Abstract
Purpose
To facilitate localization and resection of small lung nodules, we developed a prospective clinical trial (ClinicalTrials.gov number NCT01847209) for a novel surgical approach which combines placement of fiducials using intra‐operative C‐arm computed tomography (CT) guidance with standard thoracoscopic resection technique using image‐guided video‐assisted thoracoscopic surgery (iVATS).
Methods
Pretrial training was performed in a porcine model using C‐arm CT and needle guidance software. Methodology and workflow for iVATS was developed, and a multi‐modality team was trained. A prospective phase I‐II clinical trial was initiated with the goal of recruiting eligible patients with small peripheral pulmonary nodules. Intra‐operative C‐arm CT scan was utilized for guidance of percutaneous marking with two T‐bars (Kimberly‐Clark, Roswell, GA) followed by VATS resection of the tumor.
Results
Twenty‐five patients were enrolled; 23 underwent iVATS, one withdrew, and one lesion resolved. Size of lesions were: 0.6–1.8 cm, mean = 1.3 ± 0.38 cm.. All 23 patients underwent complete resection of their lesions. CT imaging of the resected specimens confirmed the removal of the T‐bars and the nodule. Average and total procedure radiation dose was in the acceptable low range (median = 1501 μGy*m2, range 665–16,326). There were no deaths, and all patients were discharged from the hospital (median length of stay = 4 days, range 2–12). Three patients had postoperative complications: one prolonged air‐leak, one pneumonia, and one ileus.
Conclusions
A successful and safe step‐wise process has been established for iVATS, combining intra‐operative C‐arm CT scanning and thoracoscopic surgery in a hybrid operating room. J. Surg. Oncol. 2015 111:18–25. © 2015 The Authors. Journal of Surgical Oncology Published by Wiley Periodicals, Inc.
Keywords: lung cancer, advanced image guided operating room, hybrid operating room, fiducials, VATS, C‐arm CT
INTRODUCTION
Lung Cancer continues to have high morbidity and mortality, and it is estimated that in 2014 in the United States there were approximately 224,210 new cases and 159,260 deaths from lung cancer, accounting for about 27% of all cancer deaths 1, 2. The National Lung Screening Trial demonstrated that screening for lung cancer with chest computed tomography (CT) results in a 20% reduction in mortality 3, 4, 5. However, most nodules detected by CT were not cancer but required interval follow‐up and/or biopsies for definitive diagnosis, translating to a large and technically challenging diagnostic burden. Proposed criteria (Early Lung Cancer Action Project) recommend biopsy and/or surgical resection for nodules larger than 1 cm and follow‐up with CT scans for smaller lesions to demonstrate stability 4, 6, 7.
In view of the recent United States Preventative Services Task Force category B recommendations for Lung Cancer Screening (LCS), it is expected that many patients with small lesions (<2 cm), enlarging lesions, and partially solid lesions that are suspicious for cancer will be discovered and will require surgical resection 5, 8, 9. The difficulty in locating, palpating, and obtaining an accurate biopsy of small nodules was the rationale for designing a clinical trial with the goal of resecting early peripheral lung cancers with minimally invasive surgery. The sensitivity of cancer diagnosis using percutaneous, image‐guided biopsy decreases with decreasing nodule size (e.g. 90% for 3 cm diameter versus 70% for 1 cm) and is substantially less accurate for Ground Glass Opacities (GGOs). Thus, patients with suspicious or enlarging lesions are often referred for surgical excision. However, the scope and extent of surgical resection depends on the ability to localize nodules, especially deep and lesions difficult to palpate. Intraoperative localization of these lesions can be challenging and can depend on factors such as size of the lesion, depth from the pleural surface, and size of the solid component 10.
Several techniques have been previously reported to assist in localizing peripheral lung lesions prior to resection, including the use of intraoperative transthoracic ultrasonography, percutaneous injection of dye (lipiodol, cyanoacrylate, “colored” collagen, barium sulphate, and methylene blue), image‐guided placement of microcoils, spring hook wires, and Kopans wire hooks 11, 12. These multidisciplinary approaches, involving image‐guided placement of radiopaque or visual markers prior to the patient being moved to the operating room, have proven to be very helpful, however they are associated with the risks for contamination, marker migration, and lung injury while transporting the patient from the CT suite to the operating room. The optimal strategy would be to provide the ability to place markers using image guidance in the hybrid OR, immediately prior to resection, with the patient already in the surgical position with lung isolation established during a single anesthetic.
Our aim was to develop high‐precision interventions by improving current surgical and diagnostic techniques using intraoperative image guidance to facilitate successful lesion identification and resection. The primary objective of the clinical trial reported herein was establishing a safe and optimal procedural workflow for combining image‐guidance with immediate surgery. The secondary objectives were to determine the peri‐operative outcomes including successful nodule localization and to complete excision of the nodule.
