Abstract
Our aim was to provide a descriptive overview of how the birth defects surveillance and folic acid fortification programs were implemented in Costa Rica—through the establishment of the Registry Center for Congenital Anomalies (Centro de Registro de Enfermedades Congénitas—CREC), and fortification legislation mandates. We estimated the overall prevalence of neural tube defects (i.e., spina bifida, anencephaly and encephalocele) before and after fortification captured by CREC. Prevalence was calculated by dividing the total number of infants born with neural tube defects by the total number of live births in the country (1987–2012).A total of 1,170 newborns with neural tube defects were identified from 1987 to 2012 (1992–1995 data excluded); 628 were identified during the baseline pre-fortification period (1987–1991; 1996–1998); 191 during the fortification period (1999–2002); and 351 during the post-fortification time period (2003–2012). The overall prevalence of neural tube defects decreased from 9.8 per 10,000 live-births (95 % CI 9.1–10.5) for the pre-fortification period to 4.8 per 10,000 live births (95 % CI 4.3–5.3) for the post–fortification period. Results indicate a statistically significant (P < 0.05) decrease of 51 % in the prevalence of neural tube defects from the pre-fortification period to the post-fortification period. Folic acid fortification via several basic food sources has shown to be a successful public health intervention for Costa Rica. Costa Rica’s experience can serve as an example for other countries seeking to develop and strengthen both their birth defects surveillance and fortification programs.
Keywords: Surveillance, Fortification, Costa Rica, Prevalence, Neural tube defects
Introduction
Approximately 300,000 neural tube defects occur each year worldwide [1]. Neural tube defects, such as spina bifida, anencephaly, and encephalocele, are severe birth defects that affect the development of the spine and brain [2, 3]. These defects occur due to the failure of the neural tube to close properly, which usually happens between the 3rd and 4th weeks of gestation [4]. Studies showed that the consumption of folic acid before pregnancy and during early stages of gestation, through vitamin supplements containing folic acid or folic acid alone, can greatly reduce a woman’s risk for having a pregnancy affected by a neural tube defect [5–7].
Costa Rica, a country in Central America bordered by Nicaragua and Panama, is home to an estimated 4.6 million people [8]. In Costa Rica, 99 % of births occur in public and private hospitals (clinics) [9]. In 2010, the World Health Organization estimated that, 37 % of deaths in children aged <5 years in Costa Rica are due to birth defects, making them the leading cause of death in this age group [10]. In 2011, there were 73,459 births, and infant mortality was estimated to be 91 deaths per 10,000 live births [8]. The infant mortality rate associated with neural tube defects in Costa Rica in 2009 was 1.7 per 10,000 live births [11]. Costa Rica has shown that targeted neural tube defects prevention efforts, such as food fortification, have led to a significant decrease in the overall prevalence of neural tube defects [11, 12] and the prevalence of infant mortality due to neural tube defects [11].
This manuscript describes how both the birth defect surveillance system and folic acid fortification programs in Costa Rica originated and developed. In 1985, the Registry Center for Congenital Anomalies (Centro de Registro de Enfermedades Congénitas—CREC) began monitoring birth defects in Costa Rica. In 1997, the first executive decree mandated the enrichment of wheat flour with folic acid. The role of folic acid fortification in the decline of neural tube defects prevalence in Costa Rica is well captured by CREC.
History of the Birth Defects Surveillance System in Costa Rica
In 1984, a birth defects research project was undertaken to understand the prevalence of birth defects and the neonatal mortality associated with birth defects in Costa Rica. The project received political support from officials and evolved into a mandatory surveillance system. In August 1985, executive decree 16488-S was established in Costa Rica [13]. This decree instituted mandatory reporting of all infants born with birth defects, detected at birth in all hospitals in the country. The mandate also led to the formation of the CREC, which serves as the central registry to which data are reported from all Costa Rican hospitals. In the early stages of the CREC, a coordinating group was formed, which included geneticists from the Costa Rica Institute of Research and Education for Nutrition and Health (Instituto Costarricense de Investigación y Enseñanza en Nutrición y Salud—INCIENSA), a branch of the Costa Rican Ministry of Health, along with a group of neonatologists from the public maternity hospitals from the Costa Rican Social Security System (Caja Costarricense del Seguro Social) and other collaborators. This coordinating group was charged with developing case definitions and designing the birth defects data collection instruments and the program’s protocol for surveillance [14]. In the beginning, CREC’s case definition for newborns with a birth defect includes both live births and stillbirths (terminations of pregnancy are not legal in Costa Rica, therefore are not part of the inclusion criteria), with a gestational weight of 500 g or more, born to resident and nonresident mothers, and born with any birth defect identified up to 7 days after delivery. The system has passive reporting of cases (i.e., hospital staff who identify babies born with birth defects report pertinent information directly to the surveillance program) [14, 15]. CREC verifies completeness of reported cases by reviewing all forms received at CREC, and by calling participating hospitals for any missing data. Coding and analysis of data are done at CREC and birth prevalence results are shared with all stakeholders of the surveillance system. In 1987, the protocol developed by CREC began fully operating in all maternity hospitals (n = 24) from the Costa Rican Social Security System. Although the surveillance system had a great deal of support from clinicians and hospital staff, in the early stages it was difficult for CREC staff to monitor reports from all hospitals, in particular those in more remote areas and those with fewer number of births. In 1991, some hospitals were having difficulty reporting, and CREC realized that the system was not ascertaining all newborns with birth defects. CREC decided to use their limited resources more strategically. In 1992, a decision was made to include only eight hospitals from the Costa Rican Social Security System to continue to participate in the surveillance program. These hospitals were chosen because of their large numbers of births (covering approximately 53 % of total births), and their ability to report complete data. Starting in 1996, CREC re-instituted reporting from all maternity hospitals in the country—both public (n = 24) and private (n = 4)—to move towards providing more accurate population data.
