Supplemental digital content is available in the text.
Key Words: systemic lupus erythematosus, Sjögren syndrome, thrombocytopenia, B-cell depletion
Objective
Recent studies suggested a potential of rituximab (RTX) in treating autoimmune thrombocytopenia (AITP) secondary to autoimmune diseases. In this study, we retrospectively evaluated the efficacy and safety of RTX therapy in patients with refractory AITP secondary to systemic lupus erythematosus (SLE) and Sjögren syndrome (SS).
Methods
Twenty-one SLE and/or SS patients with treatment-resistant AITP were treated once or repeatedly with RTX at the Rheumatology Clinic Renji Hospital, during the period March 2012 to June 2014. Clinical and laboratory variables recorded at every follow-up visit were analyzed.
Results
The median age of all patients was 37.05 ± 3.15 years (range, 13–67 years; 20 female and 1 male). The median AITP duration before RTX treatment was 5.46 years. Previous treatments of 21 patients included immunosuppressive agents such as corticosteroids (n = 19), cyclosporine (n = 9), mycophenolate mofetil (n = 2), methotrexate (n = 3), cyclophosphamide (n = 2), vincristine (n = 3), and hydroxychloroquine (n = 15), and 7 patients received concomitantly intravenous immunoglobulin therapy. Two patients had undergone splenectomy without improvement. Seventeen patients (80.95%) were treated repeatedly with RTX during the follow-up period. The overall response rate to RTX treatment (including complete response, 52.38%; partial response, 28.57%) was 80.95%. A significant increase (P < 0.05) of platelet counts was seen after 1 month (median, 32.24 × 109/mL vs 66.53 × 109/mL). Relapses occurred mostly during the first 9 months, and maintaining duration of response was 10.27 months (range, 2–17 months) on average after the first RTX infusion. Antiplatelet antibodies, especially IgG isotype, decreased significantly (P < 0.05) after RTX treatment. No adverse effects were observed among 15 patients (71.4%); however, 2 cases died of severe pneumonia, and another developed lymphoma.
Conclusions
Rituximab is an additional potent therapeutic treatment option for SLE and SS patients with AITP refractory to conventional immunosuppressive treatments. For most patients, RTX was safe and well tolerated.
Autoimmune thrombocytopenia (AITP), a common hematologic manifestation of autoimmune diseases, such as systemic lupus erythematosus (SLE) and Sjögren syndrome (SS), is found in 20% to 40% of patients1 and results from accelerated platelet destruction mediated by autoantibodies to platelet glycoproteins.1 The severe form of SLE-related immune thrombocytopenia (ITP) is relatively rare2 but is potentially life threatening and often unresponsive to standard treatment. Patients with mild and moderate AITP can present with signs of bleeding from superficial areas of the body. Petechiae, purpura, and ecchymoses are often found on examination. Severe and/or refractory AITP can lead to significant hemorrhage, including the skin, nasal and oral mucosa, gastrointestinal tract, urine, and vagina, and even intracranial hemorrhage. Severe thrombocytopenia has also been shown to be an independent predictor of damage accrual and mortality.3–5 The most common treatments for AITP are corticosteroids (CSs) and intravenous immunoglobulin (IVIG) therapy.6,7 However, in some patients, the disease becomes either resistant to this therapy or CS dependent, requiring the use of second-line agents or splenectomy.8,9 A subset of patients, despite therapy with systemic CSs and immunosuppressive agents, still remains refractory or develops unacceptable toxicity. Therefore, new effective and less toxic treatment strategies are needed.
The pathogenic role of B cells in SLE pathogenesis involves several pathways such as formation of autoantibodies and immune complexes, activation of dendritic and T cells, cytokine production, and chemokine-mediated reactions.10 Considering the multifunctional role of B cells in the pathogenesis of the autoimmune diseases, a depletion of B cells by targeting CD20 using rituximab (RTX) has emerged as a promising treatment option in autoimmune disease. Rituximab is a chimeric monoclonal antibody directed against CD20, an antigen expressed on the surface of B lymphocytes11,12 but not present on most plasma cells. Once RTX binds to the CD20 antigen on B lymphocytes, its Fc domain facilitates both complement and Ab-dependent B-cell lysis and Fc receptor–mediated clearance.13 The mechanism of RTX in treating AITP remains unclear. Rituximab depletes B cells and inhibits some B-cell pathologic functions, including autoantibody production, antigen presenting, and cytokine secretion. B-cell depletion by RTX may also reverse the TH1/TH2 ratio as well as expression of Fas ligand, Bcl-2, and Bax in T-helper cells in patients with ITP.14
Rituximab has been used to successfully treat thrombocytopenia in patients with CS-resistant disease.15 The autoimmune disorders treated with RTX in addition to ITP include SLE, vasculitis, rheumatoid arthritis, SS, autoimmune hemolytic anemia (HA), cryoglobulinemia, acquired factor VIII antibodies, IgM polyneuropathies, and thrombotic thrombocytopenic purpura.16 There is increasing evidence from clinicians showing that RTX could be effective in treating patients with SLE- or SS-related AITP. Our review of the literature revealed 29 articles including 21 case reports and 3 retrospective cohort studies documenting the outcomes of 88 RTX-treated patients with SLE-related (n = 82) or SS-related (n = 6) thrombocytopenia with an overall treatment response rate of 87.5%. There is little published experience regarding RTX in SS, and only few cases have been published regarding the use of RTX in the treatment of SS-related thrombocytopenia. The results are summarized (Table 1 in the Supplementary Online Appendix, http://links.lww.com/RHU/A52) that 66 (75%) of 88 patients achieved complete response (CR), and 11 patients (12.5%) achieved partial response (PR). There was no improvement regarding thrombocytopenia in 7 cases (7.95%), and 4 patients (4.55%) died. Relapses occurred in 9 (13.64%) of 66 patients within a median time of 7.22 months (range, 3–24 months). Recent reports on the randomized, double-blind, placebo-controlled EXPLORER (Exploratory Phase II/III SLE Evaluation of Rituximab) trial based on 16 RTX-treated patients with low baseline platelet counts failed because of loss of follow-up analysis. The follow-up periods were short, thus limiting the period available to ascertain differences objectively in therapeutic arms.17
TABLE 1.
