Abstract
Background
The aim of this study was to analyze the breast MRI characteristics of radiation-associated breast angiosarcomas (RAS).
Materials and Methods
In this institutional review board (IRB)-approved retrospective study, fifty-six (57) women were diagnosed with pathology confirmed RAS during the study period (January 1999 - May 2013). Seventeen women underwent pre-treatment breast MRI (prior to surgical resection or chemotherapy), of which 16 studies were available for review. Imaging features of these tumors were evaluated by two radiologists and correlated with clinical management and outcomes.
Results
Median age of patients at original breast cancer diagnosis was 69.3 years (range 42-84 years), with average time from initial radiation therapy to diagnosis of RAS of 7.3 years (range 5.1 – 9.5 years). Nine women had mammogram (9/16, 56%) and five had breast ultrasound (US) (5/16, 31%) prior to MRI, which demonstrated expected, non-suspicious findings of skin thickening in over half the cases. Four patients had distinct intraparenchymal masses on US, mammogram, and MRI. MRI findings included diffuse T2 high signal skin thickening (16/16, 100%). Nearly half (7/16, 44%) of patients had T2 low signal intensity lesions; all lesions rapidly enhanced on post-contrast T1 weighted imaging. All women underwent surgical resection, with 8/16 (50%) receiving neoadjuvant chemotherapy. Four women died during the study period.
Conclusion
Clinical, mammographic and sonographic findings of RAS are non-specific and may be occult on conventional breast imaging; MRI findings of RAS include rapidly enhancing dermal and intraparenchymal lesions, some of which are low signal on T2 weighted imaging.
Introduction
Breast conservation therapy (BCT) is considered the standard of care for treatment of early (stage I and II) breast cancer and consists of lumpectomy and whole-breast irradiation. Radiotherapy (RT) has been associated with the development of secondary soft tissue sarcomas with angiosarcoma being the most common[1]. Given the increasing use of BCT, the rate of secondary breast angiosarcoma has been reported with more frequency, with 0.9 per 1000 breast cancer incidence [2, 3].
The latency period of secondary breast angiosarcoma in women after BCT ranges from 2 to 23 years [4]. Secondary breast angiosarcomas can be difficult to detect clinically given that they develop in the background of radiation-induced skin changes and manifest as painless, erythematous or violaceous cutaneous nodules, which may be attributed to recent trauma [5]. Although rare, their occult clinical appearance can subsequently lead to delay in diagnosis and outcomes are overall poor [4, 5]. Treatment is surgery, often wide local excision depending on extent of disease, with or without chemotherapy [4].
Much is unknown about radiographic findings of radiation associated sarcomas (RAS) in the breast, as the current imaging literature on RAS is in the form of case reports and pictorial reviews [2, 3, 6-14], many of which describe both primary and secondary breast angiosarcoma cases, or do not differentiate pathologically these entities [2, 7, 10, 14]. Given the potential use of breast MRI in the follow-up of women with personal history of breast cancer, in addition to the non-specific findings of conventional mammography and sonography [14], the initial MRI imaging findings of secondary angiosarcoma may be useful in early detection. The purpose of this study is to describe the breast MRI imaging features of RAS in cohort of patients with histopathological and clinical correlation.
Methods and Materials
Subjects
Institutional review board approval was obtained for this HIPAA-compliant retrospective study and requirement for informed consent was waived. All available clinical, radiologic, and pathologic information of 57 patients with secondary, pathologically proven breast angiosarcoma between January 1999 and May 2013 at our institution were reviewed.
Clinical and Histopathologic Features
The clinical features of RAS, including dates of primary breast cancer diagnosis, time of radiation, time of first presentation of radiation induced breast angiosarcoma, primary presentation, treatment utilized, and overall outcome were documented. Pathologic features were recorded including mitotic rate, necrosis, and tumor margin.
