Abstract
Antiviral therapy using newer nucleos(t)ide analogues with lower resistance rates, such as entecavir or tenofovir, suppress hepatitis B virus (HBV) replication, improve liver function in patients with compensated or decompensated cirrhosis, and delay or obviate the need for liver transplantation in some patients. After liver transplantation, the combination of long-term antiviral and low-dose hepatitis B Immune globulin (HBIG) can effectively prevent HBV recurrence in greater than 90% of transplant recipients. Some forms of HBV prophylaxis need to be continued indefinitely after transplantation but, in patients with a low-risk of HBV recurrence (i.e., HBV DNA levels undetectable before transplantation), it is possible to discontinue HBIG and maintain only long-term nucleos(t)ide analogue(s) therapy. A more cautious approach is necessary for those patients with high pretransplant HBV DNA levels, those with limited antiviral options if HBV recurrence occurs (i.e., HIV or hepatitis D virus coinfection, preexisting drug resistance), those with a high risk of hepatocellular carcinoma recurrence, and those at risk of noncompliance with antiviral therapy. In this group, HBIG-free prophylaxis cannot be recommended.
The combination of long-term antiviral and low-dose Hepatitis B Immune globulin (HBIG) can effectively prevent HBV recurrence in > 90% of liver transplant recipients. In patients with low HBV DNA levels, nucleos(t)ide analogue(s) treatment without HBIG is possible.
In the initial transplant era, recurrence of hepatitis B virus (HBV) in the liver graft occurred in up to 80%, with an aggressive course resulting in graft loss.1,2 As a result, HBV-related liver disease constituted a relative and sometimes an absolute contraindication for liver transplantation (LT).2 This changed dramatically because of the effectiveness of prophylaxis regimens and then the efficacy of antiviral therapy for treating HBV infection before and after LT.1,3 Nowadays, the HBV-induced liver disease is not a contraindication to LT and accounts for 5% to 10% of indication for LT in the United States and Europe and is the leading cause in Asia. Indeed, chronic HBV infection is endemic in Asia, with an estimation of 300 million individuals infected with HBV, representing about 75% of the world’s chronic HBV carriers.
The advent of long-term intravenous (IV) hepatitis B immune globulin (HBIG) administration and the introduction of new antiviral agents against HBV infection, such as lamivudine (LAM) or adefovir (ADV), were a major breakthrough in the pre- and post-LT management of these patients. Nucleos(t)ides agents suppress HBV replication and improve liver function in patients with decompensated cirrhosis,4-9 delay or obviate the need for LT in some patients10-12 and decrease the risk of HBV recurrence after LT by reducing HBV viral load at the time of LT. Lamivudine and ADV were used in the majority of reported studies. However, they are no longer considered an optimal first-line therapy due to the high rate development of resistance. The latest guidelines suggest using new oral antiviral agents with a better safety profile, a higher efficacy, and a low rate of resistance development, such as entecavir (ETV) or tenofovir (TDV), as primary antiviral agents.13,14 When LT is indicated for hepatocellular carcinoma (HCC) or advanced liver failure, the use of nucleos(t)ides agents before transplantation and the combination prophylaxis with nucleos(t)ides agents and HBIG after transplantation prevents HBV recurrence in 90% to 100% of patients and produces survival rates at 5 years in over 80%.15,16 There is a consensus regarding the use of a lifelong HBV prophylactic therapy supported by the detection of low levels of HBV DNA in serum, liver, and peripheral blood mononuclear cells or the presence of total and covalently closed circular HBV DNA (ccc DNA) in liver tissue transiently after LT even in the absence of a positive hepatitis B surface antigen (HBsAg), whatever the prophylaxis used.17-23 However, this long-term prophylaxis using IV HBIG can be expensive and inconvenient for patients. This has led to the development of alternative strategies aimed to change the route of administration of HBIG, to reduce the dose or duration of HBIG, or to avoid the use of HBIG. However, a more cautious approach is necessary for those patients with a high risk of HBV recurrence: high pretransplant HBV DNA levels, those with limited antiviral options if HBV recurrence occurs (i.e., HIV or hepatitis D virus [HDV] coinfection, preexisting antiviral drug resistance), those with a high risk of HCC recurrence, and finally those with a risk of noncompliance with antiviral therapy. In this group, HBIG-free prophylaxis cannot be recommended.1
In this review, we will describe the significant improvements in the prevention of HBV recurrence after LT for HBV-related liver disease and the role of HBIG in the short- and long-term prophylaxis.
DIAGNOSIS, MECHANISMS AND RISK FACTORS FOR HBV RECURRENCE AFTER LT
Hepatitis B virus is a small enveloped DNA virus with a genome consisting of circular, partially double-stranded DNA containing 4 overlapping open reading frames. The envelope is composed of 3 surface proteins, large (LHBs), middle (MHBs), and small (SHBs), encoded within one open reading frame. The N-terminal part of LHBs binds at the hepatocyte membrane to the sodium taurocholate cotransporting polypeptide, recently reported as a high affinity receptor for HBV.24 All 3 proteins contain an external hydrophilic loop which carries the major antigenic determinant (a-determinant) of HBV. The 3-dimensional structure of this antigenic loop is essential for infectivity, probably through interaction with heparan sulphate proteoglycans at the hepatocyte membrane. Complete inhibition of HBV infection can be obtained by targeting LHB’s interaction with the HBV receptor25,26 or by anti-HBs antibodies. The HBV genome is converted in the cell nucleus into cccDNA which associates with histone and nonhistone proteins that control transcription of viral RNAs. Viral replication involves the retrotranscription of viral pregenomic RNA, source of significant genetic variability. During active replication, the cccDNA pool is maintained by intracellular reimport of newly synthesized viral DNA. In addition, large amounts of subviral surface antigen particles are secreted; their production only depends on the S domain of surface proteins.
Recurrence of HBV infection after LT is commonly defined as the reappearance of circulating HBsAg with or without detectable HBV DNA. However, only patients who develop persistently detectable HBV DNA are shown to be at risk for clinical disease (increase in aminotransferase levels and histological evidence of acute or chronic hepatitis) and graft loss.1 This is why certain groups consider HBV DNA reappearance as the marker of HBV reinfection. The HBV reinfection is the consequence of an immediate reinfection of the graft by circulating HBV particles or a later reinfection from HBV particles coming from extrahepatic sites, such as peripheral blood mononuclear cells21,27 or both.
Whatever the prophylaxis used, there is a direct relationship between the HBV viral load at transplantation (i.e., > 105 copies/mL or > 20 000 IU/mL with the approximate conversion factor of 5 copies per international unit for PCR methods) and the rate of HBV recurrence.15,28-32 Of note, hybridization assays used in earlier studies were much less sensitive than current real-time PCR assays (up to 10,000 times), and the viral load was not expressed in International Units as nowadays; moreover, different thresholds have been used to define a higher risk of recurrence, depending upon the HBV DNA assay. Other factors associated with low rates of recurrence are surrogate markers for low levels of viral replication and include negative hepatitis B e antigen (HBeAg) status at listing, fulminant HBV, and HDV coinfection.15,30 There is consensus for the use of antivirals before transplantation to achieve undetectable HBV DNA levels aiming at reducing the risk of HBV recurrence.
In addition, several studies have reported that HCC at LT, HCC recurrence, or chemotherapy used for HCC are independently associated with an increased risk of HBV recurrence.33-37 The association between HCC and HBV recurrence and the detection of cccDNA in HCC cells suggest the possibility of viral replication in tumor cells, which would then act as a viral reservoir.
In patients receiving antiviral monoprophylaxis with low genetic barrier agents, such as LAM, HBsAg remains positive, progressively declining over a period of a few months after transplantation to become undetectable. In compliant patients, recurrence is most often associated with HBV polymerase mutations.5,29,38-41 Infection with resistant variants before LT increases the risk of recurrence regardless of viral load at transplantation.35-42
In patients without overt recurrence, persistence or reappearance of HBsAg positivity without detection of HBV DNA can be observed.39,43 Indeed, viral mRNAs can still be produced from the cccDNA template in the infected liver graft, depending on epigenetic factors. In treated patients, the reverse-transcription step of replication is inhibited, precluding the formation of virions which requires the interaction of mature nucleocapsids containing dsDNA with the surface proteins. In contrast to virions, the formation of subviral HBsAg particles only depends on transcription and translation of the S-domain of surface proteins and their secretion follows a distinct secretory pathway.44
After the removal of the major viral reservoir, the use of HBIG at the anhepatic phase is aiming at inhibiting entry by neutralizing viral determinants of attachment. In patients receiving HBIG, HBV reinfection may be the consequence of the incomplete neutralization of viral particles at the anhepatic phase, due to high viral load, HBV overproduction coming from extrahepatic sites or an insufficiency of anti-HBs titres. Recurrence is often, but not exclusively, associated with the emergence of escape variants with mutations in the S domain of HBV, and particularly in the antigenic loop (“a” determinant).45,46 These variants may preexist before LT, even in peripheral blood mononuclear cells,27 and may emerge on HBIG selection pressure.
