Summary
Clara cells are nonciliated secretory cells lining the respiratory epithelium and are easily identified by the expression of Clara cell secretory protein (CCSP). To investigate molecular mechanism(s) regulating Clara cell function in the lungs, Cre recombinase was inserted into exon 1 of the CCSP, generating two novel mouse models, CCSPCre-Neo and CCSPCre. These two models differ only by the inclusion of the neomycin resistance gene. These mice were bred to the R26R reporter mouse to investigate the tissue and cell specificity of Cre-mediated recombination. The efficiency of Cre recombination in the CCSPCre mouse model was higher than in the CCSPCre-Neo mouse model. Recombination was detected at D 4.5 in CCSPCre-Neo/R26R mice and at D 0.5 in CCSPCre/R26R mice. The CCSPCre-Neo and CCSPCre mouse models provide valuable tools for the ablation of genes in the post-natal mouse Clara cells.
Keywords: CCSP, lung, Cre recombinase, Clara cell, knock-in
Clara cells are nonciliated, secretory cells lining the bronchioles of the lung. Clara cells can be identified in the airway by their distinct domed-shaped morphology and abundance of secretory granules (Plopper et al., 1980). Biochemically, Clara cells contain cytochrome P450 mixed function oxidase and secrete Surfactant proteins A, B, and D, a leukocyte protease inhibitor, a trypsinlike protease and a 16 kDa protein, Clara cell secretory protein, CCSP (Massaro et al., 1994). Physiologically, the Clara cells have a protective role in the airways. The major secretory product of the Clara cells is Clara cell secretory protein (CCSP) that serves to protect the lung against hyperoxic damage and inflammation (Johnston et al., 1997, 1999). Because of the cellular preference and high abundance of this protein, the mouse CCSP locus is a powerful tool to target gene expression in a cell-specific fashion to the airway (Ray et al., 1995; Stripp et al., 1992).
The expression of Cre recombinase under the control of the promoter for the CCSP gene would allow for tissue and cellular-specific gene ablation in the airways of the mouse. This animal model would allow for the investigation of the genetic control of Clara cell biology and the generation of animal models for pulmonary diseases such as lung cancer. The goal of this study was to establish a mouse model for the specific ablation and activation of genes in the Clara cells of lung. To accomplish this goal, Cre recombinase was inserted into the first exon of CCSP by homologous recombination in ES cells. Mice expressing Cre recombinase in the Clara cells were generated from these ES cell clones.
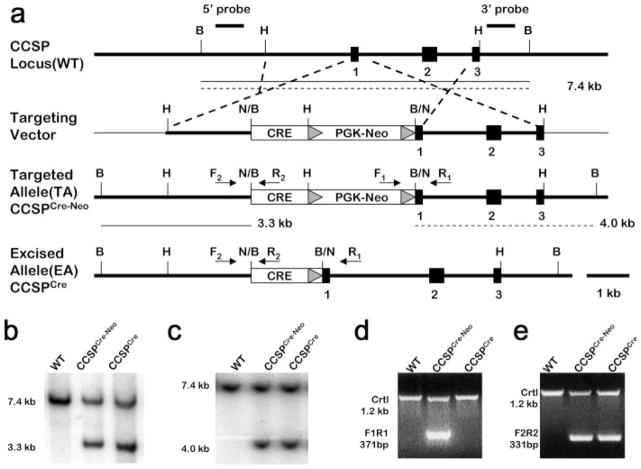
Cre recombinase was placed into the CCSP locus using the gene-targeting approach outlined in Figure 1a. The targeting vector consisted of Cre recombinase and the neomycin resistance expression cassette, neo cassette, inserted into exon 1 of the CCSP gene. The neo cassette was flanked by Frt sequences to enable Flp-mediated excision of the neo cassette. After G418 selection, 288 drug-resistant ES cell colonies were isolated. One hundred thirty-five (47.69%) colonies bearing the targeted allele were identified by Southern-blot analysis. No aberrant integration events were revealed by using 5′ and 3′ probes (Fig. 1b,c). Eight positive clones were injected into fertilized C57BL/6 blastocysts to produce chimeric mice. Of the eight positive clones injected into blastocysts, three of the resulting chimeric mice exhibited germ line transmission. The resulting mouse model was designated as CCSPCre-Neo. The CCSPCre-Neo mouse was then crossed with the FLPeR mice (R26fki) to remove the neo cassette that generated the CCSPCre mouse model (Farley et al., 2000). Removal of the neocassette was determined by PCR (Fig. 1d). The CCSPCre-Neo and CCSPCre mice were viable and fertile. The morphology of the lungs was normal (data not shown). Cre activity was determined by crossing CCSPCre-Neo and CCSPCre mice to the R26R reporter mouse line to generate the CCSPCre-Neo/ R26R and CCSPCre/R26R mice (Soriano, 1999).