MRI Protocol
Current breast MRI protocol at our institution includes prone imaging on either a GE Signa 1.5 Tesla, GE 3 Tesla HDX (General Electric Medical Systems, Milwaukee, WI), or a Siemens Trio 3 Tesla (Siemens Medical, North Carolina) scanner, using a dedicated breast surface coil (16 channel Siemens, 8 channel GE, or 7 channel InVivo (Orlando, Florida)). Sequences include a 3 plane localizing sequence, axial fat-suppressed T2W FSE or T2 STIR sequence, and axial fast spoiled gradient T1W non-fat suppressed sequence before contrast administration. Dynamic T1W fat-suppressed 3D fast spoiled gradient echo (3D FSPGR) sequences are then performed before and four times following intravenous administration of contrast (0.1 mmol/kg, Magnevist, Berlex, NJ) in the axial plane. A T1W 3D FSPGR delayed post contrast sequence is acquired in the sagittal plane. Prior to 2010, dynamic images were acquired in either the sagittal or the axial plane, with delayed imaging performed in the orthogonal plane. Post processing, including subtraction axial images, maximum intensity projections (MIP), and computer-aided diagnosis (CADSTREAM) is routinely employed.
Image and Data Analysis
Systematic review of all imaging studies, including all available mammograms, ultrasounds, and MRI (baseline and follow-up), was performed by two radiologists with 8 and 13 years of experience. The following MRI features of pathologically proven RAS were recorded: location, T1 and T2 signal intensity compared with skeletal muscle, degree and homogeneity of enhancement, and infiltration into adjacent structures.
Results
Clinical and Histopathological Findings
Fifty-seven women were diagnosed with pathology-confirmed RAS during the study period. Of these 57 women, seventeen (29.8%) underwent pre-treatment contrast-enhanced breast MRI examinations (prior to surgical resection or chemotherapy). Sixteen breast MRI studies were available for review and comprise the study cohort.
The median age at original breast cancer diagnosis of the study cohort was 69.3 years (range 42-84 years). The original breast cancer diagnosis (n=16) included invasive ductal carcinoma (IDC, n=13); invasive lobular carcinoma (ILC, n=1); invasive carcinoma with mixed lobular and ductal carcinoma (n=1); and ductal carcinoma in situ (DCIS, n=1). The average time from diagnosis of RAS and initial radiation therapy was 7.3 years (range 5.1 – 9.5 years). Initial biopsy (pre-definite treatment) demonstrated angiosarcoma (n=14), atypical post-radiation vascular proliferation [APRVP] (n=1), and de-differentiated round cell tumor (n=1). Both APRVP and de-differentiated round cell tumor were classified as angiosarcoma after definitive surgery. The indication for pre-treatment MRI imaging included assessing extent of disease after skin biopsy (n=13) and work-up of clinical skin findings (ecchymoses) without known histopathologic diagnosis (n=3).
Imaging Findings
Among these sixteen women, nine women underwent pre-MRI mammogram (9/16, 56.2%), which were available for review, as part of their imaging work-up. Five (5/9, 55.6%) were found to have expected skin thickening and post-treatment changes, and were therefore mammographically occult. The remaining four (4/9, 44.4%) were found to have underlying masses mammographically. Six women underwent pre-MRI ultrasound (6/16, 37.8%), of which three (50%) demonstrated expected skin thickening and non-specific post-treatment and clinical follow-up was recommended; the remaining three (50%) were found to have underlying irregular masses on both ultrasound and mammogram.
All cases of RAS demonstrated diffuse T2 hyperintense skin thickening (n=16). On precontrast T2 weighted images, seven out of 16 cases (7/16, 44%) demonstrated T2 low signal (hypointense) lesions within diffusely T2 hyperintense skin thickening. Five (5/16, 31%) demonstrated T2 heterogeneous intensity and four (4/16, 25%) were T2 hyperintense. On post contrast T1 weighted imaging, all masses demonstrated rapid enhancement with washout.
Four major patterns of contrast enhancement on post-contrast T1 weighted imaging were observed during image analysis (Figures 1-4). These patterns included diffuse, heterogeneous skin enhancement without discrete masses (n=7, 7/16); diffuse, heterogeneous skin enhancement with discrete masses (n=6, 6/16); multifocal small masses without diffuse skin enhancement (n=2, 2/16), and single discrete focus of enhancement (n=1, 1/16). Out of the sixteen cases, 10 (10/16, 62.5%) demonstrated disease in a quadrant away from the original lumpectomy site on MRI. No abnormal enhancement was seen at the lumpectomy sites in the sixteen cases.
Figure 1.
