Abstract
Background
Treatment-related cardiac death is the primary non-cancer cause of mortality in adult survivors of childhood malignancies. Early detection of cardiac dysfunction using modern echocardiographic techniques may identify a high risk subset of survivors for early intervention.
Objective
To determine the prevalence of cardiac dysfunction in adult survivors of childhood malignancies using state of the art echocardiographic evaluation of cardiac function including strain imaging
Methods
Echocardiographic assessment included three dimensional (3D) left ventricular ejection fraction (LVEF), global longitudinal and circumferential myocardial strain and diastolic function, graded per American Society of Echocardiography (ASE) guidelines on 1,820 adult (median age 31 [range 18-65] years) survivors of childhood cancer (median time from diagnosis 23 years [range10-48] years) exposed to either anthracycline chemotherapy (N=1,050), chest-directed radiotherapy (RT, N=306), or both therapies (N=464).
Results
Only 5.8% of survivors had an abnormal 3D LVEF (<50%). However, 32.1% of survivors with a normal 3D LVEF had evidence for cardiac dysfunction by either global longitudinal strain (28.0%), ASE graded diastolic assessment (8.7%), or both. Abnormal global longitudinal strain was associated with chest-directed RT (1-19.9 Gy, Rate Ratio (RR) 1.38, 95% Confidence Interval (CI) 1.14-1.66; 20-29.9 Gy, RR 1.65, 95% CI 1.31-2.08; >30 Gy, RR 2.39, 95% CI 1.79-3.18) and anthracycline dose >300 mg/m2 (RR 1.72, 95% CI 1.31-2.26). Survivors with metabolic syndrome were twice as likely to have abnormal global longitudinal strain (Rate Ratio [RR] 1.94, 95% CI 1.66-2.28) as well as abnormal diastolic function (RR 1.68, 95% CI 1.39-2.03), but not abnormal 3D LVEF (RR 1.07, 95% CI 0.74-1.53).
Conclusions and Relevance
Abnormal global longitudinal strain and abnormal diastolic function are more prevalent than reduced 3D LVEF and are associated with treatment exposure. They may identify a subset of survivors at higher risk for poor clinical cardiac outcome who may benefit from early medical intervention.
Keywords: Childhood Cancer, Survivor, Late effects, Cardiotoxicity, Screening
INTRODUCTION
In the modern era, more than 80% of children and adolescents diagnosed with a malignancy will become long-term cancer survivors.(1,2) However, as these individuals age, it is increasingly clear that the therapies that cured their primary malignancies place them at increased, life-long risk for adverse health conditions.(3-5) Late onset cardiac dysfunction is common, and the attribution of major cardiac events to childhood exposure to chest-directed radiotherapy (RT) and anthracycline chemotherapy is now well-established.(6,7) The cumulative incidence of congestive heart failure by thirty years from diagnosis is 12% for those exposed to both chest-directed RT and anthracycline therapy, and treatment-related cardiac death is the most common non-cancer cause of mortality in this population.(7-9)
Based on this high risk for adult onset cardiac dysfunction, early detection, when intervention can be expected to have the greatest benefit, is warranted.(10) Periodic evaluation by echocardiography is recommended by the Children's Oncology Group Long-Term Follow-Up Guidelines.(11) Left ventricular ejection fraction (LVEF) is the established parameter for evaluation of left ventricular systolic function. However, LVEF is only reliable in detection of differences in LVEF of 10%,(12,13) and often over estimates LVEF in survivors compared to cardiac MRI, the reference standard for LVEF.(14) In addition, at least 47% of heart failure in the general population is diastolic in nature, occurring with a preserved LVEF.(15)
More sensitive screening modalities for LV dysfunction are needed. Reduction in LVEF likely occurs late in the natural history of treatment-related injury as reduction in LVEF may not be overt until a substantial amount of cardiac reserve has been exhausted.(16) Global longitudinal strain is a well validated, reproducible technique for the measurement of LV deformation.(17) In non-cancer populations, reduced global longitudinal strain is a significant, independent predictor of cardiac mortality and major cardiac events, with prognostic value superior to LVEF.(18-20) In populations of adults actively receiving cancer therapy, early reduction in global longitudinal strain predicts subsequent, chemotherapy-related cardiac dysfunction.(21-23) Despite these promising findings, to date, myocardial strain for early detection of cardiac dysfunction has not been systematically evaluated in a large population of aging adult survivors of childhood cancer.
Our objectives were to: 1) determine the prevalence of late-onset cardiac dysfunction in a large population of adult, ten-year survivors of childhood malignancies using state of the art comprehensive echocardiographic evaluation of cardiac function (3D LVEF, myocardial strain imaging and comprehensive diastolic assessment); 2) identify whether abnormal myocardial strain was associated with anthracycline and chest-directed RT dose exposures; and, 3) to identify strain imaging abnormalities in survivors exposed to cardiotoxic therapy and who subsequently developed traditional cardiovascular risk factors and/or metabolic syndrome, a population at very high risk for major cardiac events.(8,24,25)
METHODS
Participants
Patients treated for childhood cancer at St. Jude Children's Research Hospital (SJCRH) who were 18 years of age or older and ten or more years from diagnosis were eligible for the St. Jude Lifetime Cohort Study (SJLIFE). SJLIFE provides lifetime, risk-based longitudinal follow-up for adult survivors of childhood cancer. The current analysis was limited to participants exposed to anthracycline chemotherapy and/or chest-directed RT who underwent a SJLIFE medical assessment including echocardiogram due to their prior exposure to cardiotoxic therapy, and reports on the baseline assessment at entry into the SJLIFE cohort. Participants who completed the SJLIFE survey only (i.e. no campus visit for direct assessment) were excluded. Details of eligibility, recruitment methods and study design have been previously published.(26) Participation involved completion of questionnaires and risk-based medical screening according to the Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers,(11) developed by the Children's Oncology Group. This investigation was approved by the Institutional Review Board at SJCRH.
Outcome Measures
Echocardiograms were performed using a VIVID-7 machine (N=1,750 [96%], General Electric Medical Systems, Milwaukee, WI) or an iE33 (N=70; Phillips Healthcare, Andover, MA). Complete systolic function by 3D echocardiogram with Doppler was performed according to American Society of Echocardiography (ASE) guidelines, (abnormal, LVEF <50%).(27) For the VIVID-7 studies, three apical views were used to obtain speckle tracking-based global longitudinal peak systolic strain and global circumferential strain using standard, commercially available software (EchoPAC PC version 10.0).
Abnormal strain was defined as a value >2SD above the mean using sex-, age- and vendor-specific strain values identified in a normative population.(28) The largest studies in a recent meta-analysis that utilized US data were extremely small (Marwick et al(29), Cleveland Clinic N=97; Saleh et al,(30) Mayo Clinic N=82; Narayanan et al,(31) University of Massachusetts, N=52). Given the known associations between age and sex with strain outcomes, as recently discussed in the Expert Consensus for Multimodal Imaging Evaluation of Adult Patients during and after Cancer Therapy(16), it was clear that these small populations would not allow us to stratify comparisons between our study population and the normative population on these key variables. In particular, our study population is generally younger than these US cohorts. Thus, we determined that use of these small cohorts that do not contain age-, sex- and vendor-specific normative data would be a particular risk to the validity of the study findings. Alternatively, the Japanese Ultrasound Speckle Tracking of Left Ventricle (JUSTICE) Study,(28) evaluated a large population (N=817) and provides age-, and sex-specific normative values, which improve the ability to provide valid comparisons between our cases and the normative standard. While this does raise the possibility that the Japanese and US populations may have different strain values based on race, we were reassured by recent evidence to the contrary based on similar sex- and vendor-specific normative values in a European population.(32)
Diastolic assessment included peak mitral flow velocity (E), mitral septal and annular early diastolic velocity (e’) their ratios with E (E/e’ ratio), and left atrial volume.(33) Diastolic function was graded as per the ASE recommendations for evaluation of left ventricular diastolic function, with any grade 1-3 considered abnormal.(34) All echocardiograms were centrally evaluated by a core echocardiography laboratory at the Cleveland Clinic.
