Although previously tested in an upfront window by the Berlin–Frankfurt–Munster group, the safety and efficacy of rituximab in combination with multiagent chemotherapy regimens in children and adolescents with advanced mature B-cell non-Hodgkin lymphoma (B-NHL) is unknown.1
The trial, COG ANHL01P1, was open to all Children’s Oncology Group (COG) centers in the United States, Canada, Australia and New Zealand. The protocol was approved at each institutional review board. Parents or patients signed informed consent in accordance with the Declaration of Helsinki. The COG-independent Data and Safety Monitoring Committee reviewed safety reports and interim analyses.
Children, adolescents and young adults (< 30 years) with intermediate risk (French-American-British (FAB) group B), St Jude Stages III/IV, CD20 positive, mature B-cell lymphoma classified by the Revised European-American Lymphoma criteria were eligible.2
The chemotherapy backbone for group B patients was similar to that reported in the FAB/LMB 96 study for the B4 arm.3 The reduction phase consisted of low-dose cyclophosphamide, Oncovin and prednisone (COP). The two induction courses (COPADM1 + 2) consisted of cyclophosphamide 1.5 g/m2 per course-fractionated, vincristine, prednisone, doxorubicin and high-dose methotrexate (HDMTX) (3 g/m2 in 3 h of infusion with intrathecal methotrexate). Patients then received two identical consolidation courses, CYM1 + 2 (continuous-infusion cytarabine and high-dose methotrexate). Patients with < 20% COP response or biopsy-proven residual disease between consolidation cycles were switched to the more intensive group C regimen as previously described.3–5
Rituximab (375 mg/m2), supplied by Genentech (San Francisco, CA, USA) through the Cancer Therapy Evaluation Program, was diluted in normal saline and infused at 0.5 mg/kg/h for the first hour of the initial infusion and then increased every 30 min per patient tolerance as we have previously described.6 Monitoring of blood pressure, pulse, respiratory rate and temperature was done every 15 min. During induction cycles (COPADM), dose-dense rituximab was administered 48 h prior (day −2) and repeated on the day of chemotherapy administration (day 0) as described by Pfreundschuh et al.7 During consolidation cycles (CYM), rituximab was administered just prior to chemotherapy administration (day 0). In the initial subpilot, the rituximab administration began with the second induction cycle (four total doses). In the pilot study, rituximab was given, beginning with the first induction cycle (six total doses) (Supplementary Figures 1A–D).
A subpilot (n = 7) of patients with Stage III/IV disease (and receiving only four doses of rituximab) preceded the pilot study, the purpose of which was to verify that there were no unexpected early toxic events.
Stevens–Johnson syndrome, toxic epidermal necrosis, frequency of grade ≥3 stomatitis and delayed recovery beyond day + 42 of the induction phase were events of concern in the subpilot and main pilot studies.
A separate rule for temporarily or permanently closing the subpilot was included. If a single toxic death was observed during the second cycle of the induction (COPADM2 + rituximab) through the end of the consolidation course (CYM2 + rituximab) among the seven subpilot patients, then the Study Committee was to review the event and determine if the study would continue forward or if it should be modified or permanently closed.
The primary objective of this study was to monitor and assess toxicity to ensure that the addition of rituximab to standard therapy would not unduly increase the rate and degree of toxic events. Two events were of particular interest, the incidence of grade ≥3 stomatitis and any occurrence of a toxic death. A three-stage stopping rule was used to terminate this study if too many group B patients experienced grade ≥3 stomatitis during either cycle of COPADM + rituximab therapy (induction). A rate >48%, the observed rate in FAB/LMB 96, was considered too high.
If there was a single incidence of Stevens–Johnson syndrome or toxic epidermal necrosis among the 44 group B patients during either induction cycle, the trial was to be temporarily closed to group B patients for the review of the event. Finally, deaths were monitored and any detectable increase in the toxic death rate above the 1.2% rate observed on FAB/LMB 96 was of concern.
Analysis of event-free survival (EFS) and overall survival (OS) was performed using the Kaplan and Meier method.5 The 95% confidence interval (CI95) for the Kaplan–Meier estimates of EFS and OS was calculated using s.e.’s according to Greenwood’s formula.8
The study opened in the subpilot phase in June 2004. Seven patients were enrolled to the subpilot. After prespecified safety closure (1.25 years), the pilot study opened in September 2005. Forty-four pilot patients were enrolled through the planned study closure date in October 2006. Five pilot patients were determined to be ineligible (all prior to receiving rituximab). In addition, one eligible pilot patient was found to have central nervous system (CNS) blasts and was switched to group C and excluded from further analysis. Thus, 38 pilot and 7 subpilot patients were included.