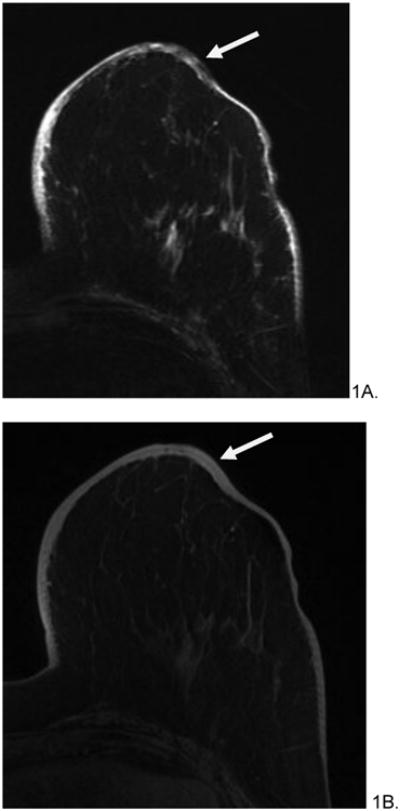
82 year old female with history of left breast invasive ductal carcinoma, status post lumpectomy and radiation treatment who presented with bruising around her nipple five years after radiation treatment. Initial mammogram (not shown) was reported as normal. Skin punch biopsy revealed postradiation angiosarcoma. Pre-surgical MRI revealed diffuse, non-mass like T2 hypointensity within skin (A, arrow), which appeared T1 isointense (B), and rapidly enhanced on post-contrast imaging (C). Subsequent mastectomy was performed and the patient has been disease free for 25 months after treatment.
Figure 4.
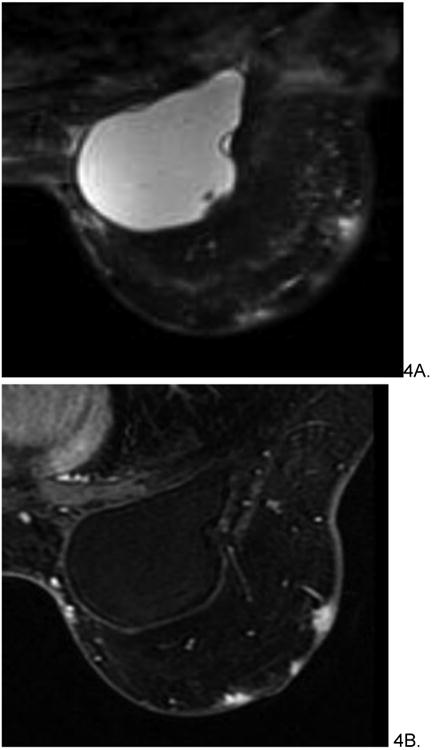
54 year old female with history of invasive ductal carcinoma, status post neoadjuvant chemotherapy, lumpectomy and radiation treatment 7 years prior who presented with bruising in left breast. Skin punch biopsy revealed post-radiation angiosarcoma involving dermis and subcutaneous tissue. Pre-treatment MRI demonstrated multifocal T2 hyperintense (A), rapidly enhancing small masses (B) without diffuse skin enhancement. The patient underwent pre-surgical chemotherapy with taxol before radical mastectomy and chest wall resection. She is currently disease free.
Of the sixteen cases, 4 demonstrated irregular, intraparenchymal masses in addition to skin involvement on MRI, of which four were demonstrated on mammography and three on ultrasound. The nipple asymmetrically enhanced and was pathologically involved in 8 cases (8/16). Imaging findings may be found in Table 1.
Table1.
