subjective states were evaluated during the test through the Visual Analogue Mood Scale (VAMS) Portuguese version (Norris, 1971; Zuardi and Karniol, 1981), grouped into four factors: (i) anxiety; (ii) sedation; (iii) cognitive impairment; and (iv) discomfort (Hallak et al., 2010). Systolic blood pressure, diastolic blood pressure, and heart rate (HR) were measured by multiparametric monitor (Monitor DX 2022, Dixtal, Brazil).
Procedure
The simulation of the public speaking test was the same as used by McNair et al. (McNair et al., 1982) with minor modifications (Hallak et al., 2010; Bergamaschi et al., 2011). Each subject participated in only one experimental session in a double-blind design. Subjects were told to have a light breakfast 2 h prior to the session. The experimental session was conducted in a sound attenuated and temperature-controlled room beginning at 08:00. After a 15-min adaptation period, baseline measurements (B) were taken followed by a single 90 mg rimonabant or placebo dose in a double-blind randomized procedure. Prestress measurements (P) were made 2 h after drug ingestion. Immediately, thereafter, the subject received instructions and had 2 min to prepare a 4-min speech about ‘the public transportation system of your city’. He/she also was told that the speech would be recorded on videotape and later analyzed by a psychologist. Anticipatory speech measurements (A) were taken before the subject started speaking. When speaking before the camera began, subjects viewed their own image on the television screen. The speech was interrupted in the middle and speech performance measurements (S) obtained. The speech was recorded for additional 2 min. Poststress measurements (F1 and F2) were made 15 and 35 min after the end of the speech, respectively. Participants were monitored 1 week and 1, 3, and 6 months after study procedure to assess occurrence of depressive symptoms.
Statistical analysis
Clinical and demographic characteristics were investigated via boxplot and Shapiro–Wilk normality tests; therefore, they were analyzed by nonparametric tests (gender, socioeconomic level, BAI, and social phobia inventory) and by analysis of variance (ANOVA) for one factor, (age and body mass index). VAMS scores, diastolic and systolic pressures and HR were calculated as previously described (Bergamaschi et al., 2011). The two treatments were compared in each phase, taking into account baseline values. Statistical tests were conducted with SPSS version 19.0 (Chicago, IL, USA) and considered significant if two-tailed p < 0.05.
RESULTS
Twenty four subjects enrolled in the study. Participants’ clinical and demographic characteristics are shown in Table 1. No significant differences were observed between groups.
Table 1.
Clinical and demographic characteristics of participant groups
| Placebo | Rimonabant | p | |
|---|---|---|---|
| Male/female | 6/6 | 6/6 | 1.00 |
| Age [mean (SD)] | 24.5 (4.9) | 24.9 (3.7) | 0.82 |
| Socioeconomic levela [Median (range)] | 2.5 (1.0–3.0) | 2.0 (1.0–4.0) | 0.93 |
| Body mass index [BMI, mean (SD)] | 23.8 (4.6) | 23.4 (3.3) | 0.85 |
| Social Phobia Inventory [SPIN, mean (SD)] | 4.3 (3.1) | 6.8 (5.9) | 0.34 |
| Beck Inventory Anxiety [BAI, mean (SD)] | 2.0 (1.7) | 3.9 (3.4) | 0.20 |
SD, standard deviation;
Socioeconomic level was assessed by the Brazil Socioeconomic Classification Criteria.