The mean age at study entry was 11 years (range 1–23 years) and 11% of the patients were less than 4 years of age. The male:female ratio was 3.5:1. Eighty-nine percent of patients were in St Jude Stage III. Forty-eight percent of patients had elevated LDH ≥2 × upper limit of institutional normal. The majority of patients had Burkitt lymphoma (56%). Diffuse large B-cell lymphoma was the second most frequent histology (22%), followed by 4 cases (9%) of mediastinal primary B-cell lymphoma. All centrally reviewed histological subtypes expressed CD20 (Supplementary Table 1).
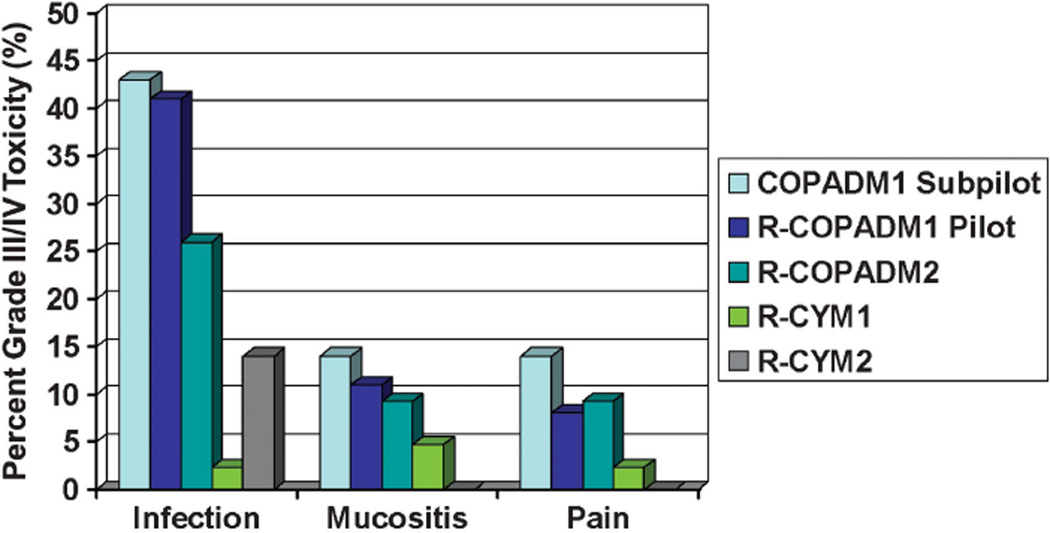
There were no toxic deaths. Figure 1 details the most common recurrent non-hematological grade III/IV toxicities by cycle of therapy. There were 19 reports in five patients of serious adverse events. No serious adverse event was probably or definitely attributed to rituximab. One episode of grade III infectious colitis occurred after the second induction cycle and was attributed as possibly related to rituximab administration. There were no grade III/IV infusion reactions reported among the 256 doses of rituximab. Prespecified stopping rules for unacceptable rates of mucositis/stomatitis and Stevens–Johnson syndrome were not met. The incidence of grade III/IV mucositis during induction cycles was 11% and 9% in COPADM 1 and COPADM 2, respectively. Human antichimeric antibody analyses were performed in seven consenting patients from 6 weeks to 1 year post completion of rituximab therapy (39 samples) with no positive results.
Figure 1.
Percentage of grade III/IV non-hematological toxicities during chemoimmunotherapy cycles. Rates of grade III/IV infection, mucositis and pain, by treatment cycle. In the initial subpilot, patients did not receive rituximab during the first induction cycle (COPADM1) and therefore toxicity for this cycle is shown separately. All other cycles combine subpilot (n = 7) and pilot (n = 38) data.
The response rate (complete response/partial response) after completion of the two induction cycles was 75% (CI95 30–95%) and 89% (CI95 73–96%) in the sub-pilot and pilot patients, respectively. After the first consolidation cycle, patients were required to undergo biopsy of any residual disease and if found positive, they were switched to a more intensive group C consolidation.4 One patient had biopsy-proven residual disease at this disease evaluation (complete response rate 98% post consolidation).