Characteristics of RTX-Treated SLE and SS Patients With ITP
Therefore, it is still controversial on the efficacy and safety of RTX. The aim of this study was to retrospectively evaluate the long-term efficacy, immunological outcomes, and safety of anti–B-cell treatment in SLE and SS patients with AITP.
MATERIALS AND METHODS
Patients
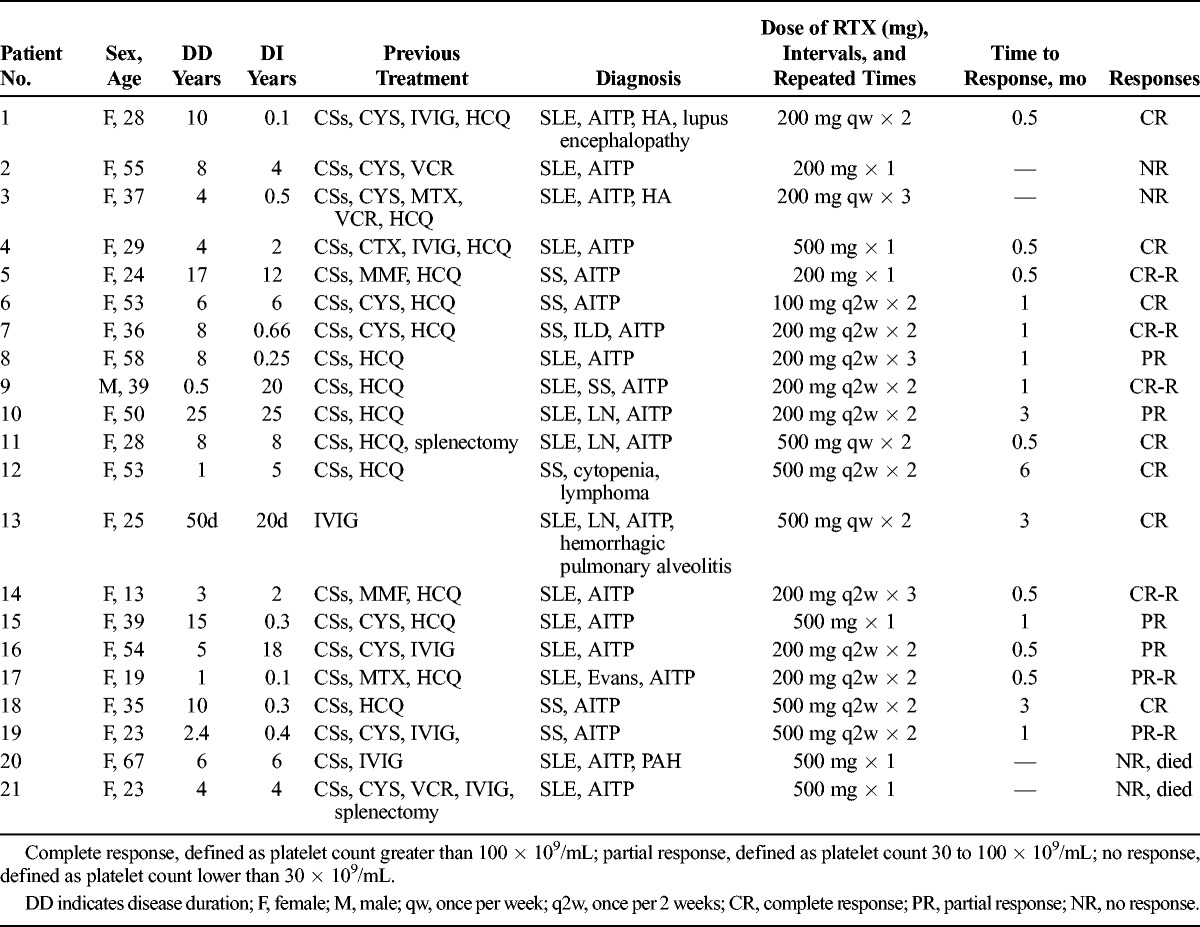
Twenty-one patients with AITP were treated with RTX at the Rheumatology Clinic, Shanghai Renji Hospital, during the period March 2012 to June 2014. This study had been approved by the institutional review board. Informed consent was obtained from each patient for the use of RTX, which was given with the agreement of the local ethics committees. Fifteen patients had SLE, diagnosed according to the American College of Rheumatology criteria.18 Seven patients fulfilled the SS diagnosis according to the European-American consensus group criteria.19 One had both of SLE and SS. Twenty patients were female, and 1 patient was male. Overall age ranged from 13 to 67 years (median, 37.05 ± 3.15 years) with treatment-resistant AITP. The median disease duration before RTX treatment was 6.95 years (range, 50 days to 25 years), and the median AITP duration before RTX treatment was 5.46 years (range, 20 days to 25 years). Part of these SLE patients also had nephritis (n = 3), lupus encephalopathy (n = 1), interstitial lung disease (n = 1), hemorrhagic pulmonary alveolitis (n = 1), pulmonary hypertension (PAH, n = 1), HA (n = 2), and Evans syndrome (n = 1). The patients’ main characteristics are summarized in Table 1.