FIG. 1.
Generation of CCSPCre-Neo and CCSPCre Knock-in Mice. (a) Targeting strategy employed to generate CCSPCre-Neo and CCSPCre knock-in mice. This gene-targeting strategy was used to insert the Cre recombinase gene into the beginning of exon 1 of the CCSP gene. The neomycin resistance cassette (neo) was flanked with Frt sites (triangles) to enable future Flp-mediated excision of this selective marker. Targeted clones were identified using a 5′ external probe and 3′ external probe (line). B, BamH I; H, Hind III; N, Not I. (b) Southern blot analysis of 5′ external probe. Mouse tail DNA from wild type, CCSPCre-Neo and CCSPCre-Neo mice were digested with BamH I. The probe is designed 439 bp outside the construct in the CCSP gene. The band size is 7.4 kb in wild type mice and 3.3 kb in CCSPCre-Neo and CCSPCre mice. (c) Southern blot analysis of 3′ external probe. Mouse tail DNA from wild type, CCSPCre-Neo and CCSPCre-Neo mice were digested with BamH I. The probe is designed 376 bp outside the construct in the CCSP gene. The band size is 7.4 kb in wild type mice and 4.0 kb in CCSPCre-Neo and CCSPCre mice. (d) Identification of neomycin gene by PCR. Using the primers crossing the conjunction of CCSP and neo gene, the wild type and CCSPCre-Neo mice show a 371-bp fragment while the CCSPCre mice show no fragment. PCR primer (F1R1) locations are indicated in (a). (e) PCR-based genotyping. Using the primers crossing the conjunction of CCSP and Cre recombinase gene, the wild type mice show no fragment while the CCSPCre-Neo and CCSPCre mice show a 331-bp fragment. PCR primer (F2R2) locations are indicated in (a).
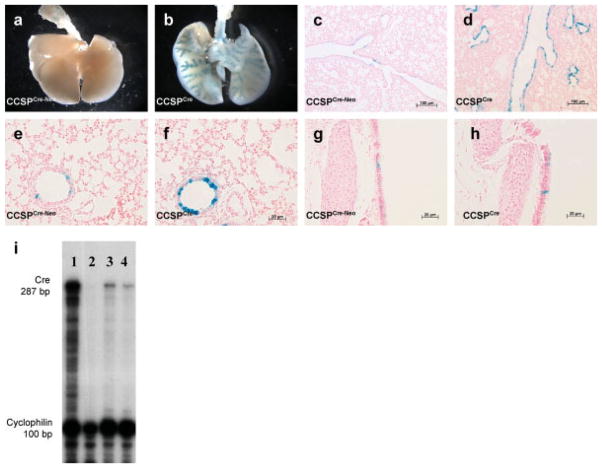
Whole mount X-gal staining of lungs from CCSPCre-Neo/ R26R and CCSPCre/R26R mice is shown in Figure 2a,b, respectively. Scant LacZ expression was observed in the lungs of CCSPCre-Neo/R26R mice while LacZ expression in CCSPCre/R26R mouse lungs showed “tree like” staining of the airways with no staining in the lung parenchyma mice. Histological sections of these lungs confirmed sporadic cell staining positive for LacZ expression in the larger airways in CCSPCre-Neo/R26R mice (Fig. 2c,e), while numerous cells stained positive for LacZ in the large airways and bronchioles of the CCSPCre/R26R lungs (Fig. 2d,f). Cells of the trachea sporadically stained positive for LacZ in both models (Fig. 2g,h).
FIG. 2.
Cre recombinase activity was assayed by X-gal staining of lungs taken from 4-week-old mice with the following genotypes: CCSPCre-Neo/R26R, CCSPCre/R26R, CCSPCre-Neo, CCSPCre, R26R and wild type mice. (a). Whole mount X-gal staining of CCSPCre-Neo/R26R and (b) CCSPCre/R26R mice. X-gal staining of lung sections showing large airways (c and d), bronchioles (e and f) and trachea (g and h) from CCSPCre-Neo/R26R and CCSPCre/R26R mice that show X-gal staining only in the airway and not in the lung parenchyma (i). RNase Protection analysis of Cre recombinase mRNA. The 287-bp protective fragment of Cre recombinase mRNA is shown in both the CCSPCre-Neo mRNA and the CCSPCre. The intensity of 287-bp band is more intense in the CCSPCre than CCSPCre-Neo mice (Lane 1: Cre + control, Lane 2: Cre-, Lane 3: CCSPCre/R26R, Lane 4: CCSPCre-Neo/R26R).
