Abstract
Introduction
Necrotizing fasciitis (NF) is an inflammatory disease of the soft tissue, which causes local tissue destruction and can lead to lethal septic shock. The therapy consists of early surgical treatment of the septic focus and an accompanying broad spectrum antibiotic therapy. Recent literature considers the additional use of immunoglobulin therapy in severe soft skin and tissue infections.
Presentation of case
In this report, we describe the case of a 33-year-old male patient treated at a university hospital intensive care unit because of an NF of his left leg. The patient rapidly developed a complicated septic disease after a minor superficial trauma. Despite intense microbiological diagnosis, no causative pathogens were identified. After non-responding to established broad anti-infective treatment, the patient received intravenous immunoglobulin, that rapidly improved his clinical condition.
Discussion
NF represents a disease processes, which is characterized by fulminant, widespread necrosis of soft tissue, systemic toxicity, and high mortality (>30%). Beside the surgical debridement and broad spectrum antibiotic therapy IVIg therapy might be an additional option in the treatment of NF. But the current literature supporting the use of IVIG in NF is largely based on retrospective or case-controlled studies, and only small randomized trials.
Conclusion
The demonstrated case suggests that IVIg treatment of patients with NF can be considered in case of hemodynamic unstable, critically ill patients. Although randomized controlled trials are missing, some patients might benefit from diminishing hyperinflammation by immunoglobins.
Keywords: Fasciitis, Necrotizing, Sepsis, Soft skin and tissue infection, Immunoglobulin, IVIg
Highlights
-
•
Necrotizing fasciitis (NF) is an inflammatory disease, which causes local tissue destruction up to lethal septic shock.
-
•
We describe the case of a 33-year-old male patient representing an NF of his left leg.
-
•
After non-responding to established broad anti-infective treatment, the patient received immunoglobulin (IVIg).
-
•
The presented case suggests that IVIg treatment of patients with NF might be considered in case of critically ill patients.
1. Introduction
Necrotizing fasciitis (NF) is defined as a rapidly expanding infection of fascia and subcutaneous tissue [1]. In general, the NF can occur ubiquitously in soft tissues, but most common areas of expansion are the extremities and in particular the lower legs [2]. Even trunk, abdomen, head, neck area, or anal region are often infected after surgery [3]. Common triggers are trivial trauma, burns, surgery, decubital ulcers, perirectal abscesses or Fournier's gangrene [4]. Risk factors for NF include diabetes mellitus, immunosuppression, malnutrition, age, intravenous drug misuse, peripheral vascular disease, renal failure, malignancy, and obesity [5]. Patients with NF usually present severe pain around the affected area and signs of skin infection (erythema, swelling, oedema, subcutaneous plaques, or surface nodes) [6]. These physical findings may rapidly evolve into a haemorrhagic infarction of the subcutis, the fascia and the dermis with the formation of areal, painless gangrene [6]. The NF is categorized into polymicrobial (type 1) and monomicrobial (type 2) infections [7]. Type 1 are mixed infections caused by anaerobic bacteria and Streptococci mainly serogroup A. Among these, Staphylococcus aureus, Bacteroides fragilis and anaerobic cocci are the most common pathogens [8]. Type 2 infections are caused by Streptococcus pyogenes alone, or in association with S. aureus or Staphylococcus epidermidis. Clostridial infections have been classified by some authors as type 3 necrotizing soft tissue infections [9]. Laboratory tests usually show pronounced leucocytosis associated with acidosis, hypocalcaemia, and anaemia [10]. The detection of pathogens should be established by blood cultures and biopsies of the affected areas. The mortality of NF is, depending on the study, up to 50% and is usually caused by a pronounced sepsis with secondary multi organ failure [11,12]. Because of the very rapid progression and high mortality, early diagnosis and effective therapy are needed [11,13]. In addition to the surgical treatment of the infection focus with repeated debridement of the affected area, intravenous administration of broad-spectrum antibiotics should be started early [2,13].