METHODS
With approval from Institutional Review Board (ClinicalTrials.gov number NCT01847209), we prospectively enrolled subjects with small pulmonary nodules suspicious for malignancy who were referred for surgery at a single academic medical center.
Patient Selection
The study cohort included patients suitable for VATS lung wedge resection due to limited lung function, prior lobectomy, small nodules, multiple bilateral nodules, and/or patient preference. Lesions included solid, partially solid, and ground glass opacities (GGOs) measuring ≤ 30 mm in diameter on preoperative evaluation of CT imaging. All patients were older than 18 years of age.
iVATS (IMAGE GUIDED VIDEO ASSISTED THORACOSCOPIC SURGERY)
Lesion Localization
Pre‐operative CT scans were used to generate three‐dimensional surface models for surgical planning (Fig. 1A, B). The images were reviewed by the team to determine optimal placement of fiducials. Patients were brought into the Advanced Multimodality Image Guided Operating (AMIGO) Room, general anesthesia was administered, bronchoscopic examination performed, a double‐lumen endo‐bronchial tube was positioned, and patients were placed in the lateral decubitus position. A C‐arm CT scan of the pre‐determined field of view that included the nodule position was acquired during an end‐inspiratoryhold maneuver using a 5 sec scan protocol with 0.36 μGy/projection and 248 projections acquired over 200°. The radiologist reviewed the C‐arm CT scan to localize the nodule and plan trajectories for percutaneous T‐bar placement using Syngo iGuide needle guidance software (Siemens Healthcare AG, Forchheim, Germany) (Fig. 1C). The planned needle pathways were integrated into the C‐arm fluoroscopic imaging system, which provided laser crosshair and guidance markers on fluoroscopy images to direct the needle pathway for T‐bar placement (Kimberly‐Clark, Roswell, GA) (Fig. 1E, F).
Figure 1.
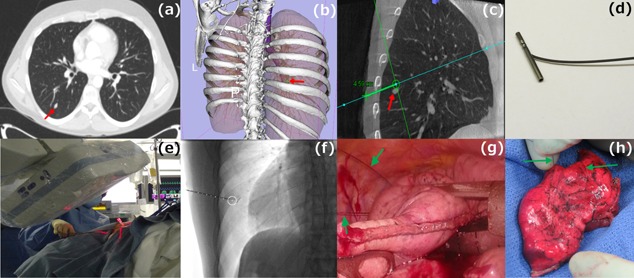
(a) Pre‐operative axial CT image depicting a 6mm solid right lower lobe nodule. (b) 3D volume rendered image showing the relationship of the nodule to the bones. (c) i‐Guide pathway planning on sagittal C‐arm CT image. (d) T‐bar (fiducial). (e) Placement of the fiducials under fluoroscopic guidance using i‐Guide. (f) Fluoroscopy image after deployment of the fiducials around the target. (g) Intra‐operative image showing the fiducial wires extending to the chest wall from the collapsed lung. (h) Resected lung specimen with both wires extending through the lung.
Surgery
Patients were prepped and draped for VATS, and a wedge resection was performed with guidance and thoracoscopic visualization of the T‐bar sutures (Fig. 1G). Lymphadenectomy or lymph node sampling was routinely performed. The specimen and T‐bars were removed using an endo‐bag. CT scan of the excised lung wedge (Fig. 1H) was acquired in an adjoining room to ensure complete excision of the T‐bars and nodule. Frozen section histological analysis provided the diagnosis and confirmed negative margins of resection. All incisions were closed, the chest was drained and the patient awoken, extubated, and transferred to recovery. (Video 1)
Study Objectives
The primary objective of the study was to establish safe and optimal procedural workflow. The secondary objectives were to determine the peri‐operative outcomes including successful nodule localization via placed T‐bar fiducials and to complete excision of the nodule and optimization of radiation dose for the procedure.
RESULTS
Twenty‐five eligible patients were enrolled in this study, and 23 patients had successful resection of their pulmonary nodules. One patient withdrew from the trial prior to surgery, and one patient was found to have complete resolution of the nodule on the intra‐operative CT scan.
Of the 23 patients who underwent surgery, seven were men and 16 were women with a median age of 65 years (Table I). Most patients were current or former cigarette smokers. In accordance with the trial's eligibility criteria, the indication for the VATS wedge resection versus lobectomy in each of these patients was the small nodule size, prior lobectomy, limited lung function, expectation for future contralateral lung resection, or patient preference. By preoperative CT scan evaluation, 15 of the nodules were either GGOs or partially solid. The median nodule size by preoperative CT scan was 1.3 cm. (Fig. 2) of the 22 cancers, 18 were primary lung cancers and four were metastatic lesions. Seven of the primary malignant nodules were minimally invasive adenocarcinoma with some degree of invasive component and one nodule was adenocarcinoma in situ. All of the resected lung cancers were partially solid or mixed ground glass lesions, and the adenocarcinoma in situ was pure ground glass attenuation.