| # | Age at Diagnosis | Original Breast Cancer | Time after radiation (years) | T2 signal | Distribution of enhancement | Discrete Intraparenchymal Masses | Treatment | Chemo? | Mets | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 67 | IDC | 7.2 | Hypointense | Diffuse, heterogeneous skin enhancement; no discrete masses | No | Radical chest wall resection and mastectomy | No | No | Alive |
| 2 | 75 | DCIS | 7.1 | Hyperintense | Diffuse, heterogeneous skin enhancement with discrete masses | Yes | Mastectomy | No | No | Deceased |
| 3 | 54 | IDC | 7.1 | Hyperintense | Multifocal small masses without diffuse skin enhancement | No | Radical chest wall resection and mastectomy | Yes | Yes | Alive |
| 4 | 84 | IDC | 8.7 | Hypointense | Diffuse, heterogeneous skin enhancement; no discrete masses | Yes | Radical chest wall resection and mastectomy | No | Yes | Deceased |
| 5 | 60 | IDC | 8.1 | Hypointense | Diffuse, heterogeneous skin enhancement with discrete masses | Yes | Radical chest wall resection and mastectomy | Yes | Yes | Alive |
| 6 | 68 | IDC | 9.5 | Heterogeneous | Diffuse, heterogeneous skin enhancement with discrete masses | No | Radical chest wall resection and mastectomy | Yes | No | Alive |
| 7 | 64 | IDC | 7.4 | Hypointense | Diffuse, heterogeneous skin enhancement; no discrete masses | Yes | Mastectomy | No | Yes | Deceased |
| 8 | 42 | IDC | 5.1 | Heterogeneous | Diffuse, heterogeneous skin enhancement with discrete masses | No | Mastectomy | No | No | Deceased |
| 9 | 67 | IDC | 6.3 | Hypointense | Diffuse, heterogeneous skin enhancement with discrete masses | No | Radical chest wall resection and mastectomy | Yes | No | Alive |
| 10 | 66 | IDLC | 7.6 | Heterogeneous | Single focus of enhancement | No | Mastectomy | Yes | No | Alive |
| 11 | 75 | IDC | 8.4 | Heterogeneous | Diffuse, heterogeneous skin enhancement with discrete masses | No | Mastectomy | No | Yes | Alive |
| 12 | 82 | IDC | 5.3 | Hypointense | Diffuse, heterogeneous skin enhancement; no discrete masses | No | Mastectomy | No | No | Alive |
| 13 | 75 | ILC | 7.2 | Hyperintense | Diffuse, heterogeneous skin enhancement; no discrete masses | No | Radical chest wall resection and mastectomy | Yes | No | Alive |
| 14 | 82 | IDC | 9.3 | Hyperintense | Diffuse, heterogeneous skin enhancement; no discrete masses | No | Radical chest wall resection and mastectomy | Yes | No | Alive |
| 15 | 82 | IDC | 5.7 | Heterogeneous | Diffuse, heterogeneous skin enhancement; no discrete masses | No | Surgical excision | Yes | No | Alive |
| 16 | 66 | IDC | 5.8 | Hypointense | Multifocal small masses without diffuse skin enhancement | No | Radical chest wall resection and mastectomy | No | No | Alive |
IDC = invasive ductal carcinoma; IDLC = invasive ductal and lobular carcinoma; ILC = invasive lobular carcinoma; Mets = metastases; Chemo = chemotherapy
Treatment and Outcome
Eight women underwent surgical management without chemotherapy. Surgical management after diagnosis included initial surgical excision with subsequent radical mastectomy with chest wall resection (n=2); mastectomy with subsequent chest wall resection (n=1); mastectomy only (n=4); and radical mastectomy with chest wall resection (n=1).
Neoadjuvant chemotherapy was administered to eight of the 16 women; chemotherapy regimens included taxol only (n=1), paclitaxil only (n=2), gemcitabine only (n=1), gemcitabine and docetaxil (n=1), gemcitabine and paclitaxil (n=2), and gemcitabine, paclitaxil and bevacizumab (n=1). Of these eight women, seven underwent radical mastectomy with chest wall resection. The remaining patient had surgical excision without further surgical treatment given she was a poor surgical candidate for other reasons; she is currently on gemcitabine palliative maintenance therapy without evidence of recurrence or metastases to date.
Of the sixteen women, 4 (4/16, 25%) developed recurrence within the chest wall in an average of 7 months (range - 3 months to 1.5 years) after initial resection. Six (6/16) developed local or metastatic disease including within the lungs (n=2), lung and bone (n=1), liver (n=1), contralateral breast (n=1) and axillary lymph node (n=1). The average time to metastatic disease from angiosarcoma diagnosis was 5.5 months (range 2-5 months).
Four women died during the study period. Three of these four women had intraparenchymal masses on presentation (3/4, 75%). Two of the four women who died had metastatic disease (lung and bones (n=1) and liver (n=1)) in addition to intraparenchymal masses.