In 2008, a modification to executive decree 16488-S increased the age of reporting of birth defects in the surveillance program to up to 1 year of age. This modification also provided for the inclusion of reporting from the National Children’s Hospital, a specialized pediatric treatment and referral center. All infants born with birth defects who are seen at this facility are crossed referenced with CREC’s database to ascertain any possible new or duplicated cases in the surveillance program. These changes to the surveillance program allowed for better ascertainment of birth defects. Since 2008, CREC has served as a national-based surveillance program that covers all 28 maternity hospitals in the country and the National Children’s Hospital.
History of Folic Acid Fortification in Costa Rica
In 1996, the third national nutrition survey in the country was undertaken, led by the Ministry of Health in collaboration with the National Reference Center of Bromatology (Centro Nacional de Referencia de Bromatología—CNRBro) at INCIENSA. This Center was given the responsibility of verifying and monitoring quality indicators and amounts of fortified staples. Similarly, the National Reference Center of Clinical-Chemistry (Centro Nacional de Referencia de Química Clínica—CNRQC) at INCIENSA was given the responsibility to collect and monitor blood folate concentrations nationally. This third national nutrition survey included measurements of serum folate concentrations from women of childbearing age (15–44 years). The mean serum folate concentration for women of childbearing age, conducted prior to fortification in the country, was reported as 10.1 ng/mL (95 % CI 9.5–10.6) (Median 8.7 ng/mL) (radioimmunoassay-RIA) [16, 17]. Data from the 1996, third national nutrition survey indicated that approximately 25 % of women of child-bearing age in the country were possible or folate deficient (i.e., folate levels <6 ng/mL) [16–18].
Since 1996, to address the concerns of nutritional deficiencies, several measures were taken to fortify basic staples of the Costa Rican diet with folic acid, iron, riboflavin, thiamine, and niacin. In October 1997, the 1st executive decree for the enrichment of wheat flour for human consumption with folic acid (1.5 mg/kg) and iron (55 mg/kg) was implemented for national, imported, and donated products [19]. In 1998, the 2nd executive decree mandated the establishment of a national commission on micronutrients [20]. In 1999, this commission developed the first comprehensive national plan and policy for the prevention of micronutrient deficiencies in the population [21]. One of the main objectives of the national plan was to prevent folate deficiency in women of childbearing age [21]. In September 1999, a 3rd executive decree mandated fortification of corn flour with 1.3 mg/kg of folic acid, based on the premise that corn flour constituted a basic staple in the Costa Rican diet [22]. In 2001, the Ministry of Health in collaboration with one Costa Rican dairy company took the initiative to enrich whole, semi-skim, skim, and powdered milk with 40 μg folic acid/250 ml of milk. As a result, a 4th executive decree in 2001 mandated all dairy products to be fortified with folic acid [23]. In 2002, an amendment to the 1st executive decree for the enrichment of wheat flour increased the amount of folic acid in wheat flour from 1.5 to 1.8 mg/kg [24]. In addition, in 2002 with the support of the rice industry, the Ministry of Health mandated rice to be fortified with folic acid (1.8 mg/kg) [25]. During the years 2008 and 2009, following fortification, a national nutrition survey in the country was undertaken by the Ministry of Health in collaboration with CNRBro and CNRQC at INCIENSA. This national nutrition survey showed that the mean serum folate concentration for women of childbearing age was 14.7 ng/mL (95 % CI 14.2–15.2) (Median 14.6 ng/mL) (chemiluminescence) [17, 26]. These data showed that although fortification was successful in raising folate concentrations, about 3.8 % of women of childbearing age in the country remained possible or folate deficient (i.e., folate levels <6 ng/mL) [17,18, 26].