Table I.
Patient Characteristics (N = 23)
| Gender (N) | |
| Male | 7 |
| Female | 16 |
| Age | |
| Median | 65 |
| Range | 19–80 |
| Comorbidities | |
| Pulmonary a | 6 |
| COPD b | 5 |
| Pulmonary hypertension b | 1 |
| Cardiac a | 3 |
| Non‐ischemic cardiomyopathy b | 1 |
| CAD b | 1 |
| CHF b | 1 |
| Prior cancer history a | 11 |
| Squamous cell carcinoma of the tongue b | 1 |
| Prostate cancer b | 2 |
| Renal cell carcinoma b | 1 |
| Breast cancer b | 5 |
| Lung cancer b | 4 |
| Hodgkin's lymphoma b | 1 |
| Endometrial cancer b | 1 |
| Smoking History (N) | |
| ≥30 pack‐years | 9 |
| (Quit >15 years ago) | (5) |
| <30 pack‐years | 6 |
| Pack‐years unknown, not current smokers | 2 |
| Never smokers | 6 |
COPD, Chronic Obstructive Pulmonary Disease; CAD, Coronary Artery Disease; CHF, Congestive Heart Failure.
Number of Patients.
Number of Cases.
Figure 2.
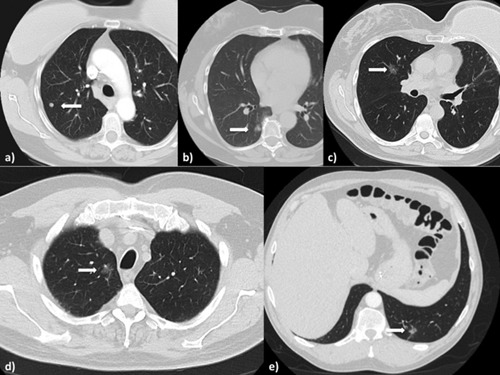
(a) Solid right upper lobe nodule in a patient with breast cancer and sarcoma, found to be consistent with a sarcoma metastasis. (b) Right lower lobe part solid nodule, on frozen section found to have micropapillary feature, prompting a decision for completion lobectomy. (c) Right upper lobe part solid lesion biopsy, was found to be consistent with lung cancer. (d) Right upper lobe part solid lesion with progressive growth, final pathology consistent with a granuloma. (e) Part solid lesion in the left lower lobe stable on two scans 3 months apart, but resolved on the day of the surgery.
Study Outcomes
Twenty‐three of 24 nodules were identified by intra‐operative C‐ arm CT scan and completely resected by VATS. The patient whose nodule had resolved had a follow up CT scan 6 months later with confirmation of complete resolution. Twenty patients (87.0%) had successful intra‐operative fluoroscopy‐guided placement of two flanking T‐bars to localize the small pulmonary nodule. The sutures attached to the two T‐bars placed around the lesions in these patients were visible on the pleural surface of the lung at thoracoscopy. Three patients underwent successful T‐bar placement, but at thoracoscopy both T‐bars were dislodged in one patient and one of the two T‐bars was dislodged in two patients. Though the T‐bars were dislodged from the lung parenchyma, all three nodules were completely excised utilizing the information from the planning C‐arm CT scan and entry marks of the needle into the lung. One patient required a completion lobectomy at the time of surgery because of micropapillary features found in the lesion during frozen pathology analysis of the wedge resection.
Median length of time for completion of each case measured from when the patient was brought into the operating room to when the patient was taken to recovery was 212 (range 144–297) min. Median duration from anesthesia induction to the first VATS incision was 130 (range 82–238) min (Table II). This period measures the time required for taking the intra‐operative C‐arm CT scan, calculating the trajectory and placing the T‐bars. The median lengths of time required for placement of the two T‐bars and for the VATS (“skin to skin”) were 39 (range 23–62) and 67 (38–156) min, respectively. The median radiation exposure from the intra‐operative C‐arm CT scan and fluoroscopy was 1501 μGy*m2 (range 665–16326). The dosimeter was non‐functional during part of one procedure, and thus only the radiation exposure from the C‐arm CT scan was recorded for this procedure.
Table II.