The level of Cre mRNA expression in both models was quantified by RNase protection assay (RPA). Figure 2i shows that the level of Cre mRNA in the CCSPCre mouse lung was higher than in the CCSPCre-Neo mouse lung. This data confirmed that the Cre recombinase gene was expressed in the lungs of both of CCSPCre-Neo and CCSPCre mice. The higher level of expression of the Cre recombinase in the CCSPCre mouse model explains the increased Cre activity in the CCSPCre model.
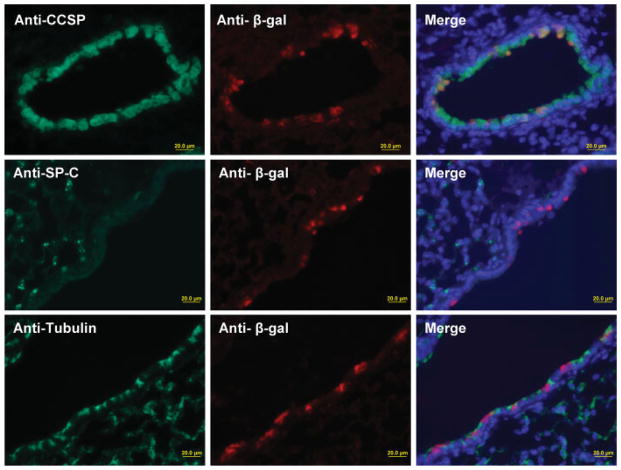
To determine the Clara cell specificity of Cre recombinase activity in the CCSPCre mouse model, anti-CCSP, anti-SPC, anti-acetylated tubulin, and anti-β-galactosidase antibodies were used to perform dual-immunofluorescence in CCSPCre/R26R mice (see Fig. 3). This analysis would determine if Cre recombination occurred in Clara cells, Alveolar type II cells, or Ciliated cells, respectively. In the lung sections of dual-immunofluorescence, the cells staining with the anti-β-galactosidase antibody colocalized only with cells staining with the anti-CCSP anti-body. Although a majority of Clara cells stained for β-galactosidase, the coincidence is not 100%. However, there was no colocalization of cells staining positive for β-galactosidase and cells staining positive for SPC or acetylated tubulin. The data indicates that Cre recombination activity is restricted to the CCSP-expressing cells.
FIG. 3.
Cellular specificity of Cre recombinase activity was analyzed by immunohistochemical staining of lung sections from 4-week-old CCSPCre/R26R mice stained for anti-CCSP (green, top row), anti-SP-C (green, middle row), and antiacetylated tubulin (green, bottom row) and anti-β-galactosidase (red, middle column). Dual immunofluorescence and DAPI staining (blue) is shown in last column.
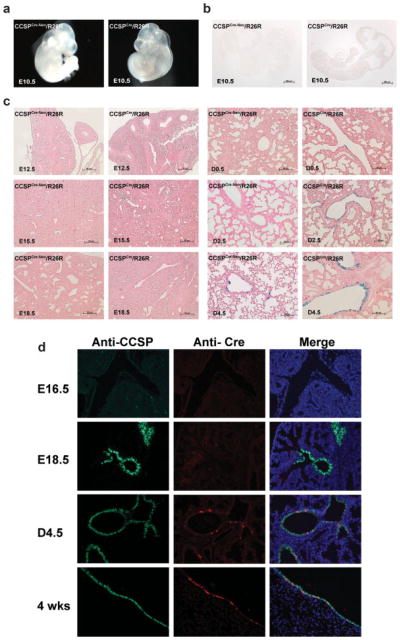
The developmental time course of Cre recombinase activity in the lung was determined by Lacz staining of whole E 10.5 embryos and the lungs of E 12.5, E 15.5, E 18.5, D 0.5, D 2.5, and D 4.5 of CCSPCre-Neo/R26R and CCSPCre/R26R mice. No LacZ staining was detected in the embryo of E 10.5 from both CCSPCre-Neo/R26R and CCSPCre/R26R mice. LacZ staining was detected in the lung from D 4.5 in the CCSPCre-Neo/R26R mice, and from D 0.5 in the CCSPCre/R26R mice (Fig. 4a–c). This data shows that Cre recombination was active in the lungs at D 4.5 in the CCSPCre-Neo mice and at D 0.5 in the CCSPCre mice. To determine if the developmental time course of Cre recombination in the CCSPCre/R26R mouse lungs correlated with CCSP or Cre expression, lungs were stained with anti-CCSP and anti-Cre antibodies. The results of this analysis are shown in Figure 4d. Analysis of CCSPCre/R26R mice lungs at E16.5, E18.5, and D4.5 and at 4 weeks of age shows that CCSP expression is observed at E18.5. However, Cre expression is not observed at this time but at D4.5. Therefore, the developmental expression of Cre is delayed in these mice when compared with the endogenous expression of CCSP. Therefore, Cre recombination is only observed after birth in these models.