Anti–B-Cell Treatment
Rituximab treatment was administrated in addition to the ongoing immunosuppressive treatment regimen in patients who did not sufficiently respond to conventional therapy. Patients were intravenously treated with RTX at 200 or 500 mg, weekly or every 2 weeks, for 1 to 3 times. We followed the standard protocol for infusion rate of RTX. As recommended, the initial infusion rate for first RTX infusion is 50 mg/h; subsequently, the rate can be escalated in increments of 50 mg/h every 30 minutes to a maximum of 400 mg/h. For subsequent infusions (numbers 2–3), RTX can be started at a rate of 100 mg/h and increased by increments of 100 mg/h every 30 minutes to a maximum of 400 mg/h. Because almost all patients included in the present study were using prednisolone orally or intravenously, they were treated with methylprednisolone at a dose of 40 mg on the day of first RTX infusion.
Laboratory Analyses
The clinical effect of RTX treatment in patients with AITP was evaluated by analyses of platelet counts, at baseline, and after 0.5, 1, 3, 6, and 12 months or further following RTX treatment. Levels of antinuclear antibodies (ANAs), anti–double-stranded DNA (anti-dsDNA) antibodies, and serum levels of antiplatelet antibody (APA) subclasses (IgG, IgM, IgA) and complements (C3, C4) were determined individually. Serum levels of APA subclasses (IgG, IgM, IgA) were detected by the competitive micro–enzyme-linked immunosorbent assay method as described previously.20 Platelet-associated IgG (PAIgG), PAIgM, and PAIgA values are shown as the IgG, IgM, and IgA value per 107 platelets. Antinuclear antibodies and anti-cardiolipid antibodies (ACLs) were detected by indirect immunofluorescence experiments. Levels of antibodies against dsDNA were determined by radioimmunoassay. CD19+ B cells were chosen for determination of the number of circulating B cells before and after anti-CD20 treatment to avoid any possible interference of RTX with the flow cytometric assay. The detection level of the method was 1%. Undetectable levels of CD19+ B (levels <1% of the total lymphocyte population) were considered to indicate B-cell depletion from the peripheral blood.
Response Criteria
Complete response was defined by achievement of a platelet count greater than 100 × 109/mL or by maintaining a platelet count greater than 100 × 109/mL. Partial response was defined if the platelet count was 30 × 109/mL to 100 × 109/mL or more than 2 times of the platelet count before RTX treatment. Both CR and PR excluded any occurrence of bleeding. Treatment failure was determined as less than 2 times of the platelet count before RTX treatment or occurrence of bleeding. Time to response was defined as the time from the first RTX administration to the achievement of any degree of response. Remission was defined as stable platelet count greater than 100 × 109/mL at least 3 months without or while tapering off CS treatment.
Statistical Analyses
Nonparametric methods were used for statistical evaluation of data in most cases because of small sample size and uneven distribution. Clinical measures and all laboratory data are presented as medians and 25th to 75th percentiles (interquartile range). Responses to RTX treatment at 0.5, 1, 3, 6, and 12 months were compared with baseline values. Student t test and 1-way analysis of variance were conducted for comparison of different variables at baseline and follow-up. P< 0.05 was considered as statistically significant. All analyses were performed using Prism software version 5.0.c (GraphPad Software, Inc, La Jolla, CA).
RESULTS
Subject Characteristics
The clinical characteristics of the patients are summarized in Table 1. All patients presented variable signs of bleeding, including the skin (n = 12), nasal and oral mucosa (n = 4), gastrointestinal tract (n = 2), urine (n = 4), vagina (n = 2), and intracranial hemorrhage (n = 1). One subject of the 2 patient deaths in the present study had skin, gastrointestinal tract, and intracranial hemorrhage simultaneously. The bone marrow samples were collected from 15 of 21 patients in the present study. All of them were filled with megakaryocytes indicating abnormal platelet destruction rather than platelet production. We also excluded other causes of thrombocytopenia such as primary or metastatic cancers, infiltration by infections, or decreased production due to drugs, radiation, or chemotherapy effect on the bone marrow. Two of 21 patients were diagnosed as HA together with SLE. Neither had positive responses to direct Coombs test. One patient had a diagnosis of Evans syndrome secondary to SLE, with positive response to direct Coombs test (against C3). All patients were treated with immunosuppressive agents such as CSs (n = 19), cyclosporine (CYS) (n = 9), mycophenolate mofetil (MMF) (n = 2), methotrexate (MTX) (n = 3), cyclophosphamide (CTX) (n = 2), vincristine (VCR) (n = 3), hydroxychloroquine (HCQ) (n = 15), and 7 patients received concomitantly IVIG. Two patients had undergone splenectomy without improvement. Seventeen patients (80.95%) were treated repeatedly with RTX during the follow-up.
Response Rate of RTX Treatment
Twenty-one patients treated with RTX were included in this study; 11 had CRs, and 6 had PRs. The median posttreatment follow-up time was 10.31 months (range, 1.5–22 months). Different from most published literature, a significant increase (P < 0.05) in platelet count was observed starting from 1 month after initial RTX treatment (median, 32.24 × 109/mL vs 66.53 × 109/mL) (Fig. 1A). Four patients (19.04%) with median platelet count 19.25 × 109/mL originally did not respond to RTX treatment. The baseline median platelet counts remained basically unchanged following 1, 3, and 6 months of RTX treatment (19.25 × 109/mL vs 18.75 × 109/mL, 26.5 × 109/mL, and 28 × 109/mL, respectively). The overall rate for response to RTX treatment (including CR of 52.38% and PR of 28.57%) was 80.95% in this study.