Operative Time Course and Radiation Exposure From Intra‐Operative CT and Fluoroscopy (Patients 6 and 22 did not Undergo Surgery)
| Patient (N = 23) | Induction to incision time (min) | Placement of first T‐bar to incision time (min) | Incision to close time (min) | Radiation exposure (mSv) |
|---|---|---|---|---|
| 1 | 135 | 35 | 62 | 36.5 |
| 2 | 150 | 23 | 72 | 17.5 |
| 3 | 131 | 41 | 84 | 3.5 |
| 4 | 119 | 36 | 156 | 5.1 |
| 5 | 141 | 26 | 105 | 2.6 |
| 7 | 142 | 52 | 83 | 8.8 |
| 8 | 136 | 39 | 116 | 3.1 |
| 9 | 132 | 36 | 65 | 6.3 |
| 10 | 158 | 48 | 58 | 4.1 |
| 11 | 111 | 41 | 100 | 4.1 |
| 12 | 99 | 41 | 55 | 2.8 |
| 13 | 142 | 43 | 76 | 2.3 |
| 14 | 108 | 33 | 59 | 4.0 |
| 15 | 130 | 37 | 50 | 2.7 |
| 16 | 124 | 40 | 67 | 2.2 |
| 17 | 88 | 36 | 90 | 5.0 |
| 18 | 82 | 34 | 79 | 2.8 |
| 19 | 140 | 62 | 54 | 3.6 |
| 20 | 122 | 42 | 63 | 4.7 |
| 21 | 116 | 51 | 100 | 6.3 |
| 23 | 238 | 29 | 40 | 3.1 |
| 24 | 118 | 52 | 38 | 3.3 |
| 25 | 91 | 30 | 40 | 3.2 |
The visible T‐bar sutures at the pleural surface of the wedge resection specimen as well as the postoperative CT scan of the resected lung confirmed the presence of the T‐bars in the lung wedge. Pathologic analysis confirmed the presence of the resected tumor and the T‐bars as well as adequacy of the margins of resection. In two patients, pneumothoraces occurred during the placement of the second T‐bars, but there was no associated hemodynamic compromise and no treatment was warranted, as the patients were about to undergo VATS with subsequent chest tube placements. All T‐bars were recovered.
Twenty‐two of the resected nodules demonstrated malignancies while one nodule was a benign granuloma (Table III).
Table III.
Radiological and Pathological Characteristics of the Resected Pulmonary Nodules
| Patient (N = 23) | Size of lesion in (cm) on pre‐operative CT scan | Actual size of lung nodule (Pathological) | Location of lesion | Associated or ancillary findings | CT appearance of the nodule and diagnosis based on history and imaging | Final pathological diagnosis | |
|---|---|---|---|---|---|---|---|
| 1 | 2.0 and 1.2 | 2.0, 1.2 (two foci) | Right upper lobe | Three less than 5 mm pure ground glass opacities in left upper lobe | Dominant part solid nodule and adjacent part solid lesions concerning for adenocarcinoma | Moderately differentiated Lung Adenocarcinoma pT3 N0 | |
| 2 | 1.7 | 2.0 | Right upper lobe | Evidence of Pulmonary Hypertension | Increase in size of pure ground glass opacity over 3 years concerning for malignancy | Granuloma | |
| 3 | 0.8 | 0.8 0.3 cm invasive component | Left upper lobe | 2 pack/year smoking history for 15 years | Part solid lesion with central 3mm solid component concerning for MIA | Minimally Invasive Lung Adenocarcinoma pT1aN0 | |
| 4 | 1.6 | 1.2 | Right Lower lobe | 1 pack /year smoking history for 20 years | Part solid lesion with increase in solid component over 2 years concerning for invasive adenocarcinoma | Moderately differentiated lung adenocarcinoma with micropapillary features pTa1N0 | |
| 5 | 1.4 | 1.3 | Right upper lobe | Past history of T1N0 Tongue cancer, moderate centrilobular emphysema | Spiculated solid nodule could represent metastasis or new Lung primary | Metastatic Tongue Squamous Cell Carcinoma | |
| 7 | 1.8 | 2.1 | Left upper lobe | 37 pack/year smoking history | Part solid lesion with increase in solid component over 2 years concerning for adenocarcinoma | Moderately differentiated lung adenocarcinoma acinar predominant pT1bN0 | |
| 8 | 1.7 | 1.9 | Right upper lobe | History breast cancer | Pure ground glass opacity stable over 1 year biopsy concerning for lung cancer | Lung Adenocarcinoma acinar (70%) and lepidic (30%) pattern pT1aN0 | |
| 9 | 1.2 | 1.5 | Right upper lobe | History renal cell carcinoma with prior resection in the lung for renal metastases | Solid right upper lobe nodule increased in size over 6 months | Metastatic Renal Cell Carcinoma | |
| 10 | 0.9 | 0.8 | Right lower lobe | Cushing's disease | Solid, oblong nodule could represent a functioning carcinoid | Carcinoid pT1a Nx | |
| 11 | 0.6 | 1.1 0.4 cm invasive component | Right upper lobe | Former smoker, upper lobe predominant emphysema | Part solid lesion concerning for MIA stable over 6 months | Minimally Invasive Lung Adenocarcinoma pT1aN0 | |
| 12 | 1.2 | 1.4 | Right upper lobe | 1.5–2 pack/year for 17 years, severe upper lobe predominant emphysema | Slow growing part solid lesion concerning for adenocarcinoma | Well‐differentiated lung adenocarcinoma acinar (10%) and lepidic (90%) pT1aN0 | |
| 13 | 1.4 | 2.5 | Right lower lobe | Previous right upper lobectomy, with multiple ground glass opacities | Part solid right lower lobe lesion growing over 6 months concerning for MIA | Moderately differentiated lung adenocarcinoma acinar, lepidic and solid | |