Psychological measures
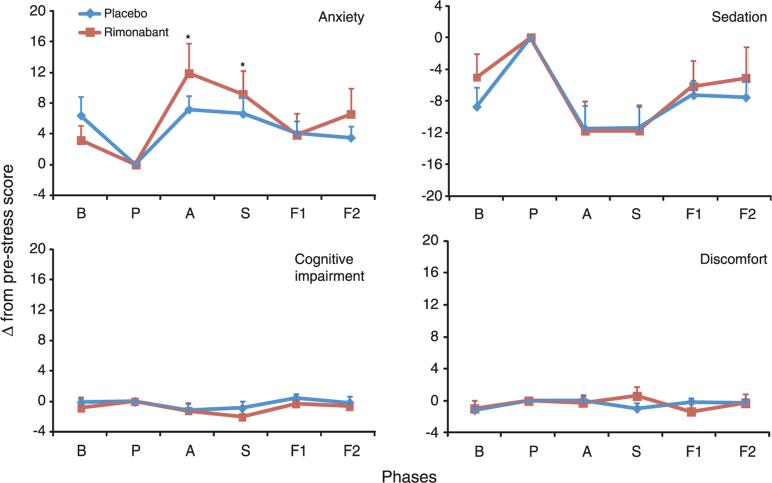
Repeated-measures ANOVA for the VAMS anxiety factor showed a significant effect of phases (F3.17, 69.68 = 9.81; p < 0.0001) and phase by group interaction between baseline and anticipatory speech (F1, 22 = 4.53; p = 0.045) and baseline and performance measurements (F1, 22 = 4.36; p = 0.049). VAMS sedation factor showed only a significant effect of phase (F3.80, 83.59 = 11.62; p < 0.0001), and no significant effects of phase and phase by group interaction were observed in the VAMS cognitive impairment and discomfort factors (Figure 1). The VAMS’ item ‘happy–sad’ was used to assess depression symptom during study procedure and showed no significant difference of phase by group interaction (F3.89, 85.51 = 1.40, p = 0.243). Participants were monitored for up to 6 months and reported no depressive symptoms.
Figure 1.
Changes in Visual Analogue Mood Scale factors induced by simulation of the public speaking test. B, baseline; P, prestress; A, anticipatory speech; S, speech performance; F1, poststress 1; and F2, poststress 2. Points indicate mean and vertical bars indicate standard error of the mean. *Indicates significant differences from placebo group (p < 0.05)
Physiological measures
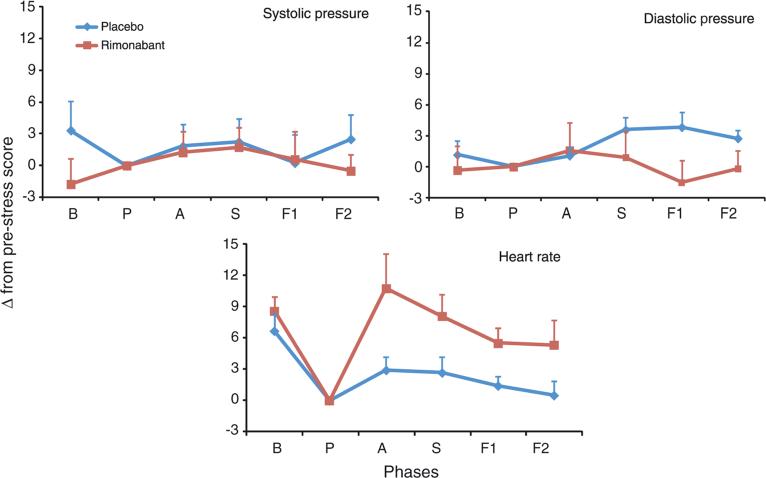
Systolic and diastolic pressure did not show significant repeated-measures ANOVA effect in phases and phase by group interaction. HR showed a significant effect of phase (F3.97, 87.31 = 6.46; p < 0.0001) (Figure 2).
Figure 2.
Changes in heart rate, systolic, and diastolic pressure induced by simulation of public speaking test. B, baseline; P, prestress; A, anticipatory speech; S, speech performance; F1, poststress 1; and F2, poststress 2. Points indicate mean and vertical bars indicate standard error of the mean
DISCUSSION
This study documents that the CB1 receptor antagonist/inverse agonist, rimonabant, increases anxiety induced by public speaking in healthy humans. The anxiogenic effects occurred selectively during anticipatory and performance speech, without interfering with the prestress phase, meaning that the drug effects occurred selectively in response to an aversive situation. Endocannabinoids implication with social anxiety is in accordance with dense expression of CB1 receptors in brain regions related to anxiety, fear, and aversion, including the medial prefrontal cortex, hippocampus, amygdala, and periaqueductal gray (Howlett et al., 2002; Mackie, 2005).