CREC estimated the overall prevalence of neural tube defects (i.e., spina bifida, anencephaly and encephalocele) before and after fortification. Currently, few publications related to birth defects surveillance and fortification programs in Latin America are available in the literature. This paper could serve as an example to countries seeking to document prevention of birth defects and infant mortality by developing and strengthening their birth defects surveillance and fortification programs.
Methods
An analysis was conducted to estimate the overall prevalence of neural tube defects (i.e., spina bifida, anencephaly, and encephalocele) included in CREC from 1987–1991 to 1996–2012. Data for the years 1992–1995 were excluded from the analysis, because not all hospitals in the country were participating during those years, as described previously. Prevalence was calculated based on the total number of infants born with neural tube defects included in CREC’s database from 1987–1991 to 1996–2012, divided by the total number of live births in the country from these same years, reported by the National Institute of Statistics and Census (Instituto Nacional de Estadistica y Censos—INEC). Although the mandate for fortification of wheat flour for human consumption with folic acid was published in the official Costa Rican newsletter in October 1997, the first infants impacted by fortification were likely not born until early 1999 when wheat fortification began to be fully implemented. Therefore, for purposes of this analysis, we considered the pre-fortification time periods from 1987–1991 to 1996–1998, the years before any folic acid fortification mandate was implemented. The fortification implementation time period from 1999 to 2002 included the years during which additional staples were fortified with folic acid. The post fortification time period from 2003 to 2012 included the years after all staples were fortified with folic acid (Table 1). A 95 % confidence interval (CI) was calculated for each prevalence estimate based on exact Poisson limits [27]. Statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC, USA). Geographical distribution of prevalence estimates by province (n = 7) in Costa Rica were performed using EPI INFO version 7 (Centers for Disease Control and Prevention, Atlanta, GA, USA). Provincial level maps were divided and grouped by different time periods: pre-fortification (1987–1991; 1996–1998), fortification implementation (1999–2002) and post-fortification (2003–2012) to show the overall prevalence of neural tube defects for these time periods. This analysis is based on public national birth defect surveillance data, for which no formal ethical review is required. All national surveillance data in Costa Rica are regulated by the executive decree 37306-S. No personal identifiers are associated with reported data.
Table 1.
Prevalence of neural tube defects, pre, during and post folic acid fortification
| Years (period) | Neural tube defects prevalence (per 10,000 live births; CI 95 %) |
Prevalence ratio (pre-fortification referent) |
Decrease relative to pre-fortification prevalence (%) |
Year published, type of fortified staples and amounts published in the official Costa Rican newsletter |
|---|---|---|---|---|
| 1987–1991; 1996–1998 (pre- fortification)* |
9.8 (9.1–10.5) |
NA | NA | 1997 (October): wheat flour (1.5 mg/kg)† |
| 1999–2002 (fortification implementation) |
6.3a (5.4–7.2) |
0.65 | 35 % | 1999: corn flour (1.3 mg/kg) 2001: dairy products (40 μg/250 ml) 2002: wheat flour (1.8 mg/kg) 2002: rice (1.8 mg/kg) |
| 2003–2012 (post- fortification) |
4.8a, b (4.3–5.3) |
0.49 | 51 % | – |
Source: Registry Center for Congenital Anomalies (Centro de Registro de Enfermedades Congénitas—CREC)
NA not applicable
1992–1995 data were excluded from the analysis because there were only 8 hospitals participating in CREC
Although published in October 1997, the first infants under this mandate were not likely born until early 1999
Values were significantly (p < 0.05) different from baseline (pre-fortification period; 1987–1991; 1996–1998)
Values were significantly (p < 0.05) different from baseline (pre-fortification period; 1987–1991; 1996–1998) and during fortification (1999–2002)
Results
Prevalence of neural tube defects pre, during and post folic acid fortification in Costa Rica
A total of 1,170 infants born with neural tube defects were ascertained in the CREC’s database from 1987–1991 to 1996–2012; 628 infants born with neural tube defects from the pre-fortification time period (1987–1991; 1996–1998); 191 infants born with neural tube defects during the fortification implementation time period (1999–2002); and 351 infants from the post-fortification time period (2003–2012). Among the 1,170 infants born with neural tube defects, 88 infants had missing or no province name, and were excluded from the geographic analysis. There were 331 (28 %) infants born with anencephaly, 714 (61 %) infants born with spina bifida, and 125 (11 %) infants born with encephalocele obtained from CREC’s database from 1987–1991 to 1996–2012. Table 1 shows the prevalence of neural tube defects before, during, and after implementation of folic acid fortification of different staples in Costa Rica. The overall prevalence of neural tube defects decreased from 9.8 per 10,000 live births (95 % CI 9.1–10.5) for the pre-fortification time period to 6.3 per 10,000 live births (95 % CI 5.4–7.2) for the time period during fortification implementation, representing a statistically significant decrease of 35 %. Furthermore, the overall prevalence of neural tube defects decreased to 4.8 per 10,000 live births (95 % CI 4.3–5.3) for the post-fortification time period, representing a statistically significant decrease of 51 % from the pre-fortification time period.