| 14 | 1.3 | 1.2 | Right lower lobe | Incidentally found lung nodule on a CT angiogram for aortic aneurysm | Well defined solid nodule could represent a carcinoid or lung cancer | Carcinoid pT1a Nx | |
| 15 | 1.6 | 1.2 0.4 cm invasive component | Right lower lobe | Previous resection of left Lung cancer and known multiple ground glass opacities | Multiple ground glass opacities with increase in size of solid component of the right lower lobe lesion concerning for MIA | Minimally Invasive Lung Adenocarcinoma pT1aN0 | |
| 16 | 1.3 | 1.0 0.4 cm invasive component | Left upper lobe | Past smoker and cardiac history | Part solid lesion concerning for MIA | Minimally Invasive Lung Adenocarcinoma pT1aN0 | |
| 17 | 0.8 | 1.5 | Left upper lobe | Past history of right lung cancer | Part solid lesion could represent metastasis or new primary lung cancer | Lung Adenocarcinoma | |
| 18 | 1.4 | 1.0 (0.2 cm invasive component) | Right upper lobe | Multiple ground glass opacities | Increase in size of the dominant part solid right upper lobe lesion concerning for MIA in the setting of multifocal adenomatous hyperplasia | Minimally Invasive Lung Adenocarcinoma pT1aN0 | |
| 19 | 0.7 | 0.7, 0.5 (two foci) | Right upper lobe | History of breast cancer and sarcoma | Multiple small solid nodules concerning for metastases | Metastatic Epithelioid Hemangioendothelioma | |
| 20 | 1.4 | 1.5 0.1 cm invasive component | Left upper lobe | Prior right lung cancer IIIA | Ground glass nodule | Minimally Invasive Lung Adenocarcinoma pT1aN0 | |
| 21 | 1.3 | 1.4 0.4 cm invasive component | Left upper lobe | Lung screening scan | Part solid ground glass opacity in | Minimally Invasive Lung Adenocarcinoma pT1aN0 | |
| 23 | 0.8 | 0.8 | Left upper lobe | Prior right upper lobe lung cancer | Part solid lesion in could represent metastases or new primary lung | Metastatic Lung Squamous Cell Carcinoma | |
| 24 | 1.3 | 1.0 | Right upper lobe | Screening CT | Multiple ground glass opacities could represent adenocarcinoma in situ | Lung Adenocarcinoma (in situ) | |
| 25 | 0.8 | 1.1 0.4 cm invasive component | Left upper lobe | 16 pack year smoking | Part solid lesion could represent MIA | Minimally Invasive Lung Adenocarcinoma pT1aN0 | |
All wedge resections were completed without intra‐operative complications. There were no operative or postoperative mortalities. The median length of hospital stay was 4 (range 2–12) days. Three patients had postoperative complications: pneumonia (in one patient who underwent lobectomy); prolonged air leak (1); and postoperative ileus (1). There were no requirements for ICU care, intubation, or any other invasive support. All complications were appropriately treated and resolved by the time of discharge from the hospital.
DISCUSSION
We describe herein a new image‐guided surgery workflow for intra‐operative marker‐guided thoracoscopic wedge resection that may be used to improve the precision of identifying and resecting small pulmonary nodules.. This approach can result in five potential benefits: (1) successful resection of early lung cancer with optimal margins and minimal resection of normal lung parenchyma, preserving lung function and allowing for future surgical therapies for second primary lung cancers; (2) a less invasive surgery without insertion of the hand or fingers into the chest to palpate for the nodule, potentially resulting in reduction in postoperative pain and time for recovery for the patient; (3) decreased total operative times and shorter hospital stays, and thus a more cost effective approach; (4) utilization of a single anesthetic for the marking and resection; and (5) shift of the treatment paradigm towards resection of more early‐stage tumors with a potential improvement in lung cancer ‐specific mortality rates.
Screening for lung cancer in at‐risk patients can lead to a reduction in cancer‐specific mortality 13, 14. It is estimated that with the implementation of lung cancer screening, indeterminate nodules will be detected in 8% to 51% of the patients screened 13, 15, and will need innovative surgical approaches for lung sparing surgery. Recent studies have demonstrated that sublobar resections, including segmentectomies and wedge resections, have comparable 5‐year survival rates to lobectomy for malignant small pulmonary nodules 16, 17. Smaller lung nodule size is associated with improved curative resection rates in lung cancer, without nodal disease 14. There is currently an on‐going prospective randomized phase III clinical trial through the Alliance Cooperative Group to compare the outcomes of patients undergoing lobectomy versus sublobar resections for <2 cm stage I lung cancers, highlighting the current equipoise in the thoracic surgical community for alternative procedures in this patient cohort.