FIG. 4.
The developmental time course of Cre activity and expression was analyzed by staining lungs for X-gal activity and immunohistochemical staining for CCSP and Cre expression, respectively. Lungs were taken from E 10.5, E 16.5, E 18.5, D 4.5, and 4 weeks with the following genotypes, CCSPCre-Neo/R26R and CCSPCre/R26R. (a) Whole mount X-gal staining of E 10.5 embryos from CCSPCre-Neo/R26R and CCSPCre/R26R mice. No blue staining is shown in the whole embryos in CCSPCre-Neo/R26R and CCSPCre/R26R mice. (b) Sections of whole mount X-gal staining of E 10.5 embryos from CCSPCre-Neo/R26R and CCSPCre/R26R mice. No blue staining is shown in the sections from CCSPCre-Neo/R26R and CCSPCre/R26R mice. (c) X-gal staining of lung sections from CCSPCre-Neo/R26R and CCSPCre/ R26R mice at E 12.5, E 15.5, E 18.5, D 0.5, D 2.5, and D 4.5. X-gal staining is detected in the lung at D 4.5 in the CCSPCre-Neo/R26R mice and at D 0.5 in the CCSPCre/R26R mice. X-gal staining is shown in the airway only and no blue staining is shown in the lung parenchyma of both CCSPCre-Neo/R26R and CCSPCre/R26R mice. (d) Lung sections of anti-CCSP (green) and anti-Cre (red) dual immunostaining at E 16.5, E 18.5, D 4.5, and 4 weeks of CCSPCre/R26R mice. The Cre expression is detected in the lung at D 4.5 in the CCSPCre/R26R mice.
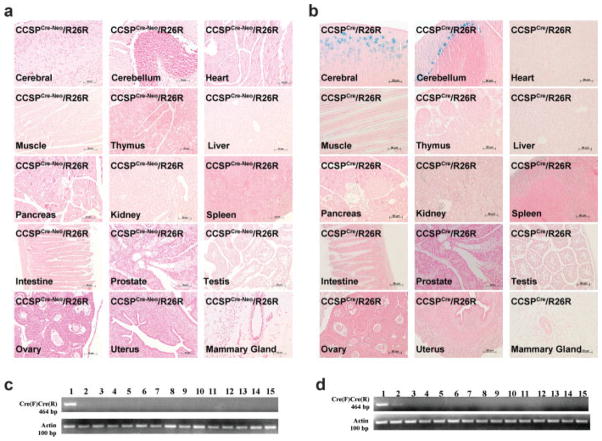
To determine tissue specificity of Cre activity in the two mouse models, X-gal staining of various organs from 4-week-old CCSPCre-Neo/R26R and CCSPCre/R26R mice was preformed. LacZ expression was observed in lungs from both CCSPCre-Neo/R26R and CCSPCre/R26R mice. No LacZ expression was observed in brain, heart, muscle, thymus, liver, pancreas, kidney, spleen, intestine, prostate, testis, ovary, uterus, and mammary gland from CCSPCre-Neo/R26R mice (Fig. 5a). In the CCSPCre/R26R mice, LacZ expression was observed in the external granular layer of cerebrum and the Purkinje cell layer of cerebellum in the brain, but was not observed in heart, muscle, thymus, liver, pancreas, kidney, spleen, intestine, prostate, testis, ovary, uterus and mammary gland (Fig. 5b).
FIG. 5.
Tissue distribution of Cre Expression. Tissues were taken from 4-week-old mice with the following genotypes, CCSPCre-Neo/ R26R and CCSPCre/R26R. Tissue distribution of Cre expression by X-gal staining in (a) CCSPCre-Neo/R26R mice and (b) CCSPCre/R26R mice. X-gal staining is shown in the external granular layer of the cerebrum and in the Purkinje cell layer of cerebellum of CCSPCre/R26R mice. Except for the brain, no other tissues show blue X-gal staining in the CCSPCre-Neo/R26R mice. Analysis of the distribution of Cre expression by RT-PCR was conducted on mRNA from tissues of CCSPCre-Neo mice (c) and CCSPCre mice (d). The size of the amplified Cre PCR product is 464 bp. The internal control, -actin, fragment amplifies a fragment of 100 bp. (Lane 1 = lung, Lane 2 = brain, Lane 3 = heart, Lane 4 = muscle, Lane 5 = thymus, Lane 6 = liver, Lane 7 = pancreas, Lane 8 = kidney, Lane 9 = spleen, Lane 10 = intestine, Lane 11 = prostate, Lane 12 = testis, Lane 13 = ovary, Lane 14 = uterus, Lane 15 = mammary gland.