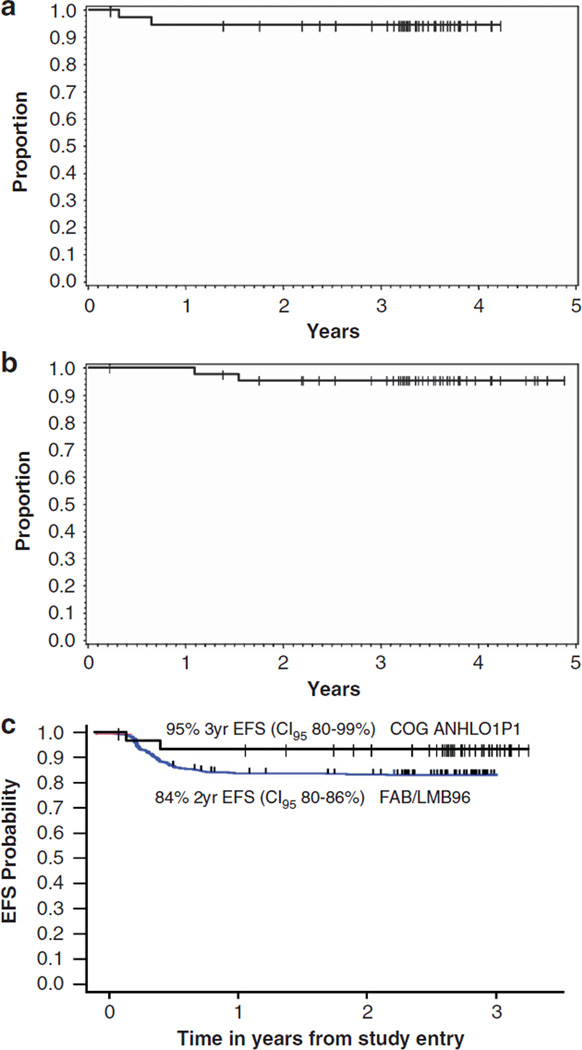
There have been three patients with reported events of recurrent disease. Two of four patients with mediastinal primary disease developed recurrent disease at 8 and 34 months, respectively, from study entry. In the remaining 41 patients, only one patient with Burkitt lymphoma developed recurrent disease at 4 months post study entry. Two deaths have occurred (both with previous recurrent disease) at 13 and 18 months from study entry, respectively. The median follow-up for the 43 patients alive at last contact is 3.5 years. The 3-year EFS among all 45 eligible patients is 93% (CI95 79–98%), the 3-year EFS among pilot patients (six doses of rituximab) is 95% (CI95 80–99%) (Figure 2a) and the three-year OS among pilot patients is 95% (CI95 83–99%) (Figure 2b).
Figure 2.
Probability of EFS and OS of all eligible intermediate-risk patients. (a) Product-limit estimate of probability of EFS in all pilot patients (six doses or rituximab plus chemotherapy) from study entry. EFS at 3 years (CI95% 80–99%). (b) Product-limit estimate of probability of OS in all patients from study entry. OS at 3 years (CI95% 83–99%). (c) Product-limit estimate of probability of EFS in all stage III/IV pilot patients on study compared to all stage III/IV patients treated on FAB/LMB 96 without rituximab.
This is the first prospective study to report the addition of targeted immunotherapy to a multiagent chemotherapy regimen in newly diagnosed children and adolescents with stage III/IV de novo mature B-NHL. Our findings demonstrate that dose-dense rituximab therapy can be safely added to chemotherapy and results in a 95% 3-year EFS. Importantly, two of the relapses occurred in patients with a mediastinal primary, which is a subgroup with a known poorer prognosis in the context of FAB/LMB therapy.7 In our previous experience in the FAB/LMB 96 trial (with 6 h of doxorubicin infusion), the incidence of grade III and IV mucositis during COPADM1 (B 1, 2, 3, 4 arms) and COPADM2 (B 3, 4 arms) was 43% and 31%, respectively.3 For this reason, we further reduced the doxorubicin infusion to ≤1 h in the current study. These safety results are in line with over a decade of use of combination chemotherapy regimens with rituximab in adult patients with B-NHL.7,9 Our study, however, was not powered to monitor for rare but highly morbid events such as progressive multifocal leukoencephalopathy (due to reactivation of latent John Cunningham virus) that have been described in adults treated with rituximab.10,11
Our outcome results demonstrate both a 3-year EFS of 95% (CI95 80–99%) in pilot patients (six doses of rituximab) and a 3-year OS of 95% (CI95 80–99%). In the prior FAB/LMB 96 study, which utilized the same FAB/LMB 96 chemotherapy only, the 5-year EFS in patients with stage III/IV B-NHL was 84% (CI95 80–86%) (Figure 2c).3,12 These current results are the best reported to date and only utilized 12 weeks of chemoimmunotherapy. In comparison, the 10-year progression-free survival in adults with Stage III/IV diffuse large B-cell lymphoma treated with R-CHOP (rituximab + cyclophosphamide, adriamycin, vincristine and prednisone) is only 37%.13 Finally, a more intriguing use of rituximab in children and adolescents with de novo mature B-NHL may be to substitute for chemotherapy, thereby potentially reducing morbidity and late effects.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Linda Rahl, Virginia Davenport and Saumya Pachauri for their assistance in the development and conduct of this trial, and Ms Amber Quinlan and Erin Morris, RN, for their editorial assistance in the development of this manuscript. We would also like to thank Dr James Lynch for his assistance with analyzing the data. This research was funded by the Division of Cancer Treatment, National Cancer Institute, and National Institutes of Health, Department of Health and Human Services (COG) (CA98543-09 and CA98413-09), Pediatric Cancer Research Foundation and the Doris Duke Charitable Foundation. NCI provided support for data collection and analysis, but had no role in data interpretation, writing of the manuscript or the decision on journal submission.