Reinfection in patients on combined therapy with HBIG and antiviral agents with a low genetic barrier are associated with mutations in both the surface and the polymerase genes.47
Whatever the prophylaxis used, measurable low levels of HBV DNA have been reported after LT in a significant proportion of patients without detectable HBsAg and without evidence of chronic hepatitis on the liver graft. The HBV DNA has been reported in serum, peripheral blood mononuclear cells and in liver both total and/or cccDNA.17-23 These findings suggest that occult HBV reinfection occurs in some HBV recipients and implies a risk of overt HBV recurrence if prophylaxis is stopped. In the study by Hussain et al,18 the longitudinal analysis of posttransplant biopsies showed a progressive decline of graft cccDNA. Indeed, HBV cccDNA is not integrated within the host genome and has no origin of replication for amplification during cell division. Clearance of cccDNA may thus be expected on long-term control of viral replication. This control involves interplay of HBV and immune responses, factors which are modified in the LT recipient by the use of antiviral prophylaxis and by immunosuppressive drugs. Conversely, for the few patients who are negative for HBV DNA and cccDNA in all compartments, the discontinuation of HBV prophylaxis might be considered.23
ROLE OF HBIG IN PREVENTION OF HBV RECURRENCE
Since the study by Samuel et al,30 HBIG has been the cornerstone of prophylaxis against HBV recurrence after LT. This study demonstrated a dramatic reduction in the rate of HBV recurrence, from 75% in patients receiving no or short-term therapy with HBIG, to 33% in those receiving long-term IV HBIG treatment (P < 0.001) and was associated with improved graft and patient survival. Recurrence of HBV occurred in 67% of patients who underwent transplantation for HBV cirrhosis, in 83% of those with detectable serum HBV DNA at the time of LT and in 58% of those with undetectable HBV DNA, in 32% of patients who underwent transplantation for HDV cirrhosis and in 17% of patients who underwent transplantation for fulminant hepatitis B. The HBV recurrence rate was related to the presence of HBV replication, which was assessed by both HBeAg and HBV-DNA detection in serum using conventional hybridization techniques at the time of LT. These results were confirmed by other clinical trials in the United States, Europe, and Asia and by long-term follow-up studies and sustained efforts to reduce HBV replication in patients with HBV cirrhosis while waiting for LT.17,48-50 The advent of antiviral therapy further changed the landscape of post-LT prophylaxis and the standard of care now is to combine HBIG with a nucleos(t)ide analog. Reduction of the pretransplant viral load with antivirals decreases the risk that high levels of HBsAg saturate the binding capacity of HBIG and the immune pressure that triggers the selection of mutation of the “a” determinant of the HBV surface protein. Antivirals inhibit HBV replication allowing a dose reduction of HBIG. Binding of HBV particles by HBIG may reduce the viral substrate available to antivirals and may thus decrease the risk for the development of resistant mutants.
Proposed Mechanisms of HBIG
The mechanism(s) by which HBIG protects the transplanted liver against HBV reinfection are not fully understood. HBIG is an anti-HBV polyclonal antibody derived from pooled plasma. In France, HBIG is mainly produced from vaccine-immunized donors, thus it only contains antibodies to the SHBs protein of HBV and not to the LHBs that interact with the HBV specific receptor. However, antibodies directed against the antigenic loop of SHBs are sufficient to completely inhibit infection. The HBIG is thus thought to bind to circulating viral and subviral particles and to prevent their attachment to proteoglycans at the hepatocyte membrane. Also, by binding to infected hepatocytes expressing HBsAg, it could enhance antibody-dependent cell-mediated cytotoxicity, as shown for other viruses.51 In addition, HBIG can be internalized in hepatocytes and has been shown to bind to intracellular HBsAg inhibiting virions and subviral HBsAg particle secretion.52
The HBIG has no effect on viral replication, in contract to antivirals that directly inhibit HBV replication in hepatocytes. However, direct antiviral agents cannot eliminate the cccDNA reservoir in the nucleus of infected cells.53 Whatever the mechanism involved, there is evidence for a dose-dependent response to HBIG treatment.54,55 Indeed, Cholongitas et al55 have reviewed that a lower frequency of HBV recurrence was associated with a high HBIG dosage (≥10,000 IU/day) versus a low HBIG dosage (<10,000 IU/day) during the first week after LT for patients receiving HBIG and nucleoside agents (LAM and/or ADV). The dose of HBIG needed during the anhepatic and the early postoperative phase to decrease HBsAg and raise anti-HBs is determined by HBsAg levels at the time of transplantation.56 Daily monitoring of HBsAg and anti-HBs levels during the initial days after LT is important to adapt HBIG doses. Evaluation of patients failing HBIG prophylaxis indicates that early recurrence of HBV post-LT is typically related to insufficient dosing of HBIG and is more frequent in patients with a high level of pre-LT HBV replication, whereas late recurrences are usually caused by the emergence of mutations involving the “a” determinant of the HBV surface protein.
Protocols for the Administration of HBIG
In the initial protocols, HBIG was used at high doses during the anhepatic phase and the first postoperative week (i.e., generally 10 000 IU/day) to neutralize HBsAg and to provide maximal protection against reinfection of the graft (Table 1).29,31,33-35,57-70 The use of antivirals and HBIG post-LT provides complementary forms of prophylaxis. Dickson et al71 reported that a combination of LAM and HBIG was associated with reduced requirements of HBIG to render the sera HBsAg negative early after LT. With combination prophylaxis regimens, the HBV recurrence rate at 1 to 2 years after transplantation has been reduced to 0% to 10% (Table 1). Combination prophylaxis also allowed a reduction in the dose of HBIG required in the long-term. In the medium- and long-term follow-up, IV HBIG has been administered in 2 different ways: at a frequency dictated by the maintenance of specific anti-HBs levels (i.e., 50‐100 IU/L); or on a fixed schedule that generally “overshoots” the target anti-HBs level. The latter approach is simpler and requires less monitoring but is more expensive.48,72 Maintenance dosing for IV HBIG is highly variable across transplant centers (Table 1). The target levels for anti-HBs titres decrease with time after LT: generally anti-HBs levels were maintained at greater than 500 IU/L during months 1 to 3, at greater than 250 IU/L until months 6 to 12 and at greater than 50 to 100 IU/L thereafter. Some studies using low-dose HBIG had successful HBV prophylaxis with target anti-HBs levels of 100 IU/L63,66 or 50 IU/L.67 The optimal anti-HBs titre needed to prevent recurrence in the medium- and long-term follow-up is unknown, probably reduced if potent antiviral therapy is associated with HBIG.
TABLE 1.
Prevention of HBV recurrence after liver transplantation with antiviral drugs and HBIG
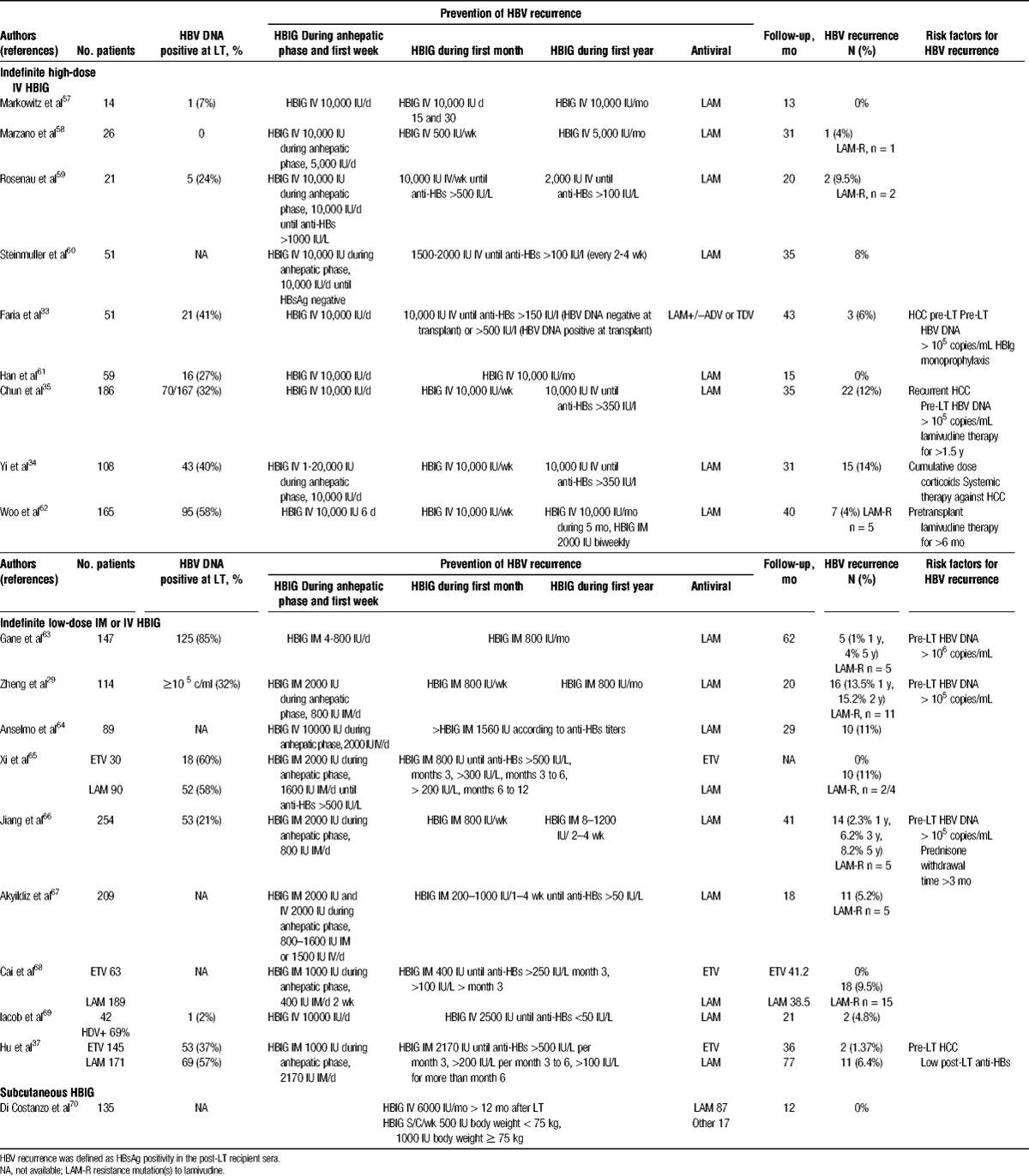
In an effort to find less costly ways of providing HBIG prophylaxis long term, alternative approaches have been studied including the use of low dose intramuscular (IM) HBIG29,37,63-68 subcutaneous HBIG,70,73,74 withdrawal of HBIG after a finite period or prophylaxis regimens without HBIG. Combination protocols are heterogeneous with regard to dosing, duration, and routes of HBIG administration (Table 1). The most cost-effective regimen reported to date is a very low IM HBIG plus LAM regimen.61,63,75 Combination prophylaxis with low dose IM HBIG (400‐800 IU IM) decreases costs by more than 90% as compared with an IV regimen, with a recurrence rate as low as 4% at 4 years.63 Hooman et al75 report the result of a crossover study comparing IV and IM HBIG administration in stable liver recipients taking LAM or ADV more than 12 months after LT. They demonstrated comparability of elimination pharmacokinetics of HBIG regardless of the antibody route of administration, maintenance of protective anti-HBs levels, and no significant difference in the elimination characteristics as a function of pretransplant replication status. Taking efficacy and cost-effectiveness into consideration, IM HBIG plus LAM seems to be superior to IV HBIG plus LAM, though there may be a subset of patients (e.g., high HBV DNA before transplantation) who may benefit from the higher doses of HBIG provided by IV route.63 More recently, subcutaneous regimens of HBIG administered 6 months after LT have proven effective as well, with some advantage regarding tolerability and the possibility of self-administration by patients at home.70,73,74 Degertekin et al15 analyzed data from 183 patients who had undergone LT between 2001 and 2007. At transplant, 29% of patients were positive for HBeAg, 38.5% had a high viral load (defined as HBV DNA >105 copies/mL). After transplantation, all except 6 patients received combination prophylaxis with antiviral therapy (mostly LAM monotherapy) plus HBIG given either IV high-dose (25%, 10,000 IU monthly), IV low-dose (21.5%, 3000‐6000 IU monthly), IM low-dose (39%, 1000‐1500 IU every 1‐2 months), or for a finite duration (14.5%; median duration, 12 months). Cumulative rates of HBV recurrence at 1, 3, and 5 years were 3%, 7%, and 9%, respectively. A multivariate analysis showed that positivity for HBeAg and a high viral load at transplant, but not the posttransplant HBIG regimen, were associated with HBV recurrence.