RNA was also isolated from various tissues, and RT-PCR analysis was performed to determine mRNA expression of the Cre-recombinase gene. Cre expression was only detected in the lung from CCSPCre-Neo mice and in the lung and brain from CCSPCre mice. No Cre expression was detected in the heart, muscle, thymus, liver, pancreas, kidney, spleen, intestine, prostate, testis, ovary, uterus, and mammary gland from CCSPCre-Neo mice and CCSPCre mice (Fig. 5c,d). These data suggest that Cre recombination is activated in the lung of both CCSPCre-Neo and CCSPCre mice with ectopic expression in the brain of CCSPCre mice.
In this study, the CCSP locus was used to allow the specific expression of Cre recombinase in the Clara cell. This approach was undertaken to ensure the highest probability of airway and Clara cells specificity of Cre recombination. As shown in these mice, Cre activity was restricted to the CCSP expressing cells lining the airway of lung but not the ciliated cells or the alveolar type II cells. The efficiency of Cre recombination in the CCSPCre mouse was much higher than that in the CCSPCre-Neo mouse. This can be attributed to adverse effects of the neocassette in the locus. The interesting aspect of this model is that Cre recombination is only detected after birth. This is most likely due to the mouse CCSP promoter not being capable of driving Cre expression to levels in which protein or Cre activity is detectable and effectively recombines loxP sites. The Cre used to generate these models was the original Cre derived from the bacteriophage P1 system and used in mice (Gu et al., 1993). This Cre has not been optimized for expression in mammalian systems as has recent derivations of this recombinase (Shimshek et al., 2002). Use of the improved Cre, iCre, may generate mice with more faithful Cre expression and activity. However, the animal models generated in this report offer the advantage of only showing Cre recombination after birth. The delay in Cre recombination to after birth makes these models ideal to investigate gene function without interfering with fetal lung development and resulting in neonatal lethality.
CCSP is expressed primarily in the lungs and female reproductive tract of the mouse with major expression in the lungs (Ray et al., 1995; Stripp et al., 1992). The CCSP locus was chosen to ensure Clara cell specificity of Cre recombinase. Analysis of the tissue distribution of Cre activity in these models showed the lung to be the major site of Cre activity. No female reproductive tract expression was observed in these mice. This was not unexpected because the level of CCSP expression in the oviduct and uterus is several orders of magnitude lower at these sites when compared with the lungs (Ray et al., 1995). However, one unexpected finding was that Cre activity was detected in the external granular layer of cerebrum and the Purkinje cell layer of cerebellum in the brain of the CCSPCre/R26R mice. Although CCSP expression has not been observed at this site, the knock-in construction may have resulted in the presence of cryptic enhancers that allowed expression at this site.
Previous mouse models in which Cre activity is directed using the CCSP promoter have been reported (Bertin et al., 2005; Ji et al., 2006; Perl et al., 2005). Perl and coworkers, using a model where the reverse tetracycline transactivator, rtTA, is expressed under the rat CCSP promoter regulated the expression of a tetracycline responsive Cre transgene in the lungs (Perl et al., 2005). In this model, Cre expression was observed as early as E12.5, and Cre acrivity was detected at E15.5. The Cre activity was not restricted to the Clara cells as was expected using the rat CCSP promoter to ultimately drive the Cre transgene. Ji and coworkers used the rat CCSP promoter to directly express Cre recombinase in the lungs. This model was used to activate a conditional KrasG12D allele. The resulting mice developed lung tumors. However, information regarding the characterization of this model is limited (Ji et al., 2006). Bertin and coworkers generated the mouse CCSP-Cre transgenic mouse strain using a 2.3-kb fragment of the mouse CCSP promoter (Bertin et al., 2005). The Cre recombinase is functionally active in nonciliated Clara cells, but not in ciliated cells nor alveolar type II cells. No information regarding the developmental timing of expression of this transgene was discussed. This model showed some ectopic expression of Cre recombinase depending on the line of transgenic mouse generated (Bertin et al., 2005). Although ectopic expression was observed in the CCSPCre model, the ectopic expression was limited in the brain.