Footnotes
CONFLICT OF INTEREST
TGG is a consultant for Genentech/Roche, Allos Therapeutics, Pfizer Oncology and Eli Lilly. JZ is an employee of Roche. All other authors declare no conflict of interest.
This work was presented in part at the American Society of Clinical Oncology, Chicago, IL, May 2010, and the American Society of Hematology, San Diego, CA, December 2011.
Supplementary Information accompanies the paper on the Leukemia website (http://www.nature.com/leu)
REFERENCES
- 1.Meinhardt A, Burkhardt B, Zimmermann M, Borkhardt A, Kontny U, Klingebiel T, et al. Phase II window study on rituximab in newly diagnosed pediatric mature B-cell non-Hodgkin’s lymphoma and Burkitt leukemia. J Clin Oncol. 2010;28:3115–3121. doi: 10.1200/JCO.2009.26.6791. [DOI] [PubMed] [Google Scholar]
- 2.Murphy SB. Classification, staging and end results of treatment of childhood non- Hodgkin’s lymphomas: dissimilarities from lymphomas in adults. Semin Oncol. 1980;7:332–339. [PubMed] [Google Scholar]
- 3.Patte C, Auperin A, Gerrard M, Michon J, Pinkerton R, Sposto R, et al. Results of the randomized international FAB/LMB96 trial for intermediate risk B-cell non-Hodgkin lymphoma in children and adolescents: it is possible to reduce treatment for the early responding patients. Blood. 2007;109:2773–2780. doi: 10.1182/blood-2006-07-036673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cairo MS, Gerrard M, Sposto R, Auperin A, Pinkerton CR, Michon J, et al. Results of a randomized international study of high-risk central nervous system B non-Hodgkin lymphoma and B acute lymphoblastic leukemia in children and adolescents. Blood. 2007;109:2736–2743. doi: 10.1182/blood-2006-07-036665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shiramizu B, Goldman S, Kusao I, Agsalda M, Lynch J, Smith L, et al. Minimal disease assessment in the treatment of children and adolescents with intermediate-risk (Stage III/IV) B-cell non-Hodgkin lymphoma: a children’s oncology group report. Br J Haematol. 2011;153:758–763. doi: 10.1111/j.1365-2141.2011.08681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Griffin TC, Weitzman S, Weinstein H, Chang M, Cairo M, Hutchison R, et al. A study of rituximab and ifosfamide, carboplatin, and etoposide chemotherapy in children with recurrent/refractory B-cell (CD20 +) non-Hodgkin lymphoma and mature B-cell acute lymphoblastic leukemia: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2009;52:177–181. doi: 10.1002/pbc.21753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zwick C, Murawski N, Pfreundschuh M. Rituximab in high-grade lymphoma. Semin Hematol. 2010;47:148–155. doi: 10.1053/j.seminhematol.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Greenwood M. A Report on the Natural Duration of Cancer. London, UK: Her Majesty’s Stationary Office; 1929. [Google Scholar]
- 9.Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 10.Carson KR, Evens AM, Richey EA, Habermann TM, Focosi D, Seymour JF, et al. Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: a report of 57 cases from the Research on Adverse Drug Events and Reports project. Blood. 2009;113:4834–4840. doi: 10.1182/blood-2008-10-186999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He YF, Li YH, Wang FH, Jiang WQ, Xu RH, Sun XF, et al. The effectiveness of lamivudine in preventing hepatitis B viral reactivation in rituximab-containing regimen for lymphoma. Ann Hematol. 2008;87:481–485. doi: 10.1007/s00277-008-0454-3. [DOI] [PubMed] [Google Scholar]
- 12.Cairo MS, Sposto R, Gerrard M, Auperin A, Goldman SC, Harrison L, et al. Advanced stage, increased lactate dehydrogenase, and primary site, but not adolescent age (> = 15 years), are associated with an increased risk of treatment failure in children and adolescents with mature B-cell non-Hodgkin’s lymphoma: results of the FAB LMB 96 study. J Clin Oncol. 2012;30:387–393. doi: 10.1200/JCO.2010.33.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coiffier B, Thieblemont C, Van Den Neste E, Lepeu G, Plantier I, Castaigne S, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood. 2010;116:2040–2045. doi: 10.1182/blood-2010-03-276246. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.