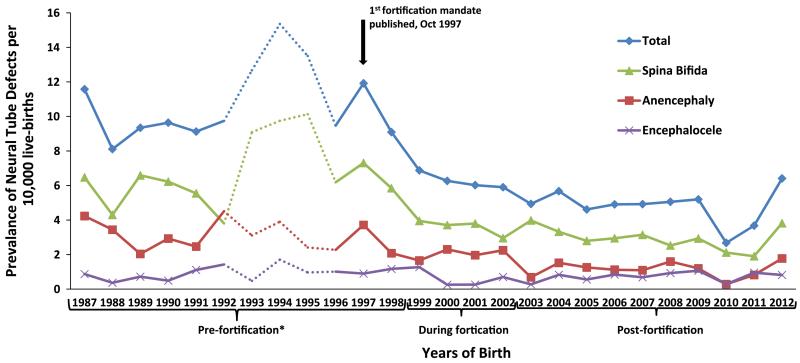
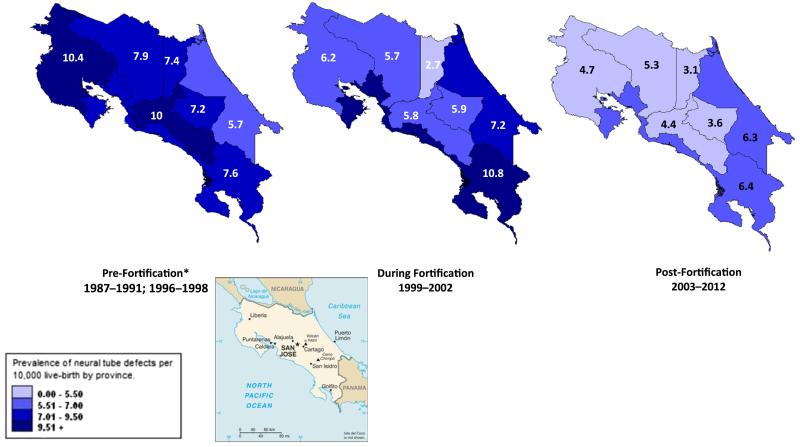
Figure 1 shows the yearly trends for the prevalence of neural tube defects registered in CREC’s database classified by time periods: pre-fortification, during fortification implementation, and post-fortification. A decrease in the overall prevalence of neural tube defects is observable throughout the span of 25 years. Figure 2 and Table 2 show the prevalence of neural tube defects in CREC’s database by province (n = 7). While the overall prevalence of neural tube defects by province declined throughout the years, there is still evidence of higher prevalence areas, specifically in the coastal areas and the areas on the border with neighboring countries (Fig. 2).
Fig. 1.
Prevalence trend for neural tube defects per 10,000 live-births in Costa Rica from 1987–1991; 1996–2012. Source: Registry Center for Congenital Anomalies (Centro de Registro de Enfermedades Congénitas—CREC). Note: *1992–1995 data were excluded from the analysis because there were only 8 hospitals participating in CREC. Dotted lines denote data from 8 hospitals participating in CREC
Fig. 2.
Prevalence of neural tube defects by province in Costa Rica 1987–1991; 1996–2012 (Pre, During and Post Folic Acid Fortification). Source: Registry Center for Congenital Anomalies (Centro de Registro de Enfermedades Congénitas—CREC). Note: *1992–1995 data was excluded from the analysis because there were only 8 hospitals participating in CREC
Table 2.