Several meta-analyses have compared the use of HBIG, antivirals, or both (Table 2).55,76-79 Despite methodological limitations of studies included in these meta-analyses, combination prophylaxis was significantly superior to antivirals or HBIG alone in preventing HBV recurrence, irrespective of the HBV DNA level at transplantation and in reducing overall and HBV-related mortality in some studies. Cholongitas et al55 found that the combination of HBIG and ADV with or without LAM is more effective than the combination of HBIG and LAM for the prevention of HBV recurrence, which developed in 2% (3/152) and 6.1% (115/1889) of patients, respectively (P = 0.024). Among the parameters of HBIG use that were evaluated in this review, only a high dose during the first week after LT was found to be significantly associated with the absence of HBV recurrence. A high HBIG dose (≥10,000 IU/day) versus a low HBIG dose (<10,000 IU/day) during the first week after LT was associated with a lower frequency of HBV recurrence (3.2% vs 6.5%, P = 0.016).
TABLE 2.
Results of meta-analyses comparing combination prophylaxis to HBIG or antiviral monoprophylaxis
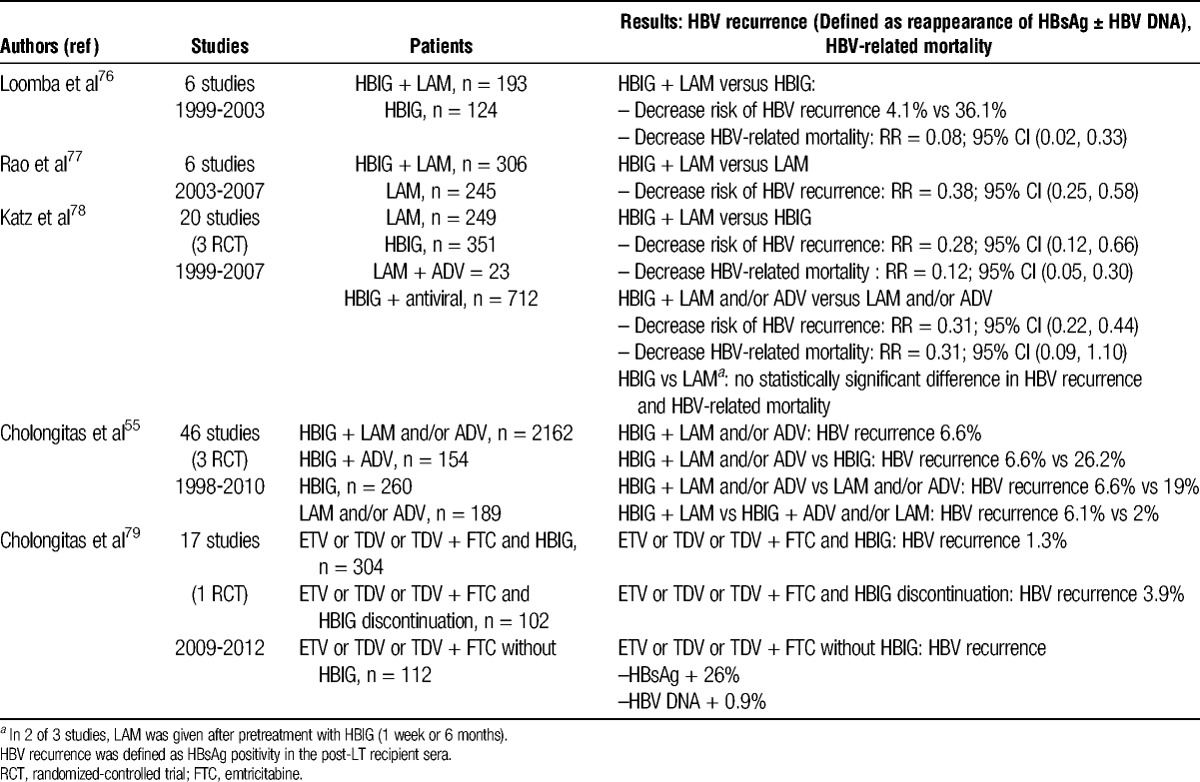
The optimal HBIG protocol is yet to be defined. Further research is needed to determine the dose and duration of HBIG after LT, appropriate titre levels of anti-HBs to prevent recurrence and whether HBIG can be stopped. The majority of published studies used a combination of HBIG and LAM and/or ADV. The role and the safety of newer nucleos(t)ide analogues with greater potency and better resistance profiles (ETV or TDF) have not yet been adequately evaluated.65,79-81 Perrillo et al81 recently assessed the safety and efficacy of ETV combined with various HBIG regimens in 65 transplant patients. Through week 72, all 61 patients evaluable for the efficacy analysis had undetectable HBV DNA. Two patients experienced a reappearance of HBsAg, but both remained HBV DNA negative. In a recent systematic review, Cholongitas et al79 evaluated the efficacy of newer nucleos(t)ide analogues with HBIG as prophylaxis against HBV recurrence. The combination of HBIG and a newer nucleos(t)ide analogue was superior to the combination of HBIG and LAM in reducing the risk of HBV recurrence (1% vs. 6.1%, P = 0.0004). The use of newer nucleos(t)ide analogues can effectively be combined with lower HBIG doses.
Limitations of HBIG
The use of IV HBIG has limitations, namely, the high cost, parenteral administration, limited supply, need for frequent clinic visits and laboratory monitoring. The IM route of administration is a cost-effective alternative to IV HBIG. However, injection is sometimes painful and local side effects occur. There are some contraindications for IM injections such as coagulopathies or oral anticoagulation medication. Subcutaneous injections improve quality of life by offering greater independence and home self-administration may contribute to decrease costs by avoiding the need for day hospitals. The HBIG has a satisfactory safety record, and adverse events observed have usually been minor. Hypersensitivity reactions or even anaphylaxis rarely occur after HBIG administration and can be controlled with antihistamines or steroids.
Prophylaxis Protocols With HBIG Discontinuation
Indefinite combination therapy with HBIG plus a nucleos(t)ide analogue may not be required in all liver transplant recipients. The replication status of the patient before the initiation of antiviral therapy and at the time of LT should guide prophylaxis. Alternative strategies to consider, especially in patients without detectable HBV DNA before transplantation are the discontinuation of HBIG after a defined period of time and continuing treatment with antivirals alone, or adding HBsAg vaccination or both.
Studies of hepatitis B vaccination as an alternative to long-term HBIG in LT recipients were conducted in patients who were serum HBV DNA-negative before LT, and during a prolonged time post-LT, who received low doses of immunosuppression, and were HBV DNA-negative by PCR at the start of vaccination.82-90 Anti-HBs titres achieved with the vaccination are highly variable and seem in part dependant on the type of vaccine: booster doses, double-dose third generation recombinant vaccines, addition of an adjuvant. Patient populations, as well as vaccine types, doses, schedules of administration, and definitions of response differed across these studies. From these data, it seems clear that successful hepatitis B vaccination and discontinuation of HBIG are feasible only in a small group of selected patients but the optimal vaccine protocol has not been established.