FIGURE 1.
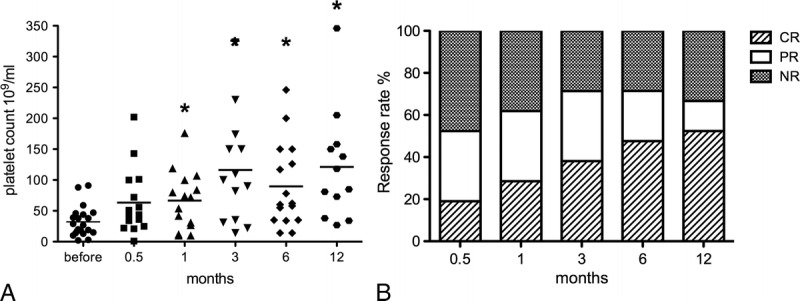
Platelet count response before and after RTX treatment. A, Box plots show the 25th and 75th percentiles. Horizontal black solid lines within boxes indicate medians; filled circles indicate means. Vertical bars indicate the fifth and 95th percentiles. *P < 0.05 as compared with levels before treatment. B, The overall response during follow-up. Each column represents response rate at a particular time point. NR indicates NR.
After the first RTX administration, at week 2 (month 0.5), 4 patients (19.05%) achieved CR, and 7 patients (33.33%) achieved PR. The overall response rate increased to 72.43% at month 3, was maintained until month 6, and began to drop after 12 months. Overall response rate was achieved in 11 (52.38%) of 21, 13 (61.90%) of 21, 15 (71.43%) of 21, 15 (71.43%) of 21, and 14 (61.67%) of 21 patients at months 0.5, 1, 3, 6, and 12, respectively (Fig. 1B).
Relapse of AITP following the first RTX treatment was observed in 6 patients (35.3%; 4 CRs and 2 PRs) occurring at a median of 5.17 months (range, 2–8 months) from initial RTX infusion. Three of them received methylprednisolone pulse therapy for 5 to 7days; the platelet counts quickly returned to CR or PR levels within 1 week and were stable since then. Two of them were treated with the second RTX cycle and achieved remission that was maintained during the follow-up period of 9 and 11 months, respectively. In 1 patient with CR to the second treatment, a third RTX cycle was needed to induce 22 months’ remission. In 11 patients (64.7%) with either CR or PR, complete remissions were achieved following the first RTX administration and maintained during the median follow-up period of 10.27 months (range, 2–17 months).
The Clinical and Immunological Effects of RTX Treatment
CD19+ B-cell counts before and after RTX treatment were collected from 10 of 21 patients. Complete depletion of B cells was achieved in 80% of cases (n = 8) 1 month later (Fig. 2A). Two patients had measurable levels of CD19+ cells (>1%) remaining in peripheral blood 1 month after treatment, 1 with PR (6.79%) and the other (1.72%) with no response (NR). However, 1 patient still had NR, even though complete depletion of B cells was achieved in the first month. Among 3 patients with complete B-cell depletion after the first RTX cycle also had relapse during the treatment. Four patients displayed undetectable levels of B cells throughout the disease, and remissions were observed. Therefore, B-cell depletion was successful in most patients with no correlation with the outcomes.
FIGURE 2.
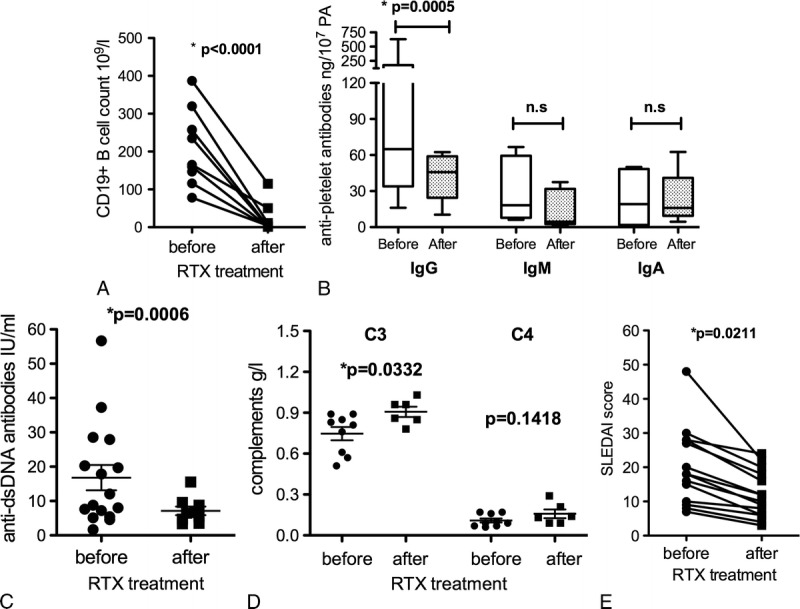
Monitoring of laboratory parameters before and after RTX treatment. A, B-cell repopulation 1 month after RTX infusion. Each dot represents CD19+ B cells percentage. Each dot represents response of single patient. Normal values are as follows: CD19+ B cells, 180–350 × 106/L. B, Changes in APAs following RTX treatment. Box plots show the 25th and 75th percentiles. Horizontal black solid lines within boxes indicate medians. Vertical bars indicate the fifth and 95th percentiles. Normal values are as follows: IgG antibodies: 0 to 120 ng/107 PA, IgM antibodies: 0 to 40 ng/107 PA, IgA antibodies: 0 to 22 ng/107 PA. C and D, Changes in anti-dsDNA antibodies and complements (C3, C4) following RTX treatment. Normal values are as follows: anti-dsDNA antibodies, 0 to 7 IU/mL; C3, 0.9 to 1.8 g/L; C4, 0.1 to 0.4 g/L. E, Changes in SLEDAI scores among 15 SLE patients following RTX treatment.