Neural tube defects prevalence per 10,000 live births by province—Costa Rica, from 1987–1991; 1996–2012
| Provinces | San Jose N P (95 % CI) |
Alajuela N P (95 % CI) |
Cartago N P (95 % CI) |
Heredia N P (95 % CI) |
Guanacaste N P (95 % CI) |
Puntarenas N P (95 % CI) |
Limon N P (95 % CI) |
|---|---|---|---|---|---|---|---|
| 1987–1991; 1996–1998 (pre-fortification)* |
225 | 94 | 51 | 37 | 48 | 54 | 34 |
| 10 (8.6–11.3) |
7.9 (6.3–9.5) |
7.2 (5.2–9.1) |
7.4 (5–9.8) |
10.4 (7.5–13.3) |
7.6 (5.6–9.7) |
5.7 (3.8–7.6) |
|
| 1999–2002 (during fortification) |
60 | 33 | 19 | 7 | 13 | 34 | 23 |
| 5.8 (4.3–7.2) |
5.7 (3.8–7.7) |
5.9 (3.3–8.6) |
2.7 (0.7–4.6) |
6.2 (2.8–9.6) |
10.8 (7.2–14.4) |
7.2 (4.2–10.1) |
|
| 2003–2012 (post-fortification) |
102 | 75 | 27 | 20 | 27 | 49 | 50 |
| 4.4 (3.5–5.2) |
5.3 (4.1–6.4) |
3.6 (2.3–5) |
3.1 (1.7–4.4) |
4.7 (2.9–6.5) |
6.4 (4.6–8.2) |
6.3 (4.5–8) |
Source: Registry Center for Congenital Anomalies (Centro de Registro de Enfermedades Congénitas—CREC)
N number of cases
P prevalence per 10,000 live births
CI confidence interval
1992–1995 data were excluded from the analysis because there were only 8 hospitals participating in CREC
Discussion
The results of this analysis demonstrate that the birth prevalence of neural tube defects in Costa Rica has declined since the implementation of folic acid fortification in 1997. We observed a statistically significant decrease of 51 % in the prevalence of neural tube defects when comparing pre-fortification and post-fortification periods. This could be attributable to folic acid fortification through the availability of multiple food sources. Declining prevalence of neural tube defects was observed in most of the seven provinces. However, there are still areas with higher prevalence of neural tube defects that deserve special monitoring by CREC and assessment of fortification status. It would also be beneficial to ascertain blood folate status of women of childbearing age.
Costa Rica’s success in assessing the decrease of the prevalence of neural tube defects can be attributed to a series of events. Costa Rica has a Social Security System—a public and centralized system—that has been responsible for providing health care services to the population since 1948. This system has played an important role in the country’s development and improvement of health outcome indicators, comparable to those in high income countries [28–30]. Improvements of basic health services throughout the years is considered to be one of the key elements that contributed to the general decline in infant mortality [28–30].
Moreover, the ongoing efforts led by the Ministry of Health and INCIENSA to increase the number of staples fortified with folic acid and to strengthen the surveillance of birth defects seem to have played an important role in contributing to the decline in the prevalence of neural tube defects and infant mortality due to neural tube defects [11,12]. Since the implementation of the folic acid fortification programs, Costa Rica has documented a decrease of neural tube defects birth prevalence, substantially diminishing the burden of neural tube defects mortality and morbidity [11,12]. Infant mortality related to neural tube defects before and after folic acid fortification was 0.64 per 1,000 live births (95 % CI 0.46–0.82) and 0.17 per 1,000 live birth (95 % CI 0.08–0.28), respectively [11]. The surveillance data support the role of folic acid fortification in the decline of neural tube defects birth prevalence. In addition, the data also show 25 years of data of birth defect surveillance and illustrate differences in the prevalence of neural tube defects by time periods (pre, during and post folic acid fortification). The effectiveness of folic acid fortification of staple foods in the prevention of neural tube defects has been well-documented in the United States, Canada, and Chile [31–34]. Costa Rica is a unique example in the implementation of folic acid fortification programs, not only fortifying wheat flour as other high income countries have done [35], but also fortifying several basic food sources (e.g., corn flour, dairy products and rice) in order to reach as much of the population as possible. The formulation, negotiation, and approval of the first policy of wheat flour fortification with folic acid in Costa Rica in 1997, led by the Ministry of Health, was made possible by the combination of several favorable conditions in place at the time, including political will, industry compliance and the collaboration and contribution of many agencies [36]. It is likely that the understanding of the importance of folic acid fortification in the reduction of the prevalence of neural tube defects, coupled with the proactive collaboration of the Costa Rican milling and food industries played a vital role in institutionalizing and sustaining folic acid fortification programs.