To estimate the inter-observer variability the lead cardiologist for this study, from the Cleveland Clinic Echo core lab (JCP, who read 625 of the 1807 evaluable studies) to review a sample of echos read by each of the other cardiologists. We randomly selected 10 studies from each of the six additional reviewers (60 total studies). Selection was stratified on EF <50% vs. ≥50% to assure there were a sufficient number of both normal and abnormal echos included in the review. Across our major echo outcomes for this manuscript, our overall agreement (normal vs. abnormal) was: EF, 76% agreement; global longitudinal strain, 76%; circumferential strain, 36%; left atrial volume, 95%; septal e’ 60%.
Demographic and Exposure Variables
Cumulative dose of anthracyclines was abstracted from the medical record along with demographic characteristics. Mean radiation dose to the heart was estimated, regardless of primary tumor site or target volume, using the primary RT record and tissue equivalent phantoms as previously described by Stovall et al.(35) Additional covariates included metabolic syndrome and its components. Metabolic syndrome was defined using the Third Report of the National Cholesterol Education Program Adult Treatment Panel (NCEP-ATP III).(36) Individuals having three or more of the following were classified as having metabolic syndrome: 1) abdominal obesity (waist circumference > 102 cm in males and > 88 cm in females); 2) triglycerides ≥ 150 mg/dL or treatment for elevated triglycerides; 3) high density lipoprotein (HDL) cholesterol < 40 mg/dL in males and <50 mg/dL in females; 4) hypertension (systolic blood pressure ≥ 130 mmHg or diastolic ≥ 85 mmHg) or treatment for hypertension; and/or, 5) fasting plasma glucose ≥ 100 mg/dL or medical therapy for diabetes.
Abdominal circumference at the narrowest point between the xiphoid process and the navel was determined with a Gullick tape measure.(37) The measure was repeated twice and recorded to nearest tenth centimeter. The highest abdominal circumference measurement was used for analysis. Resting blood pressure was taken with the participant seated with both feet on the floor following a five minute rest period. Duplicate blood pressure readings were taken to ensure accuracy; participants rested for one minute between measurements. The lowest of three blood pressure measurements was used for analysis.
Blood samples were collected following an overnight fast. Triglycerides and HDL were measured using an enzymatic spectrophotometric assay (Roche Diagnostics, Indianapolis, IN). Glucose was measured using an enzymatic spectrophotometric assay using hexokinase coupled with glucose-6-phosphate dehydrogenase (Roche Diagnostics, Indianapolis, IN). All samples were analyzed using the Roche Modular P chemistry analyzer. Exercise capacity was determined by six minute walk performed indoors, according to the guidelines established by the American Thoracic Society (abnormal, <490 meters).(38) (39) Quality of life was measured using the physical and mental component summaries of the SF-36.(40)
Statistical Methods
Descriptive statistics were used to characterize the eligible population and the study participants. The prevalence of cardiac abnormalities was estimated for the entire cohort of ten year survivors and by treatment exposure (anthracycline only, chest-directed RT only, and anthracycline and chest-directed RT combined). Associations between treatment characteristics and cardiac abnormalities were investigated using Poisson regression models with robust error variances. A similar approach was used to determine whether metabolic syndrome and its components were associated with abnormal cardiac function. These models were adjusted for current age, age at diagnosis, race/ethnicity, chest RT and anthracycline exposure and the rate ratios (RRs) and 95% confidence intervals (CIs). Frequencies and percentages of all combinations of abnormal echocardiography results were summarize and six minute walk distances were compared to normal the group with normal echocardiography results by two sample t-tests. Associations between cardiac dysfunction and impaired quality of life (defined as impaired physical and mental quality of life on the SF-36) were assessed by Poisson regression with robust error variance and adjusted for gender, education, marital status, annual household income, employment status and treatment with cranial RT. All analyses were performed in SAS version 9.3 (Cary, N.C.).
RESULTS
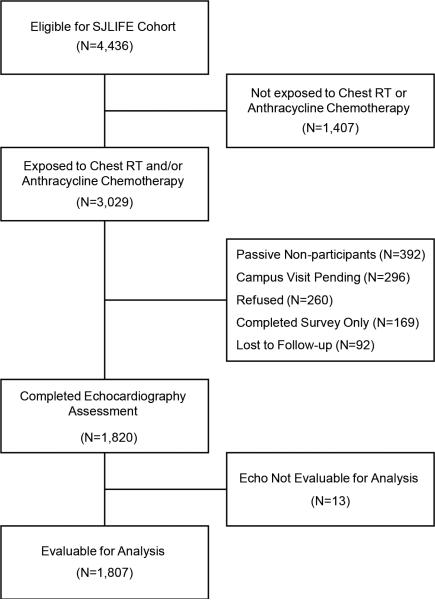
Of the 4,436 survivors eligible for SJLIFE, 3,029 were exposed to cardiotoxic therapy and eligible for echocardiography (Figure 1). At the time of this analysis, 1,820 (60% of eligible) completed SJLFE medical assessments including echocardiography. Thirteen echocardiography studies were of insufficient quality for analysis. Demographic and treatment characteristics of survivors included in this analysis and potentially eligible non-participants are summarized in Table 1. Participants were more likely to be female, but were similar for other demographic and treatment related characteristics. Median time from primary cancer diagnosis was 22.6 years (range 10.4-48.3); median age at evaluation was 31 years (range 18-65). Forty-seven survivors had been previously diagnoses with cardiomyopathy, of whom 23 were on medications for heart failure at the time of evaluation.
Figure1.
Consort diagram of SJLIFE population eligible for echocardiography evaluation
Table 1.