Antiplatelet antibodies of IgG, IgM, and IgA isotypes were measured in 9 of 21 patients before and after treatment. High levels were detected in 7 patients before RTX treatment (IgG: median 143.6 ng/107 PA [range, 16.1–629.4 ng/107 PA]; IgM: median 30.61 ng/107 PA [range, 6.1–66.7 ng/107 PA]; IgA: median 23.40 ng/107 PA [range, 1.0–50 ng/107 PA]). Only the IgG autoantibody levels decreased significantly (P = 0.0005) following RTX treatment (Fig. 2B), and 6 patients achieved CR or PR. Anti-dsDNA antibodies were evaluated from 17 patients before and/or after RTX treatment. The overall level of anti-dsDNA antibodies decreased significantly from 16.80 ± 3.69 IU/mL to 7.13 ± 1.27 IU/mL within 6 months following RTX treatment (P = 0.0006) (Fig. 2C). Complement levels of 10 patients had been detected before and/or after RTX treatment. The overall level of C3 increased significantly from 0.75 ± 0.05 g/L to 0.91 ± 0.04 g/L (P = 0.0332), whereas C4 increased from 0.11 ± 0.015 g/L to 0.16 ± 0.032 g/L within 6 months following RTX treatment (P = 0.1418) (Fig. 2D). Most patients recovered from severe bleeding. Only 1 patient still presented obvious signs of bleeding and finally died of gastrointestinal tract and intracranial hemorrhage. As shown in Figure 2E, Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) score from SLE patients were also measured before and after RTX treatment. The SLEDAI score evaluation includes a variety of clinical manifestation (psychosis, vasculitis, arthritis, hematuria, proteinuria, new rash, pericarditis, etc) and laboratory tests (low complement, anti-dsDNA antibody level, thrombocytopenia, leukopenia, etc). The overall decrease was significant from 20.13 ± 2.80 to 12.13 ± 1.70 (P = 0.0211).
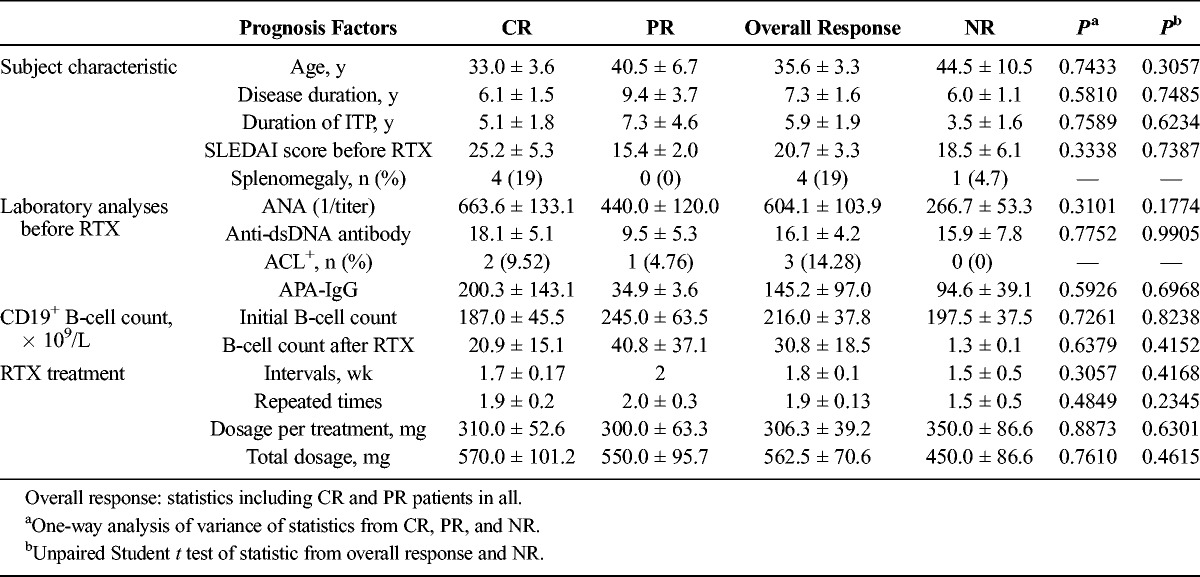
The comparison of prognosis factors in patients who achieved CR, PR, and NR is summarized in Table 2. There was no significant difference on ages and durations between patients with or without responses. All the SLE patients were assessed by SLEDAI before the first RTX administration. The initial SLEDAI score before RTX treatment was 25.2 ± 5.3 among CR patients, with 15.4 ± 2.0 among PR patients and 18.5 ± 6.1 among NR patients. Ten patients had ANA readouts both before and after RTX treatment. Decrease was observed in 8 of them, and 2 of them had the same ANA readouts as previous. However, the other 10 patients had only ANA readouts before the initial RTX treatment with no follow-up data. In addition, we included 4 patients with splenomegaly in our study. It is worth noting that these 4 patients all fulfilled CR to RTX treatment. Also, we noticed 3 patients with positive ACLs achieved CR or PR. No patients with NR had positive ACLs.