2. Presentation of case
A 33-year-old patient in good general health and nutritional status (185 cm, 80 kg) suffered from a minor, superficial injury on his lower left leg, which he acquired during a ski vacation. The patient had no pre-existing diseases and no significant medical history. Local self-treatment led to a deterioration of the wound conditions. About 10 days after the initial trauma, the outpatient visited the centre for general medicine, followed by the initiation of an antibiotic therapy with oral Cefuroxime. Because of a progressive clinical deterioration of the wound conditions with appearance of an aching hematoma, he was admitted two days later to hospital for surgical treatment of the hematoma. In the course of the next five days, two further wound debridements and the application of a vacuum-assisted closure were performed. The antibiotic therapy was escalated to Clindamycin (3 × 600 mg/d) and Tazobactam (3 × 4.5 g/d). Due to a recent clinical deterioration and the need for advanced therapeutic measures nine days after admission, the patient was transferred to a university hospital.
On hospital admission, the patient was awake, oriented and in stable respiratory and circulatory condition. During the clinical examination, his temperature was 37.2 °C, his blood pressure was 171/85 mmHg, pulse 140 beats per minute, rhythmic and his initial oxygen saturation checked by pulse oximetry was 95% in room air. He was somnolent but easily aroused and on examination presented. Secondary findings in physical examination were severe pain and swellings in both axillae and in both groins. A laboratory evaluation revealed an increase in white blood cell (WBC) count (23.1 × 103/μL; reference value 4.00 to 11.00 × 103/μL), and C-reactive protein (CRP) 298.8 mg/dL (reference value 0.0–0.5 mg/dL). On the day of admission and the day after admission, we immediately performed surgical wound excisions with large debridement in the infected area of the left lower leg (Fig. 1). Due to international recommendations [14–16] and the current antibiotic guidelines at our hospitals intensive care unit (ICU), empirical antibiotic therapy was changed to Meropenem (4 × 1000 mg/d), Penicillin G (6x 5 Mega I.E./d) and Clindamycin (3 × 600 mg/d).
Fig. 1.
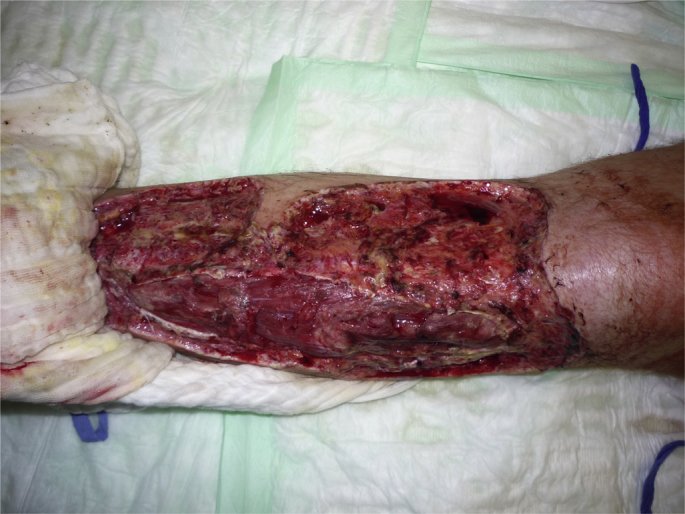
Left lower leg at day 3 after admission.