Discussion
Radiation associated secondary breast angiosarcoma is a rare, but important clinical entity whose MRI imaging characteristics have been previously described only in case reports [1, 3, 4, 6, 8-13]. Our study provides the largest cohort of pre-treatment breast MRIs on biopsy proven angiosarcomas to date, and sheds light on the entity's imaging characteristics.
Secondary angiosarcoma is clinically and pathologically distinct from primary angiosarcoma; secondary RAS arises within the skin of previously irradiated breast tissue, with variable invasion in the underlying parenchyma [2, 5, 15, 16]. This differs from primary angiosarcoma, which tends to arise in the breast parenchyma, with variable involvement of skin. Secondary angiosarcoma in the pre-BCT era was most often seen in patients with mastectomy and axillary dissection, and associated with the occurrence of lymphedema [4].
Mammographic and sonographic findings of secondary breast angiosarcoma can be non-suspicious, with expected post-treatment changes, which may lead to delays in diagnosis and treatment. Of the 9 patients who underwent pre-MRI mammograms available for review, over half (5/9, 55%) had expected skin thickening with post-radiation and lumpectomy changes. The remaining four patients (4/9, 45%) were found to have underlying masses on mammogram, but these women were also the only four who demonstrated intraparenchymal masses on subsequent breast MRI. Sonographic findings of secondary breast angiosarcoma are similar to that of mammography, as half the women who underwent breast ultrasound (3/6, 50%) were found to have non-specific skin thickening and post-treatment changes. Given the post-radiation changes of skin thickening, predominately dermal lesions may be particularly difficult to delineate on ultrasound.
The majority of patients (13/16, 81.3%) were found to have diffuse heterogeneous skin enhancement with or without cutaneous, enhancing masses. In addition, 25% (4/16) were found to have intraparenchymal involvement as demonstrated by irregular masses. These findings corroborate variety of findings published in the case report literature, which vary in description of morphological appearances; for example, Sanders et al described small (<1 cm) enhancing skin lesions [12], whereas Vuille-dit-bille et al described non-specific skin thickening and enhancement, without focal nodularity or intraparenchymal masses [13]. Only one of our cases demonstrated a single focus of enhancement. The variation in morphology found in our study more reflects what is described in the pathologic literature by Brenn and Fletcher; of the 26 cases of secondary cutaneous breast angiosarcoma, the number of lesions described varied from one to thirty [15]. Given the range of findings in RAS as seen in our study, as well as the nonspecific nature of mammography and ultrasound with features indistinguishable from expected post-treatment changes, breast MRI may provide an important imaging modality to evaluate the true extent of disease when this diagnosis is clinically suspected.
In addition, all previous case reports describe secondary breast angiosarcoma as having low signal intensity on T1 weighted imaging and high signal on T2 weighted imaging, presumably due to the vascular origin of the tumor [6, 8-13]. However, nearly half the cases in our study (7/16, 44%) which showed nodular foci of rapid early arterial enhancement with washout were hypointense on T2-weighted images. Assosciated diffuse skin thickening was T2 hyperintense and showed persistent enhancement. We hypothesize that the T2 hyperintense skin thickening with persistent enhancement could be related to post radiation inflammatory changes, and T2 hypointense foci with rapid early arterial enhancement and washout, could represent tumor foci. This warrants further investigation with imaging-pathology correlation, and if vaildated could have significant diagnostic potential.
The mortality of RAS is high, with high local recurrence rates [4, 5]. Current primary treatment options include surgery involving the whole breast and all at-risk irradiated tissue, including wide local excision and clear margins [4]. Our study demonstrated over half (10/16, 62.5%) of cases with regions of secondary angiosarcoma identified in a different quadrant from the original breast cancer and away from the lumpectomy bed. In addition, the role of chemotherapy is still under investigation with trials evaluating anti-angiogenesis agents such as Bevacizumab in progress [17]. Treatment with re-radiation is controversial, given both the toxicity associated with repeat radiation to an already treated field and the presumed role that the original radiation treatment had in the development of this malignancy [4].