TABLE 2.
Comparison of the Efficacy of RTX in Patients Who Achieved CR, PR and NR
Toxicity and Safety
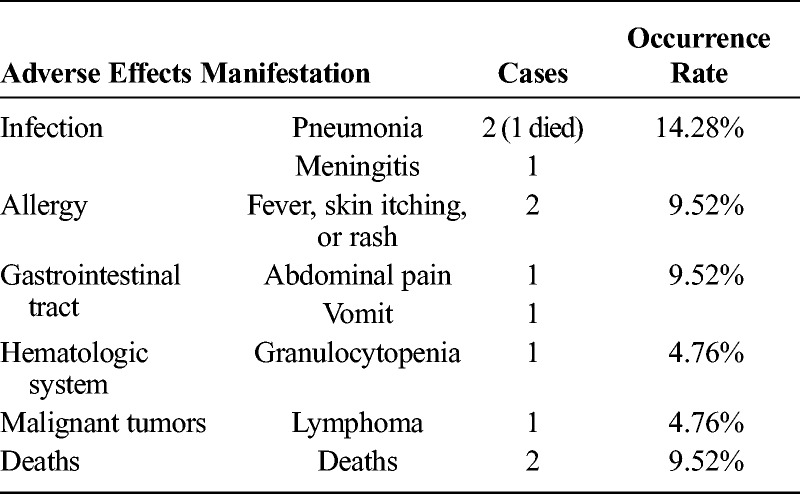
In our study, RTX treatment was generally well tolerated. Most patients (71.42%) did not develop any severe allergic reactions or adverse effects after RTX infusions. Two patients died because of severe infection (pneumonia) and refractory AITP. Five patients discontinued treatment because of adverse effects including infusion reaction or other infections (Table 3).
TABLE 3.
Adverse Effects of RTX Treatment in SLE and SS Patients With Refractory AITP
DISCUSSION
Rituximab was initially developed to treat non-Hodgkin B-cell lymphoma, and since 1997, it has been widely used in the treatment of autoantibody-mediated disorders.16 Recently, RTX also showed to be promising in treating AITP and other autoimmune diseases. The first successful case of RTX therapy in the treatment of SLE and AITP was described in 2002 by Kneitz et al.21 During the last decade, published evidence on off-label RTX treatment in SLE-associated thrombocytopenia has increased and suggests a favorable effect (Table 1 in the Supplementary Appendix, http://links.lww.com/RHU/A52).
In our retrospective cohort study, 15 patients were treated with HCQ, which was regarded as 1 of the basic treatments in SLE and SS. There are rare reports stating that HCQ might cause thrombocytopenia. Azathioprine (AZA) is the major immunosuppressive used to treat autoimmune diseases, secondary HA, ITP, and so on, rather than leading to thrombocytopenia. To our knowledge, there are few reports stating that AZA might cause bone marrow depression. However, none of the patients was treated with AZA in present study. Vincristine, the conventional therapy widely used in thrombotic thrombocytopenic purpura, is now used in refractory immune-mediated thrombocytopenia as well.22,23 It is worth noting that all the patients have been treated with these conventional immunosuppressive medications, only after diagnosis of AITP. Especially, 14 of 21 patients presented initially with signs of bleeding from variable organs. Therefore, the adverse effect of thrombocytopenia due to these medications mentioned above could be excluded. Rituximab was administered in 21 SLE or SS patients with severe thrombocytopenia who did not respond to traditional treatment including methylprednisolone pulse therapy, immunosuppressive agents, or IVIG infusion. Our study found that 80.95% of the patients responded to different doses of RTX, and the response could be as early as the first month in line with the depletion time of B cells. In our study, the response could be maintained throughout almost 2 years (22 months) despite concomitant glucocorticoids being gradually tapered and patients discontinuing previous immunosuppressants. These findings are comparable with the statistics analyzed from the previous reports we mentioned previously, although the maintaining duration could be longer as we expected because of the limited follow-up duration here.
Rituximab was again confirmed to be effective to the autoimmune diseases including SLE and primary SS. Here, we showed the disease activity indices, such as anti-dsDNA antibodies, complement levels, and SLEDAI scores from SLE patients, were all improved after RTX therapy. However, because of the small number in the study, it was impossible to extrapolate which parameter could be used to predict patient response. It is not surprising that patients with high detectable levels of APAs, especially IgG isotype, responded better to RTX therapy than did patients without antibodies. This is in line with several other, albeit few, studies in which the levels of APAs were analyzed.16,21,24–29 Rituximab treatment has shown favorable effects to patients with primary ITP as well, supporting a pathogenetic role of autoantibodies in platelet destruction. Besides APAs, here we did not find prognosis factors for effective treatment. Nevertheless, we can still expect some factors that may be correlated with the outcomes of the treatment. Patients with higher scores of SLEDAI and higher levels of ANA and APA are more likely to achieve CR or PR.
In most published studies, RTX has been given intravenously once weekly during 4 consecutive weeks at the dosage of 375 mg/m2, and in a recent prospective study by Chen et al,30 RTX at a low dose of 100 mg once weekly for 4 weeks was shown to induce a CR in 60% of SLE patients with severe refractory thrombocytopenia at week 12. Here in our study, the patients who received comparable low doses of RTX individually also showed good responses. Overall times or total dosage of RTX treatment did not differ significantly between patients responding to RTX treatment, but repeated times of RTX administration tended to be more effective. However, there is no correlation between the dosage, the intervals, and the outcomes.