Two studies evaluated the efficacy of long-term HBIG monotherapy versus HBIG followed by LAM monotherapy in patients selected on the basis of a low risk of HBV reinfection.91,92 At 1 year after the discontinuation of HBIG, the HBV reinfection rates were not significantly different; however, HBV DNA was detected by PCR in the serum of some patients without HBV recurrence. This latter finding suggests caution with this approach and the need for studies with a longer follow-up and other antiviral therapy. Another strategy is HBIG withdrawal after a defined period of combination prophylaxis (Table 3).1,23,93-103 In a study of 29 patients, high-dose HBIG and LAM were used in the first month, and patients were then randomized to receive either LAM monotherapy or LAM plus IM HBIG at 2000 IU monthly.93 None of the patients developed HBV recurrence during the first 18 months but later recurrences developed in 4 patients after 5 years of follow-up related with poor LAM compliance.94 Wong et al95 reported that HBV recurrence rates were 0% and 9% at 2 and 4 years after HBIG discontinuation. In a prospective study of 29 patients, sequential ETV monotherapy after planned discontinuation of 1-year combination therapy of IV HBIG and ETV was assessed and then followed up for 1 additional year.102 After the first 12 months, HBIG was discontinued if: liver function test within the normal range 3 times; no steroid medication; and negative HBsAg and DNA. During the first year, no HBV recurrence was reported and during the second year, HBV recurrence was noted in only 1 patient related with HCC recurrence without viral mutation. Tanaka et al103 conducted a retrospective study on 24 patients who received TDF (± LAM) and one year of low dose HBIG to prevent recurrent HBV infection. None of the patients developed recurrent HBV infection in the median follow-up period of 29.1 months (neither HBsAg nor HBV DNA levels detectable).103 An alternative approach is to switch from HBIg/LAM to a combination of antiviral agents that present a greater barrier to the development of resistance than LAM. In a randomized prospective study, 16 of 34 patients receiving low-dose IM HBIG/LAM prophylaxis who were at least 12 months after LT were switched to ADV/LAM combination therapy, whereas the remaining patients continued HBIG/LAM.97 At a median follow-up of 21 months after the switch, no patient had disease recurrence, although 1 patient in the ADV/LAM group had a low titre of HBsAg in serum but was repeatedly HBV DNA-negative. The same group has recently reported the outcome of 20 patients with an HBIG-sparing regimen using LAM + ADV initiated at the time of listing and continued after LT.100 Eight hundred IU of IM HBIG were given immediately after LT and daily during 7 days. After a median follow-up of 57 months post-LT, only 1 patient became HBsAg-positive (HBV DNA negative) at the time of HCC recurrence. Serum HBsAg became undetectable again after surgical treatment of HCC recurrence. Saab et al98 switched 61 liver transplant recipients to a combination of a nucleoside (LAM or ETV) and nucleotide analogue (ADV or TDV). At a median follow-up of 15 months after the switch, 2 patients were HBsAg-positive in serum but repeatedly HBV DNA-negative. Recently, Teperman et al99 evaluated the use of a combination of TDV with emtricitabine after HBIG discontinuation. In this study, subjects at a median of 3.4 years after LT, were treated with a combination of Emtricitabine/TDV and HBIG for 24 weeks and then randomized to continue this prophylaxis regimen (n = 19) or to discontinue HBIG (n = 18). At 72 weeks after randomization, only 1 patient in the Emtricitabine/TDV group had a transient detectability of HBV DNA related to poor compliance. Several studies demonstrated cases of seroconversion to positive HBsAg associated with undetectable HBV DNA (Table 3).95,97,98,100 A proposed mechanism for this is that HBsAg was being produced at low levels during HBIG therapy and became detectable after HBIG cessation. Longer follow-up of these patients is necessary to determine whether they will clear HBsAg or whether they are at future risk of viral breakthrough.
TABLE 3.
Prevention of HBV recurrence after liver transplantation with HBIG discontinuation and long-term antiviral therapy
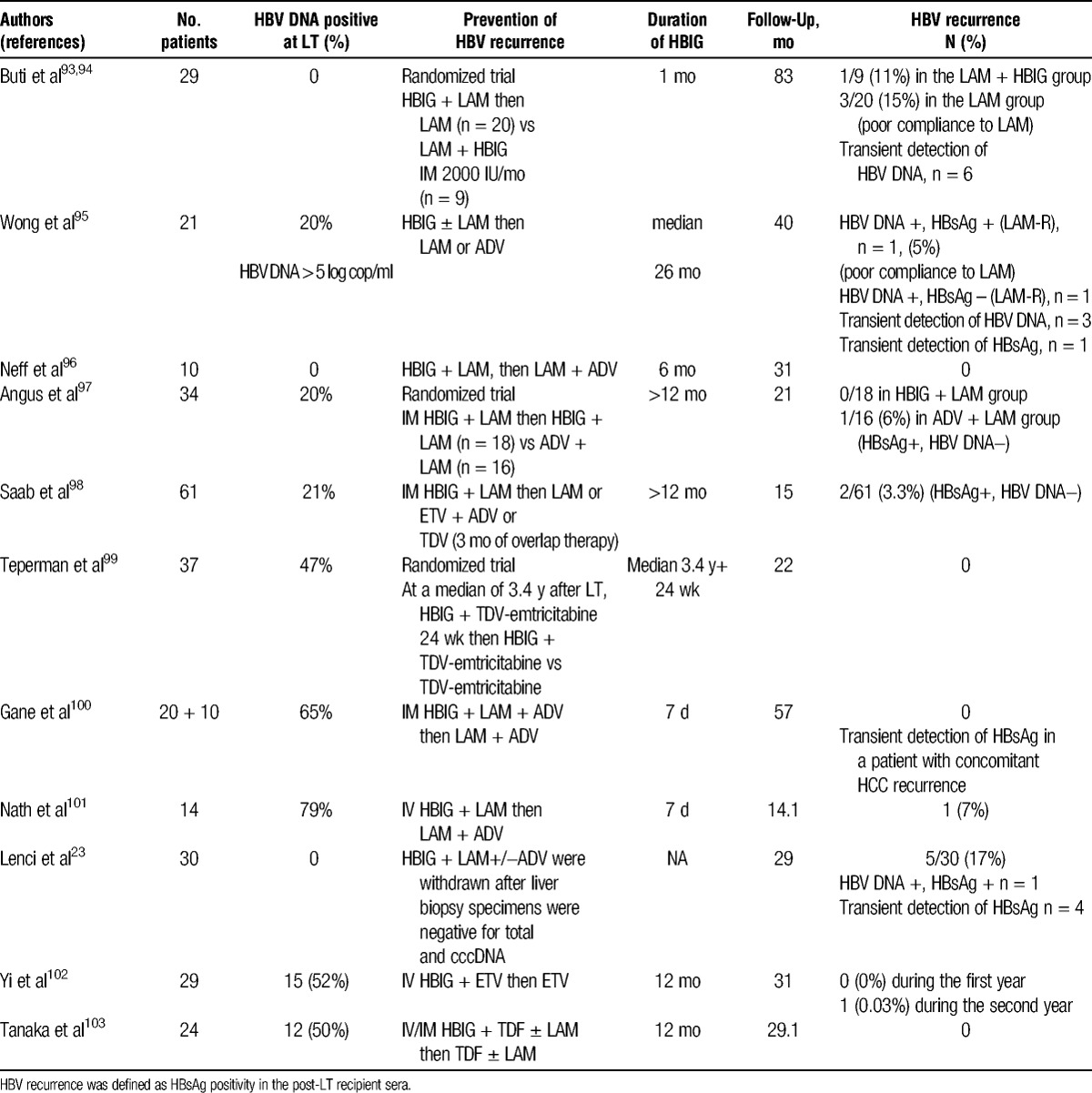
Duration of HBIG in HBIG withdrawal strategies is variable across centers and has not yet been established (Table 3). In several studies, HBIG were stopped at 1 year after LT; however, some studies report durations of HBIG as short as 7 days or 1 month with favorable results.93,100,101 Saab et al104 compared costs and outcomes of 2 strategies for HBV prophylaxis after 1 year after LT. The first strategy consisted of prophylaxis with LAM and ADV, whereas the second consisted of monthly IM HBIG and LAM. After 10 years of therapy, the decision analysis model resulted in cost savings of approximately 10 % with the first strategy.
Drug compliance during long-term antiviral therapy may be a very important issue for transplant patients who feel healthy but have a lifelong risk of HBV recurrence. Safety data on the long-term use of ETV or TDV in transplant recipients are lacking. Consideration must be given to potential side effects: nephrotoxicity associated with TDV may be enhanced in transplant patients on calcineurin inhibitor therapy, risk of decreased bone density with TDV, and mitochondrial toxicity associated with ETV.
An ultimate approach was to evaluate the safety of complete and sustained prophylaxis withdrawal in liver transplant recipients at a low risk of HBV recurrence. Lenci et al23 evaluated a cohort of 30 patients at a low risk of recurrence (HBeAg and HBV DNA negative at LT, 23% HDV coinfected) and treated with a combination of HBIG and LAM (+/− ADV) for at least 3 years. Sequential liver biopsies were performed and evaluated for the presence of intrahepatic total HBV DNA and cccDNA. Using the absence of intrahepatic total HBV DNA and cccDNA as a guide, HBIG and then antiviral therapy was withdrawn in a stepwise fashion. After a median of 28.7 months off all prophylactic therapy, 83% of the cohort remained without serologic recurrence of HBV infection. Five patients developed HBsAg recurrence but only 1 patient showed evidence of HBV disease (HBV DNA positive), in the other patients, HBsAg positivity was transient. Twenty-three of the 25 subjects without recurrence never had detectable HBV DNA in liver biopsies, whereas all 5 patients with recurrence had evidence of total HBV DNA in the liver and one had detected cccDNA. However, the ability to measure total HBV DNA and cccDNA in a liver biopsy has limitations: this strategy requires sequential liver biopsies and assays for quantification of total HBV DNA and cccDNA are not standardized.
The studies available to date highlight several key issues to consider before the discontinuation of HBIG after transplantation: First, the risk of HBV recurrence after cessation of HBIG may increase with time off HBIG either due to the development of viral resistance or due to noncompliance to antiviral therapy, and the role of antiviral combinations or antivirals, such as ETV or TDV with a high genetic barrier to resistance, should be better evaluated. Second, the patients with high levels of HBV DNA at the time of transplantation appear to be a higher risk group for recurrence when HBIG is discontinued. Third, HBV DNA persists in serum, liver, or peripheral blood mononuclear cells even 10 years after LT in a proportion of HBV-transplanted patients who are HBsAg-negative. These reservoirs may be a source of future HBV reinfection and therefore support the use of long-term prophylactic therapy in most patients.17,18,21 Fourth, we currently lack the ability to identify patients who may have cleared HBV after transplantation.