In summary, we have generated two mouse models in which Cre recombinase is expressed in a Clara cell-specific fashion in the lungs. The Cre activity is limited to after birth. Therefore, this model is most promising to investigate the impact of gene ablation on adult mouse lung physiology. Evidence for the utility of this model is that it has been recently used to investigate the interaction of Kras and Pten in the development of lung cancer (Iwanaga et al., 2008).
MATERIALS AND METHODS
Generation of CCSPCre-Neo and CCSPCre Mice
A mouse genomic sequence that contains CCSP exon 1 was isolated from the 129/sv strain. A 5.1-kb genomic fragment was used to construct the CCSPCre-Neo targeting vector. The targeting vector was constructed by placing a 2.1-kb short arm of homology and a 3.0-kb long arm of homology flanking the Cre recombinase gene and PGK-neo cassette. The Cre recombinase gene was derived from the pIC plasmid (Gu et al., 1993). The Cre recombinase gene was inserted into exon 1 of the CCSP gene. To enable drug selection, the Frt-flanked neocassette was cloned into the location between the Cre recombinase gene and the long homological arm (Fig. 1a). The cloning plasmid used in this construction was the pBluescript II Sk(−) vector (Stratagene, La Jolla, CA). Linearized vector was electroporated into R1 ES cells (129/sv × 129/sv-CP derived) (Nagy et al., 1993).
All ES cell culturing and manipulations before and after the electroporation step were performed as previously reported (Soriano et al., 1991). Drug-resistant clones were identified by Southern-blot analysis using 5′and 3′ external probes located just beyond the region of homology contained within the targeting vector. The 5′ external probe was 439 bp and identified a 7.4 kb band in wild type mice and a 3.3 kb band in targeted alleles in BamH I digested ES cell DNA. The 3′ external probe was 376 bp and identified a 7.4-kb band in wild-type mice, and a 4.0-kb band in the targeted alleles in BamH I digested ES cell DNA. Male chimeras obtained from targeted ES cell clones were used to cross with C57BL/6 mice with resultant germ line transmission of the Cre-Neo insertion; for the investigations described herein, mice with a mixed 129/sv × C57BL/6J background were used.
The CCSPCre-Neo mice were then bred with the FLPeR (R26fki) mice for in vivo Flp-mediated excision of the selection (neo) cassette to generate the CCSPCre mice (Farley et al., 2000). To distinguish the CCSPCre-Neo and CCSPCre mice, primers (F1, 5′-TGATGGCGATCTT-CATGGTA-3′ and R1, 5′-GGGGAACTTCCTGACTAGGG- 3′), which are used to identify the neocassette within the CCSP gene were designed to cross the conjunction of the neo gene and the CCSP gene. The CCSPCre-Neo mice show a 371 bp fragment while the CCSPCre mice show no fragment (Fig. 1d).
CCSPCre-Neo and CCSPCre mice then were crossed with R26R reporter mice (Soriano, 1999) on a C57BL/6J background, which harbors the ROSA26-floxed Neo-LacZ recombination substrate to obtain double-transgenic CCSPCre-Neo/R26R and CCSPCre/R26R mice on a mixed C57BL/6J genetic background.
Genotyping and Mouse Breeding
Using mouse tail DNA, the founders of CCSPCre-Neo and CCSPCre mice were identified by Southern-blot analysis and PCR analysis. Subsequent genotyping was performed by PCR analysis using genomic DNA extracted from tail biopsies. Primers (F2, 5′-GGTCCTCCACTGCCTGAATA-3′ and R2, 5′-GAGGTTCTTGCGAACCTCAT-3′), which are used to identify the Cre recombinase gene within the CCSP gene were designed to cross the conjunction of the CCSP gene and the Cre recombinase gene. Both CCSPCre-Neo and CCSPCre mice show a 331 bp fragment (Fig. 1e). Primers (F1 and R1) were used to distinguish between the CCSPCre-Neo and CCSPCre mice.
CCSPCre-Neo and CCSPCre knock-in mice were maintained on a mixed 129Sv-C57BL/6J hybrid background. R26R mice were maintained on C57BL/6J backgrounds. All mice were housed at Baylor College of Medicine animal facility in a temperature-controlled (22 ± 2°C) room with a 12:12 light–dark cycle and given rodent chow (Purina Mills, St. Louis, MO) and fresh water ad libitum. All animal experimentation was performed in accordance with an IACUC approved protocol (Baylor College of Medicine, Houston, TX).