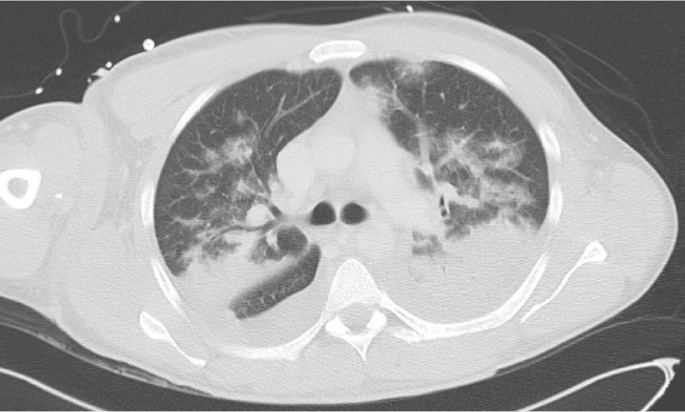
For further diagnostics, tissue samples and swabs from all infected areas were taken intraoperatively. Blood cultures were also taken at different time points. But no causative pathogen could be identified. Because of further increased inflammation markers and clinical deterioration, the anti-infective therapy was supplemented by Levofloxacin (2 × 500 mg/d) and Daptomycin (1000 mg loading-dose, 500 mg/d) for the coverage of methicillin-sensitive S. aureus and atypical pathogens two days after hospital admission. Caspofungin (70 mg loading-dose, 50 mg/d) was also added to cover invasive fungal infections. On the third day after admission, a hole-body computed tomography scan was performed. The result showed a fasciitis of the left upper and lower leg, and a symmetrical bilateral pulmonary oedema. Furthermore, bilateral pleural effusions impressed with basal atelectasis and a generalized barrier disruption. Because of a progredient lung oedema (Fig. 2) and consecutive respiratory dysfunction the patient developed the need for mechanical ventilation.
Fig. 2.
CT Image at day 3 after admission showing lung oedema, pleural effusions, compression atelectasis of both lower lung lobes, and dorsal superior lobes. Ventilated areas showing focal and lobular oedema, and thickening of the interlobular septa.
At day 4 after admission, an additional operational inspection and large debridement of both axillae were performed. Additionally, the left thigh was surgically explored and a fasciotomy was performed. The intraoperative inspection of the left leg revealed a significant increase of the necrotic area. The microbiological analyses of the collected samples were all negative. Because of a positive serological evidence of Herpes simplex virus-I a therapy using Acyclovir (6 × 250 mg/d) was initiated. The following microbiological tests using polymerase chain reaction resulted in the detection of Corynebacterium tuberculostearicum in the taken wound swab, and Acinetobacter baumanii in wound swabs, tissue biopsy, pleural-punctate and blood. A direct detection of pathogens did not succeed at any time.
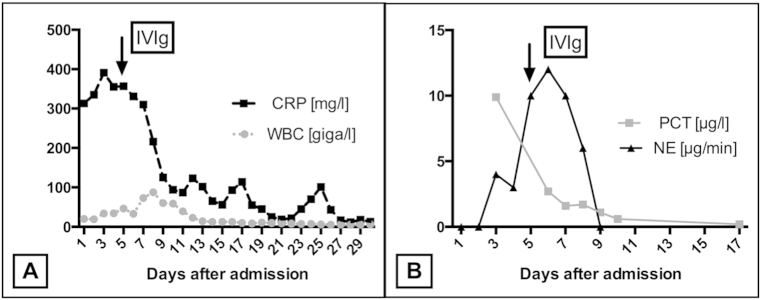
Because of the progressive deterioration of the patients' condition we decided to initiate a therapy with cortisone (Hydrocortisone 0.105 mg/kg/h) and intravenous immunoglobulin (IVIg, Pentaglobin loading dose 0.38 g/kg, 0.19 g/kg/d for the next 5 days) at the 5th day after admission. During this therapy, the aspect of hyperinflammation was diminished, the general condition improved and weaning from the respirator could be performed after 6 days of therapy.
CRP, WBC and pro-calcitonin dropped over the next days, and the need for hemodynamic support was significantly reduced (e.g. norepinephrine dose) (Fig. 3). At day 10, vacuum assisted wound closure was used to improve the wound conditions of the left lower leg and both axillae. 32 days after hospital admission, the patient could be moved from the ICU to the primary care station. Local wound conditions recovered and wounds were closed at day 39 using split-skin graft from the right thigh. At day 48 after hospital admission, the patient left the hospital in good general condition. During a follow-up examination, 6 month later, the patient was healthy and we found sufficient wound conditions.
Fig. 3.
Laboratory characteristics and need for vasopressor-therapy. A: Absolute values of C-reactive protein, CRP (mg/l) and white blood cell count, WBC (giga/l) during intensive care unit treatment. The arrow indicates the initiation of immunogloblin treatment (IVIg). B: Absolute values of procalcitonin, PCT (μg/l) and norepinephrine dose, NE (μg/min) as surrogate for the need for hemodynamic support during intensive care unit treatment. The arrow indicates the initiation of immunogloblin treatment (IVIg).