Preclinical studies showed that anxiogenic-like effects of CB1 antagonists tend to be more evident when animals are subjected to high levels of aversion (Haller et al., 2004; Jacob et al., 2012). Anandamide-hydrolysis inhibitors are more efficacious as anxiolytic drugs, when tested in a highly aversive environment (Naidu et al., 2007; Haller et al., 2009). The basal levels of endocannabinoid synthesis and release tend to be low; however, the activity of this system is enhanced in response to neural activation when experimental animals are exposed to threatening stimuli, when endocannabinoids would work to counteract fear responses (Moreira and Wotjak, 2010; Riebe et al., 2012). This would explain why CB1 antagonists tend to modify behavioral responses preferentially under high levels of aversion, without significant baseline effects.
An experimental study with healthy volunteers revealed that rimonabant reduced incidental recall of positive self-relevant adjectives (Horder et al., 2009). The role for the endocannabinoid system in anxiety emerged primarily from clinical trials of rimonabant's effect on obesity and related metabolic disorders treatment (RIO Studies). These investigations revealed that anxiety and depression are important side effects of this drug, as compared with placebo (Christensen et al., 2007). The absence of significant difference on the VAMS’ item ‘happy–sad’ is in accordance with previous studies that showed acute 90 mg rimonabant administration was well tolerated and no serious adverse events (Huestis et al., 2007), and chronic and multiple rimonabant intake increase depression incidence (Christensen et al., 2007; Mitchell and Morris, 2007).
The present work indirectly suggests that facilitating CB1 receptor signaling may alleviate the consequences of aversive stimuli with important implication in the treatment of psychiatric disorders. Rimonabant increased self-reported anxiety induced by public speaking in healthy subjects, without interfering with prestress levels, supporting the notion that the endocannabinoid system may work on-demand to counteract the consequences of aversive stimuli. Additional double-blind, placebo controlled trials are desirable to determine the precise endocannabinoids mechanism in anxiety and anxiety disorders.
ACKNOWLEDGMENTS
The authors acknowledge Sandra Bernardo and Selma Pontes for their clinical support. MMB was supported by Fundação de Amparo a Pesquisa do Estado de São Paulo (grant number 2009/11805-4). JASC (1B), AWZ (1C), JECH (1D) and AEN (1A) are the recipients of a CNPq (Brazil) fellowship award.
Footnotes
CONFLICT OF INTEREST
The authors have declared no conflict of interest.
REFERENCES
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Bergamaschi MM, Queiroz RH, Chagas MH, et al. Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-naive social phobia patients. Neuropsychopharmacology. 2011;36:1219–1226. doi: 10.1038/npp.2011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen R, Kristensen PK, Bartels EM, Bliddal H, Astrup A. Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet. 2007;370:1706–1713. doi: 10.1016/S0140-6736(07)61721-8. [DOI] [PubMed] [Google Scholar]
- Connor KM, Davidson JR, Churchill LE, Sherwood A, Foa E, Weisler RH. Psychometric properties of the Social Phobia Inventory (SPIN). New self-rating scale. Br J Psychiatry. 2000;176:379–386. doi: 10.1192/bjp.176.4.379. [DOI] [PubMed] [Google Scholar]
- Cunha JA. Manual da versão em português das escalas Beck. Casa do Psicólogo; São Paulo: 2001. [Google Scholar]
- Del-Ben CM, Vilela JaA, Crippa JaS, Hallak JEC, Labate CM, Zuardi AW. Confiabilidade da “Entrevista Clínica Estruturada para o DSM-IV - Versão Clínica” traduzida para o português. Rev Bras Psiquiatr. 2001;23:156–159. [Google Scholar]