A number of challenges exist for CREC’s surveillance system. Of particular importance is the need for a thorough evaluation of this passive surveillance system to ensure that the program’s objectives continue to be met and areas needing improvement are identified. As a passive system, CREC receives reports of birth defect cases from all hospitals without verification of cases. The program relies on local health care providers or health officers to report these data in a timely fashion. As a result, there could be variability in the completeness and accuracy of the data. To account for this, revision to the current surveillance protocol could include a process for data verification to identify completeness (i.e., possible missing cases), quality of case ascertainment (e.g., diagnosis validation through medical records review and validity audits), timeliness of reporting, and accuracy of the information reported. Reinforcement of training at participating hospitals could keep personnel motivated and assure the continuation of accurate, complete, and timely reporting. Finally, further studies are especially needed in remote areas of the country where higher prevalence of neural tube defects are reported (Fig. 2). Assessing reporting bias, potential risk factors, and barriers to obtaining fortified staples are needed to understand the higher observed prevalence of neural tube defects in those areas. Addressing these issues will be critical to ensuring CREC’s continuity in monitoring birth defects, and sustainability of folic acid fortification programs in Costa Rica. Finally, although Costa Rica has reported increases in serum folate concentrations through their fortification efforts, different methods have been used to assess serum folate concentrations in different surveys. As a result, the findings must be interpreted with caution [17]. Therefore, implementation of standardized monitoring of red blood cell folate concentrations among women of childbearing age could be helpful in providing additional information on the impact of Costa Rica’s neural tube prevention initiatives.
Lessons Learned
There are many ways other countries can learn from and apply components of the Costa Rican experience in surveillance of birth defects and folic acid fortification measures for neural tube defects prevention. These include: (1) the implementation of an executive mandate to monitor the occurrence of birth defects by the leadership of CREC that has made birth defects surveillance structured and standardized; (2) strong and well-established partnerships with public and private maternity hospitals countrywide that have allowed officials at CREC to monitor the occurrence of birth defects over a period of 25 years; (3) the implementation of executive mandates to enrich several fortified staples (e.g., wheat, corn, dairy products, and rice) of the Costa Rican diet that has proven to be a successful public health intervention in reducing birth prevalence of neural tube defects in Costa Rica; (4) strong partnerships with industry that have been vital for the establishment and implementation of folic acid prevention programs; (5) the establishment of National Reference Center of Bromatology to control and monitor all fortified products to ensure compliance with all national and regional norms; and (6) the need to implement national nutrition surveys that include blood folate measurements among women of childbearing age, to monitor folic acid prevention measures.
Conclusion
Prior to the first initiative to fortify wheat flour with folic acid in 1997, Costa Rica had already been monitoring the occurrence of birth defects through passive surveillance for a decade and had assessed folate concentration among women of childbearing age in one population-based survey. These surveillance data would later allow for the comparison and monitoring of the prevalence of neural tube defects before, during the implementation of, and after the implementation of folic acid fortification of staple foods. This allowed health officials to demonstrate the importance of folic acid fortification for the prevention of neural tube defects. Although overall declines in prevalence have been seen, there remain segments of the population—primarily in remote areas—for which the prevalence of neural tube defects is higher than that of other geographic areas. Understanding the reasons for continued higher prevalence of neural tube defects in these remote areas (e.g., lack of folic acid supplementation, lack of access to fortified staples, genetic factors, etc.) remains an important mission for CREC and public health officials. Countries considering initiating birth defects surveillance and/or fortifying certain staples with folic acid will likely need to first assess existing infrastructure and build solid foundations and partnerships with different stakeholders (e.g., hospitals, industry, government officials, lawmakers, etc.). The example of a national surveillance system in Costa Rica demonstrates that achieving gains in the prevention of neural tube defects may take several years to accomplish.
Acknowledgments
We would like to express our gratitude to all neonatologists, pediatricians and general physicians from all public and private hospitals, who has been contributing to the Center Registry for Congenital Anomalies for more than 25 years. We would also like to acknowledge Dr. Ana Laura Jimenez, Director of Spina Bifida Unit of the National Children’s Hospital (Dr. Carlos Saenz Herrera), for her continuing support to CREC. This study received no specific grant from any funding agency in the public, commercial, or nonprofit sectors.
Footnotes
Conflict of interest None.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the U.S. Centers for Disease Control and Prevention.
Contributor Information
María de la Paz Barboza-Argüello, Unidad de Enfermedades Congénitas, Centro de Registro de Enfermedades Congénitas (CREC), Instituto Costarricense de Investigación y Enseñanza en Nutrición y Salud (INCIENSA), Tres Ríos, Cartago, Costa Rica.
Lila M. Umaña-Solís, Unidad de Enfermedades Congénitas, Centro de Registro de Enfermedades Congénitas (CREC), Instituto Costarricense de Investigación y Enseñanza en Nutrición y Salud (INCIENSA), Tres Ríos, Cartago, Costa Rica
Alejandro Azofeifa, Division of Birth Defects and Developmental Disabilities, National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention (CDC), Atlanta, GA, USA.