Demographic and treatment characteristics of adult survivors of childhood cancer
| Eligible (N=3,029) | Non-participants (N=1,209) | Participants (N=1,820) | Anthracycline Alone (N=1,050) | Chest-directed RT Alone (N=306) | Anthracycline + Chest-directed RT (N=464) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | %* | N | %* | N | %* | N | %* | N | %* | N | %* | |
| Race/Ethnicity | ||||||||||||
| Non-Hispanic White | 2517 | 83.8 | 965 | 81.2 | 1552 | 85.5 | 905 | 86.4 | 260 | 85.0 | 387 | 83.8 |
| Non-Hispanic Black | 415 | 13.8 | 193 | 16.2 | 222 | 12.2 | 116 | 11.1 | 40 | 13.1 | 66 | 14.3 |
| Non-Hispanic Other | 41 | 1.4 | 18 | 1.5 | 23 | 1.3 | 12 | 1.2 | 6 | 2.0 | 5 | 1.1 |
| Hispanic | 31 | 1.0 | 13 | 1.1 | 18 | 1.0 | 14 | 1.3 | 0 | 0.0 | 4 | 0.9 |
| Sex | ||||||||||||
| Male | 1684 | 55.6 | 738 | 61.0 | 946 | 52.0 | 548 | 52.2 | 164 | 53.6 | 234 | 50.4 |
| Female | 1345 | 44.4 | 471 | 39.0 | 874 | 48.0 | 502 | 47.8 | 142 | 46.4 | 230 | 49.6 |
| Age at Diagnosis (years) | ||||||||||||
| 0-4 | 1023 | 33.8 | 404 | 33.4 | 619 | 34.0 | 416 | 39.6 | 69 | 22.6 | 134 | 28.9 |
| 5-9 | 718 | 23.7 | 296 | 24.5 | 422 | 23.2 | 246 | 23.4 | 79 | 25.8 | 97 | 20.9 |
| 10-14 | 731 | 24.1 | 286 | 23.7 | 445 | 24.5 | 242 | 23.1 | 95 | 31.1 | 108 | 23.3 |
| 15-19 | 530 | 17.5 | 210 | 17.4 | 320 | 17.6 | 138 | 13.1 | 61 | 19.9 | 121 | 26.1 |
| >19 | 27 | 0.9 | 13 | 1.1 | 14 | 0.8 | 8 | 0.8 | 2 | 0.7 | 4 | 0.9 |
| Time Since Diagnosis (years) | ||||||||||||
| 10-20 | 1027 | 34.1 | 366 | 30.3 | 661 | 36.3 | 392 | 37.5 | 80 | 26.3 | 189 | 41.3 |
| 21-30 | 1228 | 40.7 | 498 | 41.2 | 730 | 40.1 | 468 | 44.8 | 87 | 28.6 | 175 | 38.2 |
| 31-40 | 643 | 21.3 | 280 | 23.2 | 363 | 19.9 | 179 | 17.1 | 101 | 33.2 | 83 | 18.1 |
| 41-50 | 118 | 3.9 | 65 | 5.4 | 53 | 2.9 | 6 | 0.6 | 36 | 11.8 | 11 | 2.4 |
| Current Age (years) | ||||||||||||
| 18-20 | 139 | 4.6 | 51 | 4.2 | 88 | 4.8 | 69 | 6.6 | 3 | 1.0 | 16 | 3.5 |
| 21-30 | 1193 | 39.6 | 430 | 35.6 | 763 | 41.9 | 506 | 48.4 | 88 | 29.0 | 169 | 36.9 |
| 31-40 | 1112 | 36.9 | 462 | 38.2 | 650 | 35.7 | 356 | 34.1 | 84 | 27.6 | 210 | 45.9 |
| 41-50 | 484 | 16.1 | 217 | 18.0 | 267 | 14.7 | 107 | 10.2 | 103 | 33.9 | 57 | 12.5 |
| >50 | 88 | 2.9 | 49 | 4.1 | 39 | 2.1 | 7 | 0.7 | 26 | 8.6 | 6 | 1.3 |
| Primary Cancer Diagnosis | ||||||||||||
| Leukemia | 1246 | 41.1 | 479 | 39.6 | 767 | 42.1 | 601 | 57.2 | 54 | 17.7 | 112 | 24.1 |
| Acute lymphoblastic leukemia | 1053 | 34.8 | 384 | 31.8 | 669 | 36.8 | 550 | 52.4 | 31 | 10.1 | 88 | 19.0 |
| Acute myeloid leukemia | 146 | 4.8 | 72 | 6 | 74 | 4.1 | 51 | 4.9 | 1 | 0.3 | 22 | 4.7 |
| Other leukemia | 47 | 1.6 | 23 | 1.9 | 24 | 1.3 | 0 | 0.0 | 22 | 7.2 | 2 | 0.4 |
| Lymphoma | 799 | 26.4 | 332 | 27.5 | 467 | 25.7 | 136 | 13.0 | 121 | 39.5 | 210 | 45.3 |
| Non-Hodgkin Lymphoma | 307 | 10.1 | 151 | 12.5 | 156 | 8.6 | 120 | 11.4 | 11 | 3.6 | 25 | 5.4 |
| Hodgkin Lymphoma | 492 | 16.2 | 181 | 15 | 311 | 17.1 | 16 | 1.5 | 110 | 36.0 | 185 | 39.9 |
| CNS tumor | 140 | 4.6 | 62 | 5.1 | 78 | 4.3 | 2 | 0.2 | 76 | 24.8 | 0 | 0.0 |
| Bone tumor | 261 | 8.6 | 88 | 7.3 | 173 | 9.5 | 150 | 14.3 | 0 | 0.0 | 23 | 5.0 |
| Ewing sarcoma | 119 | 3.9 | 38 | 3.1 | 81 | 4.5 | 60 | 5.7 | 0 | 0.0 | 21 | 4.5 |
| Osteosarcoma | 142 | 4.7 | 50 | 4.1 | 92 | 5.1 | 90 | 8.6 | 0 | 0.0 | 2 | 0.4 |
| Other tumors | 23 | 0.8 | 13 | 1.1 | 10 | 0.5 | 0 | 0.0 | 10 | 3.3 | 0 | 0.0 |
| Germ cell tumor | 20 | 0.7 | 11 | 0.9 | 9 | 0.5 | 0 | 0.0 | 9 | 2.9 | 0 | 0.0 |
| Melanoma | 3 | 0.1 | 2 | 0.2 | 1 | 0.1 | 0 | 0.0 | 1 | 0.3 | 0 | 0.0 |
| Soft Tissue Sarcoma | 143 | 4.7 | 59 | 4.9 | 84 | 4.6 | 60 | 5.7 | 5 | 1.6 | 19 | 4.1 |
| Rhabdomyosarcoma | 97 | 3.2 | 43 | 3.6 | 54 | 3 | 42 | 4.0 | 3 | 1.0 | 9 | 1.9 |
| Non-Rhabdo Sarcoma | 46 | 1.5 | 16 | 1.3 | 30 | 1.6 | 18 | 1.7 | 2 | 0.7 | 10 | 2.2 |
| Other Malignancies | 417 | 13.8 | 176 | 14.6 | 241 | 13.2 | 101 | 9.6 | 40 | 13.1 | 100 | 21.6 |
| Neuroblastoma | 142 | 4.7 | 57 | 4.7 | 85 | 4.7 | 60 | 5.7 | 13 | 4.3 | 12 | 2.6 |
| Retinoblastoma | 16 | 0.5 | 11 | 0.9 | 5 | 0.3 | 5 | 0.5 | 0 | 0.0 | 0 | 0.0 |
| Wilms tumor | 230 | 7.6 | 97 | 8 | 133 | 7.3 | 26 | 2.5 | 20 | 6.5 | 87 | 18.8 |
| Carcinoma | 6 | 0.2 | 3 | 0.2 | 3 | 0.2 | 1 | 0.1 | 2 | 0.7 | 0 | 0.0 |