HBIG-Free Prophylactic Regimens
Lamivudine has been evaluated as a prophylactic monotherapy, the drug being started before transplantation and continued after transplantation without HBIG. The outcome at 1 year showed a 10% recurrence rate.38 However, with longer follow-up, rates of recurrence reached 20% to 41% at 3 years after LT (Table 4).5,29,38-41,105 Recurrence was due to the emergence of escape mutations in the YMDD motif of the polymerase gene and was observed mainly in patients with a high level of HBV replication before drug exposure. By reserving LAM monoprophylaxis for patients without viral replication at the time of LT, rates of recurrence can be lowered to less than 20%.107 Because prophylactic therapy using LAM alone was associated with unacceptably high rates of reinfection in patients with a high level of viral replication before drug exposure, most transplant programs do not use LAM monotherapy for prophylaxis. Schiff et al6 reported 61 LAM-resistant patients treated with ADV on the waiting list who underwent LT. Sixty percent of these patients received HBIG and ADV combination prophylaxis after LT and 40% ADV ± LAM prophylaxis. Interestingly, no patient in either group had recurrent HBV infection defined as 2 or more positive test results for HBsAg or for HBV DNA; follow-up, however, was short (18 months). These studies showed the limitations of antiviral monoprophylaxis using LAM or ADV (Table 4).106 The emergence of drug resistance before or after LT limits the efficacy of treatment. The HBV DNA levels at the time of transplantation are related to pretreatment HBV DNA levels and duration of therapy, and influence the risk of recurrence. Recently, Gane et al100 reported the results of a combination prophylaxis using LAM and ADV without HBIG in 18 patients who had documented suppression of HBV DNA below 3 log10 IU/mL before LT. No case of HBV recurrence was observed after a median follow-up of 22 months. The combination of LAM and ADV is cost-effective as compared to low-dose IM HBIG and LAM ($8290 vs $13718 per year). The availability of more potent antivirals with a higher barrier to resistance could increase the proportion of patients with undetectable HBV DNA before transplantation and decrease the risk of recurrent disease after transplantation.1 Wadhawan et al108 reported on 56 living donor recipients who received various antivirals before transplantation (LAM + ADV n = 17, ETV n = 25, TDV n = 8, ETV + TDV n = 2). Forty-seven of 56 patients achieved a HBV DNA level below 2000 IU/mL before transplant and did not receive HBIG. All were HBV DNA undetectable after a median follow-up of 20 months after transplantation. Fung et al43 investigated the efficacy of ETV as monoprophylaxis in 80 patients with chronic hepatitis B who received a liver transplant. A total of 18 patients (22.5%) had persistent HBsAg positivity after transplant without seroclearance (n = 8) or reappearance of HBsAg after initial seroclearance (n = 10). Seventeen patients had undetectable levels of HBV DNA at the time of the last follow-up. The remaining patient had a very low HBV DNA level of 217 copies/mL at 36 months after LT. The pre-LT HBsAg level was significantly higher in those who had HBV recurrence/persistence compared with those who did not. In this study, as compared with the study of Gane et al,100 the median pre-LT HBV DNA level was much higher (3.6 log10 IU/ml versus 1.9 log10 IU/mL), the rate of HBsAg clearance after LT was much slower with a median time to HBsAg loss of 4 weeks versus 1 week, and the 12-month cumulative rate of HBsAg loss was 88% versus 100%.
TABLE 4.
Prevention of HBV recurrence with antiviral(s) before and after liver transplantation
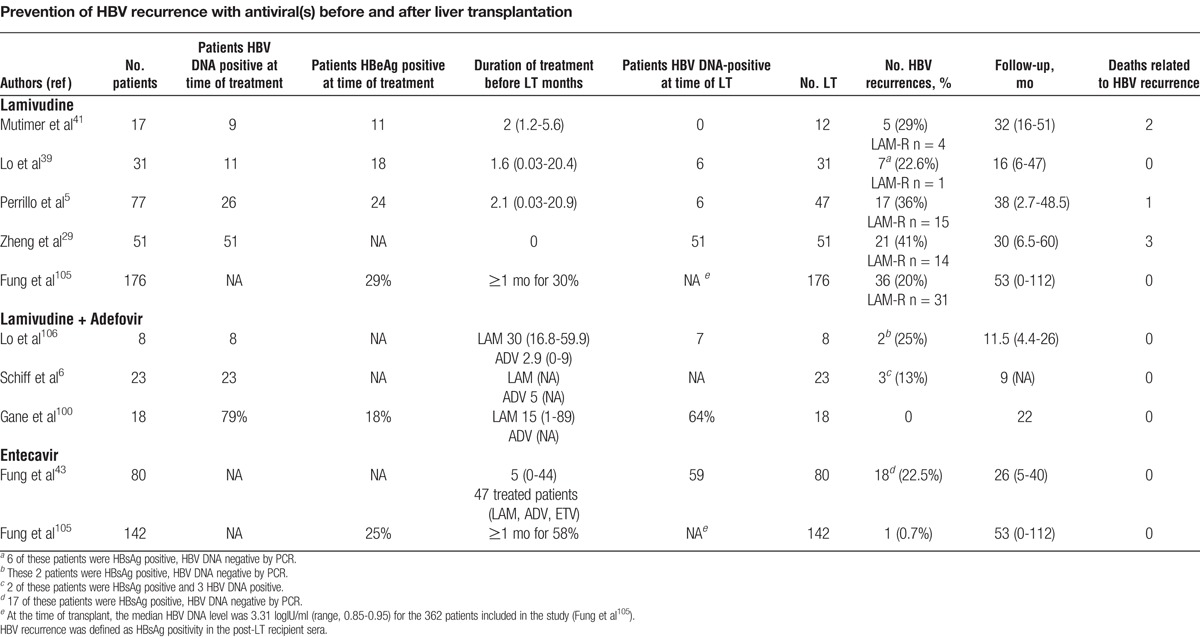
Regarding the long-term results, Fung et al105 reported a study conducted on a large population of 362 CHB patients who underwent LT, of which 176 (49% ), 142 (39% ), and 44 (12%) were on LAM, ETV, and combination therapy (predominantly LAM + ADV), respectively, without HBIG. The rate of HBsAg seronegativity and HBV DNA suppression to undetectable levels at 8 years was 88% and 98%, respectively and the overall 8-year survival was 83%, with no difference between the 3 treatment groups. The virological relapse rates, defined as greater than 1 log IU/mL increase of HBV DNA level from nadir, was 5%, 10%, 13%, and 16% at 1, 3, 5, and 8 years. The virological rebound for LAM, combination therapy, and ETV was 17%, 7%, and 0%, respectively, at 3 years. One patient required retransplantation because of fibrosing cholestatic hepatitis from HBV recurrence. Using multivariate analysis, the type of antiviral therapy, the indication for LT, and the viral load at the time of LT remained significant factors associated with virological rebound. Recently, Takaki et al53 reviewed the post-LT HBV prophylaxis with nucleos(t)ide analogues and/or HBIG. They established that a complete HBIG-free protocol may impose a risk for patients with high levels of HBV DNA at the time of LT but may be adopted for patients who are HBV DNA-negative at the time of LT. Further large and long-term studies are required to decide whether other antivirals, such as TDV or a combination of antivirals without HBIG, would provide effective prophylaxis.
Guidelines and Future Prospects for Prevention of HBV Reinfection
Viral suppression is the goal for all patients on a waiting list. For patients without detectable viral replication before transplantation, there is no evidence that preoperative antiviral therapy is useful. For patients with viral replication before transplantation, ETV, TDV or a nucleoside/nucleotide combination should be used. There is a consensus regarding the need for a lifelong prophylactic therapy supported by the detection of HBV DNA in both hepatic and extrahepatic sites in patients who are HBsAg negative on posttransplant HBIG and antivirals. In the early posttransplant period, some studies reported that a high IV HBIG dose (≥10,000 IU/day) versus a low HBIG dose (<10,000 IU/day) was associated with a lower frequency of HBV recurrence. At long-term, low-dose IM or subcutaneous HBIG in combination with a potent nucleos(t)ide analogue is the most cost-effective prophylaxis. Patients with an undetectable HBV DNA level at the time of transplant are eligible for protocols using short-term low dose IV or IM HBIG and antiviral therapy, followed by antiviral monotherapy (Figure 1). A more cautious approach to this prophylactic regimen is necessary for those patients with high pretransplant HBV DNA levels, those with limited antiviral options if HBV recurrence occurred (i.e., HIV or HDV coinfection, preexisting drug resistance or intolerance), those with a high risk of HCC recurrence and those with a risk of noncompliance with antiviral therapy. In this group, HBIG-free prophylaxis cannot be recommended.
FIGURE 1.
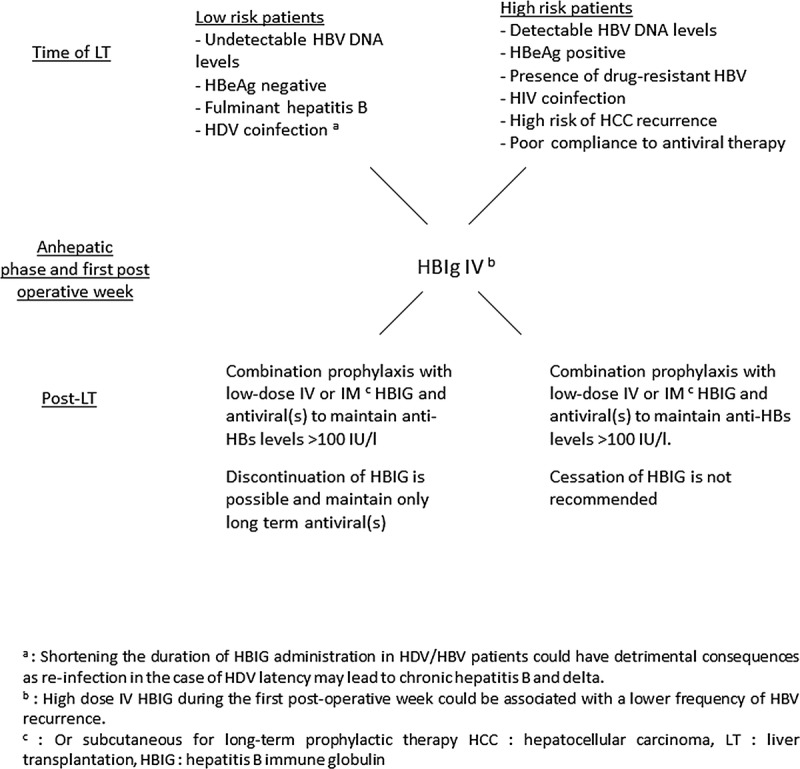
Prophylaxis for prevention of HBV graft recurrence after LT. Proposal for guideline.