3. Discussion
Necrotizing soft tissue infections (NSTI) represent a broad spectrum of disease processes, which are characterized by fulminant, widespread necrosis of soft tissue, systemic toxicity, and high mortality (>30%). Here, we describe a patient who suffered from an NF. Despite intense microbiological diagnosis, no causative pathogens were identified. After surgical treatment, we started an extended spectrum antimicrobial and supportive therapy, though without clinical improvement. Several multidrug regimens have been described in the literature. According to our internal guidelines, our initial empirical antibiotic treatment consists in a combination of penicillin, carbapenem and clindamycin. Penicillin should always be included in the treatment of NF in consideration of possible group A Streptococci etiology [13–15]. Because of the emergence of penicillin-resistant staphylococci, an additional broad spectrum antibiotic, must be considered to provide adequate cover [13,14]. According to the life threatening condition and or local resistance profile, we chose a carbapenem. The additional use of clindamycin is also recommended to inhibit M protein and exotoxin synthesis by group A Streptococcus, thus controlling the inflammatory response [16]. The described antibiotic escalation therapy was used because of the patients' rapid clinical deterioration and the lack of causative pathogens and reflects current recommendations for the antiinfective treatment of NF [9,13,14]. Despite extended empirical antibiotic therapy and large surgical debridement, we found a progressive deterioration of the patients' condition. Consequently, we decided to initiate an immunosuppressive therapy with IVIg and hydrocortisone. IVIg therapy involves the administration of pooled IVIg from human donors. Regarding its broad range of activities, IVIG has been evaluated as a therapeutic and preventive treatment for several infectious and non-infectious conditions. Previous studies reported that IVIg enhanced the bactericidal activity of serum by facilitating bacterial opsonisation, neutralizing super antigens and toxins. IVIg also modulates leukocytes by exerting a generalized anti-inflammatory effect through its effects on Fc-receptor expression, complement, cytokines, and B- and T-cells [17]. It has been suggested that the application of IVIg in treatment of NSTI may result in a binding of exotoxins produced by staphylococcal and streptococcal bacterial infections and therefore in a limitation of systemic inflammatory response [18,19]. In addition, antibodies targeting an unidentified pathogen might have been present in IVIg, which helped to clear the infection.
Neurologic and hematological disorders are the most common indications for IVIg therapy. In this regard, hypogammaglobulinemia secondary to hematological malignancies, chronic inflammatory demyelinating polyneuropathy, and primary immunodeficiency diseases remain the leading causes for IVIg use [20]. Furthermore, Guillain-Barré syndrome, NF, and toxic epidermal necrolysis are common indications for IVIg treatment in critically ill patients at ICU [21].
Retrospective studies reported efficient use of IVIg in the treatment of NF, and the US Food and Drug Administration also approved IVIg therapy for this indication. An observational cohort study of 21 patients suffering from streptococcal toxic shock syndrome treated with IVIG revealed that IVIG increased the 30-day survival rates to 67% compared to 34% in historical controls [22]. But unfortunately a double-blind, placebo-controlled trial that searched for the benefit of IVIG had to be terminated early due to slow patient recruitment [23]. Recent randomized controlled trials, however did not confirm these retrospective data [24]. Other authors suggested that IVIg use in patients suffering from NF should be restricted to necrotizing soft tissue infections of either staphylococcal or streptococcal origin because IVIg's mechanisms of action might be based on exotoxin-IVIg binding [20,25]. IVIg therapy is generally well tolerated and seems to provide a good safety profile. Beside potential risks associated with all blood products, non-infectious reactions including systemic symptoms (headache, back pain, nausea, and vomiting), renal failure, single cases of aseptic meningitis, venous thromboembolism, myocardial infarction, and retinal vein occlusion are described [21].