VATS resection is preferred to open surgery because of the use of smaller incisions and optimized postoperative recovery, including a shorter length of hospitalization 18. Studies have shown decreased operative and post‐operative morbidity with decreased operative times 19. However, in VATS wedge resection for small nodules, adequate identification of the target nodule has been be difficult, and a more significant resection or conversion to thoracotomy is occasionally needed to ensure complete resection. The current clinical trial demonstrated that intra‐operative image‐guided marking can be safely performed to effectively localize small pulmonary nodules for thoracoscopic wedge resection.
Since the 1990s a variety of methods have been proposed and described to mark lung nodules for easier identification of small nodules and help guide resection 20, 21. These ranged from percutaneous image guided injection of a dye (methylene blue) or radio‐opaque material such as barium sulphate, to percutaneously placed hookwires and micro‐coils in the radiology suite prior to surgery. The dye was generally injected under CT guidance adjacent to the nodule and was visualized by direct inspection during surgery or with fluoroscopy, as in the case of barium. The hookwires were placed adjacent to the lesion with the distal tip extending through the pleura and were secured to the skin surface. They have been found to improve lesion localization but have a tendency to dislodge during transportation to the operating room. The microcoils were similar, except that the distal end was deployed to extend along the pleural surface; the deployment is more complicated and requires interventional expertise 22. The novelty of our approach lies in the performance of this marking in real‐time, in the same suite, and proceeding immediately to resection in a hybrid operating room.
Hybrid operating rooms provide a suite using multimodality imaging with integrated hardware and software capable of 3D modeling and real time image‐ guidance for interventional and surgical procedures 23, 24. The wide availability of hybrid operating rooms, with over 1,000 reportedly in use in the US and in many other countries (originally for cardiac and vascular procedures), facilitates the rapid adoption of this approach. Many of these operating rooms are equipped with a C‐arm CT scanner that, with appropriate software, allows for excellent imaging of the lungs. Many such existing rooms were expensive to build and are under‐utilized, yet carefully planned iterations with experienced technological support from equipment and imaging vendors along with involvement of a dedicated and committed team can lead to improved utilization, and can pave the way for innovative minimally invasive thoracic procedures as described in our approach.
Unlike other protocols that utilize preoperative image‐guidance and dye or fiducial marking for nodule localization 20, 21, 22, intra‐operative nodule localization avoids the potential complications of placing markers in one procedure room and transferring the patient to another room for resection, minimizing respiratory motion, eliminating potential disruptions from anesthetic induction, airway instrumentation, and patient repositioning. The potential for marker dislodgement, bleeding, and pneumothoraces that can result from dislodgement are thus avoided. Even in the cases where the T‐bars were dislodged, the insertion points on the lung's surface, combined with intraoperative imaging, guided accurate resection. The logistics and costs of arranging two procedures in separate rooms are also mitigated.
The majority of the lesions resected in our protocol were primary lung cancers; seven of these were minimally invasive adenocarcinomas (MIA) and one adenocarcinoma in situ. All lesions were partially solid or mixed ground glass in attenuation with a small solid component, and all demonstrated growth leading to referral for surgery. Overall tumor size and the invasive component in the MIA lesions have been associated with improved overall survival rates. In fact, lymph nodeinvolvement is not usually seen in MIA lesions, even with sizes of up to 3 cm, which should further contribute to improved disease‐free survival 25, 26. However, due to the part ially solid nature of these lesions, it is often difficult to get a reliable diagnosis by image guided biopsies, and surgical resection may be difficult due to inability to palpate during surgery. Hence, historically these lesions were longitudinally followed until deemed resectable. With the implementation of Lung Cancer screening programs, a significant increase in the number of such nodules is anticipated; therefore downstream management pathways are needed. Our novel approach allows not only for targeted complete resection of small cancerous nodules but can ensure an optimal resection margin with lung sparing surgery 27, 28.
The intra‐operative imaging was optimized to minimize radiation exposure. With the exception of the first two cases, we estimated the radiation exposure in this trial ranged from 2.2 to 8.8 mSv, with a median of 3.5 mSv. The 36.5 and 17.5 mSv exposures are outliers, recorded during the first two cases when the preoperative planning involved an additional C‐arm CT scan for confirmation of the two T‐bars prior to VATS resection. For all subsequent cases only one intra‐operative C‐arm CT scan was acquired for lesion localization and fiducial placement using a lower dose acquisition protocol, with additional collimation. This allowed optimization of radiation exposure, which is in the range of a low dose lung cancer screening CT scan.