Our study demonstrates the patterns of RAS on breast MRI and introduces the modalities use in evaluating the morphologic characterization of RAS as well as extent of disease, particularly in light of the non-specific clinical and conventional mammographic imaging features. Mammograhy and sonography can be non-suspicious, and as seen in our study population, only concerning when underlying masses were present. MRI provides a more sensitive modality for detecting skin enhancement. From a radiologist's perspective, it is important to document all involved regions, including contralateral side. Smaller lesions may be obscured by diffuse skin thickening in the post-irradiated breast and therefore, it is important to scrutinize breast for any more focal, mass like skin enhancement as it may represent a small focus of angiosarcoma.
The study has some limitations. First, the study was retrospective in nature. Secondly, the study cohort had a relatively small number of patients. Finally, our institution is a tertiary care center and initial imaging (mammograms and ultrasound) was not available for review on all patients. However, RAS is a rare condition, and to our knowledge, this study is the most extensive describing the breast MRI features to date. Additional studies would be helpful for confirming the conclusions.
In conclusion, radiation associated angiosarcoma of the breast may be diffuse or focal on MRI, may be T2 low signal, and rapidly enhance on post-contrast imaging. Mammographic and sonographic findings may be non-specific, and MRI provides superior morphologic characterization and extent of disease. Further prospective studies may provide important information on the utility of MRI in evaluating this rare, however, aggressive disease.
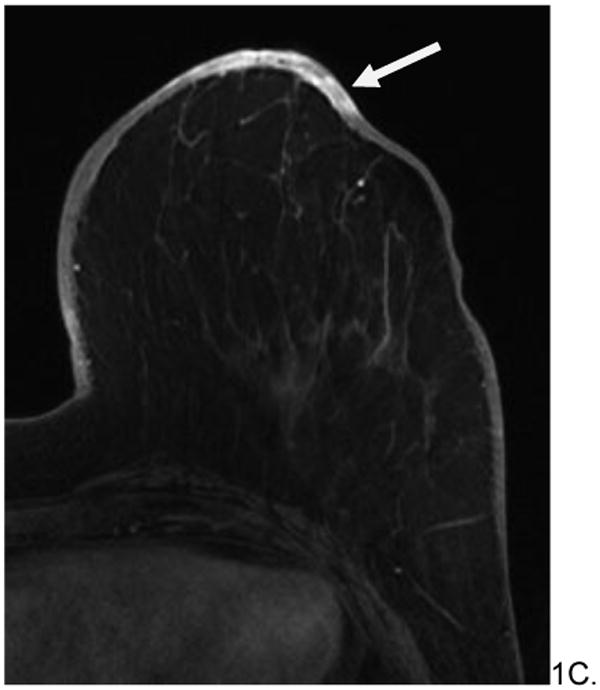
Figure 2.
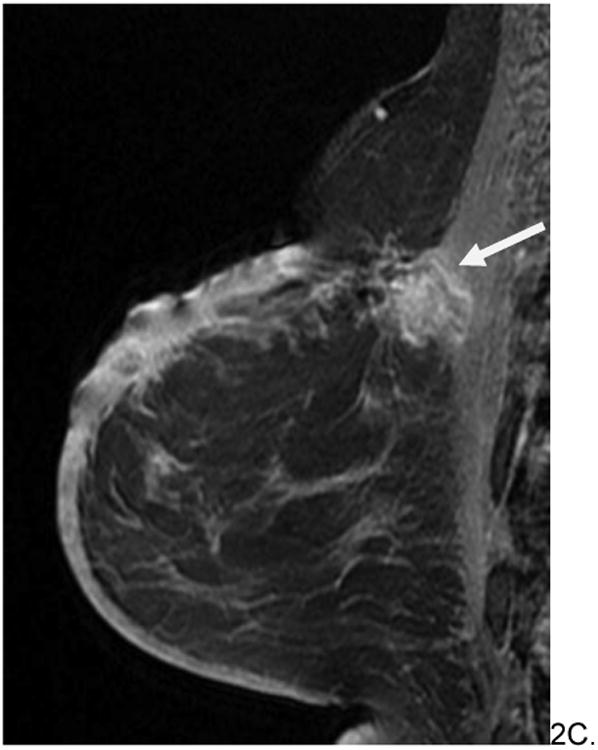
60 year old woman with history of left breast invasive ductal carcinoma status post lumpectomy and radiation eight years prior who presented with increased edema around surgical skin scar. No mammograms or ultrasounds were available for review. Skin bunch biopsy revealed post-radiation angiosarcoma. Pre-surgical MRI revealed predominately T2 hypointense non-mass skin thickening with an associated T2 hypointense posterior irregular mass (arrows, A), which were T1 hypointense (B) and enhanced rapidly on post contrast imaging (C). The patient underwent pre-surgical chemotherapy with paclitaxil and gemcitabine before radical mastectomy and chest wall resection. She is currently disease free.