Reverse Transcription-PCR
Total RNA was isolated from tissues according to the TRIzol reagent protocol (Invitrogen, Carlsbad, CA). RNA concentrations were determined by spectrophotometric absorption at 260 nm and stored at −80°C until further analysis was done. The cDNA was generated from RNA samples by SuperScript™ II reverse transcriptase (Invitrogen, Carlsbad, CA), followed by PCR amplification. The predetermined PCR product was 464 bp. The primers, Cre-F 5′-ACT ATC CAG CAA CAT TTG GGC C-3′ and Cre-R 5′-CCG GCA AAA CAG GTA GTT ATT CGG-3′, were used to identify the mRNA expression of the Cre recombinase gene in CCSPCre-Neo and CCSPCre mice.
RNase Protection Analysis
The RNase protection assay was performed as described, using the RPA III kit according to the manufacturer’s instructions (Ambion, Austin, TX). The RPA probe, for Cre-encompassing nucleotides 289–575 within the Cre gene, results in a protective fragment of 287 bp. The 32P-labeled antisense Cre riboprobes were synthesized in vitro using 32P-UTP and T7 polymerase (RPA III kit, Ambion, Austin, TX). Riboprobe was gel purified, and 1,000,000 cpm of the probe was hybridized to 1 μg of mRNA in 10 μl of hybridization solution at 45°C overnight (RPA III kit, Ambion, Austin, TX). Cyclophilin was used as the RNA-loading control. After RNase A digestion, the labelled products were separated by a 6% denaturing polyacrylamide gel. Gels were exposed to film.
Histology and X-gal Staining
Tissues were taken from mice with the following genotypes, CCSPCre-Neo/R26R, CCSPCre/R26R, CCSPCre-Neo, CCSPCre, R26R, and wild-type mice. The later four genotypes served as negative controls. Following the predetermined time, the mice were euthanized. The tracheas were isolated and cannulated with PE-50 tubing and sutured into place. The right lungs were frozen in liquid nitrogen, and the left lungs were infused with 4% fresh paraformaldehyde. Then, the left lung was removed and placed in 4% fresh paraformaldehyde for 3 h. At the same time, brain, heart, muscle, thymus, liver, pancreas, kidney, spleen, intestine, prostate, testis, ovary, uterus, and mammary gland were also removed and placed in 4% fresh paraformaldehyde for 3 h. Embryos from E 10.5 were placed in 4% fresh paraformaldehyde for 3 h. After being washed with PBS three times, tissue whole-mounts were stained with X-gal (PBS containing 1 mg/ml X-gal in DMF, 5 mM potassium ferrocyanide, 5 mM potassium ferricyanide, 2 mM MgCl2, 0.01% deoxycholate, 0.2% NP-40, pH 7.5) overnight at room temperature. Following staining, tissues were transferred to 75% ethanol, embedded in paraffin, sectioned, and stained with nuclear fast staining and H&E staining.
Dual Immunofluorescence
Dual-immunofluorescence techniques were performed to identify the specific cell lineages in the lung using anti-CCSP antibody from rabbit (Ray et al., 1996), anti-SP-C antibody from rabbit (WRAB-SPC, Seven Hills Bioreagents, Cincinnati, OH, 1:1,500), anti-acetylated tubulin from mouse (T6793, Sigma, St Louis, MO, 1:1,000), anti-Cre recombinase from rabbit (PRB-106C, Covance, Emeryville, CA, 1:1,000), and β-galactosidase antibody from goat (4600-1409, Biogenesis, Kingston, NH, 1:1,000). Sections were blocked at room temperature for 1 h in a TSA kit blocking buffer (Perkin Elmer, Boston, MA). They were then incubated at room temperature for 1 h. After washing with PBS containing 0.2% Triton X-100, the sections were incubated for 30 min with secondary antibodies using biotinylated anti-rabbit antibody (BA-1000, Vector, Burlingame, CA, 1:200), bio-tinylated anti-goat antibody (BA-5000, Vector, Burlingame, CA, 1:200), and biotinylated anti-mouse antibody (BA-9200, Vector, Burlingame, CA, 1:200). The immunofluorescence was developed using TSA kits (Perkin Elmer, Boston, MA) based on the manufacturer’s instructions.
Acknowledgments
Contract grant sponsor: NCI, Contract grant number: U01 CA105352
Meirong Gu, Jinghua Li, Jie Wang, and Janet DeMayo, M.S. gave technical assistance in the completion of this project.