To our knowledge, RTX has shown to be safe in most cases (>70%) according to most clinical trials (Table 1 in the Supplementary Appendix, http://links.lww.com/RHU/A52). In our study, 1 SLE patient with PAH died of severe pneumonia at 15 months after the initial RTX infusion. The other died of severe AITP refractory to all traditional treatments and RTX treatment. Three patients developed infections during follow-up (1 case of pneumonia as mentioned previously, 1 Cryptococcus neoformans meningitis, 1 respiratory tract infection). Other adverse effects included transient infusion reactions (serum sickness symptoms with fever and rash, abdominal pain and vomit, etc). Among the 7 SS patients here, 1 of them developed lymphoma during the follow-up. Recent studies also showed that continuous B-cell activation probably leads to the development of lymphomas in primary SS, with a 16- to 18-fold increase, as shown in recent studies.31,32 Therefore, it is worth noting that the development of lymphoma may originate from the primary SS instead of the adverse effect of RTX. Actually, the patient received RTX treatment regularly and achieved complete remission.
Among all the responding patients in our study, more than 30% had relapse during the treatment, which required retreatment of RTX or methylprednisolone pulse therapy. Relapse of AITP occurred at a median of 5.17 months (range, 2–8 months) from initial RTX infusion. These patients were treated with methylprednisolone intravenously for several days with their platelet counts quickly returning to CR or PR levels and being stable since then. Our study also showed that relapses occurred during the first 9 months, which is consistent with a previous report in 2013 by Jovancevic et al.29 Although repeating RTX administration tends to be more effective for AITP patients to achieve CR or PR, 1 to 2 retreatments were observed to be sufficient to introduce patients to long-term remission.
The tolerability of this treatment and the clinical benefit obtained are noteworthy, despite the retrospective design of this study. We could only obtain clinical statistics individually. In addition, here it is impossible to rule out the confounding effect of other antirheumatic drugs with potential implications on platelet function, because all patients in this study had failed at least 1 other therapy including glucocorticoids or immunosuppressants before RTX administration. In conclusion, our study shows that RTX therapy is promising in treating severe AITP in SLE and SS patients. In view of the paucity of effective treatment options and cost-effectiveness, standard dose of RTX should be considered in SLE and SS patients with severe thrombocytopenia who do not respond to vigorous glucocorticoids plus immunosuppressants. Nevertheless, a prospective study with randomized and controlled design including large-scale sample sizes with standard dose of RTX is warranted. Designing clinical trials to define the precise relationship between the biological effects that occur following RTX treatment and the clinical response in the long term (typically 2–5 years) would be met with the potential challenge of maintaining remission in the placebo group with conventional glucocorticoid or immunosuppressants alone.
KEY MESSAGES
Rituximab is a potent therapeutic treatment option for SLE and SS patients with refractory AITP.
The overall response rate to RTX treatment was 80.95%.
Rituximab was safe and well tolerated by most patients (71.4%).
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the patients and nurses who made this work possible. They also thank Dr Sheng Chen for invaluable assistance in the project design and submission of this manuscript; Ting Li and Guo Li for collecting the samples and analysis and interpretation of data; Dr Shuang Ye and Dr Hao Shen for the support in the preparation and revision of the manuscript. They also appreciate the great assistance from Dr Chunde Bao at Renji Hospital and Drs Ying Wang and Yanyun Zhang at Shanghai Institute of Immunology.
Footnotes
This work was supported by the National Natural Science Foundation of China (30901334), Ministry of Science and Technology, China. There is no grant from pharmaceutical agencies promoting rituximab treatments in this study.
The authors declare no conflict of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.jclinrheum.com).
REFERENCES
- 1. Kumar S, Benseler SM, Kirby-Allen M, et al. B-cell depletion for autoimmune thrombocytopenia and autoimmune hemolytic anemia in pediatric systemic lupus erythematosus. Pediatrics. 2009; 123: e159– e163. [DOI] [PubMed] [Google Scholar]
- 2. Sultan SM, Begum S, Isenberg DA. Prevalence, patterns of disease and outcome in patients with systemic lupus erythematosus who develop severe haematological problems. Rheumatology (Oxford). 2003; 42: 230– 234. [DOI] [PubMed] [Google Scholar]
- 3. Nossent JC, Swaak AJ. Prevalence and significance of haematological abnormalities in patients with systemic lupus erythematosus. Q J Med. 1991; 80: 605– 612. [PubMed] [Google Scholar]
- 4. Mok CC, Lee KW, Ho CT, et al. A prospective study of survival and prognostic indicators of systemic lupus erythematosus in a southern Chinese population. Rheumatology (Oxford). 2000; 39: 399– 406. [DOI] [PubMed] [Google Scholar]
- 5. Ziakas PD, Giannouli S, Zintzaras E, et al. Lupus thrombocytopenia: clinical implications and prognostic significance. Ann Rheum Dis. 2005; 64: 1366– 1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hepburn AL, Narat S, Mason JC. The management of peripheral blood cytopenias in systemic lupus erythematosus. Rheumatology (Oxford). 2010; 49: 2243– 2254. [DOI] [PubMed] [Google Scholar]