- Engeli S. Central and peripheral cannabinoid receptors as therapeutic targets in the control of food intake and body weight. Handb Exp Pharmacol. 2012;209:357–381. doi: 10.1007/978-3-642-24716-3_17. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM–IV Axis I Disorders-Clinician Version (SCID-CV) DC, American Psychiatric Press; Washington: 1997. [Google Scholar]
- Gorelick DA, Heishman SJ, Preston KL, Nelson RA, Moolchan ET, Huestis MA. The cannabinoid CB1 receptor antagonist rimonabant attenuates the hypotensive effect of smoked marijuana in male smokers. Am Heart J. 2006;151:754 e1–754 e5. doi: 10.1016/j.ahj.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Guimaraes FS, Zuardi AW, Graeff FG. Effect of chlorimipramine and maprotiline on experimental anxiety in humans. J Psychopharmacol. 1987;1:184–192. doi: 10.1177/026988118700100305. [DOI] [PubMed] [Google Scholar]
- Hall W, Degenhardt L. Adverse health effects of non-medical cannabis use. The Lancet. 2009;374:1383–1391. doi: 10.1016/S0140-6736(09)61037-0. [DOI] [PubMed] [Google Scholar]
- Hall W, Solowij N. Adverse effects of cannabis. Lancet. 1998;352:1611–1616. doi: 10.1016/S0140-6736(98)05021-1. [DOI] [PubMed] [Google Scholar]
- Hallak JE, Crippa JA, Quevedo J, et al. National Science and Technology Institute for Translational Medicine (INCT-TM): advancing the field of translational medicine and mental health. Revista Brasileira De Psiquiatria. 2010;32:83–90. doi: 10.1590/s1516-44462010000100016. [DOI] [PubMed] [Google Scholar]
- Haller J, Varga B, Ledent C, Barna I, Freund TF. Context-dependent effects of CB1 cannabinoid gene disruption on anxiety-like and social behaviour in mice. Eur J Neurosci. 2004;19:1906–1912. doi: 10.1111/j.1460-9568.2004.03293.x. [DOI] [PubMed] [Google Scholar]
- Haller J, Barna I, Barsvari B, et al. Interactions between environmental aversiveness and the anxiolytic effects of enhanced cannabinoid signaling by FAAH inhibition in rats. Psychopharmacology (Berl) 2009;204:607–616. doi: 10.1007/s00213-009-1494-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson R, Alwyn T, John B, Thom B, Smith A. The FAST alcohol screening test. Alcohol Alcohol. 2002;37:61–66. doi: 10.1093/alcalc/37.1.61. [DOI] [PubMed] [Google Scholar]
- Horder J, Cowen PJ, Di Simplicio M, Browning M, Harmer CJ. Acute administration of the cannabinoid CB1 antagonist rimonabant impairs positive affective memory in healthy volunteers. Psychopharmacology (Berl) 2009;205:85–91. doi: 10.1007/s00213-009-1517-4. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Barth F, Bonner TI, et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- Huestis MA, Gorelick DA, Heishman SJ, et al. Blockade of effects of smoked marijuana by the CB1-selective cannabinoid receptor antagonist SR141716. Arch Gen Psychiatry. 2001;58:322–330. doi: 10.1001/archpsyc.58.4.322. [DOI] [PubMed] [Google Scholar]
- Huestis MA, Boyd SJ, Heishman SJ, et al. Single and multiple doses of rimonabant antagonize acute effects of smoked cannabis in male cannabis users. Psychopharmacology (Berl) 2007;194:505–515. doi: 10.1007/s00213-007-0861-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob W, Marsch R, Marsicano G, Lutz B, Wotjak CT. Cannabinoid CB1 receptor deficiency increases contextual fear memory under highly aversive conditions and long-term potentiation in vivo. Neurobiol Learn Mem. 2012;98:47–55. doi: 10.1016/j.nlm.2012.04.008. [DOI] [PubMed] [Google Scholar]
- Kathuria S, Gaetani S, Fegley D, et al. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- Kirilly E, Gonda X, Bagdy G. CB1 receptor antagonists: new discoveries leading to new perspectives. Acta Physiol (Oxf) 2012;205:41–60. doi: 10.1111/j.1748-1716.2012.02402.x. [DOI] [PubMed] [Google Scholar]