| Other | 23 | 0.8 | 8 | 0.7 | 15 | 0.8 | 9 | 0.9 | 5 | 1.6 | 1 | 0.2 |
| Anthracycline Cumulative Dose (mg/m2) | ||||||||||||
| 0 | 510 | 16.9 | 204 | 17.1 | 306 | 16.9 | 0 | 0.0 | 306 | 100.0 | 0 | 0.0 |
| 1-100 | 784 | 26.0 | 296 | 24.6 | 488 | 26.9 | 419 | 40.0 | 0 | 0.0 | 69 | 15.0 |
| 101-200 | 882 | 29.2 | 364 | 30.3 | 518 | 28.6 | 292 | 27.9 | 0 | 0.0 | 226 | 49.0 |
| 201-300 | 336 | 11.1 | 141 | 11.7 | 195 | 10.8 | 105 | 10.0 | 0 | 0.0 | 90 | 19.5 |
| 301-400 | 332 | 11.0 | 107 | 8.9 | 225 | 12.4 | 163 | 15.6 | 0 | 0.0 | 62 | 13.5 |
| 401-500 | 100 | 3.3 | 41 | 3.4 | 59 | 3.3 | 51 | 4.9 | 0 | 0.0 | 8 | 1.7 |
| 501-600 | 23 | 0.8 | 5 | 0.4 | 18 | 1.0 | 13 | 1.2 | 0 | 0.0 | 5 | 1.1 |
| >600 | 48 | 1.6 | 43 | 3.6 | 5 | 0.3 | 4 | 0.4 | 0 | 0.0 | 1 | 0.2 |
| Chest-directed RT | ||||||||||||
| 0 Gy | 1765 | 60.5 | 715 | 62.1 | 1050 | 59.4 | 1050 | 100.0 | 0 | 0.0 | 0 | 0.0 |
| 1-19.9Gy | 653 | 22.4 | 250 | 21.7 | 403 | 22.8 | 0 | 0.0 | 131 | 45.0 | 272 | 63.7 |
| 20-29.9Gy | 330 | 11.3 | 121 | 10.5 | 209 | 11.8 | 0 | 0.0 | 72 | 24.7 | 137 | 32.1 |
| ≥30Gy | 172 | 5.9 | 66 | 5.7 | 106 | 6.0 | 0 | 0.0 | 88 | 30.2 | 18 | 4.2 |
| Metabolic Syndrome | ||||||||||||
| Yes | -- | -- | -- | -- | 509 | 28.6 | 280 | 27.2 | 99 | 33.5 | 130 | 28.8 |
| No | -- | -- | -- | -- | 1269 | 71.4 | 750 | 72.8 | 197 | 66.6 | 322 | 71.2 |
| Components of Metabolic Syndrome | ||||||||||||
| Abdominal Obesity | ||||||||||||
| Yes | -- | -- | -- | -- | 538 | 30.4 | 355 | 34.5 | 69 | 23.7 | 114 | 25.4 |
| No | -- | -- | -- | -- | 1232 | 69.6 | 675 | 65.5 | 222 | 76.3 | 335 | 74.6 |
| Elevated Triglycerides | ||||||||||||
| Yes | -- | -- | -- | -- | 470 | 26.0 | 234 | 22.4 | 98 | 32.2 | 138 | 30.0 |
| No | -- | -- | -- | -- | 1338 | 74.0 | 810 | 77.6 | 206 | 67.8 | 322 | 70.0 |
| Low HDL Cholesterol | ||||||||||||
| Yes | -- | -- | -- | -- | 665 | 36.8 | 379 | 36.3 | 113 | 37.2 | 173 | 37.6 |
| No | -- | -- | -- | -- | 1143 | 63.2 | 665 | 63.7 | 191 | 62.8 | 287 | 62.4 |
| Hypertension | ||||||||||||
| Yes | -- | -- | -- | -- | 816 | 45.2 | 436 | 41.8 | 160 | 53.2 | 220 | 47.8 |
| No | -- | -- | -- | -- | 989 | 54.8 | 608 | 58.2 | 141 | 46.8 | 240 | 52.2 |
| Fasting Glucose ≥100 mg/dl | ||||||||||||
| Yes | -- | -- | -- | -- | 577 | 31.9 | 295 | 28.2 | 125 | 41.1 | 157 | 34.1 |
| No | -- | -- | -- | -- | 1233 | 68.1 | 750 | 71.8 | 179 | 58.9 | 304 | 65.9 |
| Previously Diagnosed with Cardiomyopathy | -- | -- | -- | -- | 47 | 2.6 | 19 | 1.8 | 10 | 3.3 | 18 | 3.9 |
| Previously Diagnosed with Cardiomyopathy and on Medications at the Time of Evaluation | -- | -- | -- | -- | 23 | 1.3 | 7 | 0.7 | 6 | 2.0 | 10 | 2.2 |
Percentages provided for total number of participants for whom data were available for a given characteristic.
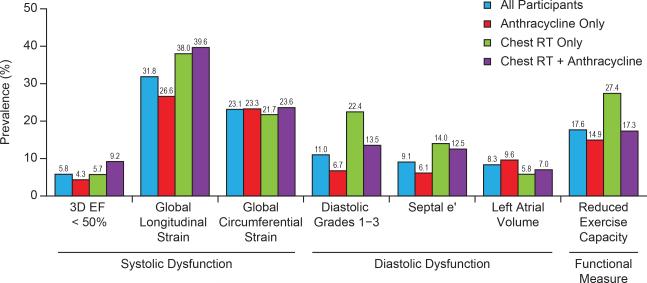
Only 5.8% of the population had a 3D LVEF<50%. However, systolic dysfunction detected by global longitudinal (31.8%) and global circumferential (23.1%) strain and diastolic dysfunction (ASE grades 1-3, 11.0%) were more prevalent (Figure 2, Supplemental Table 1) than an abnormal LVEF. Among survivors with preserved 3D LVEF (≥50%), comprehensive echocardiography identified significant systolic (28.0%, global longitudinal strain) and diastolic (8.7%, ASE Grades 1-3) dysfunction. Thus, one third (32.1%) of survivors with a normal 3D LVEF had cardiac dysfunction when both longitudinal strain and ASE grade 1-3 diastolic function were considered. Notably, among survivors exposed to chest RT only, 22.4% had evidence of diastolic dysfunction.
Figure 2.
Prevalence of Cardiac Dysfunction and Reduced Exercise Capacity in Adult, Ten Year Survivors of Childhood Cancer
Abnormal 3D LVEF was associated with chest-directed RT (20-29.9 Gy, Rate Ratio [RR] 1.86, 95% Confidence Interval [CI] 1.00-3.45; ≥30 Gy, RR 7.99, 95% CI 3.88-16.48, Table 2) and cumulative anthracycline doses > 100 mg/m2. Global longitudinal strain was associated with any dose exposure to chest-directed RT (1-19.9 Gy, OR 1.38, 95% CI 1.14-1.66; 20-29.9 Gy, RR 1.65, 95% CI 1.31-2.08; >30 Gy, RR 2.39, 95% CI 1.79-3.18) and anthracycline dose >300 mg/m2. Diastolic dysfunction was associated with chest-directed RT but not anthracycline cumulative dose.
Table 2.