For perspective, the average annual exposure from natural sources is 3.1 mSv and this does not include the additional radiation exposure from medical and industry activities 29, 30. The total effective dose for each patient was estimated by adding the dose from fluoroscopy and the C‐arm CT scan. The effective dose attributed to fluoroscopy was estimated using a dose conversion coefficient of 0.12 mSv*(Gy*cm2)−1 for the fluoroscopy KAP 31. The effective dose from the C‐arm CT acquisition was estimated using a factory measured and weighted computed tomography dose index of 7.7 mGy/100 milliamperes and the k‐factor for chest scans of 0.017 mSv/(mGy*cm).
The postoperative complication‐rates that occurred in this trial are comparable to those from standard VATS 32.
In conclusion, we described a new multimodality approach for image‐guided localization with immediate marker‐guided thoracoscopic wedge resection of small pulmonary nodules suspicious for early stage lung cancer in a hybrid OR setting. This approach is feasible, safe and efficacious with successful resection of all nodules, especially subcentimeter size or ground glass opacities.
ACKNOWLEDGMENT
We would like to dedicate this study to the memory of Dr. Ferenc A. Jolesz MD and acknowledge his support and guidance during the study.
Presented at: The Annual meeting of the America Association of Thoracic Surgery (AATS) 2014.
REFERENCES
- 1. Goldstraw P, Ball D, Jett JR, et al.: Non‐small‐cell lung cancer. [Internet]. Lancet 2011; 378:1727–1740. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21565398. [DOI] [PubMed] [Google Scholar]
- 2. Siegel R, Naishadham D, Jemal A: Cancer statistics, 2013. [Internet]. CA Cancer J Clin 2013; 63:11–30. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23335087. [DOI] [PubMed] [Google Scholar]
- 3. National Lung Screening Trial Research T, Church TR, Black WC, et al.: Results of initial low‐dose computed tomographic screening for lung cancer [Internet]. N Engl J Med 2013; 368:1980–1991. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23697514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Henschke CI, McCauley DI, Yankelevitz DF, et al.: Early lung cancer action project: Overall design and findings from baseline screening. Lancet 1999; 354:99–105. [DOI] [PubMed] [Google Scholar]
- 5. Humphrey LL, Deffebach M, Pappas M, et al.: Screening for lung cancer with low‐dose computed tomography: A systematic review to update the U.S. preventive services task force recommendation. Ann Intern Med 2013; 159:411–420. [DOI] [PubMed] [Google Scholar]
- 6. Henschke CI, Wisnivesky JP, Yankelevitz DF, et al.: Small stage I cancers of the lung: Genuineness and curability. Lung Cancer 2003; 39:327–330. [DOI] [PubMed] [Google Scholar]
- 7. Libby DM, Smith JP, Altorki NK, et al.: Managing the small pulmonary nodule discovered by CT. Chest 2004; 125:1522–1529. [DOI] [PubMed] [Google Scholar]
- 8. Moyer VA: Screening for lung cancer: U.S. preventive services task force recommendation statement. Ann Intern Med 2014; 160:330–338. [DOI] [PubMed] [Google Scholar]
- 9. Bach PB, Mirkin JN, Oliver TK, et al.: Benefits and harms of CT screening for lung cancer: A systematic review. [Internet]. JAMA 2012; Available from: 307:2418–2429. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3709596&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Suzuki K, Nagai K, Yoshida J, et al.: Video‐assisted thoracoscopic surgery for small indeterminate pulmonary nodules: Indications for preoperative marking. Chest 1999; 115:563–568. [DOI] [PubMed] [Google Scholar]
- 11. Santambrogio R, Montorsi M, Bianchi P, et al.: Intraoperative ultrasound during thoracoscopic procedures for solitary pulmonary nodules. Ann Thorac Surg 1999; 68:218–222. [DOI] [PubMed] [Google Scholar]
- 12. Zhou JH, Li WT, Chen HQ, et al.: [CT‐guided hookwire localization of small solitary pulmonary nodules in video‐assisted thoracoscopic surgery]. Zhonghua Zhong Liu Za Zhi 2009; 31:546–549. [PubMed] [Google Scholar]
- 13. Church TR, Black WC, Aberle DR, et al.: Results of initial low‐dose computed tomographic screening for lung cancer. [Internet]. N Engl J Med 2013; Available from: 368:1980–1991. http://www.ncbi.nlm.nih.gov/pubmed/23697514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ginsberg RJ, Rubinstein LV: Randomized trial of lobectomy versus limited resection for T1 N0 non‐small cell lung cancer. Ann Thorac Surg 1995; 60:615–623. [DOI] [PubMed] [Google Scholar]