- de Lima Osorio F, Vilela Mendes A, Crippa JA, Loureiro SR. Study of the discriminative validity of the PHQ-9 and PHQ-2 in a sample of Brazilian women in the context of primary health care. Perspect Psychiatr Care. 2009;45:216–227. doi: 10.1111/j.1744-6163.2009.00224.x. [DOI] [PubMed] [Google Scholar]
- Lowe B, Unutzer J, Callahan CM, Perkins AJ, Kroenke K. Monitoring depression treatment outcomes with the patient health questionnaire-9. Med Care. 2004;42:1194–1201. doi: 10.1097/00005650-200412000-00006. [DOI] [PubMed] [Google Scholar]
- Mackie K. Distribution of cannabinoid receptors in the central and peripheral nervous system. Handb Exp Pharmacol. 2005;168:299–325. doi: 10.1007/3-540-26573-2_10. [DOI] [PubMed] [Google Scholar]
- Mcintyre RS, Alsuwaidan M, Goldstein BI, et al. The Canadian Network for Mood and Anxiety Treatments (CANMAT) task force recommendations for the management of patients with mood disorders and comorbid metabolic disorders. Ann Clin Psychiatry. 2012;24:69–81. [PubMed] [Google Scholar]
- McNair DM, Frankenthaler LM, Czerlinsky T, White TW, Sasson S, Fisher S. Simulated public speaking as a model of clinical anxiety. Psychopharmacology (Berl) 1982;77:7–10. doi: 10.1007/BF00436092. [DOI] [PubMed] [Google Scholar]
- Meneses-Gaya C, Crippa JA, Zuardi AW, et al. The fast alcohol screening test (FAST) is as good as the AUDIT to screen alcohol use disorders. Subst Use Misuse. 2010;45:1542–1557. doi: 10.3109/10826081003682206. [DOI] [PubMed] [Google Scholar]
- Mitchell PB, Morris MJ. Depression and anxiety with rimonabant. Lancet. 2007;370:1671–1672. doi: 10.1016/S0140-6736(07)61705-X. [DOI] [PubMed] [Google Scholar]
- Moreira FA, Crippa JA. The psychiatric side-effects of rimonabant. Revista Brasileira De Psiquiatria. 2009;31:145–153. doi: 10.1590/s1516-44462009000200012. [DOI] [PubMed] [Google Scholar]
- Moreira FA, Wotjak CT. Cannabinoids and anxiety. Curr Top Behav Neurosci. 2010;2:429–450. doi: 10.1007/7854_2009_16. [DOI] [PubMed] [Google Scholar]
- Moreira FA, Kaiser N, Monory K, Lutz B. Reduced anxiety-like behaviour induced by genetic and pharmacological inhibition of the endocannabinoid-degrading enzyme fatty acid amide hydrolase (FAAH) is mediated by CB1 receptors. Neuropharmacology. 2008;54:141–150. doi: 10.1016/j.neuropharm.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Naidu PS, Varvel SA, Ahn K, Cravatt BF, Martin BR, Lichtman AH. Evaluation of fatty acid amide hydrolase inhibition in murine models of emotionality. Psychopharmacology (Berl) 2007;192:61–70. doi: 10.1007/s00213-006-0689-4. [DOI] [PubMed] [Google Scholar]
- Norris H. The action of sedatives on brain stem oculomotor systems in man. Neuropharmacology. 1971;10:181–191. doi: 10.1016/0028-3908(71)90039-6. [DOI] [PubMed] [Google Scholar]
- Patel S, Hillard CJ. Pharmacological evaluation of cannabinoid receptor ligands in a mouse model of anxiety: further evidence for an anxiolytic role for endogenous cannabinoid signaling. J Pharmacol Exp Ther. 2006;318:304–311. doi: 10.1124/jpet.106.101287. [DOI] [PubMed] [Google Scholar]
- Riebe CJ, Pamplona FA, Kamprath K, Wotjak CT. Fear relief-toward a new conceptual frame work and what endocannabinoids gotta do with it. Neuroscience. 2012;204:159–185. doi: 10.1016/j.neuroscience.2011.11.057. [DOI] [PubMed] [Google Scholar]
- Viveros MP, Marco EM, File SE. Endocannabinoid system and stress and anxiety responses. Pharmacol Biochem Behav. 2005;81:331–342. doi: 10.1016/j.pbb.2005.01.029. [DOI] [PubMed] [Google Scholar]
- Zuardi AW, Karniol IG. Transcultural evaluation of a self-evaluation scale of subjective states. J Bras Psiquiatr. 1981;131:403–406. [Google Scholar]
- Zuardi AW, Crippa JA, Hallak JE. Cannabis sativa: the plant that can induce unwanted effects and also treat them. Rev Bras Psiquiatr. 2010;32:S1–S2. [PubMed] [Google Scholar]