- 7. Zandman-Goddard G, Levy Y, Shoenfeld Y. Intravenous immunoglobulin therapy and systemic lupus erythematosus. Clin Rev Allergy Immunol. 2012; 42: 247– 255. [DOI] [PubMed] [Google Scholar]
- 8. Smith HR, Steinberg AD. Autoimmunity—a perspective. Annu Rev Immunol. 1983; 1: 175– 210. [DOI] [PubMed] [Google Scholar]
- 9. Arnal C, Piette JC, Léone J, et al. Treatment of severe immune thrombocytopenia associated with systemic lupus erythematosus: 59 cases. J Rheumatol. 2002; 29: 75– 83. [PubMed] [Google Scholar]
- 10. Martin F, Chan AC. B cell immunobiology in disease: evolving concepts from the clinic. Annu Rev Immunol. 2006; 24: 467– 496. [DOI] [PubMed] [Google Scholar]
- 11. Einfeld DA, Brown JP, Valentine MA, et al. Molecular cloning of the human B-cell CD20 receptor predicts a hydrophobic protein with multiple transmembrane domains. EMBO J. 1988; 7: 711– 717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Valentine MA, Meier KE, Rossie S, et al. Phosphorylation of the CD20 phosphoprotein in resting B lymphocytes. Regulation by protein kinase C. J Biol Chem. 1989; 264: 11282– 11287. [PubMed] [Google Scholar]
- 13. Reff ME, Carner K, Chambers KS, et al. Depletion of B-cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood. 1994; 83: 435– 445. [PubMed] [Google Scholar]
- 14. Stasi R, del Poeta G, Stipa E, et al. Response to B-cell depleting therapy with rituximab reverts the abnormalities of T-cell subsets in patients with idiopathic thrombocytopenic purpura. Blood. 2007; 110: 2924– 2930. [DOI] [PubMed] [Google Scholar]
- 15. Cooper N, Stasi R, Cunningham-Rundles S, et al. The efficacy and safety of B-cell depletion with anti-CD20 monoclonal antibody in adults with chronic immune thrombocytopenic purpura. Br J Haematol. 2004; 125: 232– 239. [DOI] [PubMed] [Google Scholar]
- 16. VL Patel, Mahévas M, Lee SY, et al. Outcomes 5 years after response to rituximab therapy in children and adults with immune thrombocytopenia. Blood. 2012; 119: 5989– 5995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tew GW, Rabbee N, Wolslegel K, et al. Baseline autoantibody profiles predict normalization of complement and anti-dsDNA autoantibody levels following rituximab treatment in systemic lupus erythematosus. Lupus. 2010; 19: 146– 157. [DOI] [PubMed] [Google Scholar]
- 18. Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982; 25: 1271– 1277. [DOI] [PubMed] [Google Scholar]
- 19. Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjogren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002; 61: 554– 558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nishioka T, Yamane T, Takubo T, et al. Detection of various platelet-associated immunoglobulins by flow cytometry in idiopathic thrombocytopenic purpura. Cytometry B Clin Cytom. 2005; 68: 37– 42. [DOI] [PubMed] [Google Scholar]
- 21. Kneitz C, Wilhelm M, Tony HP. Effective B cell depletion with rituximab in the treatment of autoimmune diseases. Immunobiology. 2002; 206: 519– 527. [DOI] [PubMed] [Google Scholar]
- 22. Liang Y, Zhang L, Gao J, et al. Rituximab for children with immune thrombocytopenia: a systematic review. PLoS One. 2012; 7 e36698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Patel VL, Mahévas M, Lee SY, et al. Outcomes 5 years after response to rituximab therapy in children and adults with immune thrombocytopenia. Blood. 2012; 119: 5989– 5995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tomietto P, Gremese E, Tolusso B, et al. B cell depletion may lead to normalization of antiplatelet, anti-erythrocyte and antiphospholipid antibodies in systemic lupus erythematosus. Thromb Haemost. 2004; 92: 1150– 1153. [PubMed] [Google Scholar]
- 25. Anandacoomarasamy A, Gibson J, McGill N. ‘Cure’ of life-threatening antiphospholipid syndrome with rituximab. Intern Med J. 2006; 36: 474– 475. [DOI] [PubMed] [Google Scholar]
- 26. Fukushima T, Dong L, Sakai T, et al. Successful treatment of amegakaryocytic thrombocytopenia with anti-CD20 antibody (rituximab) in a patient with systemic lupus erythematosus. Lupus. 2008; 17: 210– 214. [DOI] [PubMed] [Google Scholar]
- 27. Kittaka K, Dobashi H, Baba N, et al. A case of Evans syndrome combined with systemic lupus erythematosus successfully treated with rituximab. Scand J Rheumatol. 2008; 37: 390– 393. [DOI] [PubMed] [Google Scholar]
- 28. Lee SY, Hsu PY, Juan KC, et al. Successful treatment of autoimmune thrombocytopenic purpura with rituximab in a dialysis patient with systemic lupus erythematosus. Int Immunopharmacol. 2010; 10: 632– 634. [DOI] [PubMed] [Google Scholar]
- 29. Jovancevic B, Lindholm C, Pulleritis R. Anti B cell-therapy against refractory thrombocytopenia in SLE and MCTD patients: long-term follow-up and review of the literature. Lupus. 2013; 22: 664– 674. [DOI] [PubMed] [Google Scholar]
- 30. Chen H, Zheng W, Su J, et al. Low-dose rituximab therapy for refractory thrombocytopenia in patients with systemic lupus erythematosus—a prospective pilot study. Rheumatology (Oxford). 2011; 50: 1640– 1644. [DOI] [PubMed] [Google Scholar]
- 31. Theander E, Henriksson G, Ljungberg O, et al. Lymphoma and other malignancies in primary Sjogren’s syndrome: a cohort study on cancer incidence and lymphoma predictors. Ann Rheum Dis. 2006; 65: 796– 803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zintzaras E, Voulgarelis M, Moutsopoulos HM. The risk of lymphoma development in autoimmune diseases: a meta-analysis. Arch Intern Med. 2005; 165: 2337– 2344. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