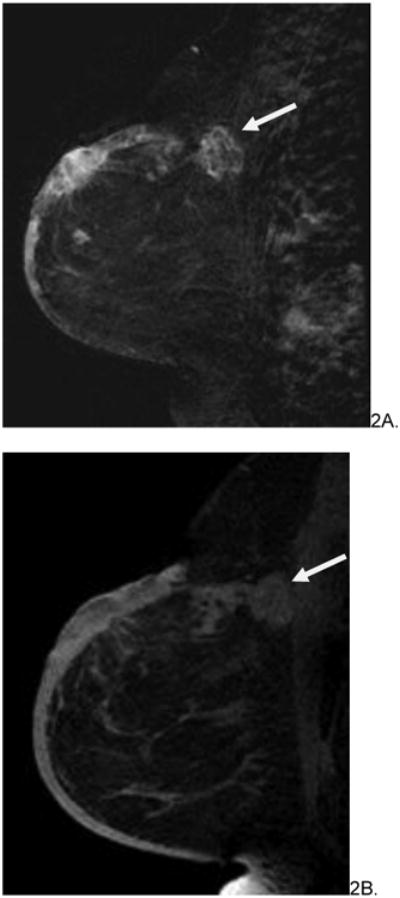
Figure 3.
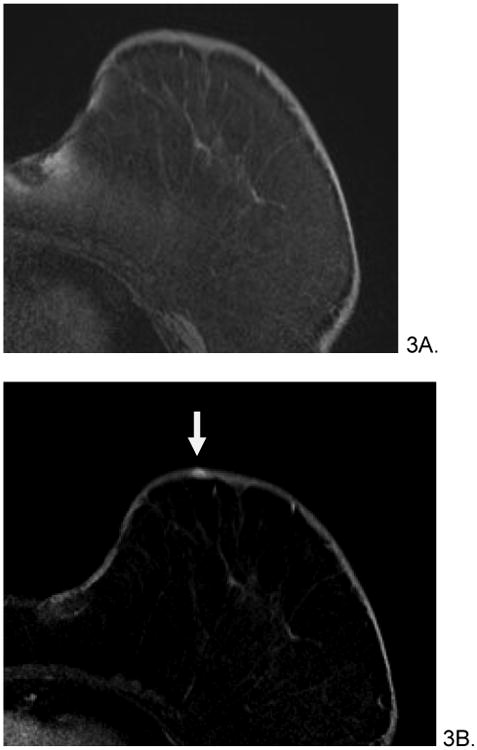
66 year old female with history of invasive ductal and lobular carcinoma of left breast, status post lumpectomy and radiation therapy 7 years prior who presented with left breast violaceous skin lesion. Skin punch biopsy revealed angiosarcoma. Pre-treatment breast MRI revealed T1 isointense (A), T2 heterogenous (not shown), focus of rapid enhancement (B, arrow). The patient underwent pre-surgical chemotherapy with taxol and gemcitabine before mastectomy. She is currently disease free.