Diana Valencia, Division of Birth Defects and Developmental Disabilities, National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention (CDC), Atlanta, GA, USA.
Alina L. Flores, Division of Birth Defects and Developmental Disabilities, National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention (CDC), Atlanta, GA, USA
Sara Rodríguez-Aguilar, Centro Nacional de Referencia en Química Clínica, Instituto Costarricense de Investigación y Enseñanza en Nutrición y Salud (INCIENSA), Tres Ríos, Cartago, Costa Rica.
Thelma Alfaro-Calvo, Centro Nacional de Referencia de Bromatología, Instituto Costarricense de Investigación y Enseñanza en Nutrición y Salud (INCIENSA), Tres Ríos, Cartago, Costa Rica.
Joseph Mulinare, Division of Birth Defects and Developmental Disabilities, National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention (CDC), Atlanta, GA, USA.
References
- 1.Christianson A, Modell B, Howson C. March of dimes global report on birth defects: The hidden toll of dying and disable children. White Plains; New York: 2006. [Google Scholar]
- 2.Wyszynski DF. Neural tube defects: from origin to treatment. Oxford University Press; New York: 2006. [Google Scholar]
- 3.Botto LD, Moore CA, Khoury MJ, et al. Neural-tube defects. New England Journal of Medicine. 1999;341(20):1509–1519. doi: 10.1056/NEJM199911113412006. [DOI] [PubMed] [Google Scholar]
- 4.Frey L, Hauser WA. Epidemiology of neural tube defects. Epilepsia. 2003;44(Suppl):13–34. doi: 10.1046/j.1528-1157.44.s3.2.x. [DOI] [PubMed] [Google Scholar]
- 5.MRC Vitamin Study Research Group Prevention of neural tube defects: Results of the Medical Research Council Vitamin Study. Lancet. 1991;338(8760):131–137. [PubMed] [Google Scholar]
- 6.Czeizel AE, Dudas I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. New England Journal of Medicine. 1992;327(26):1832–1835. doi: 10.1056/NEJM199212243272602. [DOI] [PubMed] [Google Scholar]
- 7.Berry RJ, Li Z, Erickson JD, et al. Prevention of neural-tube defects with folic acid in China. China-U.S. Collaborative Project for Neural Tube Defect Prevention. New England Journal of Medicine. 1999;341(20):1485–1490. doi: 10.1056/NEJM199911113412001. [DOI] [PubMed] [Google Scholar]
- 8.Ministerio de Salud de Costa Rica . Memoria Institucional 2011. San José, Costa Rica: 2012. [Google Scholar]
- 9.Ministerio de Salud de Costa Rica . Memoria Institucional 2008. San José, Costa Rica: 2009. [Google Scholar]
- 10.World Health Organization [Accessed 2 June 2014];Costa Rica: Health Profile. 2013 www.who.int/gho/countries/cri.pdf.
- 11.Barboza Arguello Mde L, Umana Solis LM. Impact of the fortification of food with folic acid on neural tube defects in Costa Rica. Revista Panamericana de Salud Publica. 2011;30(1):1–6. [PubMed] [Google Scholar]
- 12.Chen LT, Rivera MA. The Costa Rican experience: Reduction of neural tube defects following food fortification programs. Nutrition Reviews. 2004;62(6 Pt 2):S40–S43. doi: 10.1111/j.1753-4887.2004.tb00073.x. [DOI] [PubMed] [Google Scholar]
- 13.Creación del Centro de Registro de Enfermedades Congénitas. De Agosto De. 1985. Decreto No. 16488-S. [Google Scholar]
- 14.Benavides-Lara A, Barboza-Arguello M, Umaña-Solis LM. Manual Tecnico del Centro de Registro de Enfermedades Congénitas. INCIENSA; Costa Rica: 2008. [Google Scholar]
- 15.Instituto Costarricense de Investigación y Enseñanza en Nutrición. Centro de Registro de Enfermedades Congenita (CREC) In: Prevalencia de enfermedades congénitas por provincias y cantones: Costa Rica 1987–2000. 1 ed. INCIENSA, editor. Tres Ríos: 2002. [Google Scholar]
- 16.Ministerio de Salud de Costa Rica. Instituto Costarricense de Investigación y Enseñanza en Nutrición y Salud. Encuesta Nacional de Nutritión . Fasiculo No. 2 Micronutrientes: Ministerio de Salud-INCIENSA. San José, Costa Rica: 1996. [Google Scholar]
- 17.Rodríguez-Aguilar S, Louella-Cunnigham L. Internal quality control of determination of serum folate by immunometric methods. Rev Costarr Salúd Publica. 2012;20(2):65–69. [Google Scholar]