CONCLUSIONS
During the past 2 decades, major advances have been made in the management of HBV transplant candidates. The HBIG administration and antiviral drugs used before and after transplantation, as a prophylaxis of HBV recurrence, were major breakthroughs in the management of patients. The combination of long-term antivirals and low-dose HBIG can effectively prevent HBV recurrence in greater than 90% of transplant recipients. Some form of HBV prophylaxis needs to be continued indefinitely after transplantation. However, in patients with low HBV DNA levels before transplantation, discontinuation of HBIG, with continued long-term nucleos(t)ide analogue(s) treatment is possible.
Footnotes
This review article was supported by LFB Biomedicaments, producer and marketer of human hepatitis B immunoglobulin.
The authors declare no conflicts of interest.
Each author participated in the writing of the article.
REFERENCES
- 1. Fox AN, Terrault NA. The option of HBIG-free prophylaxis against recurrent HBV. J Hepatol. 2012; 56: 1189. [DOI] [PubMed] [Google Scholar]
- 2. Papatheodoridis GV, Cholongitas E, Archimandritis AJ, et al. Current management of hepatitis B virus infection before and after liver transplantation. Liver Int. 2009; 29: 1294. [DOI] [PubMed] [Google Scholar]
- 3. Ghaziani T, Sendi H, Shahraz S, et al. Hepatitis B and liver transplantation: molecular and clinical features that influence recurrence and outcome. World J Gastroenterol. 2014; 20: 14142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yao FY, Terrault NA, Freise C, et al. Lamivudine treatment is beneficial in patients with severely decompensated cirrhosis and actively replicating hepatitis B infection awaiting liver transplantation: a comparative study using a matched, untreated cohort. Hepatology. 2001; 34: 411. [DOI] [PubMed] [Google Scholar]
- 5. Perrillo RP, Wright T, Rakela J, et al. A multicenter United States-Canadian trial to assess lamivudine monotherapy before and after liver transplantation for chronic hepatitis B. Hepatology. 2001; 33: 424. [DOI] [PubMed] [Google Scholar]
- 6. Schiff E, Lai CL, Hadziyannis S, et al. Adefovir dipivoxil for wait-listed and post-liver transplantation patients with lamivudine-resistant hepatitis B: final long-term results. Liver Transpl. 2007; 13: 349. [DOI] [PubMed] [Google Scholar]
- 7. Liaw YF, Raptopoulou-Gigi M, Cheinquer H, et al. Efficacy and safety of entecavir versus adefovir in chronic hepatitis B patients with hepatic decompensation: a randomized, open-label study. Hepatology. 2011; 54: 91. [DOI] [PubMed] [Google Scholar]
- 8. Liaw YF, Sheen IS, Lee CM, et al. Tenofovir disoproxil fumarate (TDF), emtricitabine/TDF, and entecavir in patients with decompensated chronic hepatitis B liver disease. Hepatology. 2011; 53: 62. [DOI] [PubMed] [Google Scholar]
- 9. Shim JH, Lee HC, Kim KM, et al. Efficacy of entecavir in treatment-naive patients with hepatitis B virus-related decompensated cirrhosis. J Hepatol 2010; 52: 176. [DOI] [PubMed] [Google Scholar]
- 10. Fontana RJ, Hann HW, Perrillo RP, et al. Determinants of early mortality in patients with decompensated chronic hepatitis B treated with antiviral therapy. Gastroenterology. 2002; 123: 719. [DOI] [PubMed] [Google Scholar]
- 11. Villeneuve JP, Condreay LD, Willems B, et al. Lamivudine treatment for decompensated cirrhosis resulting from chronic hepatitis B. Hepatology. 2000; 31: 207. [DOI] [PubMed] [Google Scholar]
- 12. Kim WR, Terrault NA, Pedersen RA, et al. Trends in waiting list registration for liver transplantation for viral hepatitis in the United States. Gastroenterology. 2009; 137: 1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. European Association For The Study Of The L. EASL Clinical Practice Guidelines: management of chronic hepatitis B. J Hepatol. 2009; 50: 227. [DOI] [PubMed] [Google Scholar]
- 14. Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009; 50: 661. [DOI] [PubMed] [Google Scholar]
- 15. Degertekin B, Han SH, Keeffe EB, et al. Impact of virologic breakthrough and HBIG regimen on hepatitis B recurrence after liver transplantation. Am J Transplant. 2010; 10: 1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pauwelyn K, Cassiman D, Laleman W, et al. Outcomes of long-term administration of intravenous hepatitis B immunoglobulins for the prevention of recurrent hepatitis B after liver transplantation. Transplant Proc. 2010; 42: 4399. [DOI] [PubMed] [Google Scholar]
- 17. Roche B, Feray C, Gigou M, et al. HBV DNA persistence 10 years after liver transplantation despite successful anti-HBS passive immunoprophylaxis. Hepatology. 2003; 38: 86. [DOI] [PubMed] [Google Scholar]
- 18. Hussain M, Soldevila-Pico C, Emre S, et al. Presence of intrahepatic (total and ccc) HBV DNA is not predictive of HBV recurrence after liver transplantation. Liver Transpl. 2007; 13: 1137. [DOI] [PubMed] [Google Scholar]
- 19. Freshwater DA, Dudley T, Cane P, et al. Viral persistence after liver transplantation for hepatitis B virus: a cross-sectional study. Transplantation. 2008; 85: 1105. [DOI] [PubMed] [Google Scholar]
- 20. Yasunaka T, Takaki A, Yagi T, et al. Serum hepatitis B virus DNA before liver transplantation correlates with HBV reinfection rate even under successful low-dose hepatitis B immunoglobulin prophylaxis. Hepatol Int. 2011; 5: 918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Coffin CS, Mulrooney-Cousins PM, van Marle G, et al. Hepatitis B virus quasispecies in hepatic and extrahepatic viral reservoirs in liver transplant recipients on prophylactic therapy. Liver Transpl. 2011; 17: 955. [DOI] [PubMed] [Google Scholar]
- 22. Cheung CK, Lo CM, Man K, et al. Occult hepatitis B virus infection of donor and recipient origin after liver transplantation despite nucleoside analogue prophylaxis. Liver Transpl. 2010; 16: 1314. [DOI] [PubMed] [Google Scholar]
- 23. Lenci I, Tisone G, Di Paolo D, et al. Safety of complete and sustained prophylaxis withdrawal in patients liver-transplanted for HBV-related cirrhosis at low risk of HBV recurrence. J Hepatol. 2011; 55: 587. [DOI] [PubMed] [Google Scholar]
- 24. Yan H, Zhong G, Xu G, et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife. 2012; 1: e00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bremer CM, Sominskaya I, Skrastina D, et al. N-terminal myristoylation-dependent masking of neutralizing epitopes in the preS1 attachment site of hepatitis B virus. J Hepatol. 2011; 55: 29. [DOI] [PubMed] [Google Scholar]
- 26. Schieck A, Schulze A, Gahler C, et al. Hepatitis B virus hepatotropism is mediated by specific receptor recognition in the liver and not restricted to susceptible hosts. Hepatology. 2013; 58: 43. [DOI] [PubMed] [Google Scholar]
- 27. Brind A, Jiang J, Samuel D, et al. Evidence for selection of hepatitis B mutants after liver transplantation through peripheral blood mononuclear cell infection. J Hepatol. 1997; 26: 228. [DOI] [PubMed] [Google Scholar]
- 28. Marzano A, Gaia S, Ghisetti V, et al. Viral load at the time of liver transplantation and risk of hepatitis B virus recurrence. Liver Transpl. 2005; 11: 402. [DOI] [PubMed] [Google Scholar]
- 29. Zheng S, Chen Y, Liang T, et al. Prevention of hepatitis B recurrence after liver transplantation using lamivudine or lamivudine combined with hepatitis B Immunoglobulin prophylaxis. Liver Transpl. 2006; 12: 253. [DOI] [PubMed] [Google Scholar]
- 30. Samuel D, Muller R, Alexander G, et al. Liver transplantation in European patients with the hepatitis B surface antigen. N Engl J Med. 1993; 329: 1842. [DOI] [PubMed] [Google Scholar]
- 31. Neff GW, O'Brien CB, Nery J, et al. Outcomes in liver transplant recipients with hepatitis B virus: resistance and recurrence patterns from a large transplant center over the last decade. Liver Transpl. 2004; 10: 1372. [DOI] [PubMed] [Google Scholar]
- 32. Mutimer D, Pillay D, Dragon E, et al. High pre-treatment serum hepatitis B virus titre predicts failure of lamivudine prophylaxis and graft re-infection after liver transplantation. J Hepatol. 1999; 30: 715. [DOI] [PubMed] [Google Scholar]
- 33. Faria LC, Gigou M, Roque-Afonso AM, et al. Hepatocellular carcinoma is associated with an increased risk of hepatitis B virus recurrence after liver transplantation. Gastroenterology. 2008; 134: 1890 quiz 2155. [DOI] [PubMed] [Google Scholar]
- 34. Yi NJ, Suh KS, Cho JY, et al. Recurrence of hepatitis B is associated with cumulative corticosteroid dose and chemotherapy against hepatocellular carcinoma recurrence after liver transplantation. Liver Transpl. 2007; 13: 451. [DOI] [PubMed] [Google Scholar]
- 35. Chun J, Kim W, Kim BG, et al. High viremia, prolonged Lamivudine therapy and recurrent hepatocellular carcinoma predict posttransplant hepatitis B recurrence. Am J Transplant. 2010; 10: 1649. [DOI] [PubMed] [Google Scholar]