References
- 1.Huang J, Mackillop WJ. Increased risk of soft tissue sarcoma after radiotherapy in women with breast carcinoma. Cancer. 2001;92:172–180. doi: 10.1002/1097-0142(20010701)92:1<172::aid-cncr1306>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 2.Glazebrook KN, Magut MJ, Reynolds C. Angiosarcoma of the breast. AJR American journal of roentgenology. 2008;190:533–538. doi: 10.2214/AJR.07.2909. [DOI] [PubMed] [Google Scholar]
- 3.Cabete J, Lencastre A, Fidalgo A, Lobo L, Joao A, Serrao V. Postradiation cutaneous angiosarcoma of the breast: a diagnosis to keep in mind. The breast journal. 2014;20:89–90. doi: 10.1111/tbj.12215. [DOI] [PubMed] [Google Scholar]
- 4.Morgan EA, Kozono DE, Wang Q, et al. Cutaneous radiation-associated angiosarcoma of the breast: poor prognosis in a rare secondary malignancy. Annals of surgical oncology. 2012;19:3801–3808. doi: 10.1245/s10434-012-2563-4. [DOI] [PubMed] [Google Scholar]
- 5.Abbott R, Palmieri C. Angiosarcoma of the breast following surgery and radiotherapy for breast cancer. Nature clinical practice Oncology. 2008;5:727–736. doi: 10.1038/ncponc1242. [DOI] [PubMed] [Google Scholar]
- 6.Colwick S, Gonzalez A, Ngo N, Camuto P, Barajas D. Bilateral radiation-induced angiosarcoma of the breast. The breast journal. 2013;19:547–549. doi: 10.1111/tbj.12160. [DOI] [PubMed] [Google Scholar]
- 7.Liberman L, Dershaw DD, Kaufman RJ, Rosen PP. Angiosarcoma of the breast. Radiology. 1992;183:649–654. doi: 10.1148/radiology.183.3.1584913. [DOI] [PubMed] [Google Scholar]
- 8.Mery CM, George S, Bertagnolli MM, Raut CP. Secondary sarcomas after radiotherapy for breast cancer: sustained risk and poor survival. Cancer. 2009;115:4055–4063. doi: 10.1002/cncr.24462. [DOI] [PubMed] [Google Scholar]
- 9.Moore A, Hendon A, Hester M, Samayoa L. Secondary angiosarcoma of the breast: can imaging findings aid in the diagnosis? The breast journal. 2008;14:293–298. doi: 10.1111/j.1524-4741.2008.00577.x. [DOI] [PubMed] [Google Scholar]
- 10.O'Neill AC, D'Arcy C, McDermott E, O'Doherty A, Quinn C, McNally S. Magnetic resonance imaging appearances in primary and secondary angiosarcoma of the breast. Journal of medical imaging and radiation oncology. 2014;58:208–212. doi: 10.1111/1754-9485.12100. [DOI] [PubMed] [Google Scholar]
- 11.Rubin E, Maddox WA, Mazur MT. Cutaneous angiosarcoma of the breast 7 years after lumpectomy and radiation therapy. Radiology. 1990;174:258–260. doi: 10.1148/radiology.174.1.2152984. [DOI] [PubMed] [Google Scholar]
- 12.Sanders LM, Groves AC, Schaefer S. Cutaneous angiosarcoma of the breast on MRI. AJR American journal of roentgenology. 2006;187:W143–146. doi: 10.2214/AJR.05.1940. [DOI] [PubMed] [Google Scholar]
- 13.Vuille-dit-Bille RN, Sauter D, Pfofe D, et al. High-grade cutaneous angiosarcoma of the breast 8.5 years after radiotherapy. The breast journal. 2013;19:435–436. doi: 10.1111/tbj.12130. [DOI] [PubMed] [Google Scholar]
- 14.Yang WT, Hennessy BT, Dryden MJ, Valero V, Hunt KK, Krishnamurthy S. Mammary angiosarcomas: imaging findings in 24 patients. Radiology. 2007;242:725–734. doi: 10.1148/radiol.2423060163. [DOI] [PubMed] [Google Scholar]
- 15.Brenn T, Fletcher CD. Radiation-associated cutaneous atypical vascular lesions and angiosarcoma: clinicopathologic analysis of 42 cases. The American journal of surgical pathology. 2005;29:983–996. [PubMed] [Google Scholar]
- 16.Brenn T, Fletcher CD. Postradiation vascular proliferations: an increasing problem. Histopathology. 2006;48:106–114. doi: 10.1111/j.1365-2559.2005.02293.x. [DOI] [PubMed] [Google Scholar]
- 17.Azzariti A, Porcelli L, Mangia A, et al. Irradiation-induced angiosarcoma and anti-angiogenic therapy: A therapeutic hope? Experimental cell research. 2014;321:240–247. doi: 10.1016/j.yexcr.2013.12.018. [DOI] [PubMed] [Google Scholar]