Nevertheless, currently there is no reliable data to generally recommend the use of IVIg in NF. The current literature supporting the use of IVIG in NF is largely based on retrospective or case-controlled studies, and only small randomized trials [20–22]. Regarding recent data, further randomized controlled trials are needed to recommend the brought use of IVIG for the treatment of NF.
4. Conclusion
The demonstrated case suggests that IVIg treatment of patients with NF can be considered in case of hemodynamic unstable, critically ill patients. Although randomized controlled trials are missing, some patients might benefit from diminishing hyperinflammation by immunoglobins.
Ethical approval
This is a case report. The patient was informed that the data concerning his case would be submitted for publication.
Funding
The authors declared that this study has received no financial support.
Author contribution
CK, AH, WP, VG, MW and MH were involved in the conception, design and interpretation. CK, AH and MW collected data, reviewed relevant published literature and provided the images. All authors read and approved the final manuscript.
Conflicts of interest
The authors have no potential conflict of interest to declare.
Guarantor
Christian Koch; Andreas Hecker; Veronika Grau; Winfried Padberg; Matthias Wolff; Michael Henrich.
Consent
Written informed consent was obtained from the patient who participated in this case.
Research registration unique identifying number (UIN)
Research registry 280.
References
- 1.Wilson B. Necrotizing fasciitis. Am. Surg. 1952;18:416–431. [PubMed] [Google Scholar]
- 2.Childers B.J., Potyondy L.D., Nachreiner R., Rogers F.R., Childers E.R., Oberg K.C. Necrotizing fasciitis: a fourteen-year retrospective study of 163 consecutive patients. Am. Surg. 2002;68:109–116. [PubMed] [Google Scholar]
- 3.Lewis R.T. Soft tissue infections. World J. Surg. 1998;22:146–151. doi: 10.1007/s002689900362. [DOI] [PubMed] [Google Scholar]
- 4.Sudarsky L.A., Laschinger J.C., Coppa G.F., Spencer F.C. Improved results from a standardized approach in treating patients with necrotizing fasciitis. Ann. Surg. 1987;206:661–665. doi: 10.1097/00000658-198711000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Puvanendran R., Huey J.C.M., Pasupathy S. Necrotizing fasciitis. Can. Fam. Phys. 2009;55:981–987. [PMC free article] [PubMed] [Google Scholar]
- 6.Shimizu T., Tokuda Y. Necrotizing fasciitis. Intern. Med. 2010;49:1051–1057. doi: 10.2169/internalmedicine.49.2964. [DOI] [PubMed] [Google Scholar]
- 7.Elliott D., Kufera J.A., Myers R.A. The microbiology of necrotizing soft tissue infections. Am. J. Surg. 2000;179:361–366. doi: 10.1016/s0002-9610(00)00360-3. [DOI] [PubMed] [Google Scholar]
- 8.Singh G., Ray P., Sinha S.K., Adhikary S., Khanna S.K. Bacteriology of necrotizing infections of soft tissues. Aust. N. Z. J. Surg. 1996;66:747–750. doi: 10.1111/j.1445-2197.1996.tb00735.x. [DOI] [PubMed] [Google Scholar]
- 9.Sartelli M., Malangoni M.A., May A.K., Viale P., Kao L.S., Catena F. World society of emergency surgery (WSES) guidelines for management of skin and soft tissue infections. World J. Emerg. Surg. 2014;9:57. doi: 10.1186/1749-7922-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elliott D.C., Kufera J.A., Myers R.A. Necrotizing soft tissue infections. Risk factors for mortality and strategies for management. Ann. Surg. 1996;224:672–683. doi: 10.1097/00000658-199611000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bilton B.D., Zibari G.B., McMillan R.W., Aultman D.F., Dunn G., McDonald J.C. Aggressive surgical management of necrotizing fasciitis serves to decrease mortality: a retrospective study. Am. Surg. 1998;64:397–400. Discussion 400–1. [PubMed] [Google Scholar]