- 36. Saab S, Yeganeh M, Nguyen K, et al. Recurrence of hepatocellular carcinoma and hepatitis B reinfection in hepatitis B surface antigen-positive patients after liver transplantation. Liver Transpl. 2009; 15: 1525. [DOI] [PubMed] [Google Scholar]
- 37. Hu TH, Chen CL, Lin CC, et al. Section 14. Combination of entecavir plus low-dose on-demand hepatitis B immunoglobulin is effective with very low hepatitis B recurrence after liver transplantation. Transplantation. 2014; 97 (Suppl 8): S53. [DOI] [PubMed] [Google Scholar]
- 38. Grellier L, Mutimer D, Ahmed M, et al. Lamivudine prophylaxis against reinfection in liver transplantation for hepatitis B cirrhosis. Lancet. 1996; 348: 1212. [DOI] [PubMed] [Google Scholar]
- 39. Lo CM, Cheung ST, Lai CL, et al. Liver transplantation in Asian patients with chronic hepatitis B using lamivudine prophylaxis. Ann Surg. 2001; 233: 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Malkan G, Cattral MS, Humar A, et al. Lamivudine for hepatitis B in liver transplantation: a single-center experience. Transplantation. 2000; 69: 1403. [DOI] [PubMed] [Google Scholar]
- 41. Mutimer D, Dusheiko G, Barrett C, et al. Lamivudine without HBIg for prevention of graft reinfection by hepatitis B: long-term follow-up. Transplantation. 2000; 70: 809. [DOI] [PubMed] [Google Scholar]
- 42. Xie SB, Zhu JY, Ying Z, et al. Prevention and risk factors of the HBV recurrence after orthotopic liver transplantation: 160 cases follow-up study. Transplantation. 2010; 90: 786. [DOI] [PubMed] [Google Scholar]
- 43. Fung J, Cheung C, Chan SC, et al. Entecavir monotherapy is effective in suppressing hepatitis B virus after liver transplantation. Gastroenterology. 2011; 141: 1212. [DOI] [PubMed] [Google Scholar]
- 44. Glebe D, Bremer CM. The molecular virology of hepatitis B virus. Semin Liver Dis 2013; 33: 103. [DOI] [PubMed] [Google Scholar]
- 45. Ghany MG, Ayola B, Villamil FG, et al. Hepatitis B virus S mutants in liver transplant recipients who were reinfected despite hepatitis B immune globulin prophylaxis. Hepatology. 1998; 27: 213. [DOI] [PubMed] [Google Scholar]
- 46. Terrault NA, Zhou S, McCory RW, et al. Incidence and clinical consequences of surface and polymerase gene mutations in liver transplant recipients on hepatitis B immunoglobulin. Hepatology. 1998; 28: 555. [DOI] [PubMed] [Google Scholar]
- 47. Bock CT, Tillmann HL, Torresi J, et al. Selection of hepatitis B virus polymerase mutants with enhanced replication by lamivudine treatment after liver transplantation. Gastroenterology. 2002; 122: 264. [DOI] [PubMed] [Google Scholar]
- 48. Terrault NA, Zhou S, Combs C, et al. Prophylaxis in liver transplant recipients using a fixed dosing schedule of hepatitis B immunoglobulin. Hepatology. 1996; 24: 1327. [DOI] [PubMed] [Google Scholar]
- 49. Muller R, Gubernatis G, Farle M, et al. Liver transplantation in HBs antigen (HBsAg) carriers. Prevention of hepatitis B virus (HBV) recurrence by passive immunization. J Hepatol. 1991; 13: 90. [DOI] [PubMed] [Google Scholar]
- 50. Hwang S, Ahn CS, Song GW, et al. Posttransplantation prophylaxis with primary high-dose hepatitis B immunoglobulin monotherapy and complementary preemptive antiviral add-on. Liver Transpl. 2011; 17: 456. [DOI] [PubMed] [Google Scholar]
- 51. Shouval D, Samuel D. Hepatitis B immune globulin to prevent hepatitis B virus graft reinfection following liver transplantation: a concise review. Hepatology. 2000; 32: 1189. [DOI] [PubMed] [Google Scholar]
- 52. Schilling R, Ijaz S, Davidoff M, et al. Endocytosis of hepatitis B immune globulin into hepatocytes inhibits the secretion of hepatitis B virus surface antigen and virions. J Virol. 2003; 77: 8882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Takaki A, Yagi T, Yamamoto K. Safe and cost-effective control of post-transplantation recurrence of hepatitis B. Hepatol Res. 2015; 45: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. McGory RW, Ishitani MB, Oliveira WM, et al. Improved outcome of orthotopic liver transplantation for chronic hepatitis B cirrhosis with aggressive passive immunization. Transplantation. 1996; 61: 1358. [DOI] [PubMed] [Google Scholar]
- 55. Cholongitas E, Goulis J, Akriviadis E, et al. Hepatitis B immunoglobulin and/or nucleos(t)ide analogues for prophylaxis against hepatitis b virus recurrence after liver transplantation: a systematic review. Liver Transpl. 2011; 17: 1176. [DOI] [PubMed] [Google Scholar]
- 56. Rosenau J, Kreutz T, Kujawa M, et al. HBsAg level at time of liver transplantation determines HBsAg decrease and anti-HBs increase and affects HBV DNA decrease during early immunoglobulin administration. J Hepatol. 2007; 46: 635. [DOI] [PubMed] [Google Scholar]
- 57. Markowitz JS, Martin P, Conrad AJ, et al. Prophylaxis against hepatitis B recurrence following liver transplantation using combination lamivudine and hepatitis B immune globulin. Hepatology. 1998; 28: 585. [DOI] [PubMed] [Google Scholar]
- 58. Marzano A, Salizzoni M, Debernardi-Venon W, et al. Prevention of hepatitis B virus recurrence after liver transplantation in cirrhotic patients treated with lamivudine and passive immunoprophylaxis. J Hepatol. 2001; 34: 903. [DOI] [PubMed] [Google Scholar]
- 59. Rosenau J, Bahr MJ, Tillmann HL, et al. Lamivudine and low-dose hepatitis B immune globulin for prophylaxis of hepatitis B reinfection after liver transplantation possible role of mutations in the YMDD motif prior to transplantation as a risk factor for reinfection. J Hepatol. 2001; 34: 895. [DOI] [PubMed] [Google Scholar]
- 60. Steinmuller T, Seehofer D, Rayes N, et al. Increasing applicability of liver transplantation for patients with hepatitis B-related liver disease. Hepatology. 2002; 35: 1528. [DOI] [PubMed] [Google Scholar]
- 61. Han SH, Ofman J, Holt C, et al. An efficacy and cost-effectiveness analysis of combination hepatitis B immune globulin and lamivudine to prevent recurrent hepatitis B after orthotopic liver transplantation compared with hepatitis B immune globulin monotherapy. Liver Transpl. 2000; 6: 741. [DOI] [PubMed] [Google Scholar]
- 62. Woo HY, Choi JY, Jang JW, et al. Role of long-term lamivudine treatment of hepatitis B virus recurrence after liver transplantation. J Med Virol. 2008; 80: 1891. [DOI] [PubMed] [Google Scholar]
- 63. Gane EJ, Angus PW, Strasser S, et al. Lamivudine plus low-dose hepatitis B immunoglobulin to prevent recurrent hepatitis B following liver transplantation. Gastroenterology. 2007; 132: 931. [DOI] [PubMed] [Google Scholar]
- 64. Anselmo DM, Ghobrial RM, Jung LC, et al. New era of liver transplantation for hepatitis B: a 17-year single-center experience. Ann Surg. 2002; 235: 611 discussion 619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Xi ZF, Xia Q, Zhang JJ, et al. The role of entecavir in preventing hepatitis B recurrence after liver transplantation. J Dig Dis. 2009; 10: 321. [DOI] [PubMed] [Google Scholar]
- 66. Jiang L, Yan L, Li B, et al. Prophylaxis against hepatitis B recurrence posttransplantation using lamivudine and individualized low-dose hepatitis B immunoglobulin. Am J Transplant. 2010; 10 (8): 1861. [DOI] [PubMed] [Google Scholar]
- 67. Akyildiz M, Karasu Z, Zeytunlu M, et al. Adefovir dipivoxil therapy in liver transplant recipients for recurrence of hepatitis B virus infection despite lamivudine plus hepatitis B immunoglobulin prophylaxis. J Gastroenterol Hepatol. 2007; 22: 2130. [DOI] [PubMed] [Google Scholar]
- 68. Cai CJ, Lu MQ, Chen YH, et al. Clinical study on prevention of HBV re-infection by entecavir after liver transplantation. Clin Transplant. 2012; 26: 208. [DOI] [PubMed] [Google Scholar]
- 69. Iacob S, Hrehoret D, Matei E, et al. Costs and efficacy of “on demand” low-dose immunoprophylaxis in HBV transplanted patients: experience in the Romanian program of liver transplantation. J Gastrointestin Liver Dis. 2008; 17: 383. [PubMed] [Google Scholar]
- 70. Di Costanzo GG, Lanza AG, Picciotto FP, et al. Safety and efficacy of subcutaneous hepatitis B immunoglobulin after liver transplantation: an open single-arm prospective study. Am J Transplant. 2013; 13: 348. [DOI] [PubMed] [Google Scholar]
- 71. Dickson RC, Terrault NA, Ishitani M, et al. Protective antibody levels and dose requirements for IV 5% Nabi Hepatitis B immune globulin combined with lamivudine in liver transplantation for hepatitis B-induced end stage liver disease. Liver Transpl. 2006; 12: 124. [DOI] [PubMed] [Google Scholar]
- 72. Di Paolo D, Tisone G, Piccolo P, et al. Low-dose hepatitis B immunoglobulin given “on demand” in combination with lamivudine: a highly cost-effective approach to prevent recurrent hepatitis B virus infection in the long-term follow-up after liver transplantation. Transplantation. 2004; 77: 1203. [DOI] [PubMed] [Google Scholar]