Abbreviation
- CCSP
Clara cell secretory protein
LITERATURE CITED
- Bertin G, Poujeol C, Rubera I, Poujeol P, Tauc M. In vivo Cre/loxP mediated recombination in mouse clara cells. Transgenic Res. 2005;14:645–654. doi: 10.1007/s11248-005-7214-0. [DOI] [PubMed] [Google Scholar]
- Farley FW, Soriano P, Steffen LS, Dymecki SM. Widespread recombinase expression using FLPeR (flipper) mice. Genesis. 2000;28:106–110. [PubMed] [Google Scholar]
- Gu H, Zou YR, Rajewsky K. Independent control of immunoglobulin switch recombination at individual switch regions evidenced through Cre-loxP-mediated gene targeting. Cell. 1993;73:1155–1164. doi: 10.1016/0092-8674(93)90644-6. [DOI] [PubMed] [Google Scholar]
- Iwanaga K, Yang Y, Raso MG, Ma L, Hanna AE, Thilaganathan N, Moghaddam S, Evans CM, Li H, Cai WW, Sato M, Minna JD, Wu H, Creighton CJ, Demayo FJ, Wistuba II, Kurie JM. Pten inactivation accelerates oncogenic K-ras-initiated tumorigenesis in a mouse model of lung cancer. Cancer Res. 2008;68:1119–1127. doi: 10.1158/0008-5472.CAN-07-3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H, Houghton AM, Mariani TJ, Perera S, Kim CB, Padera R, Tonon G, McNamara K, Marconcini LA, Hezel A, El-Bardeesy N, Bronson RT, Sugarbaker D, Maser RS, Shapiro SD, Wong KK. K-ras activation generates an inflammatory response in lung tumors. Oncogene. 2006;25:2105–2112. doi: 10.1038/sj.onc.1209237. [DOI] [PubMed] [Google Scholar]
- Johnston CJ, Finkelstein JN, Oberdorster G, Reynolds SD, Stripp BR. Clara cell secretory protein-deficient mice differ from wild-type mice in inflammatory chemokine expression to oxygen and ozone, but not to endotoxin. Exp Lung Res. 1999;25:7–21. doi: 10.1080/019021499270394. [DOI] [PubMed] [Google Scholar]
- Johnston CJ, Mango GW, Finkelstein JN, Stripp BR. Altered pulmonary response to hyperoxia in Clara cell secretory protein deficient mice. Am J Respir Cell Mol Biol. 1997;17:147–155. doi: 10.1165/ajrcmb.17.2.2676. [DOI] [PubMed] [Google Scholar]
- Massaro GD, Singh G, Mason R, Plopper CG, Malkinson AM, Gail DB. Biology of the Clara cell. Am J Physiol. 1994;266:L101–L106. doi: 10.1152/ajplung.1994.266.1.L101. [DOI] [PubMed] [Google Scholar]
- Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder JC. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc Natl Acad Sci USA. 1993;90:8424–8428. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perl AK, Wert SE, Loudy DE, Shan Z, Blair PA, Whitsett JA. Conditional recombination reveals distinct subsets of epithelial cells in trachea, bronchi, and alveoli. Am J Respir Cell Mol Biol. 2005;33:455–462. doi: 10.1165/rcmb.2005-0180OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plopper CG, Mariassy AT, Hill LH. Ultrastructure of the nonciliated bronchiolar epithelial (Clara) cell of mammalian lung: I. A comparison of rabbit, guinea pig, rat, hamster, and mouse. Exp Lung Res. 1980;1:139–154. doi: 10.3109/01902148009069644. [DOI] [PubMed] [Google Scholar]
- Ray MK, Magdaleno SW, Finegold MJ, DeMayo FJ. cis-acting elements involved in the regulation of mouse Clara cell-specific 10-kDa protein gene. In vitro and in vivo analysis. J Biol Chem. 1995;270:2689–2694. doi: 10.1074/jbc.270.6.2689. [DOI] [PubMed] [Google Scholar]
- Ray MK, Wang G, Barrish J, Finegold MJ, DeMayo FJ. Immunohistochemical localization of mouse Clara cell 10-KD protein using antibodies raised against the recombinant protein. J Histochem Cytochem. 1996;44:919–927. doi: 10.1177/44.8.8756763. [DOI] [PubMed] [Google Scholar]
- Shimshek DR, Kim J, Hubner MR, Spergel DJ, Buchholz F, Casanova E, Stewart AF, Seeburg PH, Sprengel R. Codon-improved Cre recombinase (iCre) expression in the mouse. Genesis. 2002;32:19–26. doi: 10.1002/gene.10023. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Soriano P, Montgomery C, Geske R, Bradley A. Targeted disruption of the c-src protooncogene leads to osteopetrosis in mice. Cell. 1991;64:693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- Stripp BR, Sawaya PL, Luse DS, Wikenheiser KA, Wert SE, Huffman JA, Lattier DL, Singh G, Katyal SL, Whitsett JA. cis-acting elements that confer lung epithelial cell expression of the CC10 gene. J Biol Chem. 1992;267:14703–14712. [PubMed] [Google Scholar]