Multivariable associations between treatment characteristics and systolic and diastolic dysfunction in adult survivors of childhood cancer
| 3D Echo <50% | Abnormal global Longitudinal Strain | Abnormal Global Circumferential strain | Diastolic Grade 1-3 | Abnormal Septal e’ | Abnormal Left Atrial Volume | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | |
| Race/Ethnicity | ||||||||||||
| Other | 1.53 | 0.93 - 2.52 | 1.22 | 1.03 - 1.46 | 0.84 | 0.64 - 1.09 | 1.24 | 0.86 - 1.78 | 1.32 | 0.89 - 1.96 | 2.03 | 1.41 - 2.93 |
| Non-Hispanic White | 1 | 1 | 1 | 1 | 1 | |||||||
| Sex | ||||||||||||
| Female | 0.54 | 0.36 - 0.83 | 1.55 | 1.34 - 1.79 | 1.01 | 0.84 - 1.21 | 1.15 | 0.88 - 1.51 | 0.98 | 0.73 - 1.32 | 0.6 | 0.43 - 0.83 |
| Male | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Age at Diagnosis (years) | ||||||||||||
| 0-4 | 0.66 | 0.35 - 1.27 | 1.02 | 0.82 - 1.27 | 1.24 | 0.92 - 1.67 | 0.85 | 0.56 - 1.29 | 0.91 | 0.58 - 1.44 | 1.26 | 0.79 - 2.01 |
| 5-9 | 0.67 | 0.36 - 1.25 | 0.92 | 0.74 - 1.15 | 1.01 | 0.74 - 1.38 | 0.81 | 0.53 - 1.22 | 0.8 | 0.50 - 1.26 | 1.23 | 0.74 - 2.05 |
| 10-14 | 1.02 | 0.59 - 1.76 | 1.02 | 0.83 - 1.24 | 1.11 | 0.84 - 1.48 | 0.87 | 0.61 - 1.23 | 0.77 | 0.50 - 1.17 | 1.18 | 0.74 - 1.86 |
| ≥15 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Current Age (years) | ||||||||||||
| 31-40 | 1.38 | 0.81 - 2.35 | 1.25 | 1.05 - 1.48 | 0.85 | 0.69 - 1.06 | 2.43 | 1.59 - 3.71 | 1.96 | 1.31 - 2.93 | 2.4 | 1.67 - 3.45 |
| >40 | 0.98 | 0.52 - 1.84 | 1.49 | 1.20 - 1.85 | 0.98 | 0.73 - 1.33 | 4.74 | 2.90 - 7.75 | 1.52 | 0.90 - 2.54 | 3.59 | 2.25 - 5.73 |
| 18-30 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Anthracycline Cumulative Dose (mg/m2) | ||||||||||||
| 1-100 | 1.74 | 0.66 - 4.61 | 1.38 | 1.05 - 1.82 | 0.99 | 0.66 - 1.48 | 0.75 | 0.43 - 1.30 | 0.62 | 0.29 - 1.32 | 2.07 | 0.95 - 4.51 |
| 101-200 | 2.80 | 1.24 - 6.31 | 1.16 | 0.89 - 1.50 | 1.24 | 0.86 - 1.79 | 0.80 | 0.51 - 1.25 | 1.13 | 0.65 - 1.97 | 1.82 | 0.85 - 3.91 |
| 201-300 | 3.80 | 1.59 - 9.10 | 1.06 | 0.78 - 1.45 | 1.36 | 0.90 - 2.04 | 0.76 | 0.42 - 1.37 | 1.77 | 0.99 - 3.15 | 1.34 | 0.55 - 3.25 |
| 301-400 | 4.76 | 2.16 - 10.50 | 1.72 | 1.31 - 2.26 | 1.61 | 1.08 - 2.40 | 1.00 | 0.59 - 1.69 | 1.53 | 0.84 - 2.81 | 1.72 | 0.73 - 4.05 |
| >400 | 7.71 | 3.04 - 19.57 | 1.73 | 1.19 - 2.50 | 1.34 | 0.78 - 2.31 | 1.33 | 0.72 - 2.45 | 2.05 | 0.99 - 4.24 | 0.95 | 0.30 - 2.99 |
| None | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Chest RT Cumulative Dose (Gy) | ||||||||||||
| 1-19.9 | 1.24 | 0.70 - 2.22 | 1.38 | 1.14 - 1.66 | 0.86 | 0.66 - 1.11 | 1.47 | 0.99 - 2.20 | 1.09 | 0.69 - 1.72 | 0.47 | 0.29 - 0.78 |
| 20-29.9 | 1.86 | 1.00 - 3.45 | 1.65 | 1.31 - 2.08 | 1.14 | 0.83 - 1.57 | 2.03 | 1.30 - 3.17 | 2.01 | 1.27 - 3.21 | 1.1 | 0.65 - 1.87 |
| ≥30 | 7.99 | 3.88 - 16.48 | 2.39 | 1.79 - 3.18 | 1.64 | 1.05 - 2.56 | 2.44 | 1.44 - 4.14 | 4.03 | 2.22 - 7.32 | 0.63 | 0.21 - 1.89 |
| None | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
Survivors with metabolic syndrome were almost twice as likely to have an abnormal global longitudinal strain (Rate Ratio [RR] 1.94, 95% CI 1.66-2.28) as well as abnormal diastolic function (RR 1.68, 95% CI 1.39-2.03), but did not have a higher risk of abnormal 3D LVEF (RR 1.07, 95% CI 0.74-1.53). Each of the individual components of the metabolic syndrome was associated with an increased risk of abnormal global longitudinal strain and diastolic dysfunction. (Table 3).
Table 3.
Association between metabolic syndrome and systolic and diastolic echocardiography abnormalities in adult survivors of childhood cancer.
| 3D Echo <50% | Abnormal global Longitudinal Strain | Abnormal Global Circumferential strain | Diastolic Grade 1-3 | Abnormal Septal e’ | Abnormal Left Atrial Volume | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | |
| Metabolic Syndrome | ||||||||||||
| Yes | 1.07 | 0.74 - 1.53 | 1.94 | 1.66 - 2.28 | 1.02 | 0.84 - 1.24 | 1.68 | 1.39 - 2.03 | 1.65 | 1.35 - 2.02 | 1.41 | 1.14 - 1.74 |
| No | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Abdominal Obesity | ||||||||||||
| Yes | 1.34 | 0.99 - 1.82 | 1.73 | 1.48 - 2.01 | 1.1 | 0.92 - 1.32 | 1.69 | 1.39 - 2.06 | 1.49 | 1.23 - 1.82 | 1.83 | 1.53 - 2.19 |
| No | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Triglycerides ≥150 mg/dl | ||||||||||||
| Yes | 1.01 | 0.70 - 1.44 | 1.65 | 1.40 - 1.95 | 1.01 | 0.82 - 1.23 | 1.44 | 1.17 - 1.78 | 1.35 | 1.07 - 1.70 | 1.03 | 0.79 - 1.33 |
| No | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Low HDL Cholesterol | ||||||||||||
| Yes | 1.01 | 0.74 - 1.38 | 1.4 | 1.23 - 1.59 | 0.92 | 0.78 - 1.08 | 1.36 | 1.14 - 1.62 | 1.20 | 0.97 - 1.47 | 1.20 | 0.98 - 1.47 |
| No | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Hypertension | ||||||||||||
| Yes | 1.44 | 1.22 - 1.70 | 1.48 | 1.33 - 1.65 | 1.04 | 0.92 - 1.18 | 1.39 | 1.22 - 1.58 | 1.34 | 1.17 - 1.55 | 1.30 | 1.13 - 1.49 |
| No | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Fasting Glucose ≥100 mg/dl | ||||||||||||
| Yes | 1.02 | 0.75 - 1.39 | 1.37 | 1.19 - 1.59 | 1.06 | 0.89 - 1.25 | 1.42 | 1.18 - 1.70 | 1.47 | 1.22 - 1.77 | 1.00 | 0.81 - 1.25 |
| No | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
Adjusted for current age, age at diagnosis, race/ethnicity, sex, chest RT and anthracycline exposure
Survivors with global longitudinal strain as the only abnormal finding on echocardiography had a lower mean six-minute walk distance compared to survivors with normal echocardiography (560 vs. 590 meters, p=0.0002, Supplemental Table 2). Reduced exercise capacity (<490 meters, six-minute walk) was identified in 17.6% of participants (Figure 2). However, on multivariable analyses adjusting for pulmonary function, muscle strength, height and weight, no independent association between echocardiographic outcomes and reduced exercise capacity was identified. Abnormal longitudinal strain (RR 1.71, 95% CI 1.33-2.19), LVEF (RR 1.92, 95% CI 1.33-2.76) and diastolic function (grades 1-3, RR 1.83, 95% CI 1.36-2.45) were associated with reduced quality of life on the physical component summary scale but only abnormal LVEF (RR 1.53, 95% CI 1.02-2.29) and abnormal atrial volume (RR 1.37, 95% CI 1.01-1.86) were associated with the mental component summary scale of the SF-36 (Supplemental Table 3).