- 18.WHO . Vitamin and Mineral Nutrition Information System. World Health Organization; Geneva: [Accessed 2 June 2014]. 2014. Serum and red blood cell folate concentrations for assessing folate status in populations. 2012. http://apps.who.int/iris/bitstream/10665/75584/1/WHO_NMH_NHD_EPG_12.1_eng.pdf. [Google Scholar]
- 19.Reglamento para el enriquecimiento de la harina de trigo de calidad alimentaria. De Octubre De. 1997. Decreto No. 26371-S. [Google Scholar]
- 20.Creación de la Comisión Nacional de Micronutrientes. De Mayo De. 1998. Decreto No. 27086-S. [Google Scholar]
- 21.Ministerio de Salud de Costa Rica. Instituto Costarricense de Investigación y Enseñanza en Nutrición y Salud. Caja Costarricense del Seguro Social et al. In: Plan Nacional para la prevención de deficiencias de micronutrientes 1999–2002. Salud M. d., editor. San José, Costa Rica: 1999. [Google Scholar]
- 22.Reglamento para el enriquecimiento de la harina de maíz. De Setiembre De. 1999. Decreto No. 28086-S. [Google Scholar]
- 23.Reglamento para el enriqueciemito de leche de ganado vacuno. De Julio De. 2001. Decreto No. 29629-S. [Google Scholar]
- 24.Reforma al reglamento tecnico de enriquecimiento de la harina de trigo de calidad alimenantaria. De Enero De. 2002. Decreto No. 30030-S. [Google Scholar]
- 25.Reglamento para el enriquecieminto del arroz. De Enero De. 2002. Decreto No. 30031-S. [Google Scholar]
- 26.Ministerio de Salud de Costa Rica. Instituto Costarricense de Investigación y Enseñanza en Nutrición y Salud. Caja Costarricense del Seguro Social et al. Encuestas Nacional de Nutrición 2008–2009. Fascículo 2: Micronutrientes. San José, Costa Rica: 2012. pp. 31–94. [Google Scholar]
- 27.Daly L. Simple SAS macros for the calculation of exact binomial and Poisson confidence limits. Computers in Biology and Medicine. 1992;22(5):351–361. doi: 10.1016/0010-4825(92)90023-g. [DOI] [PubMed] [Google Scholar]
- 28.Miranda-Guitierrez G. La Seguridad Social y el Desarrollo en Costa Rica. 3 ed. EUNED; San José: 2003. [Google Scholar]
- 29.Miranda-Guitierrez G. La Construcción de la Seguridad Social en Costa Rica. EUNED; San José: 2004. [Google Scholar]
- 30.McGuire James W. A healthy democracy (chap3), in wealth, health and democracy in East Asia and Latin America. Cambridge University Press; Cambridge: 2010. p. 406. [Google Scholar]
- 31.Centers for Disease Control and Prevention Spina bifida and anencephaly before and after folic acid mandate—United States, 1995–1996 and 1999–2000. MMWR Morbidity and Mortality Weekly Report. 2004;53(17):362–365. [PubMed] [Google Scholar]
- 32.De Wals P, Tairou F, Van Allen MI, et al. Reduction in neural-tube defects after folic acid fortification in Canada. New England Journal of Medicine. 2007;357(2):135–142. doi: 10.1056/NEJMoa067103. [DOI] [PubMed] [Google Scholar]
- 33.Hertrampf E, Cortes F. Folic acid fortification of wheat flour: Chile. Nutrition Reviews. 2004;62(6 Pt 2):S44–8. doi: 10.1111/j.1753-4887.2004.tb00074.x. discussion S9. [DOI] [PubMed] [Google Scholar]
- 34.Lopez-Camelo JS, Orioli IM, da Graca Dutra M, et al. Reduction of birth prevalence rates of neural tube defects after folic acid fortification in Chile. American Journal of Medical Genetics A. 2005;135(2):120–125. doi: 10.1002/ajmg.a.30651. [DOI] [PubMed] [Google Scholar]
- 35.Berry RJ, Bailey L, Mulinare J, et al. Fortification of flour with folic acid. Food and Nutrition Bulletin. 2010;31(1 Suppl):S22–S35. doi: 10.1177/15648265100311S103. [DOI] [PubMed] [Google Scholar]
- 36.Ministerio de Salud de Costa Rica. Caja Costarricense del Seguro Social. Instituto Costarricense de Investigación y Enseñanza en Nutrición y Salud et al. Observatorio de Políticas de Enferemedades no Trasmisibles. Estudio de caso: Formulación de la politica de fortificación de harina de trigo con ácido fólico. Informe final; Costa Rica: 2006. [Google Scholar]