- 12.Francis K.R., Lamaute H.R., Davis J.M., Pizzi W.F. Implications of risk factors in necrotizing fasciitis. Am. Surg. 1993;59:304–308. [PubMed] [Google Scholar]
- 13.Edlich R.F., Cross C.L., Dahlstrom J.J., Long W.B. Modern concepts of the diagnosis and treatment of necrotizing fasciitis. J. Emerg. Med. 2010;39:261–265. doi: 10.1016/j.jemermed.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 14.Misiakos E.P., Bagias G., Patapis P., Sotiropoulos D., Kanavidis P., Machairas A. Current concepts in the management of necrotizing fasciitis. Front. Surg. 2014;1:36. doi: 10.3389/fsurg.2014.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mulla Z.D. Treatment options in the management of necrotising fasciitis caused by Group A Streptococcus. Expert Opin. Pharmacother. 2004;5:1695–1700. doi: 10.1517/14656566.5.8.1695. [DOI] [PubMed] [Google Scholar]
- 16.Carapetis J.R., Jacoby P., Carville K., Ang S.-J.J., Curtis N., Andrews R. Effectiveness of clindamycin and intravenous immunoglobulin, and risk of disease in contacts, in invasive group a streptococcal infections. Clin. Infect. Dis. 2014;59:358–365. doi: 10.1093/cid/ciu304. [DOI] [PubMed] [Google Scholar]
- 17.Waddington C.S., Snelling T.L., Carapetis J.R. Management of invasive Group A streptococcal infections. J. Infect. 2014;69(Suppl. 1):S63–S69. doi: 10.1016/j.jinf.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Takei S., Arora Y.K., Walker S.M. Intravenous immunoglobulin contains specific antibodies inhibitory to activation of T cells by staphylococcal toxin superantigens [see comment] J. Clin. Investig. 1993;91:602–607. doi: 10.1172/JCI116240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Norrby-Teglund A., Kaul R., Low D.E., McGeer A., Newton D.W., Andersson J. Plasma from patients with severe invasive Group A streptococcal infections treated with normal polyspecific IgG inhibits streptococcal superantigen-induced T cell proliferation and cytokine production. J. Immunol. 1996;156:3057–3064. [PubMed] [Google Scholar]
- 20.Wang J., McQuilten Z.K., Wood E.M., Aubron C. Intravenous immunoglobulin in critically ill adults: when and what is the evidence? J. Crit. Care. 2015 Jun;30(3):652.e9–652.e16. doi: 10.1016/j.jcrc.2015.01.022. [DOI] [PubMed] [Google Scholar]
- 21.Foster R., Suri A., Filate W., Hallett D., Meyer J., Ruijs T. Use of intravenous immune globulin in the ICU: a retrospective review of prescribing practices and patient outcomes. Transfus. Med. 2010;20:403–408. doi: 10.1111/j.1365-3148.2010.01022.x. [DOI] [PubMed] [Google Scholar]
- 22.Kaul R., McGeer A., Norrby-Teglund A., Kotb M., Schwartz B., O'Rourke K. Intravenous immunoglobulin therapy for streptococcal toxic shock syndrome–a comparative observational study. The Canadian Streptococcal Study Group. Clin. Infect. Dis. 1999;28:800–807. doi: 10.1086/515199. [DOI] [PubMed] [Google Scholar]
- 23.Darenberg J., Ihendyane N., Sjölin J., Aufwerber E., Haidl S., Follin P. Intravenous immunoglobulin G therapy in streptococcal toxic shock syndrome: a European randomized, double-blind, placebo-controlled trial. Clin. Infect. Dis. 2003;37:333–340. doi: 10.1086/376630. [DOI] [PubMed] [Google Scholar]
- 24.Young M.H., Engleberg N.C., Mulla Z.D., Aronoff D.M. Therapies for necrotising fasciitis. Expert Opin. Biol. Ther. 2006;6:155–165. doi: 10.1517/14712598.6.2.155. [DOI] [PubMed] [Google Scholar]
- 25.Sarani B., Strong M., Pascual J., Schwab C.W. Necrotizing fasciitis: current concepts and review of the literature. J. Am. Coll. Surg. 2009;208:279–288. doi: 10.1016/j.jamcollsurg.2008.10.032. [DOI] [PubMed] [Google Scholar]