DISCUSSION
Systematic, protocol-driven echocardiographic assessment of a large population of adult survivors has been difficult to achieve as this population has transitioned from academic pediatric centers to adult care, largely provided in a community setting.(41) However, with over 1,800 participants, we provide the largest study to date utilizing modern echocardiographic techniques (3D LVEF,(14) myocardial strain, and uniform, ASE guideline-driven grading for diastolic function) for comprehensive assessment of cardiac function in aging adult survivors of childhood cancer exposed to cardiotoxic therapy.(42-44) Only 5.8% had a 3D LVEF <50%. However, we identified that one third of survivors with a normal 3D LVEF had evidence of underlying systolic and/or diastolic cardiac dysfunction by applying comprehensive echocardiographic assessment.
Diastolic Function
It is known that chest-directed RT results in microvascular damage with subsequent myocardial interstitial fibrosis, leading to a non-compliant ventricle with resulting diastolic dysfunction, often with preserved LVEF.(45) Traditionally, diastolic dysfunction has been difficult to quantify in survivors of childhood cancer,(46,47) with more accurate assessment occurring in adults diagnosed with Hodgkin lymphoma.(48) Given the large size of our population, evaluation of the independent effect of chest-directed RT, without the confounding influence of anthracyclines was possible. Our use of the ASE consensus-based diastolic assessment, found that 22% of survivors exposed to RT-alone have evidence of diastolic dysfunction. These findings are driven by the large number of survivors of Hodgkin lymphoma treated with high doses of chest-directed RT prior to the era in which combined modality therapy with anthracyclines, and low-dose involved-field chest RT was introduced. Many of these survivors who have a well-documented increased risk for major cardiac events are now entering their fifth decade of life. (6,8) Thus, our findings underscore that screening evaluations of these survivors cannot be limited to traditional assessment of LV systolic function, but should include comprehensive diastolic assessment.
Systolic function
Strain imaging has emerged as a powerful tool to quantify myocardial mechanics including both longitudinal shortening and circumferential torsion.(17) While LVEF is the most widely used measure of systolic dysfunction, it has a number of limitations, including use of geometric assumptions (2D LVEF) of ventricular shape, load dependency, and poor reproducibility and inter-observer variability.(20) Myocardial strain is a semi-automated tool to assess multidimensional myocardial deformation that is more reproducible and not reliant on geometric assumptions.(17) In the general population, the association of LVEF with poor outcome is strongest in moderately to severely impaired ventricles.(49) Thus LVEF may not be ideal for screening asymptomatic survivors. Furthermore, in a recent meta-analysis that included almost 6000 patients with a diverse array of underlying cardiac insults (congestive heart failure, acute myocardial infarction, valvular disease included), global longitudinal strain had superior prognostic value compared to LVEF for predicting both overall mortality and major cardiac events.(20) In that meta-analysis. a one standard deviation change in global longitudinal strain was associated with a 1.62 (95% CI 1.13-2.33) times greater reduction in mortality than a comparable change in LVEF. Given that evidence from the general, non-cancer population indicates that abnormal global longitudinal strain is a valid predictor of poor outcome, our finding that 28% of survivors with a normal 3D LVEF have abnormal global longitudinal strain may identify a subset of survivors at high risk for clinical heart failure. It will be essential that future studies provide longitudinal follow-up of survivors evaluated with comprehensive echocardiographic imaging to determine whether global longitudinal strain improves prediction of major cardiac events as it does in the general population.
The current study takes important steps in validating global longitudinal strain as a clinically relevant measure in survivors. First, in this population, we established that global longitudinal strain, like LVEF, measures treatment-related injury. On the strength of detailed abstraction of cumulative dose exposure of anthracyclines and tissue-specific radiation dosimetry, we demonstrated that abnormal global longitudinal strain, but not global circumferential stain, was associated with increasing doses of both anthracyclines and chest-directed radiotherapy. Dose response relationships between exposure and major cardiac events are well established in this population,(6,10) thus, if strain is to be a meaningful early measure of cardiac injury, demonstrating a dose-response relationship is essential. Furthermore, studies in adult cancer populations have demonstrated changes in global longitudinal strain during administration of anthracyclines.(50) In these trials, early changes in global longitudinal strain precede and predict eventual reduction in LVEF and subsequent clinical heart failure.(22) These findings have resulted in an expert consensus statement from ASE that recommends strain in assessment of adult patients during and after cancer therapy.(16) In the current study, abnormal global circumferential strain, though prevalent, showed inconsistent associations with increased anthracycline or chest-directed RT exposure. This may be because the subendocardial region, which governs longitudinal left ventricular mechanics, is generally the most sensitive region to myocardial injury.(51) These findings should direct clinicians toward preferential use of global longitudinal strain over circumferential strain in screening of this population.
It is now established that the acquisition of traditional cardiovascular risk factors and metabolic syndrome potentiates risk for major cardiac events among aging survivors who received cardiotoxic therapies.(8,24) More sensitive echocardiographic measure of cardiac injury would be expected to demonstrate higher rates of abnormal function in a population with metabolic syndrome. We identified higher rates of abnormal global longitudinal strain, but not 3D LVEF in a subset of the population with metabolic syndrome. This strong association between therapeutic dose-exposure and increased rates of abnormal findings in a high risk subset of the population with metabolic syndrome suggest that global longitudinal strain may be a valid measure for detecting myocardial injury in adult survivors of childhood cancer. However, baseline assessment prior to treatment and serial, longitudinal evaluation with strain in a large population of aging survivors is needed before this can be concluded.
Limitations
Eight previous studies have reported strain evaluation among a total of 366 long-term survivors of childhood cancer (largest study population, N=111),(50,52) and identified between 6-30% of the population to have abnormal longitudinal strain. We present the most systematic assessment to date including a study population of sufficient size for a robust multivariable analysis to evaluate confounding variables contributing to cardiac outcomes. Nonetheless, limitations should be considered. The cross-sectional nature of this analysis precludes definitive assessment of the predictive nature of global longitudinal strain, or any echocardiography parameter, for major cardiac events including congestive heart failure, cardiac hospitalization or cardiac mortality. However, longitudinal follow-up of the SJLIFE Cohort will allow future assessment as survivors age. While the quality of echocardiography is inherently operator-dependent, this protocol-driven systematic assessment should limit the imprecision inherent in previous studies that have reported on echocardiography by retrospective review. Furthermore, our use of 3D LVEF eliminates assumptions regarding ventricular size inherent in 2D LVEF estimates, providing the most valid assessment of LVEF to date.(14) It is important to note that on multivariable analysis, independent associations between echocardiographic abnormalities and reduced functional performance on six minute walk were not identified. This may be a result of six minute walk be a poor surrogate for performance in this population, thus future studies will include maximal treadmill testing to assess functional performance. Additionally, calculation of mean RT dose to the heart, while providing organ-specific dosimetry, may does not fully describe the differential dose received across the heart. Finally, current circumferential strain estimations may be unreliable and future efforts should focus on improvement in reliability and validity.