- 15. Gill RR, Jaklitsch MT, Jacobson FL: Controversies in lung cancer screening. [Internet]. J Am Coll Radiol 2013; Available from: 10:931–936. http://www.ncbi.nlm.nih.gov/pubmed/24357736. [DOI] [PubMed] [Google Scholar]
- 16. McGuire AL, Hopman WM, Petsikas D, et al.: Outcomes: Wedge resection versus lobectomy for non‐small cell lung cancer at the Cancer Centre of Southeastern Ontario 1998‐2009. Can J Surg 2013; 56 E165–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schuchert MJ, Kilic A, Pennathur A, et al.: Oncologic outcomes after surgical resection of subcentimeter non‐small cell lung cancer [Internet]. Ann Thorac Surg 2011; Available from: 91:1681–1688. http://www.ncbi.nlm.nih.gov/pubmed/21536253. [DOI] [PubMed] [Google Scholar]
- 18. Wolf AS, Richards WG, Jaklitsch MT, et al.: Lobectomy versus sublobar resection for small (2 cm or Less) nonsmall cell lung cancers. Ann Thorac Surg 2011; 92:1819–1825. [DOI] [PubMed] [Google Scholar]
- 19. Swanson SJ, Miller DL, McKenna RJ, et al.: Comparing robot‐assisted thoracic surgical lobectomy with conventional video‐assisted thoracic surgical lobectomy and wedge resection: Results from a multihospital database (Premier). J Thorac Cardiovasc Surg 2014; 147:929–937. [DOI] [PubMed] [Google Scholar]
- 20. Kim YD, Jeong YJ, I H, et al.: Localization of pulmonary nodules with lipiodol prior to thoracoscopic surgery. Acta radiol 2011; 52:64–69. [DOI] [PubMed] [Google Scholar]
- 21. Garuti E, Vanzulli A, Varagona R, et al.: Use of CT‐guided metal wires in pre‐thoracoscopic localization of peripheral pulmonary nodules. Radiol Med 1995; 90:470–474. [PubMed] [Google Scholar]
- 22. Mayo JR, Clifton JC, Powell TI, et al.: Lung nodules: CT‐guided placement of microcoils to direct video‐assisted thoracoscopic surgical resection. Radiology 2009; 250:576–585. [DOI] [PubMed] [Google Scholar]
- 23. Kpodonu J, Raney A: The cardiovascular hybrid room a key component for hybrid interventions and image guided surgery in the emerging specialty of cardiovascular hybrid surgery. Interact Cardiovasc Thorac Surg 2009; 9:688–692. [DOI] [PubMed] [Google Scholar]
- 24. Kapur T, Egger J, Damato A, et al.: 3‐T MR‐guided brachytherapy for gynecologic malignancies. Magn Reson Imaging 2012; 30:1279–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kates M, Swanson S, Wisnivesky JP: Survival following lobectomy and limited resection for the treatment of stage I non‐small cell lung cancer<=1 cm in size: A review of SEER data. Chest 2011; 139:491–496. [DOI] [PubMed] [Google Scholar]
- 26. Borczuk AC: Assessment of invasion in lung adenocarcinoma classification, including adenocarcinoma in situ and minimally invasive adenocarcinoma. Mod Pathol 2012; 25:S1–S10. [DOI] [PubMed] [Google Scholar]
- 27. Takahashi M, Shigematsu Y, Ohta M, et al.: Tumor invasiveness as defined by the newly proposed IASLC/ATS/ERS classification has prognostic significance for pathologic stage IA lung adenocarcinoma and can be predicted by radiologic parameters. J Thorac Cardiovasc Surg 2014; 147:54–59. [DOI] [PubMed] [Google Scholar]
- 28. Tsutani Y, Miyata Y, Mimae T, et al.: The prognostic role of pathologic invasive component size, excluding lepidic growth, in stage i lung adenocarcinoma. J Thorac Cardiovasc Surg 2013; 146:580–585. [DOI] [PubMed] [Google Scholar]
- 29. Huda W, Rowlett WT, Schoepf UJ: Radiation dose at cardiac computed tomography: Facts and fiction. [Internet]. J Thorac Imaging 2010; Available from 25:204–212. http://www.ncbi.nlm.nih.gov/pubmed/20711036. [DOI] [PubMed] [Google Scholar]
- 30. National Council on Radiation Protection and Measurements, 2007: Ionizing radiation exposure of the population of the United States, Report 160. Bethesda, MD: 2009. [Google Scholar]
- 31. Lawson JD, Schreibmann E, Jani AB, et al.: Quantitative evaluation of a cone‐beam computed tomography‐planning computed tomography deformable image registration method for adaptive radiation therapy. J Appl Clin Med Phys 2007; 8:2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Boffa DJ, Allen MS, Grab JD, et al.: Data from The Society of Thoracic Surgeons General Thoracic Surgery database: The surgical management of primary lung tumors [Internet]. J Thorac Cardiovasc Surg 2008; Available from: 135:247–254. http://www.ncbi.nlm.nih.gov/pubmed/18242243. [DOI] [PubMed] [Google Scholar]
- 33.Please check Synopsis for Table of Contents.