- 73. Singham J, Greanya ED, Lau K, et al. Efficacy of maintenance subcutaneous hepatitis B immune globulin (HBIG) post-transplant for prophylaxis against hepatitis B recurrence. Ann Hepatol. 2011; 9: 166. [PubMed] [Google Scholar]
- 74. Yahyazadeh A, Beckebaum S, Cicinnati V, et al. Efficacy and safety of subcutaneous human HBV-immunoglobulin (Zutectra) in liver transplantation: an open, prospective, single-arm phase III study. Transpl Int. 2011; 24: 441. [DOI] [PubMed] [Google Scholar]
- 75. Hooman N, Rifai K, Hadem J, et al. Antibody to hepatitis B surface antigen trough levels and half-lives do not differ after intravenous and intramuscular hepatitis B immunoglobulin administration after liver transplantation. Liver Transpl. 2008; 14: 435. [DOI] [PubMed] [Google Scholar]
- 76. Loomba R, Rowley AK, Wesley R, et al. Hepatitis B immunoglobulin and Lamivudine improve hepatitis B-related outcomes after liver transplantation: meta-analysis. Clin Gastroenterol Hepatol. 2008; 6: 696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Rao W, Wu X, Xiu D. Lamivudine or lamivudine combined with hepatitis B immunoglobulin in prophylaxis of hepatitis B recurrence after liver transplantation: a meta-analysis. Transpl Int. 2009; 22: 387. [DOI] [PubMed] [Google Scholar]
- 78. Katz LH, Paul M, Guy DG, et al. Prevention of recurrent hepatitis B virus infection after liver transplantation: hepatitis B immunoglobulin, antiviral drugs, or both? Systematic review and meta-analysis. Transpl Infect Dis. 2009; 12: 292. [DOI] [PubMed] [Google Scholar]
- 79. Cholongitas E, Papatheodoridis GV. High genetic barrier nucleos(t)ide analogue(s) for prophylaxis from hepatitis b virus recurrence after liver transplantation: a systematic review. Am J Transplant. 2013; 13: 353. [DOI] [PubMed] [Google Scholar]
- 80. Jimenez-Perez M, Saez-Gomez AB, Mongil Poce L, et al. Efficacy and safety of entecavir and/or tenofovir for prophylaxis and treatment of hepatitis B recurrence post-liver transplant. Transplant Proc. 2010; 42: 3167. [DOI] [PubMed] [Google Scholar]
- 81. Perrillo R, Buti M, Durand F, et al. Entecavir and hepatitis B immune globulin in patients undergoing liver transplantation for chronic hepatitis B. Liver Transpl. 2013; 19: 887– 895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sanchez-Fueyo A, Rimola A, Grande L, et al. Hepatitis B immunoglobulin discontinuation followed by hepatitis B virus vaccination: a new strategy in the prophylaxis of hepatitis B virus recurrence after liver transplantation. Hepatology. 2000; 31: 496. [DOI] [PubMed] [Google Scholar]
- 83. Angelico M, Di Paolo D, Trinito MO, et al. Failure of a reinforced triple course of hepatitis B vaccination in patients transplanted for HBV-related cirrhosis. Hepatology. 2002; 35: 176. [DOI] [PubMed] [Google Scholar]
- 84. Bienzle U, Gunther M, Neuhaus R, et al. Immunization with an adjuvant hepatitis B vaccine after liver transplantation for hepatitis B-related disease. Hepatology. 2003; 38: 811. [DOI] [PubMed] [Google Scholar]
- 85. Albeniz Arbizu E, Barcena Marugan R, Oton Nieto E, et al. Prophylaxis of recurrent hepatitis B virus by vaccination after liver transplant: preliminary results. Transplant Proc. 2003; 35: 1848. [DOI] [PubMed] [Google Scholar]
- 86. Lo CM, Liu CL, Chan SC, et al. Failure of hepatitis B vaccination in patients receiving lamivudine prophylaxis after liver transplantation for chronic hepatitis B. J Hepatol. 2005; 43: 283. [DOI] [PubMed] [Google Scholar]
- 87. Rosenau J, Hooman N, Hadem J, et al. Failure of hepatitis B vaccination with conventional HBsAg vaccine in patients with continuous HBIG prophylaxis after liver transplantation. Liver Transpl. 2007; 13: 367. [DOI] [PubMed] [Google Scholar]
- 88. Weber NK, Forman LM, Trotter JF. HBIg discontinuation with maintenance oral anti-viral therapy and HBV vaccination in liver transplant recipients. Dig Dis Sci. 2010; 55: 505. [DOI] [PubMed] [Google Scholar]
- 89. Di Paolo D, Lenci I, Cerocchi C, et al. One-year vaccination against hepatitis B virus with a MPL-vaccine in liver transplant patients for HBV-related cirrhosis. Transpl Int. 2010; 23: 1105. [DOI] [PubMed] [Google Scholar]
- 90. Gunther M, Neuhaus R, Bauer T, et al. Immunization with an adjuvant hepatitis B vaccine in liver transplant recipients: antibody decline and booster vaccination with conventional vaccine. Liver Transpl. 2006; 12: 316. [DOI] [PubMed] [Google Scholar]
- 91. Dodson SF, de Vera ME, Bonham CA, et al. Lamivudine after hepatitis B immune globulin is effective in preventing hepatitis B recurrence after liver transplantation. Liver Transpl. 2000; 6: 434. [DOI] [PubMed] [Google Scholar]
- 92. Naoumov NV, Lopes AR, Burra P, et al. Randomized trial of lamivudine versus hepatitis B immunoglobulin for long-term prophylaxis of hepatitis B recurrence after liver transplantation. J Hepatol. 2001; 34: 888. [DOI] [PubMed] [Google Scholar]
- 93. Buti M, Mas A, Prieto M, et al. A randomized study comparing lamivudine monotherapy after a short course of hepatitis B immune globulin (HBIg) and lamivudine with long-term lamivudine plus HBIg in the prevention of hepatitis B virus recurrence after liver transplantation. J Hepatol. 2003; 38: 811. [DOI] [PubMed] [Google Scholar]
- 94. Buti M, Mas A, Prieto M, et al. Adherence to Lamivudine after an early withdrawal of hepatitis B immune globulin plays an important role in the long-term prevention of hepatitis B virus recurrence. Transplantation. 2007; 84: 650. [DOI] [PubMed] [Google Scholar]
- 95. Wong SN, Chu CJ, Wai CT, et al. Low risk of hepatitis B virus recurrence after withdrawal of long-term hepatitis B immunoglobulin in patients receiving maintenance nucleos(t)ide analogue therapy. Liver Transpl. 2007; 13: 374. [DOI] [PubMed] [Google Scholar]
- 96. Neff GW, Kemmer N, Kaiser TE, et al. Combination therapy in liver transplant recipients with hepatitis B virus without hepatitis B immune globulin. Dig Dis Sci. 2007; 52: 2497. [DOI] [PubMed] [Google Scholar]
- 97. Angus PW, Patterson SJ, Strasser SI, et al. A randomized study of adefovir dipivoxil in place of HBIG in combination with lamivudine as post-liver transplantation hepatitis B prophylaxis. Hepatology. 2008; 48: 1460. [DOI] [PubMed] [Google Scholar]
- 98. Saab S, Desai S, Tsaoi D, et al. Posttransplantation hepatitis B prophylaxis with combination oral nucleoside and nucleotide analog therapy. Am J Transplant. 2011; 11: 511. [DOI] [PubMed] [Google Scholar]
- 99. Teperman LW, Poordad F, Bzowej N, et al. Randomized trial of emtricitabine/tenofovir disoproxil fumarate after hepatitis B immunoglobulin withdrawal after liver transplantation. Liver Transpl. 2013; 19: 594. [DOI] [PubMed] [Google Scholar]
- 100. Gane E, Patterson S, Strasser S, et al. Combination lamivudine plus adefovir without HBIG is safe and effective prophylaxis against HBV recurrence in HBsAg + liver transplant candidates. Liver Transpl. 2013; 19: 268. [DOI] [PubMed] [Google Scholar]
- 101. Nath DS, Kalis A, Nelson S, et al. Hepatitis B prophylaxis post-liver transplant without maintenance hepatitis B immunoglobulin therapy. Clin Transplant. 2006; 20: 206. [DOI] [PubMed] [Google Scholar]
- 102. Yi NJ, Choi JY, Suh KS, et al. Post-transplantation sequential entecavir monotherapy following 1-year combination therapy with hepatitis B immunoglobulin. J Gastroenterol. 2013; 48: 1401. [DOI] [PubMed] [Google Scholar]
- 103. Tanaka T, Renner EL, Selzner N, et al. One year of hepatitis B immunoglobulin plus tenofovir therapy is safe and effective in preventing recurrent hepatitis B post-liver transplantation. Can J Gastroenterol Hepatol. 2014; 28: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Saab S, Ham MY, Stone MA, et al. Decision analysis model for hepatitis B prophylaxis one year after liver transplantation. Liver Transpl 2009; 15: 413. [DOI] [PubMed] [Google Scholar]
- 105. Fung J, Chan SC, Cheung C, et al. Oral nucleoside/nucleotide analogs without hepatitis B immune globulin after liver transplantation for hepatitis B. Am J Gastroenterol. 2013; 108: 942. [DOI] [PubMed] [Google Scholar]
- 106. Lo CM, Liu CL, Lau GK, et al. Liver transplantation for chronic hepatitis B with lamivudine-resistant YMDD mutant using add-on adefovir dipivoxil plus lamivudine. Liver Transpl 2005; 11: 807. [DOI] [PubMed] [Google Scholar]
- 107. Yoshida H, Kato T, Levi DM, et al. Lamivudine monoprophylaxis for liver transplant recipients with non-replicating hepatitis B virus infection. Clin Transplant. 2007; 21: 166. [DOI] [PubMed] [Google Scholar]
- 108. Wadhawan MVV, Goyal N, Dargan P, et al. Living related liver transplant (LRLT) in HBV DNA negative cirrhosis without hepatitis B immune globulin (HBIG). Hepatol Int. 2011; 5: 38. [Google Scholar]