CONCLUSION
In summary, these findings suggest that traditional echocardiographic evaluation of cardiac function in adult survivors of childhood cancer that focuses on LVEF as the primary measure of function may be inadequate. Evaluation that incorporates global longitudinal strain and ASE grading of diastolic function demonstrates that one in three survivors with normal LVEF has evidence of cardiac dysfunction. Long term follow up is needed to determine the predictive nature of these echocardiographic findings for major cardiac events.
Supplementary Material
PERSPECTIVES.
Competency in Medical Knowledge. Adult survivors of childhood cancer are at significant risk for cardiac morbidity and mortality as a result of therapy (chest-directed radiotherapy and anthracycline chemotherapy) they received for treatment of childhood cancer.
Competency in Patient Care. Guidelines developed by the Children's Oncology Group recommend annual evaluation for survivors exposed to cardiotoxic therapies.
Transitional Outlook 1. Comprehensive echocardiographic evaluation identifies significant rates of systolic and diastolic dysfunction in survivors, but the predictive nature of these findings for major cardiac events remains unclear.
ACKNOWLEDGEMENTS
This research was previously presented as a poster presentation at the annual meeting of the American Society of Clinical Oncology, 2014.
We would like to acknowledge James Fowler, Davi Govendaswamy, Amanda Rhinehardt, David Martin and Courtney Reid for their role in echocardiography data collection.
Funding: Support to St. Jude Children's Research Hospital provided by the Cancer Center Support (CORE) grant (CA21765, R. Gilbertson, Principal Investigator) and the American Lebanese-Syrian Associated Charities (ALSAC). Dr. Marwick reports research grants with General Electric (>$50k) and equipment support from Siemens and Philips.
Selected Abbreviations
- RT
radiotherapy
- LVEF
left ventricular ejection fraction
- ASE
American Society of Echocardiography
- RR
Rate Ratio
- CI
Confidence Interval
- SJLIFE
St. Jude Lifetime Cohort Study
Footnotes
Conflict of interest: The authors have no conflicts to disclose
REFERENCES
- 1.Howlader NNA, Krapcho M, Neyman N, Aminou R, Altekruse SF, Kosary CL, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Chen HS, Feuer EJ, Cronin KA, editors. SEER Cancer Statistics Review (CSR) National Cancer Institute; Bethesda, MD: 2012. SEER Cancer Statistics Review 1975-2009 (Vintage 2009 Populations). [Google Scholar]
- 2.Robison LL, Hudson MM. Survivors of childhood and adolescent cancer: life-long risks and responsibilities. Nature reviews Cancer. 2014;14:61–70. doi: 10.1038/nrc3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hudson MM, Ness KK, Gurney JG, et al. CLinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA. 2013;309:2371–2381. doi: 10.1001/jama.2013.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–82. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong GT, Kawashima T, Leisenring W, et al. Aging and risk of severe, disabling, life-threatening, and fatal events in the childhood cancer survivor study. J Clin Oncol. 2014;32:1218–27. doi: 10.1200/JCO.2013.51.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mulrooney DA, Yeazel MW, Kawashima T, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ. 2009;339:b4606. doi: 10.1136/bmj.b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Pal HJ, van Dalen EC, van Delden E, et al. High Risk of Symptomatic Cardiac Events in Childhood Cancer Survivors. Journal of Clinical Oncology. 2012;30:1429–1437. doi: 10.1200/JCO.2010.33.4730. [DOI] [PubMed] [Google Scholar]
- 8.Armstrong GT, Oeffinger KC, Chen Y, et al. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol. 2013;31:3673–80. doi: 10.1200/JCO.2013.49.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mertens AC, Liu Q, Neglia JP, et al. Cause-specific late mortality among 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2008;100:1368–79. doi: 10.1093/jnci/djn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lipshultz SE, Adams MJ, Colan SD, et al. Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: pathophysiology, course, monitoring, management, prevention, and research directions: a scientific statement from the American Heart Association. Circulation. 2013;128:1927–95. doi: 10.1161/CIR.0b013e3182a88099. [DOI] [PubMed] [Google Scholar]
- 11.Children's OG. The Children's Oncology Group Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers. In: Children's OG, editor. Children's Oncology Group Web Site. Chidren's Oncology Group; 2013. p. 241. [Google Scholar]
- 12.Otterstad JE. Measuring left ventricular volume and ejection fraction with the biplane Simpson's method. Heart. 2002;88:559–560. doi: 10.1136/heart.88.6.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thavendiranathan P, Grant AD, Negishi T, Plana JC, Popovic ZB, Marwick TH. Reproducibility of echocardiographic techniques for sequential assessment of left ventricular ejection fraction and volumes: application to patients undergoing cancer chemotherapy. J Am Coll Cardiol. 2013;61:77–84. doi: 10.1016/j.jacc.2012.09.035. [DOI] [PubMed] [Google Scholar]
- 14.Armstrong GT, Plana JC, Zhang N, et al. Screening adult survivors of childhood cancer for cardiomyopathy: comparison of echocardiography and cardiac magnetic resonance imaging. J Clin Oncol. 2012;30:2876–84. doi: 10.1200/JCO.2011.40.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–9. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 16.Plana JC, Galderisi M, Barac A, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27:911–39. doi: 10.1016/j.echo.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 17.Nesbitt GC, Mankad S, Oh JK. Strain imaging in echocardiography: methods and clinical applications. Int J Cardiovasc Imaging. 2009;25(Suppl 1):9–22. doi: 10.1007/s10554-008-9414-1. [DOI] [PubMed] [Google Scholar]
- 18.Cho GY, Marwick TH, Kim HS, Kim MK, Hong KS, Oh DJ. Global 2-dimensional strain as a new prognosticator in patients with heart failure. J Am Coll Cardiol. 2009;54:618–24. doi: 10.1016/j.jacc.2009.04.061. [DOI] [PubMed] [Google Scholar]
- 19.Stanton T, Ingul CB, Hare JL, Leano R, Marwick TH. Association of myocardial deformation with mortality independent of myocardial ischemia and left ventricular hypertrophy. JACC Cardiovasc Imaging. 2009;2:793–801. doi: 10.1016/j.jcmg.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 20.Kalam K, Otahal P, Marwick TH. Prognostic implications of global LV dysfunction: a systematic review and meta-analysis of global longitudinal strain and ejection fraction. Heart. 2014 doi: 10.1136/heartjnl-2014-305538. [DOI] [PubMed] [Google Scholar]
- 21.Negishi K, Negishi T, Hare JL, Haluska BA, Plana JC, Marwick TH. Independent and incremental value of deformation indices for prediction of trastuzumab-induced cardiotoxicity. J Am Soc Echocardiogr. 2013;26:493–8. doi: 10.1016/j.echo.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 22.Sawaya H, Sebag IA, Plana JC, et al. Assessment of Echocardiography and Biomarkers for the Extended Prediction of Cardiotoxicity in Patients Treated With Anthracyclines, Taxanes, and Trastuzumab. Circulation: Cardiovascular Imaging. 2012;5:596–603. doi: 10.1161/CIRCIMAGING.112.973321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stoodley PW, Richards DA, Hui R, et al. Two-dimensional myocardial strain imaging detects changes in left ventricular systolic function immediately after anthracycline chemotherapy. Eur J Echocardiogr. 2011;12:945–52. doi: 10.1093/ejechocard/jer187. [DOI] [PubMed] [Google Scholar]
- 24.Armenian SH, Sun CL, Vase T, et al. Cardiovascular risk factors in hematopoietic cell transplantation survivors: role in development of subsequent cardiovascular disease. Blood. 2012;120:4505–12. doi: 10.1182/blood-2012-06-437178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chow EJ, Baker KS, Lee SJ, et al. Influence of conventional cardiovascular risk factors and lifestyle characteristics on cardiovascular disease after hematopoietic cell transplantation. J Clin Oncol. 2014;32:191–8. doi: 10.1200/JCO.2013.52.6582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hudson MM, Ness KK, Nolan VG, et al. Prospective medical assessment of adults surviving childhood cancer: Study design, cohort characteristics, and feasibility of the St. Jude Lifetime Cohort Study. Pediatr Blood Cancer. 2011;56:825–36. doi: 10.1002/pbc.22875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 28.Takigiku K, Takeuchi M, Izumi C, et al. Normal range of left ventricular 2-dimensional strain: Japanese Ultrasound Speckle Tracking of the Left Ventricle (JUSTICE) study. Circulation journal : official journal of the Japanese Circulation Society. 2012;76:2623–32. doi: 10.1253/circj.cj-12-0264. [DOI] [PubMed] [Google Scholar]
- 29.Marwick TH, Leano RL, Brown J, et al. Myocardial strain measurement with 2-dimensional speckle-tracking echocardiography: definition of normal range. JACC Cardiovasc Imaging. 2009;2:80–4. doi: 10.1016/j.jcmg.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 30.Saleh HK, Villarraga HR, Kane GC, et al. Normal left ventricular mechanical function and synchrony values by speckle-tracking echocardiography in the transplanted heart with normal ejection fraction. J Heart Lung Transplant. 2011;30:652–8. doi: 10.1016/j.healun.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 31.Narayanan A, Aurigemma GP, Chinali M, Hill JC, Meyer TE, Tighe DA. Cardiac mechanics in mild hypertensive heart disease: a speckle-strain imaging study. Circ Cardiovasc Imaging. 2009;2:382–90. doi: 10.1161/CIRCIMAGING.108.811620. [DOI] [PubMed] [Google Scholar]
- 32.Kocabay G, Muraru D, Peluso D, et al. Normal left ventricular mechanics by two- dimensional speckle-tracking echocardiography. Reference values in healthy adults. Revista espanola de cardiologia. 2014;67:651–8. doi: 10.1016/j.rec.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 33.Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–33. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 34.Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr. 2009;10:165–93. doi: 10.1093/ejechocard/jep007. [DOI] [PubMed] [Google Scholar]
- 35.Stovall M, Weathers R, Kasper C, et al. Dose reconstruction for therapeutic and diagnostic radiation exposures: use in epidemiological studies. Radiat Res. 2006;166:141–57. doi: 10.1667/RR3525.1. [DOI] [PubMed] [Google Scholar]
- 36.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). Jama. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 37.Whaley MH, Brubaker PH, Otto RM, Armstrong LE. ACSM's Guidelines for Exercise Prescription. 7th ed. Lippincott, Williams, & Wilkins; Baltimore, MD: 2006. [Google Scholar]
- 38.ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–7. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 39.Pollentier B, Irons SL, Benedetto CM, et al. Examination of the six minute walk test to determine functional capacity in people with chronic heart failure: a systematic review. Cardiopulmonary physical therapy journal. 2010;21:13–21. [PMC free article] [PubMed] [Google Scholar]
- 40.Zeltzer LK, Lu Q, Leisenring W, et al. Psychosocial outcomes and health-related quality of life in adult childhood cancer survivors: a report from the childhood cancer survivor study. Cancer Epidemiol Biomarkers Prev. 2008;17:435–46. doi: 10.1158/1055-9965.EPI-07-2541. [DOI] [PubMed] [Google Scholar]
- 41.Nathan PC, Ford JS, Henderson TO, et al. Health behaviors, medical care, and interventions to promote healthy living in the Childhood Cancer Survivor Study cohort. J Clin Oncol. 2009;27:2363–73. doi: 10.1200/JCO.2008.21.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brouwer CA, Postma A, Vonk JM, et al. Systolic and diastolic dysfunction in long-term adult survivors of childhood cancer. Eur J Cancer. 2011;47:2453–62. doi: 10.1016/j.ejca.2011.05.023. [DOI] [PubMed] [Google Scholar]
- 43.van der Pal HJ, van Dalen EC, Hauptmann M, et al. Cardiac function in 5-year survivors of childhood cancer: a long-term follow-up study. Arch Intern Med. 2010;170:1247–55. doi: 10.1001/archinternmed.2010.233. [DOI] [PubMed] [Google Scholar]
- 44.Kremer LC, van Dalen EC, Offringa M, Voute PA. Frequency and risk factors of anthracycline-induced clinical heart failure in children: a systematic review. Ann Oncol. 2002;13:503–12. doi: 10.1093/annonc/mdf118. [DOI] [PubMed] [Google Scholar]
- 45.Lancellotti P, Nkomo VT, Badano LP, et al. Expert consensus for multi-modality imaging evaluation of cardiovascular complications of radiotherapy in adults: a report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. European heart journal cardiovascular Imaging. 2013;14:721–40. doi: 10.1093/ehjci/jet123. [DOI] [PubMed] [Google Scholar]
- 46.Adams MJ, Lipsitz SR, Colan SD, et al. Cardiovascular status in long-term survivors of Hodgkin's disease treated with chest radiotherapy. J Clin Oncol. 2004;22:3139–48. doi: 10.1200/JCO.2004.09.109. [DOI] [PubMed] [Google Scholar]
- 47.Guldner L, Haddy N, Pein F, et al. Radiation dose and long term risk of cardiac pathology following radiotherapy and anthracyclin for a childhood cancer. Radiother Oncol. 2006;81:47–56. doi: 10.1016/j.radonc.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 48.Heidenreich PA, Hancock SL, Vagelos RH, Lee BK, Schnittger I. Diastolic dysfunction after mediastinal irradiation. Am Heart J. 2005;150:977–82. doi: 10.1016/j.ahj.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 49.Curtis JP, Sokol SI, Wang Y, et al. The association of left ventricular ejection fraction, mortality, and cause of death in stable outpatients with heart failure. J Am Coll Cardiol. 2003;42:736–42. doi: 10.1016/s0735-1097(03)00789-7. [DOI] [PubMed] [Google Scholar]
- 50.Thavendiranathan P, Poulin F, Lim KD, Plana JC, Woo A, Marwick TH. Use of Myocardial Strain Imaging by Echocardiography for the Early Detection of Cardiotoxicity in Patients During and After Cancer Chemotherapy - A Systematic Review. J Am Coll Cardiol. 2014 doi: 10.1016/j.jacc.2014.01.073. [DOI] [PubMed] [Google Scholar]
- 51.Geyer H, Caracciolo G, Abe H, et al. Assessment of Myocardial Mechanics Using Speckle Tracking Echocardiography: Fundamentals and Clinical Applications. Journal of the American Society of Echocardiography. 2010;23:351–369. doi: 10.1016/j.echo.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 52.Mavinkurve-Groothuis AMC, Groot-Loonen J, Marcus KA, et al. Myocardial Strain and Strain Rate in Monitoring Subclinical Heart Failure in Asymptomatic Long-Term Survivors of Childhood Cancer. Ultrasound in Medicine & Biology. 2010;36:1783–1791. doi: 10.1016/j.ultrasmedbio.2010.08.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.